Abstract
Background
Cue-induced cocaine craving “incubates” during abstinence from cocaine self-administration. Expression of incubation ultimately depends upon elevation of homomeric GluA1 AMPA receptors (AMPARs) in the nucleus accumbens (NAc). This adaptation requires ongoing protein translation for its maintenance. Aberrant translation is implicated in CNS diseases, but nothing is known about glutamatergic regulation of translation in the drug-naïve NAc or after incubation.
Methods
NAc tissue was obtained from drug-naïve rats and after 1 or >40 days of abstinence from extended-access cocaine or saline self-administration. Newly translated proteins were labeled using 35S-Met/Cys or puromycin. We compared basal overall translation and its regulation by mGlu1, mGlu5 and NMDA receptors (NMDARs) in naïve, saline and cocaine rats, and compared GluA1 and GluA2 translation by immunoprecipitating puromycin-labeled proteins.
Results
In all groups, overall translation was unaltered by mGlu1 blockade (LY367385) but increased by mGlu5 blockade (MTEP). NMDAR blockade (APV) increased overall translation in naïve and saline, but not cocaine, rats. Cocaine/late withdrawal rats exhibited greater translation of GluA1 (but not GluA2), which was not further affected by NMDAR blockade.
Conclusions
Our results suggest that increased GluA1 translation contributes to the elevated homomeric GluA1 AMPAR levels in NAc that mediate incubation. Additional contributions to incubation-related plasticity may result from loss of the braking influence on translation normally exerted by NMDARs. Apart from elucidating incubation-related adaptations, we found a suppressive effect of mGlu5 on NAc translation regardless of drug exposure, which is opposite to results obtained in hippocampus and points to heterogeneity of translational regulation between brain regions.
Keywords: AMPA receptor, incubation of cocaine craving, metabolic labeling, nucleus accumbens, protein translation, puromycin
INTRODUCTION
A major challenge in treating addiction is the persistence of vulnerability to relapse. Even after prolonged abstinence, cocaine addicts remain susceptible to relapse, often elicited by cues previously associated with drug-taking (1–3). Aspects of this can be studied in rats using the “incubation of craving” model, in which cue-induced craving progressively intensifies (“incubates”) during withdrawal from cocaine self-administration and then remains high for months (4, 5). This models the situation of humans users when heavy cocaine use is interrupted by incarceration or prolonged hospitalization (6). During this interruption, craving may build and, when the addict returns to the “real world”, drug-related cues may present an elevated relapse risk. Incubation of craving in human drug users has now been observed during abstinence from cocaine and other drugs of abuse (3, 7–9).
In rats, incubation of cocaine craving involves time-dependent changes in multiple brain regions (5, 10). However, after >1 month of withdrawal, “incubated” craving depends upon strengthening of AMPA receptor (AMPAR) transmission onto medium spiny neurons (MSNs) in the NAc through accumulation of high-conductance GluA2-lacking, Ca2+-permeable AMPA receptors (CP-AMPARs; primarily homomeric GluA1) (5). This is accompanied by profound alterations in group I mGluR-mediated synaptic depression (11, 12). Recently, we identified a novel linkage between these adaptations and protein translation. NAc slices from “incubated” rats were exposed for ~1 h to inhibitors of translation (anisomycin, cycloheximide or rapamycin) prior to patch-clamp recordings. This normalized the state of synaptic transmission, i.e., the contribution of CP-AMPARs to synaptic transmission returned to control levels and alterations in group I mGluR-mediated synaptic depression were reversed (13). This suggests that ongoing protein translation maintains synaptic adaptations in MSNs directly linked to incubation of craving. Consistent with these findings, our recent results demonstrate that expression of incubated cocaine craving during a cue-induced seeking test requires protein translation in the NAc (14).
In many brain regions, including hippocampus, cerebellum and ventral tegmental area (VTA), group I mGluRs are well-established regulators of protein translation, with stimulation of translation by these receptors playing a critical role in long-term depression (LTD) (15). In addition, NMDA receptor (NMDAR) signaling negatively regulates translation in hippocampal and cortical neurons (16–20). Through such studies, aberrant protein translation has been implicated in CNS diseases, including fragile X syndrome and depression, and identified as a potential target for therapeutic intervention (19–25).
Previous studies have demonstrated a role for protein synthesis in the effects of drugs of abuse (e.g. (26–34)) and shown that drugs affect signaling molecules regulating translation (e.g., mTOR; (35, 36)), but nothing is known about upstream regulation of this signaling by glutamate transmission in the NAc. Based on work in other brain regions summarized above, we focused on determining how NMDAR and group I mGluRs regulate translation in the NAc under control conditions and whether this is altered after incubation of cocaine craving. As protein synthesis-mediated changes in synaptic connections are believed to underlie long-term alterations in neural circuits involved in a variety of behaviors (37), expanding our understanding of the regulation of translation in the NAc should shed light on normal and aberrant learning involving the striatum. Furthermore, if dysregulation of protein translation underlies addiction-related plasticity, normalizing translation may represent a novel therapeutic approach.
METHODS AND MATERIALS
Subjects and drug self-administration
All procedures were approved by the Rosalind Franklin University Institutional Animal Care and Use Committee in accordance with the USPHS Guide for Care and Use of Laboratory Animals. Adult male Sprague-Dawley rats underwent extended-access cocaine or saline self-administration (6 h/d) as described previously (12) and in Supplemental Information.
Metabolic labeling
Procedures were adapted from well-established protocols (22, 24) (see Supplementary Fig. 1 for time-line). Rats were decapitated and the NAc (mainly core) was punched from a 2mm brain slice obtained using a brain matrix. Each hemisphere was chopped into 4 pieces to increase surface area, placed into a net-well, and submerged in a manifold containing artificial cerebrospinal fluid (aCSF) saturated with 95% O2/5% CO2. Each hemisphere was counted as an individual sample, with hemispheres from the same rat assigned to different experimental groups, enabling paired t-tests (see Statistical Analysis). Tissue recovered in net-wells for 3 h, enabling optimal and stable protein synthesis (Supplementary Figure 2). Actinomycin D (25 µM; Sigma-Aldrich; St. Louis, MO) was added during the last 30 min of recovery to inhibit new transcription. Then net-wells were transferred to manifolds with aCSF containing actinomycin D and 10 µCi/mL 35S-Met/Cys (EasyTag™ L-[35S]-Methionine, PerkinElmer; Waltham, MA) ± drug, incubated for 1 h, and snap-frozen. To compare 35S-Met/Cys incorporation between experimental groups, labeled tissue was homogenized and processed for autoradiography as described in Supplemental Information.
Measurement of mRNA levels
RNA was extracted from NAc homogenates or synaptoneurosomes (prepared as described previously (38)) and analyzed by quantitative PCR (qPCR) as described in Supplemental Information.
Puromycin labeling
Procedures were adapted from prior studies (39, 40). Pieces of NAc tissue were prepared exactly as described for metabolic labeling experiments and placed in net-wells. Tissue was incubated with 25 µM actinomycin D (Sigma-Aldrich) during the last 30 min of the 3-h recovery period, transferred to manifolds with aCSF containing 1 µM puromycin (Sigma-Aldrich) and actinomycin D for 1 h, and then snap-frozen. As described in Supplemental Information, overall puromycin incorporation was determined by immunoblotting; puromycin incorporation into GluA1 or GluA2 was determined by Immunoprecipitation of puromycin-labeled proteins.
Statistical analyses
Data were analyzed in Prism (GraphPad Software). Student’s t-tests [paired when comparing differently treated hemispheres from a single brain (Figures 1–3), or unpaired when each rat generated a single sample (Figures 4,5)] or ANOVA followed by Tukey’s test (Figure 6) were conducted with significance set at p≤0.05. Data are presented as mean±SEM.
