Abstract
Experimental studies suggest ceramides may play a role in insulin resistance. However, the relationships of circulating ceramides and related sphingolipids with plasma insulin have been underexplored in humans. We measured 15 ceramide and sphingomyelin species in fasting baseline samples from the Strong Heart Family Study (SHFS), a prospective cohort of American Indians. We examined sphingolipid associations with both baseline and follow-up measures of plasma insulin, HOMA of insulin resistance (HOMA-IR), and HOMA of β-cell function (HOMA-B) after adjustment for risk factors. Among the 2,086 participants without diabetes, higher levels of plasma ceramides carrying the fatty acids 16:0 (16 carbons, 0 double bond), 18:0, 20:0, or 22:0 were associated with higher plasma insulin and higher HOMA-IR at baseline and at follow-up an average of 5.4 years later. For example, a twofold higher baseline concentration of ceramide 16:0 was associated with 14% higher baseline insulin (P < 0.0001). Associations between sphingomyelin species carrying 18:0, 20:0, 22:0, or 24:0 and insulin were modified by BMI (P < 0.003): higher levels were associated with lower fasting insulin, HOMA-IR, and HOMA-B among those with normal BMI. Our study suggests lowering circulating ceramides might be a target in prediabetes and targeting circulating sphingomyelins should take into account BMI.
The prevalence of type 2 diabetes is a global health issue with an estimated 347 million adults afflicted worldwide (1). Diabetes imparts a high burden of morbidity and comorbidities. Although overall diabetes incidence may have plateaued in the U.S., minority subgroups still experience high risk of diabetes with continued increases in incidence (2,3).
High levels of fasting plasma insulin, reflecting insulin resistance, are an early event in the progression to diabetes and suggested to be an underlying cause of type 2 diabetes (4–6). The search for metabolites that influence insulin levels in people without diabetes may lead to novel approaches to preventive efforts (7).
There is strong evidence from animal experimental studies and in vitro work that ceramides are implicated in insulin resistance (8,9). However, the influence of ceramides and related sphingolipids on insulin resistance in humans has received limited attention.
Experimental studies suggest that the saturated fatty acid that is acylated to ceramides influences ceramide biological activities (10). We have shown that circulating very long-chain saturated fatty acids (with 20 carbons or more) are associated with lower fasting insulin and lower risk of diabetes (11). Very long-chain saturated fatty acids are largely a component of sphingolipids, including sphingomyelins and ceramides. Whether circulating sphingolipids with very long-chain saturated fatty acids are associated with lower insulin resistance is not known.
The objective of the study was to determine the associations between circulating ceramide and sphingomyelin species with different saturated fatty acids and fasting plasma insulin and related markers of insulin homeostasis. We carried out this investigation in the Strong Heart Family Study (SHFS), a well-characterized cohort study conducted in a population at high risk of diabetes (12).
Research Design and Methods
Study Design
We used plasma samples from the SHFS cohort to measure sphingolipids and investigate associations with insulin and other markers both cross-sectionally and prospectively.
Study Population
The SHFS is a family-based cohort study of risk factors for cardiovascular disease in several American Indian communities in Arizona, North Dakota, South Dakota, and Oklahoma. The SHFS includes a baseline examination conducted in 2001–2003 and a follow-up examination in 2007–2009. Details of the study design were previously reported (13). The institutional review boards (Indian Health Services of Rapid City, SD, Phoenix, AZ, and Oklahoma City, OK; MedStar and the University of Oklahoma; and the University of Washington) and each participating tribe approved the study, and written informed consent was obtained from all participants at enrollment. At baseline, the cohort consisted of 2,768 participants in 92 families. For this investigation, we excluded 55 participants without available baseline samples, 510 with baseline diabetes, 67 without plasma fasting insulin, and 50 with missing covariate information. The remaining 2,086 participants were included in the analyses of baseline data. For the analyses of follow-up data, we further excluded 201 participants with diabetes at the follow-up examination because we were interested in the potential role of sphingolipids in hyperinsulinemia preceding the progression to diabetes, 239 participants without follow-up examination, and 86 without insulin measurements.
Data Collection
The baseline and follow-up examinations included a physical examination, laboratory testing, medication review, a 1-week pedometer log, and an in-person interview to collect information on medical conditions, education, smoking, and alcohol consumption. BMI was calculated as body weight divided by height squared (kg/m2). Diabetes was defined as use of insulin or oral antidiabetes medication or a fasting plasma glucose concentration ≥126 mg/dL. Blood samples were collected after a 12-h overnight fast and processed and stored at −70°C. Plasma insulin was measured using a modified version of the Morgan and Lazarow radioimmunoassay at both examinations (12).
Measurement of Sphingolipids
We focused on sphingolipids that carried a saturated fatty acid acylated to the sphingoid backbone, including palmitic acid (16:0 [16 carbons, 0 double bonds]), stearic acid (18:0), arachidic acid (20:0), behenic acid (22:0), and lignoceric acid (24:0). The sphingolipids were measured on baseline fasting plasma samples that had been stored at −70°C with the following method. Lipids were extracted using organic protein precipitation in a mixture of methyl tert-butyl ether, methanol, and isopropanol. For each sample, 10 µL was pipetted into the appropriate well of a 96–deep well polypropylene microtiter plate (Masterblock; Greiner Bio-One, cat. no. 780270). In a chemical fume hood, 190 µL of precipitation solvent was added to each well using a multichannel pipet. The plate was sealed with a MicroLiter silicone cap mat with sprayed-on PTFE barrier (Wheaton, cat. no. 07–0061N), placed in a plastic Ziploc bag, and mixed on a multitube vortex (VWR) for 5 min at speed 10. Subsequently, in a fume hood, a 10-μm glass filter plate (Captiva; Agilent, cat. no. A596401000) was placed above a new Masterblock plate. Using a multichannel pipet, the samples were transferred from the precipitation plate into the filter plate and allowed to flow through using gravity (approximately 50 µL flows through the membrane filter in each well). The filter plate was carefully removed and discarded. To each sample in the new Masterblock plate, 450 µL of 65% methanol/25% isopropanol (v:v) was added and mixed by pipetting up and down 10 times with an electronic multichannel pipet. The plate was sealed and then a volume of 5 µL was injected using an autosampler (samples were cooled at 8°C) and resolved using reversed-phase chromatography at 50°C on an Acquity UPLC Protein BEH C4 Column, 300Å, 1.7 µm, 2.1 mm × 50 mm analytical column (Waters, cat. no. 186004495) equipped with an Acquity UPLC Protein BEH C4 VanGuard Pre-column, 300Å, 1.7 µm, 2.1 mm × 5 mm guard column (Waters, cat. no. 186004623). Mobile phases were Optima water/0.2% formic acid (buffer A) and 60% acetonitrile/40% isopropanol/0.2% formic acid (buffer B). A linear gradient from 49% to 79% buffer B over 8.4 min at 0.4 mL/min was used to resolve the analytes. Analytes were introduced to the mass spectrometer (Sciex 6500) and analyzed using optimized mass spectrometric parameters for each compound.
