Abstract
Objectives
To evaluate joint trajectories of cognition and frailty and their association with the cumulative burden of patient-reported outcomes, including hospitalization, nursing home admission, and disability.
Design
Longitudinal study of 754 community-living persons aged 70 or older.
Participants
690 participants who had a baseline and at least one follow-up assessment of cognition and frailty between 1998 and 2009.
Measurements
Cognition was assessed using the Mini-Mental State Examination (MMSE). Frailty was defined by the 5 criteria for the Fried phenotype: muscle weakness, exhaustion, low physical activity, shrinking, and slow walking speed. A group-based, mixture modeling approach was used to fit the joint trajectories of cognition and frailty. The cumulative burden of hospitalization, nursing home admission, and disability over 141 months associated with the joint trajectories was evaluated using a series of generalized estimating equation Poisson models.
Results
Four joint trajectories were identified, including no cognitive frailty (27.8%), slow cognitive decline and progressive frailty (45.5%), rapid cognitive decline and progressive frailty (20.2%), and cognitive frailty (6.5%). For each joint trajectory group, the interval-specific incidence density rates of all patient-reported outcomes tended to increase over time, with the exception of hospitalization for which the increasing trend was apparent only for the Slow Cognitive Decline and Progressive Frailty group. The No Cognitive Frailty group had the lowest cumulative burden of all patient-reported outcomes [eg, nursing home admissions, 7.5/1000 person-months, 95% confidence interval (CI): 4.8–11.7], whereas the Cognitive Frailty group had the highest cumulative burden (eg, nursing home admissions, 381.1/1000 person-months, 95% CI: 294.5–493.1), with the exception of hospitalization. Compared with the No Cognitive Frailty group, the 3 other joint trajectory groups all had significantly greater burden of the patient-reported outcomes.
Conclusion
Community-living older persons exhibit distinct joint trajectories of cognition and frailty and experience an increasing burden of nursing home admission and disability as they age, with the greatest burden for those on a cognitive frailty trajectory.
Keywords: Older, cognition, frailty, joint trajectory, patient-reported outcome
Cognition and physical frailty (hereafter, frailty) are 2 important indicators of the aging process. Cognitive impairment ranges in severity from mild to severe because of the deterioration in domains such as memory, learning, and/or executive function. Frailty represents a state of increased vulnerability to minor stressor events resulting from cumulative decline in multiple physiological systems.1,2 Both aging indicators confer high risk for subsequent adverse outcomes, including hospitalization, nursing home admission, disability, and mortality, posing tremendous burdens on older persons, their families, and the health care systems.
Typically, cognition and frailty have been studied separately as if 2 independent processes. However, recent studies have challenged this traditional view and demonstrated a close interrelationship between cognition and frailty.3–7 A recent review article suggested that cognition and frailty interact within a cycle of age-associated decline, involving brain neuropathology, hormonal dysregulation, cardiovascular risk, and psychological factors.6 This interactive view of the 2 indicators is consistent with the multidimensional nature of health and aging,8 for which multidimensional indexes have been rigorously tested and developed to capture aggregate information across multiple domains.9–11 Cognitive frailty, a new conceptual construct proposed in 2013, is characterized by the simultaneous presence of both cognitive impairment and frailty.12 However, little is known about the natural history of cognitive frailty, including how the 2 components, that is, cognition and frailty, evolve over time. In addition, it is uncertain whether and to what extent the different subtypes or joint trajectories of cognitive frailty impact health outcomes important to older persons, such as hospitalization, nursing home admission, and disability.
Using data from a unique longitudinal study of community-living older persons that includes serial assessments of cognition and frailty over 11 years, we aimed to identify joint trajectories of cognition and frailty and to evaluate their associations with the cumulative burden of 3 patient-reported outcomes—hospitalization, nursing home admission, and disability.
