Abstract
Insulin-dependent diabetes may occur in patients with cancers who are treated with checkpoint inhibitors (CPIs). We reviewed cases occurring over a 6-year period at two academic institutions and identified 27 patients in whom this developed, or an incidence of 0.9%. The patients had a variety of solid-organ cancers, but all had received either anti–PD-1 or anti–PD-L1 antibodies. Diabetes presented with ketoacidosis in 59%, and 42% had evidence of pancreatitis in the peridiagnosis period. Forty percent had at least one positive autoantibody and 21% had two or more. There was a predominance of HLA-DR4, which was present in 76% of patients. Other immune adverse events were seen in 70%, and endocrine adverse events in 44%. We conclude that autoimmune, insulin-dependent diabetes occurs in close to 1% of patients treated with anti–PD-1 or –PD-L1 CPIs. This syndrome has similarities and differences compared with classic type 1 diabetes. The dominance of HLA-DR4 suggests an opportunity to identify those at highest risk of these complications and to discover insights into the mechanisms of this adverse event.
Introduction
Monoclonal antibodies (mAbs) that block immune inhibitory ligands CTLA-4 and PD-1, known as immune checkpoint inhibitors (CPIs), have revolutionized the treatment of cancers that are resistant to conventional cancer therapies. As a result, life expectancy of patients with malignancies such as melanoma, lung cancer, renal cell carcinoma, and several other cancers has significantly improved (1).
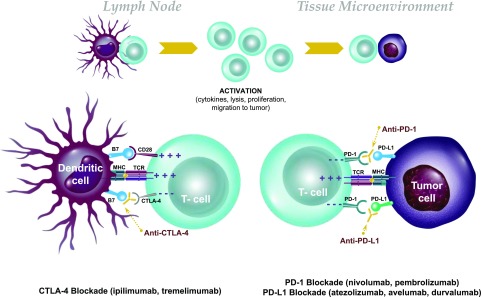
Tolerance to autoantigens expressed in the peripheral tissues, including endocrine organs, is maintained first by the deletion of highly autoreactive T and B cells from the immune repertoire during lymphocyte development and then by control mechanisms that can prevent autoreactive cells that have escaped deletion in the thymus from reactivation in the periphery. Some mechanisms are intrinsic to the immune cell, such as T-cell exhaustion, anergy, or senescence, whereas others are extrinsic. The CTLA-4 and PD-1 immune checkpoints play an integral role in maintenance of immune tolerance to self through negative regulation of the immune system (Fig. 1). Within the lymph tissue, CTLA-4 is present in naive T cells as well as regulatory T cells and binds to CD80/86 on antigen-presenting cells. Binding of CTLA-4 to CD80/86 leads to inhibition of the immune response. CTLA-4 acts as a competitive inhibitor of the key costimulatory molecule CD28, which also binds CD80/86. During normal naive T-cell activation, the levels of CD28 on the cell surface exceed those of CTLA-4, and CD28-mediated costimulation proceeds. However, as T-cell activation unfolds, the CTLA-4 levels are upregulated at the cell surface, and CTLA-4 outcompetes CD28, inhibiting the T-cell response.
Figure 1.
Immunologic actions of CPIs. Top: Blockade of negative costimulatory signals (checkpoints) leads to activation of T cells and endows their ability to kill tumor cells. Bottom: The most widely used strategies block CTLA-4, which is expressed on activated T cells and binds to B7.1 (CD80) and B7.2 (CD86), which is expressed on antigen-presenting cells (e.g., dendritic cells). In addition, other mAbs have targeted the interaction between PD-1, expressed on T cells, and PD-L1, expressed on tumor and other cells.
In addition to CTLA-4, another negative regulator of T-cell activation is the cell surface receptor PD-1. PD-1 is generally not expressed on naive T cells but rather on chronically activated T cells in peripheral tissues, particularly CD8+ T cells. By binding to its ligands PD-L1 and PD-L2, which are expressed on stromal cells, tumor cells, and antigen-presenting cells, PD-1 transmits negative signaling events in such T cells and thus promotes inhibition of the immune response (2).
The role of these peripheral tolerance mechanisms has been shown in mouse and human disease. Mice with genetic deletion of CTLA-4 develop generalized tissue infiltration by self-reactive T cells, leading to severe systemic autoimmunity, whereas mice with genetic deletion of PD-1 also develop distinct autoimmune diseases (3,4). Administration of anti–PD-1 and –PD-L1 antibodies to NOD mice results in rapid onset of diabetes (5) and reversal of tolerogenic therapies, such as anti-CD3 and tolerogenic peptide infusion (6).
CPIs are effective in reversing the mechanisms that normally block immune responses to malignancy and in maintaining control of antitumor immunity (7–9). Treatment with CPIs has shown improved prognosis over standard-of-care therapies, leading to U.S. Food and Drug Administration approval for seven cancers and for malignancies with microsatellite instability or DNA mismatch repair mutations (7,9). These therapies, such as the anti–CTLA-4 mAbs ipilimumab and tremelimumab (in trials), the anti–PD-1 mAbs nivolumab and pembrolizumab, and the anti–PD-L1 mAbs atezolizumab, avelumab, and durvalumab, benefit patients by allowing for activation of tumor-reactive T lymphocytes.
