Abstract
OBJECTIVE
To compare insulin sensitivity (M/I) and β-cell responses in youth versus adults with impaired glucose tolerance (IGT) or drug-naïve, recently diagnosed type 2 diabetes.
RESEARCH DESIGN AND METHODS
In 66 youth (80.3% with IGT) and 355 adults (70.7% IGT), hyperglycemic clamps were used to measure 1) M/I, 2) acute (0–10 min [first phase]) C-peptide (ACPRg) and insulin (AIRg) responses to glucose, 3) steady-state C-peptide and insulin concentrations at plasma glucose of 11.1 mmol/L, and 4) arginine-stimulated maximum C-peptide (ACPRmax) and insulin (AIRmax) responses at plasma glucose >25 mmol/L. The fasting C-peptide–to–insulin ratio was used as an estimate of insulin clearance.
RESULTS
Insulin sensitivity was 46% lower in youth compared with adults (P < 0.001), and youth had greater acute and steady-state C-peptide (2.3- and 1.3-fold, respectively; each P < 0.001) and insulin responses to glucose (AIRg 3.0-fold and steady state 2.2-fold; each P < 0.001). Arginine-stimulated C-peptide and insulin responses were also greater in youth (1.6- and 1.7-fold, respectively; each P < 0.001). After adjustment for insulin sensitivity, all β-cell responses remained significantly greater in youth. Insulin clearance was reduced in youth (P < 0.001). Participants with diabetes had greater insulin sensitivity (P = 0.026), with lesser C-peptide and insulin responses than those with IGT (all P < 0.001) but similar insulin clearance (P = 0.109).
CONCLUSIONS
In people with IGT or recently diagnosed diabetes, youth have lower insulin sensitivity, hyperresponsive β-cells, and reduced insulin clearance compared with adults. Whether these age-related differences contribute to declining β-cell function and/or impact responses to glucose-lowering interventions remains to be determined.
Introduction
Across the life span, the prevalence of impaired glucose tolerance (IGT) and type 2 diabetes is rising worldwide (1–3). This increase has been driven in part by an escalation in the prevalence of obesity, which afflicts no longer only middle-aged and older individuals but also youth (4,5).
Type 2 diabetes and its precursor, prediabetes, are characterized by insulin resistance and β-cell dysfunction in youth and adults (6–9). However, the progression of dysglycemia appears to be more aggressive in youth (10–12). The Restoring Insulin Secretion (RISE) Study provides a unique opportunity to compare the physiologic features that underlie dysglycemia in youth and adults. RISE is evaluating different interventions to prevent the progressive loss of β-cell function in youth and adults with prediabetes or recent-onset type 2 diabetes (13). The three RISE protocols contain many common design elements, including phenotyping of participants using the hyperglycemic clamp to measure insulin sensitivity (M/I) and three different β-cell responses: two acute and one during prolonged stimulation. The present report uses the baseline hyperglycemic clamp data to examine insulin sensitivity and β-cell function in youth versus adults with IGT or recently diagnosed type 2 diabetes.
Research Design and Methods
Participants
Individuals at high risk for IGT and type 2 diabetes (see Supplementary Appendix 2) who met other study inclusion/exclusion criteria were screened with a 75-g oral glucose tolerance test and hemoglobin A1c (HbA1c) test. Youth aged 10–19 years with pubertal development beyond Tanner stage ≥II were eligible for the RISE Pediatric Medication Study if they had a fasting plasma glucose ≥5 mmol/L plus 2-h glucose ≥7.8 mmol/L and 1) HbA1c ≤64 mmol/mol if drug naïve, 2) HbA1c ≤58.5 mmol/mol if on metformin for <3 months, or 3) ≤53 mmol/mol if on metformin for 3–6 months. Adults were eligible for the RISE Adult Medication Study if they had a fasting plasma glucose 5.3–6.9 mmol/L plus 2-h glucose ≥7.8 mmol/L and HbA1c ≤53 mmol/mol. Adults were eligible for the RISE Adult Surgery Study (BetaFat) if they had a fasting glucose >5 mmol/L plus 2-h glucose ≥7.8 mmol/L and HbA1c <53 mmol/mol. In both adult studies, individuals with known diabetes for <1 year were eligible if they had never received glucose-lowering medications and qualified otherwise.
Eighty-eight participants were randomized into the Adult Surgery Study, 267 into the Adult Medication Study, and 91 into the Pediatric Medication Study. Of the 91 pediatric participants, 25 either were on metformin at the time of randomization or had previously been exposed to it. These participants were excluded from the analyses herein to avoid potential confounding by this exposure. All participants gave written informed consent/assent, consistent with the Declaration of Helsinki and the guidelines of each center’s institutional review board.
Anthropometric Measurements
Anthropometric measurements were performed with participants wearing light clothing without shoes. Waist circumference was measured in a horizontal plane at the midpoint between the top of the iliac crest and the bottom of the costal margin in the midaxillary line using a fiberglass (nonstretching) tape. Height was measured in a fully vertical position with heels together using a calibrated stadiometer. Weight was measured using a calibrated electronic scale, zeroed before each measurement.
Procedures
After a 10-h overnight fast, a two-step hyperglycemic clamp was performed. The overall approach is outlined in Supplementary Fig. 1.
For the first step, the steady-state target blood glucose concentration of 11.1 mmol/L was achieved using an initial intravenous bolus of 20% dextrose (volume in mL calculated as weight [kg] * [200 − fasting blood glucose in mg/dL] * 1.1/180) administered over 60 seconds, after which infusion of 20% dextrose was commenced at a rate calculated as (weight [kg] * 5 * 60)/180). Starting at 10 min after the initial dextrose bolus, the rate of the infusion was modified based on a computerized algorithm combined with bedside blood glucose monitoring every 5–10 min. During this first step of the clamp, arterialized blood samples were drawn through an indwelling intravenous catheter in a warmed hand before and at 2, 4, 6, 8, 10, 100, 110, and 120 min.
For the second step, the target blood glucose of >25 mmol/L was achieved using a second bolus of 20% dextrose administered over 60 seconds (volume in mL calculated as weight [kg] * [450 − current blood glucose in mg/dL] * 1.1/180). The 20% dextrose infusion rate was then increased (typically to the pump’s maximum rate of 999 mL/h) and adjusted based on bedside blood glucose monitoring every 5 min. If the bedside blood glucose was not >22.2 mmol/L by 15 min after commencement of the second step of the clamp, an additional bolus of 50 mL of 50% dextrose was administered over 2 min. Once the target blood glucose of >25 mmol/L was attained for a minimum of 30 min, but no more than 45 min after commencement of the second step, a bolus of l-arginine (5 g) was administered over 1 min. Blood samples for subsequent assays were drawn at −5, −1, 2, 3, 4, and 5 min relative to the arginine injection.
