Abstract
BACKGOUND
Alcohol Use Disorders (AUDs) are devastating and poorly treated, and innovative targets are actively sought for prevention and treatment. The orphan G protein-coupled receptor GPR88 is enriched in mesocorticolimbic pathways, and Gpr88 knockout mice show hyperactivity and risk-taking behavior, but a potential role for this receptor in drug abuse has not been examined.
METHODS
We tested Gpr88 knockout mice for alcohol drinking and seeking behaviors. To gain system-level understanding of their alcohol endophenotype, we also analyzed whole-brain functional connectivity (FC) in naïve mice using resting-state functional magnetic resonance imaging.
RESULTS
Gpr88 knockout mice showed increased voluntary alcohol drinking at both moderate and excessive levels, with intact alcohol sedation and metabolism. Mutant mice also showed increased operant responding and motivation for alcohol, while food and chocolate operant self-administration were unchanged. Alcohol place conditioning and alcohol-induced dopamine release in the nucleus accumbens (NAC) were decreased, suggesting reduced alcohol reward in mutant mice that may partly explain enhanced alcohol drinking. Seed-based voxelwise FC analysis revealed significant remodeling of mesocorticolimbic centers, whose hallmark was predominant weakening of prefrontal cortex, ventral tegmental area (VTA) and amygdala (AMY) connectional patterns. Also, effective connectivity from VTA to NAC and AMY was reduced.
CONCLUSION
Gpr88 deletion disrupts executive, reward and emotional networks in a configuration that reduces alcohol reward and promotes alcohol seeking and drinking. The FC signature is reminiscent of alterations observed in individuals at-risk for AUDs. The Gpr88 gene, therefore, may represent a vulnerability/resilience factor for AUDs, and a potential drug target for AUDs treatment.
Keywords: Orphan G protein coupled receptor, GPR88 knockout mice, ethanol voluntary drinking, operant self-administration, resting-state functional magnetic resonance imaging, amygdala, prefrontal cortex, ventral tegmental area
INTRODUCTION
Alcohol use disorders (AUDs) are chronic relapsing disorders, characterized by excessive alcohol drinking and loss of control over consumption, and have dramatic consequences for individuals’ health and productivity, their families and society. Only few treatments are available (1–3), which target glutamatergic, GABAergic, dopaminergic or the opioid system, and efficacy is low and variable, and the search for novel therapeutic strategies is largely open. AUDs are multifactorial conditions involving both population-level (cultural and societal factors) (4) and individual-level (genetics) (5) characteristics, and family studies demonstrate that AUDs are partly heritable with genetics explaining 50–60% of phenotypic variability (6). Accordingly, neuroimaging studies show premorbid differences in brain structure and function for individuals with AUD family history considered at risk for AUDs (7). Overall AUDs show high heterogeneity (8) and psychiatric co-morbidity (9) and innovative biomarkers and drug targets are actively sought to address vulnerability factors and prevention, and to develop effective personalized treatments (8–10). In rodent research, gene knockout approaches have identified a number of genes that causally contribute to alcohol drinking-related behaviors (11, 12). Here we demonstrate that the Gpr88 gene, which encodes an orphan G protein-coupled receptor (13) expressed only in the brain (GPR88, no known native ligand) is a novel target for alcohol research.
At the neurobiological level, alcohol acts as a complex drug that modifies the activity of multiple molecular targets, and triggers broad alterations of gene expression and synaptic plasticity in neural networks responsible for reward, mood and decision-making (12, 14, 15). Remarkably, Gpr88 is essentially expressed in these brain circuits (16). The Gpr88 transcript is most enriched in the striatum of both rodent (17) and human (18) brains, and also in central amygdala (19, 20) and cortex (21) although with lower density. Gpr88 transcript levels are altered upon pharmacological treatment using antidepressants (22) and mood stabilizers (23, 24), as well as chronic exposure to drugs of abuse, including alcohol (25). To our knowledge however, a potential role of this receptor in drug consumption, seeking and dependence has not been examined.
Functional studies of GPR88 have used genetic approaches, as GPR88 drugs (26, 27) with effective in vivo activity are lacking. Gpr88 gene knockout in the mouse leads to a range of phenotypes consistent with the strong striatal GPR88 expression. In brief, these include altered dopamine signaling and enhanced medium spiny neuron excitability, increased basal activity and locomotor responses to psychostimulants, increased stereotypies and motor coordination deficits, as well as altered cue-based and procedural learning (28–30). Sensorimotor gating (28) and sensory processing (18) deficits are also observed in Gpr88 knockout mice, possibly related to cortical GPR88 expression. Finally, these mutant mice display reduced anxiety-related responses together with increased approach behaviors, leading to a risk-taking phenotype (30), perseverative (30) and compulsive-like behavior (our unpublished data). In sum, the Gpr88 expression pattern overlapping brain networks of addiction, and the phenotypic traits of Gpr88 knockout mice involving dysfunctional motivation, mood regulation and higher-order integration, prompted us to hypothesize that GPR88 may contribute to alcohol drinking behaviors.
In this report, we show that deletion of the Gpr88 gene leads to enhanced alcohol drinking and seeking behaviors. Tackling mechanisms underlying this behavior, we next show lower alcohol-induced conditioned place preference associated to reduced augmentation of extracellular dopamine levels by alcohol in the nucleus accumbens (NAC), suggesting that alcohol reward is decreased in mutant mice. Extending our study to the broader circuits of addiction, using resting-state functional Magnetic Resonance Imaging (rs-fMRI) in live animals, we finally demonstrate altered functional connectivity within the mesocorticolimbic circuitry of live knockout mice, in a pattern reminiscent to network alterations observed in individuals at risk for AUDs. Together, our data identify a circuit mechanism subserving GPR88-regulated alcohol drinking and strongly suggest that deficient GPR88 signaling is a risk factor for AUDs.
MATERIAL AND METHODS
Mice
Male Gpr88−/− mice were produced as previously described (30) (more details in Supplementary Information)
Drugs and treatments
Behavioral procedures
Two-bottle choice – continuous and intermittent access was performed as reported previously (31, 32) and measures of sucrose, quinine and saccharin consumption are described in Supplementary Information. Blood alcohol concentrations (BACs) were measured as described in (33) and details are in Supplementary Information. Loss of Righting Reflex (LORR), operant behavior to obtain alcohol, chocolate pellets and food as well as conditioned place preference are described in Supplementary Information.
In vivo microdialysis
Microdialysis was performed as described previously (34).
Resting-state functional Magnetic Resonance Imaging (Rs-fMRI)
MRI data acquisition and Resting-state functional MRI analysis were conducted as described in (35, 36) and effective connectivity (FC) analysis as in (37). Detailed methods for both the mouse experiment and human data analysis are provided in Supplementary Methods.
RESULTS
Deletion of Gpr88 increases voluntary alcohol consumption
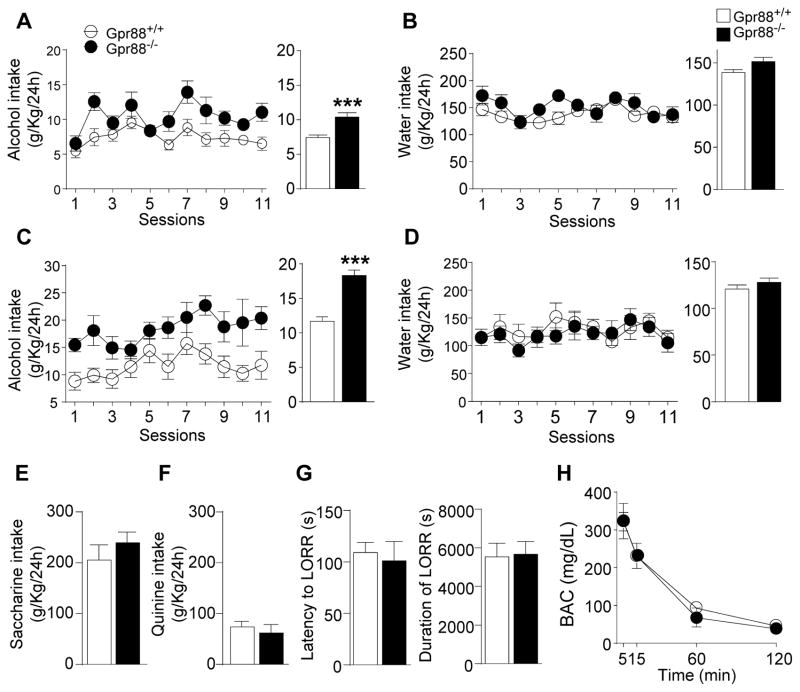
We first measured the level of moderate voluntary alcohol intake in Gpr88 knockout (Gpr88−/−) mice and their wild-type littermates (Gpr88+/+) using a 10 % alcohol continuous access two-bottle-choice drinking paradigm in the home cage. Deletion of the Gpr88 gene increased daily alcohol consumption compared to Gpr88+/+ mice (Figure 1A, left panel, see statistics in Supplementary Table S1). The mean daily alcohol intake during the entire experiment was also significantly higher in mutant mice (Figure 1A right panel, 39.9 % p < 0.001). Water intake was comparable in both groups (Figure 1B).
Figure 1.