CTLA-4 mutations in humans have been linked to multiple endocrine diseases, including type 2 diabetes, Graves disease, hypothyroidism, and Addison disease (10–13). Autoimmune endocrine diseases are seen in individuals with mutations of genes affecting thymic development (such as AIRE, APS1) or regulatory T cells (such as FoxP3, IPEX) (14,15). It is not surprising, therefore, that the treatment of patients with CPIs has led to autoimmunity in endocrine tissues (Fig. 1). Thyroiditis, hypophysitis with secondary adrenal insufficiency, secondary hypothyroidism and gonadal deficiency, primary adrenal insufficiency, and insulin-dependent diabetes have been reported following anti–CTLA-4, anti–PD-1, and/or anti–PD-L1 mAbs (16,17). These autoimmune syndromes occur with varying frequency: thyroiditis in 2.9–3.3% of patients treated with anti–PD-1 treatment (18,19) and hypophysitis in 0.5–17% of those treated with ipilimumab (18,19), with primary adrenal insufficiency and insulin-dependent diabetes being uncommon. Combination treatment is reported to induce endocrine immune-related adverse events (irAEs) in nearly half of patients (18–20). It is unclear why certain individuals develop these adverse events. Furthermore, the mechanisms behind these irAEs and their relationship to spontaneous autoimmune disease are not yet understood, nor is why certain classes of CPIs cause certain irAEs.
The literature describing autoimmune diabetes thus far is limited to case reports with variable presentations. The overall frequency of insulin-dependent diabetes as an irAE is reported to be a relatively low 0.2–11%, but the events have high clinical significance (18,19). The subjects are older than those presenting with classic type 1 diabetes, often require admission to intensive care units for treatment, and require injections of exogenous insulin for metabolic control.
Biomarkers that could identify which individuals are likely to develop diabetes would be valuable as they might allow clinicians to prevent hospitalizations or even might suggest therapies that might prevent overt onset of diabetes. Moreover, the mechanisms of this form of diabetes may identify mechanisms of other forms of insulin-dependent diabetes, including spontaneous autoimmune type 1 diabetes.
Here, we describe the largest case series to date, with data from two academic institutions, with the goal of better defining this uncommon irAE and common identifying attributes that might lead to clinical and mechanistic insights into the disease.
Research Design and Methods
Subject Identification and Case Definition
Patients were identified following inpatient consult or outpatient referral to Endocrinology at Yale New Haven Hospital and University of California, San Francisco (UCSF) Medical Center. The number of patients treated with CPIs over the same period was determined from the electronic health record (EPIC; Epic Systems Corp.). Patients who met the following criteria were included in this case series:
-
New onset of hyperglycemia requiring exogenous insulin treatment in patients
Without a history of diabetes or
With a history of type 2 diabetes who became insulin requiring and showed deterioration in glycemic control that was previously well controlled on oral medications alone.
Continued requirement for insulin treatment for more than 1 month with evidence of insulin deficiency either through presentation with diabetic ketoacidosis (DKA) or low or absent random C-peptide.
This study was approved by the institutional review boards at Yale University and the UCSF.
Autoantibodies
Diabetes autoantibodies (glutamic acid decarboxylase [GAD]65, islet antigen 2 [IA-2/ICA-512], zinc transporter 8 [ZnT8], insulin autoantibodies [IAA]) were measured in the clinical laboratories at the Yale New Haven Hospital and UCSF clinical laboratories. Biochemical autoantibodies were measured at the Department of Pathology, Immunology and Laboratory Medicine, University of Florida, in 15 of the patients; islet cell antibodies were measured by immunofluorescence.
HLA Typing
For 17 patients, HLA typing was performed at the Yale University Histocompatibility and Immune Evaluation Laboratory by reverse sequence-specific oligonucleotide HLA typing method (LIFECODES HLA SSO Typing Kit). For five patients (patients 3, 8, 13, 14, and 26), HLA-A2 was identified by flow cytometric screening using mAb BB7.1 (Abcam, Cambridge, MA). For four patients (patients 3, 4, 8, and 14), HLA-DR4 sequencing was performed by PCR with primers specific for HLA-DR:0401.
Review of Cases at UCSF Medical Center and Yale New Haven Hospital
We identified 27 cancer patients who presented with the acute onset of insulin-deficient diabetes (5 cases were previously reported) who had been treated with CPIs over the period of 2012–2018, as detailed in Table 1. Over that same period, a total of 2,960 patients received CPI therapy, indicating a prevalence of approximately 0.9%.
Table 1.