Assays
All blood samples were immediately placed on ice, separated by centrifugation, and frozen at −80°C prior to shipment to the central biochemistry laboratory at the University of Washington. Plasma glucose concentrations for use in end point calculations were measured on these samples by the glucose hexokinase method using Roche reagent on a Roche c501 autoanalyzer. The method’s interassay coefficient of variation (CV) on quality control samples with low, medium, and high concentrations was 2.0%, 1.7%, and 1.3%, respectively. C-peptide and insulin were measured by a two-site immuno-enzymometric assay performed on the Tosoh 2000 autoanalyzer (Tosoh Bioscience, Inc., South San Francisco, CA). The interassay CV for C-peptide on quality control samples with low, medium, medium-high, and high concentrations was 4.3%, 3.6%, 3.2%, and 2.6%. The assay has a minimum detectable concentration of 0.007 nmol/L, a standard curve linear to 10 nmol/L, and cross-reactivity of 0.05% with intact proinsulin, 0.02% with the proinsulin fragment containing amino acids 31–65, and zero with insulin. For the insulin assay, the interassay CV on quality control samples with low, medium, medium-high, and high concentrations was 3.5%, 3.0%, 3.3%, and 2.9%. The assay has a minimum detectable concentration of 3.7 pmol/L and the following cross-reactivities: intact proinsulin 2.0%, split 32,33 proinsulin 2.6%, des 64,65 proinsulin (which is <6% of total proinsulin) 39%, and C-peptide zero. All measures are presented in Système International (SI) units. These can be converted to conventional units using standard conversion factors with the exception of insulin, for which 0.134 should be used.
Calculations for Clamp-Derived Measurements
Insulin Sensitivity
Insulin sensitivity (M/I) was quantified as the mean of the glucose infusion rate (M) at 100, 110, and 120 min of the clamp, expressed per kilogram of body weight and corrected for urinary glucose loss, divided by the mean steady-state plasma insulin concentration at these same time points (I) (14–16). Urinary glucose loss was the product of the urinary glucose concentration and urinary volume.
C-peptide and Insulin Responses
Acute (first-phase) C-peptide (ACPRg) and insulin (AIRg) responses to glucose were calculated as the mean incremental response above baseline (average of −10 and −5 min) from samples drawn at 2, 4, 6, 8, and 10 min after intravenous dextrose administration (17). Steady-state (second-phase) C-peptide and insulin concentrations were calculated as the mean of the respective measurements at 100, 110, and 120 min of the hyperglycemic clamp (16). Acute C-peptide (ACPRmax) and insulin (AIRmax) responses to arginine at maximal glycemic potentiation (>25 mmol/L) were calculated as the mean concentrations in samples drawn 2, 3, 4, and 5 min after arginine injection minus the average concentration of the samples drawn 1 and 5 min prior to arginine (18).
Insulin Clearance
The ratio of fasting C-peptide to fasting insulin was calculated as an estimate of insulin clearance (19). The rationale for this approach is the equimolar secretion of both peptides and the lack of C-peptide extraction by the liver. Consequently, under steady-state conditions, the C-peptide–to–insulin molar ratio is proportional to the hepatic clearance rate of insulin.
Additional details on participant inclusion/exclusion criteria, procedures, and measurements have previously been published (13) and are provided in the three study protocols available at https://rise.bsc.gwu.edu/web/rise/collaborators.
Data Management and Statistical Analyses
The SAS analysis system (SAS Institute, Cary, NC) and R (The R Foundation) were used for all statistical analyses. Descriptive statistics are presented as percentages, mean ± SD, geometric means, and 95% CIs for nonnormally distributed data; for the geometric means, P values from the log-transformed data are presented. Comparisons between groups were computed using ANOVA, χ2 tests, or Student t tests. Nominal P values are presented. Except where noted, P values <0.05 were considered nominally statistically significant, with no adjustments made for multiple tests.
Linear regression models were used to evaluate the relationship of C-peptide or insulin responses with insulin sensitivity (M/I). The relationship between the steady-state insulin response and M/I was not evaluated because the steady-state insulin values are included in each calculation. All models used natural logarithmically transformed M/I and β-cell response variables owing to the skewed distribution of these data. Prior to taking logs, we added a constant of 1.06 to the ACPRg and 10.0 to the AIRg because of negative values in these β-cell response variables.
We were interested in whether the various metabolic responses differed between youth and adults and between those with IGT and type 2 diabetes at baseline. Therefore, for each C-peptide and insulin response variable, a model was constructed including the effect of M/I and both of these group terms. A three-way interaction for M/I by youth/adult and IGT/type 2 diabetes was run first to assess whether the four separate slopes of the response (e.g., C-peptide) on M/I differed, i.e., whether the slopes for adults with IGT, adults with diabetes, youth with IGT, and youth with diabetes were significantly different. In no case was this true, so subsequent models were built including two-way interaction terms for [diabetes status * M/I] and [age-group * M/I]. If both two-way interaction tests were not significant (i.e., the slopes in the two age-groups or diabetes status groups were parallel), a simple model was constructed, including terms for diabetes status and age-group without any interaction variables. Parallel slopes in these regression models indicate that differences in β-cell responses are proportionate across the range of insulin sensitivity.
Results
Demographic, Physical, and Glucose Tolerance Characteristics of the Cohort
Select baseline characteristics of the RISE cohort are presented in Table 1 for the two adult and one youth protocols and for all adults combined. Youth included a greater proportion of females and a larger proportion of nonwhite participants. There were also more women and nonwhite participants in the adult surgical protocol than in the adult medication protocol. Youth had a slightly greater BMI and triponderal index (20) than adults, but waist circumference did not differ. HbA1c did not differ significantly between the two age-groups.
Table 1.
