Abstract
Quantitative tests of vestibular function include the caloric test, cervical and ocular vestibular evoked myogenic potential (VEMP), rotary chair, and head impulse test, either at the bedside or utilizing video head impulse test (vHIT). The purpose of this article is to provide an overview of how to perform these tests in children, including which tests are recommended based on the child's age and any modifications or considerations that can be made. A variety of clinical measures have been recommended as screening measures for vestibular loss, which will be reviewed. Symptom questionnaires designed to assess the functional impact of dizziness and vestibular loss in children will also be discussed. If a child complains of dizziness or if vestibular loss is suspected (either by case history or positive screening measure), vestibular function testing is warranted. For vestibular function testing, children aged 0 to 2 years typically receive rotary chair, cervical VEMP, and vHIT if a remote system is available. For children aged 3 to 7 years, vHIT, cervical VEMP, and ocular VEMP are completed, and for children aged 8+ years, vHIT, caloric testing if vHIT is normal, and cervical and ocular VEMP are completed. For all children, modifications to testing can be made, as needed.
Keywords: Vestibular, pediatric, VEMP, vHIT, rotary chair, caloric
Learning Outcomes: As a result of this activity, the participant will be able to (1) list which tests of vestibular function are appropriate for children based on their age; (2) describe modifications that can be made to each test of vestibular function to accommodate children.
The prevalence of balance and vestibular disorders in children is estimated between 0.45 and 5.3%, with a slightly higher prevalence in females over males, which tends to rise with age. 1 2 When divided into unspecified dizziness, peripheral vestibular disorder, and central balance disorder, the majority (∼ 90%) of diagnosed pediatric disorders are categorized as unspecified dizziness, suggesting the need for increased diagnostic accuracy and differential vestibular testing. 1 Peripheral vestibular disorders are more commonly associated with sensorineural hearing loss, headaches are more commonly associated with central balance disorders, and syncope is more commonly associated with unspecified dizziness. 1 2 Other risk factors associated with increased balance/dizziness complaints include developmental delay (not intellectual), seizures, stuttering, and anemia. 2 Vestibular loss leads to delays in gross motor function; children with vestibular loss sit, walk, stand, and hold their head at a later age compared with age-matched peers who do not have vestibular loss. 3
The close anatomical relationship between the cochlea and vestibular structures explains why a large range (20–85%) of children with sensorineural hearing loss have some degree of vestibular loss. 4 5 Vestibular testing is also utilized in children pre- and post-cochlear implantation. Children who undergo cochlear implantation are especially at risk for otolith damage because of the saccule's proximity to the insertion pathway of the implant's electrode array. 6 It is estimated that between 40 and 80% of children have absent cervical vestibular evoked myogenic potential (cVEMP) responses following cochlear implantation. 7 8
The vestibular system is composed of three semicircular canals (horizontal, anterior, and posterior) and two otolith organs (saccule and utricle). The vestibular branch of the VIII cranial nerve is composed of two branches: a superior and inferior branch. The superior branch innervates the horizontal and anterior canal along with the utricle, while the inferior branch innervates the posterior canal and saccule. Depending on the etiology of dizziness, one or both branches of the nerve can be affected; therefore, vestibular evaluations should include assessments of each nerve branch. Additionally, some etiologies can selectively affect one type of rate sensor (otolith organ vs. semicircular canal); therefore, vestibular evaluations should also include assessments that evaluate each type of rate sensor. While this might seem like an easy feat when testing adults, performing an assessment for each nerve branch and rate sensor may be difficult in children.
Vestibular function testing in children can be difficult for a variety of reasons. Children may not self-report or even be aware that their symptoms are abnormal. Children can also be difficult to test due to their short attention span. From the clinician's viewpoint, deciding which vestibular tests should be performed and what modifications need to be made can also be intimidating. Quantitative tests of vestibular function include cVEMP and oVEMP (ocular vestibular evoked myogenic potential); head impulse testing, either at the bedside or utilizing video head impulse test (vHIT); rotary chair; and the caloric test. However, when vestibular testing is necessary in a child, not all tests are essential nor are all tests appropriate for all children depending on their age. Therefore, the purpose of this article is to provide an overview of pediatric vestibular testing, clinical recommendations on which tests of vestibular function should be considered based on the child's age, and modifications or considerations that can be made when testing children.
Quantitative Tests of Vestibular Function
Vestibular Evoked Myogenic Potential Testing
In 1992, it was determined that a short latency myogenic potential measured on the sternocleidomastoid (SCM) could be recorded in response to a high intensity click, 9 giving rise to what is now known as the cVEMP. Activated by the SCM, the cVEMP is an ipsilateral inhibitory response with a positive peak at approximately 13 ms (p13) followed by a negative peak at approximately 23 ms (n23), as shown in Fig. 1 (top). The pathway of the cVEMP begins when a sound, vibration, or tap stimulates the saccule and the inferior vestibular nerve to the vestibular nuclei. This information travels down the vestibular-spinal tract to the SCM. By nature of its pathway, the cVEMP response provides diagnostic information about saccular and inferior vestibular nerve function. 9 10 11
Figure 1.
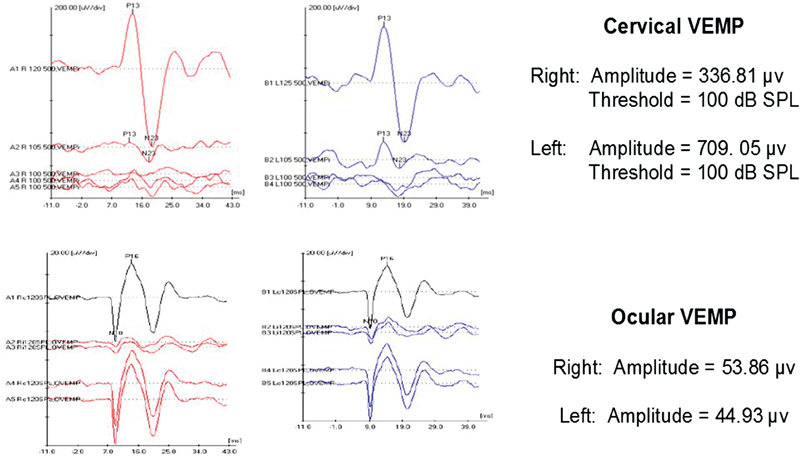
Cervical (top) and ocular (bottom) vestibular evoked myogenic potential (VEMP) responses in a child with bilateral enlarged vestibular aqueduct.
In 2003, it was determined that in response to acoustic stimulation, the vestibular system also evokes a short latency negative myogenic potential when surface electrodes are placed close to the eyes. 12 13 Later, it was found that the response could be recorded from the contracted inferior oblique eye muscle, giving rise to what is now known as the oVEMP. 14 The oVEMP is a contralateral excitatory response with a negative peak at approximately 10 ms (n10) and positive peak at approximately 16 ms (p16), as shown in Fig. 1 (bottom). The pathway of the oVEMP begins when the utricle is stimulated via sound, vibration, or taps and the superior vestibular nerve and vestibular nuclei. The signal continues on via the medial longitudinal fasciculus to the contralateral inferior oblique muscle. As such, oVEMPs predominately assess utricular and superior nerve function; however, there is speculation that saccular input is involved. 14 15 16
During cVEMP testing, patients wear a ground electrode on the chin or forehead, a reference electrode on the sternum, and active electrodes on the belly of the SCM ( Fig. 2A ). cVEMPs are typically performed with the patient lying supine. To contract the SCM, the patient can either elevate the head by pointing the nose to the ceiling or turn the head contralateral to the stimulus ear, as shown in Fig. 2A .
Figure 2.
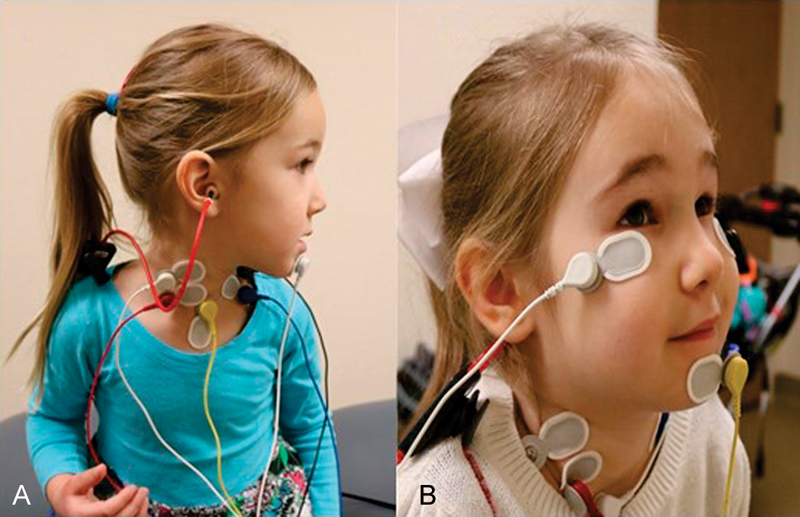
Electrode placement for ( A ) cervical vestibular evoked myogenic potential and ( B ) ocular vestibular evoked myogenic potential.