Internal standards were included in the precipitation solvent at a concentration of 19.4 nmol/L (Ceramide/Sphingolipid Internal Standard Mixture I, 25 μmol/L; Avanti Polar Lipids, LM-6002), which controls for variability in extraction efficiency, pipetting, and ion suppression. Chromatographic peak areas of the endogenous analytes and the internal standards were quantified using SkyLine software (14). Each peak area for each endogenous sphingolipid was divided by the sum of the peak area of five internal standards (ceramide C12 [CerC12], CerC25, glucosyl ceramide C12 [GluCerC12], lactosyl ceramide C12 [LacCerC12], and sphingomyelin 12 [SM12]), which was called the peak area ratio. The peak area ratio for each sphingolipid was then divided by the mean peak area ratio in the single point calibrator in the batch (precipitated and analyzed 5 times in each batch, spread across the plate). The single point calibrator was a pooled EDTA-anticoagulated plasma sample made from discarded de-identified clinical samples from the clinical laboratory at the University of Washington Medical Center. Additional details on the sphingolipid measurements and quality control procedures are provided in the Supplementary Data. The coefficients of variation for each sphingolipid species are shown in Table 1.
Table 1.
Correlations between the sphingolipid species, and coefficients of variation of the measurements
| Cer-16 | Cer-18 | Cer-20 | Cer-22 | Cer-24 | GluCer-16 | GluCer-22 | GluCer-24 | LacCer-16 | SM-14 | SM-16 | SM-18 | SM-20 | SM-22 | CV, % | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cer-16 |
1.0 |
18.4 |
|||||||||||||
| Cer-18 |
0.74 |
1.0 |
21.2 |
||||||||||||
| Cer-20 |
0.72 |
0.74 |
1.0 |
19.5 |
|||||||||||
| Cer-22 |
0.72 |
0.59 |
0.73 |
1.0 |
13.9 |
||||||||||
| Cer-24 |
0.72 |
0.58 |
0.61 |
0.87 |
1.0 |
15.5 |
|||||||||
| GluCer-16 |
0.51 |
0.39 |
0.29 |
0.31 |
0.40 |
1.0 |
13.4 |
||||||||
| GluCer-22 |
0.36 |
0.20 |
0.24 |
0.44 |
0.44 |
0.67 |
1.0 |
13.9 |
|||||||
| GluCer-24 |
0.40 |
0.23 |
0.23 |
0.37 |
0.48 |
0.72 |
0.85 |
1.0 |
16.0 |
||||||
| LacCer-16 |
0.40 |
0.27 |
0.26 |
0.29 |
0.32 |
0.59 |
0.51 |
0.49 |
1.0 |
14.4 |
|||||
| SM-14 |
0.59 |
0.47 |
0.48 |
0.62 |
0.66 |
0.48 |
0.42 |
0.45 |
0.38 |
1.0 |
18.2 |
||||
| SM-16 |
0.70 |
0.49 |
0.48 |
0.55 |
0.62 |
0.69 |
0.56 |
0.60 |
0.63 |
0.71 |
1.0 |
11.5 |
|||
| SM-18 |
0.57 |
0.71 |
0.54 |
0.49 |
0.53 |
0.55 |
0.40 |
0.41 |
0.49 |
0.65 |
0.75 |
1.0 |
12.2 |
||
| SM-20 |
0.48 |
0.44 |
0.55 |
0.65 |
0.59 |
0.35 |
0.48 |
0.40 |
0.40 |
0.78 |
0.66 |
0.72 |
1.0 |
12.6 |
|
| SM-22 |
0.51 |
0.37 |
0.52 |
0.70 |
0.59 |
0.34 |
0.51 |
0.43 |
0.42 |
0.66 |
0.67 |
0.60 |
0.89 |
1.0 |
12.4 |
| SM-24 | 0.49 | 0.32 | 0.41 | 0.60 | 0.60 | 0.38 | 0.49 | 0.52 | 0.42 | 0.64 | 0.70 | 0.57 | 0.79 | 0.92 | 13.3 |
CV, coefficient of variation.
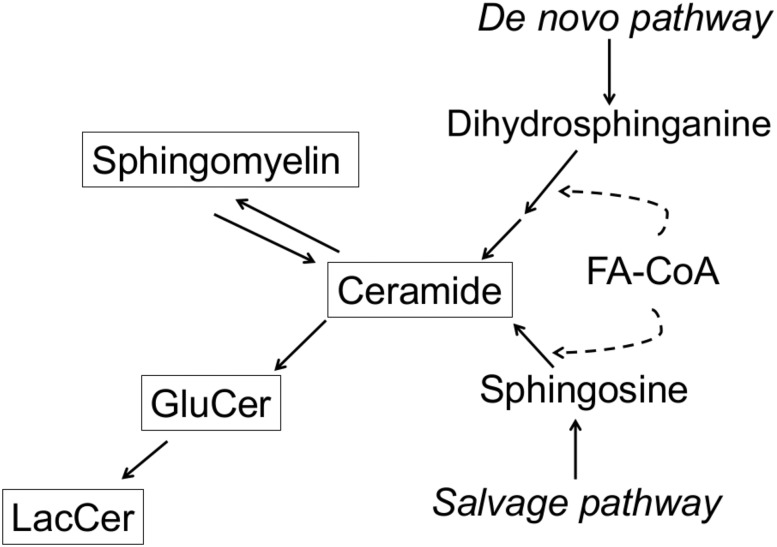
In total we measured 22 sphingolipid species. This report is restricted to the 15 species with coefficient of variation ≤21% over the whole study period. It includes five ceramides: ceramide with 16:0 (Cer-16), 18:0 (Cer-18), 20:0 (Cer-20), 22:0 (Cer-22), and a composite concentration of Cer-24 computed as the sum of the concentrations of two species of ceramides with 24:0 having the distinct “d181” and “d182” sphingoid backbones. It also includes six sphingomyelins, SM-14, SM-16, SM-18, SM-20, SM-22, and SM-24; three glucosyl ceramides, GluCer-16, GluCer-22, and GluCer-24; and one lactosyl ceramide, LacCer-16. Simplified relationships between the sphingolipid classes that were measured are shown in Fig. 1.
Figure 1.
Synthesis of ceramide and other measured sphingolipids. Shown are simplified pathways leading to ceramide, sphingomyelin, glucosyl ceramide, and lactosyl ceramide, the four sphingolipids measured in the study. In the de novo synthesis pathway and the salvage pathway, ceramide is formed by acylation of a fatty acid (FA) to a “sphingoid” backbone, dihydrosphinganine and sphingosine, respectively. There are six ceramide synthases in humans with different fatty acid specificities, resulting in multiple ceramide species carrying different fatty acids. Synthesis of ceramide by the two pathways occurs in the endoplasmic reticulum. Ceramide can also be formed by sphingomyelinases on the plasma membrane. Sphingomyelin is synthesized by sphingomyelin synthase by addition of a choline head group to ceramide transported to the Golgi. Glucosyl ceramide is synthesized by addition of a glucose head group to ceramide, and lactosyl ceramide by further addition of galactose to glucosyl ceramide, also in the Golgi.
