Abstract
OBJECTIVE
Patients with type 1 diabetes now live long enough to experience cognitive decline. During middle age, they show mild cognitive deficits, but it is unknown whether severity increases with aging or whether cognitive profiles are similar to those of age-matched peers with and without diabetes.
RESEARCH DESIGN AND METHODS
We tested and compared cognition in 82 individuals with 50 or more years of type 1 diabetes (Medalists), 31 age-matched individuals with type 2 diabetes, and 30 age-matched control subjects without diabetes. Medical histories and biospecimens were collected. We also evaluated the association of complications with cognition in Medalists only.
RESULTS
Compared with control subjects, both individuals with type 1 diabetes and individuals with type 2 diabetes performed worse on immediate and delayed recall (P ≤ 0.002) and psychomotor speed in both hands (P ≤ 0.01) and showed a trend toward worse executive function (P = 0.05). In Medalists, cardiovascular disease was associated with decreased executive function and proliferative diabetic retinopathy with slower psychomotor speed.
CONCLUSIONS
Both patients with type 1 and patients with type 2 diabetes showed overall worse cognition than control subjects. Further, in Medalists, a relationship between complications and cognition was seen. Although both groups with diabetes showed similar deficit patterns, the underlying mechanisms may be different. Now that patients with type 1 diabetes are living longer, efforts should be made to evaluate cognition and to identify modifying behaviors to slow decline.
Introduction
Two decades ago, patients with type 1 diabetes typically did not live beyond 55 years, an age before symptoms of late-onset dementia typically begin to arise (i.e., age 65 years) (1). However, as longevity with type 1 diabetes increases, it is unknown whether these individuals have the same propensity for dementia or for accelerated aging pathologies as patients with type 2 diabetes (2). Therefore, evaluation of older individuals with type 1 diabetes for deficits in psychomotor efficiency, attention, and executive function reported in young and middle-aged adults is warranted (3).
Normal aging first impacts the frontal lobe, and thereby executive function, while medial temporal decline (associated with memory loss) is more closely linked with Alzheimer disease (AD) pathology (4,5). The latter type of memory loss is more common in those with type 2 diabetes. In contrast, executive function usually declines first in vascular dementia (6). However, among those with type 1 diabetes, memory has been noted to be less affected during early-life and middle age than other forms of cognition (3). Other large cohorts, such as the Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) cognitive study, investigated the impact of glycemic control on cognition and demonstrated that intensive glucose control did not affect cognition in young or middle-aged adults (7). This finding mitigated concerns that the negative consequences of hypoglycemia would offset any benefits in glucose control. However, since the average age at study end was 46 ± 7 years and the average life span of patients with type 1 diabetes, until recently, was <54 years (8), the DCCT/EDIC study was not informative about the status of cognition in elderly patients with type 1 diabetes.
The aim of this study is to gain better insight into long-term cognitive outcomes; thus, a subset of 50-Year Medalists, a cohort of well-characterized (n = 952) individuals from across the U.S., were assessed for cognitive status. Among these individuals, 82 were compared with age-matched individuals with type 2 diabetes (n = 31) and control subjects (n = 30) using standard cognitive assessments.
After 50 years of type 1 diabetes, nephropathy, retinopathy, neuropathy, and cardiovascular disease (CVD) are believed to have already manifested; as such, a relationship with cognitive dysfunction would likely be detectable. In addition, we can assess whether small- and large-vessel diseases show a differential association with cognitive performance. Therefore, the aims of this analysis were to compare those with >50 years of type 1 diabetes duration, those with type 2 diabetes, and those without diabetes to examine which cognitive deficits may be attributable to age, long-term hyperglycemia, and insulin resistance. An additional aim was to determine whether there is an association between cognitive changes and complications in type 1 diabetes.
Research Design and Methods
Study Design
This is a comparison of three study cohorts, examining cognitive function in 82 individuals with 50 or more years of documented insulin-dependent diabetes (Medalists).
Individuals were recruited consecutively from the parent Medalist study. For comparison, we studied 31 age-matched patients with type 2 diabetes (23 Joslin patients and 8 patients recruited via craigslist) and 30 age-matched control subjects without diabetes (recruited via craigslist) (Table 1). The Medalist study has previously been described extensively (9,10). In brief, the Medalist study consists of 952 individuals from all 50 U.S. states who were characterized at the Joslin Diabetes Center by completion of medical history questionnaires and undergoing clinical and ophthalmic exams to evaluate complications with collection of biospecimens. Medical information and medication history for control subjects and for those with type 2 diabetes were gathered from medical records and questionnaires. Because some of the patients with type 2 diabetes and control subjects underwent MRI scanning as part of a separate study, exclusion criteria included existing central nervous system disorders or other illnesses affecting neurological function and antipsychotic medication use. Contraindications to MRI included BMI >40 kg/m2 owing to weight limits on the scanner and claustrophobia. Many of the patients with type 2 diabetes were part of a separate study with the following exclusion criteria: heart disease, proliferative retinopathy, and autonomic neuropathy. Those patients with type 2 diabetes who only participated in the current study had the same inclusion and exclusion criteria as the patients with type 2 diabetes who participated in the additional study so as to keep the groups uniform. Diabetes status for non-Joslin patients was verified by fasting glucose values >7.0 mmol/L.
Table 1.
