Abstract
Purpose
This randomized controlled trial examines the efficacy of INSPIRE, an INternet-based Survivorship Program with Information and REsources, with or without Problem-Solving Treatment (PST) telehealth calls, for survivors after hematopoietic cell transplantation (HCT).
Methods
All adult survivors who met eligibility criteria, were approached for consent. Participants completed patient-reported outcomes at baseline and 6 months. Those with baseline impaired scores on one or more of the outcomes were randomized to INSPIRE, INSPIRE+PST, or control with delayed INSPIRE access. Outcomes included Cancer and Treatment Distress, Symptom Checklist-90-R Depression, and Fatigue Symptom Inventory. Planned analyses compared arms for mean change in aggregated impaired outcomes and for proportion of participants improved on each outcome.
Results
Of 1306 eligible HCT recipients, 755 (58%) participated, and 344 (45%) had one or more impaired scores at baseline. We found no reduction in aggregated outcomes for either intervention (P>0.3). In analyses of individual outcomes, participants randomized to INSPIRE+PST were more likely to improve in distress than controls (45% vs. 20%, RR 2.3, CI 1.0, 5.1); those randomized to INSPIRE alone were marginally more likely to improve in distress (40% vs. 20%, RR 2.0, CI 0.9, 4.5).
Conclusions
The INSPIRE online intervention demonstrated a marginal benefit for distress that improved with the addition of telehealth PST, particularly for those who viewed the website or were age 40 or older.
Implications for Cancer Survivors
Online and telehealth programs such as INSPIRE offer opportunities to enhance HCT survivorship outcomes, particularly for mood, though methods would benefit from strategies to improve efficacy.
Introduction
Hematopoietic cell transplantation (HCT) is complicated by some of the highest rates of late effects and late mortality of any current cancer treatment [1-5]. Recovery can be lengthy, with an estimated 63% of survivors returning to pretransplant levels of physical and psychological function after 5 years [6, 7]. Many HCT survivors continue to report depression, cancer related distress and fatigue compared with their siblings or age-matched norms.[6, 8-10] The cumulative incidence of one or more major physical or mental late effects 5 years after transplant is estimated at 45% and 79% for autologous and allogeneic HCT recipients respectively [11]. Emotional distress is far more prevalent than clinical depression after HCT, with the distress prevalence estimated at 43%, and moderate to severe depressive symptoms found in 13-15% of long-term HCT survivors [12, 10, 13, 14].
Health care delivery and psychosocial interventions for HCT survivors are challenging due to the distance most live from transplant centers, their varying needs, and the lack of standard follow-up care. Few interventions have been tested to meet their health requirements. One randomized controlled trial (RCT) with 1-3 year survivors found that 10 phone-delivered sessions of cognitive behavioral treatment reduced distress, depressive and post-traumatic stress symptoms with effects sustained for 12 months [15]. A second trial comparing in-person, mindfulness-based training to a supportive telephone consultation found that mindfulness improved quality of life, depression and anxiety but not fatigue, and did not have sustained effects [16]. Both of these intensive interventions required relatively high levels of expertise, limiting their reach.
Online programs are an attractive option for HCT survivors who frequently use the internet for information [17-20]. A few online RCTs for non-HCT cancer patients have been efficacious when targeting depressive symptoms, [21-24] distress, [25] sexual function, [26, 27] fear of recurrence, [28] fatigue, [24, 29] insomnia, [30] physical function [31] or physical activity [32]. However, effect sizes of internet RCTs are generally small or not significant as confirmed by systematic reviews [33-35]. Most studies report feasibility and acceptability rather than outcomes [36-48].
Telehealth models of care delivery also have advantages for HCT survivors who live far from post-transplant expertise [15]. We effectively targeted fatigue and distress with a psychoeducational intervention using telehealth ‘booster’ calls after onsite workshops in the first year after transplant [49]. Problem-Solving Treatment (PST) delivered by phone has shown promise for treating depression in breast cancer survivors and other populations [50, 51]. PST is a brief intervention that requires less advanced training than cognitive behavioral therapy [52]. Since online interventions are not consistently effective, we predicted that PST could boost efficacy beyond an online intervention alone for HCT survivors.
The aim of the INSPIRE RCT was to determine the efficacy of an online program alone or in combination with telehealth-delivered PST to improve the primary outcomes of depression, cancer-related distress, fatigue and physical dysfunction in adult, long-term HCT survivors with impaired symptoms at baseline assessment. We have previously described the development and reach of the INSPIRE (INternet-based Survivorship Program with Information and REsources) online intervention.[53] For the RCT, we hypothesized that HCT survivors with impaired target symptoms who were randomized to the INSPIRE intervention with or without PST would have more improved aggregated primary outcomes at the 6-month assessment compared to controls. Additional hypotheses predicted higher rates of improvement in the individual outcomes for the intervention arms compared with controls, and improved response rates to the intervention for participants who were under age 40 and had higher levels of engagement as measured by pages viewed on the INSPIRE site.
Methods
Participants
All HCT survivors treated at a single transplant center were approached if they met the following criteria: age18 or older, 3-18 years since most recent transplant, U.S. or Canadian residents, with internet and email access, and adequate English to complete the baseline assessment. Exclusion criteria included recurrence or subsequent malignancy requiring treatment more than surgical excision during the two years prior to enrollment. Participants reporting suicidal ideation or severe depression were contacted by phone to ensure their safety and access to treatment in their communities. They were not randomized but were given access to the INSPIRE site.
Procedure
The RCT was registered with ClinicalTrials.gov (NCT00799461). All procedures were approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center (Fred Hutch). Eligible survivors were sent up to three letters of approach, with follow-up calls for non-responders. Interested survivors received a link to the website for registration, consent, and completion of patient-reported outcomes (PRO). Participants were assigned to group 1 if their scores were impaired on depression, distress and/or fatigue measures, and were included in the primary analyses. Randomization was carried out using an adaptive randomization algorithm and was stratified on gender, stem cell source (autologous versus allogeneic), race/ethnicity (Non-Caucasian or Hispanic vs. white and Non-Hispanic), and transplant institution (Fred Hutch vs. non-Fred Hutch). Group 1 participants were randomized to one of three arms: INSPIRE online access plus PST calls (INSPIRE+PST), INSPIRE access alone, or delayed INSPIRE access after 6-month assessment (control). Participants were assigned to group 0 if they had no impaired scores on distress, depression or fatigue measures. Group 0 participants were randomized to INSPIRE access or control and were not included in the primary analyses. After 6 months, participants completed the outcome assessment, after which those in the control group were given access to INSPIRE.
Intervention: INSPIRE
INSPIRE site content and study reach have been previously described. [53] Briefly, the INSPIRE site consisted of seven levels: 1) A greeting home page tailored to each participant, with links to sections identified as impaired at baseline. 2) Three main topics: lifting mood, reducing fatigue, and boosting health. 3) Self-care tips and tools for common complications. 4) Tailored health care guidelines, which were also mailed to survivors to take to providers or use for self-care. 5) A forum for posting survivor experiences and input. 6) Annotated national and local resources. 7) A comment box for sending secure messages to study staff. The entire site was available to intervention arm participants throughout the study.
Intervention: PST Calls
PST focused on problems and goal setting toward solutions as specified by the participant during the first call with the clinicians who were PhD psychologists (SA, JR, JY) trained and certified in PST by Dr. Mark Hegel who developed the method. [51] The first session lasted an hour and explained PST, collected an initial problem list, and applied the PST process to one problem. Subsequent sessions (3 to 7 depending on need) lasted 30 minutes and applied PST to one problem per session. PST sessions were about two weeks apart to give participants time to work on goals.
