Abstract
Patients with pancreatic ductal adenocarcinomas (PDAC) have one of the poorest survival rates of all cancers. The main reason for this is related to the unique tumor stroma and poor vascularization of PDAC. As a consequence, chemotherapeutic drugs, such as nab-paclitaxel and gemcitabine, cannot efficiently penetrate into the tumor tissue. Non-invasive radiofrequency (RF) mild hyperthermia treatment was proposed as a synergistic therapy to enhance drug uptake into the tumor by increasing tumor vascular inflow and perfusion, thus, increasing the effect of chemotherapy. RF-induced hyperthermia is a safer and non-invasive technique of tumor heating compared to conventional contact heating procedures. In this study, we investigated the short- and long-term effects (~20 days and 65 days, respectively) of combination chemotherapy and RF hyperthermia in an orthotopic PDAC model in mice. The benefit of nab-paclitaxel and gemcitabine treatment was confirmed in mice; however, the effect of treatment was statistically insignificant in comparison to saline treated mice during long-term observation. The benefit of RF was minimal in the short-term and completely insignificant during long-term observation.
Introduction
Pancreatic cancer is the fourth leading cause of cancer-related death in the United States. Both death rates and incidence for pancreatic cancer are increasing, despite a decline in overall cancer death rates over the past thirty years. The five-year survival rate for pancreatic cancer currently stands at a discouraging 8%, increasing only 5% since 1975.1 These statistics illustrate the importance of investigating novel treatments for patients with pancreatic cancer. Pancreatic ductal adenocarcinoma (PDAC) is the most common form of pancreatic tumors. Diagnosis occurs at a median age of 65 to 70 years, and since symptoms do not occur until the disease is advanced, it is usually incurable. PDAC is unusual in that it creates a large extracellular desmoplastic stroma via activation of surrounding fibroblasts and stellate cells with secretion of extracellular matrix. This desmoplastic stroma often composes the majority of the tumor, and sometimes less than 10% of the tumor volume is comprised of cancer cells.2
For eligible patients, surgical intervention offers the most reliable and durable survival benefit for patients with PDAC. However, only 15% - 20% of tumors in newly diagnosed patients are deemed resectable, in which case the malignancy cannot have metastasized, encased the celiac or superior mesenteric artery, or occluded the superior mesenteric vein. This poses a major problem since most patients present only once symptoms are evident, which typically occurs when local tumor advancement has already taken place and hence the tumor is already associating with these key vessels. Patients who are surgical candidates often receive adjuvant chemotherapy due to the high recurrence rate after surgery. Most often, six months of adjuvant chemotherapy with gemcitabine or fluorouracil is given four to six weeks after surgery and has been shown to improve median survival. Conversely, neoadjuvant chemotherapy and radiation have limited and conflicting reports of efficacy in resectable tumors, respectively.3
Systemic chemotherapy with combined regimens is recommended for patients who have good performance status and few comorbidities.4 In locally advanced non-metastatic disease, the optimal treatment regimen is still under investigation. Options include FOLFIRINOX (leucovorin, fluorouracil, irinotecan, and oxaliplatin), gemcitabine plus nab-paclitaxel (Abraxane®), and gemcitabine alone. FOLFIRINOX is generally favored over gemcitabine for patients who can tolerate the toxicity, in correclation with a good Eastern Cooperative Oncology Group (ECOG) score.[5], [6] Patients with locally advanced non-metastatic disease may undergo chemoradiotherapy or stereotactic body radiation therapy after initial chemotherapy in a number of clinical scenarios: if there is disease progression but no signs of distant metastasis after chemotherapy initiation, if chemotherapy is discontinued due to toxicity, or as an alternative to continuing chemotherapy after six months of stable disease.4
The other one third of patients who present with PDAC have metastatic disease at diagnosis.4 For patients with a favorable comorbidity profile and an ECOG performance status of 0 or 1, first-line treatment with FOLFIRINOX or gemcitabine plus nab-paclitaxel is typically offered, as these regimens have shown a median survival benefit over gemcitabine alone.[4], [5], [6] Since these chemotherapy regimens are more intensive and are associated with a higher toxicity profile, gemcitabine alone should be offered to patients with an ECOG performance status of 2 or with comorbidities that contraindicate other treatment regimens. For patients with an ECOG performance status of 3 or severe comorbid conditions, best supportive therapy alone is recommended.4
Considering the inadequacy of current therapies to improve long-term outcomes in PDAC, it is imperative to investigate novel treatments for this devastating disease. Non-invasive radiofrequency (RF) hyperthermia has shown promising results in in vivo and in situ studies as an anticancer therapy. Hyperthermia has been much studied as a method of cancer treatment, and there are multiple proposed mechanisms by which local hyperthermia may exert an anticancer effect on tumor cells. RF hyperthermia has been shown to be superior to contact-based heating because it has a higher penetration into tumor tissue and results in less damage to normal tissue compared to contact heating.7 RF hyperthermia was demonstrated to induce autophagy in malignant, but not normal pancreatic cells and potentiate the effect of gemcitabine chemotherapy.8 It has been shown to increase local vascular perfusion in the tumor and may improve chemotherapy drug-delivery to the cancer cells.9 Interestingly, RF has also been shown to exert an anticancer effect independent of hyperthermia by inducing a cytotoxic effect caused by inhibition of tumor cell proliferation and mitochondrial activity.10
RF hyperthermia shows promising pre-clinical results as a novel cancer treatment. However, the effects of RF have only been investigated in short-term studies lasting a few weeks. Thus, a long-term study is warranted to investigate any survival benefits of RF hyperthermia therapy. Here, we present a randomized control study investigating the effects of combination RF hyperthermia on PDAC in mice treated with standard-of-care gemcitabine and nab-paclitaxel (Abraxane®).