Gpr88 knockout mice show increased alcohol drinking, with no change of taste palatability, alcohol sedation and metabolism. (A–D) In the 2-bottle-choice drinking paradigm, Gpr88 knockout mice consume more alcohol than controls, while water intake is unchanged. Mice were first offered continuous access to (A) 20% alcohol (v/v) and (B) water in their home cages for 11 consecutive days (11 sessions). Next, mice underwent intermittent access procedure to (C) 20% alcohol or (D) water for one month (12 sessions). Left, curves represent the mean (±SEM) alcohol or water intake per session; right, histograms show mean (±SEM) daily alcohol or water consumption during the entire experiment. (E, F) In the two-bottle choice procedure, no difference is detected between mutant and control mice for the consumption of (E) sweet (saccharin) or (F) bitter (quinine) solutions. (G) Both latency and duration of the loss-of-righting reflex (LORR) are identical for mutant mice and their controls upon alcohol injection ((3.2 g/Kg, 20% v/v solution, i.p.), as are (H) Blood alcohol concentration (BAC) levels. A–D, n = 11–17; E–G, n = 7–11; H, n = 3 for each group. *p < 0.05; **p < 0.01; ***p < 0.001 compared with the control group, statistical analysis is shown in Supplementary Table S1.
Next, we used a 20 % alcohol intermittent two-bottles-choice drinking procedure to test whether the Gpr88 gene deletion also alters excessive alcohol intake, a hallmark of AUDs. This procedure led to enhance the mean daily alcohol intake in both GPR88−/− (76.3%) and GPR88+/+ (57 %) compared to a moderate dose in the procedure and this increase was more pronounced in Gpr88−/− mice. For Figure 1C left panel, see statistics in Supplementary Table S1. Over the entire experiment, the mean daily alcohol intake was also significantly higher in Gpr88−/− mice compared to Gpr88+/+ controls (Figure 1C right panel, 63.3 % p < 0.001). Finally, similar to our finding for moderate drinking, no difference in water consumption was found across genotypes (Figure 1D). These results together demonstrate that the Gpr88 gene deletion increases both moderate and excessive voluntary alcohol drinking. Heterozygous Gpr88 mice (Gpr88+/−) were also evaluated in the 20 % alcohol intermittent two-bottles-choice drinking procedure. We found increased alcohol consumption similar to total Gpr88−/− mice (Supplementary Figure S1A–B) indicating that a partial deletion of Gpr88 is sufficient to alter the alcohol-drinking behavior.
Finally, we found that sucrose intake (Supplementary Figure S1C), taste palatability (Figure 1E–F), sedative alcohol effects (Figure 1G), alcohol metabolism (Figure 1H) and body weights (Supplementary Figure S1D) are comparable in mutant and control groups (details in Supplementary Information), suggesting that higher alcohol consumption in Gpr88 knockout mice is mostly due to enhanced appetitive properties of alcohol (38).
Deletion of Gpr88 increases alcohol operant seeking and motivation
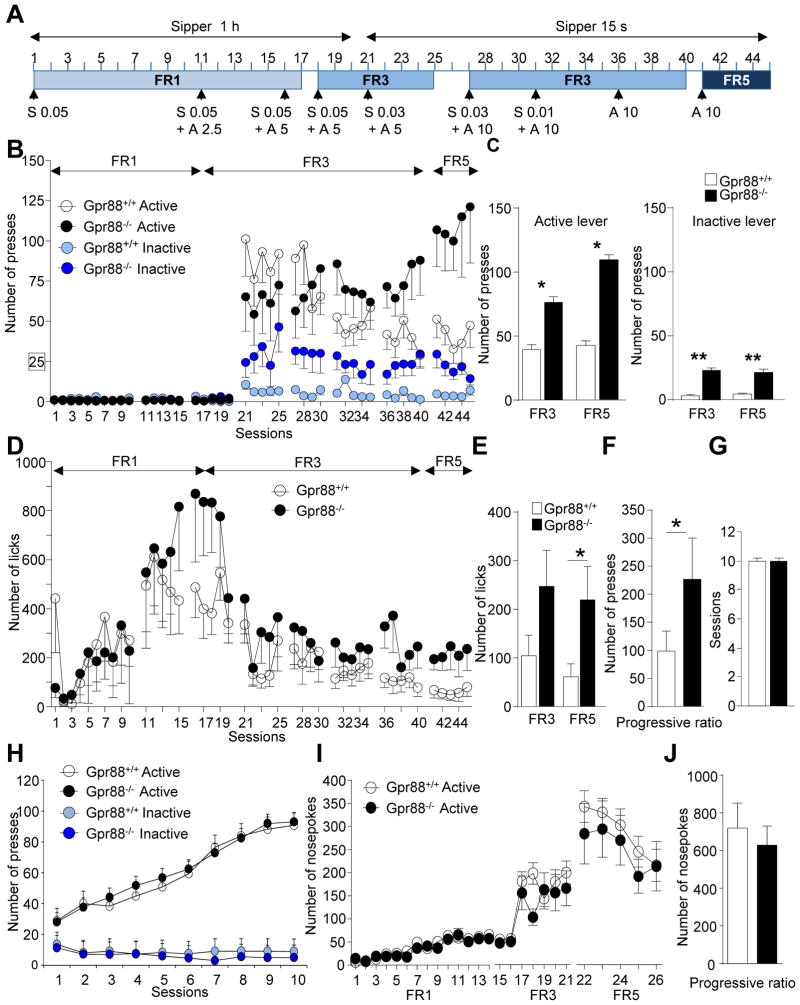
To test this, we examined both drug seeking (lever press) and taking (licks) using operant alcohol self-administration (SA; see Figure 2A and Supplementary Table S2 for statistical analysis). Gpr88−/− mice and their controls were first subjected to a saccharine fading procedure. When alcohol concentration reached 10%, saccharine was omitted and alcohol SA was examined under FR3 (sessions 36–40) and FR5 (sessions 41–46) schedules of reinforcement. During these sessions, Gpr88−/− mice made a significantly higher number of lever presses for alcohol on the active lever compared to Gpr88+/+ controls in both FR schedules (Figure 2C left panel, FR3: t(18) = 2.0, p = 0.05; FR5: t(18) = 2.4, p < 0.05). In accordance to previous findings of hyperactivity (28–30), Gpr88−/− mice also showed increased activity on the inactive lever in both FR schedules (Figure 2C right panel, FR3: t(18) = 3.6, p < 0.01; FR5: t(18) = 3.2, p < 0.01), although level of responding remained substantially lower than for the active level. Importantly also, when tested for self-administration of 10% alcohol (sessions 36–46) mutant mice showed increased number of licks compared to control counterparts in both FR schedules (Figure 2E, FR3: t(18) = 1.7, p = 0.1; FR5: t(18) = 2.2, p < 0.05), leading to more alcohol consumption and indicating that both seeking and taking were increased.
Figure 2.
Gpr88 knockout mice show increased alcohol-seeking and alcohol-taking behavior, with no change in food and chocolate self-administration. (A) Experimental timeline and history of reinforcement schedules for the acquisition and maintenance of alcohol self-administration. Alcohol was self-administered using a saccharin fading procedure on a Fixed Ratio 1 (FR1) schedule of reinforcement for sessions 1–17, then FR3 for sessions 18–41 and FR5 for days 41–45. Over sessions alcohol concentration (A, v/v) was gradually increased to reach 10% while saccharin concentration (S, w/v) was progressively eliminated. Over successive training sessions the sipper access time was reduced from 60 min to 15-sec to encourage the mice to elevate the frequency of responding. (B) Overall, the number of lever presses during 60 min sessions is higher in mutant mice compared to controls for both active and inactive levers. Also, (C) average daily active (left) and inactive (right) lever presses for 10% alcohol (sessions 36–45) are higher for Gpr88 knockout mice under both FR3 (sessions 36–40) and FR5 (sessions 41–45) schedules. (D) Number of licks/session for the whole experiment and (E) average daily licks for 10% alcohol under FR3 and FR5 schedules were also higher in mutant mice. (F) The number of active lever responses under the progressive ratio schedule of reinforcement is shown, indicating a higher breaking point for mutant animals. (G–H) Operant food self-administration does not differ between Gpr88−/− mice and their controls (see Supplementary information for method). The number of (G) sessions required for the mice to reach the criteria and (H) lever presses for food pellets across the 10 sessions of a FR1 procedure are identical across genotypes. (I–J) Operant self-administration of chocolate flavor pellets does not differ between Gpr88−/− mice and their controls. (I) Animals were trained under FR1 (1–14 sessions), FR3 (15–21 sessions) and FR5 (22–26 sessions) schedules of reinforcement in 20 minute daily sessions during 26 sessions. (J) The number of nosepoke responses under the progressive ratio schedule of reinforcement is shown, indicating a similar breaking point for both genotypes. Data are mean ± SEM. *p < 0.05; **p < 0.01; compared with control group. A–H, n = 10, I–J, n = 10–11 for each group. For B and D, statistical analysis is shown in Supplementary Table S2.
The observation of both higher lever-pressing and licking in mutant mice led us to hypothesize a stronger motivation for alcohol drinking. To test this, we conducted a progressive ratio (PR) schedule of reinforcement session. Gpr88−/− mice showed a significantly higher breaking point compared to controls (Figure 2F, t(18) = 1.7, p < 0.05), demonstrating that motivation to obtain the alcohol reward is enhanced in mutant mice.