Select baseline physical and demographic characteristics, insulin sensitivity, and β-cell responses from the hyperglycemic clamp for youth and all adults
| Pediatric medication (n = 66) [1] | Adult medication (n = 267) [2] | Adult surgery (n = 88) [3] | All adults (n = 355) [4] | P (ANOVA) (1 vs. 2 vs. 3) | P (all adults vs. pediatric) (1 vs. 4) | |
|---|---|---|---|---|---|---|
| Demographic characteristics | ||||||
| Age (years) | 14.2 ± 2.0 | 53.9 ± 8.9 | 49.1 ± 9.8 | 52.7 ± 9.4 | <0.001 | <0.001 |
| Female, n (%) | 47 (71.2) | 114 (42.7) | 69 (78.4) | 183 (51.5) | <0.001 | 0.005 |
| Race/ethnicity, n (%) | <0.001 | <0.001 | ||||
| White | 19 (28.8) | 141 (52.8) | 25 (28.4) | 166 (46.8) | ||
| Black | 14 (21.2) | 81 (30.3) | 16 (18.2) | 97 (27.3) | ||
| Hispanic | 25 (37.9) | 28 (10.5) | 40 (45.5) | 68 (19.2) | ||
| Asian | 2 (3.0) | 11 (4.1) | 7 (8.0) | 18 (5.1) | ||
| American Indian | 0 (0.0) | 1 (0.4) | 0 (0.0) | 1 (0.3) | ||
| Mixed | 6 (9.1) | 4 (1.5) | 0 (0.0) | 4 (1.1) | ||
| Other | 0 (0.0) | 1 (0.4) | 0 (0.0) | 1 (0.3) | ||
| Weight (kg) | 98.9 ± 22.6 | 102.1 ± 19.8 | 96.8 ± 11.4 | 100.8 ± 18.2 | 0.057 | 0.454 |
| BMI (kg/m2) | 36.6 ± 6.0 | 35.0 ± 5.7 | 35.4 ± 2.8 | 35.1 ± 5.1 | 0.093 | 0.035 |
| Triponderal index (kg/m3) | 22.4 ± 3.5 | 20.6 ± 3.6 | 21.5 ± 2.0 | 20.8 ± 3.3 | <0.001 | <0.001 |
| Waist circumference (cm) | 109.0 ± 14.2 | 111.8 ± 13.5 | 105.8 ± 7.5 | 110.3 ± 12.6 | <0.001 | 0.475 |
| Glycemic characteristics | ||||||
| HbA1c (mmol/mol) | 38.54 ± 6.11 | 39.30 ± 4.24 | 40.66 ± 4.55 | 39.64 ± 4.35 | 0.012 | 0.080 |
| IGT, n (%) | 53 (80.3) | 197 (73.8) | 54 (61.4) | 251 (70.7) | 0.022 | 0.147 |
| Hyperglycemic clamp parameters | ||||||
| Fasting glucose (mmol/L) | 6.03 ± 0.97 | 6.09 ± 0.56 | 6.18 ± 0.83 | 6.11 ± 0.63 | 0.416 | 0.430 |
| Fasting C-peptide (nmol/L) | 1.67 ± 0.53 | 1.22 ± 0.47 | 1.23 ± 0.38 | 1.22 ± 0.45 | <0.001 | <0.001 |
| Fasting insulin (pmol/L) | 214.1 (62.2, 736.7) | 103.6 (36.6, 293.5) | 112.9 (39.9, 319.0) | 105.8 (37.3, 299.9) | <0.001 | <0.001 |
| ACPRg (nmol/L) | 1.24 (0.14, 11.18) | 0.57 (0.10, 3.25) | 0.49 (0.07, 3.46) | 0.55 (0.09, 3.31) | <0.001 | <0.001 |
| AIRg (pmol/L) |
499.3 (55.4, 4,501.5) |
174.4 (23.7, 1,281.3) |
135.2 (11.0, 1,657.4) |
163.8 (19.3, 1,390.6) |
<0.001 |
<0.001 |
| Steady-state (second-phase) C-peptide response (nmol/L) | 5.19 (2.50, 10.74) | 3.95 (1.96, 7.98) | 3.55 (1.60, 7.84) | 3.85 (1.85, 7.99) | <0.001 | <0.001 |
| Steady-state (second-phase) insulin response (pmol/L) | 1,370.3 (298.6, 6,288.0) | 636.3 (157.4, 2,572.8) | 539.1 (123.4, 2,355.7) | 610.7 (147.4, 2,530.4) | <0.001 | <0.001 |
| ACPRmax (nmol/L) | 7.85 (3.7, 16.66) | 4.89 (2.03, 11.78) | 4.72 (1.6, 13.93) | 4.85 (1.91, 12.32) | <0.001 | <0.001 |
| AIRmax (pmol/L) | 5,409.4 (2,196.2, 13,323.6) | 3,144.1 (1,113.4, 8,878.5) | 3,084.2 (897.1, 10,603.7) | 3,129.1 (1,053.4, 9,295.3) | <0.001 | <0.001 |
| Glucose disposal rate (mmol/kg/min) | 0.025 ± 0.010 | 0.022 ± 0.010 | 0.021 ± 0.012 | 0.021 ± 0.010 | 0.020 | 0.007 |
| M/I (×10−5 mmol/kg/min per pmol/L) | 1.69 (0.37, 7.69) | 3.06 (0.72, 12.97) | 3.35 (0.89, 12.53) | 3.13 (0.76, 12.87) | <0.001 | <0.001 |
| Fasting C-peptide/fasting insulin (×10−2 nmol/pmol) | 0.75 (0.31, 1.81) | 1.1 (0.61, 1.99) | 1.04 (0.61, 1.76) | 1.09 (0.61, 1.93) | <0.001 | <0.001 |
Data are mean ± SD or geometric mean (95% CI) unless otherwise indicated. P values for nonnormally distributed data based on log-transformed values. “Other” for race/ethnicity includes mixed, Asian, American Indian, and other.
Supplementary Table 1 presents select baseline characteristics of the 304 participants with IGT and 117 with diabetes in the RISE cohort. The two groups, each comprised of both youth and adults, were well matched for physical and demographic characteristics and similar in their racial/ethnic distribution.