Characteristics of participants by diabetes group
| No diabetes** | Type 1 diabetes | Type 2 diabetes** | P* | P† | |
|---|---|---|---|---|---|
| N | 30 | 82 | 31 | ||
| Sex (female) | 40.0 (12) | 54.9 (45) | 48.4 (15) | 0.37 | 0.54 |
| Age (years) | 64.0 ± 3.9 | 66.5 ± 7.1 | 69.3 ± 8.1 | 0.02 | 0.25 |
| Age at diagnosis (years) | N/A | 11.5 ± 6.2 | 50.3 ± 11.9 | <0.001 | |
| Duration of disease (years) | N/A | 55.2 ± 5.1 | 19.1 ± 15.6 | <0.001 | |
| HbA1c (%) | 5.6 ± 0.4 | 7.1 ± 0.8 | 8.1 ± 1.4 | <0.001 | 0.001 |
| HbA1c (mmol/mol) | 38.1 ± 4.0 | 54.0 ± 8.6 | 64.7 ± 15.6 | <0.001 | 0.001 |
| BMI (kg/m2) | 27.6 ± 4.9 | 26.1 ± 4.8 | 31.3 ± 6.3 | <0.001 | <0.001 |
| Diastolic blood pressure (mmHg) | 72.5 ± 10.1 | 66.0 ± 7.5 | 72.3 ± 8.7 | <0.001 | 0.001 |
| Systolic blood pressure (mmHg) | 125.8 ± 13.6 | 134.1 ± 17.7 | 135.8 ± 13.9 | 0.02 | 0.40 |
| LDL cholesterol (mmol/L) | 2.7 ± 0.8 | 2.0 ± 0.7 | 2.2 ± 0.9 | 0.002 | 0.48 |
| HDL cholesterol (mmol/L) | 1.4 ± 0.5 | 1.8 ± 0.5 | 1.3 ± 0.5 | <0.001 | <0.001 |
| Total cholesterol (mmol/L) | 4.8 ± 0.9 | 4.2 ± 0.9 | 4.3 ± 1.3 | 0.01 | 0.83 |
| Triglycerides (mmol/L) | 1.5 ± 0.8 | 0.7 ± 0.2 | 1.7 ± 0.8 | <0.001 | <0.001 |
| Fasting glucose (mmol/L) | 4.5 ± 0.9 | 7.0 ± 2.1 | 10.7 ± 4.7 | <0.001 | 0.001 |
| CVD | 34.6 (28) | ||||
| PDR (ETDRS >53) | N/A | 41.9 (31) | 8.3 (1) | ||
| Diabetic nephropathy (eGFR ≤45 mL/min/1.73 m2) | N/A | 4.9 (4) | 8.3 (1) | ||
| Neuropathy (MNSI ≥2) | N/A | 64.0 (48) | 25.0 (3) | ||
| Hypertension | 55.6 (5) | 67.1 (53) | 66.7 (8) | ||
| Antihypertensive use | 33.3 (3) | 59.0 (46) | 58.3 (7) | ||
| Lipid-lowering medication | 11.1 (1) | 68.8 (55) | 41.7 (5) | ||
| Report physical activity | 84.2 (69) | ||||
| Report ever smoking | 44.4 (4) | 41.5 (34) | 33.3 (4) | ||
| Insulin use | N/A | 100.0 (82) | 66.7 (8) |
Values are presented as mean ± SD for continuous variables and % (n) for categorical variables unless otherwise noted. ETDRS, Early Treatment Diabetic Retinopathy Study Score; MNSI, Michigan Neuropathy Screening Instrument; N/A, not applicable.
*P value from ANOVA comparison across the three groups: no diabetes, type 1 diabetes (Medalists), and type 2 diabetes.
†P value from Kruskal-Wallis comparison of type 1 diabetes (Medalists) and type 2 diabetes.
**Some clinical characteristics unavailable or available for a subset of participants without diabetes and participants with type 2 diabetes.
All procedures were done and informed consent was obtained from all subjects prior to participation in the study in accordance with the Joslin Diabetes Center Committee on Human Subjects Institutional Review Board review.
CVD status was based on standardized validated self-reported questions regarding history of coronary artery disease, angina, heart attack, prior cardiac or leg angioplasty, or bypass graft surgery. Peripheral vascular disease consisted of self-reported history of leg angioplasty or leg bypass graft surgery. Several studies have demonstrated the reliability and validity of self-reported heart disease (11–13).
Nephropathy was determined by an estimated glomerular filtration rate (eGFR) of <45 mL/min/1.73 m2. Early Treatment Diabetic Retinopathy Study 7-field criteria was used for proliferative diabetic retinopathy (PDR) determination. The Michigan Neuropathy Screening Instrument (scores ≥2) was used to assess neuropathy. Medalists were asked about frequency and intensity of regular physical activity. Hypertension was considered as systolic blood pressure >135 mmHg or diastolic >85 mmHg at the time of visit and/or current report of blood pressure medications. HbA1c was determined by high-performance liquid chromatography (Tosoh G7 and 2.2; Tosoh Corporation, Tokyo, Japan); C-reactive protein, HDL, and C-peptide measurement methods were previously described by Sun et al. (9).
Cognitive Testing
Prior to cognitive assessment, blood glucose levels needed to exceed 4.4 mmol/L. If levels were below this value, a snack was given and glucose readings were taken every 15 min until an adequate level was reached.