Measures
Sources of data included PST audiotapes, medical records, PRO, and INSPIRE page views tracked by date and time.
PST Process and Fidelity
All PST sessions were audiotaped after consent. Dr. Hegel reviewed 36% of the tapes and rated fidelity to the treatment manual on 7 elements and global fidelity, with each item rated from 0=very poor to 5=very good. Clinicians received feedback and reviews of fidelity in monthly phone supervision by Dr. Hegel. A minimum dose of 4 sessions was required to indicate PST completion [51].
Medical and Sociodemographic Data
Medical records provided diagnosis, transplant details, years post-transplant, history of relapse, and chronic graft versus host disease (cGVHD). Medicare and Medicaid categorization of zip codes provided coding for rural versus urban residence (http://www.cms.hhs.gov/AmbulanceFeeSchedule/).
Patient-Reported Outcomes (PRO)
The PRO included demographic information, computer experience, and cGVHD treatment. Outcomes included Cancer and Treatment Distress (CTXD), Symptom Checklist-90-R depression scale (SCL-90-R), Short Form 36 Health Survey (SF-36), and Fatigue Symptom Inventory (FSI). The CTXD is a 22-item measure of distress or worry related to cancer events, with a mean score >1.10 indicating elevated distress [54]. The measure has been tested with HCT survivors as a predictor of health outcomes, and has been used in several RCTs [54, 6, 55-57]. The SCL-90-R depression scale is a widely used, psychiatrically validated, 20-item measure of depressive symptoms [58]. A mean score >1.0 indicates depressive symptoms of mild or greater severity. The SF-36 measures health-related quality of life across eight dimensions [59]. The physical function subscale used as an outcome has a standardized t-score of 50 and standard deviation of 10; a cut point of <40 indicates impaired physical function. The FSI is validated for use in cancer populations and has 13 items, with a mean score >4.7 indicating elevated fatigue [60, 61].
Statistical Analysis
The primary endpoint defined at study inception was the aggregate number of targeted problems with impaired scores at the 6-month endpoint for participants in group 1, including cancer and treatment distress, depressive symptoms, physical dysfunction and fatigue, resulting in a possible range of 0-4. To account for variability in baseline aggregate numbers of conditions, the analytic outcome used for primary analyses was the change in aggregate number of conditions between baseline and six months. The sample size was selected to allow more than 90% power to detect effect sizes in aggregate numbers of conditions of 0.5 standard deviation difference between each intervention and control arm assuming a type I overall error rate of 0.05 with Bonferroni adjustment applied to allow for two pairwise comparisons between study arms (each with 0.025 two-sided significance level). Balance between study arms was evaluated for key factors that may influence outcomes such as age, years since transplant, type of transplant (autologous or allogeneic), race/ethnicity, gender, education, income, cancer diagnosis and rural vs. urban residence as well as subjects' baseline aggregate number of targeted problems. Planned secondary analyses included subset analyses of each endpoint among participants meeting eligibility for randomization due to that endpoint (e.g. distress, depression, fatigue and physical functioning). For each of these subgroups, the relevant binary outcome of success was defined (e.g. not impaired on distress), and the proportion of participants meeting that criterion at the 6-month time point was compared between study arms. Since selection of subgroups could result in imbalances between study arms, we carefully evaluated balance between arms for each subset and found good balance between arms in all subsets. Additional exploratory analyses examined the impact of two hypothesized modifying factors on intervention efficacy, specifically engagement indicated by viewing two or more website pages, i.e., views beyond the landing page, and current age <40 or 40+ years. We evaluated interactions between these factors and the study arms. Since baseline characteristics were well balanced between study arms, primary analytic comparisons were univariate, using t-tests to compare mean change in aggregate counts between 6 month and baseline values. Chi-square tests were used to compare proportions. Additional analyses evaluating the impact of patient characteristics on relative risk estimates were carried out using generalized linear models with a log-link function and Poisson errors [62].
Results
Characteristics of the Cohort
Of the 1755 HCT survivors approached who met initial eligibility criteria, 1306 met full eligibility (Figure 1), and 755 (58% of eligible) consented, completed baseline assessment. Of the 755, 45% (n=344) met impaired symptom criteria for assignment to group 1 and randomization, while 411 did not and were assigned to group 0. Seven participants in group 1 were prospectively designated for ‘run-in’ testing for INSPIRE+PST (two completed PST cases per clinician) and therefore were not included in analyses.
Figure 1. Consort diagram for study flow.
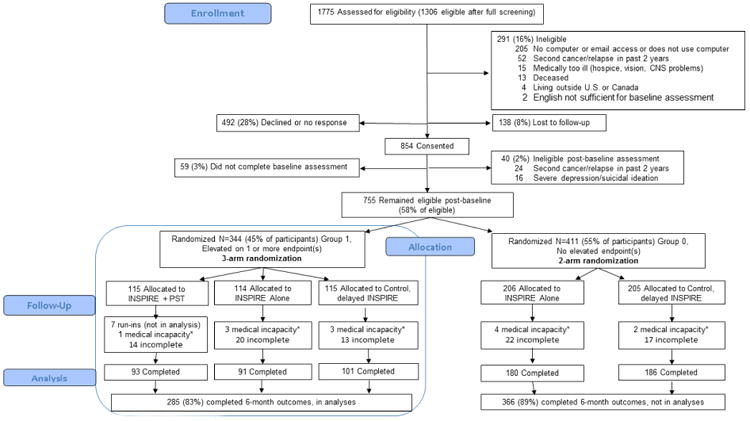
* Relapse, hospice care, hospitalized so unable to respond
Participants, compared with those eligible but not enrolled, were more likely to be over age 40, white, treated for acute leukemia or myelodysplasia, less than 10 years after HCT, and with a history of cGVHD (all P<.05, Table 1). Randomized arms in group 1 were comparable in demographic and clinical characteristics, with the exception that the control arm was more likely to have no current cGVHD (P=.02, Table 2). Group 1 participants were 18 to 76 years (mean 51, SD 12), 53% male, and over 90% white and non-Latino (Table 2). A majority, 68%, were less than 10 years from first transplant. A quarter of allogeneic survivors were in active treatment for cGVHD (n=59, 24%). Within group 1, impaired scores were reported for distress by 36% (n=120), for depression symptoms by 40% (n=134), for fatigue by 31% (n=106), and for physical dysfunction by 31% (n=104). Depression and distress were correlated (r=0.77) as were distress and fatigue (r=0.56) and fatigue and physical function (r=-0.47).
