Abstract
Coelomic fluid (CF) is the earliest dynamic and complex fluid of the gestational sac. CF contains maternal cells and proteins produced by embryonic cells, tissues and excretions. The biochemical composition of CF is modified throughout the first trimester of pregnancy and its protein profile reflects both physiological/pathological changes affecting the embryo and mother. Identification of variations in the balance of proteins might indicate particular types of pathologies, or ascertain specific genetic disorders. A platform utilizing protein enrichment procedures coupled with shotgun identification and iTRAQ differentiation provided the identification and quantitation of 88 unique embryonic proteins. It is relevant to note that chromosome X protein CXorf23 was found suggesting the embryo sex. Foetal sex was determined by Quantitative Fluorescent Polymerase Chain Reaction (QF-PCR) on coelomic cells, foetal tissues and maternal white blood cells, with a 100% concordance rate between iTRAQ-MS/MS and QF-PCR data. The functional associations among the identified proteins were investigated using STRING database. Open Targets Platform showed as significant the following therapeutic areas: nervous, respiratory, eye and head system disease.
Introduction
Coelomic fluid (CF) is the earliest fluid of the gestational sac, contained into the exocoelomic cavity (ECC) and it is in direct contact with placental villi during the first trimester of pregnancy1. Similarly to amniotic fluid (AF), CF might be amenable to prenatal testing. CF is currently used uniquely in genetic screening2. At the end of the fourth week of gestation, the ECC splits the extraembryonic mesoderm into two layers, the splanchnic mesoderm lining the secondary yolk sac and the embryo, and the somatic mesoderm lining the trophoblast3. The ECC is the largest space inside human gestational sac until the 9th week of gestation, after that time it starts contracting, while the amniotic cavity expands4. The anatomical location of ECC suggests its important role played in foetus nutrition, before the placental circulation is established5. CF contains adequate amounts of foetus cells, together proteins from embryonic tissues and excretions, with a low rate of contamination of the sample by maternal cells6. Similarly to other biological fluids, CF contains several proteins produced by the villous trophoblast, molecules that are transferred through the trophoblastic barrier (i.e. amino acids7, antioxidants, acids, metabolites8, etc.), some foetal proteins, and proteins synthesized by the secondary yolk sac, which is connected with foetal digestive and vascular systems3,9–11. In the first trimester of gestation, the CF protein concentration increases12. This alteration is not directly influenced by changes of maternal serum protein concentration, being rather closely dependent on the availability of a constant supply of amino acids for protein synthesis. Protein profile might reflect foetus and maternal physiological/pathological changes and its composition is obviously modified during the first trimester of pregnancy13. CF can selectively be aspirated under ultrasound guidance using a transvaginal route at 5–8 week gestation13. This procedure, called coelocentesis, was pioneered in 1993 by Jurkovic et al.13 and might be preferable to chorionic villous sampling (CVS), since the time to diagnosis is shortened, the risk of placental vascular damage and related foetal abnormalities are avoided. Moreover, protein profile should limit discordances resulting from pseudo-mosaicism encountered with classical chorionic preparations, facilitating in utero stem-cell therapy before the foetus becomes immunologically competent14. From a clinical point of view, detection of specific biomarkers in CF represents an interesting alternative to tissue sampling, due to the less invasive nature of collection15. Recently, the application of various advanced MS-based platforms enabled the discovery of novel biomarkers in biological fluids16 and tissues17–19. Due to their high selectivity and high sensitivity, these MS-based platforms have been widely used for the identification and quantitation of metabolites20,21, amino acids and their synthetic and non-natural analogues22–24, medicinal materials, proteins in biological fluids and tissues. Mass spectrometry-based shotgun proteomics methodology has become a standard method for characterization of proteomes from biological samples25,26, and for the comprehensive understanding of many metabolic processes in living organisms13,27,28. Many putative markers for anomalies, as premature rupture of amnion, intra-amniotic infection, and Down syndrome, have been re-analyzed or newly discovered by MS approaches13,29–32. Despite the accessibility of gestational fluids and tissues, such as CF, placenta and foetal membranes, MS investigations have seldom been applied in pregnancy research10,33. The relatively low number of embryo cells in CF, the necessity to collect only a small amount of this fluid, the risk of contamination of the sample by maternal material might limit molecular biology assays for genetic analyses34,35. The aim was to establish a differential proteomic expression profile of CF via a shotgun proteomic workflow. The presence of abundant serum proteins33,36 represents a barrier to detection of medium and low abundance proteins in proteomic analyses. This idea prompted us to develop three different analytical strategies for embryonic protein enrichment from normal CF (patients with no karyotype abnormalities) in order to increase the depth of CF proteome identification and to improve sensitivity for targeted analyses of differentially expressed proteins.
A platform utilizing protein enrichment procedures, such as chemical fractionation, hydroxyapatite (HTP), and immune depletion by “ProteoPrepBlu Albumin and IgG depletion Medium” (PROT–BA) and “Multiple Affinity Removal Spin” cartridges (MARS), coupled with shotgun identification and iTRAQ differentiation of CF was exploited. The iTRAQ-MARS was established as the most efficient approach among those tested, and provided the identification and quantitation of 88 unique embryonic proteins. Chromosome X protein CXorf23 was found suggesting embryo sex. To validate the results obtained by proteomic analysis, QF-PCR amplification using several markers for chromosomes X, Y was performed on 22 serial samples of coelomic cells (CC), foetal tissue (FT) and maternal white blood cells (MBC). Multiplex analyses of CC, FT and MBC samples allowed the distinction of foetal from maternal patterns and the identification of maternal contamination of the CF samples. Prenatal detection of feotal sex was successful in all cases. Bioinformatics elaboration of MS data was performed by STRING database and Open Targets Platform (OTP). OTP showed as significant the following therapeutic areas: “nervous system disease”, “respiratory system disease”, “eye disease” and “head disease”.
Results
Coelomic fluid (CF) is a yellow viscous fluid with lower protein concentrations. Jauniaux et al. found that total protein content is 18 times lower in CF than in maternal serum, and 54 times higher in CF than in AF (Supporting information, Table 1S)3. Giambona and co-workers adopted a direct micromanipulator pickup of the embryo-foetal cells selected on the basis of their morphology34,35 to remove maternal cell contamination and to obtain an early prenatal diagnosis of gene disorders. CF proteome reveals also maternal contamination, and the protein quantification has been reported in the updated literature data with no distinction between embryo and mother3. CF shares many proteins with maternal plasma, trophoblastic, and yolk sac33. Cindrova-Davies et al.33 identified 165 proteins from CF using a gel electrophoresis liquid chromatography (GELC)-MS/MS approach. Serum and common circulating blood proteins respectively accounted for 30% and 10% of the total number of identified and categorized proteins. Therefore, depletion of these proteins is a prerequisite for the detection of the low-abundance components. The starting point to overcome the maternal contamination should also require the removal of IgG and IgA, belonging to the mother immune system since the foetus is immunologically not competent, and HSA, playing a nutritional role during the foetal growth. It is now well accepted that no one single proteomic workflow would come even close to identifying all major proteins in action37. The introduction of LC-MS/MS or LC-MALDI MS/MS based shotgun proteomics approaches in conjunction with several pre-fractionation schemes has proven to be a valid and complementary alternative to 2-DE gel-based analysis38,39.
