Abstract
The mechanism of the low temperature autolysis of Volvariella volvacea (V. volvacea) has not been thoroughly explained, and trehalose is one of the most important osmolytes in the resistance of fungi to adversity. The present study used the low temperature sensitive V. volvacea strain V23 and the low temperature tolerant strain VH3 as test materials. Intracellular trehalose contents under low temperature stress in the two strains were measured by high performance liquid chromatography (HPLC). Quantitative real-time PCR (qPCR) analysis was carried out to study the transcriptional expression differences of enzymes related to trehalose metabolism. And trehalose solution was exogenously added during the cultivation of fruit bodies of V. volvacea. The effect of exogenous trehalose solution on the anti-hypothermia of fruit bodies was studied by evaluating the sensory changes under low temperature storage after harvest. The results showed that the intracellular trehalose content in VH3 was higher than that in V23 under low temperature stress. In the first 2 h of low temperature stress, the expression of trehalose-6-phosphate phosphatase (TPP) gene involved in trehalose synthesis decreased, while the expression of trehalose phosphorylase (TP) gene increased. The expression of TPP gene was almost unchanged in VH3, but it decreased dramatically in V23 at 4 h of low temperature stress. The expression levels of TPP and TP genes in VH3 was significantly higher than that in V23 from 6 h to 8 h of low temperature stress. TP gene may be a crucial gene of trehalose metabolism, which was more inclined to synthesize trehalose during low temperature stress. In addition, the sensory traits of V. volvacea fruit bodies stored at 4 °C were significantly improved by the application of exogenous trehalose compared with the controls. Thus, trehalose could help V. volvacea in response to low temperature stress and high content of it may be one of the reasons that why VH3 strain was more tolerant to the low temperature stress than V23 strain.
Introduction
Biological growth, reproduction, behavior, quantity and distribution are affected by temperature. Under low-temperature stimulation above zero, the fungus is under environmental osmotic stress. The physiological metabolism of the cells will lead to a series of stress responses to resist the osmotic stimulation of low temperature stress. Cells can prevent the outflow of intracellular water by promoting the accumulation of one or more specific solutes, maintaining osmotic pressure and ensuring the normal functioning of various physiological functions1. According to their characteristics, these particular solutes can be divided into two categories: inorganic ions, such as K+ and Na+, from the outside environment and organic solutes synthesized in the cell, including polyols and nitrogen compounds, such as soluble sugars (e.g., trehalose and sucrose), amino acids and their derivatives (e.g., proline and betaine)2,3. As an important osmotic adjustment substance, soluble sugar can increase the concentration of cytoplasm, reduce the freezing point and relieve the excessive dehydration of cytoplasm4. Trehalose, an important nonreducing disaccharide in soluble sugars, has a maximum content of more than 20% of the dry weight of organisms in fungi such as Saccharomycetes and Basidiomycetes5.
Trehalose is the most stable sugar in natural disaccharides6 and is widespread in organisms. This sugar was initially isolated in 1832 by WIGGERS in Claviceps purpurea from Secale cereale L.7,8. Under normal physiological conditions, the content of trehalose in the fungus is in a stable state, but the intracellular trehalose content typically changes when the cells are under osmotic stress9. The trehalose in fungi can be considered as a carbon storage source to provide energy after decomposing into glucose at spores germination or fruit body stages. This sugar can also be used as an effective cytoprotective agent under extreme temperature, radiation and dehydration environment10. However, the study of endogenous trehalose in edible fungus against the effect of low temperature stress is rare, and in the reported yeast studies, the higher levels of intracellular trehalose yeast strain often have a high tolerance of adversity caused by hypertonic conditions11,12. Trehalose is considered to be a natural protective agent in yeast13. In addition to yeast, there are also several exploratory studies using exogenous trehalose in the preservation of edible fungus strains, such as V. volvacea and Lentinula edodes, at low temperatures. However, exogenously added trehalose can also improve the frost resistance of edible fungi cells, but this effect warrants further verification.
The mushroom V. volvacea (Bull.ex.Fr.) Sing is an edible fungus with a distinctive savory flavor and high nutrient content, which is internationally recognized as “a very good protein source”14,15. Because V. volvacea is a high-temperature mushroom species, it is sensitive to low temperature. Under the conventional storage temperature of 4 °C, wilting, effluent or even decay phenomena occur, which limits the growth of the V. volvacea industry. Although there have been studies on the low-temperature autolysis of V. volvacea, its specific mechanism of action has not been clearly explained. Therefore, by reference to the amino acid sequence of trehalose metabolism-related enzymes in homology in other edibe fungus, the gene sequences of these enzymes were screened from the whole genome of V. volvacea by blast. Moreover, the metabolic pathway of trehalose in the V. volvacea was inferred, and the gene expression of the related enzymes was quantitatively analyzed. The content of intracellular trehalose in different treatments was determined. Furthermore, trehalose solution was exogenously added during the V. volvacea cultivation process, and fruit bodies were examined to explore the tolerance to low temperature. This study will provide scientific research ideas on the effect of trehalose during low temperature stress and a theoretical basis, however, the genetic mechanism of V. volvacea autolysis under low temperature, as well as genetic transformation of functional genes need to be further studied.
Materials and Methods
Test strains and collection of V. volvacea mycelia
V23 strain used in the present study, is a low temperature-sensitive strain. The VH3 strain was derived from V23 by mutagenesis, which is more tolerant to low temperature than V23. The two strains were supplied by the Institute of Edible Fungi, Shanghai Academy of Agricultural Sciences.
The V23 and VH3 strains were introduced into a solid medium and incubated at a constant temperature of 32 °C for 4 days. The strains were later transferred to a flask containing liquid medium and incubated at 150 rpm for 5 days at 32 °C.
After hypha culture, the flask was transferred to ice bath at 0 °C for 2, 4, 6 and 8 h. The blank control group was not transferred to ice bath. Sterile-filtered mycelia were rinsed several times with sterile water, blotted dry on sterile absorbent paper, collected in a sterile centrifuge tube, frozen in liquid nitrogen, and subsequently stored at −80 °C.
Trehalose extraction and quantitative determination
To determine the trehalose content, the mycelia were freeze-dried and ground to powder using a TissueLyser. 1 g of dry powder from V. volvacea mycelia was extracted by ultrasonic water bath in 100 mL distilled water for 1 h. The extracted solution was centrifuged at 13400 g for 10 min. The supernatant was filtered through a membrane filter (0.45 μm). Then a high performance liquid chromatography (HPLC) was developed for the determination of trehalose. By use of SUGAR SC1011 column (300 mm × 8 mm) as the chromatographic column and purified water as mobile phase, trehalose was separated completely at the refractive index detector with the flow rate of 0.5 mL/min at the temperature of 65 °C. The injection volume was 10 μL.
Extraction and reverse transcription RNA of V. volvacea mycelium
Total RNA from V. volvacea mycelia was extracted by using Trizol (TIANGEN, China) according to the manufacturer’s instructions. The RNA was dissolved in 50 μL water (DEPC pretreatment) and detected by 1% agarose gel electrophoresis. After removal of the genomic DNA (Table S1), the RNA was reverse transcribed into cDNA according to the instructions of the reverse transcription kit (PrimeScript RT reagent Kit with gDNA Eraser), used as a template for the fluorescence quantitative real-time PCR (qPCR) reaction, and stored at −20 °C until further use (Table S2).
