Serum ceramides have emerged as potential biomarkers of insulin resistance, diabetes, and heart disease. In this issue of Diabetes, Lemaitre et al. (1) report the largest longitudinal study to date correlating sphingolipids with insulin resistance, profiling a cohort of 2,086 American Indians at high risk for diabetes. With the analytical power that derives from profiling such a large number of samples obtained at two visits, 5 years apart, the data from the Strong Heart Family Study (SHFS) revealed that several ceramide species correlated with hyperinsulinemia and the HOMA of insulin resistance (HOMA-IR) in this at-risk population. Here, we summarize these results in the context of other preclinical and clinical studies investigating roles for ceramides as drivers of cardiometabolic dysfunction.
Ceramides are central intermediates in the biosynthetic pathway that produces the large family of sphingolipids, which includes more than 4,000 distinct molecular entities. Much of the complexity in the cellular sphingolipid pool derives from the large number of acyl chains that can be incorporated into the ceramide scaffold. Over the past 20 years, a large number of studies in preclinical models suggest that ceramides may be among the most pathogenic nutrient metabolites that accumulate in obesity, linking overnutrition to insulin resistance and its sequelae of comorbidities. In cultured cells or isolated tissues, ceramides inhibit insulin signaling and action and inhibit lipid oxidation (2). In rodents, numerous ceramide-lowering interventions have been shown to improve insulin sensitivity and ameliorate diabetes and cardiovascular pathologies (2). Because of these data, a handful of companies have started to develop ceramide-reducing interventions in hopes of producing insulin-sensitizing therapeutics.
Despite the strongly consistent findings obtained in preclinical models, the role of ceramides in human cardiometabolic pathologies has been controversial. The debate stems largely from discordance in lipidomic profiling studies, as ceramides in muscle or liver biopsies have been reported to be changed in some, but not all, insulin-resistant subjects (3–5) (Fig. 1). As these discrepant studies typically involved relatively small subject numbers, studies such as the one by Lemaitre et al. (1) are informing the debate. Lemaitre et al. profiled 15 sphingolipid species in a large cohort of Native Americans without diabetes (average age of 38 years), 24% of whom had a BMI of 35 kg/m2 or greater. Those participants with twofold higher (90th percentile) ceramide with 16:0 (Cer-16), Cer-18, Cer-20, or Cer-22 displayed hyperinsulinemia and insulin resistance (estimated using HOMA-IR, a measure of insulin resistance determined from fasting glucose and insulin concentrations). Indeed, those with twofold higher baseline concentrations of Cer-16 had 14% higher levels of insulin, revealing the increased insulin needed to maintain euglycemia owing to the insulin insensitivity. These studies are consistent with earlier, smaller studies evaluating relationships between serum ceramides and insulin resistance in both humans and nonhuman primates (6–10). However, this new study distinguishes itself from the prior ones because of 1) the large size of the cohort, 2) the novel population surveyed, and 3) the longitudinal nature of the analysis (i.e., 5-year follow-up).
