Abstract
The purpose of this study was to report the prevalence of vestibular impairment (VI) in children ( n = 2,528) referred for complete vestibular testing because of balance disorders (BD) or hearing loss (H). A VI was shown in 51.5% of the children tested (1,304/2,528). For BD (e.g., vertigo, dizziness, instability, delay in posturomotor development), VI was found in 36.5% ( n = 379/1,037). The most frequent causes of BD with VI included inner ear malformation (13.5%), delay in posturomotor development (13.4%), hearing loss revealed with vertigo (3.9%), trauma (3.9%), vestibular neuritis (3.3%), meningitis (2.5%), Meniere-like syndrome (1.1%), BPPV posttrauma (1%), labyrinthitis (0.4%), and unknown etiology (19.6%). Normal responses to the complete battery of tests ( n = 658, 63.5%) excluded a vestibular origin to BD, leading to other diagnoses: principally migraine (15.6%), ophthalmological disorders (15.1%), neurological disorders (including delay in posturomotor development; 14.4%), orthostatic hypotension, or somatoform dizziness (<1%). Of the children referred for hearing loss ( n = 1,491), 68.5% were tested without cochlear implantation (CI; n = 1,022). In this group, 54.5% presented with VI ( n = 557). This was mostly found in cytomegalovirus infection, inner ear malformation, and genetic syndromes. Profound hearing loss candidates for cochlear implants had complete bilateral vestibular loss in 20% and delay in posturomotor development, and 80% had partial or normal vestibular function and normal posturomotor development. VI was found after CI in 50% on the side of the implant (partial in 41% and complete in 9%). VI is present in 36.5% of children referred to our center for BDs and 54.5% for hearing loss. Vestibular testing permits ruling out peripheral VI and hence seeking other causes for BDs such as migraine and ophthalmological disorders and also helps lower the risk of inducing bilateral complete vestibular loss in CI protocols.
Keywords: balance disorders, vertigo, dizziness, hearing loss, etiologies, pediatric
Learning Outcomes: As a result of this activity, the participant will be able to discuss the prevalence and etiologies of vestibular impairment, and describe the need for comprehensive vestibular testing in children with dizziness and hearing loss.
The prevalence of vestibular impairments (VIs) in the pediatric population is difficult to evaluate precisely because the symptoms are not specific and often misleading. Vertigo and dizziness, for example, can be reported in migraine, intestinal disorders, ophthalmological disorders, or vestibular loss. The tolerance for and the accuracy of the reported symptoms by children experiencing VI is also very variable with age. A sudden vestibular loss in a young child can resemble gastroenteritis, while symptoms resulting from congenital complete vestibular loss can be mistaken for severe neurological impairment. 1 2 The prevalence and etiologies of VI depend also on the hospital department or clinic to which the children with balance disorders (BDs) are referred. This can vary among neurology, general pediatry, otorhinolaryngology, and general emergency departments. It also depends on the age range of the patients tested 3 and on the vestibular testing technologies available (which may partially or completely test canal and otolith function).
The prevalence of vertigo and dizziness in children (from 3 years to adolescence) has been evaluated by national questionnaires to range from 5.3% 3 (7.5% adolescents) to 8%. 4 A center specialized in balance testing found 5.7%, 5 while another study found a much higher prevalence (17%) for adolescents. 6 These numbers are probably underestimated for young children, as they tolerate dizziness better than older children and adults. Indeed, they do not complain about it unless it severely impairs their activities. Furthermore, some VIs in young children can be asymptomatic.
Previous studies have reported the distribution of the various diagnoses found for BDs in pediatric populations. 5 6 7 8 9 10 11 12 13 14 In these articles, the etiologies of dizziness and BD in children are quite similar to those of adults with some exceptions. In the authors' clinical experience, migrainous equivalents are (as in adults) the most frequent etiology for dizzy sensations in children (17–40%). Benign paroxysmal positional vertigo (BPPV; canalolithiasis and cupulolithiasis) is rare in children outside a traumatic context, whereas in adults it is the most frequent diagnosis in patients with no context of cranial trauma (53% of vertigo cases in adults). 15 The clinical picture of nonpositional benign paroxysmal vertigo of childhood (BPVC) is characteristic of young children 2 to 5 years of age but is occasionally reported in older children. However, its prevalence in the literature is variable, ranging from 6 to 20%, probably because of the lack of a strict clinical definition, and also because of their rapid and spontaneous resolution (thus not arriving in specialized clinics). Vestibular neuritis, considered as frequent in children relative to adults (up to 9.8%), 12 is not typically observed after the age of 3 years. Brain tumors are only rarely the cause of vertigo in children and more frequently their symptoms are instability and falls. They are always associated with neurological signs which develop early. This is not the case in adults where neurinomas, for example, often have no other neurological signs than deafness.
Since only few centers perform vestibular testing in young children, few publications have evaluated the prevalence of VI on a large pediatric population with a wide range of ages. For example, Sommerfleck et al 14 used videonystagmography (VNG) and the video head impulse test (VHIT) and found the prevalence of VI in children referred for BDs was 20% ( n = 206, ages 1–18 years). For hearing loss, the prevalence of VI is reportedly 50 to 70% and is independent of the severity of the hearing loss 16 17 but highly related to the etiology of the hearing loss. VI is frequently found in children with congenital cytomegalovirus infection (cCMV) to include 73% of children tested with cCMV 18 as well as evidence of inner ear malformation and syndromic hearing loss. 19 Here, we report the results from our last 5 years of clinical testing to include the prevalence of VI and the distribution of etiologies diagnosed in our department in a large population of children referred for BDs or hearing loss.
Methods
Over the last 5 years, 2,528 children from 3 months to 15 years of age were subjected to complete vestibular testing. In 1,037 cases, they were referred for BD (e.g., vertigo dizziness instability, delay in posturomotor development in walking) and in 1,491 they were referred for hearing loss: 51.6% in the context of a cochlear implantation (CI; before any implantation: 20.2% and after CI: 31.5%), 48.4% as systematic vestibular testing for patients with hearing loss.