Table 1. Demographic and medical characteristics of eligible survivors not randomized and randomized.
| Variables and Categories: | Eligible survivors not randomized (N=551) | All randomized survivors (Groups 0 & 1) (N=748) | Group 0 randomized survivors, no elevated endpoints at baseline (N=411) | Group 1 randomized survivors, with one or more elevated endpoints at baseline (N=337)a | P value Not randomized vs. All randomizedb |
|---|---|---|---|---|---|
|
| |||||
| Age, Mean (SD) | 51 (14) | 52 (12) | 52 (13) | 51 (12) | |
| Range | 18-79 | 18-78 | 18-78 | 18-76 | |
| Categories, n (%) | .001 | ||||
| 18-39 | 134 (24) | 125 (17) | 66 (16) | 59 (18) | |
| 40 or older | 417 (76) | 623 (83) | 345 (84) | 278 (82) | |
|
| |||||
| Male, n (%) | 326 (59) | 416 (56) | 239 (58) | 177 (53) | .20 |
|
| |||||
| Rural, c n (%) | 116 (21) | 150 (20) | 84 (20) | 66 (20) | .66 |
|
| |||||
| Race, n (%) | <.001 | ||||
| African American | 9 (2) | 5 (0.7) | 4 (1) | 1 (0.3) | |
| Asian | 29 (5) | 16 (2) | 7 (1.8) | 9 (2.7) | |
| Native American/Alaska Native | 8 (2) | 2 (0.3) | 1 (0.2) | 1 (0.3) | |
| White/Caucasian | 478 (87) | 695 (93) | 379 (92) | 316 (94) | |
| Other non-white or mixed | 10 (2) | 30 (4) | 20 (5) | 10 (3) | |
| Unknown | 17 (3) | ||||
|
| |||||
| Ethnicity: Hispanic or Latino, n (%) | 17 (3) | 21 (3) | 12 (3) | 9 (3) | <.74 |
| Unknown | 25 (5) | ||||
|
| |||||
| Diagnosis, n (%) | .002 | ||||
| Acute leukemia | 149 (27) | 225 (30) | 122 (30) | 103 (31) | |
| Chronic myelogenous leukemia | 198 (36) | 218 (29) | 124 (30) | 94 (28) | |
| Non-Hodgkin lymphoma | 85 (16) | 124 (17) | 64 (16) | 60 (18) | |
| Myelodysplasias | 32 (6) | 83 (11) | 52 (13) | 31 (10) | |
| Multiple Myeloma | 41 (7) | 58 (8) | 30 (7) | 28 (8) | |
| Hodgkin lymphoma | 33 (6) | 26 (3) | 11 (3) | 15 (4) | |
| Other | 13 (2) | 14 (2) | 8 (1) | 6 (1) | |
|
| |||||
| Donor Type, n (%) | .66 | ||||
| Autologous | 140 (25) | 182 (24) | 91 (22) | 91 (27) | |
| Allogeneic | 411 (75) | 566 (76) | 320 (78) | 246 (73) | |
|
| |||||
| Years post-transplant, n (%) | <.001 | ||||
| <10 years | 287 (52) | 477 (64) | 249 (61) | 228 (68) | |
| 10+ years | 264 (48) | 271 (36) | 162 (39) | 109 (32) | |
|
| |||||
| History of clinical chronic GVHD,d n (%) | 243 (59) | 421 (74) | 227 (71) | 194 (79) | .03 |
Endpoints include distress, depression, or fatigue
P value for chi-square test comparing all Group 0 and 1 participants to eligible but not randomized participants
Urban/rural coding based on zip code tables from the Department of Social and Health Services, Center for Medicare Services[84]
GVHD, graft versus host disease, reported only for those receiving allogeneic transplants
Table 2. Characteristics of participants with one or more elevated endpoints at baseline.
| Variables and Categories: | All Group 1 (N=337) | Group 1 INSPIRE+ PSTa calls (N=108) | Group 1 INSPIRE Alone (N=114) | Group 1 Controls (N=115) | P value INSPIRE+PST vs. Control | P value INSPIRE Alone vs. Control |
|---|---|---|---|---|---|---|
|
| ||||||
| Age, Mean (SD) range | 51 (12) | 51 (12) | 50 (13) | 51 (11) | ||
| Range | 18-76 | 19-76 | 19-76 | 21-76 | ||
| Categories, n (%) | ||||||
| 18-39 | 59 (18) | 17 (16) | 21 (18) | 21 (18) | .62 | .98 |
| 40 or older | 278 (82) | 91 (84) | 93 (82) | 94 (82) | ||
|
| ||||||
| Male, n (%) | 177 (53) | 58(54) | 59 (52) | 60 (52) | .82 | .95 |
|
| ||||||
| Rural,b n (%) | 66 (20) | 19 (18) | 27 (24) | 20 (17) | .97 | .24 |
|
| ||||||
| Race, n (%) | .63 | .73 | ||||
| African American | 1 (0.3) | 1 (0.9) | 0 (0) | 0 (0) | ||
| Asian | 9 (2.7) | 2 (1.9) | 3 (2.6) | 4 (3.5) | ||
| Native American/Alaska Native | 1 (0.3) | 0 (0) | 1 (0.9) | 0 (0) | ||
| White/Caucasian | 316 (94) | 102 (94) | 107 (94) | 107 (93) | ||
| Other non-white or mixed | 10 (3) | 3 (2.8) | 3 (2.6) | 4 (3.5) | ||
|
| ||||||
| Ethnicity: Hispanic or Latino, n (%) | 9 (2.7) | 3 (2.) | 3 (2.6) | 3 (2.6) | .94 | .99 |
|
| ||||||
| Education, n (%) | .71 | .09 | ||||
| High School or less | 26 (8) | 9 (8) | 6 (5) | 11 (10) | ||
| 2 year college or more | 281 (83) | 90 (83) | 98 (86) | 93 (81) | ||
| Unknown | 30 (9) | 9 (8) | 10 (9) | 1 (10) | ||
|
| ||||||
| Income, n (%) | .79 | .09 | ||||
| Below $40,000 per year | 58 (17) | 21 (19) | 21 (18) | 16 (14) | ||
| $40,000-$79,999 per year | 96 (28) | 30 (28) | 29 (25) | 37 (32) | ||
| $80,000 and above per year | 142 (42) | 45 (42) | 49 (43) | 48 (42) | ||
| Unknown | 41 (12) | 12 (11) | 15 (13) | 14 (12) | ||
|
| ||||||
| Marital status, n (%) | .94 | .12 | ||||
| Married or living with a partner | 231 (69) | 74 (69) | 73 (64) | 84 (73) | ||
| Single, separated, divorced or widowed | 80 (24) 26 (8) | 26 (24) 8 (7) | 33 (29) 8 (7) | 21 (18) 10 (9) | ||
| Unknown | ||||||
|
| ||||||
| Computer Experience, n (%) | .84 | .70 | ||||
| Beginner | 53 (16) | 17 (16) | 19 (17) | 17 (15) | ||
| Intermediate/Expert | 284 (84) | 91 (84) | 95 (83) | 98 (85) | ||
|
| ||||||
| Diagnosis, n (%) | .95 | .59 | ||||
| Acute leukemia | 103 (31) | 35 (32) | 37 (32) | 31 (27) | ||
| Chronic myelogenous leukemia | 94 (28) | 26 (24) | 35 (31) | 33 (29) | ||
| Non-Hodgkin lymphoma | 60 (18) | 19 (18) | 17 (15) | 24 (21) | ||
| Myelodysplasias | 31 (10) | 12 (10) | 8 (9) | 11 (10) | ||
| Multiple Myeloma | 28 (8) | 11 (10) | 7 (6) | 10 (9) | ||
| Hodgkin lymphoma | 15 (4) | 4 (4) | 6 (5) | 5 (4) | ||
| Other | 6 (1) | 1 (2) | 4 (2) | 1 (1) | ||
|
| ||||||
| Donor Type, n (%) | .46 | .47 | ||||
| Autologous | 91 (27) | 32 (30) | 28 (25) | 31 (27) | ||
| Allogeneic | 246 (73) | 76 (70) | 86 (75) | 84 (73) | ||
|
| ||||||
| Years post-transplant, n (%) | .48 | .93 | ||||
| <10 years | 228 (68) | 76 (70) | 76 (67) | 76 (66) | ||
| 10+ years | 109 (32) | 32 (30) | 38 (33) | 39 (34) | ||
|
| ||||||
| History of clinical chronic GVHD,c n (%) | 194 (79) | 56 (74) | 68 (79) | 70 (83) | .18 | .95 |
|
| ||||||
| Current chronic GVHD,c n (%) | .02 | .42 | ||||
| None | 133 (54) | 36 (47) | 42 (49) | 55 (66) | ||
| Mild | 74 (30) | 26 (34) | 30 (35) | 18 (21) | ||
| Moderate | 33 (13) | 9 (12) | 13 (15) | 11 (13) | ||
| Severe | 6 (2) | 5 (7) | 1 (1) | 0 (0) | ||
|
| ||||||
| Cancer and Treatment Distress, n (%) >1.10 | 120 (36) | 39 (36) | 46 (40) | 39 (34) | .36 | .12 |
| Baseline mean (SD) | 0.99 (0.53) | 0.97 (0.55) | 1.06 (0.50) | 0.94 (0.53) | ||
| 6 month mean (SD) | 0.90 (0.54) | 0.86 (0.56) | 0.94 (0.51) | 0.91 (0.55) | ||
|
| ||||||