Clamp-Derived Measurements in Youth Versus Adults
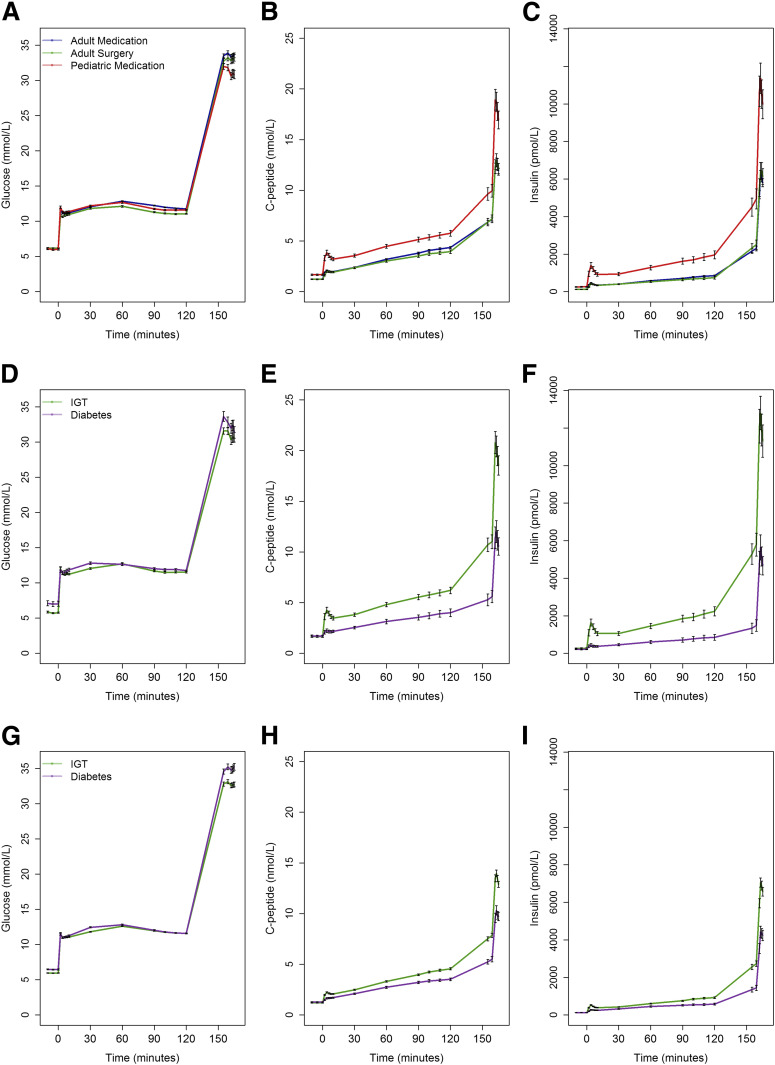
The plasma glucose, C-peptide, and insulin concentrations during the two-step hyperglycemic clamp are illustrated in Fig. 1A–C for all individuals within each protocol. Fasting glucose concentrations were lower in youth than adults (Table 1). Glucose goals were achieved and maintained in all three protocols. At all time points during the clamp, C-peptide and insulin concentrations were greater in youth than adults (all P < 0.001). Throughout the clamp procedures, plasma C-peptide and insulin concentrations were similar in adults in the medication and surgical protocols; thus, data from the two adult protocols were pooled for subsequent analyses.
Figure 1.
Plasma glucose, C-peptide, and insulin concentrations during the hyperglycemic clamps in youth and adults in the three RISE protocols. A–C: Pediatric Medication Study (n = 66 [in red]), Adult Medication Study (n = 267 [in blue]) and Adult Surgery Study (n = 88 [in green]). D–F: Youth with IGT (n = 53 [in green]) and diabetes (n = 13 [in purple]). G–I: Adults with IGT (n = 251 [in green]) and diabetes (n = 104 [in purple]). Data are mean ± SEM. At all time points after commencement of the glucose infusion for the clamp, C-peptide and insulin concentrations were greater in youth than adults (all P < 0.001) as well as in IGT vs. diabetes (all P < 0.001).
Fasting C-peptide and insulin concentrations were higher in youth than in adults (Table 1). M/I in youth was approximately half that of adults. For C-peptide and insulin, the first-phase responses, steady-state concentrations, and arginine responses were significantly greater in youth than in adults (Table 1 and Fig. 1B and C).
Clamp-Derived Measurements in IGT Versus Diabetes
Plasma glucose concentrations achieved during the clamp were the same in participants with IGT and recently diagnosed diabetes (Fig. 1D and G). Concentrations of fasting C-peptide and insulin were not different in those with IGT and diabetes (Supplementary Table 1). In both youth (Fig. 1E and F) and adults (Fig. 1H and I), C-peptide and insulin concentrations were greater in participants with IGT than in those with diabetes at all time points after commencement of the glucose infusion.
M/I was significantly lower in those with IGT, while all β-cell responses were greater in those with IGT (Supplementary Table 1). The differences in β-cell responses remained significant after adjustment for M/I (all P < 0.001).
Relationship of Insulin Sensitivity With β-Cell Responses in Youth Versus Adults
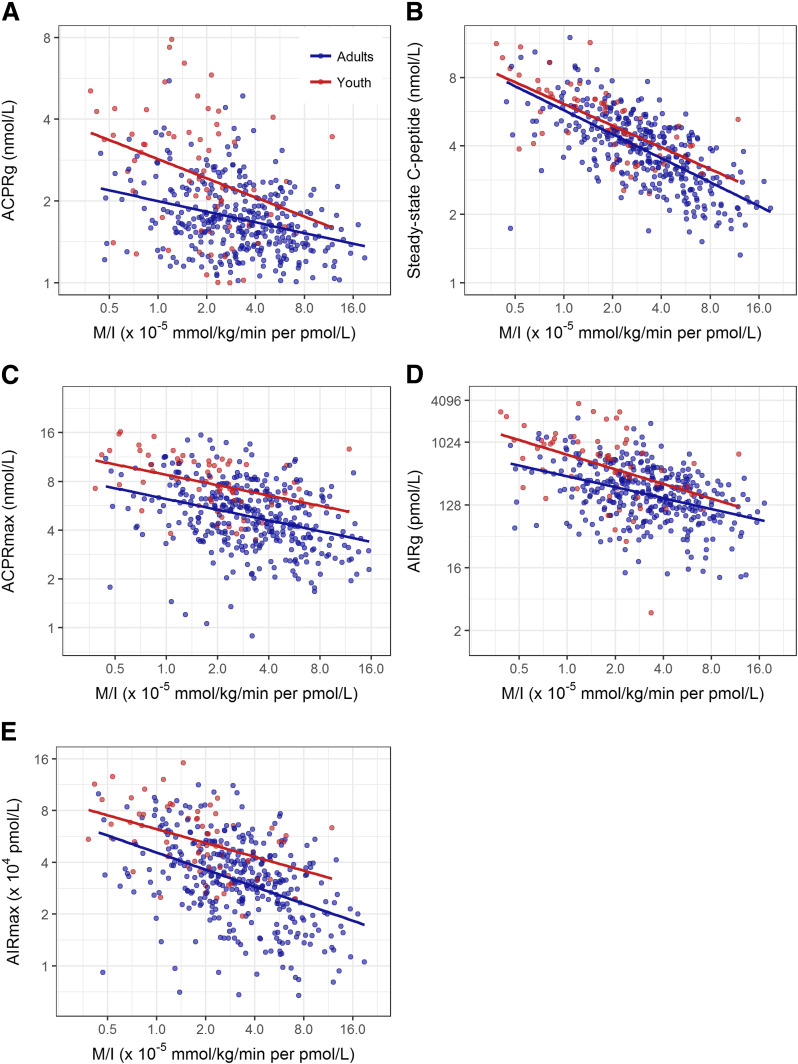
The relationship between log-transformed M/I and log-transformed first-phase, steady-state, and maximal C-peptide responses demonstrated significant inverse linear relationships in both youth and adults (all P < 0.001) (Fig. 2A–C [these panels illustrate the differences in distribution of M/I and β-cell responses between youth and adults]). The slopes relating log M/I with log ACPRg, log steady-state C-peptide, and log ACPRmax did not differ between youth and adults (P = 0.200, P = 0.357, and 0.780, respectively) (Fig. 2A–C). Across the range of M/I, ACPRg, steady-state C-peptide, and ACPRmax were greater in youth than adults (P < 0.001 for both ACPRg and ACPRmax and P = 0.047 for steady-state C-peptide). Details of these relationships are provided in Supplementary Table 2. Supplementary Fig. 2A–C illustrates these relationships using natural scale data.