Statistical Analysis
The statistical methods were identical for the outcomes of fasting plasma insulin, HOMA of insulin resistance (HOMA-IR), and HOMA of β-cell function (HOMA-B). The analyses are described using insulin as example. In the analyses, sphingolipid species concentrations and outcomes were log-transformed. We investigated the association of each sphingolipid with baseline plasma insulin, follow-up plasma insulin, and changes in insulin between baseline and follow-up examinations. Participants with diabetes at baseline were excluded from all analyses. For the prospective analyses and analyses of changes between baseline and follow-up, we further excluded participants with diabetes at the follow-up exam. The analyses of baseline and follow-up insulin used linear mixed models that included a family-specific random effects to account for familial aggregation and a subject-specific random effects with covariance among family members proportional to the kinship coefficient to account for genetic similarity among family members. Each model included prespecified adjustments for age, sex, geographic area, education, smoking, log(BMI), waist circumference, and physical activity. The analyses of change in insulin between baseline and follow-up did not include random effects with covariance proportional to the kinship matrix but were additionally adjusted for baseline insulin and used a random-effects longitudinal model to correct for measurement error in baseline insulin (15), as measurement error in an adjustment variable may introduce bias (16). To correct for multiple comparisons within each type of outcome model, we applied a Bonferroni correction and used a significance threshold of 0.0033 (0.05/15 sphingolipid species).
Missing values of baseline physical activity measures (n = 179) were multiply imputed (20 replicates) using information on age, sex, education, body fat, and triglycerides in models that accounted for possible family effect. The fully conditional expectation method implemented by the MICE package in R was used with predictive mean matching method (17). Variables included in the imputation were selected by minimizing the Bayesian information criterion in models predicting physical activity in the complete case data. Twenty imputed data sets were generated and model fitting results were pooled using standard methods (18).
We examined whether associations between sphingolipids and baseline insulin concentration were modified by differences in age, sex, and BMI by adding product interaction terms to the models above. To correct for multiple comparisons, we used a significance threshold of 0.0033 for interaction tests (0.05/15 sphingolipid species).
Multivariate model results are presented per twofold higher concentration of each sphingolipid. This twofold difference is comparable to the difference between the 90th and 10th percentiles of each sphingolipid species (Table 2). We saw no departure from linearity when modeling the sphingolipids with cubic splines (not shown).
Table 2.
Sphingolipid concentrations, expressed in terms of normalized peak area ratios
| Species | Log of normalized peak ratio, mean ± SD | Normalized peak ratio, median of Q1 | Normalized peak ratio, median of Q5 | Fold difference in normalized peak ratio: Q5 median/Q1 median |
|---|---|---|---|---|
| Cer-16 |
−0.61 ± 0.30 |
0.37 |
0.79 |
2.14 |
| Cer-18 |
−0.81 ± 0.44 |
0.26 |
0.77 |
2.96 |
| Cer-20 |
−0.31 ± 0.40 |
0.46 |
1.18 |
2.57 |
| Cer-22 |
0.28 ± 0.36 |
0.85 |
2.10 |
2.46 |
| Cer-24 |
0.79 ± 0.32 |
1.49 |
3.31 |
2.22 |
| GluCer-16 |
−0.07 ± 0.28 |
0.66 |
1.31 |
1.98 |
| GluCer-22 |
0.34 ± 0.29 |
0.97 |
2.04 |
2.10 |
| GluCer-24 |
0.25 ± 0.31 |
0.86 |
1.88 |
2.19 |
| LacCer-16 |
−0.12 ± 0.25 |
0.65 |
1.21 |
1.86 |
| SM-14 |
−0.01 ± 0.39 |
0.60 |
1.59 |
2.65 |
| SM-16 |
0.12 ± 0.19 |
0.89 |
1.44 |
1.62 |
| SM-18 |
−0.18 ± 0.24 |
0.62 |
1.13 |
1.82 |
| SM-20 |
0.08 ± 0.26 |
0.78 |
1.51 |
1.94 |
| SM-22 |
0.36 ± 0.26 |
1.05 |
2.00 |
1.90 |
| SM-24 | 0.31 ± 0.27 | 0.98 | 1.92 | 1.96 |
Q, quintile.
Results
Baseline characteristics of the cohort participants included in the study are shown in Table 3. Participants were on average 38 years old, 41% were men, and 24% had a BMI of 35 kg/m2 or greater. Levels of the sphingolipid species, especially species from the same class, were correlated with each other (Table 1).
Table 3.
Baseline characteristics of the 2,086 SHFS participants in the study
| Mean or % | |
|---|---|
| Age, years |
37.88 ± 16.46 |
| Male sex |
40.70 |
| Education, years |
12.19 ± 2.28 |
| BMI, kg/m2 |
30.57 ± 7.22 |
| Waist circumference, cm |
99.98 ± 17.34 |
| Smoking, current |
37.44 |
| LDL, mg/dL |
99.90 ± 30.06 |
| HDL, mg/dL |
52.29 ± 14.83 |
| Triglycerides, mg/dL |
147.84 ± 92.24 |
| Baseline outcomes |
|
| Fasting insulin, μU/mL |
15.62 ± 15.49 |
| Fasting glucose, mg/dL |
93.96 ± 10.40 |
| HOMA-IR |
3.75 ± 4.14 |
| HOMA-B | 178 ± 130 |
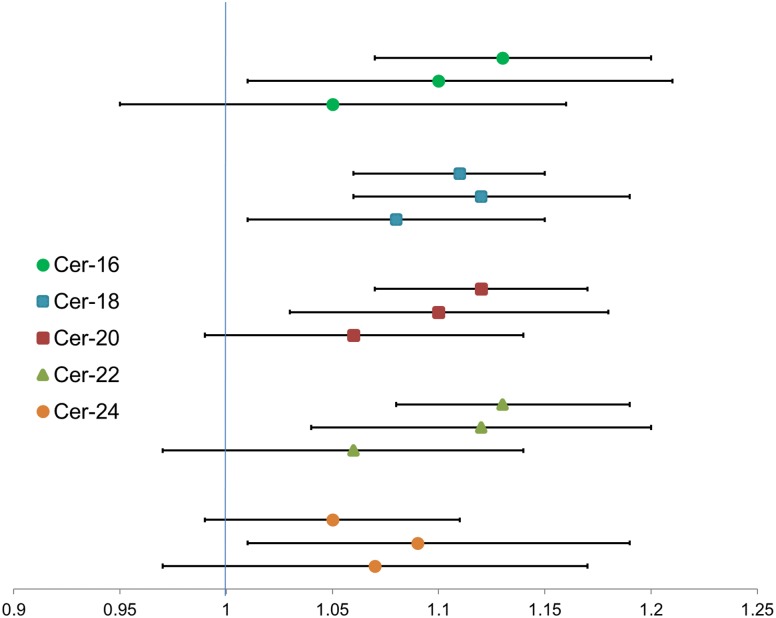
In multivariable linear mixed-effects models that included adjustment for potential confounders and accounted for the familial relationship between study participants, we observed that twofold higher concentrations of four ceramide species, Cer-16, Cer-18, Cer-20, and Cer-22, were each associated with 11–16% higher concentrations of baseline fasting plasma insulin and HOMA-IR (Table 4). Similar associations were observed with follow-up fasting plasma insulin and HOMA-IR measures, collected an average of 5.4 years after baseline (Fig. 2, middle estimates, and Supplementary Tables 1 and 2). Models of ceramides with baseline and follow-up HOMA-B resulted in associations that were similar to but slightly weaker than associations of ceramides with insulin and HOMA-IR (Table 4 and Supplementary Table 3). We did not observe significant associations of ceramides with change in insulin between baseline and follow-up, change in HOMA-IR, or change in HOMA-B (Fig. 2, bottom estimates, and Supplementary Tables 1–3).
