Abstract
Type 1 diabetes (T1D) arises from a failure to maintain tolerance to specific β-cell antigens. Antigen-specific immunotherapy (ASIT) aims to reestablish immune tolerance through the supply of pertinent antigens to specific cell types or environments that are suitable for eliciting tolerogenic responses. However, antigen-presenting cells (APCs) in T1D patients and in animal models of T1D are affected by a number of alterations, some due to genetic polymorphism. Combination of these alterations, impacting the number, phenotype, and function of APC subsets, may account for both the underlying tolerance deficiency and for the limited efficacy of ASITs so far. In this comprehensive review, we examine different aspects of APC function that are pertinent to tolerance induction and summarize how they are altered in the context of T1D. We attempt to reconcile 25 years of studies on this topic, highlighting genetic, phenotypic, and functional features that are common or distinct between humans and animal models. Finally, we discuss the implications of these defects and the challenges they might pose for the use of ASITs to treat T1D. Better understanding of these APC alterations will help us design more efficient ways to induce tolerance.
Introduction
Type 1 diabetes (T1D) results from T-cell–mediated destruction of insulin-producing pancreatic β-cells, leading to hyperglycemia and associated complications (1). The etiology of T1D is not completely understood, but both genetic and environmental factors are known contributors in conjunction with a decline of central and peripheral tolerance mechanisms. T1D susceptibility genes substantially overlap with other polygenic autoimmune and autoinflammatory diseases (Supplementary Table 1), and T1D patients may develop other autoimmune diseases such as thyroiditis and celiac disease (2). Therefore, although a number of genetic traits may predispose to multiple autoimmune diseases, specific precipitating events may serve as a trigger and dictate which tissue(s) becomes targeted by autoreactive T cells. Whether it is deletion, anergy, or induction of regulatory T cells (Tregs), all mechanisms of tolerance require presentation of self-antigens to thymocytes or peripheral T cells by tolerogenic antigen-presenting cells (APCs), which are equipped to deliver, upon engagement of T cells, appropriate signals to prevent or shut down unwanted responses. The most studied are professional (hematopoietic) APCs, such as dendritic cells (DCs), macrophages (MΦs), and B cells. However, these APCs constitute double-edged swords in T1D because they may be inappropriately swayed toward immunogenic functions. Here, we comprehensively review APC features and functions that are relevant to tolerance (Fig. 1) and how they are altered or defective in humans and animals with T1D, including genetic associations that may influence these functions (Table 1 and Supplementary Tables 2 and 3). We then discuss the implications of such APC alterations on the efficacy of antigen-specific immunotherapies (ASITs), which aim to deliver particular self-antigens to the patient’s endogenous populations of APCs for tolerance induction (Fig. 2 and Table 2). In the context of this review, we define APC as any cell that can present self-antigens and may potentially be the target of ASITs.
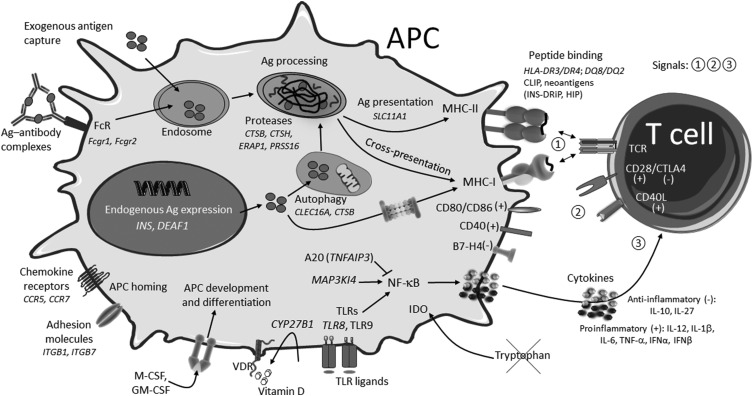
Figure 1.
Summary of APC biological processes affected in T1D with examples. Processes shown are from multiple APCs (DCs, MΦs, B cells, mTECs, stromal cells) and may not all be found in a given type of APC. (+) and (-) denote immunogenic and tolerogenic signals, respectively. Ag, antigen; DRiP, defective ribosomal product; FcR, Fc receptors; HIP, hybrid insulin peptide; VDR, vitamin D receptor.
Table 1.
Summary of APC functions affected in T1D
| APC functions and pathways | T1D (human) | T1D (rodents) | ||
|---|---|---|---|---|
| APC development (Supplementary Tables 4–6) | Cell number and yield | Fewer blood DCs (young subjects); reduced moDC yield in vitro | Fewer splenic DCs in vivo; reduced BM-DC yield in vitro | |
| APC generation and expansion | IKZF1* DC subsets?; SH2B3* DC numbers? GAB3*/ZFP36L1* monocyte and MΦ generation? GAB3*/PTPN2* response to M-CSF | Csf1M/Csf2M; responsiveness to M-CSF ↓ in MΦs Ptpn2M? | ||
| Antigen presentation | Antigen expression (Supplementary Table 7) | β-Cell autoantigens | INS*; IA-2/IGRP (splicing) | IappM; InsR |
| PTA regulation | DEAF1 (splicing) | Deaf1 (splicing) | ||
| MHC (Supplementary Tables 8–10) | MHC-II haplotype | HLA-DR3/4; DQ8/2 | I-Ag7 (M); RT1u (R) | |
| MHC-II expression | HLA-DR ↑ | MHC-II ↓ | ||
| SLC11A1* | Slc11a1M (↑ Nramp) | |||
| MHC-I | HLA-B; HLA-A | β2-microglobulinM/R | ||
| Antigen capture | Phagocytosis | FCGR2A* | Fcgr2M in MΦs; impaired clearance of apoptotic cells | |
| Antigen processing and loading (Supplementary Table 8) | Autophagy | CLEC16A*; CTSB* | ||
| Proteolysis | CTSB*; CTSH*; ERAP1*; PRSS16* | CtshM? | ||
| Peptide binding | CLIP; neoantigens (DRiP, HIP) | CLIP, HIP | ||
| Cross-presentation | RAC2*?; MAP3K14*? | Impaired in CD8α+ DCs; RAC2R? | ||
| APC activation and function | Maturation (Supplementary Table 11) | NF-κB pathway hyperactivity (monocytes, moDCs); MAP3K14* TNFAIP3* A20 ↓ | NF-κB pathway dysregulation and hyperactivity; Nfkb1R | |
| Costimulation (Supplementary Tables 9 and 10) | No consensus on costimulatory molecule expression; CD226* | No consensus on costimulatory molecule expression | ||
| Cytokines (Supplementary Tables 12 and 13) | IL10*; C1QTNF6*?; SLC11A1*? IL-10↓ in B cells | Il10M?; CD101M? C1qtnf6R? Slc11a1M? | ||
| IL27* | Il27R | |||
| IL-12 ↓ or ≡ in DCs; TYK2*?; STAT4*? SLC11A1*? | IL-12 ↓ in DCs; ↑ in MΦs; no consensus in BM-DCs; Slc11a1M? | |||
| IL-1β ↑ or ≡ in monocytes | Il1a/Il1bM/R | |||
| IFN-α/IFN-β ↑; IFIH1* TLR8*; TAGAP*; PTPN22* | IFN-α/IFN-β ↑ TagapM?; Ptpn22M | |||
| GM-CSF ↑ (monocytes) | GM-CSF ↑ (MΦs) | |||
| Tolerogenic function (Supplementary Table 14) | Defective Treg induction by lamina propria DCs; NRP1*?; B7-H4 ↓; galectin-1 ↓; CYP27B1* | Defective tolerance induction by CD8α+ DCs; B7-H4 ↓; IDO ↓ in DCs and fibroblasts | ||
| APC adhesion and homing (Supplementary Table 15) | Cell adhesion | ITGB1*; ITGB7*; ICAM-1 ↓ (monocytes) | Fibronectin adhesion ↑; SLAM ↓ | |
| Chemotaxis | CCR2 ↓; CCR5*; CCR7*; CXCL12*; GPR183*; SKAP2*; CD69* | CCR2 ↓; CCR5 ↓; CCR7M? CXCL12 ↑; Skap2R | ||
Genes associated with the disease are denoted by * for humans, M for NOD mice, or R for diabetes-prone BB rats; they are described in more details in Supplementary Tables 1, 2 (humans), and 3 (rodents). For more details about the genes or functions in each category, refer to the indicated supplementary tables. All functions listed under rodents are for NOD mice. Commonalities between patients and rodent models are in boldface type. A question mark indicates that the role of the gene in a particular function is speculative or that the gene association is not refined. When the same gene is linked to T1D in both humans and animal models, it is nonetheless possible that the function of that gene is affected differently between the two species. ↑, increased; ↓, decreased; ≡, unchanged. DRiP, defective ribosomal product; HIP, hybrid insulin peptide.
