Abstract
Background
Screening for undiagnosed type 2 diabetes mellitus is recommended for Asian Americans with a body mass index ≥23. However, the optimal body mass index cut-off score for predicting the risk of diabetes mellitus in Japanese people is not well known. The aim of this study was to determine the best body mass index cut-off score for predicting insulin resistance and diabetes mellitus in the Japanese population.
Methods
This study had two parts, a clinical investigation and a retrospective observational investigation. In the clinical part of the study, 58 participants (26 with type 2 diabetes mellitus and 32 non-diabetics) underwent a hyperinsulinemic-euglycemic clamp from which their glucose disposal rate was measured. For the retrospective part of the study, medical check-up data from 88,305 people in the Tottori Prefecture were analyzed for clinical evidence of diabetes mellitus. The optimal BMI cut-off scores for prediction of insulin resistance and diabetes mellitus were determined.
Results
In the clamp study, the optimal body mass index cut-off score to predict insulin resistance in non-diabetic patients was 22.7. All participants with type 2 diabetes mellitus were insulin resistant, and the optimal body mass index cut-off score for prediction of severe insulin resistance was 26.2. When the data from the type 2 diabetic and the non-diabetic participants were combined, the optimal body mass index cut-off score for prediction of insulin resistance was 23.5. Analysis of 88,305 medical check-up records yielded an optimal body mass index cut-off score for prediction of diabetes mellitus of 23.6.
Conclusions
These results suggest that having a body mass index ≥23 is a risk factor for insulin resistance and diabetes mellitus in the Japanese population.
Introduction
The pathophysiology of type 2 diabetes mellitus (T2DM) involves insulin resistance and impaired insulin secretion [1]. Obesity is a condition strongly associated with the development of insulin resistance and T2DM. The World Health Organization (WHO) defines obesity as a body mass index (BMI) >30 and overweight as a BMI of 25–30 [2]. The Japan Society for the Study of Obesity defines obesity as a BMI ≥25 [3]. Therefore, the risk of insulin resistance is an important consideration for those with a BMI ≥25. However, a WHO panel reported that Asian populations have a lower BMI cut-off point for T2DM and cardiovascular disease risk than the WHO criteria of BMI 25 [4]. The American Diabetes Association recently recommended that testing for diabetes should be considered for all Asian American adults who present with a BMI of ≥23 [5]. However, the BMI range for overweight has not been defined for Japanese people, and there are no guidelines that define optimal BMI cut-offs for predicting diabetes in the Japanese population.
The most precise method for assessing insulin resistance is the hyperinsulinemic-euglycemic clamp test; however, this test is very complicated [6]. Instead, the homeostasis model assessment for insulin resistance (HOMA-IR) is widely used in clinical practice and in clinical studies [7]. However, the reliability of HOMA-IR is limited in the patients with low BMI values, decreased beta-cell function, and/or high fasting glucose levels [8]. Since Asian and Japanese patients often have decreased beta-cell function [9], a clamp study is required for accurate evaluation of insulin resistance in these populations.
In this study, we aimed to determine the optimal BMI cut-off score for prediction of insulin resistance and diabetes mellitus (DM) in Japanese people using a glucose clamp test in patients with T2DM and in non-diabetic volunteers. We also analyzed the prevalence of diabetes in approximately 90,000 participants in Tottori Prefecture using data from medical check-ups.
Research design and methods
Subjects
This study consisted of a clinical investigation and a retrospective analysis of medical check-up records. A total of 58 volunteers participated in the clinical investigation. Twenty-six of these were patients treated at the Tottori University Hospital between 2014 and 2017 who were diagnosed with T2DM using the WHO criteria [10]. Prospective participants with pancreatic disease, liver disease, or renal failure, and those taking diabetogenic medications such as corticosteroids, were excluded from this study. All participants in the T2DM group were on diet therapy alone and were not taking any medications aimed at treating their T2DM. Thirty-two non-diabetic volunteers (Non-DM group) also participated in this study. The Non-DM participants were residents of Tottori Prefecture who received a routine medical check-up at our institute; they were recruited to participate in the glucose clamp study by written request after their check-up showed that they did not have, and were not taking medication for, T2DM, hypertension, or dyslipidemia. This study was conducted according to the principles expressed in the Declaration of Helsinki, and approved by the Ethics Committee of the Faculty of Medicine at Tottori University (approval number G161). Written informed consent was obtained from all of the participants prior to their participation in this study.
