Abstract
Sclerotinia stem rot (SSR), caused by the fungal pathogen Sclerotinia sclerotiorum, is a devastating disease resulting in yield losses and decreases in seed quality in oilseed rape (Brassica napus) worldwide. However, the molecular mechanisms underlying the response of oilseed rape to S. sclerotiorum infection at the transcriptional and post-transcriptional levels are poorly understood. Here, we used an integrated omics approach (transcriptome, sRNAome, and degradome sequencing) on the Illumina platform to compare the RNA expression and post-transcriptional profiles of oilseed rape plants inoculated or not with S. sclerotiorum. In total, 7,065 differentially expressed genes (DEGs) compared with the mock-inoculated control at 48 hours post inoculation were identified. These DEGs were associated with protein kinases, signal transduction, transcription factors, hormones, pathogenesis-related proteins, secondary metabolism, and transport. In the sRNA-Seq analysis, 77 known and 176 novel miRNAs were identified; however, only 10 known and 41 novel miRNAs were differentially expressed between the samples inoculated or not with S. sclerotiorum. Degradome sequencing predicted 80 cleavage sites with 64 miRNAs. Integrated mRNA, sRNA and degradome sequencing analysis reveal oilseed rape complex responses to S. sclerotiorum infection. This study provides a global view of miRNA and mRNA expression profiles in oilseed rape following S. sclerotiorum infection.
Introduction
Oilseed rape (Brassica napus L.), one of the most economically important crops worldwide, is a valued source of vegetable oil, animal feed, and bioenergy1. Among the fungal diseases affecting oilseed rape, Sclerotinia stem rot, caused by Sclerotinia sclerotiorum (Lib.) de Bary, poses a significant threat to yield and seed quality. S. sclerotiorum is a necrotrophic pathogen, which causes severe disease in over 400 plant species, such as soybean (Glycine max), sunflower (Hellianthus annuus), and chickpea (Cicer arietinum)2. Infection occurs through senescing or injured plant tissues, lower stems, and flower petals3. Necrotic lesions develop quickly throughout the stem and leaves, resulting in wilting, necrosis, stem breakage, yield losses, and decreases in seed quality. S. sclerotiorum can persist in the soil for many years and it is not a good way to suppress the pathogen using fungicides and biocontrol agents. Breeding of resistant oilseed rape cultivars is the most viable option for controlling the disease; however, the molecular mechanisms underlying the interaction between S. sclerotiorum and B. napus are not clear.
Forward genetic approaches, such as quantitative trait loci (QTL) and genome-wide association studies (GWAS), have been used to map candidate genes for S. sclerotiorum resistance, and pathogen-resistant QTLs have been identified on almost every chromosome4–11. Genes encoding mitogen-activated protein kinases (MAPKs)12, WRKY transcription factors (TFs)13,14, and germin-like proteins15 and several plant hormones (e.g., jasmonic acid, salicylic acid, and ethylene)16,17 have been shown to play important roles in the resistance to S. sclerotiorum. RNA sequencing (RNA-Seq) is a powerful tool for characterizing the pathogen response pathways and genes involved in resistance in many crops, including Oryza sativa (rice)18, Triticum aestivum (wheat)19, Sorghum bicolor (sorghum)20, sugarcane21, and cucumber22. Transcriptome studies have shown that B. napus genes involved in the response to pathogen invasion (e.g., genes encoding receptor-like kinases, proteins harboring nucleotide binding site leucine-rich repeats, proteins that function in mitogen-activated protein kinase cascades, G-proteins, calcium binding proteins, hormones, TFs, pathogenesis-related (PR) proteins, proteins involved in phenylpropanoid and glucosinolate metabolism, and transport proteins) were significantly altered following infection10,23,24.
MicroRNAs (miRNAs) are endogenous, non-coding RNAs that play crucial roles in various biological processes at the transcriptional and post-transcriptional level by negatively regulating gene expression25. miRNAs act as key components in gene regulatory pathways associated with many processes, such as germination25, development26, organ maturation27, signal transduction28, and stress responses29. Moreover, several studies have shown that miRNAs function in responses to pathogen invasion29,30. miR393 contributed to the defense response in Arabidopsis thaliana against Pseudomonas syringae pv. tomato (Pst) by suppressing target genes, including auxin receptors, transport inhibitor response 1 (TIR1), auxin signaling F-box protein 2 (AFB2), and AFB3, which negatively regulate auxin signaling29. Furthermore, miR393 acts as a key regulator in the glucosinolate pathway, which is involved in plant responses to pathogens31. In Brassica rapa, miR158 and miR1885 were induced after infection by Turnip mosaic virus (TuMV) and suppressed disease resistance protein genes -Nucleotide-binding site leucine-rich repeat32. MiR396 from A. thaliana functions as a positive regulator during cyst nematode infection33. In Nicotiana tabacum (tobacco), miR6019 and miR6020 guided to incise N genes, which increases resistance to the Tobacco mosaic virus (TMV)34.
Several studies have been conducted to identify miRNAs in oilseed rape. Xie et al.35 first identified 21 potential B. napus miRNAs using computational methods. Later, next generation sequencing technologies were used to identify miRNAs in different tissues or in response to different stresses36–41. There are only 92 mature miRNA sequences of B. napus in the miRBase database (version: 21.0). Compared with the number of miRNAs that have been identified in other species, such as A. thaliana, Arabidopsis lyrata, and Oryza sativa. It is likely that there are many B. napus miRNAs that have not yet been discovered.
The molecular mechanisms underlying the interaction between S. sclerotiorum and B. napus are only partially understood, due to the lack of resistant lines. We identified five relatively resistant lines in a previous study10. To investigate the response of oilseed rape to S. sclerotiorum infection at the mRNA and miRNA levels, we performed RNA-Seq, sRNA-Seq, and degradome deep sequencing on both mock-inoculated and S. sclerotiorum inoculated stems at 48 hours post-inoculation (hpi). We identified differentially expressed genes associated with protein kinases, signal transduction (CDPKs, G proteins, and MAPKs), TFs, hormones, PR proteins, secondary metabolism, and transport proteins. In sRNA-Seq analysis, 10 known and 41 novel miRNAs were differentially expressed at 48 hpi with S. sclerotiorum. Furthermore, 80 cleavage sites with 64 miRNAs were predicted by degradome sequencing. Our data provide a broad view of gene expression changes afterS. sclerotiorum infection in oilseed rape.
