Abstract
Glucagon-like peptide 1 receptor (GLP-1R) agonists are U.S. Food and Drug Administration–approved weight loss drugs. Despite their widespread use, the sites of action through which GLP-1R agonists (GLP1RAs) affect appetite and body weight are still not fully understood. We determined whether GLP-1Rs in either GABAergic or glutamatergic neurons are necessary for the short- and long-term effects of the GLP1RA liraglutide on food intake, visceral illness, body weight, and neural network activation. We found that mice lacking GLP-1Rs in vGAT-expressing GABAergic neurons responded identically to controls in all parameters measured, whereas deletion of GLP-1Rs in vGlut2-expressing glutamatergic neurons eliminated liraglutide-induced weight loss and visceral illness and severely attenuated its effects on feeding. Concomitantly, deletion of GLP-1Rs from glutamatergic neurons completely abolished the neural network activation observed after liraglutide administration. We conclude that liraglutide activates a dispersed but discrete neural network to mediate its physiological effects and that these effects require GLP-1R expression on glutamatergic but not GABAergic neurons.
Introduction
The incretin glucagon-like peptide 1 (GLP-1) is produced mainly in intestinal L cells and a discrete population of hindbrain neurons. GLP-1 is released from the intestine after a meal and acts through its receptor (GLP-1R) to increase insulin and decrease glucagon secretion in a glucose-dependent manner (1). As such, long-acting synthetic GLP-1R agonists (GLP1RAs) are useful therapeutic agents to treat type 2 diabetes (2). Of note, long-acting GLP1RAs, such as liraglutide, not only improve blood glucose homeostasis but also cause significant weight loss (3). In rodents and humans, the weight loss is associated with a reduction in food intake with little effect on energy expenditure (4,5). Despite these clinically significant effects on body weight, the cellular mechanisms by which GLP1RAs modulate feeding are unclear, and understanding these mechanisms remains an important research goal.
GLP-1R is expressed in the pancreas, heart, kidney, gastrointestinal tract, and brain (1). We and others have shown that GLP-1R–expressing cells in the central nervous system (CNS) are required for the full anorectic and body weight effects of peripherally administered liraglutide in both mice and rats (6,7). GLP-1Rs are distributed across the CNS, including in areas known to be critical for the regulation of food intake and body weight (8,9). The fact that local microinfusion of GLP1RAs into numerous CNS nuclei is sufficient to decrease food intake (10) suggests that many GLP-1R–expressing neuronal populations contribute to the anorectic effects of peripherally administered liraglutide. To date, genetic deletion of GLP-1R from specific brain nuclei known to be involved in energy balance has shown that GLP-1Rs in the paraventricular hypothalamus (PVH), arcuate nucleus, and ventromedial nucleus of the hypothalamus are not necessary for the anorectic effects of peripherally administered GLP1RAs (11–13).
These results are consistent with a model in which the anorectic effects of GLP1RAs are distributed across the CNS rather than contained in a single anatomic site (10). Because cell-specific targeting of GLP-1R expression has yet to identify critical neural populations required for GLP1RA action, we used established neuron-specific Cre lines to target broad and mostly nonoverlapping neuronal populations: vGlut2-expressing glutamatergic and vGAT-expressing GABAergic neurons (14). With the use of Cre-dependent reporter animals, we examined and cataloged the brain regions and neuronal subtypes activated by liraglutide and found a discrete pattern of neural activation that encompasses both glutamatergic and GABAergic neurons. We then deleted GLP-1R from each population and examined the behavioral and physiological outcomes at baseline and in response to short- and long-term liraglutide treatment in both lean and obese mouse models. We found that GLP-1Rs expressed in GABAergic neurons are dispensable for liraglutide-induced anorexia, weight loss, and neural network activation, whereas GLP-1Rs in glutamatergic neurons are required for these effects.
Research Design and Methods
Animals
Glp1r-flox (6), vGAT-Cre (14), vGlut2-Cre (14), Glp1r-Cre (15), and L10-GFP reporter (16) mice have been described previously (see Supplementary Data for breeding strategies). All studies were approved by the University of Michigan institutional animal care and use committee.
Immunohistochemistry
Pair-housed vGAT-GFP, vGlut2-GFP, and Glp1r-GFP (n = 7–8 per genotype and treatment, sexes combined) and singly housed male Glp1r-flox, vGATΔGlp1r, and vGlut2ΔGlp1r (n = 5 per genotype and treatment) were used for Fos analysis. Mice were fasted at 9:00 a.m. and subcutaneously injected with either liraglutide (400 μg/kg; Novo Nordisk) or saline (10 mL/kg body weight) at 11:00 a.m. At 1:00 p.m., mice were perfused and brains processed for immunostaining as previously described (17) (see Supplementary Data for antibodies used and protocol).
Microscopy and Image Analysis
Coordinates and landmarks for regions of interest were identified by counterstaining and the mouse brain atlas (18) (Supplementary Table 1). For each nucleus, counts from the left and right side were added, and then those from each coronal plane were averaged to yield one count per nucleus per mouse (see Supplementary Data regarding image acquisition and analysis).
Model Characterization
Knockdown of Glp1r in the hypothalami of vGATΔGlp1r and vGlut2ΔGlp1r mice was confirmed by semiquantitative real-time PCR (RT-PCR). Hypothalamic tissue was microdissected from male mice in the long-term liraglutide study (see below), with treatment groups collapsed (n = 13–14 per genotype) and processed and analyzed as previously described (19) (Supplementary Data).
For in situ hybridization (ISH), Glp1r-flox, vGATΔGlp1r, and vGlut2ΔGlp1r mice were decapitated under anesthesia; whole brains were dissected, flash-frozen in isopentane chilled on dry ice, and stored at −80°C. Sixteen-micrometer-thick cryostat coronal sections were thaw-mounted to slides and stored at −80°C. Slides were processed for ISH using RNAScope per the manufacturer’s protocol (Advanced Cell Diagnostics), and the multiplex fluorescent assay (320850) was used to visualize Glp1r (418851) and Cre (312281-C3) probes using Amp 4 Alt-A.
Animal Studies
Dosages of liraglutide were chosen on the basis of previous studies (6). For the longitudinal study, mice (n = 10 per sex and genotype) were singly housed at age 4 weeks, and body weight and food intake were monitored weekly. At age 13–15 weeks, mice were fasted at 10:00 a.m. and then subcutaneously injected with either liraglutide (400 μg/kg) or saline at 5:00 p.m. Preweighed food was given at 6:00 p.m., and food intake was measured 1, 2, 4, and 24 h later. Body weight was measured at the 24-h time point. One week later, all mice were given the opposite treatment (saline or liraglutide) in a crossover design, and the study was repeated. At age 15–17 weeks, mice were fasted at 9:00 a.m., and basal fasting blood glucose was measured at 1:00 p.m. with a glucometer (Contour Next EZ; Bayer). Mice were then injected with liraglutide (400 μg/kg) or saline as above and blood glucose measured at 3:00 p.m. The crossover treatment was performed 1 week later. At age 18 weeks, brains from the male Glp1r-flox, vGATΔGlp1r, and vGlut2ΔGlp1r mice were collected for Fos immunohistochemistry (IHC) analysis.
