Abstract
Rationale
Clonal hematopoiesis has been associated with increased mortality and cardiovascular disease (CVD). This condition can arise from somatic mutations in pre-leukemic driver genes within hematopoietic stem/progenitor cells (HSPC). Approximately 40 candidate driver genes have been identified, but mutations in only one of these genes, Ten-Eleven Translocation-2 (TET2), has been shown to casually contribute to CVD in murine models.
Objective
To develop a facile system to evaluate the disease characteristics of different clonal hematopoiesis driver genes using lentivirus vector and CRISPR/Cas9 methodology. Using this methodology, evaluate whether DNA (Cytosine-5)-methyltransferase 3a (Dnmt3a), a commonly occurring clonal hematopoiesis driver gene, causally contributes to CVD.
Methods and Results
Lentivirus vectors were used to deliver Cas9 and guide RNA (gRNA) to introduce inactivating mutations in Tet2 and Dnmt3a in lineage-negative bone marrow cells. Following implantation into lethally-irradiated mice, these cells were engrafted and gave rise to labeled blood cell progeny. When challenged with an infusion of angiotensin II (AngII), mice with inactivating mutations in Tet2 or Dnmt3a displayed greater cardiac hypertrophy, diminished cardiac function, and greater cardiac and renal fibrosis. In comparison to Tet2, inactivation of Dnmt3a did not lead to detectable expansion of the mutant hematopoietic cells during the time course of these experiments. Tet2 inactivation promoted the expression of IL1β, IL-6 and Ccl5 whereas Dnmt3a inactivation promoted the expression of Cxcl1, Cxcl2, IL-6 and Ccl5 in a LPS-stimulated macrophage cell line.
Conclusions
Experiments employing lentivirus vector/CRISPR methodology provided evidence suggesting that inactivating DNMT3A mutations in hematopoietic cells contributes to CVD. Comparative analyses showed that inactivation of Tet2 and Dnmt3 were similar in their ability to promote AngII-induced cardiac dysfunction and renal fibrosis in mice. However, gene-specific actions were indicated by differences in kinetics of HSPC expansion and different patterns of inflammatory gene expression.
Keywords: Clonal hematopoiesis, heart failure, CRISPR, Dnmt3a, stem cell, genetics, transgenic models
INTRODUCTION
Somatic DNA mutations accumulate over time in many tissues, and this is a hallmark of the aging process1. In particular, somatic mutations in preleukemic “driver” genes within HSPC can confer “fitness” advantages leading to their clonal amplification. This process is referred to as clonal hematopoiesis2. This condition is prevalent in the elderly population and it has been termed clonal hematopoiesis of indeterminate potential (CHIP)3 or age-related clonal hematopoiesis (ARCH)4. A number of recent studies have associated advanced clonal hematopoiesis with increased mortality4–6 and with increased frequencies of cardiovascular disease and stroke4, 7. Recently, we provided causal evidence for a link between somatic mutations in TET2 and CVD in models of murine atherosclerosis and heart failure, and provided details about its mechanism8, 9. TET2 is one of approximately 40 candidate driver genes that have been associated with clonal hematopoiesis10. To date, there is an absence of information about the roles of driver genes, other than Tet2, in chronic disease. Thus, the development of procedures to facilitate the analysis of additional candidate driver genes is warranted.
METHODS
The data, analytical methods and study materials will be/have been made available to other researchers for the purposes of reproducing the results or replicating the procedures. The authors declare that all supporting data are available within the article and its online supplementary files.
An expanded Methods section is available in the Online Data Supplement and includes detailed information regarding standard methods involving plasmids, lentivirus production, validation of gRNA, isolation of lineage-negative cells and lentivirus transduction, mice, bone marrow transplantation, flow cytometry, angiotensin-II infusion, echocardiography, histology, quantitative PCR analysis, and statistics.
