Abstract
Fungal infections remain a significant global health problem in humans. Fungi infect millions of people worldwide and cause from acute superficial infections to life-threatening systemic disease to chronic illnesses. Trying to decipher the complex innate and adaptive immune mechanisms that protect humans from pathogenic fungi is therefore a key research goal that may lead to immune-based therapeutic strategies and improved patient outcomes. In this review, we summarize how the cells and molecules of the innate immune system activate the adaptive immune system to elicit long-term immunity to fungi. We present current knowledge and exciting new advances in the context of organ-specific immunity, outlining the tissue-specific tropisms for the major pathogenic fungi of humans, the antifungal functions of tissue-resident myeloid cells, and the adaptive immune responses required to protect specific organs from fungal challenge.
Every organ is endowed with resident immune cells that are specially equipped for immune surveillance and homeostatic maintenance of organ functions [1]. Many of these resident immune cell populations are macrophages or close relations, including alveolar macrophages in the lung and microglia in the brain. Organs such as the skin, gastrointestinal (GI) tract and other mucosal barriers are also rich in other innate cells that provide additional homeostatic control, and include dendritic cells (DCs) and non-conventional lymphoid cells such as innate lymphoid cells (ILCs), mucosal-associated invariant T-cells (MAIT) cells and gamma-delta T-cells. Based on their close association with the tissue parenchyma and vasculature, these innate cells are often the first responders to tissue damage and infection and thus they have a remarkable influence over the resulting immune response. For example, their production of chemokines drives accumulation of inflammatory cells [2], while expression of MHC molecules by these cells can regulate adaptive immune responses [3]. Therefore, resident immune cells can act as a bridge between innate and adaptive immunity and shape organ-specific immune responses.
Human fungal diseases continue to represent a significant global health problem [4]. Despite antifungal therapy being widely available, mortality and morbidity rates associated with fungal infections remain unacceptably high. Moreover, there are worrying recent reports of emerging fungal pathogens with inherent resistance to our arsenal of available antifungal drugs [5]. Vaccines and immunotherapies to treat fungal diseases are therefore a priority. In the last few decades, we have significantly enhanced our understanding of the molecular pathways controlling innate fungal recognition and the antifungal responses elicited by myeloid cells. However, we understand less with regard to the generation and regulation of the adaptive immune response to many of these pathogens, and even less about the organ-specific pathways controlling fungal growth. Yet, the goal of antifungal immunotherapies will be to elicit long-term memory and protection, thus it will be important to determine the signals required to generate effective antifungal memory responses. Moreover, the specificity of these immunotherapeutic approaches will also need to be fine-tuned to reduce the potential side effects, thus delineating antifungal immune mechanisms in an organ-specific context will also be important.
In this review, we will cover how cells and molecules considered part of the innate immune system regulate the adaptive immune response during fungal infections, presenting these exciting new advances in the context of organ-specific immunity (Figure 1). Finally, we will highlight gaps in knowledge and indicate at major new developments in other fields that may help to bridge these gaps in the fungal immunology field.
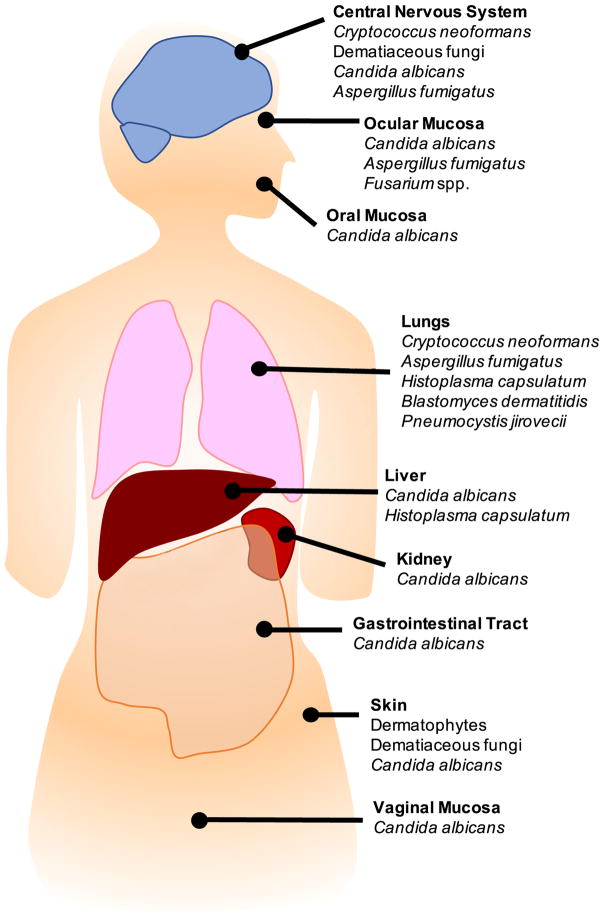
Figure 1.
Overview of the predominant fungal species causing clinical infections of the labelled organs in humans.
The Central Nervous System (CNS)
Fungal CNS infections are rare and most often associate with acquired immunodeficiency caused by HIV infection [6]. Less often, inborn errors in immunity predispose to fungal CNS disease [2]. The majority of fungal meningitis cases are caused by Cryptococcus neoformans, an infection that is critically dependent on CD4+ T-cells since HIV infection is the predominant risk factor for developing infections with this species [7; 8]. Cerebral infection by dematiaceous fungi (e.g. Exophiala species, Curvularia species) is the most common form of systemic disease for this group of pathogenic fungi, and primarily affects “putatively” immunocompetent people who become traumatically inoculated through the skin or mucous membranes, although cerebral cases without apparent mucocutaneous inoculation are not uncommon [9]. Other fungi that cause meningitis include Candida albicans and Aspergillus fumigatus, both rare causes of brain infection that most commonly occur following complications after neurosurgery or with iatrogenic immunosuppression, but have also been associated with some anti-cancer treatments [10] and mutations in the signaling molecule, CARD9 [2; 11].
Microglia are the most numerous innate immune cells in the CNS and are important for neuronal function, immune surveillance and synaptic pruning [12]. The ontogeny of microglia, along with several other tissue-resident macrophage populations, has recently been revealed to be dependent on embryonic precursors deriving from the fetal yolk sac that seed the developing brain and give rise to long-lived cells that divide in situ [1; 12; 13]. Microglia exhibit strong responses to various species of fungi, including C. albicans and C. neoformans [14; 15], utilizing the receptor GPR43 [16], PI3 kinase signaling [17], and inducible nitric oxide synthase (iNOS) [18] for C. neoformans phagocytosis and killing. Using bone-marrow derived macrophages, several studies have shown that classically-activated macrophages (M1) are required for protection against C. neoformans, since these cells elicit IFNγ-producing Th1 cells which control cryptococcal infections in the lung and brain whereas alternatively-activated macrophages (M2) and Th2 cells do not [6; 19]. Microglia may also polarize towards ‘M1’ and ‘M2’-like activation states, however it is not yet clear if the M1/M2 paradigm is accurately applicable to microglia [20] and if the same clear protective phenotypes are generated in the brain as observed with bone-marrow derived M1 macrophages. Likewise, our understanding of how the protective antifungal Th1 response is generated in the brain is not well understood. Microglia express MHC Class II and produce inflammatory cytokines such as IL-12 during brain inflammation [21; 22], and are therefore capable of activating CD4+ T-cells and driving Th1 differentiation; this has never formally been shown for fungal-induced meningitis although it has been demonstrated for parasitic meningitis [23]. Moreover, there are other antigen-presenting cells (APCs) in the brain, such as astrocytes and recruited DCs, that may also be involved in the induction of antifungal T-cell immunity, but are as yet not explored.
Lastly, human CARD9 deficiency is a primary immunodeficiency disorder associated with the spontaneous development of fungal brain infections caused by C. albicans and A. fumigatus, as well as dermatophytes and dematiaceous yeast-like fungi [24]. CARD9 is a signaling adaptor protein expressed mainly by myeloid cells, including high expression by microglia [2], and is critical for the activation for antifungal immune responses to a variety of fungal pathogens in mice and humans [24]. We recently showed that CARD9 deficiency predisposes to fungal brain infections because of defects in neutrophil chemoattractant production by glial cells in the brain, resulting in absent recruitment of neutrophils during C. albicans infection and uncontrolled fungal growth (Figure 2); these mechanisms were specific to both the brain and fungal infection [2]. Whether CARD9 promotes the generation of adaptive immune responses in the brain, and to which fungal species, remains unexplored. In other organs, however, CARD9 has been indicated to play key roles in the induction of T-cell responses in a variety of settings (discussed in detail below).
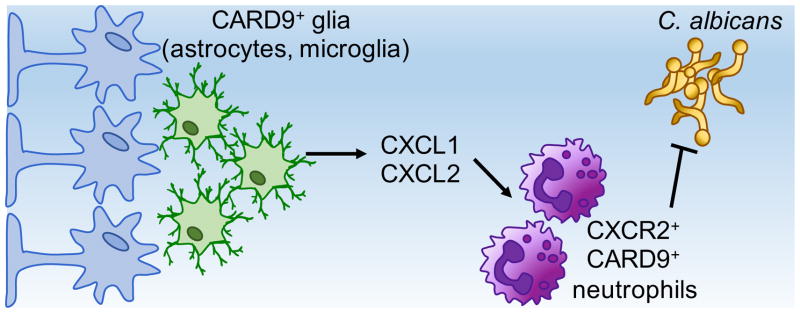
Figure 2. CARD9-Dependent Control of C. albicans Infection of the Brain.
Glial cells in the brain, including astrocytes (shown in blue) and microglia (shown in green), produce CXCL1 and CXCL2 upon C. albicans infection in a CARD9-dependent manner. This in turn recruits CXCR2+ neutrophils, which are required to control C. albicans infection in this tissue.
The Liver
Fungal infections of the liver are usually a complication of disseminated infections, such as those caused by Candida albicans and Histoplasma capsulatum [25; 26]. The tissue-resident macrophages of the liver are called Kupffer cells, and they make up the largest population of tissue-resident macrophages in the body. Kupffer cells are found along the sinusoidal endothelium and closely interact with these cells and hepatocytes. Kupffer cells are required for hepatocyte health and liver function, as well as clearing microbial products and toxins from the blood that filter through the liver via the portal vein [27].
The role of Kupffer cells in sensing and responding to fungi is very poorly understood. Liver macrophages vastly increase in number following systemic C. albicans infection and the liver clears yeast cells quickly in immunocompetent mice [28], suggesting that Kupffer cells are proficient at fungal phagocytosis and killing. Indeed, early work showed that Kupffer cells were able to phagocytose C. albicans yeast with similar kinetics to peritoneal macrophages, although killing of phagocytosed yeasts by Kupffer cells was not as efficient [29]. Other studies have shown a dependency on integrin αxβ2 and tyrosine kinases for antifungal defense by Kupffer cells [30; 31]. In terms of stimulating adaptive antifungal immunity, Kupffer cells are highly tolerogenic and preferentially induce regulatory T-cells (Treg) rather than effector cells [3]. Instead, activation of effector T-cells falls onto the shoulders of liver-resident DCs, a highly heterogeneous population which separates into several subsets characteristic of those found in both lymphoid and non-lymphoid organs, each with distinct functions [32]. For example, human CD141+ liver DCs (equivalent to CD103+ DCs in the mouse) are abundant in healthy liver and preferentially expand Th1 and Th17 effector cells [33], as well as activating anti-viral CD8+ T-cells [32]. Whether these functions are activated during a systemic fungal infection, and the mechanisms controlling such a response, have yet to be defined.
The Kidneys
Systemic candidiasis is associated with profound kidney invasion and renal failure in mice [28; 34]. As such, the majority of studies analyzing disseminated C. albicans infections in mice have focused on the kidney as the primary target organ. Multiple studies have shown a strong induction of the innate immune response in the kidney during C. albicans infection [35; 36], associated with a large influx of neutrophils and Ly6Chi monocytes [28] (Figure 3). This innate inflammatory response is required to control infection early on [37; 38], yet also drives immunopathology through the action of CCR1-expressing neutrophils late in the course of infection [39].
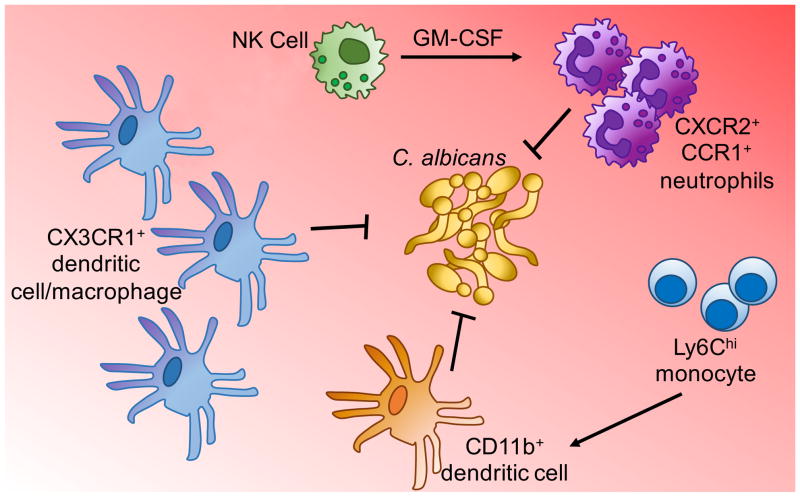
Figure 3. Myeloid Cell Mediated Protection Against Renal C. albicans Infections.