All study participants were administered the Wechsler Abbreviated Scale of Intelligence (WASI) (14), the Trail Making Number-Letter Switching subtest from the Delis-Kaplan Executive Function System (D-KEFS), the Letter-Number Sequencing subtest from the Wechsler Memory Scale III, the Rey Auditory Verbal Learning Test, and the Grooved Pegboard for both the dominant and nondominant hand. This battery allowed us to assess general intelligence, executive function, working memory, long-term memory, and psychomotor speed, respectively.
WASI provides a reliable, psychometrically sound estimate of intelligence using two (as in the current study) or four subtests, yielding three composite intelligence quotient (IQ) estimates (Verbal, Performance, and Full Scale IQ [FSIQ]). Correlations with other tests of the same domains (in the range of 0.66–0.88) help support the validity of the scales. The WASI manual provides normative references and extensive documentation of psychometric properties (14). The number-letter switching subtest of the D-KEFS is a measure of executive function. Participants must connect scrambled letters and numbers in ascending alternating order as quickly as possible and are scored by time to complete plus errors. Validity of these measures has been assessed by comparisons with other similar tests (15). The Rey Auditory Verbal Learning Test is a 15-item word test measuring auditory-verbal learning and memory and is presented five consecutive times in the same order with a recall test after each trial. After a 30-min delay, a free-recall test is administered without repeating the list. The validity of this measure is demonstrated by its high correlation with other standardized memory tests such as Logical Memory of the Wechsler Memory Scale–Revised (15). The Letter-Number Sequencing subtest of the Wechsler Memory Scale III was used to assess working memory. Its validity as a test of auditory working memory is noted by confirmatory factor analyses (15). The Grooved Pegboard is a psychomotor speed and efficiency task, scored according to time for completion combined with the number of dropped pegs. The validity of this task as a measure of motor speed and psychomotor processing comes from its demonstrated relationship with other tests of motor speed (e.g., tapping) as well as with measures of attention and perceptual speed and nonverbal reasoning (15).
Statistical Analyses
The distributions of relevant variables were examined for outliers and to determine appropriate statistics for use. χ2, Kruskal-Wallis, and ANOVA tests were used to examine categorical differences. Linear models were constructed to adjust for covariates and for effect modification. All variables significant in the bivariable comparisons at the α = 0.1 levels were tested in the multivariable model with the main effect and remained in the model if significant at P < 0.05 with other covariates, or were confounders using the 15% change rule or significant as part of an interaction term. Confounders tested in the model include age, HbA1c, BMI, diastolic and systolic blood pressure, LDL and HDL, total cholesterol, and triglycerides. Descriptive statistics are presented as mean (SD) or percentage (n) as appropriate. Cohen’s delta (d) was also used to assess effect size between groups. SPSS, version 19 (SPSS, Chicago, IL), Stata/SE (StataCorp, College Station, TX), and SAS, version 9.4 (SAS Institute, Cary, NC), were used to perform analyses.
Results
A subset of the Medalist population (n = 82) was tested between February 2013 and August 2016. The mean ± SD age was 66.5 ± 7.1 years, age at diagnosis 11.5 ± 6.2 years, duration of type 1 diabetes 55.2 ± 5.1 years, and HbA1c 7.1 ± 0.8% (54.0 ± 8.6 mmol/mol). The prevalence of CVD was 34.6%, PDR 41.9%, and diabetic nephropathy 4.9%. Key diabetes characteristics (i.e., HbA1c, diabetes duration, and age at diagnosis) did not differ meaningfully between the subgroup and the overall Medalist cohort (Supplementary Table 1). CVD risk factors were less favorable in patients with type 2 diabetes than Medalists including HDL, triglycerides, HbA1c, diastolic blood pressure, and fasting glucose (Table 1). The control group showed a trend toward being younger (64 ± 3.9 vs. 66.5 ± 7.1 years for control subjects vs. Medalists, respectively; P = 0.05), and sex distribution was 60% male for control subjects vs. 45.1% for Medalists.
Cognitive Findings
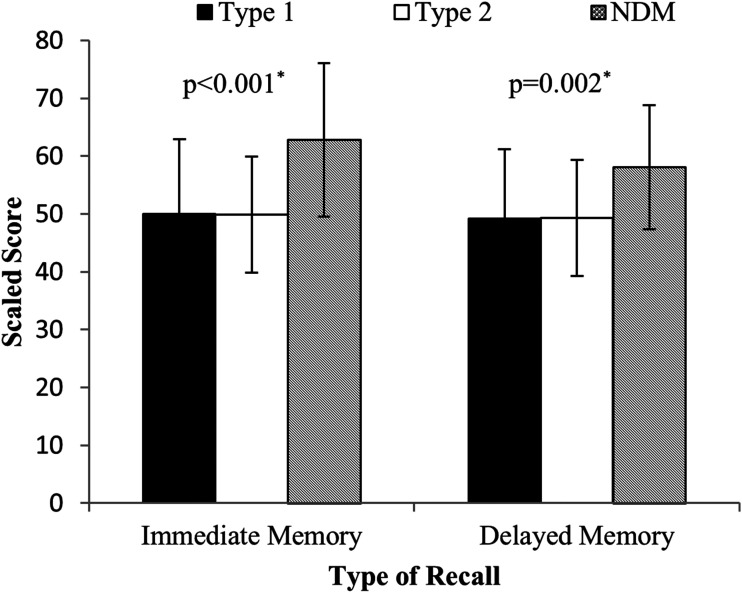
The mean ± SD WASI FSIQ score was 120.3 ± 8.7, 117.1 ± 12.5, and 111.8 ± 12.8 for the control subjects, Medalists, and patients with type 2 diabetes, respectively. Initial analyses indicate that diabetes status differentiates measures of immediate and delayed recall (P ≤ 0.002), psychomotor speed both in dominant and nondominant hands (P ≤ 0.01) (Table 2), and a trend for a difference in executive function (P = 0.05) between all groups with varying degrees of glycemic tolerance. This was confirmed by the estimate of effect using Cohen’s d, which suggests that there were large differences between Medalists and those without diabetes, particularly in the areas of immediate and delayed memory (Cohen’s d = 0.98 and 0.78, respectively) (Supplementary Table 2). In an unadjusted comparison, those with diabetes (type 1 and type 2) were similar on most facets. This remained true for immediate and delayed recall with adjustment for the potential confounders of age and IQ among Medalists (unadjusted: immediate β = −0.018, P = 0.0001, and delayed β = −0.016, P = 0.0034; age and IQ adjusted: immediate β = −0.014, P = 0.004, and delayed β = −0.011, P = 0.05) (data not shown). Individuals with type 2 diabetes also performed worse than control subjects on these tests, with immediate recall reaching significance (adjusted for age and IQ, P = 0.02) and delayed recall approaching significance (adjusted for age and IQ, P = 0.11 [Fig. 1]) (unadjusted [Table 2]).