Hyperinsulinemic-euglycemic clamp
We performed glucose clamp tests as previously reported [11]. Briefly, the hyperinsulinemic-euglycemic clamp was performed using an artificial endocrine pancreas (STG 55; Nikkiso, Shizuoka, Japan) to evaluate insulin sensitivity [1]. A primed constant infusion of insulin (100 mU/m2/min) was administered to each participant and plasma glucose levels were maintained at 5.2 mmol/L (95 mg/dL). According to previous studies, this method achieves a steady-state plasma insulin level of 1200 pmol/L in patients with T2DM [12, 13]. The steady-state glucose infusion rate (GIR) from 90–120 min after the start of the infusion. The mean GIR during this time was defined as the glucose disposal rate (GDR), which was used as a marker of peripheral insulin sensitivity. The glucose clamp method is a well-established procedure at our hospital [11, 14].
At the insulin infusion rate used in this protocol, we defined GDR >10 mg/kg/min as normal, GDR <10 mg/kg/min as insulin resistance [15, 16, 17], and GDR <5 mg/kg/min as severe insulin resistance [18].
Calculation of insulin resistance indices
Insulin resistance was calculated as follows: HOMA-IR [8] = [fasting plasma glucose (mmol/L)] × [fasting plasma insulin (pmol/L)]/135.
DM prevalence in patients undergoing medical check-ups in Tottori Prefecture
We detected the prevalence of DM in Tottori Prefecture using medical check-up data from patients in this region in 2013. In diabetic patients, the characteristics of the diabetes were not always indicated in the portion of the record that we examined; therefore, patients were classified as having DM or not but were not specifically classified as having T2DM. This retrospective, observational, epidemiological study was approved by the Insurer Council of Tottori Prefecture, who gave their written informed consent allowing use of their anonymized data. No personal identifying information was present in any of the medical check-up records used. According to the Japanese Ethical Guidelines for Epidemiological Research, informed consent does not necessarily need to be obtained from research subjects in observational studies using only existing materials, particularly when subjects are not identifiable in the records used [19]. DM was defined as follows: fasting plasma glucose (FPG) ≥126 mg/dl or glycated hemoglobin (HbA1c) ≥6.5%, or (because not all participants were assessed for FPG and HbA1c) a history of treatment for DM. The numbers of patients assessed for each measure were: FPG, not HbA1c, 45,411; HbA1c, not FPG, 18,314; both FPG and HbA1c, 22,851.
Statistical analysis
Data are expressed as means ± standard deviation of the mean. Differences in mean values of characteristics between T2DM and Non-DM participants were assessed using unpaired t-tests. We determined BMI cut-off values using receiver operating characteristic (ROC) analysis. To evaluate the ability of BMI to predict insulin resistance in the clamp study, and DM in the medical check-up study, we plotted ROC curves. Diagnostic properties of BMI cut-off scores were defined by maximizing the sensitivity and specificity to identify GDR <10 and the presence of DM. The optimal cut-off points were obtained using ROC curves and the Youden Index (maximum [sensitivity + specificity − 1]) [20]. The cut-off point on the ROC curve was closest to (0,1), and was calculated as the minimum value of the square root of ([1 − sensitivity]2 + [1 − specificity]2) [21]. Values were considered statistically significant if P <0.05. SPSS software version 26.0 (IBM Corp., Armonk, NY, USA) was used for all analyses.
Results
Cut-off BMI scores for detection of insulin resistance determined by hyperinsulinemic-euglycemic clamp
The characteristics of the participants in the T2DM and Non-DM groups are shown in Table 1. Mean age, BMI, waist circumstance, FPG, HbA1c, and HOMA-IR were all significantly higher in the T2DM group than in the Non-DM group, and the mean GDR was significantly lower in the DM group than the non-DM group.