Obtaining the target gene coding region, design and synthesis of qPCR primers
According to the accession number of the genes related to trehalose metabolism in the homologous species, the sequence of the coding region of each gene was obtained from NCBI, and the complete genome sequence information of V. volvacea (https://www.ncbi.nlm.nih.gov/) was aligned. In GenBank, blastx was used to compare the DNA sequence and eukaryotic protein to remove introns (GT-AG). The potential introns were removed to obtain the coding region of the reference and target genes. According to the stability and high copy number of the internal standard genes of V. volvacea strains under low temperature, tubulin alpha (TUB) was used as an internal standard to quantify the target gene according to prevous study16. Primer 5.0 software was used to design primers based on the nucleotide sequence of the coding region of genes involved in trehalose metabolism. The length of the primers was typically between 18 and 25 bp.
Amplification of the target fragment and the preparation of standard plasmid
The target fragment was amplified by conventional PCR (Table S3) and the product was examined by 1% agarose electrophoresis. The purified PCR product was ligated into the pGEM®-T vector to construct a standard plasmid to generate a standard curve for qPCR17 (Table S4).
Fluorescent quantitation of genes
The cDNA of V. volvacea mycelia treated at 0 °C for different time was used as the template, and the quantitative amplification of the target gene and internal standard gene was performed. Three replicates were set for each sample, and ddH2O was used as a blank control (amplification reaction system shown in Table 1). qPCR was performed using the ΔΔCT method.
Table 1.
Reaction system of qPCR.
| Reagent | Volume |
|---|---|
| SYBR Premix Ex TaqTM (2×) | 10 μL |
| Forward primer (10 μmo1/L) | 0.4 μL |
| Reverse primer (10 μmo1/L) | 0.4 μL |
| Rox | 0.4 μL |
| ddH2O | 6.8 μL |
| cDNA | 2 μL |
| Total | 20 μL |
The reaction conditions: 95 °C 20 s; 95 °C 5 s, 60 °C 15 s, 72 °C 15 s, total 40 cycles. The reaction fluid was prepared in an ice bath.
Exogenous trehalose spray treatment
Next, 95% cottonseed hulls and 5% lime were weighed according to the mass ratio, and the pH value was 10. After loading and inoculation, the culture materials were used for the fruit test.
Trehalose treatment group (T): spray 10% trehalose solution; each basket sprayed with approximately 100 mL. After 6 days of vaccination, water was added once. Each basket of water added was approximately 120 mL.
Blank control group (CK): spray water; each basket sprayed with approximately 100 mL. After 6 days of vaccination, water was added once. Each basket of water added was approximately 120 mL.
Storage after harvest
V. volvacea is in the egg shape, and the membrane is not cracked prior to harvesting. The fruit body was picked up and stored in a plastic tissue culture flask with a diameter of 9 cm and a height of 8 cm. The kraft paper was sealed and stored at 4 °C. Under a humidity of 80% for 0, 6, 12, 18, 24, 30, 36, 42, and 48 h.
Index determination and data analysis
Sensory index determination
Sensory index: sensory evaluation using the digital scoring method, according to the indicators listed in Table 2. The sum of the scores of each index is the final score.
Table 2.
Sensory evaluation criteria.
| Sensory index | Scores | |||
|---|---|---|---|---|
| 4 | 3 | 2 | 1 | |
| Hardness | Texture is full | Slightly inelastic | Soft texture | Soft texture |
| Hard | Hardness is acceptable | Not edible | ||
| Flavor | Odorless | Fresh mushroom flavor | Slightly fresh | No fresh mushroom flavors |
| Typical mushroom flavor | Slight odor | Mushrooms have obvious smell | Serious odor | |
| Market is acceptable | Limited application | Not edible | ||
| Color | No change | Small part of browning | Most browning | Whole mushroom browning |
| Effluent condition | None | Slight water | Water | Large amount of water |
| The surface is not sticky | The surface slightly sticky | The surface is sticky | The surface is very sticky | |
Weight loss rate determination
Weight loss rate = (fresh weight before storage-fresh weight after storage)/fresh weight before storage × 100%.
Morphometric determination
To measure the changes in the morphological trend of the fruit bodies during storage, the diameter of the bottom is defined as the bottom diameter in the erect state of the conical fruit body. The diameter of the boundary between brown and white in the middle of the fruit body is defined as the middle diameter. Each index measurement was obtained using vernier calipers. Each treatment group was selected after the determination of the average of 10 fruit bodies for statistics.
Bottom diameter reduction = (initial bottom diameter-post-storage bottom diameter)/initial bottom diameter × 100%.
Reduced middle diameter = (initial middle diameter-middle diameter after storage)/initial bottom diameter × 100%.
Data analysis of differences
All extracts and determinations were performed in 3 replicates, and the data were analyzed using SPSS (Version 11.0) software with an analysis difference at P = 0.05.
Data availablity
The dataset supporting the conclusions of this study is available and we have agreed to share the dataset. You can contact us by email to obtain the raw data in our manuscript.
Results
Changes of trehalose content in V. volvacea mycelia subjected to low temperature treatments at different times
The mycelia of V. volvacea exhibited optimal growth at 28–32 °C. Trehalose has been reported to play an important role in sustaining the growth of microorganisms under temperature stress18. However, little is known regarding the role of trehalose in the response to low temperature stress in V. volvacea. Therefore, we messured the contents of trehalose during low temperature stress by HPLC. During exposure to low temperature, the trehalose content in V23 and VH3 kept unchanged at the first 2 h compared with their respective control (0 h). However, the trehalose content in both strains decreased with storage time, especially decreased dramatically in V23 at 4 h of low temperature stress (Fig. 1). Under the same time of low temperature stress, the trehalose content in VH3 strain was significantly higher than that in V23 strain.
Figure 1.
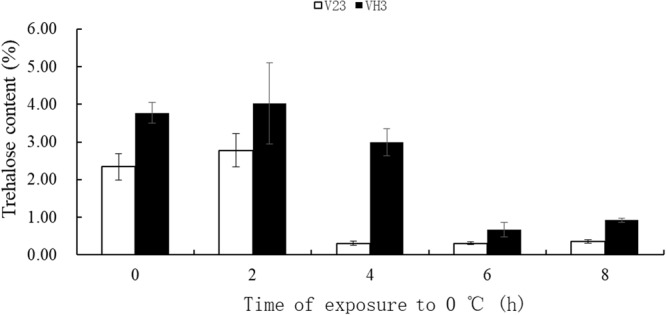
Changes in the trehalose content in mycelia of V. volvacea at 0 °C for 0, 2, 4, 6 and 8 h. The data are provided as the means ± SD from three independent replicates (n = 3).
