Abstract
The use of nanoparticle-stabilized nanocapsules for cytosolic siRNA delivery for immunomodulation in vitro and in vivo is reported. These NPSCs deliver siRNA directly to the cytosol of macrophages in vitro with concomitant knockdown of gene expression. In vivo studies showed directed delivery of NPSCs to the spleen, enabling gene silencing of macrophages, with preliminary studies showing 70% gene knockdown at a siRNA dose of 0.28 mg/kg. Significantly, the delivery of siRNA targeting tumor necrosis factor-α efficiently silenced TNF-α expression in LPS-challenged mice, demonstrating efficacy in modulating immune response in an organ-selective manner. This research highlights the potential of the NPSC platform for targeted immunotherapy and further manipulation of the immune system.
Keywords: cytokine, cytosolic delivery, siRNA delivery, immunomodulation, inflammation
Graphical Abstract:
Nanoparticle-stabilized nanocapsules (NPSCs) are used to deliver TNF-α targeted siRNA for inflammation therapy. NPSC siRNA delivery effectively prevents cytokine production in LPS-stimulated mice.
1. Introduction
Inflammation is a complex biological reaction of the immune system in response to microbial, autoimmune, metabolic or physical insults.[1-2] Uncontrolled inflammation, however, is responsible for numerous autoimmune disorders.[3-4] Macrophages play a critical role in the production of inflammation by secreting proinflammatory cytokines.[5] Suppressing cytokine expression has proven to be beneficial for the treatment of inflammatory diseases.[6] For example, up-regulation of tumor necrosis factor-α (TNF-α) is frequently involved in autoimmune disorders. TNF-α monoclonal antibodies or recombinant TNF-α receptors have been used to interfere with the inflammation cascade.[7] RNA interference (RNAi) is an alternative approach to modulate the immune system, with potential to treat immune disorders by knocking down proinflammatory cytokines using small interfering RNA (siRNA).[8] Cytosolic siRNA delivery, however, remains a challenge to realizing RNAi-based immunotherapy.[9] Though a number of nanocarriers have recently been developed for siRNA delivery relying on endocytosis of siRNA to enter cells, endosomal escape is quite inefficient, which decreases RNAi efficiency.
Recently, we have reported that nanoparticle-stabilized nanocapsules (NPSCs) are capable of direct cytosolic siRNA delivery, avoiding siRNA endosomal entrapment.[10] The NPSC/siRNA assembly relies on stabilization through interaction between the cationic gold nanoparticle shell and hydrophobic/anionic “oil” component. These capsules are also stabilized through lateral electrostatic interactions at the NPSC surface between NPs and siRNA, resulting in nanocapsules as small as 150 nm, preventing siRNA degradation and delivering siRNA directly to the cytosol, bypassing endocytosis.
In this study, we report NPSCs for siRNA delivery to macrophages, and modulation of cellular immune response in vitro and in vivo (Figure 1). In vitro studies showed NPSCs deliver siRNA to the cytosol of RAW 264.7 cells with transfection efficiency up to 90%. Delivery of TNF-α targeted siRNA knocked down TNF-α expression of RAW cells stimulated with lipopolysaccharide (LPS). In vivo bio-distribution studies show that >80% of systematically administrated NPSC/siRNA accumulated in the spleen, with siRNA targeting GAPDH resulting in effective gene knockdown. TNF-α targeted NPSC/siRNA dosing lowers TNF-α expression after LPS challenge, with higher efficiency than other in vivo siRNA studies, which utilize concentrations 5-10 fold higher for effective gene knockdown.[11] The efficacy of NPSCs for immune system delivery presents a promising approach for the treatment of inflammatory disease.
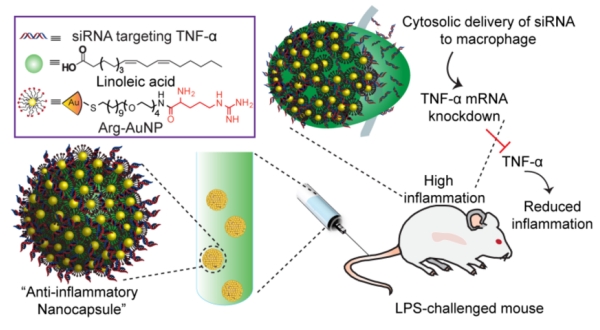
Figure 1.