Table 1. Participant characteristics in the glucose clamp study.
| Total | T2DM | Non-DM | P value | |
|---|---|---|---|---|
| n | 58 | 26 | 32 | |
| Sex (male/female) | 25/23 | 16/10 | 19/13 | |
| Age (years) | 41.9 ± 14.9 | 54.0 ± 12.0 | 32.1 ± 8.3 | <0.001 |
| BMI (kg/m2) | 24.0 ± 4.4 | 26.9 ± 4.1 | 21.8 ± 2.9 | <0.001 |
| Waist circumference (cm) | 85.8 ± 13.7 | 94.3 ± 11.0 | 76.2 ± 9.8 | <0.001 |
| Fasting plasma glucose (mmol/L) | 5.6 ± 1.6 | 6.9 ± 1.0 | 4.5 ± 1.2 | <0.001 |
| HbA1c (%) | 6.3 ± 1.2 | 7.4 ± 0.9 | 5.3 ± 0.3 | <0.001 |
| HbA1c (mmol/mol) | 45 ± 58.5 | 57 ± 10 | 34 ± 4 | <0.001 |
| HOMA-IR | 2.4 ± 2.0 | 3.5 ± 2.7 | 1.5 ± 1.0 | <0.001 |
| GDR (mg/kg/min) | 7.9 ± 3.0 | 5.7 ± 2.1 | 9.6 ± 2.5 | <0.001 |
Data are means ± standard deviation. P-values were determined using unpaired t-tests, T2DM vs. non-DM. BMI, body mass index; GDR, glucose disposal rate; HbA1c, glycated hemoglobin; HOMA-IR, homeostasis model assessment for insulin resistance; Non-DM, non-diabetic study participants; T2DM, study participants with type 2 diabetes mellitus