RESULTS
CRISPR/Cas9-mediated Tet2 gene disruption promotes hematopoietic cell expansion
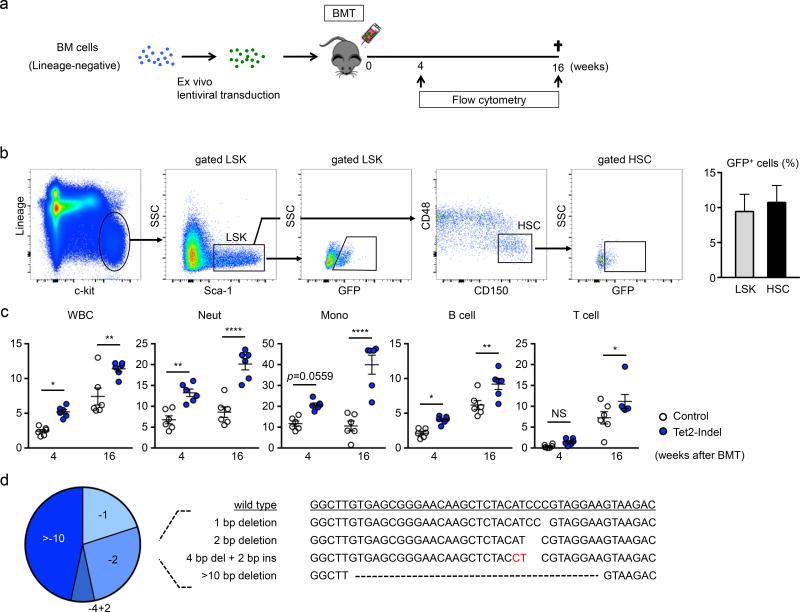
To evaluate the efficiency of the lentivirus system to mutate driver genes in HSPCs, lineage-negative cells were isolated from wild-type mice and transduced ex vivo with a lentivirus vector encoding Cas9-GFP and transplanted into lethally-irradiated wild-type mice (Figure 1a). At 8 weeks post-transplantation, the detection of GFP-positive cells in LSK- or LT-HSC-gated populations revealed successful HSPC transduction and engraftment with the lentivirus system (Figure 1b). Guide RNA (gRNA) candidates targeting Tet2 were selected after evaluation in NIH/3T3 by the T7 endonuclease I mismatch cleavage assay (Online Figure I). To assess whether ex vivo Tet2 gene-edited HSPC replicate the expansion characteristics of bone marrow cells from Tet2-KO mice, the fractions of GFP-positive cells in the different peripheral blood cell linages were determined at the 4 and 16 week time points (Figure 1c). This analysis revealed expansion characteristics that were similar to what was observed in our prior studies with Tet2-KO mice8, 9. To determine the degree of Tet2 editing by the lentivirus vector, DNA sequencing of the Tet2 locus was performed on DNA isolated GFP-positive white blood cells. All the sequence reads revealed out-of-frame insertion and deletion (indel) mutations (3n+1, 3n+2) within exon 3 (Figure 1d), that are predicted to lead to a loss of gene function.
Figure 1. CRISPR/Cas9-mediated Tet2 gene disruption confers a competitive advantage to the hematopoietic stem/progenitor cells.
a. Bone marrow lineage-negative cells from wild type mice were transduced with lentivirus particles expressing Cas9/eGFP and delivered to lethally irradiated wild type mice. b. Flow cytometry analysis of HSPC transduction by lentivirus. Cells are defined as LSK cells (lineage−, c-kit+, Sca-1+) and HSC (CD48−, CD150+ in LSK cells). Transduced cells are GFP positive (n=4). c. Flow cytometry analysis of the peripheral blood at 4 and 16 weeks after reconstitution with bone marrow transduced with Tet2-targeted and control (no Tet2 guide RNA) lentivirus vectors. The percentage of GFP+ cells in both experimental groups are shown (n=6 in both Tet2-indel mice and control mice). Statistical analysis was evaluated by two-way repeated measure ANOVA with Sidak’s multiple comparison tests. d. Results of the TA cloning procedure showing that GFP+ peripheral white blood cells harbor edited Tet2 genes. The wild-type Tet2 sequence is shown for reference. *p<0.05, **p<0.01, ****p<0.0001.