For the long-term liraglutide study, male Glp1r-flox, vGATΔGlp1r, and vGlut2ΔGlp1r mice (n = 6–8 per genotype and treatment) were weaned into group housing with standard rodent chow. At age 5 weeks, the food was switched to high-fat chow (D12451; Research Diets), and the mice were singly housed at age 9 weeks. At age 11 weeks, mice were randomly assigned to either the saline control or liraglutide group and subcutaneously injected once per day (5:00 p.m.) for 2 weeks using a previously described ascending dosing schedule (6). Body weight and food intake were measured daily. On day 15, mice were decapitated under anesthesia and the hypothalamus was isolated, snap-frozen on dry ice, and stored at −80°C for semiquantitative RT-PCR analysis (see above). Gonadal and perirenal fat pads were removed and weighed. To assay conditioned taste avoidance (CTA), male and female Glp1r-flox, vGATΔGlp1r, and vGlut2ΔGlp1r mice (n = 8 per genotype and treatment) were singly housed at age 10–12 weeks, and the assay was performed as previously described (20) (Supplementary Data).
Statistics
Data are presented as mean ± SEM, and statistical analyses were performed with GraphPad Prism 7 software. Specific tests performed for each experiment, along with n values, are included in the figure legends. Post hoc tests after ANOVA were performed only if the interaction between the two variables was significant at P < 0.05.
Results
Liraglutide Activates Both GABAergic and Glutamatergic Neurons
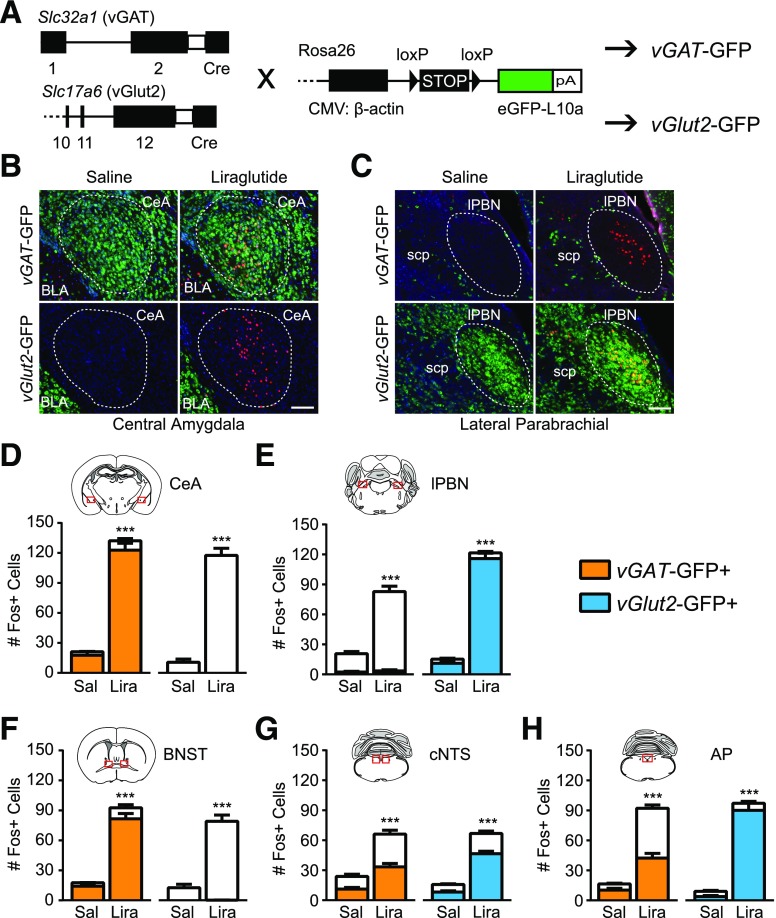
To characterize the subtypes of neurons activated by liraglutide, we generated vGAT- and vGlut2-GFP mice to label GABAergic and glutamatergic neurons, respectively (Fig. 1A). We then performed IHC for Fos, an early marker of neural activation, after liraglutide or saline injection. Representative images from brain regions known to be activated by GLP1RAs (21,22) show specific GFP expression in the GABAergic central amygdala (CeA) versus the glutamatergic basolateral amygdala and lateral parabrachial nucleus (lPBN) (Fig. 1B and C). Liraglutide induced a significant increase in Fos+ cells in five regions: CeA, lPBN, bed nucleus of the stria terminalis (BNST), caudal nucleus of the solitary tract (cNTS), and area postrema (AP) (Fig. 1D–H). No effect of liraglutide on Fos activation in the lateral septum, paraventricular thalamus, PVH, arcuate, ventromedial hypothalamus, lateral dorsal tegmental nucleus, or rostral NTS were found (Supplementary Fig. 1).
Figure 1.
Characterization of neuronal subtypes activated by liraglutide. A: Breeding schematic with genetic constructs used to generate vGAT-GFP and vGlut2-GFP mice. B and C: Representative images from the CeA (B) and lPBN (C), showing Fos IHC (red) after saline or liraglutide injection in vGAT-GFP (green) and vGlut2-GFP (green) neurons. Blue is DAPI. Scale bars = 100 μm. Regions of interest for quantification are highlighted by a dotted line. D–H: Quantification of Fos+ neurons per section in five regions. The proportion of Fos+ neurons positive for vGAT-GFP or vGlut2-GFP is indicated (n ≥ 6). Data were analyzed by unpaired Student t test between drug treatments. White bars indicate GFP-negative neurons. ***P < 0.001. BLA, basolateral amygdala; CMV, cytomegalovirus; eGFP, enhanced green fluorescent protein; Lira, liraglutide; Sal, saline; scp, superior cerebellar peduncle.
The CeA and BNST are largely GABAergic regions, and 93.6 ± 1.2% and 88.9 ± 2.7%, respectively, of the Fos+ neurons in these regions were vGAT-GFP+. Conversely, in the glutamatergic lPBN, 95.4 ± 1.1% of Fos+ neurons were vGlut2-GFP+. The cNTS and AP were more heterogeneous in the expression of vGlut2- and vGAT-GFP. In the cNTS, 50.9 ± 3.5% of Fos+ cells were vGAT-GFP+, and 69.9 ± 2.8% were vGlut2-GFP+. In the AP, 45.4 ± 3.3% of Fos+ cells were vGAT-GFP+, and 93.2 ± 1.5% were vGlut2-GFP+. These combined percentages exceed 100, suggesting that a fraction of cNTS and AP neurons express both vGAT and vGlut2 during development. Thus, peripheral liraglutide injection leads to activation of specific brain regions containing both GABAergic and glutamatergic neurons, consistent with a model of distributed GLP1RA action (10).