Materials and Methods
Materials
Corning Matrigel Growth Factor Reduced Basement Membrane Matrix was used for tumor inoculation. Gemcitabine – Gemzar® (stock solution: 38 mg/mL): the range of doses from 30 to 60 mg/kg BW was provided i.v. after reconstitution of stock solution in sterile saline solution (0.9% Sodium Chloride). Abraxane® (stock: 100 mg paclitaxel, 900 mg albumin): dose range from 15 to 30 mg/kg BW was applied i.v. after preparation of drug solution in sterile saline solution. Dosing was calculated with paclitaxel only. All drug solutions were prepared freshly under sterile conditions and used within 12 h after dilution.
Ethic Statement and General Mice Conditions
Experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Baylor College of Medicine (No. AN-6448). Female Balb/c Nude mice were housed in standard temperature and lighting conditions with free access to food and water. All procedures which required anesthesia were performed under 0.7% to 2.5% isoflurane in medical air. During anesthesia, mouse core temperature was monitored with rectal probes and the breathing frequency was established around 1 Hz. Heating pads were used to establish mouse body temperature during each experimental step. After anesthesia, the animal was kept in a pre-warmed recovery chamber.
Tumor Model
PANC-1, a human PDAC line was obtained from American Type Culture Collection (ATCC). Cells were maintained in DMEM with 10% FBS. Cells were maintained in a 95% humidified atmosphere of 5% CO2 at 37 °C and 2% penicillin–streptomycin solution and grown to confluence. They were harvested with trypsin–EDTA (Lonza) when needed. When Balb/c nude mice were at least 7 weeks old, 106 PANC-1 cells mixed with matrigel (1:1) were injected (30G needle) into the pancreatic tissue under ultrasound guidance. Approximately 80% of total injected mice reached expected tumor size and were promoted to treatment phase (for 20% of all animals injected with PANC-1 cells, tumors did not appear in the expected time window or growth kinetics were too slow or too fast). Four weeks after injection, ultrasound imaging of the pancreas (B-mode, VEVO 2100) was performed weekly to monitor for tumor growth. Treatment was started 6 to 8 weeks after PANC-1 injection. The average tumor size upon initiation of treatment was 59 ± 40 mm3. Mouse weight was also monitored to investigate possible side effects on the animal's general health condition. Tumor volume was calculated according to Equation 1 based on three perpendicular diameters collected during 3D ultrasound imaging.
| (1) |
Equation to calculate tumor volume where a, b, c are three perpendicular tumor diameters.
Chemotherapy
For chemotherapy-treated mice, a regimen of nab-paclitaxel (Abraxane®) and gemcitabine was used. Control group mice were injected with similar volumes of saline solution. For different drug doses, the same injectable volume per mouse was preserved resulting in different compound concentrations. For example, a 25 g mouse would receive 119 μL of gemcitabine solution and 150 μL of nab-paclitaxel. This was done to avoid confounding blood flow and physiological changes related to the injection volume. The following treatment scheme was used for each mouse: (1) nab-paclitaxel was injected intravenously (2) and was given 110 minutes prior to RF field exposure to exert its effect on the tumor, (3) the tumors were irradiated with RF radiation and maintained at 39°C for 10 minutes, (4) followed by immediate intravenous injection of gemcitabine. The time interval between nab-paclitaxel and gemcitabine injection was exactly two hours. The entire treatment scheme (steps 1 through 4) was repeated a total of 6 times at 72 hour time intervals (Q3Dx6 protocol) resulting in 16 days of treatment. To study the dose effects, the following combination of chemotherapy doses were used: (a) 15 mg/kg of nab-paclitaxel followed with 30 mg/kg of gemcitabine, (b) 20 mg/kg of nab-paclitaxel followed by 40 mg/kg of gemcitabine, and (c) 30 mg/kg of nab-paclitaxel followed by 60 mg/kg of gemcitabine. Each of the protocols used the same chemotherapy and RF regimen discussed above.
Hyperthermia
The portable-RF system described elswhere[11], [12] but highlighted in the Supplementary Information (S1) was used to produce local hyperthermia in the tumor tissue. Essentially, the device outputs a high-power (200 W) RF electric field at an operating frequency of 13.56 MHz. We have added extra, automated components to the general device to allow for better loading and manipulation of the mouse both before and during RF irradiation. This setup allows us to constantly control the tumor temperature to allow for a nice even heating profile. The influence of RF radiation and induced tumor temperature increase on pseudo-drug uptake into tumors was investigated earlier,9 the results of which led us to choose 39°C as the general tumor temperature limit for enhanced drug uptake. Hyperthermia of the tumor tissue was maintained for 10 minutes at 39°C. The superficial skin surface (measured using infrared cameras, FLIR) was not allowed to reach a temperature greater than 41°C. Control mice (RF-) were placed on a heated platform to match the rectal temperature of the treatment mouse concurrently, but without locally applied contact heating of the tumor. The results of contact-heating hyperthermia are presented elsewhere.9
Ultrasound Imaging
A Vevo 2100 ultrasonographic imager (VisualSonics®, Toronto, Canada) was used for non-invasive investigation of tumor size. An MS-550S (32-56 MHz) transducer was used for abdominal imaging of the mice, with a focus on the pancreatic tissue. Imaging was performed weekly to quantify tumor size (as described above) and to observe potential side effects.