Control of C. albicans growth in the kidney requires multiple populations of myeloid cells, including resident CX3CR1-expressing macrophages and dendritic cells, and recruitment of inflammatory monocytes and neutrophils. The antifungal functions of neutrophils are further enhanced by GM-CSF, produced by recruited NK cells.
The kidneys have an extensive network of CX3CR1+ phagocytes that interact with renal endothelial cells and actively probe their environment to provide immune surveillance [40; 41]. However, determining the difference between a kidney-resident macrophage versus a kidney-resident DC has proven difficult and is a key issue in the renal immunology field [42; 43]. Kidney-resident macrophages and DCs express similar surface markers, such as CX3CR1, CD11b and CD11c, and share developmental pathways [43; 44]. These cells also share functional qualities such as phagocytosis and antigen presentation, whereas splenic macrophages and DCs preferentially perform either phagocytosis or antigen presentation, respectively [45; 46]. As a result, many studies indicate that their results are due to the action of either kidney macrophages or DCs, when their methods do not allow for this distinction. The use of microscopy to define morphology and spatial localization, combined with surface marker expression, has proven more useful to distinguish between these renal mononuclear cell types [36; 42]. Moreover, the advent of sophisticated sequencing-based technologies has recently been used to separate complex subtypes of immune cells in non-lymphoid organs [47], and may provide further clarity on the different subpopulations of phagocytes in the kidney in the near future. Nomenclature issues aside, most studies agree that there is a large population of CX3CR1+ macrophages found throughout the healthy kidney cortex and medulla regions [40]. The majority of kidney-resident DCs are also CX3CR1+, and their function appears to be closely related to their spatial localization within the kidney [48; 49]. CX3CR1+ DCs in the kidney cortex, for example, are the main conductors of adaptive immunity, while DCs in the kidney medulla are more involved in the induction of innate immune responses through chemokine production [50]. Finally, there is a small population of CD103+CD11b− DCs, no CD103+CD11b+ DCs, and small numbers of lymphocytes resident in the healthy kidney [42; 43].
The role of kidney-resident phagocytes in control of fungal infection has been explored in a small number of studies (Figure 3). Early on in a C. albicans infection, there are significant fluctuations in the myeloid cell pool in the kidney [51], including a large expansion of macrophages [28; 36] and accumulation of CD103+CD11b− DCs [52]. CX3CR1+ macrophages are the main phagocytic cells for C. albicans during infection and as a result, decreased accumulation of CX3CR1+ macrophages in vivo results in significantly increased kidney fungal burdens associated with poor survival of CX3CR1-deficient macrophages. In line with this, humans with the dysfunctional CX3CR1-M280 allele are more likely to develop systemic candidiasis [36] and human CX3CR1-M280 mononuclear phagocytes exhibit impaired survival [53]. Thus, kidney-resident macrophages are critical for early innate control of C. albicans infection.
Kidney-resident DCs are also required for control of renal fungal infections. CD11c+ DCs recruit GM-CSF-producing NK cells, which enhance the antifungal activity of accumulating neutrophils in the kidney [51] (Figure 3). Similarly, IL-15-producing Ly6Chi monocytes in the spleen have also been shown to drive GM-CSF+ NK cell activation and neutrophil function during systemic C. albicans infection [54]. In addition to their role in innate immunity, kidney-resident DCs are also critical for the activation of adaptive immune responses [43]. However, the induction and control of antifungal lymphocyte responses in the kidney is far less well studied. Early studies demonstrated that IL-4 was detrimental to systemic C. albicans control, and that IFNγ+ Th1 cells were protective [37; 55]. CD103+CD11b− DCs in the kidney are required for the production of the Th1-polarizing cytokine, IL-12, during C. albicans infection [52] and thus may be important for inducing these T-cell populations. CD103+ DCs in the kidney have also been shown to mediate Treg recruitment and activation of cytotoxic T-cells in models of kidney injury [56; 57], indicating that this population has diverse functions that are context-dependent. However, mice lacking T-cells do not show an increased susceptibility to systemic C. albicans infections [58] and thus the relevance of these DC functions in the context of a fungal infection is uncertain. Mice lacking CD103+ DCs do not have increased kidney fungal burdens and exhibit normal survival rates when challenged with C. albicans [52], supporting the lack of a role for T-cell-activating DCs. However, while T-cells do accumulate within infected kidneys [28], these cells do not carry the appropriate fungal-specific T-cell receptors [59]. This suggests that there is a defect in the ability to recruit the relevant antigen-specific T-cell populations to the C. albicans-infected kidney. Indeed, restoration of this defect leads to a significant reduction in kidney fungal burdens [59], indicating that antigen-specific T-cells can play protective roles in the kidney, but their recruitment becomes disrupted during fungal infection.
The Lungs
Pulmonary fungal infections are among the most common, partly because fungal spores are ubiquitous in the environment and it is estimated that we inhale hundreds of fungal spores daily. As a result, immunosuppressed patients are highly susceptible to pulmonary infections caused by A. fumigatus, C. neoformans, H. capsulatum, Blastomyces dermatitidis and Pneumocystis jirovecii. Some of these species along with several others (for example, Alternaria species) have also been shown to exacerbate underlying lung diseases including asthma and cystic fibrosis [60; 61; 62; 63]. As such, fungi cause a wide range of pulmonary disorders that are challenging to diagnose and treat.
Alveolar macrophages (AMs) are, like microglia and Kupffer cells, long-lived resident immune cells that derive from fetal precursors and self-renew [64]. AMs are found in the alveolar airspaces, and are distinct from interstitial macrophage subpopulations in the lung, which are found in the lung parenchyma. AMs have been shown to phagocytose Pneumocystis carinii yeasts through a GM-CSF-dependent pathway and innate recognition by the C-type lectin receptor Dectin-1, leading to fungal killing and protection [65; 66]. AMs are also adept at phagocytosis and killing of A. fumigatus conidia [67], which again is predominantly dependent on expression of Dectin-1 by AMs [68] (Figure 4A). In contrast, some fungi can readily survive inside AMs, using these host cells as a means of escaping immune recognition and causing latent infection (Figure 4B). For example, B. dermatitidis, H. capsulatum and C. neoformans have all been shown to subvert AM oxidative killing mechanisms through inhibition of iNOS [69].
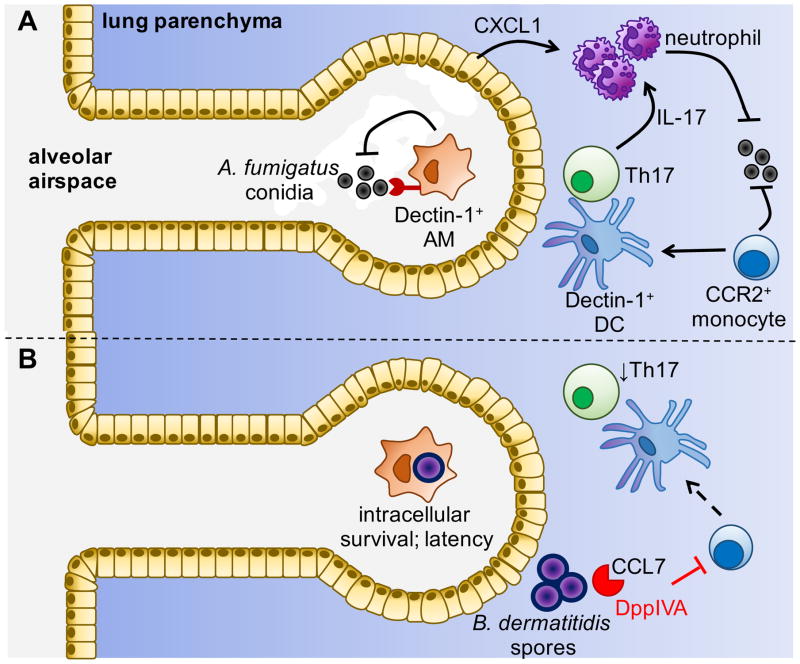
Figure 4. Mechanisms Controlling Immunity to Pulmonary Fungal Pathogens.
(A) A. fumigatus conidia are inhaled and phagocytosed by resident alveolar macrophages (AMs) via Dectin-1. Escape into the lung parenchyma activates epithelial cells to produce CXCL1, which recruits neutrophils that help fight infection. A. fumigatus infection also stimulates the recruitment of CCR2+ monocytes, which differentiate into Dectin-1-expressing DCs that elicit protective Th17 cells. (B) In contrast, other fungi such as B. dermatitidis are able to survive intracellularly within AMs, and can further subvert immunity via the production of proteases (e.g. dipeptidylpeptidase IVA; DppIVA) that cleave chemokines to prevent monocyte/DC recruitment and function.
How AMs affect antifungal T-cell responses is not well understood. AMs express low levels of co-stimulatory molecules and MHC Class II, and are thus considered poor conductors of adaptive immunity [64]. Like Kupffer cells in the liver, the poor ability of AMs to activate T-effector cells could be to help maintain a tolerogenic environment, especially since the lung is constantly dealing with microbial and environmental insults. Instead, antifungal T-cell responses are most likely activated by lung-resident DCs. There are at least 3 subsets of DCs resident in the lung (reviewed extensively in [70]), which have been shown to mediate distinct and overlapping functions. For example, CD103+ DCs are superior at cross-presentation and activation of anti-viral CD8+ T-cells, while CD11b+ DCs promote Th2 and Th17 responses, depending on the initial insult [70; 71]. In the A. fumigatus-infected lung, CD11b+CD24+ DCs are required to drive protective Th17 responses [72], while CD103+ DCs have been shown to activate Th1 responses in the H. capsulatum-infected lung via Type 1 IFN production and Toll-like receptor (TLR) 7 and 9 signaling [73]. CCR2+ monocyte-derived DCs are also recruited to the lung upon fungal infection to provide additional protection [74] and prime CD4+ T-cells [75] (Figure 4A). Their recruitment has been found to depend on the scavenger receptor MARCO during C. neoformans infections, since deletion of this receptor in mice leads to uncontrolled lung infection with reduced recruitment of monocyte-derived DCs [76], similar to what has been described for mycobacterial infections [77]. Recruitment of CCR2+ monocytes to the lung has also been found to be targeted by some fungi to subvert and evade clearance. For example, B. dermatitidis produces a proteolytic enzyme, DppIVA, which cleaves monocyte-recruiting chemokines, such as CCL7, rendering them inactive leading to reduced monocyte recruitment and differentiation [78] (Figure 4B). Moreover, B. dermatitidis can also induce host metalloproteinase MMP-2, which additionally cleaves CCL7 and disrupts the CCR2+ monocyte response and subsequent T-cell responses [79].
The molecular control of IL-17-dependent responses, which appear to be generally non-redundant for protection against pulmonary fungal infections [80; 81; 82], are only partially understood. During A. fumigatus infection, Dectin-1 is required for Th17 polarization (Figure 4A), since genetic deficiency of Dectin-1 or depletion of Dectin-1+ monocyte-derived DCs leads to enhanced Th1 polarization of A. fumigatus-specific T-cells due to aberrant T-bet expression [83]. On the other hand, Dectin-1 is redundant for Th17 immunity during infection with H. capsulatum and B. dermatitidis, which requires the TLRs instead [80]. Indeed, while Dectin-1 is certainly capable of driving Th17 immunity, other receptors have been shown to be superior at this function, such as the related receptor, Dectin-2 [84] and the Mannose Receptor (MR; CD206) [85]. The role of Dectin-2 and MR in pulmonary antifungal immunity has only been analyzed in a small number of studies, which have shown that Dectin-2 plays species-specific roles in antifungal defense in the lung [86], and MR signaling is required for CD4+ T-cell proliferation in the C. neoformans-infected lung [87]. Both Dectin-1 and Dectin-2 signal using CARD9 [88], which has also been indicated to drive Th17 immunity [24]. In the lung, deletion of Card9 causes a reduction in Th17 polarization during pulmonary infection with C. neoformans [89], H. capsulatum and B. dermatitidis [86]. However, while the C-type lectin-CARD9 signaling axis appears to be critical for the induction of protective T-cell immunity in the murine lung, it is important to note that mutations in these molecules in humans have thus far not been found to enhance susceptibility to pulmonary fungal infections. Similarly, patients with mutations in IL-17F, IL-17RA, IL-17RC and ACT1 that lack IL-17 receptor signaling have not been reported to develop pulmonary fungal disease to date [90; 91].
Lastly, lung epithelial cells can also significantly contribute to control of pulmonary infection and intimately associate with the lung-resident myeloid cells discussed above. Epithelial cells produce inflammatory cytokines and neutrophil chemoattractants using a MyD88-dependent pathway during acute A. fumigatus pulmonary challenge, which is critical for inducing protective neutrophil recruitment and fungal clearance [92] (Figure 4A). How epithelial cells influence antifungal T-cells in the lung remains to be determined, although their interaction with lung-resident DCs and indirect effects on T-cell polarization are well characterized in other models. For example, epithelial production of TSLP has been shown to profoundly affect DC responses, resulting in the production of Th2-polarising cytokines such as IL-5 and IL-13 through a pathway dependent on OX40L [93], which in turn drives pathogenic Th2 responses and contributes towards the severity of asthma [94].