Table 2.
Cognitive scores by diabetes group
| Cognitive Test | No diabetes | Type 1 diabetes | Type 2 diabetes | P* | P† | P‡ | P** |
|---|---|---|---|---|---|---|---|
| N | 30 | 82 | 31 | ||||
| Grooved Pegboard (dominant hand) time (s) | 92.6 ± 20.5 | 108.2 ± 27.9 | 114.4 ± 41.6 | 0.009 | 0.001 | 0.05 | 0.93 |
| Grooved Pegboard (nondominant hand) time (s) | 99.8 ± 23.3 | 125.6 ± 51.2 | 117.4 ± 42.1 | 0.01 | 0.002 | 0.21 | 0.34 |
| Letter-Number Sequencing (scaled) | 12.2 ± 3.2 | 11.1 ± 3.2 | 11.2 ± 2.4 | 0.17 | 0.10 | 0.08 | 0.88 |
| Immediate Memory (scaled) | 62.8 ± 13.3 | 50.0 ± 12.9 | 49.9 ± 10.0 | <0.001 | <0.001 | <0.001 | 0.97 |
| Delayed Memory (scaled) | 58.1 ± 10.7 | 49.2 ± 12.0 | 49.3 ± 9.9 | 0.002 | 0.001 | 0.003 | 0.91 |
| Wechsler Abbreviated Scale of Intelligence FSIQ | 120.3 ± 8.7 | 117.1 ± 12.5 | 111.8 ± 12.8 | 0.03 | 0.19 | 0.008 | 0.08 |
| D-KEFS: Number-Letter Switching (scaled) | 12.0 ± 2.1 | 10.9 ± 2.3 | 11.2 ± 2.8 | 0.05 | 0.02 | 0.22 | 0.37 |
Data are means ± SD unless otherwise noted. Data are not adjusted.
*P value from ANOVA comparison across the three groups: no diabetes, type 1 diabetes (Medalists), and type 2 diabetes.
†P value from Kruskal-Wallis comparison of no diabetes and type 1 diabetes (Medalists).
‡P value from Kruskal-Wallis comparison of no diabetes and type 2 diabetes.
**P value from Kruskal-Wallis comparison of type 1 diabetes (Medalists) and type 2 diabetes.
Figure 1.
Comparison of immediate and delayed memory assessed by the Rey Auditory Verbal Learning Test across study groups. Medalists (filled), individuals with type 2 diabetes (white), and control subjects without diabetes (NDM) (cross-hatched). Means ± SD are presented. *Adjusted for age and IQ.
Complications
Complications data were only available on Medalists. The largest differences in cognitive function were found between those with and without CVD (Cohen’s d = −0.62 for D-KEFS Trail Making task [Number-Letter Switching]) and for diabetic retinopathy (Grooved Pegboard [dominant hand]: Cohen’s d = 0.58) (Table 3 and Supplementary Table 3). Traits associated with cognitive deficits included those associated with insulin resistance including higher BMI (mean ± SD 27.8 ± 5.1 vs. 25.1 ± 4.5 kg/m2, P = 0.02), higher prevalence of hypertension (89.3% vs. 54.0%, P = 0.002), and statin use (82.1% vs. 60.8%, P = 0.05).
Table 3.
