Abstract
Purpose
Neovascularization (NV) and retinal vascular leakage are major causes of impaired vision in ocular diseases. The purpose of this study was to identify novel phenylphthalimide analogs with therapeutic effects on NV and vascular leakage and to explore the mechanism of action.
Methods
Antiangiogenic activities of novel phenylphthalimide analogs were assessed in vitro by using VEGF ELISA and endothelial cell proliferation assay. Their efficacies on retinal vascular leakage were evaluated using rat models of oxygen-induced retinopathy (OIR) and streptozotocin (STZ)-induced diabetes. The in vivo antiangiogenic activity was evaluated using topical administration in the alkali burn-induced corneal NV model. The expression of VEGF and intercellular adhesion molecule-1 (ICAM-1) were measured using ELISA.
Results
Thalidomide and three novel analogs all showed inhibitory effects on endothelial cell proliferation and VEGF expression in vitro. Through intravitreal injection, all of the compounds reduced retinal vascular leakage in the OIR and STZ-induced diabetic models. Among these compounds, (2,6-diisopropylphenyl)-5-amino-1H-isoindole-1,3-dione (DAID) displayed the most potent efficacy and reduced retinal vascular leakage in a dose-dependent manner in both the OIR and STZ-diabetes models. Topical administration of DAID also inhibited alkali burn-induced corneal NV. Furthermore, DAID attenuated the overexpression of VEGF and ICAM-1 in the retina of the OIR model. Intravitreal injection of DAID did not result in any detectable side effects, as shown by electroretinogram and retinal histological analysis.
Conclusions
DAID is a novel phenylphthalimide analog with potent effects on NV and retinal vascular leakage through downregulation of VEGF and inflammatory factors and has therapeutic potential.
Keywords: angiogenesis, inflammation, neovascularization, phenylphthalimide analog, retinal vascular leakage, thalidomide, vascular endothelial growth factor
Ocular neovascularization (NV) is associated with a number of ocular diseases such as various corneal diseases, diabetic retinopathy (DR), retinopathy of prematurity (ROP), and age-related macular degeneration (AMD). NV is the leading cause of severe vision loss and blindness.1–3 Corneal NV is characterized by the invasion of new capillaries into the cornea arising from the pericorneal plexus. The abnormal growth of new vessels in corneal NV may lead to lipid exudation, persistent inflammation, and scarring, thus threatening corneal transparency and visual acuity.4 Retinal NV in proliferative DR can ultimately cause severe vitreous cavity hemorrhage and/or retinal detachment. Diabetic macular edema (DME), which can occur at any stage of DR, results from retinal vascular leakage and is associated with vascular inflammation.5 Unwanted vessels in AMD, ROP, and DR have high permeability, which can lead to macular edema and macrophage infiltration, thereby exacerbating retinal inflammation.6
The imbalance between endogenous angiogenic factors and angiogenic inhibitors is responsible for the formation of pathologic vessels.7 It has been shown that multiple angiogenic factors, such as VEGF, insulin-like growth factor, and erythropoietin, are implicated in the pathogenesis of DR and AMD.8,9 VEGF expression is upregulated in DR, ROP, AMD, and inflammation-associated corneal NV.10–12 Alterations of these growth factors and their receptors have been identified in corneal NV, DR, ROP, and AMD in both experimental models and clinical studies.13–15
In recent years, large-molecule VEGF inhibitors have shown beneficial effects on corneal NV. Anti-VEGF agents have demonstrated efficacies in reducing ocular NV in both animal models and clinical trials.16 In particular, topical administration of an anti-VEGF antibody attenuated corneal NV in experimental animal models.17,18 Previous studies indicate that VEGF is one of the key mediators of retinal vascular hyperpermeability in diabetes.19–21 Retinal VEGF levels are correlated with high retinal vascular permeability in oxygen-induced retinopathy (OIR) and streptozotocin (STZ)-induced diabetic rats.22–25 Overexpression of VEGF and its receptors is associated with vascular hyperpermeability in the retina of STZ-induced diabetes.26 Regarding choroidal neovascularization (CNV) in AMD, several lines of evidence have indicated that growth factors, such as VEGF, also play pivotal roles. Elevated VEGF levels in the plasma have been reported in AMD patients.27,28 NV and retinal vascular leakage were effectively ameliorated by anti-VEGF treatment in AMD patients.29
Overexpression of VEGF is believed to play a critical role in the development of abnormal angiogenesis and vascular leakage.26,30,31 Targeting VEGF or VEGF signaling has proven to be a promising strategy for pharmacologic interventions of ocular NV.32–35 Therefore, searching for and developing new small-molecule drugs to block retinal vascular leakage and NV via targeting VEGF or the VEGF receptor represent a major effort for the treatment of ocular NV and vascular leakage.
Thalidomide (α-[N-phthalimido]-glutarimide, C13H10N2O4) and some of its existing analogs have displayed antiangiogenic activities and have been used for the treatment of a number of inflammatory diseases and cancer.36–39 Thalidomide can attenuate the increase of VEGF in ocular fluid and inhibit the thickening of retinal capillary basement membrane in STZ-induced diabetic rats, thus representing a potential therapeutic drug for DR.40 However, in addition to teratogenicity, thalidomide also has other adverse effects, including peripheral neuropathy, hyperglycemia, and impairment of insulin action.41–43 The application of thalidomide analogs in the treatment of retinal vascular leakage and ocular NV has not been reported. This is mainly because thalidomide and the existing analogs possess weak antiangiogenic activities, and, thus, high doses are required for the effects on NV and vascular leakage, which may consequently result in adverse effects.
In the present study, we have designed and synthesized a series of novel phenylphthalimide analogs by substituting the glutarimide ring with an aromatic group.44 In vitro studies have shown that some of these analogs have antiproliferative and antimitotic activities.45 Here, we evaluated the antiangiogenic effects of three analogs. Our findings demonstrated that the compound DAID has more potent effects on inflammation and NV than thalidomide and other compounds, suggesting that it is a promising drug candidate for ocular NV.
Materials and Methods
Animal Experiments
The animal study was conducted in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The protocol was approved by Institutional Animal Care and Use Committee of University of Oklahoma Health Sciences Center.
Synthesis of Phenylphthalimide Analogs
Thalidomide was purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA). The compound 5-hydroxy-(2,6-diisopropylphenyl)-1H-isoindole-1,3-dione (HDID), compound (2,6-Diisopropylphenyl)-isoindole-1,3-dione (DID), and compound (2,6-diisopropylphenyl)-5-amino-1H-isoindole-1,3-dione (DAID) were synthesized as described previously.44
Preparation of DAID Eye Drops
The 0.25% DAID microemulsion eyedrops were prepared to treat corneal NV in the cornea alkali burn rat model. All of the ingredients included polysorbate 20 (Millipore Co., Bellerica, MA, USA), polysorbate 80 (Nanjing Well Chemical Co., Ltd, Nanjing, China), and isopropyl myristate (Millipore Co.). They were mixed together according to the percentage of weight (100-ml eyedrops include 0.25 g DAID, 3.6 g isopropyl myristate, 22 g polysorbate 20, and 22 g polysorbate 80) and stirred overnight. The average size of the nanoparticles of DAID was 2.505 nm.
Cell Culture
All cell culture media and supplements were purchased from Cellgro (Mediatech, Inc., Tewksbury, MA, USA) unless otherwise indicated. Bovine retinal endothelial cells (BRECs) and pericytes were isolated according to a modified method, as described previously.46 Bovine eyes were obtained from a local slaughterhouse (Country Home Meats, Oklahoma City, OK, USA). The retinas were removed, washed four times in Dulbecco's modified Eagle's medium (DMEM), dispersed, and centrifuged at 400g for 10 minutes. The resultant pellet was resuspended in an isolation medium (DMEM with 100 IU/ml penicillin, 100 μg/ml streptomycin, and 250 ng/ml amphotericin). Microvessels were trapped on an 85-μm nylon mesh and transferred to a petri dish (Falcon; Life Science, Corning, NY, USA) containing 10 ml of an enzyme cocktail, which contains 600 μg/ml DNase I, 165 μg/ml collagenase, and 700 μg/ml pronase E (Sigma-Aldrich Corp.) and were incubated at 37°C for 20 minutes. The resultant vessel fragments were trapped on a 53-μm nylon mesh, washed with the isolation medium, and centrifuged at 400g for 5 minutes. For selective culture of pericytes, the resultant pellet was resuspended in 10 ml of the pericyte growth medium and transferred into 75-cm2 plastic tissue culture flasks.
For selective culture of BRECs, the resultant cell pellet was resuspended in 10 ml of the BREC growth medium and transferred into 75-cm2 collagen-coated plastic tissue culture flasks. The BREC growth medium consisted of DMEM supplemented with 10% human serum, 1% glutamine, 1 mg/ml insulin, 550 μg/ml transferrin, 670 ng/ml selenium, 100 IU/ml penicillin, 100 μg/ml streptomycin, 250 ng/ml amphotericin, 90 μg/ml heparin (Sigma-Aldrich Corp.), and 15 μg/ml endothelial cell growth supplement. Cells were cultured at 37°C and 5% CO2. Confluent cultures were passaged by detaching the cells with 0.25% trypsin and plated at a split 1:3. The purity of BRECs and pericytes were confirmed by binding of Dil-Ac-LDL (Biomedical Technologies, Inc., Stoughton, MA, USA) to the LDL receptor on the surface of BRECs and immunolabeling with an anti-smooth muscle actin antibody (Sigma-Aldrich Corp.), respectively. At passage 2, BRECs and pericytes were stored in liquid nitrogen for future use.
MTT Assay
Cells were seeded in 24-well plates or gelatin-coated 24-well plates at a density of 5 × 104 cells per well in 400 μl of growth medium in triplicate. Twenty-four hours after seeding, the growth medium was replaced by a medium containing 1% fetal bovine serum, with or without different concentrations of thalidomide or phenylphthalimide analogs. After the cells were treated for 48 to 72 hours, MTT was added to a final concentration of 0.5% and incubated for 4 hours at 37°C in 5% CO2. An equal volume of solubilization buffer was then added, following the protocol recommended by the manufacturer (Roche Molecular Biochemicals, Mannheim, Germany) and incubated with the cells overnight at 37°C in 5% CO2. The absorbance of the formazan product was measured at a wavelength of 570 nm.
