Abstract
OBJECTIVE
Pediatric type 2 diabetes prevalence is increasing, with β-cell dysfunction key in its pathogenesis. The RISE Pediatric Medication Study compared two approaches—glargine followed by metformin and metformin alone—in preserving or improving β-cell function in youth with impaired glucose tolerance (IGT) or recently diagnosed type 2 diabetes during and after therapy withdrawal.
RESEARCH DESIGN AND METHODS
Ninety-one pubertal, overweight/obese 10–19-year-old youth with IGT (60%) or type 2 diabetes of <6 months duration (40%) were randomized to either 3 months of insulin glargine with a target glucose of 4.4–5.0 mmol/L followed by 9 months of metformin or to 12 months of metformin alone. β-Cell function (insulin sensitivity paired with β-cell responses) was assessed by hyperglycemic clamp at baseline, 12 months (on treatment), and 15 months (3 months off treatment).
RESULTS
No significant differences were observed between treatment groups at baseline, 12 months, or 15 months in β-cell function, BMI percentile, HbA1c, fasting glucose, or oral glucose tolerance test 2-h glucose results. In both treatment groups, clamp-measured β-cell function was significantly lower at 12 and 15 months versus baseline. HbA1c fell transiently at 6 months within both groups. BMI was higher in the glargine followed by metformin versus metformin alone group between 3 and 9 months. Only 5% of participants discontinued the interventions, and both treatments were well tolerated.
CONCLUSIONS
In youth with IGT or recently diagnosed type 2 diabetes, neither 3 months of glargine followed by 9 months of metformin nor 12 months of metformin alone halted the progressive deterioration of β-cell function. Alternate approaches to preserve β-cell function in youth are needed.
Introduction
The SEARCH for Diabetes in Youth (SEARCH) epidemiological study has highlighted a continued increase in the incidence of type 2 diabetes in adolescents aged 10–19 years, particularly among ethnic minority groups. The Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study demonstrated that in 50% of youth with type 2 diabetes, initial assigned treatment failed, requiring the initiation of insulin therapy after an average follow-up of 3.9 years, which is in keeping with faster disease progression than reported in adults (1–3). In TODAY, lower β-cell function at baseline as well as a greater decline in β-cell function over time predicted worse glycemic control and treatment failure (4). Thus, interventions to preserve or improve β-cell function in youth are critically needed.
Metformin and insulin each has been shown to improve β-cell function in adults with impaired glucose tolerance (IGT) or recent-onset type 2 diabetes. The Diabetes Prevention Program (DPP) demonstrated that metformin improves β-cell function and reduces diabetes progression by 31% over 3 years in adults with IGT (5,6). Weng et al. (7) showed in adults with newly diagnosed type 2 diabetes that <2 weeks of intensive insulin therapy improves and maintains β-cell function, resulting in prolonged remission from requiring diabetes medication. Whether such interventions have similar benefits in youth is unclear. The Restoring Insulin Secretion (RISE) Pediatric Medication Study was designed as a proof-of-principle trial to test in youth across a continuum of β-cell dysfunction from IGT to mild, recently diagnosed type 2 diabetes the hypothesis that initial short-term treatment with insulin glargine for 3 months followed by metformin for 9 months would preserve or improve β-cell function compared with metformin alone, with a sustained effect after withdrawal of therapy.
Research Design and Methods
Study Protocol
The RISE Pediatric Medication Study was a four-center, randomized, open-label clinical trial funded by the National Institute of Diabetes and Digestive and Kidney Diseases. The rationale and methods have been described in detail elsewhere (8–10); additional specifics are provided in the Supplementary Data. Each center’s institutional review board approved the protocol. Written parental consent and child assent were obtained consistent with the Declaration of Helsinki and each center’s institutional review board guidelines. The protocol can be found at https://rise.bsc.gwu.edu/web/rise/collaborators.
Participants
A summary of study visits is shown in Supplementary Fig. 1, and a summary of participants enrolled and their rates of completion of study visits is shown in a CONSORT (Consolidated Standards of Reporting Trials) diagram (Supplementary Fig. 2). Enrollment occurred between July 2013 and April 2016. Youth aged 10–19 years with a BMI ≥85th percentile for age and sex but <50 kg/m2, IGT or recently diagnosed (<6 months duration) type 2 diabetes, and Tanner stage ≥II (females, Tanner stage ≥II breast development; and males, testicular volume >3mL) were eligible. Screening included a history and physical examination, laboratory tests, and a 75-g oral glucose tolerance test (OGTT). Eligibility criteria included the simultaneous presence of all the following: negative GAD and IA2 autoantibodies, fasting glucose ≥5 mmol/L, OGTT 2-h glucose ≥7.8 mmol/L, and HbA1c ≤8.0% (64 mmol/mol) if drug naïve. In youth with type 2 diabetes already taking metformin, eligibility was modified to HbA1c ≤7.5% (58 mmol/mol) if on metformin for <3 months and ≤7.0% (53 mmol/mol) if on metformin for 3–6 months.
Final eligibility required ≥80% compliance with 3 weeks of placebo medication (or study-provided metformin if already taking metformin) and attendance at scheduled run-in visits. Eligible participants underwent a baseline hyperglycemic clamp and OGTT followed by random 1:1 treatment assignment by study site, stratified by glycemic status.
Interventions
The glargine followed by metformin group received 3 months of insulin glargine titrated at least twice weekly by a preset algorithm (Supplementary Study Methods) to achieve a fasting glucose of 4.4–5.0 mmol/L on the basis of daily self-monitored blood glucose (SMBG) (Freestyle Lite, Abbott Laboratories, Lake Bluff, IL), which was followed immediately by 9 months of metformin (titrated to 1,000 mg twice daily). Youth already taking metformin and randomized to glargine therapy discontinued active metformin at the time of initiation of glargine treatment. The metformin group received metformin alone (titrated to 1,000 mg twice daily) for 12 months.