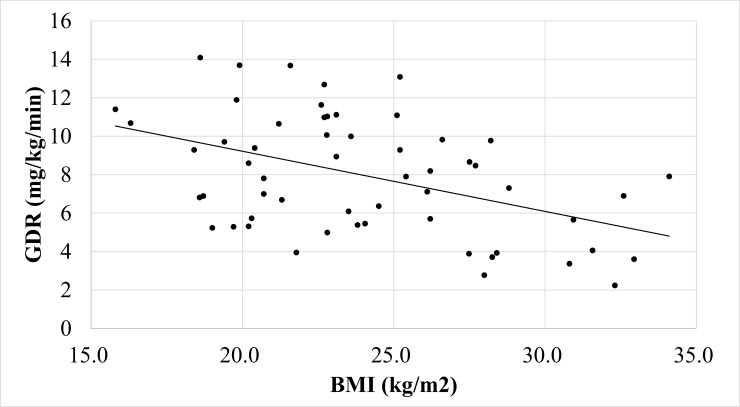
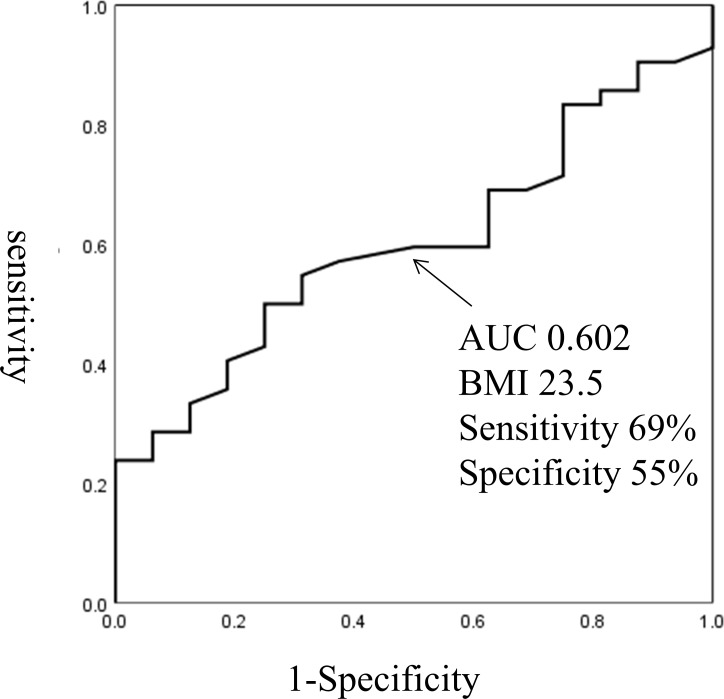
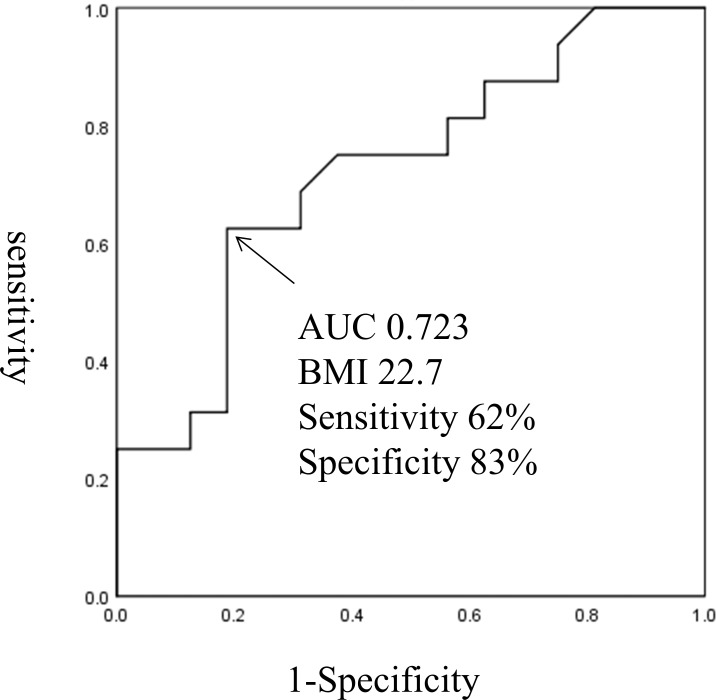
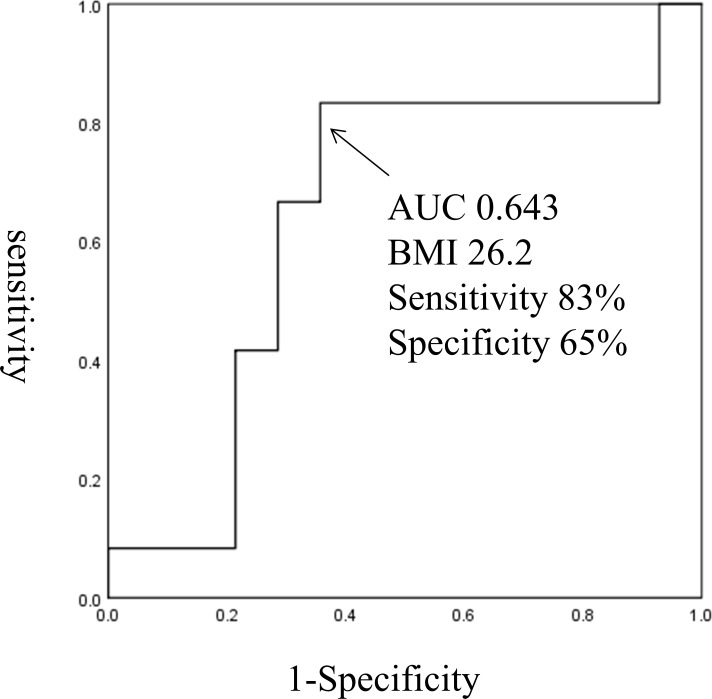
BMI was significantly correlated with GDR (R = 0.67, P<0.0001; Fig 1). Of the 58 participants in the clamp study, 42 were insulin resistant (defined as having a GDR <10). The optimal BMI cut-off score for prediction of insulin resistance was 23.5 (69% sensitivity, 55% specificity, AUC 0.602; Fig 2). Of the 32 Non-DM participants, 16 had insulin resistance, defined as GDR <10. The optimal BMI cut-off score to predict insulin resistance in the Non-DM participants was 22.7 (62% sensitivity, 83% specificity, AUC 0.723; Fig 3). Of the 26 T2DM participants, all had insulin resistance and 12 had severe insulin resistance defined as GDR <5.0. The optimal BMI cut-off score to predict severe insulin resistance in the T2DM group was 26.2 (83% sensitivity, 65% specificity, AUC 0.643; Fig 4).