Methods
Study Population
Participants were members of the Yale Precipitating Events Project (PEP), a longitudinal study of 754 initially nondisabled, community-living persons, 70 years or older.13–16 In brief, potential participants were identified from a computerized list of 3157 age-eligible members of a large health plan in greater New Haven, CT. Exclusion criteria included significant cognitive impairment with no available proxy, inability to speak English, diagnosis of a terminal illness with a life expectancy less than 12 months, or a plan to move out of the area. Only 4.6% of the 2735 health plan members who were alive and could be contacted refused to complete the screening telephone interview, and 75.2% of the 1002 eligible members agreed to participate in the project and were enrolled between March 1998 and October 1999. Persons who refused to participate did not differ significantly from those who were enrolled in terms of age or sex. The Yale Human Investigation Committee approved the study protocol, and all participants provided verbal informed consent.
The current study used data collected through December 31, 2009, with home-based comprehensive assessments completed every 18 months for 108 months and monthly telephone interviews completed up to 141 months.17 The completion rates of the comprehensive assessments ranged from 94.2% at 90 months to 100% at baseline. During the comprehensive assessments, data were collected on demographic characteristics, 9 self-reported, physician-diagnosed chronic conditions (ie, hypertension, myocardial infarction, congestive heart failure, stroke, diabetes mellitus, arthritis, hip fracture, chronic lung disease, and cancer), depressive symptoms [sex-adjusted Center for Epidemiologic Studies–Depression scale (CES-D) score ≥20],18 cognition,19 and frailty.20
Deaths were ascertained from local obituaries or an informant during a subsequent interview. Participants having only a baseline assessment of cognition and frailty (n = 64) were excluded because no corresponding trajectory could be modeled. Through December 31, 2009, a total of 396 (57.4%) of the remaining study participants (n = 690) had died after a median of 81 months.
Cognition
Cognition was assessed by a trained research nurse during the comprehensive assessments using the Mini-Mental State Examination (MMSE).19 The MMSE is the most widely used instrument for assessing global cognitive function in both clinical and research settings, with higher scores indicating better performance (range: 0–30). Studies of its psychometric properties show moderate to high levels of short-term test-retest reliability, construct and criterion validity, and adequate responsiveness to cognitive change over time.21,22
Frailty
Data from the comprehensive assessments were used to define each of the 5 criteria for the Fried phenotype: muscle weakness, exhaustion, low physical activity, shrinking, and slow walking speed. As described previously,20,23 our operational definitions for the last 3 criteria differed modestly from those previously described by Fried and colleagues for use in the Cardiovascular Health Study.2 The physical activity criterion was met for men who scored less than 64 and women who scored less than 52 on the Physical Activity Scale for the Elderly.24 The shrinking criterion was met if the participant answered yes when asked, “In the past year, have you lost more than 10 pounds?”20 Finally, the slow walking speed criterion was met if the participant scored greater than 10 seconds on the rapid gait test [walk back and forth over a 10-ft (3-m) course as quickly as possible].16 These modified criteria have been previously validated.25 Among a subgroup of 24 participants who were evaluated independently within a 3-day period by different nurse researchers, the reliability of our frailty assessment was substantial, with a weighted κ equal to 0.78.20,23
Hospitalization, Nursing Home Admission, and Disability
During the monthly telephone interviews, participants were asked whether they had stayed at least overnight in a hospital (κ = 0.94)26,27 and whether they had been admitted to a nursing home (κ = 0.96)26,28 since the last interview. Participants were also asked, “At the present time, do you need help from another person to (complete the task)?” for each of 4 basic activities of daily living (ADL) (bathing, walking, dressing, and transferring), 5 instrumental ADL (IADL) (shopping, housework, meal preparation, taking medications, and managing finances), and 3 mobility activities (walk ¼ mile, climb flight of stairs, and lift/carry 10 lb). Disability in the 3 functional domains (ADL, IADL, and mobility) was operationalized as the need for personal assistance in performing 1 or more of the corresponding activities.29 Participants were also asked about a fourth mobility activity, “Have you driven a car during the past month?” Participants who respond no were deemed to have stopped driving. To maintain consistency with the other activities, these participants were classified as “needing for personal assistance” in driving.30 The reliability of our disability assessment was high, with κ ranging from 0.75 to 1.31
Statistical Analyses
To identify common patterns of concurrent changes in cognition and frailty, we used a group-based, joint trajectory modeling approach developed by Jones and coworkers via an SAS macro PROC TRAJ.32 This approach fits a semiparametric (discrete) mixture model to longitudinal data using the maximum likelihood function where each resultant joint trajectory is a combination of a trajectory for cognition and a trajectory for frailty.