During oVEMP testing, patients wear a ground electrode on the forehead, a reference electrode on the side of the nose or on the chin, and active electrodes mediolateral to the infraorbital midline ( Fig. 2B ). oVEMPs can be performed with the patient lying supine or sitting upright, with gaze elevated to a fixed target. oVEMPs recorded in a seated position with a target set at 30 degrees up-gaze yield higher oVEMP amplitudes compared with the traditional supine position. 17
When completing cVEMP or oVEMP, it is common to use a 500-Hz air-conducted tone burst stimulus; however, bone conduction is an option as well. The presentation level of the stimulus ranges from 110 to 130 dB SPL with stimulus durations from 4 to 10 ms. For each cVEMP and oVEMP, it is recommended to present a minimum of 100 to 150 stimuli and obtain two trials for replication. 18
The primary outcome parameters of cVEMP and oVEMP are their peak latencies, peak-to-peak amplitudes, and thresholds. Peak latencies refer to the time in which the p13/n23 and n10/p16 peaks occur for cVEMP and oVEMP, respectively. Peak-to-peak amplitude is the absolute difference between the peak amplitude response at p13 and n23 for cVEMP and n10 and p16 for oVEMP. Most often, an asymmetry ratio is computed between the peak-to-peak amplitudes for the left and right ears, and a difference greater than 40% between the ears is considered significant. 19 For cVEMP, a positive relationship between level of SCM contraction and amplitude of the response exists and is most linear between 50 and 300 μV. 20 Variability in SCM contraction can be high, and patients (especially children) often vary their SCM contraction throughout testing. Therefore, evoked potential units with the use of EMG monitoring can be useful for limiting variability. 21 22 An example of EMG monitoring is using a visual stimulus as biofeedback. In this method, patients monitor their own tonic EMG activity and make adjustments to their neck elevation to meet the EMG level requirement, usually between 50 and 300 μV. With EMG monitoring, the corrected peak-to-peak amplitudes are calculated by dividing the average EMG by the peak-to-peak amplitude. Due to the smaller size of the inferior oblique muscle compared with the SCM, oVEMP amplitudes are smaller than cVEMP amplitudes. Muscle tension is regulated with up-gaze; therefore, EMG monitoring is not needed with oVEMP.
Both cVEMP and oVEMP testings have been completed across the age range in children. Early development of the vestibular colic reflex (VCR) facilitates the ability to use cVEMP testing in children younger than 12 months. Wang et al determined that repeatable cVEMP responses could be elicited in full-term (72% response rate) and pre-term (26% response rate) neonates by 5 days of birth as long as they met a weight of 2.82 kg (6.2 lb) and 2.26 kg (5 lb), respectively. 23 Sheykholeslami et al also successfully attained 100% cVEMP responses in infants (ages 1–12 months) with similar waveform morphology compared with adults. 24 By age 3, cVEMP testing is well tolerated in children, given they are able to independently sustain SCM contraction. 25 For these reasons, cVEMP testing is recommended in all children, regardless of age. Pediatric normative amplitude ranges are approximately 208. 5 to 285.00 μV and do not differ significantly from young adults; 26 27 however, more variability in amplitude may be observed. 24 In children, normative cVEMP threshold responses have been reported at approximately 105 to 110 dB SPL, 26 28 which is consistent with data from our laboratory. 29 Most often cVEMPs are considered abnormal if responses are absent or low in amplitude; however, with third window disorders, such as enlarged vestibular aqueduct or superior canal dehiscence, large amplitudes and low thresholds are considered abnormal. 30 31 cVEMP responses in a child with bilateral enlarged vestibular aqueduct are shown in Fig. 1 (top), and demonstrate low cVEMP thresholds (100 dB SPL) that are below the normal range.
Consistently, children have shorter p13 or n23 latencies as compared with adults. 26 32 33 The cVEMP latencies also appear to prolong as children get older. 24 25 26 32 The significantly shorter cVEMP latencies in children are attributed to shorter neck length and thus shorter length of the VCR pathway. 34
oVEMPs emerge in children at a later age. This is largely because translational vestibulo-ocular reflex (VOR) pathways are not completely developed before 12 months of age, as represented by poor oVEMP response rates in newborns. 35 However, oVEMP response rates improve when infants achieve the milestone of walking independently. 35 By the age of 2 years, clear oVEMPs can be detected, with 100% reliability by 4 years of age. 34 35 Following maturation of the oVEMP pathways, amplitudes and latencies are adult-like by 3 years of age. 36 37 For these reasons, oVEMP testing is recommended in all children at age 3. Normative oVEMP amplitude ranges for children are approximately 7.0 to 15.8 μV. 27 35 36 For children, oVEMP thresholds are approximately 110 to 115 dB SPL and similar to that of young adults, 38 which is consistent with findings from our laboratory. 29 Similar to cVEMP, oVEMPs are considered abnormal if responses are absent or low in amplitude; however, with third window disorders, large amplitudes and low thresholds are considered abnormal. oVEMP responses in a child with bilateral enlarged vestibular aqueduct are shown in Fig. 1 (bottom), and demonstrate high oVEMP amplitudes (44–53 μV) that are well outside the normal range.
There are many advantages in completing VEMP testing in children. Most importantly, the VEMP test provides the clinician with diagnostic information about otolith function that cannot be identified by rotary chair, caloric, or head impulse testing, as described later. This allows the clinician to identify isolated otolith loss when the rest of the vestibular battery that assess semicircular canal function is unremarkable. Second, VEMP testing is well received by children because the testing procedure does not require that they be in the dark, does not induce symptoms of dizziness, and they can sit with or close to their parent. The VEMP test is also a quick assessment (∼10–15 minutes) that clinicians and children can perform if time or compliance is an issue.
Despite the benefits of VEMP testing, factors such as age, variability, inability to maintain adequate muscle contraction, intolerance of the electrodes, and unsafe sound exposure could reduce the utility of the VEMP test in children. Because of these reasons, clinicians could misinterpret abnormal responses as otolith loss as opposed to poor test reliability or avoid performing the test entirely. 39 Data collected from our laboratory suggest that cVEMP testing is reliable in children ages 4 to 19 years who were tested on two separate sessions using air conduction and reflex hammer stimuli; oVEMP amplitudes were more reliable using air conduction compared with reflex hammer stimuli (Marler E, Rodriguez AI, Thomas MLA, Cruetz T, Fitzpatrick D, and Janky KL, unpublished data).
While this suggests that VEMP testing is feasible in the majority of children, young children (< 4 years) may have difficulty sustaining muscle contraction. cVEMP and oVEMP responses are contingent on sufficient contraction of the SCM and inferior oblique eye muscle; therefore, clinicians may find it difficult to complete both cVEMP and oVEMP or replicate trials without the child becoming fatigued. Younger children (below the age of 10 years) or those with smaller faces may be aversive to having electrodes on their neck and face due to discomfort or fear. This is especially true for oVEMP testing where the active electrodes sit close to the eyes. As such, children may try to pull them off during testing or move their face, which results in noisy recordings.
Another consideration when testing children is that the VEMP response is elicited with a high-intensity air-conducted signal (i.e., 125 dB SPL) that may result in harmful sound exposure. In adults, changes in cochlear function and an increase in otologic symptoms have been reported post-VEMP. 40 41 42 43 Because children have smaller ear canal volume (ECV) and may require more repetitions of stimuli to see a reliable response compared with adults, they could be at an even greater risk for unsafe sound exposure during traditional VEMP testing. 44
However, modifications to VEMP testing for the pediatric population can improve the reliability, clinical efficiency, and safety of the test procedure. These modifications will be described below; also see Table 1 for a summary of modifications. For young children with a short attention span, the use of toys, stickers, or videos can be helpful to watch while they are performing the task. One method for decreasing the time it takes to complete VEMP is to complete bilateral and simultaneous cVEMP and oVEMP recordings; Hsu et al recorded cVEMP and oVEMP in children (ages 3–13 years) while sitting with their heads rotated away from the stimulating ear and gazing at a target 2 m upward. 36
Table 1. VEMP Testing Modifications for Children.
| Test Procedure Characteristic | Modification |
|---|---|
| Shorten testing time | – Perform bilateral, simultaneous cVEMP and oVEMP – Use a bone conduction stimulus |
| Increase attention span | – Use interesting toys, stickers, or videos as distractors and targets |
| Improve sustained muscle contraction |
cVEMP:
– Rotate the head and stimulate rooting reflex for newborns – Sit child on parent's lap or have him/her lie on a table with head turned toward a toy or video |
|
oVEMP:
– Use of a sitting position with target (light bar or video adhered to the wall) elevated at ∼ 30 degrees – Use eye-closed testing for small children who cannot perform testing with eyes open | |
| Utilize EMG monitoring | – Use an animated cartoon that plays when contraction level is met |
| Improve electrode tolerance | – Use one reference electrode (e.g., chin) – Put oVEMP active electrodes on after cVEMP testing |
| Improve safety for air-conduction VEMP | – Present at 120 dB SPL if ear canal volume is ≤ 0.8 mL – Use a 750-Hz tone burst stimulus – Use an ascending threshold search approach – Use a bone-conduction stimulus |
Abbreviations: cVEMP, cervical vestibular evoked myogenic potential; oVEMP, ocular vestibular evoked myogenic potential.