The aim was to establish a differential proteomic expression profile of CF via a shotgun proteomic workflow. The practicality in the rapid detection of low abundant classes of protein families was used to critically evaluate the pitfalls and strengths of the approach. Three different analytical strategies for embryonic protein enrichment from CF were designed wherein information with respect to the readiness of the protein entry being detected by any one approach were used as an indicator of the specificity. The first step was to establish an efficient methodology to perform, monitor, and compare different pre-fractionation schemes, for the development of a distinctive approach in which efforts were not directed primarily towards identifying markers, but rather in establishing a proteomic expression profile of CF. The protein profile of CF obtained by direct MALDI mass spectrometry in the linear mode showed the presence of multicharged species of HSA (Fig. 1). The five ion peaks of m/z 131663, 65754, 32865, 21899 and 16414 can be ascribed to [2HSA]+, [HSA]+, [HSA]2+, [HSA]3+and [HSA]4+, respectively. IgG and serotransferrin gave peaks at m/z 52675, 98704, and 39493 respectively. Spectrum showed also the presence of other less intense peaks, nevertheless the attribution was not so simple at this level. Three different protocols were used for sample preparation and variations were “tried-and-tested” within each procedure (Scheme 1, Experimental Section). Protocol I involved a chemical fractionation of proteins carried out by a simple procedure based on their different physicochemical properties. The acid isoelectric point of HSA (IP 5.2) suggested the protein to be soluble under basic conditions. The experimental design was planned in order to obtain three fractions: the supernatant fraction (S) and two hydrosoluble fractions (basic H1 and acidic H2). All fractions (S, H1 and H2) were directly analyzed by MALDI mass spectrometry and checked by SDS-PAGE (Supporting Information, Figs 1S and 2S).
Figure 1.
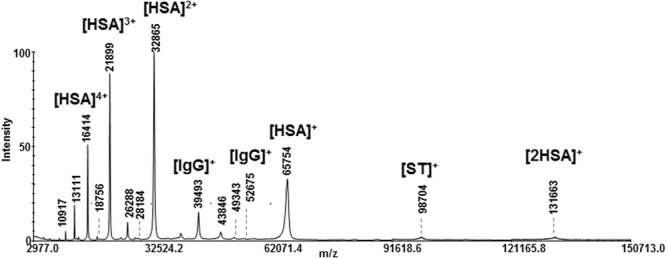
Linear MALDI MS of CF sample.
Scheme 1.
Sample preparation protocols used in this study. Protocol I: chemical fractionation; Protocol II: HTP purification; Protocols III: immunodepletion. All fractions appearing in blue were monitored by SDS-PAGE and/or linear MALDI MS.
Analyses showed the presence of albumin and similar protein profile in all fractions, indicating the non-specificity of the method. Finally, the three fractions were subjected to in-solution protein digestion and chromatographic fractionation, then analyzed by MALDI MS/MS. The Protein Pilot software allowed the identification of 104 proteins from fractions H1 and H2 (Supporting Information, Table 2S). Protocol II was based on depletion by home-made HTP spin column40–43. HTP is a crystalline form of calcium phosphate which is widely used in biochemistry because its specificity for fractionation and purification of monoclonal antibodies and proteins. HTP chromatography was performed by applying a salt and pH gradient (Fig. 6S, Protocol II) in order to obtain an efficient separation according to the different protein isoelectric points44. All steps were monitored by SDS-Page and mass spectrometry. MALDI and SDS-Page protein profile of HTP fractions revealed the ubiquitous presence of HSA (Supporting Information, Figs 3S and 4S), demonstrating the non-specificity of the method. In order to check all fractions by direct mass spectrometry, a novel MS-compatible experimental procedure based on the use of immunoaffinity devices was designed (Experimental session)18. MARS cartridge (IIIa) can selectively remove high-abundant proteins from human serum, plasma, and cerebrospinal fluid, offering the opportunity to analyze up to 200 samples with no memory effect. The selective immunodepletion provides an enriched pool of low-abundant proteins for downstream proteomics analysis. PROT-BA device (IIIb) is specific for albumin and IgG depletion from human serum (25–50 μL).The immunoaffinity medium in the prepacked spin column is a mixture of two beaded mediums containing recombinant expressed small single-chain antibody ligands, resulting in low non-specific binding and high capacity.
The depletion efficiency of protocols IIIa and IIIb was compared by linear MALDI mass spectrometry. MS/MS analysis was used to further evaluate the most efficient setup to deliver the highest number of identified low-abundant proteins. Linear MALDI spectra of fractions collected from PROT-BA showed residual HSA (Supporting information, Fig. 5SA,B). MS/MS analysis of tryptic peptides from the PROT-BA depleted fraction allowed the identification of 48 proteins and several different isoforms (Supporting Information, Table 3S). MARS led to an excellent HSA depletion. Figure 2A shows the linear MALDI spectra of the depleted fraction. In this case, 95 proteins were identified by MS/MS analysis (Table 4S, Supporting Information). Therefore, MARS was reputed to be the best analytical device for CF protein quantitation.
Figure 2.
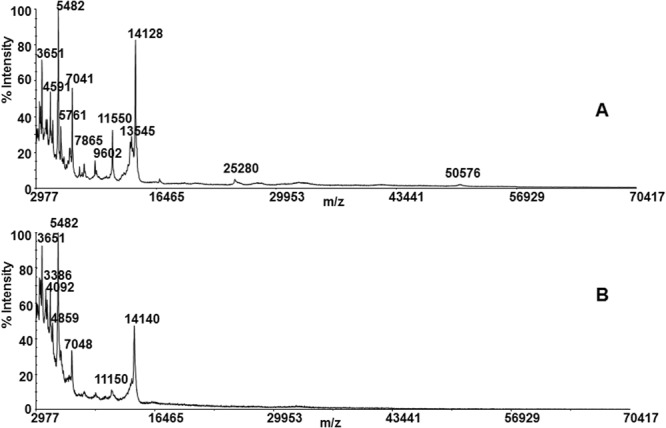
Linear MALDI spectra of CF from Protocol IIIa MARS: (A) after the depletion procedure, and (B) after the deglycosylation step by PNGase F.
Shotgun identification and iTRAQ differentiation of CF were carried out on twelve individual samples (DCFA-N). To improve the sequence coverage and the number of identified peptides, a deglycosylation step was performed after the MARS protocol application. The iTRAQ-MS/MS analysis of DCFA-N revealed 88 differentially expressed proteins using an ion ratio ≥2 or ≤0.5. Only 49 UniProtKB validated sequences were evaluated (Table 1). At least three peptides were used to identify and quantify CF proteins. Table 1 lists iTRAQ-MARS differentiation data for individual samples A-C. All experiments were performed in triplicate.
Table 1.
Proteins quantified by iTRAQ-MARS. Identification and quantification of proteins were performed using the Protein Pilot Paragon Method.