Based on the distributions of the MMSE scores (minimum = 0, maximum = 30)17 and the frailty score (range: 0–5), we used a censored normal model for cognition and a zero-inflated Poisson (ZIP) model for frailty. After comparing the goodness of fit across alternative models with varied high-order growth terms, a quadratic time for cognition and a linear time for frailty provided the best fit to the data based on the Bayesian information criterion (BIC). We assessed model adequacy using the average posterior probability of assignment (a probability of assignment ≥0.9 was considered an excellent fit and a value <0.7 was considered a poor fit), proportion of group membership with a posterior probability of assignment <0.7, and the differences between the predicted group probability and observed group proportions.33 We selected the final model based on these model adequacy indices, plus practical considerations of distinctiveness and interpretability of the estimated trajectories.32,33
Next, we estimated incidence density rates as the total number of events per 1000 person-months based on the monthly telephone interviews across the seven 18-month intervals, using separate models for each outcome.17 An “event” occurred when participants reported a hospitalization, nursing home admission, or ADL, IADL, or mobility disability, during a specific month. We then determined the observed interval-specific incidence density rates of the patient-reported outcomes over the seven 18-month intervals by the identified joint trajectories. Subsequently, we applied Poisson models invoking generalized estimating equations34 to evaluate the associations between the identified joint trajectories and the cumulative burden of the patient-reported outcomes over the 11-year follow-up. Global model fit was checked using the quasi-likelihood information criterion. The models were adjusted for the following covariates: age, sex, race, education, living alone, number of chronic conditions, and depressive symptoms.
P values <.05 were considered as statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Characteristics of Study Participants
The baseline characteristics of the study participants are shown in Table 1. About 12% of participants were ≥85 years old, and about one-half had 2 or more chronic conditions. The majority of participants were female, non-Hispanic white, had more than 12 years of education, and did not live alone, whereas a relatively small minority had significant depressive symptoms.
Table 1.
Characteristics of the Study Participants at Baseline According to the Joint Trajectories of Cognition and Frailty
| Participants | Age ≥ 85 Years | Female | Non-Hispanic White | Education <12 Years | Living Alone | No. of Chronic Conditions‡ ≥ 2 | Depression§ | |
|---|---|---|---|---|---|---|---|---|
| Total | 690 | 82 (11.9) | 451 (65.4) | 622 (90.1) | 228 (33.0) | 274 (39.7) | 360 (52.2) | 88 (12.8) |
| Trajectory group* | ||||||||
| No cognitive frailty | 194 | 6 (3.1) | 124 (63.9) | 178 (91.7) | 38 (19.6) | 62 (32.0) | 78 (40.2) | 11 (5.7) |
| Slow cognitive decline and progressive frailty | 320 | 40 (12.5) | 210 (65.6) | 292 (91.2) | 89 (27.8) | 143 (44.7) | 190 (59.4) | 43 (13.4) |
| Rapid cognitive decline and progressive frailty | 131 | 25 (19.1) | 84 (64.1) | 113 (86.3) | 74 (56.5) | 51 (38.9) | 74 (56.5) | 26 (19.9) |
| Cognitive frailty | 45 | 11 (24.4) | 33 (73.3) | 39 (86.7) | 27 (60.0) | 18 (40.0) | 18 (40.0) | 8 (17.8) |
| P value† | <.001 | .673 | .279 | <.001 | .042 | <.001 | .001 | |
Characteristics are presented as number (percentage).
Estimated using a joint trajectory modeling approach of cognition and frailty over seven 18-month intervals (see text for details).