Next, we examined whether the alcohol phenotype is substance-specific. Gpr88−/− mice were measured for operant responding for food under FR1 schedule and for highly palatable chocolate-flavored pellets under FR1, FR3 and FR5 schedules. The criteria for acquisition of food operant responding were reached upon the same number of sessions in both genotypes (Figure 2G). In addition, knockout animals acquired and maintained operant responding for food (Figure 2H) and chocolate pellets (Figure 2I and Supplementary Figure S2) similarly to control animals. Motivation for natural rewards as measured in PR training was also preserved in Gpr88−/− mice (Figure 2J). These data suggest that the general motivational state of mutant animals remains unchanged.
In conclusion, operant SA experiments reveal that deletion of the Gpr88 gene increases incentive properties of alcohol, and that this phenotype is not generalizable to all appetitive substances.
Deletion of Gpr88 decreases alcohol-induced reward
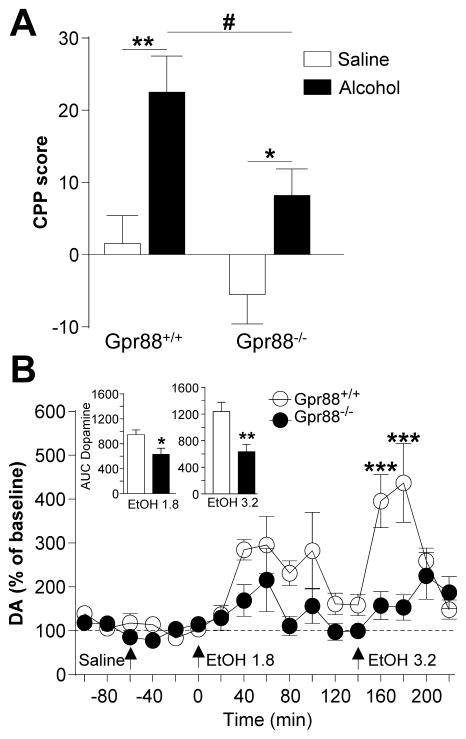
To understand mechanisms underlying increased alcohol seeking and drinking, we examined whether rewarding properties of alcohol are altered in mutant mice using alcohol-induced conditioned place preference (CPP) (39) (Figure 3A). No side preference was observed for any genotype during conditioning (data not shown). After conditioning, CPP scores differed between mutant and control animals. Two-way ANOVA revealed a significant effect of genotype (F(1,46) = 6.8, p < 0.05) and treatment (F(1,46) = 17.7, p < 0.001) and no significant interaction (F(1,46) = 0.76, p = 0.39). Despite the lack of interaction in the more stringent analysis, planned comparison analysis showed that both Gpr88+/+ (t(21) = 3.7, p < 0.01) and Gpr88−/− mice (t(25) = 2.5, p < 0.05) showed a significant alcohol-induced CPP and that mutant mice spent less time in the alcohol-paired compartment than wild-type littermates (t(23) = 2.4, p <0.05). These data indicate that mutant mice show reduced development and/or expression of alcohol CPP, an indicator of reduced alcohol reward.
Figure 3.
Gpr88 knockout mice show lower place conditioning to alcohol and reduced alcohol-induced dopamine (DA) release in the NAC. (A) Alcohol conditioned place preference (CPP) was induced by alternating alcohol (1.8 g/kg i. p.) or saline administration in the drug- or non-drug-paired compartment for 5 min (daily sessions, 8 days total). CPP scores are expressed as the percent time spent in alcohol- or saline- paired compartment during post- minus pre-conditioning session, and show the expression of alcohol CPP for the two genotypes with a significantly lower score for mutant mice. (B) Extracellular DA and DOPAC levels were determined by in vivo microdialysis and HPLC. After the collection of basal fractions, saline and both alcohol concentrations (1.8 and 3.2 g/kg) were administrated at times 0, 60 and 140 min, respectively. Alcohol-induced changes in DA were normalized to the percent change over baseline, and insets show the area under the curve of cumulative dialysate DA or DOPAC levels from the four samples following alcohol injection (EtOH 1.8: 0–80 min; EtOH 3.2: 140–200 min). Data are mean ± SEM, (A) n=11–14, (B) n=11 for each group. *p < 0.05, **p < 0.01 and ***p < 0.001 compared with control group. #p < 0.05 Alcohol Gpr88+/+ vs Alcohol Gpr88−/− group.
Drug reward is typically associated with drug-induced dopamine (DA) release in the NAC. We therefore tested consequences of the Gpr88 gene deletion on basal and alcohol-enhanced extracellular levels of NAC DA (Figure 3B) in response to two alcohol doses (1.8 and 3.2 g/kg). The mean baseline dialysate DA concentration was not significantly different between the groups (Gpr88+/+ 0.098±0.014 nM, Gpr88−/− 0.102±0.022 nM) and systemic injection of saline did not alter DA for any group (Gpr88+/+ 0.081±0.008 nM; Gpr88−/− 0.085±0.007 nM). Alcohol administration increased extracellular DA in both Gpr88−/− and Gpr88+/+ mice. Notably however, Gpr88−/− mice exhibited significantly lower DA-elevating response to the high alcohol dose, and areas under the curve for cumulative dialysates following alcohol injection (inserts for Figure 3B) showed lower increase of DA levels in mutant mice for the two alcohol doses (see complete statistical analysis in Supplemental Table 3).
Together, the significant reduction of both alcohol place conditioning and NAC DA response to alcohol strongly suggests that alcohol reward is diminished in mutant mice, a mechanism that could contribute partly to their enhanced alcohol consumption.
Deletion of Gpr88 weakens functional connectivity of the mesocorticolimbic circuitry
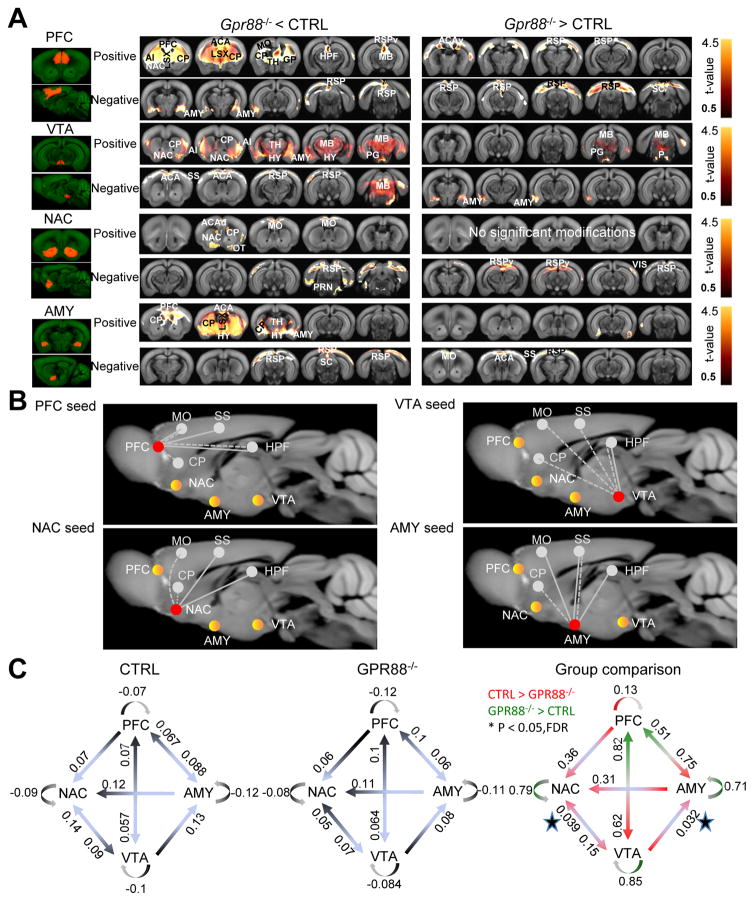
In addition to their alcohol phenotype, Gpr88 knockout mice show a number of other behavioral deficits (28–30). Most likely therefore, reduced alcohol reward is not the only mechanism underlying high alcohol seeking and taking in these animals. To gain a broader circuit-level view of brain function deficits, which may lead to the high alcohol-drinking phenotype, we compared the functional connectivity (FC) patterns of key mesocorticolimbic players in live mutant mice and their controls (Figure 4).
Figure 4.
Gpr88 knockout mice show weakened functional connectivity (FC) for four key centers of the mesocorticolimbic circuitry. (A) Quantification of brain-wide FC alterations for PFC, VTA, NAC, and AMY seeds. The figure shows group statistical significance of FC differences between mutant (Gpr88−/−) and control (CTRL) mice using voxel-level general linear model corrected for multiple comparison (GLM, p < 0.001) from group data shown in Supplementary Figure S3. Positively correlated and anti-correlated voxels, with annotated brain regions, are shown for each seed, and group differences are found with higher FC for either CTRL (left panels) or mutant (right panels). The color scale indicates the corresponding t-values. (B) Schematic representation of major significant FC modifications for each of the four selected seeds (PFC, VTA, NAC and AMY) with the other regions of the mesocoticolimbic circuit, and also including CP, HPF, MO and SS related to previously described behavioral and cognitive characteristics of mutant mice. The seed used for the voxelwise analysis is shown in red, the three other mesocorticolimbic regions are shown in yellow, and CP, HPF, MO and SS are in grey. Dashed and solid lines represent weakened (Gpr88−/− < CTRL) or strengthened (Gpr88−/− > CTRL) FC, respectively. White lines represent FC with mesocorticolimbic regions, and grey lines show FC with CP, HPF, MO and SS. Hallmarks of the Gpr88 mesocorticolimbic signature are weaker PFC-AMY, VTA-NAC and altered VTA-AMY FC. (C) Effective connectivity analysis using spectral dynamic causal modeling shows significant modification of information flow from VTA to NAC and VTA to AMY in mutant mice. Optimal causal models are shown for CTRL (left) and Gpr88−/− (middle) mice. Numbers represent mean strengths of directional information transfer using Bayesian parameter averaging (BPA) following t-test (p<0.001, FDR correction). Group comparison is shown in the right panel, with numbers indicating p values. Stars show the significant group difference directions by paired t-test at p<0.05. n=14 for each group.