Changes of genes expression related to trehalose metabolism
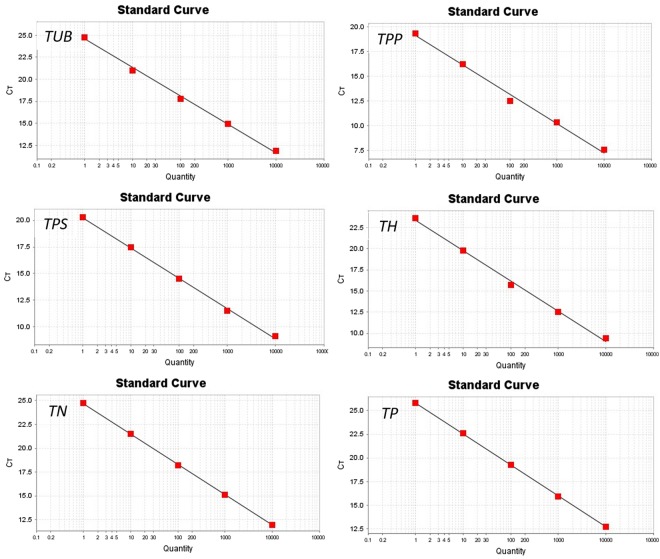
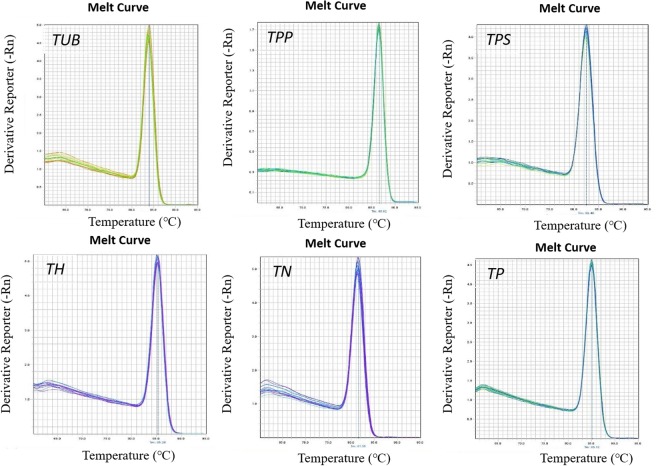
The quality of total RNA extraction directly affects the quality of template cDNA and the accuracy of quantitative PCR results. The quality of extracted total RNA electrophoresis test is shown in Fig. 2, revealing an intact, clear band, indicating that the RNA has not been degraded. After routine PCR amplification, the band of the target gene was consistent with the length of the primer-specific fragment (Table 3), and a single band was detected for each gene, indicating that the primer has good specificity and can be used for qPCR amplification. Cloning and sequencing results (Table 4) showed that the amplified target gene fragment sequence and the original sequence were precisely the same. The standard curve R2 of the target gene was greater than 0.99, indicating a good linear relationship (Fig. 3 and Table S5). The standard curve can be used as an accurate quantification of unknown samples. The single peak of the melting curve of the target gene and the internal standard gene (Fig. 4) indicated that the specificity of the amplification was strong and that the result was feasible.
Figure 2.
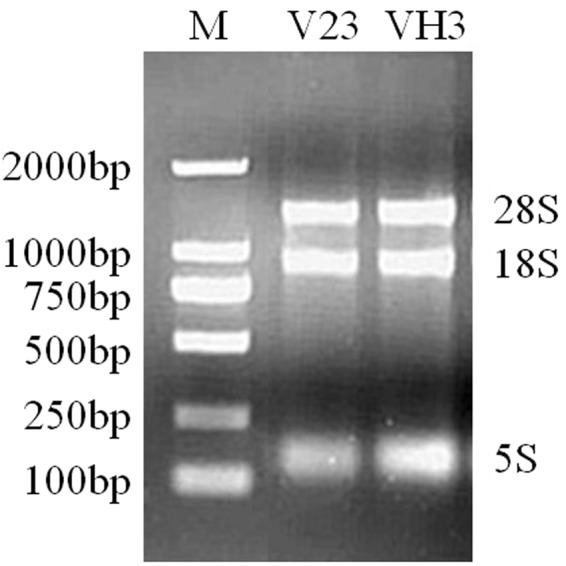
Electrophoresis of the total RNA of V. volvacea mycelia. The extracted total RNA was intact, the strip was clear, 28S was twice as bright as 18S, and the 5S dispersion was not obvious, indicating that the RNA was not degraded. Figure was edited as described in methods, for original figure referred to Figure S1.
Table 3.
Primers used for qPCR.
| Primer | Base number (bp) | Gene sequence |
|---|---|---|
| TPS-F | 20 | 5′-GATTACCACCTGTTACTTGC-3′ |
| TPS-R | 19 | 5′-GACGAGCGTAGGAGTATGT-3′ |
| TPP-F | 21 | 5′-GATCTGGAAACGGGATCTGTC-3′ |
| TPP-R | 21 | 5′-CCCACTCAACAACCAAACCTC-3′ |
| TH-F | 21 | 5′-GGCGAAGAAGGAAACACACAC-3′ |
| TH-R | 25 | 5′-CGTAGCATTACCACCATTAACAACA-3′ |
| TN-F | 24 | 5′-GTATTCCGTTTATTGTGCCTGGTG-3′ |
| TN-R | 20 | 5′-TATGAGCGACTGCCGTTGAG-3′ |
| TP-F | 20 | 5′-TCAGGTTCCTCCGTGCTCTT-3′ |
| TP-R | 22 | 5′-TTGTGGTTGTTCTTGGTGGTTC-3′ |
Table 4.
Cloning and sequencing results.
| Gene | Cloning and sequencing results | Sequence length (bp) |
|---|---|---|
| TUB | CCAACACTACCGCTATCTCCTCGGCTTGGAGTCGCCTTGATCACAAGTTCGACCTCCTCTATTCGAAGCGTGCTTTCGTGCATTGGTACGTTGGTGAGGGTATGGAGGAAGGTGAA | 116 |
| TPP | GATTACCACCTGTTACTTGCCCCGAGGATGATCCGTGAACTCATCCCTGAAGCTGTCCTCGGCTTGTTCGTGCACACACCATTCCCAAGCAGCGAAGTTTTCCGATGCCTACCTCGCCGCAAGGAGATACTCGATGGCATGCTAGGCGCCAACCTTGTTTGCTTCCAAACATACTCCTACGCTCGTC | 187 |
| TPS | GATCTGGAAACGGGATCTGTCGAGGCCGTTGGTCAATGATACGACCGGGCTGCCGCTGGAGGTGTTGGAGGATCTGAAAAAGTTGGCGGAGGATGAGAGGAGGAATGAGGTTTGGTTGTTGAGTGGG | 127 |
| TH | GGCGAAGAAGGAAACACACACAATGCAACCCTCACCGGACCTGAAGCTGATATCAACACTTTGGAAGGCACTGTTGTTAATGGTGGTAATGCTACG | 96 |
| TN | GTATTCCGTTTATTGTGCCTGGTGCCCGCTTCAATGAGCTTTACAACTGGGATTCCTACTTCATCTCGTTGGGCCTGCTGGTCGACGGACAAGTGAGCATGGCCAAAGGCATGGTCGACCACTTCATCTTTGAAATCAAACACTACGGCAAAATCCTCAACGGCAGTCGCTCATA | 175 |
| TP | TCAGGTTCCTCCGTGCTCTTGACGTTAATGCAGCTTGGTATGTGCCAAACCCATCACCAAGCGTCTTTCGAACCACCAAGAACAACCACAA | 91 |
Figure 3.