Clinical histories of patients with CPI-induced insulin-dependent diabetes
| Patient | Age, years | Sex | Type of cancer | CPI | Cycles of treatment at diagnosis, n | Other therapies | Other CPI irAE | Relevant PMH |
|---|---|---|---|---|---|---|---|---|
| 1 |
57 |
F |
Melanoma |
I, N, I/N |
3 |
None |
Thyroiditis, hypothyroidism, hypopituitarism, hepatitis |
NC |
| 2 |
61 |
M |
Melanoma |
I/N |
3 |
None |
Hypopituitarism, nephritis |
NC |
| 3 |
55 |
F |
Ocular melanoma |
I/N, I |
2 |
None |
Thyroiditis, hypothyroidism, pancreatitis |
Hashimoto thyroiditis |
| 4 |
64 |
F |
Melanoma |
P |
5 |
None |
Hypothyroidism, pancreatitis, hepatitis, arthritis |
Hashimoto thyroiditis |
| 5 |
80 |
F |
NSCLC |
N |
20 |
Carboplatin, gemcitabine |
None |
Sarcoidosis |
| 6 |
67 |
M |
RCC |
N |
10 |
Axitinib, sunitinib |
None |
Hypercalcemia |
| 7 |
64 |
F |
Melanoma |
I/N |
1 |
None |
Thyroiditis, hypothyroidism, hepatitis |
NC |
| 8 |
63 |
M |
RCC |
N |
78 |
IL-2, bevacizumab, IFN |
Hypothyroidism |
NC |
| 9 |
67 |
M |
GI adenocarcinoma |
I/N, N |
6 |
Irinotecan, docetaxel |
Hepatitis, pancreatitis |
NC |
| 10 |
83 |
M |
Melanoma |
I, P |
11 |
None |
None |
Prediabetes |
| 11 |
63 |
F |
RCC |
Atezo |
1 |
IL-2, IFN |
Hypothyroidism, pancreatitis |
NC |
| 12 |
64 |
F |
Ocular melanoma |
I, P |
16 |
Imatinib |
Hypothyroidism, vitiligo, colitis |
NC |
| 13 |
64 |
M |
NSCLC |
I/N |
1 |
None |
Thyroiditis, pancreatitis |
NC |
| 14 |
83 |
F |
NSCLC |
N |
3 |
None |
None |
NC |
| 15 |
49 |
M |
Pancreatic cancer (Lynch syndrome) |
P |
24 |
None |
Colitis, pancreatitis |
Vitamin D deficiency |
| 16 |
68 |
F |
Melanoma |
I/N |
2 |
Fluorouracil, leucovorin, irinotecan, oxaliplatin |
Hypothyroidism, pneumonitis |
Hashimoto thyroiditis |
| 17 |
64 |
M |
Melanoma |
P, I/N, N |
12 |
None |
None |
NC |
| 18 |
53 |
M |
Melanoma |
P, I/N, N |
3 |
None |
Hypothyroidism, hypophysitis, hepatitis, vitiligo |
Prediabetes, hypothyroidism |
| 19 |
87 |
F |
Ocular melanoma |
P |
8 |
None |
None |
Prediabetes, hypothyroidism |
| 20 |
62 |
M |
Neuroendocrine tumor of the colon (Lynch syndrome) |
20 |
Carboplatin, etoposide |
Thyroiditis, myocarditis, hepatitis, arthritis |
Prediabetes, hypothyroidism |
|
| 21 |
70 |
M |
SCC (tongue) |
P |
12 |
None |
Polyarthralgia, pneumonitis |
Hypothyroidism |
| 22 |
64 |
M |
Cholangiocarcinoma |
P, GM-CSF |
4 |
Gemcitabine, cisplatin |
Myasthenia gravis |
Hypothyroidism |
| 23 |
60 |
M |
Melanoma |
N, epacadostat |
10 |
None |
None |
NC |
| 24 |
60 |
M |
Melanoma |
I/N, N |
12 |
None |
Colitis |
NC |
| 25 |
79 |
M |
Melanoma |
P |
2 |
None |
Neurotoxicity |
NC |
| 26 |
58 |
M |
SCLC |
N |
1 |
Carboplatin, etoposide, paclitaxel |
Limbic encephalitis |
Type 2 diabetes, lung adenocarcinoma |
| 27 | 80 | M | NSCLC | N | 14 | Carboplatin-pemetrexed, pemetrexed maintenance | None | Type 2 diabetes, toxic MNG |
Atezo, atezolizumab; GI, gastrointestinal; GM-CSF, granulocyte-macrophage colony-stimulating factor; I, ipilimumab; INF, interferon; Lynch syndrome, hereditary nonpolyposis colorectal cancer; MNG, multinodular goiter; N, nivolumab; NC, noncontributory; NSCLC, non–small-cell lung cancer; P, pembrolizumab; PMH, past medical history; RCC, renal cell carcinoma; SCC, squamous cell carcinoma; SCLC, small-cell lung cancer.
CPI Therapy
The majority of the patients had been treated with CPI for metastatic melanoma (cutaneous, 11; ocular, 3), which may reflect the earlier introduction of the CPI therapies and clinical trials for the treatment of these malignancies. The remaining patients had been diagnosed with six other types of malignancies.
The patients were exposed to different individual and combination CPIs. Fourteen patients received only anti–PD-1 mAb (nivolumab or pembrolizumab) and one received only anti–PD-L1 mAb (atezolizumab). The most common combination was nivolumab and ipilimumab. Interestingly, all of the case subjects were exposed to anti–PD-1 or anti–PD-L1 therapy, but many were not treated with an anti–CTLA-4 mAb. There were no cases of CPI-induced diabetes in patients treated with anti–CTLA-4 mAb alone.
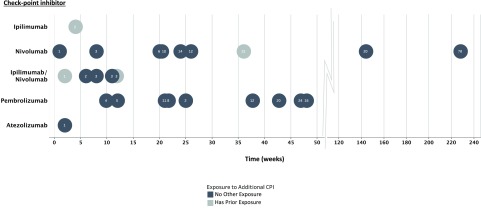
The median time of onset of CPI-induced diabetes was 20 weeks after the first treatment cycle, but the range was wide (1–228 weeks). The number of CPI treatments given prior to presentation also varied widely (1–78 cycles). The patient with the longest time from treatment to diagnosis (228 weeks and 78 cycles) had a treatment holiday between two rounds of therapy.