The Skin and Subcutaneous Tissues
The human skin is readily colonized by fungi, particularly Malassezia species and, to a lesser degree, Candida species [95]. These fungi, while considered commensals, have been suggested to influence and exacerbate underlying skin disorders such as alopecia and psoriasis. Significant improvements in psoriatic symptoms have been reported in some patients treated with antifungal drugs, although the underlying reasons for this are not well understood since no clear correlations between Malassezia and Candida colonization and severity of psoriasis have been found [96]. Superficial fungal infections of the skin are relatively common and are most often caused by the dermatophytes, such as Trichophyton. However, some fungal species can cause extreme, sometimes deforming, chronic infections of the skin and subcutaneous tissue. These tropical diseases include chromoblastomycosis (most often caused by Fonsecaea pedrosoi), phaeohyphomycosis (caused by a variety of dematiaceous fungi) and eumycetoma (most often caused by Madurella species). In all cases, the immune responses and resident immune cells in the skin controlling fungal growth and clearance are not well understood.
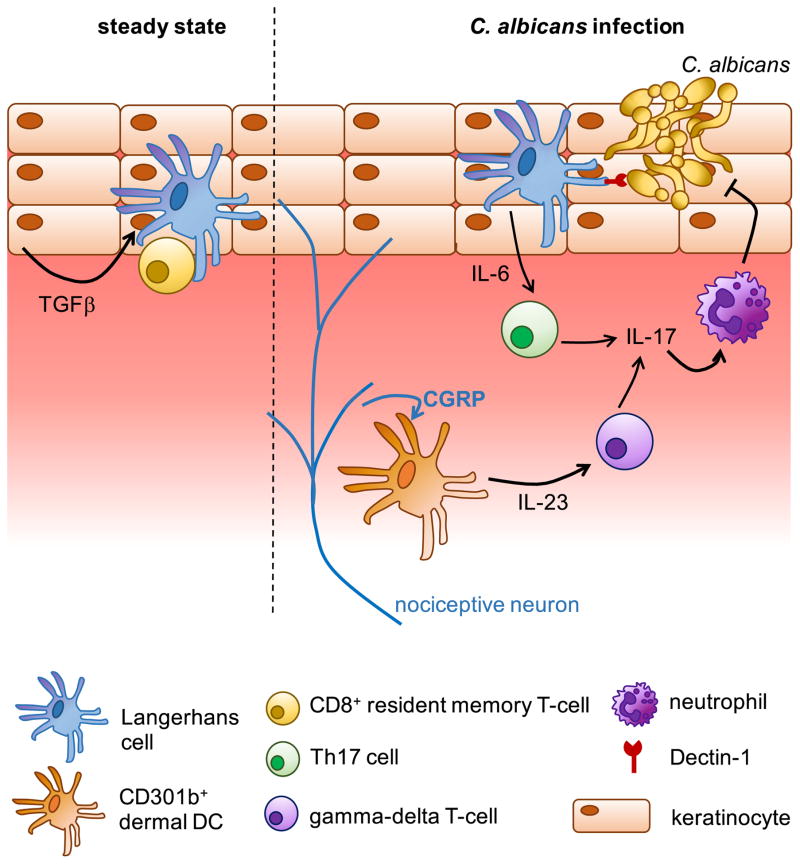
Langerhans cells (LC) are the best-defined DC population resident in the skin, found in the epidermal layer. They express large amounts of the C-type lectin Langerin, which is the major antifungal receptor in human LC by binding to both mannose and β-glucan within Candida and Malassezia cell walls [97]. In the deeper dermis layer, dermal DCs are found and can be split into several subsets based on expression of Langerin and CD103 [98]. The role of skin DC subsets in antifungal immunity has been investigated extensively by the Kaplan laboratory, in which an epicutaneous C. albicans infection model has been used to probe the relative contributions of different skin DC subsets to driving IL-17-mediated immune responses [99]. IL-17 is required to protect against C. albicans skin infections by recruiting neutrophils which phagocytose and kill the fungus [100] (Figure 5). Using this model, the authors demonstrated that LC drove Th17 differentiation through Dectin-1-mediated IL-6 production [101] (Figure 5), whereas Th1 and CD8+ T-cell responses predominantly relied on Langerin+ dermal DCs [99]. In addition to controlling antifungal T-cell responses, LC have also been shown to modulate the behavior of NK cells. Using an intradermal model of fungal-induced sensitization, Kaplan and colleagues showed that LC were required to dampen inappropriate inflammatory responses mediated by CXCR6+ NK cells recruited from the liver [102], which have also been shown to mediate contact hypersensitivity reactions and memory-like responses to these allergic insults [103].
Figure 5. Protection Against Candida albicans Infection of the Skin.
In the steady-state, TGFβ production by keratinocytes promotes localization of resident DC and T-cell populations to the epidermal layer. Upon infection, neurons in the skin release neuropeptides such as calcitonin gene-related peptide (CGRP) which activates Langerhans cells and dermal DCs to secrete cytokines that activate IL-17-producing lymphoid cells, which in turn recruits and promotes neutrophil effector function.
In addition to Th17 cells, gamma-delta T-cells and CD8+ T-cells can also contribute towards protection through IL-17 production. Gamma-delta T-cells are activated by a subset of dermal DCs expressing CD301b, which produce IL-23 to activate gamma-delta T-cells in response to neuropeptides produced by nociceptive neurons in the skin [104] (Figure 5). IL-17+ CD8+ T-cells also protect against C. albicans invasion of the skin, and are activated by CD103+ resident DCs which become activated by specific bacterial commensals living on the skin [105]. Thus, skin-resident DCs exquisitely tailor multiple lymphoid populations for protection against fungal skin infections. These responses can also be further fine-tuned by keratinocytes, specialized epithelial cells which make the uppermost layer of the skin. Keratinocytes are responsible for maintaining epidermal localization of LC and memory T-cells through TGFβ signaling [106] (Figure 5), and have also been shown to produce antimicrobial peptides and chemokines that affect developing immune responses in the skin [107].
The roles of CARD9 in skin immunity have been emerging in recent years; several clinical cases of human CARD9-deficiency associating with the development of rare, debilitating fungal skin diseases have now been described [24]. In particular, CARD9 deficiency predisposes to phaeohyphomycosis and deep dermatophytosis which appears to be linked to severe defects in the generation of Th17 responses, since these CARD9-deficient patients were reported to have reduced numbers of circulating Th17 cells [108; 109]. However, not all CARD9-deficient patients have decreased peripheral Th17 cells, and a direct role for IL-17 signaling in controlling these diseases has not been shown, thus the relevance of these defects in CARD9-deficient patients remains to be determined. CARD9 has also been indicated to play critical roles in sterile skin inflammation. For example, during atopic dermatitis, CARD9-signaling in dermal DCs was shown to drive IL-1α/β production following application of the hapten TNCB, which in turn activated T-cells through the IL-1R/MyD88 pathway to make IFNγ and IL-17 [110]. Similarly, the CARD9-coupled receptor Mincle was shown to have natural ligands in the skin, which bound and activated Mincle following chemical-induced skin damage leading to activation of skin-resident myeloid cells and the subsequent production of multiple pro-inflammatory cytokines [111]. Mincle is also a key receptor for F. pedrosoi, the causative agent of chromoblastomycosis. Recognition of F. pedrosoi by Mincle and signaling through the Syk-CARD9 pathway is required for sterilizing immunity, but also depends on co-operation with the TLRs [112]. However, TLR-mediated recognition of F. pedrosoi by macrophages was found to be defective [112]. Application of the TLR7 agonist, Imiquimod, was shown to help control infection in both mice and humans, by restoring these recognition defects [112; 113].
The Oral Mucosa
Fungal infections of the oral mucosa are most often caused by C. albicans, usually occurring as a result of HIV infection [114], inborn errors in IL-17 immunity [115] and mutations in AIRE resulting in APECED [116]. Most of these risk factors affect T-cell responses and thus these cells are critical for antifungal immunity at this site, particularly Th17 cells [117] which are naturally abundant in the oral mucosa and develop independently of microbial signals [118]. Mice deficient in IL-17 or T-cells are highly susceptible to oropharyngeal candidiasis (OPC), whereas deficiencies in IFNγ signaling do not affect susceptibility [119]. IL-17 is predominantly produced by natural Th17 cells and gamma-delta T-cells in the oral mucosa in response to C. albicans [120; 121], with lesser contributions by ILC type 3 cells [122], which is required for the production of antimicrobial peptides and fungal clearance [123] (Figure 6). Activation of conventional Th17 cells after re-infection at this site depends on CARD9 signaling [124] and monocyte-derived DCs and Flt3-dependent resident DCs, which traffic fungal antigens to draining cervical lymph nodes in a CCR7-dependent manner [125], while CARD9 is dispensable for innate natural Th17 dependent immunity [124]. Another crucial factor for protection against OPC is the IL-1R. Damaged keratinocytes, caused by the action of the C. albicans mycotoxin Candidalysin [121; 126], release IL-1α which stimulates G-CSF production from endothelial cells, this in turn promotes neutrophil development and recruitment to the oral mucosa [127], independently of IL-17 [128] (Figure 6).
Figure 6. IL-17-Dependent and Independent Mechanisms Controlling Immunity to C. albicans at the Oral Mucosa.
C. albicans hyphae produce the mycotoxin Candidalysin, which causes damage to keratinocytes and allows invasion of the oral mucosa. Damaged keratinocytes release IL-1α, which in turn stimulates G-CSF from neighboring cells to recruit neutrophils that act to control C. albicans infection. IL-17 is the predominant protective cytokine at the oral mucosa and has multiple cellular sources, including Th17 cells and a variety of innate-like lymphocytes (gamma-delta, innate αβ T-cells [nTh17], ILC3). IL-17 production stimulates the production of antimicrobial peptides, such as b-defensins, which protects against C. albicans growth and invasion of oral epithelium.
While the protective capacity of Th17, IL-17+ gamma-delta T-cells and neutrophils in antifungal defense at the oral barrier is relatively well established, the functional contributions of other resident cells at this site are not well understood and are currently understudied. For example, most mucosal barriers are also heavily populated with non-conventional lymphoid cells such as ILCs and MAIT cells. ILCs are grouped by their expression of key cytokines and master transcription factors; ILC1 express T-bet and IFNγ (these include NK cells), ILC2 express GATA-3 and IL-5/13, and ILC3 express RORγt and IL-17/IL-22 [129]. The role for ILCs at the oral mucosa in antifungal immunity is somewhat controversial, with some studies demonstrating a role for ILC3 [122] and others showing no role for ILCs [120]. This is likely in part due to the experimental intractability of studying ILCs, which has since massively evolved and led to multiple key developments in our understanding of the behavior and function of these cells in the GI tract (see below). Like ILCs, MAIT cells are also innate-like lymphocytes whose function is not well understood in the context of fungal infections. MAIT cells express an invariant T-cell receptor that recognizes microbial vitamin B2 metabolites in the context of MHC-related molecule, MR1. MAIT cells are particularly enriched in mucosal barriers and have been shown to strongly respond to bacteria and fungi, including C. albicans [130]. Whether MAIT cells protect against C. albicans infections in vivo is not known, although they have been shown to be critically involved in protection against disseminated bacterial infections [130]. Interestingly, human mutations in STAT3, which predispose to oral C. albicans infections, have recently been shown to cause MAIT cell dysfunction and reduced circulating numbers [131; 132]. Thus, it will be important to determine whether these MAIT cell disturbances have any functional relevance for the development of C. albicans infections in these patients.
The Gastrointestinal (GI) Tract
Commensal fungi in the GI tract are very poorly understood, in part because they have not received the same degree of attention as bacterial commensals and also because sequencing databases and methodologies for studying the ‘mycobiome’ are in their infancy relative to sequencing bacteria [133]. Yeast belonging to the Saccharomyces and Candida genera appear to be the dominant species found in the human GI tract [134], however other studies have suggested that Candida colonization of the human gut may be related to Western diets and antibiotic usage, since Candida colonization is less common in non-Western communities [135]. In germ-free mice, C. albicans is able to colonize the entire length of the GI tract equally well [136], where it exists in a specialized cell-type called the ‘GUT’ cell, characterized by enhanced expression of WOR1 transcription factor and cell wall changes [137]. Mice are useful animal models to study C. albicans colonization of the mammalian GI tract, since they are naturally resistant to colonization with this fungus and can be rendered susceptible with short-courses of antibiotics. These models have allowed for the identification of key host signaling pathways and bacterial commensals which mediate C. albicans colonization resistance [138; 139]. Understanding the interaction between commensal fungi and the GI mucosal immune system is important, since it is often thought that these commensal populations act as reservoirs for life-threatening disseminated infections [140; 141], and dysbiosis of the mycobiome has been repeatedly linked with inflammatory bowel diseases (IBD) [142; 143]. Yet, we understand very little about how intestinal fungi are recognized and how their potential invasion of gut tissues is handled by the immune system.