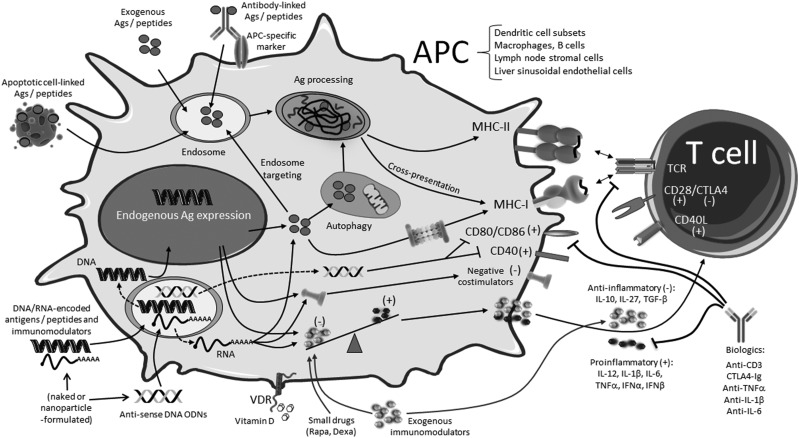
Figure 2.
Summary of ASIT approaches and associated therapies for T1D. (+) and (-) denote immunogenic and tolerogenic signals, respectively. Not shown: Exogenous antigens/peptides may be formulated for codelivery with small drugs or other immunomodulators or conjugated with molecules other than the antibody for specific cell targeting. Ag, antigen; Dexa, dexamethasone; ODNs, oligodeoxynucleotides; Rapa, rapamycin; VDR, vitamin D receptor.
Table 2.
Challenges ascribed to APC alterations and approaches to overcome them
| Function affected | Challenge | Approaches |
|---|---|---|
| APC development | Some populations of tolerogenic DCs may be reduced in number |
|
| Antigen expression and distribution | Insufficient thymic expression; insufficient distribution of β-cell antigens beyond draining lymph nodes |
|
| Antigen capture | Defective acquisition of exogenous antigens |
|
| Antigen processing and presentation | Inability to generate certain neoepitopes from native antigens outside the islets |
|
| Limited autophagy may limit Treg induction from endogenous antigens |
|
|
| Defective cross-presentation |
|
|
| APC maturation | Excessive DC or MΦ maturation |
|
| Costimulation | Imbalance between positive and negative costimulatory molecules |
|
| Cytokines | Imbalance between proinflammatory and suppressive cytokines |
|
| Tolerogenic function | Defective stimulation or induction of Tregs, defective pathways (IDO, vitamin D) due to insufficient expression or responsiveness |
|
| Homing | Same chemokines may recruit both proinflammatory and regulatory APCs (and T cells) to islets |
|
| Defective homing of DCs to lymph nodes may also limit tolerance induction |
|
We distinguish between current approaches that have already been evaluated clinically and possible future approaches to improve the current ones and to better address challenges from APC alterations. HIPs, hybrid insulin peptides.
APC Development and Frequencies
The relative proportion of different types of APCs may influence the frequency of their interactions with self-reactive T cells and the subsequent phenotype of these T cells. Peripheral blood DCs are differentially altered in their proportions depending on the age and disease stage (new-onset or long-term T1D vs. control group) of subjects (Supplementary Table 4A). The youngest patients appear to have a deficit in both myeloid DCs (mDCs) and plasmacytoid DCs (pDCs), although this difference is not apparent when evaluating a broader age range. The term mDC is now obsolete, but in most studies listed (Supplementary Table 4), it refers to CD11c+CD123− DCs, and in a few studies, specifically to migratory CD1c+ DCs. Furthermore, fewer monocyte-derived DCs (moDCs) were obtained from T1D or at-risk patients compared with control subjects (Supplementary Table 4B). Polymorphism in several human susceptibility genes may impact the development and number of APCs, including SH2B3 for DCs, IKZF1 for mDCs and pDCs, and GAB3 and ZFP36L1 for monocytes and MΦs (Supplementary Table 2).
Studies in NOD mice have made it possible to analyze APC frequency, phenotype, and function beyond the peripheral blood. NOD mice have consistently yielded fewer splenic DCs, particularly splenic CD8α+ DCs (Supplementary Table 5A). Similarly, the yield of bone marrow-derived DCs (BM-DCs) generated in vitro was reduced in NOD mice in the great majority of studies (Supplementary Table 5B). Defects affecting in vivo DC subsets, as well as pDC precursors, could be corrected by treatment with FLT3L (Supplementary Table 6). However, it is unclear if insufficient levels of FLT3L or other growth factors are responsible for the reduced number of certain DC subsets. Candidate genes for other pertinent growth factors in NOD susceptibility regions include Csf1 (Idd18.3, encoding macrophage colony-stimulating factor [M-CSF]) and Csf2 (Idd4.3, encoding granulocyte-macrophage colony-stimulating factor [GM-CSF]) (Supplementary Table 3). Polymorphism on these genes (and perhaps others) or altered responsiveness to these cytokines may affect the development and differentiation of myeloid APC progenitors or the function of myeloid cells (Supplementary Table 6). On the one hand, MΦs from NOD mice and monocytes from T1D patients and high-risk subjects express higher basal levels of GM-CSF, leading to persistent STAT5 stimulation. This increased GM-CSF production is somewhat surprising given the generally reported lower frequency of DCs and the therapeutic benefit of GM-CSF in T1D (3). On the other hand, responsiveness to M-CSF is reduced in NOD mice (Supplementary Table 6) and may be influenced in humans by the susceptibility genes GAB3 and PTPN2, which encode signaling components downstream of the M-CSF receptor (Supplementary Table 2).
Merocytic DCs, a lesser-known population of CD11c+CD8α−CD11b−/low DCs that can break the tolerance to apoptotic cell–derived antigens, are increased in NOD mice compared with other strains (4). Pancreatic islets contain several populations of DCs and MΦs (5–7). The majority of islet DCs are CD11b+CX3CR1+ DCs that are potentially proinflammatory and increase over time, whereas CD103+ DCs represent a minority that typically migrate to pancreatic lymph nodes (PLNs) to present antigens (6) but are reduced in the islets of prediabetic NOD mice (8). Islet MΦs also take part in the initiation of disease (9). It is unclear if the frequency or phenotype of DCs and MΦs in NOD islets is abnormal prior to the inflammatory leukocytic infiltration since this process starts very early.
Antigenic Signals
Antigen Expression
In order to prevent autoimmunity, peripheral tissue antigens (PTAs) must be presented by tolerogenic APCs. Migratory DCs continuously acquire antigens in the tissue of origin to later present them to T cells in draining lymph nodes and, to some extent, in the thymus. Some antigens may also flow to local lymph nodes via lymphatics for uptake by local APCs. However, the predominant mechanism of tolerance varies per tissue. In mice, T cells that are specific to a pancreatic antigen and not deleted in the thymus tend to be ignorant and may become activated upon vaccination with this antigen and adjuvant (10). This is in contrast to ubiquitous antigens that primarily lead to deletion and to gut or lung antigens that induce Tregs (10). Shedding of self-antigens by the tissue does not apply to all PTAs, and many PTAs may also be ectopically expressed at very low levels by other cells, some of which have antigen-presenting and tolerogenic properties. Most importantly, medullary thymic epithelial cells (mTECs) express a variety of PTAs, owing to the activity of transcription regulators such as AIRE and FEZF2 (11,12), and play a crucial role in mediating deletion of autoreactive T cells and/or Treg induction while also serving as a local source of antigen for thymic DCs. Among β-cell antigens, insulin is expressed in mTECs under AIRE’s control and also in a variety of other cells in the periphery, including subsets of DCs that also express AIRE (Supplementary Table 7). Lower expression of the INS gene in the thymus, due to risk variants affecting its promoter, may result in insulin-reactive T cells being less efficiently engaged for deletion or deviation toward Tregs. The INS gene is also associated with T1D in diabetes-prone BioBreeding (BB) rats (Supplementary Tables 1–3). Other β-cell antigens, including islet antigen 2 (IA-2), islet amyloid polypeptide (IAPP), and islet-specific glucose-6-phosphate related protein (IGRP), are also ectopically expressed in the thymus and peripheral lymphoid tissues (Supplementary Table 7), and IAPP is the only other β-cell autoantigen found in a susceptibility region (Idd6.2 of NOD mice) (Supplementary Table 3). The expression of some PTAs in lymph nodes is controlled by DEAF1, a transcriptional regulator that resembles AIRE. Both NOD mice and T1D patients have excessive splicing of DEAF1 mRNA in PLNs, leading to loss of DEAF1 function, which correlates with reduced local expression of PTAs (13). Overall, defects in ectopic PTA expression could limit the availability of self-antigens in sites such as the thymus or lymph nodes for tolerance induction.