Fig 1. The correlation between BMI and GDR in the all participants in the clamp study.
Fig 2. The optimal BMI cut-off score for prediction of insulin resistance in all participants in the T2DM and Non-DM groups combined.
Fig 3. The optimal BMI cut-off score for prediction of insulin resistance in the Non-DM participants.
Fig 4. The optimal BMI cut-off score for prediction of severe insulin resistance in the T2DM group.
Cut-off BMI scores for detection of DM determined from analysis of medical check-up records
We analyzed the medical check-up records of 88,305 people in Tottori Prefecture and detected 8,311 people with T2DM (Table 2). The mean age of people with DM was 62.7 years, and mean BMI was 22.8 kg/m2, 5,229 had a history of DM treatment, 2,231 had FPG ≥126 mg/dl, 1,303 had HbA1c ≥6.5%, and 452 had FPG ≥126 mg/dl and HbA1c ≥6.5%.
Table 2. Participant characteristics in the medical check-up study.
| Total | DM | Non-DM | P value | |
|---|---|---|---|---|
| n | 88,305 | 8,316 | 79,989 | |
| Sex (male/female) | 41,518/46,787 | 5,116/3,200 | 36,402/43,587 | |
| Age (years) | 62.7 ± 13.3 | 67.0 ± 11.4 | 62.3 ± 13.4 | <0.001 |
| BMI (kg/m2) | 22.8 ± 3.3 | 24.2 ± 3.8 | 22.6 ± 3.2 | <0.001 |
| Waist circumference (cm) | 82.1 ± 9.5 | 87.3 ± 10.1 | 81.6 ± 9.3 | <0.001 |
| Fasting plasma glucose (mmol/L) | 5.3 ± 1.4 | 7.7 ± 2.3 | 5.1 ± 1.0 | <0.001 |
| HbA1c (%) | 5.5 ± 1.2 | 6.9 ± 2.0 | 5.4 ± 1.0 | <0.001 |
| HbA1c (mmol/mol) | 37 ± 13 | 52 ± 22 | 36 ± 10 | <0.001 |
Data are means ± standard deviation. BMI, body mass index; HbA1c, glycated hemoglobin; DM, study participants with diabetes mellitus; Non-DM, non-diabetic study participants. P-values were determined using unpaired t-tests, DM vs. non-DM.
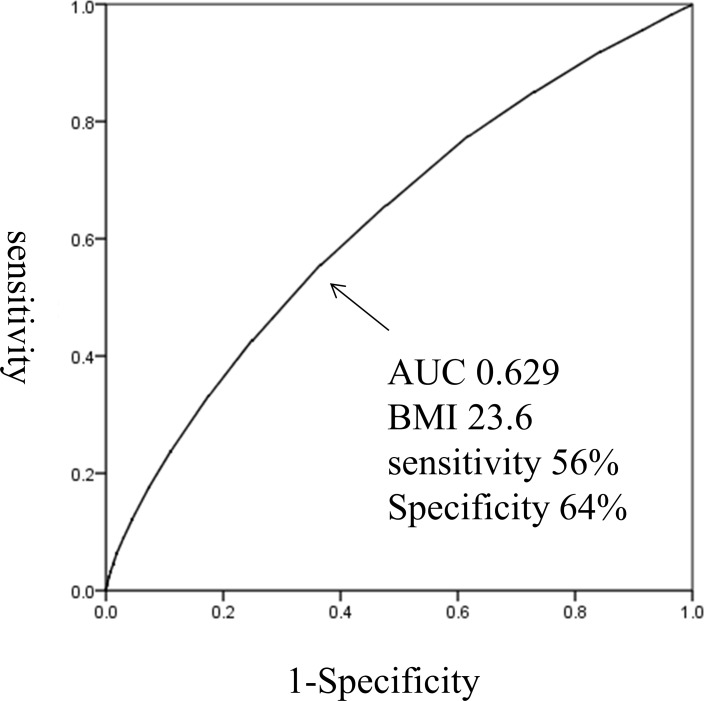
The optimal BMI cut-off score for detection of DM in this population was 23.6 (56% sensitivity, 64% specificity, AUC 0.629; Fig 5).