Schematic of nanoparticle stabilized nanocapsule/siRNA-mediated in vivo TNF-α silencing in lipopolysaccharide-induced inflammation. The anti-inflammatory nanocapsule was prepared by assembling TNF-α targeted siRNA with arginine functionalized gold nanoparticles, with the ensemble self -assembled onto the surface of fatty acid nanodroplets to form a NPSC/siRNA nanocomplex.
2. Materials and Methods
2.1 General:
All reagents or chemicals used were purchased from Fisher Scientific or Sigma -Aldrich. Chloroauric acid used for gold nanoparticle synthesis was bought from Strem Chemicals Inc. (Newburyport, MA). si_GADPH and si_TNF-α with the following sequence, 5’-CAAGAGAGGCCCUAUCCCA[dT][dT]-3’(sense strand); and 5′-GUCUCAGCCUCUUCUCAUUCCUGct-3’ (sense strand), respectively, were synthesized by Sigma-Aldrich. Scramble siRNA (sense strand: 5’-UUCUCCGAACGUGUCACGU-3′) and Cy3-labeled scramble siRNA, were both purchased from Life Technologies (Carlsbad, CA). Confocal microscopy images were obtained on an Eclipse Ti-E microscope using a 40× objective. Flow cytometry analysis was performed on a BD LSR-II flow cytometer equipped with FACSDiva (BD Sciences, USA) by counting 10000 events.
2.2 NPSC/siRNA preparation:
The arginine-functionalized AuNP (Arg-AuNPs) and NPSC were synthesized according to our previous report.[10] Briefly, 1 μL of linoleic acid was mixed with 500 μL of phosphate buffer (5 mM, pH = 7.4) containing 1 μM Arg-AuNPs and agitated by an amalgamator at 5000 rpm for 100 s to form emulsions. Then, 10 μL of the emulsion was added into 90 μL of 5 mM phosphate buffer containing pre-mixed 2.5 μM Arg-AuNPs and 1 μM siRNA and incubated for 10 min. at room temperature to afford NPSC/siRNA complex.
2.3 Cytotoxicity of NPS C/siRNA complex:
For the Alamar Blue assay, RAW 264.7 cells (2.0 × 105 cells) were seeded in a 48-well plate 24 h prior to the experiment. At the day of experiment, cells were washed by cold PBS and treated with varied concentration of NPSC and scramble siRNA complexes for 2 h, followed by an incubation of additional 24 h with fresh culture medium. The cell viability was determined using Alamar Blue assay reagent (Invitrogen, CA) according to manufacturer’s instruction. Three biological replicates were performed for viability determination.
2.4 Fluorescently labeled siRNA delivery:
For confocal laser scanning microscopy (CLSM) imaging of the cellular uptake of NPSC/siRNA complex, RAW 264.7 cells (4.0 * 105 cells) were seeded in each well of a 4 chamber Lab-Tek II chambered coverglass system (Nunc, NY) one day prior to the experiment. At the day of delivery, the culture medium was removed and replaced with Opti-MEM containing 40 nM NPSC/Cy3-siRNA, followed by 1 h of incubation at 37 °C. After removing medium, the cells were washed once with cold phosphate buffer saline (PBS), fluorescence imaging was performed in PBS using an Eclipse Ti-E microscope. For timelapse imagery, RAW 264.7 cells (2.5 * 105) were plated into a 35 mm petri dish (MatTek) one day prior to the experiment. At the day of delivery, the culture medium was removed, the cells were washed once with cold PBS and replaced with DMEM containing 100 nm NPSC/Cy3 -siRNA. Cells were imaged following transfection every 30 seconds for two hours using an Eclipse Ti-E microscope. For the inhibitor assay, RAW 264.7 cells (7.5 * 104) were seeded in each well of a 4 chamber Lab-Tek II chambered coverglass system one day prior to the experiment. At the day of delivery, the culture medium was removed and replaced with media containing the appropriate inhibitor. The following concentrations were utilized, wortmannin: 150 ng/mL, chlorpromazine: 1.5 μg/mL, and MβCD: 7.5 mg/mL. The cells were incubated at 37 °C for one hour and then the media was replaced with DMEM containing 40 nM NPSC/FAM-siRNA, followed by another 1 h of incubation at 37 °C. The media was then replaced with PBS and fluorescence imaging was performed using an Eclipse Ti-E microscope. For flow cytometry analysis, RAW 264.7 cells (4.0 * 105 cells) were seeded in a 24-well plate for 24 h prior to delivery, and the cells were washed with PBS for three times before siRNA delivery. At the day of transfection, various concentrations of siRNA formulations were added to cells and incubated for 2 h in Opti-MEM, The cells were harvested and resuspended in PBS for flow cytometry analysis on FACS LSR II (BD Biosciences). Cells suspensions were analyzed under the same parameter setting, and at least 10000 events were analyzed for each sample.
