Abstract
OBJECTIVE
To compare oral glucose tolerance test (OGTT) glucose, C-peptide, and insulin responses and insulin sensitivity in youth and adults with impaired glucose tolerance (IGT) or recently diagnosed type 2 diabetes.
RESEARCH DESIGN AND METHODS
A total of 66 youth (80.3% with IGT) and 355 adults (70.7% with IGT) underwent a 3-h OGTT to assess 1) insulin sensitivity (1/fasting insulin), 2) C-peptide index (CPI) and insulinogenic index (IGI) over the first 30 min, and 3) glucose, C-peptide, and insulin incremental areas above fasting over the 3-h post-ingestion (incremental glucose [G-iAUC], incremental C-peptide [CP-iAUC], and incremental insulin area under the curve [I-iAUC] responses, respectively).
RESULTS
Fasting, 2-h glucose, and G-iAUC were similar in both age-groups, but youth had ∼50% lower 1/fasting insulin (P < 0.001), 75% higher CPI (mean [95% CI] 0.703 [0.226, 2.183] vs. 0.401 [0.136, 1.183] nmol/mmol; P < 0.001), and more than twofold higher IGI (257.3 [54.5, 1,215.8] vs. 114.8 [28.0, 470.8] pmol/mmol; P < 0.001). Two-hour C-peptide and insulin concentrations, CP-iAUC, and I-iAUC were all higher in youth (all P < 0.001). C-peptide and insulin responses remained significantly greater in youth after adjustment for insulin sensitivity. Within each age-group, individuals with type 2 diabetes versus IGT had significantly lower CPI and IGI with no difference in insulin sensitivity.
CONCLUSIONS
The balance between insulin sensitivity and β-cell responses differs between youth and adults with IGT or recently diagnosed type 2 diabetes. Despite similar postload glucose levels, youth demonstrate greater C-peptide and insulin responses that exceed what is needed to compensate for their lower insulin sensitivity. Longitudinal studies are required to determine whether this feature contributes to a more rapid decline in β-cell function in youth with dysglycemia.
Introduction
With the epidemic of pediatric obesity (1), the number of youth with prediabetes and type 2 diabetes continues to increase rapidly (2). Cross-sectional, observational, and therapeutic trials suggest that type 2 diabetes in these younger individuals might represent a more severe and rapidly progressive condition than in adults (3). These observations provide urgency to better understand the similarities and differences in disease pathogenesis between youth and adults in order to design appropriate intervention strategies to slow or halt diabetes progression.
The Restoring Insulin Secretion (RISE) Study is examining interventional approaches to preserve or improve β-cell function in youth and adults with impaired glucose tolerance (IGT) or recently diagnosed type 2 diabetes (4). These different approaches—medical (metformin, insulin glargine, and liraglutide) and lap band surgery—are being tested in a seven-center study of youth and adults in the U.S. using common outcome measurements and a central laboratory. In this manuscript, we describe results from the oral glucose tolerance test (OGTT) performed at baseline. We compare indices of insulin sensitivity and β-cell function derived from the integrated physiologic responses to glucose ingestion in both youth and adults. These analyses parallel, complement, and expand on those made using hyperglycemic clamps that are presented in an accompanying article (5).
Research Design and Methods
Participants
Youth aged 10–19 years with pubertal development assessed by a RISE Study pediatric endocrinologist to be Tanner stage ≥II (females, Tanner stage ≥II breast development; and males, testicular volume >3 mL) and adults at high risk for IGT and type 2 diabetes were screened with a 75-g OGTT and hemoglobin A1c (HbA1c). To qualify, participants needed to have an elevated 2-h glucose concentration (≥7.8 mmol/L) and meet additional specific protocol-based criteria for fasting glucose and HbA1c as detailed in a companion article (5). Youth were required to have a BMI ≥85th percentile for age and for adults, ≥25 kg/m2 (6). Additional details on participant recruitment and eligibility criteria have been described (4), and information is available for all three protocols at the RISE website (https://rise.bsc.gwu.edu/web/rise/collaborators).
For this analysis, we combined all participants in the Adult Surgery Study (BetaFat; n = 88) and Adult Medication Study (n = 267) and compared them to 66 drug-naïve (53 with IGT and 13 with type 2 diabetes) participants in the Pediatric Medication Study. Twenty-five youth treated with metformin at baseline or previously were excluded from the present analysis to obviate potential confounding from this exposure. All adults (n = 355; 251 with IGT and 104 with type 2 diabetes) were, per protocol, naïve for glucose-lowering medications. Classification of glucose tolerance in youth and adults was based on American Diabetes Association OGTT criteria for fasting and 2-h glucose (7).
All participants gave written informed consent/assent, consistent with the Declaration of Helsinki and the guidelines of each center’s institutional review board.
Anthropometric Measurements
Anthropometric measurements were performed with participants wearing light clothing without shoes. Waist circumference was measured in a horizontal plane at the midpoint between the top of the iliac crest and the bottom of the costal margin in the midaxillary line using a fiberglass (nonstretching) tape. Height was measured in a fully vertical position using a calibrated stadiometer with the heels together. Weight was measured using a calibrated electronic scale, zeroed before each measurement. From these measurements, BMI and triponderal index (8) were calculated.
Procedures
Following a 10-h overnight fast, a 3-h, 75-g OGTT was performed. An indwelling intravenous catheter was placed for venous sampling. After 15 min of rest, venous blood samples were drawn 10 and 5 min prior to ingesting the glucose solution, which was consumed within 5 min. Additional blood samples were obtained 10, 20, 30, 60, 90, 120, 150, and 180 min after commencing glucose ingestion. Blood samples were immediately placed on ice prior to being separated and frozen at −80°C for shipment to the central biochemistry laboratory at the University of Washington for subsequent measurement of plasma glucose, C-peptide, and insulin.
Assays
Glucose was measured by the glucose hexokinase method using Roche reagent on a c501 autoanalyzer (Roche). C-peptide and insulin were measured by a two site immunoenzymometric assay performed on the Tosoh 2000 autoanalyzer (Tosoh Bioscience, Inc., South San Francisco, CA). The interassay coefficients of variation on quality control samples with low, medium, medium-high, and high concentrations were ≤2.0% for glucose, ≤4.3% for C-peptide, and ≤3.5% for insulin. All measures are presented in Système International units. These can be converted to conventional units using standard conversion factors with the exception of insulin, for which 0.134 should be used.
Calculations for OGTT-Derived Measurements
Insulin Sensitivity and Clearance
The 1/fasting insulin (1/FI) was calculated and used as a surrogate estimate of insulin sensitivity (9,10). The ratio of fasting C-peptide to fasting insulin was used as an estimate of insulin clearance (11).
C-Peptide and Insulin Responses
The C-peptide index (CPI; ∆C30/∆G30) and insulinogenic index (IGI; ∆I30/∆G30) were calculated using the 0- and 30-min samples (12,13). The incremental glucose (G-iAUC), C-peptide (CP-iAUC), and insulin area under the curve (I-iAUC) responses above the fasting concentration over the entire 3-h sampling period were calculated as the AUC using the trapezoidal method. The ratio of the iAUC for each β-cell peptide to that for glucose (CP-iAUC/G-iAUC and I-iAUC/G-iAUC) was calculated as an additional measure of the β-cell response accounting for the prevailing glucose stimulus.