Results
Overview of transcriptome sequencing and analysis of gene expression
To identify the expression levels of B. napus genes, cDNA libraries were constructed from the stems of the inoculated plants (48 hours after inoculation with S. sclerotiorum (T48)) and the mock-inoculated control (CK) and paired-end (PE150) sequenced by HiSeqTM 2500. After filtering low quality reads and removing sequences with N, 44,817,708 reads from the T48 and 42,143,988 reads from the CK libraries were obtained. Furthermore, 67.37% of the reads (30,192,484 reads) from the T48 library and 70.26% of the reads (29,611,119 reads) from the CK library were uniquely mapped to the B. napus genome (Table 1).
Table 1.
Summary of Illumina transcriptome sequencing data mapping on B. napus reference genome for CK and T48 libraries.
| Sample | Total Reads | mapped Reads | Uniq Map | Multiple Map | Pair Map | Single Map | Only Map Plus Strand | Only Map Minus Strand |
|---|---|---|---|---|---|---|---|---|
| T48 | 44817708 | 32180724 | 30192484 | 1988240 | 27463572 | 4717152 | 15775761 | 15779373 |
| 100% | 71.80% | 67.37% | 4.44% | 61.28% | 10.53% | 35.20% | 35.21% | |
| CK | 42143988 | 31191889 | 29611119 | 1580770 | 27143836 | 4048053 | 15276040 | 15303754 |
| 100% | 74.01% | 70.26% | 3.75% | 64.41% | 9.61% | 36.25% | 36.31% |
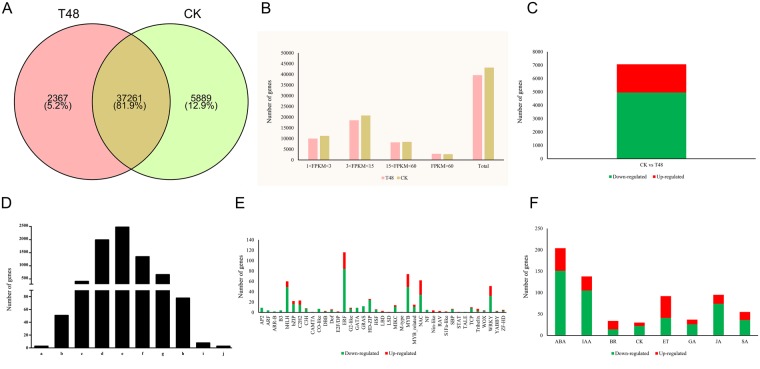
In total, 39,628 and 43,150 genes were detected in the T48 and CK libraries, respectively, using a FPKM (fragments per kilobase of transcript per million fragments mapped) value ≥1 with 37,261 genes in common between the two libraries, and 2,367 and 5,889 unique genes detected in the T48 and CK libraries, respectively (Fig. 1A). Moreover, almost all of the genes in the libraries showed moderate expression levels with only a small percentage of the genes expressed in high levels (Fig. 1B).
Figure 1.
Gene expression profiles in two samples and DEGs identified at 48 hpi after S. sclerotiorum infection. (A) Venn Diagram of genes detected in the two samples. (B) Statistical analysis of gene expressions identified in two samples; (C) Statistical analysis of DEGs identified at 48 hpi after S. sclerotiorum infection; (D) Fold changes in the DEGs identified at 48 hpi after S. sclerotiorum infection. [(a) Number of DEGs with a log2 fold change ≤−9; (b) number of DEGs with −9 < log2 fold change ≤−7; (c) number of DEGs with −7 < log2 fold change ≤−5; (d) number of DEGs with −5 < log2 fold change ≤−3; (e) number of DEGs with −3 < log2 fold change ≤−1; (f) number of DEGs with 1 < log2 fold change ≤3; (g) number of DEGs with 3 <log2 fold change ≤5; (h) number of DEGs with 5 < log2 fold change ≤7; (i) number of DEGs with 7 < log2 fold change ≤9; (j) number of DEGs with log2 fold change ≥9]; (E) Statistical analysis of genes encoding TFs identified in the DEGs; (F) Statistical analysis of genes encoding hormones identified in the DEGs. [ABA: abscisic acid; IAA: auxin; BR: brassinosteroids; CK: cytokinin; GA: gibberellin JA: jasmonic acid; SA: Salicylic acid].
Transcriptomic changes in response to S. sclerotiorum infection
In total, 7,065 genes were identified as DEGs with 4,950 down-regulated and 2,115 up-regulated genes between the inoculated and mock-inoculated samples with a cut-off FDR (false discovery rate) value of <0.001 and |log2 (fold change) | ≥1 (Fig. 1C). As shown in Fig. 1D, more than 92% of the DEGs had a fold change ≤32 and only a few DEGs had a fold change ≥128.
Transcription factors (TFs) play important roles in plant biotic stress responses14. To further analyze the function of the DEGs, we aligned all DEGs against known TFs in the A. thaliana genome. In total, 602 DEGs belonging to 39 TF families were identified with 434 being down-regulated and 168 being up-regulated and the |log2 (fold change)| of most TF genes were between 1 and 5 (Fig. 1E). The TF family members were unevenly distributed; five TF families, ERF (116), MYB (74), NAC (62), bHLH (60), and WRKY (51) accounted for more than 60% (363/602) of all TFs. Furthermore, most members of ERF (84/116), MYB (49/74), WRKY (32/51), bHLH (49/60), bZIP (16/22), and HD-ZIP (24/26) were down-regulated. Plant hormones, such ethylene (ET), jasmonic acid (JA), and salicylic acid (SA), play vital roles in biotic stresses. In total, 685 DEGs belonging to eight classifications of plant hormones were detected with 469 being down-regulated and 216 being up-regulated. Almost all of the ABA (151/204), IAA (105/138), JA (74/95), SA (36/55), GA (26/37), and CK (22/30) genes were down-regulated and the ET and BR genes were up- and down-regulated to similar extents (Fig. 1F).