GLP-1R–Expressing Glutamatergic Neurons Mediate the Short-term Feeding Effects of Liraglutide
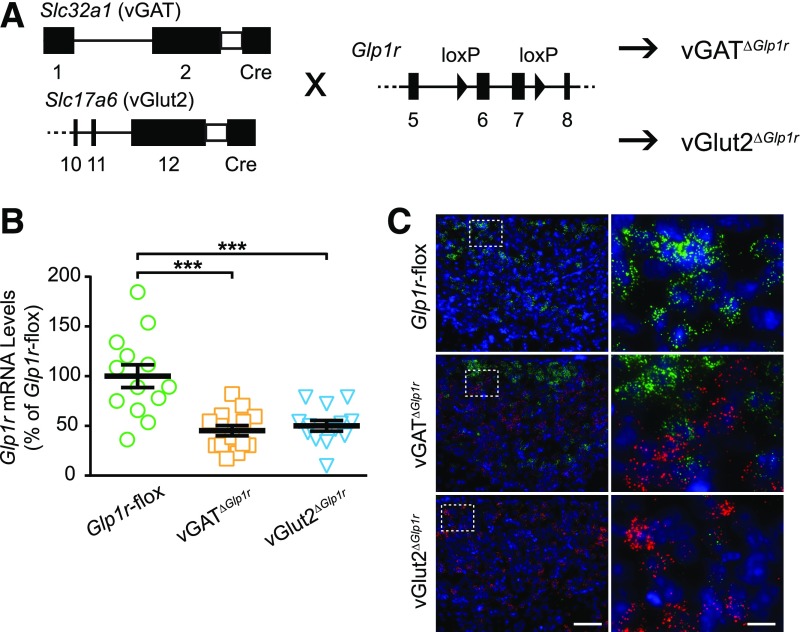
To test the role of GLP-1Rs expressed in GABAergic versus glutamatergic neurons in response to GLP1RAs, we generated mice lacking Glp1r in either vGlut2- or vGAT-expressing cells (Fig. 2A). In the hypothalamus, Glp1r mRNA expression levels were 45.3 ± 5.1% and 50.2 ± 5.3% of controls in vGATΔGlp1r and vGlut2ΔGlp1r mice, respectively (Fig. 2B). To confirm that this knockdown was cell-type specific, we performed double ISH for Glp1r and Cre transcripts in the liraglutide-responsive AP, which comprises both glutamatergic and GABAergic cell types. Glp1r-flox control mice had robust expression of Glp1r and no Cre throughout the AP. In vGATΔGlp1r mice, no overlap of Cre and Glp1r in the AP was found. In vGlut2ΔGlp1r mice, we observed many neurons expressing Cre, but Glp1r expression was absent throughout the AP (Fig. 2C). The absence of Glp1r from the AP of vGlut2ΔGlp1r mice is consistent with transient developmental vGlut2 expression across this region.
Figure 2.
Generation and validation of vGATΔGlp1r and vGlut2ΔGlp1r mice. A: Breeding schematic with genetic constructs. B: Semiquantitative RT-PCR of Glp1r in the hypothalamus of male mice (n ≥ 13 per group). Data analyzed by one-way ANOVA. ***P < 0.001 by Tukey multiple comparisons test. C: Representative ISH images showing efficient knockdown of Glp1r (green) in cells containing Cre recombinase (red) in the AP. Blue is DAPI. Inset in left panels are shown at higher magnification in the right panels. Scale bars = 50 μm (left panels) and 10 μm (right panels).
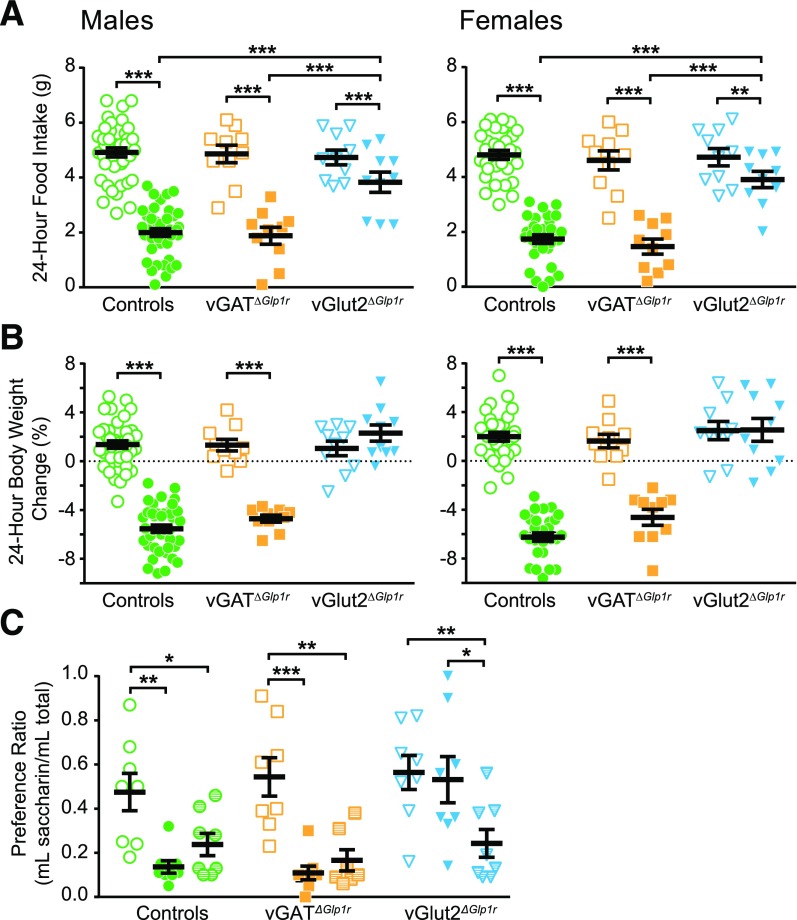
vGATΔGlp1r and vGlut2ΔGlp1r mice exhibited normal body weight, food intake, and blood glucose at baseline (Supplementary Fig. 2). We then assessed the response of these genotypes to GLP1RA administration with regard to food intake, body weight, and blood glucose. After liraglutide injection, control and vGATΔGlp1r mice of both sexes decreased their 24-h food intake by 60–70% compared with saline injection. Liraglutide produced a significant but dramatically attenuated anorectic effect in vGlut2ΔGlp1r mice, with 24-h food intake decreasing by only 15–20% compared with saline treatment (Fig. 3A). Of note, liraglutide-induced anorexia was delayed in vGlut2ΔGlp1r mice relative to vGATΔGlp1r and control mice (Supplementary Fig. 3), suggesting that some aspect of the effects of GLP1RAs may be mediated by non-vGlut2–expressing neurons. These results indicate that GLP-1Rs in glutamatergic neurons are required for the full anorectic effect of liraglutide.