Table 4.
Association of plasma sphingolipids with baseline fasting plasma insulin, HOMA-IR, and HOMA-B
| Sphingolipid | Outcome |
||
|---|---|---|---|
| Insulin | HOMA-IR | HOMA-B | |
| Cer-16 |
1.13 (1.07–1.20), 1.9 × 10−5 |
1.15 (1.08–1.22), 4.7 × 10−6 |
1.04 (0.99–1.11), 0.13 |
| Cer-18 |
1.11 (1.06–1.15), 1.0 × 10−6 |
1.12 (1.08–1.17), 6.3 × 10−8 |
1.04 (1.00–1.09), 0.03 |
| Cer-20 |
1.12 (1.07–1.17), 1.5 × 10−7 |
1.13 (1.08–1.18), 1.3 × 10−7 |
1.06 (1.02–1.11), 0.003 |
| Cer-22 |
1.13 (1.08–1.19), 3.9 × 10−7 |
1.15 (1.09–1.21), 1.0 × 10−7 |
1.07 (1.02–1.12), 0.004 |
| Cer-24 |
1.05 (0.99–1.11), 0.08 |
1.06 (1.00–1.13), 0.04 |
1.00 (0.94–1.05), 0.86 |
| SM-14 |
1.00 (0.96–1.05), 0.91 |
1.01 (0.96–1.06), 0.79 |
0.97 (0.93–1.01), 0.19 |
| SM-16 |
0.89 (0.81–0.97), 0.009 |
0.87 (0.79–0.96), 0.006 |
0.89 (0.82–0.98), 0.01 |
| SM-18 |
0.95 (0.89–1.02), 0.19 |
0.96 (0.89–1.03), 0.24 |
0.93 (0.87–0.99), 0.03 |
| SM-20 |
0.95 (0.89–1.02), 0.17 |
0.95 (0.89–1.02), 0.19 |
0.94 (0.88–1.00), 0.05 |
| SM-22 |
1.00 (0.93–1.07), 0.97 |
1.00 (0.93–1.08), 0.91 |
0.96 (0.90–1.03), 0.30 |
| SM-24 |
0.96 (0.90–1.02), 0.22 |
0.97 (0.90–1.03), 0.31 |
0.93 (0.87–0.99), 0.02 |
| GluCer-16 |
0.96 (0.90–1.02), 0.16 |
0.95 (0.89–1.02), 0.15 |
0.95 (0.89–1.00), 0.07 |
| GluCer-22 |
0.93 (0.87–0.98), 0.013 |
0.93 (0.87–0.98), 0.01 |
0.91 (0.86–0.97), 0.002 |
| GluCer-24 |
0.92 (0.86–0.97), 0.0023 |
0.92 (0.86–0.97), 0.004 |
0.90 (0.85–0.95), 0.0002 |
| LacCer-16 | 0.87 (0.82–0.94), 0.0001 | 0.86 (0.80–0.92), 3.7 × 10−5 | 0.91 (0.85–0.98), 0.007 |
Data are ratio of geometric means associated with twofold higher sphingolipid (95% CI), P value.
Figure 2.
Association of plasma ceramides with plasma fasting insulin and change in plasma fasting insulin. Shown is the ratio of insulin geometric means associated with twofold higher ceramide. Within each ceramide, top estimate represents the association with insulin at baseline, middle estimate the association with insulin at follow-up, and bottom estimate the association with change in insulin between baseline and follow-up.
In contrast to ceramides, higher concentrations of GluCer-24 and LacCer-16 were associated with lower baseline plasma insulin concentrations, lower HOMA-IR, and lower HOMA-B (Table 4). However, these ceramide derivatives were not associated with follow-up outcomes or change between baseline and follow-up (Supplementary Tables 1–3).
Sphingomyelin species concentrations were not associated with the outcomes at the prespecified significance threshold of 0.0033, although higher SM-16 concentrations showed marginal evidence of an association with lower baseline concentrations of insulin, HOMA-IR, and HOMA-B (Table 4). In sensitivity analyses, further adjustments for LDL and HDL cholesterol did not change the study results (Supplementary Table 4).
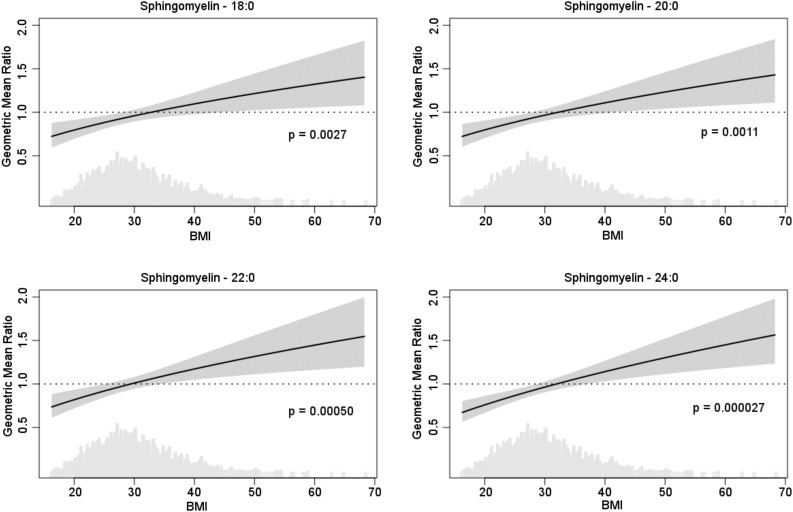
We did not observe any modification of the association between the sphingolipid species and outcomes by age or sex. However, we observed evidence of interactions between BMI and four of the sphingomyelin species, SM-18, SM-20, SM-22, and SM24, at the prespecified significance threshold of 0.0033 (Table 5). To illustrate the modification of sphingomyelin species associations with baseline insulin by BMI, we produced plots of the geometric mean insulin ratio corresponding to twofold higher sphingomyelin species as a function of BMI based on estimates from these interaction models (Fig. 3). At normal BMI, higher concentrations of the sphingomyelins were associated with lower insulin concentrations, whereas at very high BMIs, higher concentrations were associated with higher insulin. For example, a twofold higher concentration in SM-24 was associated with 24% lower geometric mean baseline insulin at BMI of 20 and with 14% higher insulin at BMI of 40 (P for interaction: 2.7 × 10−5) (Table 5). Excluding BMI >45 kg/m2 did not change the findings of interactions (not shown). Similar interactions were observed with the outcomes of HOMA-IR and HOMA-B and with each outcome at the follow-up exam (Table 5). For example, a twofold higher concentration in SM-24 was associated with 27% lower geometric mean follow-up HOMA-B at BMI of 20 kg/m2 and with 24% higher HOMA-B at BMI of 40 kg/m2 (P for interaction: 0.0002).
