Abstract
OBJECTIVE
The aim is to report 1-year outcomes of the Supporting Teens Problem Solving (STePS) study, a randomized controlled trial comparing a distress and depression prevention program with a diabetes education program for adolescents with type 1 diabetes.
RESEARCH DESIGN AND METHODS
With 264 adolescents in two locations (Chicago and San Francisco Bay Area), a randomized controlled trial was conducted comparing the Penn Resilience Program for type 1 diabetes (PRP T1D) to Advanced Diabetes Education. Interventions lasted 4.5 months, and assessments were conducted at baseline, and 4.5, 8, 12, and 16 months. Outcomes of interest were diabetes distress (DD), depressive symptoms, resilience, diabetes self-management, and glycemic control. Latent growth curve modeling was used to test between-group differences over time.
RESULTS
Results indicate that there was acceptable randomization and exposure to interventions, and that exposure to PRP T1D was associated with substantial reductions in DD. In addition, stable glycemic control, resilience characteristics, and depressive symptoms were observed 1 year post-treatment. Diabetes management deteriorated in both groups.
CONCLUSIONS
Intervening before symptoms of psychological distress start can prevent the development of the DD commonly seen in adolescents with type 1 diabetes. The STePS program represents a promising prevention program, and future reports on 2- and 3-year outcomes will explore benefits over longer periods of time.
Introduction
Diabetes distress (DD) is common, disruptive to diabetes management, and associated with poor health outcomes (1,2). It is commonly described as experiencing depression-like symptoms, but they are specific to the burden of managing diabetes. Prevalence and incidence data are available largely for adults with diabetes and indicate that close to half of adults will experience DD in an 18-month period (3). Less is known about DD in youths with type 1 diabetes (T1D), but estimates of distress and depressive symptoms indicate heightened risk (4–6). It is likely that DD and depressive symptoms complicate diabetes management by suppressing the ability to engage in daily tasks, resulting in higher hemoglobin A1c (A1C) values and recurrent hospital admissions for diabetic ketoacidosis (7,8).
Few interventions have specifically targeted the reduction of DD or depressive symptoms in youths with T1D, although some have investigated the collateral effect from coping or problem solving interventions (9,10). Further, no programs to date focus on preventing DD or depressive symptoms in youths with T1D who have not yet received a diagnosis of depression. Given the dearth of preventive interventions and the clear need to address DD and depressive symptoms, the Supporting Teens Problem Solving (STePS) study was initiated (11). The goal of the STePS randomized controlled trial (RCT) was to test the efficacy of a resilience-promoting, depression-preventing intervention and to compare the effects of this intervention with those of an advanced diabetes education intervention. The resilience promotion arm was adapted from the University of Pennsylvania Penn Resilience Program (PRP) (12), which is an evidence-based program for preventing depression in youths and young adults in the general population. Theoretically, the PRP prevents depression and reduces distress by promoting resilience, which consists of the following four key constructs: a sense of hopefulness, an optimistic explanatory style, effective coping strategies, and positive problem-solving skills. The PRP promotes resilience by teaching one to challenge hopeless thoughts, think flexibly and accurately about challenges, develop adaptive problem-solving strategies, and use social supports. The cognitive-behavioral and the social-problem-solving components are complementary and integrated throughout the prevention program (13,14). The comparator arm for the STePS study was advanced diabetes-specific education focused on adolescents (educational intervention [EI]). When designing the trial, potential comparison groups included standard diabetes education or no treatment at all. Given the evidence that diabetes education alone does not translate into significant change in the outcomes measured in this study, there was little interest in demonstrating the superiority of STePS over what is known to not impact these outcomes. Advanced diabetes education, which includes more detail on the application of education in to daily life, seemed more appropriate and also fit the framework of comparative effectiveness research, which was desirable.
The aim of this article is to report 1-year outcomes of the STePS program for adolescents with T1D who participated in this multisite RCT. The trial focused on both psychosocial and health outcomes (11); this current article reports on the impact of PRP T1D on DD, depressive symptoms, resilience, diabetes self-management, and glycemic control. We hypothesized that youths receiving the PRP T1D intervention would demonstrate fewer depressive symptoms, lower levels of diabetes-specific emotional distress, and improved resilience skills compared with youths receiving EI. We also hypothesized that youths receiving the PRP T1D intervention would demonstrate improved self-management behaviors and lower A1C levels compared with youths receiving the EI.