Children referred for vestibular function assessment receive a complete vestibular battery of tests for VI. A complete otoneurovestibular clinical examination with postural testing, eye movements and gaze stabilization system evaluation, search for spontaneous or revealed nystagmus, head impulse test (HIT), and videoscopy. A complete vestibular canal testing was performed including the bithermal caloric test, computer-driven rotatory chair test, and VHIT, and vestibular otolith testing includes the subjective vertical, cervical vestibular evoked myogenic potentials by bone conduction and off-vertical axis rotation (OVAR), as well as neurological examination and oculomotor testing. In our department, all the available vestibular tests were adapted to a broad range of ages (i.e., 2–3 months to 15 years of age). See the following references for procedures and technical details. 1 16 18 20 21
Results
Prevalence and Etiologies of Vestibular Impairment in Children with Balance Disorders
We found VI in 36.5% (379/1,037) of the children referred for BD ( Fig. 1 and Table 1 ). VI was the result of several etiologies in this group to include inner ear malformation (13.5%), vestibular neuritis (3.3%), delayed posturomotor syndrome (13.4%), traumatic causes (3.9%), meningitis (2.5%), Meniere-like syndrome (1.1%), labyrinthitis (0.4%), and rarely inflammatory inner ear disorder (Cogan's syndrome: five cases diagnosed in 20 years). 22 In 19% of the children with BD, etiology remained unknown.
Figure 1.
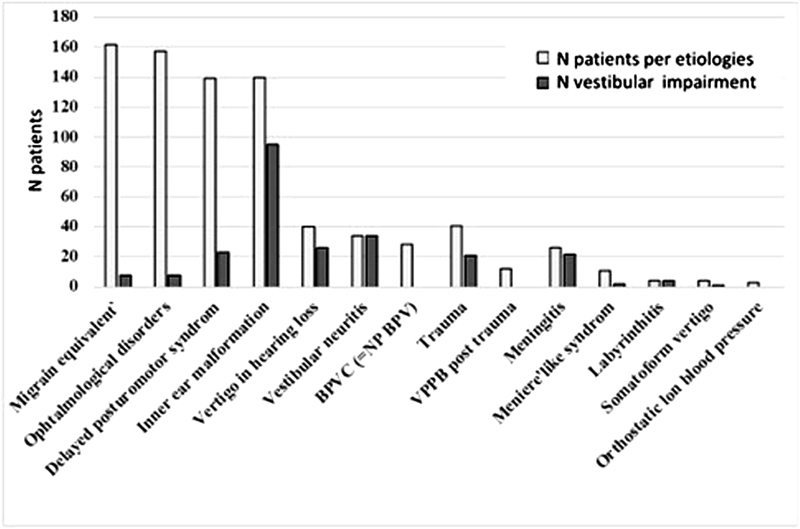
Prevalence of etiologies and vestibular impairment in the children referred for balance disorders. Etiologies are indicated by the textured columns and vestibular impairment by the black columns.
Table 1. Repartition of the Vestibular Impairment versus Normal Vestibular Function for the Principal Etiologies found in Children Referred for Balance Disorders.
| Diagnoses for balance disorders referred over 5 y period (2013–2017) | % of the children referred for balance disorders ( n = 1,037) | Vestibular impairment | Normal vestibular function |
|---|---|---|---|
| Migraine equivalent | 15.6 | 8 | 154 |
| Ophthalmological disorders | 8 | 149 | |
| Inner ear malformation | 13.5 | 95 | 45 |
| Delayed posturomotor syndrome | 13.4 | 23 | 116 |
| Neurological disorder | 3.1 | 0 | 33 |
| Vertigo revealing hearing loss | 3.9 | 26 | 14 |
| Trauma | 3.9 | 21 | 20 |
| VPPB posttrauma | 1.2 | 0 | 12 |
| Vestibular neuritis | 3.3 | 34 | 0 |
| BPVC (= NP BPV) | 2.7 | 0 | 28 |
| Meningitis | 2.5 | 22 | 4 |
| Meniere-like syndrome | 1.1 | 2 | 9 |
| Labyrinthitis | 0.4 | 4 | 0 |
| Somatoform vertigo | 0.4 | 0 | 4 |
| Orthoptic hypotension | 0.3 | 0 | 3 |
| Other rare etiologies for unknown | 19.6 | 136 | 67 |
| Total | 379 | 658 |
Abbreviations: BPVC, benign paroxysmal vertigo of childhood; NP BPV, non positional benign paroxysmal vertigo.
Normal responses to the complete battery of vestibular tests excluded a vestibular origin to the BD in 63.5%. In these patients, other diagnoses included migraine (15.6%), ophthalmological disorders (15.1%), neurological disorders (e.g. encephalitis, degenerative diseases, brain tumor; 3.1%), delayed posturomotor development syndrome (13.4%), benign paroxysmic vertigo in childhood (or nonpositional benign paroxysmal vertigo; 2.7%), BPPV posttrauma (benign positional paroxysmal vertigo due to migration of otoliths in the semicircular canals; 1.2%), Meniere-like syndrome (1.1%), orthostatic hypotension (0.4%), somatoform dizziness (0.4%), and rare etiologies or unknown (19.6%).
The different etiologies of dizziness and BDs in children are quite similar to those of adults with some exceptions. Migrainous equivalent or migraine vertigo (following the criteria of the International Headache Society) 23 is one of the most frequent causes of dizzy sensations in our pediatric population (15.6%). If not treated, 44.4% of them presented with ophthalmological problems that worsened the migrainous symptoms. Benign positional paroxysmal vertigo (i.e., canalolithiasis and cupulolithiasis) is the most frequent diagnosis for vertigo in adults but is rare in children (1.2%) and, in our experience, always occurs in a traumatic context. Vertigo and dizziness of ophthalmological origin, with or without headache, can result from vergence insufficiency and/or refraction problems. This represents 15.1% of our children referred for balance problems and is associated with migraine equivalents in 44.4%. This is not reported in many of the epidemiological studies for children or adults.
The clinical picture of nonpositional BPVC (as described by Basser in 1964) 24 occurs only in children aged 2 to 5 years. It represents 2.7% of the children referred for BDs in our study. Neurological causes were found in 14.3% ( n = 149) of patients referred for BDs. A majority of them (77.8%) corresponded to delay in posturomotor development syndrome with normal vestibular testing and 22.2% included encephalitis, degenerative diseases, and very rarely brain tumor.
Prevalence and Etiologies of Vestibular Impairment in Children Referred for Hearing Loss
A total of 1,491 children with hearing loss were tested. Children tested after CI (31.5%; n = 468) are not included in the calculation for prevalence of VI in hearing loss because CI modifies vestibular function in 50%. 24
Children with hearing loss with no CI (i.e., not candidates for cochlear implant or before any CI) showed VI in 54.5% (557/1,022; Table 2 ). VI was mostly found in children with cytomegalovirus infection, inner ear malformation, and genetic syndromes (e.g., Usher, CHARGE, Waardenburg, Pendred). Twenty percent of cases with profound bilateral congenital hearing loss were associated with a complete bilateral vestibular loss and a delay in development of posturomotor control, while the remaining 80% had partial loss or normal vestibular function and normal posturomotor development ( Fig. 2 ). Cochlear implantation indicated for profound sensorineural hearing loss (SNHL) induced impaired vestibular function on the side of the implant (partially 40% and completely 10%). 20 Of the 468 children tested after CI (unilateral or bilateral), we found 78.6% had abnormal vestibular testing. Many children with no balance problems after CI (particularly when they were referred by other implantation centers) were not followed up for vestibular function and this can partly explain this high percentage of VI.