Functional classifications of the DEGs
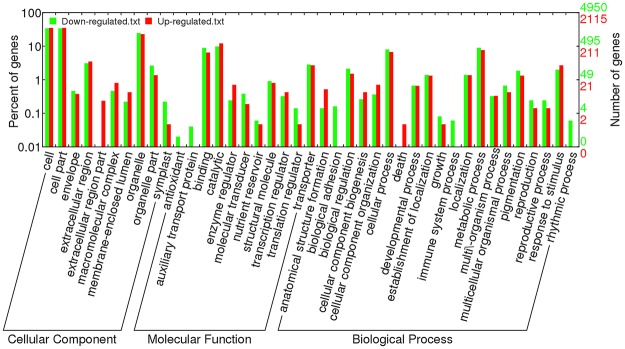
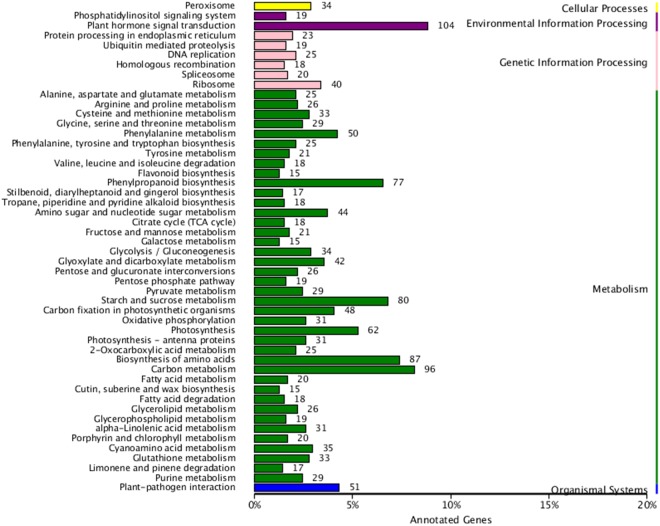
To further explore the functions of the DEGs in response to S. sclerotiorum infection, Gene Ontology (GO) enrichment analysis was conducted on the down- and up-regulated DEGs. We found 10, 11, and 20 functional groups in cellular components (CC), molecular functions (MF), and biological processes (BP), respectively (Fig. 2). Moreover, the dominant functions in each of the three main categories were cellular processes (GO: 0009987) and metabolic processes (GO: 0008152) in the BP category, cell (GO: 0005623), cell part (GO: 0044464), and organelle (GO: 0043226) in the CC category, and binding (GO: 0005488) and catalytic activity (GO: 0008152) in the MF category (Fig. 2). In addition, the DEGs were aligned against the KEGG pathways database to identify pathways that were responsive to S. sclerotiorum infection. In total, 50 pathways were detected in our study including five branches: cellular processes, environmental information processing, genetic information processing, metabolism, and organismal systems. The most abundant pathway in each branch was peroxisome (34), plant hormone signal transduction (104), ribosome (40), carbon metabolism (96), and plant-pathogen interaction (51), respectively (Fig. 3 and Table S1).
Figure 2.
Gene Ontology (GO) classification of DEGs identified at 48 hpi after S. sclerotiorum infection.
Figure 3.
KEGG pathways of DEGs identified at 48 hpi after S. sclerotiorum infection.
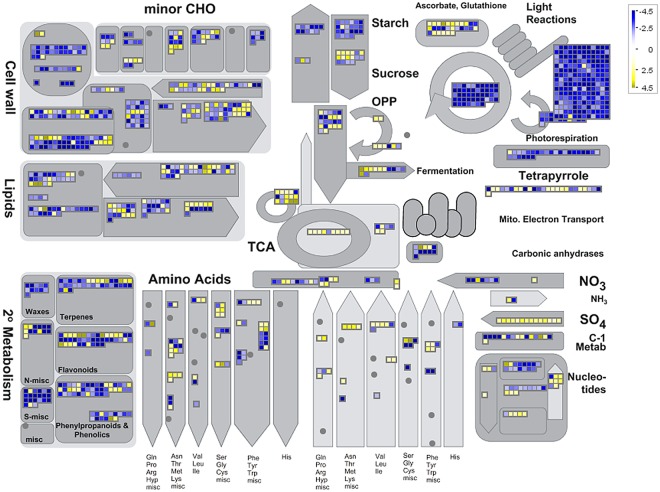
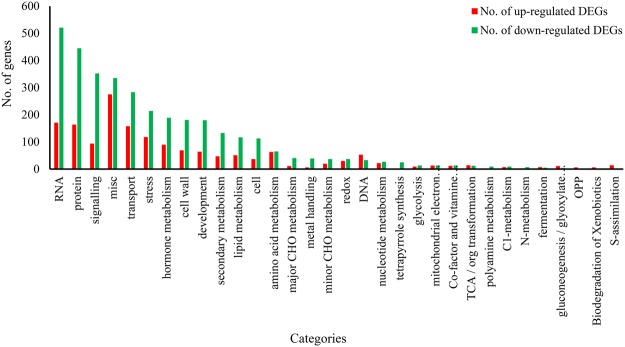
To decipher the mechanisms of the resistance response to S. sclerotiorum infection in B. napus, MapMan software was used to classify and determine the metabolic pathways of the DEGs. As shown in Fig. 4, photosynthesis, glyoxalic acid, and carbon metabolism were suppressed, while cell wall, lipids, and metabolism (sulphur metabolism and amino acid metabolism) were mostly activated. An overview of the DEGs represented in the different processes is provided in Fig. 5 and Table S2.
Figure 4.
Overview of DEGs involved in different metabolic processes at 48 hpi after S. sclerotiorum infection. The images were drew using MapMan and show different functional categories of all DEGs identified in this study. Each box depicts an individual gene. Yellow and blue were represented up-DEGs and down-DEGs, respectively. The scale bar represents fold change values.
Figure 5.
Overview of the total number of DEGs distribution in the changed pathways using the MapMan. The number of up-regulated and down-regulated genes within each category is represented by red and green bars, respectively. See S3 Tables for the detailed information of the changed pathways.
Validation of RNA-Seq results by qRT-PCR
qRT-PCR was used to validate the data from RNA-Seq. In total, 55 genes (hormones, TFs, MAPKs, NBS-LRRs, MLO, and lignin synthesis) were selected for the qPCR assays (Table S3). High correlations were obtained between qPCR and RNA-Seq techniques. The correlation coefficient between qPCR and RNA-Seq data obtained from resistant lines or ZS11 were 0.921 and 0.770 (Fig. 6), emphasizing the reproducibility and reliability of the RNA-Seq data.
Figure 6.