Participants took their assigned intervention for 12 months, after which it was withdrawn, and follow-up continued while off study medication for an additional 3 months to determine the 15-month primary outcome of treatment durability. Anthropometrics, HbA1c, and safety evaluations were performed every 3 months, and hyperglycemic clamps and OGTTs were repeated at 12 and 15 months (8–10) (Supplementary Fig. 1). Laboratory assessments were performed in a central laboratory at the University of Washington as summarized below and described in greater detail elsewhere (9).
Medication adverse effects, symptoms of hypoglycemia and hyperglycemia, and adherence (returned medication bottle pill count, returned insulin pen residual volume) were assessed at all study visits. A detailed algorithm was used for addressing hypoglycemia, hyperglycemia, and acute metabolic decompensation at each visit (Supplementary Study Methods). If protocol-specified HbA1c safety thresholds were exceeded at any visit, outcome assessments were performed whenever possible. The participant was then withdrawn from the study and referred for additional diabetes treatment as appropriate. Safety was monitored by an independent data and safety monitoring board.
Procedures and Calculations
A two-step hyperglycemic clamp (11.1 mmol/L, >25 mmol/L) paired with the insulin secretagogue arginine at the second step was performed at baseline, 12 months, and 15 months as previously described (8–10). If the participant was on metformin, the last dose of metformin was taken the evening before the hyperglycemic clamp. The hyperglycemic clamp was used to quantify simultaneously insulin sensitivity (M/I) and three β-cell response measures: 1) steady-state (second-phase) C-peptide, 2) acute C-peptide response to arginine at maximal glycemic potentiation (ACPRmax), and 3) acute (first-phase) C-peptide response to glucose (ACPRg). M/I was calculated as the mean glucose infusion rate (M) at 100, 110, and 120 min of the clamp divided by the corresponding mean steady-state plasma insulin concentration (I) (11–13). Mean steady-state (second-phase) C-peptide and insulin concentrations were calculated at 100, 110, and 120 min (13). ACPRmax was calculated as the mean incremental response above concentrations before the arginine bolus (14) and ACPRg as the mean incremental response above baseline for the first 10 min after glucose bolus (15).
The 15-month (3 months after discontinuation of metformin) steady-state C-peptide and ACPRmax, each paired with 15-month insulin sensitivity, represent the coprimary outcomes. These were paired to express the magnitude of the β-cell response as a function of the prevailing insulin sensitivity (15). Secondary outcomes from the hyperglycemic clamp included the 15-month ACPRg paired with the 15-month M/I and the 12-month C-peptide response measures (steady-state C-peptide, ACPRmax, ACPRg) each paired with M/I at the same time point.
A 3-h 75-g OGTT also was performed on a separate day and used to determine fasting and 2-h glucose at baseline, 12 months, and 15 months. If the participant was on metformin, the last dose of metformin was taken the evening before the OGTT. Additional HbA1c testing was done at baseline and 3, 6, 9, 12, and 15 months for robust phenotyping of participants over time.
Assays
Glucose was measured using the glucose hexokinase method on a Roche c501 autoanalyzer. C-peptide and insulin were measured by a two-site immunoenzymometric assay performed on the Tosoh 2000 autoanalyzer (Tosoh Bioscience, Inc., South San Francisco, CA). Interassay coefficients of variation on quality control samples with low, medium, medium-high, and high concentrations were ≤2.0% for glucose, ≤4.3% for C-peptide, and ≤3.5% for insulin.
Statistics
Data were accumulated centrally, and analyses were performed according to a prespecified analysis plan. For this proof-of-principle trial, we chose two measures of β-cell function as coprimary outcomes: clamp-derived steady-state C-peptide and ACPRmax at 15 months (both evaluated jointly with M/I). These two measures evaluate different components of β-cell function and were selected because there were no data in youth to determine whether one or the other was likely to change after the interventions.
No preliminary data were available in youth on the expected differences in either primary outcome after a period of intervention withdrawal (washout). Several unpublished analyses provided a range of possible differences between individuals taking medication and those taking placebo as well as expected correlations between baseline and follow-up measures. Therefore, sample size was based on a minimum effect size equal to the difference between the two groups divided by the SD in favor of the glargine followed by metformin group at a power of 80%. Because we targeted improvements in β-cell function, a one-sided significance level of 0.05 to detect a benefit of glargine followed by metformin was used for each comparison, with a conservative correlation of 0.5 between baseline and follow-up measures. Thirty-nine participants per arm (78 total) at the end of the washout provided ≥80% power to detect a minimum effect size of 0.57 in favor of glargine followed by metformin for either primary outcome measurement using baseline-adjusted ANCOVA. Ninety participants were sought, allowing for an ∼10% loss to follow-up.
The primary analysis was the comparison of β-cell responses paired with M/I between treatment groups at 15 months, adjusted for baseline β-cell response and M/I. Joint models for β-cell response and M/I were fit simultaneously using seemingly unrelated regression techniques (16–18), which provide a two degrees of freedom χ2 test of the treatment arm difference in the joint values of β-cell response and M/I between treatment groups. The same methods were used to compare treatment groups at 12 months.
To evaluate changes within each treatment arm over time, Hotelling T2 distribution was used to test changes in β-cell responses and M/I simultaneously (19). Additional secondary analyses compared means across treatment arms at specific time points by using ANCOVA adjusted for the baseline value of the measure and by repeated-measures ANCOVA across the active 12-month intervention period adjusted for the baseline value of the measure.