Histology
When any of the survival criteria (e.g. invasive tumor, tumor size >200 mm3, weight loss greater than 15%, fluid in the abdomen) were met, the animals were euthanized and examined for potential metastases. The tumor mass was removed and placed in 4% formalin solution, after which the paraffin imbedded slices were prepared and stained with standard hematoxylin and eosin staining (H&E) and Picro Sirius (used to stain collagen I and III fibers as well as muscle tissue).
Experimental Design and Statistical Analysis
Statistica12® software (StatSoft, Tulsa, USA) was used to design the experiment and randomize all treated animals. The Design of Experiment (DOE) module was used with full factorial design option to randomize chemotherapy/saline and RF+/− procedures. The mice were divided into fully randomized groups. For statistical data processing, Statistica12® was used and factorial ANOVA (including Kurskal-Wallis, Kendall's, and median test) and U test were performed.
Results
The orthotopic PANC-1 tumors in our study had similar growth rates compared to those presented in similar studies that utilized MRI imaging.13 Ultrasound analysis of the pancreas allowed us to accurately identify and measure tumors as small as 15 mm3 with minimal error and with excellent inter-measurement consistency, before, during, and after treatment (Figure 1). Weekly monitoring of tumor growth started at approximately 4 weeks post-inoculation. Mice with tumors that displayed extreme growth kinetics (i.e., very slow or very fast growth rates) were excluded before randomization. All mice began treatment once their tumors had reached a similar size (59 ± 40 mm3) and underwent a Q3D6 treatment schedule (six cycles of treatment three days apart). Mice receiving chemotherapy received a regimen of gemcitabine and nab-paclitaxel (Abraxane®) at one of three doses (A15/G30, A20/G40, and A30/G60). This nab-paclitaxel and gemcitabine regimen for mice has been derived from the FDA-approved chemotherapy regimen for pancreatic cancer in humans and is based on other studies on mouse models.[14], [15], [16], [17] Mice receiving RF treatment had their tumors subjected to local hyperthermia of 39°C for 10 minutes (applied just before gemcitabine injection), which minimizes superficial burns and is adequate in increasing extravasation of compound (i.e., chemotherapy) from the vasculature.12
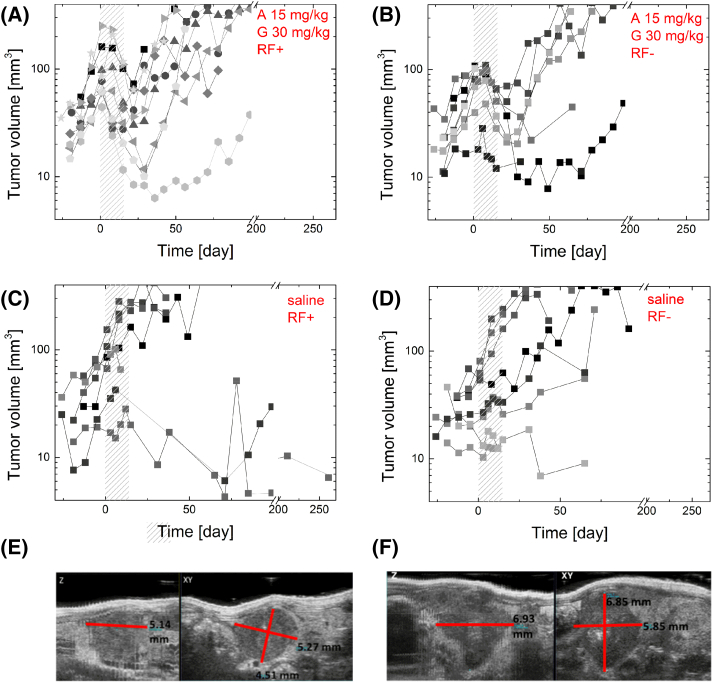
Figure 1.
Select tumor growth data from mice treated with: (A) Abraxane 15 mg/kg followed by gemcitabine 30 mg/kg with RF (n = 9); (B) Abraxane 15 mg/kg followed by gemcitabine 30 mg/kg without RF (n = 8); (C) intravenous saline injection with RF (n = 7); and (D) intravenous saline injection without RF (n = 9). Certain mice were excluded based on criteria outlined below. The shaded areas indicate the Q3D6 treatment time. (E and F) Random USG images of tumors with Z and XY axes marked. Total tumor volume is (E) 63.97 mm3 and (F) 145.40 mm3. Tumor volumes were calculated in 3D B-Mode of ultrasound image (VEVO2100) by selection of three perpendicular diameters and total tumor volume was calculated using Equation 1.