Glucose Tolerance
Individuals were classified as having IGT or diabetes based on the 2-h glucose concentration (7). The 3-h G-iAUC above fasting was also used as a measure of glucose tolerance.
Data Management and Statistical Analysis
All analyses were performed using SAS (SAS Institute, Cary, NC) and R (The R Foundation). Descriptive statistics include percentages, mean ± SD, or geometric means and 95% CI for nonnormally distributed data. For the latter, P values were calculated from log-transformed data. Comparisons between any two groups were computed using χ2 tests or Student t tests. Nominal P values are presented. Except where noted, P values <0.05 were considered statistically significant, with no adjustments made for multiple comparisons.
Linear regression models were used to evaluate the relationship of CPI, IGI, CP-iAUC/G-iAUC, and I-iAUC/G-iAUC (dependent variable) with the inverse of fasting insulin (independent variable). Models included terms to compare adults with youth and those with IGT to those with diabetes. All models used the log of β-cell response variables due to the skewedness of these data. Parallel slopes in these regression models indicate that differences in β-cell responses are proportionate across the range of insulin sensitivity.
Results
Demographic and Physical Characteristics of Youth Versus Adults and IGT Versus Type 2 Diabetes
Table 1 lists the characteristics of youth (n = 66) and adult (n = 355) RISE participants included in this analysis. Among youth, there were fewer males and fewer white participants. Despite comparable weight, BMI and triponderal index were higher in youth than adults because they were shorter. Waist circumference, however, did not differ. Among youth participants, 78.8% were Tanner stages IV or V and 21.2% stages II or III. By protocol, no participating youth were Tanner stage I (i.e., prepubertal). Among youth, 80.3% had IGT vs. 70.7% of adults. The remainder had diabetes at screening. HbA1c did not differ between youth and adults.
Table 1.
Baseline physical characteristics, demographic characteristics, and OGTT-based measures in drug-naïve youth vs. adults and in IGT vs. type 2 diabetes
| Youth (n = 66) (1) | Adults (n = 355) (2) | Youth vs. adults, P value (1 vs. 2) | IGT (n = 304) (3) | Type 2 diabetes (n = 117) (4) | IGT vs. type 2 diabetes, P value (3 vs. 4) | ||
|---|---|---|---|---|---|---|---|
| Demographic characteristics | |||||||
| Age (years) | 14.2 ± 2.0 | 52.7 ± 9.4 | <0.001 | 45.7 ± 16.9 | 49.0 ± 15.1 | 0.069 | |
| Male, n (%) | 19 (28.8) | 172 (48.5) | 0.005 | 140 (46.1) | 51 (43.6) | 0.730 | |
| Race/ethnicity, n (%) | <0.001 | 0.197 | |||||
| White | 19 (28.8) | 166 (46.8) | 140 (46.1) | 45 (38.5) | |||
| Black | 14 (21.2) | 97 (27.3) | 83 (27.3) | 28 (23.9) | |||
| Hispanic | 25 (37.9) | 68 (19.2) | 62 (20.4) | 31 (26.5) | |||
| Other | 8 (12.1) | 24 (6.8) | 19 (6.2) | 13 (11.1) | |||
| Weight (kg) | 98.9 ± 22.6 | 100.8 ± 18.2 | 0.454 | 101.1 ± 18.9 | 98.9 ± 19.1 | 0.281 | |
| Height (cm) | 163.7 ± 9.0 | 169.2 ± 9.7 | <0.001 | 168.3 ± 9.8 | 168.4 ± 9.8 | 0.934 | |
| BMI (kg/m2) | 36.6 ± 6.0 | 35.1 ± 5.1 | 0.035 | 35.6 ± 5.3 | 34.8 ± 5.2 | 0.148 | |
| Triponderal index (kg/m3) | 22.4 ± 3.5 | 20.8 ± 3.3 | <0.001 | 21.2 ± 3.4 | 20.7 ± 3.3 | 0.166 | |
| Waist circumference (cm) | 109.0 ± 14.2 | 110.3 ± 12.6 | 0.475 | 110.9 ± 13.2 | 108.3 ± 11.8 | 0.070 | |
| Glycemic characteristics | |||||||
| HbA1c (mmol/mol) | 38.54 ± 6.11 | 39.64 ± 4.35 | 0.080 | 38.42 ± 4.11 | 42.17 ± 4.99 | <0.001 | |
| Diabetes at screening, n (%) | 0.147 | — | |||||
| IGT | 53 (80.3) | 251 (70.7) | — | — | |||
| Diabetes | 13 (19.7) | 104 (29.3) | — | — | |||
| OGTT parameters | |||||||
| Fasting glucose (mmol/L) | 5.93 ± 0.93 | 6.15 ± 0.65 | 0.019 | 5.94 ± 0.56 | 6.56 ± 0.84 | <0.001 | |
| Fasting C-peptide (nmol/L) | 1.64 ± 0.587 | 1.24 ± 0.479 | <0.001 | 1.29 ± 0.479 | 1.34 ± 0.606 | 0.392 | |
| Fasting insulin (pmol/L) | 218.2 (70.4, 676.5) | 106.4 (35.6, 318.4) | <0.001 | 120.8 (36.8, 396.7) | 115.1 (32.1, 412.6) | 0.473 | |
| Two-hour glucose (mmol/L) | 9.89 ± 2.46 | 10.15 ± 2.37 | 0.404 | 9.34 ± 1.78 | 12.1 ± 2.59 | <0.001 | |
| Two-hour C-peptide (nmol/L) | 5.8 ± 2.34 | 4.7 ± 1.55 | <0.001 | 5.02 ± 1.76 | 4.57 ± 1.64 | 0.018 | |
| Two-hour insulin (pmol/L) | 1,418.7 (358.8, 5,608.9) | 788.8 (231.7, 2,685.5) | <0.001 | 907.1 (237.1, 3,469.5) | 764.9 (225.3, 2,597.3) | 0.020 | |
| Incremental OGTT AUC (0–180 min) | |||||||
| G-iAUC (mmol/L · min) | 595.9 ± 234.9 | 613.6 ± 250.7 | 0.600 | 538.7 ± 192.4 | 794.1 ± 278.6 | <0.001 | |
| CP-iAUC (nmol/L · min) | 528.7 (249.5, 1,120.1) | 424.4 (214.2, 840.7) | <0.001 | 460.4 (236.9, 894.8) | 387.8 (179.8, 836.7) | <0.001 | |
| I-iAUC (pmol/L · min) | 171,453 (49,351, 595,658) | 91,284 (28,568, 291,676) | <0.001 | 108,638 (32,184, 366,705) | 82,775 (23,038, 297,408) | <0.001 | |
| Estimates of insulin sensitivity, insulin clearance, and β-cell function | |||||||
| 1/FI [× 10−3 1/(pmol/L)] | 5.45 ± 3.88 | 10.94 ± 6.41 | <0.001 | 9.85 ± 5.96 | 10.67 ± 7.41 | 0.237 | |
| Fasting C-peptide/insulin (× 10−2 nmol/pmol) | 0.71 (0.36, 1.37) | 1.09 (0.59, 2.03) | <0.001 | 1.00 (0.50, 2.01) | 1.07 (0.53, 2.16) | 0.075 | |
| CPI (nmol/mmol) | 0.703 (0.226, 2.183) | 0.401 (0.136, 1.183) | <0.001 | 0.508 (0.171, 1.51) | 0.297 (0.111, 0.796) | <0.001 | |
| IGI (pmol/mmol) | 257.3 (54.5, 1,215.8) | 114.8 (28.0, 470.8) | <0.001 | 154.1 (34.1, 697.4) | 84.7 (23, 311.9) | <0.001 | |
| CP-iAUC/G-iAUC (nmol/mmol) | 0.972 (0.332, 2.846) | 0.762 (0.261, 2.218) | 0.001 | 0.927 (0.368, 2.335) | 0.526 (0.184, 1.505) | <0.001 | |
| I-iAUC/G-iAUC (pmol/mmol) | 312.6 (72.7, 1,343.3) | 163.9 (40.9, 657.0) | <0.001 | 218.7 (58.7, 814.4) | 112.3 (26.8, 470.1) | <0.001 | |
Data are mean ± SD or geometric mean (95% CI). P values for nonnormally distributed data based on log-transformed values. “Other” for self-reported race/ethnicity includes, mixed, Asian, American Indian, and other.