| Symptom Checklist 90-R Depression, n (%) >1.0 | 134 (40) | 45 (42) | 47 (41) | 42 (37) | .43 | .47 |
| Baseline mean (SD) | 0.94 (0.52) | 0.96 (0.57) | 0.98 (0.51) | 0.88 (0.49) | ||
| 6 month mean (SD) | 0.89 (0.56) | 0.88 (0.62) | 0.91 (0.51) | 0.88 (0.55) | ||
|
| ||||||
| Fatigue Symptom Inventory, n (%) >4.7 | 106 (31) | 34 (31) | 39 (34) | 33 (29) | .65 | .37 |
| Baseline mean (SD) | 3.68 (1.87) | 3.73 (1.79) | 3.75 (1.99) | 3.57 (1.83) | ||
| 6 month mean (SD) | 3.50 (1.90) | 3.50 (1.88) | 3.74 (1.94) | 3.30 (1.87) | ||
|
| ||||||
| SF-36 Physical Function, n (%) T score <40 | 104 (31) | 38 (35) | 31 (27) | 35 (30) | .45 | .59 |
| Baseline mean (SD) | 44.04 (10.73) | 43.57 (11.00) | 44.70 (10.74) | 43.84 (10.51) | ||
| 6 month mean (SD) | 44.69 (10.52) | 43.76 (11.36) | 44.83 (10.19) | 45.41 (10.03) | ||
|
| ||||||
| INSPIRE site number of pages viewed, n (%) | .67 | |||||
| 0-1 page | 73 (32) | 33 (31) | 38 (33) | NAd | ||
| 2+ pages | 151 (68) | 75 (69) | 76 (67) | NA | ||
PST, Problem Solving Treatment, seven participants randomized to the PST group were treated as practice run-ins and were not included in analyses
Urban/rural coding based on zip code tables from the Department of Social and Health Services, Center for Medicare Services[84]
GVHD, graft versus host disease, reported only for those receiving allogeneic transplants
NA, not applicable
Process Measures: INSPIRE Page Views, PST Calls, and PST Fidelity
For the n=222 given immediate access to INSPIRE and in analyses (not including the seven run-in cases), median number of page views was 9, with an interquartile range of 0-23, and a full range of 0-179. A third (32%, n=71) viewed no pages or only visited the home page of the site. The intervention arms did not differ in page views (P=.67, Table 2). Among INSPIRE+PST participants, n=15 (14%) declined PST calls but continued with INSPIRE online; n=19 (18%) started but did not complete PST. On average, participants received 4.5 calls (SD=2.8). Mean ratings of clinician fidelity to the PST manual ranged from 4.2-4.8; global ratings had a mean score of 4.0 (SD=1.0), equivalent to “good.”
Primary Outcome Analysis of the Aggregated Outcome
There were no differences in the mean change in aggregated endpoint score from baseline to six months between the three study arms (all P >0.3). Mean (Standard Deviation [SD]) change in aggregate endpoints from baseline to 6 months were 0.30 (1.23), 0.38 (1.28) and 0.29 (1.15) for the INSPIRE+PST, INSPIRE alone and control arms, respectively.
In the primary analysis and secondary analyses below we evaluated adjusted analyses for the aggregate score and for individual outcomes, and found no factors that affected point estimates between study arms. Inclusion of other factors reduced power and decreased precision of the estimates. Factors considered included: current age (<40 vs. 40+ years), education (<4 vs. 4+ years of college), gender, rural/urban residence, autologous versus allogeneic HCT, years since diagnosis, cGVHD, and within the intervention arms the number of pages of INSPIRE visited.
Planned Secondary Analyses of Individual Outcomes
Table 3 provides results of analyses comparing the proportion of survivors achieving a successful outcome for each measure among participants with impaired scores for that outcome at baseline. Compared to controls, INSPIRE+PST recipients demonstrated improvement in distress (RR=2.3, CI 1.0, 5.1, P=.032); INSPIRE alone participants demonstrated a trend toward improvement (RR=2.0, CI 0.9, 4.5, P=.075). We found no differences between intervention arms and controls in rates of change in depressive symptoms, fatigue or physical functioning (RR's 0.6 to 1.4).
Table 3. Relative risks (RR) comparing rates of improvement in the 3 study arms for the primary outcomes at 6 months among group 1 participants impaired on that outcome at baseline assessment.
| Outcome measure and cut point indicating improved score | INSPIRE +PSTa | INSPIRE | Control | ||
|---|---|---|---|---|---|
| n/N (%)b | RR (95% CI)c | n/N (%) | RR (95% CI)d | n/N % | |
| Cancer and Treatment Distress <1.11 | 15/33 (45) | 2.3 (1.0, 5.1) | 16/40 (40) | 2.0 (0.9, 4.5) | 6/30 (20) |
| Symptom Checklist 90-R Depression <1.0 | 12/38 (32) | 0.9 (0.5, 1.7) | 20/39 (51) | 1.4 (0.9, 2.4) | 14/39 (36) |
| Fatigue Symptom Inventory <4.7 | 14/31 (45) | 0.9 (0.8, 1.0) | 12/33 (36) | 0.6 (0.4, 1.1) | 17/30 (57) |
| SF-36 Physical Function >40 | 8/33 (24) | 0.9 (0.4, 1.9) | 9/36 (25) | 0.9 (0.4, 1.9) | 12/43 (28) |
PST, problem-solving treatment
n = number improved on outcome at 6 months of those impaired on the outcome at baseline (N), % = percent improved
INSPIRE+PST vs. Control: Distress: P = 0.032, Depression: P = 0.69, Fatigue: P =0.37, PF: P = 0.72
INSPIRE Alone vs. Control: Distress: P = 0.075, Depression: P = 0.17, Fatigue: P =0.11, PF: P = 0.77
Although the study had limited power to assess interactions, we explored whether selected subgroups improved more in distress or depression within the arms. In the INSPIRE arm, those with impaired depression scores at baseline who viewed two or more pages of the site had improved depression compared to controls (60% vs. 36%, RR=1.7, CI 1.0, 2.8, P=.047); distress was marginally improved for this subgroup: (40% vs. 20%, RR=2.0, CI 0.9, 4.6, P=.091). In the INSPIRE+PST arm, those who viewed two or more pages had improved distress compared to controls (42% vs. 20%, RR=2.7, CI 1.2, 6.1, P=.009), but not improved depression. Relatively few participants had fewer than two page views, which was set as a cut-point indicative of views of the website beyond the landing page. However, in the INSPIRE arm those with two or more page views had a marginally higher rate of improvement in depression (RR=2.7, CI 0.8, 9.5, P=.065) compared with those with one or no page views. There were no age differences across arms in improvement in depression. However, contrary to our hypothesis, for survivors age 40 or older rather than under age 40, distress was more likely to improve for those in the INSPIRE+PST arm (RR=4.2, CI 1.4, 12.8, P=.003) and the INSPIRE arm (RR=3.9, CI 1.3, 12.0, P =.006) compared to controls. Although there were few participants below age 40, we found significant interactions for distress outcomes between age and each intervention arm (INSPIRE+PST P=.025; INSPIRE P=.009), indicating that the interventions were potentially more effective among survivors over age 40 than under age 40.