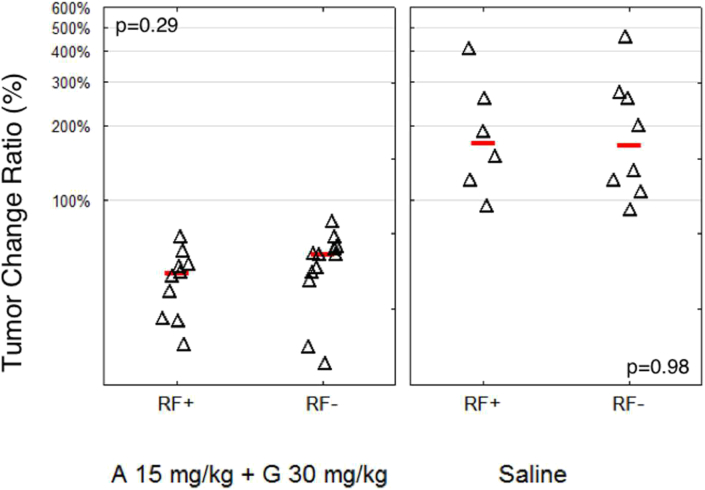
The study of tumor growth kinetics during the treatment time (shaded regions, Figure 1) indicates that chemotherapy treatment combined with RF hyperthermia (Figure 1A) leads to a fast tumor response to therapy (generally tumor regression was visible after the first treatment day). In contrast, tumors in mice treated with chemotherapy only (Figure 1B) need around two/three treatment days (3-9 days from beginning of therapy) to display tumor regression. To analyze the effect of treatment on tumor volume, we describe the tumor change ratio, which is defined here as the ratio of tumor size at the end of the treatment schedule (day 16) to tumor size at the beginning of the treatment schedule (day 1). The tumor change ratio for mice treated with chemotherapy (with or without RF) was significantly smaller than that of mice treated with saline (P < .0001), (Figure 2). All mice treated with all dosages of chemotherapy benefited from a decrease in their tumor volume in relation to their initial tumor volume prior to initiation of treatment. This validates that the chosen chemotherapy regimen of nab-paclitaxel plus gemcitabine in our study was an effective treatment for PDAC in mice. The addition of RF hyperthermia in combination with chemotherapy had a weak tendency to decrease tumor volume immediately after treatment. Chemotherapy alone produced a tumor change ratio of 0.61, while combination of RF and chemotherapy had a tumor change ratio of 0.50. However, this change was not significant (P = .29). Analysis of relative tumor volume (RTV), which is relative to initial tumor volume, leads to similar statistical conclusions.
Figure 2.
Change of pancreatic tumor volume caused by chemotherapy (gemcitabine plus Abraxane) treatment (Q3D6, 16 days total) with and without RF. The tumor volume ratio (after/before treatment) was calculated for each mouse (expressed as %). The lines indicate the mean for each selected group while triangles represent data from a single mouse. The four groups are mice treated with chemotherapy (A 15 mg/kg + G 30 mg/kg) and RF (n = 10), chemotherapy without RF (n = 12), saline with RF (n = 6), and saline without RF (n = 8). Chemotherapy treatment led to a highly significant decrease in tumor volume compared to saline-injected mice (P < .0001). RF irradiation did not lead to a statistically significant difference in tumor growth for either saline or chemotherapy groups.
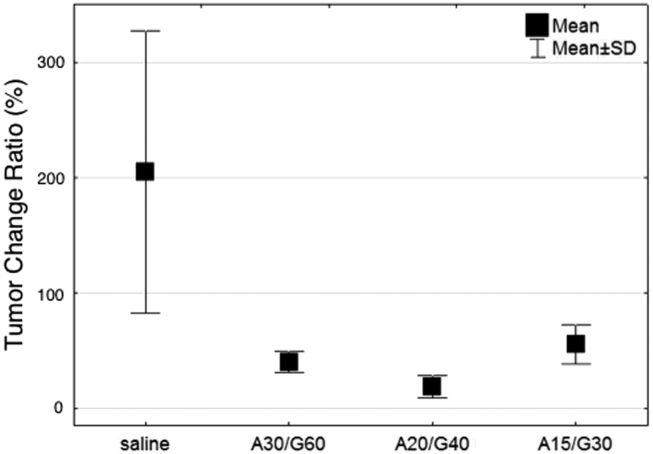
Length of time in regression was also investigated between the treatment groups. Regression is defined as the period of time after completion of treatment before which a tumor begins to increase in size again. Tumors treated with chemotherapy (A15/G30) had regression of their tumor volume that lasted approximately 20 days after treatment initiation (Figure 1, A and B). Interestingly, there was no significant difference in tumor regression between the additional two tested chemotherapy doses. For example, mice treated with a 50% higher dose of chemotherapy (i.e. A30/G60) had a 12% decrease in tumor change ratio that was not statistically significant. This suggests that the selected range of chemotherapy dosing used in this study surpassed the greatest achievable efficacy of this regimen, and a further increase in dosing would not result in a benefit in tumor volume regression or survival (Figure 3). Combination chemotherapy-RF did not produce a significant difference in length of time in regression when compared to mice treated with chemotherapy alone. However, there were some notable tendencies observed in complete remission rates between treatment groups. Complete remission is defined here as no detectable tumors on ultrasound and no evidence of primary cancer or metastasis on necropsy. The combination chemotherapy (A15/G30) and RF group had a complete remission rate of 10%, compared to 7% in mice receiving chemotherapy alone. The control group, which received neither chemotherapy nor RF, had a complete remission rate of 5%. Application of a higher chemotherapy dose resulted in a higher complete remission rate.
Figure 3.
Tumor change ratio (%) versus treatment method: saline (n = 10), A30/G60 (n = 5), A20/G40 (n = 5), and A15/G30 (n = 12). Chemotherapy (gemcitabine plus Abraxane) leads to a significant decrease in tumor volume immediately after treatment in comparison to mice injected only with saline solution (P = .00008). During the whole treatment schedule (Q3D6) control tumors (saline injected mice) double in size on average. The highest dose of Abraxane (30 mg/kg) and gemcitabine (60 mg/kg) led to an average decrease of 61% in tumor volume immediately after treatment, while the lowest chemotherapy dose (Abraxane 15 mg/kg + gemcitabine 30 mg/kg) led to a 49% decrease in tumor volume (P = .12). This indicates the selected chemotherapy dosing is effective against PANC-1 tumors in Balb/c nude mice. The 50% difference in chemotherapy dosing led to a 12% change in tumor size. The therapy effectiveness was confirmed via histological analysis.