Abbreviations: ACA: Anterior cingulate area, ACAd: Anterior cingulate area – dorsal part, ACAv: Anterior cingulate area – ventral part, AUD: Auditory area, aco: Anterior commissure, AI: Agranular insular Area, AMY: Amygdala, CP: Caudate putamen, ECT: Ectorhinal cortex, ENT: Entorhinal area, GP, globus Pallidus HPF: Hippocampal formation, HY: Hypothalamus, LSX: Lateral septal complex, MB: Midbrain, MO: Motor area, NAC: Nucleus accumbens, OFC: Orbito-frontal cortex, OT: Olfactory tubercle, P: Pons, PG: Pontine gray, PFC: Prefrontal cortex, PRN: Pontine reticular nucleus, PTLp: Posterior parietal association area, RSP: Retrosplenial area, RSPv: Retrosplenial area – ventral part, SC: Superior colliculus, SS: Somatosensory area, TEa: Temporal association area, TH: Thalamus, TRN: Tegmental reticular nucleus, VIS: Visual area, VTA: Ventral tegmental area.
Resting-state (Rs) functional resonance magnetic imaging (fMRI) is based on the statistical analysis of low-frequency fluctuations in blood-oxygenation-level-dependent signals at rest (40), and is now highly used in human research emotional responses. We have adapted RsfMRI to mice (41), and our initial data-driven analysis of mice lacking the mu opioid receptor gene revealed major reshaping of reward/aversion networks (35), consistent with the known role of this receptor in pain and drug abuse. Very recently, we also used Rs-fMRI to compare whole brain FC of Gpr88−/− and Gpr88+/+ mice (36) and here, we further analyzed the Rs-fMRI datasets using seed-based analysis with a focus on key mesocorticolimbic centers(16), and mapped their connectivity patterns across the whole brain.
We selected four seeds most relevant to the high alcohol seeking and drinking behavior of mutant mice. These include the prefrontal cortex (PFC) as a key center for executive functions, the ventral tegmental area (VTA) and NAC considered the core of reward circuitry, and the amygdala (AMY) central to mood. RsFC data from Gpr88−/− and Gpr88+/+ mice were acquired and preprocessed as described previously (35) (and see methods). Bilateral seeds were anatomically defined by co-registration with the Allen Mouse Brain Atlas and voxelwise seed-based correlation analysis was conducted for each seed. The correlation maps revealed well-detectable cortical and sub-cortical rsFC modifications in the Gpr88−/− group for PFC, VTA and AMY while group differences for the NAC seed were less obvious (Supplementary Figure S3).
We further quantified the statistical significance of connectivity alterations in Gpr88−/− mice using voxel-level general linear model corrected for multiple comparisons (GLM, p < 0.001), and significant group differences are mapped in Figure 4A for both positive (from 0 to +1) and negative (from −1 to 0) correlations. For all the seeds (PFC, NAC, VTA and AMY), voxelwise FC connectivity was predominantly weakened (Gpr88−/− < CTRL) in mutant mice, with only rare and mainly cortical strengthened correlated targets (Gpr88−/− > CTRL). See Supplementary Results for a detailed description.
Network diagrams in Figure 4B summarize significant voxelwide modifications observed for each of the four PFC, NAC, VTA and AMY seeds, with a focus on their correlated activity with selected brain areas. These include (i) the four seeds, (ii) Somatosensory area (SS), which is related to sensorimotor gating deficiency reported in mutant animals (18), (iii) Motor area (MO), which is pivotal for sensorimotor integration and the control of voluntary movements (42) and likely contribute to the hyperactivity phenotype of Gpr88−/− mice (29, 30, 36, 43) and (iv) hippocampal formation (HPF) and CP both relevant to a specific learning deficit that we previously reported in Gpr88−/− animals (29, 30, 43). Together, this analysis reveals a dysfunctional RsFC pattern within the mesocorticolimbic network.
FC analysis provides information on whole-brain connectional patterns for selected seeds, without addressing how these brain regions may influence each other. Effective connectivity (EF) further evaluates the causal influence of one region of interest on other regions within a predefined small network (44). To gain further insight into abnormal patterns of distributed activity in the mesocorticolimbic circuitry of mutant mice, we measured statistic dependencies within and among regional dynamics of the four PFC, NAC, VTA and AMY seeds, as described previously (37). Spectral Dynamic Causal Modelling (spDCM) was performed using datasets from Gpr88−/− mice and wild-type littermates. We specified a model (Figure 4C) where connected regions are based on known anatomical projections for the four seeds (45, 46), hence unreported or minor physical projections were deleted (PFC-to-VTA, NAC-to-PFC and NAC-to-AMY AMY-to-VTA). Average EF parameters (t-test, p< 0.001, FDR corrected) of the model are shown for wild-type (CTRL) and mutant (Gpr88−/−) mice (left and middle panels). In general, EF strength values were lower in the Gpr88−/− group for all the selected directions, with the exception of the NAC-to-VTA direction. Group comparison (right panel) showed significantly reduced EF strength in mutant animals for both VTA-to-AMY and VTA-to-NAC connections (paired t-test, p< 0.05).
Therefore, in addition to reducing correlated activities within mesocorticolimbic circuitry (FC analysis), deletion of the Gpr88 gene limits information flow from VTA to NAC and AMY. The latter finding is in line with reduced DA release in the NAC upon alcohol treatment in mutant mice.
DISCUSSION
In this study, we first demonstrate that genetic deletion of Gpr88 in mice increases alcohol seeking and taking behavior. We next show that alcohol reward is reduced in these mice and that further, functional connectivity is weakened throughout reward, executive, and emotional networks that are all involved in substance abuse disorders.
A first conclusion from this study is that activity of the GPR88 receptor, an orphan G protein-coupled receptor encoded by the Gpr88 gene, influences behaviors related to AUDs. Mice lacking Gpr88 exhibited higher levels of voluntary alcohol drinking and higher alcohol intake in operant SA, which together indicate significant alteration of processes that promote approach behaviors to alcohol. These phenotypes could not be attributed to a general modification of appetitive learning or taste sensitivity, as no genotype differences in daily sucrose intake were found. Also both mutant and control mice similarly acquired and maintained stable operant responding for food and chocolate pellets, and showed comparable preference for non-alcohol tastes (saccharine and quinine). In addition, food and chocolate operant responding, as well as sucrose intake were unchanged, indicating that neither hyperactivity nor generalized responding to rewarding stimuli could explain the higher motivation for alcohol in SA experiments. Future studies will determine whether Gpr88 knockout mice also show enhanced preference and/or intake behavior for other drugs of abuse.
The PR breakpoint during alcohol SA, considered a measure of motivation for the reward, was also enhanced in Gpr88−/− mice. Increased motivation for alcohol may be due to higher or lower rewarding effects of alcohol, as SA studies show that higher drug seeking behavior can be associated to either higher or lower drug reward (see (47–50)). Here we find that, parallel to increased motivation for alcohol, mutant mice show reduced alcohol place preference in a conditioning paradigm and, importantly also, reduced DA extracellular levels release in the NAC upon alcohol administration. Because extracellular DA levels in the NAC classically reflect drug reward related to abuse potential (51–53), we propose that alcohol reward is indeed reduced in Gpr88−/− mice. This, in turn, would contribute to augment both voluntary intake and operant responding for alcohol, in order to reach in mutant mice alcohol rewarding effects similar to those achieved by control animals. Paralleling our findings, previous rodent studies showed that reduced drug reward together with reduced drug-induced DA responses are associated to higher motivation for cocaine (48, 49). In humans, reduced DA response to psychostimulant (54), or low response to an alcohol challenge in young humans with a family history of AUDs (55), are both predictive of a higher risk for addiction. The Gpr88 KO mouse phenotype may therefore be interpreted along a similar line (56).
This mechanism, however, is unlikely to be the only cause for higher alcohol seeking and taking in Gpr88 knockout mice, and a second conclusion from this study is that GPR88 is critical in regulating functional activity of a number of brain networks. Rs-fMRI is increasingly used in human research to address how disease conditions and genes influence FC of brain networks (57, 58). RsFC alterations are associated with many brain disorders (see for example (59–63)), including drug abuse (64, 65), and have already provided a host of information and biomarkers for alcohol research (7). In a prior study (36) and further in this study, we have investigated the Gpr88−/− mice phenotype at brain circuitry level using Rs-fMRI neuroimaging adapted to mice (35). Our initial structural and functional analysis provided evidence for altered cortical microstructure, as well as cortical remodeling in live mutant animals (36). In particular, resting-state connectional patterns of sensorimotor cortical areas were significantly altered, consistent with sensory processing and sensorimotor gating deficits, as well as hyperactivity in these mice (18, 28–30). Also, amygdala connectivity with motor and sensory cortices was modified, and we suggested that these alterations may subserve enhanced exploratory and “risk-taking” phenotypes in these animals (30). The same AMY-MO/SS modifications may also contribute to increased alcohol drinking behavior observed in this study.