Figure 2.
Relationship of log-transformed M/I and log-transformed ACPRg (A), steady-state (second phase) C-peptide concentration (B), ACPRmax (C), AIRg (D), and AIRmax (E) in youth (n = 66 [in red]) and adults (n = 355 [in blue]). The axes are logged with the values on each being natural numbers. Lines were fit by linear regression on the log-log scale. The slopes relating the five β-cell response measures to M/I were all significant (P < 0.001), and the group differences were also all significant (all P < 0.001 except steady-state C-peptide [P = 0.047]). The slopes for youth and adults did not differ (all P ≥ 0.200).
The insulin responses are presented in Fig. 2D and E and Supplementary Fig. 2D and E; as for C-peptide, the figures highlight the difference in distribution of the data in youth and adults. Values for coefficients for the relationships of log-transformed data are shown in Supplementary Table 2. For both AIRg and AIRmax, the slopes for youth and adults were significantly inversely related (all P < 0.001) and parallel (P = 0.465 and 0.343, respectively). Across the range of M/I, these two responses were significantly greater in youth (each P < 0.001).
In summary, across the range of insulin sensitivity, the acute C-peptide and insulin responses to glucose and arginine at maximal glycemic potentiation, as well as steady-state C-peptide concentrations, were greater in youth than in adults.
Relationship of Insulin Sensitivity With β-Cell Responses in IGT Versus Diabetes
After log transformation, the relationships between M/I and first-phase, steady-state, and maximal C-peptide responses were significantly inversely related in both IGT and diabetes (all P < 0.001) (Fig. 3A–C). The slope relating M/I to ACPRg was significantly different between those with IGT and diabetes (P = 0.031), with the slope for IGT slightly steeper than that for diabetes (Fig. 3A). Slopes for steady-state C-peptide and ACPRmax were not significantly different between IGT and diabetes (P = 0.204 and P = 0.965, respectively) (Fig. 3B and C). For steady-state C-peptide and ACPRmax, across the range of M/I the responses were lower in those with diabetes versus IGT (both P < 0.001). Supplementary Fig. 3A–C illustrates these same relationships for C-peptide using nontransformed data.
Figure 3.
Relationship of log-transformed M/I and log-transformed ACPRg (A), steady-state (second phase) C-peptide concentration (B), ACPRmax (C), AIRg (D), and AIRmax (E) in participants with IGT (n = 304 [in green]) and diabetes (n = 117 [in purple]). The axes are logged with the values on each being natural numbers. Lines were fit by linear regression on the log-log scale. The slopes relating the five β-cell response measures to M/I were all significant (P < 0.001). Slopes for M/I and ACPRg in individuals with IGT or diabetes were significantly different (P = 0.031). For all other β-cell response measures, the slopes with M/I were not significantly different between participants with IGT or diabetes (all P > 0.200), and participants with IGT had higher β-cell responses across the range of M/I (all P < 0.001).
In consideration of insulin responses, the slopes of log M/I versus log AIRg and log AIRmax were significantly inversely related (all P < 0.001) and did not differ between the IGT and diabetes groups (P = 0.633 and P = 0.757, respectively) (Fig. 3D and E). These parallel slopes were associated with reduced responses in those with diabetes—lower across the range of M/I for AIRg and AIRmax (P < 0.001 for both). Supplementary Fig. 2D and E presents these same relationships plotted on the natural scale.
In summary, for youth and adults, across the range of insulin sensitivity, the acute and maximal C-peptide and insulin responses and the steady-state C-peptide response were greater in individuals with IGT compared with those with diabetes.