To minimize variability or difficulty with SCM muscle contraction, various studies have incorporated alternative methods for children. For cVEMP in a newborn, the SCM is contracted by rotating their head to the side and down toward the shoulder, contralateral to the ear being stimulated. 35 45 To facilitate contraction of the SCM in newborns, the clinician can stimulate the rooting reflex, which is elicited by stroking the left or right cheek, causing newborns to turn and rotate their heads, searching for the stimulating object. 35 45 46 For a child, he/she can be seated on the parent's lap, sitting in a chair, or semirecumbent on an exam table while the head is turned watching an engaging visual stimulus (e.g., video, toy). 35 36 47 Methods to monitor the level of SCM contraction in children are important to verify that adequate SCM contraction is made during cVEMP testing and reduce intersubject variability when interpreting cVEMP amplitudes and asymmetry ratios. Yang et al described a visual feedback system to allow children to view an animated cartoon if they were holding the SCM contraction within the designated range. 47 If the EMG activity is not met, the cartoon pauses and response averaging stops until the contraction returns to the target EMG level. For children, the use of EMG monitoring may improve repeatability of the waveform and subsequently require fewer repetitions.
For oVEMP, the optimal elevated gaze on a target is approximately 20 degrees; however, this may be difficult for a child to sustain. 48 A sitting position for children can be more comfortable than lying supine. In this position, the child can look up at a target on the light bar of a VNG system that is turned vertically, or the child can look up at an iPod (Apple, Inc., Cupertino, CA) adhered to the wall, and watch a short video during testing. Alternatively, oVEMPs have been elicited while the eyes are closed in adults 49 and children (ages 2 days to 3 years). 35 By way of Bell's phenomena, the eyes naturally rotate upward (contracting the inferior oblique muscle) for a short timeframe when the eyes close. 49 However, in an eye's closed condition, longer n10/p16 latencies and smaller peak-to-peak amplitude are noted when compared with a traditional eyes open, up-gaze condition. 49 For children who do not like the oVEMP electrode placement, using only one-reference electrode on the chin can reduce the amount of electrodes around the eyes or placing the active electrodes on after cVEMP testing can reduce the time the child has to wear them.
Children may receive unsafe sound exposure during VEMP testing. On average, children receive an additive approximately 3 dB (or more) in the ear compared with adults when presented with a 500-Hz tone burst at 125 dB SPL; therefore, children are exposed to 128 dB SPL (or as high as 132 dB SPL). 27 Equivalent ECV (as measured by diagnostic tympanometry) predicts the amount of SPL delivered to the ear. When using the European Union standards, children with ECVs less than or equal to 0.8 mL are at risk for unsafe sound energy exposure from VEMP testing. 27 50 51
However, there are a few ways to reduce sound exposure for VEMP and thereby reduce adverse changes in cochlear function for children. First, clinicians can initiate testing at a lower presentation level. When a child's ECV is less than or equal to 0.8 mL, the presentation level should be reduced to 120 dB SPL. The approximately 3-dB increase from their smaller ECVs means children are actually receiving approximately 123 dB SPL. Second, children have similar cVEMP and oVEMP responses at both 500 and 750 Hz, suggesting similar frequency tuning. 29 Therefore, a 750-Hz tone burst stimulus can be used, which has a shorter duration and subsequently less sound exposure than the traditionally used 500-Hz tone burst. Third, when performing VEMP threshold testing in children, testing can be achieved without affecting cochlear function by using an ascending approach. 29 Lastly, because cVEMP and oVEMP threshold values are noted below standard high-intensity levels (i.e., 125 dB SPL) in children, testing can be initiated at an even lower level (∼115 or 110 dB SPL) if several repetitions are necessary.
In addition to air-conducted stimuli, bone-conduction VEMP using either an inertial triggered reflex hammer or mini-shaker offers several other advantages. First, the risk of cochlear trauma is reduced using a bone-conducted stimulus. Second, middle ear issues can be bypassed that often impede the ability to perform air-conduction VEMP testing in a pediatric clinic. Third, VEMP responses are achieved for the right and left simultaneously, thus cutting testing time in half. Results from our laboratory suggest that the reflex hammer is well tolerated by children and yields similar response rates compared with air-conducted VEMPs (Rodriguez AI, Marler E, Creutz T, Fitzpatrick D, and Janky KL, unpublished data).
In summary, VEMP testing is recommended as a vestibular assessment in children whenever peripheral vestibular involvement is suspected. cVEMP testing can be completed in newborns; however, oVEMP testing is not routinely completed until children are 3 years. 36 52 For cVEMP testing, a variety of methods can be used to attain sustained SCM contraction; however, to ensure adequate and comparable SCM contraction between the right and left sides, EMG monitoring is recommended. For oVEMP testing, sustained up-gaze is required; therefore, use of an interesting visual target such as a wall-mounted video player is recommended. An alternative is to complete oVEMP in an eyes-closed condition. For children with ECV less than or equal to 0.8 mL, a 750 Hz TB presented at 120 dB SPL would be recommended for cVEMP and oVEMP testing or use of a bone conduction stimulus for safe exposure.
Video Head Impulse Testing
The vHIT is a relatively new assessment of vestibular function. Head impulse testing originated as a vestibular bedside test; 53 however, use of a video camera now allows for objective vestibular evaluation. 54 In particular, vHIT is an assessment of individual semicircular canal (right and left horizontal, anterior, and posterior canal) VOR. Therefore, vHIT also evaluates both branches of the vestibular nerve; posterior canal vHIT is an assessment of the inferior portion of the vestibular nerve and anterior and horizontal canal vHITs are assessments of the superior portion of the vestibular nerve. During vHIT, patients wear tight fitting goggles, while high-acceleration, low-amplitude head impulses are delivered in the plane of each semicircular canal, as shown in Fig. 3 . Patients are required to fixate on a stable visual target during the head impulses. Within the goggles are a camera and a gyroscope, which simultaneously measure eye and head velocity, respectively. An example of normal and abnormal vHIT findings are shown in Fig. 4A and B , where eye velocity is shown in green and head velocity is shown in blue and red for left and right head impulses, respectively. When completing vHIT, it is recommended that head velocities exceed 150 degrees/second for horizontal head impulses and 100 degrees/second for vertical head impulses to overcome assistance from the smooth pursuit system. 55
Figure 3.
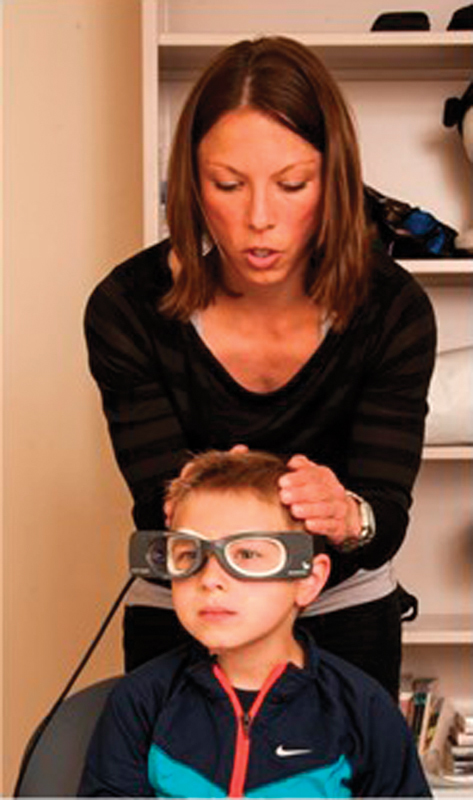
Video head impulse testing in a pediatric patient.
Figure 4.
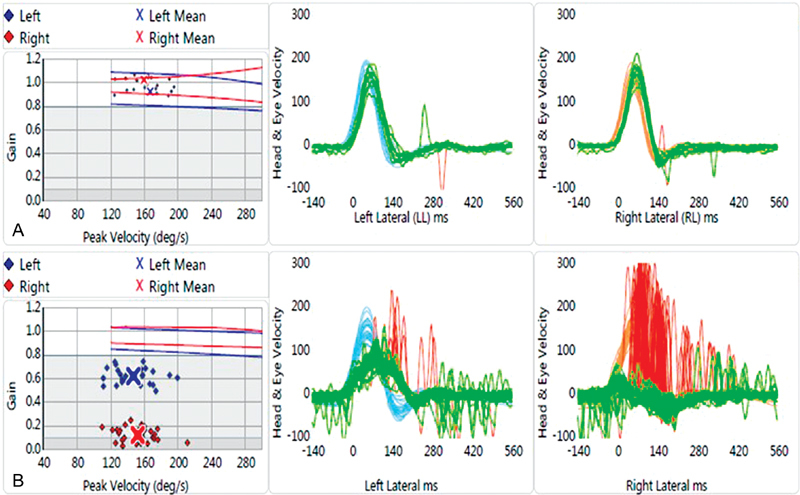
Example video head impulse test findings in ( A ) a child with normal vestibular function and ( B ) a child with asymmetric bilateral vestibular loss. Eye velocity is shown in green and head velocity is shown in blue and red for left and right head impulses, respectively.