RESULTS
Measuring protein translation in the NAc
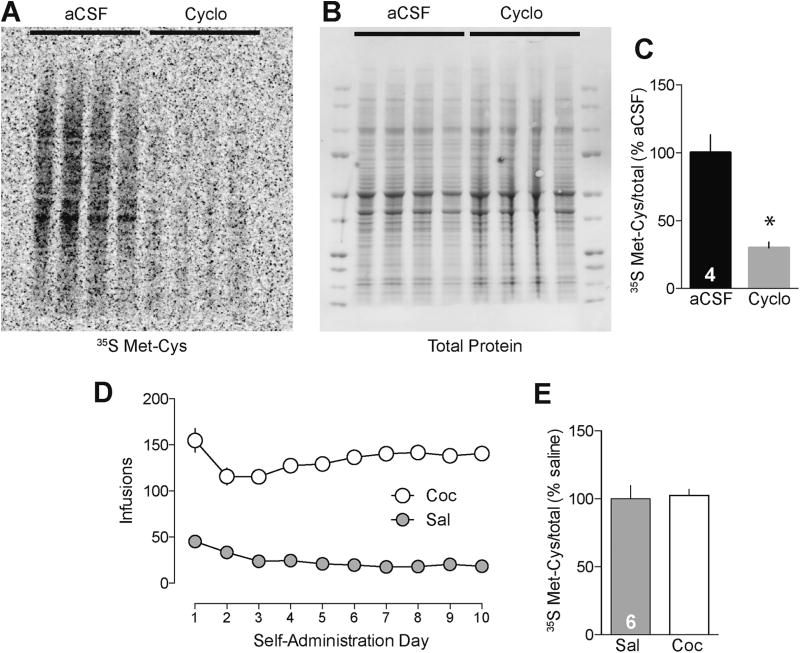
To confirm that 35S-Met/Cys incorporates into newly translated proteins in the NAc, we pre-exposed tissue from naïve animals to the translation inhibitor cycloheximide (60 µM) for 30 min, before moving the tissue into a chamber containing cycloheximide and 35S-Met/Cys for an additional 60 min. We then processed tissue for SDS-PAGE/autoradiography. Figures 1A and 1B show incorporation of 35S-Met/Cys into newly synthesized proteins across multiple molecular weights and demonstrate that cycloheximide significantly reduced 35S-Met/Cys incorporation (Figure 1C, t3=5.46, p<0.05), validating the assay in NAc tissue.
Figure 1.
Monitoring protein translation in the NAc using 35S-Met/Cys incorporation reveals no differences in overall translation between saline controls and rats that have undergone incubation of cocaine craving. (A) Representative autoradiograph shows 35S-Met/Cys incorporation into proteins of various molecular weights in freshly dissected NAc tissue. (B) Ponceau S stain of the same gel demonstrates equal protein loading in each lane. (C) Quantification of 35S-labeled proteins in A, normalized to total protein in each lane determined with Ponceau S staining, shows that 30 min of incubation with the protein translation inhibitor cycloheximide (Cyclo) significantly reduces protein translation. (D) Mean ± SEM number of cocaine infusions per day over the ten 6-hour daily self-administration training sessions for all animals (163 total rats). (E) Quantification of 35S-labeled proteins (normalized to total protein in each lane) shows that overall translation did not differ between cocaine and saline self-administering rats killed after withdrawal day 40. Numbers in black bars (panels C and E) represent the number of rats in the experiment; for each rat, the NAc from one hemisphere was used for the control group and the NAc from the other hemisphere was used for the experimental group. *p<0.05 versus aCSF control (paired t-test).
Regulation of translation in the NAc under control conditions and after incubation
We then tested the hypothesis that the overall rate of protein translation is altered in the NAc of rats after incubation of cocaine craving. Tissue was obtained after extended-access self-administration of saline or cocaine (Figure 1D) and >40 days of abstinence, a period sufficient for maximal incubation of craving and stable CP-AMPAR elevation in NAc core (10, 42–44). Rats subjected to this cocaine regimen and withdrawal period will hereafter be referred to as “incubated” rats. After 3 h of recovery, tissue was incubated with 35S-Met/Cys (60 min) and processed for SDS-PAGE/autoradiography. Measurements of total protein translation, made by scanning the entire lane, were not different between saline controls and “incubated” rats (Figure 1E, t5=0.30, p=0.77). This result indicates that altered translation of a subset of proteins, rather than gross changes in protein translation, must contribute to maintenance of incubation-related neuroadaptations in the NAc (13). Furthermore, it leaves open the possibility that mechanisms regulating protein translation are altered in the NAc after incubation of craving.
To test the latter possibility, we examined two glutamate receptor subtypes – NMDARs and group I mGluRs – that are well-established as regulators of protein translation in several brain regions (see Introduction). We utilized the strategy of incubating tissue with receptor antagonists to detect tonic regulation of translation by endogenous glutamate. This strategy has been used to study the regulation of protein synthesis in other brain regions (17, 22) and was preferred to incubating with agonists because NMDAR activation can be associated with toxicity, while effects of the group I mGluR agonist DHPG in our hands were quite variable depending on concentration and time of incubation (data not shown), as has been found previously (Emily Osterweil, personal communication).
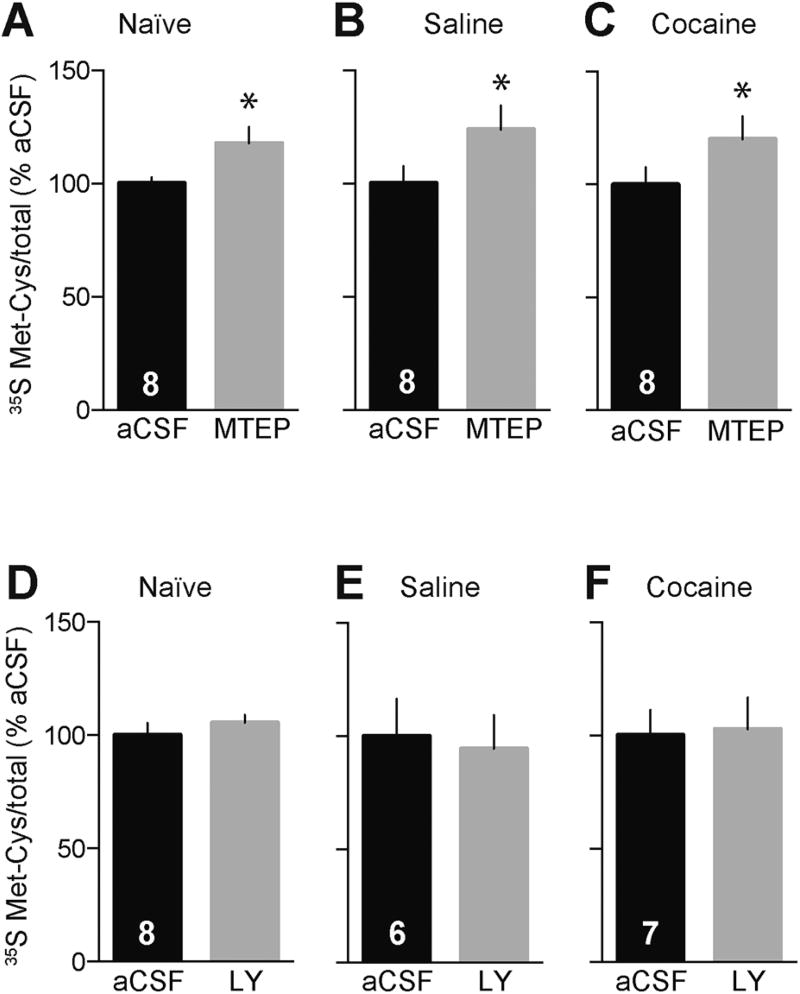
First, we examined mGlu5 by co-exposing NAc tissue (from drug-naïve, saline, or “incubated” rats) to vehicle or MTEP, a selective mGlu5 negative allosteric modulator (NAM) (45), and 35S-Met/Cys for 60 min. In contrast to previous studies in hippocampus, where mGlu5 activation increases protein translation (46), inhibition of mGlu5 with MTEP increased 35S-Met/Cys incorporation in both naïve and saline NAc tissue (Figure 2A,B, naïve: t7=2.20, saline: t7=2.12, p<0.05). This is an important result because it shows that protein translation is differentially regulated in these two regions, and thus that regulatory mechanisms operating in the NAc cannot necessarily be inferred from prior studies in hippocampus. Furthermore, the magnitude of the MTEP-induced increase in translation was similar between saline and cocaine animals (Figure 2B,C, cocaine: t7=2.69, p<0.05). These results suggest that mGlu5 activity suppresses protein translation in the NAc and this is not changed after incubation of cocaine craving.