Enzyme-Linked Immunosorbent Assay
Cell-free conditioned media were collected 24 hours after the treatment with the compounds. The retinas were dissected from experimental rats, sonicated, and centrifuged at 4°C and 3000g for 10 minutes. The equal amounts of proteins from cell-free conditioned media of each treatment and from the retina homogenates from normal rats, vehicle-treated, and DAID-treated OIR rats were used for VEGF ELISA using an ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's protocols.
Corneal Alkali Burn in Rats
Sprague Dawley rats (male, body weight 250–275 g; obtained from Charles River Laboratories International, Inc., Wilmington, MA, USA) were anesthetized by intraperitoneal injection of ketamine (75 mg/kg, Zetamine; MWI, Boise, ID, USA) and xylazine (10 mg/kg, AnaSed LA; MWI, Boise, ID, USA). A corneal alkali burn was generated in the right eye of each anesthetized rat. A piece of 4-mm diameter Whatman GF/A (Whatman International Ltd., Maidstone, England) filter paper was soaked in NaOH (1 N) and applied to the center of the right cornea for 40 seconds. The ocular surface was then immediately rinsed with 60 ml of normal saline.47
All rats were randomly assigned into two groups. The first group (n = 7) was treated topically with the DAID eye drops on the right corneas immediately after alkali burn of the cornea. The animals were held on for at least 1 minute after eye drop instillation to prevent from claw scratching. The instillation was continued twice daily for 7 days. Rats in the second group (n = 7) were instilled with the same volume of vehicle eye drops in the same way.
Evaluation of Corneal NV
On the third and seventh day post–alkali burn, all animals were anesthetized and the corneal opacity and NV were examined. All observations were performed by an experienced ophthalmologist who was blinded to the allocation of the animals in each group. The pupils were dilated with a phenylephrine hydrochloride ophthalmic solution, USP 10% (Paragon BioTeck, Inc., Portland, OR, USA). The corneal opacity, NV, and hyphema were observed and recorded. Moreover, all animals were examined under a microscope. The lengths of new corneal vessels were measured with regard to sclera-corneal limbus in the four quadrants, that is superior, inferior, temporal, and nasal. Corneal NV was quantified by calculating the wedge-shaped area (S) of the vessel growth by using the following formula: S = C/12 × 3.1416 × [r2 − (r − L)2], where S is the area, C is the number of clock hours, L is the length of new vessels from the limbus, and r is the radius of individual rat cornea.48–50
Perfusion With India Ink
On day 7 postinjury, the rats were euthanized. The chest cavity was carefully opened and a 25-G perfusion needle (Safety-Lok; Becton and Dickinson Company, Franklin Lakes, NJ, USA) was introduced into the left ventricle. The drainage was achieved by cutting the edge of the right atrium with the blockage of abdominal aorta. Animals were perfused using a pump (P-1, Pharmacia Biotech; Pharmacia Biotech, Inc., Piscataway, NJ, USA) firstly with phosphate-buffered saline for 3 minutes at the physiologic pressure (flow rate, 0.15 ml/sec), which was prewarmed to 37°C to prevent vasoconstriction, followed by perfusion with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA) for 2 minutes, and lastly with a mixture of red India ink (Salis International Corporation, Golden, CO, USA; 85 ml of phosphate-buffered saline and 15 ml of ink) for 3 minutes.51 Immediately after perfusion, the eyes were enucleated, and the corneas were carefully dissected at 1 mm from limbus under a stereoscopic microscope.
Assessment of Flat-Mounted Corneas
Corneas were flattened with four cuts at 12, 3, 6 and 9 o'clock positions, into four quadrants. The corneas were mounted with mounting media (Immu-Mount; Thermo Fisher Scientific, Inc. Kalamazoo, MI, USA) on slides, and photographs were taken under an Olympus Microscope (BX43; Olympus, Tokyo, Japan) attached to a digital camera (U-TV0.5XC-3; Olympus). The digital images of flat-mounted corneas were analyzed using the Olympus CellSens Standard 1.17 software (Olympus). The total area of the cornea was encircled by drawing a freehand region, including the innermost vessels of limbal arcade. The new vessel sprouts were connected using a freehand selection and then calculated. The total corneal area and the avascular area were quantified with the assistance of software. The percentages of NV area in the total cornea were calculated.
Oxygen-Induced Retinopathy
The OIR model, which adequately reproduces the vascular obliteration and NV phases of ROP, is a commonly used model of ischemic retinopathies. Induction of OIR followed the procedure as described by Smith et al.52 with some modifications. Briefly, newborn brown Norway (BN) rats (Charles River Laboratories International, Inc.) at postnatal day 7 (P7) were exposed to hyperoxia (75% O2) for 5 days (P7–12) and then returned to normoxia (room air) to induce retinopathy.
Induction of Diabetes by Using STZ
BN rats at 8 weeks of age (Charles River Laboratories International, Inc.) received a single intraperitoneal injection of freshly made STZ (Sigma-Aldrich Corp.; 50 mg/kg in 10 mM of citrate buffer, pH 4.5) following overnight fasting. Control rats received an injection of citrate buffer alone. Blood glucose levels were measured at 48 hours following the STZ injection and 2 weeks thereafter, and only the animals with glucose levels higher than 350 mg/dl were considered diabetic. Rats with hyperglycemia for 2 weeks were used for these experiments (Supplementary Table S1).
Intravitreal Injection of Compounds
Thalidomide and phenylphthalimide analogs HDID, DID, and DAID were dissolved in vehicle (BN rat serum) and sterilized by filtration. OIR and STZ-diabetic BN rats received a single intravitreal injection of 0.5 to 2.0 μg/eye of (5 μl/eye, 0.1–0.4 mg/ml in BN rat serum) of the compounds into the right eye and the equal volume of the vehicle into the left eye.
Measurement of Retinal Vascular Permeability
Vascular permeability changes were evaluated by extravascular albumin accumulation and leakage of intravenous-injected fluorescein isothiocyanate-bovine serum albumin (FITC-albumin) as described previously.53 Briefly, FITC-albumin was injected through the femoral vein and circulated for 2 hours while the rats were kept on a warm pad. The rats were then perfused via the left ventricle to remove FITC-albumin from the circulation. The retinas were carefully dissected and homogenized. The concentrations of FITC-albumin were measured in a fluorometer and normalized by the total protein concentration in each retina and by plasma concentration of FITC-albumin.
Retinal vascular permeability was also measured using Evans blue-albumin as tracer, following a documented procedure with minor modifications.54 Evans blue dye (Sigma-Aldrich Corp.) was dissolved in 0.9% saline (30 mg/ml), sonicated for 5 minutes, and filtered through a 0.45-μm filter. The rats were then anesthetized, and Evans blue (30 mg/kg) was injected over 10 seconds through the femoral vein under microscopic inspection. Evans blue noncovalently binds to plasma albumin in the blood stream.55 The rats were kept on a warm pad for 2 hours to ensure the complete circulation of the dye. Then, the chest cavity was opened, and the rats were perfused via the left ventricle with 1% paraformaldehyde in citrate buffer (pH 4.2), which was prewarmed to 37°C to prevent vasoconstriction. The perfusion lasted for 10 minutes under the physiologic pressure of 120 mm Hg, in order to clear the dye from the vessel. Immediately after the perfusion, the eyes were enucleated and the retinas were carefully dissected under an operating microscope and homogenized. Evans blue dye was extracted by incubating each retinal homogenate in 150 μl of formamide for 18 hours at 70°C. The extract was centrifuged at 200,000g (rotor type, TLA 100.3) for 20 minutes at 4°C. Absorbance was measured using 100 μl of the supernatant at 620 nm using Spectrophotometer DU800 (Beckman Coulter, Inc. Indianapolis, IN, USA). The concentration of Evans blue in the extract was calculated from a standard curve of Evans blue in formamide and normalized by the total protein concentration in each sample. Results were expressed in μg of Evans blue per mg of total proteins.
Electroretinogram (ERG) Recording
Full-field ERGs were recorded using Espion E2 ERG system (Diagnosys LLC., Lowell, MA, USA), as described previously56 in two protocols: (1) 10-ms flashes of increasing light intensities under scotopic and photopic conditions and (2) 2-Hz flicker ERG under photopic conditions. BN rats received an intravitreal injection of DAID (2.0 μg/eye, 5 μl/eye of 0.4 mg/ml in BN rat serum) or an equal amount of BN rat serum. At various intervals after injection, a- and b-wave amplitudes were measured.
Histologic Analysis
To evaluate potential ocular toxicities of DAID, 8-week-old normal BN rats received an intravitreal injection of DAID (2.0 μg/eye, 5 μl/eye of 0.4 mg/ml in BN rat serum), with the equal amount of BN rat serum as vehicle control. At various intervals after the injection, the animals were killed. The eyes were then removed, fixed in 4% formaldehyde, embedded in paraffin, and cut into 6-μm cross sections containing the whole retina. Paraffin-embedded sections were stained with hematoxylin-eosin and examined.
Statistical Analysis
All statistical analyses were carried out using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA). Data were expressed as mean ± SD. The paired Student's t-test was applied to compare differences between two groups. For comparison of multiple groups, differences were analyzed using the 1-way ANOVA with post hoc contrasts by least significant difference. Differences were considered statistically significant at P < 0.05.
Results
Chemical Structures of Phenylphthalimide Analogs
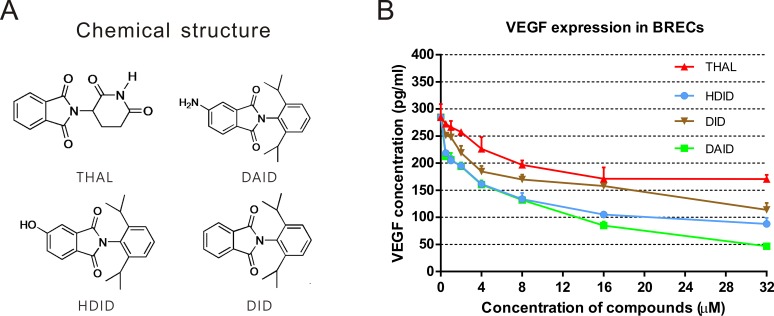
Three novel phenylphthalimide analogs, HDID, DID, and DAID, were used in this study, and thalidomide was used for comparison. The chemical structures of these compounds are shown in Figure 1A.
Figure 1.