A total of 304 participants had IGT, and 117 had type 2 diabetes (Table 1). The two groups did not differ in their racial/ethnic distribution and were well matched for physical and demographic characteristics.
OGTT Glucose, C-Peptide, and Insulin Concentrations in Youth and Adults
The fasting glucose concentration was lower, whereas the fasting C-peptide and insulin concentrations were higher in youth compared with adults (Table 1). The mean OGTT 2-h glucose concentration was not different between youth and adults, but the 2-h C-peptide and insulin concentrations were significantly higher in youth. These differences in the 2-h C-peptide and insulin concentrations remained significant after adjusting for differences in BMI or triponderal index, sex, and racial/ethnic distribution between youth and adults.
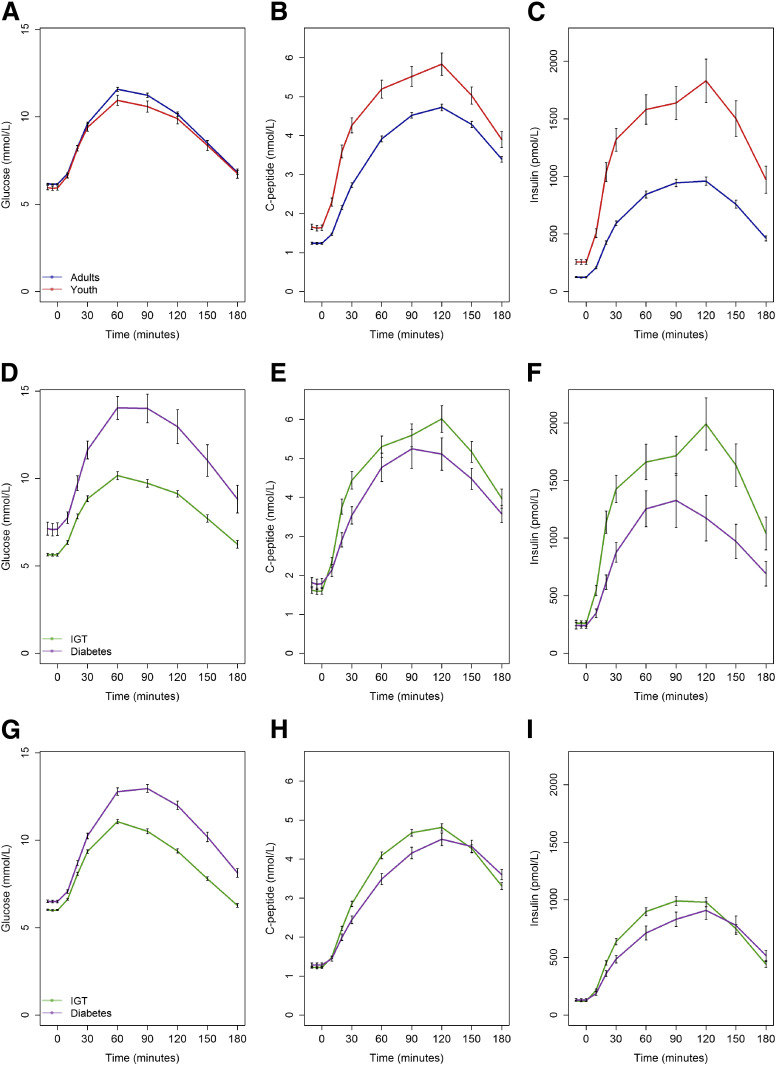
Figure 1A–C shows glucose, C-peptide, and insulin concentrations during the 3-h OGTT in youth and adults. Postload glucose concentrations did not differ significantly between youth and adults except for the 60-min plasma glucose, which was lower in youth (10.9 ± 2.4 vs. 11.6 ± 2.2 mmol/L; P = 0.037). G-iAUC did not differ between the two age-groups (Table 1). Despite these comparable OGTT glucose excursions, the C-peptide and insulin concentrations were significantly greater at all time points in youth compared with adults. These increases were reflected in the 25 and 88% greater iAUC for C-peptide and insulin, respectively (Table 1).
Figure 1.
Plasma glucose, C-peptide, and insulin concentrations during the OGTT in youth and adults (A–C), youth with IGT and diabetes (D–F), and adults with IGT and diabetes (G–I). Youth are shown in red, adults in blue, IGT in green, and diabetes in purple. Data are mean ± SEM. In youth and adults, at all time points following glucose ingestion, glucose concentrations were similar except at 60 min (P = 0.037). C-peptide and insulin were greater in youth at all time points (P ≤ 0.009). In IGT and diabetes, in both youth and adults, the glucose values were greater in diabetes compared with IGT (all P < 0.001). In youth, although the differences were large, they only reached significance at 20 min for C-peptide (P = 0.040) and 10–30 min for insulin (P < 0.05). In adults, both C-peptide and insulin were greater in IGT from 20 to 90 min (P < 0.05).
OGTT Glucose, C-Peptide, and Insulin Concentrations in IGT and Type 2 Diabetes
By definition, fasting and 2-h glucose concentrations were higher in participants with recently diagnosed, drug-naïve type 2 diabetes (Table 1). Despite these differences, fasting C-peptide and insulin concentrations did not differ significantly between those with IGT and type 2 diabetes. However, both the 2-h C-peptide and insulin concentrations were significantly greater in the IGT group (Table 1).