| Accessiona | Namea | Gene Namea | B115:P114b | A116:P114b | C117:P114b | |
|---|---|---|---|---|---|---|
| 1 | RM10_HUMAN | 39 S ribosomal protein L10, mitochondrial | MRPL10 | 1.08 | 1.03 | 0.66 |
| 2 | APCL_HUMAN | Adenomatous polyposis coli protein 2 | APC2 | 3.67 | 6.62 | 4.88 |
| 3 | SIA7E_HUMAN | Alpha-N-acetylgalactosaminide alpha-2,6-sialyltransferase 5 | ST6GALNAC5 | 1.42 | 2.14 | 1.74 |
| 4 | RNPL1_HUMAN | Arginyl aminopeptidase-like 1 | RNPEPL1 | 0.21 | 0.68 | 0.75 |
| 5 | ANPRB_HUMAN | Atrial natriuretic peptide receptor 2 | NPR2 | 1.38 | 3.67 | 2.76 |
| 6 | CTF8A_HUMAN | Chromosome transmission fidelity protein 8 homolog isoform 2 | CHTF8 | 2.44 | 4.11 | 3.30 |
| 7 | CC183_HUMAN | Coiled-coil domain-containing protein 183 (Isoform 4) | CCDC183 | 2.35 | 2.05 | 1.43 |
| 8 | CC186_HUMAN | Coiled-coil domain-containing protein 186 | CCDC186 | 1.55 | 3.86 | 1.94 |
| 9 | CO5A1_HUMAN | Collagen alpha-1(V) chain | COL5A1 | 1.98 | 3.36 | 2.92 |
| 10 | CR2_HUMAN | Complement receptor type 2 (Isoform D) | CR2 | 1.81 | 3.22 | 2.47 |
| 11 | NEUL1_HUMAN | E3 ubiquitin-proteinligase NEURL1 | NEURL1 | 1.49 | 3.96 | 2.57 |
| 12 | FLNC_HUMAN | Filamin-C | FLNC | 1.23 | 3.94 | 3.93 |
| 13 | GRASP_HUMAN | General receptor for phosphoinositides 1-associated scaffold protein (isoforms 2) | GRASP | 1.24 | 2.56 | 2.20 |
| 14 | GLHA_HUMAN | Glycoprotein hormones alpha chain | CGA | 1.52 | 1.71 | 1.72 |
| 15 | GCC2_HUMAN | GRIP and coiled-coil domain-containing protein 2 | GCC2 | 1.61 | 4.83 | 3.08 |
| 16 | H3C_HUMAN | Histone H3.3C | H3F3C | 2.06 | 4.63 | 3.39 |
| 17 | HV311_HUMAN | Ig heavy chain V-III region KOL | IGHV3–33 | 1.37 | 2.52 | 0.81 |
| 18 | IPO8_HUMAN | Importin-8 | IPO8 | 1.36 | 1.60 | 1.73 |
| 19 | G137C_HUMAN | Integral membrane protein GPR137C | GPR137C | 2.00 | 4.20 | 2.60 |
| 20 | ITIH1_HUMAN | Inter-alpha-trypsin inhibitor heavy chain H1 | ITIH1 | 2.02 | 1.34 | 1.31 |
| 21 | DCP2_HUMAN | m7GpppN-mRNA hydrolase | DCP2 | 1.10 | 3.52 | 2.50 |
| 22 | MPEG1_HUMAN | Macrophage-expressed gene 1 protein | MPEG1 | 2.33 | 6.14 | 2.93 |
| 23 | KISS1_HUMAN | Metastasis-suppressor KiSS-1 | KISS1 | 3.38 | 2.63 | 1.64 |
| 24 | MIIP_HUMAN | Migration and invasion-inhibitory protein | MIIP | 2.36 | 4.22 | 3.47 |
| 25 | MINT_HUMAN | Msx2-interacting protein | SPEN | 1.73 | 3.85 | 4.55 |
| 26 | MUC16_HUMAN | Mucin-16 | MUC16 | 2.40 | 5.37 | 4.02 |
| 27 | MUC19_HUMAN | Mucin-19 | MUC19 | 1.87 | 4.26 | 2.44 |
| 28 | MCTP1_HUMAN | Multiple C2 and transmembrane domain-containing protein 1 | MCTP1 | 3.11 | 7.33 | 2.57 |
| 29 | ACM1_HUMAN | Muscarinic acetylcholine receptor M1 | CHRM1 | 1.00 | 2.49 | 1.43 |
| 30 | NDUA5_HUMAN | NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 5 (Isoform2) | NDUFA5 | 1.09 | 2.85 | 3.08 |
| 31 | PK3CG_HUMAN | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform | PIK3CG | 2.36 | 2.39 | 1.86 |
| 32 | PMF1_HUMAN | Polyamine-modulated factor 1 (Isoform 2) | PMF1 | 1.40 | 0.00 | 0.86 |
| 33 | PSME2_HUMAN | Proteasome activator complex subunit 2 | PSME2 | 1.50 | 5.20 | 1.24 |
| 34 | K1456_HUMAN | Putative methyltransferase KIAA1456 | KIAA1456 | 1.57 | 2.94 | 1.97 |
| 35 | SHSA8_HUMAN | Putative protein shisa-8 | SHISA8 | 2.24 | 4.32 | 2.90 |
| 36 | RANB9_HUMAN | Ran-binding protein 9 (Isoform 2) | RANBP9 | 2.28 | 2.35 | 2.82 |
| 37 | RALB_HUMAN | Ras-related protein Ral-B | RALB | 0.26 | 1.28 | 0.07 |
| 38 | EWS_HUMAN | RNA-binding protein EWS | EWSR1 | 1.03 | 1.89 | 1.06 |
| 39 | SPCS3_HUMAN | Signal peptidase complex subunit 3 | SPCS3 | 1.33 | 3.49 | 2.63 |
| 40 | SLF2_HUMAN | SMC5-SMC6 complex localization factor protein 2 | SLF2 | 1.29 | 0.78 | 2.63 |
| 41 | SPEG_HUMAN | Striated muscle preferentially expressed protein kinase | SPEG | 1.30 | 3.85 | 1.41 |
| 42 | TPC12_HUMAN | Traffickingproteinparticlecomplexsubunit 12 | TRAPPC12 | 0.81 | 2.30 | 0.99 |
| 43 | TRIM3_HUMAN | Tripartite motif-containing protein 3 (Isoform Gamma) | TRIM3 | 3.14 | 6.92 | 4.49 |
| 44 | UBP37_HUMAN | Ubiquitin carboxyl-terminal hydrolase 37 | USP37 | 1.54 | 2.48 | 1.58 |
| 45 | CX023_HUMAN | Uncharacterized protein CXorf23 | CXorf23 | 1.90 | 3.49 | 2.91 |
| 46 | WDR97_HUMAN | WD repeat-containing protein KIAA1875 | WDR97 | 2.78 | 2.42 | 3.45 |
| 47 | XKR7_HUMAN | XK-related protein 7 | XKR7 | 0.95 | 2.47 | 2.07 |
| 48 | ZC11A_HUMAN | Zinc finger CCCH domain-containing protein 11A | ZC3H11A | 2.17 | 4.67 | 2.06 |
| 49 | ZN592_HUMAN | Zinc finger protein 592 | ZNF592 | 2.96 | 6.72 | 6.03 |
The MS/MS data were processed using a mass tolerance of 10 ppm and 0.2 Da for the precursor and fragment ions, respectively. aAccording to “UniProtKB” (http://www.uniprot.org/). bThe relative quantification was calculated according to Protein Pilot, and based on the ratio of the reporter ions corresponding to the A116, B115 and C117 tryptic peptides, over the ratio of the P114 reporter ions. Proteins giving tryptic peptides with an average reporter ion ratio ≥2 or ≤0.5 were classified as up- or down-regulated, respectively.
Quantitative Fluorescent Polymerase Chain Reaction (QF-PCR). Evaluation of foetal sex
Embryo-foetal nucleated red cells in CF were identified by optical phase contrast microscopy. These are roundish cells with a diameter of 12–16 μm, high cytoplasmic nuclear ratio, and the nucleus polarized to one side of the cell near the wall. Coelomic cells isolated from CF were successfully analyzed to obtain information on foetal sex. Quantitative Fluorescent Polymerase Chain Reaction (QF-PCR) was used to evaluate foetal sex. Specific short tandem repeats (STR) of highly variable chromosomal markers (STR) located on chromosome X and Y chromosomes (AMXY, HPRT, SRY, DXS1187, DXS8377, DXS6803, DXS6809) were used to obtain information on foetal sex. Figures 3 and 4 display parts of electropherograms of QF-PCR polymorphic STR markers for samples from a female and a male foetus. Specific markers of X and Y chromosomes are showed. Patterns of DNA derived from coelomic cells, fetal tissues and maternal white blood cells are reported (Figs 3 and 4).