In this study, we have focused analysis of Rs-fMRI data on mesocorticolimbic networks. The most salient finding is a broad reduction of brain-wide FC for VTA, PFC and AMY seeds, providing circuit-level mechanisms to explain excessive alcohol seeking and taking in mutant animals. First, VTA seed-based connectivity showed decreased correlation/anticorrelation with voxels covering NAC and AMY regions and, further, information flow from VTA to NAC (EF) was significantly reduced in Gpr88−/− mice. These data are consistent with neurochemical analysis showing lower increase of NAC DA levels upon alcohol treatment, and support the notion that reduced alcohol reward in mutant mice promotes increased alcohol drinking behavior. Second, the PFC seed also showed reduced FC with voxels belonging to NAC and AMY seeds, as well as SS, MO, CP and HPF which remarkably correlate with previously reported behavioral deficits of Gpr88 deficient mice (28–30, 43). This finding strongly suggests that top-down controls are disrupted in mutant mice, a hallmark of behavioral modification in addiction research (66). Third, the AMY seed showed strong abnormalities, as correlated voxels were reduced with PFC and CP. Conversely, PFC and VTA seeds showed either decreased or increased FC with voxels belonging to AMY. Also, EF from VTA to AMY was strongly reduced, and together, these multiple modifications of AMY FC are suggestive of altered emotional processing. In sum, the genetic deletion of Gpr88 leads to significant modifications of brain networks contributing to reward processing, executive controls and emotional regulation, and all concur to regulate addiction-related behaviors. Whether GPR88 activity regulates neuronal connectivity and effectiveness of these circuits during development, and/or is an active brain modulator in the adult, remains to be established. The observation of developmental stage-dependent Gpr88 expression (18, 21) certainly includes the former. In the future inducible gene knockout experiments may clarify the respective contributions of developmental and tonic GPR88 activities in shaping addiction-related networks. Alternatively, pharmacology may adequately address this question, should specific and bioavailable agonists/antagonists become available.
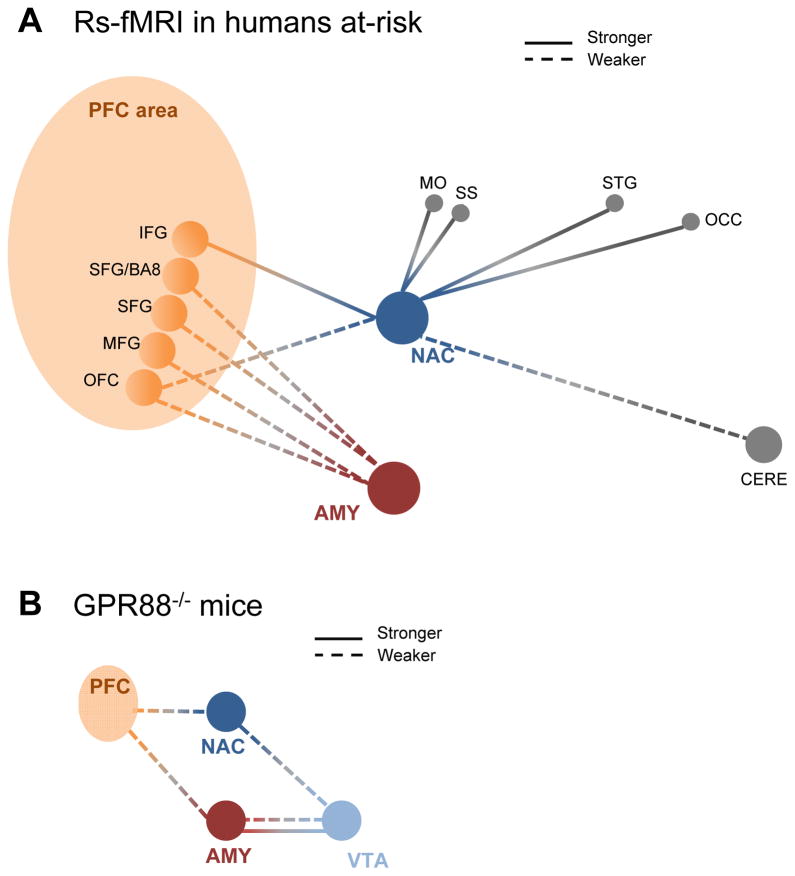
Our behavioral, neurochemical and functional connectivity analyses of Gpr88 knockout mice together suggest that deletion of the Gpr88 gene creates an alcohol vulnerability phenotype in mice. This endophenotype, and in particular the brain-level functional architecture of mutant mice, is reminiscent of dysfunctional connectivity reported in individuals with a family history of AUDs but who are not alcoholics (7, 67), or abstinent individuals with high risk of relapse (68, 69). Review of the human Rs-fMRI literature for alcohol research (see Supplementary Methods) identifies a complex set of brain network abnormalities in at risk individuals, which we summarize in Figure 5A. These include predominant alterations of networks responsible for reward/emotion processing and inhibitory controls, generally considered risk markers for substance abuse in the human neuroimaging literature (70). Although the reductionist mouse model does not, by far, recapitulate the complexity of human brain connectivity, homologous deficits of Rs brain connectivity can be noted for the Gpr88 knockout mouse model and humans at-risk (see Figure 5B). In particular, our study shows altered correlated activity of NAC with cortical areas in Gpr88 knockout animals (weaker with PFC and MO, and stronger with MO). Impaired Rs synchrony between the NAC and executive brain centers was also reported in youths with a family history of alcoholism (71). Notable also is the strong reduction of PFC-AMY synchrony in our study, and findings of poorer amygdala-frontal cortex Rs connectivity in vulnerable individuals (72, 73). In brief, the impaired interplay between reward, emotional and executive functioning in Gpr88 mutant mice also characterizes the premorbid condition of at-risk human subjects. Our study represents a first step towards the establishment of translatable FC signatures, or biomarkers that may also provide mechanistic clues for abnormal alcohol-related behavior.
Figure 5.
Review of the human Rs-fMRI literature identifies a complex set of network abnormalities in individuals at risk for AUDs (71–74), with homology to the Gpr88 knockout mice FC signature. (A) Summary of the human RsfMRI literature. Dashed and solid lines represent weakened and strengthened FC, respectively. PFC (orange), NAC (blue), AMY (red), PPC (grey) and CERE (grey) seeds are shown. Lateralization details: Left (L)-NAC showed increased functional connectivity with L-STG and Right (R)/L-IFG (71). R-NAC showed decreased functional connectivity with R-CERE, L-OFC (71). L-AMY showed decreased FC with L-SFG and L-SFG/BA8, and R-MFG (72). R-AMY showed decreased FC to R-MFG (72). (B) Summary of FC modifications in this study. The Rs FC signature of the Gpr88 gene is adapted from Figure 4. The scheme highlights homology with human findings in (A), namely reduced PFC-NAC and PFC-AMY correlations. The altered connectional pattern for VTA reported in this study was been described in the human literature. Abbreviations: R/L amygdala (AMY), L.SFG-Brodmann area 8 (BA8), R/L. cerebellum (CERE), R/L inferior frontal gyrus (IFG), R. middle frontal gyrus (MFG), motor cortex (MO), R/L nucleus accumbens (NAC), R. occipital cortex (OCC), orbitofrontal cortex (OFC), all part of the prefrontal cortex area (PFC area), L. superior frontal gyrus (SFG), somatosensory (SS) and R/L. superior temporal gyrus (STG).
In conclusion, our study positions the Gpr88 gene as a target for alcohol research. In the future, this gene may be considered a risk factor, though the search for genetic association with alcoholism has not yet started. The combined gene/connectome information may also be useful for diagnostic and prevention. Finally, current drug development efforts will likely provide GPR88 drugs that may hold promise for the treatment of alcohol and drug addiction.
Supplementary Material
Supplementary Figure S1. Heterozygous (Gpr88+/−), and Gpr88−/− mice show increased alcohol drinking, with no change of water and sucrose intake. (A–B) Mice underwent intermittent access 2-bottle-choice drinking paradigm for 8 sessions., Gpr88−/− and Gpr88+/− mice consume more alcohol than controls (Figure S1A Left panel, Two-way ANOVA with repeated measures (RM) showed significant main effects of Genotype (F(2, 32) = 3.7, p < 0.05) and Sessions (F(7, 224) = 7.3, p < 0.001) and no significant interactions between Genotype x Session (F(7, 224) = 0.89, p = 0.56). The mean daily alcohol intake during the entire experiment was also significantly higher in mutant and heterozygote mice (Figure S1A Right panel, one way ANOVA showed a group effect (F(7, 14) = 7.8, p < 0.001). Newman-Keuls post-hoc comparisons showed a strong increase on alcohol intake in Gpr88−/− and Gpr88+/− compared to Gpr88+/+ animals. Water (Figure S1B) intake was comparable in all groups. (C) Mice were tested for sucrose (2%) intake. Sucrose solution and water were offered for 4 consecutive days and the amount of fluid intake was recorded every day. No change on sucrose intake between the three mice genotypes. (D) Gpr88 knockout mice show no alteration of body weights. Gpr88−/− mice showed similar evolution of body weights compared to control animals over the alcohol drinking experiments showed in Figure 1A–D. A–B Left, curves represent the mean (±SEM) alcohol or water intake per session; right, histograms show mean (±SEM) daily alcohol or water or sucrose consumption during the entire experiment. A–C, n=10–13, D n=11–17, ***p < 0.001 compared with control group.