Antigen Capture and Phagocytosis
A reduced ability to capture self-antigen for presentation may hinder tolerance induction. Defective clearance and persistence of immune complexes or apoptotic cells may also cause local inflammation and aberrant reactivity against self-antigens. The Fcgr2 allele in NOD mice was associated with lower expression on MΦs (14), whereas the human gene FCGR2A is linked to T1D, though not as significantly as celiac disease (15). Furthermore, NOD MΦs are inefficient in the phagocytosis of apoptotic cells (16–18), which was linked to Idd5. This may contribute to the earliest stages of the disease pathogenesis by defective clearance: first, of dying β-cells, resulting in accumulation of DNA and antigens, and second, of immune complexes between natural antibodies and apoptotic components that can stimulate the production of IFN-α and other inflammatory cytokines by pDCs (19). Whether defects in clearance and antigen capture are implicated in human T1D has not yet been established, though they are typically found in systemic lupus erythematosus, a disease with a substantial overlap of susceptibility genes with T1D (Supplementary Table 1).
Antigen Processing
Endogenously expressed or exogenously acquired antigens must be processed into peptides within APCs for presentation to T cells. Defects that affect antigen processing can influence the abundance and diversity of generated peptides and their availability for presentation. Presentation of endogenously expressed antigens on MHC-II molecules can be achieved via autophagy, as seen in mTECs (20). The susceptibility genes CLEC16A and CTSB regulate autophagy, including in mTECs where they influence the thymic selection of autoreactive T cells (Supplementary Table 8). The processing of proteins into peptides involves the activity of various proteases that influence the repertoire of self-antigens presented for tolerance induction. Genetic variations in CTSB and CTSH, which encode cathepsins B and H, two lysosomal cysteine proteases expressed in DCs and MΦs, are associated with T1D (Supplementary Table 8). The risk allele of CTSH has also been linked to lower insulin expression and production in the islets (21), but whether it also affects the ectopic expression of insulin elsewhere is unknown. Two other protease-encoding genes are also associated with T1D/autoimmunity in humans: ERAP1, which controls the trimming of peptides for MHC-I loading, thereby influencing recognition by CD8+ T cells, and PRSS16, which has been found to impact the deletion of several diabetogenic CD4+ T cells in the thymus (Supplementary Table 8). Overall, impaired mechanisms of antigen processing can lead to qualitative and quantitative changes in the generation of epitopes derived from self-antigens that are presented to T cells.
Antigen Presentation
Different antigenic peptides are subject to presentation to T cells based on their ability to fit on MHC molecules. The MHC region confers the greatest genetic susceptibility to T1D, with particular MHC-II haplotypes playing an essential role. In humans, combinations of HLA-DR3/DR4 and DQ8/DQ2 predispose to disease to different degrees, a lot more than other haplotypes (22). Rodent models have a unique MHC-II haplotype (I-Ag7 in NOD mice and RT1u in BB rats) that is required for spontaneous disease development. The mouse I-Ag7 MHC molecule structurally resembles HLA-DQ8 with a nonnegatively charged amino acid at position 57 of the β-chain that allows more promiscuous peptide binding (23,24). Poor HLA-DM editing leads to increased levels of CLIP peptide on MHC-II in both NOD mice and T1D patients (Supplementary Table 8). These molecules may also preferentially bind neoepitopes, such as insulin’s defective ribosomal product on HLA-DQ8/DQ2 or hybrid insulin peptides on I-Ag7 and HLA-DQ8/DQ2 (Supplementary Table 8).
Although various haplotypes are responsible for qualitative differences in their ability to bind key peptides, quantitative differences have also been reported on the amount of MHC molecules expressed on the surface of APCs. There is overwhelming evidence that both splenic DCs and BM-DCs from NOD mice express abnormally low levels of MHC-II relative to many different strains of mice, with or without stimulation (Supplementary Table 9). BM-DCs from diabetes-prone BB rats also express lower levels of MHC-II than those from control strains (25). In contrast, a few studies reported unchanged or increased HLA-DR expression on DCs from T1D patients (Supplementary Table 10), but these findings are mainly from the same group. MHC-II expression and antigen presentation can also be affected by polymorphism in the SLC11A1 gene in humans and NOD mice (Supplementary Tables 1 and 2).
Diabetogenic CD8+ T cells have also been identified both in humans and rodent models. T1D predisposition by MHC-I haplotypes within HLA-A and HLA-B loci has been observed in humans (26), whereas in rodents, it is the gene for the β2-microglobulin component of MHC-I that has been found in susceptibility regions in NOD mice and BB rats that can influence disease in NOD mice (Supplementary Table 3).
Cross-presentation refers to the ability of limited subsets of APCs, primarily Batf3-dependent DCs (CD8α+ resident DCs and CD103+ migratory DCs), to process and present exogenously acquired antigens on MHC-I molecules to CD8+ T cells. In NOD mice, splenic CD8α+ DCs show a reduced capacity to cross-present MHC-I–restricted antigens to diabetogenic CD8+ T cells (27). This defect may limit the presentation of certain islet cell-derived epitopes to induce tolerance. Cross-presentation of certain β-cell antigens by B cells also exacerbates T1D (28). In humans, cross-presentation may be affected by polymorphism in the RAC2 and MAP3K14 genes (Supplementary Table 2).
Tolerogenic Versus Immunogenic Signals
The type of immune response initiated by DCs ultimately depends on the context in which antigens were acquired, which influences the balance of immunogenic and tolerogenic functions of APCs. Once autoreactive T cells are engaged by specific antigens (signal 1), their response will be dictated by the sum of signals that are delivered to T cells in the form of contact-dependent costimulation (signal 2) and cytokine-mediated immunomodulation (signal 3) (Fig. 1).
APC Maturation and Regulatory Function
Mature DCs express higher levels of MHC and costimulation molecules, which are required to stimulate effector cells. Many studies have compared levels of costimulatory molecules on APC populations between NOD mice and control strains (Supplementary Table 9) or between T1D patients and control subjects (Supplementary Table 10), reporting variable and conflicting results, possibly due to a large variety of tested conditions. As an important regulator of APC maturation, the nuclear factor-κB (NF-κB) pathway was found to be altered in T1D in various ways (Supplementary Table 11). Several genes associated with T1D in humans may impact NF-κB activation, including NIK-encoding MAP3K14 and A20-encoding TNFAIP3 (Supplementary Table 2), the latter being an important negative regulator of DC immunogenicity associated with multiple autoimmune diseases (Supplementary Table 1). Other genes associated with DC maturation include ERBB3 and CD226 in humans (Supplementary Table 2) and genes within the Idd10/17/18 region in NOD mice (Supplementary Table 3).
Loss of tolerogenic function has been reported in multiple APC subsets from NOD mice. First, CD8α+ DCs (CD40hi) are defective at inducing CD4+ T-cell tolerance after antigen delivery targeted to these DCs, but after CD40 blockade, they improve in their ability to mediate T-cell deletion and reduce Th1 stimulation (29). A deficiency of DCs to mediate T-cell deletion in PLNs was previously mapped to Idd3 (including Il2 and Acadl, underexpressed in stimulated NOD DCs) and Idd5 (Slc11a1, overexpressed in stimulated NOD DCs and MΦs) (Supplementary Table 2) (30). Second, both pancreatic CD8α+CD103+Langerin+ and CD11c+CD8α− DC populations also appear less tolerogenic than those from control strains based on phenotype and gene expression (8,31). Irradiated splenocytes from NOD mice also induced less suppressive Tregs than their B6 counterparts (32). Similarly in humans, both APCs from peripheral blood mononuclear cells and DCs from the lamina propria of T1D patients were defective in the induction of Foxp3+ Tregs (33,34). T1D patients with suboptimal glycemic control produce moDCs that have reduced tolerogenic potential (35), indicating a negative influence of hyperglycemia. This loss of tolerogenic function is not understood but may be contributed by imbalance between positive and negative costimulatory signals or, as we will see later, between proinflammatory and immunoregulatory cytokines. For instance, NOD mice exhibit a gradual loss of the negative costimulatory molecule B7-H4 on the surface of APCs (compared with other strains) due to proteolytic cleavage, which correlates with increased levels of circulating soluble B7-H4; about half of T1D patients have high levels of soluble B7-H4 as well (36).
Cytokines and TLR Signaling
Full DC maturation commonly results in production of proinflammatory cytokines that support T-cell differentiation into effector T-cell subsets (IL-12 for Th1, IL-1β and/or IL-6 for Th17), whereas semimature DCs may express relatively high levels of costimulatory molecules without secreting proinflammatory cytokines and regulatory DCs may correspond to a terminally differentiated state with switch toward suppressive cytokines to regulate the elicited T-cell response. Cytokine imbalance may contribute to inappropriate T-cell responses to self-antigens (Supplementary Tables 12 and 13). Furthermore, aberrant Toll-like receptor (TLR) response to viruses, bacteria, and other microbes may lead to excessive secretion of proinflammatory cytokines.