2.5 In vitro TNF-α knockdown:
RAW 264.7 cells (2.0 × 105 cells/well) were cultured in a 48-well plate for 24 h prior to the experiment. At the day of experiment, cells were washed by cold PBS and treated with PBS, NPSC/si_TNF-α complexes at the indicated si_TNF-α concentration, NPSC/si_Scr (40 nM), and free si_TNF-α (40 nM) for 24 hours, followed by 3 hours LPS stimulation (1 μg/mL). At the end of incubation, culture media was collected for TNF-α level measurement by ELISA (R&D Systems, MN, USA). The silencing efficiency was denoted as the percentage of TNF-α levels of the control cells without nanoparticle treatment. Experiments were performed in triplicate.
2.6 Animal care:
All animal experiments were conducted in accordance with the guidelines of Institutional Animal Care and Use Committee (IACUC) at University of Massachusetts Amherst. Female BALB/c mice at least 6 weeks of age used for biodistribution and GAPDH knockdown study, and TNF-α knockdown study were purchased from The Jackson Laboratory (Bar Harbor, ME), and generously provided by Prof. Michelle Farkas (University of Massachusetts Amherst), respectively. All mice were allowed to rest at least one week in the animal facilities before any procedure was performed. Food and water intake were assessed.
2.7 In vivo gene silencing:
After one week of acclimatization, Female BALB/c mice at least 6 weeks of age, received lateral tail vein injections of PBS (negative control), or NPSC containing either non -targeting siRNA (NPSC/si_SCr), or anti-GAPDH siRNA (NPSC/si_GADPH), or anti-TNF-α siRNA (NPSC/si_ TNF-α) diluted in PBS at a volume of 0.01 ml/g. For biodistribution and GADPH knockdown study, BALB/c mice were i.v. injected twice with NPSC/siRNA complexes at a siRNA dose of 0.14 mg/kg at a 2 day interval, blood and organs were collected and harvested 3 days after final injection. Blood was centrifuged in serum separator tubes at 5,000 r.p.m. for 10 min, and the supernatant serum was carefully collected for TNF-α and IFN-γ analysis by ELISA (R&D Systems, MN, USA). Serum total bilirubin, alkaline phosphatase, aspartate aminotransferase, and alanine aminotransferase were measured using commercial kits (Teco Diagnostics, Anaheim, CA). For TNF-α knockdown study, BALB/c mice were i.v. injected twice with NPSC/siRNA complexes at a siRNA dose of 0.28 mg/kg at a 6 hour interval, followed by LPS (10 μg/kg or 5 mg/kg, i.p.) 24 hours later. Serum TNF-α was measured by ELISA 1.5 hours after administration of LPS.
2.8 Real Time Polymerase Chain Reaction (RT-PCR) analysis:
Once mice were sacrificed, organs including spleen, liver, lung, kidney, and heart were harvested, cut into small pieces, washed with saline, and homogenized with Trizol reagent. RNA extraction was performed. Briefly, approximately 1.5 μg of RNA was isolated using the Pure Link RNA Mini kit (Ambion) following the manufacturer’s instructions. Superscript IV reverse transcriptase was used for conversion of approximately 150 ng of RNA to cDNA, along with RNaseOut, 10 mM dNTPs, and 50 μm random hexamers (Thermo Fisher Scientific), also following manufacturer’s instructions. RT-PCR was performed on cDNA as prepared above using a CFX Connect Real Time System with iTaq Universal SYBR Green Supermix (Biorad). All DNA primers were purchased from Integrated DNA Technologies (Caralville, Iowa). The following sequences were used: GAPDH Forward (5’-ACC ACA GTC CAT GCC ATC AC-3’), Reverse (5’-TCC ACC ACC CTG TTG CTG TA-3’); β-actin Forward (5’-GAT CAG CAA GCA GGA GTA CGA -3’), Reverse (5’-AAA ACG CAG CTC AGT AAC AGT C-3’). Three biological replicates were performed for each control group and three technical replicates were used for each biological replicate. All GAPDH mRNA measurement was normalized to β-actin, with the NPSC/si_GAPDH values presented relative to the NPSC/si_Scr controls.