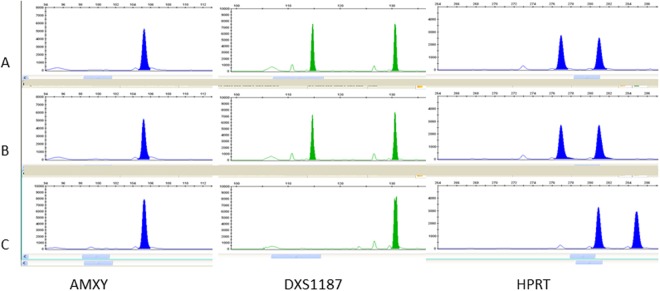
Figure 3.
Coelomic fluid of sample A. Evaluation of CF for sex determination by quantitative fluorescent PCR. Electropherograms of amplification product from CF without maternal contamination using small tandem repeat (STR) markers specific for chromosome X. A part of the total electropherograms is displayed in Panel A–C (A) coelomic cells pattern; (B) foetal tissue pattern; (C) maternal pattern. AMXY: is present with a peak of 105 bp; DXS1187: STR located in the X chromosome. Coelomic cell DNA and foetal DNA showed two X peaks, one of maternal origin and one of paternal origin. HPRT: STR located in the X chromosome. Coelomic cell DNA and foetal DNA showed two X peaks, one of maternal origin and one of paternal origin.
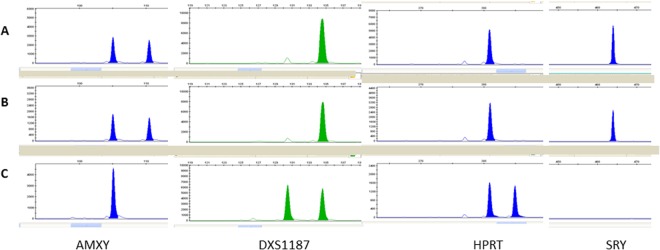
Figure 4.
Coelomic fluid of sample B. Evaluation of CF for sex determination by quantitative fluorescent PCR. Electropherograms of amplification product from CF without maternal contamination using small tandem repeat (STR) markers specific for chromosome Y. A part of the total electropherograms are displayed in Panel A–C (A) coelomic cell pattern; (B) foetal tissue pattern; (C) maternal pattern. AMXY: are visible two different peaks, one peak of 105 bp specific for X chromosome, and one of 110 bp specific for Y chromosome. DXS1187: STR located in the X chromosome. Coelomic cell DNA and foetal DNA showed only one peak of maternal origin. HPRT: STR located in the X chromosome. Coelomic cell DNA and foetal DNA showed only one peak of maternal origin. SRY: STR located in the Y chromosome, absent in the profile of the mother and present as one peak in the profiles of coelomic cell DNA and foetal DNA.
Discussion
Prenatal screening tests play an important role in the diagnosis for affected pregnancies. Pregnancy progression and birth involve foetal/maternal biochemical processes that depend on complex interactions at multiple levels. The balance among these interactions is disturbed at more than one level when a major problem arises. Proteins represent the functional complements of genes, therefore disorders, as well as changes in the number of gene copies and/or gene regulatory mechanisms, are reflected at the level of protein production and expression. The complex nature of biological fluids requires well established analytical methodologies for the enrichment of low abundance proteins or efficient sample fractionation steps before proteomics analysis. There are no currently preferred or standardized protocols to separate proteins from body fluid proteomes, and gel electrophoresis and chromatography are considered to be complementary. Characterization of CF proteome might be a key point for an innovative prenatal screening. CF is quite different in comparison to the other biological fluids which are usually adopted for clinical screening (plasma, serum, urine). The protein content of CF is profusely due to the maternal contamination, that include serum albumin and immunoglobulins in high concentrations (1.7 g/L and 35 mg/L, respectively)3. Therefore, immunodepletion avoids the masking effect of high abundance proteins for obtaining information about the whole CF proteome. Protocols I and II were planned taking into account the physico-chemical properties of HSA. Protocol I led to the identification of 104 proteins, distributed between acidic and basic proteins. The most part of proteins were identified from only one or three peptides, and the method showed a low specificity towards HSA. Moreover, several isoforms and thirty peptides of HSA were also detected, confirming the masking effect exerted by high abundance proteins. In protocol II, based on the use of HTP resin, a series of gradients were applied in order to achieve an efficient protein separation. The elution of basic and acidic proteins required the use of KCl, TRIS and EDTA solutions which caused difficult in monitoring all fractions by MALDI mass spectrometry. The fractions collected from the column were pooled according to the elution profile and analyzed by SDS-PAGE. The last fractions eluted by water were checked by mass spectrometry. Spectra revealed the HSA isolation to be unsuccessful, confirming the non-specificity of the method (Figs 3S and 4S, Supporting Information). The other two protocols (IIIa and IIIb), based on the use of immunoaffinity devices, yielded the best results. Notwithstanding, the PROT-BA method was characterized by low recovery of the total protein content, and the nature as mono use device limited its practicality. Under the adopted experimental conditions, the non-complete depletion of HSA was observed and only 48 proteins were identified (Fig. 5S and Table 3S, Supporting Information). HSA removal was strongly improved by the MARS protocol and this method provided the identification of 94 proteins. The reusability of the same device without memory effects was considered striking. The MARS protocol was then adopted for the quantitative analysis. The use of the deglycosylating enzyme PNGase F was a ploy to improve the number of detectable peptides for protein sequence coverage. Figure 2B shows several little mass shifts related to sugar removal from proteins. The CF develops during the 4th week of gestation, and it can be aspirated starting from the 5th week making coelocentesis the earliest possible method of prenatal diagnosis. It is reasonable to consider coelocentesis to be a source of foetal progenitor and stem cells, and CF to be associated to the foetal system45. The proteomics outlook becomes a complementary and fundamental source of information. Quantitative proteomics analysis of CF might open an ideal route to the discovery of more efficient and specific biomarkers, elucidating changes mostly related to physiological and pathological conditions in the foetus in the first trimester of pregnancy. The iTRAQ-MARS protocol provided the identification and quantification of 88 proteins, 49 having reviewed UniProtKB sequences (Table 1). It is worthy of note that literature reports realistic associations between specific diseases recognized by maternal serum/plasma or amniotic fluid diagnostics and several proteins detected and quantified by our iTRAQ-MARS protocol. APC2 (Adenomatous polyposis coli protein 2, APCL_HUMAN; Table 1, lane 2) was identified in maternal plasma and amniotic fluid by LC-MS/MS. APC2 is involved in actin-associated events influencing cell motility or adhesion through interaction with actin filaments46. Mutations of the gene expressing this protein are related to a form of Sotos syndrome, characterized by prenatal and postnatal childhood overgrowth, developmental delay, mental retardation, advanced bone age, and abnormal craniofacial morphology. Fragments of COL5A1 (Collagen alpha-1(V) chain, CO5A1_HUMAN; Table 1, lane 8), have been found up-regulated in amniotic fluid from patients in pregnancies with Down Syndrome affected foetus30. ITIH1 (Inter-alpha-trypsin inhibitor heavy chain H1, ITIH1; Table 1, lane 17) has been characterized by LC-MS and predictive analysis of microarrays, and reported to be correlated to pregnancy-related complications, such as pre-eclampsia and preterm birth47. Since the analyzed samples were from patients with no karyotype abnormalities, at the moment it is not possible to establish strict relationships with diseases. However, it is important to note that chromosome X protein CXorf23 (Uncharacterized protein CXorf23, CX023_HUMAN; Table 1, lane 45) was found over-expressed in samplesA116 and C117, suggesting an embryo female sex. The same protein was found down regulated in sample B115. Genomic DNA analysis from corionic villus and maternal blood confirmed the sex of foetus; in particular, female for A116 and C117, and male for B115, proving that CF can certainly be used in prenatal screening. The proteomic approach used here led to a differential identification of the catalogue of CF proteins. These data can further be used for bioinformatics elaboration. The biological associations among the identified proteins were investigated using the STRING database. The predicted protein-protein associations were queried through a vast number of databases derived in different ways (e.g., experimentally determined interactions, protein neighborhood data, or data acquired via text mining)48.