Identification and characterization of intestinal myeloid cells is an intense area of investigation and there are many excellent reviews on the subject [144; 145]. In brief, mononuclear phagocytes in the gut can be broadly divided by CD103 expression. CD103+ cells are generally accepted as migratory DCs, and can be further divided based on CD11b expression. CD103+CD11b+ DCs depend on IRF4 for their development and are essential for the induction of Th17 immunity in the GI tract [146], but not Th1, Treg or the stability of bacterial commensal communities [147]. CD103− cells contain both macrophages and DCs; a subset of these cells are CD103− DCs that migrate in the lymphatics to the mesenteric lymph nodes [148], and there is also a rare population of CCR2+ monocyte-derived DCs that selectively induce Th17 cells [149]. The antifungal activities of intestinal DCs and macrophages is not well understood. Thus far, Dectin-1 is the only antifungal receptor to be studied in detail in this tissue. All populations of DCs in the GI tract express Dectin-1, with the highest expression on CD103+ populations [150]. Dectin-1 is not required to control colonization of the GI tract by C. albicans [151], but is required to prevent invasion of intestinal tissues in mice on a variety of genetic backgrounds [151; 152]. During intestinal infection with C. albicans, Dectin-1 promotes CD4+ T-cell survival specifically in the mesenteric lymph nodes and lamina propia, since genetic deletion of Dectin-1 results in mass CD4+ T-cell apoptosis and disruption of fungal-specific CD4+ T-cell proliferation and activation within these tissues [150]. In line with a critical role for Dectin-1 in controlling fungal-induced inflammation of the intestine, human polymorphisms in CLEC7A (encoding Dectin-1) have been shown to associate with ulcerative colitis [153]. In animal models, mice deficient in either Dectin-1 or Dectin-3 develop worse DSS-induced colitis when first colonized with Candida tropicalis [150; 153; 154] indicating that fungi in the gut can have a profound influence over IBD, especially when combined with dysregulation of host immunity. Indeed, other work has shown that antifungal treatment in mice enhances their susceptibility to colitis by causing an expansion of antifungal-resistant fungi [155]. Moreover, human polymorphisms in CARD9 have also been repeatedly linked to IBD [156; 157]. Using Card9−/− mice and models of DSS-induced colitis, Card9 has been shown to be critical for the recovery phase of colitis by promoting IL-22 production and epithelial cell health [158]. However, the role of fungi in CARD9-dependent control of colitis appears marginal. Although Card9−/− animals have increased levels of commensal fungi [158], the relative proportions of different fungal genera are not significantly different compared to Card9+/+ mice and antifungal treatment does not alleviate colitis symptoms in Card9−/− animals [159]. In humans, however, CARD9-deficiency has been linked to the development of fungal colitis associated with enhanced Candida colonization of the gut [160]. Thus, while it is clear that Dectin-1/CARD9 signaling has a major influence in mucosal immunity of the GI tract, many questions remain as to their molecular control and how fungal commensals feature into these roles.
Finally, ILCs form a large, highly heterogeneous resident population in the GI tract [47] and have been shown to provide homeostatic control of the GI immune system. For example, ILC3 are critical for preventing dissemination of commensal bacteria through a pathway dependent on IL-22 [161], control intestinal Treg numbers through GM-CSF production [162], and have also been shown to mediate deletion of bacteria-specific CD4+ T-cells in the lamina propia through MHC Class II-TCR interactions, thus preventing unwarranted inflammation in response to commensals [163]. How ILC3 recognize and uptake bacterial antigens is unclear, although some work has shown a synergistic response of a specific subset of human ILC3 to TLR2 ligands and IL-2 [164], suggesting that these cells could sense bacteria and fungi via TLR2. Whether ILC3 in the gut respond to commensal fungi is unknown. ILC3 produce IL-22 and IL-17, cytokines that are thought to promote antifungal resistance at mucosal barriers. Thus, these cells could potentially be required to control fungal overgrowth in the GI tract and be important for controlling infection in this tissue as they are for the oral mucosa [122]. However, the function of ILCs in the context of fungal colonization and infection of the GI tract remains to be fully explored.
The Ocular Mucosa
Fungal keratitis is a leading cause of vision loss, and is predominantly caused by Aspergillus and Fusarium species. These infections typically occur following traumatic inoculation, have also been associated with the use of contaminated contact lenses, and can also occur in the setting of disseminated infections in profoundly immunosuppressed individuals [165]. The ocular mucosa includes the tear ducts and conjunctiva, which are associated lymphoid tissues that harbor lymphocytes and macrophages [166]. We recently showed that γδ T-cells in the conjunctiva are important sources of IL-17, which is made in response to commensal bacteria living on the eye surface [166]. As in the oral mucosa, IL-17 is an important component of antifungal defense by driving production of antimicrobial peptides, which are shed in the tears and promote protection against invasive C. albicans infection of the cornea [166].
Future Perspectives
The majority of studies analyzing antifungal immunity have utilized generic macrophage and DC populations to better understand mechanisms of myeloid cell control of fungal growth. These studies have provided seminal insights into the critical receptors and molecules needed to prevent fungal overgrowth and activate the appropriate T-cell responses. However, it is clear that our immune system exhibits organ-specific restrictions. For example, mutations in key antifungal molecules like CLEC7A and CARD9 do not affect susceptibility to all fungal diseases equally, indicating that there are species- and organ-specific roles for these molecules. Many recent studies have demonstrated the sheer complexity of tissue-resident cells, with their diverse functions and phenotypes, and have indicated that rare subpopulations can have profound effects in health and disease. The powerful new technologies used to make these advances have not yet been strongly utilized in the context of fungal disease, yet there is great opportunity to better understand organ-specific antifungal immune responses. Improving our knowledge of antifungal immunity and the cells required to implement protection is critical for the development of sorely-needed adjunctive immune-based therapies, to help tackle the global burden of human fungal disease.
Acknowledgments
RAD and MSL are supported by the Intramural Research Program of the National Institute of Allergy and Infectious Disease, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ginhoux F, Guilliams M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity. 2016;44:439–449. doi: 10.1016/j.immuni.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 2.Drummond RA, Collar AL, Swamydas M, Rodriguez CA, Lim JK, Mendez LM, Fink DL, Hsu AP, Zhai B, Karauzum H, Mikelis CM, Rose SR, Ferre EMN, Yockey L, Lemberg K, Kuehn HS, Rosenzweig SD, Lin X, Chittiboina P, Datta SK, Belhorn TH, Weimer ET, Hernandez ML, Hohl TM, Kuhns DB, Lionakis MS. CARD9-Dependent Neutrophil Recruitment Protects against Fungal Invasion of the Central Nervous System. PLoS Pathog. 2015;11:e1005293. doi: 10.1371/journal.ppat.1005293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heymann F, Peusquens J, Ludwig-Portugall I, Kohlhepp M, Ergen C, Niemietz P, Martin C, van Rooijen N, Ochando JC, Randolph GJ, Luedde T, Ginhoux F, Kurts C, Trautwein C, Tacke F. Liver inflammation abrogates immunological tolerance induced by Kupffer cells. Hepatology. 2015;62:279–291. doi: 10.1002/hep.27793. [DOI] [PubMed] [Google Scholar]
- 4.Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG, White TC. Hidden Killers: Human Fungal Infections. Sci Transl Med. 2012;4:165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 5.Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, Colombo AL, Calvo B, Cuomo CA, Desjardins CA, Berkow EL, Castanheira M, Magobo RE, Jabeen K, Asghar RJ, Meis JF, Jackson B, Chiller T, Litvintseva AP. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clinical Infectious Diseases. 2017;64:134–140. doi: 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panackal AA, Wuest SC, Lin Y-C, Wu T, Zhang N, Kosa P, Komori M, Blake A, Browne SK, Rosen LB, Hagen F, Meis J, Levitz SM, Quezado M, Hammoud D, Bennett JE, Bielekova B, Williamson PR. Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis. PLOS Pathogens. 2015;11:e1004884. doi: 10.1371/journal.ppat.1004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aguirre K, Crowe J, Haas A, Smith J. Resistance to Cryptococcus neoformans infection in the absence of CD4(+) T cells. Medical Mycology. 2004;42:15–25. [PubMed] [Google Scholar]
- 8.Rohatgi S, Pirofski LA. Host immunity to Cryptococcus neoformans. Future Microbiol. 2015;10:565–581. doi: 10.2217/fmb.14.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandt ME, Warnock DW. Epidemiology, Clinical Manifestations, and Therapy of Infections Caused by Dematiaceous Fungi. J Chemother. 2003;15:36–47. doi: 10.1179/joc.2003.15.Supplement-2.36. [DOI] [PubMed] [Google Scholar]
- 10.Lionakis MS, Dunleavy K, Roschewski M, Widemann BC, Butman JA, Schmitz R, Yang Y, Cole DE, Melani C, Higham CS, Desai JV, Ceribelli M, Chen L, Thomas CJ, Little RF, Gea-Banacloche J, Bhaumik S, Stetler-Stevenson M, Pittaluga S, Jaffe ES, Heiss J, Lucas N, Steinberg SM, Staudt LM, Wilson WH. Inhibition of B Cell Receptor Signaling by Ibrutinib in Primary CNS Lymphoma. Cancer Cell. 2017;31:833–843.e5. doi: 10.1016/j.ccell.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rieber N, Gazendam RP, Freeman AF, Hsu AP, Collar AL, Sugui JA, Drummond RA, Rongkavilit C, Hoffman K, Henderson C, Clark L, Mezger M, Swamydas M, Engeholm M, Schüle R, Neumayer B, Ebel F, Mikelis CM, Pittaluga S, Prasad VK, Singh A, Milner JD, Williams KW, Lim JK, Kwon-Chung KJ, Holland SM, Hartl D, Kuijpers TW, Lionakis MS. Extrapulmonary Aspergillus infection in patients with CARD9 deficiency. JCI Insight. 2016;1 doi: 10.1172/jci.insight.89890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ginhoux F, Prinz M. Origin of Microglia: Current Concepts and Past Controversies. Cold Spring Harbor Perspectives in Biology. 2015 doi: 10.1101/cshperspect.a020537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Réu P, Khosravi A, Bernard S, Mold JE, Salehpour M, Alkass K, Perl S, Tisdale J, Possnert G, Druid H, Frisén J. The Lifespan and Turnover of Microglia in the Human Brain. Cell Reports. 2017;20:779–784. doi: 10.1016/j.celrep.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blasi E, Mazzolla R, Barluzzi R, Bistoni F. MICROGLIAL CELL-MEDIATED ANTICANDIDA ACTIVITY - TEMPERATURE, IONS, PROTEIN-KINASE-C AS CRUCIAL ELEMENTS. J Neuroimmunol. 1991;34:53–60. doi: 10.1016/0165-5728(91)90098-r. [DOI] [PubMed] [Google Scholar]
- 15.Barluzzi R, Brozzetti A, Delfino D, Bistoni F, Blasi E. Role of the capsule in microglial cell-Cryptococcus neoformans interaction: impairment of antifungal activity but not of secretory functions. Medical Mycology. 1998;36:189–197. [PubMed] [Google Scholar]
- 16.Preissler J, Grosche A, Lede V, Le Duc D, Krugel K, Matyash V, Szulzewsky F, Kallendrusch S, Immig K, Kettenmann H, Bechmann I, Schoneberg T, Schulz A. Altered Microglial Phagocytosis in GPR34-Deficient Mice. Glia. 2015;63:206–215. doi: 10.1002/glia.22744. [DOI] [PubMed] [Google Scholar]