Relationship of Fasting Glucose With β-Cell Responses
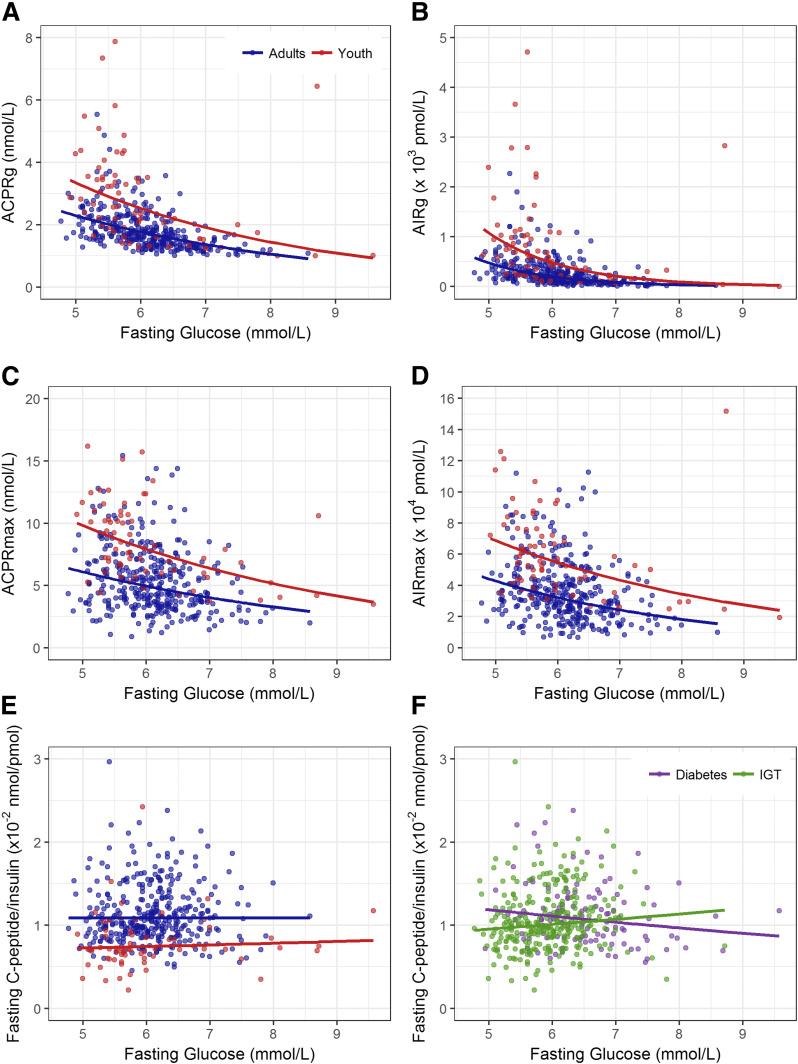
As fasting glucose is associated with the first-phase insulin response, we next examined whether the relationship of fasting glucose with ACPRg and AIRg differed in youth and adults. As depicted in Fig. 4A and B, the magnitude of both ACPRg and AIRg declined as the fasting glucose concentration increased, with the response being markedly diminished in those with a fasting glucose in the diabetes range. A negative ACPRg was observed in five youth and seven adults. The relationships between fasting glucose and both ACPRg and AIRg were significant in youth (each P < 0.001) and adults (each P < 0.001). The responses in youth were greater than in adults across the range of glucose concentration, even after adjustment for M/I (P < 0.001).
Figure 4.
Relationship of fasting glucose and ACPRg (A) and AIRg (B), ACPRmax (C), and AIRmax (D) and fasting glucose and the ratio of fasting C-peptide to insulin in youth (n = 66 [in red]) and adults (n = 355 [in blue]) (E), and IGT (n = 304 [in green]) and diabetes (n = 117 [in purple]) (F). Lines were fit by linear regression of the log-transformed variables and then transformed back to the original scale for plotting. Prior to taking logs, a constant of 1.06 was added to ACPRg and 10.0 to AIRg because of negative values in these variables. Therefore, all plotted values appear greater than 0. The slopes relating the β-cell response measures to fasting glucose were all significant (all P < 0.001), and the group differences were also all significant (all P < 0.001). The slopes for youth and adults did not differ (all P ≥ 0.21). For the relation of the fasting C-peptide–to–insulin ratio to fasting glucose, there were no significant relationships. Youth had a ratio that was lower than adults across the fasting glucose range (P < 0.001), while there was no difference across the same glucose range between IGT and diabetes.
The C-peptide and insulin responses to arginine (ACPRmax and AIRmax) also decreased with increasing fasting glucose (Fig. 4C and D). Unlike the first-phase responses, these maximal responses were still largely present, although diminished, in those with a fasting glucose in the diabetes range. The inverse relationships between fasting glucose and both log ACPRmax and log AIRmax were significant in youth and adults (all P < 0.001). The maximal responses in youth were greater than in adults across the range of fasting glucose concentration and remained so after the difference in M/I was accounted for (P < 0.001).
These observations indicate that even after fasting glucose and insulin sensitivity are accounted for, β-cells in youth are more responsive to acute stimulation with intravenous glucose or arginine than are β-cells of adults.
Estimation of Insulin Clearance in Youth Versus Adults and IGT Versus Diabetes
The ratio of C-peptide to insulin was calculated in the fasting state as an estimate of insulin clearance. This ratio was significantly lower in youth (Table 1), suggesting lesser insulin clearance in youth compared with adults. This difference in the ratio was not related to the glucose concentration, being consistently lower in youth across the fasting glucose range (Fig. 4E). Insulin clearance was not statistically different in people with IGT or diabetes (Table 1 and Fig. 4F).
Conclusions
The pathogenesis of type 2 diabetes in youth and adults has been examined separately in cross-sectional studies (6–9). RISE is the first large study that included youth and adults and has applied identical, sophisticated, and quantitative methodologies including performance of all assays in a central laboratory, thereby allowing direct comparisons of β-cell function and insulin sensitivity between youth and adults. Using this approach, we report many novel observations that advance our understanding of apparent differences between youth and adults in the progression of dysglycemia (10–12).
In RISE, insulin sensitivity in youth was 46% lower than in comparably overweight dysglycemic adults. Differences in body adiposity and/or effects of puberty (21,22) may explain some of this lower insulin sensitivity in youth. Acute C-peptide and insulin responses to glucose and the nonglucose secretagogue arginine were greater in youth, as well as greater than required to compensate for measured differences in insulin sensitivity. Interestingly, C-peptide concentrations measured at a steady-state glucose of ∼11.1 mmol/L were also significantly greater in youth than adults, but the relative difference was smaller than for the acute responses. These observations suggest that in youth, the β-cell is hyperresponsive to acute stimulation by glucose and nonglucose (arginine) secretagogues, releasing greater amounts of C-peptide and insulin from the readily releasable pool (23). The difference in response between groups is less evident after prolonged glucose exposure, a condition of continuous stimulation that is more dependent on release of more recently synthesized peptide (23). Our findings are compatible with a recent study that used oral glucose tolerance test modeling of pooled data and showed that youth with impaired glucose metabolism were insulin resistant with increased parameters of β-cell responsiveness compared with adults (24). In the same study, on the other hand, youth and adults with normal glucose tolerance or type 2 diabetes did not differ for insulin sensitivity, while certain, but not all, parameters of β-cell responsiveness were greater in youth (24). Longitudinal studies are necessary to determine whether the changes in β-cell function and insulin sensitivity over time differ in youth and adults and whether they explain why the disease appears to be more aggressive in youth (10–12).