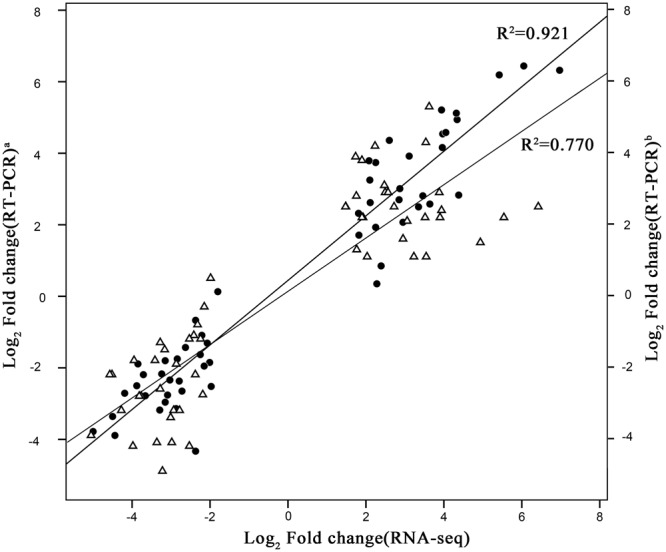
Correlation of gene expression between qPCR and RNA-seq approaches. The qPCR log2 value of the expression ratio (inoculated/mock-inoculated) (y-axis) was plotted against the value from the RNA-seq (x-axis). a, data from resistant lines; b, data from ZS11. All qPCR data were collected from three biological replicates and three technical replicates for each sample.
sRNA library construction
To elucidate the regulatory roles of the miRNAs responsive to S. sclerotiorum infection, two sRNA libraries were constructed using total RNA isolated from the inoculated and mock-inoculated samples. After sequencing, a total of 42,806,114 and 32,205,058 high-quality, raw sequence reads were obtained from the T48 and CK libraries, respectively. After removing adapters, poly-A sequences, and short RNA reads smaller than 18 nucleotides (nt), 26,570,562 and 23,051,268 clean reads were obtained from the T48 and CK libraries, respectively. After further filtering the RFam and Repbase sequences, 15,459,745 (58.18%) and 17,767,719 (77.08%) small RNA sequences from the T48 and CK libraries, respectively, were mapped to the B. napus genome using SOAP2 (Table 2). Similar patterns of length distribution of the sRNAs between the two libraries are shown in Fig. 7, which revealed that the majority of the reads were 21–24 nt in length, and 24-nt reads were the most abundant (Fig. 7). Furthermore, the common and specific of total and unique small RNA sequences were compared between the two libraries), showing that 63.87% and 10.07% of the total sRNAs and unique sRNA were shared, respectively.
Table 2.
Distribution of small RNAs among different categories.
| Types | T48 | CK | ||
|---|---|---|---|---|
| Number | Percentage | Number | Percentage | |
| Total | 26570562 | 100.00% | 23051268 | 100.00% |
| rRNA | 8767858 | 33.00% | 4296731 | 18.64% |
| scRNA | 0 | 0.00% | 0 | 0.00% |
| snRNA | 0 | 0.00% | 0 | 0.00% |
| snoRNA | 21392 | 0.08% | 27068 | 0.12% |
| tRNA | 2231675 | 8.40% | 844134 | 3.66% |
| Repbase | 89892 | 0.34% | 115616 | 0.50% |
| Unannotated | 15459745 | 58.18% | 17767719 | 77.08% |
Figure 7.
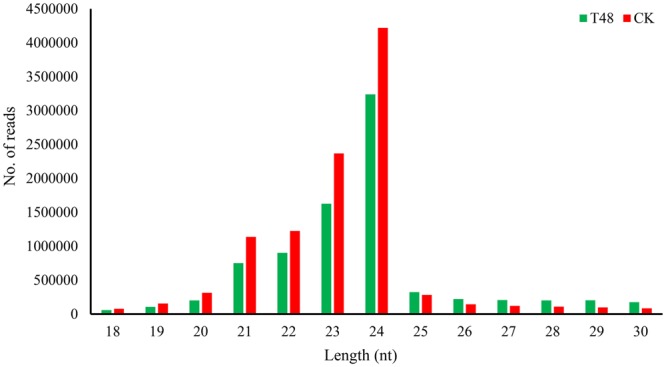
Length distribution of sRNAs detected in two libraries.
Identification of known and novel miRNAs in B. napus
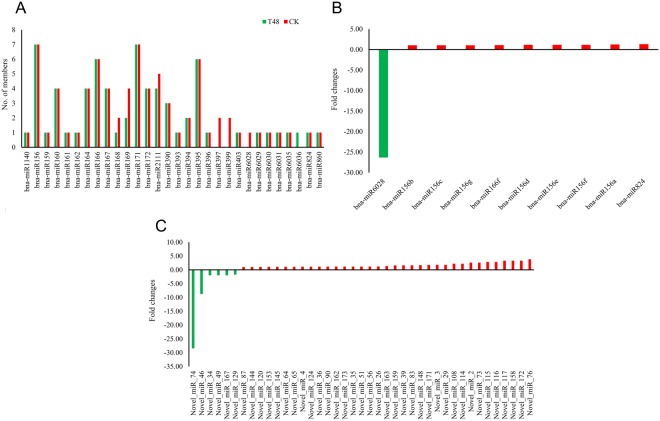
To identify known miRNAs in the two libraries, sRNA sequences obtained from each library were aligned with mature sequences of B. napus miRNAs deposited in miRBase v21.0. A total of 77 known miRNAs belonging to 30 families were identified in the T48 (68 miRNAs) and CK (76 miRNAs) libraries (Table 3); among them, 67 (87.01%) were detected in both libraries. Among the 30 families, bna-miR156 and bna-miR171 had seven members in both libraries, comprising the largest groups, followed by bna-miR166 and bna-miR395 with six members (Fig. 8A). In our study, 33.82% (23 miRNAs) and 38.16% (29 miRNAs) of the miRNAs had more than 100 reads in the T48 and CK libraries, respectively, and 16.18% (11 miRNAs) and 17.11% (13 miRNAs) of the miRNAs had more than 1,000 reads in the T48 and CK libraries, respectively. Among them, bna-miR159 had the highest expression level in both libraries (48,352 and 71,828 reads in the T48 and CK libraries, respectively, Table 3).
Table 3.