- 17.Song XY, Tanaka S, Cox D, Lee SC. Fc gamma receptor signaling in primary human microglia: differential roles of PI-3K and Ras/ERK MAPK pathways in phagocytosis and chemokine induction. J Leukoc Biol. 2004;75:1147–1155. doi: 10.1189/jlb.0403128. [DOI] [PubMed] [Google Scholar]
- 18.Adami C, Sorci G, Blasi E, Agneletti AL, Bistoni F, Donato R. S100B expression in and effects on microglia. Glia. 2001;33:131–142. [PubMed] [Google Scholar]
- 19.Hardison SE, Herrera G, Young ML, Hole CR, Wozniak KL, Wormley FL. Protective Immunity against Pulmonary Cryptococcosis Is Associated with STAT1-Mediated Classical Macrophage Activation. J Immunol. 2012;189:4060–4068. doi: 10.4049/jimmunol.1103455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ransohoff RM. A polarizing question: do M1 and M2 microglia exist? Nat Neurosci. 2016;19:987–991. doi: 10.1038/nn.4338. [DOI] [PubMed] [Google Scholar]
- 21.Redwine JM, Buchmeier MJ, Evans CF. In Vivo Expression of Major Histocompatibility Complex Molecules on Oligodendrocytes and Neurons during Viral Infection. The American Journal of Pathology. 2001;159:1219–1224. doi: 10.1016/S0002-9440(10)62507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aguirre K, Miller S. MHC class II-positive perivascular microglial cells mediate resistance to Cryptococcus neoformans brain infection. Glia. 2002;39:184–188. doi: 10.1002/glia.10093. [DOI] [PubMed] [Google Scholar]
- 23.Sa Q, Ochiai E, Tiwari A, Perkins S, Mullins J, Gehman M, Huckle W, Eyestone WH, Saunders TL, Shelton BJ, Suzuki Y. Cutting Edge: IFN-γ Produced by Brain-Resident Cells Is Crucial To Control Cerebral Infection with Toxoplasma gondii. The Journal of Immunology. 2015 doi: 10.4049/jimmunol.1500814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drummond RA, Lionakis MS. Mechanistic insights into the role of C-type lectin receptor/CARD9 signaling in human antifungal immunity. Front Cell Infect Microbiol. 2016;6 doi: 10.3389/fcimb.2016.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cornely OA, Bangard C, Jaspers NI. Hepatosplenic candidiasis. Clinical Liver Disease. 2015;6:47–50. doi: 10.1002/cld.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rihana NA, Kandula M, Velez A, Dahal K, Neill EB. Histoplasmosis Presenting as Granulomatous Hepatitis: Case Report and Review of the Literature. Case Reports in Medicine. 2014;2014:4. doi: 10.1155/2014/879535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol. 2017;17:306–321. doi: 10.1038/nri.2017.11. [DOI] [PubMed] [Google Scholar]
- 28.Lionakis MS, Lim JK, Lee CCR, Murphy PM. Organ-Specific Innate Immune Responses in a Mouse Model of Invasive Candidiasis. J Innate Immun. 2011;3:180–199. doi: 10.1159/000321157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Redmond HP, Shou J, Gallagher HJ, Kelly CJ, Daly JM. Macrophage-dependent candidacidal mechanisms in the murine system. Comparison of murine Kupffer cell and peritoneal macrophage candidacidal mechanisms. The Journal of Immunology. 1993;150:3427. [PubMed] [Google Scholar]
- 30.Jawhara S, Pluskota E, Verbovetskiy D, Skomorovska-Prokvolit O, Plow EF, Soloviev DA. Integrin αXβ2 Is a Leukocyte Receptor for Candida albicans and Is Essential for Protection against Fungal Infections. The Journal of Immunology. 2012;189:2468. doi: 10.4049/jimmunol.1200524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Øverland G, Stuestøl JF, Dahle MK, Myhre AE, Netea MG, Verweij P, Yndestad A, Aukrust P, Kullberg BJ, Warris A, Wang JE, Aasen AO. Cytokine Responses to Fungal Pathogens in Kupffer Cells are Toll-like Receptor 4 Independent and Mediated by Tyrosine Kinases. Scandinavian Journal of Immunology. 2005;62:148–154. doi: 10.1111/j.1365-3083.2005.01653.x. [DOI] [PubMed] [Google Scholar]
- 32.Krueger PD, Kim TS, Sung S-SJ, Braciale TJ, Hahn YS. Liver-Resident CD103+ Dendritic Cells Prime Antiviral CD8+ T Cells In Situ. The Journal of Immunology. 2015;194:3213. doi: 10.4049/jimmunol.1402622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly A, Fahey R, Fletcher JM, Keogh C, Carroll AG, Siddachari R, Geoghegan J, Hegarty JE, Ryan EJ, O’Farrelly C. CD141+ myeloid dendritic cells are enriched in healthy human liver. Journal of Hepatology. 2014;60:135–142. doi: 10.1016/j.jhep.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Parker JC, McCloskey JJ, Knauer KA. Pathobiologic features of human candidiasis - common deep mycosis of brain, heart and kidney in altered host. Am J Clin Pathol. 1976;65:991–1000. doi: 10.1093/ajcp/65.6.991. [DOI] [PubMed] [Google Scholar]
- 35.MacCallum DM. Massive induction of innate immune response to Candida albicans in the kidney in a murine intravenous challenge model. Fems Yeast Research. 2009;9:1111–1122. doi: 10.1111/j.1567-1364.2009.00576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lionakis MS, Swamydas M, Fischer BG, Plantinga TS, Johnson MD, Jaeger M, Green NM, Masedunskas A, Weigert R, Mikelis C, Wan W, Lee C-CR, Lim JK, Rivollier A, Yang JC, Laird GM, Wheeler RT, Alexander BD, Perfect JR, Gao J-L, Kullberg B-J, Netea MG, Murphy PM. CX3CR1-dependent renal macrophage survival promotes Candida control and host survival. J Clin Invest. 2013;123:5035–5051. doi: 10.1172/JCI71307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spellberg B, Johnston D, Phan QT, Edwards JE, French SW, Ibrahim AS, Filler SG. Parenchymal organ, and not splenic, immunity correlates with host survival during disseminated candidiasis. Infect Immun. 2003;71:5756–5764. doi: 10.1128/IAI.71.10.5756-5764.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ngo LY, Kasahara S, Kumasaka DK, Knoblaugh SE, Jhingran A, Hohl TM. Inflammatory Monocytes Mediate Early and Organ-Specific Innate Defense During Systemic Candidiasis. Journal of Infectious Diseases. 2014;209:109–119. doi: 10.1093/infdis/jit413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lionakis MS, Fischer BG, Lim JK, Swamydas M, Wan W, Richard Lee C-C, Cohen JI, Scheinberg P, Gao J-L, Murphy PM. Chemokine Receptor Ccr1 Drives Neutrophil-Mediated Kidney Immunopathology and Mortality in Invasive Candidiasis. PLoS Pathog. 2012;8:e1002865. doi: 10.1371/journal.ppat.1002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soos TJ, Sims TN, Barisoni L, Lin K, Littman DR, Dustin ML, Nelson PJ. CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int. 2006;70:591–596. doi: 10.1038/sj.ki.5001567. [DOI] [PubMed] [Google Scholar]
- 41.Stamatiades Efstathios G, Tremblay M-E, Bohm M, Crozet L, Bisht K, Kao D, Coelho C, Fan X, Yewdell William T, Davidson A, Heeger Peter S, Diebold S, Nimmerjahn F, Geissmann F. Immune Monitoring of Trans-endothelial Transport by Kidney-Resident Macrophages. Cell. 2016;166:991–1003. doi: 10.1016/j.cell.2016.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gottschalk C, Kurts C. The Debate about Dendritic Cells and Macrophages in the Kidney. Frontiers in Immunology. 2015;6:435. doi: 10.3389/fimmu.2015.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rogers NM, Ferenbach DA, Isenberg JS, Thomson AW, Hughes J. Dendritic cells and macrophages in the kidney: a spectrum of good and evil. Nat Rev Nephrol. 2014;10:625–643. doi: 10.1038/nrneph.2014.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kawakami T, Lichtnekert J, Thompson LJ, Karna P, Bouabe H, Hohl TM, Heinecke JW, Ziegler SF, Nelson PJ, Duffield JS. Resident Renal Mononuclear Phagocytes Comprise Five Discrete Populations with Distinct Phenotypes and Functions. The Journal of Immunology. 2013;191:3358. doi: 10.4049/jimmunol.1300342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferenbach D, Hughes J. Macrophages and dendritic cells: what is the difference? Kidney Int. 2008;74:5–7. doi: 10.1038/ki.2008.189. [DOI] [PubMed] [Google Scholar]
- 46.Krüger T, Benke D, Eitner F, Lang A, Wirtz M, Hamilton-Williams EE, Engel D, Giese B, Müller-Newen G, Floege J, Kurts C. Identification and Functional Characterization of Dendritic Cells in the Healthy Murine Kidney and in Experimental Glomerulonephritis. Journal of the American Society of Nephrology. 2004;15:613–621. doi: 10.1097/01.asn.0000114553.36258.91. [DOI] [PubMed] [Google Scholar]
- 47.Gury-BenAri M, Thaiss Christoph A, Serafini N, Winter Deborah R, Giladi A, Lara-Astiaso D, Levy M, Salame Tomer M, Weiner A, David E, Shapiro H, Dori-Bachash M, Pevsner-Fischer M, Lorenzo-Vivas E, Keren-Shaul H, Paul F, Harmelin A, Eberl G, Itzkovitz S, Tanay A, Di Santo James P, Elinav E, Amit I. The Spectrum and Regulatory Landscape of Intestinal Innate Lymphoid Cells Are Shaped by the Microbiome. Cell. 2016;166:1231–1246.e13. doi: 10.1016/j.cell.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 48.Heymann F, Meyer-Schwesinger C, Hamilton-Williams EE, Hammerich L, Panzer U, Kaden S, Quaggin SE, Floege J, Gröne H-J, Kurts C. Kidney dendritic cell activation is required for progression of renal disease in a mouse model of glomerular injury. J Clin Invest. 2009;119:1286–1297. doi: 10.1172/JCI38399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rabb H. The T cell as a bridge between innate and adaptive immune systems: Implications for the kidney. Kidney Int. 2002;61:1935–1946. doi: 10.1046/j.1523-1755.2002.00378.x. [DOI] [PubMed] [Google Scholar]
- 50.Hochheiser K, Heuser C, Krause TA, Teteris S, Ilias A, Weisheit C, Hoss F, Tittel AP, Knolle PA, Panzer U, Engel DR, Tharaux P-L, Kurts C. Exclusive CX(3)CR1 dependence of kidney DCs impacts glomerulonephritis progression. The Journal of Clinical Investigation. 2013;123:4242–4254. doi: 10.1172/JCI70143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whitney PG, Bär E, Osorio F, Rogers NC, Schraml BU, Deddouche S, LeibundGut-Landmann S, Reis e Sousa C. Syk Signaling in Dendritic Cells Orchestrates Innate Resistance to Systemic Fungal Infection. PLoS Pathog. 2014;10:e1004276. doi: 10.1371/journal.ppat.1004276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Break TJ, Hoffman KW, Swamydas M, Lee C-CR, Lim JK, Lionakis MS. Batf3-dependent CD103(+) dendritic cell accumulation is dispensable for mucosal and systemic antifungal host defense. Virulence. 2016;7:826–835. doi: 10.1080/21505594.2016.1186324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Collar AL, Swamydas M, O’Hayre M, Sajib MS, Hoffman KW, Singh SP, Mourad A, Johnson MD, Ferre EMN, Farber JM, Lim JK, Mikelis CM, Gutkind JS, Lionakis MS. The Homozygous CX3CR1-M280 Mutation Impairs Human Monocyte Survival. JCI Insight. 2018 doi: 10.1172/jci.insight.95417. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dominguez-Andres J, Feo-Lucas L, de la Escalera MM, Gonzalez L, Lopez-Bravo M, Ardavin C. Inflammatory Ly6C(high) Monocytes Protect against Candidiasis through IL-15-Driven NK Cell/Neutrophil Activation. Immunity. 2017;46:1059-+. doi: 10.1016/j.immuni.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 55.Romani L, Mencacci A, Grohmann U, Mocci S, Mosci P, Puccetti P, Bistoni F. Neutralizing antibody to interleukin 4 induces systemic protection and T helper type 1-associated immunity in murine candidiasis. J Exp Med. 1992;176:19–25. doi: 10.1084/jem.176.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cao Q, Lu J, Li Q, Wang C, Wang XM, Lee VWS, Wang C, Nguyen H, Zheng G, Zhao Y, Alexander SI, Wang Y, Harris DCH. CD103+ Dendritic Cells Elicit CD8+ T Cell Responses to Accelerate Kidney Injury in Adriamycin Nephropathy. Journal of the American Society of Nephrology. 2016;27:1344–1360. doi: 10.1681/ASN.2015030229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Evers BDG, Engel DR, Böhner AMC, Tittel AP, Krause TA, Heuser C, Garbi N, Kastenmüller W, Mack M, Tiegs G, Panzer U, Boor P, Ludwig-Portugall I, Kurts C. CD103+ Kidney Dendritic Cells Protect against Crescentic GN by Maintaining IL- 10–Producing Regulatory T Cells. Journal of the American Society of Nephrology. 2016;27:3368–3382. doi: 10.1681/ASN.2015080873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones-Carson J, Vazquez-Torres A, Warner T, Balish E. Disparate Requirement for T Cells in Resistance to Mucosal and Acute Systemic Candidiasis. Infect Immun. 2000;68:2363–2365. doi: 10.1128/iai.68.4.2363-2365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Drummond RA, Wallace C, Reid DM, Way SS, Kaplan DH, Brown GD. Cutting Edge: Failure of Antigen-Specific CD4(+) T Cell Recruitment to the Kidney during Systemic Candidiasis. J Immunol. 2014;193:5381–5385. doi: 10.4049/jimmunol.1401675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goldman DL, Davis J, Bommarito F, Shao X, Casadevall A. Enhanced Allergic Inflammation and Airway Responsiveness in Rats with Chronic Cryptococcus neoformans Infection: Potential Role for Fungal Pulmonary Infection in the Pathogenesis of Asthma. J Infect Dis. 2006;193:1178–1186. doi: 10.1086/501363. [DOI] [PubMed] [Google Scholar]