Table 2. Repartition of the Vestibular Impairment versus Normal Vestibular Function for the Children Referred for Hearing Loss (with no Cochlear Implantation and after Cochlear Implantation).
| Children referred for HL over 5 y period (2013–2017) | Children with vestibular impairment | Children with normal vestibular function | Percentage of children referred for HL ( n = 1,491) |
|---|---|---|---|
| Vestibular status in HL with no CI: |
n
= 557/1,022
54.5% |
n
= 466/1,022
45.5% |
n
= 1,022
68.5% |
| Hearing loss with no CI program | n = 386 | n = 335 | |
| Hearing loss before CI | n = 171 | n = 131 | |
| Follow-up post-CI | n = 368 | n = 100 |
n
= 468
31.5% |
Abbreviations: CI, cochlear implantation; HL, hearing loss.
Figure 2.
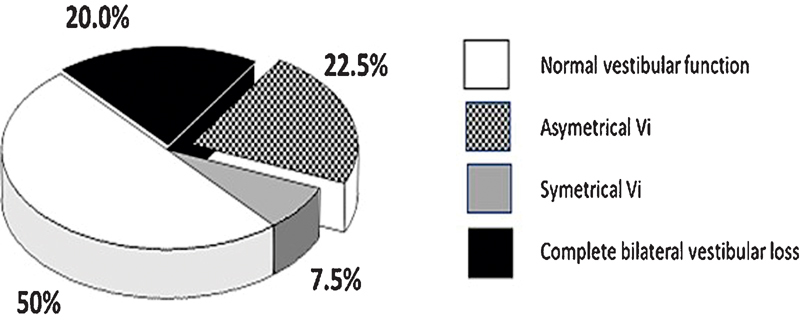
Vestibular impairment in profound congenital hearing loss candidates for cochlear implantation ( n = 291).
Discussion
Vertigo and balance problems in children often lead to too many unnecessary and costly tests (e.g., magnetic resonance imaging [MRI], computed tomography [CT]) because of insufficient understanding of the most typical underlying pathologies and their respective symptomatology. In our experience, balance problems in children represent half of the pediatric population referred for vestibular testing in our center, the other half corresponding to the diagnosis, treatment, and follow-up of children with hearing loss.
In our clinic, all children first undergo clinical otoneurovestibular testing to search for VI signs or neurological signs before prescribing brain imaging. If there is no neurological sign requiring an urgent referral to neurology and brain imaging, it will be efficient to perform complete vestibular testing to determine VI (36.5% of the cases) and ophthalmological testing (i.e., orthoptic testing and eye refraction measurement) to detect ophthalmological disorders (the only cause of BDs in 15.1% of the cases and aggravating factor for migraine equivalent in 44.4%).
In the following paragraphs, we will discuss the epidemiological results found in our pediatric population and compare them to the data found in the literature to include controversies about some diagnoses.
Posttraumatic Vertigo
Posttraumatic vertigo represents 5.1% of the children referred for balance testing ( n = 53/1,037): 12 of them had BPPV with no VI and 41 of them had no positional vertigo and VI in 51.2% of them (21/20). This prevalence rate is highly variable depending on the centers (6–20%). 10 11 14
BPPV (i.e., brief, intense, and positional vertigo) is the most frequent etiology of acute vertigo in adults, but it is rare in children (1.2% in our series) and always occurs in the context of cranial trauma after a fall or a road accident. The characteristics are the same in children and in adults: vertigo occurring during a change of position (e.g., Hallpike-Dix position) with adaptable and fatigable geotropic nystagmus (i.e., rapid phase of the nystagmus beating toward the ground) occurring with a latency. The dizzy sensation is often better tolerated by children than by adults. Otological, vestibular, and neurological assessments are typically normal. Given the rarity of BPPV in children, care must be taken not to reach this diagnosis too easily, especially if the clinical picture is atypical; always seek signs of central origins. Some malformations of the inner ear, especially large dilatations of the vestibular aqueduct, may also give atypical positional nystagmus. In these cases, there is often associated hearing loss and the CT scan will detect the malformation.
In a traumatic context, dizziness may be a sign of fracture of the temporal bone with perilymphatic fistula. Perilymphatic fistulae may lead to progressive deafness and vestibular loss or recurrent meningitis and requires urgent surgical treatment (clogging this leak) to limit these risks. Any child who, immediately or in a few hours after a head trauma, has a BD or vertigo, otorrhagia, hypoacousia, deafness, continuous tinnitus, and/or permanent torticollis should have at least an otological and vestibular clinical examination. This examination will search for signs of temporal bone fracture to include hemotympanum, otorrhagia, sensorineural or conductive hearing loss, and spontaneous nystagmus with vestibular partial or complete loss. These signs should lead urgently to the realization of a CT scan. The CT scan can show a fracture line on the temporal bone which may or may not pass through the inner ear. It can show air inside the labyrinth (transitory pneumolabyrinth, which disappears in <8 days), signaling the opening of the inner ear in the middle ear and the existence of a fistula. The CT scan can show a dislocation of the ossicular chain usually associated with an opening of the inner ear. It is interesting to note that in young children, the fracture line may not be visible and yet be accompanied by significant functional impairment of the vestibular or auditory function (e.g., due to intralabyrinthine hemorrhage or labyrinthine contusion).