2.9 ICP-MS sample preparation and conditions:
Each harvested organ was weighed and transferred to metal ion-free tubes, followed by overnight digestion using a 3:1 (v/v) mixture of HNO3 (68%) and H2O2 (30%). On the next day, ~ 0.5 mL of fresh aqua regia was added, the sample was then diluted to 10 mL with de-ionized water.[14] A series of standard solutions (gold concentration: 20, 10, 5, 2, 1, 0.5, 0.2, 0 ppb) was prepared for each experiment. The ICP-MS analyses were performed on a Perkin-Elmer NexION 300X ICP mass spectrometer. 197Au was measured under standard mode. Operating conditions are listed as below: nebulizer flow rate: 0.95-1 L/min; rf power: 1600 W; plasma Ar flow rate: 18 L/min; dwell time: 50 ms.
3. Results and Discussion
3.1 NPSC deliver fluorescent siRNA to RAW 264.7 macrophages
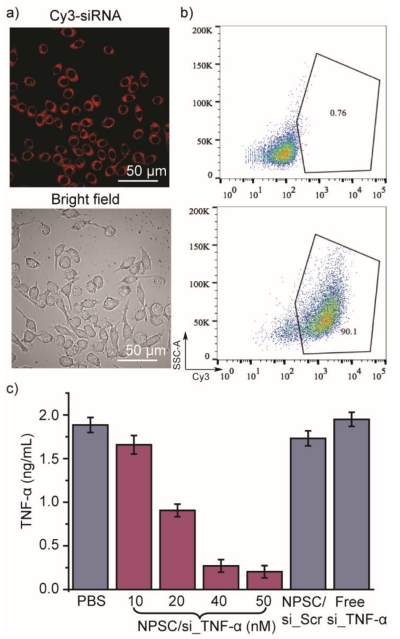
We explored siRNA delivery to RAW 264.7 cells, and studied gene knockdown efficiency following TNF-α targeted siRNA delivery. NPSCs carrying different siRNA sequences were synthesized and characterized by DLS (Figure S1). We treated RAW 264.7 cells with Cy3 labeled siRNA-loaded NPSCs to track cellular localization. Both confocal laser scanning microscopy (CLSM) imaging and flow cytometry analysis (Figure 2a and 2b) confirmed that NPSC/Cy3-siRNA complex was efficiently internalized by RAW 264.7 cells. Diffusion of Cy3 red fluorescence in the cytosol was observed for cells treated with 40 nM NPSC/Cy3-siRNA, indicating cytosolic siRNA delivery via a non-endocytic route associated with NPSC delivery (Figure 2a).[10] A comparison of free siRNA versus NPSC complexed siRNA fluorescence demonstrated that the lack of punctate fluorescence was not due to nanoparticle quenching (Figure S2). NPSCs were also used to deliver FAM-siRNA and the rate of uptake was analyzed by time-lapse CLSM. Imaging every 30 seconds demonstrated that the siRNA is dispersed completely through the cytosol near instantaneously (Figure S3). The rapid transfection rate and absence of punctate fluorescence provide evidence of a non-endocytotic entry route of NPSC/ siRNA into RAW 264.7 cells. This non-endocytotic uptake mechanism was also confirmed by delivery of FAM-siRNA into RAW 264.7 cells pretreated with endocytosis inhibitors (wortmannin and chlorpromazine) and membrane fusion inhibitor, methyl-β-cyclodextrin (MβCD). We have found that siRNA delivery was not inhibited by endocytosis inhibition, however, membrane fusion inhibition using MβCD significantly decreased siRNA transfection efficacy (Figure S4). By bypassing endosomal entrapment, siRNA transfection efficiency of RAW 264.7 cells was as high as 90% (40 nM siRNA, Figure 2b), with dose-dependent siRNA uptake observed (Figure S5).
Figure 2.