Chemical structures of the compounds and their effects on VEGF expression. (A) Chemical structures of thalidomide and phenylphthalimide analogs of HDID, DID, and DAID. (B) Downregulation of hypoxia-induced VEGF expression. BRECs were incubated with or without cobalt chloride plus thalidomide, HDID, DID, and DAID at concentrations indicated. The cell-free conditioned medium was collected after a 24-hour treatment, and VEGF levels were measured by ELISA.
DAID is Substantially More Potent than Thalidomide and the Other Two Analogs in Inhibition of Proliferation of Endothelial Cells and VEGF Expression
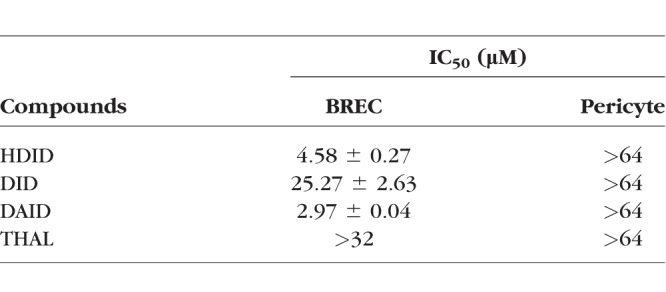
BRECs and pericytes were treated with the compounds at increasing concentrations of 1, 2, 4, 8, 16, 32, and 64 μM for 3 days. Viable cells were quantified using the MTT assay, and the half maximal inhibitory concentration (IC50) of each compound was calculated. Compounds HDID, DID, and DAID inhibited the proliferation of BRECs in a concentration-dependent manner, with IC50s of 4.58, 25.27, and 2.97 μM, respectively, which were more potent than thalidomide (IC50 > 32 μM) in BRECs (Table 1). The compound did not show a significant inhibition of pericyte growth in the concentration range of 1 to 64 μM. These results indicated that DAID had more potent antiangiogenic effects than thalidomide and the other two compounds.
Table 1.
Effect of Phenylphthalimide Analogs on Cell Proliferation
To address if the antiangiogenic activities of the new phenylphthalimide analogs are via blocking VEGF overexpression, we measured levels of VEGF in the conditioned medium from BRECs after treatment. BRECs were seeded at a density of 2 × 104 cells/well in 400 μl of growth medium in triplicate in gelatin-coated 24-well plates 24 hours prior to the experiments. VEGF expression was induced by 200 μM of cobalt chloride plus thalidomide, HDID, DID, and DAID at the concentrations of 0.5, 1, 2, 4, 8, 16, and 32 μM for 24 hours. Equal amounts of proteins from the BREC conditioned medium of each treatment were used for ELISA to measure VEGF (R&D Systems). VEGF levels were significantly downregulated by thalidomide, HDID, DID, and DAID (n = 3, P < 0.05). HDID and DAID displayed more potent inhibitory effects on VEGF expression than thalidomide at all of the concentrations (Fig. 1B) (n = 3, P < 0.05).
DAID Has a More Potent Effect on Retinal Vascular Leakage in OIR Rats
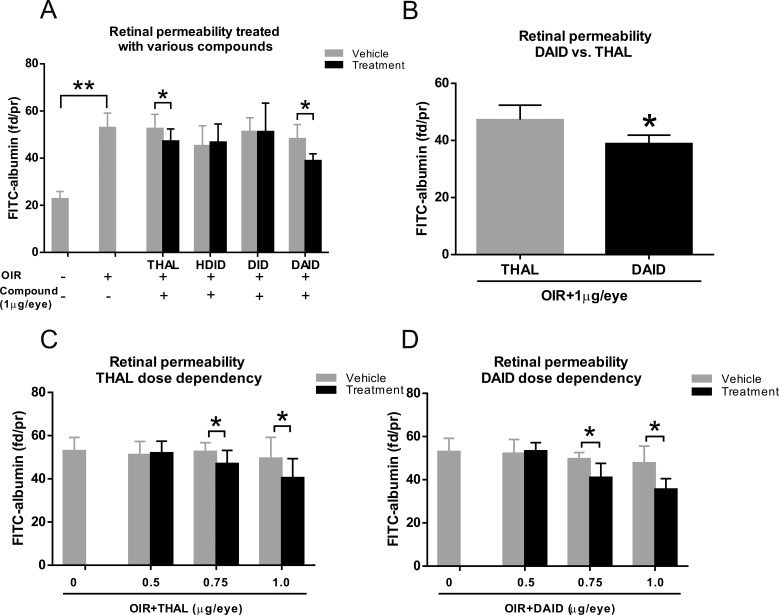
At P14, rats with OIR received an intravitreal injection of 1.0 μg/eye of thalidomide, HDID, DID, or DAID into the right eye and the equal volume of the vehicle into the contralateral eye as a control. At 48-hours postinjection, retinal vascular leakage was measured using FITC-albumin as a tracer. Retinal vascular leakage in normal rats (n = 6), which were maintained in constant room air, was measured at P16 as the normal baseline. Compared to non-OIR rats, there was significantly increased retinal permeability in OIR rats at P16 (Fig. 2A). Injection of thalidomide decreased retinal vascular leakage by 18% in the OIR rats, when compared with that in the contralateral eyes injected with vehicle (P < 0.05, n = 6). DAID decreased the retinal vascular leakage by 40% (P < 0.05, n = 6). The result showed that DAID had a more potent effect on retinal vascular leakage compared with thalidomide at the dose of 1.0 μg/eye (Fig. 2B). At the same dose, HDID and DID did not significantly reduce the retinal vascular leakage (Fig. 2A).
Figure 2.
Effects of thalidomide (THAL), HDID, DID, and DAID on retinal vascular leakage in OIR rats. (A) OIR rats received an intravitreal injection of 1.0 μg/eye (5 μl/eye, 0.2 mg/ml in BN rat serum) of thalidomide, HDID, DID, and DAID into the right eye and an equal volume of the vehicle into the left eye at P14. Vascular leakage was measured using the FITC-albumin leakage method at P16 and expressed as fluorescent dye/protein (fd/pr) in the retina. Vascular permeability in non-OIR rats (P16) treated with vehicle injection was used as baseline of permeability. Compared to vehicle-treated non-OIR rats, there were significant increases in retinal leakages in vehicle-treated OIR rats. (B) DAID-treated rats showed significantly lower retinal permeability than thalidomide-treated rats at the dose of 1.0 μg/eye. (C, D) OIR rats received an intravitreal injection of DAID or thalidomide at doses as indicated at P14. Permeability was measured at P16 and expressed as fd/pr in the retina. All values are mean ± SD, n = 6. *P < 0.05, **P < 0.01 (1-way ANOVA).
To determine if the effect of DAID on retinal vascular leakage was dose-dependent, the OIR rats at P14 received a single injection of DAID at doses of 0.5, 0.75, and 1.0 μg/eye. DAID and thalidomide significantly reduced vascular leakage at doses of 0.75 and 1.0 μg/eye (P < 0.05, n = 6) but not at the dose of 0.5 μg/eye (Figs. 2C, 2D).
DAID Has a More Potent Effect on Retinal Vascular Leakage in STZ-Induced Diabetic Rats
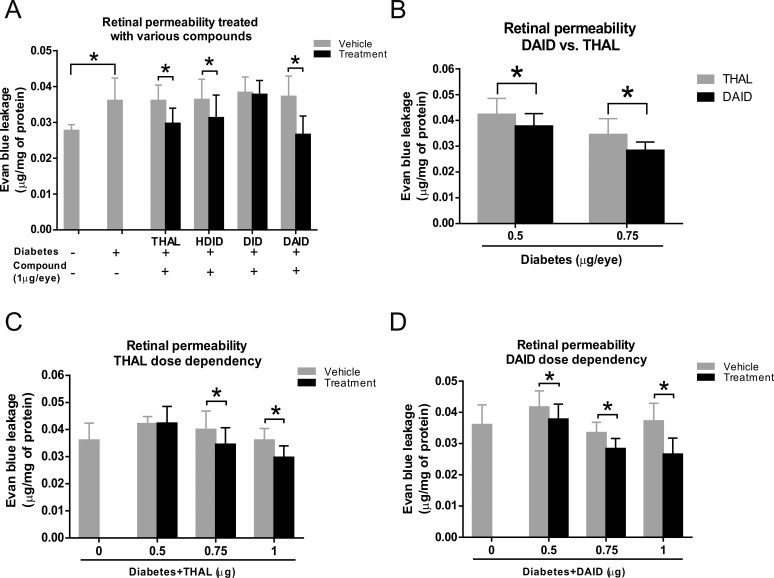
Thalidomide, HDID, DID, and DAID were separately injected into the vitreous cavity of the right eye in STZ-induced diabetic rats 2 weeks after the onset of diabetes. Forty-eight hours after the injection, retinal vascular leakage was measured using the Evans blue dye-albumin complex leakage method. Significantly increased retinal vascular permeability in diabetic rats was demonstrated at 2 weeks after STZ injection compared with nondiabetic rats (Fig. 3A). The result showed that in diabetic rats, the eyes injected with thalidomide, HDID, and DAID had a significant reduction in vascular leakage in the retinas, compared with the contralateral eyes injected with the vehicle (P < 0.05, n = 6).
Figure 3.
Effect of thalidomide (THAL), HDID, DID, and DAID on retinal vascular leakage in STZ-induced diabetes rats. (A) Two weeks after the induction of diabetes by STZ, diabetic rats received an intravitreal injection of 1.0 μg/eye (5 μl/eye, 0.2 mg/ml in BN rat serum) of thalidomide, HDID, DID, and DAID into the right eye and an equal volume of the vehicle into the left eye. Retinal vascular permeability was measured using Evans blue dye-albumin complex leakage method, 2 days after the injection and normalized by the total protein concentration in the retina. The data showed significantly higher vascular permeability in diabetic rats at 2 weeks after STZ injection compared to nondiabetic rats. (B) DAID induced more potent reduction of retinal vascular leakage compared with thalidomide at the doses of 0.5 and 0.75 μg/eye. (C, D) STZ-induced diabetic rats received an intravitreal injection of DAID or thalidomide at doses as indicated 2 weeks after the induction of diabetes. Permeability was measured 48 hours after the injection and expressed as μg of Evans blue per mg of protein in the retina. All values are mean ± SD, n = 6, *P < 0.05 (1-way ANOVA).