Inner Ear Malformations
In our data, the most frequent etiologies of VI are inner ear malformations. They represent 13.5% ( n = 140/1,037) of the children referred for BDs. Inner ear malformations are known to induce VI (67.8% of them presented VI in our study). They are discovered in a context of recurrent episodes of vertigo or secondary to a mild trauma followed by dizziness, or a sudden hearing loss associated with vertigo or frequent falls and delayed posturomotor development. The CT scan will reveal the inner ear malformation. 25 Inner ear malformations also have a greater risk of VI after CI (35%) compared with anatomically normal ears (10%). 16
Vestibular Neuritis
This type of acute unilateral vestibular loss is a common diagnosis in children (3.3% here) as in adults (4 and 9.8%). 11 26 Vestibular neuritis however, has a better prognosis in children than adults. A study on a group of 47 children with typical acute unilateral vestibular loss, aged from 18 months to 15 years with 9 of them younger than 3 years with a follow up to 10 years shows 93% of recovery at 1 year (Majer et al, unpublished data, 2017) compared to a 50% recovery rate in adults. Vestibular neuritis occurs during epidemics of nasopharyngeal viral infections and is most likely of viral origin in children. Vestibular neuritis is manifested by a sudden and severe rotatory vertigo, with instability and vomiting, occurring during an infectious viral episode. This picture can be misleading in young children of prelingual age, because the association of sudden vomiting and abdominal pain in a young child is a picture that resembles gastroenteritis. However, on clinical examination, there are clear signs of peripheral isolated vestibular deficit to include the tendency to always fall on the same side when the child stands on the mattress or on the floor with eyes closed. Nystagmus is also observed, if it is sought while preventing the fixation of gaze, for example, with Frenzel glasses. Such signs indicate the need for an audiovestibular assessment which will prove and quantify the vestibular loss and confirm the absence of hearing impairment. The neurological examination of these children is strictly normal, as is the otological examination which excludes the differential diagnosis of complicated acute otitis media with labyrinthitis. An MRI may show a hypersignal flair of the vestibular nerve, but this sign is very inconsistent.
VI in middle ear pathologies is rare in children (< 1% in our data). Otitis media can induce VI in the case of meningitis (2.5% of the BDs referred to us) or secondary labyrinthitis, with a long-term risk of ossifying labyrinthitis and progressive lesions with cochleovestibular destruction. Similarly, chronic ear infections (particularly cholesteatoma) can erode the inner wall of the tympanic cavity and induce audio VI. Vertigo may be the first and only sign of chronic otitis. The otoscopic examination shows the perforation of the eardrum, an epidermal plug or a retraction pocket or, more rarely, a congenital cholesteatoma. The vestibular function can be normal or impaired depending on the extension of the cholesteatoma. Simple middle ear effusion, especially in young children who are learning to walk, may be accompanied by instability with an increase in the frequency of falls. The vestibular and neurological assessment is normal. The imbalance disappears with the resolution of the middle ear effusion (often spontaneously or with transtympanic tubes).
Meniere-Like Syndrome
Meniere-like syndrome is rare in children in our experience (1.1%) compared to 13.8% in adults. 15 Meniere's syndromes associated with vertigo, hypoacousia, and tinnitus with a clogged ear have only been observed in our center since the past 8 years. Perhaps it is an underestimated diagnosis because all the symptoms are not always found during the interview: tinnitus and mild hearing loss are rarely reported spontaneously by the child. The recurrence of invalidating vertigo, associated with nausea and vomiting, without headache, lasting several hours to days, will evoke Meniere-like syndrome in the absence of any other cause. The otological and neurological examinations are normal, and the vestibular assessment outside the attacks is usually normal. The audiogram may show a SNHL predominant on the low frequencies and the vestibular tests will reveal a mild VI on the side of the hearing loss. Care should be taken not to make this diagnosis during an initial attack, to monitor the child regularly, and to be prepared to reconsider the diagnosis if symptoms change. Delayed vertigo is a Meniere-like syndrome (often incomplete without tinnitus or aggravation of the hypoacousia) associated with a history of a preexisting lesion of the inner ear (hearing loss after trauma and temporal bone fracture, viral infection–type mumps).
Autoimmune Inner Ear Impairment (Cogan's Syndrome)
This is very rare in adults and children and is often misdiagnosed in its initial phase. We did not diagnose any new cases during the last 5 years but published three pediatric cases in 2001. 22 Cogan's syndrome can be announced by conjunctivitis (i.e., red eyes) often preceding vertigo and deafness. This could be interpreted as a benign viral conjunctivitis if the association of these signs is not recognized. Laboratory analysis will reveal a substantial inflammatory syndrome and the MRI will sometimes show a contrasting hyper signal of inflammatory nature of the inner ear fluids. The ophthalmological examination will show interstitial keratitis or uveitis and vestibular and audiological impairments. Steroid treatment at high doses is urgent to avoid a complete loss of hearing and vestibular function.
When the vestibular functional assessment is normal (excluding a peripheral VI at the origin of the symptoms) other etiologies can be considered for a child with BDs.
Headache and Vertigo, Migraine Equivalent, Vestibular Migraine
There is consensus in the literature that the most common diagnoses in children with vertigo, dizziness, and headache is vestibular migraine usually in 20 to 28% of cases 11 13 14 27 and even in some studies 40 and 60%. 5 9 In our data, the diagnosis of migraine was retained in 15.6% of the children with normal vestibular function assessment and normal neurological examination. Many children with headache and vertigo or dizziness did not fulfill all the criteria of vestibular migraine defined by the International Headache Society 23 and were classified as vertigo migraine equivalent. Many of these children (44.4%) were found to have ophthalmological problems (e.g., vergence insufficiency and pathological refraction). These ophthalmological disorders were worsening the symptoms of migraine and the control of the migraine episodes were always easier as soon as the ophthalmological problems were solved. That led us to request systematic ophthalmological assessment for all children diagnosed with migraine.
Ophthalmological Disorders
In our study, vergence insufficiency and refraction disorders are the second most frequent cause (15.1%) of dizziness and BDs. Such ophthalmological problems also are found as an aggravating factor in 44.4% of the typical migraine equivalent patients. To our knowledge, this diagnosis is not cited in any published articles on prevalence of vertigo and dizziness in children. Their incidence is constantly increasing with the ever-growing popularity of all types of recreational activities based on small video screens (e.g., video games, mobile phones, TV, etc.). Refractive disorders (e.g., myopia, hyperopia, and astigmatism) or vergence insufficiency (e.g., poor convergence or divergence) can lead to dizziness. 28 29 They are never expressed by a severe attack of rotatory vertigo but rather by brief but repeated sensation of rotation or pitching often related to eye strain (e.g., at school, at the end of the day, after long sessions of computer or television, or reading). The dizziness can sometimes occur during sleep or upon waking in the morning. Ophthalmological disorders can be isolated or associated with headache (50%) or nausea but rarely vomiting. Vestibular testing and clinical neurological examination are normal. There is often individual or family history of migraine. This diagnosis must be suggested to avoid wide ranging diagnoses that lead to unnecessary testing such as MRI or psychiatric examination, and often school absenteeism. The diagnosis is assessed with an orthoptic evaluation and refractometry with cycloplegic treatment. The correction of abnormal refraction by prescription of glasses and orthoptic training will make the symptoms disappear and any migraine will be stabilized with minor pharmaceutical treatment (Wiener Vacher et al, unpublished data).