Discussion
Online programs and telehealth calls provide resource-conserving access to survivorship knowledge and tools for HCT recipients who may live far from their transplant centers. This RCT of an internet and telehealth intervention addressing distress, depression and fatigue demonstrated a high enrollment rate (58%) relative to other internet-based RCTs. [53, 63] Although we found no differences between the study arms on the primary endpoint of aggregated outcomes, the secondary endpoint of distress improved significantly for those in the INSPIRE+PST arm at six months, with a more modest effect for INSPIRE alone. As hypothesized, survivors receiving INSPIRE alone who viewed two or more pages were more likely to report improved depression and a trend toward improved distress. Conversely, those receiving INSPIRE+PST who viewed two or more pages reported improved distress but not depressive symptoms. Of note, distress and depression were strongly correlated (r=0.77). Contrary to hypothesis, survivors 40 or older, rather than under age 40, also had improved distress with either intervention.
Online interventions for chronic diseases and cancer have proliferated although with mixed success [64, 34, 65]. Challenges include recruiting those with more severe chronic symptoms to enroll, [66, 67] and maintaining engagement for those who enroll [68]. Effect sizes are often modest at best since many of those who remain engaged with the program are already doing well, and improvement is therefore difficult to measure [69]. Nonetheless, cancer survivors, including after HCT, remain interested in using these modalities [70, 71].
High attrition rates are major reasons for reduced effect sizes of online interventions compared with face-to-face treatments [72]. Our attrition rate of 32% is consistent with other oncology online studies that include one for fatigue that had a 38% dropout and another for coping skills in cancer survivors that had a 32% attrition rate [29, 73]. It is also worth noting that a meta-analysis of dropouts from in-person psychotherapy for anxiety is 17%, with no definable modifiers based on patient, therapist, or treatment variables and can range up to at least 38% for some in-person treatments [74, 75].
Because technology-based RCTs with cancer survivors remain infrequent, this study provides needed information regarding strategies that may improve efficacy. The site did not provide for direct participant interactions, which could improve engagement [76, 77]. Although the INSPIRE site was mobile enabled, it did not incorporate social media or texting which may improve the appeal to younger adults,[78] although other factors such as wanting to put cancer behind them or a focus on other aspects of life also contribute to their low participation rates in other studies [79]. As noted by Mohr and colleagues, to be effective, technology-based interventions must be integrated into the user's lifestyle and familiar manner of use of their devices [80, 81]. Therefore, particularly for younger adults, mobile applications and texting focused on shorter and more frequent interactions may be more effective [80]. Flexible methods that adapt to individual needs, including phone contact options, seem necessary to engage some survivors [82, 83]. While PST improved the efficacy of the intervention for distress, a relatively large proportion (22%) of those randomized to PST declined participation in calls. This suggests that a stepped care model adding telehealth calls only for those who do not improve with the online site alone may direct utilization of resources more efficiently [24]. Lack of intervention efficacy for fatigue and physical dysfunction highlights the need for a more interactive methodology to increase activity and reduce fatigue. The open-access online site may not be sufficiently powerful to alter exercise habits or alternatively the focus on physical activity may not be adequate to improve fatigue in these HCT survivors.
This study has several strengths and limitations. As strengths, we approached all survivors who were potentially eligible, and enrollment was high for an internet intervention. The sample size was large, whereas many online studies with cancer survivors are small pilot studies. The design was risk-based, focusing resources on survivors with the problems targeted by the intervention. Limitations included the requirement for internet access, a low rate of eligible minority survivors to approach and among enrollees, and enrollment was conducted at a single center. A multi-center trial may highlight different needs among a more sociodemographically diverse group of survivors.
In conclusion, this research demonstrates the promise of online and telehealth modalities for benefiting some HCT survivors, particularly for mood-based interventions. More work is needed to realize the full potential of technology-enhanced interventions, by optimizing delivery to those with needs and engaging diverse survivors, as well as defining effective intervention models for health needs other than mood. Technology-facilitated care has the potential to reach many more survivors than in-person interventions. Adding more tailored content and increasing interactive options, along with strategies for directing, tracking and motivating healthy behaviors may extend and improve the efficacy of online interventions.
Acknowledgments
Funding: K Syrjala was supported by grants from the National Cancer Institute, R01 CA112631 and R01 CA160684. W Leisenring was supported in part by the NIH/NCI Cancer Center Support Grant P30 CA015704.
Footnotes
Conflict of Interest Disclosures: none
References
- 1.Syrjala KL, Martin PJ, Lee SJ. Delivering care to long-term adult survivors of hematopoietic cell transplantation. Journal of Clinical Oncology. 2012;30(30):3746–51. doi: 10.1200/JCO.2012.42.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Majhail NS, Tao L, Bredeson C, Davies S, Dehn J, Gajewski JL, et al. Prevalence of hematopoietic cell transplant survivors in the United States. Biol Blood Marrow Transplant. 2013;19(10):1498–501. doi: 10.1016/j.bbmt.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin PJ, Counts GW, Appelbaum FR, Lee SJ, Sanders JE, Deeg HJ, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. Journal of Clinical Oncology. 2010;28(6):1011–6. doi: 10.1200/JCO.2009.25.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashton LJ, Le Marsney RE, Dodds AJ, Nivison-Smith I, Wilcox L, O'Brien TA, et al. A population-based cohort study of late mortality in adult autologous hematopoietic stem cell transplant recipients in Australia. Biol Blood Marrow Transplant. 2014;20(7):937–45. doi: 10.1016/j.bbmt.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Sun CL, Kersey JH, Francisco L, Armenian SH, Baker KS, Weisdorf DJ, et al. Burden of morbidity in 10+ year survivors of hematopoietic cell transplantation: report from the bone marrow transplantation survivor study. Biol Blood Marrow Transplant. 2013;19(7):1073–80. doi: 10.1016/j.bbmt.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Syrjala KL, Langer SL, Abrams JR, Storer B, Sanders JE, Flowers ME, et al. Recovery and long-term function after hematopoietic cell transplantation for leukemia or lymphoma. JAMA. 2004;291(19):2335–43. doi: 10.1001/jama.291.19.2335. [DOI] [PubMed] [Google Scholar]