The P values were derived from the chi-square test across the joint trajectory groups.
Based on 9 self-reported, physician-diagnosed conditions, including hypertension, myocardial infarction, heart failure, stroke, diabetes mellitus, arthritis, hip fracture, chronic lung disease, and cancer.
Based on a sex-adjusted score ≥ 20 on the Center for Epidemiologic Studies–Depression scale (CES-D).
Joint Trajectories of Cognition and Frailty
Among the joint trajectory models evaluated, a 4-group solution was selected as the optimal model. A 4-group model achieved acceptable average posterior probability of assignment, with values ranging from 0.90 to 0.96 across the 4 trajectory groups (Appendix 1, Table S1).33 The BIC index was less than that for alternative models having fewer groups. Subsequent models with more than 4 groups had lower average posterior probability of assignment and a higher proportion of group membership with a posterior probability of assignment <0.7 (eg, 20% for the model with 5 groups). The maximum likelihood estimates for the final 4-group joint trajectory model are summarized in Table S2 (Appendix 2).
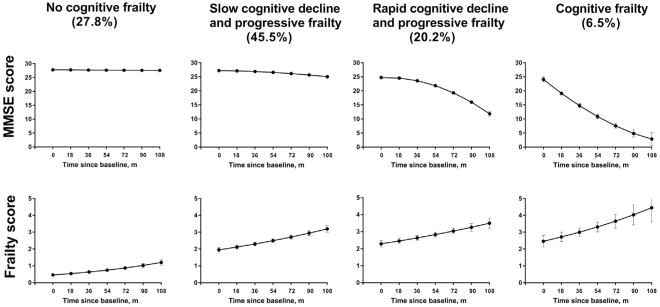
As shown in Figure 1, the 4 distinct joint trajectories were identified as no cognitive frailty, slow cognitive decline and progressive frailty, rapid cognitive decline and progressive frailty, and cognitive frailty. The predicted group probabilities for the 4 groups were 27.8%, 45.5%, 20.2%, and 6.5%, respectively. Participants in the No Cognitive Frailty group had no cognitive impairment (MMSE score < 2414,19) and low frailty scores throughout the follow-up period. In contrast, those in the Cognitive Frailty group exhibited an accelerated cognitive decline and developed worsening frailty over time. The remaining participants fell into 2 intermediate groups, each with progressive frailty but one exhibiting a slow cognitive decline and the other showing a rapid cognitive decline.
Fig. 1.
Distinct joint trajectories of cognition and frailty. Cognition was assessed using the Mini-Mental State Examination (MMSE, score range: 0–30). A lower score indicates greater impairment. Frailty was defined by the 5 criteria for the Fried phenotype: muscle weakness, exhaustion, low physical activity, shrinking, and slow walking speed. A higher score indicates greater frailty. Values represent the predicted MMSE score and frailty score, respectively. The error bars represent 95% confidence intervals for the predicted value of MMSE score and frailty score, respectively.
Characteristics by the Joint Trajectories
Baseline characteristics of the study participants by the joint trajectories of cognition and frailty are presented in Table 1. Across the 4 trajectory groups, the proportions of these factors differed significantly (all P < .05), except female (P = .673) and non-Hispanic white (P = .279).
Associations Between the Joint Trajectories and the Cumulative Burden of Hospitalization, Nursing Home Admission, and Disability
Supplementary Figure S1 (Appendix 3) provides the observed interval-specific incidence density rates of the patient-reported outcomes over the seven 18-month intervals for each of the 4 joint trajectories. The values tended to increase over time, with the exception of hospitalization, for which the increasing trend was apparent only for the Slow Cognitive Decline and Progressive Frailty group.