Phenotypes of Tet2 gene disruption in the Angiotensin-II infusion model
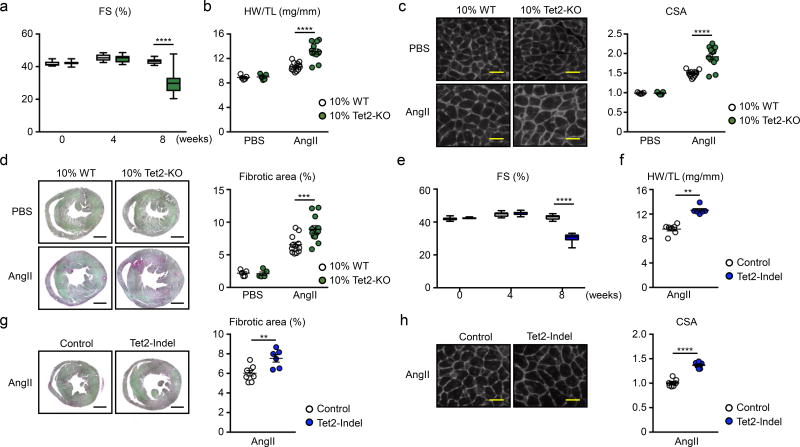
Having demonstrated the efficiency of the lentivirus delivery system on HSPCs expansion, we next sought to examine whether Tet2 gene editing in HSPCs promotes pathological cardiac remodeling. The AngII infusion model was chosen because it does not involve a major surgical manipulation. To validate this new model for studies of clonal hematopoiesis, AngII was infused to mice that had undergone a competitive bone marrow transplantation using 10% conventional CD45.2+ Tet2-KO mice and 90% CD45.1+ wild-type mice (Online Figure II), as in our prior studies8, 9. Flow cytometry analysis of the different blood cell lineages, according to the gating strategy shown in Online Figure III, revealed the selective expansion of the Tet2-KO hematopoietic cells (Online Figure IV). In this model, mice transplanted with 10% Tet2-deficient bone marrow cells displayed greater deterioration of cardiac function by echocardiography after 8 weeks of systemic AngII infusion compared with mice transplanted with 100% wild-type cells that were comprised of 10% CD45.2+ cells (Figure 2a). At the termination of the experiment, hearts in mice receiving 10% Tet2-KO bone marrow displayed an increase in cardiac weight (Figure 2b), an increase in cardiac myocyte cross sectional area (Figure 2c), and an increase in interstitial fibrosis (Figure 2d and Online Figure V). Finally, a similar cardiac phenotype could also be detected in myeloid-specific, Tet2-deficient mice that were infused with AngII (Online Figure VI), consistent with observations in other models of myocardial dysfunction9.
Figure 2. Phenotype comparison of Angiotensin-II infusion-induced cardiac dysfunction between conventional competitive BMT model and lenti-CRISPR/Cas9 model.
a–d. Data from conventional competitive BMT model. e–h. Data from lenti-CRISPR/Cas9 model. a. Echocardiographic analysis at the indicated time points after AngII infusion (12 mice per group). Statistical analysis was evaluated by two-way repeated measure ANOVA with Sidak’s multiple comparison tests. b. HW adjusted by TL at the end of the study (8 weeks). Statistical analysis was evaluated by two-way ANOVA with Sidak’s multiple comparison tests. c. Representative images and analysis of WGA staining of the heart sections from hearts of 10% KO-BMT mice and 10% WT-BMT mice at the end of the study. Statistical analysis was evaluated by two-way ANOVA with Sidak’s multiple comparison tests. Scale bar: 25µm. d. Representative images and analysis of picrosirius red staining of the heart sections from hearts of 10% KO-BMT mice and 10% WT-BMT mice at the end of the study. Statistical analysis was evaluated by two-way ANOVA with Sidak’s multiple comparison tests. Scale bar: 1mm. In b–d, n=12 for AngII groups and n= 5 for PBS groups were analyzed. e. Echocardiographic analysis at indicated time points after AngII infusion (6 mice per group). Statistical analysis was evaluated by two-way repeated measure ANOVA with Sidak’s multiple comparison tests. f. HW adjusted by TL at the end of the study (8 weeks). Statistical analysis was evaluated by Mann-Whitney U test. g. Representative images and analysis of WGA staining of the heart sections from hearts of Tet2-indel mice and control mice at the end of the study. Scale bar: 1mm. Statistical analysis was evaluated by two-tailed unpaired Student’s t test. h. Representative images and analysis of Picro sirius staining of the heart sections from hearts of Tet2-indel mice and control mice at the end of the study. Scale bar: 25µm. Statistical analysis was evaluated by two-tailed unpaired Student’s t test. In e–h, n=6 per group were analyzed. **p<0.01, ***p<0.001, ****p<0.0001.