Figure 2.
mGlu5 blockade increases protein translation in the NAc of naïve rats, saline controls, and “incubated” rats while mGlu1 blockade does not influence overall translation in the NAc. Including the mGlu5 antagonist MTEP (25 µM) during the 35S-Met/Cys incorporation period increases protein translation in NAc tissue from naïve rats (A), as well as rats that self-administered saline (B) or cocaine (C) and were killed after withdrawal day 40. Including the mGlu1 antagonist LY367385 (LY; 50 µM) during the 35S incorporation period does not significantly alter protein translation in NAc tissue from the same experimental groups (D–F). Numbers in black bars represent the number of rats in the experiment; for each rat, the NAc from one hemisphere was used for the control group and the NAc from the other hemisphere was used for the experimental group. *p<0.05 versus aCSF control (paired t-test).
To examine mGlu1, NAc tissue from naïve, saline, or “incubated” rats was labeled with 35S-Met/Cys in the presence or absence of LY367385 (50 µM), a selective mGlu1 competitive antagonist (47). LY367385 did not influence overall translation in any group (Figure 2D,E,F, naïve: t7=0.73, saline: t5=0.59, cocaine: t6=0.22). Thus, group I mGluR-mediated translational regulation in the NAc is primarily driven by mGlu5.
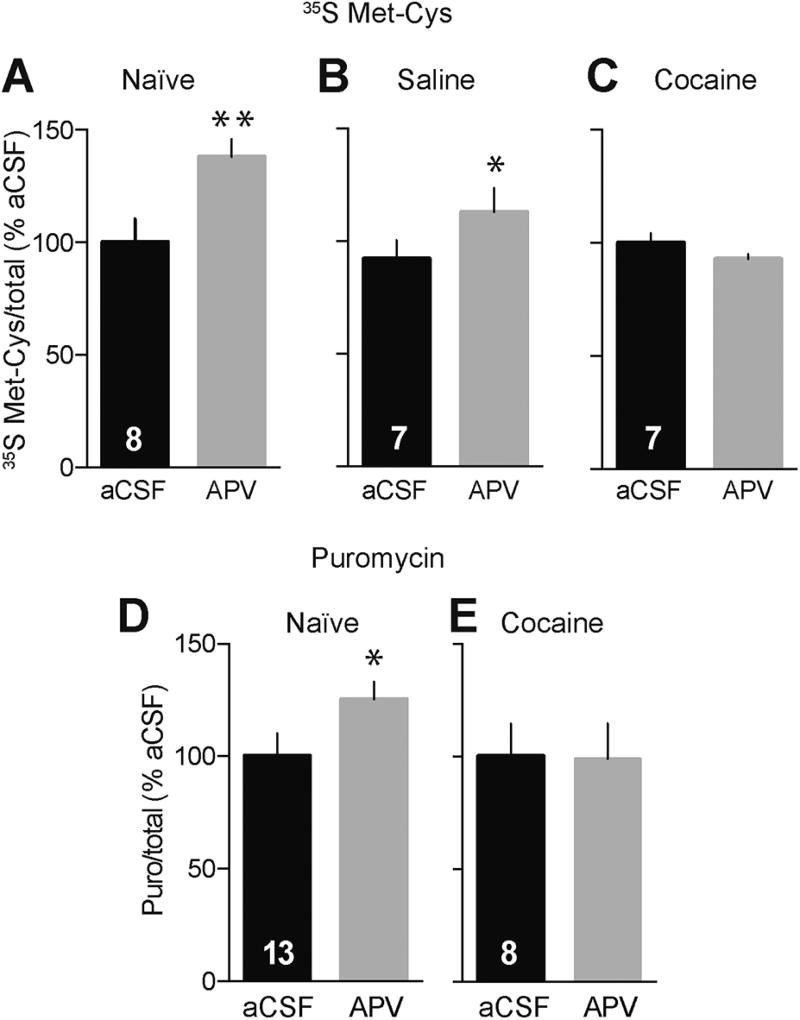
We next asked whether NMDAR transmission regulates protein synthesis in the NAc and if this is altered following incubation of craving. In other brain regions, NMDAR transmission can exert a suppressive influence on translation by stimulating phosphorylation of eukaryotic elongation factor 2 (eEF2; see Discussion). NAc tissue from naïve, saline, or “incubated” rats was labeled with 35S-Met/Cys in the presence or absence of the competitive NMDAR antagonist APV (50 µM). APV increased protein synthesis in naïve and saline rats (Figure 3A,B, naïve: t7=2.26, saline: t6=1.90, p<0.05). This suggests that tonic NMDAR signaling suppresses translation in the NAc, consistent with findings in other regions. Interestingly, this NMDAR-mediated inhibitory tone was lost in the “incubated” rats (t6=1.70, p=0.14; Figure 3C).
Figure 3.
NMDAR blockade increases translation in control rats, but not following prolonged withdrawal from cocaine self-administration. Including the NMDAR antagonist APV (50 µM) during the 35S-Met/Cys or puromycin incorporation period increases protein translation in NAc tissue dissected from naïve rats (A, D) and saline self-administering rats killed after withdrawal day 40 (B), but not NAc tissue from cocaine self-administering rats killed after withdrawal day 40 (C, E). Numbers in black bars represent the number of rats in the experiment. For rats in these metabolic labeling and puromycin experiments, the NAc from one hemisphere was used for the control group and the NAc from the other hemisphere was used for the experimental group. In both cases, data were normalized to total protein in each lane, determined with Ponceau S staining. **p<0.01 and *p<0.05 versus aCSF control (paired t-test).
GluA1 and GluA2 translation under control conditions and after incubation
As described in the Introduction, homomeric GluA1 CP-AMPARs accumulate in NAc core after ~30 days of withdrawal from our cocaine regimen and thereafter are required for expression of “incubated” craving (5, 12, 42). Their accumulation is associated with increased GluA1 protein levels in NAc homogenates, LP1 fractions and on the cell surface (42, 48), suggesting a role for increased GluA1 translation.
Testing this hypothesis requires measuring the translation rate of individual proteins. Using GluA1 as a test case, we labeled tissue using 35S Met/Cys, performed a highly efficient immunoprecipitation of GluA1 (~95% recovery), subjected the immunoprecipitated material to SDS-PAGE/immunoblotting, and exposed the membrane to a phosphorimager screen for 3–4 weeks (Supplementary Figure 3). Even under these conditions, we could detect only faint isotope incorporation into the GluA1 band that was insufficient for reliable quantification. We therefore explored an alternative method based on the ability of the antibiotic puromycin, which resembles tyrosyl-transfer RNA, to incorporate into newly translated proteins (39, 40, 49, 50). When puromycin incorporates into the growing peptide chain, it interrupts elongation; however, if used at a low concentration (1 µM), it labels proteins without substantially altering overall protein synthesis (40).