Standard curves of the target genes. The standard curve can be used as an accurate quantification of unknown samples. The standard curve R2 of the target gene was greater than 0.99, the slope K value satisfies |ΔK| ≤ 0.1 (Table S6). qRT-PCR was therefore performed using the ΔΔCT method. Biology duplications are three times.
Figure 4.
Melting curves of the target genes and the internal standard gene. The single peak of the melting curve of the target gene and the internal standard gene indicated that the specificity of the amplification was strong and that the result was feasible.
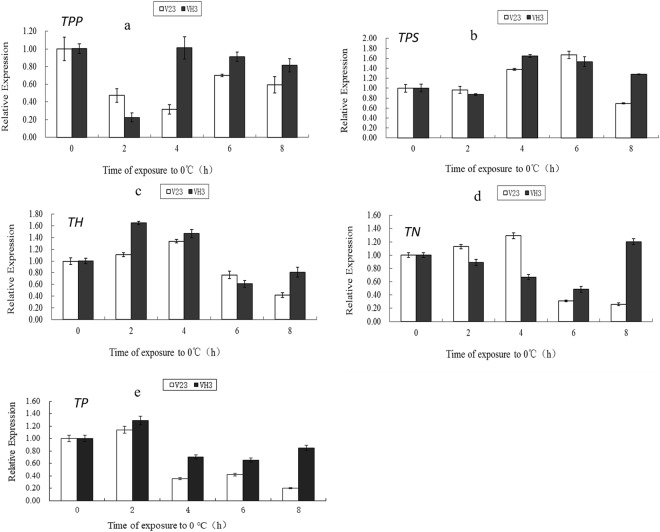
The relative expression of each gene in the trehalose metabolic pathway was determined for strains V23 and VH3. The results (Fig. 5) showed that the expression of TPP gene (Fig. 6a and Table S7) in strain V23 decreased significantly after low temperature treatment for 2 h. The gene expression decreased with prolonged low temperature treatment. However, the expression of TPP gene increased at 6 h but it was still lower than that at 0 h. At low temperature for 8 h, the gene expression also decreased. During the entire low temperature treatment, the expression level of TPP gene in V23 strain was lower than that in the 0 h untreated strain. The TPP gene of V. volvacea strain VH3 decreased significantly after 2 h at low temperature. However, the gene expression significantly increased after cold treatment for 4 h. With the extension of the low temperature treatment time, TPP gene expression showed a slight downward trend. As shown in Fig. 6b and Table S8, the expression level of trehalose-6-phosphate synthase (TPS) gene of the V23 strain of V. volvacea slightly decreased at 2 h compared with the 0 h. With the extension of low temperature treatment, the gene expression increased. At low temperature for 8 h, TPS gene expression reduced significantly. The expression level of TPS gene of strain VH3 decreased at 2 h of low temperature treatment. With the extension of low temperature treatment, the gene expression increased at 4 h or 6 h. At low temperature for 8 h, TPS gene expression decreased but it was still higher than that at 0 h. As shown in Fig. 6c and Table S9, the trehalase (TH) gene expression of the V23 strain of V. volvacea slightly increased at 2 h. The gene expression increased with prolonged cold treatment at 4 h. However, the TH gene expression decreased at 6 and 8 h. The TH gene of V. volvacea strain VH3 was significantly increased after 2 h at low temperature. The gene expression was reduced at 4 h but it was still higher than that at 0 h. With the extension of low temperature treatment, TH gene expression decreased at 6 h or 8 h. As shown in Fig. 6d and Table S10, the alpha-trehalose-neutral trehalase (TN) gene expression in the V23 strain of V. volvacea increased after 2 and 4 h compared to that at 0 h and later decreased with the prolongation of low temperature treatment. With the delay of low temperature, the TN gene in V. volvacea strain VH3 showed a decreasing trend, and the expression level at 6 h decreased to 0.48 times of that detected at 0 h. As shown in Fig. 6e and Table S11, the expression of trehalose phosphorylase (TP) gene was highest at 2 h both in V23 strain and VH3 strain. Compared with their respective control (0 h), the expression of TP gene had a significant decline after 2 h of low temperature stress, however, the TP gene expression in VH3 strain was significantly higher than that in V23 strain during 4–8 h of low temperature stress.
Figure 5.
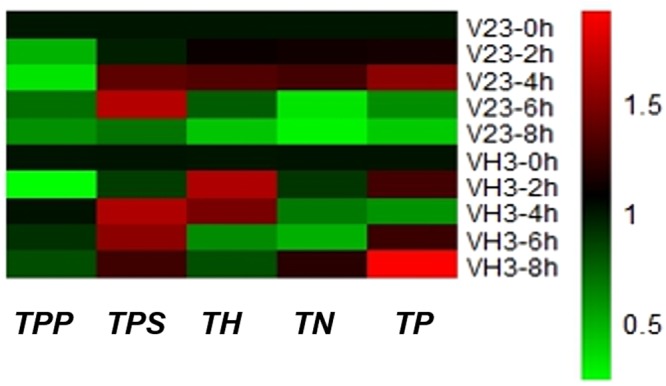
Overall changes in the expression of each gene in the trehalose metabolic pathway under low temperature stress. Red means up-regulation, green means down-regulation.
Figure 6.
Relative expression of each gene in the trehalose metabolic pathway of strains V23 and VH3. Values are means ± standard errors (n = 3). The letters in the picture represent expression of each gene, i.e. (a) TPP; (b) TPS; (c) TH; (d) TN; (e) TP.
Effect of exogenous trehalose on the low temperature tolerance of V. volvacea fruit bodies
Sensory
The sensory quality of the V. volvacea fruit bodies under different treatment conditions at 4 °C is shown in Fig. 7. The morphological changes of strains V23 and VH3 were observed and recorded every six hours, and the evaluation indexes in Table 2 were adopted. The sensory quality score is shown in Fig. 8a,b. The sensory score of trehalose treatment group (T) were significantly higher than that of the blank control (CK) (P < 0.05) both in V23 and VH3, indicating that exogenous trehalose treatment had a good effect on the V. volvacea fruit bodies during storage.
Figure 7.
Sensory quality of the V. volvacea fruit bodies under different treatment conditions at 4 °C. Every six hours, we took a picture of the sensory images of the fruit bodies and observed the morphological changes of V23 and VH3 at 4 °C. Among them, T was the trehalose treatment group, and CK was the control group.
Figure 8.