The primary outcome parameter of vHIT is gain, which is a comparison of eye velocity with head velocity. In patients with normal vestibular and VOR function, as shown in Fig. 4A , the eyes move in an equal and opposite direction relative to head movement, which results in a gain of 1.0. In the event of vestibular loss and abnormal VOR function, the eyes will momentarily move in the direction of head movement, which results in a repeatable corrective (or refixation) saccade to put the eyes back on target. The result is reduced vHIT gain (< 0.68–0.7) and repeatable corrective saccades. 56 57 An example of a child with vestibular loss (i.e., deficient VOR) is shown in Fig. 4B , where the eye movement occurs at a reduced velocity compared with head movement and repeatable corrective saccades are noted in red. In this example, the child was diagnosed with bilateral Mondini's malformation. Based on these vHIT findings, gain is abnormal for both left (0.62) and right (0.12) horizontal canals, suggesting a bilateral weakness. However, based on the asymmetry in vHIT gain, it appears that vestibular function is better in the left horizontal canal compared with the right horizontal canal.
Findings are conflicting regarding whether vHIT gain changes with age in children. In some children, vHIT gain has been found to be unchanging from 4 to 18 years. 58 Similarly, others have found no significant differences in vHIT gain between older children and young adults. 33 56 59 However, lower gain values have been noted for children younger than 3 years, with a rapid increase in vHIT gain up to age 6 years, and then a slower increase in vHIT gain up to age 16 years. 60 Additionally, variability in vHIT gain also decreases with age. 60 From age 16, vHIT gain appears to stabilize until the eighth or ninth decade, where we begin to see a decline in vHIT gain. 55 61 62 While age-related changes in vHIT gain should be considered when determining normative cutoff values for children, the presence of a corrective saccade should also be present whenever vHIT is considered to be abnormal.
There are a variety of benefits to using vHIT for vestibular function testing in children over traditional methods, such as rotary chair and caloric testing. One of the major benefits is that vHIT does not induce dizziness like rotary chair and caloric testing; therefore, vHIT is significantly less intimidating. Similarly, vision is not occluded during vHIT, which is not only less frightening for the child but also makes it easier to communicate, especially when the child has hearing loss. Test administration is approximately 10 to 15 minutes and provides ear-specific information about all six semicircular canals and each branch of the vestibular nerve. This is an advantage over rotary chair, which does not provide ear-specific information, and is a reflection of superior nerve function only. Compared with caloric testing, vHIT can be completed regardless of middle ear status, including the presence of pressure equalization tubes, perforations, or a mastoid cavity.
In spite of the benefits of vHIT, there are some challenges when testing children. Some reported pitfalls include loose goggles, inability to follow directions, frequent eye blinks, wandering gaze, decreased attention span, noncompliance, and apprehension for receiving head impulses. 56 60 63 vHIT goggles require a snug fit to alleviate slippage during the high acceleration head impulses, which can be difficult in children who have smaller faces and heads. Sustained eye gaze is also necessary to measure the VOR during these high head accelerations; therefore, inability to sustain eye gaze or frequent eye blinks can be problematic in children. Similar to completing vHIT in adults, clinicians find that head impulses in the horizontal canals are easier to complete in children compared with the vertical canals. vHIT tends to take longer to complete in children compared with adults. 63 Lastly, it can be difficult to achieve the recommended number of head impulses (∼20) as well as maximum head velocities (> 150 degrees/second) in children due to compliance. 60
While pitfalls exist, there are some solutions for combatting these issues when testing children. To alleviate the issue of appropriately fitting goggles, remote video detection is an option. 60 As opposed to commercial vHIT systems which utilize tight fitting goggles for measuring eye and head velocity, remote video detection can be used where eye and head velocities are recorded via a remote camera that is placed in front of the child. We have also utilized a large piece of foam, inserted between the back of the child's head and the video goggle strap to help with a snug fit. vHIT has been recorded in children as young as 3 months with a remote video method 60 and as young as 3 years in vHIT systems that require the use of goggles. 56 Videos played on a cell phone, flashing lights, toys, or interesting stickers can be used to sustain eye gaze on a fixed target. 60 Small children (< 5 years of age) can also sit on the lap of a parent while the head impulses are being delivered. 60
In summary, vHIT is recommended as a vestibular assessment in children whenever peripheral vestibular involvement is suspected. vHIT can be completed in children as young as 3 months using a remote system, and 3 years using a traditional goggle system. 56 60 63 An interesting visual target is recommended to sustain visual fixation and attention. Due to their lower attention span, fewer head impulses can be obtained for each canal (∼ 10 head impulses as opposed to 20 head impulses for adults). Regardless of patient's age (child vs. adult), it is recommended to review data and remove head impulse data with artifacts. 64 Overall, vHIT has been found to be reliable test of vestibular function in children. 59
Rotary Chair Testing
While VEMP and vHIT are gaining popularity in pediatric vestibular testing due to their ability to provide site of lesion vestibular information with a short testing time (< 15 minutes each), rotary chair has been a long-standing test of vestibular function in children. Rotary chair testing takes approximately 10 to 15 minutes in duration and is a midfrequency (0.01–0.64 Hz) assessment of the horizontal canal and superior branch of the vestibular nerve. During rotary chair testing, children sit with a parent, in a car seat, or by themselves in the motorized chair. While most rotary chairs are enclosed within a light tight booth using an infrared camera monitor, as shown in Fig. 5 , some rotary chairs are open and vision is occluded by either a pair of infrared goggles, eye closure, or a blindfold. One of two rotary chair paradigms is typically used: sinusoidal harmonic acceleration (SHA) or step testing. With SHA testing, the rotary chair oscillates back and forth at frequencies from 0.01 to 0.64 Hz, typically at a set velocity of 50 to 60 degrees/second. With step testing, the chair accelerates at approximately 100/second 2 up to approximately 100 degrees/second where it rotates at this velocity for 45 to 60 seconds and then decelerates at approximately 100/second 2 to a complete stop while eye movements are measured for 45 to 60 seconds. The test is then repeated in the opposite direction. For either paradigm, eye movements are recorded by electrodes or infrared goggles.
Figure 5.
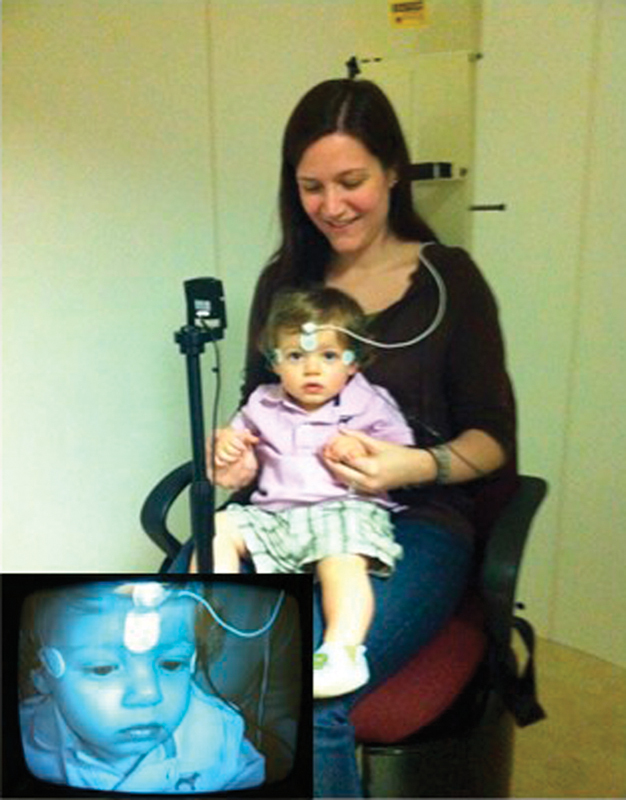
Rotary chair testing in an infant. The infant is seated on a parent or caregiver's lap. The infant can be monitored during testing using an infrared camera (inset).
There are three rotary chair outcomes: gain, phase (or time constant), and symmetry. Gain is the ratio of chair velocity to eye velocity. Phase (or time constant) is the relationship in timing between eye and chair movement. Third, symmetry is a comparison between the magnitude of right and left beating nystagmus. Patients with unilateral vestibular loss usually present with borderline low gain and marginal phase lead, while patients with bilateral vestibular loss present with reduced gain across most, if not all, frequencies, and a pronounced phase lead. Asymmetry is an indicator of compensation and can provide information on the likely weaker side. Rotary chair is generally referred to as an assessment of overall vestibular responsiveness and is excellent for diagnosing bilateral vestibular loss and providing information on the degree of bilateral vestibular loss. An example of rotary chair data is shown in Fig. 6 ; these data are from the same child referenced previously with bilateral vestibular loss from Mondini's malformation. As shown, gain normalizes at 0.32 Hz, which suggests some residual function. This is likely a reflection of the left better than right vHIT gain, which is also consistent with the clockwise (right) weaker asymmetry at 0.32 Hz.
Figure 6.
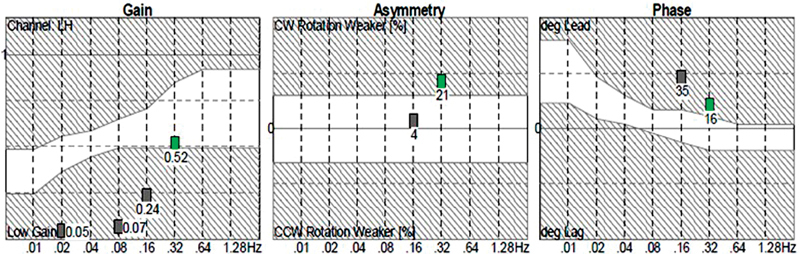
Rotary chair data of a child with bilateral vestibular loss from Mondini's malformation. Gain normalizes at 0.32 Hz, which suggests some residual function. The clockwise (right) weaker asymmetry at 0.32 Hz suggests this bilateral loss is asymmetric. This is confirmed with video head impulse test (vHIT) findings ( Fig. 4 ), which show left better than right vHIT gain.