Clinical and Laboratory Features
There were 17 men and 10 women with the average age at diabetes diagnosis of 66 years. All patients are Caucasian non-Hispanic, except for patients 18, 22, and 27, who are Hispanic, Asian, and other and non-Hispanic, respectively.
Four of the patients had a personal history of prediabetes, and two of type 2 diabetes. Prior to treatment with CPI, two patients with type 2 diabetes were well controlled using 1,000 mg of metformin daily or less. There was a personal history of other autoimmune diseases in 30% (8/27) of patients. Although no patients had a first-degree family history of type 1 diabetes, 2 of the patients had a second-degree family history of type 1 diabetes, 10 of type 2 diabetes, 1 of both type 1 diabetes and type 2 diabetes, and 1 of unspecified diabetes. Five of the patients were being treated with steroids (but ≤10 mg/day of prednisone) for other irAEs at the time of diabetes diagnosis.
Fifty-nine percent of the patients (16/27) presented with DKA, with an average glucose of 653 mg/dL (range 240–1,765). The average BMI was 26.07 kg/m2 (range 17–40). The clinical presentations were acute based on symptoms and review of the random glucoses (Fig. 2). However, the average A1C was 7.95% (63 mmol/mol) at diagnosis (range 6.0–10.5% [42–91 mmol/mol], n = 25), suggesting that some degree of hyperglycemia had been present prior to the acute presentation.
Figure 2.
Timing of hyperglycemia after CPI treatment. The symbols indicate the weeks between the initial treatment with CPI and the time of diagnosis of insulin-dependent diabetes. Black symbols indicate exposure to a single CPI indicated on the y-axis. Gray symbols indicate whether additional CPIs were used. The numbers in the circles refer to the treatment cycles that were administered.
In most subjects (23/27; 85%), there was rapid loss of β-cell function evidenced by the acute progression to hyperglycemia and low or undetectable levels of C-peptide at time of diagnosis (i.e., <1.1 ng/mL; normal = 1.1–4.4 ng/mL). We considered whether the autoimmune destruction in the islet involved other islet cells but found that random glucagon levels were within the normal range (average 98.5 pg/mL, range 79–136 pg/mL; normal range <134 pg/mL) in a small sample of 4 patients in whom glucagon was measured.
Interestingly, the levels of lipase and/or amylase were elevated (2- to >10-fold above upper limit of normal) in 32% of the patients on the day of diagnosis, and in one, the enzymes were more than eightfold elevated from 1 month prior to diagnosis until presentation with DKA. Patient 11 had pancreatic edema identified by computed tomography of the abdomen obtained at diabetes diagnosis. This observation suggests that ongoing pancreatic inflammation may be a factor in the precipitation of the disease.
The average insulin use at the first follow-up visit after diagnosis was 0.56 units/kg/day, suggesting insulin sensitivity similar to patients with type 1 diabetes.
Immunologic Features
We measured at least 1 autoantibody in 25 of the patients (Table 2) and 3 or more in 24 of the patients. Of these 25, at least 1 autoantibody was positive in 40% (10/25), and 2 or more autoantibodies were positive in 21% (5/24) of cases. We also found a single positive autoantibody in 25% (3/12) of patients who were treated with CPI that did not develop diabetes but had similar cancer diagnoses. None of the patients without diabetes had more than one positive autoantibody.
Table 2.
Autoantibodies in patients with CPI-induced insulin-dependent diabetes
| Frequency of autoantibodies | |||||||
|---|---|---|---|---|---|---|---|
| CPI-treated patients with diabetes, n/N | CPI-treated patients without diabetes, n/N | ||||||
| Anti-GAD65 | 9/25 | 2/12 | |||||
| Anti–IA-2 | 5/24 | 1/12 | |||||
| Anti–ZnT8 | 2/20 | 0/12 | |||||
| Islet cell antibody |
2/19 |
0/12 |
|||||
| Autoantibodies before and after CPI treatment | |||||||
| Autoantibodies before treatment |
Autoantibodies after treatment |
||||||
| GAD |
IA-2 |
ZnT8 |
GAD |
IA-2 |
ZnT8 |
IAA |
|
| Patient 5 | NEG | NEG | NEG | NEG | NEG | N/A | NEG |
| Patient 9 | POS | POS | POS | POS | NEG | N/A | NEG |
| Patient 10 | NEG | NEG | NEG | POS | POS | NEG | POS |
We investigated possible associations between antibody status and other clinical features. Patients with any positive type 1 diabetes autoantibody at the time of presentation develop CPI-induced diabetes after fewer cycles than those without autoantibodies (Wilcoxon rank sum test, median cycles 2.5 for those with any positive autoantibody and 13 for those with negative autoantibodies, P = 0.024). There was also a shorter number of weeks on CPI therapy, 14 for those with any positive autoantibody and 21 for those with negative autoantibodies, but this did not reach statistical significance (P = 0.18). Presentation with DKA, age, and BMI were not associated with autoantibodies.
In three patients, autoantibodies before treatment with CPI and after diagnosis of diabetes were tested (Table 2). In one, autoantibodies were present before and after treatment. A second had negative autoantibodies prior to treatment, and two of the three originally tested autoantibodies became positive after treatment. The third was negative before and after treatment.