In vitro siRNA delivery into RAW 264.7 cells. a) Confocal microscopy images of RAW 264.7 cells treated with 40 nM Cy3-labeled siRNA delivered with NPSCs. b) Flow cytometry plots of Cy3-siRNA positive RAW 264.7 cells. RAW 264.7 cells treated with PBS (top), or 40 nM NPSC/Cy3-siRNA (bottom) for 2 hours were harvested for flow cytometry analysis. c) In vitro delivery of NPSC/si_TNF-α decreased TNF-α production from LPS stimulation. RAW 264.7 cells were treated with PBS, NPSC/si_TNF-α complexes at indicated si_TNF-α concentration, NPSC/si_Scr (50 nM), and free si_TNF-α (50 nM) for 24 hours, followed by 3 hours LPS stimulation (1 μg/mL). Supernatant TNF-α was measured by ELISA. Error bars represent the standard deviations of three parallel measurements.
3.2 RNAi mediated knockdown of TNF-α expression in vitro
We next investigated the efficacy of NPSC-facilitated siRNA delivery to knockdown TNF-α expression. The secretion of TNF-α from RAW 264.7 cells challenged with LPS (1 μg/mL) was used as the determinant for silencing efficiency.[12] NPSCs/si_TNF-α (50 nM siRNA) treatment resulted in a 90% decrease in TNF-α secretion, with suppression occurring in a concentration dependent manner (Figure 2c). si_TNF-α alone or NPSCs containing scrambled siRNA had no effect on suppressing TNF-α secretion. Meanwhile, no cytotoxicity or induction of apoptosis occurred at the siRNA concentrations used for these studies (Figure S6 and Figure S7), suggesting the efficient yet safe gene silencing using NPSC.
3.3 NPSC delivery of siRNA in vivo
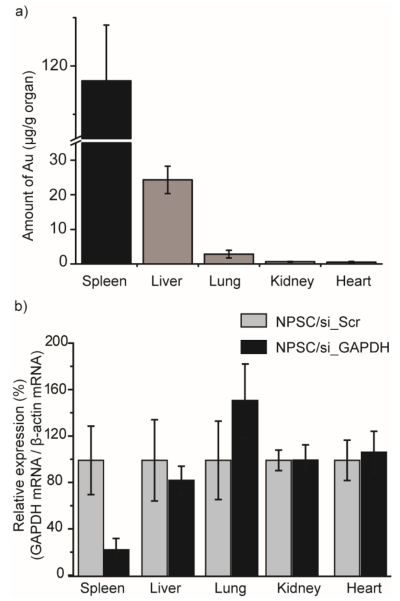
We next investigated NPSC/siRNA delivery in vivo. To characterize tissue distribution and efficacy of RNAi of NPSC/siRNA in vivo, we chose glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a target.[13] NPSCs loaded with siRNA targeting GAPDH (si_GAPDH) were injected intravenously into BALB/c mice twice, with a 2 day interval in between, at a dose of 0.14 mg/kg siRNA each. Tissues were harvested 3 days after the second injection for analysis. Two parallel analyses were performed to assess in vivo distribution of NPSC/siRNA, one using inductively coupled plasma mass spectrometry (ICP-MS) to determine gold accumulation in different tissues,[14] and the other using reverse transcription polymerase chain reaction (RT-PCR) to quantify GAPDH mRNA following the injections of different formulations.[15] As a percentage of the total injected dose, both spleen and liver showed predominant accumulation of NPSC/siRNA (Figure S8). The liver accumulated ~10% more of the total dose than the spleen, indicating reasonable selectivity to the spleen since the mouse liver is several-fold larger than the spleen. However, quantifying the concentration of gold in different tissues (μg of Au/g of organ) revealed that gold concentration accumulated in spleen were nearly 6-fold higher than in liver, and 20-fold higher than that were accumulated in other tissues, including kidney, lung, and heart (Figure 3a). The combination of vehicle accumulation in the spleen and direct cytosolic delivery provided potent silencing in the spleen after systematic injection of NPSC/si_GAPDH. The efficacy of in vivo GAPDH silencing in different tissues was analyzed by quantifying GAPDH mRNA, with β-actin expression as an internal comparison. The systematic injection of si_GAPDH NPs into BALB/c mice at a total siRNA dose of 0.28 mg/kg, resulted in 80% decrease of GAPDH mRNA in spleen (Figure 3b) compared to mice injected with scramble NPSCs/siRNA. In contrast, no GAPDH silencing was observed in other organs, including the liver. Importantly, no significant bodyweight change was noted for mice injected with NPSC compared to that with PBS injection (Figure S9). Moreover, testing clinical chemistry parameters demonstrated that NPSCs have low liver toxicity and low immune response for in vivo siRNA delivery (Figure S10).[18] There was no significant change of TNF-α, and IFN-γ levels in serum (Figure S11), demonstrating that NPSCs did not induce an innate immune response. Due to the high stability of AuNPs in vitro, there is potential concern about toxicity from over-accumulation in vivo. However, there is evidence in recent literature that 2 nm AuNPs are rapidly degraded in the spleen and are cleared by the renal system, ameliorating this possible toxicity.[19,20]
Figure 3.