Similar to vHIT, trends are conflicting regarding the relationship between age and rotary chair gain in children. Decreasing gain, increasing gain, and no change in gain have been reported with age in children. Chan et al (2016, n = 100) and Charpiot et al (2010, n = 147) report decreasing gain with increasing age. Both groups report significantly higher gains in their youngest (∼ 6 years) subjects. 65 66 This trend was more pronounced in the low frequencies (0.01–0.04 Hz). 66 More importantly, in children 6 to 12 years, rotary chair gains exceeded the manufacturer's normative references, suggesting that high gains should not be considered pathologic in children. 66 Some groups have failed to demonstrate maturational changes in rotary chair gain in children; however, they have noted higher gains in children compared with adults. 26 32 Conversely, increasing rotary chair gains with increasing age (∼ 0.05–0.06 per year) have been noted in children 3 to 9 years of age. 67 In these children, phase was relatively stable across age with the exception of 0.05 Hz, where phase decreased with an increase in age from 3 to 8 years. 67 Some infants less than 9 months of age did not generate nystagmus in response to rotation, particularly to high-frequency rotations (0.16 Hz), and in children younger than 4 years, 19% did not elicit nystagmus at 0.01 Hz. 68 Lack of responses is more likely to occur in infants with low birth weight. 69 These differences in trends could be the result of different testing paradigms, equipment, or level of attentiveness. Some studies evaluated responses at various frequencies, while others evaluated responses at just one frequency. Given the conflicting findings for gain and age, it is recommended to collect normative data for your current equipment and protocol. For infants less than 9 months of age, especially those with a low birth weight, consider repeat testing if nystagmus is not noted in response to rotation to rule out maturation.
There are a variety of benefits to using rotary chair in children. For many clinics, rotary chair may be the only test of canal function for children less than 3 years of age. Rotary chair can be completed regardless of middle ear status, and is not prohibited in cases where pressure equalization tubes or perforations are present. However, it should be noted that rotary chair responses can be affected by active middle ear effusion or even a history of middle ear effusion; 70 therefore, tympanometry is recommended with rotary chair testing. Rotary chair is tolerated by most children, faster to complete compared with caloric testing, and has been found to be reliable in children. 32
While rotary chair is a suitable test of vestibular function for children, particularly infants, there are pitfalls. First, goggles are often not small enough to adequately fit children's smaller faces. While some manufacturers have pediatric goggles, electrodes are typically used in this instance, transitioning to goggles as children get older. However, infants have a tendency of pulling electrodes off during rotation. Second, infants can be placed in a car seat during rotation; however, it is more frequent that children sit on their parent's lap during testing, which removes the child from the center of the axis of rotation and can artificially inflate rotary chair gain. Third, some children simply do not tolerate rotary chair testing. Infants occasionally will not tolerate sitting still on a parent's lap during rotation and some older children are fearful of riding in the dark. Lastly, and most importantly, rotary chair gain is affected by attention. The VOR can disappear if the child is drowsy or falls asleep during testing; 69 therefore, keeping the child awake and alert during testing is vital.
In spite of these pitfalls, there are effective strategies for overcoming these issues. While riding in the chair on a parent's lap places the child outside the axis of rotation, parents can be helpful for overcoming several issues. Parents can place a hand over the child's head to maintain head placement, ensure that electrodes are not removed, and assist with keeping the child awake and alert. If infants do not tolerate wearing electrodes, a subjective assessment of nystagmus can be completed via the infrared video monitor. While subjective assessment cannot determine if a phase lead is present, it can rule out bilateral vestibular loss. For older children who choose to ride alone but are scared to rotate in the dark, the rotary chair door can be kept open while the child closes their eyes during rotation, using electrodes to monitor eye movements. Lastly, to maintain alertness, the examiner or a co-riding parent can sing children's songs to young children. In older children, alertness is maintained by engaging in conversation or asking them to sing a song to you. Maintaining alertness can be difficult in children with significant hearing loss who may have a difficult time sustaining conversation in a dark environment without visual cues. In these cases, it is recommended that a co-riding parent provide alerting tasks to the child at a close range or that alerting tasks are discussed prior to closing the rotary chair door.
In summary, rotary chair is recommended as a vestibular assessment in children whenever peripheral vestibular involvement is suspected. Rotary chair can be completed in children as young as 2 months; however, maturation should be considered. If nystagmus is not measured in children less than 9 months of age, rotary chair should be repeated. At minimum, it is recommended that rotary chair at a low (0.01 Hz), mid (0.08 Hz), and high (0.32 Hz) frequency be completed. Attention and alertness can significantly affect rotary chair gain; therefore, clinicians should use strategies to keep the child awake and alert.
Caloric Testing
Caloric testing is considered the gold standard of peripheral vestibular system testing, although it is rarely used in younger children. During caloric testing, children lie supine, with their head elevated approximately 30 degrees while water or air irrigations are delivered to the ear canal. Cool (30°C) and warm (44°C) irrigations are completed in each ear. Irrigations are typically 30 seconds in response to water and 60 seconds in response to air. Electrodes or infrared goggles are used to record eye movements during the irrigation and then for 60 seconds following the irrigation. Caloric testing takes approximately 20 to 25 minutes to complete and is a low-frequency (0.002 Hz) assessment of the horizontal canal and superior branch of the vestibular nerve.
There are two main outcomes to the caloric test: caloric weakness and directional preponderance. The caloric weakness is a comparison of right and left irrigations (cool and warm). The directional preponderance is similar to the asymmetry outcome in rotary chair, and is a comparison of irrigations eliciting right versus left beating nystagmus. For each of these comparisons, the peak slow-phase velocity of each irrigation is inserted into the formula of Jongkees et al. 71 Most laboratories use a cutoff between 20 and 30% for caloric weakness and directional preponderance.
Caloric responses have been reported in children as young as 2 months of age; 69 however, more commonly caloric testing is not routine in pediatric vestibular testing until children are greater than 6 to 7 years. In infants, the caloric response is thought to be mature by 6 to 12 months, and the likelihood of obtaining a normal response increases as children gain weight. 69 In children 2 to 10 years of age, the magnitude of the slow-phase velocities in response to caloric stimulation decreases with age. 72
There are some benefits to using caloric testing in children. Caloric testing provides ear-specific, low-frequency information about the superior branch of the vestibular nerve and horizontal semicircular canals. While both rotary chair and vHIT testing provide similar information, results between these tests can be conflicting. Both vHIT and rotary chair are insensitive to mild vestibular loss. In the event of a unilateral weakness, vHIT and rotary chair abnormalities are typically not present until the caloric weakness exceeds 40 to 45%; 73 therefore, when vestibular involvement is suspected and rotary chair and/or vHIT are normal, caloric testing could be completed to rule out mild, unilateral vestibular loss.
There are some drawbacks to caloric testing in children. One of the biggest challenges is that caloric testing can be scary for children as vision is occluded, caloric irrigations induce dizziness, hearing is temporarily disrupted in the ear receiving the irrigation, and children are expected to lie still for several minutes during and following caloric irrigations. Use of a papoose board has been reported when testing infant children, 69 which can also contribute to the test being frightening. Another drawback is that caloric testing is prohibited by middle ear pathology (e.g., pressure equalization tubes, perforations, middle ear effusion, etc.). Similar to both rotary chair and vHIT, the infrared goggles are often not small enough to adequately fit children's small faces and electrodes are needed. Caloric testing is also affected by attention. Lastly, it can be difficult to calibrate young children as they are not adequately placed in front of the visual target.
Some strategies for overcoming these issues include reinforcement and reassurance from the clinician and parent. Parents can hold the hand of their child while undergoing caloric responses. For children who are fearful of testing, the duration of the caloric irrigation can be reduced (e.g., reducing irrigation time from 30 to 20 seconds) or monothermal irrigations can be completed instead of bithermal. Similar to rotary chair, alertness is maintained by singing songs or engaging in conversation. However, maintaining alertness can be difficult in children with significant degrees of hearing loss who cannot continue verbal dialog once fixation is removed; therefore, it is recommended that any mental tasking be explained prior to removing visual fixation.
In summary, caloric testing is recommended as a vestibular assessment in children whenever peripheral vestibular involvement is suspected. Caloric testing can be completed in infants; however, it is typically not part of the pediatric vestibular battery until children are greater than 6 to 7 years. In comparison to caloric testing, vHIT is recommended as a first-tier assessment because it is fast, provides ear-specific information, and does not induce dizziness. When vHIT is normal, monothermal irrigations (warm or cool) at minimum would be recommended to rule out mild, unilateral vestibular involvement.