Benign Paroxysmal Vertigo in Childhood or Nonpositional Benign Paroxysmal Vertigo
In our study, BPVC is responsible for only 2.7% of the dizziness in children, which is different from the prevalence reported in the literature (up to 20%). We follow the description published by Basser in 1964 for recurrent nonpositional paroxysmal benign vertigo. Its etiology is unknown, but it is benign and resolves itself spontaneously without special treatment. Unlike migraine, it almost always affects very young children, from 2 to 5 years of age. Some children have this type of symptomatology around the age of 5 to 8 years, but are found, in our experience, to be manifesting an ophthalmological problem decompensated during the acquisition of reading. In the literature, there is a great confusion in the criteria used for diagnosing vertigo in a child as BPVC which can include any brief and recurrent sensation of positional or nonpositional vertigo, associated or not associated with headache. 30 These criteria often stray far from the strict definition of Basser. 24
For diagnosis, BPVC was characterized by short, brief episodes, usually lasting only a few seconds (generally < 10 minutes), marked by a sudden imbalance while the child was playing, forcing him to stop, or even sit down. The child, if he can speak, says that “the house turns,” and in general the child does not fall. There is no positional trigger for this vertigo and no nystagmus. These episodes are typically well tolerated without pain, nausea, or vomiting, and no postcrisis drowsiness or sleepiness. Sometimes parents describe a slight pallor and are surprised to see the child resume normal activities after the attacks. The repetition of the crises makes these episodes worrisome and motivates visits to the clinic. The episodes repeat for several months and disappear spontaneously, justifying no treatment and no invasive tests. The clinical otoneurovestibular assessment is normal. It is prudent to make ophthalmological and vestibular assessments of these children, to find another cause for the dizziness, but MRI is not justified from the start because it is expected to be normal. These children should be reviewed in consultation 4 to 5 months later to confirm the disappearance of the vertigo attacks. Parents need to be informed that if the symptoms change they must immediately return for consultation to reevaluate the diagnosis.
Neurological Impairment
Neurological impairment was found in 14.4% of the children referred for BDs. This diagnosis was already suggested in 3.3% of cases where the clinical otoneurovestibular examination showed other neurological signs of central nervous system impairment (e.g., cerebellar syndrome, cranial nerve paralysis) leading to brain imaging and a complete neurological screening. Tumors of the posterior fossa are very rare and occurred in less than 1% in our study. They are generally responsible for instability rather than dizziness or vertigo, expressed as recent walking difficulties (while autonomous walking has long been acquired), and always associated with neurological signs. Posterior fossa tumors can be responsible for abnormal eye movements (e.g., torsional, multidirectional, and flutter nystagmus) whose recent appearance and persistence are warning signs. A neurological disorder was evoked as a cause for a delay in posturomotor development with normal vestibular function (83.4% of the posturomotor delay syndrome tested in our center, n = 116/139) as reported by Wiener-Vacher et al. 1 18
Somatoform Vertigo
Psychogenic balance problems in children are rare (< 1% in our series), and usually occur in girls aged 8 to 10 years. Ketola et al 7 reported a prevalence of 2.5% in children and adolescents and Langhagen et al 31 reported a prevalence of 14%. These cases are easily recognizable by their atypical appearance: symptoms are caricaturally mimicked by patients defying the normal laws of equilibrium, while maintaining very good balance when performing automatic tasks. For example, a child arriving at the clinic in a wheelchair might claim to be unable to stand or walk but gesticulates and is able to unlatch his shoes while standing, or suddenly walks to pick up an object of interest that had fallen to the ground then changes direction without any problem. Vestibular and neurological tests are normal. Often somatoform vertigo follows a real episode of VI (e.g., vestibular neuritis) by weeks or months. The child draws a benefit from this experience where his/her parents were more attentive and worried about him/her and will unconsciously attempt to repeat it. The interview with the child and the parents often reveals underlying psychological problems (e.g., school violence, family conflicts). Awareness of the problem usually helps the child to get out of this impasse, but professional psychological care is necessary to avoid relapse. Such psychogenic vertigo must be taken seriously because they are the source of frustration, school absenteeism for the child, and professional absenteeism for the parents. Somatoform disorders are the cause of unjustified medical expenses (MRI is not required) and a cascade of unnecessary specialized medical interventions that merely crystallize the anguish of the child and his or her parents.
Orthostatic Hypotension
Langhagen et al 12 reported on a cohort of 1,661 adolescents that 72% of them had mild to severe episodes of dizziness or vertigo occurring when either getting up or standing for a long time. In our imbalance patient population, it was rare (< 1%). This can be partially explained by the younger age distribution of our population. 21 Orthostatic hypotension occurs more often in children during rapid growth periods while the cardiovascular system has not adapted to the new height of the body to counteract the effects of gravity on blood pressure following abrupt position changes. Dizziness related to orthostatic hypotension is usually positional (e.g., on standing up or during prolonged standing) with sensations of an empty head, falling, head turns and imminent loss of consciousness, phosphenes, and headaches. This discomfort lasts from a few seconds to a few minutes, but the headache may last longer and may be accompanied by lipothymia and, more rarely, loss of consciousness. All tests are typically normal except blood pressure that drops when a standing position is suddenly taken after 20 minutes of dorsal decubitus.
Epileptic Vertigo
We did not find any cases of epileptic vertigo over the last 5 years. However, we previously found two cases of recurrent episodes of epileptic eye movements due to occipital cortical lesions (i.e., pial angioma and neuroepithelial dysplasia), but these two children did not complain of vertigo neither of instability during the crisis. Comitial vertigo is rare and is part of the auras of comitial seizures, usually associated with a series of signs suggestive of comitiality: hallucinations of complex movements (more than simple isolated rotatory vertigo), auditory hallucinations, neurological signs of localization, epileptic absence with loss of consciousness, seizures of abnormal ocular movements (which can sometimes mimic nystagmus). These vertigos are remarkably well tolerated, do not bring on great instability despite their amplitude, are not associated with neurovegetative signs, and are characterized by their recurrence. The neurological examination may be normal outside the crisis. Such a clinical pattern should lead to a referral to a neurological clinic generally followed by an electroencephalogram and brain imaging. The brain imaging in epilepsy is often normal; however, in a few cases, it may reveal an acortical lesion such as neuroepithelial dysplasia or cortical angioma.