Cognitive assessment score by complication status in 50-Year Medalists
| No CVD | CVD | No PDR | PDR | No nephropathy | Nephropathy | No neuropathy | Neuropathy | |
|---|---|---|---|---|---|---|---|---|
| N | 53 | 28 | 43 | 31 | 78 | 4 | 27 | 48 |
| Grooved Pegboard (dominant hand) time (s) | 106.4 ± 24.4 | 112.5 ± 34.0 | 101.7 ± 24.9 | 117.9 ± 30.5* | 106.8 ± 26.4 | 130.3 ± 44.2 | 102.4 ± 27.9 | 110.2 ± 26.1 |
| Grooved Pegboard (nondominant hand) time (s) | 125.9 ± 52.6 | 126.3 ± 50.5 | 119.2 ± 53.3 | 134.5 ± 51.2 | 124.3 ± 51.4 | 146.0 ± 49.2 | 109.9 ± 34.0 | 129.2 ± 54.0 |
| Letter-Number Sequencing (scaled) | 11.5 ± 3.3 | 10.3 ± 3.0 | 10.9 ± 3.4 | 10.9 ± 3.1 | 11.1 ± 3.2 | 10.5 ± 2.1 | 11.3 ± 2.6 | 10.7 ± 3.5 |
| Immediate Memory (scaled) | 51.4 ± 13.4 | 48.3 ± 12.0 | 49.3 ± 11.7 | 49.2 ± 15.1 | 50.0 ± 13.2 | 51.0 ± 7.9 | 51.2 ± 14.0 | 48.2 ± 12.3 |
| Delayed Memory (scaled) | 50.0 ± 12.0 | 48.3 ± 12.4 | 47.4 ± 11.0 | 49.9 ± 13.5 | 49.4 ± 12.0 | 44.9 ± 14.7 | 49.2 ± 11.3 | 48.8 ± 12.0 |
| Wechsler Abbreviated Scale of Intelligence FSIQ | 117.9 ± 11.7 | 115.1 ± 14.0 | 120.0 ± 11.3 | 112.5 ± 13.0* | 118.0 ± 12.1 | 101.0 ± 7.4* | 120.2 ± 11.9 | 115.5 ± 12.5 |
| D-KEFS: Number-Letter Switching (scaled) | 11.4 ± 2.3 | 10.0 ± 2.2* | 11.0 ± 2.3 | 10.7 ± 2.5 | 10.9 ± 2.4 | 10.7 ± 1.5 | 11.1 ± 2.4 | 10.8 ± 2.4 |
Data are presented as means ± SD unless otherwise noted.
*Significant P value (<0.05) from Kruskal-Wallis comparison of complication(s) and no complications.
A higher proportion of those without CVD reported regular physical activity. Among those with PDR, there was a borderline difference in duration (mean ± SD 56.7 ± 6.3 vs. 54.0 ± 3.6 years, P = 0.10) and a significant difference in eGFR; however, mean levels were not <60 mL/min/1.73 m2 in either group. Additionally, those with PDR showed a similar pattern of hypertension and statin use (data not shown).
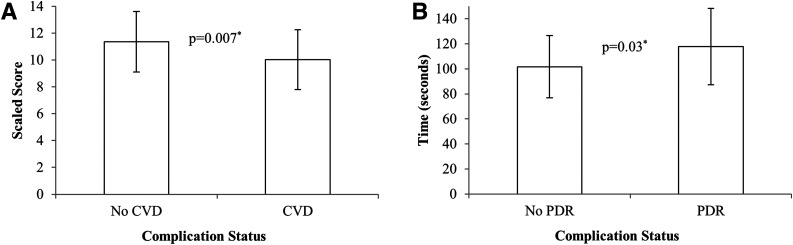
CVD was independently associated with executive function as measured by D-KEFS Trail Making task (Number-Letter Switching) (Fig. 2A), even after adjustment for factors associated with insulin resistance (lipids, statin use, and BMI). HbA1c was significant in the adjusted model (CVD: β = −1.2, P = 0.03; HbA1c: β = −0.73, P = 0.04). Additionally, psychomotor speed in the dominant hand was significantly associated with PDR (101.7 vs. 117.9, P = 0.03) (Fig. 2B), and this effect was independent of age (PDR: β = 14.79, P = 0.03; age: β = 1.66, P = 0.03). While a significant difference was found between nephropathy status (eGFR ≤45 mL/min/1.73 m2) and IQ, there were only n = 4 in the nephropathy group, and this association was independent of adjustment for age and CVD. Neuropathy status was not associated with differences in any of the cognitive facets measured on either a univariable or multivariable level (data not shown).
Figure 2.
A: Executive function performance assessed by D-KEFS Trail Making task (Number-Letter Switching) and depicted according to presence or absence of CVD in Medalists. Means ± SD are presented. *Adjusted for lipids, statin use, and BMI. B: Psychomotor speed measured by Grooved Pegboard assessment and depicted according to presence or absence of PDR in Medalists. Means ± SD are presented. *Adjusted for age.
Conclusions
In this comparison, both those with type 1 diabetes and those with type 2 diabetes show deficits in cognitive function, particularly in memory, compared with those without diabetes. We note that IQ in the three groups studied was higher than what is found in the general population and could affect baseline cognitive function. This suggests that the generalizability of these findings may be limited to those with IQ in the higher range. To address the possible confounding effect of IQ, we adjusted for IQ in the analysis of cognitive performance. Our findings vary from what was previously documented (3) in that those with shorter-duration type 1 diabetes experience deficits in cognitive function, including psychomotor speed, attention, and executive function. In Medalists, the pattern of cognitive performance is similar to that seen in the age-matched individuals with type 2 diabetes, affecting primarily memory. The underlying mechanism is likely to be weighted more to hyperglycemia than insulin resistance, which is often the underlying culprit in type 2 diabetes. In Medalists, long-term hyperglycemia may have led to the accumulation of damage to both the parenchyma and vasculature of the brain (16). Our data showing association with vascular complications support this theory.