In vivo distribution and gene silencing of NPSC/siRNA. a) Biodistribution of gold after intravenous injection of NPSC/siRNA. Gold content was analyzed by ICP-MS (n = 3). b) In vivo delivery of NPSC/si_GAPDH decreased splenic GAPDH mRNA levels. BALB/c mice were i.v. injected twice with NPSC/siRNA complexes at a siRNA dose of 0.14 mg/kg at a 2 day interval, and organs were harvested 3 days after final injection. NPSC/si_GAPDH values are presented relative to the NPSC/si_Scr control (n = 3).
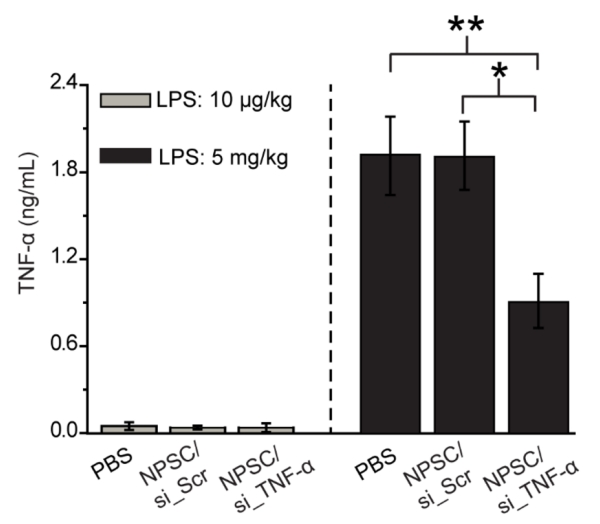
Finally, we studied whether splenic NPSC/siRNA delivery could regulate TNF-α secretion and modulate immune response in a LPS-challenged mouse model.[21] BALB/c mice were i.v. injected with PBS, NPSC/si_TNF-α or NPSC/si_Scr twice at a total siRNA dose of 0.56 mg/kg, 24 hours prior to LPS administration.[21c] Mice were sacrificed 1.5 h post LPS treatment and serum was processed for a TNF-α ELISA assay. NPSC/si_TNF-α treated mice displayed 60% reduced serum TNF-α secretion compared to PBS and NPSC/si_Scr treated mice (LPS: 5 mg/kg, Figure 4). We observed effective TNF-α gene knockdown at a 9-fold lower dose of siRNA than prior systems,[21c] demonstrating the efficacy of NPSC/si_TNF-α for in vivo RNAi. There was no significant difference in serum TNF-α level between PBS and NPSC/si_Scr injected mice, indicating that NPSCs are biocompatible, and attenuation of LPS-induced inflammation in vivo resulted only from RNAi. At a concentration of 5 mg/kg LPS, the generation of TNF-α was significant and robust, but there was no noticeable production of blood TNF-α at a lower concentration LPS treatment (10 μg/kg, Figure 4). Our results indicate that LPS-induced inflammation and TNF-α secretion can be suppressed by in vivo delivery of NPSCs/siRNA targeting TNF-α. Given the high accumulation of NPSC/siRNA in spleen, we speculated the potent systematic TNF-α knockdown resulted from siRNA delivery to splenic macrophages, which lead to TNF-α depletion in a LPS-induced mouse inflammation model.
Figure 4.
In vivo delivery of NPSC/si_TNF-α decreased serum TNF-α production from LPS-induced inflammation. BALB/c mice were i.v. injected twice with NPSC/siRNA at a siRNA dose of 0.28 mg/kg in 6 hour intervals, followed by LPS administration (10 μg/kg, or 5 mg/mouse, i.p.) 24 hours later. Serum TNF -α was measured by ELISA 1.5 hours after LPS dosing (n = 3). * = p < 0.05 through unpaired t-test; **= p < 0.005 through unpaired t-test between control group and siRNA group.