IL-10 and IL-27 are two major anti-inflammatory cytokines produced by APCs and whose genes are associated with T1D in humans, though the markers associated with the human genes are in noncoding regions and it is not clear how the expression and/or function of these cytokines is affected by these polymorphisms. The same genes are found in susceptibility regions in NOD mice (Il10) and BB rats (Il27) (Supplementary Table 3). Other susceptibility genes can influence the production of IL-10 by APCs, including Cd101 in NOD mice (Supplementary Table 3) and C1QTNF6 in humans (Supplementary Table 2) and BB rats (Supplementary Table 3). Studies performed in NOD mice did not provide a consensus on whether IL-10 production is defective, although both CD8α+ and CD8α− DCs in the pancreas seem to express less IL-10 (Supplementary Table 12A). IL-10 production does not appear to be defective in DCs and monocytes from T1D patients, and if anything, it may be increased in some cases (Supplementary Table 13). B cells can also serve as APCs and as a source of regulatory cytokines. T1D patients have altered frequency of different B-cell subsets, in particular they have fewer CD19+CD5+CD1dhi cells, which are thought to be IL-10–producing regulatory B cells (B10 cells), and B cells from T1D patients are defective in IL-10 production in response to TLR ligands (Supplementary Table 13).
IL-12 is secreted by DCs and MΦs to promote the differentiation of Th1 cells, the main type of pathogenic CD4+ T cells in T1D. Although studies in NOD mice consistently point to a defective ability of splenic CD8α+ DCs to produce IL-12, this defect is overcompensated by increased expression of IL-12 by MΦs (Supplementary Table 12A), attributed to abnormal NF-κB signaling (Supplementary Table 11) and linked to the Idd4 region (Supplementary Table 3). Thus, assessing IL-12 production in only one type of APC may not provide a full picture of the milieu to which T cells are exposed. Studies using BM-DCs from NOD mice yielded variable results, though most studies reported increased production of IL-12, linked to excessive NF-κB activity and to the Idd10/17/18 regions (Supplementary Tables 3, 11, and 12). In T1D patients, APCs express normal amounts of IL-12, although DCs may produce less IL-12 under certain conditions (Supplementary Table 13). Dysregulated IL-12 production may be influenced by variants of the TYK2 and STAT4 genes, whereas SLC11A1-encoded NRAMP1 may also affect the IL-10/IL-12 balance (Supplementary Table 2).
Proinflammatory cytokines TNF-α, IL-1, and IL-6 are usually undesirable in autoimmune diseases and commonly targeted by neutralizing biologics. When assessed in NOD mice, the production of these cytokines has been variable and inconsistent depending on the analyzed APCs, the control strains used for comparison, and the type of stimulation (Supplementary Table 12B). The Il1a and Il1b genes are associated with T1D in both NOD mice and BB rats (Supplementary Table 3). In T1D patients, there is also no clear consensus on how the expression of these cytokines is altered in DCs and monocytes (Supplementary Table 13).
Type I interferons are typically overexpressed when APCs from NOD mice or T1D patients are stimulated with resiquimod, CpG, or flu virus (Supplementary Tables 12B and 13). As cytokine expression by APCs is generally assessed after stimulation with TLR ligands, such as peptidoglycan (TLR2), polyinosinic:polycytidylic acid (TLR3), lipopolysaccharide (TLR4), resiquimod (TLR7/8), and CpG (TLR9), the observed alterations of cytokine expression also point toward abnormal TLR expression (37) or TLR signaling (38–40). The risk variant of the human IFIH1 gene is associated with a greater production of type I interferons and responsiveness to self-RNA ligands (Supplementary Table 2). Other risk alleles associated with T1D and TLR signaling include TLR8, PTPN22, and TAGAP (Supplementary Table 2). Certain infections and changes in the microbiome can alter the function of APCs through TLR stimulation, at least in certain tissue compartments. The role of TLRs in T1D has been more extensively reviewed elsewhere (41).
Other Tolerance-Regulating Mechanisms
Defects affecting other mechanisms of tolerance maintenance have also been reported. Monocytes from T1D patients produce less galectin-1, an immunosuppressive molecule, than control subjects (Supplementary Table 14). Indoleamine 2,3-dioxygenase (IDO) limits T-cell activation by depriving the milieu of the tryptophan necessary for cell growth and contributes to Treg development and to the protective role of pDCs in NOD mice (Supplementary Table 14). However, DCs and stromal cells (fibroblasts) from NOD mice are impaired in the induction of IDO in response to IFN-γ (Supplementary Table 14). Finally, vitamin D regulates the tolerogenic function of DCs and the maintenance of Tregs. Therefore, alterations in the production of or responsiveness to vitamin D may contribute to autoimmunity. The genes CYP27B1 (encoding an enzyme converting vitamin D into its active form) and VDR (encoding the vitamin D receptor) are associated with T1D in humans and BB rats, respectively (Supplementary Tables 2, 3, and 14). The expression of CYP27B1 is reduced in APCs of T1D patients (Supplementary Table 14).
Cell Adhesion and Chemotaxis
Adhesion molecules facilitate contact between cells and help with the proper transmission of regulatory signals to and from APCs. Integrin genes ITGB1 and ITGB7 are associated with human T1D (Supplementary Table 2), with ITGB7 playing an important role in T-cell (and likely DC) recruitment to islets and homing of CD103+ migratory DCs (Supplementary Table 15). Both integrins are involved in the binding of DCs to fibronectin, and mature BM-DCs from NOD mice show increased adhesion to fibronectin in vitro (Supplementary Table 15). Other altered adhesion molecules include ICAM-1 (reduced in moDCs of T1D patients) and SLAM (reduced in mature mDCs of NOD mice), the latter resulting in an impaired induction of “regulatory” natural killer T cells (Supplementary Table 15). SIRPα is expressed on MΦs and DC subsets to block cell phagocytosis via the SIRPα–CD47 interaction. Although not regarded as an adhesion molecule, the protein encoded by the NOD variant of Sirpa binds more strongly to CD47, resulting in enhanced T-cell proliferation induced by DCs (Supplementary Tables 3 and 15). It is unclear if this effect results from signaling on either side of the SIRPα–CD47 axis or simply from a tighter contact between the two cells. Variations in the strength and duration of adhesion may impact the potency of T-cell stimulation (APC–T-cell interaction) and the ability of APCs to exit one tissue (attachment to extracellular matrix) or access another (e.g., transendothelial migration).
Chemokine receptors also play a crucial role in the trafficking of migratory APCs to lymph nodes and to sites of inflammation. Both CCR2 and CCR5 play distinct but important roles in the recruitment of leukocytes to inflamed islets, reflecting differential attraction of proinflammatory versus regulatory APCs (Supplementary Table 15). CXCL12 may also control homing of certain APCs to the pancreas (Supplementary Table 15). CCR7 is required for the homing of migratory DCs to draining lymph nodes. In humans, CCR7 is associated with T1D, and in NOD mice, mature BM-DCs have a defect in migration toward CCL19, one of CCR7 ligands (Supplementary Table 15). Polymorphism in the SKAP2 and GPR183 genes may also alter MΦ and DC migration (Supplementary Table 2). Finally, CD69 is also associated with human T1D, and in addition to regulating retention of T cells in tissues, it may also control the ability of DCs to leave peripheral tissues to lymph nodes (Supplementary Table 2). However, all the aforementioned chemokine receptors and other homing/retention molecules are expressed in many immune cells, so their specific role in positioning APCs in the context of T1D and tolerance is still not clearly established.
APC Alterations in T1D: Cause or Consequence?
Some of the changes seen in APC development, phenotype, and function may contribute to disease, result from disease, or simply occur in parallel as a reflection of the genetic makeup of the individual. As mentioned above, many aspects of APC frequency and function are affected by susceptibility genes, and genetically controlled APC alterations are more likely to be causative as they predate the initiation of disease. However, it is plausible that some of these alterations manifest themselves only in the context of inflammation. In T1D patients, changes in APC frequency or function (e.g., excessive secretion of proinflammatory cytokines or overreaction to such cytokines or TLR ligands) are most often evaluated after disease onset. The heterogeneity of patients, discussed below, leads to data inconsistencies, whereby alterations in APC parameters vary with disease stage, age-group, and criteria to measure responsiveness and maturation of APCs (examples can be found in Supplementary Tables 4, 10, and 13). This complicates our understanding of the contribution of APC alterations to predisposing to or perpetuating disease. Persistent inflammation and uncontrolled hyperglycemia can also have adverse repercussions on APC functions in mice (42) and patients (35).