Fig 5. The optimal BMI cut-off for detection of DM in patient in Tottori Prefecture.
Discussion
The results of this study indicate that the optimal BMI cut-off score for prediction of insulin resistance in all of the Non-DM and T2DM participants in the glucose clamp study was 23.5. The optimal BMI cut-off score for prediction of insulin resistance in Non-DM participants using the glucose clamp method was 22.7. In the analysis of medical check-up data, the optimal BMI cut-off score to predict DM was 23.6. These results suggest that having a BMI ≥23 is a risk factor for insulin resistance and DM in the Japanese population. This is consistent with the recommendation of the American Diabetes Association that testing for diabetes should be considered for all Asian American adults who present with a BMI of ≥23 [5]. A recent study also suggested that, among Japanese men with BMI ranging from 23–25, muscle insulin resistance was present in those with even one cardiometabolic risk factor (hypertension, hyperglycemia, or dyslipidemia) [22]. Because the Non-DM participants in our study did not have hypertension, hyperglycemia, or dyslipidemia, our results show that there is a risk of insulin resistance in Japanese people who have a BMI ≥23 even without the presence of cardiometabolic risk factors. In addition, all T2DM participants in the clamp study were insulin resistant, and the BMI cut-off score for detecting severe insulin resistance was 26.2. These results suggest that most Japanese people with T2DM are insulin resistant and that having a BMI >25 is a risk factor for severe insulin resistance. These results have important implications for clinical practice in Japan.
There are few Japanese population-based reports of BMI cut-off scores for predicting diabetes diagnosed by the oral glucose tolerance test (OGTT). The Japan Diabetes Complication Study showed that the mean BMI of 2,205 patients with T2DM was 23.1; however, this study did not perform OGTTs [23]. A pilot study reported that the optimal BMI cut-off value for prediction of worsening glucose metabolism diagnosed by OGTT was 23.1 kg/m2 in 604 Japanese participants [24]. These results and our results indicate that BMI >23 is a risk factor for diabetes in Japanese people. However, further studies using OGTTs and larger numbers of subjects are needed.
Our study had several limitations. The relatively small number of participants who underwent the glucose clamp and the differences in age and BMI between the T2DM and Non-DM groups indicate that our results require confirmation in a larger study. There are several important risk factors for insulin resistance that we were unable to control for in our study. These include age, body composition, and gender, all of which could have affected our BMI cut-off analyses. In our clamp study there was no significant difference in the sex ratio between the T2DM and Non-DM group; however, participants in the T2DM group were significantly older than those in the Non-DM group. It is possible that this could have influenced our results. However, it has been previously shown that, when age is taken into account, BMI is still a significant risk factor for T2DM [25]. Thus, we feel that our BMI cut-off values are still relevant even with our relatively small sample size. We are currently working on a larger study, the results of which we plan to publish in the future.
In the analysis of medical check-up records, it was not always possible to determine the type of DM (type 1, type 2, or other) from the portion of the record that we had access to. However, type 1 DM is rare in the Japanese population; the prevalence rate in Japan has been reported to be 10–15 per 100,000 [26]. Therefore, most participants with diabetes can be assumed to have T2DM. Moreover, a subset of participants with diabetes was diagnosed using FPG or HbA1c, and the AUC value of ROC analysis was low. Because there was no information on medication use in the records that we analyzed, it is possible that the use of medications such as insulin or insulin-sensitizers may have affected our study outcome. It is also important to consider other confounding factors such as age, body composition, and gender differences. However, despite these limitations we consider the results generated from this population-based analysis with a large number of participants to be meaningful. In the future, we plan to build on these results by conducting a population-based OGTT study. Despite its limitations, we believe that our study contributes information that may be important to screening strategies for insulin resistance and diabetes in the Japanese population.
In summary, this study showed that the optimal BMI cut-off score for prediction of insulin resistance in Non-DM participants diagnosed using a glucose clamp was 22.7. For the Non-DM and T2DM participants combined, the optimal BMI cut-off score for prediction of insulin resistance was 23.5. In the population-based medical record analysis, the optimal BMI cut-off score for prediction of DM was 23.6. These results suggest that having a BMI ≥23 is a risk factor for insulin resistance and DM in the Japanese population.