To determine the dose-response efficacy, STZ-induced diabetic rats received an intravitreal injection of DAID or thalidomide with doses of 0.5, 0.75, and 1.0 μg/eye. Two days after the injection, the retinal vascular permeability assay showed that DAID had a more significant reduction of retinal vascular leakage, compared with thalidomide at the doses of 0.5 and 0.75 μg/eye (Fig. 3B). In addition, thalidomide showed an inhibitory effect only at the doses of 0.75 and 1.0 μg/eye (P < 0.05, n = 6) but not at 0.5 μg/eye (P > 0.05, n = 6) (Fig. 3C). DAID significantly decreased vascular permeability in the retina at all of the doses, when compared with the vehicle group (P < 0.05, n = 6) (Fig. 3D).
These results indicate, to some extent, that DAID has a more potent effect on reducing retinal vascular leakage not only in the OIR model but also in the STZ-induced diabetes model, compared with thalidomide.
DAID Inhibits Alkali Burn-Induced Corneal NV
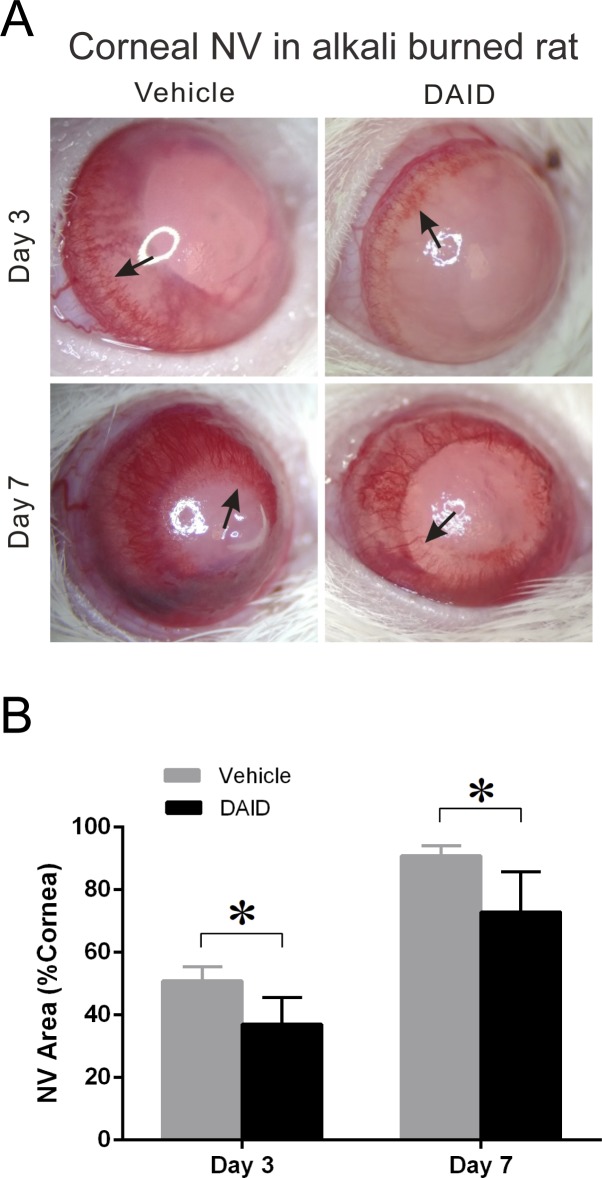
To evaluate the antiangiogenic activity of DAID, Sprague Dawley rats with the alkali burn-induced corneal NV were treated topically with eye drops containing 0.25% DAID or a vehicle control. The eye drop instillation was continued twice a day for 7 days. The new vessel sprouts began to grow from the limbus on day 3 after the corneal injury and reached the center of the cornea on day 7 in rats without treatment (Fig. 4A). On days 3 and 7, the lengths of corneal NV were measured with a caliper from four different quadrants, that is superior, inferior, temporal, and nasal. The wedge-shaped area (S) of the vessel growth was calculated with the formula: S = C/12 × 3.1416 × [r2 − (r − L)2] to quantify the total corneal NV area. The whole corneal area was quantified with the measured radius of each rat cornea. The ratio of corneal NV area/total cornea area was calculated and analyzed between the two groups (Fig. 4B). The percentage of NV area in the cornea was significantly lower in the DAID-treated group than that in the vehicle control group (P = 0.011 on day 3, P = 0.019 on day 7), suggesting an inhibitory effect of DAID on corneal NV.
Figure 4.
Antiangiogenic effect of DAID on alkali burn-induced corneal NV. (A) The photographs of the rat corneas were taken on days 3 and 7 after the alkali burn. The representative photographs of rat corneas with indicated treatments are shown. Arrows indicated corneal NV areas. (B) The comparison of the percentage of the NV area in the total cornea area on days 3 and 7. The NV area was expressed in % of cornea area, which was significantly lower in the DAID group than in the vehicle control group at both time points (mean ± SD, n = 7, P = 0.011 on day 3, P = 0.019 on day 7).
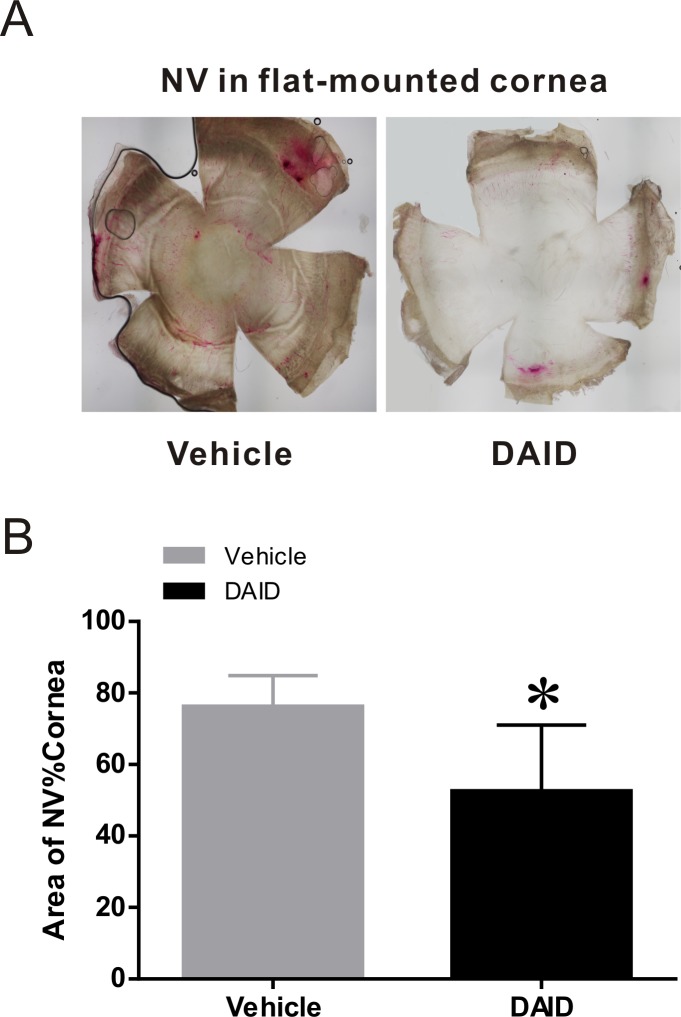
The ocular NV was also assessed in the flat-mounted corneas stained with India ink on day 7. The vessels in the cornea were stained with India ink perfusion, and the flat-mounted corneas were photographed under a stereomicroscope (Fig. 5A). The areas of corneal NV and the whole cornea were measured and analyzed using the Olympus CellSens Standard 1.17 software. The ratios of NV area/total cornea area in the DAID group and vehicle group were 52.6% ± 18.4% and 76.3% ± 8.6%, respectively, showing a remarkable decrease by DAID (P = 0.019) (Fig. 5B). These results suggested that DAID topical treatment had a significantly therapeutic effect on corneal NV.
Figure 5.
Cornea NV analysis in India ink-stained corneas. The flat-mounted corneas were stained with India ink and photographed under stereomicroscope on day 7 after the alkali burn. (A) Representative photos showed the new vessels stained by India ink. (B) The comparison of NV area/total cornea (%) between the two groups. The areas of corneal NV and the total cornea were measured and calculated with the Olympus CellSens Standard 1.17 software. The mean percentages of area of NV/total cornea were 52.6% ± 18.4% and 76.3% ± 8.6% in the treated and vehicle control groups, respectively. The NV area of rat cornea in the DAID group was significantly decreased compared to that in vehicle group (mean ± SD, n = 7, *P = 0.019).
DAID Attenuates the Overexpression of VEGF in the Retinas of OIR Rats
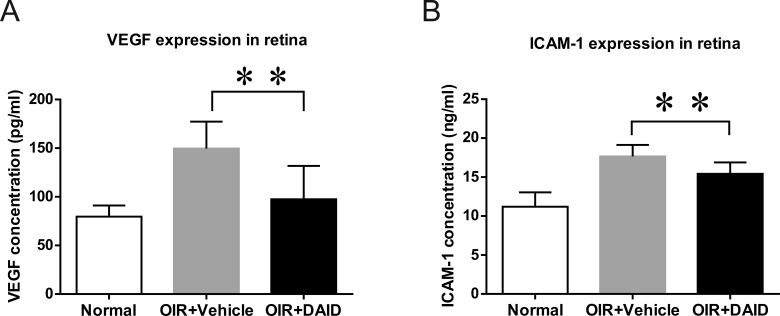
To explore the mechanism underlying the beneficial effects of DAID, levels of VEGF in the retinas of OIR rats were measured following DAID administration. OIR rats received an intravitreal injection of DAID (1.0 μg/eye, 5 μl/eye of 0.2 mg/ml in BN rat serum) and vehicle (5 μl/eye of BN rat serum). Forty-eight hours after the injection, VEGF levels in the retinas from normal rats, vehicle-treated, and DAID-treated OIR rats were measured by ELISA. The expression of VEGF was downregulated in the retinas of DAID-treated eyes, compared with the contralateral vehicle control (n = 6, P < 0.01) (Fig. 6A).
Figure 6.
Effect of DAID on the expression of VEGF and ICAM-1 in the retina of OIR rats. OIR rats received an intravitreal injection of DAID and vehicle at P14. (A) VEGF levels in the retinas from normal rats, vehicle-treated, and DAID-treated OIR rats were measured by ELISA at P16. (B) ICAM-1 levels in the retinas from normal rats and vehicle-treated and DAID-treated OIR rats were determined by ELISA. Values are mean ± SD, n = 6, **P < 0.01.