Two participants had an HbA1c elevation requiring study withdrawal before their 15-month primary outcome visit. The clamp-derived outcome data for these participants were imputed using the midpoint between zero and the lowest value in their respective treatment arm.
Results
Demographic, Physical, and Metabolic Characteristics
We screened 236 youth and randomized 91 (Supplementary Fig. 2). Table 1 lists baseline demographic and metabolic characteristics of participants by treatment arm. The metformin alone group was slightly younger but not different in Tanner stage. All other baseline physical, metabolic, β-cell response, and insulin sensitivity measures were similar between treatment groups. Of the 91 participants, 21 with type 2 diabetes were receiving metformin at randomization or were previously exposed to it, with a similar distribution of metformin exposure and IGT versus type 2 diabetes status between treatment groups.
Table 1.
Baseline demographic and physical characteristics, insulin sensitivity, and β-cell responses from the hyperglycemic clamp and OGTT by treatment group
| Glargine followed by metformin (n = 44) | Metformin alone(n = 47) | |
|---|---|---|
| Female, n (%) | 27 (61.4) | 38 (80.9) |
| Age (years) | 14.9 ± 2.0 | 13.9 ± 2.1* |
| Tanner stage V, n (%) | 32 (72.7) | 25 (53.2) |
| Race/ethnicity, n (%) | ||
| White | 13 (29.5) | 12 (25.5) |
| Black | 14 (31.8) | 9 (19.1) |
| Hispanic | 14 (31.8) | 20 (42.6) |
| Other | 3 (6.8) | 6 (12.8) |
| Weight (kg) | 102.0 ± 25.7 | 97.7 ± 23.3 |
| BMI (kg/m2) | 36.5 ± 6.4 | 36.9 ± 6.4 |
| BMI percentile | 98.4 ± 2.5 | 98.8 ± 1.3 |
| BMI z score | 2.3 ± 0.4 | 2.4 ± 0.3 |
| Waist circumference (cm) | 109.8 ± 15.8 | 109.6 ± 12.4 |
| HbA1c | ||
| % | 5.7 ± 0.6 | 5.7 ± 0.6 |
| mmol/mol | 39.2 ± 6.6 | 38.6 ± 6.3 |
| IGT, n (%) | 26 (59) | 28 (60) |
| Type 2 diabetes, n (%) | 18 (41) | 19 (40) |
| Metformin use at baseline, n (%) | 10 (22.7) | 11 (23.4) |
| Systolic blood pressure (mmHg) | 120.7 ± 7.8 | 119.5 ± 8.7 |
| Diastolic blood pressure (mmHg) | 67.6 ± 7.7 | 70.1 ± 7.9 |
| Hypertension, n (%) | 9 (20.5) | 15 (31.9) |
| Triglycerides (mmol/L) | 1.11 (0.39, 3.14) | 1.17 (0.45, 3.06) |
| HDL cholesterol (mmol/L) | 1.0 ± 0.3 | 1.0 ± 0.2 |
| LDL cholesterol (mmol/L) | 2.3 ± 0.8 | 2.1 ± 0.6 |
| Hyperglycemic clamp variables | ||
| Fasting glucose (mmol/L) | 6.04 ± 0.85 | 6.11 ± 1.10 |
| Fasting C-peptide (nmol/L) | 1.63 ± 0.55 | 1.82 ± 0.58 |
| Fasting insulin (pmol/L) | 211.1 (54.8, 813.5) | 247.4 (74.1, 825.4) |
| Steady-state C-peptide (nmol/L) | 5.22 (2.44, 11.15) | 5.09 (2.32, 11.19) |
| ACPRmax (nmol/L) | 7.29 (3.29, 16.16) | 8.12 (3.42, 19.26) |
| ACPRg (nmol/L) | 1.06 (0.10, 11.66) | 1.14 (0.14, 9.02) |
| Glucose infusion rate (M) (mmol/kg/min) | 0.025 ± 0.013 | 0.023 ± 0.010 |
| Steady-state insulin (I) (pmol/L) | 1,391 (281, 6,874) | 1,371 (286, 6,572) |
| M/I (× 10e−5 mmol/kg/min per pmol/L) | 1.60 (0.35, 7.38) | 1.54 (0.35, 6.76) |
| OGTT variables | ||
| Fasting glucose (mmol/L) | 6.0 ± 0.8 | 6.1 ± 1.1 |
| 2-h glucose (mmol/L) | 10.2 ± 2.5 | 10.2 ± 2.8 |
Data are mean ± SD or geometric mean (95% CI) for nonnormally distributed variables.
*P < 0.03; all other P values were not significant.