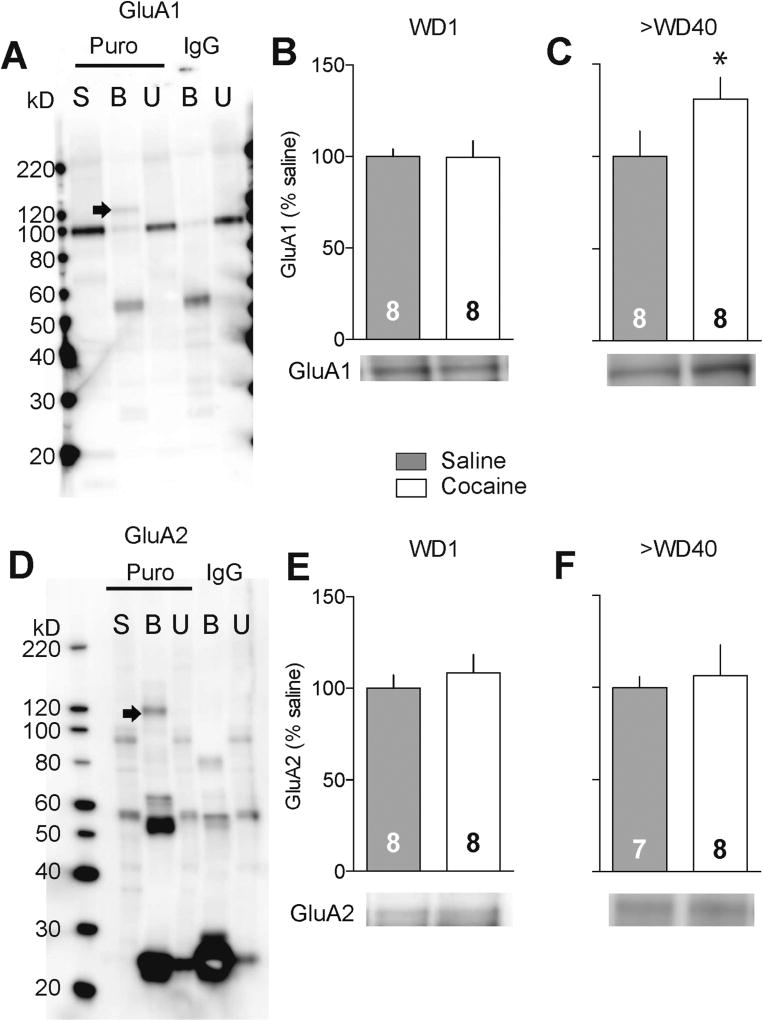
We began by verifying that puromycin and metabolic labeling yielded similar results, using NMDAR regulation of translation as a test case. As observed with metabolic labeling (Figure 3A–C), we found that APV increased translation, measured with puromycin labeling, in NAc tissue from naïve but not cocaine rats (Figures 3D and 3E, naïve: t12=2.25, p=0.04; cocaine: t7=0.08, p=0.94). To examine whether this method could be used to study the rate of translation of individual proteins, we prepared puromycin-labeled NAc tissue and immunoprecipitated with puromycin antibody to isolate all newly translated (puromycin-containing) proteins. Then, we used immunoblotting to measure GluA1 and GluA2 protein levels in starting material and the puromycin-immunoprecipitated fraction. By comparison to samples immunoprecipitated with IgG (control condition), we identified puromycin-labeled GluA1 and GluA2 bands (Figure 4A,D). They migrated slightly above their predicted molecular weight, which is not surprising as structural changes due to puromycin incorporation likely affect protein unfolding/SDS binding and thereby alter migration during SDS-PAGE. As expected, the newly translated pool of each protein was small compared to the amount present in starting material (Figure 4A,E). Perhaps for this reason, the alternative strategy – immunoprecipitating with GluA1 antibody and immunoblotting for puromycin – did not yield a quantifiable signal, paralleling results described above for 35S-labeling (see legend to Supplemental Fig. 3).
Figure 4.
GluA1 translation in the NAc is unchanged one day after discontinuing cocaine self-administration but is increased after incubation of craving has occurred. NAc tissue was labeled with puromycin and then immunoprecipitated using puromycin antibody or IgG (control condition). Equal amounts (40 µg) of starting material (S), bound material (B; immunoprecipitated material purified by Protein A/G resin), and unbound material (U) were separated by SDS-PAGE and immunoblotted for GluA1 (A) or GluA2 (D). Comparison of bound fractions after puromycin versus IgG immunoprecipitation reveals newly translated GluA1 and GluA2 bands that migrate above their predicted molecular weight (arrowheads in panels A and D), presumably due to structural changes associated with puromycin incorporation (see Results). Using this approach, we demonstrated that, compared to saline controls, GluA1 translation is unchanged after 1 day of withdrawal from cocaine self-administration (B) but increased after prolonged withdrawal (>40 days) (C), whereas GluA2 translation is unchanged at both withdrawal times (E,F). For these puromycin experiments, each sample consisted of NAc pooled from both hemispheres of one rat. Numbers in bars represent number of samples/rats in each group. *p<0.05 versus saline controls (unpaired t-test).
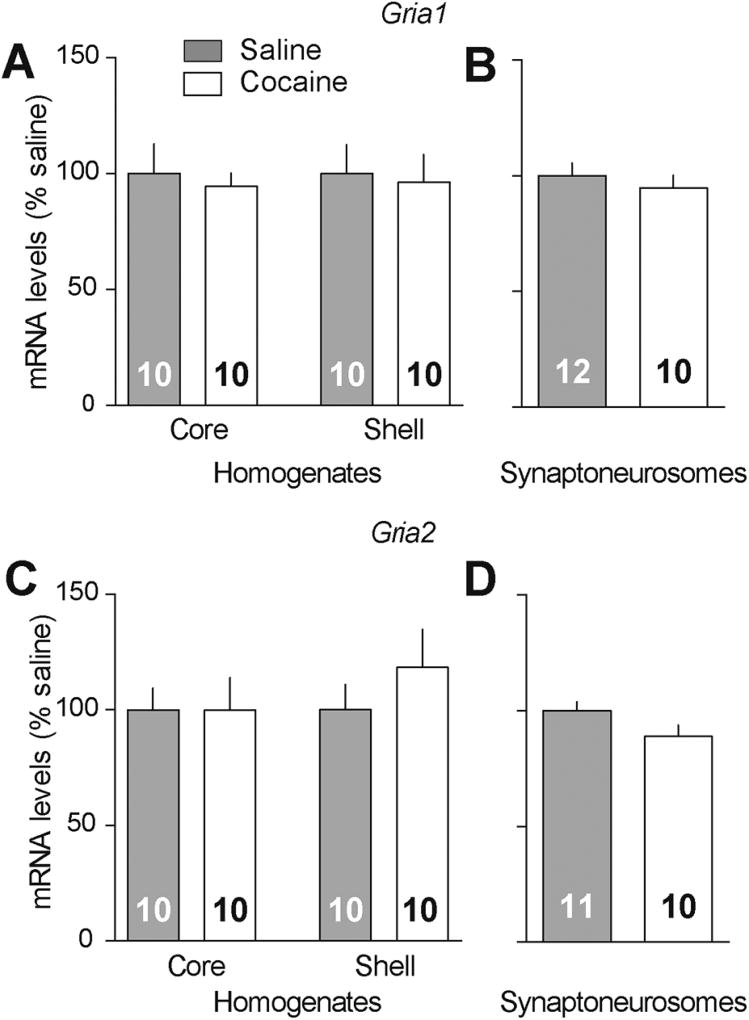
Puromycin labeling/pull-down followed by GluA immunoblotting was then used to compare GluA1 and GluA2 translation in the NAc of rats that self-administered saline or cocaine. Rats were killed on WD1 (prior to CP-AMPAR elevation) or on >WD40 (maximal incubation and CP-AMPAR accumulation). No group differences were observed on WD1 (Fig. 4B and E, GluA1: t14=0.04, p=0.97, GluA2 t14=0.69, p=0.50). However, “incubated” rats (>WD40) exhibited a significant increase in GluA1 but not GluA2 translation (Figure 4C and F, GluA1: t14=1.72, p=0.05, GluA2: t13=0.34, p=0.74). To evaluate a possible role for altered transcription, we used additional “incubated” rats and saline controls. We measured levels of Gria1 and Gria2 mRNA extracted from NAc core or shell homogenates, and from synaptoneurosomes prepared from the entire NAc. We found no group differences in mRNA from homogenates (Figure 5A,C, Gria1: Core t18=0.39, p=0.70, Shell, t18=0.21, p=0.83; Gria2: Core t18=0.01, p=0.99, Shell t18=0.93, p=0.36). In synaptoneurosomes, we found no group difference in Gria1 mRNA levels (Figure 5B, t20=0.68, p=0.50) but observed a trend towards decreased Gria2 mRNA in cocaine rats (Figure 5D, t19=1.77, p=0.09). Results for Gria3 mRNA were similar to those for Gria2 (Supplementary Table 1). The most important finding is absence of a change in Gria1 mRNA in synaptoneurosomes because this suggests that the enhanced GluA1 translation observed after incubation of cocaine craving (Figure 4C) is not explained by increased Gria1 mRNA levels in dendritic compartments. Together, these findings suggest that elevated translation of GluA1 contributes to CP-AMPAR accumulation in the NAc of “incubated” rats.