- 7.Bevans M, El-Jawahri A, Tierney DK, Wiener L, Wood WA, Hoodin F, et al. National Institutes of Health Hematopoietic Cell Transplantation Late Effects Initiative: Consensus Recommendations for Patient-Centered Outcomes. Biol Blood Marrow Transplant. 2016 doi: 10.1016/j.bbmt.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun CL, Francisco L, Baker KS, Weisdorf DJ, Forman SJ, Bhatia S. Adverse psychological outcomes in long-term survivors of hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study (BMTSS) Blood. 2011;118(17):4723–31. doi: 10.1182/blood-2011-04-348730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrykowski MA, Bishop MM, Hahn EA, Cella DF, Beaumont JL, Brady MJ, et al. Long-term health-related quality of life, growth, and spiritual well-being after hematopoietic stem-cell transplantation. Journal of Clinical Oncology. 2005;23(3):599–608. doi: 10.1200/JCO.2005.03.189. [DOI] [PubMed] [Google Scholar]
- 10.Mosher CE, DuHamel KN, Rini C, Corner G, Lam J, Redd WH. Quality of life concerns and depression among hematopoietic stem cell transplant survivors. Supportive Care in Cancer. 2011;19(9):1357–65. doi: 10.1007/s00520-010-0958-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khera N, Storer B, Flowers ME, Carpenter PA, Inamoto Y, Sandmaier BM, et al. Nonmalignant late effects and compromised functional status in survivors of hematopoietic cell transplantation. Journal of Clinical Oncology. 2012;30(1):71–7. doi: 10.1200/JCO.2011.38.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jim HS, Sutton SK, Jacobsen PB, Martin PJ, Flowers ME, Lee SJ. Risk factors for depression and fatigue among survivors of hematopoietic cell transplantation. Cancer. 2016;122(8):1290–7. doi: 10.1002/cncr.29877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rusiewicz A, DuHamel KN, Burkhalter J, Ostroff J, Winkel G, Scigliano E, et al. Psychological distress in long-term survivors of hematopoietic stem cell transplantation. Psychooncology. 2008;17(4):329–37. doi: 10.1002/pon.1221. [DOI] [PubMed] [Google Scholar]
- 14.Hefner J, Kapp M, Drebinger K, Dannenmann A, Einsele H, Grigoleit GU, et al. High prevalence of distress in patients after allogeneic hematopoietic SCT: fear of progression is associated with a younger age. Bone Marrow Transplant. 2014;49(4):581–4. doi: 10.1038/bmt.2013.228. [DOI] [PubMed] [Google Scholar]
- 15.DuHamel KN, Mosher CE, Winkel G, Labay LE, Rini C, Meschian YM, et al. Randomized clinical trial of telephone-administered cognitive-behavioral therapy to reduce post-traumatic stress disorder and distress symptoms after hematopoietic stem-cell transplantation. Journal of Clinical Oncology. 2010;28(23):3754–61. doi: 10.1200/JCO.2009.26.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grossman P, Zwahlen D, Halter JP, Passweg JR, Steiner C, Kiss A. A mindfulness-based program for improving quality of life among hematopoietic stem cell transplantation survivors: feasibility and preliminary findings. Support Care Cancer. 2015;23(4):1105–12. doi: 10.1007/s00520-014-2452-4. [DOI] [PubMed] [Google Scholar]
- 17.Blanch-Hartigan D, Blake KD, Viswanath K. Cancer survivors' use of numerous information sources for cancer-related information: does more matter? Journal of cancer education : the official journal of the American Association for Cancer Education. 2014;29(3):488–96. doi: 10.1007/s13187-014-0642-x. [DOI] [PubMed] [Google Scholar]
- 18.Finney Rutten LJ, Agunwamba AA, Wilson P, Chawla N, Vieux S, Blanch-Hartigan D, et al. Cancer-Related Information Seeking Among Cancer Survivors: Trends Over a Decade (2003-2013) Journal of cancer education : the official journal of the American Association for Cancer Education. 2016;31(2):348–57. doi: 10.1007/s13187-015-0802-7. [DOI] [PubMed] [Google Scholar]
- 19.Shea-Budgell MA, Kostaras X, Myhill KP, Hagen NA. Information needs and sources of information for patients during cancer follow-up. Curr Oncol. 2014;21(4):165–73. doi: 10.3747/co.21.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jansen F, van Uden-Kraan CF, van Zwieten V, Witte BI, Verdonck-de Leeuw IM. Cancer survivors' perceived need for supportive care and their attitude towards self-management and eHealth. Support Care Cancer. 2015;23(6):1679–88. doi: 10.1007/s00520-014-2514-7. [DOI] [PubMed] [Google Scholar]
- 21.Stanton AL, Thompson EH, Crespi CM, Link JS, Waisman JR. Project connect online: randomized trial of an internet-based program to chronicle the cancer experience and facilitate communication. Journal of Clinical Oncology. 2013;31(27):3411–7. doi: 10.1200/JCO.2012.46.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cleary EH, Stanton AL. Mediators of an Internet-based psychosocial intervention for women with breast cancer. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2015;34(5):477–85. doi: 10.1037/hea0000170. [DOI] [PubMed] [Google Scholar]
- 23.Steel JL, Geller DA, Kim KH, Butterfield LH, Spring M, Grady J, et al. Web-based collaborative care intervention to manage cancer-related symptoms in the palliative care setting. Cancer. 2016;122(8):1270–82. doi: 10.1002/cncr.29906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willems RA, Bolman CA, Mesters I, Kanera IM, Beaulen AA, Lechner L. Short-term effectiveness of a web-based tailored intervention for cancer survivors on quality of life, anxiety, depression, and fatigue: randomized controlled trial. Psychooncology. 2016 doi: 10.1002/pon.4113. [DOI] [PubMed] [Google Scholar]
- 25.van den Berg SW, Gielissen MF, Custers JA, van der Graaf WT, Ottevanger PB, Prins JB. BREATH: Web-Based Self-Management for Psychological Adjustment After Primary Breast Cancer--Results of a Multicenter Randomized Controlled Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(25):2763–71. doi: 10.1200/JCO.2013.54.9386. [DOI] [PubMed] [Google Scholar]
- 26.Schover LR, Yuan Y, Fellman BM, Odensky E, Lewis PE, Martinetti P. Efficacy trial of an Internet-based intervention for cancer-related female sexual dysfunction. Journal of the National Comprehensive Cancer Network. 2013;11(11):1389–97. doi: 10.6004/jnccn.2013.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wootten AC, Meyer D, Abbott JM, Chisholm K, Austin DW, Klein B, et al. An Online Psychological Intervention Can Improve The Sexual Satisfaction Of Men Following Treatment For Localised Prostate Cancer: Outcomes Of An Rct Evaluating My Road Ahead. Psychooncology. 2016 doi: 10.1002/pon.4244. [DOI] [PubMed] [Google Scholar]