Implications for Asit
The Hurdles of Translating Therapies From Mice to Patients
In this review, we have identified multiple layers of APC function that are defective or simply vary in NOD mice or T1D patients (Fig. 1). These alterations are influenced by genetic polymorphism, response to infections, changes in microbiota, and other environmental factors. Although there are appreciable similarities between animal models and T1D patients (Table 1), the latter constitute a heterogeneous population in terms of genetic risk factors. The NOD mouse model is very valuable to understand the role of certain human genes when the genes of both species are associated with T1D. However, there are also limitations to the animal models when testing therapeutic approaches. Despite the numerous alterations in APC function reported in NOD mice, it has been possible to achieve antigen-specific tolerance by delivering antigens in different forms (protein, peptide, DNA) and via different routes (oral, subcutaneous, intramuscular, etc.). However, the most promising of these approaches that were evaluated in clinical trials had no or little beneficial effect in secondary prevention and treatment (recent onset) settings. Ineffective induction of tolerance may be due to a number of reasons, including insufficient antigen coverage or inadequate choice of antigens, inability to generate certain epitopes from delivered protein antigens or to properly bind them on MHC (e.g., inadequate HLA haplotype), improper expression of positive and negative costimulatory molecules on APCs, inability to target critical subsets of APCs, or resistance of antigen-specific T cells to the tolerogenic effect of APCs and/or Tregs, for example. The approaches currently in clinics may fail at different levels (Table 2). For example, the current practice of administering a single antigen (or multiple peptides of the same antigen) by a single route may not achieve the in vivo antigen distribution and broad engagement of diabetogenic T cells that may be required for complete tolerance induction (further discussed below). Moreover, low and sustained antigen levels may be preferable over antigen levels that fluctuate between different administrations. Less conventional approaches, including nanoparticle-based delivery and apoptosis-associated uptake (43), may facilitate immunomodulation or targeting of atypical tolerogenic APCs (44) but have not yet been translated to T1D patients. Occasionally, a transient effect (delayed loss of C-peptide) has been observed in particular subsets of patients or with unconventional delivery strategies (Table 3). These few studies suggest that 1) antigens selected based on high autoantibody reactivity may have a greater therapeutic impact, 2) HLA haplotype may influence the efficiency of antigen presentation for T-cell deletion, and 3) efficient delivery to lymph nodes may have a more profound effect. As we enter the new era of precision medicine to address patient heterogeneity, understanding patient-specific patterns and defects will enable us to apply ASIT more effectively with relevant antigens and appropriate delivery platforms.
Table 3.
Partial successes in antigen-specific prevention and intervention in humans: lessons learned
| Trial | Treatment | Results | Lessons learned | Ref. |
|---|---|---|---|---|
| Oral insulin (DPT-1) | Secondary prevention in high-risk individuals with autoantibodies (372 treated subjects) | No prevention, except in a subgroup of patients with highest insulin autoantibody levels where loss of C-peptide was delayed | Unlike in NOD mice, proinsulin may not be a driving antigen in all patients; selecting antigens based on strong evidence of autoreactivity may be required | 75,76 |
| Proinsulin DNA (BHT-3021) | Phase 1 study in T1D patients with 5 years of onset and with residual C-peptide, involving intramuscular delivery of proinsulin-encoding plasmid (80 T1D patients) | Significant delay in C-peptide loss up to 15 weeks after treatment with 1-mg dose; significant decrease of proinsulin-reactive CD8+ T cells in treated HLA-A3+ patients | Presentation of proinsulin-derived peptides (at least HLA-A3 restricted) may mediate peripheral deletion of some autoreactive CD8+ T cells and delay CD8+ T cell–mediated β-cell destruction | 77 |
| GAD65-Alum (DIAGNODE-1) | Ongoing pilot study involving intralymphatic delivery of GAD65-alum and oral vitamin D (6 new-onset patients, all with GAD65 autoantibodies) | Promising results of C-peptide preservation relative to historical studies with GAD65-alum or anti-CD3; these data remain very preliminary | Intralymphatic delivery may provide better exposure of antigens and leverage nonmigratory subsets of APCs | 78 |
DIAGNODE-1, GAD-Alum (Diamyd) Administered into Lymph Nodes in Combination with Vitamin D in Type 1 Diabetes; DPT-1, Diabetes Prevention Trial–Type 1 Diabetes.
APC Heterogeneity and the Need for Better Profiling
The same APC population may have contrasting roles depending on the stage of disease. For example, pDCs are critical for the initiation of T1D (19,45) but also play a tolerogenic/regulatory role later on (46,47). Most data from patients and NOD mice suggest that this population is reduced in later stages of the disease (Supplementary Tables 4 and 5). The frequency and phenotype of certain human APCs may vary depending on the compared parameters, such as age range (children vs. adults) and disease status (autoantibody positive, new-onset T1D, long-term T1D, etc.) (Supplementary Tables 4, 10, and 13). Since these early studies on patient APCs, multiparametric technologies have come a long way (spectral flow cytometry or mass cytometry, high-throughput single cell sequencing) and would now allow for a very detailed profiling of different APCs before and after therapy. Revisiting blood APCs using these new technologies, for example, is warranted to stratify groups of patients and better correlate responsiveness to therapy (or lack thereof) with subgroups of patients. Although blood APCs are not perfect surrogates of lymphoid tissue APCs, they may nonetheless reveal genetically imprinted impairments that may be extrapolated and taken into account. This information, combined with genetic and serological data, as well as a similar multiparametric profiling of T-cell populations, will help us to better appreciate the heterogeneity of T1D patients and, as far as ASIT is concerned, to deliver pertinent antigens to appropriate APCs. Approaches to bolster the number of certain underrepresented APCs populations (e.g., pDCs) and/or to dampen APC hyperactivity (e.g., excessive NF-kB induction and related susceptibility genes) (Supplementary Table 11) may then be considered alongside ASIT.
Expanding the Panoply of Self-antigens for Tolerance Induction
The need to deliver exogenous antigens for ASIT is fueled by the insufficient or lack of expression/presentation of relevant β-cell antigens outside the islet-draining PLNs, which becomes an unsuitable environment for tolerance induction in T1D. The INS gene is a good example of a β-cell antigen that may not be sufficiently expressed outside the pancreas. Although insulin in its mature form is available systematically for uptake and processing, certain epitopes unique to (pre)proinsulin can only be produced from endogenously expressed protein. This provided a rationale to use proinsulin in ASIT, supported by promising results in inducing tolerance in mice. Similarly, GAD65, which is not expressed in mTECs (48), has been an autoantigen of choice for delivery to T1D patients based on preclinical efficacy. However, clinical studies have not used insulin and GAD65 in combination, nor have other β-cell antigens been used so far. When antigens are generated exclusively in the islets, for example neoepitopes under excessive stress (posttranslational modifications [49]) or as a result of high concentrations of peptides from different β-cell proteins (hybrid peptides [50]), tolerance would rely entirely on tolerogenic presentation of these antigens by DCs in draining lymph nodes. However, the recent evidence that islet-infiltrating pathogenic T cells recognize all these antigens (51–53) suggests that this mechanism of autoantigen presentation fails to achieve tolerance in T1D individuals and provides a rationale to include these new antigens in ASITs and deliver them to sites that are more conducive for tolerance. Modified epitopes (mimotopes) have been produced to stabilize binding to MHC-II molecules. Mimotopes of InsB9–23 that bind better to HLA-DQ8 (or I-Ag7 in NOD mice) are able to better engage populations of diabetogenic T cells (54,55), and preclinical studies were indicative of a superior therapeutic benefit of mimotopes (56–58), though not consistently (59). More studies are needed to ascertain their safety and benefit in patients. The use of soluble peptides or multiepitopes polypeptides can circumvent antigen-processing defects and make it possible to include neoepitopes that are otherwise not present in native proteins (56,60). Reassuringly, the efficacy of therapies that rely on apoptotic cell uptake (61) does not appear to be hindered by the phagocytosis defects reported in NOD mice (16–18). Finally, the consistent and robust decrease of MHC-II expression on DCs from NOD mice (Supplementary Table 9) may possibly be a factor in their better responsiveness to ASIT. At an equivalent antigen dose, NOD DCs may engage T cells with lower avidity than their human counterparts, which may be beneficial for Treg induction. Indeed, it has been proposed that lower/subimmunogenic stimulation favors Tregs in NOD mice (56,57,62).