Acknowledgments
All authors read and approved the final manuscript.
We thank Ms. Megumi Endo and Ms. Yoshiko Oda for their excellent technical assistance. We also thank Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript. This work was carried out at the Tottori University Faculty of Medicine, Tottori, Japan.
TO is the guarantor of this work and takes responsibility for the integrity of the data and the accuracy of the data analyses.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported by the JSPS KAKENHI Grant-in-Aid for Scientific Research (C) grant number 16K08935.
References
- 1.DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 1988; 37: 667–687 [DOI] [PubMed] [Google Scholar]
- 2.WHO. Obesity: preventing and managing the global epidemic. Report of a WHO Consultation. WHO Technical Report Series 894. Geneva: World Health Organization, 2000 [PubMed]
- 3.Examination Committee of Criteria for 'Obesity Disease' in Japan; Japan Society for the Study of Obesity. New criteria for 'obesity disease' in Japan. Circ J. 2002; 66: 987–92 [DOI] [PubMed]
- 4.WHO expert consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363: 157–63 [DOI] [PubMed]
- 5.Hsu WC, Araneta MR, Kanaya AM, Chiang JL, Fujimoto W, BMI cut points to identify at-risk Asian Americans for type 2 diabetes screening. Diabetes Care. 2015; 38: 150–8 10.2337/dc14-2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979; 237: 214–223 [DOI] [PubMed] [Google Scholar]
- 7.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419 [DOI] [PubMed] [Google Scholar]
- 8.Kang ES, Yun YS, Park SW, Kim HJ, Ahn CW, Song YD, Cha BS, Lim SK, Kim KR, Lee HC. Limitation of the validity of the homeostasis model assessment as an index of insulin resistance in Korea. Metabolism 2005; 54: 206–211 10.1016/j.metabol.2004.08.014 [DOI] [PubMed] [Google Scholar]
- 9.Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care 2013; 36: 1789–1796 10.2337/dc12-1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alberti KG, Zimmet PZ: Definition, diagnosis and classification of diabetes mellitus and its complications. Part1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998; 15: 539–553 [DOI] [PubMed] [Google Scholar]
- 11.Ohkura T, Shiochi H, Fujioka Y, Sumi K, Yamamoto N, Matsuzawa K, Izawa S, Kinoshita H, Ohkura H, Kato M, Taniguchi SI, Yamamoto K. 20/(fasting C-peptide x fasting plasma glucose) is a simple and effective index of insulin resistance in patients with type 2 diabetes mellitus: a preliminary report. Cardiovasc Diabetol 2013;12:21 10.1186/1475-2840-12-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawamori R, Matsuhisa M, Kinoshita J, Mochizuki K, Niwa M, Arisaka T, Ikeda M, Kubota M, Wada M, Kanda T, Ikebuchi M, Tohdo R, Yamasaki Y. Pioglitazone enhances splanchnic glucose uptake as well as peripheral glucose uptake in non-insulin-dependent diabetes mellitus. AD-4833 Clamp-OGL Study Group. Diabetes Res Clin Pract 1998; 41: 35–43 [DOI] [PubMed] [Google Scholar]
- 13.Tamura Y, Tanaka Y, Sato F, Choi JB, Watada H, Niwa M, Kinoshita J, Ooka A, Kumashiro N, Igarashi Y, Kyogoku S, Maehara T, Kawasumi M, Hirose T, Kawamori R. Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab 2005; 90: 3191–3196 10.1210/jc.2004-1959 [DOI] [PubMed] [Google Scholar]