Longitudinal studies show that cognition is negatively affected in type 1 diabetes (17). The mechanisms proposed include insulin resistance, inflammation, oxidative stress (18), and neurovascular dysfunction (19). There has been less research in older individuals with type 1 diabetes but, in two studies, individuals showed mild decrements in cognition relative to control subjects, especially in information processing speed (20,21). One longitudinal study with a 4-year follow-up period demonstrated that patients with type 1 diabetes with significant comorbidities continued to decline in several cognitive facets relative to control subjects, whereas there was not a significant decline among those without complications compared with control subjects (21).
It is difficult to pinpoint the underlying cause of this elevated risk in either group, although neuroimaging studies comparing modest to relatively large-sized groups of patients with diabetes with individuals without diabetes have brought us closer to understanding the impact that diabetes has on the brain (20,22,23). Brain imaging techniques allow us to measure glucose metabolism, brain activity, and brain structure to identify vulnerable brain regions and susceptible forms of cognition. Cutting edge techniques, such as dynamic contrast imaging that allow us to visualize the integrity of the blood-brain barrier, can carry risks to the patient and are highly specialized and not widely available (24), and the research done to determine the molecular underpinnings of oxidative stress or disruptions in insulin signaling have required animal models. Still, brain imaging and cognitive testing can help inform molecular biologists of where to shine the spotlight.
The present analysis indicates that despite memory scores within normal limits, performance is worse in individuals with 50 or more years of type 1 diabetes compared with age-matched control subjects without diabetes. Executive function and working memory do not differ significantly between type 1 and type 2 diabetes, and both groups performed more poorly than participants without diabetes. Immediate and delayed memory were the most affected cognitive functions, as these forms of memory showed statistical significance or trended toward higher performance in control subjects relative to patients with type 1 or type 2 diabetes, as demonstrated by effect size estimates and adjusted analyses.
Reduced performance in Medalists does not necessarily indicate a propensity for dementia. It is difficult to ascertain whether memory decline signals normal aging or accelerated decline due to the aging process (25). Using hospital visits to determine risk for AD or vascular dementia in both patients with type 1 and patients with type 2 diabetes, Smolina et al. (26) found that each population showed an elevated and similar risk for any form of dementia (in particular, vascular). Careful examinations are needed to evaluate whether memory is disproportionately affected by each type of diabetes, especially in recall, relative to other forms of cognition (e.g., executive function). Further, both functional and structural MRI studies have been helpful in identifying abnormalities, as some functional MRI studies suggest that patients with amnestic mild cognitive impairment have hippocampal hyperactivation during active learning as a means of compensating for poorer memory (27). Further, patterns of regional volumetric loss in frontal versus medial temporal lobes may assist in differentiating normal aging from AD, as the normal aging process impacts the frontal lobes first. This is in contrast to AD in which the hippocampus and entorhinal cortex are affected first (25). Further, those at risk for AD show disproportionate problems in autobiographical memory. When standard mnemonic devices, such as retrieval cues, are ineffective but other forms of cognition, such as executive function, are relatively unaffected, this may indicate that memory impairment is due to the dementing process and not to normal aging (28). Longitudinal studies examining these neurocognitive and MRI patterns should be conducted to distinguish dementia from accelerated aging. The latter may be modifiable, as it is due to persistent hyperglycemia that can be managed with better glycemic control (29).
We found that psychomotor speed was slower in both older individuals with type 1 and older individuals with type 2 diabetes compared with control subjects. Psychomotor speed is typically impaired in type 1 diabetes and may be tied to basal ganglia or white matter integrity (30). In our earlier research using voxel-based morphometry, we showed hyperglycemia-associated reduced gray matter density in posterior cingulate, superior temporal gyrus, and parahippocampal gyri (23). These regions are important for learning and memory and may be a possible contributor to poorer memory performance.
The association between CVD and executive function signifies the importance of the integrity of the large brain vessels that serve the frontal lobes (e.g., anterior cerebral artery), which are important in decision-making and attention, which are key components of executive function.
Similar associations between psychomotor performance and macrovascular risk factors were also found in the shorter-duration (24.5 years) EDIC cohort, but further follow-up data are not yet available on this population. Individuals with type 1 diabetes often have early atherosclerotic calcification, which combined with the aging process in the older Medalists may accelerate cerebrovascular disease (31). Importantly, an advantage of the diabetes duration among Medalists is that it is long enough to evaluate the association of complications on the brain in a way that is not possible in younger cohorts, as complications have likely fully manifested after 50 years of disease duration. This is critical as not only the incidence of type 1 diabetes increases but also as increasing longevity with the disease has now been documented by several groups (32–34). This line of research allows us to contrast the ways in which glycemic dysregulation in type 1 diabetes is linked to cognitive dysfunction compared with type 2 diabetes. We recognize that insulin resistance may also play a role, although it would be expected to be less pervasive owing to relatively lean body mass and low insulin doses in Medalists. There is little previous research on cognition in elderly patients with type 1 diabetes; thus, a concerted effort must be made to longitudinally assess elderly patients with type 1 diabetes on a neurocognitive battery. Repeated testing could provide an early screening tool that can be used for development of potential therapies as is used in those with type 2 diabetes (35). If cognition does decline more rapidly in patients with type 1 diabetes compared with individuals without diabetes, the contributory factors need to be identified so that symptom-ameliorating therapies can be implemented.