- 61.Hadebe S, Kirstein F, Fierens K, Chen K, Drummond RA, Vautier S, Sajaniemi S, Murray G, Williams DL, Redelinghuys P, Reinhart TA, Fallert Junecko BA, Kolls JK, Lambrecht BN, Brombacher F, Brown GD. Microbial Ligand Costimulation Drives Neutrophilic Steroid-Refractory Asthma. PLoS One. 2015;10:e0134219. doi: 10.1371/journal.pone.0134219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Overton NL, Simpson A, Bowyer P, Denning DW. Genetic susceptibility to severe asthma with fungal sensitization. International Journal of Immunogenetics. 2017 doi: 10.1111/iji.12312. n/an/a. [DOI] [PubMed] [Google Scholar]
- 63.Williams C, Ranjendran R, Ramage G. Pathogenesis of Fungal Infections in Cystic Fibrosis. Current Fungal Infection Reports. 2016;10:163–169. doi: 10.1007/s12281-016-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol. 2014;14:81–93. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 65.Paine R, Preston AM, Wilcoxen S, Jin H, Siu BB, Morris SB, Reed JA, Ross G, Whitsett JA, Beck JM. Granulocyte-Macrophage Colony-Stimulating Factor in the Innate Immune Response to Pneumocystis carinii Pneumonia in Mice. The Journal of Immunology. 2000;164:2602. doi: 10.4049/jimmunol.164.5.2602. [DOI] [PubMed] [Google Scholar]
- 66.Steele C, Marrero L, Swain S, Harmsen AG, Zheng MQ, Brown GD, Gordon S, Shellito JE, Kolls JK. Alveolar macrophage-mediated killing of Pneumocystis carinii f. sp muris involves molecular recognition by the dectin-1 beta-glucan receptor. J Exp Med. 2003;198:1677–1688. doi: 10.1084/jem.20030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Philippe B, Ibrahim-Granet O, Prévost MC, Gougerot-Pocidalo MA, Sanchez Perez M, Van der Meeren A, Latgé JP. Killing of Aspergillus fumigatus by Alveolar Macrophages Is Mediated by Reactive Oxidant Intermediates. Infect Immun. 2003;71:3034–3042. doi: 10.1128/IAI.71.6.3034-3042.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhatia S, Fei M, Yarlagadda M, Qi Z, Akira S, Saijo S, Iwakura Y, van Rooijen N, Gibson GA, St Croix CM, Ray A, Ray P. Rapid Host Defense against Aspergillus fumigatus Involves Alveolar Macrophages with a Predominance of Alternatively Activated Phenotype. PLoS One. 2011;6:e15943. doi: 10.1371/journal.pone.0015943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rocco NM, Carmen JC, Klein BS. Blastomyces dermatitidis Yeast Cells Inhibit Nitric Oxide Production by Alveolar Macrophage Inducible Nitric Oxide Synthase. Infect Immun. 2011;79:2385–2395. doi: 10.1128/IAI.01249-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kopf M, Schneider C, Nobs SP. The development and function of lung-resident macrophages and dendritic cells. Nat Immunol. 2015;16:36–44. doi: 10.1038/ni.3052. [DOI] [PubMed] [Google Scholar]
- 71.Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, Vanhoutte L, Neyt K, Killeen N, Malissen B, Hammad H, Lambrecht Bart N. Conventional and Monocyte-Derived CD11b+ Dendritic Cells Initiate and Maintain T Helper 2 Cell-Mediated Immunity to House Dust Mite Allergen. Immunity. 2013;38:322–335. doi: 10.1016/j.immuni.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 72.Schlitzer A, McGovern N, Teo P, Zelante T, Atarashi K, Low D, Ho Adrian W, See P, Shin A, Wasan Pavandip S, Hoeffel G, Malleret B, Heiseke A, Chew S, Jardine L, Purvis Harriet A, Hilkens Catharien M, Tam J, Poidinger M, Stanley ER, Krug Anne B, Renia L, Sivasankar B, Ng Lai G, Collin M, Ricciardi-Castagnoli P, Honda K, Haniffa M, Ginhoux F. IRF4 Transcription Factor-Dependent CD11b(+) Dendritic Cells in Human and Mouse Control Mucosal IL-17 Cytokine Responses. Immunity. 2013;38:970–983. doi: 10.1016/j.immuni.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Van Prooyen N, Henderson CA, Hocking Murray D, Sil A. CD103+ Conventional Dendritic Cells Are Critical for TLR7/9-Dependent Host Defense against Histoplasma capsulatum, an Endemic Fungal Pathogen of Humans. PLOS Pathogens. 2016;12:e1005749. doi: 10.1371/journal.ppat.1005749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Espinosa V, Jhingran A, Dutta O, Kasahara S, Donnelly R, Du P, Rosenfeld J, Leiner I, Chen C-C, Ron Y, Hohl TM, Rivera A. Inflammatory Monocytes Orchestrate Innate Antifungal Immunity in the Lung. PLOS Pathogens. 2014;10:e1003940. doi: 10.1371/journal.ppat.1003940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hohl TM, Rivera A, Lipuma L, Gallegos A, Shi C, Mack M, Pamer EG. Inflammatory Monocytes Facilitate Adaptive CD4 T Cell Responses during Respiratory Fungal Infection. Cell host & microbe. 2009;6:470–481. doi: 10.1016/j.chom.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu J, Flaczyk A, Neal LM, Fa Z, Eastman AJ, Malachowski AN, Cheng D, Moore BB, Curtis JL, Osterholzer JJ, Olszewski MA. Scavenger Receptor MARCO Orchestrates Early Defenses and Contributes to Fungal Containment during Cryptococcal Infection. The Journal of Immunology. 2017 doi: 10.4049/jimmunol.1700057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martinez N, Ketheesan N, West K, Vallerskog T, Kornfeld H. Impaired Recognition of Mycobacterium tuberculosis by Alveolar Macrophages From Diabetic Mice. The Journal of Infectious Diseases. 2016;214:1629–1637. doi: 10.1093/infdis/jiw436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sterkel Alana K, Lorenzini Jenna L, Fites JS, Subramanian Vignesh K, Sullivan Thomas D, Wuthrich M, Brandhorst T, Hernandez-Santos N, Deepe George S, Klein Bruce S. Fungal Mimicry of a Mammalian Aminopeptidase Disables Innate Immunity and Promotes Pathogenicity. Cell Host & Microbe. 2016;19:361–374. doi: 10.1016/j.chom.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wüthrich M, Ersland K, Sullivan T, Galles K, Klein Bruce S. Fungi Subvert Vaccine T Cell Priming at the Respiratory Mucosa by Preventing Chemokine-Induced Influx of Inflammatory Monocytes. Immunity. 2012;36:680–692. doi: 10.1016/j.immuni.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wüthrich M, Gern B, Hung CY, Ersland K, Rocco N, Pick-Jacobs J, Galles K, Filutowicz H, Warner T, Evans M, Cole G, Klein B. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J Clin Invest. 2011;121:554–568. doi: 10.1172/JCI43984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Werner JL, Gessner MA, Lilly LM, Nelson MP, Metz AE, Horn D, Dunaway CW, Deshane J, Chaplin DD, Weaver CT, Brown GD, Steele C. Neutrophils Produce Interleukin 17A (IL-17A) in a Dectin-1-and IL-23-Dependent Manner during Invasive Fungal Infection. Infect Immun. 2011;79:3966–3977. doi: 10.1128/IAI.05493-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Werner JL, Metz AE, Horn D, Schoeb TR, Hewitt MM, Schwiebert LM, Faro-Trindade I, Brown GD, Steele C. Requisite Role for the Dectin-1 beta-Glucan Receptor in Pulmonary Defense against Aspergillus fumigatus. J Immunol. 2009;182:4938–4946. doi: 10.4049/jimmunol.0804250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rivera A, Hohl TM, Collins N, Leiner I, Gallegos A, Saijo S, Coward JW, Iwakura Y, Pamer EG. Dectin-1 diversifies Aspergillus fumigatus–specific T cell responses by inhibiting T helper type 1 CD4 T cell differentiation. J Exp Med. 2011;208:369–381. doi: 10.1084/jem.20100906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gringhuis SI, Wevers BA, Kaptein TM, van Capel TMM, Theelen B, Boekhout T, de Jong EC, Geijtenbeek TBH. Selective C-Rel Activation via Malt1 Controls Anti-Fungal Th17 Immunity by Dectin-1 and Dectin-2. Plos Pathog. 2011;7 doi: 10.1371/journal.ppat.1001259. Article No: e1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van de Veerdonk FL, Marijnissen RJ, Kullberg BJ, Koenen HJPM, Cheng S-C, Joosten I, van den Berg WB, Williams DL, van der Meer JWM, Joosten LAB, Netea MG. The Macrophage Mannose Receptor Induces IL-17 in Response to Candida albicans. Cell Host Microbe. 2009;5:329–340. doi: 10.1016/j.chom.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 86.Wang H, LeBert V, Hung CY, Galles K, Saijo S, Lin X, Cole GT, Klein BS, Wüthrich M. C-Type Lectin Receptors Differentially Induce Th17 Cells and Vaccine Immunity to the Endemic Mycosis of North America. The Journal of Immunology. 2014;192:1107. doi: 10.4049/jimmunol.1302314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dan JM, Kelly RM, Lee CK, Levitz SM. Role of the mannose receptor in a murine model of Cryptococcus neoformans infection. Infect Immun. 2008;76:2362–2367. doi: 10.1128/IAI.00095-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Drummond RA, Saijo S, Iwakura Y, Brown GD. The role of Syk/CARD9 coupled C-type lectins in antifungal immunity. Eur J Immunol. 2011;41:276–281. doi: 10.1002/eji.201041252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yamamoto H, Nakamura Y, Sato K, Takahashi Y, Nomura T, Miyasaka T, Ishii K, Hara H, Yamamoto N, Kanno E, Iwakura Y, Kawakami K. Defect of CARD9 Leads to Impaired Accumulation of Gamma Interferon-Producing Memory Phenotype T Cells in Lungs and Increased Susceptibility to Pulmonary Infection with Cryptococcus neoformans. Infect Immun. 2014;82:1606–1615. doi: 10.1128/IAI.01089-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lionakis MS, Netea MG, Holland SM. Mendelian Genetics of Human Susceptibility to Fungal Infection. Cold Spring Harbor Perspect Med. 2014;4 doi: 10.1101/cshperspect.a019638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lévy R, Okada S, Béziat V, Moriya K, Liu C, Chai LYA, Migaud M, Hauck F, Al Ali A, Cyrus C, Vatte C, Patiroglu T, Unal E, Ferneiny M, Hyakuna N, Nepesov S, Oleastro M, Ikinciogullari A, Dogu F, Asano T, Ohara O, Yun L, Della Mina E, Bronnimann D, Itan Y, Gothe F, Bustamante J, Boisson-Dupuis S, Tahuil N, Aytekin C, Salhi A, Al Muhsen S, Kobayashi M, Toubiana J, Abel L, Li X, Camcioglu Y, Celmeli F, Klein C, AlKhater SA, Casanova J-L, Puel A. Genetic, immunological, and clinical features of patients with bacterial and fungal infections due to inherited IL-17RA deficiency. Proceedings of the National Academy of Sciences. 2016;113:E8277–E8285. doi: 10.1073/pnas.1618300114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jhingran A, Kasahara S, Shepardson KM, Junecko BAF, Heung LJ, Kumasaka DK, Knoblaugh SE, Lin X, Kazmierczak BI, Reinhart TA, Cramer RA, Hohl TM. Compartment-Specific and Sequential Role of MyD88 and CARD9 in Chemokine Induction and Innate Defense during Respiratory Fungal Infection. PLoS Pathog. 2015;11:e1004589. doi: 10.1371/journal.ppat.1004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ito T, Wang Y-H, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX-F, Yao Z, Cao W, Liu Y-J. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. The Journal of Experimental Medicine. 2005;202:1213. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ying S, O’Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, Robinson D, Zhang G, Zhao J, Lee TH, Corrigan C. Thymic Stromal Lymphopoietin Expression Is Increased in Asthmatic Airways and Correlates with Expression of Th2-Attracting Chemokines and Disease Severity. The Journal of Immunology. 2005;174:8183. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 95.Findley K, Oh J, Yang J, Conlan S, Deming C, Meyer JA, Schoenfeld D, Nomicos E, Park M, Kong HH, Segre JA. Topographic diversity of fungal and bacterial communities in human skin. Nature. 2013;498:367–370. doi: 10.1038/nature12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clinics in Dermatology. 2007;25:606–615. doi: 10.1016/j.clindermatol.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 97.de Jong MAWP, Vriend LEM, Theelen B, Taylor ME, Fluitsma D, Boekhout T, Geijtenbeek TBH. C-type lectin Langerin is a β-glucan receptor on human Langerhans cells that recognizes opportunistic and pathogenic fungi. Molecular Immunology. 2010;47:1216–1225. doi: 10.1016/j.molimm.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stingl G, Brüggen M-C, Vázquez-Strauss M . Intramural Sequencing Ctr NIH. Innate and Adaptive Components of the Cutaneous Immune Barrier: The Central Role of Dendritic Cells. In: Gaspari AA, Tyring SK, Kaplan DH, editors. Clinical and Basic Immunodermatology. Springer International Publishing; Cham: 2017. pp. 1–10. [Google Scholar]