Adherence to Interventions
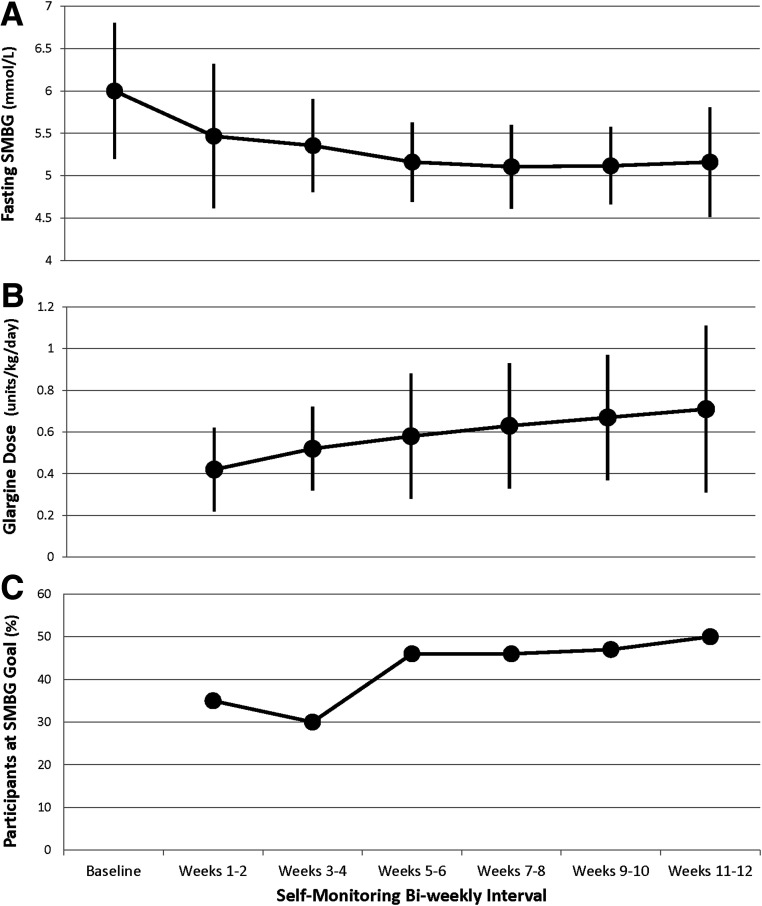
During weeks 11 and 12, the mean ± SD glargine dose in the glargine followed by metformin group was 0.7 ± 0.4 units/kg/day, and fasting blood glucose had decreased from a mean of 6.0 ± 0.8 mmol/L at the baseline OGTT to a fasting SMBG mean of 5.2 ± 0.7 mmol/L, with 50% achieving the 4.4–5.0 mmol/L goal (Fig. 1). As assessed by residual volume, 68% of participants were >80% compliant with the insulin. Metformin adherence by pill count was 88 ± 18% over the entire study, not differing by treatment group.
Figure 1.
Insulin doses and corresponding fasting glucose values over time while on glargine treatment. A: Mean fasting morning SMBG values every 2 weeks over 12 weeks. B: Mean glargine dose corresponding to each SMBG value. C: Corresponding percentage of participants who achieved the goal fasting SMBG of 4.4–5.0 mmol/L every 2 weeks. Data are mean (95% CI).
Treatment Effects on Temporal Changes in β-Cell Function
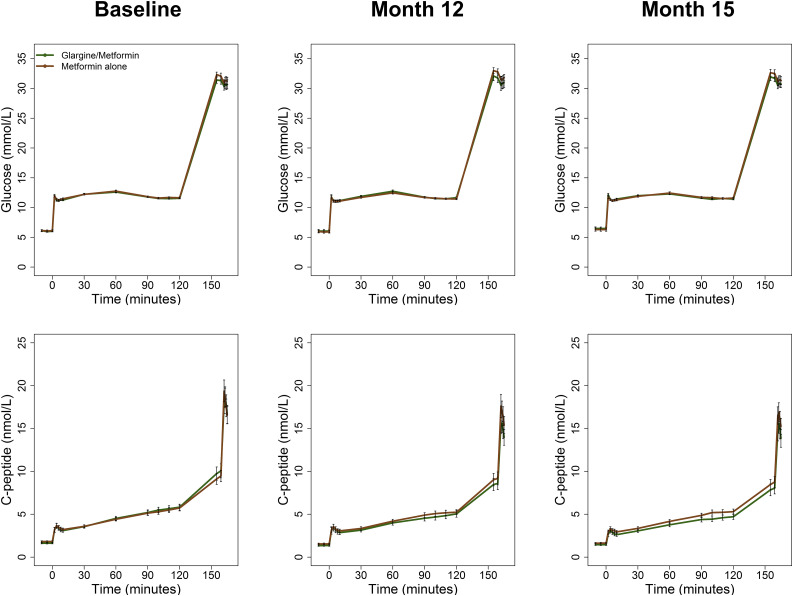
Figure 2 illustrates glucose and C-peptide concentrations during the hyperglycemic clamps performed at baseline, 12 months, and 15 months. Of note, identical target glucose concentrations of 11.1 and >25 mmol/L were achieved and not different between treatment groups at baseline, 12 months, and 15 months. Supplementary Table 1 shows β-cell responses and M/I by treatment group.
Figure 2.
Glucose and C-peptide values during the hyperglycemic clamp at baseline, after 12 months of treatment, and 3 months after discontinuing the intervention (15 months). The glargine followed by metformin group (n = 44 [green]) and the metformin alone group (n = 47 [brown]) are shown. The steady-state glucose targets were 11.1 mmol/L between 90 and 120 min and >25 mmol/L at 150 min. Data are mean ± SEM.