DAID Inhibits the Intracellular Adhesion Molecule 1 (ICAM-1) Expression in the Retinas of OIR Rats
ICAM-1 has been shown to play a key role in inflammatory processes and NV. To address if the effects of DAID on retinal NV and vascular leakage are via the attenuation of ICAM-1 overexpression, we measured the retinal levels of ICAM-1 after an intravitreal injection of DAID in OIR rats. Equal amounts of retinal proteins from normal rats, vehicle-treated, and DAID-treated OIR rats were used for ELISA to measure ICAM-1 (R&D Systems). The results demonstrated that levels of ICAM-1 were significantly reduced in the retinas of DAID-treated eyes, compared with the contralateral control injected with the vehicle (n = 6, P < 0.01) (Fig. 6B).
DAID Does Not Show Any Detectable Ocular Toxicities in Rats
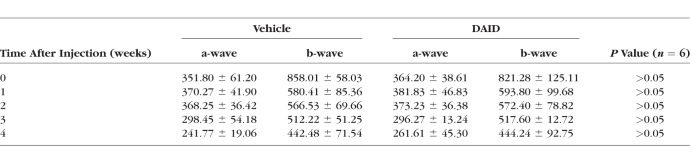
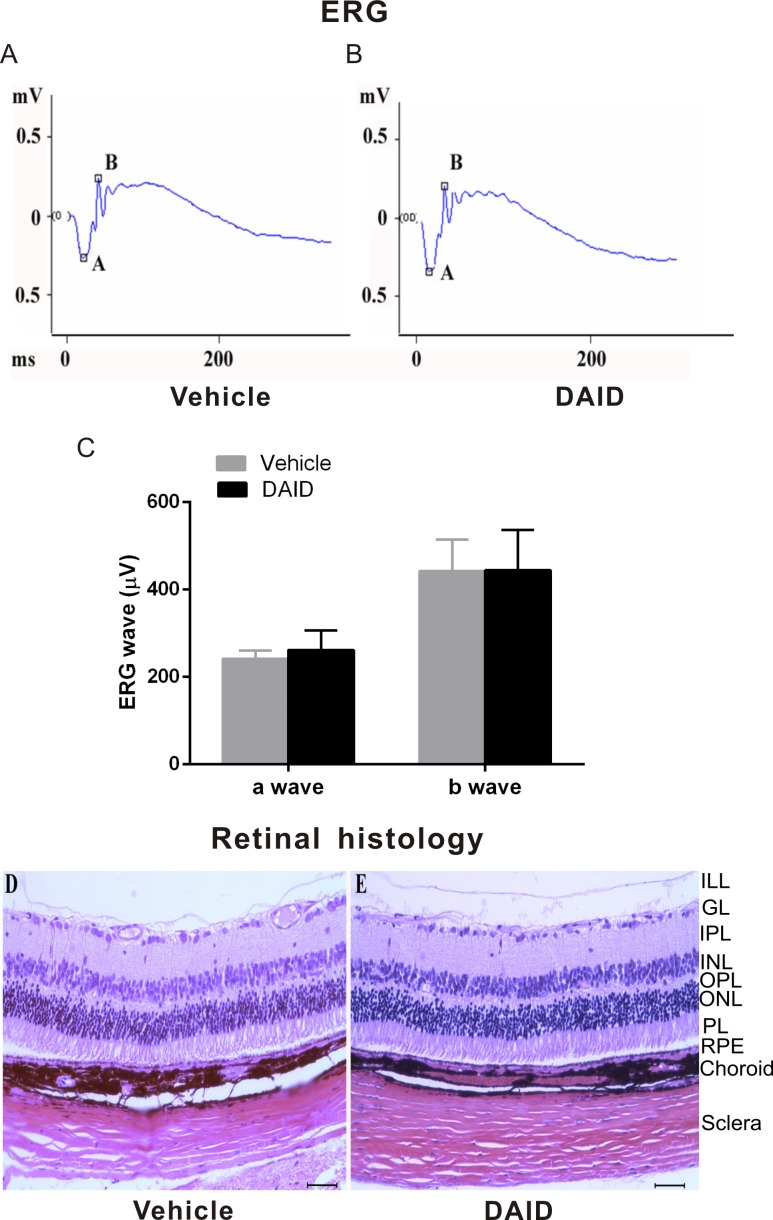
To test the potential ocular toxicities of DAID, normal rats at the age of 8 weeks received an intravitreal injection of a high dose of DAID, 2 μg/eye (5 μl/eye of 0.4 mg/ml in BN rat serum) or an equal amount of BN rat serum as the vehicle control. The possible impacts of DAID on visual function were evaluated by ERG recording, prior to study initiation, and weekly for 4 weeks following the injection. The detailed ERG recording showed no detectable change in the scotopic a-wave and b-wave amplitudes in DAID-injected rats compared with vehicle-injected eyes (Table 2). The typical waveform of a dark-adapted ERG was recorded in response to a high intensity flash at 4 weeks after treatment (Figs. 7A–C).
Table 2.
Effect of DAID on ERG a-wave and b-wave Amplitudes (μV) in Rats (n = 6), Mean ± SD
Figure 7.
Functional and morphologic analyses of the retina treated by DAID. BN rats (8 weeks of age) received an intravitreal injection of DAID (2.0 μg/eye, 5 μl/eye 0.4 mg/ml in BN rat serum) or equal volume of BN rat serum (n = 6). (A, B) Representative waveforms of dark-adapted ERG recorded in response to a high intensity flash at 4 weeks after the injection. (C) No significant changes in the a-wave and b-wave amplitudes in DAID-injected rats compared with vehicle-injected rats 4 weeks after the treatment. (D, E) The animals were killed 4 weeks after the injection. The eye sections were stained with hematoxylin and eosin and examined under a light microscope (Scale bar: 100 μm).
Possible toxicities of DAID were also evaluated using histologic examination 4 weeks after the drug administration. Retinal cross sections stained with hematoxylin-eosin were examined under a light microscope. No detectable morphologic change was found in the retinas treated with 2 μg/eye DAID in any of the rats analyzed (n = 6), compared with the contralateral retinas treated with the vehicle (Figs. 7D, 7E).
Discussion
Ocular NV is a widespread destructive process that is involved in nearly all major eye diseases and is a major cause of blindness.57 It can affect a number of tissues in the eye, including the cornea and retina. Corneal NV is characterized by the invasion of new blood vessels into the cornea, which may lead to scarring and corneal opacity.58 The immature new blood vessels in the retina greatly elevate the risk of retinal vascular leakage diffusely from a more generalized breakdown of the blood–retina barrier. The subsequent macular edema manifests as intraretinal and subretinal accumulation of fluid resulting from increased vascular permeability, which is the main cause of visual loss and blindness in eye diseases such as AMD and DR.59–61
The common mechanism of NV in corneal and retinal disorders is the disturbed balance between angiogenic and antiangiogenic factors.4 Numerous studies have shown that VEGF plays a key role in stimulating angiogenesis by acting as a mitogen for vascular endothelial cells.62,63 Further, VEGF was reported to be significently overexpressed in the limbal area during the early stages of corneal NV, and VEGF overexpression gradually progresses toward the corneal center in inflammation-associated corneal NV.64 Moreover, a growing body of evidence demonstrates that VEGF plays an important role in DME and macular edema associated with AMD.26,30,31 The increased vascular permeability and pathologic angiogenesis observed in DR and AMD are mediated, at least partially, by local inflammation and overexpression of VEGF. Clinically, inhibition of VEGF is becoming the preferred choice of treatment for DME.65,66 VEGF has been confirmed as a major cytokine mediating the corneal and retinal NV.67–69 Therefore, numerous agents have been developed to inhibit VEGF.58,70,71 The current treatment focuses on intravitreous injection of anti-VEGF antibodies.72–74 Although they have achieved encouraging effects in the treatment of NV and DME, the repetitive injections are associated with high costs, risks of trauma, infection, and other side effects.66,75
One advantage of small molecule drugs (SMDs) over “large molecule” antibodies is that many small molecules can be administered systemically as well as topically. This feature avoids repetitive intravitreal injections, and, thus, is especially suitable for diseases that require long-term treatment such as DR and AMD. SMDs are able to penetrate into cells easily, acting on molecules or pathway inside cells.76 In addition, SMDs are relatively easy to synthesize, transport, and formulate with low costs, compared with therapeutic antibodies. Furthermore, SMDs are characterized by mostly well-defined physicochemical and pharmacokinetic properties. These features of SMDs represent a huge advantage over large biologics as drugs.77,78 To develop SMD candidates for the treatment of NV and vascular leakage, in the present study, we synthesized a series of new analogs of phenylphthalimide and evaluated their efficacies on retinal vascular leakage and corneal NV.
Thalidomide is a glutamic acid derivative, which has a broad spectrum of pharmacologic and immunologic effects holding a reputable position due to its inhibitory effect on angiogenesis. In view of thalidomide possessing antiangiogenic activities, thalidomide and its existing analogs have been used experimentally to treat various cancers, dermatologic diseases, inflammatory diseases, and other neovascular disorders such as AMD and DR.36,79–82 Thalidomide blocked the increase of VEGF in the ocular fluid of STZ-diabetic rats, thus suggesting a therapeutic potential for DME caused by vascular leakage or breakdown of the blood–retina barrier.40
As shown by previous studies, the mechanisms of action of thalidomide include antiangiogenic activity via regulating expression of angiogenic factors such as VEGF83–87 and mediating the degradation of the VEGF receptors.80 In addition, it decreases the expression of TNF-α by enhancing the degradation of the TNF-α mRNA and increasing the effect of α1-acid glycoproteins, which possess an intrinsic anti-TNF-α activity.85,88–91 Other immunomodulatory effects are achieved by stimulating cytotoxic T cell and NK cell proliferation; inducting secretion of TNF-α, interferon-γ, and interleukin-2 (IL-2)92; modulating the expression of cell surface adhesion molecules such as ICAM-1, VCAM-1 (CD106), E-selectin, and L-selectin (CD62L)93; and inhibiting nuclear factor-κB activity through suppression of I-κB kinase activity and inhibition of the cyclooxygenases 1 and 2.94,95
However, the antiangiogenic activity of thalidomide requires high doses, which is associated with severe side effects.96,97 In order to improve the antiangiogenic activities, we have designed and synthesized a series of novel phenylphthalimide analogs by substituting the glutarimide ring with an aromatic group and a series of other novel functional groups. Our in vitro screening using the endothelial cell proliferation assay has identified three new compounds with potent antiproliferative activities, as they selectively inhibited endothelial cell growth with higher potency than that of thalidomide. In addition, these phenylphthalimide analogs did not inhibit the growth of pericytes, suggesting the presence of endothelial cell-specific inhibitory activities. Similar to thalidomide, these new phenylphthalimide analogs also inhibit VEGF expression induced by hypoxia in endothelial cells. One of the phenylphthalimide analogs, DAID, displayed more potent effects on the growth of BRECs and inhibition of VEGF expression than other compounds.