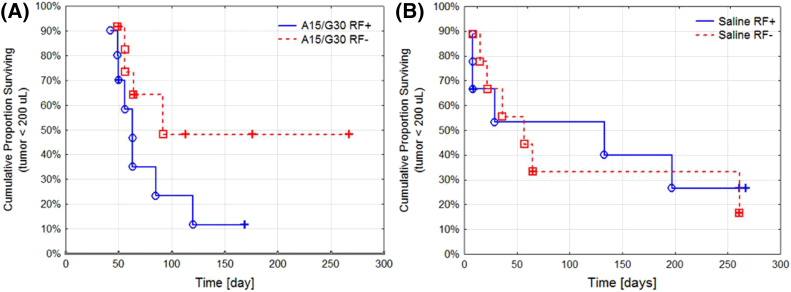
While there is prior evidence of the short-term benefits of RF hyperthermia in cancer,18 there is no data for PDAC long-term therapeutic outcomes and survival benefit. Here we describe the long-term (i.e. ~65 days) therapeutic pre-clinical model outcome of RF and chemotherapy. Kaplan-Mayer analysis was performed to study the long-term effectiveness of each treatment (Figure 4). Chemotherapy produced a short-term (~20 days) benefit in survival and improved outcomes for animals treated with chemotherapy compared to saline until a certain time point. We estimate this time point to be around 65 days, after which there was no significant survival difference between mice in the chemotherapy and saline groups that had reached that time point. After day 65, the remaining mice in the chemotherapy and saline control groups had no significant difference in overall survival (P = .09) (Supplemental Information S2A). This leads to the conclusion that the effect of chemotherapy is transient, and treatment must be extended and/or repeated after 2 months. Furthermore, there was no statistically significant benefit in survival from increasing chemotherapy dosing (Figure 3). Additionally, RF did not lead to a significant improvement in mouse survival up to this time point at day 65 (P = .30). After day 65, mice treated with RF seem to have poorer survival compared to mice not receiving RF (Supplemental Information S2B).
Figure 4.
Survival analysis of mice treated with: (A) Abraxane 15 mg/kg followed by gemcitabine 30 mg/kg with RF (n = 10) and without RF (n = 12), (P = .12); and (B) saline injection control with RF (n = 9) and without RF (n = 9), (P = .86). Mice were censored based on certain survival criteria (e.g. tumor size < 200 uL, mouse weight < 85 % of starting weight, or euthanasia due to reasons unrelated to cancer or treatment.
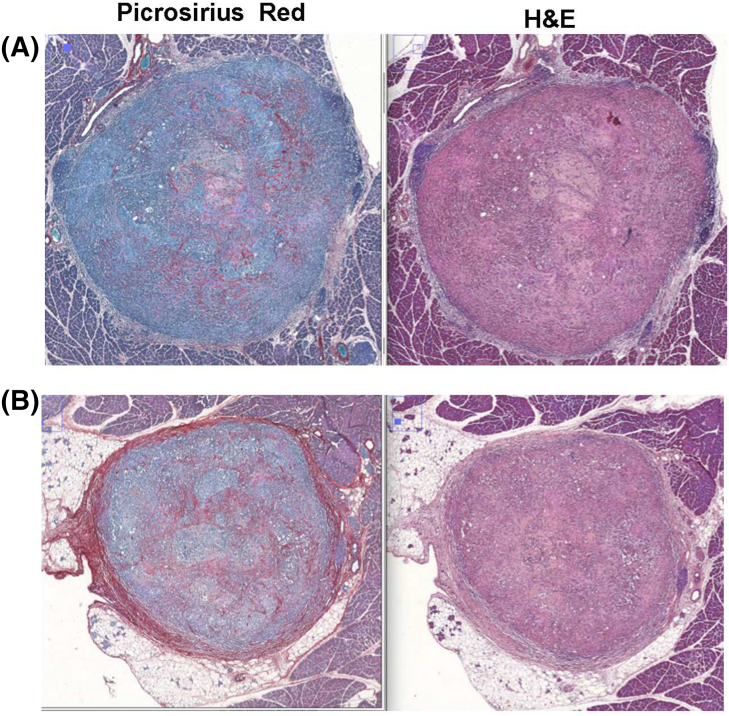
Histological analysis of tumors at the end of observation time suggests the applied chemotherapy treatment leads to decrease in stroma-compartment tissue in comparison to saline treated tumors (P = .12), (Figure 5). Observation of tumor necrosis suggests that selected chemotherapy treatment leads to more intense tumor necrosis than in tumors from control group animals (saline treated). Statistical analysis does not indicate that RF induced hyperthermia as a relevant factor for connective tissue and necrotic fraction concentration.
Figure 5.
Histological analysis revealed that applied chemotherapy treatment led to a decrease in stroma-compartment tissue in comparison to saline-treated tumors (P = .12). (A) Mouse treated with A30/G60 and RF, (B) mice treated only with saline solution.