The information of known miRNAs identified in CK and T48 libraries.
| Name of miRNAs | Read Counts | Length | Expression Normalization | Mature Sequence | ||
|---|---|---|---|---|---|---|
| T48 | CK | T48 | CK | |||
| bna-miR1140 | 3 | 7 | 21 | 2.69 | 3.46 | ACAGCCTAAACCAATCGGAGC |
| bna-miR156a | 25 | 19 | 21 | 22.41 | 9.40 | TGACAGAAGAGAGTGAGCACA |
| bna-miR156b | 55 | 48 | 21 | 49.29 | 23.74 | TTGACAGAAGATAGAGAGCAC |
| bna-miR156c | 55 | 48 | 21 | 49.29 | 23.74 | TTGACAGAAGATAGAGAGCAC |
| bna-miR156d | 21 | 17 | 20 | 18.82 | 8.41 | TGACAGAAGAGAGTGAGCAC |
| bna-miR156e | 21 | 17 | 20 | 18.82 | 8.41 | TGACAGAAGAGAGTGAGCAC |
| bna-miR156f | 21 | 17 | 20 | 18.82 | 8.41 | TGACAGAAGAGAGTGAGCAC |
| bna-miR156g | 55 | 48 | 21 | 49.29 | 23.74 | TTGACAGAAGATAGAGAGCAC |
| bna-miR159 | 48352 | 71828 | 21 | 43334.40 | 35518.82 | TTTGGATTGAAGGGAGCTCTA |
| bna-miR160a | 42 | 116 | 21 | 37.64 | 57.36 | TGCCTGGCTCCCTGTATGCCA |
| bna-miR160b | 42 | 116 | 21 | 37.64 | 57.36 | TGCCTGGCTCCCTGTATGCCA |
| bna-miR160c | 42 | 116 | 21 | 37.64 | 57.36 | TGCCTGGCTCCCTGTATGCCA |
| bna-miR160d | 42 | 116 | 21 | 37.64 | 57.36 | TGCCTGGCTCCCTGTATGCCA |
| bna-miR161 | 7 | 10 | 21 | 6.27 | 4.94 | TCAATGCACTGAAAGTGACTA |
| bna-miR162a | 1 | 1 | 21 | 0.90 | 0.49 | TCGATAAACCTGTGCATCCAG |
| bna-miR164a | 1075 | 3252 | 21 | 963.44 | 1608.11 | TGGAGAAGCAGGGCACGTGCA |
| bna-miR164b | 235 | 744 | 21 | 210.61 | 367.91 | TGGAGAAGCAGGGCACGTGCG |
| bna-miR164c | 235 | 744 | 21 | 210.61 | 367.91 | TGGAGAAGCAGGGCACGTGCG |
| bna-miR164d | 235 | 744 | 21 | 210.61 | 367.91 | TGGAGAAGCAGGGCACGTGCG |
| bna-miR166a | 5126 | 10238 | 21 | 4594.06 | 5062.67 | TCGGACCAGGCTTCATTCCCC |
| bna-miR166b | 5126 | 10238 | 21 | 4594.06 | 5062.67 | TCGGACCAGGCTTCATTCCCC |
| bna-miR166c | 5126 | 10238 | 21 | 4594.06 | 5062.67 | TCGGACCAGGCTTCATTCCCC |
| bna-miR166d | 5126 | 10238 | 21 | 4594.06 | 5062.67 | TCGGACCAGGCTTCATTCCCC |
| bna-miR166e | 5126 | 10238 | 21 | 4594.06 | 5062.67 | TCGGACCAGGCTTCATTCCCC |
| bna-miR166f | 94 | 80 | 21 | 84.25 | 39.56 | TCGGACCAGGCTTCATCCCCC |
| bna-miR167a | 44 | 89 | 22 | 39.43 | 44.01 | TGAAGCTGCCAGCATGATCTAA |
| bna-miR167b | 44 | 89 | 22 | 39.43 | 44.01 | TGAAGCTGCCAGCATGATCTAA |
| bna-miR167c | 43 | 89 | 21 | 38.54 | 44.01 | TGAAGCTGCCAGCATGATCTA |
| bna-miR167d | 30 | 77 | 20 | 26.89 | 38.08 | TGAAGCTGCCAGCATGATCT |
| bna-miR168a | 1088 | 1681 | 21 | 975.10 | 831.25 | TCGCTTGGTGCAGGTCGGGAA |
| bna-miR168b | 0 | 3 | 21 | 0.00 | 1.48 | TCGCTTGGTGCAGGTCGAGAA |
| bna-miR169a | 0 | 1 | 21 | 0.00 | 0.49 | CAGCCAAGGATGACTTGCCGA |
| bna-miR169b | 0 | 1 | 21 | 0.00 | 0.49 | CAGCCAAGGATGACTTGCCGA |
| bna-miR169m | 24 | 83 | 21 | 21.51 | 41.04 | TGAGCCAAAGATGACTTGCCG |
| bna-miR169n | 10 | 6 | 21 | 8.96 | 2.97 | CAGCCAAGGATGACTTGCCGG |
| bna-miR171a | 3 | 17 | 21 | 2.69 | 8.41 | TTGAGCCGTGCCAATATCACG |
| bna-miR171b | 3 | 17 | 21 | 2.69 | 8.41 | TTGAGCCGTGCCAATATCACG |
| bna-miR171c | 3 | 17 | 21 | 2.69 | 8.41 | TTGAGCCGTGCCAATATCACG |
| bna-miR171d | 3 | 17 | 21 | 2.69 | 8.41 | TTGAGCCGTGCCAATATCACG |
| bna-miR171e | 3 | 17 | 21 | 2.69 | 8.41 | TTGAGCCGTGCCAATATCACG |
| bna-miR171f | 5 | 4 | 21 | 4.48 | 1.98 | TGATTGAGCCGCGCCAATATC |
| bna-miR171g | 5 | 4 | 22 | 4.48 | 1.98 | TGATTGAGCCGCGCCAATATCT |
| bna-miR172a | 1024 | 2651 | 21 | 917.74 | 1310.91 | AGAATCTTGATGATGCTGCAT |
| bna-miR172b | 78 | 204 | 21 | 69.91 | 100.88 | GGAATCTTGATGATGCTGCAT |
| bna-miR172c | 78 | 204 | 21 | 69.91 | 100.88 | GGAATCTTGATGATGCTGCAT |
| bna-miR172d | 290 | 836 | 21 | 259.91 | 413.40 | AGAATCTTGATGATGCTGCAG |
| bna-miR2111a-3p | 0 | 1 | 21 | 0.00 | 0.49 | GTCCTCGGGATGCGGATTACC |