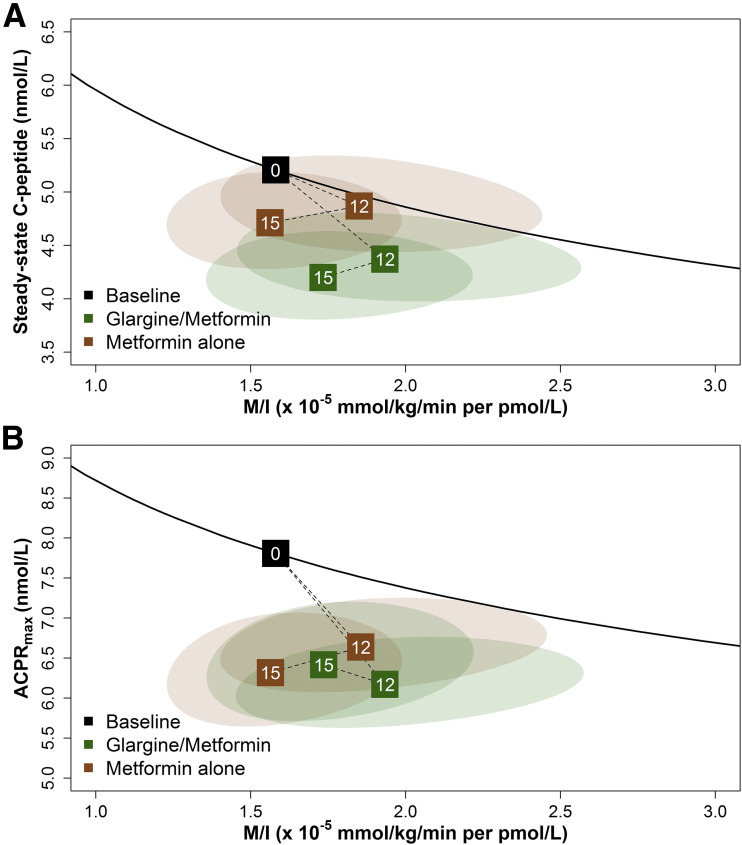
No significant differences were found between treatment groups at 12 or 15 months in either primary measure of β-cell function (e.g., response [steady-state C-peptide, ACPRmax] paired with M/I) or in the secondary measure of β-cell function (ACPRg paired with M/I). Figure 3 shows a vector plot of changes from baseline to 12 and 15 months for each of the two coprimary clamp-derived β-cell responses (steady-state C-peptide, ACPRmax) paired with M/I, and Supple-mentary Fig. 3 shows the vector plot for the secondary β-cell response (ACPRg paired with M/I). Explanations of the vector plots appear in the respective figure legends. Two participants in the metformin alone arm met HbA1c criteria for study withdrawal at 9 months; thus they had a 12-month visit but did not complete the 15-month visit. The inclusion of imputed values for their β-cell responses and M/I at 15 months did not alter the results.
Figure 3.
Relationship of the two coprimary outcomes: hyperglycemic clamp-derived β-cell responses (steady-state C-peptide and ACPRmax) paired with M/I. Changes are shown from baseline to 12 and 15 months for the clamp-derived β-cell responses of steady-state C-peptide (A) and ACPRmax (B) paired with M/I. The black line depicts the joint relationship between β-cell response and M/I at baseline for the full cohort, with the mean value at baseline for the full cohort indicated by the black box with a 0. The dotted lines to boxes at months 12 and 15 show the trajectory of values from baseline to 12 months of intervention and then to 3 months after discontinuation of the intervention (15 months) for glargine followed by metformin (green) and metformin alone (brown). Values above the black line represent improved β-cell function, and values below the line represent poorer β-cell function. The ellipses depict the 95% confidence bands around the points at months 12 and 15. No significant differences were observed at any time point between treatment arms; however, significant within-group declines were seen from baseline while on active treatment through 12 months for steady-state C-peptide with M/I (glargine followed by metformin P = 0.019, metformin alone P = 0.025) and ACPRmax with M/I (glargine followed by metformin P < 0.001, metformin alone P = 0.001) and through 15 months for steady-state C-peptide with M/I (glargine followed by metformin P < 0.001, metformin alone P = 0.031) and ACPRmax with M/I (glargine followed by metformin P = 0.001, metformin alone P < 0.001).
Neither treatment improved nor maintained β-cell function at the end of or after active treatment (Fig. 3 and Supplementary Fig. 3). Rather, β-cell function declined significantly from baseline while on active treatment through 12 months within each treatment group for steady-state C-peptide with M/I (glargine followed by metformin P = 0.019, metformin alone P = 0.025) and ACPRmax with M/I (glargine followed by metformin P < 0.001, metformin alone P = 0.001). β-Cell function remained significantly worse at 15 months compared with baseline within both treatments for steady-state C-peptide with M/I (glargine followed by metformin P < 0.001, metformin alone P = 0.031), ACPRmax with M/I (glargine followed by metformin P = 0.001, metformin alone P < 0.001), and ACPRg with M/I (glargine followed by metformin P < 0.001, metformin alone P = 0.014).
Approximately 60% of participants had IGT at baseline; by study design, none had received metformin before randomization. In this IGT subgroup, we observed patterns similar to those seen in the full cohort, with no evidence of a beneficial effect on β-cell function of glargine followed by metformin versus metformin alone, and a worsening of β-cell function through 12 and 15 months in both treatment groups (Supplementary Fig. 4).
Temporal Patterns of BMI and Glycemic Measures Over 15 Months
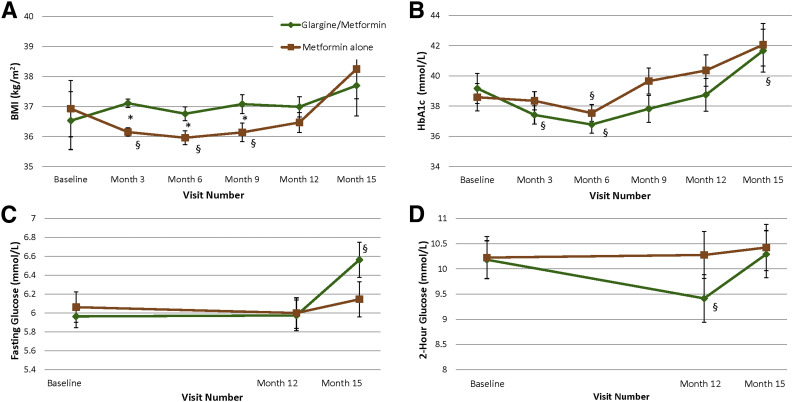
Several transient changes were seen in body size and glycemic parameters. BMI did not differ between treatment groups at 12 or 15 months. However, BMI declined from baseline within the metformin alone group over the first 9 months, resulting in a significantly lower BMI across the 12-month intervention period in the metformin alone versus glargine followed by metformin group (P = 0.008) and specifically at 3 (P < 0.001), 6 (P < 0.02), and 9 (P < 0.04) months.