Supplementary Figure S2. Gpr88 knockout mice show no change in Chocolate self-administration. Number of nosepokes in the inactive side in operant self-administration of chocolate flavor pellets does not differ between Gpr88−/− mice and their controls. n = 10–11 for each group.
Supplementary Figure S3. Remodeled resting state FC patterns of PFC, NAC, AMY and VTA in Gpr88−/− mouse brains. BOLD rsfMRI correlation maps for (A) PFC and (B) VTA, (C) NAC and (D) AMY. Corresponding correlation maps of control (left) and Gpr88−/− (right) groups were over-laid on a T2-weighted anatomical brain slices (two-tailed t-test, p < 0.001). The color scale indicates the T- value (positive correlations from 0 to +1: dark red to yellow and negative correlations from 0 to −1: dark blue to turquoise). n=14 for each group.
Supplementary Table S1. Statistical analysis of home cage alcohol intake data.
(A) Analysis for continuous access (Figure 1A Left). Two-way ANOVA with repeated measures (RM) showed significant main effects of Genotype (F(1, 28) = 7.2, p < 0.05) and Sessions (F(10, 280) = 4.1, p < 0.001) and no significant interactions between Genotype x Session (F(10, 280) = 7.2, p = 1.4). Subsequent analyses using the method of contrasts detected a significant difference in alcohol intake during sessions 2, 7 and 11 (p’s<0.05) between Gpr88−/− and Gpr88+/+ mice. (B) Analysis for intermittent access protocol (Figure 1C left). Two-way RM ANOVA showed significant main effects of Genotype (F(1, 24) = 10.0, p < 0.01) and Sessions (F(10, 240) = 2.6, p < 0.01) and no significant Genotype x Session interaction effect (F(10, 240) = 0.6, p = 0.7). Subsequent analyses using the method of contrasts revealed a significant difference between Gpr88−/− and Gpr88+/+ mice during sessions 1, 2, and 11 (p’s<0.05) and a trend towards higher alcohol consumption in Gpr88−/− mice for sessions 6, 8 and 9 (p’s=0.06).
Supplementary Table S2. Statistical analysis of alcohol self-administration data.
Gpr88−/− mice and their controls were first subjected to a saccharine fading procedure, and then had access to 10% alcohol (Figure 2A). In the first phase, there was no difference in the pattern of saccharine intake alone (number of licks) between Gpr88−/− mice (168±84) and their controls (177±72). During mixed saccharine/alcohol exposure, we observed increased lever responding (Figure 2B) and licks (Figure 2D) for all the saccharine/alcohol mixtures in Gpr88−/− mice compared to controls. During this acquisition phase, statistical analysis (see tables below) showed significant lever or licks, session and genotype effects, indicating successful acquisition of alcohol SA for both groups, and also enhanced responding to saccharine/alcohol mixtures for mutant mice. (Table A) Analysis for level pressing (Figure 2B), three-way ANOVA with genotype and lever presses as between-subjects factor and repeated measures in the factor sessions was conducted. (Table B) Analysis for licking (Figure 2D), two-way ANOVA with genotype as between-subjects factor and repeated measures in the factor sessions was conducted.
Supplementary Table S3. Statistical analysis of alcohol-induced dopamine release in the NAC.
Gpr88 gene deletion reduces alcohol-enhanced extracellular levels of NAC DA (Figure 3B). Two-way ANOVA with RM showed an significant effect of Genotype (F(1, 20) = 12.0, p<0.01), significant effect of time (F(16, 320) = 7.6, p<0.001) and significant interaction F(16, 320) = 3.2, p<0.001). Subsequent analyses using Newman-Keuls post hoc analysis detected a significant reduction of DA release in the NAC at 160 and 180 min in Gpr88−/− mice compared to control animals. Areas under the curve for the four cumulative dialysates following alcohol injection (inserts for Figure 3B) also showed lower increase of DA levels in mutant mice for the two alcohol doses (EtOH 1.8: t(20) = 2.6, p < 0.05; EtOH 3.2: t(20) = 3.4, p < 0.01).
Acknowledgments
We thank the staff at the animal facility of the Neurophenotyping Center Douglas Research Center (Montréal, Canada), as well as the Institut de Génétique et de Biologie Moléculaire et Cellulaire and the Mouse Clinic Institute (Illkirch, France) for technical support and housing of the animals. We are grateful to Audrey Matifas, Elise Le Marchand, Thomas Favier, Gilles Duval and Dzemailj Memedov, Aude Villemain, Eujin Kim, Annie Salesse, Karine Lachapelle, Aimee Lee Luco for animal care and genotyping. This work was supported by National Institute of Health (National Institute of Drug Abuse Grant No. 05010 to BLK and National Institute on Alcohol Abuse and Alcoholism, Grant No. 16658 to BLK), the Canada Fund for Innovation and the Canada Research Chairs to BLK. This work was also supported by the Spanish Ministerio de Economía y Competitividad-MINECO (#SAF2014-59648-P/FEDER), Instituto de Salud Carlos III, RETICS-RTA (#RD12/0028/0023/FEDER) and Ministerio de Sanidad, Servicios Sociales e Igualdad, Plan Nacional sobre Drogas (#PNSD-2013-068), and the Generalitat de Catalunya, AGAUR (#2014-SGR-1547) and ICREA (2015-ICREA Acadèmia Award), to R.M. S. M-N was supported by the Brazilian government's CAAP scholarship (Programa Ciência Sem Froteiras). This project was finally funded with support from the NeuroTime Erasmus+: Erasmus Mundus program of the European Commission. This publication/communication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.
Footnotes
CONFLICT OF INTEREST
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108:275–293. doi: 10.1111/j.1360-0443.2012.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson BA. Medication treatment of different types of alcoholism. Am J Psychiatry. 2010;167:630–639. doi: 10.1176/appi.ajp.2010.08101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muller CA, Geisel O, Pelz P, Higl V, Kruger J, Stickel A, et al. High-dose baclofen for the treatment of alcohol dependence (BACLAD study): a randomized, placebo-controlled trial. Eur Neuropsychopharmacol. 2015;25:1167–1177. doi: 10.1016/j.euroneuro.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Castro FG, Barrera M, Jr, Mena LA, Aguirre KM. Culture and alcohol use: historical and sociocultural themes from 75 years of alcohol research. J Stud Alcohol Drugs Suppl. 2014;75(Suppl 17):36–49. doi: 10.15288/jsads.2014.s17.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buscemi L, Turchi C. An overview of the genetic susceptibility to alcoholism. Med Sci Law. 2011;51(Suppl 1):S2–6. doi: 10.1258/msl.2010.010054. [DOI] [PubMed] [Google Scholar]
- 6.Reilly MT, Noronha A, Goldman D, Koob GF. Genetic studies of alcohol dependence in the context of the addiction cycle. Neuropharmacology. 2017 doi: 10.1016/j.neuropharm.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cservenka A. Neurobiological phenotypes associated with a family history of alcoholism. Drug Alcohol Depend. 2016;158:8–21. doi: 10.1016/j.drugalcdep.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heilig M, Goldman D, Berrettini W, O’Brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nat Rev Neurosci. 2011;12:670–684. doi: 10.1038/nrn3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helton SG, Lohoff FW. Pharmacogenetics of alcohol use disorders and comorbid psychiatric disorders. Psychiatry Res. 2015;230:121–129. doi: 10.1016/j.psychres.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Heilig M, Sommer WH, Spanagel R. The Need for Treatment Responsive Translational Biomarkers in Alcoholism Research. Curr Top Behav Neurosci. 2016;28:151–171. doi: 10.1007/7854_2015_5006. [DOI] [PubMed] [Google Scholar]
- 11.Mayfield J, Arends MA, Harris RA, Blednov YA. Genes and Alcohol Consumption: Studies with Mutant Mice. Int Rev Neurobiol. 2016;126:293–355. doi: 10.1016/bs.irn.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ron D, Barak S. Molecular mechanisms underlying alcohol-drinking behaviours. Nat Rev Neurosci. 2016;17:576–591. doi: 10.1038/nrn.2016.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davenport AP, Alexander SP, Sharman JL, Pawson AJ, Benson HE, Monaghan AE, et al. International Union of Basic and Clinical Pharmacology. LXXXVIII. G protein-coupled receptor list: recommendations for new pairings with cognate ligands. Pharmacol Rev. 2013;65:967–986. doi: 10.1124/pr.112.007179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- 15.Mulholland PJ, Chandler LJ, Kalivas PW. Signals from the Fourth Dimension Regulate Drug Relapse. Trends Neurosci. 2016;39:472–485. doi: 10.1016/j.tins.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizushima K, Miyamoto Y, Tsukahara F, Hirai M, Sakaki Y, Ito T. A novel G-protein-coupled receptor gene expressed in striatum. Genomics. 2000;69:314–321. doi: 10.1006/geno.2000.6340. [DOI] [PubMed] [Google Scholar]
- 18.Ehrlich AT, Semache M, Bailly J, Wojcik S, Arefin TM, Colley C, et al. Mapping GPR88-Venus illuminates a novel role for GPR88 in sensory processing. Brain Struct Funct. 2017 doi: 10.1007/s00429-017-1547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becker JA, Befort K, Blad C, Filliol D, Ghate A, Dembele D, et al. Transcriptome analysis identifies genes with enriched expression in the mouse central extended amygdala. Neuroscience. 2008;156:950–965. doi: 10.1016/j.neuroscience.2008.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Befort K, Filliol D, Ghate A, Darcq E, Matifas A, Muller J, et al. Mu-opioid receptor activation induces transcriptional plasticity in the central extended amygdala. Eur J Neurosci. 2008;27:2973–2984. doi: 10.1111/j.1460-9568.2008.06273.x. [DOI] [PubMed] [Google Scholar]