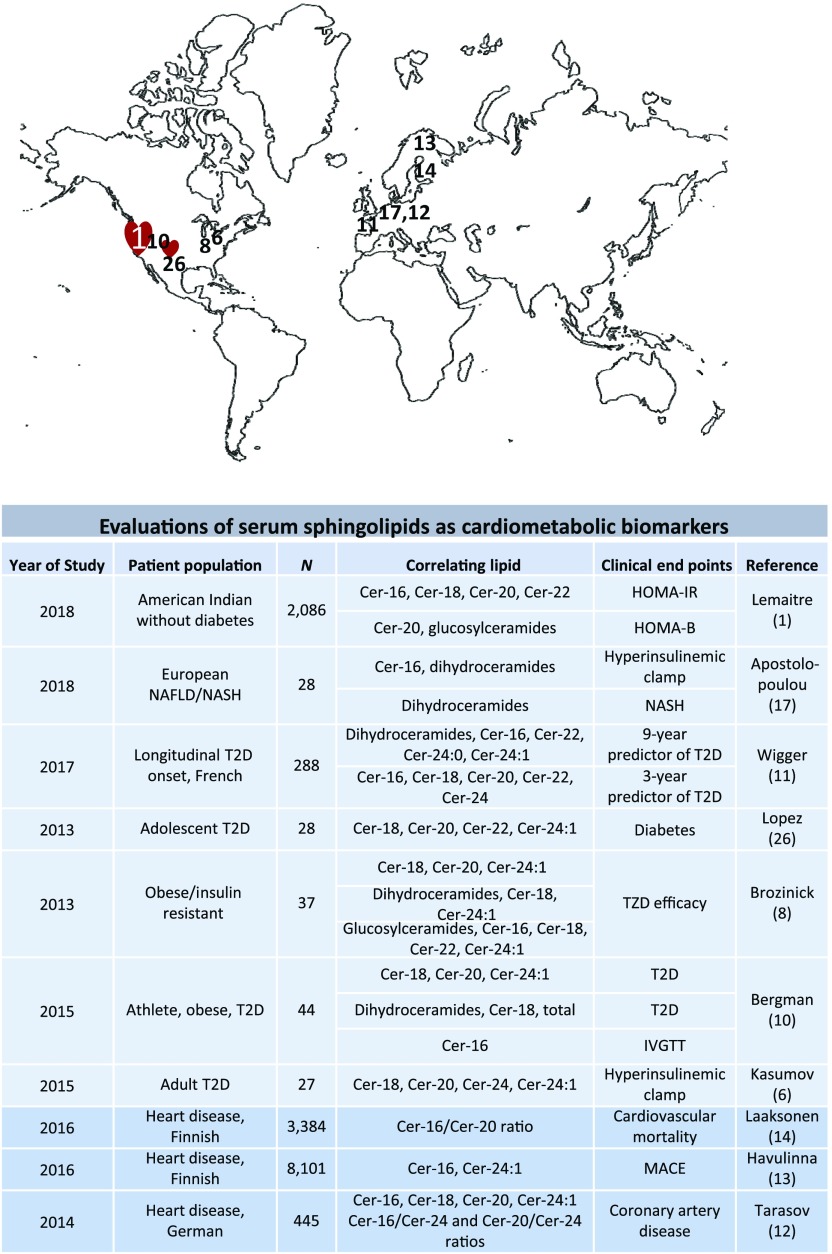
Figure 1.
Abbreviated summary of published studies relating serum ceramides to various measures of cardiometabolic disease. Upper panel denotes sites where the studies were performed. Lower panel denotes study population, number of subjects, lipids analyzed, and clinical end points assessed. IVGTT, intravenous glucose tolerance test; MACE, major adverse cardiovascular events; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; T2D, type 2 diabetes.
Insulin resistance is a major risk factor for diabetes and cardiovascular disease, and the findings of Lemaitre et al. (1) are interesting to consider in relation to other recent studies evaluating relationships between ceramides and clinical indices of cardiometabolic dysfunction. For example, Wigger et al. (11) identified relationships between dihydroceramides, the precursor to ceramides, that revealed increased rates of ceramide synthesis as markers for and predictors of diabetes development. Other groups have found relationships between circulating ceramides and future cardiovascular events. Tarasov et al. (12) found that specific ceramides associated with fatality in patients with coronary artery disease. Laaksonen and colleagues (13,14) found that distinct plasma ceramide ratios were predictors of cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes. Yu et al. (15) reported that plasma ceramide levels correlated with the severity of chronic heart failure and were an independent risk factor of mortality and reduced left ventricular systolic function. The authors of each of these studies highlighted that circulating ceramide levels might provide additional predictive value for cardiovascular events beyond conventional risk measures, and the studies served as the foundation for a diagnostic test being marketed by the Mayo clinic that uses a ceramide score to predict future adverse cardiovascular events. We will not summarize all of the studies that profiled tissue ceramides, but a subset have also shown associations between muscle, liver, or adipose ceramides and insulin resistance (reviewed by Summers and Goodpaster [3]). We highlight the largest of these, a particularly robust study by Luukkonen et al. (16) that profiled 125 liver biopsies, finding that Cer-16 and other “saturated” ceramides correlated strongly with insulin resistance independent of steatosis. Nonetheless, this finding is not universal, as several smaller studies have identified no such relationship between tissue ceramides and insulin sensitivity (5). The reason for the discrepancy is unclear but could reveal differences in statistical power, as the larger studies have generally revealed relationships between ceramides and clinical pathologies. Alternatively, hepatic ceramides may be more tightly linked with progression to nonalcoholic steatohepatitis, with plasma ceramides serving as a predictive biomarker in progression of the disease (17).