The next series of AngII-infusion experiments involved mice transplanted with HSPC that underwent Tet2 editing using the lentivirus/CRISPR system (Online Figure II). Similar to mice subjected to the conventional, competitive bone marrow transplantation with Tet2-KO cells, mice transplanted with CRISPR-edited HSPC displayed greater reductions in cardiac function as determined by echocardiography (Figure 2e). These mice also displayed an increase in cardiac mass, an increase in cardiac fibrosis, and an increase in cardiac myocyte cross sectional area (Figure 2f–2h and Online Figure Vb). Histological analyses also revealed greater kidney fibrosis in mice that were implanted with the CRISPR-edited HSPC (Online Figure VII).
CRISPR/Cas9-mediated hematopoietic Dnmt3a gene disruption promotes cardiac dysfunction
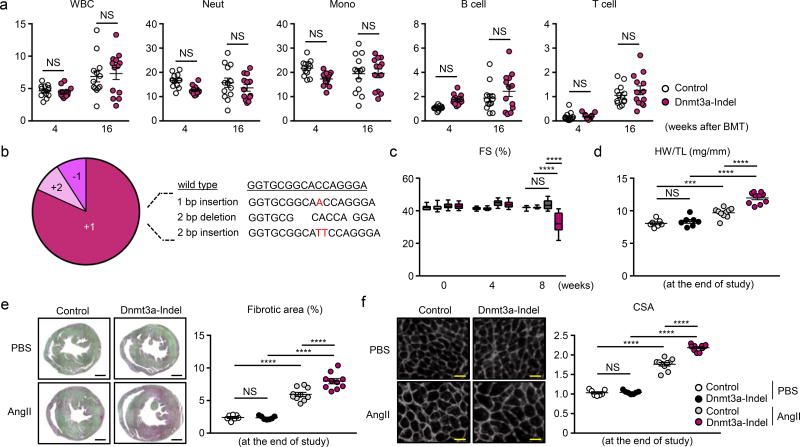
Having demonstrated that the lentivirus/CRISPR/AngII system can be used to analyze the role of clonal hematopoiesis in CVD processes, mice with Dnmt3a-edited HSPC were evaluated in the AngII model. The validation of guide RNA (gRNA) targeting Dnmt3a was performed using the T7 endonuclease I mismatch cleavage assay and immunoblotting (Online Figure Id,e). Consistent with previous reports11–13, Dnmt3a-disrupted HSPCs did not undergo selective expansion over the 4 month time course of these experiments (Figure 3a). Thus, a relatively low level of chimerism, similar to that of non-edited cells, was observed in of the peripheral blood populations. To determine the status of Dnmt3a editing by the lentivirus vector, sequencing of the Dnmt3a locus was performed on DNA isolated from GFP-positive white blood cells. All sequence reads revealed out-of-frame mutations (3n+1, 3n+2), that lead to premature stop codons in exon 17 of Dnmt3a gene (Figure 3b), and a loss of enzymatic activity14. Mice transplanted with Dnmt3a-edited HSPC showed a reduction in cardiac function by echocardiographic analysis and an increase in cardiac mass 8 weeks after systemic AngII administration (Figure 3c, 3d). Mice receiving Dnmt3a-edited HSPC also displayed greater cardiac fibrosis and greater cardiac myocyte cross sectional area in tissue sections (Figure 3e, 3f and Online Figure Vc). The Dnmt3a-edited HSPC condition led to greater renal fibrosis (Online Figure VIIc).
Figure 3. CRISPR/Cas9-mediated hematopoietic Dnmt3a gene disruption promotes cardiac dysfunction after AngII infusion.
a. Flow cytometry analysis of the peripheral blood at 4 and 16 weeks after bone marrow reconstitution. The percentages of GFP+ cells in both experimental groups are shown (n=13 in both Dnmt3a-indel mice and control mice). Statistical analysis was evaluated by two-way repeated measure ANOVA with Sidak’s multiple comparison tests. b. The result of the TA cloning procedure showing that GFP+ peripheral white blood cells harbors edited Dnmt3a genes. The wild-type Dnmt3a sequence is shown for reference. c. Echocardiographic analysis at indicated time points after AngII infusion (10 mice per group). Statistical analysis was evaluated by two-way repeated measure ANOVA with Tukey’s multiple comparison tests. d. HW adjusted by TL at the end of the study (2 months). Statistical analysis was evaluated by two-way ANOVA with Tukey’s multiple comparison tests. e. Representative images and analysis of picrosirius staining of the heart sections from hearts of Dnmt3a-indel mice and control mice at the end of the study. Scale bar: 1mm. Statistical analysis was evaluated by two-way ANOVA with Tukey’s multiple comparison tests. f. Representative images and analysis of WGA staining of the heart sections from hearts of Dnmt3a-indel mice and control mice at the end of the study. Scale bar: 25µm. Statistical analysis was evaluated by two-way ANOVA with Tukey’s multiple comparison tests. In d–f, n=10 for AngII groups and n=7 for PBS groups were analyzed. ***p<0.001, ****p<0.0001, NS: not significant.