- 99.Igyarto BZ, Haley K, Ortner D, Bobr A, Gerami-Nejad M, Edelson BT, Zurawski SM, Malissen B, Zurawski G, Berman J, Kaplan DH. Skin-Resident Murine Dendritic Cell Subsets Promote Distinct and Opposing Antigen-Specific T Helper Cell Responses. Immunity. 2011;35:260–272. doi: 10.1016/j.immuni.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kagami S, Rizzo HL, Kurtz SE, Miller LS, Blauvelt A. IL-23 and IL-17A, but Not IL-12 and IL-22, Are Required for Optimal Skin Host Defense against Candida albicans. Journal of immunology (Baltimore, Md: 1950) 2010;185:5453–5462. doi: 10.4049/jimmunol.1001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kashem Sakeen W, Igyártó Botond Z, Gerami-Nejad M, Kumamoto Y, Mohammed J, Jarrett E, Drummond Rebecca A, Zurawski Sandra M, Zurawski G, Berman J, Iwasaki A, Brown Gordon D, Kaplan Daniel H. Candida albicans Morphology and Dendritic Cell Subsets Determine T Helper Cell Differentiation. Immunity. 2015;42:356–366. doi: 10.1016/j.immuni.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Scholz F, Naik S, Sutterwala FS, Kaplan DH. Langerhans Cells Suppress CD49a+ NK Cell–Mediated Skin Inflammation. The Journal of Immunology. 2015;195:2335–2342. doi: 10.4049/jimmunol.1500935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Peng H, Jiang X, Chen Y, Sojka DK, Wei H, Gao X, Sun R, Yokoyama WM, Tian Z. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. The Journal of Clinical Investigation. 2013;123:1444–1456. doi: 10.1172/JCI66381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kashem Sakeen W, Riedl Maureen S, Yao C, Honda Christopher N, Vulchanova L, Kaplan Daniel H. Nociceptive Sensory Fibers Drive Interleukin-23 Production from CD301b+ Dermal Dendritic Cells and Drive Protective Cutaneous Immunity. Immunity. 2015;43:515–526. doi: 10.1016/j.immuni.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Naik S, Bouladoux N, Linehan JL, Han S-J, Harrison OJ, Wilhelm C, Conlan S, Himmelfarb S, Byrd AL, Deming C, Quinones M, Brenchley JM, Kong HH, Tussiwand R, Murphy KM, Merad M, Segre JA, Belkaid Y. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature. 2015;520:104–108. doi: 10.1038/nature14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mohammed J, Beura LK, Bobr A, Astry B, Chicoine B, Kashem SW, Welty NE, Igyarto BZ, Wijeyesinghe S, Thompson EA, Matte C, Bartholin L, Kaplan A, Sheppard D, Bridges AG, Shlomchik WD, Masopust D, Kaplan DH. Stromal cells control the epithelial residence of DCs and memory T cells by regulated activation of TGF-[beta] Nat Immunol. 2016;17:414–421. doi: 10.1038/ni.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kashem SW, Kaplan DH. Skin Immunity to Candida albicans. Trends in Immunology. 2016;37:440–450. doi: 10.1016/j.it.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lanternier F, Pathan S, Vincent QB, Liu L, Cypowyj S, Prando C, Migaud M, Taibi L, Ammar-Khodja A, Boudghene Stambouli O, Guellil B, Jacobs F, Goffard J-C, Schepers K, del Marmol V, Boussofara L, Denguezli M, Larif M, Bachelez H, Michel L, Lefranc G, Hay R, Jouvion G, Chretien F, Fraitag S, Bougnoux M-E, Boudia M, Abel L, Lortholary O, Casanova J-L, Picard C, Grimbacher B, Puel A. Deep Dermatophytosis and Inherited CARD9 Deficiency. N Engl J Med. 2013;369:1704–1714. doi: 10.1056/NEJMoa1208487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wu W, Zhang R, Wang X, Song Y, Liu Z, Han W, Li R. Impairment of Immune Response against Dematiaceous Fungi in Card9 Knockout Mice. Mycopathologia. 2016:1–12. doi: 10.1007/s11046-016-0029-0. [DOI] [PubMed] [Google Scholar]
- 110.Yasukawa S, Miyazaki Y, Yoshii C, Nakaya M, Ozaki N, Toda S, Kuroda E, Ishibashi K-i, Yasuda T, Natsuaki Y, Mi-ichi F, Iizasa Ei, Nakahara T, Yamazaki M, Kabashima K, Iwakura Y, Takai T, Saito T, Kurosaki T, Malissen B, Ohno N, Furue M, Yoshida H, Hara H. An ITAM-Syk-CARD9 signalling axis triggers contact hypersensitivity by stimulating IL-1 production in dendritic cells. Nature Communications. 2014;5:3755. doi: 10.1038/ncomms4755. [DOI] [PubMed] [Google Scholar]
- 111.Kostarnoy AV, Gancheva PG, Lepenies B, Tukhvatulin AI, Dzharullaeva AS, Polyakov NB, Grumov DA, Egorova DA, Kulibin AY, Bobrov MA, Malolina EA, Zykin PA, Soloviev AI, Riabenko E, Maltseva DV, Sakharov DA, Tonevitsky AG, Verkhovskaya LV, Logunov DY, Naroditsky BS, Gintsburg AL. Receptor Mincle promotes skin allergies and is capable of recognizing cholesterol sulfate. Proceedings of the National Academy of Sciences. 2017 doi: 10.1073/pnas.1611665114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Da Glória Sousa M, Reid DM, Schweighoffer E, Tybulewicz V, Ruland J, Langhorne J, Yamasaki S, Taylor PR, Almeida SR, Brown GD. Restoration of Pattern Recognition Receptor Costimulation to Treat Chromoblastomycosis, a Chronic Fungal Infection of the Skin. Cell Host Microbe. 2011;9:436–443. doi: 10.1016/j.chom.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.de Sousa MdGT, Belda JW, Spina R, Lota PR, Valente NS, Brown GD, Criado PR, Benard G. Topical Application of Imiquimod as a Treatment for Chromoblastomycosis. Clinical Infectious Diseases. 2014;58:1734–1737. doi: 10.1093/cid/ciu168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Auclair S, Liu F, Hu H. Loss of immune control in HIV-infected patients: how does mucosal candidiasis occur? Future Microbiol. 2016;12:5–8. doi: 10.2217/fmb-2016-0194. [DOI] [PubMed] [Google Scholar]
- 115.Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, Migaud M, Israel L, Chrabieh M, Audry M, Gumbleton M, Toulon A, Bodemer C, El-Baghdadi J, Whitters M, Paradis T, Brooks J, Collins M, Wolfman NM, Al-Muhsen S, Galicchio M, Abel L, Picard C, Casanova J-L. Chronic Mucocutaneous Candidiasis in Humans with Inborn Errors of Interleukin-17 Immunity. Science. 2011 doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ferre EMN, Rose SR, Rosenzweig SD, Burbelo PD, Romito KR, Niemela JE, Rosen LB, Break TJ, Gu W, Hunsberger S, Browne SK, Hsu AP, Rampertaap S, Swamydas M, Collar AL, Kong HH, Lee C-CR, Chascsa D, Simcox T, Pham A, Bondici A, Natarajan M, Monsale J, Kleiner DE, Quezado M, Alevizos I, Moutsopoulos NM, Yockey L, Frein C, Soldatos A, Calvo KR, Adjemian J, Similuk MN, Lang DM, Stone KD, Uzel G, Kopp JB, Bishop RJ, Holland SM, Olivier KN, Fleisher TA, Heller T, Winer KK, Lionakis MS. Redefined clinical features and diagnostic criteria in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. JCI Insight. 2016;1 doi: 10.1172/jci.insight.88782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hernández-Santos N, Huppler AR, Peterson AC, Khader SA, McKenna KC, Gaffen SL. Th17 cells confer long-term adaptive immunity to oral mucosal Candida albicans infections. Mucosal Immunol. 2013;6:900–910. doi: 10.1038/mi.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dutzan N, Abusleme L, Bridgeman H, Greenwell-Wild T, Zangerle-Murray T, Fife ME, Bouladoux N, Linley H, Brenchley L, Wemyss K, Calderon G, Hong B-Y, Break TJ, Bowdish DME, Lionakis MS, Jones SA, Trinchieri G, Diaz PI, Belkaid Y, Konkel JE, Moutsopoulos NM. On-going Mechanical Damage from Mastication Drives Homeostatic Th17 Cell Responses at the Oral Barrier. Immunity. 2017;46:133–147. doi: 10.1016/j.immuni.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, Filler SG, Masso-Welch P, Edgerton M, Gaffen SL. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Conti HR, Peterson AC, Brane L, Huppler AR, Hernández-Santos N, Whibley N, Garg AV, Simpson-Abelson MR, Gibson GA, Mamo AJ, Osborne LC, Bishu S, Ghilardi N, Siebenlist U, Watkins SC, Artis D, McGeachy MJ, Gaffen SL. Oral-resident natural Th17 cells and γδ T cells control opportunistic Candida albicans infections. The Journal of Experimental Medicine. 2014;211:2075–2084. doi: 10.1084/jem.20130877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Verma AH, Richardson JP, Zhou C, Coleman BM, Moyes DL, Ho J, Huppler AR, Ramani K, McGeachy MJ, Mufazalov IA, Waisman A, Kane LP, Biswas PS, Hube B, Naglik JR, Gaffen SL. Oral epithelial cells orchestrate innate type 17 responses to Candida albicans through the virulence factor candidalysin. Science Immunology. 2017;2 doi: 10.1126/sciimmunol.aam8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gladiator A, Wangler N, Trautwein-Weidner K, LeibundGut-Landmann S. Cutting Edge: IL-17-Secreting Innate Lymphoid Cells Are Essential for Host Defense against Fungal Infection. J Immunol. 2013;190:521–525. doi: 10.4049/jimmunol.1202924. [DOI] [PubMed] [Google Scholar]
- 123.Conti HR, Baker O, Freeman AF, Jang WS, Holland SM, Li RA, Edgerton M, Gaffen SL. New mechanism of oral immunity to mucosal candidiasis in hyper-IgE syndrome. Mucosal Immunol. 2011;4:448–455. doi: 10.1038/mi.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bishu S, Hernández-Santos N, Simpson-Abelson MR, Huppler AR, Conti HR, Ghilardi N, Mamo AJ, Gaffen SL. The Adaptor CARD9 Is Required for Adaptive but Not Innate Immunity to Oral Mucosal Candida albicans Infections. Infect Immun. 2014;82:1173–1180. doi: 10.1128/IAI.01335-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Trautwein-Weidner K, Gladiator A, Kirchner FR, Becattini S, Rülicke T, Sallusto F, LeibundGut-Landmann S. Antigen-Specific Th17 Cells Are Primed by Distinct and Complementary Dendritic Cell Subsets in Oropharyngeal Candidiasis. PLoS Pathog. 2015;11:e1005164. doi: 10.1371/journal.ppat.1005164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moyes DL, Wilson D, Richardson JP, Mogavero S, Tang SX, Wernecke J, Höfs S, Gratacap RL, Robbins J, Runglall M, Murciano C, Blagojevic M, Thavaraj S, Förster TM, Hebecker B, Kasper L, Vizcay G, Iancu SI, Kichik N, Häder A, Kurzai O, Luo T, Krüger T, Kniemeyer O, Cota E, Bader O, Wheeler RT, Gutsmann T, Hube B, Naglik JR. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature advance online publication. 2016 doi: 10.1038/nature17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Altmeier S, Toska A, Sparber F, Teijeira A, Halin C, LeibundGut-Landmann S. IL-1 Coordinates the Neutrophil Response to C. albicans in the Oral Mucosa. PLOS Pathogens. 2016;12:e1005882. doi: 10.1371/journal.ppat.1005882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Trautwein-Weidner K, Gladiator A, Nur S, Diethelm P, LeibundGut-Landmann S. IL-17-mediated antifungal defense in the oral mucosa is independent of neutrophils. Mucosal Immunol. 2015;8:221–231. doi: 10.1038/mi.2014.57. [DOI] [PubMed] [Google Scholar]