HLA Genotypes
HLA genotypes were determined in 23 of the 27 subjects, and the haplotype frequencies are shown in Table 3. There was a predominance of HLA-DR4 (16/21, 76%), which is significantly higher than reported frequencies in U.S. Caucasians (17.3%; χ2 test, P < 0.0001) or even patients with spontaneous type 1 diabetes (χ2 test, P = 0.002) (21). HLA-A2 also was frequent (59%, 13/22), but not significantly different from the reported frequencies in U.S. Caucasians (47.4%). HLA-DR3, which is also increased in frequency among patients with type 1 diabetes (34.1%), was at a similar frequency in the CPI diabetes group (35%, 6/17). HLA-DQ8 (DQB1*0302), which is in linkage disequilibrium with HLA-DR4 and is also increased in type 1 diabetes, was found in 38% (6/16) of the patients with extended sequencing and the frequency is similar to patients with type 1 diabetes (χ2 test, P = 0. 77) (21). Two of the patients were DR3/4 heterozygotes. None of the subjects expressed the type 1 diabetes protective allele HLA-DR2.
Table 3.
HLA genotypes in patients with CPI-induced diabetes and in patients treated with CPIs who did not develop diabetes
| HLA genotype | Patients, n/N (%) | |
|---|---|---|
| Patients with diabetes |
A*02:01 (A2) |
13/22 (59) |
| DR17 |
7/17 (41.1) |
|
| DR7 |
3/17 (17.6) |
|
| DR11 |
6/17 (35) |
|
| DR12 |
1/17 (5.8) |
|
| DR3 |
6/17 (35) |
|
| DR4 |
16/21 (76) |
|
| Patients without diabetes | A*02:01 (A2) |
5/9 (56) |
| DR3 |
1/9 (11) |
|
| DR4 | 2/9 (22) |
n/N, positive/total N of patients checked.
Relationship Between Autoimmune Diabetes, Other Endocrinopathies, and Tumor Responses to the CPIs
After diabetes presentation, 37% (10/27) of the patients continued CPI therapy (Table 1). Overall 73% (8/11) of those patients with diabetes and cutaneous melanoma had partial or complete responses to CPI therapy defined by Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria (Table 4). These response rates compare favorably with those reported previously: for anti–PD-1 mAb (nivolumab) alone, the response rate in the frontline setting is 43.7%, and for combined ipilimumab/nivolumab in the frontline setting it is 57.6% (22).
Table 4.
Tumor responses in patients treated with CPIs
| Type of cancer | Patients, n/N (%) |
|---|---|
| Cutaneous melanoma |
8/11 (73) |
| Ocular melanoma |
1/3 (33) |
| Non–small-cell lung cancer |
3/4 (75) |
| Renal cell carcinoma |
3/3 (100) |
| Other cancers* | 4/6 (67) |
n/N, n with diabetes with partial or complete tumor response/total N of patients.
*Other cancers include gastrointestinal adenocarcinoma, cholangiocarcinoma, small-cell lung cancer, primitive neuroectodermal tumor (PNET), Lynch syndrome (hereditary nonpolyposis colorectal cancer), pancreatic cancer, squamous cell carcinoma (tongue).
Seventy percent (19/27) of the patients with CPI-induced diabetes had other irAEs, and 44% (12/27) had an endocrine irAE prior or concurrent to the development of diabetes. The majority (11/12) had primary thyroid dysfunction that presented as hypothyroidism or thyroiditis (thyrotoxicosis followed by hypothyroidism).
Clinical and Biologic Significance of CPI-Induced Diabetes
Treatment with CPIs has shown improved prognosis over standard-of-care therapies and has been approved for seven cancers and for malignancies with microsatellite instability or DNA mismatch repair mutations. These therapies benefit patients by allowing for activation of tumor-reactive T lymphocytes.
CPI-induced insulin-dependent diabetes is an uncommon but clinically significant event. Since our first description of CPI-induced diabetes (23), there have been more than 39 cases of CPI-induced diabetes reported in 22 different publications (for example, 24–35). Melanoma was the most frequently represented form of cancer, and the most commonly used CPIs were either PD-1 or PD-L1 mAbs. DKA was the presentation in 81%, indicating the severe nature of this adverse event. Also consistent with our findings was the frequent co-occurrence of thyroid disease (28%). Although these cases also reported frequent anti-GAD65 antibodies (47%), HLA-DR4 was only present in 40% (8/20), which is still higher than the expected rate in the general population (12.7%).
Similar to these previous reports, we defined CPI-induced diabetes as new-onset insulin-dependent diabetes following treatment of a malignancy with a CPI. In our series from two academic institutions, the overall incidence of this form of diabetes was 0.9% among those treated with CPIs in the 6-year period that we examined. This may be an underestimate as patients at both Yale University and UCSF received care in multiple medical settings. The time of diabetes onset can be long after the initial CPI treatment, and therefore the link of new-onset diabetes to the CPI may not have been appreciated in some cases. The presence of preexisting type 2 diabetes, which is a common diagnosis in this same age range, has a different pathogenesis but does not preclude the development of CPI-induced diabetes. Indeed, two of our patients had this prior history but developed new insulin dependence and worsening of metabolic control.
There are clinical and laboratory features of this form of diabetes that are similar to but also clearly different from spontaneous type 1 diabetes. Most striking is the difference in age of the time of onset, which was 66 years. The time between initial exposure to CPI and clinical presentation with diabetes was variable but more rapid than thought for type 1 diabetes. In two patients, in particular, diabetes diagnosis occurred after prolonged exposure to CPI therapy. Within the existing literature, the median time from CPI initiation to presentation with diabetes was shorter, 6.2 weeks, but the range was still large (1–52 weeks). The time to diabetes presentation was longer than for other irAEs, such as thyroiditis, which on average occurs between 3 and 8 weeks after treatment (36,37).