The profiles for glucose, C-peptide, and insulin in IGT or type 2 diabetes are illustrated separately for youth (Fig. 1D–F) and adults (Fig. 1G–I). By definition, in both youth and adults, all glucose concentrations were significantly greater throughout the OGTT in those with type 2 diabetes than with IGT (all P < 0.001) (Fig. 1D and G and Table 2). In both youth and adults, fasting and 2-h C-peptide and insulin concentrations were not different between the glycemia groups; however, they were greater at other time points postglucose ingestion in the IGT groups (P < 0.05). Thus, CP-iAUC and I-iAUC were greater in IGT compared with diabetes (Table 2).
Table 2.
OGTT-based measures in drug-naïve youth and adults subdivided based on glucose tolerance category (IGT vs. type 2 diabetes)
| Youth (n = 66) |
Adults (n = 355) |
Youth IGT vs. adults IGT, P value (1 vs. 3) | Youth with type 2 diabetes vs. adults with type 2 diabetes, P value (2 vs. 4) | |||||
|---|---|---|---|---|---|---|---|---|
| IGT (n = 53) (1) | Type 2 diabetes (n = 13) (2) | P value (1 vs. 2) | IGT (n = 251) (3) | Type 2 diabetes (n = 104) (4) | P value (3 vs. 4) | |||
| Glycemic characteristic | ||||||||
| HbA1c (mmol/mol) | 36.78 ± 4.57 | 45.70 ± 6.52 | <0.001 | 38.77 ± 3.93 | 41.73 ± 4.62 | <0.001 | 0.001 | 0.006 |
| OGTT parameters | ||||||||
| Fasting glucose (mmol/L) | 5.64 ± 0.53 | 7.10 ± 1.29 | <0.001 | 6.01 ± 0.55 | 6.5 ± 0.75 | <0.001 | <0.001 | 0.014 |
| Fasting C-peptide (nmol/L) | 1.601 ± 0.608 | 1.791 ± 0.483 | 0.299 | 1.223 ± 0.420 | 1.281 ± 0.598 | 0.303 | <0.001 | 0.004 |
| Fasting insulin (pmol/L) | 215.9 (63.9, 729.4) | 227.7 (111.9, 463.4) | 0.768 | 106.8 (37.9, 300.6) | 105.6 (30.7, 363) | 0.870 | <0.001 | 0.002 |
| Two-hour glucose (mmol/L) | 9.13 ± 1.34 | 12.97 ± 3.51 | <0.001 | 9.39 ± 1.86 | 11.99 ± 2.45 | <0.001 | 0.338 | 0.202 |
| Two-hour C-peptide (nmol/L) | 6.01 ± 2.48 | 5.11 ± 1.5 | 0.216 | 4.81 ± 1.5 | 4.51 ± 1.65 | 0.089 | <0.001 | 0.214 |
| Two-hour insulin (pmol/L) | 1,527.5 (362.1, 6,444.3) | 1,049.8 (432.9, 2,545.8) | 0.084 | 812.2 (240.5, 2,742.6) | 735.2 (212.8, 2,540) | 0.173 | <0.001 | 0.248 |
| Incremental OGTT AUC (0–180 min) | ||||||||
| G-iAUC (mmol/L · min) | 528.9 ± 164.4 | 858.8 ± 288.2 | <0.001 | 540.7 ± 197.9 | 786 ± 277.8 | <0.001 | 0.690 | 0.377 |
| CP-iAUC (nmol/L · min) | 555 (258.4, 1,192.1) | 429.8 (248.7, 742.9) | 0.036 | 442.9 (239.0, 820.7) | 383.2 (174.0, 843.8) | <0.001 | <0.001 | 0.339 |
| I-iAUC (pmol/L · min) | 185,932 (51,812, 667,233) | 124,745 (50,683, 307,030) | 0.042 | 97,185 (32,857, 287,458) | 78,599 (21,703, 284,646) | 0.002 | <0.001 | 0.016 |
| Estimates of insulin sensitivity, insulin clearance, and β-cell function | ||||||||
| 1/FI × [10−3 1/(pmol/L)] | 5.65 ± 4.25 | 4.65 ± 1.56 | 0.408 | 10.74 ± 5.89 | 11.43 ± 7.52 | 0.359 | <0.001 | 0.002 |
| Fasting C-peptide/insulin (× 10−2 nmol/pmol) | 0.69 (0.34, 1.42) | 0.76 (0.53, 1.09) | 0.373 | 1.08 (0.60, 1.95) | 1.12 (0.57, 2.22) | 0.356 | <0.001 | <0.001 |
| CPI (nmol/mmol) | 0.815 (0.293, 2.263) | 0.385 (0.183, 0.81) | <0.001 | 0.46 (0.169, 1.248) | 0.287 (0.106, 0.779) | <0.001 | <0.001 | 0.048 |
| IGI (pmol/mmol) | 301.8 (66.8, 1,363.4) | 134.4 (49.0, 368.8) | <0.001 | 133.4 (34.4, 516.4) | 79.8 (21.9, 291.7) | <0.001 | <0.001 | 0.007 |
| CP-iAUC/G-iAUC (nmol/mmol) | 1.119 (0.447, 2.798) | 0.536 (0.214, 1.343) | <0.001 | 0.891 (0.359, 2.211) | 0.525 (0.18, 1.529) | <0.001 | 0.002 | 0.897 |
| I-iAUC/G-iAUC (pmol/mmol) | 374.7 (102.4, 1,370.4) | 153.5 (44.9, 525) | <0.001 | 195.6 (58.3, 656.3) | 108 (25.5, 456.8) | <0.001 | <0.001 | 0.102 |
Data are mean ± SD or geometric mean (95% CI). P values for nonnormally distributed data based on log-transformed values.
Among youth and adults with IGT, fasting glucose and HbA1c were lower in youth, whereas the 2-h glucose concentration and G-iAUC did not differ significantly (Table 2). Fasting and 2-h C-peptide and insulin concentrations as well as CP-iAUC and I-iAUC were greater in youth with IGT versus adults with IGT.
Among youth and adults with type 2 diabetes, fasting glucose and HbA1c were greater in youth, whereas G-iAUC did not differ significantly (Table 2). Fasting C-peptide and insulin concentrations were greater in youth with type 2 diabetes, whereas the 2-h values for these peptides did not differ between the two age-groups. The iAUC for insulin was greater in youth, whereas that for C-peptide did not differ significantly between youth with type 2 diabetes and adults with type 2 diabetes.
Insulin Sensitivity, Insulin Clearance, and β-Cell Responses in Youth and Adults
The inverse of fasting insulin, a surrogate estimate of insulin sensitivity, was 50% lower in youth compared with adults (Table 1). The lower insulin sensitivity in youth persisted after adjusting for the higher proportion of nonwhite participants, greater number of females, higher BMI, and greater triponderal index in youth. To minimize the impact of puberty-related insulin resistance at Tanner stages II–IV, an analysis that included Tanner stage V youth only (n = 40) was performed: insulin sensitivity was still significantly lower in youth than adults (1/FI: 5.63 ± 4.65 vs. 10.94 ± 6.41 1/[pmol/L]; P < 0.001).
The fasting C-peptide/insulin ratio was significantly lower in youth compared with adults (Table 1), indicative of lower insulin clearance in youth. This difference persisted after adjusting for BMI, triponderal index, sex, and racial/ethnic differences between youth and adults.