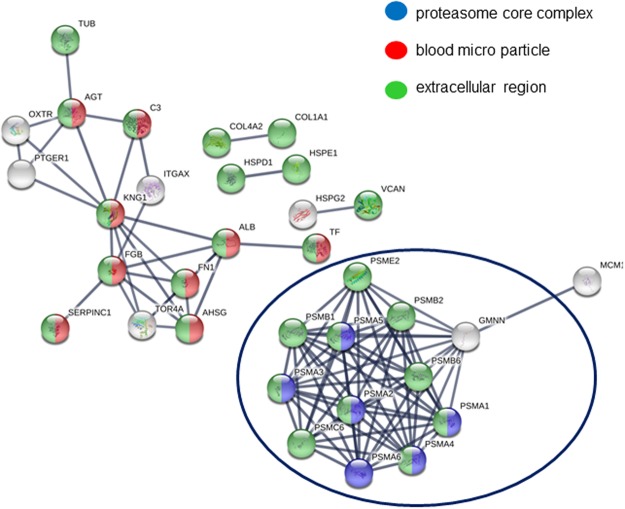
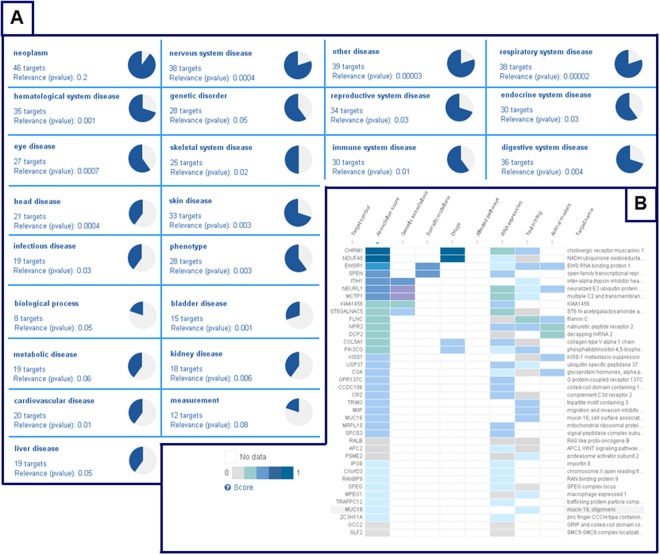
As shown in Fig. 5 three main networks of cellular components (GO) were identified: proteasome core complex (GO:0019773, blu), blood micro particle (GO:0072562, red), and extracellular region (GO:0005576, green). For the 49 differentially expressed proteins, functional enrichment analysis showed two major pathways in INTERPRO Protein domains and features networks: proteasome, subunit alpha/beta (IPR001353), nucleophile aminohydrolases, N-terminal (IPR029055) (Table 5S, Supporting Information). It is not surprising to observe the relevance of proteasome in GO and INTERPRO networks because proteasome constitutes the central proteolytic machinery of the highly conserved ubiquitin/proteasome system, the major cellular tool for extralysosomal protein degradation. Proteasome can play opposite roles in the regulation of cell proliferation and apoptosis, these roles are apparently defined by the cell environment and proliferative state. During early embryogenesis, proteasomes perform proteolytic functions and also stored as a maternal supply of proteasomes for the developing embryo. Changes in proteasome distribution during fertilization and further stages of development, could be associated to the replacement of maternal proteasome by proteasomes expressed by the embryo itself49. Furthermore, we adopted Open Targets (http://www.opentargets.org) as a tool for the verification of the congruency of results. Open Targets is a public-private partnership to establish an informatics platform, the Target Validation Platform. The Open Targets Platform (OTP) is a comprehensive and robust data integration for access to and visualisation of potential drug targets associated with diseases50. A drug target can be a protein, protein complex or RNA molecule, and it is displayed by its gene name according to the Human Gene Nomenclature Committee. OTP links multiple data types and assists users in identifying and prioritizing targets, in our case proteins, for further investigation. We checked a list of 49 targets (Table 1) and the OTP output gave back a summary report, in which therapeutic areas of interest were sorted by relevance to our list. Part of OTP output is reported in Fig. 6, where panel A displays the summary page for 49 targets together with the corresponding therapeutic areas. Some areas did not show correlations with the targets, for example “neoplasm” (p-value 0.2), “liver disease” (p-value 0.05) or “metabolic disease” (p-value 0.06). Nevertheless, other areas showed good correlations with the submitted proteins, for example “nervous system disease” (p-value 0.0004), “respiratory system disease” (p-value 0.00002), “eye disease” (p-value 0.0007) and “head disease” (p-value 0.0004). It is not surprising to observe the relevance of targets related to nervous system disease, since humans have considerably more prenatal maturation of their nervous systems.
Figure 5.
Interaction network analysis of proteins identified by iTRAQ-MARS MS/MS approach (STRING database).
Figure 6.
Open Target Platform output. (A) Summary page for 49 targets. (B) Platform workflow for nervous system diseases.
Panel B in Fig. 6 displays the summary report obtained for 38 targets related to “nervous system disease”. Colour variations are directly connected with scores. Values of 0.5 and 1 indicate that genes are weakly or strongly involved in diseases, respectively. Several proteins involved in neuronal diseases (Fig. 6, Panel B) were detected, although they can indicate a normal state of the central nervous system (CNS) embryonic evolution. In the first month of gestation specific areas of the CNS begin to form following a sequence of developmental processes including proliferation, migration, differentiation, synaptogenesis, apoptosis, and myelination51. In particular, cell precursors of brain and spinal cord in humans start to develop early in embryogenesis, approximately two weeks of gestation, through the process called neurulation. Ending the third week of gestation, the neural folds begin to move together and fuse forming the neural tube, leading to a complete neural tube formation approximately from gestation day 26 to 28. Interruption of neural development during this early period can result in severe abnormalities of the brain and spinal cord.
A quantitative shotgun proteomics analysis strategy was successfully used for the identification and differentiation of embryo proteins from CF. Several putative AF/maternal serum markers were identified. The proposed experimental approach furnished a powerful tool for achieving deeper insight into the CF protein composition in early stage of pregnancy, offering a novel perspective in the investigation of molecular constitution and dynamics of this gestational fluid. However, it should be noted that data reported are specific for gestational ages of 8 weeks and for samples from chromosomally normal pregnancies. In all cases, a concordance rate of 100% was found for sex determination between iTRAQ-MARS and Genomic DNA analysis from corionic villus, or amniotic liquid and maternal blood.
Methods
Collection of coelomic fluid
This study is part of an on-going investigation examining the feasibility of analysis on DNA extracted from CF for earlier prenatal diagnosis of foetal diseases. The study was conducted in accordance with the Declaration of Helsinki (Hospital Ethical Committee authorization), protocol number 26-01-2005, No 80 approved by the institutional Review Board of “Ospedali Riuniti Villa Sofia-Cervello”, Palermo, Italia. Informed consent forms were obtained from all the study participants and all methods were performed in accordance with relevant guidelines and regulations. The details of such protocols have been previously described33,34. Following written consent, women were recruited between 7–10 weeks of gestation. CF from pregnancies with chromosomally normal foetus was obtained by ultrasound-guided transvaginal puncture, as reported33,34. Twenty-two samples of CF were selected for proteomic studies. CF cells were used for morphological and genetic analysis33,34, while the fluid was used for proteomic experiments.