Mouse weights were recorded as a surrogate measure of chemotherapy toxicity (Supplementary Information S2). Generally, an animal weight ratio of 0.85 indicates significant toxicity of the treatment to the animal. Animal weight ratios in this study were never below 0.85 for any mouse. RF hyperthermia treatment did not have any significant effect on the animal weight ratios. Moreover, mouse weights were stable for even the highest dosing of chemotherapy throughout the treatment schedule. The average animal weight ratios for all treatment groups were close to 1 (Supplementary Information S3). The major adverse effect of RF hyperthermia in this study was related to superficial burns. This problem was discussed by us earlier,9 and we showed that contact-based heating led to significantly greater burns when compared to RF. In our optimization protocol for RF radiation, hyperthermia at 39°C for 10 minutes successfully prevented superficial burns and major organ damage related to hyperthermia. In our previous studies, we did not observe any significant difference between hyperthermia at 39°C and 41°C, and we found no significant difference for hyperthermia lasting for 10 minutes versus 30 minutes. Furthermore, in our previous study utilizing intravital microscopy, we showed that hyperthermia at 39°C for 5 minutes is sufficient in increasing drug and fluorescent extravasation from blood vessels.12
Discussion
Based on Sun's work in 2015, we demonstrated that additional chemotherapy dosing is not beneficial for murine survival in our xenograft PDAC model. The 50% survival of PANC-1 tumor-bearing mice in Sun et al. is similar to the mouse survival data presented here. We used a Q3D6 treatment scheme in comparison to Sun's Q3D5 regimen (60 mg/kg intraperitoneal and nab-paclitaxel 30 mg/kg intravenous) and achieved similar results until approximately 65 days post-treatment.16
Koshkina et al. performed a similar study to ours but obtained differing results.8 In their study, orthotopic PANC-1 tumors were exposed to the combination of chemotherapy and RF radiation. Standard gemcitabine dosing of 70 mg/kg was given via intraperitoneal injections and followed by next-day RF exposure for 10 min (600 W, 13.56 MHz), with treatments occurring once weekly. They found that combination RF and gemcitabine resulted in a significantly greater inhibition of tumor progression when compared to RF or chemotherapy alone.8 It is not clear how long animals were observed after the treatment in their study, but based on other data presented by the authors, the assumption can be made that post-treatment investigation was short-term and up to 1 month. Different RF parameters, chemotherapy scheduling, and length of post-treatment observation can explain these inconclusive results.
The findings in this PDAC model should be investigated in other PDAC models. There are several types of immortalized PDAC cell lines available for inducing in vivo models such as AsPC-1, BxPC-3, Capan-1, Capan-2, HPAC, HPAF-II, Hs766T, CFPAC-1, MIA PaCa-2, and SU.86.86. Each cell line has inherently different properties in regards to their in vivo and in vitro proliferation characteristics, phenotypic characteristics such as invasion, tumorigenesis, migration, and adhesion, as well as the genotypic expression of altered genes such as p53, Kras, and SMAD4.19 There are also several transgenic, genetically engineered mouse models (GEMMS) of PDAC available including combining the ‘sleeping beauty’ (SB) transposon system with an ongogenic Kras allele to allow for highly metastatic PDAC.20 A comprehensive review of GEMMs can be found in the literature21 and is outside the scope of this paper.
The rationale for selecting the PANC-1 cell-line was based on previous successful efforts and experience in our lab in developing a quick and easy PDAC orthotopic model to evaluate the effects of RF on, as well as the relatively large success-rate in tumor establishment. In addition, previous work utilizing antibody conjugated gold nanoparticles and quantum dots as thermally active RF-agents was also performed on PANC-1 cell lines.[22], [23] Tumor selective hyperthermia induced by RF-radiation was also previously investigated in our lab using a PANC-1 orthotopic model of PDAC.7
Given that PANC-1 tumors are inherently highly vascularized, additional treatment with RF may not increase perfusion in an already highly vascular tumor (especially in comparison to poorly vascularized human PDAC tumors).24 Furthermore, our tumor population had differing growth rates, with some being evidently delayed. This leads us to conclude that the investigated tumor population in our study is heterogeneous and may account for the differing treatment responses between mice of the same group. Alternatively, previous work by Ware et al. published in 20159 suggested that there may be various sub-populations within the PANC-1 cancer cell populations that respond differently to RF treatment. These subpopulations may evolve to become somewhat ‘resistant’ to RF therapy after multiple RF treatments have occurred. This observation may explain why long-term survival in mice treated multiple times with RF is poorer or equal to non-treated animals.
Future work should thus be focused on evaluating the effects of combined drug delivery and RF therapy on other PDAC mouse models, particularly the ones that are inherently hypo-vascularized. On another note, although we have also investigated the use of ectopic, superficial cancer models in previous studies, this model has a tendency to distort and confine the electric field around its tumor mass due to the protruding nature of the tumor and as such does not mimic adequately the tumor conditions found in human models. More experiments should also be performed to optimize both the RF parameters (e.g., frequency, dosages, deposited energy) and drug delivery mechanism (i.e. intravenous versus intraperitoneal, etc.) needed to both enhance tumor response and avoid tumor drug resistance. It is hypothesized that different operational frequencies, better matched to the heterogeneous tumor tissue electrical properties can selectively and effectively target cancer and also reduce the adverse effects of superficial burns and organ damage, as has been preliminary investigated in past studies.7
Conclusion
The short-term (20 days) benefit of nab-paclitaxel and gemcitabine treatment of PDAC tumors was confirmed in a murine model of orthotopic PDAC. The selected dosages of chemotherapeutics led to a significant decrease of tumor volume during and immediately after the treatment time. In the long-term observation (65 days), the benefit of chemotherapy application becomes insignificant in comparison to saline treated mice. In contrast to previous findings, there was no significant short or long-term survival benefit observed with adjuvant RF-induced hyperthermia.
Acknowledgments
Acknowledgments
SJC and SAC acknowledge support the Kanzius Cancer Research Foundation, Baylor College of Medicine, and NIH (U54CA143837). JMN acknowledges financial support from the National Institute of General Medical Sciences T32 predoctoral training grant (T32GM088129) and the National Institute of Dental and Craniofacial Research F31 NRSA training grant (F31DE026682) both of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. JCH acknowledges support from Baylor College of Medicine Oncology Scholars (T32CA174647).
Authors’ Contribution and Declarations
The study was conceived by SJC, MKS, MJW and SAC. MKS, JCH, MJW and SJC designed the experiments. MKS, MA, JL, MJW, JCH, JMN and LN and performed the experiments. MKS and MA performed the data analysis. The manuscript was written by MA, SJC, and MKS and edited by all authors before submission. The authors declare that they have no competing interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tranon.2018.02.023.