Table 5.
Ratio of geometric mean ratios (95% CI) of baseline and follow-up insulin, HOMA-IR, and HOMA-B, associated with doubling of sphingomyelin species at different BMIs
| BMI (kg/m2) |
P for interaction | ||||||
|---|---|---|---|---|---|---|---|
| 20 | 25 | 30 | 35 | 40 | 45 | ||
| Baseline insulin | |||||||
| SM-18 |
0.80 (0.69–0.91) |
0.88 (0.81–0.96) |
0.96 (0.89–1.03) |
1.03 (0.95–1.12) |
1.10 (0.98–1.23) |
1.16 (1.00–1.34) |
0.0027 |
| SM-20 |
0.80 (0.70–0.91) |
0.89 (0.82–0.96) |
0.97 (0.90–1.04) |
1.04 (0.95–1.13) |
1.11 (0.99–1.24) |
1.17 (1.02–1.35) |
0.0011 |
| SM-22 |
0.82 (0.72–0.93) |
0.92 (0.85–1.00) |
1.01 (0.94–1.08) |
1.09 (1.00–1.19) |
1.17 (1.05–1.31) |
1.25 (1.08–1.44) |
0.0005 |
| SM-24 |
0.76 (0.67–0.86) |
0.87 (0.80–0.94) |
0.96 (0.90–1.03) |
1.06 (0.98–1.14) |
1.14 (1.03–1.27) |
1.22 (1.07–1.39) |
2.7 × 10−5 |
| Follow-up insulin | |||||||
| SM-18 |
0.85 (0.69–1.05) |
0.96 (0.84–1.09) |
1.05 (0.94–1.17) |
1.14 (0.99–1.31) |
1.23 (1.02–1.47) |
1.30 (1.03–1.64) |
0.028 |
| SM-20 |
0.79 (0.65–0.96) |
0.92 (0.81–1.04) |
1.05 (0.94–1.17) |
1.17 (1.02–1.35) |
1.29 (1.07–1.55) |
1.41 (1.12–1.77) |
0.0020 |
| SM-22 |
0.80 (0.65–0.98) |
0.94 (0.83–1.07) |
1.08 (0.97–1.20) |
1.21 (1.05–1.39) |
1.34 (1.11–1.61) |
1.46 (1.15–1.84) |
0.0019 |
| SM-24 |
0.76 (0.63–0.93) |
0.90 (0.79–1.01) |
1.02 (0.92–1.13) |
1.14 (1.01–1.30) |
1.26 (1.06–1.49) |
1.37 (1.10–1.70) |
0.0014 |
| Baseline HOMA-IR | |||||||
| SM-18 |
0.79 (0.69–0.92) |
0.88 (0.81–0.97) |
0.96 (0.89–1.04) |
1.04 (0.94–1.14) |
1.10 (0.98–1.25) |
1.17 (1.00–1.36) |
0.0039 |
| SM-20 |
0.78 (0.68–0.89) |
0.88 (0.81–0.96) |
0.97 (0.90–1.04) |
1.05 (0.96–1.15) |
1.13 (1.00–1.27) |
1.20 (1.03–1.40) |
0.0006 |
| SM-22 |
0.81 (0.71–0.93) |
0.92 (0.84–1.00) |
1.02 (0.95–1.09) |
1.11 (1.01–1.21) |
1.19 (1.06–1.34) |
1.27 (1.09–1.48) |
0.0005 |
| SM-24 |
0.76 (0.66–0.87) |
0.87 (0.80–0.95) |
0.97 (0.91–1.04) |
1.06 (0.98–1.16) |
1.15 (1.03–1.29) |
1.24 (1.08–1.42) |
5.2 × 10−5 |
| Follow-up HOMA-IR | |||||||
| SM-18 |
0.86 (0.69–1.07) |
0.96 (0.83–1.10) |
1.05 (0.93–1.18) |
1.13 (0.98–1.31) |
1.21 (0.99–1.47) |
1.28 (1.00–1.64) |
0.052 |
| SM-20 |
0.78 (0.63–0.95) |
0.91 (0.80–1.04) |
1.04 (0.93–1.17) |
1.17 (1.01–1.35) |
1.29 (1.06–1.56) |
1.40 (1.10–1.79) |
0.0030 |
| SM-22 |
0.80 (0.64–0.99) |
0.94 (0.82–1.07) |
1.07 (0.96–1.20) |
1.20 (1.04–1.40) |
1.33 (1.09–1.62) |
1.45 (1.13–1.86) |
0.0036 |
| SM-24 |
0.77 (0.62–0.94) |
0.90 (0.79–1.02) |
1.02 (0.92–1.14) |
1.14 (1.00–1.31) |
1.26 (1.05–1.51) |
1.37 (1.09–1.72) |
0.0029 |
| Baseline HOMA-B | |||||||
| SM-18 |
0.77 (0.68–0.88) |
0.86 (0.79–0.93) |
0.93 (0.87–1.00) |
1.00 (0.92–1.09) |
1.07 (0.95–1.19) |
1.13 (0.98–1.30) |
0.0020 |
| SM-20 |
0.84 (0.74–0.95) |
0.90 (0.83–0.97) |
0.95 (0.88–1.01) |
0.99 (0.91–1.08) |
1.03 (0.92–1.15) |
1.07 (0.93–1.23) |
0.033 |
| SM-22 |
0.82 (0.72–0.93) |
0.90 (0.83–0.98) |
0.98 (0.91–1.04) |
1.04 (0.96–1.14) |
1.11 (0.99–1.24) |
1.17 (1.02–1.34) |
0.0022 |
| SM-24 |
0.75 (0.66–0.84) |
0.84 (0.78–0.91) |
0.93 (0.87–0.99) |
1.01 (0.94–1.09) |
1.09 (0.99–1.21) |
1.16 (1.02–1.32) |
5.3 × 10−5 |
| Follow-up HOMA-B | |||||||
| SM-18 |
0.80 (0.67–0.97) |
0.93 (0.83–1.05) |
1.05 (0.95–1.16) |
1.17 (1.03–1.32) |
1.27 (1.08–1.51) |
1.38 (1.11–1.70) |
0.0026 |
| SM-20 |
0.81 (0.68–0.97) |
0.94 (0.84–1.05) |
1.05 (0.95–1.16) |
1.16 (1.02–1.31) |
1.26 (1.07–1.49) |
1.35 (1.10–1.67) |
0.0029 |
| SM-22 |
0.80 (0.66–0.96) |
0.94 (0.84–1.05) |
1.07 (0.97–1.18) |
1.19 (1.05–1.35) |
1.31 (1.11–1.56) |
1.43 (1.16–1.77) |
0.0009 |
| SM-24 | 0.73 (0.61–0.88) | 0.87 (0.78–0.97) | 1.00 (0.91–1.09) | 1.12 (1.00–1.26) | 1.24 (1.06–1.45) | 1.36 (1.12–1.65) | 0.0002 |
Figure 3.