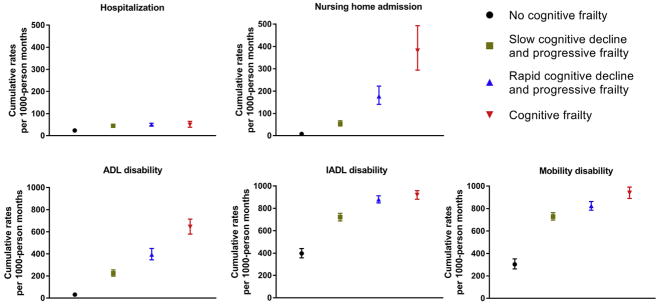
Figure 2 provides the adjusted values for cumulative burden of hospitalization, nursing home admission, and disability (ADL, IADL, mobility, respectively) for each of the joint trajectories groups. The No Cognitive Frailty group had the lowest burden of all patient-reported outcomes [eg, nursing home admissions, 7.5/1000 person-months, 95% confidence interval (CI): 4.8–11.7], whereas the Cognitive Frailty group had the highest burden (eg, nursing home admissions, 381.1/ 1000 person-months, 95% CI: 294.5–493.1), with the exception of hospitalization. Compared with the No Cognitive Frailty group, the 3 other joint trajectories groups all had significantly greater burdens of the patient-reported outcomes (see Appendix 4, Supplementary Material, for details). For example, the adjusted rate ratios (aRRs) for nursing home admission were 7.2 (95% CI: 4.4–11.8) for Slow Cognitive Decline and Progressive Frailty, 23.5 (95% CI: 14.4–38.4) for Rapid Cognitive Decline and Progressive Frailty, and 50.7 (30.5–84.1) for Cognitive Frailty, respectively, compared with No Cognitive Frailty.
Fig. 2.
Adjusted values for cumulative burden of hospitalization, nursing home admission, and disability by the joint trajectories of cognition and frailty. ADL, activities of daily living; IADL, instrumental activities of daily living. The bars denote the 95% confidence intervals (CIs). Values represent predicted number of events per 1000 person-months based on separate generalized estimating equation Poisson models for each patient-reported outcome. These values were estimated using a 4-group trajectory model of cognition and frailty, adjusting for the following covariates as group-specific population means: age, sex, race, education, living alone, number of chronic conditions, and depression symptoms.
Discussion
Based on longitudinal data for more than 11 years, we identified 4 distinct joint trajectories of cognition and frailty in a large sample of community-living older persons. The most favorable group (ie, No Cognitive Frailty) experienced no cognitive impairment and low frailty scores throughout the follow-up period, whereas the least favorable group (ie, Cognitive Frailty) exhibited an accelerated cognitive decline and developed worsening frailty over time. The 2 intermediate groups experienced progressive frailty with slow and rapid cognitive decline, respectively. The No Cognitive Frailty group had the lowest burden of the 3 patient-reported outcomes (hospitalization, nursing home admission, and disability), whereas the Cognitive Frailty group had the highest burden of nursing home admission and disability, but not hospitalization. Compared with the No Cognitive Frailty group, the 3 other joint trajectories groups all had significantly greater burden of the patient-reported outcomes. By advancing our understanding of cognitive frailty, these findings have the potential to inform clinical decision making for a highly vulnerable subset of older persons.
The largely parallel changes of cognition and frailty over time and their joint contributions to the increasing burden of patient-reported outcomes provide support for the construct of cognitive frailty. In the Cognitive Frailty group, worsening frailty closely tracked accelerated cognitive decline over time, suggesting the possibility of a common underlying etiology.6,35 Moreover, after about 36 months of follow-up, participants in this group had both cognitive impairment and frailty and, therefore, met criteria for cognitive frailty according to the standard definition.12 The Cognitive Frailty group experienced the greatest cumulative burden of nursing home admission and disability, a finding that is consistent with prior studies,36,37 suggesting that these older persons represent a unique subpopulation with extremely high risk of adverse outcomes.
The identification of 4 distinct joint trajectories of cognition and frailty suggest that the development of cognitive frailty is not uniform. Our study extends previous findings by jointly modeling cognition and frailty as potentially related domains and by demonstrating a clear gradient across the 4 trajectories for several patient-reported outcomes. Preventing or slowing decline in frailty and/or cognition has the potential to reduce the burden of these outcomes in older persons. Although many clinical trials have evaluated the effect of interventions such as nutritional supplements (eg, vitamin E, and omega 3 fatty acids) and/or exercise (eg, aerobic and resistance training, and multicomponent training) on preventing or slowing cognitive decline, little benefit has been demonstrated.38–43 Therefore, the Slow Cognitive Decline and Progressive Frailty group may currently be the most suitable target subpopulation for implementing preventive interventions.