Appendix A. Supplementary data
Figure S1 Portable RF system setup and generated electric field. (A) Portable RF system consists of the transmitting unit (TX) and receiving head (RX) that generates a high-power electric field across the specimen (e.g. mouse). The system is driven by a variable power fixed RF amplifier (0–200 W, 13.56 MHz) that is cooled during operation by a water chiller. Heat production is monitored using an infrared (IR) camera or direct insertion of fiber optical probes. (B) Circuit representation of the portable RF system. (C) Setup for extracting electric-field intensities. An electric-field probe (EFP) is placed at specific points along the x- and z-axis in between the TX and RX heads and measures the voltage at each point for 20WRF-power. (D) The electric field is derived from the voltage data and is plotted as an intensity contour plot. Figures and Caption used with permission by Stuart J. Corr et al under the Creative Commons Attribution (CC-YB) License (A New Imaging Platform for Visualizing Biological Effects of Non-Invasive Radiofrequency Electric-Field Cancer Hyperthermia, PLoS One, 10, 8. e0136382, 2015.)
Figure S2 Survival analysis of PANC-1 tumor bearing mice after: (A) general chemotherapy treatment with all studied dosages vs saline injections (p = 0.09), (B) general RF influence presented on RF+ and RF- mice (p = 0.30). As survival criteria the tumor size (< 200 uL) and change of mouse weight was selected, animals were censored (+) when either of those criteria was adequate.
Figure S3 Change of animals weight caused by the treatment (Q3Dx6, 18 days total). The after/before treatment weight ratio was calculated per each mouse. Line indicate the mean for selected group while tringles represents data from single mouse. All animals were injected i.v. with saline solution or Abraxane (15 mg/kg) and 2 h after second i.v. injection of saline or gemcitabine (30 mg/kg) was performed, just before second injection tumor was irradiated 10 min by RF (39°C) (or NHC for control group), those treatment protocol was repeated 6 times (every 72 h). Mice treated with chemotherapy (A 15 mg/kg + G 30 mg/kg) and RF (N=10) and without RF (N=12), mice treated with saline and RF (N = 6) and without (N = 8). The RF treatment and chemotherapy are not leading to weight lose bigger then 15%, which can be a indicator of lack of overall toxic effects on mice.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Bartholin L. Transworld Research Network; 2012. Pancreatic cancer and the tumor microenvironment: Mesenchyme's role in pancreatic carcinogenesis. [PubMed] [Google Scholar]
- 3.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039–1049. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- 4.Balaban EP, Mangu P.B., Yee N.S. Locally Advanced, Unresectable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34:2654–2668. doi: 10.1200/JCO.2016.67.5561. [DOI] [PubMed] [Google Scholar]
- 5.Conroy T, Desseigne F., Ychou M., Bouché O., Guimbaud R., Bécouarn Y., Adenis A., Raoul J.-L., Gourgou-Bourgade S., de la Fouchardière C. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 6.Von Hoff DD, Ervin T., Arena F.P., Chiorean E.G., Infante J., Moore M., Seay T., Tjulandin S.A., Ma W.W., Saleh M.N. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raoof M, Cisneros B.T., Corr S.J., Palalon F., Curley S.A., Koshkina N.V. Tumor Selective Hyperthermia Induced by Short-Wave Capacitively-Coupled RF Electric-Fields. PLoS One. 2013;8:1–9. doi: 10.1371/journal.pone.0068506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koshkina NV, Briggs K, Palalon F, Curley SA. Autophagy and enhanced chemosensitivity in experimental pancreatic cancers induced by noninvasive radiofrequency field treatment. Cancer. 2014;120:480–491. doi: 10.1002/cncr.28453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ware MJ, Krzykawska-Serda M., Ho J.C.-S., Newton J.M., Suki S., Law J.J., Nguyen L., Keshishian V., Serda M., Taylor K. Optimizing non-invasive radiofrequency hyperthermia treatment for improving drug delivery in 4T1 mouse breast cancer model. Sci Rep. 2017;7:43961. doi: 10.1038/srep43961. [1-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curley S, Palalon F, Sanders K, Koshkina N. The Effects of Non-Invasive Radiofrequency Treatment and Hyperthermia on Malignant and Nonmalignant Cells. Int J Environ Res Public Health. 2014;11:9142–9153. doi: 10.3390/ijerph110909142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corr SJ, Shamsudeen S., Vergara L.A., Ho J.C.-S., Ware M.J., Keshishian V., Yokoi K., Savage D.J., Meraz I.M., Kaluarachchi W. A New Imaging Platform for Visualizing Biological Effects of Non-Invasive Radiofrequency Electric-Field Cancer Hyperthermia. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lapin NA, Krzykawska-Serda M, Ware MJ, Curley SA, Corr SJ. Intravital microscopy for evaluating tumor perfusion of nanoparticles exposed to non-invasive radiofrequency electric fields. Cancer Nanotechnol. 2016;7:5. doi: 10.1186/s12645-016-0016-7. [1-19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samkoe KS, Chen A., Rizvi I., O'Hara J.A., Hoopes P.J., Pereira S.P., Hasan T., Pogue B.W. Imaging Tumor Variation in Response to Photodynamic Therapy in Pancreatic Cancer Xenograft Models. Int J Radiat Oncol Biol Phys. 2010;76:251–259. doi: 10.1016/j.ijrobp.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cividalli A, Mauro F., Livdi E., Ceciarelli F., Altavista P., Cruciani G., Tirindelli Danesi D. Schedule dependent toxicity and efficacy of combined gemcitabine/paclitaxel treatment in mouse adenocarcinoma. J Cancer Res Clin Oncol. 2000;126:461–467. [PubMed] [Google Scholar]