A theme that has emerged from the multiple profiling studies is that the acyl composition of the ceramide species likely influences their contribution to metabolic disease. Indeed, long-chain Cer-16 and Cer-18 often show stronger associations with disease pathologies than very long-chain ceramides such as Cer-24. These findings are consistent with determinations made in preclinical models involving the genetic ablation of ceramide synthase isoforms. Indeed, these studies in mice have identified roles for Cer-16–containing ceramides produced by the liver, adipose tissue, and heart (18–22) and Cer-18–containing ceramides generated in skeletal muscle (18–22) as antagonists of insulin action and lipid oxidation. By comparison, the very long-chain ceramides, such as the Cer-24–containing ones produced in excess in the liver, were deemed to be unlikely to contribute to metabolic disorders (20,21). Lemaitre et al. (1) found that Cer-20– and Cer-22–containing ceramides, in addition to Cer-16 and Cer-18, correlated with insulin resistance. Of note, only Cer-20 and some hexosylceramide species correlated with impaired HOMA of β-cell function, an indicator of impaired β-cell function. A limitation of this work is that only static (fasting) measures were taken, which limit the capacity to fully distinguish insulin resistance from β-cell dysfunction.
Most ceramides are converted into sphingomyelins, which represent approximately 70% of sphingolipid mass. However, these more abundant species had less predictive value than ceramides, as relationships with insulin resistance were evident only when the data were stratified by BMI. This is consistent with speculation of many investigators, including us (23), that intermediates in the sphingolipid synthesis cascade rather than sphingomyelins contribute to insulin resistance.
Although the study by Lemaitre et al. (1) adds strongly supportive evidence for roles of ceramides in insulin resistance, it is unlikely by itself to provide full resolution to the debate about the relevance of ceramides to insulin resistance, which has percolated for a long time. Nonetheless, in our opinion, the preponderance of data supports roles for these sphingolipids in insulin resistance and its related comorbidities. Two additional lines of evidence are worth noting. First, recent studies have revealed associations between mutations in the coding region of genes required for ceramide synthesis and cardiometabolic pathologies in humans (24). Second, mutations in adiponectin receptors that negate its intrinsic ceramidase activity negate the cardioprotective and antidiabetes actions of the adipokine in rodents (25). Human observations support the inverse association of adiponectin with plasma ceramides (26). As pharmaceutical interventions to limit sphingolipid abundance progress, we will edge ever closer to knowing whether ceramides will indeed prove to be bona fide mediators of insulin resistance.
Article Information
Funding. The authors receive research support from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (DK112826 and DK108833 to W.L.H. and DK115824 to S.A.S.), JDRF (to W.L.H.), American Diabetes Association (to S.A.S.), and American Heart Association (to S.A.S.).
Duality of Interest. S.A.S. is a consultant for and shareholder of Centaurus Therapeutics. No other potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying article, p. 1663.