| bna-miR2111a-5p | 3 | 3 | 21 | 2.69 | 1.48 | TAATCTGCATCCTGAGGTTTA |
| bna-miR2111b-3p | 2 | 9 | 21 | 1.79 | 4.45 | ATCCTCGGGATACAGATTACC |
| bna-miR2111b-5p | 3 | 3 | 21 | 2.69 | 1.48 | TAATCTGCATCCTGAGGTTTA |
| bna-miR2111d | 3 | 3 | 21 | 2.69 | 1.48 | TAATCTGCATCCTGAGGTTTA |
| bna-miR390a | 202 | 328 | 21 | 181.04 | 162.20 | AAGCTCAGGAGGGATAGCGCC |
| bna-miR390b | 202 | 328 | 21 | 181.04 | 162.20 | AAGCTCAGGAGGGATAGCGCC |
| bna-miR390c | 202 | 328 | 21 | 181.04 | 162.20 | AAGCTCAGGAGGGATAGCGCC |
| bna-miR393 | 1 | 6 | 21 | 0.90 | 2.97 | TCCAAAGGGATCGCATTGATC |
| bna-miR394a | 935 | 1948 | 20 | 837.97 | 963.28 | TTGGCATTCTGTCCACCTCC |
| bna-miR394b | 935 | 1948 | 20 | 837.97 | 963.28 | TTGGCATTCTGTCCACCTCC |
| bna-miR395a | 4 | 17 | 21 | 3.58 | 8.41 | CTGAAGTGTTTGGGGGAACTC |
| bna-miR395b | 4 | 17 | 21 | 3.58 | 8.41 | CTGAAGTGTTTGGGGGAACTC |
| bna-miR395c | 4 | 17 | 21 | 3.58 | 8.41 | CTGAAGTGTTTGGGGGAACTC |
| bna-miR395d | 5 | 9 | 21 | 4.48 | 4.45 | CTGAAGTGTTTGGGGGGACTC |
| bna-miR395e | 5 | 9 | 21 | 4.48 | 4.45 | CTGAAGTGTTTGGGGGGACTC |
| bna-miR395f | 5 | 9 | 21 | 4.48 | 4.45 | CTGAAGTGTTTGGGGGGACTC |
| bna-miR396a | 1193 | 2099 | 21 | 1069.20 | 1037.95 | TTCCACAGCTTTCTTGAACTT |
| bna-miR397a | 0 | 5 | 22 | 0.00 | 2.47 | TCATTGAGTGCAGCGTTGATGT |
| bna-miR397b | 0 | 5 | 22 | 0.00 | 2.47 | TCATTGAGTGCAGCGTTGATGT |
| bna-miR399a | 0 | 1 | 21 | 0.00 | 0.49 | TGCCAAAGGAGATTTGCCCGG |
| bna-miR399b | 0 | 1 | 21 | 0.00 | 0.49 | TGCCAAAGGAGATTTGCCCGG |
| bna-miR403 | 3727 | 5914 | 21 | 3340.24 | 2924.46 | TTAGATTCACGCACAAACTCG |
| bna-miR6028 | 0 | 17 | 21 | 0.00 | 8.41 | TGGAGAGTAAGGACATTCAGA |
| bna-miR6029 | 295 | 396 | 21 | 264.39 | 195.82 | TGGGGTTGTGATTTCAGGCTT |
| bna-miR6030 | 145 | 159 | 22 | 129.95 | 78.63 | TCCACCCATACCATACAGACCC |
| bna-miR6031 | 27 | 61 | 24 | 24.20 | 30.16 | AAGAGGTTCGGAGCGGTTTGAAGC |
| bna-miR6035 | 3 | 4 | 21 | 2.69 | 1.98 | TGGAGTAGAAAATGCAGTCGT |
| bna-miR6036 | 1 | 0 | 24 | 0.90 | 0.00 | ATAGTACTAGTACTTGCATGATCA |
| bna-miR824 | 270 | 196 | 21 | 241.98 | 96.92 | TAGACCATTTGTGAGAAGGGA |
| bna-miR860 | 7 | 2 | 21 | 6.27 | 0.99 | TCAATACATTGGACTACATAT |
Figure 8.
The number of known miRNAs members and differentially expressed miRNAs between CK and T48 libraries. (A) Number of family members per miRNA family detected in the two libraries. (B) expressed differentially of known miRNAs; (C) expressed differentially of novel miRNAs.
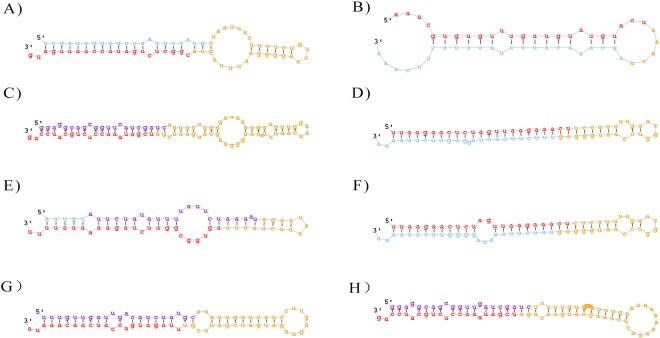
To predict novel miRNAs in B. napus from the two libraries, “unannotated” reads were aligned with the B. napus genome. A total of 176 novel miRNA candidates were obtained from the T48 (175 miRNAs) and CK (176 miRNAs) libraries; among them, Novel_miR_74 was expressed only in the CK library. The mature miRNAs were 20–25 nt in length with 21-nt reads being the most abundant (Table S4). The minimal folding energy (MFE) of these predicted pre-miRNAs ranged from −30.4 kcal/mol to −208.3 kcal/mol (Table S4). Among them, 36 (20.6%) and 55 (31.3%) miRNA candidates had more than 1,000 reads in the T48 and CK libraries, respectively. Most of these novel miRNAs were more highly expressed than the known miRNAs. Information about the miRNA candidates identified from the two libraries is summarized in Table S4 and the hairpin structures for the precursors of eight novel miRNAs were chosen as examples and are shown in Fig. 9.
Figure 9.
Predicted secondary structures of novel miRNAs in our study. The mature miRNAs are in red. (A) Novel_miR_2; (B) Novel_miR_4; (C) Novel_miR_11; (D) Novel_miR_22; (E) Novel_miR_119; (F) Novel_miR_133; (G) Novel_miR_137; (H) Novel_miR_147.