Conclusion
NPSCs are an effective platform for spleen-directed siRNA delivery and immunomodulation in vivo. The direct cytosolic delivery of siRNA, coupled with the splenic directed ability, contributes to potent RNAi in vivo, enabling silencing of proinflammatory cytokines with a total siRNA dose as low as 0.56 mg/kg. The ability to dose at levels significantly lower than prior studies decreases off-target effects, overcoming a critical barrier in in vivo siRNA delivery. The modular nature of the NPSC system allows for further modification for targeting of other organs and different parts of the immune system. PEGylation of the NPSC surface would result in immune “stealth” which would allow the NPSCS to circulate to other organs beyond the reticuloendothelial system.22 Additionally, nanoparticle functionalization with macrophage-specific antibodies or long hydrophobic alkyl chains could potentially increase the specificity of the NPSC system to macrophage uptake.23 The NPSC system provides a means to enhance the potential for therapeutic siRNA in the treatment of autoimmune disorders and other inflammatory conditions.
Supplementary Material
Highlights.
Immunomodulation is an effective strategy to treat a wide range of immune diseases
RNA interference (RNAi) is a safe, specific method for reducing the cytokine expression
Nanocapsules deliver siRNA direct to the cytosol c for effective RNAi
Nanocapsules loaded with anti-inflammatory siRNA diminished immune response in mice
Anti-inflammatory NPSCs have potential to ameliorate inflammatory diseases
Acknowledgements
We are grateful to Prof. Barbara A. Osborne of University of Massachusetts Amherst for her advice. This research was supported by the NIH (GM077173 and EB023369 (VR) and the Chemistry -Biology Interface Training grant (JH, 5T32GM008515). We also thank Dr. Chambers at the Light Microscopy Facility and Nikon Center of Excellence at the Institute for Applied Life Sciences, UMass Amherst with support from the Massachusetts Life Sciences Center for assistance gathering light microscopy data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no competing financial interests.
References
- [1]. Medzhitov R Origin and physiological roles of inflammation Nature 2008. 454 428–435 [DOI] [PubMed] [Google Scholar]
- [2]. Chovatiya R Medzhitov R Stress, Inflammation, and Defense of Homeostasis Mol. Cell 2014. 54 281–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Czapski GA Cakala M Chalimoniuk M Gajkowska B Strosznajder JB Role of nitric oxide in the brain during lipopolysaccharide-evoked systemic inflammation J. Neurosci. Res 2007. 85 1694–1703 [DOI] [PubMed] [Google Scholar]
- [4]. Feldmann M Maini RN Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu. Rev. Immunol 2001. 19 163–96 [DOI] [PubMed] [Google Scholar]
- [5]. Gordon S Taylor PR Monocyte and macrophage heterogeneity Nat. Rev. Immunol 2005. 5 953–964 [DOI] [PubMed] [Google Scholar]
- [6]. Tabas I Glass CK Anti-inflammatory therapy in chronic disease: challenges and opportunities Science 2013. 339 166–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Shealy DJ Visvanathan S Anti-TNF antibodies: lessons from the past, roadmap for the future Handb. Exp. Pharmacol 2008. 181 101–129 [DOI] [PubMed] [Google Scholar]
- [8]. Aouadi MG Tesz J Nicoloro SM Wang M Chouinard M Soto E Ostroff GR Czech MP Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation Nature 2009. 458 1180–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].a) Whitehead KA Langer R Anderson DG Knocking down barriers: advances in siRNA delivery Nat. Rev. Drug Discovery 2009. 8 129–138 [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Jiang Y Huo S Hardie J Liang XJ Rotello VM Progress and perspective of inorganic nanoparticle-based siRNA delivery systems Expert Opin. Drug Deliv 2016. 13 547–559 [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Wang YH Huang L A window onto siRNA delivery Nat. Biotechnol 2013. 31 611–612 [DOI] [PubMed] [Google Scholar]