- 129.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ, Mebius RE, Powrie F, Vivier E. Innate lymphoid cells - a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 130.Le Bourhis L, Martin E, Peguillet I, Guihot A, Froux N, Core M, Levy E, Dusseaux M, Meyssonnier V, Premel V, Ngo C, Riteau B, Duban L, Robert D, Rottman M, Soudais C, Lantz O. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010;11:701–708. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- 131.Gao Y, Rae W, Ramakrishnan KA, Barcenas-Morales G, Döffinger R, Eren E, Faust SN, Ottensmeier CH, Williams AP. Mucosal-Associated Invariant T (MAIT) Cells Are Impaired in Th17 Associated Primary and Secondary Immunodeficiencies. PLoS One. 2016;11:e0155059. doi: 10.1371/journal.pone.0155059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wilson RP, Ives ML, Rao G, Lau A, Payne K, Kobayashi M, Arkwright PD, Peake J, Wong M, Adelstein S, Smart JM, French MA, Fulcher DA, Picard C, Bustamante J, Boisson-Dupuis S, Gray P, Stepensky P, Warnatz K, Freeman AF, Rossjohn J, McCluskey J, Holland SM, Casanova J-L, Uzel G, Ma CS, Tangye SG, Deenick EK. STAT3 is a critical cell-intrinsic regulator of human unconventional T cell numbers and function. The Journal of Experimental Medicine. 2015;212:855. doi: 10.1084/jem.20141992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Underhill DM, Iliev ID. The mycobiota: interactions between commensal fungi and the host immune system. Nature reviews Immunology. 2014;14:405–416. doi: 10.1038/nri3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hoffmann C, Dollive S, Grunberg S, Chen J, Li H, Wu GD, Lewis JD, Bushman FD. Archaea and Fungi of the Human Gut Microbiome: Correlations with Diet and Bacterial Residents. PLoS One. 2013;8:e66019. doi: 10.1371/journal.pone.0066019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Angebault C, Djossou F, Abélanet S, Permal E, Ben Soltana M, Diancourt L, Bouchier C, Woerther P-L, Catzeflis F, Andremont A, d’Enfert C, Bougnoux M-E. Candida albicans Is Not Always the Preferential Yeast Colonizing Humans: A Study in Wayampi Amerindians. The Journal of Infectious Diseases. 2013;208:1705–1716. doi: 10.1093/infdis/jit389. [DOI] [PubMed] [Google Scholar]
- 136.Hoffmann TW, Pham H-P, Bridonneau C, Aubry C, Lamas B, Martin-Gallausiaux C, Moroldo M, Rainteau D, Lapaque N, Six A, Richard ML, Fargier E, Le Guern M-E, Langella P, Sokol H. Microorganisms linked to inflammatory bowel disease-associated dysbiosis differentially impact host physiology in gnotobiotic mice. ISME J. 2015 doi: 10.1038/ismej.2015.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pande K, Chen C, Noble SM. Passage through the mammalian gut triggers a phenotypic switch that promotes Candida albicans commensalism. Nat Genet. 2013;45:1088–1091. doi: 10.1038/ng.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fan D, Coughlin LA, Neubauer MM, Kim J, Kim MS, Zhan X, Simms-Waldrip TR, Xie Y, Hooper LV, Koh AY. Activation of HIF-1[alpha] and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nat Med advance online publication. 2015 doi: 10.1038/nm.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vautier S, Drummond RA, Chen K, Murray GI, Kadosh D, Brown AJP, Gow NAR, MacCallum DM, Kolls JK, Brown GD. Candida albicans colonization and dissemination from the murine gastrointestinal tract: the influence of morphology and Th17 immunity. Cellular Microbiology. 2014 doi: 10.1111/cmi.12388. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Odds FC, Davidson AD, Jacobsen MD, Tavanti A, Whyte JA, Kibbler CC, Ellis DH, Maiden MCJ, Shaw DJ, Gow NAR. Candida albicans strain maintenance, replacement, and microvariation demonstrated by multilocus sequence typing. J Clin Microbiol. 2006;44:3647–3658. doi: 10.1128/JCM.00934-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Koh AY, Köhler JR, Coggshall KT, Van Rooijen N, Pier GB. Mucosal Damage and Neutropenia Are Required for Candida albicans Dissemination. PLoS Pathog. 2008;4:e35. doi: 10.1371/journal.ppat.0040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chehoud CAB, Albenberg LGDO, Judge CAB, Hoffmann CP, Grunberg SBA, Bittinger KP, Baldassano RNMD, Lewis JDMD, Bushman FDP, Wu GDMD. Fungal Signature in the Gut Microbiota of Pediatric Patients With Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015 doi: 10.1097/MIB.0000000000000454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lewis James D, Chen Eric Z, Baldassano Robert N, Otley Anthony R, Griffiths Anne M, Lee D, Bittinger K, Bailey A, Friedman Elliot S, Hoffmann C, Albenberg L, Sinha R, Compher C, Gilroy E, Nessel L, Grant A, Chehoud C, Li H, Wu Gary D, Bushman Frederic D. Inflammation, Antibiotics, and Diet as Environmental Stressors of the Gut Microbiome in Pediatric Crohn’s Disease. Cell Host & Microbe. 2015;18:489–500. doi: 10.1016/j.chom.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Milling S, Yrlid U, Cerovic V, MacPherson G. Subsets of migrating intestinal dendritic cells. Immunol Rev. 2010;234:259–267. doi: 10.1111/j.0105-2896.2009.00866.x. [DOI] [PubMed] [Google Scholar]
- 145.Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol. 2014;14:667–685. doi: 10.1038/nri3738. [DOI] [PubMed] [Google Scholar]
- 146.Persson Emma K, Uronen-Hansson H, Semmrich M, Rivollier A, Hägerbrand K, Marsal J, Gudjonsson S, Håkansson U, Reizis B, Kotarsky K, Agace William W. IRF4 Transcription-Factor-Dependent CD103+CD11b+ Dendritic Cells Drive Mucosal T Helper 17 Cell Differentiation. Immunity. 2013;38:958–969. doi: 10.1016/j.immuni.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 147.Welty NE, Staley C, Ghilardi N, Sadowsky MJ, Igyártó BZ, Kaplan DH. Intestinal lamina propria dendritic cells maintain T cell homeostasis but do not affect commensalism. J Exp Med. 2013;210:2011–2024. doi: 10.1084/jem.20130728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cerovic V, Houston SA, Scott CL, Aumeunier A, Yrlid U, Mowat AM, Milling SWF. Intestinal CD103- dendritic cells migrate in lymph and prime effector T cells. Mucosal Immunol. 2013;6:104–113. doi: 10.1038/mi.2012.53. [DOI] [PubMed] [Google Scholar]
- 149.Scott CL, Bain CC, Wright PB, Sichien D, Kotarsky K, Persson EK, Luda K, Guilliams M, Lambrecht BN, Agace WW, Milling SW, Mowat AM. CCR2+CD103- intestinal dendritic cells develop from DC-committed precursors and induce interleukin-17 production by T cells. Mucosal Immunol. 2015;8:327–339. doi: 10.1038/mi.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Drummond RA, Dambuza IM, Vautier S, Taylor JA, Reid DM, Bain CC, Underhill DM, Masopust D, Kaplan DH, Brown GD. CD4+ T-cell survival in the GI tract requires dectin-1 during fungal infection. Mucosal Immunol. 2015 doi: 10.1038/mi.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vautier S, Drummond RA, Redelinghuys P, Murray GI, MacCallum DM, Brown GD. Dectin-1 Is not required for controlling Candida albicans colonization of the gastrointestinal tract. Infect Immun. 2012;80:4216–4222. doi: 10.1128/IAI.00559-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, Brown GD. Dectin-1 is required for β-glucan recognition and control of fungal infection. Nat Immunol. 2007;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Iliev ID, Funari VA, Taylor KD, Quoclinh N, Reyes CN, Strom SP, Brown J, Becker CA, Fleshner PR, Dubinsky M, Rotter JI, Wang HL, McGovern DPB, Brown GD, Underhill DM. Interactions Between Commensal Fungi and the C-Type Lectin Receptor Dectin-1 Influence Colitis. Science. 2012;336:1314–1317. doi: 10.1126/science.1221789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang T, Pan D, Zhou Z, You Y, Jiang C, Zhao X, Lin X. Dectin-3 Deficiency Promotes Colitis Development due to Impaired Antifungal Innate Immune Responses in the Gut. PLoS Pathog. 2016;12:e1005662. doi: 10.1371/journal.ppat.1005662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wheeler Matthew L, Limon Jose J, Bar Agnieszka S, Leal Christian A, Gargus M, Tang J, Brown J, Funari Vincent A, Wang Hanlin L, Crother Timothy R, Arditi M, Underhill David M, Iliev Iliyan D. Immunological Consequences of Intestinal Fungal Dysbiosis. Cell Host & Microbe. 19:865–873. doi: 10.1016/j.chom.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rivas MA, Beaudoin M, Gardet A, Stevens C, Sharma Y, Zhang CK, Boucher G, Ripke S, Ellinghaus D, Burtt N, Fennell T, Kirby A, Latiano A, Goyette P, Green T, Halfvarson J, Haritunians T, Korn JM, Kuruvilla F, Lagace C, Neale B, Lo KS, Schumm P, Torkvist L, Dubinsky MC, Brant SR, Silverberg MS, Duerr RH, Altshuler D, Gabriel S, Lettre G, Franke A, D’Amato M, McGovern DPB, Cho JH, Rioux JD, Xavier RJ, Daly MJ K. Natl Inst Diabet Digestive, B. United Kingdom Inflammatory, and D. Int Inflammatory Bowel. Deep resequencing of GWAS loci identifies independent rare variants associated with inflammatory bowel disease. Nat Genet. 2011;43:1066-U50. doi: 10.1038/ng.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cao Z, Conway Kara L, Heath Robert J, Rush Jason S, Leshchiner Elizaveta S, Ramirez-Ortiz Zaida G, Nedelsky Natalia B, Huang H, Ng A, Gardet A, Cheng S-C, Shamji Alykhan F, Rioux John D, Wijmenga C, Netea Mihai G, Means Terry K, Daly Mark J, Xavier Ramnik J. Ubiquitin Ligase TRIM62 Regulates CARD9-Mediated Anti-fungal Immunity and Intestinal Inflammation. Immunity. 2015;43:715–726. doi: 10.1016/j.immuni.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sokol H, Conway KL, Zhang M, Choi M, Morin B, Cao Z, Villablanca EJ, Li C, Wijmenga C, Yun SH, Shi HN, Xavier RJ. Card9 Mediates Intestinal Epithelial Cell Restitution, T-Helper 17 Responses, and Control of Bacterial Infection in Mice. Gastroenterology. 2013;145:591–601. doi: 10.1053/j.gastro.2013.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lamas B, Richard ML, Leducq V, Pham H-P, Michel M-L, Da Costa G, Bridonneau C, Jegou S, Hoffmann TW, Natividad JM, Brot L, Taleb S, Couturier-Maillard A, Nion-Larmurier I, Merabtene F, Seksik P, Bourrier A, Cosnes J, Ryffel B, Beaugerie L, Launay J-M, Langella P, Xavier RJ, Sokol H. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med advance online publication. 2016 doi: 10.1038/nm.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lanternier F, Mahdaviani SA, Barbati E, Chaussade H, Koumar Y, Levy R, Denis B, Brunel A-S, Martin S, Loop M, Peeters J, de Selys A, Vanclaire J, Vermylen C, Nassogne M-C, Chatzis O, Liu L, Migaud M, Pedergnana V, Desoubeaux G, Jouvion G, Chretien F, Darazam IA, Schäffer AA, Netea MG, De Bruycker JJ, Bernard L, Reynes J, Amazrine N, Abel L, Van der Linden D, Harrison T, Picard C, Lortholary O, Mansouri D, Casanova J-L, Puel A. Inherited CARD9 deficiency in otherwise healthy children and adults with Candida species–induced meningoencephalitis, colitis, or both. J Allergy Clin Immunol. 2015;135:1558–1568. doi: 10.1016/j.jaci.2014.12.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, Shibata N, Grunberg S, Sinha R, Zahm AM, Tardif MR, Sathaliyawala T, Kubota M, Farber DL, Collman RG, Shaked A, Fouser LA, Weiner DB, Tessier PA, Friedman JR, Kiyono H, Bushman FD, Chang K-M, Artis D. Innate Lymphoid Cells Promote Anatomical Containment of Lymphoid-Resident Commensal Bacteria. Science. 2012;336:1321–1325. doi: 10.1126/science.1222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y, Merad M. Microbiota-Dependent Crosstalk Between Macrophages and ILC3 Promotes Intestinal Homeostasis. Science. 2014;343 doi: 10.1126/science.1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hepworth MR, Fung TC, Masur SH, Kelsen JR, McConnell FM, Dubrot J, Withers DR, Hugues S, Farrar MA, Reith W, Eberl G, Baldassano RN, Laufer TM, Elson CO, Sonnenberg GF. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria–specific CD4+ T cells. Science. 2015;348:1031–1035. doi: 10.1126/science.aaa4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Crellin NK, Trifari S, Kaplan CD, Satoh-Takayama N, Di Santo JP, Spits H. Regulation of Cytokine Secretion in Human CD127+ LTi-like Innate Lymphoid Cells by Toll-like Receptor 2. Immunity. 2010;33:752–764. doi: 10.1016/j.immuni.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 165.Taylor PR, Roy S, Meszaros EC, Sun Y, Howell SJ, Malemud CJ, Pearlman E. JAK/STAT regulation of Aspergillus fumigatus corneal infections and IL-6/23-stimulated neutrophil, IL-17, elastase, and MMP9 activity. J Leukoc Biol. 2016;100:213–222. doi: 10.1189/jlb.4A1015-483R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.St Leger AJ, Desai JV, Drummond RA, Kugadas A, Almaghrabi F, Silver P, Raychaudhuri K, Gadjeva M, Iwakura Y, Lionakis MS, Caspi RR. An Ocular Commensal Protects against Corneal Infection by Driving an Interleukin-17 Response from Mucosal γδ T Cells. Immunity. 47:148–158.e5. doi: 10.1016/j.immuni.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]