Differentially expressed miRNAs after S. sclerotiorum infection
To screen for S. sclerotiorum-responsive miRNAs, differential expression analysis of the miRNAs was performed between the T48 and CK libraries, based on the normalized read count for each identified miRNA. In our study, 10 known and 41 novel miRNAs were identified as differentially expressed (DE) miRNAs based on the following criteria: fold change (≥−2 or ≤2) and FDR-value (≤0.001). Among them, only one known (bna-miR6028) and six novel (Novel_miR_34, Novel_miR_46, Novel_miR_49, Novel_miR_74, Novel_miR_129, and Novel_miR_167) DE miRNAs were down-regulated after S. sclerotiorum infection. To confirm the reliability of the data produced through deep sequencing, qRT-PCR was conducted. Fifteen miRNAs including two conserved miRNAs (miR6028 and miR156f) and 13 novel miRNAs (Novel_miR_7, Novel_miR_11, Novel_miR_13, Novel_miR_44, Novel_miR_45, Novel_miR_52, Novel_miR_76, Novel_miR_93, Novel_miR_119, Novel_miR_140, Novel_miR_167, Novel_miR_172, and Novel_miR_176) from miRNAs detected in our study were selected (Fig. 10). As expected, the qRT-PCR results were consistent with the deep sequencing results.
Figure 10.
RT-qPCR validation of the miRNAs. The expression values presented are the means of three technical replicates. U6 was used for each sample as an endogenous control. (A,B) Represent the expression pattern of mature conserved and novel miRNAs, respectively.
Target prediction of DE miRNAs
To gain insights into functions of DE miRNAs, corresponding target genes were predicted using bioinformatics analysis. Among these target genes, only 15 genes were DEG in our RNA-Seq analysis (Table 4). Of them, eight targets (encoding squamosa promoter-like proteins and phototropic-responsive NPH3 family protein) were targeted by miR156, four genes (encoding revolute and homeobox gene 8 proteins) were targeted by miR166f and the rest three were novel miRNAs (Novel_miR_87/126/158). Interestingly, all 15 targets were reverse expression patterns with corresponding miRNAs but BnaA08g11090D, which was encoding CAN OF WORMS1 (COW1).
Table 4.
DE miRNAs with corresponding DE genes in our study.
| DE-miRNAs | log2FCa | Targets | log2FCb | AGI accession | Functions |
|---|---|---|---|---|---|
| bna-miR156a-g | 1.05–1.25 | BnaA04g27550D | −2.54 | AT3G57920 | squamosa promoter binding protein-like 15 (SPL15) |
| bna-miR156a-g | 1.05–1.25 | BnaA06g36780D | −2.15 | AT5G43270 | squamosa promoter binding protein-like 2 (SPL2) |
| bna-miR156a-g | 1.05–1.25 | BnaA09g27960D | −2.45 | AT1G27360 | squamosa promoter-like 11 (SPL11) |
| bna-miR156a-g | 1.05–1.25 | BnaC04g02520D | −4.13 | AT2G42200 | squamosa promoter binding protein-like 9 (SPL9) |
| bna-miR156a-g | 1.05–1.25 | BnaC07g17030D | −1.83 | AT5G43270 | squamosa promoter binding protein-like 2 (SPL2) |
| bna-miR156d/e/f | 1.16 | BnaAnng03450D | −2.59 | AT3G44820 | Phototropic-responsive NPH3 family protein |
| bna-miR156d/e/f | 1.16 | BnaC01g23990D | −2.00 | AT3G44820 | Phototropic-responsive NPH3 family protein |
| bna-miR156d/e/f | 1.16 | BnaC06g32630D | −3.83 | AT1G71690 | Protein of unknown function (DUF579) |
| bna-miR166f | 1.09 | BnaA02g06170D | −2.50 | AT5G60690 | REVOLUTA (REV) |
| bna-miR166f | 1.09 | BnaA08g11980D | −4.24 | AT4G32880 | homeobox gene 8 (HB-8) |
| bna-miR166f | 1.09 | BnaA10g13520D | −2.06 | AT5G60690 | REVOLUTA (REV) |
| bna-miR166f | 1.09 | BnaAnng30670D | −3.68 | AT4G32880 | homeobox gene 8 (HB-8) |
| Novel_miR_87 | 1.02 | BnaC03g36760D | −2.13 | AT3G10740 | alpha-L-arabinofuranosidase 1 (ASD1) |
| Novel_miR_129 | −1.72 | BnaA08g11090D | −2.30 | AT4G34580 | CAN OF WORMS1 (COW1) |
| Novel_miR_158 | 3.32 | BnaC02g06060D | −5.89 | AT5G15450 | casein lytic proteinase B3 (CLPB3) |
Note: a and b: log2FC values of miRNAs and genes, respectively.