HbA1c did not significantly differ between treatment groups across the 12-month intervention period or at any individual time point (Fig. 4). However, compared with baseline, HbA1c declined significantly within the glargine followed by metformin group at 3 months while on glargine treatment (P < 0.03) and within both groups at 6 months (glargine followed by metformin P = 0.001, metformin alone P = 0.01) before rebounding to baseline levels in both groups at months 9 and 12, despite continued active metformin treatment. By 15 months, HbA1c had increased significantly from baseline in the glargine followed by metformin group (P = 0.03), ending at ∼6% (42 mmol/mol) in both treatment groups.
Figure 4.
BMI and glycemia over time. BMI (A), HbA1c (B), fasting glucose (C), and OGTT 2-h glucose over 15 months (D). In the glargine followed by metformin group (n = 44 [green]), glargine insulin was given from baseline to 3 months followed by metformin from months 3 through 12. In the metformin alone group (n = 47 [brown]), metformin was given from baseline through 12 months. Treatment was discontinued in both groups at 12 months. Data are mean ± SEM. *P < 0.05 for the difference between treatment groups at the specified time point; §P < 0.05 for the difference within treatment group between the specified time point and baseline.
No differences were found between treatment groups in fasting or OGTT 2-h glucose concentrations at 12 or 15 months. Within the glargine followed by metformin group, fasting glucose was not different at 12 months compared with baseline but rose higher than baseline by 15 months (off treatment) (P = 0.02). Within the glargine followed by metformin group, 2-h glucose was significantly lower than baseline at 12 months (P = 0.02) but no longer different from baseline at 15 months. Within the metformin alone group, no significant changes were found in fasting or 2-h glucose at 12 months while on active treatment or at 15 months.
Safety Outcomes
Seven serious adverse events occurred (one hospitalization each for suicidal ideation/anxiety, newly diagnosed Ewing sarcoma, otitis externa, appendicitis/appendectomy, and pneumonia and two hospitalizations for tonsillectomy/adenoidectomy), all deemed unrelated to the study interventions. Other nonserious adverse events are summarized in Supplementary Table 2. No participants had severe hypoglycemia or acute metabolic decompensation as defined in Supplementary Study Methods. Accordingly, no participants required rescue therapy with insulin.
Over the 15 months, three participants in the glargine followed by metformin group with IGT at baseline and none in the metformin alone group with IGT at baseline developed diabetes as defined by OGTT criteria and HbA1c ≥6.5% (48 mmol/mol) (20). In each treatment group, three participants met hyperglycemic criteria for study withdrawal. The last outcome visits for these participants were three at 15 months in the glargine followed by metformin group and two at 12 and one at 15 months in the metformin alone group, after which the participants were withdrawn from the study and returned to their primary diabetes provider for additional diabetes treatment.
Conclusions
In youth along the glycemic continuum of IGT to mild, recently diagnosed type 2 diabetes, we tested whether initial short-term treatment with insulin glargine for 3 months followed by metformin for 9 months would preserve or improve β-cell function compared with metformin alone for 12 months and a sustained effect after withdrawal of therapy. No significant differences were found between groups in β-cell function at 12 or 15 months, and all β-cell measures at 12 months (on treatment) and 15 months (off treatment) were worse than at baseline. Thus, the only two agents approved for treating type 2 diabetes in youth both failed to preserve or improve β-cell function during or after withdrawal of treatment in youth with IGT or recently diagnosed type 2 diabetes.
Trials of insulin therapy in adults with IGT or early type 2 diabetes support the concept that early use of insulin may have a beneficial long-term effect on dysglycemia. In the Outcome Reduction With Initial Glargine Intervention (ORIGIN Trial), adults with high cardiovascular disease risk and prediabetes were treated with insulin glargine to a similar target glucose (≤5.3 mmol/L) as we achieved (21). A median of 3.3 months after insulin discontinuation, a similar duration of durability as we evaluated, those with prediabetes randomized to glargine treatment had a 20% reduction in the odds of developing diabetes on the basis of OGTT criteria compared with standard care (21). In adults with recently diagnosed type 2 diabetes, Weng et al. (7) found that up to 2 weeks of intensive insulin therapy as either multiple daily injections or continuous subcutaneous infusion, an even shorter treatment duration than we tested, resulted in remission and no need for diabetes medications 1 year later in 45% and 51% of participants, respectively. Furthermore, after the 2-week insulin treatment, the β-cell response increased, and this improvement was sustained in the participants who remained in remission while off medications. Although the study by Weng et al. suggested benefit from <2 weeks of insulin treatment, we saw no effect from a 3-month duration of treatment, which is longer than that used by Weng et al. yet brief enough to realistically consider in youth. Moreover, we chose a target glucose lower than that used in the ORIGIN Trial to maximize the effect of the insulin treatment. To further increase the likelihood of insulin having an effect, we added the oral glucose-lowering agent metformin shown in the DPP to be effective in preventing the development of diabetes in adults with IGT (6). In contrast to the adult studies, we found no durable effect of insulin glargine targeted to achieve a plasma glucose of 4.4–5.0 mmol/L followed by metformin on measures of β-cell function or glycemia in youth. Furthermore, the lack of a beneficial effect of metformin alone on β-cell function, even when youth were still on the medication at 12 months, contrasts with observations after 1 year of therapy in adults with IGT (5) or type 2 diabetes (22). Although a longer duration of insulin treatment may have had more of an effect on β-cell function, longer-term treatment also may have led to additional weight gain, with an uncertain net balance of benefit and harm. Moreover, the injections and glucose monitoring required for longer-term insulin treatment might be met with resistance by youth, particularly those who are not yet diagnosed with diabetes.