- 15.Meng H, Wang M., Liu H., Liu X., Situ A., Wu B., Ji Z., Hyun Chang C., Nel A.E. Use of a lipid-coated mesoporous silica nanoparticle platform for synergistic gemcitabine and Paclitaxel delivery to human pancreatic cancer in mice. ACS Nano. 2015;9:3540–3557. doi: 10.1021/acsnano.5b00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun JD, Liu Q., Ahluwalia A., Li W., Meng F., Wang Y., Bhupathi D., Ruprell A.S., Hart C.P. Efficacy and safety of the hypoxia-activated prodrug TH-302 in combination with gemcitabine and nab-paclitaxel in human tumor xenograft models of pancreatic cancer. Cancer Biol Ther. 2015;16:438–449. doi: 10.1080/15384047.2014.1003005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Awasthi N, Zhang C., Schwarz A.M., Hinz S., Wang C., Williams N.S., Schwarz M.A., Schwarz R.E. Comparative benefits of nab-paclitaxel over gemcitabine or polysorbate-based docetaxel in experimental pancreatic cancer. Carcinogenesis. 2013;34:2361–2369. doi: 10.1093/carcin/bgt227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirui DK, Celia C., Molinaro R., Bansal S.S., Cosco D., Fresta M., Shen H., Ferrari M. Mild Hyperthermia Enhances Transport of Liposomal Gemcitabine and Improves In Vivo Therapeutic Response. Adv Healthc Mater. 2015;4:1092–1103. doi: 10.1002/adhm.201400738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deer EL, González-Hernández J., Coursen J.D., Shea J.E., Ngatia J., Scaife C.L., Firpo M.A., Mulvihill S.J. Phenotype and Genotype of Pancreatic Cancer Cell Lines. Pancreas. 2010;39:425–435. doi: 10.1097/MPA.0b013e3181c15963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mann KM, Ward J.M., Yew C.C., Kovochich A., Dawson D.W., Black M.A., Brett B.T., Sheetz T.E., Dupuy A.J., Chang D.K. Sleeping Beauty mutagenesis reveals cooperating mutations and pathways in pancreatic adenocarcinoma. Proc Natl Acad Sci. 2012;109:5934–5941. doi: 10.1073/pnas.1202490109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pérez-Mancera PA, Guerra C, Barbacid M, Tuveson DA. What We Have Learned About Pancreatic Cancer From Mouse Models. Gastroenterology. 2012;142:1079–1092. doi: 10.1053/j.gastro.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Glazer ES, Curley SA. Radiofrequency field-induced thermal cytotoxicity in cancer cells treated with fluorescent nanoparticles. Cancer. 2010;116:3285–3293. doi: 10.1002/cncr.25135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glazer ES, Massey KL, Zhu C, Curley SA. Pancreatic carcinoma cells are susceptible to noninvasive radio frequency fields after treatment with targeted gold nanoparticles. Surgery. 2010;148:319–324. doi: 10.1016/j.surg.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuzuki Y., Mouta Carreira C., Bockhorn M., Xu L., Jain R.K., Fukumura D. Pancreas microenvironment promotes VEGF expression and tumor growth: novel window models for pancreatic tumor angiogenesis and microcirculation. Lab Invest. 2001;81:1439–1451. doi: 10.1038/labinvest.3780357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Portable RF system setup and generated electric field. (A) Portable RF system consists of the transmitting unit (TX) and receiving head (RX) that generates a high-power electric field across the specimen (e.g. mouse). The system is driven by a variable power fixed RF amplifier (0–200 W, 13.56 MHz) that is cooled during operation by a water chiller. Heat production is monitored using an infrared (IR) camera or direct insertion of fiber optical probes. (B) Circuit representation of the portable RF system. (C) Setup for extracting electric-field intensities. An electric-field probe (EFP) is placed at specific points along the x- and z-axis in between the TX and RX heads and measures the voltage at each point for 20WRF-power. (D) The electric field is derived from the voltage data and is plotted as an intensity contour plot. Figures and Caption used with permission by Stuart J. Corr et al under the Creative Commons Attribution (CC-YB) License (A New Imaging Platform for Visualizing Biological Effects of Non-Invasive Radiofrequency Electric-Field Cancer Hyperthermia, PLoS One, 10, 8. e0136382, 2015.)
Figure S2 Survival analysis of PANC-1 tumor bearing mice after: (A) general chemotherapy treatment with all studied dosages vs saline injections (p = 0.09), (B) general RF influence presented on RF+ and RF- mice (p = 0.30). As survival criteria the tumor size (< 200 uL) and change of mouse weight was selected, animals were censored (+) when either of those criteria was adequate.
Figure S3 Change of animals weight caused by the treatment (Q3Dx6, 18 days total). The after/before treatment weight ratio was calculated per each mouse. Line indicate the mean for selected group while tringles represents data from single mouse. All animals were injected i.v. with saline solution or Abraxane (15 mg/kg) and 2 h after second i.v. injection of saline or gemcitabine (30 mg/kg) was performed, just before second injection tumor was irradiated 10 min by RF (39°C) (or NHC for control group), those treatment protocol was repeated 6 times (every 72 h). Mice treated with chemotherapy (A 15 mg/kg + G 30 mg/kg) and RF (N=10) and without RF (N=12), mice treated with saline and RF (N = 6) and without (N = 8). The RF treatment and chemotherapy are not leading to weight lose bigger then 15%, which can be a indicator of lack of overall toxic effects on mice.