Sample preparation of embryo-foetal cells and Quantitative Fluorescent Polymerase Chain Reaction (QF-PCR)
Embryo-foetal cells were one by one aspirated by a micromanipulator using a 45 μm glass micropipette (BioCare Europe). Cells were placed into a drop of 0.9% NaCl in the same Petri disk. Each drop containing a group of embryo-foetal erythroid precursor cells was centrifuged at 10000 rpm for 7 min and supernatants discarded. All samples were subjected to DNA extraction by alkaline method. QF-PCR was used to evaluate the foetal sex. Specific short tandem repeat (STR) of highly variable chromosomal markers located on X and Y chromosomes (AMXY, HPRT, SRY, DXS1187, DXS8377, DXS6803, DXS6809) were used to obtain information on foetal sex. Each primer was labelled with fluorescent dyes (PET, VIC, NED, 6-FAM) to obtain fluorescent PCR amplicons. DNA (2.5 μL; 10–20 ng) was added to the Master Mix (10 μL), and the mixture was used for amplification according to the manufacturer’s instructions. Denaturation was performed for 15 min at 95 °C, then 26 cycles of 30 s at 95 °C, 90 s at 59 °C, 90 s at 72 °C, and a final extension of 30 min at 72 °C were applied. PCR products (2 μL) were mixed with 15 μL of HiDi and 0.5 μL of LIZ 500 (Applied Biosystems), as the internal-lane size standard. Capillary electrophoresis was performed by an automated ABI Prism 3130 Genetic Analyzer and data were analyzed by the Gene Mapper 4.0 software (Applied Biosystems). Genomic DNA was analyzed from corionic villus or amniotic liquid and maternal blood. DNA extraction was carried out by standard protocols.
Sample preparation of coelomic fluid proteome
Individual samples of CF were used to check sample preparation procedures (Protocols I–III, Scheme 1). An aliquot of 200 μL from ten CF samples were pooled to get analytical robustness and consistence (CFp, pooled coelomic fluid). Individual CF (twelve) was manipulated for the comparative quantification experiments.
Protocol I
CF (200 μL) was precipitated with 600 μL of CHCl3/CH3OH 1:3 (v/v), yielding the supernatant fraction S and a pellet, which was successively partitioned with 200 μL of 50 mM NH4HCO3 (H1), and 50 μL of H2O/CH3CN 3:2 (v/v, TFA 0.3%) (H2).
Protocol II
Hydroxyapatite (100 mg) was packed in a spin column. CF (200 μL) was added together with 25 μL of equilibration buffer (10 mM TRIS, 1 mM EDTA, pH 7), and loaded onto HTP spin column. After 30 min of incubation at room temperature, the column was spun at 6000 rpm for 2 min. Bound proteins were eluted with 200 μL of elution buffer (100 mM KCl, 20 mM TRIS, 2 mM EDTA, pH 8) for five times, giving 30 min incubation each time, followed by centrifugation at 6000 rpm for 2 min. The column was then washed with 200 μL of (NH4)2CO3 (pH 10), and with 200 μL of H2O.
Immunodepletion
CF proteins were depleted of high abundant proteins using (a) “Multiple Affinity Removal Spin cartridge-MARS” (Agilent Technologies, Italy), or (b) “ProteoPrepBlu Albumin and IgG depletion Medium” (PROT-BA; Sigma-Aldrich, Italy).
Protocol IIIa
The cartridge was treated four times with 400 µl of 50 mM NH4HCO3, (pH 8). CF (200 µl) was applied on column, centrifuged for 2 min at 3000 rpm, then collected. The cartridge was washed with 400 µl of 50 mM NH4HCO3 and the obtained flow-through fractions were collected and concentrated (50 µl). High abundant proteins were eluted with buffer B (5 times, pH 2.5). The collected fraction was dried, than solubilized with 100 µl of buffer (0.375 M TRIS, 0.1% SDS, pH 8.8).
Protocol IIIb
The cartridge PROT-BA was treated with 200 μl of 50 mM NH4HCO3, (pH 8). CF (200 μl) was applied on column and incubated for 10 min. After centrifugation at 3000 rpm for 1 min, the flow-through fraction was collected. The cartridge was washed with 200 μl of 50 mM NH4HCO3 and the collected flow-through fractions were combined. The retained proteins were eluted with (NH4)2CO3 (pH 10), after 10 min of incubation and centrifugation at 3000 rpm for 2 min.
Tryptic digestion
Each lyophilized fraction was solubilized with 100 µl of 50 mM NH4HCO3. Trypsin (20 pmol) was then added to the protein mixture, and the digestion step was performed in a home microwave (MWD 246 SL, Whirlpool, Italy) at 250 W irradiation power (12 treatments, each one lasting 2 min). Tryptic peptide mixtures were subjected to reversed phase chromatography fractionation52.
Sample preparation for comparative quantification
200 µL from 10 samples of CF were pooled together (CFp) and used for comparative quantification experiments with twelve individual CFs. CFp and the twelve CFs (A-N) were depleted of high abundant proteins using MARS approach (Protocol IIIa).The depleted fractions were collected and dried. The depleted CFp sample (DCFp) was treated with 100 µl of 50 mM NH4HCO3 and incubated with 4 µL of PNGase F (0.5 unit/μL). The microwave assisted deglycosylation step was performed at 250 W irradiation power (20 treatments each lasting 1 min). The protein mixture was subsequently treated with 200 µl of buffer (0.375 M TRIS, 0.1% SDS, pH 8.8) and boiled in a bain-marie for 5 min. After this time, trypsin (120 pmol) was added. The microwave assisted tryptic digestion was carried as above reported. All tryptic peptide mixtures were purified on a SPE Strata C18-E column (Phenomenex Inc,USA) to eliminate salts53 and interferences with the iTRAQ reagent procedure. Column was washed with CH3OH, and then conditioned with 2 mL of CH3CN/TFA 0.1% (50:50, v/v) and 2 mL of TFA 0.1%. Samples were made acidic by adding 300 µl of TFA 0.5% and loaded on column. The washing step was performed using 4 mL of TFA 0.1%, and the flow-through fractions were wasted. Collection of peptides was performed by using (a) 4 mL of CH3CN/TFA 0.1% (50:50, v/v), (b) 4 mL of CH3CN/TFA 0.1% (80:20, v/v), and (c) 6 mL of CH3CN. The flow-through fractions from steps (a)-(c) were combined and concentrated under vacuum. The dried samples were re-suspended in 50 µl of 500 mM triethyl ammonium bicarbonate buffer (TEAB), and tryptic peptides were labelled with the iTRAQ reagents (m/z 114.1, 115.1, 116.1 and 117.1) following the manufacturer’s protocol. The iTRAQ labels were added to the digested samples, in particular m/z 114.1 reporter ions was added to DCFP (sample P114), m/z 116.1 reporter ions to DCFA,D,G,L (samples A116, D116, G116, L116), m/z 115.1 reporter ions to DCFB,E,H,M (samples B115, E115, H115, M115), and m/z 117.1 reporter ions to DCFC,F,I,N (samples C117, F117, I117, N117). Labeled samples were combined in four groups (i.e., P114, A116, B115 and C117) and dried prior cation exchange and reversed phase chromatography fractionation. A series of 180 chromatographic fractions were collected for each group.
MALDI MS and MS/MS analysis
Linear MALDI-TOF spectra were acquired using a 5800 MALDI-TOF/TOF analyzer (AB SCIEX, Germany). All spectra were acquired in default calibration mode averaging 2500 laser shots with a mass accuracy of 500 ppm. MS and MS/MS analyses were performed in reflectron positive-ion mode. All chromatographic fractions were solubilized in 10 µl of matrix (α-CHCA 10 mg/mL, CH3CN/0.3% TFA in water, 50:50, v/v). MS spectra were acquired with a laser pulse rate of 400 Hz and at least 4000 laser shots, and CID-MS/MS experiments were performed at collision energy of 1 kV, using ambient air as the collision gas (10−6 Torr). CID-MS/MS spectra required up to 5000 laser shots and a pulse rate of 1000 Hz.