The RISE Consortium (9) reported that youth with IGT or recently diagnosed, drug-naïve type 2 diabetes are profoundly more insulin resistant, have greater insulin responses for any degree of insulin sensitivity, and have lower hepatic insulin clearance than adults. This finding, combined with the poor response of youth to the RISE interventions, suggests that although β-cell dysfunction is the foundation for dysglycemia in both age-groups, greater β-cell demand may place youth at a higher risk for more rapid β-cell damage. Additional support for this interpretation comes from the apparently more rapid progression of type 2 diabetes observed in youth in the TODAY study than in a similar study in adults treated with the same glucose-lowering medications (2,3). Furthermore, the current observation of a lack of a beneficial effect of metformin on β-cell function in youth after 12 months of therapy contrasts the improvement in β-cell function observed in adults with IGT (5) or type 2 diabetes (22). The RISE Adult Medication Study, which includes these same two intervention arms as part of a four-arm study, should provide additional insight into these differences (8).
The current study has several strengths, including the randomized design and a robust, multicenter approach to quantification of insulin sensitivity and β-cell responses to both glucose and nonglucose secretagogue arginine performed to provide thorough, longitudinal phenotyping of participants. By quantifying both insulin sensitivity and β-cell responses simultaneously, we were able to account for the well-recognized relationship between insulin sensitivity and β-cell responsivity (15) and thus gain mechanistic insight into the effect of the interventions on β-cell function over time. Of key importance for this multicenter study, we reached matching levels of hyperglycemia during each of three repeated hyperglycemic clamp tests, showing a high degree of both reproducibility and reliability for the outcome measures across time points and across study sites. Study retention and metformin adherence also were excellent despite adolescents presenting a challenging study population. Limitations include the absence of a placebo arm because of the inclusion of youth with type 2 diabetes who could not remain untreated. Although a decline in β-cell function might have been even greater in a placebo group, the marked decline in β-cell function evident during and after stopping the interventions suggests that the medications largely were masking disease progression. In addition, despite the high reported insulin doses, 50% of the participants in the insulin glargine followed by metformin arm did not fully meet the fasting goal of 4.4–5.0 mmol/L, regardless of IGT or type 2 diabetes status. Of note, adults with prediabetes or type 2 diabetes in the ORIGIN Trial were treated with insulin glargine to a target fasting glucose of ≤5.3 mmol/L, a slightly less-aggressive target than we chose, yet we still observed no significant beneficial effect on β-cell function despite reaching a mean fasting glucose of 5.2 mmol/L (21). Moreover, after 1 year in the ORIGIN Trial, only 50% of the adult participants achieved the targeted glucose, similar to the proportion of youth in the current study, yet positive effects still were seen on diabetes development in these adults (21). Finally, the sample size was too small to obtain reliable estimates of the effects of the interventions in the subgroups of IGT versus type 2 diabetes. However, in a sensitivity analysis of the subgroup with IGT who had not received metformin before randomization and comprised the majority of participants in each arm, results were similar to the full cohort (Supplementary Fig. 4). Specifically, in the IGT group, treatment with glargine followed by metformin did not have a beneficial effect on β-cell function outcomes compared with metformin alone, and β-cell function declined within both treatment groups.
In conclusion, neither 3 months of insulin glargine followed by 9 months of metformin nor 12 months of metformin alone improved or preserved β-cell function when administered to youth with IGT or recently diagnosed type 2 diabetes. β-Cell function deteriorated with both treatments, the only treatments approved for use in type 2 diabetes in youth, highlighting an urgent need for alternate approaches to preserve β-cell function in youth and prevent the progression of dysglycemia.
Supplementary Material
Article Information
Acknowledgments. The RISE Consortium thanks the RISE Data and Safety Monitoring Board and Barbara Linder, the National Institute of Diabetes and Digestive and Kidney Diseases Program Official for RISE (Rockville, MD), for support and guidance. The Consortium also thanks the participants who, by volunteering, are furthering the ability to reduce the burden of diabetes.
Funding and Duality of Interest. RISE is supported by grants from the National Institutes of Health (U01-DK-094406, U01-DK-094430, U01-DK-094431, U01-DK-094438, U01-DK-094467, P30-DK-017047, P30-DK-020595, P30-DK-045735, P30-DK-097512, UL1-TR-000430, UL1-TR-001082, UL1-TR-001108, UL1-TR-001855, UL1-TR-001857, UL1-TR-001858, UL1-TR-001863), the Department of Veterans Affairs, and Kaiser Permanente Southern California. Additional financial and material support from the American Diabetes Association, Allergan, Apollo Endosurgery, Abbott Laboratories, and Novo Nordisk was received. S.A.A. and S.E.K. serve as paid consultants on advisory boards for Novo Nordisk. S.A.A. is a participant in a Novo Nordisk–sponsored clinical trial. T.A.B. has received research support from Allergan and Apollo Endosurgery. K.J.M. holds an investigator-initiated research grant from Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. Members of the RISE Consortium recruited participants and collected study data. K.J.N. wrote the first draft. K.J.N., S.L.E., S.A.A., S.C., E.W.L., T.A.B., D.A.E., K.J.M., and S.E.K. (chair) comprise the steering committee (principal investigator at each site, the data coordinating center, and the National Institute of Diabetes and Digestive and Kidney Diseases project scientist) and designed and implemented the study, contributed to the discussion, and edited the manuscript. K.J.N., T.S.H., S.L.E., S.A.A., S.C., E.W.L., P.S.Z., T.A.B., D.A.E., K.J.M., and S.E.K. researched data. S.L.E. performed all data analyses. K.J.N., S.L.E., and S.E.K. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in oral form at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018.