- 21.Massart R, Mignon V, Stanic J, Munoz-Tello P, Becker JA, Kieffer BL, et al. Developmental and adult expression patterns of the G-protein-coupled receptor GPR88 in the rat: Establishment of a dual nuclear-cytoplasmic localization. J Comp Neurol. 2016;524:2776–2802. doi: 10.1002/cne.23991. [DOI] [PubMed] [Google Scholar]
- 22.Conti B, Maier R, Barr AM, Morale MC, Lu X, Sanna PP, et al. Region-specific transcriptional changes following the three antidepressant treatments electro convulsive therapy, sleep deprivation and fluoxetine. Mol Psychiatry. 2007;12:167–189. doi: 10.1038/sj.mp.4001897. [DOI] [PubMed] [Google Scholar]
- 23.Ogden CA, Rich ME, Schork NJ, Paulus MP, Geyer MA, Lohr JB, et al. Candidate genes, pathways and mechanisms for bipolar (manic-depressive) and related disorders: an expanded convergent functional genomics approach. Mol Psychiatry. 2004;9:1007–1029. doi: 10.1038/sj.mp.4001547. [DOI] [PubMed] [Google Scholar]
- 24.Brandish PE, Su M, Holder DJ, Hodor P, Szumiloski J, Kleinhanz RR, et al. Regulation of gene expression by lithium and depletion of inositol in slices of adult rat cortex. Neuron. 2005;45:861–872. doi: 10.1016/j.neuron.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Le Merrer J, Befort K, Gardon O, Filliol D, Darcq E, Dembele D, et al. Protracted abstinence from distinct drugs of abuse shows regulation of a common gene network. Addict Biol. 2012;17:1–12. doi: 10.1111/j.1369-1600.2011.00365.x. [DOI] [PubMed] [Google Scholar]
- 26.Dzierba CD, Bi Y, Dasgupta B, Hartz RA, Ahuja V, Cianchetta G, et al. Design, synthesis, and evaluation of phenylglycinols and phenyl amines as agonists of GPR88. Bioorg Med Chem Lett. 2015;25:1448–1452. doi: 10.1016/j.bmcl.2015.01.036. [DOI] [PubMed] [Google Scholar]
- 27.Jin C, Decker AM, Huang XP, Gilmour BP, Blough BE, Roth BL, et al. Synthesis, pharmacological characterization, and structure-activity relationship studies of small molecular agonists for the orphan GPR88 receptor. ACS Chem Neurosci. 2014;5:576–587. doi: 10.1021/cn500082p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Logue SF, Grauer SM, Paulsen J, Graf R, Taylor N, Sung MA, et al. The orphan GPCR, GPR88, modulates function of the striatal dopamine system: a possible therapeutic target for psychiatric disorders? Mol Cell Neurosci. 2009;42:438–447. doi: 10.1016/j.mcn.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Quintana A, Sanz E, Wang W, Storey GP, Guler AD, Wanat MJ, et al. Lack of GPR88 enhances medium spiny neuron activity and alters motor- and cue-dependent behaviors. Nat Neurosci. 2012;15:1547–1555. doi: 10.1038/nn.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meirsman AC, Le Merrer J, Pellissier LP, Diaz J, Clesse D, Kieffer BL, et al. Mice Lacking GPR88 Show Motor Deficit, Improved Spatial Learning, and Low Anxiety Reversed by Delta Opioid Antagonist. Biol Psychiatry. 2016;79:917–927. doi: 10.1016/j.biopsych.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warnault V, Darcq E, Levine A, Barak S, Ron D. Chromatin remodeling--a novel strategy to control excessive alcohol drinking. Transl Psychiatry. 2013;3:e231. doi: 10.1038/tp.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, et al. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zapata A, Gonzales RA, Shippenberg TS. Repeated ethanol intoxication induces behavioral sensitization in the absence of a sensitized accumbens dopamine response in C57BL/6J and DBA/2J mice. Neuropsychopharmacology. 2006;31:396–405. doi: 10.1038/sj.npp.1300833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charbogne P, Gardon O, Martin-Garcia E, Keyworth HL, Matsui A, Mechling AE, et al. Mu Opioid Receptors in Gamma-Aminobutyric Acidergic Forebrain Neurons Moderate Motivation for Heroin and Palatable Food. Biol Psychiatry. 2017;81:778–788. doi: 10.1016/j.biopsych.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mechling AE, Arefin T, Lee HL, Bienert T, Reisert M, Ben Hamida S, et al. Deletion of the mu opioid receptor gene in mice reshapes the reward-aversion connectome. Proc Natl Acad Sci U S A. 2016;113:11603–11608. doi: 10.1073/pnas.1601640113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arefin TM, Mechling AE, Meirsman C, Bienert T, Hübner N, Lee HL, et al. Remodeling of Sensorimotor Brain Connectivity in Gpr88 deficient mice. In revision. 2017 doi: 10.1089/brain.2017.0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friston KJ, Kahan J, Biswal B, Razi A. A DCM for resting state fMRI. Neuroimage. 2014;94:396–407. doi: 10.1016/j.neuroimage.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samson HH, Slawecki CJ, Sharpe AL, Chappell A. Appetitive and consummatory behaviors in the control of ethanol consumption: a measure of ethanol seeking behavior. Alcohol Clin Exp Res. 1998;22:1783–1787. [PubMed] [Google Scholar]
- 39.Gremel CM, Cunningham CL. Role of test activity in ethanol-induced disruption of place preference expression in mice. Psychopharmacology (Berl) 2007;191:195–202. doi: 10.1007/s00213-006-0651-5. [DOI] [PubMed] [Google Scholar]
- 40.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 41.Mechling AE, Hubner NS, Lee HL, Hennig J, von Elverfeldt D, Harsan LA. Fine-grained mapping of mouse brain functional connectivity with resting-state fMRI. Neuroimage. 2014;96:203–215. doi: 10.1016/j.neuroimage.2014.03.078. [DOI] [PubMed] [Google Scholar]
- 42.Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- 43.Meirsman AC, Robe A, de Kerchove d’Exaerde A, Kieffer BL. GPR88 in A2AR Neurons Enhances Anxiety-Like Behaviors. eNeuro. 2016:3. doi: 10.1523/ENEURO.0202-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friston KJ. Functional and effective connectivity: a review. Brain Connect. 2011;1:13–36. doi: 10.1089/brain.2011.0008. [DOI] [PubMed] [Google Scholar]
- 45.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, et al. A mesoscale connectome of the mouse brain. Nature. 2014;508:207–214. doi: 10.1038/nature13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lack CM, Jones SR, Roberts DC. Increased breakpoints on a progressive ratio schedule reinforced by IV cocaine are associated with reduced locomotor activation and reduced dopamine efflux in nucleus accumbens shell in rats. Psychopharmacology (Berl) 2008;195:517–525. doi: 10.1007/s00213-007-0919-4. [DOI] [PubMed] [Google Scholar]
- 49.Song R, Zhang HY, Li X, Bi GH, Gardner EL, Xi ZX. Increased vulnerability to cocaine in mice lacking dopamine D3 receptors. Proc Natl Acad Sci U S A. 2012;109:17675–17680. doi: 10.1073/pnas.1205297109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blum K, Gardner E, Oscar-Berman M, Gold M. “Liking” and “wanting” linked to Reward Deficiency Syndrome (RDS): hypothesizing differential responsivity in brain reward circuitry. Curr Pharm Des. 2012;18:113–118. doi: 10.2174/138161212798919110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Howe MW, Tierney PL, Sandberg SG, Phillips PE, Graybiel AM. Prolonged dopamine signalling in striatum signals proximity and value of distant rewards. Nature. 2013;500:575–579. doi: 10.1038/nature12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adamantidis AR, Tsai HC, Boutrel B, Zhang F, Stuber GD, Budygin EA, et al. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J Neurosci. 2011;31:10829–10835. doi: 10.1523/JNEUROSCI.2246-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abrahao KP, Quadros IM, Andrade AL, Souza-Formigoni ML. Accumbal dopamine D2 receptor function is associated with individual variability in ethanol behavioral sensitization. Neuropharmacology. 2012;62:882–889. doi: 10.1016/j.neuropharm.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 54.Casey KF, Benkelfat C, Cherkasova MV, Baker GB, Dagher A, Leyton M. Reduced dopamine response to amphetamine in subjects at ultra-high risk for addiction. Biol Psychiatry. 2014;76:23–30. doi: 10.1016/j.biopsych.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 55.Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- 56.de Wit H, Phillips TJ. Do initial responses to drugs predict future use or abuse? Neurosci Biobehav Rev. 2012;36:1565–1576. doi: 10.1016/j.neubiorev.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Richiardi J, Altmann A, Milazzo AC, Chang C, Chakravarty MM, Banaschewski T, et al. BRAIN NETWORKS. Correlated gene expression supports synchronous activity in brain networks. Science. 2015;348:1241–1244. doi: 10.1126/science.1255905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fornito A, Zalesky A, Breakspear M. The connectomics of brain disorders. Nat Rev Neurosci. 2015;16:159–172. doi: 10.1038/nrn3901. [DOI] [PubMed] [Google Scholar]
- 59.Takamura T, Hanakawa T. Clinical utility of resting-state functional connectivity magnetic resonance imaging for mood and cognitive disorders. J Neural Transm (Vienna) 2017 doi: 10.1007/s00702-017-1710-2. [DOI] [PubMed] [Google Scholar]