The loss of β-cells is acute, as illustrated by the rapid progression from normoglycemia to hyperglycemia. In Type 1 Diabetes TrialNet studies of new-onset type 1 diabetes, 88% had a stimulated C-peptide level of at least 0.6 ng/mL (0.2 pmol/mL), but in our subjects, random C-peptide levels were undetectable or very low in 88% at the initial onset of hyperglycemia (38). The A1C levels at the time of diagnosis are similar to those found in patients with new-onset type 1 diabetes (in our case series [7.95%, or 63 mmol/mol] and in the reported cases [7.7%, or 61 mmol/mol]). It may be that the A1C elevation in these patients is due to significant hyperglycemia over a short period rather than a more mild hyperglycemia over a longer period, e.g., one subject had an A1C of 5.8% (40 mmol/mol) 1 week prior to diagnosis and an A1C of 6.8% (51 mmol/mol) at the time of diagnosis.
About 40% of our subjects had biochemical autoantibodies that are found in spontaneous type 1 diabetes. This is similar to previous reports in which anti-GAD65 autoantibody was positive in 47% (18/38) but different from spontaneous type 1 diabetes where over 95% have developed at least one positive autoantibody by the time of diagnosis (39). The islet cell antibody assay was used to identify additional targets of the immune response in the islets, but most subjects who had antibody positivity had autoantibodies to known antigens. In addition, random glucagon levels were not reduced, suggesting that α-cells had not been affected. Effects on B cells in CPI-treated patients who develop irAEs have been reported by others, and a role for them in spontaneous type 1 diabetes has been shown (40,41). Our data are consistent with a role for this arm of the adaptive immune response.
HLA typing revealed a striking predominance of HLA-DR4–positive cases. Other spontaneous type 1 diabetes high-risk alleles were not overrepresented, including DR3, DQ2, and DQ8. The frequency of the HLA-DR4 genotypes was higher than in the background population but was also higher compared with patients with type 1 diabetes in which 42% were reported to be positive for any of the DR4 alleles (χ2 test, P < 0.001). Interestingly, we did not find a dominance of HLA-DR4 among the CPI-treated patients with diabetes. The very high rates of DR4 in this group warrants further studies with more extended cohorts to determine whether prescreening for these HLA alleles should be considered prior to initiation of CPI treatment.
These affected patients received a number of other medications, e.g., glucocorticoids, IL-2, and interferon, and it is possible that those medications contributed to the diabetes presentations, particularly because of the relatively long time interval between CPI exposure and diabetes onset. High-dose IL-2 has been associated with deterioration in C-peptide levels in patients with new-onset type 1 diabetes (42). Interferon-α mediates human β-cell overexpression of HLA class I, endoplasmic reticulum stress, and β-cell apoptosis in humans, hallmarks of early type 1 diabetes development (43). It is unlikely that glucocorticoids played a significant role in diabetes presentation in these patients, as they are not a cause of insulin deficiency.
At this time, there are no treatments that are known to stop the development of this irAE, and patients who develop diabetes do not undergo spontaneous remission, which may occur in some patients with CPI-induced thyroiditis. Glucocorticoid treatment did not reverse the diabetes in one patient treated with high (50 mg/day) or three patients treated with low (10 mg/day) doses of prednisone.
CPI-induced diabetes was only seen in patients that had received anti–PD-1 or –PD-L1 therapies, which is consistent with most other reports in the literature. This predilection for affecting particular endocrine organs with certain CPI classes is notable and may hint at the mechanism of the irAEs. Overall, endocrine adverse events are more common with combination therapies than monotherapies, but thyroid dysfunction is more frequent with anti–PD-1/L1 mAbs compared with anti–CTLA-4 mAbs and hypophysitis is more common with ipilimumab. The reasons for the different rates of irAE with different CPIs are not known but may involve the response of the target tissue to injury or inflammation or the effects of the CPIs on a repertoire of autoreactive T cells. Expression of CTLA-4 on pituitary cells is associated with activation of complement after binding of anti–CTLA-4 antibody (ipilimumab) (44). We previously reported finding increased expression of PD-L1, but not CD80 or CD86, on β-cells from NOD mice during the progression of autoimmune diabetes (45). The rate of pancreatitis in those with DKA exceeds the reported rates in the general population of CPI-treated patients (46,47). We speculate that blockade of the cellular response to inflammatory mediators, possibly resulting from pancreatitis or other inflammatory processes, may contribute to the disease development and explain the absence of diabetes in patients treated with anti–CTLA-4 mAb, whose ligands are CD80 and CD86 alone (6). A similar mechanism has been suggested for protection of cardiac tissue and the development of myocarditis with anti–PD-1 mAb (48).
Finally, the mechanisms that lead to CPI-induced diabetes are expected to reflect the same mechanisms involved in the antitumor responses. In this regard, the tumor response rate was satisfactory in our patients, but our sample size is too small for comparison and a suitable control group is not available at this time. Nonetheless, elucidating these mechanisms may identify strategies for prevention of autoimmunity without inhibiting the anticancer activity of the therapy and for treatment of type 1 diabetes.