Screening Measures
It is recommended that all children with dizziness be evaluated for vestibular loss. Given the high incidence of vestibular loss in children with sensorineural hearing loss, vestibular loss should be suspected whenever hearing loss is present. However, not all children with hearing loss will have vestibular loss; therefore, several different functional tests have been suggested as screening measures in children with hearing loss. Clinicians can utilize these measures to help them decide whether or not a child potentially has vestibular loss and if further vestibular testing is needed. These measures include the modified clinical test of sensory integration on balance, the bedside head thrust test, the Emory clinical vestibular chair test, the dynamic visual acuity test, single-leg stance, tandem standing, age of gross motor attainment, and severity of hearing loss. 74 75 76 77 See Table 2 for a summary of how these screening measures can be used. If the child has a positive score for any of the measures, vestibular loss would be suspected and vestibular testing recommended.
Table 2. Screening Measures for Vestibular Loss in Children.
| Measure | Description | Cutoff score | Sensitivity | Specificity |
|---|---|---|---|---|
| mCTSIB a | Children maintain balance with arms crossed against chest for 30 s while (1) standing, eyes open; (2) standing, eyes closed; (3) standing on foam, eyes open; (4) standing on foam, eyes closed. Maximum score is 120 s | 110 s | 88% | 85% |
| HTT a | The head is tilted 30 degrees downward and high acceleration, unpredictable head thrusts are delivered in the plane of each horizontal canal | Corrective saccade | 75% | 91% |
| ECVCT a | Children are rotated in an office chair with eyes closed for 30 s at 0.5 Hz. After 30 s, Frenzel lenses are placed over the child's eyes and nystagmus is timed | < 29.2 s | 75% | 100% |
| DVA a | Children read letters/symbols from an eye chart with the head still and again with head in motion (2 Hz or 120 degrees/s). The number of missed letters/symbols is recorded | 10 optotypes | 88% | 69% |
| Single-leg stance b | Children stand on their dominate leg with their nondominant leg raised, knee bent to 90 degrees, hands on hips, and eyes closed for a maximum of 10 s. Timing is stopped if eyes open, foot is put down, or standing leg is moved | < 4 s | 90% | 100% |
| Tandem standing b | Children stand with one foot placed in front of the other, hands on hips, eyes closed. Timing is stopped if they take a step, move hands from hips or open eyes | < 8 s | 95% | 69% |
| Age to sit c | During case history, parents report age child sat independently | > 7.25 mo | 62% | 81% |
| Age to walk c | During case history, parents report age child walked independently | > 14.5 mo | 78% | 77% |
| Hearing loss c | Compute the bilateral pure tone average for 250, 1,000, 2,000, and 4,000 Hz | > 40 dB HL > 66 dB HL |
80% 33% |
55% 91% |
Pediatric Questionnaires
Children with dizziness and vestibular loss may present with diverse subjective complaints. Additionally, children may not be able to verbally describe their symptoms in a manner that suggests their dizziness or vestibular loss is affecting their daily activities. Therefore, clinicians can quantify the severity and impact of dizziness or vestibular loss using recently developed questionnaires. For example, the Dizziness Handicap Inventory for parents and caregivers (DHI-pc) is a 25-question inventory that asks parents to quantify difficulties his/her child may be experiencing related to dizziness or unsteadiness (e.g., Does your child's problem make him/her feel tired?). 78 The Pediatric Vestibular Symptom Questionnaire (PVSQ) was developed as a child-reported 11-item questionnaire where the child is asked to quantify (on a Likert scale) the severity of their vestibular symptoms (e.g., How often in the past month have you felt a feeling that things are spinning or moving around?). 79 Lastly, the Pediatric Visually Induced Dizziness Questionnaire (PVID) aims to quantify the presence and severity of visually induced dizziness, such as symptoms induced by scrolling on a computer screen. 80 Similar to the PVSQ, the PVID is an 11-item questionnaire that is completed by the child. Any one of these questionnaires may be helpful in further characterizing the character and/or impact of symptoms in children.
Conclusion
Vestibular testing can be valuable in children at risk for vestibular loss or dizziness. For children with hearing loss, cochlear implants, and gross motor delay, the risk of vestibular loss is increased. If a child complains of dizziness or if vestibular loss is suspected (either by case history or positive screening measure), vestibular function testing is warranted. Screening measures can help build the case for possible vestibular loss. Symptom questionnaires can be used to quantify the impact of dizziness or vestibular loss in children. For vestibular function testing, children 0 to 2 years of age typically receive rotary chair, cVEMP, and vHIT if a remote system is available. For children 3 to 7 years of age, vHIT, cVEMP, and oVEMP are completed, and for children 8+ years of age, vHIT, caloric testing (if vHIT is normal), cVEMP, and oVEMP are completed. Vestibular testing can be achieved with modifications tailored for the pediatric population.
Footnotes
Conflicts of Interest K.L.J.:
• Supported by the National Institute on Deafness and Other Communication Disorders under award numbers R03DC015318 and P30DC004662.
• Provides consulting for Audiology Systems regarding the clinical use of vestibular evoked myogenic potential testing and video head impulse testing (vHIT).
• Board Member, President Elect, American Balance Society.
• Editorial Board Member, American Journal of Audiology.
A.I.R.:
• Supported by the National Institute on Deafness and Other Communication Disorders under award number 5T32DC00013–36.
• American Speech, Language, and Hearing Association, Financial Planning Board Member.
References
- 1.O'Reilly R C, Morlet T, Nicholas B D et al. Prevalence of vestibular and balance disorders in children. Otol Neurotol. 2010;31(09):1441–1444. doi: 10.1097/MAO.0b013e3181f20673. [DOI] [PubMed] [Google Scholar]
- 2.Li C M, Hoffman H J, Ward B K, Cohen H S, Rine R M.Epidemiology of dizziness and balance problems in children in the United States: a population-based study J Pediatr 2016171240–70., 3 [DOI] [PubMed] [Google Scholar]
- 3.Inoue A, Iwasaki S, Ushio M et al. Effect of vestibular dysfunction on the development of gross motor function in children with profound hearing loss. Audiol Neurootol. 2013;18(03):143–151. doi: 10.1159/000346344. [DOI] [PubMed] [Google Scholar]
- 4.Kaga K, Shinjo Y, Jin Y, Takegoshi H. Vestibular failure in children with congenital deafness. Int J Audiol. 2008;47(09):590–599. doi: 10.1080/14992020802331222. [DOI] [PubMed] [Google Scholar]
- 5.O'Reilly R C, Greywoode J, Morlet T et al. Comprehensive vestibular and balance testing in the dizzy pediatric population. Otolaryngol Head Neck Surg. 2011;144(02):142–148. doi: 10.1177/0194599810393679. [DOI] [PubMed] [Google Scholar]
- 6.Basta D, Todt I, Goepel F, Ernst A. Loss of saccular function after cochlear implantation: the diagnostic impact of intracochlear electrically elicited vestibular evoked myogenic potentials. Audiol Neurootol. 2008;13(03):187–192. doi: 10.1159/000113509. [DOI] [PubMed] [Google Scholar]
- 7.Cushing S L, Papsin B C, Rutka J A, James A L, Gordon K A. Evidence of vestibular and balance dysfunction in children with profound sensorineural hearing loss using cochlear implants. Laryngoscope. 2008;118(10):1814–1823. doi: 10.1097/MLG.0b013e31817fadfa. [DOI] [PubMed] [Google Scholar]
- 8.Licameli G, Zhou G, Kenna M A. Disturbance of vestibular function attributable to cochlear implantation in children. Laryngoscope. 2009;119(04):740–745. doi: 10.1002/lary.20121. [DOI] [PubMed] [Google Scholar]
- 9.Colebatch J G, Halmagyi G M. Vestibular evoked potentials in human neck muscles before and after unilateral vestibular deafferentation. Neurology. 1992;42(08):1635–1636. doi: 10.1212/wnl.42.8.1635. [DOI] [PubMed] [Google Scholar]
- 10.Colebatch J G, Halmagyi G M, Skuse N F. Myogenic potentials generated by a click-evoked vestibulocollic reflex. J Neurol Neurosurg Psychiatry. 1994;57(02):190–197. doi: 10.1136/jnnp.57.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robertson D D, Ireland D J. Vestibular evoked myogenic potentials. J Otolaryngol. 1995;24(01):3–8. [PubMed] [Google Scholar]