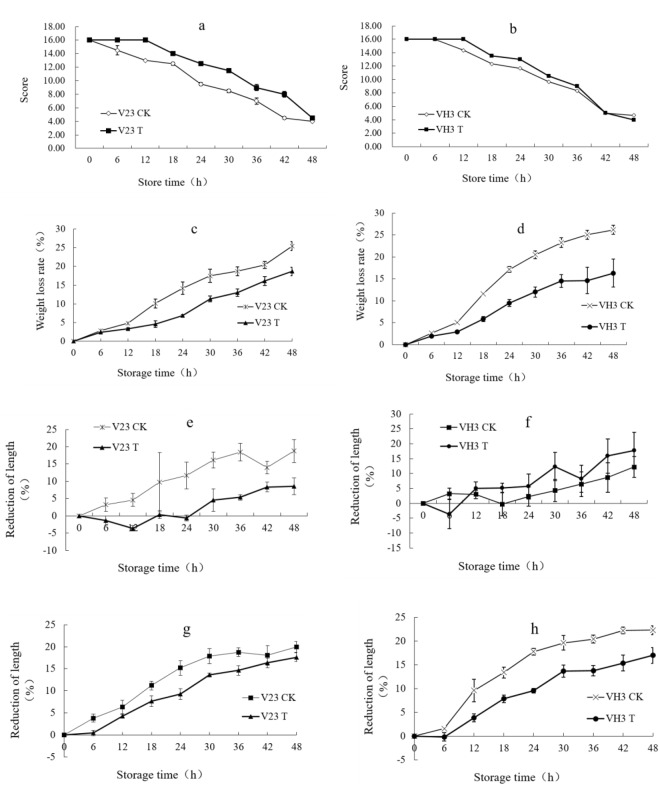
Sensory indices of the V23 and VH3 strains: trehalose treatment group (T), and blank treatment group (CK). Trend chart of sensory quality. (a, b) Weight loss rate. (c, d) Bottom diametershortening rate. (e, f) Middle diameter shortening rate (g, h). Values are means ± standard errors (n = 3). Vertical bars indicate the standard errors of the means, where the bars exceed the size of the symbol used.
Weight loss rate
Under 4 °C storage, the weight loss rate of the trehalose treatment group (T) and blank treatment group (CK) of V23 strain fruit bodies both showed an increasing trend (Fig. 8c). The weight loss rate of blank treatment group (CK) significantly increased at 12 h and stabilized at 30 h. The weight loss rate of trehalose treatment (T) significantly increased at 18 h and remained stable at 30 h. The weight loss rate of the blank treatment group (CK) was higher than that of the trehalose treatment group at all time points except at 6 h.
The weight loss rate of the trehalose treatment group (T) and blank treatment group (CK) of VH3 strain fruit bodies both showed an increasing trend (Fig. 8d). The weight loss rate of the blank treatment group significantly increased at 6 h and reached a stable trend at 42 h. The weight loss rate of the trehalose treatment group significantly increased at 12 h and reached a stable trend at 36 h. The weight loss rate of the blank treatment group (CK) was higher than that of the trehalose treatment group (T) at all time points except at 6 h.
Bottom diameter shortening rate
Under the storage at 4 °C, the shortening rate of the bottom diameter of the trehalose-treated group (T) and blank-treated group (CK) of V23 strain showed an upward trend (Fig. 8e). The blank treatment group showed a gradual increase at each time point, of which the shortening rate decreased at 42 h. The shortening rate of the trehalose-treated group after the first 18 h of storage was negative, and it increased from 30 h to 48 h of storage. The shortening rate of the blank treatment group was higher than that of trehalose treatment group at all time points.
The shortening rate of the bottom diameter of the trehalose-treated group (T) and blank-treated group (CK) of VH3 strain showed an upward trend (Fig. 8f). The blank treatment group showed a non-significant increase at each time point, of which the shortening rate decreased at 18 h. The trehalose treatment group had a negative shortening rate at the first 6 h of storage, and then increased at all time points. However, shortening rate decreased at 36 h. The shortening rate of the blank treatment group had no significant difference with the trehalose treatment group at all time points.
Middle diameter shortening rate
Under the storage at 4 °C, the shortening rate of the middle diameter of treatment group (T) and blank treatment group (CK) of V23 strain showed an upward trend (Fig. 8g). The blank control group was significantly increased at 6 h and reached a stable trend after 30 h. The trehalose treatment group was significantly increased at 12 h, indicating that the fruit bodies began to significantly change after 12 h. V23 strains of fruit bodies, trehalose treatment group before 36 h at various time points, middle diameter shortening rate were significantly lower than those in the blank control group. After 36 h, both sets of fruit bodies atrophied to an unacceptable state, with no significant difference.
The middle diameter shortening rate of VH3 strain fruit bodies of treatment group (T) and blank treatment group (CK) showed an upward trend (Fig. 8h). The blank control group at 12 h significantly increased and subsequently maintained a steady trend after 30 h. The trehalose treatment group also significantly increased at 12 h and after 30 h to maintain a relatively stable trend. All time points were significantly lower than the blank group except at 6 h of storage, indicating that the trehalose treatment of fruit bodies of VH3 strain has a positive effect on, reducing the middle diameter shortening rate both in V23 and VH3.
Discussion
V. volvacea is a high-temperature mushroom, therefore the mycelia growth and the development of fruit body need relatively high temperatures. Such phenomena as softening, seepage, browning and other autolysis are observed after the fruit bodies in conventional 4 °C reservations. The strong respiration of V. volvacea cells at the maturity stage and the action of polyphenol oxidase and protease lead to autolysis. However, there are no reports that clearly explain the mechanism of action of this phenomenon19. Therefore, we analyzed the data of the low-temperature performance of the V. volvacea and examined that the genes expression involved in trehalose-related enzymes. Trehalose exists in a variety of different metabolic pathways20–23. According to the amino acid sequences of enzymes related to trehalose metabolism in homologous species, such as Coprinus cinereus and Laccania bicolor, the amino acid sequences of the enzymes related to trehalose metabolism were compared in NCBI, and the gene sequences of these enzymes were screened from the genome of V. volvacea. The trehalose metabolic pathway in V. volvacea, as shown in Fig. 9, was estimated. On the one hand, the relative transcriptional expression levels of enzymes involved in the trehalose metabolic pathway were determined in mycelia of V. volvacea treated with low temperature for various periods of time. On the other hand, the sensory evaluation indices of fruit bodies stored at 4 °C after harvest were examined, which were applied by exogenous trehalose during the cultivation period.
Figure 9.
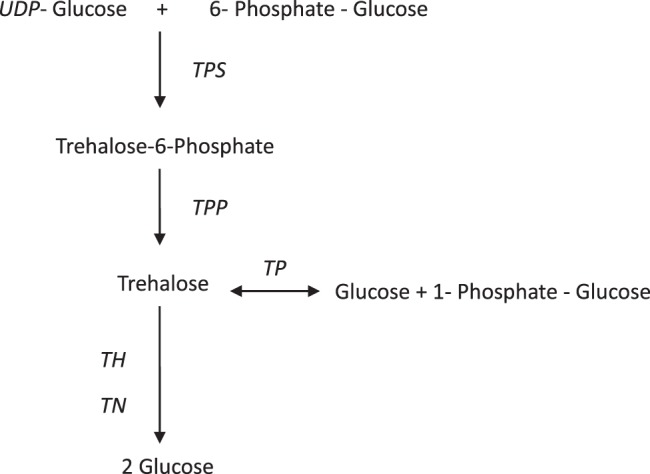
Trehalose metabolic pathway of V. volvacea.
The relationship between the key genes of trehalose metabolic pathway and the low-temperature tolerance of V. volvacea
The mushroom V23 strain is a low-temperature sensitive strain. The low-temperature tolerance of VH3 strain is better than that of V23 strain. The two strains were different in the expression of each enzyme involved in trehalose metabolism when stimulated by hypothermia. The analysis of gene expression changes of V. volvacea mycelia under low temperature at different times showed that the metabolism of trehalose may have a postive effect on the adaptation of V. volvacea to low temperature stress.