It has long been recognized that the first-phase insulin response to intravenous glucose decreases as the fasting glucose concentration increases and that this response is essentially absent in adults with diabetes (25). In adults who are hyperglycemic, this calculated response can even be negative (26). We have extended these observations. First, across the range of fasting glucose concentrations and after adjustment for insulin sensitivity, the first-phase response remains greater in youth than adults, in keeping with β-cells of youth responding more vigorously. Second, we describe for the first time in youth that negative first-phase responses occur, as with adults, in some individuals who are hyperglycemic. Third, for β-cell secretory capacity (ACPRmax and AIRmax), there is an inverse relationship with fasting glucose, and these responses are greater in youth than in adults. However, unlike the first-phase response, in both youth and adults, the responses to arginine at maximal glycemic potentiation were measurably greater than zero, even in individuals with the highest fasting glucose concentrations (9.6 mmol/L). This difference in relationships between fasting glucose and the acute responses to glucose and arginine suggests that the lack of a β-cell response to intravenous stimulation is limited to glucose in both age-groups. Further, it suggests the two parameters are measuring different β-cell characteristics.
Our data also suggest that the liver is playing an important role in regulating glucose homeostasis and that this effect is different in the two age-groups, with a lower fasting C-peptide–to–insulin ratio in youth. This observation suggests that hepatic insulin clearance is reduced in youth, thus allowing a greater amount of insulin to appear in the periphery. We can only provide conjecture on the genesis of this difference. First, it seems likely that there exists an as yet undefined feedback system linking reduced peripheral insulin sensitivity with hepatic clearance, and since youth with dysglycemia have a marked reduction in insulin sensitivity (24,27,28), this feedback mechanism may be contributing to differences in hepatic clearance. The consistent decrease in insulin clearance in youth compared with adults across the range of fasting glucose suggests that glucose is not this putative factor. Second, it is possible that a protective mechanism exists that modulates hepatic extraction in order to mitigate the increased workload on β-cells in circumstances of high secretion, with differential actions in youth and adults or in circumstances of hypersecretion. It is known in adults that hepatic glucose production is more sensitive to insulin than is glucose utilization by the peripheral tissues (29), but whether this is also the case in youth is unclear. Further work is warranted to explore determinants or regulators of insulin extraction in youth versus adults.
We observed parallel but shifted relationships between insulin sensitivity and β-cell responses in IGT and diabetes, with lower C-peptide and insulin responses in those with diabetes. This is consistent with the concept that hyperglycemia results from impaired β-cell function in recently diagnosed type 2 diabetes. Of note, it is apparent in these data that this difference in β-cell function is not due to differences in insulin clearance as we observed in youth compared with adults.
There are some limitations to our study. First, we did not directly quantify body fat or its distribution to evaluate possible contributions to the greater insulin resistance in youth. Future assessment of biomarkers of fat mass could shed some light on this issue. Second, we used the hyperglycemic clamp to simultaneously quantify β-cell responsiveness and insulin sensitivity, but this approach did not explore differences in tissue-specific responses to insulin between youth and adults. Third, we used the fasting C-peptide–to–insulin ratio as a surrogate estimate of insulin clearance and did not measure it directly. Fourth, we have focused on the two major β-cell products, C-peptide and insulin; however, there are a number of islet cell peptides of potential interest that we did not measure such as proinsulin, islet amyloid polypeptide (IAPP), or glucagon. Samples have been stored to allow for the measurement of analytes such as these should funding become available.
In conclusion, we found that youth with IGT or recently diagnosed type 2 diabetes are markedly more insulin resistant and have β-cells that are hyperresponsive to acute stimulation compared with adults with a similar degree of dysglycemia. Further, these β-cell responses in youth were enhanced even after insulin sensitivity was accounted for, suggesting that the workload their β-cells are experiencing is greater than that observed in adults. To the extent that increased workload contributes to β-cell decline (11,30), these findings may represent fundamental differences between youth and adults in the pathogenesis of type 2 diabetes or in the rates at which diabetes develops and progresses. The two groups also exhibited differences in insulin clearance that may, in youth, be a compensatory mechanism to provide sufficient insulin to the peripheral tissues to aid in appropriate and similar glucose disposal while simultaneously reducing secretory demand on the β-cell. Whether these observed differences between youth and adults will also result in differences in the response to the same medication interventions (metformin alone or basal insulin followed by metformin) in their β-cell function will be answered by the RISE Study.
Supplementary Material
Article Information
Acknowledgments. The RISE Consortium thanks the RISE Data and Safety Monitoring Board and Barbara Linder, the National Institute of Diabetes and Digestive and Kidney Diseases Program Official for RISE (Rockville, MD), for support and guidance. The Consortium also thanks the participants who, by volunteering, are furthering the ability to reduce the burden of diabetes.
Funding and Duality of Interest. RISE is supported by grants from the National Institutes of Health (U01-DK-094406, U01-DK-094430, U01-DK-094431, U01-DK-094438, U01-DK-094467, P30-DK-017047, P30-DK-020595, P30-DK-045735, P30-DK-097512, UL1-TR-000430, UL1-TR-001082, UL1-TR-001108, UL1-TR-001855, UL1-TR-001857, UL1-TR-001858, and UL1-TR-001863), the Department of Veterans Affairs, and Kaiser Permanente Southern California. Additional financial and material support from the American Diabetes Association, Allergan, Apollo Endosurgery, Abbott Laboratories, and Novo Nordisk was received is gratefully acknowledged. S.E.K. and S.A.A. serve as paid consultants on advisory boards for Novo Nordisk. S.A.A. is a participant in a Novo Nordisk–sponsored clinical trial. T.A.B. has received research support from Allergan and Apollo Endosurgery. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. Members of the RISE Consortium recruited participants and collected study data. S.E.K. and S.A.A. proposed the analysis, interpreted data, and wrote and edited the manuscript, which was also reviewed and edited by members of the writing group. The RISE Steering Committee reviewed and edited the manuscript and approved its submission. S.E.K. and S.L.E. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in oral form at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018.
Appendix
Writing Group:
Steven E. Kahn (co-chair), Silva A. Arslanian (co-chair), Thomas A. Buchanan, Sharon L. Edelstein, David A. Ehrmann, Kristen J. Nadeau, Jerry P. Palmer, and Kristina M. Utzschneider.
Footnotes
Clinical trial reg. nos. NCT01779362, NCT01779375, and NCT01763346, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-0244/-/DC1.
*A complete list of the RISE Consortium Investigators can be found in the Supplementary Data online.