Targeting and Modulating Endogenous APCs
It may be challenging to control which APCs participate in the presentation of antigens after ASIT. Many delivery methods may involve any type of APC that is capable of taking up local or systemic antigen, whereas others target specific DC subsets (e.g., antigens conjugated to DEC205 or DCIR-targeting antibodies) or specific compartments (e.g., nasal or oral mucosa). However, some DC populations may be functionally deficient, and dysbiosis (microbiota imbalance) may cause the mucosal interface to be more inflammatory in some patients. Changes in gut microbiome have been observed in T1D in patients (63,64). Interestingly, T1D-associated genes most overlap with gut-associated diseases (Crohn disease, ulcerative colitis, celiac disease) (Supplementary Table 1), and PLNs also drain regions of the gut, at least in mice (65). Thus, gut leakiness of bacterial products may also enhance the immunogenicity of APCs in PLNs (66). Lingering inflammation, whether it is in the pancreas, draining lymph nodes, or the gut, can alter the phenotype of DCs and MΦs in a way that hinders effective tolerance induction. Unlike patients, NOD mice are subject to minimal microbiome variation within a colony, as they need to be kept under specific pathogen-free conditions for them to develop T1D. This needs to be taken into account when transitioning ASITs based on oral tolerance from preclinical to clinical studies. Importantly, the delivery of immunomodulators in conjunction with antigens will likely be needed to rectify the signals provided by APCs to T cells (Fig. 2). Such immunomodulators include regulatory cytokines, negative costimulatory molecules, and metabolic modifiers. Biologics that neutralize proinflammatory cytokines (TNF-α, IL-1β, IL-6) or costimulatory molecules (CTLA4-Ig, antisense oligonucleotides against CD80, CD86, and CD40) have been assessed on their own but should be evaluated in combination with ASIT (Fig. 2). Alternatively, certain inhibitory ligands and/or immunoregulatory molecules may be overexpressed along with antigens when using nucleic acid–based strategies. New vehicles and routes for antigen delivery continue to be investigated to target alternative APCs and delivery sites to substitute or mitigate functionally altered APCs that may otherwise interfere with the tolerogenic process. Although protein antigens are primarily used by APCs that have efficient endocytosis and endosomal degradation (DCs and MΦs), nucleic acid–based delivery may be more efficient for other nonprofessional APCs that have reduced endosomal degradation. Examples of such APCs include lymph node stromal cells and a variety of other nonhematopoietic APCs that can present antigens without positive costimulation and that have been implicated in tolerance induction and maintenance (44,67). Endogenous and specific antigen expression in these cells has been achieved with vectors using tissue-specific promoters and miR-142 target sites, preventing expression in professional APCs (68,69). At present, the extent of nonprofessional APCs’ contribution to tolerance induction under steady-state conditions is unclear. Further, whether their function is defective in T1D and whether they constitute worthy targets as alternative APCs in ASIT remain to be determined.
The Case of Exogenous APCs
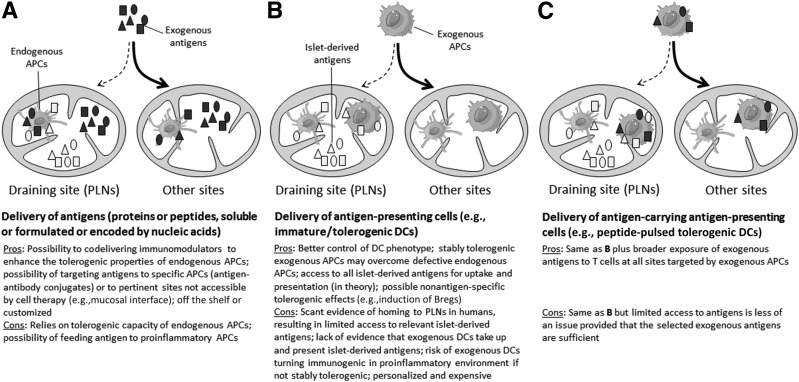
As an alternative ASIT strategy, antigens may be provided to ex vivo generated DCs that have been conditioned to adopt robust and stable tolerogenic properties before being reintroduced into patients in a more personalized approach, which is reviewed elsewhere (70–72). Such ex vivo “engineered” exogenous APCs (typically DCs) may overcome deficiencies of endogenous APCs to induce tolerance. In this respect, an outstanding question remains as to whether exogenous DCs need to be provided with antigens in order to effectively engage diabetogenic T cells or whether they are able to acquire these antigens in situ. Although antigen provision has been used in DC therapy for rheumatoid arthritis and multiple sclerosis, this has not yet been tested clinically in T1D (72). When DCs with and without antigen provision were compared side by side in preclinical models, DCs with antigen were more often protective (Supplementary Table 16). Other studies have shown that tolerogenic DCs without antigens or with irrelevant antigens can nonetheless be protective. In the most recent study, provision of relevant (GAD65) or irrelevant (OVA) antigen surprisingly abrogated the therapeutic benefit of unpulsed tolerogenic DCs (73). Given the limited number of such studies and the inconsistency of conditions used (e.g., DC generation and treatment, dose, route, antigen(s), preclinical model, age of mice at treatment) (Supplementary Table 16), it is difficult to draw solid conclusions. The merits and caveats of antigen provision to exogenous APCs are summarized in Fig. 3. Although most of the islet-derived antigens are expected to be found in islet-draining PLNs, the majority of cells or antigens administered in ASIT likely end up in other sites. In mice, intraperitoneal (and to a lesser extent intravenous) delivery best achieves targeting to PLNs (65,74). One important caveat is the lack of CCR7 expression on immature DCs that limits homing to that site, and ex vivo treatments that enable upregulation of CCR7 (and inhibitory ligands such as PD-L1) without increasing costimulatory molecules will be essential. Although it is undeniable that DCs have therapeutic potential without antigen provision, it remains unclear whether this effect involves uptake and presentation of islet-derived antigens in situ or is basically antigen-independent and primarily immunomodulatory in nature. These important considerations should be addressed experimentally, particularly in the context of the delivery routes used in humans.
Figure 3.
Different ASIT approaches relying on endogenous vs. exogenous APCs and on islet-derived vs. exogenously provided antigens. A: Delivery of exogenous antigens to endogenous APCs. Delivery of exogenous APCs without (B) or with (C) exogenous antigens. Bregs, regulatory B cells.
Conclusions
Overall, the simple provision of antigens in T1D patients for ASITs faces challenges related to the possible limited ability of endogenous APCs to properly induce tolerance with these antigens. A number of approaches may be implemented to overcome these limitations and improve their efficacy (Table 2). Defects affecting T cells, not covered in this review, will also need to be taken into consideration, particularly those that make T cells resistant to regulation by APCs or Tregs. Although successful outcomes have been achieved in NOD mice despite a considerable number of reported APC defects, the heterogeneity of T1D patients makes it unlikely that an off-the-shelf ASIT strategy that suits all patients will be available soon. Instead, combination therapies will be required to tackle multiple defects and to better control the way T cells are being stimulated. We will need to better delineate APC defects in patients and, in particular, how the function of a particular gene in APCs is affected by risk alleles. Eventually, once a sufficient number of contributing genes have been characterized, their polymorphism may be tested as part of an assay panel to better customize the type of intervention that patients may require. Identification of targeted epitopes in human, which has undergone a renaissance in the past few years, will continue to play a critical role in the effort to sort out patient-specific epitopes from those that are commonly shared. Genomic evaluation should include HLA typing to ensure that epitopes selected are tailored for the patient’s HLA if not using the whole protein as antigen. ASIT is a safe approach to treat tissue-specific autoimmune diseases such as T1D. As we enter the era of precision medicine, ASIT can also be tweaked to address specific deficiencies, thereby making the treatment even safer and more effective.
Supplementary Material
Article Information
Acknowledgments. The authors thank Drs. Stephan Kissler (Joslin Diabetes Center, Harvard Medical School, Boston, MA), Mark Anderson (Diabetes Center at University of California, San Francisco, San Francisco, CA), and Rebuma Firdessa (Columbia University, New York, NY) for critical reading of the manuscript.
Funding. Studies involving ASIT in the laboratory are currently funded by grants from the National Institutes of Health National Institute of Allergy and Infectious Diseases (R21AI110812) and JDRF (2-SRA-2017-312-S-B [subcontract]). Studies on the analysis for APC subsets from PLNs of T1D patients were funded by The Leona M. and Harry B. Helmsley Charitable Trust, in a subcontract from the Network for Pancreatic Organ Donors with Diabetes (2015PG-T1D052). J.P.-F. has been supported by the Russell Berrie Foundation (Berrie Fellowship in Diabetes Research) and by an American Diabetes Association Postdoctoral Fellowship (1-18-PDF-151).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. All authors contributed in the literature review, assembling of the data, and writing and editing of the manuscript.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db17-1564/-/DC1.