β-Cell responses determined as a function of the glucose stimulus following ingestion highlighted that the younger age-group had greater responses compared with adults, reflected by both the early C-peptide and insulin responses (CPI and IGI, respectively) and the integrated measures over the duration of the test (CP-iAUC/G-iAUC and I-iAUC/G-iAUC). The C-peptide responses in youth were 75% greater in the initial phase and 28% greater over the whole OGTT, whereas the insulin responses were about twofold greater for both time periods. These responses remained significantly greater in youth following adjustment for insulin sensitivity.
Insulin Sensitivity, Insulin Clearance, and β-Cell Responses in IGT and Type 2 Diabetes
Comparing individuals with IGT to those with type 2 diabetes showed no difference in insulin sensitivity (1/FI; Table 1). However, all β-cell responses (CPI, IGI, CP-iAUC/G-iAUC, and I-iAUC/G-iAUC) were significantly lower in the group with type 2 diabetes. In contrast with the observation in youth versus adults, insulin clearance was not different between those with IGT versus diabetes.
Further analysis of those with IGT or type 2 diabetes among youth and adults separately yielded some differences (Table 2). In both IGT and type 2 diabetes, the inverse of fasting insulin was ∼50% lower in youth compared with adults. Early phase C-peptide and insulin responses were significantly greater in youth with either IGT or type 2 diabetes, although relative differences were smaller in those with diabetes (CPI: 77% higher in youth than adults with IGT and 34% higher in youth than adults with diabetes; IGI: 126% higher in youth than adults with IGT and 68% higher in youth than adults with diabetes). Similar patterns were observed for β-cell responses over the whole OGTT (CP-iAUC/G-iAUC and I-iAUC/G-iAUC), but in this instance, only the C-peptide and insulin responses in youth and adults with IGT differed significantly.
Relationship of Insulin Sensitivity to C-Peptide and Insulin Responses in Youth and Adults and in IGT and Diabetes
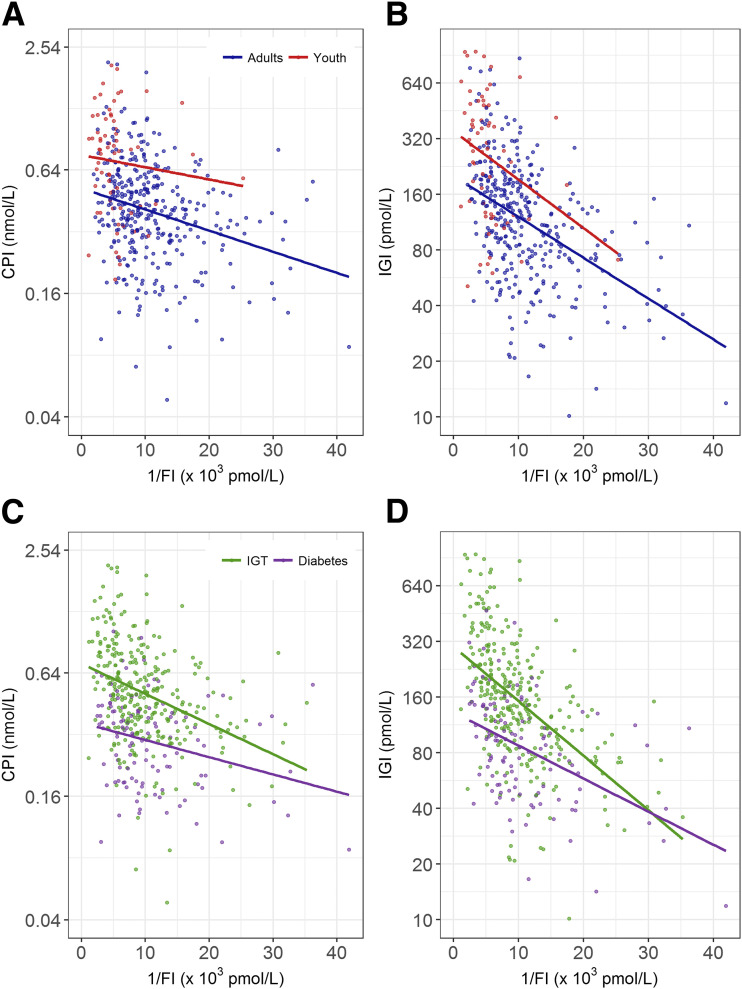
The relationships of insulin sensitivity with CPI and IGI for youth and adults are depicted in Fig. 2A and B. Both CPI and IGI were inversely related to insulin sensitivity; although the slopes in youth and adults did not differ (P = 0.577 and P = 0.563, respectively), both CPI and IGI were significantly higher at a given level of insulin sensitivity in youth than in adults (both P < 0.001).
Figure 2.
Relationship of insulin sensitivity (1/FI) with the CPI and IGI from the OGTT in youth and adults (A and B, respectively) and those with IGT and diabetes (C and D, respectively). Youth are shown in red, adults in blue, IGT in green, and diabetes in purple. CPI and IGI are presented on a log scale. The slopes relating early β-cell response measures to insulin sensitivity were all significant (P < 0.001), and the group differences were all significant (youth vs. adults: P < 0.001 for CPI and IGI; IGT vs. diabetes: P < 0.001 for CPI and IGI). The slopes for youth and adults did not differ (P = 0.577 for CPI and P = 0.563 for IGI), whereas in IGT and diabetes, that for CPI did not differ (P = 0.074) but for IGI did (P = 0.007).
When participants were categorized based on glucose tolerance status (IGT and diabetes) (Fig. 2C and D), the difference in the slopes relating insulin sensitivity to CPI in IGT versus diabetes approached significance (P = 0.074), whereas that relating insulin sensitivity to IGI was significant (P = 0.007). CPI and IGI were both lower in diabetes than IGT at a given level of insulin sensitivity (both P < 0.001). Thus, across the range of insulin sensitivity, CPI and IGI were greater in those with IGT compared with type 2 diabetes, although this difference decreased with increasing insulin sensitivity.
Similar relationships existed between insulin sensitivity and the β-cell responses measured over the 3 h of the test (CP-iAUC/G-iAUC and I-iAUC/G-iAUC) in youth and adults as well as IGT and diabetes (Supplementary Fig. 1).