Dnmt3a disruption enhances inflammation
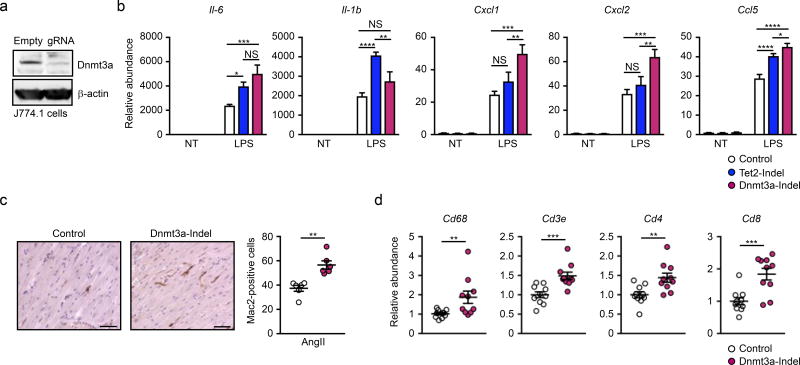
The recruitment and activation of peripheral myeloid cells contributes to cardiac dysfunction in the AngII infusion model15, 16. To address the role of Dnmt3a gene disruption in myeloid cells, we established Dnmt3a-deficient J774.1 myeloid cell line using the lentivirus/CRISPR system. After sorting and amplification, GFP-positive cells displayed a marked reduction in Dnmt3a protein expression by immunoblotting (Figure 4a). Using the same system, Tet2-deficiency was also established in J774.1 cells for comparative analyses. Consistent with our previous results, Tet2-disruption led to increased inflammatory chemokine/cytokine transcript expression after LPS stimulation8, 9. Dnmt3a-deficiency in J774.1 cells also displayed greater inflammatory responses after LPS stimulation (Figure 4b). However, the patterns of inflammatory mediator induction in the Dnmt3a- and Tet2-deficient states differed. Whereas IL-6 and Ccl5 were similarly induced in both conditions, IL-1β was significantly upregulated in Tet2-edited cells, but there was only a trend in Dnmt3a-edited cells. Conversely, Cxcl1 and Cxcl2 were upregulated only in Dnmt3a-deficient cells compared to the control condition.
Figure 4. Hematopoietic Dnmt3a loss-of-function enhances cardiac inflammation.
a. Western blot analysis revealing decreased in Dnmt3a expression in J774.1 cells treated with lentivirus-mediated Dnmt3a knock out. The lentivirus without sgRNA was used as control. b. Gene expression analysis of WT, Tet2-indel, and Dnmt3a-indel J774.1 cells at 6 hours after stimulation with10 ng/ml LPS. Statistical analysis was evaluated by two-way ANOVA with Tukey’s multiple comparison tests. c. Representative images and analysis of Mac2 staining of the sections of hearts from Dnmt3a-indel mice and control mice at 8 weeks after AngII infusion (n=6 per group). Scale bar: 100µm. Statistical analysis was evaluated by Mann-Whitney U test. d. Gene expression analysis of heart from Dnmt3a-indel and Control mice (n=10 per group) 8 weeks after AngII infusion. Statistical analysis was evaluated by two-tailed unpaired Student’s t test or Mann-Whitney U test. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Consistent with observations of greater inflammatory responses in the Dnmt3a-deficient state, greater macrophage accumulation was observed in the myocardium following AngII infusion (Figure 4c). This was accompanied by elevated levels of transcripts of immune cell markers including CD68, CD3e, CD4, and CD8, suggesting that hematopoietic Dnmt3a-deficiency leads to an increase in myocardial inflammation (Figure 4d).