Figure 3.
Short-term effects of liraglutide in vGATΔGlp1r and vGlut2ΔGlp1r chow-fed mice. Males and females are displayed separately (A and B) and combined (C). Change in total food intake (A) and body weight (B) 24 h after liraglutide or saline control injection. CTA test results (C) display the taste preference for saccharin after pairing with either saline, liraglutide, or lithium chloride (positive control). n ≥ 10 (A and B) and n = 8 (C). Repeated-measures two-way ANOVA (A and B) or standard two-way ANOVA (C) was performed followed by Sidak (A and B) or Tukey (C) multiple comparisons test. Open symbols indicate saline treatment, partially filled symbols indicate lithium chloride treatment, and closed symbols indicate liraglutide treatment. *P < 0.05, **P < 0.01, ***P < 0.001.
Twenty-four hours after liraglutide injection, control and vGATΔGlp1r mice lost ∼5–6% of their body weight, whereas vGlut2ΔGlp1r mice maintained normal body weight (Fig. 3B). Liraglutide lowered blood glucose equally in males of all three genotypes, but in females, only control and vGATΔGlp1r mice responded (Supplementary Fig. 4). Because liraglutide is known to cause visceral illness, we tested whether this effect requires GLP-1R in GABAergic or glutamatergic neurons by using a CTA assay. After learning to associate a novel taste (saccharin water) with lithium chloride (positive control), mice of all genotypes demonstrated a decreased preference for the saccharin water, indicating that the lithium chloride was aversive (Fig. 3C). In contrast, liraglutide induced CTA in control and vGATΔGlp1r but not vGlut2ΔGlp1r mice, thus revealing that GLP-1Rs in glutamatergic neurons are also necessary for the visceral illness response to liraglutide.
Long-term Liraglutide-Induced Weight Loss Is Mediated by GLP-1R–Expressing Glutamatergic Neurons
To model the clinical use of liraglutide as an obesity therapy, we fed a second cohort of male Glp1r-flox, vGATΔGlp1r, and vGlut2ΔGlp1r mice a high-fat diet to induce obesity. After 6 weeks, all three genotypes had gained significant weight (15–20%) compared with genotype- and age-matched controls from the previous study, with no differences between groups (Glp1r-flox 29.5 ± 0.6 g, vGATΔGlp1r 29.6 ± 0.6 g, vGlut2ΔGlp1r 31.2 ± 0.7 g; not significant by one-way ANOVA).
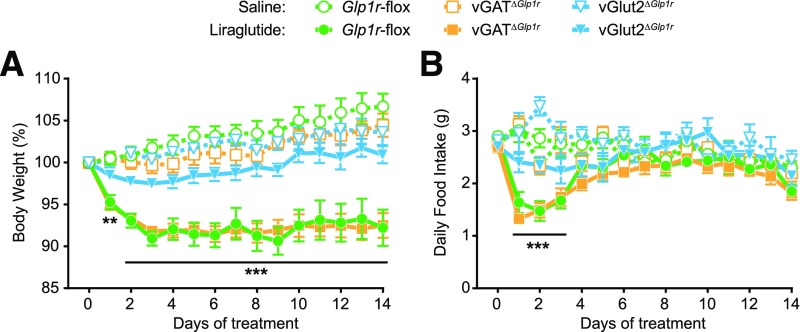
Obese mice were then treated daily with liraglutide or saline for 14 days. As seen in the short-term studies, Glp1r-flox control and vGATΔGlp1r mice showed a decrease in food intake and body weight 24 h after liraglutide injection, whereas no effect was observed in vGlut2ΔGlp1r mice. Body weights of Glp1r-flox control and vGATΔGlp1r mice plateaued at ∼90% of original weight after 3 days of liraglutide treatment, whereas saline-treated mice steadily gained weight (Fig. 4A). In contrast, liraglutide had no effect on weight gain in vGlut2ΔGlp1r mice. The sustained body weight loss in Glp1r-flox and vGATΔGlp1r mice was accompanied by a significant but transient drop in food intake that returned to normal after 4 days of treatment (Fig. 4B). Food intake of vGlut2ΔGlp1r mice was not affected by liraglutide during the study. Long-term liraglutide reduced visceral fat pad weights in Glp1r-flox controls and vGATΔGlp1r mice but had no effect on fat pad weights in vGlut2ΔGlp1r mice (Supplementary Fig. 5). Together, these results suggest that GLP-1R–expressing glutamatergic neurons mediate both the short-term anorexia and the long-term weight loss effects of liraglutide.
Figure 4.
Long-term effects of liraglutide in vGATΔGlp1r and vGlut2ΔGlp1r high-fat diet–fed male mice. Body weight change from baseline (A) and daily food intake (B) during a 14-day treatment with once daily liraglutide or saline control injection. All mice were fed a 45% high-fat diet 6 weeks before and during the study (n ≥ 6). Data were analyzed by repeated-measures two-way ANOVA followed by Dunnett multiple comparisons test. **P < 0.01, ***P < 0.001 at each time point compared with saline-treated controls of the same genotype.