Determinants of the OGTT 2-h Glucose Concentration and Glucose Excursion
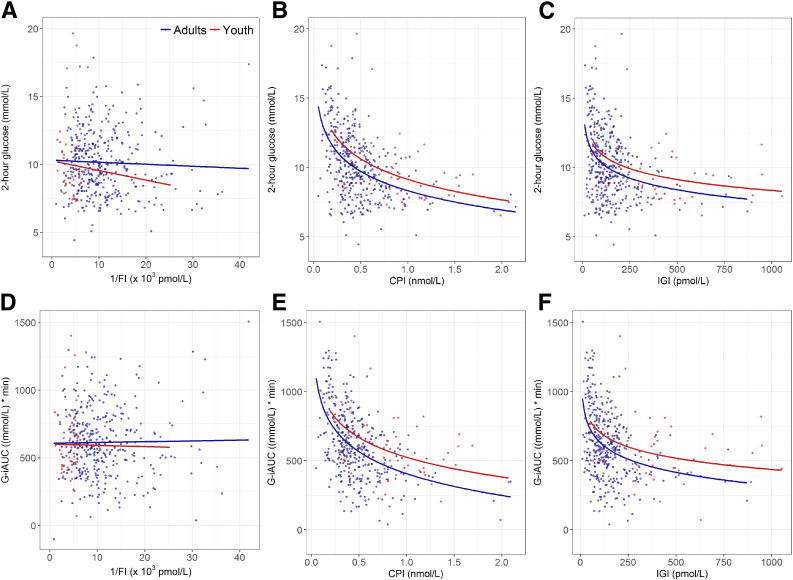
To assess the relative importance of insulin sensitivity and/or β-cell responses in determining glucose tolerance, we examined the relationships of 1/FI, CPI, and IGI with the 2-h glucose concentration (Fig. 3A–C). Insulin sensitivity was not related to the 2-h glucose concentration in either youth (r = −0.11; P = 0.373) or adults (r = −0.039; P = 0.456) (Fig. 3A). In contrast, both CPI (youth: r = −0.50, P < 0.001; adults: r = −0.47, P < 0.001) and IGI (youth: r = −0.37, P = 0.003; adults: r = −0.37, P < 0.001) were inversely related to the 2-h glucose concentration (Fig. 3B and C). Although the slopes for youth and adults were not significantly different for the relationship between the 2-h glucose and CPI or IGI, youth had higher peptide responses than adults for both CPI (P = 0.004) and IGI (P = 0.034) across the range of 2-h glucose concentration.
Figure 3.
Relationship in youth and adults of the OGTT 2-h glucose concentration (A–C) and glucose excursion determined as G-iAUC (D–F) with insulin sensitivity (1/FI; A and D), CPI (B and E), and IGI (C and F), respectively. Youth are shown in red and adults in blue. The slopes relating the 2-h glucose concentration and G-iAUC to insulin sensitivity were not significant in youth or adults (P = 0.373–0.900). For calculation of the slopes relating the 2-h glucose concentration and G-iAUC to CPI and IGI, the β-cell responses were log transformed. Slopes relating the 2-h glucose concentration to CPI and IGI were all significant (P < 0.001 for youth and adults for CPI and adults for IGI; P = 0.003 for youth for IGI), and the group differences were significant (P = 0.004 for CPI and P = 0.034 for IGI). Slopes relating G-iAUC to CPI and IGI were all significant (P < 0.001 for youth and adults for CPI and adults for IGI; P = 0.001 for youth for IGI), and the group differences were significant (P < 0.001 for CPI and P = 0.009 for IGI). For the 2-h glucose, the slopes for youth and adults did not differ (P = 0.476 for 1/FI; P = 0.806 for CPI; and P = 0.852 for IGI). Likewise, for G-iAUC, the slopes for youth and adults did not differ (P = 0.858 for 1/FI; P = 0.665 for CPI; and P = 0.623 for IGI).
Similarly, total glucose excursion during the OGTT (G-iAUC) was not related to insulin sensitivity in youth or adults (youth: r = −0.015, P = 0.90; adults: r = 0.014, P = 0.79) (Fig. 3D). In contrast, G-iAUC was related to early β-cell responses (CPI in youth: r = −0.506, P < 0.001 and adults: r = −0.500, P < 0.001; IGI in youth: r = −0.400, P = 0.001 and adults: r = −0.400, P < 0.001) (Fig. 3E and F). Slopes relating G-iAUC and CPI and IGI were not different between youth and adults; however, again youth had higher peptide responses for CPI (P < 0.001) and IGI (P = 0.009) across the range of total glucose excursion.
Conclusions
The pathogenesis of type 2 diabetes in both youth and adults involves dual defects: insulin resistance and β-cell dysfunction (14–16). We have used OGTT data from the RISE Study to perform direct comparisons between youth and adults using shared measurement methodologies. Following glucose ingestion, youth exhibited higher C-peptide and insulin responses despite matched glucose excursions. These increased C-peptide and insulin responses in youth exceeded what would be needed to compensate for their markedly lower insulin sensitivity. Further, these OGTT data not only mirror what we observed using the hyperglycemic clamp (5), but also demonstrate that this effect is also present in response to physiologic delivery of glucose. This suggests that in youth, this hyperresponsiveness is occurring with food intake every day. With these analyses, we have also extended the clamp observations by demonstrating that the β-cell change responsible for the hyperresponsiveness in youth is lost with disease progression.
The differences observed in β-cell responsiveness between the two age-groups and the difference in responses within glucose tolerance categories are novel. Not only did we observe the expected inverse relationship between insulin sensitivity and β-cell responses (9), but also an enhancement of the β-cell responses in youth across the range of insulin sensitivity in keeping with it not simply being due to the difference in insulin sensitivity. Further, the lack of a difference in glucose concentrations between groups during the OGTT argues that glucose is not mediating this effect. As we also observed increased β-cell responsiveness in these youth with intravenous administration of glucose and the nonglucose secretagogue arginine (5), this effect seems to be generalized rather than specific to a secretagogue or route of administration. Importantly, the current observations using oral glucose delivery support the assumption that this hyperresponsiveness is occurring in youth in response to all nutrients on a daily basis. Among the possible explanations for this enhanced responsiveness are: 1) increased circulating incretins or β-cell sensitivity to these peptides, 2) genetic defects or epigenetic changes resulting in hyperresponsiveness, and/or 3) altered central regulation resulting in increased parasympathetic/vagal tone and insulin secretion. In the latter two instances, it is possible that a primary β-cell lesion resulting in hypersecretion will also lead to peripheral downregulation of insulin action to avoid hypoglycemia (17). Further research will be needed to explore these possibilities.
The C-peptide and insulin responses in youth with IGT were significantly greater than in adults in both the early responses and total response over the 3-h test. Among those with diabetes, the early responses were also greater in youth, but this was not the case over the entire test for either C-peptide or insulin. Thus, the ratio of each β-cell response in youth to adults was less in those with diabetes than IGT (diabetes vs. IGT, respectively; CPI: 1.34- vs. 1.77-fold; IGI: 1.68- vs. 2.26-fold; CP-iAUC/G-iAUC: 1.02- vs. 1.26-fold; and I-iAUC/G-iAUC 1.42- vs. 1.92-fold). This mirrors our observations with the clamp studies, in which youth had greater differences compared with adults in the acute (first-phase) response to intravenous glucose than in the steady-state (second-phase) response (5). Our observation of differences in the magnitude and nature of the β-cell responses by age and category of glucose tolerance are also consistent with a recent report that used mathematical modeling of OGTT data obtained from pooled studies (18).