In summary, we have identified features of CPI-induced diabetes, the recognition of which is increasing with wider use of these drugs to treat cancers. Glucose levels and, in patients with known type 2 diabetes, A1C levels should be followed carefully in cancer patients treated with CPIs and appropriate referrals instituted as suggested. The providers should be alarmed and check baseline glucose prior to the initiation of treatment in all patients, as suggested in the consensus recommendations for management of CPI-induced diabetes by the Society for Immunotherapy of Cancer Toxicity Management Working Group (49). Our studies provide insight into the mechanisms that may be involved in this new form of insulin-deficient diabetes. CPI treatment may induce autoantibodies in some, but in those patients, particularly those who are HLA-DR4–positive, β-cell killing may progress. In addition to their importance in identifying individuals at risk for this outcome, studies of this form of diabetes may shed light on immune mechanisms that drive spontaneous type 1 diabetes.
Supplementary Material
Article Information
Acknowledgments. The authors thank Doreen Sese (Yale University), Nalini Vudattu (Yale University), and Hideki Ogura (Hyogo College of Medicine) for assistance with HLA typing and William Winter and David Pittman (University of Florida) for analysis of autoantibodies. They also thank the Yale SPORE (Specialized Programs of Research Excellence) in Skin Cancer team for providing serum samples.
Funding. This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK057846 and UC4 DK116290), the Leona M. and Harry B. Helmsley Charitable Trust (2018PG-T1D059), and JDRF.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.M.S. and Z.Q. wrote the manuscript and researched data. A.L.P., P.L.C., H.K., S.A.W., S.G., M.S., A.Y., R.R., and J.L. reviewed and edited the manuscript. J.A.B., M.A., and K.C.H. contributed to the discussion and reviewed and edited the manuscript.
Prior Presentation. Parts of this article were presented at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/dbi18-0002/-/DC1.
A.M.S. and Z.Q. are co–first authors, and M.A. and K.C.H. are co–senior authors.
See accompanying article, p. 1461.
References
- 1.Azoury SC, Straughan DM, Shukla V. Immune checkpoint inhibitors for cancer therapy: clinical efficacy and safety. Curr Cancer Drug Targets 2015;15:452–462 [DOI] [PubMed] [Google Scholar]
- 2.Bour-Jordan H, Esensten JH, Martinez-Llordella M, Penaranda C, Stumpf M, Bluestone JA. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/ B7 family. Immunol Rev 2011;241:180–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995;3:541–547 [DOI] [PubMed] [Google Scholar]
- 4.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science 1995;270:985–988 [DOI] [PubMed] [Google Scholar]
- 5.Ansari MJ, Salama AD, Chitnis T, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med 2003;198:63–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fife BT, Guleria I, Gubbels Bupp M, et al. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med 2006;203:2737–2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest 2015;125:3384–3391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyi C, Postow MA. Checkpoint blocking antibodies in cancer immunotherapy. FEBS Lett 2014;588:368–376 [DOI] [PubMed] [Google Scholar]
- 9.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol 2015;36:265–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chistiakov DA, Turakulov RI. CTLA-4 and its role in autoimmune thyroid disease. J Mol Endocrinol 2003;31:21–36 [DOI] [PubMed] [Google Scholar]
- 11.Kavvoura FK, Ioannidis JP. CTLA-4 gene polymorphisms and susceptibility to type 1 diabetes mellitus: a HuGE Review and meta-analysis. Am J Epidemiol 2005;162:3–16 [DOI] [PubMed] [Google Scholar]
- 12.Rioux JD, Abbas AK. Paths to understanding the genetic basis of autoimmune disease. Nature 2005;435:584–589 [DOI] [PubMed] [Google Scholar]
- 13.Wolff AS, Mitchell AL, Cordell HJ, et al. CTLA-4 as a genetic determinant in autoimmune Addison’s disease. Genes Immun 2015;16:430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity 2005;23:227–239 [DOI] [PubMed] [Google Scholar]
- 15.Verbsky JW, Chatila TA. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) and IPEX-related disorders: an evolving web of heritable autoimmune diseases. Curr Opin Pediatr 2013;25:708–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barroso-Sousa R, Ott PA, Hodi FS, Kaiser UB, Tolaney SM, Min L. Endocrine dysfunction induced by immune checkpoint inhibitors: practical recommendations for diagnosis and clinical management. Cancer 2018;124:1111–1121 [DOI] [PubMed] [Google Scholar]
- 17.Sznol M, Postow MA, Davies MJ, et al. Endocrine-related adverse events associated with immune checkpoint blockade and expert insights on their management. Cancer Treat Rev 2017;58:70–76 [DOI] [PubMed] [Google Scholar]
- 18.Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol 2018;4:173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byun DJ, Wolchok JD, Rosenberg LM, Girotra M. Cancer immunotherapy - immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol 2017;13:195–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med 2015;13:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erlich H, Valdes AM, Noble J, et al.; Type 1 Diabetes Genetics Consortium . HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes 2008;57:1084–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes J, Vudattu N, Sznol M, et al. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care 2015;38:e55–e57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aleksova J, Lau PK, Soldatos G, McArthur G. Glucocorticoids did not reverse type 1 diabetes mellitus secondary to pembrolizumab in a patient with metastatic melanoma. BMJ Case Rep 2016;2016:pii: bcr2016217454 [DOI] [PMC free article] [PubMed]