Liraglutide Activates a Neuronal Network Through GLP-1R–Expressing Glutamatergic Neurons
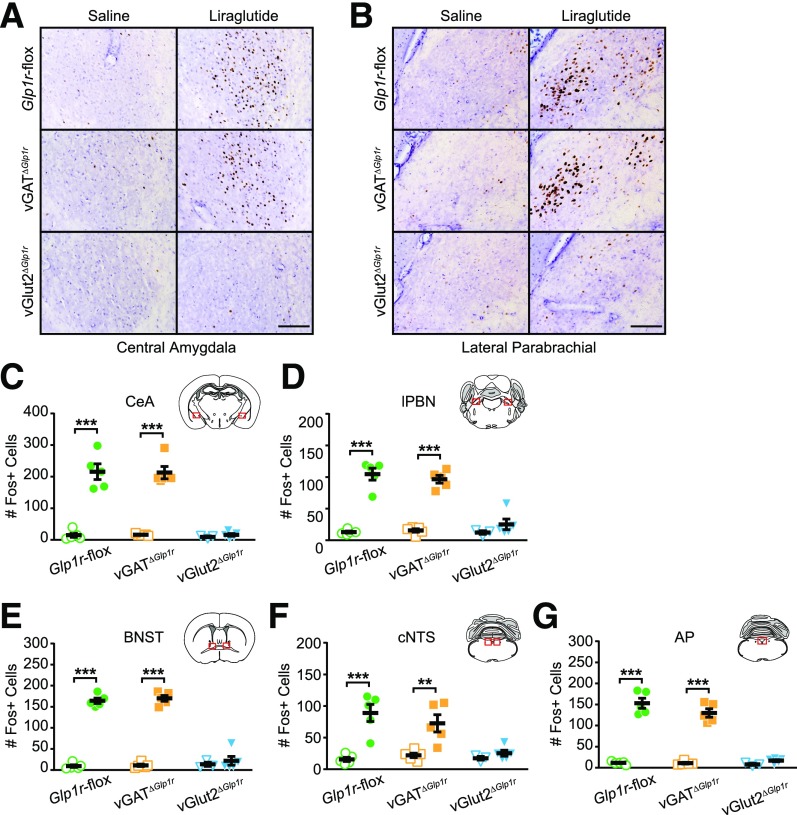
To identify neural responses associated with these different behavioral phenotypes, we examined Fos IHC after saline and liraglutide treatment in male Glp1r-flox, vGATΔGlp1r, and vGlut2ΔGlp1r mice. In both Glp1r-flox controls and vGATΔGlp1r mice, liraglutide treatment significantly increased Fos+ cells in the same regions identified previously (i.e., CeA, BNST, lPBN, cNTS, AP). Somewhat surprisingly, Fos activation in all of these regions (both glutamatergic and GABAergic) was completely absent in vGlut2ΔGlp1r mice (Fig. 5). These data suggest that a population of glutamatergic GLP-1R–expressing neurons are activated directly by liraglutide and then engage a downstream neural network that is both glutamatergic and GABAergic to elicit potent physiological effects.
Figure 5.
Neuronal activation in vGATΔGlp1r and vGlut2ΔGlp1r mice after liraglutide injection. A and B: Representative images from the CeA (A) and lPBN (B) showing Fos IHC (brown) after saline or liraglutide injection. Sections were counterstained with hematoxylin. Scale bars = 100 μm. C–G: Quantification of Fos+ neurons in each of five brain regions (n ≥ 3). Data were analyzed by two-way ANOVA followed by Tukey multiple comparisons test. Open symbols indicate saline treatment, and closed symbols indicate liraglutide treatment. **P < 0.01, ***P < 0.001.
Glp1r–Expressing Neurons in the lPBN and AP Are Activated by Liraglutide
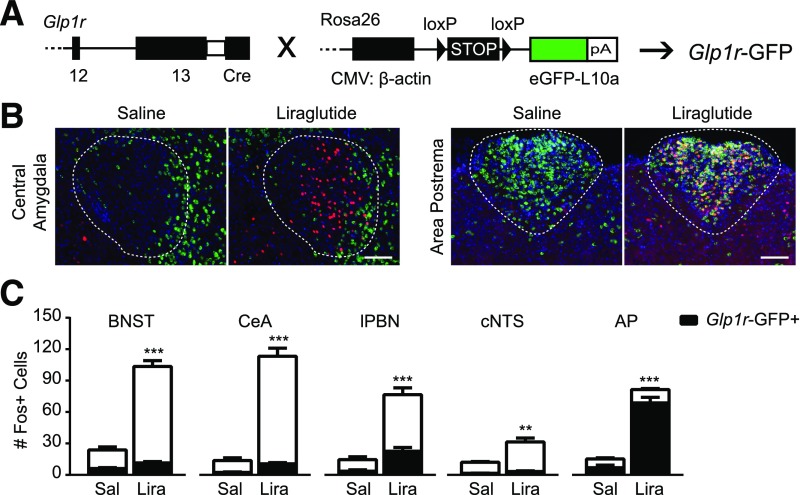
To identify which GLP-1R–expressing neurons are directly activated by liraglutide, we generated Glp1r-GFP mice (Fig. 6A). The pattern of Glp1r-GFP expression across the brain generally agreed with that reported for Glp1r expression using other methods (8,9) (Supplementary Fig. 6). Glp1r-GFP mice were injected with saline or liraglutide for subsequent Fos analysis. Although Glp1r-GFP+ neurons were found in all five identified regions of interest, most were not activated by liraglutide. In the CeA, BNST, and cNTS, only 9.4 ± 1.1%, 10.7 ± 1.0%, and 9.4 ± 1.2%, respectively, of Fos+ neurons also expressed Glp1r-GFP. On the other hand, 28.6 ± 2.2% and 84.3 ± 1.3% of lPBN and AP Fos+ neurons, respectively, were Glp1r-GFP+ (Fig. 6B and C). Thus, the lPBN and AP contained the highest percentage of liraglutide-activated Fos+ neurons that also expressed Glp1r-GFP, suggesting that glutamatergic neurons in these regions are critical for GLP1RA actions.
Figure 6.
Identification of liraglutide-activated neurons expressing Glp1r. A: Breeding schematic with genetic constructs used to generate Glp1r-GFP mice. B: Representative images from the CeA and AP showing Fos IHC (red) after saline or liraglutide injection in Glp1r-GFP (green) neurons. Blue is DAPI. Scale bars = 100 μm. Regions of interest for quantification are highlighted by a dotted line. C: Quantification of Fos+ neurons per section in each region. In each column, the proportion of Fos+ neurons positive for Glp1r-GFP is indicated in black (n ≥ 6). Data were analyzed by unpaired Student t test between drug treatments. White bars indicate GFP-negative neurons. **P < 0.01, ***P < 0.001. CMV, cytomegalovirus; eGFP, enhanced green fluorescent protein; Lira, liraglutide; Sal, saline.
Discussion
Consistent with previously published data on other GLP1RAs (21–24), we found that peripheral liraglutide activates a rather limited network of cells in the BNST, CeA, lPBN, cNTS, and AP. Of the five liraglutide-activated nuclei that we identified, two (BNST, CeA) are primarily composed of vGAT-positive neurons, one (lPBN) almost exclusively is composed of vGlut2-positive neurons, and two (cNTS, AP) contain a mixture of both cell types. To test the role of these neuron groups in mediating the effects of liraglutide, we deleted Glp1r specifically in neurons expressing vGAT or vGlut2. We found that mice lacking GLP-1R in vGlut2-expressing neurons had a blunted anorectic response to liraglutide and did not lose body weight after short- or long-term treatment, whereas loss of Glp1r from vGAT-expressing neurons had no effect on liraglutide responsiveness. These data indicate that GLP-1Rs in vGlut2-expressing neurons are required for the majority of the anorectic and weight loss effects of liraglutide. Furthermore, we found that GLP1RA administration was associated with a distinct pattern of neuronal activation across both glutamatergic and GABAergic cell groups that depends on a direct effect of liraglutide on GLP-1R–expressing vGlut2+ neurons.