We observed 50% lower insulin sensitivity in youth using the inverse of fasting insulin. This difference is similar to the 46% reduction in insulin sensitivity we quantified using the hyperglycemic clamp (5) and results from two recent retrospective analyses of cohorts studied for other purposes (18,19). The basis for the lower insulin sensitivity in youth in RISE does not seem to be due simply to dissimilarities in sex (20,21), race/ethnicity (14,22), or body size (23) that are known to affect insulin sensitivity, as this difference persisted in our data after accounting for any potential effect of these differences. Consistent with our findings, when obese youth with IGT were matched for BMI, sex, and race to adults with IGT, youth exhibited 40–50% lower peripheral and hepatic insulin sensitivity, twofold higher fasting insulin concentrations, and lower insulin clearance compared with adults (19). Although physiological transition through puberty is known to reduce insulin sensitivity, it returns to near prepubertal levels once puberty is complete (24–26). However, a difference in insulin sensitivity was still present when we limited the comparator group to youth at Tanner stage V, which is postpubertal and more akin to adulthood. Our findings may differ from previous studies, however, as studies of pubertal insulin resistance have been performed in normal weight youth or those across a wide spectrum of weight/BMI and not specifically in obese youth. It is also possible that as obese prepubertal youth transition through puberty, they might sustain a greater decrease in insulin sensitivity consequent to the combined effect of puberty and obesity. Also, it remains unknown if their puberty-related insulin resistance recovers with completion of puberty. We did not collect data on other factors that could affect insulin sensitivity, which include environmental factors such as nutrient balance (27), exercise (28), and stress (29) as well as physiological factors such as menstrual status (30) and body fat topography (31); therefore, we cannot determine whether these or other unmeasured factors are contributing to the observed differences. Finally, whether a unique genetic component(s) could also be causative cannot be excluded.
Type 2 diabetes appears to be more aggressive in youth, with a faster rate of deterioration of β-cell function (32) and poorer response to glucose-lowering medications (33). Our observation of a smaller relative difference in the C-peptide and insulin responses between youth and adults with diabetes versus those with IGT suggests that the β-cell change responsible for the hyperresponsiveness in youth is lost with disease progression from IGT to type 2 diabetes. It also suggests the possibility that the deterioration in β-cell function that occurs with progression from IGT to diabetes is greater, more rapid, and/or may also continue beyond the time of diabetes diagnosis. Such a difference could possibly also explain why type 2 diabetes presents at an earlier age and the response to glucose-lowering therapy is poorer so that hyperglycemia appears to progress more rapidly in youth (33,34). Although studies have demonstrated the possibility to preserve β-cell function in adults with IGT or recent-onset type 2 diabetes (35,36), future longitudinal and intervention studies, including the results of the RISE Study evaluating the effects of β-cell rest in this cohort of youth, will be needed to define and provide greater insight into these distinctions between the two age-groups.
In our data in both youth and adults, the β-cell’s early response (CPI and IGI) was a more important determinant of the 2-h glucose concentration than insulin sensitivity. Also, the early β-cell response in youth was greater than adults across the achieved 2-h glucose concentration and the indices of total glucose excursion. These observations are in line with a previous report of OGTT data in 531 first-degree relatives of individuals from four different ethnic groups with normal glucose tolerance, IGT, and newly identified diabetes, which found that the early insulin response explained threefold more of the variance in the 2-h glucose concentration than did insulin sensitivity measured as HOMA of insulin resistance (14).
There are limitations to the current analyses. Our comparisons are cross-sectional, and it is unclear whether observed differences between youth and adults represent underlying group differences or transient differences that will resolve with maturation. Because most of the youth had completed puberty in Tanner stage V, it is unlikely that the observed differences are due to puberty, as discussed above in detail. Longitudinal studies that include youth with normal glucose tolerance, IGT, and type 2 diabetes are needed to evaluate temporal changes in β-cell hyperresponsiveness with maturation. The current findings are based on a relatively small number of youth who had diabetes and were drug naïve. Further, they differed from adults in sex and race/ethnicity distribution, both of which were adjusted for in our analyses. Nevertheless, we saw noteworthy group differences between youth and adults.
In conclusion, this analysis of OGTT data from RISE highlights the fundamental differences in insulin sensitivity and β-cell function in youth and adults with IGT and recently diagnosed, drug-naïve type 2 diabetes. These data complement parallel analyses in the same cohort using clamp studies and underscore the likely relevance of these phenomena to daily meal ingestion. The observed hyperresponsiveness of β-cells may be a critical factor in the observed accelerated progression to diabetes at a young age and youth’s more aggressive disease.
Supplementary Material
Article Information
Acknowledgments. The RISE Consortium thanks the RISE Data and Safety Monitoring Board and Barbara Linder, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Program Official for RISE (Rockville, MD), for support and guidance. The Consortium also thanks the participants who, by volunteering, are furthering the ability to reduce the burden of diabetes.
Funding and Duality of Interest. RISE is supported by grants from the National Institutes of Health (U01-DK-094406, U01-DK-094430, U01-DK-094431, U01-DK-094438, U01-DK-094467, P30-DK-017047, P30-DK-020595, P30-DK-045735, P30-DK-097512, UL1-TR-000430, UL1-TR-001082, UL1-TR-001108, UL1-TR-001855, UL1-TR-001857, UL1-TR-001858, and UL1-TR-001863), the Department of Veterans Affairs, and Kaiser Permanente Southern California. Additional financial and material support from the American Diabetes Association, Allergan, Apollo Endosurgery, Abbott Laboratories, and Novo Nordisk was received. S.A.A. and S.E.K. serve as paid consultants on advisory boards for Novo Nordisk. S.A.A. is a participant in a Novo Nordisk–sponsored clinical trial. T.A.B. has received research support from Allergan and Apollo Endosurgery. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. Members of the RISE Consortium recruited participants and collected study data. S.A.A. and S.E.K. proposed the analysis, interpreted data, and wrote and edited the manuscript, which was also reviewed and edited by members of the writing group. The RISE Steering Committee reviewed and edited the manuscript and approved its submission. S.E.K. and S.L.E. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in oral form at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018.
Appendix
Writing Group:
Silva A. Arslanian (co-chair), Steven E. Kahn (co-chair), Thomas A. Buchanan, Sharon L. Edelstein, David A. Ehrmann, Kristen J. Nadeau, Jerry P. Palmer, and Kristina M. Utzschneider.
Footnotes
Clinical trial reg. nos. NCT01779362, NCT01779375, and NCT01763346, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-0243/-/DC1.
A complete list of the RISE Consortium Investigators can be found in the Supplementary Data online.