Research Design and Methods
STePS Program
The STePS study was an RCT with two intervention arms. One was an adaptation of the PRP (12), referred to as PRP T1D, which incorporates the complex and demanding nature of T1D management into the curriculum. The adaptation to PRP T1D was performed by a working group that included the investigators (K.K.H. and J.W.-B.), nurses, certified diabetes educators (CDEs), and feedback from 39 high school–aged individuals with T1D as part of a nonrandomized, pilot, and feasibility study. PRP T1D teaches cognitive-behavioral and social problem–solving skills in a group format. The cognitive processes and risk factors implicated in depression and targeted in PRP T1D include linking beliefs, feelings, and behaviors and challenging negative thinking by evaluating the accuracy of one’s beliefs. PRP T1D also aims to promote problem-solving techniques such as negotiating, assertiveness, and decision-making, and to teach coping skills such as relaxation techniques and seeking social support. The PRP T1D intervention arm was led by masters-level clinicians. The comparator arm (i.e., EI) was led by CDEs. Group leaders received extensive training on T1D, typical adolescent developmental demands, and active-listening skills, and were also audio recorded and supervised on 25% of randomly drawn sessions. They were supervised by the primary investigators at each site (K.K.H. and J.W.-B.). Advanced diabetes education focused on issues such as nutrition for teenagers, the importance of exercise, a review of insulin action, and a review of diabetes technologies.
Interventionists for both study arms worked from an instructor’s guide to help them proceed through their own session topics and promote group discussion and interaction throughout. Table 1 displays session focus and content. All participants were given a student workbook specific to their intervention that reviewed the concepts from each session and contained homework that reinforced the concepts discussed each week. Both conditions consisted of nine biweekly sessions lasting 90–120 min. Active treatment lasted ∼4.5 months.
Table 1.
Components of the PRP T1D and EI groups in the STePS study
| PRP T1D sessions | EI sessions |
|---|---|
| 1. Resilience through connecting adversity, beliefs, and consequences | 1. A diabetes overview, including symptoms and treatment of out of range numbers |
| 2. Catching errors in logic and understanding thinking errors | 2. A review of general nutrition, strategies for carbohydrate counting, and managing challenging foods |
| 3. Learning self-disputing skills by understanding explanatory styles and creating alternative explanations | 3. Physical activity and its impact on glucose, and strategies for preventing hypoglycemia |
| 4. Learning self-disputing skills by evaluating the evidence and generating alternatives | 4. A review of the different types and actions of insulin and the importance of site rotation and strategies for storage |
| 5. Learning to put beliefs into perspective by understanding the worst case, best case, and most likely scenarios | 5. Strategies for blood glucose monitoring and pattern management |
| 6. Learning relaxation and focusing techniques | 6. Strategies for prevention, detection, and treatment of acute complications |
| 7. Applying resilience skills in real-time situations | 7. A review of diabetes technology including insulin pumps and continuous glucose monitoring |
| 8. Learning assertiveness skills in social communication | 8. A review of current research for a biologic cure |
| 9. Reviewing and consolidating resilience skills | 9. Reviewing and consolidating diabetes education skills |
Patient Population
Inclusion criteria for this study were an age range of 14–18 years (selected to match the typical age of high school students in the U.S.), the presence of T1D for at least 1 year, and daily insulin dosing of at least 0.5 units/kg/day. Exclusion criteria for this depression prevention intervention included a current diagnosis of major depressive disorder or current treatment with an antidepressant agent. Further, adolescents with a psychotic disorder or a major developmental disorder such as autism, or who had received a diagnosis of an eating disorder (e.g., anorexia nervosa) were excluded. In total, 264 adolescents with T1D were randomized. The CONSORT diagram is presented as Fig. 1.