- 60.Hull JV, Jacokes ZJ, Torgerson CM, Irimia A, Van Horn JD. Resting-State Functional Connectivity in Autism Spectrum Disorders: A Review. Front Psychiatry. 2016;7:205. doi: 10.3389/fpsyt.2016.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tahmasian M, Bettray LM, van Eimeren T, Drzezga A, Timmermann L, Eickhoff CR, et al. A systematic review on the applications of resting-state fMRI in Parkinson’s disease: Does dopamine replacement therapy play a role? Cortex. 2015;73:80–105. doi: 10.1016/j.cortex.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 62.Colombo B, Rocca MA, Messina R, Guerrieri S, Filippi M. Resting-state fMRI functional connectivity: a new perspective to evaluate pain modulation in migraine? Neurol Sci. 2015;36(Suppl 1):41–45. doi: 10.1007/s10072-015-2145-x. [DOI] [PubMed] [Google Scholar]
- 63.Vargas C, Lopez-Jaramillo C, Vieta E. A systematic literature review of resting state network--functional MRI in bipolar disorder. J Affect Disord. 2013;150:727–735. doi: 10.1016/j.jad.2013.05.083. [DOI] [PubMed] [Google Scholar]
- 64.Ma X, Qiu Y, Tian J, Wang J, Li S, Zhan W, et al. Aberrant default-mode functional and structural connectivity in heroin-dependent individuals. PLoS One. 2015;10:e0120861. doi: 10.1371/journal.pone.0120861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fedota JR, Stein EA. Resting-state functional connectivity and nicotine addiction: prospects for biomarker development. Ann N Y Acad Sci. 2015;1349:64–82. doi: 10.1111/nyas.12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med. 2006;12:559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 67.Squeglia LM, Cservenka A. Adolescence and Drug Use Vulnerability: Findings from Neuroimaging. Curr Opin Behav Sci. 2017;13:164–170. doi: 10.1016/j.cobeha.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Volkow ND, Baler RD. Brain imaging biomarkers to predict relapse in alcohol addiction. JAMA Psychiatry. 2013;70:661–663. doi: 10.1001/jamapsychiatry.2013.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Camchong J, Stenger A, Fein G. Resting-state synchrony during early alcohol abstinence can predict subsequent relapse. Cereb Cortex. 2013;23:2086–2099. doi: 10.1093/cercor/bhs190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heitzeg MM, Cope LM, Martz ME, Hardee JE. Neuroimaging Risk Markers for Substance Abuse: Recent Findings on Inhibitory Control and Reward System Functioning. Curr Addict Rep. 2015;2:91–103. doi: 10.1007/s40429-015-0048-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cservenka A, Casimo K, Fair DA, Nagel BJ. Resting state functional connectivity of the nucleus accumbens in youth with a family history of alcoholism. Psychiatry Res. 2014;221:210–219. doi: 10.1016/j.pscychresns.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cservenka A, Fair DA, Nagel BJ. Emotional processing and brain activity in youth at high risk for alcoholism. Alcohol Clin Exp Res. 2014;38:1912–1923. doi: 10.1111/acer.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peters S, Peper JS, Van Duijvenvoorde AC, Braams BR, Crone EA. Amygdala-orbitofrontal connectivity predicts alcohol use two years later: a longitudinal neuroimaging study on alcohol use in adolescence. Dev Sci. 2016 doi: 10.1111/desc.12448. [DOI] [PubMed] [Google Scholar]
- 74.Peters S, Jolles DJ, Van Duijvenvoorde AC, Crone EA, Peper JS. The link between testosterone and amygdala-orbitofrontal cortex connectivity in adolescent alcohol use. Psychoneuroendocrinology. 2015;53:117–126. doi: 10.1016/j.psyneuen.2015.01.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Heterozygous (Gpr88+/−), and Gpr88−/− mice show increased alcohol drinking, with no change of water and sucrose intake. (A–B) Mice underwent intermittent access 2-bottle-choice drinking paradigm for 8 sessions., Gpr88−/− and Gpr88+/− mice consume more alcohol than controls (Figure S1A Left panel, Two-way ANOVA with repeated measures (RM) showed significant main effects of Genotype (F(2, 32) = 3.7, p < 0.05) and Sessions (F(7, 224) = 7.3, p < 0.001) and no significant interactions between Genotype x Session (F(7, 224) = 0.89, p = 0.56). The mean daily alcohol intake during the entire experiment was also significantly higher in mutant and heterozygote mice (Figure S1A Right panel, one way ANOVA showed a group effect (F(7, 14) = 7.8, p < 0.001). Newman-Keuls post-hoc comparisons showed a strong increase on alcohol intake in Gpr88−/− and Gpr88+/− compared to Gpr88+/+ animals. Water (Figure S1B) intake was comparable in all groups. (C) Mice were tested for sucrose (2%) intake. Sucrose solution and water were offered for 4 consecutive days and the amount of fluid intake was recorded every day. No change on sucrose intake between the three mice genotypes. (D) Gpr88 knockout mice show no alteration of body weights. Gpr88−/− mice showed similar evolution of body weights compared to control animals over the alcohol drinking experiments showed in Figure 1A–D. A–B Left, curves represent the mean (±SEM) alcohol or water intake per session; right, histograms show mean (±SEM) daily alcohol or water or sucrose consumption during the entire experiment. A–C, n=10–13, D n=11–17, ***p < 0.001 compared with control group.
Supplementary Figure S2. Gpr88 knockout mice show no change in Chocolate self-administration. Number of nosepokes in the inactive side in operant self-administration of chocolate flavor pellets does not differ between Gpr88−/− mice and their controls. n = 10–11 for each group.
Supplementary Figure S3. Remodeled resting state FC patterns of PFC, NAC, AMY and VTA in Gpr88−/− mouse brains. BOLD rsfMRI correlation maps for (A) PFC and (B) VTA, (C) NAC and (D) AMY. Corresponding correlation maps of control (left) and Gpr88−/− (right) groups were over-laid on a T2-weighted anatomical brain slices (two-tailed t-test, p < 0.001). The color scale indicates the T- value (positive correlations from 0 to +1: dark red to yellow and negative correlations from 0 to −1: dark blue to turquoise). n=14 for each group.
Supplementary Table S1. Statistical analysis of home cage alcohol intake data.
(A) Analysis for continuous access (Figure 1A Left). Two-way ANOVA with repeated measures (RM) showed significant main effects of Genotype (F(1, 28) = 7.2, p < 0.05) and Sessions (F(10, 280) = 4.1, p < 0.001) and no significant interactions between Genotype x Session (F(10, 280) = 7.2, p = 1.4). Subsequent analyses using the method of contrasts detected a significant difference in alcohol intake during sessions 2, 7 and 11 (p’s<0.05) between Gpr88−/− and Gpr88+/+ mice. (B) Analysis for intermittent access protocol (Figure 1C left). Two-way RM ANOVA showed significant main effects of Genotype (F(1, 24) = 10.0, p < 0.01) and Sessions (F(10, 240) = 2.6, p < 0.01) and no significant Genotype x Session interaction effect (F(10, 240) = 0.6, p = 0.7). Subsequent analyses using the method of contrasts revealed a significant difference between Gpr88−/− and Gpr88+/+ mice during sessions 1, 2, and 11 (p’s<0.05) and a trend towards higher alcohol consumption in Gpr88−/− mice for sessions 6, 8 and 9 (p’s=0.06).
Supplementary Table S2. Statistical analysis of alcohol self-administration data.
Gpr88−/− mice and their controls were first subjected to a saccharine fading procedure, and then had access to 10% alcohol (Figure 2A). In the first phase, there was no difference in the pattern of saccharine intake alone (number of licks) between Gpr88−/− mice (168±84) and their controls (177±72). During mixed saccharine/alcohol exposure, we observed increased lever responding (Figure 2B) and licks (Figure 2D) for all the saccharine/alcohol mixtures in Gpr88−/− mice compared to controls. During this acquisition phase, statistical analysis (see tables below) showed significant lever or licks, session and genotype effects, indicating successful acquisition of alcohol SA for both groups, and also enhanced responding to saccharine/alcohol mixtures for mutant mice. (Table A) Analysis for level pressing (Figure 2B), three-way ANOVA with genotype and lever presses as between-subjects factor and repeated measures in the factor sessions was conducted. (Table B) Analysis for licking (Figure 2D), two-way ANOVA with genotype as between-subjects factor and repeated measures in the factor sessions was conducted.
Supplementary Table S3. Statistical analysis of alcohol-induced dopamine release in the NAC.
Gpr88 gene deletion reduces alcohol-enhanced extracellular levels of NAC DA (Figure 3B). Two-way ANOVA with RM showed an significant effect of Genotype (F(1, 20) = 12.0, p<0.01), significant effect of time (F(16, 320) = 7.6, p<0.001) and significant interaction F(16, 320) = 3.2, p<0.001). Subsequent analyses using Newman-Keuls post hoc analysis detected a significant reduction of DA release in the NAC at 160 and 180 min in Gpr88−/− mice compared to control animals. Areas under the curve for the four cumulative dialysates following alcohol injection (inserts for Figure 3B) also showed lower increase of DA levels in mutant mice for the two alcohol doses (EtOH 1.8: t(20) = 2.6, p < 0.05; EtOH 3.2: t(20) = 3.4, p < 0.01).