Nausea is a common early side effect of GLP1RAs in humans and may contribute to the initial appetite loss and subsequent weight loss (25). In rodents, drug-induced visceral illness can be inferred by the formation of a CTA to a novel taste paired with the drug (with the caveat that rats will sometimes exhibit CTA even to substances that they find rewarding, such as amphetamines [26]) and/or by pica (consumption of a nonnutritive clay after drug treatment). Long-term liraglutide treatment induced pica in rats only at the onset of treatment, mirroring reports of nausea in patients (27). Although well characterized in rats, the validity of the pica assay has been questioned in mice (28). In both rats and mice, peripheral administration of GLP1RAs also led to the formation of a CTA that is mediated by GLP-1R–expressing cells in the brain (6,21,27). Some evidence has suggested that the nausea and anorexia caused by GLP1RAs are achieved by separate and dissociable mechanisms. For example, in rats, direct administration of GLP1RAs into the fourth ventricle (29), the PVH (30), and the nucleus accumbens (31) reduces food intake without inducing CTA, although this may be dose dependent.
The CeA has been implicated as a neural mediator for the aversive effects of GLP1RAs because GLP1RA infusion into the CeA induced CTA without reducing food intake (27,29). The current results suggest that the CeA is not a direct site of action for peripherally administered liraglutide (although it could be a downstream target) because mice lacking Glp1r in the GABAergic CeA still formed a CTA to liraglutide. On the other hand, bona fide species differences between mice and rats, particularly pertaining to the GLP-1 system and CTA, have been identified previously (32). Thus, we cannot exclude the possibility that the CeA is an important direct site for GLP-1–induced aversion in rats but not in mice. Nevertheless, the current results suggest that although the sites of action may be separable, both the aversive and the anorectic effects of liraglutide require GLP-1R expression on vGlut2+ neurons.
Studies that used various methodological approaches in rats have implicated vagal GLP-1Rs in the anorectic effect of intraperitoneally administered GLP1RAs (7,33,34). However, rats subjected to subdiaphragmatic vagal afferent deafferentation responded normally to intravenous and subcutaneous liraglutide injection (35,36). Taken together, these studies suggest that the sites (peripheral vs. central) of action of GLP1RAs may differ depending on the route of drug administration as well as on the dosage. The current study was not specifically designed to test the role of vagal versus central GLP-1R. Because GLP-1R–expressing neurons in the nodose ganglion are glutamatergic (15) and would be deleted in vGlut2ΔGlp1r mice, a contribution of vagal afferent glutamatergic GLP-1R–expressing neurons in the anorectic effect of liraglutide cannot be fully excluded. However, mice lacking GLP-1Rs on vagal afferents responded normally to the same dose of subcutaneous liraglutide used in this study (6), underscoring the importance of central GLP-1R–expressing neurons in our paradigm.
GLP-1–expressing neurons in the brainstem are glutamatergic and project to the hypothalamus (37,38). Activation of brainstem GLP-1 neurons induces Fos expression in hypothalamic regions (39), suggesting that the hypothalamus mediates some of the effects of endogenous brainstem-derived GLP-1. However, the role of the hypothalamus in the physiological response to peripherally administered GLP1RAs is less clear. Some studies have found hypothalamic neuronal activation after short-term peripheral GLP1RA injection, whereas others, including the current study, have not (21,22,40). Mice with GLP-1R deleted from the majority of the hypothalamus (Nkx2.1-Cre), the PVH (Sim1-Cre), or the ventromedial nucleus (SF1-Cre) had normal anorectic responses to peripherally administered GLP1RAs (11–13). In addition, decerebrate rats, in which hindbrain-forebrain connectivity is severed, reduced their food intake comparably with control rats after peripheral GLP1RA administration (41). The current data support a model in which hypothalamic GLP-1Rs are not required for the anorectic effects of peripherally administered GLP1RAs.
In this study, mice lacking GLP-1R in either GABAergic or glutamatergic neurons did not differ from controls in body weight or food intake under basal conditions or when fed a high-fat diet, suggesting that GLP-1R in these neural populations is not necessary for normal appetite and body weight regulation. Indeed, whole-body Glp1r-knockout as well as pan-neuronal Glp1r-knockout mice exhibit normal food intake and body weight (6,42). However, the possibility of developmental compensation for loss of this receptor should not be overlooked. In rats, intracerebroventricular injection of a specific GLP-1R antagonist, exendin(9-39) has been shown to increase food intake in the short and long term, suggesting that neuronal GLP-1Rs contribute to normal feeding behavior (43,44). Site-specific manipulations, such as antagonist microinfusion or RNA interference–mediated Glp1r knockdown are further unraveling the roles of specific Glp1r-expressing populations in normal physiology (45–47). Future studies in mice should use site-specific viral delivery for Cre-mediated excision of Glp1r in adult mice or tamoxifen-inducible mouse strains to circumvent developmental compensation.
Endogenous GLP-1 can regulate feeding in the short term by signaling satiety as well as by inhibiting gastric emptying (34,48). Because synthetic GLP1RAs have been engineered for prolonged biological activity, it is plausible that the actions of GLP1RA will mimic those of endogenous GLP-1 in addition to inducing visceral illness. In the current study, control animals ate significantly less food within 1 h of liraglutide injection, and this effect was maintained for 24 h. Of note, vGlut2ΔGlp1r mice also exhibited a blunted food intake reduction at the 4- and 24-h time points after liraglutide administration, suggesting a role for nonglutamatergic GLP-1R–expressing cells in some aspect of the anorectic response. Future meal pattern analyses and gastric emptying studies will clarify the role of specific neuronal subsets in these physiological responses.
Long-term liraglutide administration decreased food intake for only 3 days in control and vGATΔGlp1r mice, whereas body weight was reduced throughout the study. Persistent weight reduction in the setting of transient anorexia suggests establishment of a new body weight set point. This phenomenon is seen with multiple weight loss regimens (e.g., lorcaserin [49], vertical sleeve gastrectomy [50]), and the mechanism underlying this shared response requires additional investigation. Future studies are needed to determine whether this transient anorexia reflects cellular desensitization or changes in neuronal response patterns after long-term liraglutide administration.
Liraglutide induces a characteristic pattern of Fos expression in a mixture of GABAergic and glutamatergic cell groups; moreover, this pattern requires GLP-1Rs on vGlut2+ but not vGAT+ neurons, which supports the hypothesis that a population of GLP-1R–expressing glutamatergic neurons serves as a gateway to transduce the signal of peripheral liraglutide into the brain. To identify the critical population of neurons, we treated Glp1r-GFP reporter mice with liraglutide and examined Fos activation specifically in Glp1r+ cells. We found that the greatest overlap of GLP-1R-GFP expression and Fos activation was within the AP and to a lesser extent in the lPBN, suggesting that glutamatergic cells in these two brain regions may be particularly important for liraglutide action.