Because retinal vascular leakage is a major cause of DME and is associated with VEGF overexpression, the present study evaluated the effects of these compounds on retinal vascular leakage in animal models. The OIR model is known to develop abnormal vascular leakage in the retina and VEGF overexpression.98 As shown by the vascular permeability assay using FITC-albumin as tracer, all three analogs significantly reduced retinal vascular leakage in OIR model. Among them, DAID displayed more potent effects on retinal vascular leakage than the other compounds. Similarly, we also evaluated the effects of these compounds on diabetes-induced retinal vascular leakage in STZ-induced diabetic rats, which are a widely used model for nonproliferative DR, including retinal vascular leakage.99 Consistent with that in the OIR model, our permeability assay showed that DAID possesses the most potent effect on diabetes-induced retinal vascular leakage. As DAID showed the highest efficacies in the inhibition of BREC proliferation, VEGF expression, and in reduction of retinal vascular leakage in both of the STZ-induced diabetic and OIR models, we identified DAID as the leading compound for further analysis. We treated OIR rats and STZ-induced diabetic rats with various doses of DAID and measured the efficacies on retinal vascular leakage. The results indicated that DAID reduced retinal vascular leakage in a dose-dependent manner. These results suggest a potential therapeutic application of this compound for the treatment of DME.
As VEGF is also a key pathogenic factor for NV, we also evaluated the effect of DAID on NV in a corneal NV model. Corneal NV significantly diminishes corneal transparency and subsequently causes impaired vision. Various treatments, including drugs and surgical operations such as radiotherapy, laser therapy, and photodynamic therapy, are applied in treating corneal NV.4,15,100 Topical administration is an advantageous route for drug delivery to the cornea because it is noninvasive and results in minimal adverse effects compared with systemic administration. Bock et al.101 reported that bevacizumab (Avastin) eye drops decreased the NV area in the cornea. The present study showed that DAID eye drops significantly reduced corneal NV area, compared with the vehicle eye drop control, demonstrating an inhibitory effect of this compound on corneal NV. Thus, DAID eye drop instillation should have the potential to become a noninvasive treatment for corneal NV. In addition, this compound may have therapeutic potential for other ocular neovascular diseases, such as DR, AMD, and ROP.
To explore whether the mechanism of action of DAID is through inhibition of VEGF, we investigated the regulatory effect of DAID on the expression of VEGF. DAID downregulated the expression of VEGF in cultured cells and in the retina of the OIR model. On the other hand, DAID downregulated levels of ICAM-1 that plays a key role in leukostasis and breakdown of the blood–retina barrier.102 It has been reported that VEGF-induced retinal vascular leakage is partly mediated by ICAM-1 because VEGF can induce ICAM-1 expression.103 The vascular permeability changes correlate with the upregulation of ICAM-1.104 The upregulation of VEGF and ICAM-1 in the retina of STZ-induced diabetic rats has also been reported.105 Our results showed that DAID attenuated the overexpression of both VEGF and ICAM-1 in the retina of OIR rat model, suggesting that DAID reduces retinal vascular leakage and NV at least in part, through downregulation of VEGF and ICAM-1. However, it remains to be defined how DAID suppresses the expression of VEGF and ICAM-1.
In consideration of DAID being the leading compound, we further assessed its ocular toxicities in rats. Both ERG recording and histopathologic examination demonstrated that DAID, at a high dose, did not result in detectable changes in the retinal function and histology in rats. Topical application of DAID eye drops did not result in detectable side effects in the cornea, suggesting that DAID lacks severe toxicities at doses required for its antiangiogenic activities.
Although the present study demonstrated the therapeutic potential of DAID, some studies remain to be conducted to develop it as a drug candidate. Careful pharmacokinetic studies are needed to identify its tissue distribution and duration of availability in ocular tissues. The delivery route and regimen of administration remain to be optimized. For topical administration, the eye drop formulation needs to be optimized to prolong the drug availability and penetration in the cornea. The systemic exposure and systemic side effects after topical instillation need to be examined. Toward the mechanism of action, it remains to be studied how DAID downregulates VEGF and ICAM-1.
In summary, the present study identified a novel phenylphthalimide analog and showed that it has potent inhibitory effects on retinal vascular leakage and ocular NV without apparent ocular toxicity. These results suggest that DAID is a promising drug candidate for ocular diseases associated with angiogenesis and vascular leakage, including corneal NV, DR, AMD, and ROP.
Supplementary Material
Acknowledgments
The authors thank Rafal A. Farjo, PhD, Ronald A. Wassel, PhD, and Temmy Sasore, PhD, for critical review of the manuscript.
Supported by National Institutes of Health grants EY016627, EY018659, EY019309, GM104934, and GM122744.
Disclosure: B. Wang, None; P.-K. Li, None; J.-X. Ma, None; D. Chen, None
References
- 1.Bhutto IA, McLeod DS, Hasegawa T., et al. Pigment epithelium-derived factor (PEDF) and vascular endothelial growth factor (VEGF) in aged human choroid and eyes with age-related macular degeneration. Exp Eye Res. 2006;82:99–110. doi: 10.1016/j.exer.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li T., Hu A., Li S., et al. KH906, a recombinant human VEGF receptor fusion protein, is a new effective topical treatment for corneal neovascularization. Mol Vis. 2011;17:797–803. [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang W., Ito Y., Berlin E., Roberts R., Berkowitz BA. Role of hypoxia during normal retinal vessel development and in experimental retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2003;44:3119–3123. doi: 10.1167/iovs.02-1122. [DOI] [PubMed] [Google Scholar]
- 4.Azar DT. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2006;104:264–302. [PMC free article] [PubMed] [Google Scholar]
- 5.Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Levison SW. Diabetic retinopathy: more than meets the eye. Surv Ophthalmol. 2002;47(suppl 2):253–262. doi: 10.1016/s0039-6257(02)00387-9. [DOI] [PubMed] [Google Scholar]
- 6.Provis JM, Penfold PL, Cornish EE, Sandercoe TM, Madigan MC. Anatomy and development of the macula: specialisation and the vulnerability to macular degeneration. Clin Exp Optom. 2005;88:269–281. doi: 10.1111/j.1444-0938.2005.tb06711.x. [DOI] [PubMed] [Google Scholar]
- 7.Carmeliet P., Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiedemann P. Growth factors in retinal diseases: proliferative vitreoretinopathy, proliferative diabetic retinopathy, and retinal degeneration. Surv Ophthalmol. 1992;36:373–384. doi: 10.1016/0039-6257(92)90115-a. [DOI] [PubMed] [Google Scholar]
- 9.Robinson GS, Aiello LP. Angiogenic factors in diabetic ocular disease: mechanisms of today, therapies for tomorrow. Int Ophthalmol Clin. 1998;38:89–102. [PubMed] [Google Scholar]
- 10.Aiello LP, Robert L., Avery PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 11.Lopez PF, Sippy BD, Lambert HM, Thach AB, Hinton DR. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1996;37:855–868. [PubMed] [Google Scholar]
- 12.Cursiefen C., Rummelt C., Kuchle M. Immunohistochemical localization of vascular endothelial growth factor, transforming growth factor alpha, and transforming growth factor beta1 in human corneas with neovascularization. Cornea. 2000;19:526–533. doi: 10.1097/00003226-200007000-00025. [DOI] [PubMed] [Google Scholar]
- 13.Gerhardinger C., McClure KD, Romeo G., Podesta F., Lorenzi M. IGF-I. mRNA and signaling in the diabetic retina. Diabetes. 2001;50:175–183. doi: 10.2337/diabetes.50.1.175. [DOI] [PubMed] [Google Scholar]
- 14.Casey R., Li WW. Factors controlling ocular angiogenesis. Am J Ophthalmol. 1997;124:521–529. doi: 10.1016/s0002-9394(14)70868-2. [DOI] [PubMed] [Google Scholar]
- 15.Gan L., Fagerholm P., Palmblad J. Vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in the regulation of corneal neovascularization and wound healing. Acta Ophthalmol Scand. 2004;82:557–563. doi: 10.1111/j.1600-0420.2004.00312.x. [DOI] [PubMed] [Google Scholar]
- 16.Cheng SF, Dastjerdi MH, Ferrari G., et al. Short-term topical bevacizumab in the treatment of stable corneal neovascularization. Am J Ophthalmol. 2012;154:940–948. doi: 10.1016/j.ajo.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bock F., König Y., Dietrich T., Zimmermann P., Baier M., Cursiefen C. Inhibition of angiogenesis in the anterior chamber of the eye. Ophthalmology. 2007;104:336–344. doi: 10.1007/s00347-007-1512-2. [DOI] [PubMed] [Google Scholar]
- 18.Habot-Wilner Z., Barequet IS, Ivanir Y., Moisseiev J., Rosner M. The inhibitory effect of different concentrations of topical bevacizumab on corneal neovascularization. Acta Ophthalmol. 2010;88:862–867. doi: 10.1111/j.1755-3768.2009.01571.x. [DOI] [PubMed] [Google Scholar]