Figure 1.
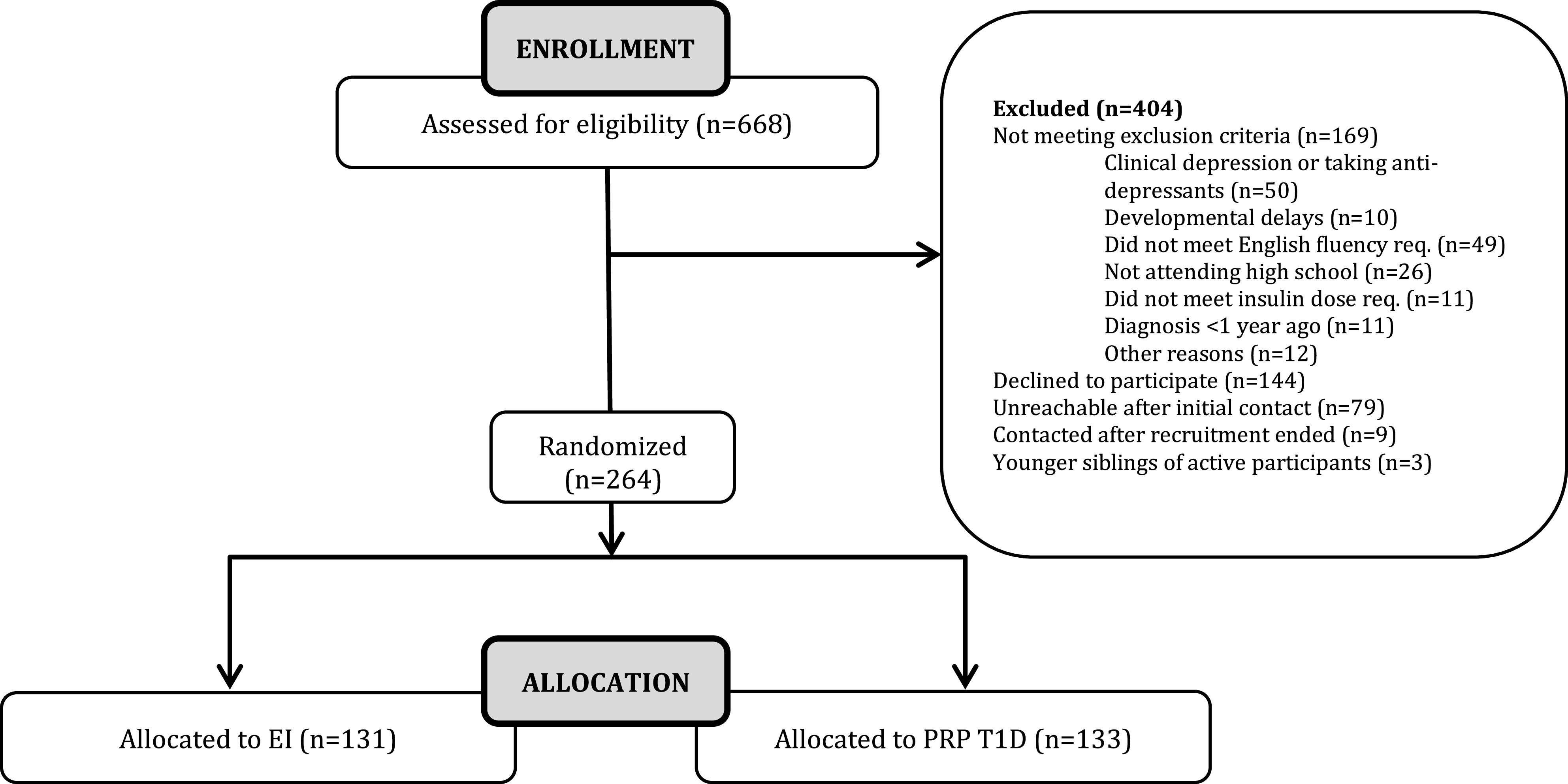
CONSORT diagram for STePS study.
Study Procedures
Study participants were drawn from two geographic regions in the U.S.: the Chicago, IL, metropolitan area and the San Francisco, CA, Bay Area. Recruitment materials were posted in area pediatric diabetes clinics as well as on a website created for this study. Once participants indicated an interest in the study, a study coordinator screened them in person or over the phone based on the eligibility criteria noted. They were also screened for a depression diagnosis with a structured clinical interview. Eligible, willing participants were enrolled and completed the informed consent process, which included parental consent for all those under the age of 18 years. Participants were then randomized.
The STePS program aims to follow participants up to 3 years post-treatment. This report focuses on the exposure to the program and outcomes through 1 year post-treatment. The following five outcomes were of interest: DD, depressive symptoms, resilience, diabetes self-management, and glycemic control. Study visits were conducted at baseline (0 months), immediately at the end of the intervention (4.5 months from baseline), and then at three follow-up visits (8, 12, and 16 months from baseline; that is, up to 1 year post-treatment). At each study visit, questionnaires were completed electronically using Health Insurance Portability and Accountability Act of 1996–compliant Snap Survey software, and blood sample collection was performed for the A1C. Efforts to optimize participation and retention in the study included providing options to complete surveys remotely, intervention sessions delivered in local buildings such as libraries, compensation for public transportation when needed, and frequent check-ins by study staff to update contact information via texts and emails.
Measures
DD
The Problem Areas in Diabetes–Teen (PAID-T) survey (15,16) was used to assess DD. The PAID-T survey has 26 items, and the baseline interitem reliability was high (coefficient α = 0.95). Higher scores indicate more DD.
Depressive Symptoms
The Children’s Depression Inventory (17) is a 27-item, widely used, psychometrically strong measure of depressive symptoms. Higher scores are indicative of depression. At baseline, the coefficient α value was 0.87 for this sample.
Resilience
Five different measures were selected to capture the complex construct of resilience (18–22). Instead of using a single measure or multiple independent measures of resilience, a one-factor model was estimated using the following five indicators: the Resiliency Scales for Children Mastery and Relatedness total score, Automatic Thoughts Questionnaire total score, Coping Efficacy Questionnaire total score, Diabetes Strengths and Resilience-Teen total score, and Social Problem Solving Inventory-Revised short form total score. All surveys have been described in detail previously (11) and are reliable and valid measures capturing the broad concept of resilience; coefficient α values ranged from 0.72 to 0.97 at baseline. The measurement model indicated good fit (Bentler’s comparative fit index [CFI] = 0.97, Tucker-Lewis Index [TLI] = 0.96, root mean squared error of approximation [RMSEA] = 0.05, standardized root mean squared residual [SRMR] = 0.07) with absolute standardized factor loadings ranging from 0.62 to 0.92; factor parameters were constrained to be equal across time points and residual covariances adjusted for shared measurement error (23). The rationale for measuring resilience in this manner was that the investigators determined that it was best to have both general and diabetes-specific resilience represented by measures, in addition to having both cognitive (i.e., automatic thoughts) and active (e.g., problem solving) constructs that demonstrate resilience.