Database Searching and Bioinformatics
Protein identification was performed by the Protein Pilot 4.0 software, using the Paragon protein database search algorithm (AB Sciex)37. Data analysis parameters for samples were as follows: Sample type: iTRAQ 4plex (Peptide Labelled); Cys Alkylation: None; Digestion: Trypsin; Instrument: 5800; Special Factors: Phosphorylation emphasis, Species: Homo Sapiens; Quantitate tab: checked; ID Focus: Biological modification and Amino acid substitutions; Database: UniProt_taxonomy_Eukaryota [2759]; Search Effort: Thorough ID; Minimum Detected Protein Threshold [Unused ProtScore (Conf)]: 1 (90.0%); Run. False Discovery Rate Analysis Tab: checked. The relative quantification was based on the ratio of the reporter ions corresponding to the 116.1, 115.1 and 117.1 tryptic peptides, over the ratio of the P114 reporter ions (114.1 Da).
Electronic supplementary material
Acknowledgements
This work was supported by a Post-Doctoral Research Fellowship from the MIUR (Bando di Concorso DR 2648/2014) and PRIN 2015 (Prot. 201545245K_002).
Author Contributions
F.L., C.P., A.G. and A.M. were involved in patient enrollment and performed experiments of QF-PCR. G.D. collected samples of CF by coelocentesis. A.D., C.S. and A.N. have contributed to the study design, performed all proteomics experiments and bioinformatics data analysis. A.N. wrote the manuscript with help from A.D.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-29384-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jones CPJ, Jauniaux E. Ultrastructure of the materno-embryonic interface in the first trimester of pregnancy. Micron. 1995;2:145–173. doi: 10.1016/0968-4328(95)00002-L. [DOI] [PubMed] [Google Scholar]
- 2.Jouannic JM, Costa JM, Ernault P, Bénifla JL. Very early prenatal diagnosis of genetic diseases based on coelomic fluid analysis: a feasibility study. Hum. Reprod. 2006;21:2185–2188. doi: 10.1093/humrep/del143. [DOI] [PubMed] [Google Scholar]
- 3.Jauniaux E, Gulbis B. Fluid compartments of the embryonic environment. Hum. Reprod. Update. 2000;6:268–278. doi: 10.1093/humupd/6.3.268. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton WJ, Boyd JD. Development of the human placenta in the first three months of gestation. J. Anat. 1960;94:297–328. [PMC free article] [PubMed] [Google Scholar]
- 5.Jauniaux E, Gulbis B, Burton GJ. The Human First Trimester Gestational Sac Limits Rather than Facilitates Oxygen Transfer to the Foetus-A Review. Placenta. 2003;24:S86–93. doi: 10.1053/plac.2002.0932. [DOI] [PubMed] [Google Scholar]
- 6.Jurkovic D, Jauniaux E, Campbell S. Detection of sickle gene by coelocentesis in early pregnancy: A new approach to prenatal diagnosis of single gene disorders. Hum. Reprod. 1995;10:1287–1289. doi: 10.1093/oxfordjournals.humrep.a136135. [DOI] [PubMed] [Google Scholar]
- 7.Di Gioia ML, et al. Alternative and Chemoselective Deprotection of the α-Amino and Carboxy Functions of N-Fmoc-Amino Acid and N-Fmoc-Dipeptide Methyl Esters by Modulation of the Molar Ratio in the AlCl3/N,N-Dimethylaniline Reagent System. Eur. J. Org. Chem. 2004;21:4437–4441. doi: 10.1002/ejoc.200400321. [DOI] [Google Scholar]
- 8.Siciliano C, et al. Synthesis of d-erythro-Sphinganine through Serine-Derived α-Amino Epoxides. J. Org. Chem. 2014;79:5320–5326. doi: 10.1021/jo500493c. [DOI] [PubMed] [Google Scholar]
- 9.Jauniaux E, et al. Relationship between protein concentrations in embryological fluids and maternal serum and yolk sacsize during human early pregnancy. Hum. Reprod. 1994;9:161–166. doi: 10.1093/oxfordjournals.humrep.a138308. [DOI] [PubMed] [Google Scholar]
- 10.Virgiliou C, et al. Metabolic profile of human coelomic fluid. Bioanalysis. 2017;9:37–51. doi: 10.4155/bio-2016-0223. [DOI] [PubMed] [Google Scholar]
- 11.Steegers-Theunissen RP, Wathen NC, Eskes TK, Van Raaij-Selten B, Chard T. Maternal and fetal levels of methionine and homocysteine in early human pregnancy. Br. J. Obstet. Gynaecol. 1997;104:20–24. doi: 10.1111/j.1471-0528.1997.tb10642.x. [DOI] [PubMed] [Google Scholar]
- 12.Jauniaux E, Cirigliano V, Adinolfi M. Very early prenatal diagnosis on coelomic cells using quantitative fluorescent polymerase chain reaction. Reprod Biomed Online. 2003;6:494–498. doi: 10.1016/S1472-6483(10)62173-6. [DOI] [PubMed] [Google Scholar]
- 13.Jurkovic D, et al. Coelocentesis: a new technique for early prenatal diagnosis. Lancet. 1993;341:1623–1624. doi: 10.1016/0140-6736(93)90761-5. [DOI] [PubMed] [Google Scholar]
- 14.Le Blanc K, et al. Fetal Mesenchymal Stem-Cell Engraftment in Bone after In Utero Transplantation in a Patient with Severe Osteogenesis Imperfecta. Transplantation. 2005;79:1607–1614. doi: 10.1097/01.TP.0000159029.48678.93. [DOI] [PubMed] [Google Scholar]
- 15.Villar-Garea A, Griese M, Imhof A. Biomarker discovery from body fluids using mass spectrometry. J. Chromat. B. 2007;849:105–114. doi: 10.1016/j.jchromb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Mavrou A, et al. Proteomic Analysis of Amniotic Fluid in Pregnancies with Turner Syndrome Fetuses. J. Proteome Res. 2008;7:1862–1866. doi: 10.1021/pr700588u. [DOI] [PubMed] [Google Scholar]
- 17.Park J. Comparative Proteomic Analysis of Human Amniotic Fluid Supernatants with Down Syndrome Using Mass Spectrometry. J. Microbiol. Biotechnol. 2010;20:959–967. doi: 10.4014/jmb.0912.12035. [DOI] [PubMed] [Google Scholar]
- 18.Aiello D, et al. Targeted proteomic approach in prostatic tissue: a panel of potential biomarkers for cancer detection. Oncoscience. 2016;3:220–241. doi: 10.18632/oncoscience.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tu C, et al. Depletion of abundant plasma proteins and limitations of plasma proteomics. J. Proteome Res. 2010;9:4982–4991. doi: 10.1021/pr100646w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Donna L, et al. Determination of ketosteroid hormones in meat by liquid chromatography tandem mass spectrometry and derivatization chemistry. Anal. Bioanal. Chem. 2015;407:5835–5842. doi: 10.1007/s00216-015-8772-5. [DOI] [PubMed] [Google Scholar]
- 21.Mazzotti F, et al. N-hydroxysuccinimidyl p-methoxybenzoate as suitable derivative reagent for isotopic dilution assay of biogenic amines in food. J. Mass Spectrom. 2014;49:802–810. doi: 10.1002/jms.3417. [DOI] [PubMed] [Google Scholar]
- 22.Di Gioia ML, et al. An efficient and highly selective deprotection of N-Fmoc-alpha-amino acid and lipophilic N-Fmoc-dipeptide methyl esters with aluminium trichloride and N,N-dimethylaniline. J. Pept. Res. 2004;63:383–387. doi: 10.1111/j.1399-3011.2004.00104.x. [DOI] [PubMed] [Google Scholar]