Of the patient-reported outcomes, hospitalization warrants additional comment because its incidence density rates and cumulative burden did not track the joint trajectories as closely as the other outcomes. These differences may reflect diverse pathways towards or risk factor profiles of these patient-reported outcomes. For example, hospitalization is commonly due to acute illness or injury rather than to cognitive impairment or frailty itself.
Our study has several strengths. The serial assessments of cognition and frailty provided a unique opportunity to evaluate the natural history (or trajectory) of these 2 domains that often underlie vulnerability in older persons. The patient-reported outcomes, including hospitalization, nursing home admission, and disability, were obtained through monthly interviews with high reliability. Furthermore, the validity of our results is strengthened by the low rate of attrition and missing data due to losses to follow-up. Our study, however, has at least 3 limitations. First, the reported associations between the joint trajectories and the cumulative burden of patient-reported outcomes cannot be construed as causal. Second, ceiling effects were observed for mobility and IADL disability, for example, at about 36 months for the Cognitive Frailty group, making any subsequent changes difficult to ascertain. Finally, because our study participants were members of a single health plan in a small urban area and were oversampled for slow gait speed,13 the findings may not be generalizable to older persons in other settings.
In summary, community-living older persons exhibit distinct joint trajectories of cognition and frailty and experience an increasing burden of nursing home admission and disability as they age, with the greatest burden for those on a cognitive frailty trajectory. The findings highlight the unique characteristics of concurrent changes of cognition and frailty over time and provide a better understanding of cognitive frailty.
Supplementary Material
Acknowledgments
Dr Liu is the recipient of a James Hudson Brown–Alexander B. Coxe Fellowship from Yale School of Medicine, and he also received support from U01AG022376. The study was conducted at the Yale Claude D. Pepper Older Americans Independence Center (P30AG21342) and was supported by a grant from the National Institute on Aging (R01AG17560). Dr. Gill is the recipient of an Academic Leadership Award (K07AG043587) from the National Institute on Aging.
We thank Drs George Agogo, Darce Costello, and Xiao Xu for their help with the analyses and writing. We thank Linda Leo-Summers for help with data cleaning. We thank Denise Shepard, BSN, MBA, Shirley Hannan, RN, Andrea Benjamin, BSN, Martha Oravetz, RN, Amy Shelton, MPH, Alice Kossack, Barbara Foster, Shari Lani, and Alice Van Wie for assistance with data collection; Wanda Carr and Geraldine Hawthorne, BS, for assistance with data entry and management; Peter Charpentier, MPH, for development of the participant tracking system; and Joanne McGloin, MDiv, MBA, for leadership and advice as the Project Director.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 3.Alencar MA, Dias JM, Figueiredo LC, et al. Frailty and cognitive impairment among community-dwelling elderly. Arq Neuropsiquiatr. 2013;71:362–367. doi: 10.1590/0004-282X20130039. [DOI] [PubMed] [Google Scholar]
- 4.Han ES, Lee Y, Kim J. Association of cognitive impairment with frailty in community-dwelling older adults. Int Psychogeriatr. 2014;26:155–163. doi: 10.1017/S1041610213001841. [DOI] [PubMed] [Google Scholar]