Diabetes Management
The Self-Care Inventory (24) was used to obtain a broad measure of diabetes management behaviors and tasks completed over the past 1–2 months. The Self-Care Inventory includes 14 items, and the baseline α was 0.77.
Glycemic Control
A small fingerstick capillary sample of blood was collected at each assessment and was sent to the central laboratory for processing. The central laboratory—the Diabetes Diagnostic Laboratory at the University of Missouri—then provided a report of A1C values.
Clinical and Sociodemographic Characteristics
Adolescents and their primary caregivers provided information about sex, race/ethnicity, family income, and educational attainment of caregivers. A chart review was conducted to obtain diabetes duration, insulin delivery regimen, and prescreening eligibility criteria.
Analytic Plan
To assess the effects on outcomes over time, we conducted latent growth curve modeling (LGCM) (25). Based on structural equation modeling, LGCM captures participants’ longitudinal trajectories in terms of two dimensions of change: the rate of individuals’ change over time (slope) and individuals’ level of a given outcome (intercept). The slope and intercept are treated as latent variables, which are both quantified in terms of the mean across individuals (fixed effects) and the variability across individuals (variances or random effects). LGCM offers several advantages over traditional preanalysis and postanalysis or repeated-measures ANOVA to evaluate treatment effects, including the use of all available data across time points and increased statistical power (26).
The five outcome variables were tested in individual models using outcome measures from baseline to 1 year post-treatment. In LGCM, the intercept represents the level of the outcome at time zero. To allow the intercept to reflect outcome levels at the end of the follow-up period, assessment 5 (1 year post-treatment) was set as the zero point via specification of the slope factor loadings (e.g., −4, −3, −2, −1, and 0 for a linear curve). Possible nonlinear slopes were assessed in preliminary analyses through the use of quadratic and cubic growth terms and selectively freed slope factor loadings. We tested group differences by modeling treatment assignment as a predictor of the slope and intercept (0.5 = resilience, −0.5 = education). Thus, the treatment-slope effect represented the difference between the rate of change for the PRP T1D compared with the EI group, whereas the treatment-intercept effect estimated the mean difference between groups at 1 year post-treatment.
Consistent with an intent-to-treat framework, all individuals who were randomized into the study were included in analyses. Missing data were addressed through full information maximum likelihood estimation, which derives estimates from individuals’ entire response pattern. Model fit was evaluated based on recommended fit indices and cutoffs (27): CFI ≥ 0.95; TLI ≥ 0.95; RMSEA ≤ 0.06; and SRMR ≤ 0.08. Models were tested using MPlus version 7 with maximum likelihood estimation with robust SE to mitigate skewness in the outcome variables.
Results
Table 2 displays complete participant characteristics. In brief, 264 adolescents with T1D between 14 and 18 years of age were randomized. This participant sample had a mean age of 15.7 ± 1.1 years, a mean duration of T1D 6.9 ± 4.0 years, and a mean A1C level at baseline of 9.1 ± 1.9%. The sample included more females (60%) than males and was diverse in that nearly one-third identified as being from racial or ethnic minority groups.
Table 2.
Participant characteristics for the overall STePS study sample (N = 264)
| Baseline variables | N | % | Mean | SD |
|---|---|---|---|---|
| Age (years) | 15.74 | 1.09 | ||
| Sex | ||||
| Male | 106 | 40.2 | ||
| Female | 158 | 59.8 | ||
| Race or ethnicity | ||||
| White, non-Hispanic | 173 | 65.5 | ||
| African American | 38 | 14.4 | ||
| Hispanic | 29 | 11.0 | ||
| Asian or Pacific Islander | 6 | 2.3 | ||
| Native American or Alaska Native | 3 | 1.1 | ||
| Reported as “Other” | 15 | 5.7 | ||
| Family income | ||||
| <$50,000 | 39 | 14.8 | ||
| $50,000–$75,000 | 38 | 14.4 | ||
| $76,000–$100,000 | 43 | 16.3 | ||
| $101,000–$150,000 | 50 | 18.9 | ||
| >$150,000 | 63 | 23.9 | ||
| Not reported | 19 | 7.2 | ||
| Mother’s education (college graduate) | 162 | 61.4 | ||
| Two-parent home | 158 | 59.8 | ||
| Intervention assignment | ||||
| Resilience | 133 | 50.4 | ||
| Education | 131 | 49.6 | ||
| Diabetes duration (years) | 6.88 | 4.03 | ||
| Insulin regimen | ||||
| Injections | 79 | 29.9 | ||
| Insulin pump | 185 | 70.1 | ||
| A1C (%) | 9.14 | 1.92 | ||
| Blood glucose monitoring frequency | 3.71 | 2.36 |
Randomization
Of the 264 randomized participants, 133 were randomized to PRP T1D and 131 to EI. There were no differences on baseline demographic or clinical characteristics between the groups (P > 0.05).