Contributor Information
Collaborators: David A. Ehrmann, Karla A. Temple, Abby Rue, Elena Barengolts, Babak Mokhlesi, Eve Van Cauter, Susan Sam, M. Annette Miller, Steven E. Kahn, Karen M. Atkinson, Jerry P. Palmer, Kristina M. Utzschneider, Tsige Gebremedhin, Abigail Kernan-Schloss, Alexandra Kozedub, Brenda K. Montgomery, Emily J. Morse, Kieren J. Mather, Tammy Garrett, Tamara S. Hannon, Amale Lteif, Aniket Patel, Robin Chisholm, Karen Moore, Vivian Pirics, Linda Pratt, Kristen J. Nadeau, Susan Gross, Philip S. Zeitler, Jayne Williams, Melanie-Cree Green, Yesenia Garcia Reyes, Krista Vissat, Silva A. Arslanian, Kathleen Brown, Nancy Guerra, Kristin Porter, Sonia Caprio, Mary Savoye, Bridget Pierpont, Thomas A. Buchanan, Anny H. Xiang, Enrique Trigo, Elizabeth Beale, Fadi N. Hendee, Namir Katkhouda, Krishan Nayak, Mayra Martinez, Cortney Montgomery, Xinhui Wang, Sharon L. Edelstein, John M. Lachin, Ashley N. Hogan, Santica Marcovina, Jessica Harting, John Albers, Dave Hill, Peter J. Savage, and Ellen W. Leschek
References
- 1.GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, Reitsma MB, et al.. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017;377:13–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al.; SEARCH for Diabetes in Youth Study . Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med 2017;376:1419–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hannon TS, Arslanian SA. The changing face of diabetes in youth: lessons learned from studies of type 2 diabetes. Ann N Y Acad Sci 2015;1353:113–137 [DOI] [PubMed] [Google Scholar]
- 4.The RISE Consortium Restoring Insulin Secretion (RISE): design of studies of β-cell preservation in prediabetes and early type 2 diabetes across the life span. Diabetes Care 2014;37:780–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The RISE Consortium Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: I. Observations using the hyperglycemic clamp. Diabetes Care 2018;41:1696–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000;320:1240–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 8.Peterson CM, Su H, Thomas DM, et al. Tri-ponderal mass index vs body mass index in estimating body fat during adolescence. JAMA Pediatr 2017;171:629–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and β-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 1993;42:1663–1672 [DOI] [PubMed] [Google Scholar]
- 10.George L, Bacha F, Lee S, Tfayli H, Andreatta E, Arslanian S. Surrogate estimates of insulin sensitivity in obese youth along the spectrum of glucose tolerance from normal to prediabetes to diabetes. J Clin Endocrinol Metab 2011;96:2136–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castillo MJ, Scheen AJ, Letiexhe MR, Lefèbvre PJ. How to measure insulin clearance. Diabetes Metab Rev 1994;10:119–150 [DOI] [PubMed] [Google Scholar]
- 12.Seltzer HS, Allen EW, Herron AL Jr., Brennan MT. Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J Clin Invest 1967;46:323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med 1994;11:286–292 [DOI] [PubMed] [Google Scholar]
- 14.Jensen CC, Cnop M, Hull RL, Fujimoto WY, Kahn SE; American Diabetes Association GENNID Study Group . β-Cell function is a major contributor to oral glucose tolerance in high-risk relatives of four ethnic groups in the U.S. Diabetes 2002;51:2170–2178 [DOI] [PubMed] [Google Scholar]
- 15.Weiss R, Caprio S, Trombetta M, Taksali SE, Tamborlane WV, Bonadonna R. β-Cell function across the spectrum of glucose tolerance in obese youth. Diabetes 2005;54:1735–1743 [DOI] [PubMed] [Google Scholar]
- 16.Gungor N, Bacha F, Saad R, Janosky J, Arslanian S. Youth type 2 diabetes: insulin resistance, β-cell failure, or both? Diabetes Care 2005;28:638–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care 2008;31(Suppl. 2):S262–S268 [DOI] [PubMed] [Google Scholar]
- 18.Chen ME, Chandramouli AG, Considine RV, Hannon TS, Mather KJ. Comparison of β-cell function between overweight/obese adults and adolescents across the spectrum of glycemia. Diabetes Care 2018;41:318–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arslanian S, Kim JY, Nasr A, et al. Insulin sensitivity across the lifespan from obese adolescents to obese adults with impaired glucose tolerance: who is worse off? Pediatr Diabetes 2018;19:205–211 [DOI] [PubMed] [Google Scholar]
- 20.Nuutila P, Knuuti MJ, Mäki M, et al. Gender and insulin sensitivity in the heart and in skeletal muscles. Studies using positron emission tomography. Diabetes 1995;44:31–36 [DOI] [PubMed] [Google Scholar]
- 21.Travers SH, Jeffers BW, Bloch CA, Hill JO, Eckel RH. Gender and Tanner stage differences in body composition and insulin sensitivity in early pubertal children. J Clin Endocrinol Metab 1995;80:172–178 [DOI] [PubMed] [Google Scholar]
- 22.Goran MI, Bergman RN, Cruz ML, Watanabe R. Insulin resistance and associated compensatory responses in African-American and Hispanic children. Diabetes Care 2002;25:2184–2190 [DOI] [PubMed] [Google Scholar]
- 23.Kolterman OG, Reaven GM, Olefsky JM. Relationship between in vivo insulin resistance and decreased insulin receptors in obese man. J Clin Endocrinol Metab 1979;48:487–494 [DOI] [PubMed] [Google Scholar]
- 24.Amiel SA, Caprio S, Sherwin RS, Plewe G, Haymond MW, Tamborlane WV. Insulin resistance of puberty: a defect restricted to peripheral glucose metabolism. J Clin Endocrinol Metab 1991;72:277–282 [DOI] [PubMed] [Google Scholar]
- 25.Arslanian SA, Kalhan SC. Correlations between fatty acid and glucose metabolism. Potential explanation of insulin resistance of puberty. Diabetes 1994;43:908–914 [DOI] [PubMed] [Google Scholar]
- 26.Moran A, Jacobs DR Jr., Steinberger J, et al. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes 1999;48:2039–2044 [DOI] [PubMed] [Google Scholar]
- 27.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest 2008;118:2992–3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soman VR, Koivisto VA, Deibert D, Felig P, DeFronzo RA. Increased insulin sensitivity and insulin binding to monocytes after physical training. N Engl J Med 1979;301:1200–1204 [DOI] [PubMed] [Google Scholar]
- 29.Rizza RA, Mandarino LJ, Gerich JE. Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor detect of insulin action. J Clin Endocrinol Metab 1982;54:131–138 [DOI] [PubMed] [Google Scholar]
- 30.Valdes CT, Elkind-Hirsch KE. Intravenous glucose tolerance test-derived insulin sensitivity changes during the menstrual cycle. J Clin Endocrinol Metab 1991;72:642–646 [DOI] [PubMed] [Google Scholar]
- 31.Cnop M, Landchild MJ, Vidal J, et al. The concurrent accumulation of intra-abdominal and subcutaneous fat explains the association between insulin resistance and plasma leptin concentrations: distinct metabolic effects of two fat compartments. Diabetes 2002;51:1005–1015 [DOI] [PubMed] [Google Scholar]
- 32.TODAY Study Group Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY. Diabetes Care 2013;36:1749–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.TODAY Study Group A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012;366:2247–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kahn SE, Haffner SM, Heise MA, et al.; ADOPT Study Group . Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–2443 [DOI] [PubMed] [Google Scholar]
- 35.Diabetes Prevention Program Research Group Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the Diabetes Prevention Program: effects of lifestyle intervention and metformin. Diabetes 2005;54:2404–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weng J, Li Y, Xu W, et al. Effect of intensive insulin therapy on β-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet 2008;371:1753–1760 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.