- 23.Furia E, Napoli A, Tagarelli A, Sindona G. Speciation of 2-hydroxybenzoic acid with Calcium(II), Magnesium(II), andNickel(II) Cations in self-medium. Journal of Chemical & Engineering Data. 2013;58(5):1349–1353. doi: 10.1021/je400105c. [DOI] [Google Scholar]
- 24.De Marco R, et al. N-Alkylation of N-arylsulfonyl-α-amino acid methyl esters by trialkyloxonium tetrafluoroborates. Tetrahedron. 2011;67:9708–9714. doi: 10.1016/j.tet.2011.10.042. [DOI] [Google Scholar]
- 25.Napoli A, et al. Profiling of Hydrophilic Proteins from Olea europaea Olive Pollen by MALDI TOF Mass Spectrometry. Anal. Chem. 2006;78:3434–3443. doi: 10.1021/ac0600508. [DOI] [PubMed] [Google Scholar]
- 26.Reddy CE, et al. Multisite phosphorylation of c-Jun at threonine 91/93/95 triggers the onset of c-Jun pro-apoptotic activity in cerebellar granule neurons. Cell Death Dis. 2013;4:e852. doi: 10.1038/cddis.2013.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aiello D, et al. A major allergen in rainbow trout (Oncorhynchus mykiss): complete sequences of parvalbumin by MALDI tandem mass spectrometry. Mol. BioSyst. 2015;11:2373–2381. doi: 10.1039/C5MB00148J. [DOI] [PubMed] [Google Scholar]
- 28.Diamandis EP. Mass Spectrometry as a Diagnostic and a Cancer Biomarker Discovery Tool. Opportunities and Potential Limitations. Mol. Cell. Proteomics. 2004;3:367–378. doi: 10.1074/mcp.R400007-MCP200. [DOI] [PubMed] [Google Scholar]
- 29.Cho CJ, Shan SJ, Winsor EJ, Diamandis EP. Proteomics Analysis of Human Amniotic Fluid. Mol. Cell. Proteomics. 2007;6:1406–1415. doi: 10.1074/mcp.M700090-MCP200. [DOI] [PubMed] [Google Scholar]
- 30.Tsangaris GT, et al. Proteomic analysis of amniotic fluid in pregnancies with Down syndrome. Proteomics. 2006;6:4410–4419. doi: 10.1002/pmic.200600085. [DOI] [PubMed] [Google Scholar]
- 31.Thadikkaran L, et al. The role of proteomics in the assessment of premature rupture of fetal membranes. Clin. Chim. Acta. 2005;360:27–36. doi: 10.1016/j.cccn.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 32.Vuadens F, et al. Identification of biologic markers of the premature rupture of fetal membranes: Proteomic approach. Proteomics. 2003;3:1521–1525. doi: 10.1002/pmic.200300455. [DOI] [PubMed] [Google Scholar]
- 33.Cindrova-Davies TE, Jauniaux MG, Elliot S, Gong GJ, Burton DS, Charnck-Jones RNA-seq reveals conservation of function among the yolk sacs of human, mouse, and chicken. Proc. Natl. Acad. Sci. USA. 2017;114:E4753–E4761. doi: 10.1073/pnas.1702560114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giambona A, et al. Embryo-fetal erythroid cell selection from celomic fluid allows earlier prenatal diagnosis of hemoglobinopathies. Prenat. Diagn. 2016;36:375–381. doi: 10.1002/pd.4793. [DOI] [PubMed] [Google Scholar]
- 35.Giambona A, et al. Identification of embryo–fetal cells in celomic fluid using morphological and short-tandem repeats analysis. Prenat. Diagn. 2016;36:973–978. doi: 10.1002/pd.4922. [DOI] [PubMed] [Google Scholar]
- 36.Jauniaux E, et al. Protein and steroid levels in embryonic cavities in early human pregnancy. Hum. Reprod. 1993;8:782–787. doi: 10.1093/oxfordjournals.humrep.a138141. [DOI] [PubMed] [Google Scholar]
- 37.Aebersold R, Mann M. Mass spectrometry-based proteomics. Nature. 2003;422:198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- 38.McDonald WH, Yates JR., III Shotgun proteomics and biomarker discovery. Dis. Markers. 2002;18:99–105. doi: 10.1155/2002/505397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolters DA, Washburn MP, Yates JR., III An automated multidimensional protein identification technology for shotgun proteomics. Anal. Chem. 2001;73:5683–5690. doi: 10.1021/ac010617e. [DOI] [PubMed] [Google Scholar]
- 40.Fountoulakis M, Takas M, Berndt P, Langen H, Takacs B. Enrichment of low abundance proteins of Escherichia coli by hydroxyapatite chromatography. Electrophoresis. 1999;20:2181–2195. doi: 10.1002/(SICI)1522-2683(19990801)20:11<2181::AID-ELPS2181>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 41.Gorbunoff MJ. The interaction of proteins with hydroxyapatite. I. Role of protein charge and structure. Anal. Biochem. 1984;136:425–432. doi: 10.1016/0003-2697(84)90239-2. [DOI] [PubMed] [Google Scholar]
- 42.Gorbunoff MJ. The interaction of proteins with hydroxyapatite. II. Role of acidic and basic groups. Anal. Biochem. 1984;136:433–439. doi: 10.1016/0003-2697(84)90240-9. [DOI] [PubMed] [Google Scholar]
- 43.Madeo M, et al. Abundant expression and purification of biologically active mitochondrial citrate carrier in baculovirus-infected insect cells. J Bioenerg Biomembr. 2009;41(3):289–97. doi: 10.1007/s10863-009-9226-6. [DOI] [PubMed] [Google Scholar]
- 44.Gorbunoff MJ. Protein chromatography on hydroxyapatite columns. Methods Enzymol. 1985;182:329–339. doi: 10.1016/0076-6879(90)82028-Z. [DOI] [PubMed] [Google Scholar]
- 45.Fauza D. Amniotic fluid and placental stem cells. Clin. Obstet. Gynecol. 2004;18:877–891. doi: 10.1016/j.bpobgyn.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Michel PE, et al. Proteome analysis of human plasma and amniotic fluid by Off-Gel™ isoelectric focusing followed by nano-LC-MS/MS. Electrophoresis. 2006;27:1169–1181. doi: 10.1002/elps.200500680. [DOI] [PubMed] [Google Scholar]
- 47.Anderson NL. The Human Plasma Proteome A Non redundant List Developed by Combination of Four Separate Sources. Mol. Cell. Proteomics. 2004;3:311–326. doi: 10.1074/mcp.M300127-MCP200. [DOI] [PubMed] [Google Scholar]
- 48.Szklarczyk, D. et al. The STRING database in 2011: functional interaction networks of proteins, globallyintegrated and scored. Nucleic Acids Res. 39(Database issue), D561–D568 (2011). [DOI] [PMC free article] [PubMed]
- 49.Wójcik C, Benchaib M, Lornage J, Czyba JC, Guerin JF. Localization of proteasomes in human oocytes and preimplantation embryos. Mol. Hum. Reprod. 2000;6:331–336. doi: 10.1093/molehr/6.4.331. [DOI] [PubMed] [Google Scholar]
- 50.Koscielny G, et al. Open Targets: a platform for therapeutic target identification and validation. Nucleic Acids Res. 2017;45:D985–D994. doi: 10.1093/nar/gkw1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rice’ D, Barone S., Jr. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ. Health Perspect. 2000;108:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Napoli A, et al. Mass Spectrometry-Based Proteomic Approach in Oenococcus oeni Enological Starter. J. Proteome Res. 2014;13:2856–2866. doi: 10.1021/pr4012798. [DOI] [PubMed] [Google Scholar]
- 53.Di Gioia ML, et al. SPE–GC–MS Analysis of Chloroform in Drinking Water. Chromatographia. 2004;60:319–322. doi: 10.1365/s10337-004-0387-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.