Figure 5.
GluA1 and GluA2 mRNA levels are unchanged in NAc homogenates or synaptoneurosomes after incubation of cocaine craving. (A and C) Quantification of Gria1 and Gria2 mRNA levels in NAc core and NAc shell homogenates indicates no differences between rats killed after >40 days of withdrawal from saline versus cocaine self-administration. (B and D) Likewise, there was no significant difference in Gria1 or Gria2 mRNA levels in synaptoneurosomes prepared from the whole NAc of identically treated saline and cocaine rats, although a trend towards a reduction in Gria2 mRNA (p=0.09) was observed in the cocaine group. For these experiments, each sample consisted of NAc pooled from both hemispheres of one rat. Numbers in bars represent number of samples/rats in each group. Data analyzed with unpaired t-tests.
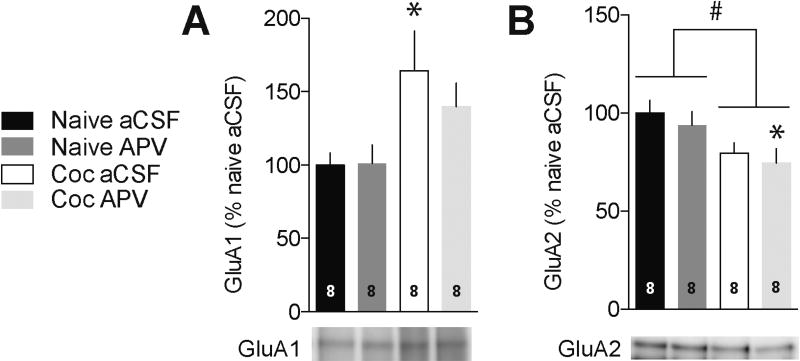
Finally, given its robust effect on overall translation in the NAc, we asked whether NMDAR blockade, shown to increase dendritic GluA1 translation in hippocampal neurons (17), influenced the translation of GluA1 or GluA2 in NAc, and whether this effect was changed following incubation. Using the puromycin immunoprecipitation approach described above, we compared GluA1 and GluA2 translation in NAc tissue from “incubated” and naïve rats (naïve rats were chosen as the control because the effect of APV was most robust in this group) labeled with puromycin in the presence of either aCSF (vehicle control) or APV. Results in Figure 6 confirm increased GluA1 translation in ”incubated” rats (naïve aCSF versus Coc aCSF, Tukey’s post hoc, p=0.04). However, in contrast to its ability to enhance overall translation (Figure 3), APV had no effect on GluA1 translation in naïve rats (naïve aCSF versus naïve APV; p=0.39) and did not further augment GluA1 translation in cocaine rats (cocaine aCSF versus cocaine APV; p=0.75). For GluA2 translation, two-way ANOVA revealed a significant main effect of drug history, i.e., cocaine exposure tended to reduce GluA2 translation relative to the drug-naïve condition (F(1,28)=9.04, p<0.01). However, Tukey’s post hoc analysis revealed that GluA2 translation did not differ between naïve and cocaine aCSF groups (p=0.15), similar to results in Figure 4F showing no significant difference between saline and cocaine groups. Likewise, there was no difference between cocaine aCSF and cocaine APV groups (p=0.95), although there was a marginally significant decrease in newly translated GluA2 in cocaine APV tissue compared to naïve aCSF tissue (Figure 5B, p=0.05). Together, these results suggest that cocaine exposure and NMDAR blockade differentially influence protein translation in the NAc.
Figure 6.
APV does not influence GluA1 translation in the NAc of naïve or “incubated” rats. NAc tissue from drug-naïve rats or rats killed after >40 days of withdrawal from cocaine self-administration was labeled with puromycin in the presence of aCSF or APV (50 µM). Tissue was immunoprecipitated with puromycin antibody to isolate newly translated proteins, and the immunoprecipitated fraction was immunoblotted for GluA1 (A) or GluA2 (B). (A) Comparison of GluA1 translation in naïve aCSF and cocaine aCSF tissue confirmed results in Figure 4 demonstrating elevated GluA1 translation after cocaine withdrawal, but APV did not influence GluA1 translation in either naive or cocaine groups. (B) A two-way ANOVA revealed a main effect of drug pre-exposure on GluA2 translation, as indicated by lines over bars (#p<0.05) (see Results for discussion of post hoc comparisons). For these puromycin experiments, each sample consisted of NAc pooled from both hemispheres of one rat. Numbers in bars represent number of samples/rats in each group. *p<0.05 compared to naïve aCSF group (Tukey test).
DISCUSSION
This study is the first to characterize regulation of protein translation in the NAc by glutamate transmission. In addition, we tested the hypothesis that this regulation was altered after incubation of cocaine craving. Our results reveal mGlu5 and NMDAR regulation of translation under control conditions, loss of NMDAR regulation of translation after incubation, and increased GluA1 translation after incubation.
We suggest that our results primarily reflect translation in MSNs, as these comprise ~90% of neurons in the NAc (48). However, contributions of other cell types cannot be excluded. Furthermore, we suggest that dendritic translation may contribute to our measures of protein translation based on the fact that glutamate inputs synapse on dendrites/spines of MSNs rather than the soma (48) and on similarities between the present mGlu1/5 results and those we have recently obtained by monitoring translation in processes of cultured MSNs (see next section).
mGlu5 and translational regulation
In hippocampus, it is well established that group I mGluRs stimulate translation (23, 46). In contrast, we found an MTEP-induced increase in protein translation in NAc tissue from both drug-naïve and “incubated” rats (Figure 3), consistent with results obtained in cultured NAc neurons (51). Since MTEP is an mGlu5 NAM, we cannot determine whether it acts by opposing mGlu5 transmission stimulated by tissue glutamate or by opposing constitutive mGlu5 activity (52), although our results in cultured NAc neurons support the latter possibility (51). Regardless, an important conclusion is that mGlu5 transmission exerts an inhibitory influence on protein translation in NAc tissue, opposite from the direction observed in hippocampus. Different “wiring” may reflect the fact that the NAc is comprised mainly of GABAergic MSNs, whereas hippocampal principal neurons are glutamatergic. While this underscores the importance of characterizing mGluR regulation of translation in the NAc, such differences are not entirely unprecedented. For example, mGlu5 stimulation in hippocampus leads to LTD that depends upon dendritic translation in the postsynaptic cell and AMPAR endocytosis (15, 46, 53), while the predominant form of mGlu5-induced LTD in striatum is presynaptically expressed via CB1R stimulation (54, 55). The only study implicating protein translation in striatal mGluR LTD indicated a locus for this translation outside the MSN itself (56), whereas another found no effect of translation inhibition (57).