Exposure to Treatment and Retention Rates
The average number of sessions attended approached seven of nine (mean number of sessions 6.85 ± 3.05), and there were no differences between groups in the number of sessions attended or retention rates (P > 0.05). Of note, nearly two-thirds of participants completed all of the treatment sessions (sessions 1–8; session 9 was a review). At 1 year post-treatment (16 months after baseline), the study retention rate was 92%, with 244 of 264 individuals actively participating. Only 4 participants had formally withdrawn from the study, and the other 16 were unable to be reached.
Group Means and Baseline Correlations
Table 3 shows survey means for the entire sample and within each treatment group. Baseline values demonstrate no differences between groups (P > 0.05) on any of the outcome variables. In general, values were stable over time for three of the five outcomes—diabetes management, resilience, and A1C. Depressive symptoms and DD showed the most variability, and DD changed nearly 10 points in a favorable direction for PRP T1D participants. Of note, DD was correlated with depressive symptoms (r = 0.64, P < 0.001), diabetes management (r = −0.46, P < 0.001), resilience (r = −0.69, P < 0.001), and A1C level (r = 0.36, P < 0.001).
Table 3.
Means over time by STePS study group—PRP T1D and advanced diabetes education
| Measure | PRP T1D |
Advanced diabetes education |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 4 months | 8 months | 12 months | 16 months | Baseline | 4 months | 8 months | 12 months | 16 months | |
| DD | 72.1 ± 28.3 | 67.4 ± 26.2 | 66.2 ± 26.2 | 62.5 ± 26.8 | 62.8 ± 26.5 | 74.2 ± 25.0 | 70.1 ± 26.1 | 69.9 ± 27.2 | 68.4 ± 29.1 | 68.7 ± 27.6 |
| Depressive symptoms | 7.6 ± 6.1 | 7.9 ± 7.0 | 7.8 ± 7.4 | 8.5 ± 8.7 | 8.5 ± 8.5 | 7.9 ± 6.3 | 7.7 ± 6.9 | 7.9 ± 7.0 | 7.3 ± 6.9 | 8.5 ± 8.0 |
| Diabetes management | 53.4 ± 8.5 | 54.0 ± 8.2 | 53.1 ± 9.4 | 52.7 ± 9.3 | 52.8 ± 9.6 | 54.7 ± 7.1 | 54.2 ± 8.4 | 53.2 ± 9.2 | 52.4 ± 9.2 | 52.4 ± 9.3 |
| A1C (%) | 9.1 ± 1.9 | 9.1 ± 1.9 | 9.0 ± 1.8 | 9.2 ± 2.0 | 9.3 ± 1.9 | 9.1 ± 2.0 | 9.0 ± 2.0 | 9.1 ± 1.9 | 9.0 ± 1.8 | 9.1 ± 1.9 |
| Resilience characteristics | ||||||||||
| Mastery and relatedness | 178.4 ± 26.7 | 181.1 ± 26.8 | 181.6 ± 28.4 | 182.2 ± 31.4 | 179.4 ± 33.2 | 179.4 ± 24.7 | 180.6 ± 27.1 | 180.4 ± 28.4 | 180.3 ± 27.0 | 178.9 ± 28.4 |
| Automatic thoughts | 49.4 ± 21.9 | 48.1 ± 21.6 | 48.8 ± 25.6 | 47.7 ± 26.4 | 49.9 ± 25.1 | 49.2 ± 20.3 | 48.1 ± 21.9 | 49.2 ± 22.9 | 47.7 ± 22.4 | 50.3 ± 24.1 |
| Coping efficacy | 24.6 ± 4.4 | 25.4 ± 4.3 | 25.1 ± 4.5 | 25.3 ± 4.7 | 25.5 ± 4.9 | 24.8 ± 4.0 | 25.7 ± 3.8 | 25.6 ± 4.2 | 25.6 ± 4.2 | 25.6 ± 4.2 |
| Diabetes resilience | 48.9 ± 8.6 | 49.3 ± 8.6 | 48.6 ± 9.0 | 48.4 ± 9.9 | 49.1 ± 9.6 | 49.0 ± 7.1 | 49.0 ± 8.1 | 48.1 ± 8.4 | 48.4 ± 8.9 | 48.5 ± 8.4 |
| Social problem solving | 13.0 ± 2.8 | 12.9 ± 2.8 | 13.2 ± 2.7 | 13.0 ± 3.0 | 13.1 ± 3.1 | 12.8 ± 2.5 | 12.7 ± 2.7 | 12.8 ± 2.6 | 12.7 ± 3.0 | 12.7 ± 2.7 |
Data are means ± SD.