References
- 1.Lemaitre RN, Yu C, Hoofnagle A, et al. Circulating sphingolipids, insulin, HOMA-IR, and HOMA-B: the Strong Heart Family Study. Diabetes 2018;67:1663–1672 [DOI] [PMC free article] [PubMed]
- 2.Chaurasia B, Summers SA. Ceramides - lipotoxic inducers of metabolic disorders. Trends Endocrinol Metab 2015;26:538–550 [DOI] [PubMed] [Google Scholar]
- 3.Summers SA, Goodpaster BH. CrossTalk proposal: intramyocellular ceramide accumulation does modulate insulin resistance. J Physiol 2016;594:3167–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen MC, Jurczak MJ. CrossTalk opposing view: intramyocellular ceramide accumulation does not modulate insulin resistance. J Physiol 2016;594:3171–3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen MC, Shulman GI. Roles of diacylglycerols and ceramides in hepatic insulin resistance. Trends Pharmacol Sci 2017;38:649–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasumov T, Solomon TP, Hwang C, et al. Improved insulin sensitivity after exercise training is linked to reduced plasma C14:0 ceramide in obesity and type 2 diabetes. Obesity (Silver Spring) 2015;23:1414–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haus JM, Kashyap SR, Kasumov T, et al. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 2009;58:337–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brozinick JT, Hawkins E, Hoang Bui H, et al. Plasma sphingolipids are biomarkers of metabolic syndrome in non-human primates maintained on a Western-style diet. Int J Obes 2013;37:1064–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warshauer JT, Lopez X, Gordillo R, et al. Effect of pioglitazone on plasma ceramides in adults with metabolic syndrome. Diabetes Metab Res Rev 2015;31:734–744 [DOI] [PubMed] [Google Scholar]
- 10.Bergman BC, Brozinick JT, Strauss A, et al. Serum sphingolipids: relationships to insulin sensitivity and changes with exercise in humans. Am J Physiol Endocrinol Metab 2015;309:E398–E408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wigger L, Cruciani-Guglielmacci C, Nicolas A, et al. Plasma dihydroceramides are diabetes susceptibility biomarker candidates in mice and humans. Cell Reports 2017;18:2269–2279 [DOI] [PubMed] [Google Scholar]
- 12.Tarasov K, Ekroos K, Suoniemi M, et al. Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and PCSK9 deficiency. J Clin Endocrinol Metab 2014;99:E45–E52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Havulinna AS, Sysi-Aho M, Hilvo M, et al. Circulating ceramides predict cardiovascular outcomes in the population-based FINRISK 2002 cohort. Arterioscler Thromb Vasc Biol 2016;36:2424–2430 [DOI] [PubMed] [Google Scholar]
- 14.Laaksonen R, Ekroos K, Sysi-Aho M, et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur Heart J 2016;37:1967–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu J, Pan W, Shi R, et al. Ceramide is upregulated and associated with mortality in patients with chronic heart failure. Can J Cardiol 2015;31:357–363 [DOI] [PubMed] [Google Scholar]
- 16.Luukkonen PK, Zhou Y, Sädevirta S, et al. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J Hepatol 2016;64:1167–1175 [DOI] [PubMed] [Google Scholar]
- 17.Apostolopoulou M, Gordillo R, Koliaki C, et al. Specific hepatic sphingolipids relate to insulin resistance, oxidative stress, and inflammation in nonalcoholic steatohepatitis. Diabetes Care 2018;41:1235–1243 [DOI] [PubMed] [Google Scholar]
- 18.Hla T, Kolesnick R. C16:0-ceramide signals insulin resistance. Cell Metab 2014;20:703–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turpin SM, Nicholls HT, Willmes DM, et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab 2014;20:678–686 [DOI] [PubMed] [Google Scholar]
- 20.Chaurasia B, Kaddai VA, Lancaster GI, et al. Adipocyte ceramides regulate subcutaneous adipose browning, inflammation, and metabolism. Cell Metab 2016;24:820–834 [DOI] [PubMed] [Google Scholar]
- 21.Raichur S, Wang ST, Chan PW, et al. CerS2 haploinsufficiency inhibits β-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance [published correction appears in Cell Metab 2014;20:919]. Cell Metab 2014;20:687–695 [DOI] [PubMed] [Google Scholar]
- 22.Russo SB, Baicu CF, Van Laer A, et al. Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J Clin Invest 2012;122:3919–3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park M, Kaddai V, Ching J, et al. A role for ceramides, but not sphingomyelins, as antagonists of insulin signaling and mitochondrial metabolism in C2C12 myotubes. J Biol Chem 2016;291:23978–23988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Summers SA. Could ceramides become the new cholesterol? Cell Metab 2018;27:276–280 [DOI] [PubMed] [Google Scholar]
- 25.Sharma AX, Holland WL. Adiponectin and its hydrolase-activated receptors. J Nat Sci 2017;3:3. [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez X, Goldfine AB, Holland WL, Gordillo R, Scherer PE. Plasma ceramides are elevated in female children and adolescents with type 2 diabetes. J Pediatr Endocrinol Metab 2013;26:995–998 [DOI] [PubMed] [Google Scholar]