We previously showed that mGlu5-mediated synaptic depression is disabled in the NAc of “incubated” rats (11). This is accompanied by decreased coupling of long Homer proteins to mGlu5 (12). Homers are critical for regulating group I mGluR association with downstream signaling pathways (58), and changes in association with long Homers can shift mGlu5 signaling (59–61), including signaling regulating protein translation (46, 62). Despite loss of mGlu5 synaptic depression and reduced association with long Homers after incubation (11), the MTEP-induced increase in translation in all groups demonstrates that mGlu5 remains capable of regulating protein translation (Figure 3). This dissociation is consistent with evidence that mGlu5-synaptic depression in NAc MSNs involves the canonical Gq-linked pathway (11, 55) and is independent of protein translation within the MSN (56, 57), whereas mGlu5 regulation of translation in hippocampus is mediated through a distinct beta-arrestin pathway (63, 64). Interestingly, after cocaine exposure and extinction training, mGlu5 remains capable of modulating reinstatement (65–69). It will be important to determine the signaling pathways mediating mGlu5’s disparate roles in regulating MSN function (e.g. (70)).
mGlu1 and translational regulation
Both mGlu1 activation and exposure to protein synthesis inhibitors remove CP-AMPARs from NAc synapses of “incubated rats” (11, 13), raising the possibility of overlapping mechanisms. We showed previously that mGlu1 removes CP-AMPARs via a PKC-dependent mechanism (11) and here we show no effect of the mGlu1 antagonist LY367385 on overall translation in any group (Figure 4). Nevertheless, we cannot rule out a role for mGlu1 in regulating translation of key proteins. We note that protein translation is implicated in the mGlu1-mediated removal of CP-AMPARs from the VTA of cocaine-exposed animals (71). In the VTA, as in the NAc of “incubated” rats, this mGlu1 LTD involves a swap in which high-conductance CP-AMPARs are removed and replaced with lower conductance GluA2-containing AMPARs (11, 28, 71–73). In VTA, the swap depends upon mGlu1-stimulated translation of GluA2 (28). Future studies should examine potential mGlu1 regulation of GluA1 or GluA2 translation in the NAc, as we have done here for NMDAR regulation.
NMDAR and translational regulation
NMDAR transmission, including spontaneous transmission (miniature EPSCs), can exert a tonic suppressive effect on translation (16–20). In hippocampus and superior colliculus, this was shown to depend on Ca2+ influx through the NMDAR channel and Ca2+-induced Ca2+ release, ultimately leading to phosphorylation of eEF2 (74, 75). Phosphorylation of eEF2 inhibits its activity and reduces general translation, a regulated process that contributes to synaptic plasticity (76, 77). In cultured NAc neurons, the NMDAR antagonist APV did not alter translation, probably due to low glutamate tone at NMDARs in this system (51). However, in NAc tissue from drug-naïve animals, our results with APV suggest a suppressive influence of NMDARs on overall protein translation similar to that observed in other regions (Figure 3A,B,D). Interestingly, the ability of APV to increase overall translation is absent in NAc tissue from “incubated” rats (Figure 3C,E). This alteration in NMDAR function may be related to emergence of GluN3-containing NMDARs in NAc MSN during incubation (78). The presence of this atypical subunit confers low Ca2+ permeability on NMDARs (79), and, as noted above, Ca2+ entry is part of the pathway by which NMDARs reduce translation (74, 75).
What is the functional consequence of loss of NMDAR regulation of translation? In hippocampal neurons, one of the proteins negatively regulated by NMDAR transmission is GluA1, such that NMDAR blockade increases dendritic GluA1 translation and synaptic insertion of homomeric GluA1 CP-AMPARs (17). Therefore, loss of NMDAR negative regulation could in theory account for increased homomeric GluA1 CP-AMPARs in the NAc after incubation of craving. However, in experiments using puromycin labeling to selectively measure GluA1 translation, we found no effect of APV in NAc tissue from drug-naïve or “incubated” rats (Figure 6). These results suggest that the functional significance of the loss of NMDAR regulation is not related to CP-AMPAR upregulation during cocaine withdrawal. Rather, our recent results (14) suggest that its significance may be manifest during the expression of incubated craving, i.e., during a cue-induced seeking test in which rats perform an operant response to deliver cues previously paired with cocaine. Specifically, we conducted immunoblotting studies of signaling molecules regulating translation in the NAc after long withdrawal (>47 days) from saline or cocaine self-administration (i.e., saline and cocaine groups identical to those in the present study) or identically treated rats subjected to a cue-induced seeking test (saline/test and cocaine/test groups). Several robust changes were observed in the cocaine/test group, including dephosphorylation of eEF2 relative to the cocaine group and the saline/test group. This is indicative of increased translation. Indeed, we also found that expression of incubated craving by the cocaine/test group depends on translation in the NAc (14). It is possible that NMDAR plasticity during incubation (78; see previous paragraph) lessens the ability of NMDAR transmission to maintain eEF2 in a phosphorylated/inactivated state, setting the stage for its dephosphorylation/activation during a cue-induced seeking test (14). Other evidence, however, indicates that altered signaling downstream of mTOR may underlie eEF2 dephosphorylation in this group (14). The picture is further complicated by other changes in translation pathways in the cocaine/test group. Most notably, dephosphorylation of eukaryotic initiation factor 2α (eIF2α), which promotes translation, is observed in the cocaine/test group and this dephosphorylation is required for expression of incubation (14). Future studies will probe the relationship between incubation-related alterations in NMDAR regulation of translation, downstream signaling cascades, and cue-induced cocaine seeking.
Conclusions
These experiments are the first to characterize how glutamatergic transmission regulates protein translation in the NAc under control conditions and after incubation of cocaine craving. Under control conditions, translation in the NAc showed similar regulation by NMDARs but different regulation by group I mGluRs compared to hippocampus, revealing cell-type differences in regulatory mechanisms. While metabolic labeling revealed no difference in overall protein translation between saline and cocaine animals, the ability of translation inhibition to normalize the elevation of homomeric GluA1 CP-AMPARs that mediates expression of incubation (13) indicates that some proteins must be differentially translated in the NAc of “incubated” rats. Indeed, we found elevated translation of GluA1 after incubation, suggesting a simple explanation for increased CP-AMPAR formation. Incubation was also accompanied by loss of NMDAR-mediated suppression of protein translation in the NAc. The functional consequences of this adaptation remain to be determined, but our results suggest that it is not related to increased GluA1 translation.