Compared with the untreated group (0 h), the changes of various genes expression under low temperature stress in V23 and VH3 were shown in Fig. 6. During the first 2 h of low temperature stress, there was no significant difference in the expression of TPS gene involved in trehalose synthesis, while the expression of TPP gene decreased significantly in both strains. The expression of TN gene in V23 strain and TH gene in VH3 strain, involved in the degradation of trehalose, showed an upward trend in 4 h of low temperature stress. However, the intracellular trehalose was not significantly changed at 2 h of low temperature stress. Perhaps because the expression of the TP gene, which increased both in V23 and VH3, was more biased towards the synthesis pathway20,24, that is to say the TP enzyme may be a key enzyme involved in the trehalose synthesis during the early stage of exposure to low temperature stress, as reported that the TPS/TPP pathway plays an important role during the middle and later stages20. Notably, the TPS gene significantly increased both in V23 and VH3 at 4 h of low temperature stress. What is more, the expression of TPS gene in VH3 was significantly higher than that in V23. The expression of TPP gene was almost unchanged in VH3, but it decreased dramatically in V23. The expression of TH gene increased significantly in both strains, while the expression of TN gene increased in V23 strain and decreased in VH3 strain except for at 8 h. The expression level of TP gene decreased both in V23 and VH3 with time, and the expression level of it was higher in VH3 than that in V23. When V. volvacea mycelia was treated under low temperature at 4 h, there was no significant change in trehalose accumulation in VH3 strain, while the decrease of trehalose content in V23 strain was observed, which was consistent with changes of genes expression of enzymes related to trehalose metabolism. For 6 h and 8 h of low temperature stress, the expression level of TPS gene increased significantly (except for V23 at 8 h). The expression of TPP gene showed a significant decrease in V23 compared with its untreated group (0 h), and the expression level of it in VH3 was significantly higher than that in V23. The expression of TH gene and TN gene began to decrease in V23 and VH3. However, at the low temperature treatment of 8 h, the expression level of TN gene increased significantly in VH3 strain. The expression level of TP gene decreased significantly in both strains during that time, while it was higher in VH3 than that in V23. During the whole process, the expression of key genes in trehalose metabolic pathway was consistent with the changes of trehalose content in mycelia of V. volvacea. The expression of trehalose synthesis related genes (TPS, TPP and TP) in VH3 strain was higher than that in V23 strain at most time of low temperature stress, and the trehalose content in VH3 strain was higher than that in V23 strain, which may be one of the reasons that why VH3 strain was more tolerant to the low temperature stress than V23 strain, and it could also prove that trehalose is beneficial for fungi in response to low temperature stress.
From the recent results of the enzymatic functions of trehalose metabolism in fungi, the above results on the function of key genes in the trehalose metabolic pathway of V. volvacea were confirmed. For example, Guo Yonghao25 and Chen Hongge et al.26 constructed the pUC18 and pET-30a vectors of the trehalose synthase gene TPS1 from Saccharomyces uvarum and Saccharomyces cerevisiae, respectively, which were successfully expressed in Escherichia coli. Song et al.27 constructed antagonistic yeast strains overexpressing TPP to enhance the ability of resisting unfavorable environments. Due to the independent catalytic action of TH and TN, the fungal extracellular and cytoplasmic trehalose molecules were respectively decomposed. Therefore, the case that these two types of enzymes coexist in the organism and work together to ensure the utilization of cells for the decomposition of exogenous trehalose is typical28. NWAKA et al.29 found a TH mutant in S. cerevisiae. Trehalose utilization within the cell was not affected. However, exogenous trehalose could not grow normally as a carbon source. In contrast, TN gene mutants were normally grown when trehalose was contained in the medium. However, the TH mutant of the filamentous fungus Neuropora crassa did not grow on the substrate of the trehalose carbon source. This mutation effectively degrades cytoplasmic trehalose as wild-type strains30. FOSTER et al.28 showed that both TN of Aspergillus nidulans and Magnaporthe grisea can decompose intracellular trehalose. In the 1990s, to demonstrate the relationship between trehalose content and yeast tolerance, studies investigated the effect of trehalose hydrolase gene regulation on yeast cell tolerance by genetic engineering, for example, by employing gene knockout. The logarithmic growth phase strain and normal strain of the TH-deficient mutant, TN-deficient mutant and mutant strain with simultaneous deletion of TH and TN were frozen at −20 °C for 3 weeks, and the viability count and the frozen dough fermentation ability were respectively measured. The results showed that the trehalose accumulation and cell density of all mutant strains were higher than those of the parental strain. Frozen yeast dough fermentation mutant strains was higher than that in the parent, indicating that mutant strains greatly improved resistance to freezing31. HAN et al.32 cloned the Pleurotus sajorcaju TP gene. The gene in mycelium and fruit bodies of the cap and stigma were expressed and transferred the TPS gene and TPP gene double mutant to yeast cells, and these yeast cells grew in the glucose matrix. Moreover, the amount of trehalose in cells transfected with the TP gene was 2 to 2.5 times higher than that of the TPS gene, and double mutant yeast cells lacking TPP gene or empty vector control yeast cells. However, the catalytic activity of TP is reversible. Studies have reported that its catalytic orientation is more likely inclined to the trehalose synthesis process24, which is consistent with the results of the present study. However, the specific catalysis of trehalose in V. volvacea still needs to be further studied.
Effect of intracellular trehalose on the low-temperature tolerance of the mycelia of V. volvacea
Trehalose can help organisms resist adverse circumstances33. Once the organism perceives external signals, mechanisms related to trehalose metabolism are triggered and the metabolic pathways are adjusted34. In this study, the observed changes in trehalose content indicated that the level of intracellular trehalose in the mycelia of V. volvacea distinctively decreased during this time period when the fungi were exposed to low temperature (0 °C). High trehalose content was found in mycelia of V. volvacea during the first 2 hours of low temperature stress. However, the expression of trehalose synthesis related genes (TPS/TPP) was not very high in the initial stage of low temperature stress. The expression of TP gene increased significantly at 2 h of low temperature stress compared to their respective blank control both in V23 and VH3, which may contribute to the high trehalose content determined during early period of low temperature stress. In terms of the change of fruit body corruption after the low temperature of V. volvacea, VH3 is more durable freshness than V23. The content of trehalose in VH3 was always higher than that of V23, which may contribute to the stronger tolerance of VH3 to low temperature stress. The trehalose content in the mycelia of Pleurotus eryngii var. tuoliensis and F. velutipes exposed to 37 °C heat shock increased during the early stage of processing34,35. So the trehalose in edible fungi may have a positive effect on tolerance to temperature stress.
Effect of extrogenous trehalose on the low-temperature tolerance of the fruit body of V. volvacea
Several studies have suggested that exogenously added trehalose solution can enhance the tolerance of organism to temperature stress36,37. In the present study, for the first time, we attempted to apply exogenous trehalose solution during the cultivation of fruit bodies of V. volvacea and to explore whether the anti-hypothermia of the fruit body can be improved.
With the prolongation of storage time, the comprehensive scores of sensory qualities, including hardness, flavor, color and water effluent, showed a declining trend when the V. volvacea fruit bodies were stored at 4 °C. In the untreated blank group, the comprehensive score of sensory quality was significantly lower than that of trehalose solution-treated group at 4 °C both in V23 and VH3. In the blank control group, the comprehensive score of various sensory indices of V23 strain was lower than that of the VH3 strain. The sensory quality results showed that the addition of trehalose played a positive role on the fruit bodies under low temperature storage.