- 19.Aiello LP, Wong JS. Role of vascular endothelial growth factor in diabetic vascular complications. Kidney Intl Suppl. 2000;77:113–119. doi: 10.1046/j.1523-1755.2000.07718.x. [DOI] [PubMed] [Google Scholar]
- 20.Murata T., Nakagawa K., Khalil A., Ishibashi T., Inomata H., Sueishi K. The relation between expression of vascular endothelial growth factor and breakdown of the blood-retinal barrier in diabetic rat retinas. Lab Invest. 1996;74:819–825. [PubMed] [Google Scholar]
- 21.Hammes HP, Lin J., Bretzel RG, Brownlee M., Breier G. Upregulation of the vascular endothelial growth factor/vascular endothelial growth factor receptor system in experimental background diabetic retinopathy of the rat. Diabetes. 1998;47:401–406. doi: 10.2337/diabetes.47.3.401. [DOI] [PubMed] [Google Scholar]
- 22.Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118:445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- 23.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci U S A. 1995;92:905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Werdich XQ, McCollum GW, Rajaratnam VS, Penn JS. Variable oxygen and retinal VEGF levels: correlation with incidence and severity of pathology in a rat model of oxygen-induced retinopathy. Exp Eye Res. 2004;79:623–630. doi: 10.1016/j.exer.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Pe'er J., Shweiki D., Itin A., Hemo I., Gnessin H., Keshet E. Hypoxia-induced expression of vascular endothelial growth factor by retinal cells is a common factor in neovascularizing ocular diseases. Lab Invest. 1995;72:638–645. [PubMed] [Google Scholar]
- 26.Qaum T., Xu Q., Joussen AM, et al. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci. 2001;42:2408–2413. [PubMed] [Google Scholar]
- 27.Lip PL, Blann AD, Hope-Ross M., Gibson JM, Lip GY. Age-related macular degeneration is associated with increased vascular endothelial growth factor, hemorheology and endothelial dysfunction. Ophthalmology. 2001;108:705–710. doi: 10.1016/s0161-6420(00)00663-1. [DOI] [PubMed] [Google Scholar]
- 28.Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res. 2003;22:1–29. doi: 10.1016/s1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- 29.Shienbaum G., Gupta OP, Fecarotta C., Patel AH, Kaiser RS, Regillo CD. Bevacizumab for neovascular age-related macular degeneration using a treat-and-extend regimen: clinical and economic impact. Am J Ophthalmol. 2012;153:468–473. doi: 10.1016/j.ajo.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Antonetti DA, Lieth E., Barber AJ, Gardner TW. Molecular mechanisms of vascular permeability in diabetic retinopathy. Semin Ophthalmol. 1999;14:240–248. doi: 10.3109/08820539909069543. [DOI] [PubMed] [Google Scholar]
- 31.Aiello LP, Bursell SE, Clermont A., et al. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes. 1997;46:1473–1480. doi: 10.2337/diab.46.9.1473. [DOI] [PubMed] [Google Scholar]
- 32.Duh E., Aiello LP. Vascular endothelial growth factor and diabetes: the agonist versus antagonist paradox. Diabetes. 1999;48:1899–1906. doi: 10.2337/diabetes.48.10.1899. [DOI] [PubMed] [Google Scholar]
- 33.Chiarelli F., Santilli F., Mohn A. Role of growth factors in the development of diabetic complications. Horm Res. 2000;53:53–67. doi: 10.1159/000023515. [DOI] [PubMed] [Google Scholar]
- 34.Caldwell RB, Bartoli M., Behzadian MA, et al. Vascular endothelial growth factor and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Diabetes Metab Res Rev. 2003;19:442–455. doi: 10.1002/dmrr.415. [DOI] [PubMed] [Google Scholar]
- 35.Gao G., Li Y., Zhang D., Gee S., Crosson C., Ma J. Unbalanced expression of VEGF and PEDF in ischemia-induced retinal neovascularization. FEBS Lett. 2001;489:270–276. doi: 10.1016/s0014-5793(01)02110-x. [DOI] [PubMed] [Google Scholar]
- 36.Wu JJ, Huang DB, Pang KR, Tyring SK. Thalidomide: dermatological indications, mechanisms of action and side-effects. Br J Dermatol. 2005;153:254–273. doi: 10.1111/j.1365-2133.2005.06747.x. [DOI] [PubMed] [Google Scholar]
- 37.Bartlett JB, Dredge K., Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer. 2004;4:314–322. doi: 10.1038/nrc1323. [DOI] [PubMed] [Google Scholar]
- 38.Hattori Y., Kakimoto T., Okamoto S., Sato N., Ikeda Y. Thalidomide-induced severe neutropenia during treatment of multiple myeloma. Int J Hematol. 2004;79:283–288. doi: 10.1532/ijh97.03136. [DOI] [PubMed] [Google Scholar]
- 39.Masiero L., Figg WD, Kohn EC. New anti-angiogenesis agents: review of the clinical experience with carboxyamido-triazole (CAI), thalidomide, TNP-470 and interleukin-12. Angiogenesis. 1997;1:23–35. doi: 10.1023/A:1018301031580. [DOI] [PubMed] [Google Scholar]
- 40.Bosco AA, Lerario AC, Santos RF, Wajchenberg BL. Effect of thalidomide and rosiglitazone on the prevention of diabetic retinopathy in streptozotocin-induced diabetic rats. Diabetologia. 2003;46:1669–1675. doi: 10.1007/s00125-003-1234-1. [DOI] [PubMed] [Google Scholar]
- 41.Arne-Bes MC. Neurotoxic effects of medications: an update. Rev Med Liege. 2004;59(suppl 1):118–123. [PubMed] [Google Scholar]
- 42.Pathak RD, Jayaraj K. Blonde L. Thalidomide-associated hyperglycemia and diabetes: case report and review of literature. Diabetes Care. 2003;26:1322–1323. doi: 10.2337/diacare.26.4.1322-a. [DOI] [PubMed] [Google Scholar]
- 43.Iqbal N., Zayed M., Boden G. Thalidomide impairs insulin action on glucose uptake and glycogen synthesis in patients with type 2 diabetes. Diabetes Care. 2000;23:1172–1176. doi: 10.2337/diacare.23.8.1172. [DOI] [PubMed] [Google Scholar]
- 44.Xiao Z., Schaefer K., Firestine S., Li PK. Solid-phase synthesis of thalidomide and its analogues. J. Comb. Chem. 2002;4:149–153. doi: 10.1021/cc010038n. [DOI] [PubMed] [Google Scholar]
- 45.Li PK, Pandit B., Sackett DL, et al. A thalidomide analogue with in vitro antiproliferative, antimitotic, and microtubule-stabilizing activities. Mol Cancer Ther. 2006;5:450–456. doi: 10.1158/1535-7163.MCT-05-0254. [DOI] [PubMed] [Google Scholar]
- 46.Wong HC, Boulton M., Marshall J., Clark P. Growth of retinal capillary endothelia using pericyte conditioned medium. Invest Opthalmol Vis Sci. 1987;28:1767–1775. [PubMed] [Google Scholar]
- 47.Lee SH, Kim KW, Joo K., Kim JC. Angiogenin ameliorates corneal opacity and neovascularization via regulating immune response in corneal fibroblasts. BMC Ophthalmol. 2016;16:1–13. doi: 10.1186/s12886-016-0235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dana MR, Streilein JW. Loss and restoration of immune privilege in eyes with corneal neovascularization. Invest Ophthalmol Vis Sci. 1996;37:2485–2494. [PubMed] [Google Scholar]
- 49.Anderson C., Zhou Q., Wang S. An alkali-burn injury model of corneal neovascularization in the mouse. J Vis Exp. 2014;86:1–6. doi: 10.3791/51159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.D'Amato RJ, Loughnan MS, Flynn E., Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci U S A. 1994;91:4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Figueroa-Ortiz LC. Martin Rodriguez O, Garcia-Ben A, Garcia-Campos J. Neovascular growth in an experimental alkali corneal burn model. Arch Soc Esp Oftalmol. 2014;89:303–307. doi: 10.1016/j.oftal.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 52.Smith LE, Wesolowski E., McLellan A., et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 53.Shyong MP, Lee FL, Kuo PC, et al. Reduction of experimental diabetic vascular leakage by delivery of angiostatin with a recombinant adeno-associated virus vector. Mol Vis. 2007;13:133–141. [PMC free article] [PubMed] [Google Scholar]
- 54.Xu Q., Qaum T., Adamis AP. Sensitive blood-retinal barrier breakdown quantitation using Evans blue. Invest Ophthalmol Vis Sci. 2001;42:789–794. [PubMed] [Google Scholar]
- 55.Bae DG, Gho YS, Yoon WH, Chae CB. Arginine-rich anti-vascular endothelial growth factor peptides inhibit tumor growth and metastasis by blocking angiogenesis. J Biol Chem. 2000;275:13588–13596. doi: 10.1074/jbc.275.18.13588. [DOI] [PubMed] [Google Scholar]
- 56.Rohrer B., Korenbrot JI, LaVail MM, Reichardt LF, Xu B. Role of neurotrophin receptor TrkB in the maturation of rod photoreceptors and establishment of synaptic transmission to the inner retina. J Neurosci. 1999;19:8919–8913. doi: 10.1523/JNEUROSCI.19-20-08919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee P., Wang CC, Adamis AP. Ocular neovascularization: an epidemiologic review. Surv Ophthalmol. 1998;43:245–269. doi: 10.1016/s0039-6257(98)00035-6. [DOI] [PubMed] [Google Scholar]
- 58.Chang JH, Garg NK, Lunde E., Han KY, Jain S., Azar DT. Corneal neovascularization: an anti-VEGF therapy review. Surv Ophthalmol. 2012;57:415–429. doi: 10.1016/j.survophthal.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bresnick GH. Diabetic maculopathy: a critical review highlighting diffuse macular edema. Ophthalmology. 1983;90:1301–1317. doi: 10.1016/s0161-6420(83)34388-8. [DOI] [PubMed] [Google Scholar]
- 60.Campochiaro PA, Hackett SF. Ocular neovascularization: a valuable model system. Oncogene. 2003;22:6537–6548. doi: 10.1038/sj.onc.1206773. [DOI] [PubMed] [Google Scholar]
- 61.Ingeborg K., Cornelis JF. Van N, Reinier OS. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res. 2013;34:19–48. doi: 10.1016/j.preteyeres.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 62.Holger G., Matthew G., Marcus F., et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;16:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peter C., Rakesh KJ. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 64.Kvanta A., Sarman S., Fagerholm P., Seregard S., Steen B. Expression of matrix metalloproteinase-2 (MMP-2) and vascular endothelial growth factor (VEGF) in inflammation-associated corneal neovascularization. Exp Eye Res. 2000;70:419–428. doi: 10.1006/exer.1999.0790. [DOI] [PubMed] [Google Scholar]