Geometric mean ratio of insulin levels associated with twofold difference in sphingomyelin species as a function of BMI (kg/m2). Each plot shows the ratio of baseline insulin geometric means associated with twofold higher sphingomyelin species as a function of BMI (solid line) and 95% CI (shaded area). A geometric mean ratio of 1.0 indicates no association. Values less than 1 indicate an association with lower insulin, and values above 1 indicate an association with higher insulin. The density plot shows the distribution of BMI values in the cohort.
Discussion
In this large family-based cohort of American Indians, higher levels of plasma ceramides were associated with higher fasting plasma insulin and HOMA-IR and less consistently with higher HOMA-B, cross-sectionally as well as prospectively. These associations were observed for most measured ceramide species, with saturated fatty acids of different length.
The association of circulating ceramides with higher plasma insulin complements a large body of evidence from animal experimental studies supporting a role of ceramides in insulin resistance and diabetes (8). For example, in mice fed a high-fat diet and in obese mice, inhibition of ceramide synthesis or enhanced degradation by genetic engineering or pharmacological means improves insulin sensitivity (19,20). Importantly, infusion of LDL-containing ceramides into lean mice reduces insulin-stimulated glucose uptake (21), suggesting that ceramides might be delivered from the circulation thereby influencing glucose homeostasis. In addition, in nonhuman primates, plasma ceramide levels increase in parallel with a reduction in insulin sensitivity in response to a high-fat, high-fructose diet (22).
In humans, the role of ceramide in insulin resistance is not as well established. Treatments that improve muscle insulin sensitivity, such as diet-induced weight loss and exercise training, lower muscle ceramides in some but not all studies (23,24). The evidence relating circulating ceramides and insulin resistance is limited. In small cross-sectional studies, with fewer than 50 subjects, plasma ceramide species correlate with insulin sensitivity (25,26). We show for the first time in a large population without diabetes that circulating ceramide species with saturated fatty acids of different lengths are associated with higher insulin levels and higher HOMA-IR.
Circulating dihydroceramide species, precursors of ceramides, were associated prospectively with diabetes risk in two nested case-control studies from the Data from an Epidemiological Study on the Insulin Resistance Syndrome (DESIR) cohort and the Cohorte Lausannoise (CoLaus) study (27). In addition, in a large prospective cohort in Finland, circulating Cer-16 and Cer-18 were associated with risk of incident major cardiovascular events (28). Our study complements these by suggesting that ceramides may associate early with insulin resistance, prior to its progression to diabetes and its complications.
The fatty acid 16:0 appears unique in its ability to promote endogenous ceramide synthesis and insulin resistance in experimental systems such as myotubes in culture (8,29). In the circulation, 16:0 is the most abundant saturated fatty acid, and total circulating 16:0 is associated with diabetes risk (30–33). In contrast, circulating concentrations of the fatty acids 20:0, 22:0, and 24:0 are associated with lower diabetes risk (11,30). However, we found similar associations of ceramides with different saturated fatty acids with insulin and HOMA-IR, suggesting similar potential biological activities of different ceramides with saturated fatty acids on insulin resistance.
Although sphingomyelins did not show significant associations with any of the outcomes, we provide strong evidence that BMI modifies the associations of the sphingomyelin species SM-18, SM-20, SM-22, and SM-24 with fasting insulin, HOMA-IR, and HOMA-B in both cross-sectional and prospective analyses. Likely as a result of these interactions, overall associations of sphingomyelins were not detected.
Basic studies paint a complex picture of sphingomyelin and insulin sensitivity. In β-cells in culture, increasing sphingomyelin by inhibiting its hydrolysis protects against palmitate-induced lipotoxicity (34), and inhibiting sphingomyelin synthase in myotubes, also resulting in a decrease in sphingomyelin, impairs insulin signaling (35). In contrast, the lowering of sphingomyelin by inhibition of sphingomyelin synthase in knockout mice prevents high-fat–induced obesity and increases insulin sensitivity (36,37). Whether adipogenesis or another aspect of the animal’s metabolism influences the consequences of decreased sphingomyelin is not known.
Studies of sphingomyelins and insulin in humans are limited to cross-sectional studies. Among metabolomics studies that measured sphingomyelins, findings for species with saturated fatty acids ranged from positive to negative associations to no associations with HOMA-IR (38–41). The largest such study measured 47 sphingomyelins among 1,100 young adults; none of the sphingomyelin species, including saturated sphingomyelins, were associated with HOMA-IR (38), mirroring our findings of no main associations. In a smaller study, patients with insulin resistance and those with diabetes had lower SM-16 (39), whereas two studies that included obese individuals observed positive associations of sphingomyelins with saturated fatty acids with HOMA-IR (40,41). Our findings of interactions with BMI may explain these apparently contradictory findings.
Plasma phospholipid levels of 20:0, 22:0, and 24:0 are associated with lower risk of incident diabetes (11,30), lower triglyceride levels, and a better insulin sensitivity score (11). Furthermore, we reported modification of the associations between plasma phospholipid 22:0 and 24:0 levels and diabetes risk by BMI: the association of these fatty acids with lower risk of diabetes was most pronounced among participants with normal BMI (11). The fatty acids 20:0, 22:0, and 24:0 are primarily found in sphingolipids (42), and sphingomyelins are the primary sphingolipids in phospholipids. Our findings that sphingomyelin species with 20:0, 22:0, and 24:0 are associated with lower levels of insulin among those with normal BMI raise the possibility that these sphingomyelins were the lipids associated with lower risk of diabetes in the previous study. Further studies are needed to investigate if circulating sphingomyelin species with 20:0, 22:0, and 24:0 are in fact associated with lower diabetes risk.