- 12.Rosengren S M, McAngus Todd N P, Colebatch J G. Vestibular-evoked extraocular potentials produced by stimulation with bone-conducted sound. Clin Neurophysiol. 2005;116(08):1938–1948. doi: 10.1016/j.clinph.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Todd N P, Rosengren S M, Colebatch J G.A short latency vestibular evoked potential (VsEP) produced by bone-conducted acoustic stimulation J Acoust Soc Am 2003114(6, Pt 1):3264–3272. [DOI] [PubMed] [Google Scholar]
- 14.Todd N P, Rosengren S M, Aw S T, Colebatch J G. Ocular vestibular evoked myogenic potentials (OVEMPs) produced by air- and bone-conducted sound. Clin Neurophysiol. 2007;118(02):381–390. doi: 10.1016/j.clinph.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 15.Weber K P, Rosengren S M, Michels R, Sturm V, Straumann D, Landau K. Single motor unit activity in human extraocular muscles during the vestibulo-ocular reflex. J Physiol. 2012;590(13):3091–3101. doi: 10.1113/jphysiol.2011.226225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curthoys I S, Iwasaki S, Chihara Y, Ushio M, McGarvie L A, Burgess A M. The ocular vestibular-evoked myogenic potential to air-conducted sound; probable superior vestibular nerve origin. Clin Neurophysiol. 2011;122(03):611–616. doi: 10.1016/j.clinph.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 17.Makowiec K, McCaslin D L, Jacobson G P, Hatton K, Lee J. Effect of electrode montage and head position on air-conducted ocular vestibular evoked myogenic potential. Am J Audiol. 2017;26(02):180–188. doi: 10.1044/2017_AJA-16-0108. [DOI] [PubMed] [Google Scholar]
- 18.Papathanasiou E S, Murofushi T, Akin F W, Colebatch J G. International guidelines for the clinical application of cervical vestibular evoked myogenic potentials: an expert consensus report. Clin Neurophysiol. 2014;125(04):658–666. doi: 10.1016/j.clinph.2013.11.042. [DOI] [PubMed] [Google Scholar]
- 19.McCaslin D L, Jacobson G P, Hatton K, Fowler A P, DeLong A P. The effects of amplitude normalization and EMG targets on cVEMP interaural amplitude asymmetry. Ear Hear. 2013;34(04):482–490. doi: 10.1097/AUD.0b013e31827ad792. [DOI] [PubMed] [Google Scholar]
- 20.Bogle J M, Zapala D A, Criter R, Burkard R. The effect of muscle contraction level on the cervical vestibular evoked myogenic potential (cVEMP): usefulness of amplitude normalization. J Am Acad Audiol. 2013;24(02):77–88. doi: 10.3766/jaaa.24.2.2. [DOI] [PubMed] [Google Scholar]
- 21.McCaslin D L, Fowler A, Jacobson G P. Amplitude normalization reduces cervical vestibular evoked myogenic potential (cVEMP) amplitude asymmetries in normal subjects: proof of concept. J Am Acad Audiol. 2014;25(03):268–277. doi: 10.3766/jaaa.25.3.6. [DOI] [PubMed] [Google Scholar]
- 22.Isaradisaikul S, Navacharoen N, Hanprasertpong C, Kangsanarak J.Cervical vestibular-evoked myogenic potentials: norms and protocols Int J Otolaryngol 20122012913515. doi: 10.1155/2012/913515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S J, Chen C N, Hsieh W S, Young Y H. Development of vestibular evoked myogenic potentials in preterm neonates. Audiol Neurootol. 2008;13(03):145–152. doi: 10.1159/000112422. [DOI] [PubMed] [Google Scholar]
- 24.Sheykholeslami K, Megerian C A, Arnold J E, Kaga K. Vestibular-evoked myogenic potentials in infancy and early childhood. Laryngoscope. 2005;115(08):1440–1444. doi: 10.1097/01.mlg.0000167976.58724.22. [DOI] [PubMed] [Google Scholar]
- 25.Kelsch T A, Schaefer L A, Esquivel C R. Vestibular evoked myogenic potentials in young children: test parameters and normative data. Laryngoscope. 2006;116(06):895–900. doi: 10.1097/01.mlg.0000214664.97049.3e. [DOI] [PubMed] [Google Scholar]
- 26.Maes L, De Kegel A, Van Waelvelde H, Dhooge I. Rotatory and collic vestibular evoked myogenic potential testing in normal-hearing and hearing-impaired children. Ear Hear. 2014;35(02):e21–e32. doi: 10.1097/AUD.0b013e3182a6ca91. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez A T, Thomas M LA, Fitzpatrick D, Janky K L. Effects of high sound pressure exposure during air-conducted vestibular evoked myogenic potential testing in children and young adults. Ear Hear. 2018;39(02):269–277. doi: 10.1097/AUD.0000000000000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou G, Kenna M A, Stevens K, Licameli G. Assessment of saccular function in children with sensorineural hearing loss. Arch Otolaryngol Head Neck Surg. 2009;135(01):40–44. doi: 10.1001/archoto.2008.508. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez A, Thomas M LA, Janky K.Air-conducted vestibular evoked myogenic potential testing in children, adolescents, and young adults: thresholds, frequency tuning, and effects of sound exposure Ear Hear 2018[Epub ahead of print] 10.1097/AUD0000000000000607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor R L, Bradshaw A P, Magnussen J S, Gibson W P, Halmagyi G M, Welgampola M S. Augmented ocular vestibular evoked myogenic potentials to air-conducted sound in large vestibular aqueduct syndrome. Ear Hear. 2012;33(06):768–771. doi: 10.1097/AUD.0b013e31825ce613. [DOI] [PubMed] [Google Scholar]
- 31.Taylor R L, Bradshaw A P, Halmagyi G M, Welgampola M S. Tuning characteristics of ocular and cervical vestibular evoked myogenic potentials in intact and dehiscent ears. Audiol Neurootol. 2012;17(04):207–218. doi: 10.1159/000336959. [DOI] [PubMed] [Google Scholar]
- 32.Valente M. Maturational effects of the vestibular system: a study of rotary chair, computerized dynamic posturography, and vestibular evoked myogenic potentials with children. J Am Acad Audiol. 2007;18(06):461–481. doi: 10.3766/jaaa.18.6.2. [DOI] [PubMed] [Google Scholar]
- 33.Janky K L, Givens D. Vestibular, visual acuity, and balance outcomes in children with cochlear implants: a preliminary report. Ear Hear. 2015;36(06):e364–e372. doi: 10.1097/AUD.0000000000000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young Y H. Assessment of functional development of the otolithic system in growing children: a review. Int J Pediatr Otorhinolaryngol. 2015;79(04):435–442. doi: 10.1016/j.ijporl.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 35.Wang S J, Hsieh W S, Young Y H. Development of ocular vestibular-evoked myogenic potentials in small children. Laryngoscope. 2013;123(02):512–517. doi: 10.1002/lary.23535. [DOI] [PubMed] [Google Scholar]
- 36.Hsu Y S, Wang S J, Young Y H. Ocular vestibular-evoked myogenic potentials in children using air conducted sound stimulation. Clin Neurophysiol. 2009;120(07):1381–1385. doi: 10.1016/j.clinph.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Chou C H, Hsu W C, Young Y H. Ocular vestibular-evoked myogenic potentials via bone-conducted vibration in children. Clin Neurophysiol. 2012;123(09):1880–1885. doi: 10.1016/j.clinph.2012.02.059. [DOI] [PubMed] [Google Scholar]
- 38.Piker E G, Jacobson G P, McCaslin D L, Hood L J. Normal characteristics of the ocular vestibular evoked myogenic potential. J Am Acad Audiol. 2011;22(04):222–230. doi: 10.3766/jaaa.22.4.5. [DOI] [PubMed] [Google Scholar]
- 39.Cushing S L, Papsin B C, Rutka J A, James A L, Blaser S L, Gordon K A. Vestibular end-organ and balance deficits after meningitis and cochlear implantation in children correlate poorly with functional outcome. Otol Neurotol. 2009;30(04):488–495. doi: 10.1097/MAO.0b013e31819bd7c8. [DOI] [PubMed] [Google Scholar]
- 40.Young Y H. Vestibular evoked myogenic potentials: optimal stimulation and clinical application. J Biomed Sci. 2006;13(06):745–751. doi: 10.1007/s11373-006-9106-6. [DOI] [PubMed] [Google Scholar]
- 41.Krause E, Mayerhofer A, Gürkov R et al. Effects of acoustic stimuli used for vestibular evoked myogenic potential studies on the cochlear function. Otol Neurotol. 2013;34(07):1186–1192. doi: 10.1097/MAO.0b013e31829ce7b4. [DOI] [PubMed] [Google Scholar]
- 42.Strömberg A K, Olofsson Å, Westin M, Duan M, Stenfelt S. Changes in cochlear function related to acoustic stimulation of cervical vestibular evoked myogenic potential stimulation. Hear Res. 2016;340:43–49. doi: 10.1016/j.heares.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 43.Mattingly J K, Portnuff C D, Hondorp B M, Cass S P. Sudden bilateral hearing loss after cervical and ocular vestibular evoked myogenic potentials. Otol Neurotol. 2015;36(06):961–964. doi: 10.1097/MAO.0000000000000764. [DOI] [PubMed] [Google Scholar]
- 44.Thomas M LA, Fitzpatrick D, McCreery R, Janky K L. Big stimulus, little ears: safety in administering vestibular-evoked myogenic potentials in children. J Am Acad Audiol. 2017;28(05):395–403. doi: 10.3766/jaaa.15097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen C N, Wang S J, Wang C T, Hsieh W S, Young Y H. Vestibular evoked myogenic potentials in newborns. Audiol Neurootol. 2007;12(01):59–63. doi: 10.1159/000097248. [DOI] [PubMed] [Google Scholar]
- 46.Salloway S P. New York, NY: Springer; 2011. Rooting reflex; pp. 161–173. [Google Scholar]