As this is not a longitudinal study, we cannot tell the order of complication development and cognitive changes cannot be determined. However, as we know the incidence curve for microvascular complications plateaus around 30 years’ duration and we now see subclinical symptomology, we may be able to say that nephropathy and PDR predate the cognitive deficits. As there are many aspects to cognitive function critical to daily life, a wider battery would have been optimal. However, to limit confounding from fatigue, the number and length of the cognitive testing were limited, as the total study visit was 5 h. Further, our executive function test had a psychomotor speed component and may not reflect purely executive function. We had fewer patients with type 2 diabetes and control subjects and were not able to collect as much clinical data for these participants as for the Medalists. However, despite these shortcomings, given that the Medalists are exceptionally well suited to answer questions concerning cognitive decline in the elderly and in long-duration type 1 diabetes, we thought the endeavor well worth pursuing. We view it as a way to gain some insight into cognitive abilities in this population to help design a larger study that can fill in the gaps.
In summary, this is the first study to measure cognition in patients with type 1 diabetes with minimum duration of 50 years. These patients were compared with similarly aged patients with type 2 diabetes and age-matched control subjects. Patients with either type 1 or type 2 diabetes performed worse than control subjects on measures of memory and psychomotor speed, with a trend toward poorer executive function despite scores in the normal range. When we considered patients with type 1 diabetes only, patients with CVD, a macrovascular complication, showed worse performance on our measure of executive function than those without CVD, and those with PDR or diabetic nephropathy (microvascular complications) showed worse performance on psychomotor speed than those without these complications.
Addressing the potential of a growing population with neurodegeneration requires both further study and broader clinical awareness for those aging with type 1 diabetes. Efforts should be made to further study this population and screen patient populations and to conduct imaging studies, including those using MRI, to better understand these processes. Ultimately, examination of postmortem specimens may also help clarify the relative roles of hyperglycemia, hypoglycemia, and insulin resistance and enable therapeutic intervention.
Supplementary Material
Article Information
Acknowledgments. This study would not have been possible without the staff of the Joslin Clinical Research Center and the 50-Year Medalists and their families.
Funding. The Joslin Medalist study is supported by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (P30-DK-036836, UL1-RR-025758-03, R24-DK-083957-01, and DP3-DK-094333-01); JDRF (17-2013-310); and contributions by Tom Beatson and many Medalists.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. G.M., L.J.T., K.A.M., D.P., J.K.S., M.K., R.H., A.L., G.L.K., and H.A.K. reviewed and edited the manuscript. G.M., L.J.T., K.A.M., D.P., J.K.S., G.L.K., and H.A.K. conceived the analysis and manuscript. G.M., L.J.T., K.A.M., D.P., and H.A.K. performed data analysis. G.M., L.J.T., and H.A.K. prepared the manuscript. M.K., R.H., and A.L. collected data and performed study procedures/conducted study operations. H.A.K. had final authority over manuscript preparation and the decision to submit the manuscript for publication. H.A.K. is the guarantor of this work, and as such, had full access to the data, takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-1955/-/DC1.
References
- 1.Krolewski AS. Epidemiology of late complications of diabetes. In Joslin's Diabetes Mellitus. 13th ed. Kahn CR, Weir GC, Eds. Philadelphia, PA, Lea & Febiger, 1994, p. 605–619 [Google Scholar]
- 2.Biessels GJ, Strachan MW, Visseren FL, Kappelle LJ, Whitmer RA. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. Lancet Diabetes Endocrinol 2014;2:246–255 [DOI] [PubMed] [Google Scholar]
- 3.Brands AM, Biessels GJ, de Haan EH, Kappelle LJ, Kessels RP. The effects of type 1 diabetes on cognitive performance: a meta-analysis. Diabetes Care 2005;28:726–735 [DOI] [PubMed] [Google Scholar]
- 4.Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron 2004;44:195–208 [DOI] [PubMed] [Google Scholar]
- 5.Elias MF, Beiser A, Wolf PA, Au R, White RF, D’Agostino RB. The preclinical phase of Alzheimer disease: a 22-year prospective study of the Framingham Cohort. Arch Neurol 2000;57:808–813 [DOI] [PubMed] [Google Scholar]
- 6.Schindler RJ. Dementia with cerebrovascular disease: the benefits of early treatment. Eur J Neurol 2005;12(Suppl. 3):17–21 [DOI] [PubMed] [Google Scholar]
- 7.Jacobson AM, Musen G, Ryan CM, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group . Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med 2007;356:1842–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes 2006;55:1463–1469 [DOI] [PubMed] [Google Scholar]
- 9.Sun JK, Keenan HA, Cavallerano JD, et al. Protection from retinopathy and other complications in patients with type 1 diabetes of extreme duration: the Joslin 50-Year Medalist study. Diabetes Care 2011;34:968–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He ZH, D’Eon SA, Tinsley LJ, et al. Cardiovascular disease protection in long-duration type 1 diabetes and sex differences. Diabetes Care 2015;38:e73–e74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergmann MM, Byers T, Freedman DS, Mokdad A. Validity of self-reported diagnoses leading to hospitalization: a comparison of self-reports with hospital records in a prospective study of American adults. Am J Epidemiol 1998;147:969–977 [DOI] [PubMed] [Google Scholar]