Contributor Information
Collaborators: David A. Ehrmann, Karla A. Temple, Abby Rue, Elena Barengolts, Babak Mokhlesi, Eve Van Cauter, Susan Sam, M. Annette Miller, Steven E. Kahn, Karen M. Atkinson, Jerry P. Palmer, Kristina M. Utzschneider, Tsige Gebremedhin, Abigail Kernan-Schloss, Alexandra Kozedub, Brenda K. Montgomery, Emily J. Morse, Kieren J. Mather, Tammy Garrett, Tamara S. Hannon, Amale Lteif, Aniket Patel, Robin Chisholm, Karen Moore, Vivian Pirics, Linda Pratt, Kristen J. Nadeau, Susan Gross, Philip S. Zeitler, Jayne Williams, Melanie Cree-Green, Yesenia Garcia Reyes, Krista Vissat, Silva A. Arslanian, Kathleen Brown, Nancy Guerra, Kristin Porter, Sonia Caprio, Mary Savoye, Bridget Pierpont, Thomas A. Buchanan, Anny H. Xiang, Enrique Trigo, Elizabeth Beale, Fadi N. Hendee, Namir Katkhouda, Krishan Nayak, Mayra Martinez, Cortney Montgomery, Xinhui Wang, Sharon L. Edelstein, John M. Lachin, Ashley N. Hogan, Santica Marcovina, Jessica Harting, John Albers, Dave Hill, Peter J. Savage, and Ellen W. Leschek
References
- 1.Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. . IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 2017;128:40–50 [DOI] [PubMed] [Google Scholar]
- 2.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al.; SEARCH for Diabetes in Youth Study . Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med 2017;376:1419–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017 [article online], 2017. Available from https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed 18 July 2017
- 4.World Health Organzation. Global Health Observatory (GHO) data. Overweight and obesity [Internet], 2017. Available from http://www.who.int/gho/ncd/risk_factors/overweight/en/. Accessed 24 August 2017
- 5.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. JAMA 2018;319:1723–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen CC, Cnop M, Hull RL, Fujimoto WY, Kahn SE; American Diabetes Association GENNID Study Group . β-Cell function is a major contributor to oral glucose tolerance in high-risk relatives of four ethnic groups in the U.S. Diabetes 2002;51:2170–2178 [DOI] [PubMed] [Google Scholar]
- 7.Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest 2005;115:485–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss R, Caprio S, Trombetta M, Taksali SE, Tamborlane WV, Bonadonna R. β-Cell function across the spectrum of glucose tolerance in obese youth. Diabetes 2005;54:1735–1743 [DOI] [PubMed] [Google Scholar]
- 9.Burns SF, Bacha F, Lee SJ, Tfayli H, Gungor N, Arslanian SA. Declining β-cell function relative to insulin sensitivity with escalating OGTT 2-h glucose concentrations in the nondiabetic through the diabetic range in overweight youth. Diabetes Care 2011;34:2033–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.TODAY Study Group A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012;366:2247–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahn SE, Haffner SM, Heise MA, et al.; ADOPT Study Group . Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–2443 [DOI] [PubMed] [Google Scholar]
- 12.TODAY Study Group Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY. Diabetes Care 2013;36:1749–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The RISE Consortium Restoring Insulin Secretion (RISE): design of studies of β-cell preservation in prediabetes and early type 2 diabetes across the life span. Diabetes Care 2014;37:780–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andres R, Swerdloff R, Posefsky T, Coleman DL. Manual feedback technique for the control of blood glucose concentration. In Automation in Analytical Chemistry. Skeegs LT, Jr., Ed. New York, Mediad, 1966, p. 486–491 [Google Scholar]
- 15.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 16.Elahi D. In praise of the hyperglycemic clamp. A method for assessment of β-cell sensitivity and insulin resistance. Diabetes Care 1996;19:278–286 [DOI] [PubMed] [Google Scholar]
- 17.Kahn SE, Prigeon RL, McCulloch DK, et al. . Quantification of the relationship between insulin sensitivity and β-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 1993;42:1663–1672 [DOI] [PubMed] [Google Scholar]
- 18.Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D Jr. Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest 1984;74:1318–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castillo MJ, Scheen AJ, Letiexhe MR, Lefèbvre PJ. How to measure insulin clearance. Diabetes Metab Rev 1994;10:119–150 [DOI] [PubMed] [Google Scholar]
- 20.Peterson CM, Su H, Thomas DM, et al. . Tri-ponderal mass index vs body mass index in estimating body fat during adolescence. JAMA Pediatr 2017;171:629–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amiel SA, Caprio S, Sherwin RS, Plewe G, Haymond MW, Tamborlane WV. Insulin resistance of puberty: a defect restricted to peripheral glucose metabolism. J Clin Endocrinol Metab 1991;72:277–282 [DOI] [PubMed] [Google Scholar]
- 22.Hannon TS, Janosky J, Arslanian SA. Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr Res 2006;60:759–763 [DOI] [PubMed] [Google Scholar]
- 23.Curry DL, Bennett LL, Grodsky GM. Dynamics of insulin secretion by the perfused rat pancreas. Endocrinology 1968;83:572–584 [DOI] [PubMed] [Google Scholar]
- 24.Chen ME, Chandramouli AG, Considine RV, Hannon TS, Mather KJ. Comparison of β-cell function between overweight/obese adults and adolescents across the spectrum of glycemia. Diabetes Care 2018;41:318–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunzell JD, Robertson RP, Lerner RL, et al. . Relationships between fasting plasma glucose levels and insulin secretion during intravenous glucose tolerance tests. J Clin Endocrinol Metab 1976;42:222–229 [DOI] [PubMed] [Google Scholar]
- 26.Metz SA, Halter JB, Robertson RP. Paradoxical inhibition of insulin secretion by glucose in human diabetes mellitus. J Clin Endocrinol Metab 1979;48:827–835 [DOI] [PubMed] [Google Scholar]
- 27.Kelsey MM, Forster JE, Van Pelt RE, Reusch JE, Nadeau KJ. Adipose tissue insulin resistance in adolescents with and without type 2 diabetes. Pediatr Obes 2014;9:373–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arslanian S, Kim JY, Nasr A, et al. . Insulin sensitivity across the lifespan from obese adolescents to obese adults with impaired glucose tolerance: who is worse off? Pediatr Diabetes 2018;19:205–211 [DOI] [PubMed] [Google Scholar]
- 29.Rizza RA, Mandarino LJ, Gerich JE. Dose-response characteristics for effects of insulin on production and utilization of glucose in man. Am J Physiol 1981;240:E630–E639 [DOI] [PubMed] [Google Scholar]
- 30.Buchanan TA, Xiang AH, Peters RK, et al. . Preservation of pancreatic β-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes 2002;51:2796–2803 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.