The weight loss rate of V. volvacea fruit bodies showed an upward trend with storage time prolonged at 4 °C. The weight loss rate of fruit bodies in trehalose treatment group was significantly lower than that of the blank control group. These results indicated that exogenous trehalose had a relevance to the decreased weight loss rate during the low temperature storage period of V. volvacea fruit bodies.
During the initial 24 h, the bottom diameter shortening rate of trehalose treatment was negative in V23, presumably due to the cotinued growth of fruit bodies; after 24 h, the fruit bodies further wilted. As a result, the bottom diameter shortening rate steadily increased. The bottom diameter shortening rate of the fruit bodies of VH3 strain in trehalose treatment group had no significant difference with the blank group at each time point. The bottom diameter shortening rate of V23 strain fruit bodies in the trehalose treatment group was lower than that in the blank control group, which indicated trehalose treatment had a more positive effect on the reduction of the diameter at the bottom of the fruit bodies of low temperature sensitive strain (V23) than low temperature tolerant strain (VH3).
With the 4 °C low-temperature storage time, the middle diameter shortening rate of V. volvacea fruit bodies showed an upward trend, indicating that fruit bodies shrinkage after dehydration. The middle diameter shortening rate of trehalose treated groups in V23 or VH3 strain was significantly lower than that of the respective blank control group. This finding indicated that the exogenous trehalose added had a good effect on the decrease of middle diameter shortening rate of V. volvacea fruit bodies stored at 4 °C.
In summary, during cultivation, V. volvacea was sprayed with water containing the osmolyte trehalose, which improved the tolerance of fruit bodies stored under 4 °C. Sensory traits of various indicators were slower reduced to varying degrees in the trehalose treatment group compared with the control group. The autolysis of fruit bodies under low temperatures is primarily manifested as softening, liquefaction, odor, and weight loss. Bottom diameter shortening rate measured the softening level of fruit bodies. Data analysis showed that the sensory quality of fruit bodies in the trehalose treatment group decreased to levels less than that in the blank control group. Speculated trehalose treatment of fruit bodies of V. volvacea tolerance to 4 °C has a role, consistent with the findings of HIRASAWA et al.38, that the direct addition of exogenous trehalose increased the freeze tolerance of yeast. In addition, DINIZ-MENDES et al.39 also found that treatment with exogenous trehalose, Saccharomyces cerevisiae also showed enhanced cell viability under freezing conditions. MERIC40 and SHIMA et al.13 showed that the intracellular trehalose content of 4~5% protected yeast cells from freezing injury. Thus, the addition of exogenous trehalose could improve the tolerance of V. volvacea fruit bodies to low temperature stress, especially for the low temperature sensitive strain V23.
Conclusions
The trehalose metabolic pathway-related genes, and the content of trehalose in different treatments was determined. Furtherly, the addition of exogenous trehalose to examine the role of this sugar under the low temperature stress of V. volvacea fruit bodies revealed that trehalose could enhance the tolerance of this mushroom to low temperature stress. The intracellular trehalose content was significantly higher in VH3 strain than that in V23 strain, which may help VH3 to be more tolerant to low temperature stress. And TP gene may be a key gene of trehalose metabolism, which could be inclined to synthesize trehalose during low temperature stress. The application of exogenous trehalose solution during the cultivation of V. volvacea could improve the anti-hypothermia of fruit bodies stored at 4 °C, which further verified the positive correlation between trehalose and V. volvacea tolerance to low temperatures, which laid the foundation for further research on prolongation of the preservation of V. volvacea strains and fresh keeping of its fruit bodies after harvest under conventional low temperature.
Electronic supplementary material
Acknowledgements
This work was financially supported through grants from the Shanghai Agricultural Commission Program (2014-7-1-5 and 2015-5-6) and the National Natural Science Foundation of China (Grant No. 31301828).
Author Contributions
Xu Zhao, Xiaoxia Song, Yapeng Li, Changxia Yu, Yan Zhao, Ming Gong, Xuexiang Shen and Mingjie Chen developed the study idea, concept, and the overall study design in addition to planning and coordinating the study. Xu Zhao, Xiaoxia Song and Yapeng Li performed the plans. Xu Zhao wrote the paper. Yan Zhao edited the paper. Changxia Yu, Ming Gong and Xuexiang Shen contributed to the sample analysis. Yan Zhao and Mingjie Chen supervised the study. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Xu Zhao and Xiaoxia Song contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-29116-z.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yan Zhao, Email: jiandan289@126.com.
Mingjie Chen, Email: mjchen@saas.sh.cn.
References
- 1.Vinocur B, Altman A. Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr Opin Biotechnol. 2005;16:123–132. doi: 10.1016/j.copbio.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Zhang XQ, Cao JW, Zhai C, Cheng JJ. Cloning and expression of a osmoregulatory gene proB from halotolerant Bacillus subtilis. Acta Microbiologica Sinica. 2002;42:163–168. [PubMed] [Google Scholar]
- 3.Seki M, Umezawa T, Urano K, Shinozaki K. Regulatory metabolic networks in drought stress responses. Current Opinion in Plant Biology. 2007;10:296. doi: 10.1016/j.pbi.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Peng LX, Li DQ, Shu HR. Osmotic adjust action of plant under osmotic stress. Tianjin Agricultural Sciences. 2002;8:40–43. [Google Scholar]
- 5.Li, Q. & Yuan, Q. S. The property of trehalose and its application. Chinese Journal Biochemical Pharmaceutics, 231–233 (1995).
- 6.Crowe JH, Crowe LM, Chapman D. Preservation of membranes in anhydrobiotic organisms: the role of trehalose. Science. 1984;223:701–703. doi: 10.1126/science.223.4637.701. [DOI] [PubMed] [Google Scholar]
- 7.Higashiyama T. Novel functions and applications of trehalose. Pure & Applied Chemistry. 2002;74:1263–1269. doi: 10.1351/pac200274071263. [DOI] [Google Scholar]
- 8.Elbein AD, Pan YT, Pastuszak I, Carroll D. New insights on trehalose: a multifunctional molecule. Glycobiology. 2003;13:17R–27R. doi: 10.1093/glycob/cwg047. [DOI] [PubMed] [Google Scholar]
- 9.Gancedo C, Flores CL. The importance of a functional trehalose biosynthetic pathway for the life of yeasts and fungi. FEMS Yeast Res. 2004;4:351–359. doi: 10.1016/S1567-1356(03)00222-8. [DOI] [PubMed] [Google Scholar]
- 10.Wannet WJ, et al. Purification and characterization of trehalose phosphorylase from the commercial mushroom Agaricus bisporus. Antonie Van Leeuwenhoek. 2000;77:215–222. doi: 10.1023/A:1002450221778. [DOI] [PubMed] [Google Scholar]
- 11.Gancedo C, Flores CL. The importance of a functional trehalose biosynthetic pathway for the life of yeasts and fungi. Fems Yeast Research. 2010;4:351–359. doi: 10.1016/S1567-1356(03)00222-8. [DOI] [PubMed] [Google Scholar]
- 12.Hounsa CG, Brandt EV, Thevelein J, Hohmann S, Prior BA. Role of trehalose in survival of Saccharomyces cerevisiae under osmotic stress. Microbiology. 1998;144(Pt 3):671. doi: 10.1099/00221287-144-3-671. [DOI] [PubMed] [Google Scholar]
- 13.Shima J, et al. Breeding of freeze-tolerant baker’s yeast by the regulation of trehalose metabolism. Japan Agricultural Research Quarterly. 2000;34:257–260. [Google Scholar]
- 14.Luo, G. L. The nutritional value of V. volvacea. Sichuan Food and Fermentation, 49–53 (1995).