Prevalence and Etiologies of Vestibular Impairment in Sensorineural Hearing Loss
For patients with hearing loss without cochlear implants, the prevalence of VI was 54.5%. Abnormal vestibular responses associated with hearing loss were more frequently found in patients with cytomegalovirus infection, inner ear malformation, and genetic syndromes (e.g., Usher, CHARGE, Waardenburg, and Pendred; see Table 3 ). Profound bilateral congenital SNHL occurred in 20% of cases and was associated with a complete bilateral vestibular loss and delay in posturomotor control. The remaining 80% of cases had partial or normal vestibular function and normal posturomotor development. In patients with profound hearing loss, CI can lead to impairment of vestibular function on the side of the implant. Jacot et al 16 found partial impairment in 41.0% and complete impairment in 10% of cases (see Fig. 3 protocol).
Table 3. Summary of Syndromic Hearing Loss (=H) Frequently Associated with Vestibular Impairment.
| Dysmorphy | Level of hearing loss | Cochlea CT scan images | Vestibule CT scan images | Vestibular function | NMR central nervous system | Diagnosis to suspect | What to look for to confirm the diagnosis? |
|---|---|---|---|---|---|---|---|
| No dysmorphy +/− microcephaly, RCIU | HL to SP, progressive ++ | Normal | Normal | Normal to bilateral complete loss, progressive ++ | Hypersignals white matter, temporal cysts, calcifications | CMV fetopathy | Maternal CMV serology? PCR on Guthrie |
| No dysmorphy | Congenital HP | Normal | Normal | Bilateral complete loss | Normal | USHER 1 | Retinitis pigmentosa fundus examination? ERG |
| No dysmorphy | HM to HS, progressive +/− | Normal | Normal | Normal to bilateral complete loss, +/− progressive | Normal | USHER 2 or 3 | Retinitis pigmentosa fundus examination? ERG |
| No dysmorphy | HM to HP | Normal | Normal | Normal to bilateral complete loss, +/− Progressive | Normal | Jervell and Lange Nielsen | Malaise, ECG: long QT |
| Facial dysmorphy | HL to HP, +/− asymmetry | Normal or possible malformations | SCC aplasia | Bilateral canal areflexia, possible otolith impairment | Agenesis of olfactory bulbs | CHARGE | Coloboma, cardiac malformation, choanal atresia, growth retardation, not always present |
| Canthal dystopia, abnormal pigmentation (white hair wick) | HM to HP, progressive +/− | Possible malformations | Dysmorphy of the SCC | Normal to bilateral complete loss | Normal, possible agenesis of olfactory bulbs | Waardenburg I | Pigmentation abnormalities, vertebral and cardiac malformation, cleft palate? |
| Pigmentation disorder +/− discrete (heterochromia) | HM to HP, progressive +/− | Possible malformations | Dysmorphy of the SCC | Normal to bilateral complete loss | Agenesis of olfactory bulbs | Waardenburg II | Anosmia, parotid hypoplasia, pigmentation abnormalities? |
| Canthal dystopia, abnormal pigmentation (white hair wick) | HM to HP, progressive +/− | Possible malformations | Dysmorphy of the SCC | Normal to bilateral complete loss | Normal, or possible cerebellar hypoplasia | Waardenburg III | Muscle contractions, finger and toes fusion |
| Pigmentation disorder +/− discrete (heterochromia) | HM to HP, progressive +/− | Possible malformations | Dysmorphy of the SCC | Normal to bilateral complete loss | Normal, possible agenesis of olfactory bulbs | Waardenburg IV | Hirschsprung, digestive neonatal surgery? |
| No dysmorphy | Progressive H | Incomplete cochlear partition type II (Sennaroglu L, 2002) 25 | EVA | Normal to bilateral complete loss | Normal | Pendred | Hypo or euthyroidism, goiter (perchlorate test) |
| Possible asymmetry, enchondroma | Progressive H | Microcochlea with incomplete cochlear partition | Horizontal SCC dysmorphy +/− EVA + absence of central bony island | Normal to bilateral complete loss | Normal | BOR syndrome | Cervical cyst or fistula, kidney malformation |
| Facial asymmetry, mandibular hypoplasia, external ear hypo or aplasia | Conductive H | Middle ear malformation, possible cochlear hypoplasia | Possible enlarged or hypoplasic vestibule | Normal to complete loss | Possible hypomyelination cerebellum atrophy | Goldenhar syndrome, ocular auricular vertebral dysplasia | Spinal malformation |
| No dysmorphy | Progressive H | Cochlear incomplete partition type II (Sennaroglu L, 2002) 25 | EVA | Normal to bilateral complete loss | Normal | rDTA | Kidney disorders, Na + /K + treatment? |
| Mild facial dysmorphy | HL to HP, +/− progressive | Normal cochlea | Possible malformations of the horizontal SCC (small or absent central bony island) | Normal to bilateral complete loss | Possible abnormalities (cerebellum) | Deletion 22q11 (DiGeorge, velocardio facial syndromes) | Velar insufficiency, labiopalatine cleft, cardiac problems, calcemic disorders, infection+++ |
| Dysmorphy | Congenital HP | Possible malformations | Possible malformations of SCC | Normal to bilateral complete loss | Possible abnormalities (ventricles, cerebellum) | DOOR | Fingers and nails abnormalities? |
Abbreviations: BOR, branchiootorenal; CMV, cytomegalovirus infection; DOOR, deafness onycho-osteodystrophy retardation; ECG, electrocardiogram; ERG, electroretinogram; EVA, enlarged vestibular aqueduct; HL, mild hearing loss; HM, moderate hearing loss; HP, profound hearing loss; HS, severe hearing loss; IUGD, in-utero growth delay; rDTA, renal distal tubular acidosis; SCC, semicircular canals.
Figure 3.
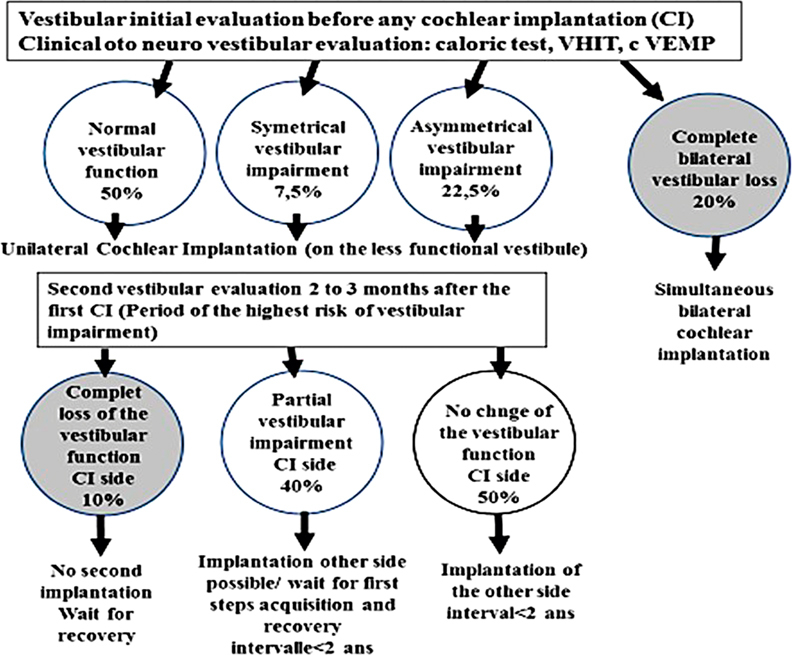
Flowchart showing the protocol that reduces to zero the risk of inducing bilateral complete vestibular loss with cochlear implantation. CI, cochlear implantation; cVEMP, cervical vestibular evoked myogenic potential; VHIT, video head impulse test.