Change Over Time
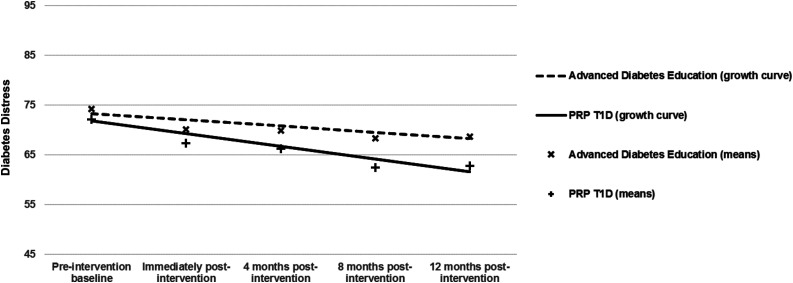
LGCM was conducted to determine the treatment effects for five outcomes—DD, depressive symptoms, resilience, diabetes management, and A1C. Each model was run separately. Prior to testing final models, potential interaction effects, and covariates (e.g., site, sessions attended, sex) were tested; none were significant and were not included in the final models presented. Full results are presented in Table 4. The DD analysis showed excellent model fit (CFI = 0.995, TLI = 0.995, RMSEA = 0.03, SRMR = 0.05). Overall, DD decreased over time regardless of group assignment (slope effect P < 0.001); however, group differences were also found. A significant treatment-intercept effect (P < 0.05) indicated less DD 1 year post-treatment in the PRP T1D group compared with the EI group, and a trend-level (P = 0.066) treatment-slope effect indicated that the PRP T1D group changed more rapidly (in a favorable direction) compared with the EI group (Fig. 2). The DD treatment-slope effect was examined for its magnitude given the trend-level significance, which was in line with recommendations and found to have a 0.20 effect size using methods described by Feingold (28).
Table 4.
Results of LGCM analyses for the overall STePS study sample
| Parameters | DD |
Depressive symptoms |
Diabetes management |
A1C |
Resilience^ |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | |
| Main effects | ||||||||||
| Intercept | 64.99*** | 1.67 | 8.09*** | 0.51 | 52.28*** | 0.59 | 9.22*** | 0.13 | ||
| Intercept variance | 605.89*** | 53.42 | 55.50*** | 6.85 | 75.27*** | 7.54 | 3.44*** | 0.37 | 428.26*** | 50.80 |
| Slope | −1.90*** | 0.37 | 0.10 | 0.11 | −0.43*** | 0.12 | 0.03 | 0.03 | 0.24 | 0.37 |
| Slope variance | 14.17** | 4.32 | 1.69*** | 0.35 | 1.47*** | 0.41 | 0.09** | 0.03 | 14.21*** | 4.12 |
| Intercept-slope covariance | 36.26** | 11.44 | 6.46*** | 1.21 | 6.51*** | 1.56 | 0.19** | 0.07 | 6.54 | 10.91 |
| Treatment effects | ||||||||||
| Treatment-intercept effect | −6.67* | 3.34 | 0.24 | 1.02 | 0.13 | 1.18 | 0.12 | 0.25 | −0.97 | 2.97 |
| Treatment-slope effect | −1.32† | 0.72 | 0.08 | 0.22 | 0.31 | 0.20 | 0.05 | 0.05 | 0.46 | 0.72 |
^Intercept effect not available because of the multiple-indicator model.
***P < 0.001; **P < 0.01; *P < 0.05; †P = 0.066.
Figure 2.
Change in diabetes distress from baseline to 12 months postintervention by treatment group.
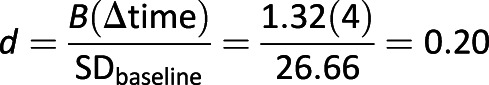 |
Models for depressive symptoms, A1C levels, and diabetes management met thresholds for good model fit. Depressive symptoms and A1C values remained stable over time across groups (slope effect P values > 0.05), and no significant group differences were found for these outcomes (treatment-intercept and treatment-slope effect P values > 0.05). For both treatment groups, diabetes management deteriorated over time (slope effect P < 0.001), without any significant differences between groups on intercept or slope (P values > 0.05). The multiple indicator growth curve model for resilience showed good fit (CFI = 0.96, TLI = 0.96, RMSEA = 0.05, SRMR = 0.08), but neither the slope nor the treatment-slope effects were significant (P > 0.05), indicating stability over time and no group differences in this construct.
Conclusions
Results indicate that exposure to the STePS program was associated with substantial reductions in DD. In this large, diverse sample of adolescents with T1D, those exposed to the PRP T1D versus EI fared better on DD. Youths, regardless of intervention, experienced stable glycemic control, resilience characteristics, and depressive symptoms 1 year post-treatment. It may be that symptoms of DD were reduced and depressive symptoms remained stable because of the diabetes-specific nature of the adapted intervention. Plus, individuals with a diagnosis of depression were excluded; thus, we may also have a restricted sample in terms of the occurrence of depressive symptoms. Results also indicated that a large percentage of participants received the intended dose of each program—PRP T1D and EI—and retention was very high (92%) 1 year after treatment ended. Overall, the 1-year post-treatment results of this trial suggest that PRP was successfully adapted to a T1D population and likely prevented DD and common deteriorations seen in adolescence.