- 47.Yang C J, Lavender V, Meinzen-Derr J K et al. Vestibular pathology in children with enlarged vestibular aqueduct. Laryngoscope. 2016;126(10):2344–2350. doi: 10.1002/lary.25890. [DOI] [PubMed] [Google Scholar]
- 48.Govender S, Rosengren S M, Colebatch J G. The effect of gaze direction on the ocular vestibular evoked myogenic potential produced by air-conducted sound. Clin Neurophysiol. 2009;120(07):1386–1391. doi: 10.1016/j.clinph.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 49.Huang Y C, Yang T L, Young Y H. Feasibility of ocular vestibular-evoked myogenic potentials (oVEMPs) recorded with eyes closed. Clin Neurophysiol. 2012;123(02):376–381. doi: 10.1016/j.clinph.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 50.Colebatch J G, Rosengren S M. Safe levels of acoustic stimulation: comment on “effects of acoustic stimuli used for vestibular evoked myogenic potential studies on the cochlear function”. Otol Neurotol. 2014;35(05):932–933. doi: 10.1097/MAO.0000000000000289. [DOI] [PubMed] [Google Scholar]
- 51.European Union. Directive 2003/10/EC of the European Parliament and of the Council on the minimum health and safety requirements regarding the exposure of workers to the risks arising from physical agents (noise) (Seventeenth individual Directive within the meaning of Article 16(1) of Directive 89/391/EEC). Official Journal L 2003;042:0038–0044
- 52.Chihara Y, Iwasaki S, Ushio M, Murofushi T. Vestibular-evoked extraocular potentials by air-conducted sound: another clinical test for vestibular function. Clin Neurophysiol. 2007;118(12):2745–2751. doi: 10.1016/j.clinph.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 53.Halmagyi G M, Curthoys I S. A clinical sign of canal paresis. Arch Neurol. 1988;45(07):737–739. doi: 10.1001/archneur.1988.00520310043015. [DOI] [PubMed] [Google Scholar]
- 54.MacDougall H G, Weber K P, McGarvie L A, Halmagyi G M, Curthoys I S. The video head impulse test: diagnostic accuracy in peripheral vestibulopathy. Neurology. 2009;73(14):1134–1141. doi: 10.1212/WNL.0b013e3181bacf85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McGarvie L A, MacDougall H G, Halmagyi G M, Burgess A M, Weber K P, Curthoys I S. The video head impulse test (vHIT) of semicircular canal function - Age-dependent normative values of VOR gain in healthy subjects. Front Neurol. 2015;6:154. doi: 10.3389/fneur.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hamilton S S, Zhou G, Brodsky J R. Video head impulse testing (VHIT) in the pediatric population. Int J Pediatr Otorhinolaryngol. 2015;79(08):1283–1287. doi: 10.1016/j.ijporl.2015.05.033. [DOI] [PubMed] [Google Scholar]
- 57.Janky K L, Patterson J, Shepard N et al. Video head impulse test (vHIT): The role of corrective saccades in identifying patients with vestibular loss. Otol Neurotol. 2018;39(04):467–473. doi: 10.1097/MAO.0000000000001751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lehnen N, Ramaioli C, Todd N S et al. Clinical and video head impulses: a simple bedside test in children. J Neurol. 2017;264(05):1002–1004. doi: 10.1007/s00415-017-8450-y. [DOI] [PubMed] [Google Scholar]
- 59.Ross L M, Helminski J O. Test-retest and interrater reliability of the video head impulse test in the pediatric population. Otol Neurotol. 2016;37(05):558–563. doi: 10.1097/MAO.0000000000001040. [DOI] [PubMed] [Google Scholar]
- 60.Wiener-Vacher S R, Wiener S I. Video head impulse tests with a remote camera system: normative values of semicircular canal vestibulo-ocular reflex gain in infants and children. Front Neurol. 2017;8:434. doi: 10.3389/fneur.2017.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davalos-Bichara M, Agrawal Y. Normative results of healthy older adults on standard clinical vestibular tests. Otol Neurotol. 2014;35(02):297–300. doi: 10.1097/MAO.0b013e3182a09ca8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matiño-Soler E, Esteller-More E, Martin-Sanchez J C, Martinez-Sanchez J M, Perez-Fernandez N. Normative data on angular vestibulo-ocular responses in the yaw axis measured using the video head impulse test. Otol Neurotol. 2015;36(03):466–471. doi: 10.1097/MAO.0000000000000661. [DOI] [PubMed] [Google Scholar]
- 63.Hülse R, Hörmann K, Servais J J, Hülse M, Wenzel A. Clinical experience with video Head Impulse Test in children. Int J Pediatr Otorhinolaryngol. 2015;79(08):1288–1293. doi: 10.1016/j.ijporl.2015.05.034. [DOI] [PubMed] [Google Scholar]
- 64.Mantokoudis G, Saber Tehrani A S, Kattah J C et al. Quantifying the vestibulo-ocular reflex with video-oculography: nature and frequency of artifacts. Audiol Neurootol. 2015;20(01):39–50. doi: 10.1159/000362780. [DOI] [PubMed] [Google Scholar]
- 65.Charpiot A, Tringali S, Ionescu E, Vital-Durand F, Ferber-Viart C. Vestibulo-ocular reflex and balance maturation in healthy children aged from six to twelve years. Audiol Neurootol. 2010;15(04):203–210. doi: 10.1159/000255338. [DOI] [PubMed] [Google Scholar]
- 66.Chan F M, Galatioto J, Amato M, Kim A H. Normative data for rotational chair stratified by age. Laryngoscope. 2016;126(02):460–463. doi: 10.1002/lary.25497. [DOI] [PubMed] [Google Scholar]
- 67.Casselbrant M L, Mandel E M, Sparto P J et al. Longitudinal posturography and rotational testing in children three to nine years of age: normative data. Otolaryngol Head Neck Surg. 2010;142(05):708–714. doi: 10.1016/j.otohns.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Staller S J, Goin D W, Hildebrandt M. Pediatric vestibular evaluation with harmonic acceleration. Otolaryngol Head Neck Surg. 1986;95(04):471–476. doi: 10.1177/019459988609500409. [DOI] [PubMed] [Google Scholar]
- 69.Eviatar L, Eviatar A.The normal nystagmic response of infants to caloric and per rotatory stimulation Laryngoscope 197989(7, Pt 1):1036–1045. [PubMed] [Google Scholar]
- 70.Casselbrant M L, Furman J M, Mandel E M, Fall P A, Kurs-Lasky M, Rockette H E.Past history of otitis media and balance in four-year-old children Laryngoscope 2000110(5, Pt 1):773–778. [DOI] [PubMed] [Google Scholar]
- 71.Jongkees L B, Maas J P, Philipszoon A J. Clinical nystagmography. A detailed study of electro-nystagmography in 341 patients with vertigo. Pract Otorhinolaryngol (Basel) 1962;24:65–93. [PubMed] [Google Scholar]
- 72.Andrieu-Guitrancourt J, Peron J M, Dehesdin D, Aubet J, Courtin P. Normal vestibular responses to air caloric tests in children. Int J Pediatr Otorhinolaryngol. 1981;3(03):245–250. doi: 10.1016/0165-5876(81)90007-0. [DOI] [PubMed] [Google Scholar]
- 73.McCaslin D L, Jacobson G P, Bennett M L, Gruenwald J M, Green A P. Predictive properties of the video head impulse test: measures of caloric symmetry and self-report dizziness handicap. Ear Hear. 2014;35(05):e185–e191. doi: 10.1097/AUD.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 74.Brookhouser P E, Cyr D G, Peters J E, Schulte L E.Correlates of vestibular evaluation results during the first year of life Laryngoscope 1991101(7, Pt 1):687–694. [DOI] [PubMed] [Google Scholar]
- 75.Christy J B, Payne J, Azuero A, Formby C. Reliability and diagnostic accuracy of clinical tests of vestibular function for children. Pediatr Phys Ther. 2014;26(02):180–189. doi: 10.1097/PEP.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 76.Oyewumi M, Wolter N E, Heon E, Gordon K A, Papsin B C, Cushing S L. Using balance function to screen for vestibular impairment in children with sensorineural hearing loss and cochlear implants. Otol Neurotol. 2016;37(07):926–932. doi: 10.1097/MAO.0000000000001046. [DOI] [PubMed] [Google Scholar]
- 77.Janky K L, Thomas M LA, High R R, Schmid K K, Ogun O A. Predictive factors for vestibular loss in children with hearing loss. Am J Audiol. 2018;27(01):137–146. doi: 10.1044/2017_AJA-17-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McCaslin D L, Jacobson G P, Lambert W, English L N, Kemph A J. The development of the Vanderbilt pediatric dizziness handicap inventory for patient caregivers (DHI-PC) Int J Pediatr Otorhinolaryngol. 2015;79(10):1662–1666. doi: 10.1016/j.ijporl.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 79.Pavlou M, Whitney S, Alkathiry A A et al. The Pediatric Vestibular Symptom Questionnaire: a validation study. J Pediatr. 2016;168:171–70. doi: 10.1016/j.jpeds.2015.09.075. [DOI] [PubMed] [Google Scholar]
- 80.Pavlou M, Whitney S L, Alkathiry A A et al. Visually induced dizziness in children and validation of the pediatric visually induced dizziness questionnaire. Front Neurol. 2017;8:656. doi: 10.3389/fneur.2017.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Janky K L, Thomas M LA, High R R, Schmid K K, Ogen A. Predictive factors for vestibular loss in children with hearing loss. Am J Audiol. 2018;27(01):137–146. doi: 10.1044/2017_AJA-17-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]