- 28.Otto AK, Szczesny EC, Soriano EC, Laurenceau JP, Siegel SD. Effects of a randomized gratitude intervention on death-related fear of recurrence in breast cancer survivors. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2016;35(12):1320–8. doi: 10.1037/hea0000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruggeman-Everts FZ, Wolvers MDJ, van de Schoot R, Vollenbroek-Hutten MMR, Van der Lee ML. Effectiveness of Two Web-Based Interventions for Chronic Cancer-Related Fatigue Compared to an Active Control Condition: Results of the “Fitter na kanker” Randomized Controlled Trial. J Med Internet Res. 2017;19(10):e336. doi: 10.2196/jmir.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ritterband LM, Bailey ET, Thorndike FP, Lord HR, Farrell-Carnahan L, Baum LD. Initial evaluation of an Internet intervention to improve the sleep of cancer survivors with insomnia. Psycho-Oncology. 2012;21(7):695–705. doi: 10.1002/pon.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz AL, Biddle-Newberry M, de Heer HD. Randomized trial of exercise and an online recovery tool to improve rehabilitation outcomes of cancer survivors. Phys Sportsmed. 2015;43(2):143–9. doi: 10.1080/00913847.2015.1005547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanera IM, Willems RA, Bolman CA, Mesters I, Verboon P, Lechner L. Long-term effects of a web-based cancer aftercare intervention on moderate physical activity and vegetable consumption among early cancer survivors: a randomized controlled trial. Int J Behav Nutr Phys Act. 2017;14(1):19. doi: 10.1186/s12966-017-0474-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Damholdt MF, Mehlsen M, O'Toole MS, Andreasen RK, Pedersen AD, Zachariae R. Web-based cognitive training for breast cancer survivors with cognitive complaints-a randomized controlled trial. Psychooncology. 2016 doi: 10.1002/pon.4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McAlpine H, Joubert L, Martin-Sanchez F, Merolli M, Drummond KJ. A systematic review of types and efficacy of online interventions for cancer patients. Patient Educ Couns. 2015;98(3):283–95. doi: 10.1016/j.pec.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Slev VN, Mistiaen P, Pasman HR, Verdonck-de Leeuw IM, Uden-Kraan CF, Francke AL. Effects of eHealth for patients and informal caregivers confronted with cancer: A meta-review. Int J Med Inform. 2016;87:54–67. doi: 10.1016/j.ijmedinf.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Carpenter KM, Stoner SA, Schmitz K, McGregor BA, Doorenbos AZ. An online stress management workbook for breast cancer. J Behav Med. 2014;37(3):458–68. doi: 10.1007/s10865-012-9481-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanera IM, Bolman CA, Willems RA, Mesters I, Lechner L. Lifestyle-related effects of the web-based Kanker Nazorg Wijzer (Cancer Aftercare Guide) intervention for cancer survivors: a randomized controlled trial. J Cancer Surviv. 2016 doi: 10.1007/s11764-016-0535-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Northouse L, Schafenacker A, Barr KL, Katapodi M, Yoon H, Brittain K, et al. A tailored Web-based psychoeducational intervention for cancer patients and their family caregivers. Cancer Nurs. 2014;37(5):321–30. doi: 10.1097/NCC.0000000000000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zernicke KA, Campbell TS, Speca M, McCabe-Ruff K, Flowers S, Carlson LE. A randomized wait-list controlled trial of feasibility and efficacy of an online mindfulness-based cancer recovery program: the eTherapy for cancer applying mindfulness trial. Psychosom Med. 2014;76(4):257–67. doi: 10.1097/PSY.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 40.Tamminga SJ, van Hezel S, de Boer AG, Frings-Dresen MH. Enhancing the Return to Work of Cancer Survivors: Development and Feasibility of the Nurse-Led eHealth Intervention Cancer@Work. JMIR research protocols. 2016;5(2):e118. doi: 10.2196/resprot.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Cocker K, Charlier C, Van Hoof E, Pauwels E, Lechner L, Bourgois J, et al. Development and usability of a computer-tailored pedometer-based physical activity advice for breast cancer survivors. Eur J Cancer Care (Engl) 2015;24(5):673–82. doi: 10.1111/ecc.12225. [DOI] [PubMed] [Google Scholar]
- 42.Hill-Kayser CE, Jacobs LA, Gabriel P, Palmer SC, Hampshire MK, Vachani C, et al. Feasibility Study of an Electronic Interface Between Internet-Based Survivorship Care Plans and Electronic Medical Records. Journal of oncology practice / American Society of Clinical Oncology. 2016;12(4):e380–7. doi: 10.1200/JOP.2015.006841. [DOI] [PubMed] [Google Scholar]
- 43.Lounsberry JJ, Macrae H, Angen M, Hoeber M, Carlson LE. Feasibility study of a telehealth delivered, psychoeducational support group for allogeneic hematopoietic stem cell transplant patients. Psycho-Oncology. 2010;19(7):777–81. doi: 10.1002/pon.1617. [DOI] [PubMed] [Google Scholar]
- 44.Ramachandra P, Booth S, Pieters T, Vrotsou K, Huppert FA. A brief self-administered psychological intervention to improve well-being in patients with cancer: results from a feasibility study. Psycho-Oncology. 2009;18(12):1323–6. doi: 10.1002/pon.1516. [DOI] [PubMed] [Google Scholar]
- 45.Duffecy J, Sanford S, Wagner L, Begale M, Nawacki E, Mohr DC. Project onward: an innovative e-health intervention for cancer survivors. Psycho-Oncology. 2012;22(4):947–51. doi: 10.1002/pon.3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duman-Lubberding S, van Uden-Kraan CF, Jansen F, Witte BI, van der Velden LA, Lacko M, et al. Feasibility of an eHealth application “OncoKompas” to improve personalized survivorship cancer care. Support Care Cancer. 2016;24(5):2163–71. doi: 10.1007/s00520-015-3004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alberts NM, Hadjistavropoulos HD, Dear BF, Titov N. Internet-delivered cognitive-behaviour therapy for recent cancer survivors: a feasibility trial. Psychooncology. 2015 doi: 10.1002/pon.4032. [DOI] [PubMed] [Google Scholar]
- 48.Forbes CC, Blanchard CM, Mummery WK, Courneya KS. Feasibility and Preliminary Efficacy of an Online Intervention to Increase Physical Activity in Nova Scotian Cancer Survivors: A Randomized Controlled Trial. JMIR Cancer. 2015;1(2):e12. doi: 10.2196/cancer.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Syrjala KL, Abrams JR, Roth-Roemer SR, Dikmen S, Heiman J, Thomas AM. Efficacy of a Randomized Controlled Trial (RCT) to enhance recovery from Hemaopoietic Stem Cell Transplantation (HSCT) Psycho-Oncology. 2004;13(8):397. [Google Scholar]
- 50.Lyons KD, Hull JG, Kaufman PA, Li Z, Seville JL, Ahles TA, et al. Development and initial evaluation of a telephone-delivered, behavioral activation, and problem-solving treatment program to address functional goals of breast cancer survivors. J Psychosoc Oncol. 2015;33(2):199–218. doi: 10.1080/07347332.2014.1002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hegel MT, Lyons KD, Hull JG, Kaufman P, Urquhart L, Li Z, et al. Feasibility study of a randomized controlled trial of a telephone-delivered problem-solving-occupational therapy intervention to reduce participation restrictions in rural breast cancer survivors undergoing chemotherapy. Psychooncology. 2011;20(10):1092–101. doi: 10.1002/pon.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nezu AM, Nezu CM, Perri MG. Problem-Solving Therapy for Depression: Theory, Research, and Clinical Guidelines. New York, NY: Wiley; 1989. [Google Scholar]
- 53.Syrjala KL, Stover AC, Yi JC, Artherholt SB, Romano EM, Schoch G, et al. Development and implementation of an Internet-based survivorship care program for cancer survivors treated with hematopoietic stem cell transplantation. Journal of Cancer Survivorship. 2011;5(3):292–304. doi: 10.1007/s11764-011-0182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Syrjala KL, Yi JC, Langer SL. Psychometric properties of the Cancer and Treatment Distress (CTXD) measure in hematopoietic cell transplantation patients. Psychooncology. 2016;25(5):529–35. doi: 10.1002/pon.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schulz-Kindermann F, Hennings U, Ramm G, Zander AR, Hasenbring M. The role of biomedical and psychosocial factors for the prediction of pain and distress in patients undergoing high-dose therapy and BMT / PBSCT. Bone Marrow Transplantation. 2002;29(4):341–51. doi: 10.1038/sj.bmt.1703385. [DOI] [PubMed] [Google Scholar]