References
- 1.Katsarou A, Gudbjörnsdottir S, Rawshani A, et al. . Type 1 diabetes mellitus. Nat Rev Dis Primers 2017;3:17016. [DOI] [PubMed] [Google Scholar]
- 2.Hughes JW, Riddlesworth TD, DiMeglio LA, Miller KM, Rickels MR, McGill JB; T1D Exchange Clinic Network . Autoimmune diseases in children and adults with type 1 diabetes from the T1D Exchange Clinic Registry. J Clin Endocrinol Metab 2016;101:4931–4937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhattacharya P, Thiruppathi M, Elshabrawy HA, Alharshawi K, Kumar P, Prabhakar BS. GM-CSF: an immune modulatory cytokine that can suppress autoimmunity. Cytokine 2015;75:261–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katz JD, Ondr JK, Opoka RJ, Garcia Z, Janssen EM. Cutting edge: merocytic dendritic cells break T cell tolerance to beta cell antigens in nonobese diabetic mouse diabetes. J Immunol 2010;185:1999–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calderon B, Suri A, Miller MJ, Unanue ER. Dendritic cells in islets of Langerhans constitutively present beta cell-derived peptides bound to their class II MHC molecules. Proc Natl Acad Sci U S A 2008;105:6121–6126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin N, Xu J, Ginhoux F, et al. . Functional specialization of islet dendritic cell subsets. J Immunol 2012;188:4921–4930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calderon B, Carrero JA, Ferris ST, et al. . The pancreas anatomy conditions the origin and properties of resident macrophages. J Exp Med 2015;212:1497–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welzen-Coppens JM, van Helden-Meeuwsen CG, Leenen PJ, Drexhage HA, Versnel MA. Reduced numbers of dendritic cells with a tolerogenic phenotype in the prediabetic pancreas of NOD mice. J Leukoc Biol 2012;92:1207–1213 [DOI] [PubMed] [Google Scholar]
- 9.Carrero JA, McCarthy DP, Ferris ST, et al. . Resident macrophages of pancreatic islets have a seminal role in the initiation of autoimmune diabetes of NOD mice. Proc Natl Acad Sci U S A 2017;114:E10418–E10427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Legoux FP, Lim JB, Cauley AW, et al. . CD4+ T cell tolerance to tissue-restricted self antigens is mediated by antigen-specific regulatory t cells rather than deletion. Immunity 2015;43:896–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson MS, Venanzi ES, Klein L, et al. . Projection of an immunological self shadow within the thymus by the aire protein. Science 2002;298:1395–1401 [DOI] [PubMed] [Google Scholar]
- 12.Takaba H, Morishita Y, Tomofuji Y, et al. . Fezf2 orchestrates a thymic program of self-antigen expression for immune tolerance. Cell 2015;163:975–987 [DOI] [PubMed] [Google Scholar]
- 13.Yip L, Su L, Sheng D, et al. . Deaf1 isoforms control the expression of genes encoding peripheral tissue antigens in the pancreatic lymph nodes during type 1 diabetes. Nat Immunol 2009;10:1026–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luan JJ, Monteiro RC, Sautès C, et al. . Defective Fc gamma RII gene expression in macrophages of NOD mice: genetic linkage with up-regulation of IgG1 and IgG2b in serum. J Immunol 1996;157:4707–4716 [PubMed] [Google Scholar]
- 15.Alizadeh BZ, Valdigem G, Coenen MJ, et al. . Association analysis of functional variants of the FcgRIIa and FcgRIIIa genes with type 1 diabetes, celiac disease and rheumatoid arthritis. Hum Mol Genet 2007;16:2552–2559 [DOI] [PubMed] [Google Scholar]
- 16.O’Brien BA, Huang Y, Geng X, Dutz JP, Finegood DT. Phagocytosis of apoptotic cells by macrophages from NOD mice is reduced. Diabetes 2002;51:2481–2488 [DOI] [PubMed] [Google Scholar]
- 17.O’Brien BA, Geng X, Orteu CH, et al. . A deficiency in the in vivo clearance of apoptotic cells is a feature of the NOD mouse. J Autoimmun 2006;26:104–115 [DOI] [PubMed] [Google Scholar]
- 18.Marée AF, Komba M, Dyck C, Łabecki M, Finegood DT, Edelstein-Keshet L. Quantifying macrophage defects in type 1 diabetes. J Theor Biol 2005;233:533–551 [DOI] [PubMed] [Google Scholar]
- 19.Diana J, Simoni Y, Furio L, et al. . Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat Med 2013;19:65–73 [DOI] [PubMed] [Google Scholar]
- 20.Aichinger M, Wu C, Nedjic J, Klein L. Macroautophagy substrates are loaded onto MHC class II of medullary thymic epithelial cells for central tolerance. J Exp Med 2013;210:287–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fløyel T, Brorsson C, Nielsen LB, et al. . CTSH regulates β-cell function and disease progression in newly diagnosed type 1 diabetes patients. Proc Natl Acad Sci U S A 2014;111:10305–10310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noble JA. Immunogenetics of type 1 diabetes: a comprehensive review. J Autoimmun 2015;64:101–112 [DOI] [PubMed] [Google Scholar]
- 23.Lee KH, Wucherpfennig KW, Wiley DC. Structure of a human insulin peptide-HLA-DQ8 complex and susceptibility to type 1 diabetes. Nat Immunol 2001;2:501–507 [DOI] [PubMed] [Google Scholar]
- 24.Stratmann T, Apostolopoulos V, Mallet-Designe V, et al. . The I-Ag7 MHC class II molecule linked to murine diabetes is a promiscuous peptide binder. J Immunol 2000;165:3214–3225 [DOI] [PubMed] [Google Scholar]
- 25.Sommandas V, Rutledge EA, Van Yserloo B, Fuller J, Lernmark A, Drexhage HA. Defects in differentiation of bone-marrow derived dendritic cells of the BB rat are partly associated with IDDM2 (the lyp gene) and partly associated with other genes in the BB rat background. J Autoimmun 2005;25:46–56 [DOI] [PubMed] [Google Scholar]
- 26.Nejentsev S, Howson JM, Walker NM, et al.; Wellcome Trust Case Control Consortium . Localization of type 1 diabetes susceptibility to the MHC class I genes HLA-B and HLA-A. Nature 2007;450:887–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee CN, Lew AM, Shortman K, Wu L. NOD mice are functionally deficient in the capacity of cross-presentation. Immunol Cell Biol 2015;93:548–557 [DOI] [PubMed] [Google Scholar]
- 28.Mariño E, Tan B, Binge L, Mackay CR, Grey ST. B-cell cross-presentation of autologous antigen precipitates diabetes. Diabetes 2012;61:2893–2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price JD, Beauchamp NM, Rahir G, et al. . CD8+ dendritic cell-mediated tolerance of autoreactive CD4+ T cells is deficient in NOD mice and can be corrected by blocking CD40L. J Leukoc Biol 2014;95:325–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamilton-Williams EE, Martinez X, Clark J, et al. . Expression of diabetes-associated genes by dendritic cells and CD4 T cells drives the loss of tolerance in nonobese diabetic mice. J Immunol 2009;183:1533–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beumer W, Welzen-Coppens JM, van Helden-Meeuwsen CG, Gibney SM, Drexhage HA, Versnel MA. The gene expression profile of CD11c+ CD8α- dendritic cells in the pre-diabetic pancreas of the NOD mouse. PLoS One 2014;9:e103404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alard P, Manirarora JN, Parnell SA, Hudkins JL, Clark SL, Kosiewicz MM. Deficiency in NOD antigen-presenting cell function may be responsible for suboptimal CD4+CD25+ T-cell-mediated regulation and type 1 diabetes development in NOD mice. Diabetes 2006;55:2098–2105 [DOI] [PubMed] [Google Scholar]
- 33.Jin Y, Chen X, Podolsky R, et al. . APC dysfunction is correlated with defective suppression of T cell proliferation in human type 1 diabetes. Clin Immunol 2009;130:272–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Badami E, Sorini C, Coccia M, et al. . Defective differentiation of regulatory FoxP3+ T cells by small-intestinal dendritic cells in patients with type 1 diabetes. Diabetes 2011;60:2120–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dáňová K, Grohová A, Strnadová P, et al. . Tolerogenic dendritic cells from poorly compensated type 1 diabetes patients have decreased ability to induce stable antigen-specific t cell hyporesponsiveness and generation of suppressive regulatory T cells. J Immunol 2017;198:729–740 [DOI] [PubMed] [Google Scholar]
- 36.Radichev IA, Maneva-Radicheva LV, Amatya C, et al. . Nardilysin-dependent proteolysis of cell-associated VTCN1 (B7-H4) marks type 1 diabetes development. Diabetes 2014;63:3470–3482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Devaraj S, Dasu MR, Rockwood J, Winter W, Griffen SC, Jialal I. Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: further evidence of a proinflammatory state. J Clin Endocrinol Metab 2008;93:578–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyers AJ, Shah RR, Gottlieb PA, Zipris D. Altered Toll-like receptor signaling pathways in human type 1 diabetes. J Mol Med (Berl) 2010;88:1221–1231 [DOI] [PubMed] [Google Scholar]