- [10]. Jiang Y Tang R Duncan B Jiang Z Yan B Mout R Rotello VM Direct cytosolic delivery of siRNA using nanoparticle-stabilized nanocapsules Angew. Chem. Int. Ed 2015. 54 506–510 [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem 2015. 54 506–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Lee H Lytton-Jean AKR Chen Y Love KT Park AI Karagiannis ED Sehgal A Querbes W Zurenko CS Jayaraman M Peng CG Charisse K Borodovsky A Manoharan M Donahoe JS Truelove J Nahrendorf M Langer R Anderson DG Molecularly self-a ssembled nucleic acid nanoparticles for targeted in vivo siRNA delivery Nat. Nanotechnol 2012. 7 389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. MacKenzie S Fernandez-Troy N Espel E Post-transcriptional regulation of TNF-alpha during in vitro differentiation of human monocyte s/macrophages in primary culture J. Leukoc. Biol 2002. 71 1026–1032 [PubMed] [Google Scholar]
- [13]. Joseph R Srivastava OP Pfister RR Downregulation of β-actin and its regulatory gene HuR affect cell migration of human corneal fibroblasts Mol. Vis 2014. 20 593–605 [PMC free article] [PubMed] [Google Scholar]
- [14]. Yan B Kim ST Kim CS Saha K Moyano DF Xing Y Jiang Y Roberts AL Alfonso FS Rotello VM Vachet RW Quantitative characterization of surface ligands on functionalized magnetic nanoparticles using la ser de sorption/Ionization mass spectrometry (LDI-MS) J. Am. Chem. Soc 2013. 135 12564–12567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Alvarez-Erviti L Seow Y Yin H Betts C Lakhal S Wood MJ Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes Nat. Biotechnol 2011. 29 341–345 [DOI] [PubMed] [Google Scholar]
- [16]. Braet F Wisse E Bomans P Frederik P Geerts W Koster A Soon L Ringer S Contribution of high-resolution correlative imaging techniques in the study of the liver sieve in three -dimensions Microsc. Res. Tech 2007. 70 230–242 [DOI] [PubMed] [Google Scholar]
- [17]. Blanco E Shen H Ferrari M Principles of nanoparticle design for overcoming biological barriers to drug delivery Nat. Biotechnol 2015. 33 941–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Whitehead KA, Dorkin JR, Vegas AJ, Chang PH, Veiseh O, Matthews J, Fenton OS, Zhang Y, Olejnik KT, Yesilyurt V, Chen D, Barros S, Klebanov B, Novobrantseva T, Langer R, Anderson DG. Degradable lipid nanoparticles with predictable in vivo siRNA delivery activity. Nat. Commun. 2014;5:4277. doi: 10.1038/ncomms5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Elci SG Tonga GY Yan B Kim ST Kim CS Jiang Y Saha K Moyano DF Marsico ALM Rotello VM Vachet RW Dual-mode mass spectrometric imaging for determination of in vivo stability of nanoparticle monolayers ACS Nano 2017. 11 7424–7430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Zhou C Long M Qin Y Sun X Zheng J Lumine scent gold nanoparticles with efficient renal clearance Angew. Chem., Int. Ed 2011. 50 3168–3172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].a) Yin L Song Z Qu Q Kim KH Zheng N Yao C Chaudhury I Tang H Gabrielson NP Uckun FM Cheng J Supramolecular self-assembled nanoparticles mediate oral delivery of therapeutic TNF-α siRNA against systemic inflammation Angew. Chem. Int. Ed Angew. Chem 2013. 2013. 52 52 5757–5761 [DOI] [PMC free article] [PubMed] [Google Scholar]; b) He H Zheng N Song Z Kim KH Yao C Zhang R Zhang C Huang Y Uckun FM Cheng J Zhang Y Yin L Suppression of hepatic inflammation via systemic siRNA delivery by membrane-disruptive and endosomolytic helical polypeptide hybrid nanoparticles ACS Nano 2016. 10 1859–1870 [DOI] [PubMed] [Google Scholar]; c) Kim SS Ye C Kumar P Chiu I Subramanya S Wu H Shankar P Manjunath N Targeted delivery of siRNA to macrophage s for anti-inflammatory treatment Mol. Ther 2010. 18 993–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Lee SJ Lee A Hwang SR Park JS Jang J Huh MS Jo DG Yoon SY Byun Y Kim SH Kwon IC Youn I Kim K TNF-α gene silencing using polymerized siRNA/thiolated glycol chitosan nanoparticles for rheumatoi d arthritis Mol. Ther 2014. 22 397–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Jevsevar S Kunstelj M Porekar VG PEGylation of therapeutic proteins Bio. Tech. Meth. Adv 2010. 5 113–128 [DOI] [PubMed] [Google Scholar]
- [23]. Ahsan F Rivas IP Khan MA Suarez AIT Targeting to macrophages: role of physicochemical properties of particulate carriers-liposome s and microspheres-on the phagocytosis by macrophages J. Control Release 2002. 79 29–40 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.