Congenital cytomegalovirus (cCMV) infection is the leading cause of psychomotor retardation and non-genetic neurosensory hearing loss. It is responsible for 0.2 to 1.3 cases of deafness per 1,000 births, that is 10 to 50% of all hearing loss, all grades combined. In a study involving 52 children with cCMV infection and neurosensory hearing loss who underwent vestibular assessment, 92.3% had vestibular involvement, and 33.3% of these were complete and bilateral deficits, 43.7% partial and bilateral and 22.9% partial and unilateral. 18 The risk of hearing loss is higher if the child shows symptoms of CMV infection at birth (32.8%, usually bilateral, severe to profound) and if hearing threshold degrades over time (50% of cases).
Syndromic Hearing Loss and Vestibular Impairment
Hearing loss associated with special malformative syndrome is often accompanied by VI and may not be associated with inner ear malformation. 19 Vestibular loss is always present and complete in Usher 1, while it is inconstant, partial and progressive in Usher 2 and 3. The VI is variable in Jervell and Lange-Nielsen Syndrome. Some syndromic hearing loss also shows vestibular loss related to malformation of the inner ear. For example, the CHARGE syndrome is characterized by an absence of semicircular canals. Other syndrome-associated hearing loss with a cochlea-vestibular malformation is found in Waardenburg syndrome 1, 2 and 3; Pendred syndrome, Branchio-oto-renal syndrome (BOR), Goldenhar syndrome, renal distal tubular acidosis syndrome (rDTA), 22q11 deletion syndrome, and Deafness Onycho-Osteodystrophy mental Retardation syndrome (DOOR).
Nonsyndromic Hearing Loss
Non-syndromic hearing loss is less often associated with VI. DFNB1 (Connexin 26), the most frequently occurring form of congenital pediatric hearing loss, rarely affects vestibular function. However, DFNB3 (POUF 4) is associated with an enlarged internal meatus with a progressive VI. DFNB9 (otoferlin) does not affect vestibular function. DFNB 4 is associated with the same inner ear malformation as the Pendred syndrome (see syndromic hearing loss section). In children with profound deafness who were candidates for CI, Vi was found in 56.7% of the cases (165/291). This Vi can be complete (20%), partial (38%) or absent (42%). 16 VI can be induced by the cochlear implant surgery when vestibular function is present. Jacot and Wiener-Vacher. 16 first showed that after unilateral CI, there was a complete loss of vestibular function on the side of the implant in 10% of cases, a change in vestibular responses in 40% and no modification of vestibular function in 48%. In case of inner ear malformation, the prevalence of induction of a complete loss of vestibular function after implantation increases to 35%. 16
Considering the severe impact of a complete bilateral vestibular loss in young children on posturomotor and cognitive performances, 32 it is advisable to limit the risk for inducing bilateral vestibular loss by adopting sequential bilateral implantation rather than bilateral simultaneous implantation. Bilateral simultaneous implantation should be reserved for patients with no vestibular function on either side (e.g., patients with meningitis or Usher 1; see Fig. 3 ) at the first vestibular testing before CI. For the sequential bilateral implantation, the first CI will be done on the side of the less functional vestibule and the second CI only if after a complete vestibular testing the first implant did not induce a complete vestibular loss. In the case of a complete vestibular loss on the side of the first CI, the contralateral implant will not be performed to keep efficient vestibular function on the other side.
Conclusion
Our clinical data over 5 years revealed that the prevalence of VI for children with BDs was 36.5% and for children with hearing loss was 54.5% of patients referred for vestibular testing. Vestibular testing permits to prove or to exclude a peripheral VI. In the case of normal vestibular function, other causes of BDs were detected, such as migraine and ophthalmological disorders. In the case of hearing loss, vestibular testing helps in lowering the risk of complete bilateral vestibular loss in CI protocols for profound hearing loss, reserving bilateral simultaneous CI to the children with already complete bilateral vestibular loss and preferring bilateral sequential CI with a vestibular testing after the first.