- 25.Alzenaidi AA, Dendy J, Rejjal L. Autoimmune diabetes presented with diabetic ketoacidosis induced by immunotherapy in an adult with melanoma. J La State Med Soc 2017;169:49. [PubMed] [Google Scholar]
- 26.Araújo M, Ligeiro D, Costa L, et al. A case of fulminant type 1 diabetes following anti-PD1 immunotherapy in a genetically susceptible patient. Immunotherapy 2017;9:531–535 [DOI] [PubMed] [Google Scholar]
- 27.Capitao R, Bello C, Fonseca R, Saraiva C. New onset diabetes after nivolumab treatment. BMJ Case Rep 2018;2018:pii: bcr-2017-220999 [DOI] [PMC free article] [PubMed]
- 28.Gauci ML, Laly P, Vidal-Trecan T, et al. Autoimmune diabetes induced by PD-1 inhibitor-retrospective analysis and pathogenesis: a case report and literature review. Cancer Immunol Immunother 2017;66:1399–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaudy C, Clévy C, Monestier S, et al. Anti-PD1 pembrolizumab can induce exceptional fulminant type 1 diabetes. Diabetes Care 2015;38:e182–e183 [DOI] [PubMed] [Google Scholar]
- 30.Godwin JL, Jaggi S, Sirisena I, et al. Nivolumab-induced autoimmune diabetes mellitus presenting as diabetic ketoacidosis in a patient with metastatic lung cancer. J Immunother Cancer 2017;5:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer 2016;60:190–209 [DOI] [PubMed] [Google Scholar]
- 32.Kapke J, Shaheen Z, Kilari D, Knudson P, Wong S. Immune checkpoint inhibitor-associated type 1 diabetes mellitus: case series, review of the literature, and optimal management. Case Rep Oncol 2017;10:897–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lowe JR, Perry DJ, Salama AK, Mathews CE, Moss LG, Hanks BA. Genetic risk analysis of a patient with fulminant autoimmune type 1 diabetes mellitus secondary to combination ipilimumab and nivolumab immunotherapy. J Immunother Cancer 2016;4:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin-Liberal J, Furness AJ, Joshi K, Peggs KS, Quezada SA, Larkin J. Anti-programmed cell death-1 therapy and insulin-dependent diabetes: a case report. Cancer Immunol Immunother 2015;64:765–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mellati M, Eaton KD, Brooks-Worrell BM, et al. Anti-PD-1 and Anti-PDL-1 monoclonal antibodies causing type 1 diabetes. Diabetes Care 2015;38:e137–e138 [DOI] [PubMed] [Google Scholar]
- 36.Orlov S, Salari F, Kashat L, Walfish PG. Induction of painless thyroiditis in patients receiving programmed death 1 receptor immunotherapy for metastatic malignancies. J Clin Endocrinol Metab 2015;100:1738–1741 [DOI] [PubMed] [Google Scholar]
- 37.de Filette J, Jansen Y, Schreuer M, et al. Incidence of thyroid-related adverse events in melanoma patients treated with pembrolizumab. J Clin Endocrinol Metab 2016;101:4431–4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greenbaum CJ, Beam CA, Boulware D, et al.; Type 1 Diabetes TrialNet Study Group . Fall in C-peptide during first 2 years from diagnosis: evidence of at least two distinct phases from composite Type 1 Diabetes TrialNet data. Diabetes 2012;61:2066–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bingley PJ. Clinical applications of diabetes antibody testing. J Clin Endocrinol Metab 2010;95:25–33 [DOI] [PubMed] [Google Scholar]
- 40.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al.; Type 1 Diabetes TrialNet Anti-CD20 Study Group . Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med 2009;361:2143–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Das R, Bar N, Ferreira M, et al. Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J Clin Invest 2018;128:715–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Long SA, Buckner JH, Greenbaum CJ. IL-2 therapy in type 1 diabetes: “trials” and tribulations. Clin Immunol 2013;149:324–331 [DOI] [PubMed] [Google Scholar]
- 43.Marroqui L, Dos Santos RS, Op de Beeck A, et al. Interferon-α mediates human beta cell HLA class I overexpression, endoplasmic reticulum stress and apoptosis, three hallmarks of early human type 1 diabetes. Diabetologia 2017;60:656–667 [DOI] [PubMed] [Google Scholar]
- 44.Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med 2014;6:230ra45. [DOI] [PubMed] [Google Scholar]
- 45.Rui J, Deng S, Arazi A, Perdigoto AL, Liu Z, Herold KC. β cells that resist immunological attack develop during progression of autoimmune diabetes in NOD mice. Cell Metab 2017;25:727–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nair S, Yadav D, Pitchumoni CS. Association of diabetic ketoacidosis and acute pancreatitis: observations in 100 consecutive episodes of DKA. Am J Gastroenterol 2000;95:2795–2800 [DOI] [PubMed] [Google Scholar]
- 47.Friedman CF, Clark V, Raikhel AV, et al. Thinking critically about classifying adverse events: incidence of pancreatitis in patients treated with nivolumab + ipilimumab. J Natl Cancer Inst 2016;109:pii: djw260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tarrio ML, Grabie N, Bu DX, Sharpe AH, Lichtman AH. PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J Immunol 2012;188:4876–4884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Puzanov I, Diab A, Abdallah K, et al.; Society for Immunotherapy of Cancer Toxicity Management Working Group . Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 2017;5:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.