DISCUSSION
Studies have associated the clonal expansion of HSPC with mortality and increased CVD risk4, 5, 7. Clonal hematopoiesis can be caused by somatic mutations in 40 or more candidate driver genes, and it is likely that these different drivers will confer gene-specific effects on pathological processes. To date, studies focused on Tet2, that functions to promote a DNA hydroxymethylation and to recruit HDACs to gene regulatory sequences2, have provided the only evidence that these somatic mutations can causally contribute to CVD. Given the challenge of evaluating many other driver genes, we have developed procedures to facilitate the analysis of clonal hematopoiesis in CVD. The described system employs lentiviral vectors to transduce HSPC and CRISPR methodology to create indel mutations in candidate driver gene. While off-target mutations are a potential concern, this system allows the ex vivo manipulation of HSPC that can then be implanted into wild-type mice and thereby provide a more versatile and rapid approach to assess HSPC clonal expansion and its systemic consequences than the conventional murine transgenic/knockout technology. Similar techniques have been used to edit driver genes in a model of myeloid malignancy17. To evaluate CVD, gene-edited mice were infused with AngII, a peptide hormone involved in the pathologies of vascular, renal and cardiac diseases18. Under these conditions, CRISPR-mediated indels in Tet2 of HSPC led to the expansion of mutant cells and worsened cardiac remodeling, serving to validate the system and extend our prior findings8, 9.
We then applied this technology to evaluate the consequences of inactivating mutations in Dnmt3a. Mutations in DNMT3A represent the most prevalent driver gene associated with clonal hematopoiesis in the elderly population4–6, and it can conservatively be estimated that millions of Americans are afflicted with DNMT3A-related clonal hematopoiesis. This protein is a de novo DNA methyltransferase and it is expressed in long-term HSPC19. Here, we show that lentivirus/CRISPR-mediated disruption of hematopoietic Dnmt3a results in greater cardiac hypertrophy, diminished ejection fraction, and increased fibrosis after AngII administration. Cardiac dysfunction was associated with greater macrophage accumulation and the elevated expression of immune cell markers in the myocardium. Consistent with previous studies on Tet28, 9, these effects tended to be observed late in the course of the pathological process, suggesting that clonal hematopoiesis impairs the resolution of inflammation. In cell culture studies, the inactivation of either Dnmt3a or Tet2 led to increased cytokine expression, but the effects differed qualitatively and quantitatively suggesting that they confer gene-specific effects resulting from mechanistic differences between these two proteins.
Inactivating mutations in Dnmt3a and Tet2 led to markedly different HSPC expansion characteristics. The robust expansion of Tet2-deficient into multiple blood cell lineages was observed following the implantation of CRISPR-edited HSPC, and these results are consistent with observations from experiments that employing a competitive transplantation approach employing a 1:9 ratio of Tet2-KO:wild-type bone marrow8, 9 (Figure 1b and Online Figure II). In contrast, there was no detectable expansion of Dnmt3a-deficient peripheral blood cells over the time course of our study. This finding is consistent with previous reports showing that Dnmt3a-null HSPC are observed to expand in aged mice or only after sequential bone marrow transplantations 12, 13, 20. While Dnmt3a and Tet2 appeared to similarly affect cardiac pathology in the AngII model, the lack of Dnmt3a hematopoietic cell expansion suggests that it may be more potent than Tet2 in promoting CVD.
Dnmt3a has been demonstrated to have multiple roles in the regulation of inflammation. Cao et al. reported that Dnmt3a controls inflammation in peritoneal macrophage by acting on HADC9 to cause TBK1 deacetylation. It is also reported that Dnmt3a controls inflammatory cytokine expression by regulating the expression of the scaffold protein IQGAP2 in mast cell21, and the expression of DNMT3A is associated with the methylation status and the activity of IL-6 gene in synovial fibroblasts from osteoarthritis patients22. In the current study, we show that Dnmt3a-deficiency can alter the function of myeloid cells, and promote the inflammation via the up-regulation of specific cytokines and chemokines. These findings suggest that Dnmt3a-mediated clonal hematopoiesis could be causally associated with cardiovascular diseases by promoting prolonged inflammatory responses in myeloid cells. However, we do not rule out the possibility that other blood cells could also contribute to the pathogenesis of CVD associated with the loss of Dnmt3a (on Tet2) in hematopoietic cells.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Clonal hematopoiesis may represent a new causal risk factor for cardiovascular disease.
Clonal hematopoiesis can result from somatic mutations in any of a number of pre-leukemic driver genes within hematopoietic stem cells.