The AP is a sensory circumventricular organ known to play important roles in autonomic output, nausea, and the response to emetic drugs (51). It is activated in response to peripheral administration of GLP1RAs (24,52), and liraglutide binds the AP in a GLP-1R–dependent manner (35). The current results suggest that the AP is an important site mediating the anorectic effects of GLP1RAs. In contrast, AP lesions in rats did not decrease the short-term anorectic or long-term weight loss effects of GLP1RAs (35,53). Although the apparent contradiction of these results with the current study might be explained by differences in drug, dose, species, and/or experimental paradigm, this discrepancy suggests the existence of both AP-dependent and AP-independent responses to peripheral GLP1RAs. The lPBN could be a candidate site for AP-independent actions of GLP1RAs because 1) it is glutamatergic, 2) it expresses GLP-1R and is activated by peripheral GLP1RAs (23,24), and 3) direct lPBN infusion of exendin-4 decreases food intake and body weight (46). The NTS also has been identified as a potential site of action of GLP1RAs (10). Although the current study cannot completely exclude an NTS contribution, we found little liraglutide-induced Fos in NTS Glp1r-GFP cells. Furthermore, ISH revealed very few neurons expressing Glp1r mRNA in this region (data not shown). Determining the relative contribution of each of these regions to GLP1RA action will require future nucleus-specific manipulations.
In conclusion, we have used genetic mouse models to clarify the mechanisms whereby liraglutide decreases food intake and body weight. We found that short-term peripheral liraglutide injection activates several brain regions that comprise both GABAergic and glutamatergic neurons. By inactivating Glp1r in each population, we determined that liraglutide acts through GLP-1R exclusively expressed on glutamatergic, vGlut2-expressing neurons to activate a specific neuronal circuit, induce visceral illness, and reduce food intake and body weight in both the short and the long term. Moreover, GLP-1R expressed in GABAergic, vGAT-expressing neurons is not required for these effects. GLP-1R–expressing neurons in the AP and lPBN may be important direct targets through which peripherally administered liraglutide engages the CNS. Understanding the neural mechanisms by which liraglutide exerts its beneficial effects not only will help to elucidate its mechanism of action but also may reveal neural circuit components that might be exploited for more effective weight loss therapies.
Supplementary Material
Article Information
Acknowledgments. The authors acknowledge the assistance of all the members of D.P.O.’s laboratory (University of Michigan), especially Korri Burnett and Allen Zhu, for mouse colony maintenance and genotyping.
Funding. These studies were funded by the following National Institute of Diabetes and Digestive and Kidney Diseases grants: T32-DK-101357 (to J.M.A.), DK-082480 (to D.A.S.), DK-093848 and DK-107652 (to R.J.S.), DK-103703 (to S.D.L.), and DK-104999 (to D.P.O.).
Duality of Interest. J.M.A., D.A.S., R.J.S., and D.P.O. received funding from Novo Nordisk. This work was supported in part by Novo Nordisk, which also supplied the liraglutide. The investigators also received support from Boehringer Ingelheim (to D.A.S. and R.J.S.), Ethicon Endo-Surgery (to D.A.S. and R.J.S.), MedImmune (to D.A.S., R.J.S., and D.P.O.), Sanofi (to R.J.S.), Janssen/Johnson & Johnson (to R.J.S.), Kallyope (to R.J.S.), and Zafgen (to R.J.S.). D.A.S. has served on a scientific advisory board for Novo Nordisk. R.J.S. has served on scientific advisory boards for Ethicon Endo-Surgery, Daiichi Sankyo, Janssen, Orexigen, Novartis, Nestlé, Takeda, Boehringer Ingelheim, Kallyope, Scohia, Sanofi, and Novo Nordisk. R.J.S. is also a paid speaker for Ethicon Endo-Surgery. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. J.M.A. and H.P. performed experiments and acquired the data. J.M.A., D.A.S., R.J.S., and D.P.O. designed the research studies. J.M.A. and D.P.O. analyzed and interpreted the data and wrote the manuscript. D.A.S., R.J.S., R.B.C., and S.D.L. provided reagents. D.A.S., R.J.S., and S.D.L. reviewed and edited the manuscript. D.P.O. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented at the Keystone Symposia Conference, Copenhagen, Denmark, 9–13 May 2017.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db17-1385/-/DC1.
References
- 1.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 2007;87:1409–1439 [DOI] [PubMed] [Google Scholar]
- 2.Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 2012;8:728–742 [DOI] [PubMed] [Google Scholar]
- 3.Pi-Sunyer X, Astrup A, Fujioka K, et al.; SCALE Obesity and Prediabetes NN8022-1839 Study Group . A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015;373:11–22 [DOI] [PubMed] [Google Scholar]
- 4.van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WHM. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes 2014;38:784–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Bloemendaal L, Ten Kulve JS, la Fleur SE, Ijzerman RG, Diamant M. Effects of glucagon-like peptide 1 on appetite and body weight: focus on the CNS. J Endocrinol 2014;221:T1–T16 [DOI] [PubMed] [Google Scholar]
- 6.Sisley S, Gutierrez-Aguilar R, Scott M, D’Alessio DA, Sandoval DA, Seeley RJ. Neuronal GLP1R mediates liraglutide’s anorectic but not glucose-lowering effect. J Clin Invest 2014;124:2456–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology 2011;152:3103–3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 1999;403:261–280 [DOI] [PubMed] [Google Scholar]
- 9.Cork SC, Richards JE, Holt MK, Gribble FM, Reimann F, Trapp S. Distribution and characterisation of Glucagon-like peptide-1 receptor expressing cells in the mouse brain. Mol Metab 2015;4:718–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanoski SE, Hayes MR, Skibicka KP. GLP-1 and weight loss: unraveling the diverse neural circuitry. Am J Physiol Regul Integr Comp Physiol 2016;310:R885–R895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosal S, Packard AEB, Mahbod P, et al. Disruption of glucagon-like peptide 1 signaling in Sim1 neurons reduces physiological and behavioral reactivity to acute and chronic stress. J Neurosci 2017;37:184–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burmeister MA, Ayala JE, Smouse H, et al. The hypothalamic glucagon-like peptide 1 receptor is sufficient but not necessary for the regulation of energy balance and glucose homeostasis in mice. Diabetes 2017;66:372–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burmeister MA, Brown JD, Ayala JE, et al. The glucagon-like peptide-1 receptor in the ventromedial hypothalamus reduces short-term food intake in male mice by regulating nutrient sensor activity. Am J Physiol Endocrinol Metab 2017;313:E651–E662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vong L, Ye C, Yang Z, Choi B, Chua S Jr., Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 2011;71:142–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, Liberles SD. Sensory neurons that detect stretch and nutrients in the digestive system. Cell 2016;166:209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krashes MJ, Shah BP, Madara JC, et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 2014;507:238–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sutton AK, Pei H, Burnett KH, Myers MG Jr., Rhodes CJ, Olson DP. Control of food intake and energy expenditure by Nos1 neurons of the paraventricular hypothalamus. J Neurosci 2014;34:15306–15318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Amsterdam, Elsevier Academic Press, 2008 [Google Scholar]