- 65.Gupta N., Mansoor S., Sharma A., et al. Diabetic retinopathy and VEGF. Open Ophthalmol J. 2013;7:4–10. doi: 10.2174/1874364101307010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eugene WM, David TS, Perry C., Emmett TC, David RG, Anthony PA. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov. 2006;5:123–132. doi: 10.1038/nrd1955. [DOI] [PubMed] [Google Scholar]
- 67.Philipp W., Speicher L., Humpel C. Expression of vascular endothelial growth factor and its receptors in inflamed and vascularised human corneas. Invest Ophthalmol Vis Sci. 2000;41:2514–2522. [PubMed] [Google Scholar]
- 68.Kvanta A., Algvere PV, Berglin L., Seregard S. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci. 1996;37:1929–1934. [PubMed] [Google Scholar]
- 69.Boulton M., Foreman D., Williams G., McLeod D. VEGF localisation in diabetic retinopathy. Br J Ophthalmol. 1998;82:561–568. doi: 10.1136/bjo.82.5.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lynch SS, Cheng CM. Bevacizumab for neovascular ocular diseases. Ann Pharmacother. 2007;41:614–625. doi: 10.1345/aph.1H316. [DOI] [PubMed] [Google Scholar]
- 71.Nguyen QD, Shah SM, Hafiz G., et al. A phase I trial of an IV-administered vascular endothelial growth factor trap for treatment in patients with choroidal neovascularization due to age-related macular degeneration. Ophthalmology. 2006;113:1522–1532. doi: 10.1016/j.ophtha.2006.05.055. [DOI] [PubMed] [Google Scholar]
- 72.Fung AE, Rosenfeld PJ, Reichel E. The International Intravitreal Bevacizumab Safety Survey: using the Internet to assess drug safety worldwide. Br J Ophthalmol. 2006;90:1344–1349. doi: 10.1136/bjo.2006.099598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu L., Martinez-Castellanos MA, Quiroz-Mercado H., et al. Twelve-month safety of intravitreal injections of bevacizumab (Avastin): results of the Pan-American Collaborative Retina Study Group (PACORES) Graefes Arch Clin Exp Ophthalmol. 2008;246:81–87. doi: 10.1007/s00417-007-0660-z. [DOI] [PubMed] [Google Scholar]
- 74.Simó R., Hernández C. Intravitreous anti-VEGF for diabetic retinopathy: hopes and fears for a new therapeutic strategy. Diabetologia. 2008;51:1574–1580. doi: 10.1007/s00125-008-0989-9. [DOI] [PubMed] [Google Scholar]
- 75.Eugene WM, Anthony PA. Anti-VEGF aptamer (pegaptanib) therapy for ocular vascular diseases. Ann N Y Acad Sci. 2006;1082:151–171. doi: 10.1196/annals.1348.062. [DOI] [PubMed] [Google Scholar]
- 76.Ong SE, Monica S., Adam A., et al. Identifying the proteins to which small-molecule probes and drugs bind in cells. Proc Natl Acad Sci U S A. 2009;106:4617–4622. doi: 10.1073/pnas.0900191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wan H. An overall comparison of small molecules and large biologics in ADME testing. ADMET DMPK. 2016;4:1–22. [Google Scholar]
- 78.Li L., Tian D., Chen F., Yang J., Yu K., Sun Y. Strategies for improving the quantitative bioanalytical performance of LC-MS in pharmacokinetic studies. Curr Drug Metab. 2012;13:1206–1212. doi: 10.2174/138920012803341320. [DOI] [PubMed] [Google Scholar]
- 79.Kumar S., Rajkumar SV. Thalidomide and lenalidomide in the treatment of multiple myeloma. Eur J Cancer. 2006;42:1612–1622. doi: 10.1016/j.ejca.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 80.Teo SK. Properties of thalidomide and its analogues: implications for anticancer therapy. AAPS J. 2005;22:14–19. doi: 10.1208/aapsj070103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mujagic H., Chabner BA, Mujagic Z. Mechanisms of action and potential therapeutic uses of thalidomide. Croat Med J. 2002;43:274–285. [PubMed] [Google Scholar]
- 82.Von Moos R., Stolz R., Cerny T., Gillessen S. Thalidomide: from tragedy to promise. Swiss Med Wkly. 2003;133:77–87. doi: 10.4414/smw.2003.09947. [DOI] [PubMed] [Google Scholar]
- 83.D'Amato RJ, Loughnan MS, Flynn E., Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci U S A. 1994;91:4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kenyon BM, Browne F., D'Amato RJ. Effects of thalidomide and related metabolites in a mouse corneal model of neovascularization. Exp Eye Res. 1997;64:971–978. doi: 10.1006/exer.1997.0292. [DOI] [PubMed] [Google Scholar]
- 85.Hideshima T., Chauhan D., Shima Y., et al. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood. 2000;96:2943–2950. [PubMed] [Google Scholar]
- 86.Komorowski J., Jerczynska H., Siejka A., et al. Effect of thalidomide affecting VEGF secretion, cell migration, adhesion and capillary tube formation of human endothelial EA.hy 926 cells. Life Sciences. 2006;78:2558–2563. doi: 10.1016/j.lfs.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 87.Yabu T., Tomimoto H., Taguchi Y., Yamaoka S., Igarashi Y., Okazaki T. Thalidomide-induced antiangiogenic action is mediated by ceramide through depletion of VEGF receptors, and is antagonized by sphingosine-1-phosphate. Blood. 2005;106:125–134. doi: 10.1182/blood-2004-09-3679. [DOI] [PubMed] [Google Scholar]
- 88.Moreira AL, Sampaio EP, Zmuidzinas A., Frindt P., Smith KA, Kaplan G. Thalidomide exerts its inhibitory action on tumor necrosis factor alpha by enhancing mRNA degradation. J Exp Med. 1993;177:1675–1680. doi: 10.1084/jem.177.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Niwayama S., Turk BE, Liu JO. Potent inhibition of tumor necrosis factor-alpha production by tetrafluorothalidomide and tetrafluorophthalimides. J Med Chem. 1996;39:3044–3045. doi: 10.1021/jm960284r. [DOI] [PubMed] [Google Scholar]
- 90.Powers MR, Qu Z., O'Brien B., Wilson DJ, Thompson JE, Rosenbaum JT. Immunolocalization of bFGF in pterygia: association with mast cells. Cornea. 1997;16:545–549. [PubMed] [Google Scholar]
- 91.Yagyu T., Kobayashi H., Matsuzaki H., et al. Thalidomide inhibits tumor necrosis factor-alpha-induced interleukin-8 expression in endometriotic stromal cells, possibly through suppression of nuclear factor-kappaB activation. J Clin Endocrinol Metab. 2005;90:3017–3021. doi: 10.1210/jc.2004-1946. [DOI] [PubMed] [Google Scholar]
- 92.Haslett PA. Anticytokine approaches to the treatment of anorexia and cachexia. Semin Oncol. 1998;25:53–57. [PubMed] [Google Scholar]
- 93.Geitz H., Handt S., Zwingenberger K. Thalidomide selectively modulates the density of cell surface molecules involved in the adhesion cascade. Immunopharmacology. 1996;31:213–221. doi: 10.1016/0162-3109(95)00050-x. [DOI] [PubMed] [Google Scholar]
- 94.Keifer JA, Guttridge DC, Ashburner BP, Baldwin AS., Jr. Inhibition of NF-kappa B activity by thalidomide through suppression of IkappaB kinase activity. J Biol Chem. 2001;276:22382–22387. doi: 10.1074/jbc.M100938200. [DOI] [PubMed] [Google Scholar]
- 95.Noguchi T., Shimazawa R., Nagasawa K., Hashimoto Y. Thalidomide and its analogues as cyclooxygenase inhibitors. Bioorg Med Chem Lett. 2002;12:1043–1046. doi: 10.1016/s0960-894x(02)00084-7. [DOI] [PubMed] [Google Scholar]
- 96.Ghobrial IM, Rajkumar SV. Management of thalidomide toxicity. J Support Oncol. 2003;1:194–205. [PMC free article] [PubMed] [Google Scholar]
- 97.Rajkumar SV, Witzig TE. A review of angiogenesis and antiangiogenic therapy with thalidomide in multiple myeloma. Cancer Treat Rev. 2000;26:351–362. doi: 10.1053/ctrv.2000.0188. [DOI] [PubMed] [Google Scholar]
- 98.Zhang SX, Sima J., Shao C., et al. Plasminogen kringle 5 reduces vascular leakage in the retina in rat models of oxygen-induced retinopathy and diabetes. Diabetologia. 2004;47:124–131. doi: 10.1007/s00125-003-1276-4. [DOI] [PubMed] [Google Scholar]
- 99.Ayalasomayajula SP, Kompella UB. Celecoxib, a selective cyclooxygenase-2 inhibitor, inhibits retinal vascular endothelial growth factor expression and vascular leakage in a streptozotocin-induced diabetic rat model. Eur J Pharmacol. 2003;458:283–289. doi: 10.1016/s0014-2999(02)02793-0. [DOI] [PubMed] [Google Scholar]
- 100.Hosseini H., Nejabat MA. Potential therapeutic strategy for inhibition of corneal neovascularization with new anti-VEGF agents. Med Hypotheses. 2007;68:799–801. doi: 10.1016/j.mehy.2006.06.063. [DOI] [PubMed] [Google Scholar]
- 101.Bock F., Konig Y., Kruse F., Baier M., Cursiefen C. Bevacizumab (Avastin) eye drops inhibit corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2008;246:281–284. doi: 10.1007/s00417-007-0684-4. [DOI] [PubMed] [Google Scholar]
- 102.Joussen AM, Poulaki V., Qin W., et al. Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expression and initiates early diabetic retinal leukocyte adhesion in vivo. Am J Pathol. 2002;60:501–509. doi: 10.1016/S0002-9440(10)64869-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim I., Moon SO, Kim SH, Kim HJ, Koh YS, Koh GY. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-κB activation in endothelial cells. J Biol Chem. 2001;276:7614–7620. doi: 10.1074/jbc.M009705200. [DOI] [PubMed] [Google Scholar]
- 104.Miyamoto K., Khosrof S., Bursell SE, et al. Vascular endothelial growth factor (VEGF)-induced retinal vascular permeability is mediated by intercellular adhesion molecule-1 (ICAM-1) Am J Pathol. 2000;156:1733–1739. doi: 10.1016/S0002-9440(10)65044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miyamoto K., Khosrof S., Bursell SE, et al. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci U S A. 1999;96:10836–10841. doi: 10.1073/pnas.96.19.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.