- 56.Jacobsen PB, Le-Rademacher J, Jim H, Syrjala K, Wingard JR, Logan B, et al. Exercise and stress management training prior to hematopoietic cell transplantation: Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0902. Biol Blood Marrow Transplant. 2014;20(10):1530–6. doi: 10.1016/j.bbmt.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Syrjala KL, Sutton SK, Jim HSL, Knight JM, Wood WA, Lee SJ, et al. Cancer and Treatment Distress (CTXD) Psychometric Evaluation over Time: a BMT CTN 0902 Secondary Analysis. Cancer. 2016 doi: 10.1002/cncr.30454. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Derogatis LR. SCL-90-R Administration, Scoring, and Procedures Manual. Third. P.O. Box 1416; Minneapolis, MN: 1994. [Google Scholar]
- 59.Ware JE, Kosinski M, Dewey JE. How to Score Version 2 of the SF-36 Health Survey. Lincoln, RI: QualityMetric Incorporated; 2000. [Google Scholar]
- 60.Hann DM, Jacobsen PB, Azzarello LM, Martin SC, Curran SL, Fields KK, et al. Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Quality of Life Research. 1998;7(4):301–10. doi: 10.1023/a:1024929829627. [DOI] [PubMed] [Google Scholar]
- 61.Hann DM, Denniston MM, Baker F. Measurement of fatigue in cancer patients: further validation of the Fatigue Symptom Inventory. Quality of Life Research. 2000;9(7):847–54. doi: 10.1023/a:1008900413113. [DOI] [PubMed] [Google Scholar]
- 62.Zou G. A modified poisson regression approach to prospective studies with binary data. American Journal of Epidemiology. 2004;159(7):702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 63.Shaw PA, Yancy WS, Jr, Wesby L, Ulrich V, Troxel AB, Huffman D, et al. The design and conduct of Keep It Off: An online randomized trial of financial incentives for weight-loss maintenance. Clinical trials. 2016 doi: 10.1177/1740774516669679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fredericks S, Martorella G, Catallo C. A systematic review of web-based educational interventions. Clinical nursing research. 2015;24(1):91–113. doi: 10.1177/1054773814522829. [DOI] [PubMed] [Google Scholar]
- 65.Kim AR, Park HA. Web-based Self-management Support Interventions for Cancer Survivors: A Systematic Review and Meta-analyses. Studies in health technology and informatics. 2015;216:142–7. [PubMed] [Google Scholar]
- 66.Bantum EO, Albright CL, White KK, Berenberg JL, Layi G, Ritter PL, et al. Surviving and thriving with cancer using a Web-based health behavior change intervention: randomized controlled trial. J Med Internet Res. 2014;16(2):e54. doi: 10.2196/jmir.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kjaer T, Johansen C, Andersen E, Karlsen R, Nielsen AL, Frederiksen K, et al. Do we reach the patients with the most problems? Baseline data from the WebCan study among survivors of head-and-neck cancer, Denmark. J Cancer Surviv. 2016;10(2):251–60. doi: 10.1007/s11764-015-0471-x. [DOI] [PubMed] [Google Scholar]
- 68.Anguera JA, Jordan JT, Castaneda D, Gazzaley A, Arean PA. Conducting a fully mobile and randomised clinical trial for depression: access, engagement and expense. BMJ Innov. 2016;2(1):14–21. doi: 10.1136/bmjinnov-2015-000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kuijpers W, Groen WG, Aaronson NK, van Harten WH. A systematic review of web-based interventions for patient empowerment and physical activity in chronic diseases: relevance for cancer survivors. Journal of Medical Internet Research. 2013;15(2):e37. doi: 10.2196/jmir.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Edwards L, Thomas C, Gregory A, Yardley L, O'Cathain A, Montgomery AA, et al. Are people with chronic diseases interested in using telehealth? A cross-sectional postal survey. J Med Internet Res. 2014;16(5):e123. doi: 10.2196/jmir.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jim HS, Quinn GP, Gwede CK, Cases MG, Barata A, Cessna J, et al. Patient education in allogeneic hematopoietic cell transplant: what patients wish they had known about quality of life. Bone Marrow Transplant. 2014;49(2):299–303. doi: 10.1038/bmt.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Penate W, Fumero A. A meta-review of Internet computer-based psychological treatments for anxiety disorders. Journal of telemedicine and telecare. 2016;22(1):3–11. doi: 10.1177/1357633×15586491. [DOI] [PubMed] [Google Scholar]
- 73.Smith SK, O'Donnell JD, Abernethy AP, MacDermott K, Staley T, Samsa GP. Evaluation of Pillars4life: a virtual coping skills program for cancer survivors. Psychooncology. 2015;24(11):1407–15. doi: 10.1002/pon.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gersh E, Hallford DJ, Rice SM, Kazantzis N, Gersh H, Gersh B, et al. Systematic review and meta-analysis of dropout rates in individual psychotherapy for generalized anxiety disorder. J Anxiety Disord. 2017;52:25–33. doi: 10.1016/j.janxdis.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 75.Barrera TL, Mott JM, Hofstein RF, Teng EJ. A meta-analytic review of exposure in group cognitive behavioral therapy for posttraumatic stress disorder. Clin Psychol Rev. 2013;33(1):24–32. doi: 10.1016/j.cpr.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 76.Chen Z, Koh PW, Ritter PL, Lorig K, Bantum EO, Saria S. Dissecting an online intervention for cancer survivors: four exploratory analyses of internet engagement and its effects on health status and health behaviors. Health Educ Behav. 2015;42(1):32–45. doi: 10.1177/1090198114550822. [DOI] [PubMed] [Google Scholar]
- 77.Gorlick A, Bantum EO, Owen JE. Internet-based interventions for cancer-related distress: exploring the experiences of those whose needs are not met. Psychooncology. 2014;23(4):452–8. doi: 10.1002/pon.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yoon H, Sohn M, Jung M. Media Use and the Cancer Communication Strategies of Cancer Survivors. J Cancer Prev. 2016;21(3):127–34. doi: 10.15430/JCP.2016.21.3.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pugh G, Gravestock HL, Hough RE, King WM, Wardle J, Fisher A. Health Behavior Change Interventions for Teenage and Young Adult Cancer Survivors: A Systematic Review. Journal of adolescent and young adult oncology. 2016;5(2):91–105. doi: 10.1089/jayao.2015.0042. [DOI] [PubMed] [Google Scholar]
- 80.Ben-Zeev D, Schueller SM, Begale M, Duffecy J, Kane JM, Mohr DC. Strategies for mHealth research: lessons from 3 mobile intervention studies. Adm Policy Ment Health. 2015;42(2):157–67. doi: 10.1007/s10488-014-0556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mohr DC, Weingardt KR, Reddy M, Schueller SM. Three Problems With Current Digital Mental Health Research … and Three Things We Can Do About Them. Psychiatr Serv. 2017;68(5):427–9. doi: 10.1176/appi.ps.201600541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peiris D, Miranda JJ, Mohr DC. Going beyond killer apps: building a better mHealth evidence base. BMJ Glob Health. 2018;3(1):e000676. doi: 10.1136/bmjgh-2017-000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mohr DC, Schueller SM, Riley WT, Brown CH, Cuijpers P, Duan N, et al. Trials of Intervention Principles: Evaluation Methods for Evolving Behavioral Intervention Technologies. J Med Internet Res. 2015;17(7):e166. doi: 10.2196/jmir.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Centers for Medicare & Medicaid Services. Ambulance Fee Schedule. [Accessed April 29, 2010]; https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AmbulanceFeeSchedule/index.html?redirect=/AmbulanceFeeSchedule.