- 19.Adams JM, Otero-Corchon V, Hammond GL, Veldhuis JD, Qi N, Low MJ. Somatostatin is essential for the sexual dimorphism of GH secretion, corticosteroid-binding globulin production, and corticosterone levels in mice. Endocrinology 2015;156:1052–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding HK, Teixeira CM, Frankland PW. Inactivation of the anterior cingulate cortex blocks expression of remote, but not recent, conditioned taste aversion memory. Learn Mem 2008;15:290–293 [DOI] [PubMed] [Google Scholar]
- 21.Baraboi ED, St-Pierre DH, Shooner J, Timofeeva E, Richard D. Brain activation following peripheral administration of the GLP-1 receptor agonist exendin-4. Am J Physiol Regul Integr Comp Physiol 2011;301:R1011–R1024 [DOI] [PubMed] [Google Scholar]
- 22.Parker JA, McCullough KA, Field BCT, et al. Glucagon and GLP-1 inhibit food intake and increase c-fos expression in similar appetite regulating centres in the brainstem and amygdala. Int J Obes 2013;37:1391–1398 [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto H, Lee CE, Marcus JN, et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest 2002;110:43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baggio LL, Huang Q, Brown TJ, Drucker DJ. A recombinant human glucagon-like peptide (GLP)-1-albumin protein (albugon) mimics peptidergic activation of GLP-1 receptor-dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis. Diabetes 2004;53:2492–2500 [DOI] [PubMed] [Google Scholar]
- 25.Buse JB, Rosenstock J, Sesti G, et al.; LEAD-6 Study Group . Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009;374:39–47 [DOI] [PubMed] [Google Scholar]
- 26.Parker LA, Rana SA, Limebeer CL. Conditioned nausea in rats: assessment by conditioned disgust reactions, rather than conditioned taste avoidance. Can J Exp Psychol 2008;62:198–209 [DOI] [PubMed] [Google Scholar]
- 27.Kanoski SE, Rupprecht LE, Fortin SM, De Jonghe BC, Hayes MR. The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology 2012;62:1916–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu YL, Malik N, Sanger GJ, Friedman MI, Andrews PLR. Pica--a model of nausea? Species differences in response to cisplatin. Physiol Behav 2005;85:271–277 [DOI] [PubMed] [Google Scholar]
- 29.Kinzig KP, D’Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci 2002;22:10470–10476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMahon LR, Wellman PJ. PVN infusion of GLP-1-(7-36) amide suppresses feeding but does not induce aversion or alter locomotion in rats. Am J Physiol 1998;274:R23–R29 [DOI] [PubMed] [Google Scholar]
- 31.Dossat AM, Lilly N, Kay K, Williams DL. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci 2011;31:14453–14457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lachey JL, D’Alessio DA, Rinaman L, Elmquist JK, Drucker DJ, Seeley RJ. The role of central glucagon-like peptide-1 in mediating the effects of visceral illness: differential effects in rats and mice. Endocrinology 2005;146:458–462 [DOI] [PubMed] [Google Scholar]
- 33.Abbott CR, Monteiro M, Small CJ, et al. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res 2005;1044:127–131 [DOI] [PubMed] [Google Scholar]
- 34.Krieger JP, Arnold M, Pettersen KG, Lossel P, Langhans W, Lee SJ. Knockdown of GLP-1 receptors in vagal afferents affects normal food intake and glycemia. Diabetes 2016;65:34–43 [DOI] [PubMed] [Google Scholar]
- 35.Secher A, Jelsing J, Baquero AF, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest 2014;124:4473–4488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rüttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology 2009;150:1174–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng H, Stornetta RL, Agassandian K, Rinaman L. Glutamatergic phenotype of glucagon-like peptide 1 neurons in the caudal nucleus of the solitary tract in rats. Brain Struct Funct 2015;220:3011–3022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Llewellyn-Smith IJ, Reimann F, Gribble FM, Trapp S. Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience 2011;180:111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaykema RP, Newmyer BA, Ottolini M, et al. Activation of murine pre-proglucagon-producing neurons reduces food intake and body weight. J Clin Invest 2017;127:1031–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baggio LL, Huang Q, Brown TJ, Drucker DJ. Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology 2004;127:546–558 [DOI] [PubMed] [Google Scholar]
- 41.Hayes MR, Skibicka KP, Grill HJ. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology 2008;149:4059–4068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scrocchi LA, Brown TJ, MaClusky N, et al. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat Med 1996;2:1254–1258 [DOI] [PubMed] [Google Scholar]
- 43.Turton MD, O’Shea D, Gunn I, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 1996;379:69–72 [DOI] [PubMed] [Google Scholar]
- 44.Meeran K, O’Shea D, Edwards CMB, et al. Repeated intracerebroventricular administration of glucagon-like peptide-1-(7-36) amide or exendin-(9-39) alters body weight in the rat. Endocrinology 1999;140:244–250 [DOI] [PubMed] [Google Scholar]
- 45.Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology 2009;150:2654–2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alhadeff AL, Baird JP, Swick JC, Hayes MR, Grill HJ. Glucagon-like peptide-1 receptor signaling in the lateral parabrachial nucleus contributes to the control of food intake and motivation to feed. Neuropsychopharmacology 2014;39:2233–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barrera JG, Jones KR, Herman JP, D’Alessio DA, Woods SC, Seeley RJ. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci 2011;31:3904–3913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology 2009;150:1680–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomsen WJ, Grottick AJ, Menzaghi F, et al. Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: in vitro and in vivo pharmacological characterization. J Pharmacol Exp Ther 2008;325:577–587 [DOI] [PubMed] [Google Scholar]
- 50.Stefater MA, Pérez-Tilve D, Chambers AP, et al. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology 2010;138:2426–2436, 2436.e1–2436.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Price CJ, Hoyda TD, Ferguson AV. The area postrema: a brain monitor and integrator of systemic autonomic state. Neuroscientist 2008;14:182–194 [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto H, Kishi T, Lee CE, et al. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci 2003;23:2939–2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baraboi ED, Smith P, Ferguson AV, Richard D. Lesions of area postrema and subfornical organ alter exendin-4-induced brain activation without preventing the hypophagic effect of the GLP-1 receptor agonist. Am J Physiol Regul Integr Comp Physiol 2010;298:R1098–R1110 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.