- 39.Alkanani AK, Rewers M, Dong F, Waugh K, Gottlieb PA, Zipris D. Dysregulated Toll-like receptor-induced interleukin-1β and interleukin-6 responses in subjects at risk for the development of type 1 diabetes. Diabetes 2012;61:2525–2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jagannathan M, McDonnell M, Liang Y, et al. . Toll-like receptors regulate B cell cytokine production in patients with diabetes. Diabetologia 2010;53:1461–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tai N, Wong FS, Wen L. The role of the innate immune system in destruction of pancreatic beta cells in NOD mice and humans with type I diabetes. J Autoimmun 2016;71:26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yip L, Fuhlbrigge R, Taylor C, et al. . Inflammation and hyperglycemia mediate Deaf1 splicing in the pancreatic lymph nodes via distinct pathways during type 1 diabetes. Diabetes 2015;64:604–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baekkeskov S, Hubbell JA, Phelps EA. Bioengineering strategies for inducing tolerance in autoimmune diabetes. Adv Drug Deliv Rev 2017;114:256–265 [DOI] [PubMed] [Google Scholar]
- 44.Kambayashi T, Laufer TM. Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell? Nat Rev Immunol 2014;14:719–730 [DOI] [PubMed] [Google Scholar]
- 45.Hansen L, Schmidt-Christensen A, Gupta S, et al. . E2-2 dependent plasmacytoid dendritic cells control autoimmune diabetes. PLoS One 2015;10:e0144090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saxena V, Ondr JK, Magnusen AF, Munn DH, Katz JD. The countervailing actions of myeloid and plasmacytoid dendritic cells control autoimmune diabetes in the nonobese diabetic mouse. J Immunol 2007;179:5041–5053 [DOI] [PubMed] [Google Scholar]
- 47.Beaudoin L, Diana J, Ghazarian L, Simoni Y, Boitard C, Lehuen A. Plasmacytoid dendritic cells license regulatory T cells, upon iNKT-cell stimulation, to prevent autoimmune diabetes. Eur J Immunol 2014;44:1454–1466 [DOI] [PubMed] [Google Scholar]
- 48.Gotter J, Brors B, Hergenhahn M, Kyewski B. Medullary epithelial cells of the human thymus express a highly diverse selection of tissue-specific genes colocalized in chromosomal clusters. J Exp Med 2004;199:155–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marré ML, Profozich JL, Coneybeer JT, et al. . Inherent ER stress in pancreatic islet β cells causes self-recognition by autoreactive T cells in type 1 diabetes. J Autoimmun 2016;72:33–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delong T, Wiles TA, Baker RL, et al. . Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science 2016;351:711–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pathiraja V, Kuehlich JP, Campbell PD, et al. . Proinsulin-specific, HLA-DQ8, and HLA-DQ8-transdimer-restricted CD4+ T cells infiltrate islets in type 1 diabetes. Diabetes 2015;64:172–182 [DOI] [PubMed] [Google Scholar]
- 52.Michels AW, Landry LG, McDaniel KA, et al. . Islet-derived CD4 T-cells targeting proinsulin in human autoimmune diabetes. Diabetes 2017;66:722–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Babon JA, DeNicola ME, Blodgett DM, et al. . Analysis of self-antigen specificity of islet-infiltrating T cells from human donors with type 1 diabetes. Nat Med 2016;22:1482–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crawford F, Stadinski B, Jin N, et al. . Specificity and detection of insulin-reactive CD4+ T cells in type 1 diabetes in the nonobese diabetic (NOD) mouse. Proc Natl Acad Sci U S A 2011;108:16729–16734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakayama M, McDaniel K, Fitzgerald-Miller L, et al. . Regulatory vs. inflammatory cytokine T-cell responses to mutated insulin peptides in healthy and type 1 diabetic subjects. Proc Natl Acad Sci U S A 2015;112:4429–4434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daniel C, Weigmann B, Bronson R, von Boehmer H. Prevention of type 1 diabetes in mice by tolerogenic vaccination with a strong agonist insulin mimetope. J Exp Med 2011;208:1501–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Serr I, Fürst RW, Achenbach P, et al. . Type 1 diabetes vaccine candidates promote human Foxp3(+)Treg induction in humanized mice. Nat Commun 2016;7:10991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kobayashi M, Abiru N, Arakawa T, et al. . Altered B:9-23 insulin, when administered intranasally with cholera toxin adjuvant, suppresses the expression of insulin autoantibodies and prevents diabetes. J Immunol 2007;179:2082–2088 [DOI] [PubMed] [Google Scholar]
- 59.Bergman ML, Lopes-Carvalho T, Martins AC, Grieco FA, Eizirik DL, Demengeot J. Tolerogenic insulin peptide therapy precipitates type 1 diabetes. J Exp Med 2017;214:2153–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dastagir SR, Postigo-Fernandez J, Xu C, Stoeckle JH, Firdessa-Fite R, Creusot RJ. Efficient presentation of multiple endogenous epitopes to both CD4+ and CD8+ diabetogenic T cells for tolerance. Mol Ther Methods Clin Dev 2016;4:27–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prasad S, Xu D, Miller SD. Tolerance strategies employing antigen-coupled apoptotic cells and carboxylated PLG nanoparticles for the treatment of type 1 diabetes. Rev Diabet Stud 2012;9:319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Turner MS, Isse K, Fischer DK, Turnquist HR, Morel PA. Low TCR signal strength induces combined expansion of Th2 and regulatory T cell populations that protect mice from the development of type 1 diabetes. Diabetologia 2014;57:1428–1436 [DOI] [PubMed] [Google Scholar]
- 63.Kostic AD, Gevers D, Siljander H, et al.; DIABIMMUNE Study Group . The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe 2015;17:260–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alkanani AK, Hara N, Gottlieb PA, et al. . Alterations in intestinal microbiota correlate with susceptibility to type 1 diabetes. Diabetes 2015;64:3510–3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Turley SJ, Lee JW, Dutton-Swain N, Mathis D, Benoist C. Endocrine self and gut non-self intersect in the pancreatic lymph nodes. Proc Natl Acad Sci U S A 2005;102:17729–17733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vaarala O, Atkinson MA, Neu J. The “perfect storm” for type 1 diabetes: the complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes 2008;57:2555–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fletcher AL, Malhotra D, Turley SJ. Lymph node stroma broaden the peripheral tolerance paradigm. Trends Immunol 2011;32:12–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Akbarpour M, Goudy KS, Cantore A, et al. . Insulin B chain 9-23 gene transfer to hepatocytes protects from type 1 diabetes by inducing Ag-specific FoxP3+ Tregs. Sci Transl Med 2015;7:289ra81. [DOI] [PubMed] [Google Scholar]
- 69.Cire S, Da Rocha S, Ferrand M, Collins MK, Galy A. In vivo gene delivery to lymph node stromal cells leads to transgene-specific CD8+ T cell anergy in mice. Mol Ther 2016;24:1965–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Creusot RJ, Giannoukakis N, Trucco M, Clare-Salzler MJ, Fathman CG. It’s time to bring dendritic cell therapy to type 1 diabetes. Diabetes 2014;63:20–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suwandi JS, Toes RE, Nikolic T, Roep BO. Inducing tissue specific tolerance in autoimmune disease with tolerogenic dendritic cells. Clin Exp Rheumatol 2015;33(Suppl. 92):S97–S103 [PubMed] [Google Scholar]
- 72.Phillips BE, Garciafigueroa Y, Trucco M, Giannoukakis N. Clinical tolerogenic dendritic cells: exploring therapeutic impact on human autoimmune disease. Front Immunol 2017;8:1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Funda DP, Goliáš J, Hudcovic T, Kozáková H, Špíšek R, Palová-Jelínková L. Antigen loading (e.g., glutamic acid decarboxylase 65) of tolerogenic DCs (tolDCs) reduces their capacity to prevent diabetes in the non-obese diabetes (NOD)-severe combined immunodeficiency model of adoptive cotransfer of diabetes as well as in NOD mice. Front Immunol 2018;9:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Creusot RJ, Yaghoubi SS, Chang P, et al. . Lymphoid-tissue-specific homing of bone-marrow-derived dendritic cells. Blood 2009;113:6638–6647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Skyler JS, Krischer JP, Wolfsdorf J, et al. . Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial-Type 1. Diabetes Care 2005;28:1068–1076 [DOI] [PubMed] [Google Scholar]
- 76.Skyler JS; Type 1 Diabetes TrialNet Study Group . Update on worldwide efforts to prevent type 1 diabetes. Ann N Y Acad Sci 2008;1150:190–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roep BO, Solvason N, Gottlieb PA, et al.; BHT-3021 Investigators . Plasmid-encoded proinsulin preserves C-peptide while specifically reducing proinsulin-specific CD8⁺ T cells in type 1 diabetes. Sci Transl Med 2013;5:191ra82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ludvigsson J, Wahlberg J, Casas R. Intralymphatic injection of autoantigen in type 1 diabetes. N Engl J Med 2017;376:697–699 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.