It is typical for adolescents with T1D to experience rising A1C values and diminishing diabetes management across this age span. Data from the T1D Exchange (29) highlight that there is a gradual increase in A1C levels from an average for preadolescents of 8.5% to the peak of 9.0% for 13- to 17-year-old individuals. A1C levels go down only slightly for 18- to 25-year-old individuals (8.7%). Similarly, rates of blood glucose monitoring diminish over this period by nearly three fewer checks per day (29). The STePS study sample was very similar on these markers at baseline but saw little deterioration at 1 year post-treatment on A1C levels, suggesting that there may be a protective effect for youths in the PRP T1D group as well as for the comparison group offered in this RCT. The route to stabilization of A1C level at 1 year may be different for those exposed to EI versus PRP-T1D, and future analyses of 2- and 3-year outcomes will explore drivers of those differences.
The large sample increases the likelihood of representativeness and generalizability to the larger population of youths with T1D, but the sample did have a relatively restricted age range (14–18). It is possible that PRP T1D will work well in youths and young adults around this age range, but future analyses should examine whether there are age and social contextual variables that predict better (or worse) uptake of the program in particular subgroups. This type of analysis would inform efforts to implement the program across diverse populations. Further, we had to exclude individuals who reported they were not fluent in English. This also limits the generalizability of the findings to English-fluent individuals. A limitation of this study, given its preventive nature, in that a number of youths were excluded who were experiencing, or had experienced, elevated depressive symptoms or depression treatment. The results of this study suggest that even with a prevention focus, this intervention may have produced robust effects for them. It may be important to try this intervention in youths with documented DD and elevated depressive symptoms to see whether there is a benefit.
In sum, future plans include examining outcomes at 2 and 3 years post-treatment as those data become available in addition to examining intervening variables (moderators and mediators) such as sociodemographic and diabetes treatment variables. Given the effect size of 0.20 1 year out from treatment, and the evidence that PRP effects tend to grow over time, it is likely that more robust effects will be seen as future analyses are conducted. Further, inspection of total personnel efforts to enroll, deliver, and retain participants as an estimate of the total cost of the program will be examined. It is possible that future applications of the STePS program could be performed remotely or with established modules, thus reducing some personnel costs. However, participants noted very high satisfaction with face-to-face meetings, so this will have to be balanced with cost and staffing concerns. Overall, this research demonstrates that preventing a common problem facing adolescents with T1D is possible and future refinements of a program like STePS will aim to optimize management and glycemic outcomes as well.
Article Information
Acknowledgments. The authors thank the site research teams for research contributions, including the following team members: Katie Savin, Sarah Woods, Sarah Hanes, Bianca Agustin (all from the San Francisco Bay Area), and Aneta Jedraszko (Chicago metropolitan area).
Funding. This study was supported by funding from the National Institute of Diabetes and Digestive and Kidney Diseases (grant R01-090030).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. K.K.H. and J.W.-B. designed the study; obtained funding; trained staff; oversaw the conduct of the study; and wrote, reviewed, and edited the manuscript. E.I. and J.R. conducted statistical analyses; wrote sections of and reviewed and edited the manuscript; and approved the final manuscript. K.K.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 75th Scientific Sessions of the American Diabetes Association, Boston, MA, 5–9 June 2015, and the 76th Scientific Sessions of the American Diabetes Association, New Orleans, LA, 10–14 June 2016.
Footnotes
Clinical trial reg. no. NCT01490619, clinicaltrials.gov.
References
- 1.Nicolucci A, Kovacs Burns K, Holt RI, et al.; DAWN2 Study Group . Diabetes Attitudes, Wishes and Needs second study (DAWN2™): cross-national benchmarking of diabetes-related psychosocial outcomes for people with diabetes. Diabet Med 2013;30:767–777 [DOI] [PubMed] [Google Scholar]
- 2.Hagger V, Hendrieckx C, Sturt J, Skinner TC, Speight J. Diabetes distress among adolescents with type 1 diabetes: a systematic review. Curr Diab Rep 2016;16:9. [DOI] [PubMed] [Google Scholar]
- 3.Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care 2010;33:1034–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaser SS, Patel N, Xu M, Tamborlane WV, Grey M. Stress and coping predicts adjustment and glycemic control in adolescents with type 1 diabetes. Ann Behav Med 2017;51:30–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hood KK, Huestis S, Maher A, Butler D, Volkening L, Laffel LM. Depressive symptoms in children and adolescents with type 1 diabetes: association with diabetes-specific characteristics. Diabetes Care 2006;29:1389–1391 [DOI] [PubMed] [Google Scholar]