- 12.Martin LM, Leff M, Calonge N, Garrett C, Nelson DE. Validation of self-reported chronic conditions and health services in a managed care population. Am J Prev Med 2000;18:215–218 [DOI] [PubMed] [Google Scholar]
- 13.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol 2004;57:1096–1103 [DOI] [PubMed] [Google Scholar]
- 14.Wechsler D. WASI Manual. San Antonio, TX, Psychological Corporation, 1999 [Google Scholar]
- 15.Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Oxford University Press, Oxford, U.K., 2006 [Google Scholar]
- 16.Biessels GJ, Reagan LP. Hippocampal insulin resistance and cognitive dysfunction. Nat Rev Neurosci 2015;16:660–671 [DOI] [PubMed] [Google Scholar]
- 17.Davis WA, Zilkens RR, Starkstein SE, Davis TM, Bruce DG. Dementia onset, incidence and risk in type 2 diabetes: a matched cohort study with the Fremantle Diabetes Study Phase I. Diabetologia 2017;60:89–97 [DOI] [PubMed] [Google Scholar]
- 18.Blázquez E, Velázquez E, Hurtado-Carneiro V, Ruiz-Albusac JM. Insulin in the brain: its pathophysiological implications for states related with central insulin resistance, type 2 diabetes and Alzheimer’s disease. Front Endocrinol (Lausanne) 2014;5:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Popa-Wagner A, Buga AM, Popescu B, Muresanu D. Vascular cognitive impairment, dementia, aging and energy demand. A vicious cycle. J Neural Transm (Vienna) 2015;122(Suppl. 1):S47–S54 [DOI] [PubMed] [Google Scholar]
- 20.Brands AM, Kessels RP, Hoogma RP, et al. Cognitive performance, psychological well-being, and brain magnetic resonance imaging in older patients with type 1 diabetes. Diabetes 2006;55:1800–1806 [DOI] [PubMed] [Google Scholar]
- 21.Duinkerken Ev, Brands AM, van den Berg E, Henselmans JM, Hoogma RP, Biessels GJ; Utrecht Diabetic Encephalopathy Study Group . Cognition in older patients with type 1 diabetes mellitus: a longitudinal study. J Am Geriatr Soc 2011;59:563–565 [DOI] [PubMed] [Google Scholar]
- 22.Brands AM, Biessels GJ, Kappelle LJ, et al.; Utrecht Diabetic Encephalopathy Study Group . Cognitive functioning and brain MRI in patients with type 1 and type 2 diabetes mellitus: a comparative study. Dement Geriatr Cogn Disord 2007;23:343–350 [DOI] [PubMed] [Google Scholar]
- 23.Musen G, Lyoo IK, Sparks CR, et al. Effects of type 1 diabetes on gray matter density as measured by voxel-based morphometry. Diabetes 2006;55:326–333 [DOI] [PubMed] [Google Scholar]
- 24.Nelson AR, Sweeney MD, Sagare AP, Zlokovic BV. Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer’s disease. Biochim Biophys Acta 2016;1862:887–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tromp D, Dufour A, Lithfous S, Pebayle T, Despres O. Episodic memory in normal aging and Alzheimer disease: insights from imaging and behavioral studies. Ageing Res Rev 2015;24:232–262 [DOI] [PubMed] [Google Scholar]
- 26.Smolina K, Wotton CJ, Goldacre MJ. Risk of dementia in patients hospitalised with type 1 and type 2 diabetes in England, 1998-2011: a retrospective national record linkage cohort study. Diabetologia 2015;58:942–950 [DOI] [PubMed] [Google Scholar]
- 27.Trivedi MA, Murphy CM, Goetz C, et al. fMRI activation changes during successful episodic memory encoding and recognition in amnestic mild cognitive impairment relative to cognitively healthy older adults. Dement Geriatr Cogn Disord 2008;26:123–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peña-Casanova J, Sánchez-Benavides G, de Sola S, Manero-Borrás RM, Casals-Coll M. Neuropsychology of Alzheimer’s disease. Arch Med Res 2012;43:686–693 [DOI] [PubMed] [Google Scholar]
- 29.Tan ZS, Beiser AS, Fox CS, et al. Association of metabolic dysregulation with volumetric brain magnetic resonance imaging and cognitive markers of subclinical brain aging in middle-aged adults: the Framingham Offspring Study. Diabetes Care 2011;34:1766–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nunley KA, Ryan CM, Aizenstein HJ, et al. Regional gray matter volumes as related to psychomotor slowing in adults with type 1 diabetes. Psychosom Med 2017;79:533–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernandez SL, Gong JH, Chen L, et al. Characterization of circulating and endothelial progenitor cells in patients with extreme-duration type 1 diabetes. Diabetes Care 2014;37:2193–2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tinsley LJ, Kupelian V, D'Eon SA, Pober D, Sun JK, King GL, Keenan HA. Association of glycemic control with reduced risk of large vessel disease after more than 50 years of type 1 diabetes. J Clin Endocrinol Metabol 2017;102:3704–3711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bain SC, Gill GV, Dyer PH, et al. Characteristics of type 1 diabetes of over 50 years duration (the Golden Years Cohort). Diabet Med 2003;20:808–811 [DOI] [PubMed] [Google Scholar]
- 34.Weisman A, Lovblom LE, Keenan HA, et al. Diabetes care disparities in long-standing type 1 diabetes in Canada and the U.S.: a cross-sectional comparison. Diabetes Care 2018;41:88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts RO, Cha RH, Mielke MM, et al. Risk and protective factors for cognitive impairment in persons aged 85 years and older. Neurology 2015;84:1854–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.