- 14.Okura T, Ueta E, Nakamura R, Fujioka Y, Sumi K, Matsumoto K, Shoji K, Matsuzawa K, Izawa S, Nomi Y, Mihara H, Otsuka Y, Kato M, Taniguchi SI, Yamamoto K. High Serum Advanced Glycation End Products Are Associated with Decreased Insulin Secretion in Patients with Type 2 Diabetes: A Brief Report. J Diabetes Res. 2017;2017:5139750 10.1155/2017/5139750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakurai Y, Tamura Y, Takeno K, Kumashiro N, Sato F, Kakehi S, Ikeda S, Ogura Y, Saga N, Naito H, Katamoto S, Fujitani Y, Hirose T, Kawamori R, Watada H. Determinants of intramyocellular lipid accumulation after dietary fat loading in non-obese men. J Diabetes Invest 2011; 4: 310–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawaguchi M, Tamura Y, Kakehi S, Takeno K, Sakurai Y, Watanabe T, Funayama T, Sato F, Ikeda S, Ogura Y, Saga N, Naito H, Fujitani Y, Kanazawa A, Kawamori R, Watada H. Association between expression of FABPpm in skeletal muscle and insulin sensitivity in intramyocellular lipid-accumulated nonobese men. J Clin Endocrinol Metab. 2014;99:3343–52 10.1210/jc.2014-1896 [DOI] [PubMed] [Google Scholar]
- 17.Kakehi S, Tamura Y, Takeno K, Sakurai Y, Kawaguchi M, Watanabe T, Funayama T, Sato F, Ikeda S, Kanazawa A, Fujitani Y, Kawamori R, Watada H. Increased intramyocellular lipid/impaired insulin sensitivity is associated with altered lipid metabolic genes in muscle of high responders to a high-fat diet. Am J Physiol Endocrinol Metab. 2016;310:E32–40 10.1152/ajpendo.00220.2015 [DOI] [PubMed] [Google Scholar]
- 18.Sato F, Tamura Y, Watada H, Kawamori R, Kumashiro N, Igarashi Y, Uchino H, Maehara T, Kyogoku S, Sunayama S, Sato H, Hirose T, Tanaka Y, Kawamori R. Effects of diet-induced moderate weight reduction on intrahepatic and intramyocellular triglycerides and glucose metabolism in obese subjects. J Clin Endocrinol Metab 2007; 92: 3326–3329 10.1210/jc.2006-2384 [DOI] [PubMed] [Google Scholar]
- 19.The Ministry of Health, Labor and Welfare, Ministry of Education, Culture, Sports, Science and Technology. Ethical guidelines for epidemiological research. 2002. Available from: http://www.niph.go.jp/wadai/ekigakurinri/guidelines.pdf
- 20.Youden WJ. Index for rating diagnostic tests. Cancer 1950; 3: 32–35 [DOI] [PubMed] [Google Scholar]
- 21.Perkins NJ, Schisterman EF. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol 2006; 163: 670–675 10.1093/aje/kwj063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeno K, Tamura Y, Kawaguchi M, Kakehi S, Watanabe T, Funayama T, Furukawa Y, Kaga H, Yamamoto R, Kim M, Nishitani-Yokoyama M, Shimada K, Daida H, Aoki S, Taka H, Fujimura T, Sawada SS, Giacca A, Kanazawa A, Fujitani Y, Kawamori R, Watada H. Relation Between Insulin Sensitivity and Metabolic Abnormalities in Japanese Men With BMI of 23–25 kg/m2. J Clin Endocrinol Metab. 2016; 101: 3676–3684 10.1210/jc.2016-1650 [DOI] [PubMed] [Google Scholar]
- 23.Sone H, Ito H, Ohashi Y, Akanuma Y, Yamada N; Japan Diabetes Complication Study Group. Obesity and type 2 diabetes in Japanese patients. Lancet 2016; 361: 85. [DOI] [PubMed] [Google Scholar]
- 24.Yamauchi K, Oka R, Yagi K, Hayashi K, Kawashiri MA, Yamagishi M, Shimbo T, Aizawa T. Population impact of increased body mass index and attenuated beta-cell function on worsening of glucose metabolism in subjects with normal glucose tolerance: a pilot study. Acta Diabetol. 2014; 51: 441–5 10.1007/s00592-013-0535-1 [DOI] [PubMed] [Google Scholar]
- 25.Perry IJ, Wannamethee SG, Walker MK, Thomson AG, Whincup PH, Shaper AG. Prospective study of risk factors for development of non-insulin dependent diabetes in middle aged British men. BMJ. 1995; 310: 560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akazawa Y. Prevalence and incidence of diabetes mellitus by WHO criteria. Diabetes Res Clin Pract. 1994; 24: 23–7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.