- 6.Hood KK, Beavers DP, Yi-Frazier J, et al. . Psychosocial burden and glycemic control during the first 6 years of diabetes: results from the SEARCH for Diabetes in Youth study. J Adolesc Health 2014;55:498–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garrison MM, Katon WJ, Richardson LP. The impact of psychiatric comorbidities on readmissions for diabetes in youth. Diabetes Care 2005;28:2150–2154 [DOI] [PubMed] [Google Scholar]
- 8.Hessler DM, Fisher L, Polonsky WH, et al. . Diabetes distress is linked with worsening diabetes management over time in adults with type 1 diabetes. Diabet Med 2017;34:1228–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riley AR, Duke DC, Freeman KA, Hood KK, Harris MA. Depressive symptoms in a trial behavioral family systems therapy for diabetes: a post hoc analysis of change. Diabetes Care 2015;38:1435–1440 [DOI] [PubMed] [Google Scholar]
- 10.Grey M, Whittemore R, Tamborlane W. Depression in type 1 diabetes in children: natural history and correlates. J Psychosom Res 2002;53:907–911 [DOI] [PubMed] [Google Scholar]
- 11.Weissberg-Benchell J, Rausch J, Iturralde E, Jedraszko A, Hood K. A randomized clinical trial aimed at preventing poor psychosocial and glycemic outcomes in teens with type 1 diabetes (T1D). Contemp Clin Trials 2016;49:78–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillham JE, Hamilton J, Freres DR, Patton K, Gallop R. Preventing depression among early adolescents in the primary care setting: a randomized controlled study of the Penn Resiliency Program. J Abnorm Child Psychol 2006;34:203–219 [DOI] [PubMed] [Google Scholar]
- 13.Reivich KJ, Gillham J, Chaplin TM, Seligman ME. From helplessness to optimism: the role of resilience in treating and preventing depression in youth. In Handbook of Resilience in Children. Goldstein S, Brooks RB, Eds. New York, Kluwer Academic/Plenum Publishers, 2005, p. 223–237 [Google Scholar]
- 14.Hilliard ME, Harris MA, Weissberg-Benchell J. Diabetes resilience: a model of risk and protection in type 1 diabetes. Curr Diab Rep 2012;12:739–748 [DOI] [PubMed] [Google Scholar]
- 15.Weissberg-Benchell J, Antisdel-Lomaglio J. Diabetes-specific emotional distress among adolescents: feasibility, reliability, and validity of the Problem Areas In Diabetes-Teen version. Pediatr Diabetes 2011;12:341–344 [DOI] [PubMed] [Google Scholar]
- 16.Weissberg-Benchell J, Hood K, Antisdel-Lomaglio J. Psychometric properties of the parent version of the Problem Areas In Diabetes-Teens. Late-breaking abstract presented at the 74th Annual Scientific Sessions of the American Diabetes Association, 13–17 June 2014, at the Moscone Center, San Francisco, California [Google Scholar]
- 17.Kovacs M. The Children’s Depression Inventory (CDI): Technical Manual. North Tonawanda, NY, Multi-Health Systems, 2003 [Google Scholar]
- 18.Harrell TH, Ryon NB. Cognitive-behavioral assessment of depression: clinical validation of the automatic thoughts questionnaire. J Consult Clin Psychol 1983;51:721–725 [DOI] [PubMed] [Google Scholar]
- 19.Hollon S, Kendall P. Cognitive self-statements in depression: development of an automatic thoughts questionnaire. Cognit Ther Res 1980;4:383–395 [Google Scholar]
- 20.Kazdin AE. Evaluation of the automatic thoughts questionnaire: negative cognitive processes and depression among children. Psychol Assess 1990;2:73–79 [Google Scholar]
- 21.Sandler IN, Tein JY, Mehta P, Wolchik S, Ayers T. Coping efficacy and psychological problems of children of divorce. Child Dev 2000;71:1099–1118 [DOI] [PubMed] [Google Scholar]
- 22.Maydeu-Olivares A, D’Zurilla T. A factor analytic study of the social problem-solving inventory: an integration of theory and data. Cognit Ther Res 1996;20:115–133 [Google Scholar]
- 23.Wu A, Liu Y, Gadermann AM, Zumbo BD. Multiple-indicator multilevel growth model: a solution to multiple methodological challenges in longitudinal studies. Soc Indic Res 2010;97:123–142 [Google Scholar]
- 24.Weinger K, Butler HA, Welch GW, La Greca AM. Measuring diabetes self-care: a psychometric analysis of the Self-Care Inventory-Revised with adults. Diabetes Care 2005;28:1346–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McArdle JJ. Latent variable modeling of differences and changes with longitudinal data. Annu Rev Psychol 2009;60:577–605 [DOI] [PubMed] [Google Scholar]
- 26.Stull DE. Analyzing growth and change: latent variable growth curve modeling with an application to clinical trials. Qual Life Res 2008;17:47–59 [DOI] [PubMed] [Google Scholar]
- 27.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling 1999;6:1–55 [Google Scholar]
- 28.Feingold A. Effect sizes for growth-modeling analysis for controlled clinical trials in the same metric as for classical analysis. Psychol Methods 2009;14:43–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller KM, Foster NC, Beck RW, et al.; T1D Exchange Clinic Network . Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care 2015;38:971–978 [DOI] [PubMed] [Google Scholar]