Supplementary Material
Acknowledgments
This research was supported by R01 DA015835 to MEW, postdoctoral NRSA DA040414 to MTS, and predoctoral NRSA DA036950 to CTW. We thank Dr. Emily Osterweil for generously sharing protocols and otherwise advising us on the metabolic labeling experiments, and Dr. Xuan (Anna) Li for help with design of qPCR experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures: The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. The American journal of psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, et al. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- 3.Parvaz MA, Moeller SJ, Malaker P, Sinha R, Alia-Klein N, Goldstein RZ. Abstinence reverses EEG-indexed attention bias between drug-related and pleasant stimuli in cocaine-addicted individuals. J Psychiatry Neurosci. 2016;41:150358. doi: 10.1503/jpn.150358. [DOI] [PubMed] [Google Scholar]
- 4.Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47(Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 5.Wolf ME. Synaptic mechanisms underlying persistent cocaine craving. Nature reviews Neuroscience. 2016;17:351–365. doi: 10.1038/nrn.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reichel CM, Bevins RA. Forced abstinence model of relapse to study pharmacological treatments of substance use disorder. Current drug abuse reviews. 2009;2:184–194. doi: 10.2174/1874473710902020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, et al. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol Psychiatry. 2011;69:708–711. doi: 10.1016/j.biopsych.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li P, Wu P, Xin X, Fan YL, Wang GB, Wang F, et al. Incubation of alcohol craving during abstinence in patients with alcohol dependence. Addict Biol. 2015;20:513–522. doi: 10.1111/adb.12140. [DOI] [PubMed] [Google Scholar]
- 9.Wang G, Shi J, Chen N, Xu L, Li J, Li P, et al. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS One. 2013;8:e68791. doi: 10.1371/journal.pone.0068791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends in neurosciences. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCutcheon JE, Loweth JA, Ford KA, Marinelli M, Wolf ME, Tseng KY. Group I mGluR activation reverses cocaine-induced accumulation of calcium-permeable AMPA receptors in nucleus accumbens synapses via a protein kinase C-dependent mechanism. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:14536–14541. doi: 10.1523/JNEUROSCI.3625-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loweth JA, Scheyer AF, Milovanovic M, LaCrosse AL, Flores-Barrera E, Werner CT, et al. Synaptic depression via mGluR1 positive allosteric modulation suppresses cue-induced cocaine craving. Nature neuroscience. 2014;17:73–80. doi: 10.1038/nn.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheyer AF, Wolf ME, Tseng KY. A protein synthesis-dependent mechanism sustains calcium-permeable AMPA receptor transmission in nucleus accumbens synapses during withdrawal from cocaine self-administration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:3095–3100. doi: 10.1523/JNEUROSCI.4940-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Werner CT, Stefanik MT, Milovanovic M, Caccamise A, Wolf ME. Protein translation in the nucleus accumbens is dysregulated during cocaine withdrawal and required for expression of incubation of cocaine craving. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2018 Feb 5;:pii: 2412–17. doi: 10.1523/JNEUROSCI.2412-17.2018. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luscher C, Huber KM. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron. 2010;65:445–459. doi: 10.1016/j.neuron.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sutton MA, Wall NR, Aakalu GN, Schuman EM. Regulation of dendritic protein synthesis by miniature synaptic events. Science. 2004;304:1979–1983. doi: 10.1126/science.1096202. [DOI] [PubMed] [Google Scholar]
- 17.Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125:785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 18.Sutton MA, Taylor AM, Ito HT, Pham A, Schuman EM. Postsynaptic decoding of neural activity: eEF2 as a biochemical sensor coupling miniature synaptic transmission to local protein synthesis. Neuron. 2007;55:648–661. doi: 10.1016/j.neuron.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 19.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kavalali ET, Monteggia LM. Synaptic mechanisms underlying rapid antidepressant action of ketamine. The American journal of psychiatry. 2012;169:1150–1156. doi: 10.1176/appi.ajp.2012.12040531. [DOI] [PubMed] [Google Scholar]
- 21.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osterweil EK, Krueger DD, Reinhold K, Bear MF. Hypersensitivity to mGluR5 and ERK1/2 leads to excessive protein synthesis in the hippocampus of a mouse model of fragile X syndrome. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:15616–15627. doi: 10.1523/JNEUROSCI.3888-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhakar AL, Dolen G, Bear MF. The pathophysiology of fragile X (and what it teaches us about synapses) Annual review of neuroscience. 2012;35:417–443. doi: 10.1146/annurev-neuro-060909-153138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michalon A, Sidorov M, Ballard TM, Ozmen L, Spooren W, Wettstein JG, et al. Chronic pharmacological mGlu5 inhibition corrects fragile X in adult mice. Neuron. 2012;74:49–56. doi: 10.1016/j.neuron.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richter JD, Bassell GJ, Klann E. Dysregulation and restoration of translational homeostasis in fragile X syndrome. Nature reviews Neuroscience. 2015;16:595–605. doi: 10.1038/nrn4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karler R, Finnegan KT, Calder LD. Blockade of behavioral sensitization to cocaine and amphetamine by inhibitors of protein synthesis. Brain research. 1993;603:19–24. doi: 10.1016/0006-8993(93)91294-3. [DOI] [PubMed] [Google Scholar]
- 27.Sorg BA, Ulibarri C. Application of a protein synthesis inhibitor into the ventral tegmental area, but not the nucleus accumbens, prevents behavioral sensitization to cocaine. Synapse. 1995;20:217–224. doi: 10.1002/syn.890200305. [DOI] [PubMed] [Google Scholar]
- 28.Mameli M, Balland B, Lujan R, Luscher C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science. 2007;317:530–533. doi: 10.1126/science.1142365. [DOI] [PubMed] [Google Scholar]
- 29.Bailey J, Ma D, Szumlinski KK. Rapamycin attenuates the expression of cocaine-induced place preference and behavioral sensitization. Addict Biol. 2012;17:248–258. doi: 10.1111/j.1369-1600.2010.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu F, Zhong P, Liu X, Sun D, Gao HQ, Liu QS. Metabotropic glutamate receptor I (mGluR1) antagonism impairs cocaine-induced conditioned place preference via inhibition of protein synthesis. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:1308–1321. doi: 10.1038/npp.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith LN, Jedynak JP, Fontenot MR, Hale CF, Dietz KC, Taniguchi M, et al. Fragile X mental retardation protein regulates synaptic and behavioral plasticity to repeated cocaine administration. Neuron. 2014;82:645–658. doi: 10.1016/j.neuron.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi X, Miller JS, Harper LJ, Poole RL, Gould TJ, Unterwald EM. Reactivation of cocaine reward memory engages the Akt/GSK3/mTOR signaling pathway and can be disrupted by GSK3 inhibition. Psychopharmacology. 2014;231:3109–3118. doi: 10.1007/s00213-014-3491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jian M, Luo YX, Xue YX, Han Y, Shi HS, Liu JF, et al. eIF2alpha dephosphorylation in basolateral amygdala mediates reconsolidation of drug memory. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:10010–10021. doi: 10.1523/JNEUROSCI.0934-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang W, Placzek AN, Viana Di Prisco G, Khatiwada S, Sidrauski C, Krnjevic K, et al. Translational control by eIF2alpha phosphorylation regulates vulnerability to the synaptic and behavioral effects of cocaine. Elife. 2016;5 doi: 10.7554/eLife.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dayas CV, Smith DW, Dunkley PR. An emerging role for the Mammalian target of rapamycin in "pathological" protein translation: relevance to cocaine addiction. Front Pharmacol. 2012;3:13. doi: 10.3389/fphar.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neasta J, Barak S, Hamida SB, Ron D. mTOR complex 1: a key player in neuroadaptations induced by drugs of abuse. Journal of neurochemistry. 2014;130:172–184. doi: 10.1111/jnc.12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Most D, Leiter C, Blednov YA, Harris RA, Mayfield RD. Synaptic microRNAs Coordinately Regulate Synaptic mRNAs: Perturbation by Chronic Alcohol Consumption. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2016;41:538–548. doi: 10.1038/npp.2015.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt EK, Clavarino G, Ceppi M, Pierre P. SUnSET, a nonradioactive method to monitor protein synthesis. Nature methods. 2009;6:275–277. doi: 10.1038/nmeth.1314. [DOI] [PubMed] [Google Scholar]