Appendix
Writing Group:
Kristen J. Nadeau (chair), Tamara S. Hannon, Sharon L. Edelstein, Silva A. Arslanian, Sonia Caprio, Ellen W. Leschek, Philip S. Zeitler, Thomas A. Buchanan, David A. Ehrmann, Kieren J. Mather, and Steven E. Kahn.
Footnotes
Clinical trial reg. no. NCT01779375, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-0787/-/DC1.
A complete list of the RISE Consortium Investigators can be found in the Supplementary Data online.
Contributor Information
Collaborators: Kristen J. Nadeau, Tamara S. Hannon, Sharon L. Edelstein, Silva A. Arslanian, Sonia Caprio, Ellen W. Leschek, Philip S. Zeitler, Thomas A. Buchanan, David A. Ehrmann, Kieren J. Mather, Steven E. Kahn, Susan Gross, Jayne Williams, Melanie Cree-Green, Yesenia Garcia Reyes, Krista Vissat, Kathleen Brown, Nancy Guerra, Kristin Porter, Mary Savoye, Bridget Pierpont, Tammy Garrett, Amale Lteif, Aniket Patel, Robin Chisholm, Karen Moore, Vivian Pirics, Linda Pratt, Karla A. Temple, Abby Rue, Elena Barengolts, Babak Mokhlesi, Eve Van Cauter, Susan Sam, M. Annette Miller, Karen M. Atkinson, Jerry P. Palmer, Kristina M. Utzschneider, Tsige Gebremedhin, Abigail Kernan-Schloss, Alexandra Kozedub, Brenda K. Montgomery, Emily J. Morse, Anny H. Xiang, Enrique Trigo, Elizabeth Beale, Fadi N. Hendee, Namir Katkhouda, Krishan Nayak, Mayra Martinez, Cortney Montgomery, Xinhui Wang, John M. Lachin, Ashley N. Hogan, Santica Marcovina, Jessica Harting, John Albers, Dave Hill, and Peter J. Savage
References
- 1.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. ; SEARCH for Diabetes in Youth Study. Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med 2017;376:1419–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.TODAY Study Group A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012;366:2247–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahn SE, Haffner SM, Heise MA, et al.; ADOPT Study Group . Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–2443 [DOI] [PubMed] [Google Scholar]
- 4.TODAY Study Group Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY. Diabetes Care 2013;36:1749–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diabetes Prevention Program Research Group Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the Diabetes Prevention Program: Effects of lifestyle intervention and metformin. Diabetes 2005;54:2404–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weng J, Li Y, Xu W, et al. Effect of intensive insulin therapy on β-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet 2008;371:1753–1760 [DOI] [PubMed] [Google Scholar]
- 8.The RISE Consortium Restoring Insulin Secretion (RISE): Design of studies of β-cell preservation in prediabetes and early type 2 diabetes across the life span. Diabetes Care 2014;37:780–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The RISE Consortium Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: I. Observations using the hyperglycemic clamp. Diabetes Care 2018;41:1696–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hannon TS, Kahn SE, Utzschneider KM, et al.; The RISE Consortium . Review of methods for measuring β-cell function: Design considerations from the Restoring Insulin Secretion (RISE) Consortium. Diabetes Obes Metab 2018;20:14–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andres R, Swerdloff R, Posefsky T, Coleman DL. Manual feedback technique for the control of blood glucose concentration. In Automation in Analytical Chemistry. Skeegs LT, Jr, Ed. New York, Mediad, 1966, p. 486–491 [Google Scholar]
- 12.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 13.Elahi D. In praise of the hyperglycemic clamp. A method for assessment of β-cell sensitivity and insulin resistance. Diabetes Care 1996;19:278–286 [DOI] [PubMed] [Google Scholar]
- 14.Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D Jr. Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest 1984;74:1318–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and β-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 1993;42:1663–1672 [DOI] [PubMed] [Google Scholar]
- 16.Zellner A. An efficient method of estimating seemingly unrelated regression equations and tests for aggregation bias. J Am Stat Assoc 1962;57:348–368 [Google Scholar]
- 17.Srivastava VK, Giles DEA. Seemingly Unrelated Regression Equations Models: Estimation and Inference. New York, Marcel Dekker, 1987 [Google Scholar]
- 18.Henningsen A, Hamann J. Systemfit: a package for estimating systems of simultaneous equations in R. J Stat Softw 2007;23:1–40 [Google Scholar]
- 19.Hotelling H. The generalization of student’s ratio. Ann Math Stat 1931;2:360–378 [Google Scholar]
- 20.American Diabetes Association 2 Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41(Suppl. 1):S13–S27 [DOI] [PubMed] [Google Scholar]
- 21.The ORIGIN Trial Investigators Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012;367:319–328 [DOI] [PubMed] [Google Scholar]
- 22.Kahn SE, Lachin JM, Zinman B, et al.; ADOPT Study Group . Effects of rosiglitazone, glyburide, and metformin on β-cell function and insulin sensitivity in ADOPT. Diabetes 2011;60:1552–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.