To date, the only driver gene evaluated for its impact on cardiovascular disease in an experimental setting is Tet2.
What New Information Does This Article Contribute?
Using lentivirus/CRISPR methodology, a system is developed that allows for the rapid introduction of mutations into driver genes of hematopoietic stem cells in mice.
This system was used to show that inactivating mutations in Tet2 or Dnmt3a within hematopoietic stem cells can promote experimental heart failure.
Inactivating mutations in Tet2 and Dnmt3a differed in their ability to promote hematopoietic cell expansion in vivo and in patterns of inflammatory gene expression in macrophages in vitro, suggesting gene-specific actions.
DNMT3A is recognized to be the most prevalent clonal hematopoiesis driver gene in the elderly population, conservatively estimated to affect millions of individuals in the USA alone. Here it is shown that an inactivating mutation in Dnmt3a will promote pathological remodeling in experimental heart failure. These studies suggest multiple driver genes, beyond Tet2, can impact cardiovascular disease, and that gene-specific effects will be observed. These data provide further support hypothesis that there is a common mechanistic basis between age-associated cardiovascular disease and hematological cancer.
Acknowledgments
SOURCES OF FUNDING
Dr. Walsh was funded by NIH grants HL131006, HL138014 and Dr. Sano was supported by an American Heart Association post-doctoral fellowship.
Nonstandard Abbreviations and Acronyms
- ARCH
age-related clonal hematopoiesis
- AngII
angiotensin II
- CVD
cardiovascular disease
- CHIP
clonal hematopoiesis of indeterminate potential
- Dnmt3a
DNA (Cytosine-5)-methyltransferase 3a
- gRNA
guide RNA
- HSPC
hematopoietic stem/progenitor cells
- Indel
insertion and deletion
- Tet2
Ten-Eleven Translocation-2
Footnotes
DISCLOSURES
None.
References
- 1.Vijg J. Somatic mutations, genome mosaicism, cancer and aging. Curr Opin Genet Dev. 2014;26:141–9. doi: 10.1016/j.gde.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuster JJ, Walsh K. Somatic Mutations and Clonal Hematopoiesis: Unexpected Potential New Drivers of Age-Related Cardiovascular Disease. Circ Res. 2018;122:523–532. doi: 10.1161/CIRCRESAHA.117.312115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP, Ebert BL. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126:9–16. doi: 10.1182/blood-2015-03-631747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, Higgins JM, Moltchanov V, Kuo FC, Kluk MJ, Henderson B, Kinnunen L, Koistinen HA, Ladenvall C, Getz G, Correa A, Banahan BF, Gabriel S, Kathiresan S, Stringham HM, McCarthy MI, Boehnke M, Tuomilehto J, Haiman C, Groop L, Atzmon G, Wilson JG, Neuberg D, Altshuler D, Ebert BL. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–98. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genovese G, Kahler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, Chambert K, Mick E, Neale BM, Fromer M, Purcell SM, Svantesson O, Landen M, Hoglund M, Lehmann S, Gabriel SB, Moran JL, Lander ES, Sullivan PF, Sklar P, Gronberg H, Hultman CM, McCarroll SA. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371:2477–87. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zink F, Stacey SN, Norddahl GL, Frigge ML, Magnusson OT, Jonsdottir I, Thorgeirsson TE, Sigurdsson A, Gudjonsson SA, Gudmundsson J, Jonasson JG, Tryggvadottir L, Jonsson T, Helgason A, Gylfason A, Sulem P, Rafnar T, Thorsteinsdottir U, Gudbjartsson DF, Masson G, Kong A, Stefansson K. Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood. 2017 doi: 10.1182/blood-2017-02-769869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, Baber U, Mehran R, Fuster V, Danesh J, Frossard P, Saleheen D, Melander O, Sukhova GK, Neuberg D, Libby P, Kathiresan S, Ebert BL. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med. 2017 doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu CL, Sano S, Muralidharan S, Rius C, Vuong J, Jacob S, Muralidhar V, Robertson AA, Cooper MA, Andres V, Hirschi KK, Martin KA, Walsh K. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355:842–847. doi: 10.1126/science.aag1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sano S, Oshima K, Wang Y, MacLauchlan S, Katanasaka Y, Sano M, Zuriaga MA, Yoshiyama M, Goukassian D, Cooper MA, Fuster JJ, Walsh K. Tet2-Mediated Clonal Hematopoiesis Accelerates Heart Failure Through a Mechanism Involving the IL-1beta/NLRP3 Inflammasome. J Am Coll Cardiol. 2018;71:875–886. doi: 10.1016/j.jacc.2017.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie M, Lu C, Wang J, McLellan MD, Johnson KJ, Wendl MC, McMichael JF, Schmidt HK, Yellapantula V, Miller CA, Ozenberger BA, Welch JS, Link DC, Walter MJ, Mardis ER, Dipersio JF, Chen F, Wilson RK, Ley TJ, Ding L. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med. 2014;20:1472–8. doi: 10.1038/nm.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeong M, Park HJ, Celik H, Ostrander EL, Reyes JM, Guzman A, Rodriguez B, Lei Y, Lee Y, Ding L, Guryanova OA, Li W, Goodell MA, Challen GA. Loss of Dnmt3a Immortalizes Hematopoietic Stem Cells In Vivo. Cell Rep. 2018;23:1–10. doi: 10.1016/j.celrep.2018.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Challen GA, Sun D, Jeong M, Luo M, Jelinek J, Berg JS, Bock C, Vasanthakumar A, Gu H, Xi Y, Liang S, Lu Y, Darlington GJ, Meissner A, Issa JP, Godley LA, Li W, Goodell MA. Dnmt3a is essential for hematopoietic stem cell differentiation. Nat Genet. 2011;44:23–31. doi: 10.1038/ng.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole CB, Russler-Germain DA, Ketkar S, Verdoni AM, Smith AM, Bangert CV, Helton NM, Guo M, Klco JM, O'Laughlin S, Fronick C, Fulton R, Chang GS, Petti AA, Miller CA, Ley TJ. Haploinsufficiency for DNA methyltransferase 3A predisposes hematopoietic cells to myeloid malignancies. J Clin Invest. 2017;127:3657–3674. doi: 10.1172/JCI93041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang L, Rau R, Goodell MA. DNMT3A in haematological malignancies. Nat Rev Cancer. 2015;15:152–65. doi: 10.1038/nrc3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, Schilling JD, Schwendener R, Sergin I, Razani B, Forsberg EC, Yokoyama WM, Unanue ER, Colonna M, Randolph GJ, Mann DL. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hulsmans M, Sam F, Nahrendorf M. Monocyte and macrophage contributions to cardiac remodeling. J Mol Cell Cardiol. 2016;93:149–55. doi: 10.1016/j.yjmcc.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heckl D, Kowalczyk MS, Yudovich D, Belizaire R, Puram RV, McConkey ME, Thielke A, Aster JC, Regev A, Ebert BL. Generation of mouse models of myeloid malignancy with combinatorial genetic lesions using CRISPR-Cas9 genome editing. Nat Biotechnol. 2014;32:941–6. doi: 10.1038/nbt.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danilczyk U, Penninger JM. Angiotensin-converting enzyme II in the heart and the kidney. Circ Res. 2006;98:463–71. doi: 10.1161/01.RES.0000205761.22353.5f. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Zhang Q, Ding Y, Liu Y, Zhao D, Zhao K, Shen Q, Liu X, Zhu X, Li N, Cheng Z, Fan G, Wang Q, Cao X. Methyltransferase Dnmt3a upregulates HDAC9 to deacetylate the kinase TBK1 for activation of antiviral innate immunity. Nat Immunol. 2016;17:806–15. doi: 10.1038/ni.3464. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Su J, Jeong M, Ko M, Huang Y, Park HJ, Guzman A, Lei Y, Huang YH, Rao A, Li W, Goodell MA. DNMT3A and TET2 compete and cooperate to repress lineage-specific transcription factors in hematopoietic stem cells. Nat Genet. 2016;48:1014–23. doi: 10.1038/ng.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leoni C, Montagner S, Rinaldi A, Bertoni F, Polletti S, Balestrieri C, Monticelli S. Dnmt3a restrains mast cell inflammatory responses. Proc Natl Acad Sci U S A. 2017;114:E1490–E1499. doi: 10.1073/pnas.1616420114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang F, Zhou S, Wang C, Huang Y, Li H, Wang Y, Zhu Z, Tang J, Yan M. Epigenetic modifications of interleukin-6 in synovial fibroblasts from osteoarthritis patients. Sci Rep. 2017;7:43592. doi: 10.1038/srep43592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.