- 40.Goodman CA, Hornberger TA. Measuring protein synthesis with SUnSET: a valid alternative to traditional techniques? Exerc Sport Sci Rev. 2013;41:107–115. doi: 10.1097/JES.0b013e3182798a95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferrario CR, Loweth JA, Milovanovic M, Wang X, Wolf ME. Distribution of AMPA receptor subunits and TARPs in synaptic and extrasynaptic membranes of the adult rat nucleus accumbens. Neuroscience letters. 2011;490:180–184. doi: 10.1016/j.neulet.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolf ME, Tseng KY. Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why? Frontiers in molecular neuroscience. 2012;5:72. doi: 10.3389/fnmol.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loweth JA, Tseng KY, Wolf ME. Adaptations in AMPA receptor transmission in the nucleus accumbens contributing to incubation of cocaine craving. Neuropharmacology. 2014;76 Pt B:287–300. doi: 10.1016/j.neuropharm.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cosford ND, Tehrani L, Roppe J, Schweiger E, Smith ND, Anderson J, et al. 3-[(2-Methyl-1,3-thiazol-4-yl)ethynyl]-pyridine: a potent and highly selective metabotropic glutamate subtype 5 receptor antagonist with anxiolytic activity. J Med Chem. 2003;46:204–206. doi: 10.1021/jm025570j. [DOI] [PubMed] [Google Scholar]
- 46.Waung MW, Huber KM. Protein translation in synaptic plasticity: mGluR-LTD, Fragile X. Current opinion in neurobiology. 2009;19:319–326. doi: 10.1016/j.conb.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clark BP, Baker RS, Goldsworthy J, Harris JR, Kingston AE. (+)-2-methyl-4-caboxyphenylglycine (LY367385) selectively antagonises metabotropic glutamate mGluR1 receptors. Bioorg Med Chem Lett. 1997;7:2777–2780. [Google Scholar]
- 48.Ferrario CR, Loweth JA, Milovanovic M, Ford KA, Galinanes GL, Heng LJ, et al. Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca(2)(+)-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology. 2011;61:1141–1151. doi: 10.1016/j.neuropharm.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Starck SR, Green HM, Alberola-Ila J, Roberts RW. A general approach to detect protein expression in vivo using fluorescent puromycin conjugates. Chem Biol. 2004;11:999–1008. doi: 10.1016/j.chembiol.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 50.Ge J, Zhang CW, Ng XW, Peng B, Pan S, Du S, et al. Puromycin Analogues Capable of Multiplexed Imaging and Profiling of Protein Synthesis and Dynamics in Live Cells and Neurons. Angew Chem Int Ed Engl. 2016;55:4933–4937. doi: 10.1002/anie.201511030. [DOI] [PubMed] [Google Scholar]
- 51.Stefanik MT, Sakas C, Lee D, Wolf ME. The regulation of protein translation by ionotropic and metabotropic glutamate receptors in co-cultured nucleus accumbens and prefrontal cortex neurons. Submitted. 2017 doi: 10.1016/j.neuropharm.2018.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ango F, Prezeau L, Muller T, Tu JC, Xiao B, Worley PF, et al. Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature. 2001;411:962–965. doi: 10.1038/35082096. [DOI] [PubMed] [Google Scholar]
- 53.Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- 54.Choi S, Lovinger DM. Decreased frequency but not amplitude of quantal synaptic responses associated with expression of corticostriatal long-term depression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:8613–8620. doi: 10.1523/JNEUROSCI.17-21-08613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yin HH, Davis MI, Ronesi JA, Lovinger DM. The role of protein synthesis in striatal long-term depression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:11811–11820. doi: 10.1523/JNEUROSCI.3196-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jung KM, Sepers M, Henstridge CM, Lassalle O, Neuhofer D, Martin H, et al. Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nat Commun. 2012;3:1080. doi: 10.1038/ncomms2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Szumlinski KK, Ary AW, Lominac KD. Homers regulate drug-induced neuroplasticity: implications for addiction. Biochem Pharmacol. 2008;75:112–133. doi: 10.1016/j.bcp.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kammermeier PJ, Xiao B, Tu JC, Worley PF, Ikeda SR. Homer proteins regulate coupling of group I metabotropic glutamate receptors to N-type calcium and M-type potassium channels. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:7238–7245. doi: 10.1523/JNEUROSCI.20-19-07238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kammermeier PJ, Worley PF. Homer 1a uncouples metabotropic glutamate receptor 5 from postsynaptic effectors. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:6055–6060. doi: 10.1073/pnas.0608991104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kammermeier PJ. Endogenous homer proteins regulate metabotropic glutamate receptor signaling in neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:8560–8567. doi: 10.1523/JNEUROSCI.1830-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ronesi JA, Collins KA, Hays SA, Tsai NP, Guo W, Birnbaum SG, et al. Disrupted Homer scaffolds mediate abnormal mGluR5 function in a mouse model of fragile X syndrome. Nature neuroscience. 2012;15:431–440. S431. doi: 10.1038/nn.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eng AG, Kelver DA, Hedrick TP, Swanson GT. Transduction of group I mGluR-mediated synaptic plasticity by beta-arrestin2 signalling. Nat Commun. 2016;7:13571. doi: 10.1038/ncomms13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stoppel LJ, Auerbach BD, Senter RK, Preza AR, Lefkowitz RJ, Bear MF. beta-Arrestin2 Couples Metabotropic Glutamate Receptor 5 to Neuronal Protein Synthesis and Is a Potential Target to Treat Fragile X. Cell Rep. 2017;18:2807–2814. doi: 10.1016/j.celrep.2017.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kufahl PR, Hood LE, Nemirovsky NE, Barabas P, Halstengard C, Villa A, et al. Positive Allosteric Modulation of mGluR5 Accelerates Extinction Learning but Not Relearning Following Methamphetamine Self-Administration. Front Pharmacol. 2012;3:194. doi: 10.3389/fphar.2012.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang X, Moussawi K, Knackstedt L, Shen H, Kalivas PW. Role of mGluR5 neurotransmission in reinstated cocaine-seeking. Addict Biol. 2013;18:40–49. doi: 10.1111/j.1369-1600.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Knackstedt LA, Trantham-Davidson HL, Schwendt M. The role of ventral and dorsal striatum mGluR5 in relapse to cocaine-seeking and extinction learning. Addict Biol. 2014;19:87–101. doi: 10.1111/adb.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bird MK, Lohmann P, West B, Brown RM, Kirchhoff J, Raymond CR, et al. The mGlu5 receptor regulates extinction of cocaine-driven behaviours. Drug Alcohol Depend. 2014;137:83–89. doi: 10.1016/j.drugalcdep.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 69.Knackstedt LA, Schwendt M. mGlu5 Receptors and Relapse to Cocaine-Seeking: The Role of Receptor Trafficking in Postrelapse Extinction Learning Deficits. Neural Plast. 2016;2016:9312508. doi: 10.1155/2016/9312508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mao L, Yang L, Tang Q, Samdani S, Zhang G, Wang JQ. The scaffold protein Homer1b/c links metabotropic glutamate receptor 5 to extracellular signal-regulated protein kinase cascades in neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:2741–2752. doi: 10.1523/JNEUROSCI.4360-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bellone C, Luscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nature neuroscience. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- 72.Bellone C, Luscher C. mGluRs induce a long-term depression in the ventral tegmental area that involves a switch of the subunit composition of AMPA receptors. The European journal of neuroscience. 2005;21:1280–1288. doi: 10.1111/j.1460-9568.2005.03979.x. [DOI] [PubMed] [Google Scholar]
- 73.Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, et al. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nature neuroscience. 2009;12:1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- 74.Scheetz AJ, Nairn AC, Constantine-Paton M. NMDA receptor-mediated control of protein synthesis at developing synapses. Nature neuroscience. 2000;3:211–216. doi: 10.1038/72915. [DOI] [PubMed] [Google Scholar]
- 75.Reese AL, Kavalali ET. Spontaneous neurotransmission signals through store-driven Ca(2+) transients to maintain synaptic homeostasis. Elife. 2015;4 doi: 10.7554/eLife.09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heise C, Gardoni F, Culotta L, di Luca M, Verpelli C, Sala C. Elongation factor-2 phosphorylation in dendrites and the regulation of dendritic mRNA translation in neurons. Front Cell Neurosci. 2014;8:35. doi: 10.3389/fncel.2014.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Taha E, Gildish I, Gal-Ben-Ari S, Rosenblum K. The role of eEF2 pathway in learning and synaptic plasticity. Neurobiology of learning and memory. 2013;105:100–106. doi: 10.1016/j.nlm.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 78.Christian DT, Tseng KY, Wolf ME. Extended access cocaine self-administration leads to increased GluN3-containing NMDA receptor function in the rat nucleus accumbens. Society for Neuroscience 2017 [Google Scholar]
- 79.Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nature reviews Neuroscience. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.