- 5.Raji MA, Al Snih S, Ostir GV, et al. Cognitive status and future risk of frailty in older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2010;65:1228–1234. doi: 10.1093/gerona/glq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment—A review of the evidence and causal mechanisms. Ageing Res Rev. 2013;12:840–851. doi: 10.1016/j.arr.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Canevelli M, Cesari M, van Kan GA. Frailty and cognitive decline: How do they relate? Curr Opin Clin Nutr Metab Care. 2015;18:43–50. doi: 10.1097/MCO.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 8.Jiang S, Li P. Current development in elderly comprehensive assessment and research methods. Biomed Res Int. 2016;2016:3528248. doi: 10.1155/2016/3528248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pilotto A, Ferrucci L, Franceschi M, et al. Development and validation of a multidimensional prognostic index for one-year mortality from comprehensive geriatric assessment in hospitalized older patients. Rejuvenation Res. 2008;11:151–161. doi: 10.1089/rej.2007.0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swindell WR, Ensrud KE, Cawthon PM, et al. Indicators of “healthy aging” in older women (65–69 years of age). A data-mining approach based on prediction of long-term survival. BMC Geriatr. 2010;10:55. doi: 10.1186/1471-2318-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santoni G, Marengoni A, Calderon-Larranaga A, et al. Defining health trajectories in older adults with five clinical indicators. J Gerontol A Biol Sci Med Sci. 2017;72:1123–1129. doi: 10.1093/gerona/glw204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelaiditi E, Cesari M, Canevelli M, et al. Cognitive frailty: Rational and definition from an (I.A.N.A./I.A.G.G) international consensus group. J Nutr Health Aging. 2013;17:726–734. doi: 10.1007/s12603-013-0367-2. [DOI] [PubMed] [Google Scholar]
- 13.Gill TM. Disentangling the disabling process: Insights from the precipitating events project. Gerontologist. 2014;54:533–549. doi: 10.1093/geront/gnu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gill TM, Allore HG, Holford TR, et al. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;292:2115–2124. doi: 10.1001/jama.292.17.2115. [DOI] [PubMed] [Google Scholar]
- 15.Hardy SE, Gill TM. Recovery from disability among community-dwelling older persons. JAMA. 2004;291:1596–1602. doi: 10.1001/jama.291.13.1596. [DOI] [PubMed] [Google Scholar]
- 16.Gill TM, Desai MM, Gahbauer EA, et al. Restricted activity among community-living older persons: Incidence, precipitants, and health care utilization. Ann Intern Med. 2001;135:313–321. doi: 10.7326/0003-4819-135-5-200109040-00007. [DOI] [PubMed] [Google Scholar]
- 17.Han L, Gill TM, Jones BL, et al. Cognitive aging trajectories and burdens of disability, hospitalization and nursing home admission among community-living older persons. J Gerontol A Biol Sci Med Sci. 2016;71:766–771. doi: 10.1093/gerona/glv159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohout FJ, Berkman LF, Evans DA, et al. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Gill TM, Gahbauer EA, Allore HG, et al. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166:418–423. doi: 10.1001/archinte.166.4.418. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell AJ. A meta-analysis of the accuracy of the Mini-Mental State Examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res. 2009;43:411–431. doi: 10.1016/j.jpsychires.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 22.Tombaugh TN, McIntyre NJ. The Mini-Mental State Examination: A comprehensive review. J Am Geriatr Soc. 1992;40:922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 23.Gill TM, Gahbauer EA, Han L, et al. The relationship between intervening hospitalizations and transitions between frailty states. J Gerontol A Biol Sci Med Sci. 2011;66:1238–1243. doi: 10.1093/gerona/glr142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Washburn RA, Smith KW, Jette AM, et al. The Physical Activity Scale for the Elderly (PASE): Development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 25.Rothman MD, Leo-Summers L, Gill TM. Prognostic significance of potential frailty criteria. J Am Geriatr Soc. 2008;56:2211–2216. doi: 10.1111/j.1532-5415.2008.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gill TM, Gahbauer EA, Han L, et al. Factors associated with recovery of pre-hospital function among older persons admitted to a nursing home with disability after an acute hospitalization. J Gerontol A Biol Sci Med Sci. 2009;64:1296–1303. doi: 10.1093/gerona/glp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gill TM, Allore H, Holford TR, et al. The development of insidious disability in activities of daily living among community-living older persons. Am J Med. 2004;117:484–491. doi: 10.1016/j.amjmed.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 28.Gill TM, Allore HG, Han L. Bathing disability and the risk of long-term admission to a nursing home. J Gerontol A Biol Sci Med Sci. 2006;61:821–825. doi: 10.1093/gerona/61.8.821. [DOI] [PubMed] [Google Scholar]