References
- 1.Wiener-Vacher S R, Obeid R, Abou-Elew M. Vestibular impairment after bacterial meningitis delays infant posturomotor development. J Pediatr. 2012;161(02):246–510. doi: 10.1016/j.jpeds.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Rine R M, Wiener-Vacher S. Evaluation and treatment of vestibular dysfunction in children. NeuroRehabilitation. 2013;32(03):507–518. doi: 10.3233/NRE-130873. [DOI] [PubMed] [Google Scholar]
- 3.Li C M, Hoffman H J, Ward B K, Cohen H S, Rine R M.Epidemiology of dizziness and balance problems in children in the United States: a population-based study J Pediatr 2016171240–70., 3 [DOI] [PubMed] [Google Scholar]
- 4.Niemensivu R, Pyykkö I, Wiener-Vacher S R, Kentala E. Vertigo and balance problems in children--an epidemiologic study in Finland. Int J Pediatr Otorhinolaryngol. 2006;70(02):259–265. doi: 10.1016/j.ijporl.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 5.Humphriss R L, Hall A J. Dizziness in 10 year old children: an epidemiological study. Int J Pediatr Otorhinolaryngol. 2011;75(03):395–400. doi: 10.1016/j.ijporl.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 6.Filippopulos F M, Albers L, Straube A et al. Vertigo and dizziness in adolescents: risk factors and their population attributable risk. PLoS One. 2017;12(11):e0187819. doi: 10.1371/journal.pone.0187819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ketola S, Niemensivu R, Henttonen A, Appelberg B, Kentala E. Somatoform disorders in vertiginous children and adolescents. Int J Pediatr Otorhinolaryngol. 2009;73(07):933–936. doi: 10.1016/j.ijporl.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 8.O'Reilly R C, Morlet T, Nicholas B D et al. Prevalence of vestibular and balance disorders in children. Otol Neurotol. 2010;31(09):1441–1444. doi: 10.1097/MAO.0b013e3181f20673. [DOI] [PubMed] [Google Scholar]
- 9.Jahn K, Langhagen T, Schroeder A S, Heinen F. Vertigo and dizziness in childhood - update on diagnosis and treatment. Neuropediatrics. 2011;42(04):129–134. doi: 10.1055/s-0031-1283158. [DOI] [PubMed] [Google Scholar]
- 10.Langhagen T, Lehnen N, Krause E, Jahn K.Vertigo in children and adolescents. Part 1: Epidemiology and diagnosis of peripheral vestibular disorders [in German] HNO 20136109791–802., quiz 803–804 [DOI] [PubMed] [Google Scholar]
- 11.Gioacchini F M, Alicandri-Ciufelli M, Kaleci S, Magliulo G, Re M. Prevalence and diagnosis of vestibular disorders in children: a review. Int J Pediatr Otorhinolaryngol. 2014;78(05):718–724. doi: 10.1016/j.ijporl.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Langhagen T, Albers L, Heinen F et al. Period prevalence of dizziness and vertigo in adolescents. PLoS One. 2015;10(09):e0136512. doi: 10.1371/journal.pone.0136512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raucci U, Vanacore N, Paolino M C et al. Vertigo/dizziness in pediatric emergency department: five years' experience. Cephalalgia. 2016;36(06):593–598. doi: 10.1177/0333102415606078. [DOI] [PubMed] [Google Scholar]
- 14.Sommerfleck P A, González Macchi M E, Weinschelbaum R, De Bagge M D, Bernáldez P, Carmona S. Balance disorders in childhood: main etiologies according to age. Usefulness of the video head impulse test. Int J Pediatr Otorhinolaryngol. 2016;87:148–153. doi: 10.1016/j.ijporl.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 15.Lüscher M, Theilgaard S, Edholm B. Prevalence and characteristics of diagnostic groups amongst 1034 patients seen in ENT practices for dizziness. J Laryngol Otol. 2014;128(02):128–133. doi: 10.1017/S0022215114000188. [DOI] [PubMed] [Google Scholar]
- 16.Jacot E, Wiener-Vacher S. Potential value of vestibular evoked myogenic potentials in paediatric neuropathies. J Vestib Res. 2008;18(04):231–237. [PubMed] [Google Scholar]
- 17.Cushing S L, Gordon K A, Rutka J A, James A L, Papsin B C. Vestibular end-organ dysfunction in children with sensorineural hearing loss and cochlear implants: an expanded cohort and etiologic assessment. Otol Neurotol. 2013;34(03):422–428. doi: 10.1097/MAO.0b013e31827b4ba0. [DOI] [PubMed] [Google Scholar]
- 18.Bernard S, Wiener-Vacher S, Van Den Abbeele T, Teissier N. Vestibular disorders in children with congenital cytomegalovirus infection. Pediatrics. 2015;136(04):e887–e895. doi: 10.1542/peds.2015-0908. [DOI] [PubMed] [Google Scholar]
- 19.Noel-Petroff N. France: EDP Sante; 2014. Cent Syndromes ORL Avec Surdite. [Google Scholar]
- 20.Jacot E, Van Den Abbeele T, Debre H R, Wiener-Vacher S R. Vestibular impairments pre- and post-cochlear implant in children. Int J Pediatr Otorhinolaryngol. 2009;73(02):209–217. doi: 10.1016/j.ijporl.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 21.Wiener-Vacher S R, Wiener S I. Video head impulse tests with a remote camera system: normative values of semicircular canal vestibulo-ocular reflex gain in infants and children. Front Neurol. 2017;8:434. doi: 10.3389/fneur.2017.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ndiaye I C, Rassi S J, Wiener-Vacher S R. Cochleovestibular impairment in pediatric Cogan's syndrome. Pediatrics. 2002;109(02):E38. doi: 10.1542/peds.109.2.e38. [DOI] [PubMed] [Google Scholar]
- 23.Lempert T, Olesen J, Furman J et al. Vestibular migraine: diagnostic criteria. J Vestib Res. 2012;22(04):167–172. doi: 10.3233/VES-2012-0453. [DOI] [PubMed] [Google Scholar]
- 24.Basser L S. Benign paroxysmal vertigo of childhood. A variety of vestibular neuronitis. Brain. 1964;87:141–152. doi: 10.1093/brain/87.1.141. [DOI] [PubMed] [Google Scholar]
- 25.Sennaroglu L, Saatci I.A new classification for cochleovestibular malformations Laryngoscope 2002112122230–2241..PMID:12461346 [DOI] [PubMed] [Google Scholar]
- 26.Guerra-Jiménez G, Arenas Rodríguez A, Falcón González J C, Pérez Plasencia D, Ramos Macías Á. Epidemiology of vestibular disorders in the otoneurology unit. Acta Otorrinolaringol Esp. 2017;68(06):317–322. doi: 10.1016/j.otorri.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Langhagen T, Landgraf M N, Huppert D, Heinen F, Jahn K. Vestibular migraine in children and adolescents. Curr Pain Headache Rep. 2016;20(12):67. doi: 10.1007/s11916-016-0600-x. [DOI] [PubMed] [Google Scholar]
- 28.Anoh-Tanon M J, Bremond-Gignac D, Wiener-Vacher S R. Vertigo is an underestimated symptom of ocular disorders: dizzy children do not always need MRI. Pediatr Neurol. 2000;23(01):49–53. doi: 10.1016/s0887-8994(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 29.Gaertner C, Bucci M P, Ajrezo L, Wiener-Vacher S. Binocular coordination of saccades during reading in children with clinically assessed poor vergence capabilities. Vision Res. 2013;87:22–29. doi: 10.1016/j.visres.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Lee J D, Kim C H, Hong S M et al. Prevalence of vestibular and balance disorders in children and adolescents according to age: a multi-center study. Int J Pediatr Otorhinolaryngol. 2017;94:36–39. doi: 10.1016/j.ijporl.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Langhagen T, Schroeder A S, Rettinger N, Borggraefe I, Jahn K. Migraine-related vertigo and somatoform vertigo frequently occur in children and are often associated. Neuropediatrics. 2013;44(01):55–58. doi: 10.1055/s-0032-1333433. [DOI] [PubMed] [Google Scholar]
- 32.Wiener-Vacher S R, Hamilton D A, Wiener S I. Vestibular activity and cognitive development in children: perspectives. Front Integr Nuerosci. 2013;7:92. doi: 10.3389/fnint.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]