- 15.Orilto CA, Carangal A. Nitrogenous constituents of Volvariella volvacea. Phip Agr. 1961;45:29–35. [Google Scholar]
- 16.Wan, P. Analysis of Volvariella volvacea expression profile and the key gene of related cold-stress metabolic pathways expression. Nanjing Agricultural University (2012).
- 17.Wang H, Chen MJ. The establishment of the standard quality granule and standard curve of Cor3 gene in real-time fluorescence quantitative PCR. Acta Edulis Fungi. 2007;14:16–19. [Google Scholar]
- 18.Singer M, Lindquist S. Thermotolerance in Saccharomyces cerevisiae: the Yin and Yang of trehalose. Trends in Biotechnology. 1998;16:460–468. doi: 10.1016/S0167-7799(98)01251-7. [DOI] [PubMed] [Google Scholar]
- 19.Xu ZX, Li GW, Zhen YL, Bao J. Cloning and expression of grifola frondosa trehalose synthase gene in Escherichia coli. Acta Microbiologica Sinica. 2004;44:540–542. [Google Scholar]
- 20.Liu JH, Shang XD, Liu JY, Tan Q. Changes in trehalose content, enzyme activity and gene expression related to trehalose metabolism in Flammulina velutipes under heat shock. Microbiology. 2016;162:1274–1285. doi: 10.1099/mic.0.000251. [DOI] [PubMed] [Google Scholar]
- 21.Cross M, et al. Enzyme characteristics of pathogen-specific trehalose-6-phosphate phosphatases. Sci Rep. 2017;7(2015):2. doi: 10.1038/s41598-017-02220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garg AK, et al. Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:15898–15903. doi: 10.1073/pnas.252637799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu XM, Huang CY, Chen Q, Xiang-Li WU, Zhang JX. Study on the metabolic pathway of trehalose in Pleurotus pulmonarius during heat stress recovery. Scientia Agricultura Sinica. 2013;46:5188–5195. [Google Scholar]
- 24.Ren YYEA. Study on the gene expression and molecular biological properties of the hydrophobic trehalose phosphorylase in Tengchong. Science in China Ser. C Life Sciences. 2004;34:409–415. [Google Scholar]
- 25.Guo, Y. H. The expression and cloning reseach of gene tps1 in yeast. Henan Agricultural University (2005).
- 26.Chen HG, et al. Expression of trehalose-6-phophase synthase gene in Escherichia coli and tests of its function. Jourmal of Agricultural Biotechnology. 2005;13:402–403. [Google Scholar]
- 27.Song HC, Zhang Y, Fu WY, Lin MH, Shi XQ. Construction of vector for high trehalase by overexpression of tps1 in antagonistic yeast Pichia membranefaciens. Chinese Agricultural Science Bulletin. 2011;27:352–357. [Google Scholar]
- 28.Foster AJ, Jenkinson JM, Talbot NJ. Trehalose synthesis and metabolism are required at different stages of plant infection by Magnaporthe grisea. Embo Journal. 2003;22:225–235. doi: 10.1093/emboj/cdg018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nwaka S, Mechler B, Holzer H. Deletion of the ATH1 gene in Saccharomyces cerevisiae prevents growth on trehalose. Febs Letters. 1996;386:235–238. doi: 10.1016/0014-5793(96)00450-4. [DOI] [PubMed] [Google Scholar]
- 30.Bonini BM, Neves MJ, Jorge JA, Terenzi HF. Effects of temperature shifts on the metabolism of trehalose in Neurospora crassa wild type and a trehalase-deficient (tre) mutant. Evidence against the participation of periplasmic trehalase in the catabolism of intracellular trehalose. Biochimica Et Biophysica Acta General Subjects. 1995;1245:339–347. doi: 10.1016/0304-4165(95)00098-4. [DOI] [PubMed] [Google Scholar]
- 31.Lv Y, Xiao DG, He DQ, Guo XW. Construction and stress tolerance of trehalase mutant in Saccharomyces cerevisiae. Acta Microbiologica Sinica. 2008;48:1301–1307. [PubMed] [Google Scholar]
- 32.Han SE, et al. Cloning and characterization of a gene encoding trehalose phosphorylase (TP) from Pleurotus sajor-caju. Protein Expression & Purification. 2003;30:194–202. doi: 10.1016/S1046-5928(03)00104-9. [DOI] [PubMed] [Google Scholar]
- 33.Liang Z, et al. Expression of the spinach betaine aldehyde dehydrogenase (BADH) gene in transgenic tobacco plants. Chinese Journal of Biotechnology. 1997;13:153–159. [PubMed] [Google Scholar]
- 34.Liu J, Shang X, Liu J, Tan Q. Changes in trehalose content, enzyme activity and gene expression related to trehalose metabolism in Flammulina velutipes under heat shock. Microbiology. 2016;162:1274. doi: 10.1099/mic.0.000251. [DOI] [PubMed] [Google Scholar]
- 35.Kong WW, et al. Nitric oxide is involved in the regulation of trehalose accumulation under heat stress in Pleurotus eryngii var. tuoliensis. Biotechnology Letters. 2012;34:1915–1919. doi: 10.1007/s10529-012-0988-2. [DOI] [PubMed] [Google Scholar]
- 36.Luo Y, Yang XH, Wang W. Trehalose-mediated signal transduction and stress tolerance in plants. Acta Ecologica Sinica. 2007;27:5382–5389. [Google Scholar]
- 37.Liu XM, Huang CY, Chen Q, Wu XL, Zhang JX. Alleviative effects of exogenous trehalose on oxidative damage metabolism in Pleurotus pulmonarius under heatstress. Acta Horticulturae Sinica. 2013;40:1501–1508. [Google Scholar]
- 38.Hirasawa R, Yokoigawa K, Isobe Y, Kawai H. Improving the freeze tolerance of bakers’ yeast by loading with trehalose. Bioscience Biotechnology & Biochemistry. 2001;65:522–526. doi: 10.1271/bbb.65.522. [DOI] [PubMed] [Google Scholar]
- 39.Diniz-Mendes L, Bernardes E, de Araujo PS, Panek AD, Paschoalin VM. Preservation of frozen yeast cells by trehalose. Biotechnology & Bioengineering. 2015;65:572–578. doi: 10.1002/(SICI)1097-0290(19991205)65:5<572::AID-BIT10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 40.Meric L, Lambertguilois S, Neyreneuf O, Richardmolard D. Cryoresistance of baker’s yeast Saccharomyces cerevisiae in frozen dough: contribution of cellular trehalose. Cereal Chemistry. 1995;72:609–615. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.