- 29.Gill TM, Murphy TE, Gahbauer EA, et al. Association of injurious falls with disability outcomes and nursing home admissions in community-living older persons. Am J Epidemiol. 2013;178:418–425. doi: 10.1093/aje/kws554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gill TM, Gahbauer EA, Murphy TE, et al. Risk factors and precipitants of long-term disability in community mobility: A cohort study of older persons. Ann Intern Med. 2012;156:131–140. doi: 10.1059/0003-4819-156-2-201201170-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gill TM, Hardy SE, Williams CS. Underestimation of disability in community-living older persons. J Am Geriatr Soc. 2002;50:1492–1497. doi: 10.1046/j.1532-5415.2002.50403.x. [DOI] [PubMed] [Google Scholar]
- 32.Jones BL, Nagin DS. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociol Methods Res. 2007;35:542–571. [Google Scholar]
- 33.Nagin DS. Group-Based Modeling of Development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- 34.Diggle PJ, Liang KY, Zeger S. Analysis of Longitudinal Data. Oxford: Clarendon Press; 1994. [Google Scholar]
- 35.Halil M, Cemal Kizilarslanoglu M, Emin Kuyumcu M, et al. Cognitive aspects of frailty: Mechanisms behind the link between frailty and cognitive impairment. J Nutr Health Aging. 2015;19:276–283. doi: 10.1007/s12603-014-0535-z. [DOI] [PubMed] [Google Scholar]
- 36.Feng L, Zin Nyunt MS, Gao Q, et al. Cognitive frailty and adverse health outcomes: Findings from the Singapore Longitudinal Ageing Studies (SLAS) J Am Med Dir Assoc. 2017;18:252–258. doi: 10.1016/j.jamda.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Roppolo M, Mulasso A, Rabaglietti E. Cognitive frailty in Italian community-dwelling older adults: Prevalence rate and its association with disability. J Nutr Health Aging. 2017;21:631–636. doi: 10.1007/s12603-016-0828-5. [DOI] [PubMed] [Google Scholar]
- 38.Saez de Asteasu ML, Martinez-Velilla N, Zambom-Ferraresi F, et al. Role of physical exercise on cognitive function in healthy older adults: A systematic review of randomized clinical trials. Ageing Res Rev. 2017;37:117–134. doi: 10.1016/j.arr.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 39.Schattin A, Baur K, Stutz J, et al. Effects of physical exercise combined with nutritional supplements on aging brain related structures and functions: A systematic review. Front Aging Neurosci. 2016;8:161. doi: 10.3389/fnagi.2016.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andrieu S, Guyonnet S, Coley N, et al. Effect of long-term omega 3 poly-unsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): A randomised, placebo-controlled trial. Lancet Neurol. 2017;16:377–389. doi: 10.1016/S1474-4422(17)30040-6. [DOI] [PubMed] [Google Scholar]
- 41.Valls-Pedret C, Sala-Vila A, Serra-Mir M, et al. Mediterranean diet and age-related cognitive decline: A randomized clinical trial. JAMA Intern Med. 2015;175:1094–1103. doi: 10.1001/jamainternmed.2015.1668. [DOI] [PubMed] [Google Scholar]
- 42.Sink KM, Espeland MA, Castro CM, et al. Effect of a 24-month physical activity intervention vs health education on cognitive outcomes in sedentary older adults: The LIFE randomized trial. JAMA. 2015;314:781–790. doi: 10.1001/jama.2015.9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Forbes SC, Holroyd-Leduc JM, Poulin MJ, et al. Effect of nutrients, dietary supplements and vitamins on cognition: A systematic review and meta-analysis of randomized controlled trials. Can Geriatr J. 2015;18:231–245. doi: 10.5770/cgj.18.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.