Higher levels of LacCer-16 and GluCer-24 were associated with lower fasting insulin, HOMA-IR, and HOMA-B, although these associations were only observed when examined cross-sectionally. Glucosyl ceramide and lactosyl ceramide are the precursors of larger glycosphingolipids such as gangliosides (43). Inhibition of glycosylceramide synthase, which affects the whole pathway, enhances insulin sensitivity in rodents (44). This effect appears to be due to gangliosides interfering with insulin action in adipocytes but not in myotubes, where gangliosides actually enhance insulin sensitivity (45). Biological properties of glucosyl and lactosyl ceramides and their metabolites in humans need to be pursued.
Study strengths include its large size; the study setting of a family-based, well-phenotyped cohort, with recorded data on many potential confounders; and the longitudinal study design in additional to cross-sectional analyses. Study limitations include its observational nature, which precludes assessment of causality. We were not able to investigate all the ceramide species containing saturated fatty acids, such as lactosyl and glycosyl ceramides, due to measurement error with the laboratory assay; however, we measured most underivatized ceramides and sphingomyelins with saturated fatty acids with good precision. The study included a single ethnicity; however, the relatively young cohort, at high risk of diabetes, is an ideal setting for the discovery of early risk factors for diabetes. We cannot eliminate the possibility that drift in the laboratory assay for insulin between baseline and follow-up influenced our measurements of change in insulin over time. However, we had no evidence of drift and it would be unlikely any drift would be differential by sphingolipid level.
Like many others (46,47), we used HOMA-IR and insulin as proxies for insulin resistance because only these measures were available in the SHFS population. It is not surprising that associations with HOMA-IR were very similar to those with fasting insulin, suggesting that values of HOMA-IR are largely driven by insulin levels in this relatively young population with an excess of obesity (48–50). Further, these basal estimates of insulin resistance, calculated only using fasting insulin and glucose values, may differ from estimates based on dynamic measurements of insulin and glucose responses or those derived from clamp experiments with uncertainties regarding contributions of peripheral, hepatic, or whole-body insulin resistance to these measures.
In summary, we have shown that higher plasma levels of ceramides with saturated fatty acids are prospectively associated with higher fasting insulin and HOMA-IR; and, in contrast, higher sphingomyelins with saturated fatty acids are associated with lower fasting insulin, HOMA-IR, and HOMA-B in those with normal BMI. Similar associations were seen for species within the same class, with different saturated fatty acids. The study suggests the lowering of circulating ceramides with saturated fatty acids might be a target in prediabetes, and targeting an increase in circulating sphingomyelins should take into account a person’s BMI.
Supplementary Material
Article Information
Funding. This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases grants R01-DK103657 and P30 DK035816.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. R.N.L. designed the study, obtained funding, and wrote the manuscript. C.Y. contributed to the statistical analyses. A.H. obtained funding, developed the laboratory method for the sphingolipid measurements, and contributed to the writing of the manuscript. N.H. contributed to the sphingolipid measurements. P.N.J. contributed to the statistical analyses and the writing of the manuscript. A.M.F., C.M.S., D.S.S., I.B.K., and N.S. contributed to the interpretation of the results. J.G.U. and B.V.H. obtained funding, contributed plasma samples and data from the SHFS cohort for the study, and contributed to the interpretation of the study results. B.M. oversaw the statistical analyses and contributed to the interpretation of the study results and the writing of the manuscript. All the authors revised the manuscript for important intellectual content and approved the final version of the manuscript. R.N.L. and B.M. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018.
Footnotes
Clinical trial reg. no. NCT00005134, clinicaltrials.gov.
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db17-1449/-/DC1.
See accompanying article, p. 1457.
References
- 1.Danaei G, Finucane MM, Lu Y, et al.; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose) . National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011;378:31–40 [DOI] [PubMed] [Google Scholar]
- 2.Kirtland KA, Cho P, Geiss LS. Diabetes among Asians and native Hawaiians or other Pacific Islanders—United States, 2011-2014. MMWR Morb Mortal Wkly Rep 2015;64:1261–1266 [DOI] [PubMed] [Google Scholar]
- 3.Geiss LS, Wang J, Cheng YJ, et al. . Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980-2012. JAMA 2014;312:1218–1226 [DOI] [PubMed] [Google Scholar]
- 4.Pories WJ, Dohm GL. Diabetes: have we got it all wrong? Hyperinsulinism as the culprit: surgery provides the evidence. Diabetes Care 2012;35:2438–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corkey BE. Diabetes: have we got it all wrong? Insulin hypersecretion and food additives: cause of obesity and diabetes? Diabetes Care 2012;35:2432–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weyer C, Hanson RL, Tataranni PA, Bogardus C, Pratley RE. A high fasting plasma insulin concentration predicts type 2 diabetes independent of insulin resistance: evidence for a pathogenic role of relative hyperinsulinemia. Diabetes 2000;49:2094–2101 [DOI] [PubMed] [Google Scholar]
- 7.Franks PW, McCarthy MI. Exposing the exposures responsible for type 2 diabetes and obesity. Science 2016;354:69–73 [DOI] [PubMed] [Google Scholar]
- 8.Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab 2012;15:585–594 [DOI] [PubMed] [Google Scholar]
- 9.Meikle PJ, Summers SA. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat Rev Endocrinol 2017;13:79–91 [DOI] [PubMed] [Google Scholar]
- 10.Grösch S, Schiffmann S, Geisslinger G. Chain length-specific properties of ceramides. Prog Lipid Res 2012;51:50–62 [DOI] [PubMed] [Google Scholar]
- 11.Lemaitre RN, Fretts AM, Sitlani CM, et al. . Plasma phospholipid very-long-chain saturated fatty acids and incident diabetes in older adults: the Cardiovascular Health Study. Am J Clin Nutr 2015;101:1047–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee ET, Welty TK, Fabsitz R, et al. . The Strong Heart Study. A study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol 1990;132:1141–1155 [DOI] [PubMed] [Google Scholar]
- 13.North KE, Howard BV, Welty TK, et al. . Genetic and environmental contributions to cardiovascular disease risk in American Indians: the Strong Heart Family Study. Am J Epidemiol 2003;157:303–314 [DOI] [PubMed] [Google Scholar]
- 14.MacLean B, Tomazela DM, Shulman N, et al. . Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 2010;26:966–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrison L, Dunn DT, Green H, Copas AJ. Modelling the association between patient characteristics and the change over time in a disease measure using observational cohort data. Stat Med 2009;28:3260–3275 [DOI] [PubMed] [Google Scholar]
- 16.Yanez ND 3rd, Kronmal RA, Shemanski LR, Psaty BM; Cardiovascular Health Study . A regression model for longitudinal change in the presence of measurement error. Ann Epidemiol 2002;12:34–38 [DOI] [PubMed] [Google Scholar]
- 17.Van Buuren S, Groothuis-Oudshoorn K. MICE: multivariate imputation by chained equations in R. J Stat Softw 2011;45:3 [Google Scholar]
- 18.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res 1999;8:3–15 [DOI] [PubMed] [Google Scholar]
- 19.Holland WL, Brozinick JT, Wang LP, et al. . Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 2007;5:167–179 [DOI] [PubMed] [Google Scholar]
- 20.Ussher JR, Koves TR, Cadete VJ, et al. . Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes 2010;59:2453–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boon J, Hoy AJ, Stark R, et al. . Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes 2013;62:401–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brozinick JT, Hawkins E, Hoang Bui H, et al. . Plasma sphingolipids are biomarkers of metabolic syndrome in non-human primates maintained on a Western-style diet. Int J Obes 2013;37:1064–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petersen MC, Jurczak MJ. CrossTalk opposing view: intramyocellular ceramide accumulation does not modulate insulin resistance. J Physiol 2016;594:3171–3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Summers SA, Goodpaster BH. CrossTalk proposal: intramyocellular ceramide accumulation does modulate insulin resistance. J Physiol 2016;594:3167–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergman BC, Brozinick JT, Strauss A, et al. . Serum sphingolipids: relationships to insulin sensitivity and changes with exercise in humans. Am J Physiol Endocrinol Metab 2015;309:E398–E408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haus JM, Kashyap SR, Kasumov T, et al. . Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 2009;58:337–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wigger L, Cruciani-Guglielmacci C, Nicolas A, et al. . Plasma dihydroceramides are diabetes susceptibility biomarker candidates in mice and humans. Cell Reports 2017;18:2269–2279 [DOI] [PubMed] [Google Scholar]
- 28.Havulinna AS, Sysi-Aho M, Hilvo M, et al. . Circulating ceramides predict cardiovascular outcomes in the population-based FINRISK 2002 cohort. Arterioscler Thromb Vasc Biol 2016;36:2424–2430 [DOI] [PubMed] [Google Scholar]
- 29.Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem 1999;274:24202–24210 [DOI] [PubMed] [Google Scholar]
- 30.Forouhi NG, Koulman A, Sharp SJ, et al. . Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol 2014;2:810–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zong G, Zhu J, Sun L, et al. . Associations of erythrocyte fatty acids in the de novo lipogenesis pathway with risk of metabolic syndrome in a cohort study of middle-aged and older Chinese. Am J Clin Nutr 2013;98:319–326 [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Folsom AR, Zheng ZJ, Pankow JS, Eckfeldt JH; ARIC Study Investigators . Plasma fatty acid composition and incidence of diabetes in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr 2003;78:91–98 [DOI] [PubMed] [Google Scholar]
- 33.Ma W, Wu JH, Wang Q, et al. . Prospective association of fatty acids in the de novo lipogenesis pathway with risk of type 2 diabetes: the Cardiovascular Health Study. Am J Clin Nutr 2015;101:153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boslem E, Weir JM, MacIntosh G, et al. . Alteration of endoplasmic reticulum lipid rafts contributes to lipotoxicity in pancreatic β-cells. J Biol Chem 2013;288:26569–26582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park M, Kaddai V, Ching J, et al. . A role for ceramides, but not sphingomyelins, as antagonists of insulin signaling and mitochondrial metabolism in C2C12 myotubes. J Biol Chem 2016;291:23978–23988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Z, Zhang H, Liu J, et al. . Reducing plasma membrane sphingomyelin increases insulin sensitivity. Mol Cell Biol 2011;31:4205–4218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitsutake S, Zama K, Yokota H, et al. . Dynamic modification of sphingomyelin in lipid microdomains controls development of obesity, fatty liver, and type 2 diabetes. J Biol Chem 2011;286:28544–28555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rauschert S, Uhl O, Koletzko B, et al. . Lipidomics reveals associations of phospholipids with obesity and insulin resistance in young adults. J Clin Endocrinol Metab 2016;101:871–879 [DOI] [PubMed] [Google Scholar]
- 39.Xu F, Tavintharan S, Sum CF, Woon K, Lim SC, Ong CN. Metabolic signature shift in type 2 diabetes mellitus revealed by mass spectrometry-based metabolomics. J Clin Endocrinol Metab 2013;98:E1060–E1065 [DOI] [PubMed] [Google Scholar]
- 40.Hanamatsu H, Ohnishi S, Sakai S, et al. . Altered levels of serum sphingomyelin and ceramide containing distinct acyl chains in young obese adults. Nutr Diabetes 2014;4:e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tulipani S, Palau-Rodriguez M, Miñarro Alonso A, et al. . Biomarkers of morbid obesity and prediabetes by metabolomic profiling of human discordant phenotypes. Clin Chim Acta 2016;463:53–61 [DOI] [PubMed] [Google Scholar]
- 42.Hannun YA, Obeid LM. Many ceramides. J Biol Chem 2011;286:27855–27862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jennemann R, Gröne HJ. Cell-specific in vivo functions of glycosphingolipids: lessons from genetic deletions of enzymes involved in glycosphingolipid synthesis. Prog Lipid Res 2013;52:231–248 [DOI] [PubMed] [Google Scholar]
- 44.Aerts JM, Ottenhoff R, Powlson AS, et al. . Pharmacological inhibition of glucosylceramide synthase enhances insulin sensitivity. Diabetes 2007;56:1341–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chavez JA, Siddique MM, Wang ST, Ching J, Shayman JA, Summers SA. Ceramides and glucosylceramides are independent antagonists of insulin signaling. J Biol Chem 2014;289:723–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hurtado-Roca Y, Bueno H, Fernandez-Ortiz A, et al. . Oxidized LDL is associated with metabolic syndrome traits independently of central obesity and insulin resistance. Diabetes 2017;66:474–482 [DOI] [PubMed] [Google Scholar]
- 47.Neergaard JS, Dragsbæk K, Christiansen C, et al. . Metabolic syndrome, insulin resistance, and cognitive dysfunction: does your metabolic profile affect your brain? Diabetes 2017;66:1957–1963 [DOI] [PubMed] [Google Scholar]
- 48.Abbasi F, Okeke Q, Reaven GM. Evaluation of fasting plasma insulin concentration as an estimate of insulin action in nondiabetic individuals: comparison with the homeostasis model assessment of insulin resistance (HOMA-IR). Acta Diabetol 2014;51:193–197 [DOI] [PubMed] [Google Scholar]
- 49.Ader M, Stefanovski D, Richey JM, et al. . Failure of homeostatic model assessment of insulin resistance to detect marked diet-induced insulin resistance in dogs. Diabetes 2014;63:1914–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reaven GM. What do we learn from measurements of HOMA-IR? Diabetologia 2013;56:1867–1868 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.