Degradome analyses
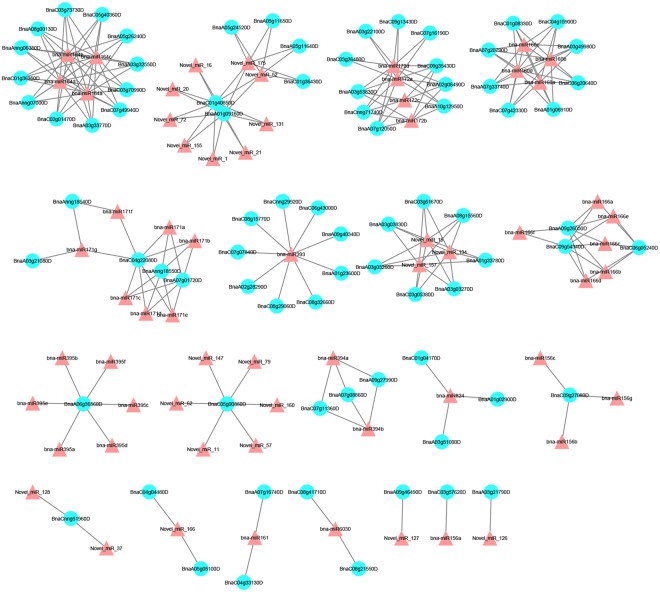
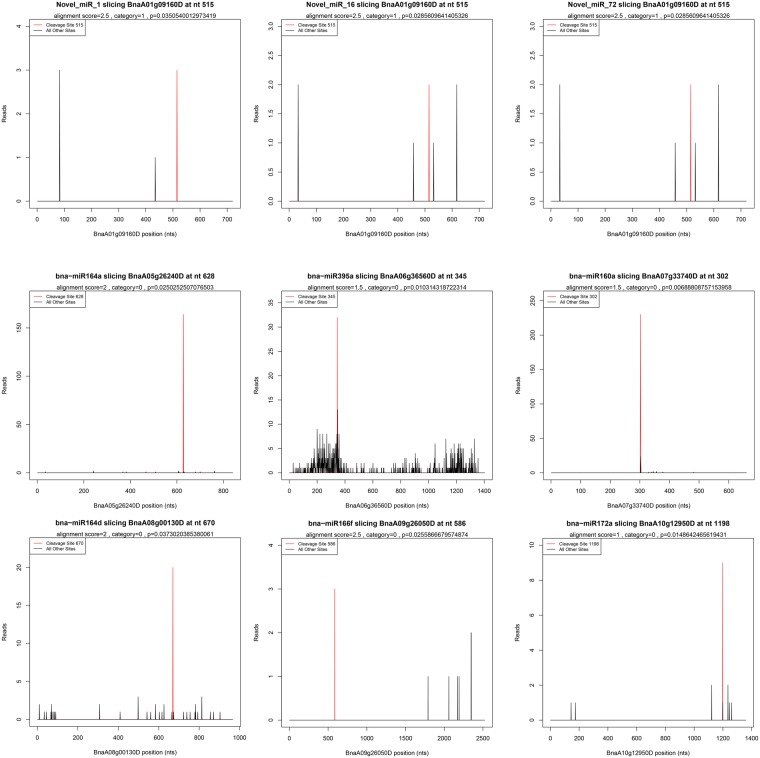
To identify the potential functions of the miRNAs found in our study, two degradome libraries were constructed to identify the targets of the miRNAs. After sequencing, a total of 13,835,867 and 12,764,316 raw reads with 12,936,736 (93.50%) and 11,910,513 (93.31%) clean reads were obtained in the T48 and CK libraries, respectively. The clean tags were mapped to the B. napus reference genome database and Rfam database using SOAP2.20 software42. Using the Cleave-Land v3.0.1 pipeline43, a total of 80 (49 in the T48 and 57 in the CK libraries) cleavage sites with 64 miRNAs were predicted with a P-value < 0.05 (Table S5). Most of the targets of the known miRNAs were TFs (Table S5). For example, SBP (bna-miR156), ARF (bna-miR160), NAC (bna-miR164), TOE (bna-miR172), bHLH (bna-miR393), AGL16 (bna-miR824), AFB2/3 (bna-miR393), GRAS (bna-miR171), TIR1 (bna-miR393), and RAP (bna-miR172) were detected in our study (Table S5). Many TFs were also detected in the targets of the novel miRNAs, including DCL1 (Novel_miR_11/57/62/79/147/160), TCP (Novel_miR_1/16/20/21/52/72/131/155/175), and MYB (Novel_miR_37/126/128) (Table S5). Additionally, eight disease resistance protein (TIR-NBS-LRR class) family genes were cleaved by three novel miRNAs (Novel_miR_15/134/157). The interactions between the miRNAs and their targets were presented using cytoscape software (Fig. 11). Nine random miRNA:mRNA pairs were chosen as examples and corresponding t-plots were constructed (Fig. 12).
Figure 11.
A miRNA-mediated S. sclerotiorum-responsive regulatory network. Triangles represent the differentially expressed miRNAs and Blue circles represent the target genes of differentially expressed miRNAs.
Figure 12.
Target plots (t-plots) of miRNAs targets confirmed by degradome sequencing. In t-plots, the red lines indicate the miRNA-directed cleaved transcript. The X axis indicates the nucleotide position in target cDNA. The Y axis indicates the number of reads of cleaved transcripts detected in the degradome cDNA library. “alignment score” is the score for mismatch. Score = 0 represents perfect match and G:U = 0.5.
Discussion
Due to a lack of resistant germplasm, Sclerotinia stem rot, caused by the pathogen S. sclerotiorum, results in yield and economic losses in oilseed rape worldwide each year. A better understanding of the molecular mechanisms of resistance to the S. sclerotiorum pathogen is imperative for long-term, sustainable production of oilseed rape. In our previous study, five relatively resistant B. napus lines were identified10. In this study, we conducted deep sequencing at the transcriptional and post-transcriptional levels to explore the defense response to S. sclerotiorum infection in the relatively resistant lines. Our data showed that a coordination among different pathways is involved in the resistance response in oilseed rape at the transcriptional and post-transcriptional levels and further analysis is needed regarding candidate genes, miRNAs, or targets identified in our study.
Previous studies on the transcriptomic changes in oilseed rape in response to S. sclerotiorum infection were primarily conducted using oligonucleotide microarrays. More than 300 DEGs involved in JA biosynthesis and signaling, reactive oxygen species metabolism, and cell wall structure and function were identified using microarrays44. Later, 686 and 1,547 DEGs in resistant and susceptible B. napus genotypes, respectively, associated with S. sclerotiorum infection using an Arabidopsis microarray were detected39. Changes in the expression of PR proteins, kinases, transporters, JA, ET, cell wall proteins, and unknown proteins were identified. Researchers further investigated altered gene expression in ZhongYou 821 (ZY821, a partially resistant line) and Westar (a susceptible cultivar) after S. sclerotiorum infection using a B. napus microarray17. Differential gene expression was identified in genes encoding defense proteins such as osmotins, chitinases, lectins, glucanases, JA, ET, auxin, zinc fingers, WRKYs, AP2, and MYB TFs. Genes involved in molecular processes, such as carbohydrate and energy metabolism, and glucosinolate and phenylpropanoid biosynthesis were also discussed. In our study, genes encoding protein kinases, signal transduction proteins (CDPKs, G proteins, and MAPKs), TFs (WRKYs and MYBs), hormones (JA and ET), PR proteins, secondary metabolism, transporters, P450, UGTs, and lipid metabolism were found to be differentially expressed between the S. sclerotiorum inoculated and mock-inoculated samples (Tables S6–S13). In other plant-pathogen interactions, such as flax-Fusarium oxysporum45, banana-F. oxysporum46 and cabbage-F. oxysporum47, the defense proteins or pathways discussed above were also changed.
Protein kinase genes involved in the recognition of S. sclerotiorum
The receptor-like kinase (RLK) family plays important roles in the B. napus-S. sclerotiorum interaction. More than 1,200 RLK homologs genes in B. napus were identified using 300 AtRLKs (http://www.arabidopsis.org/browse/genefamily/Receptor_kinase. jsp) as queries24. In our study, the expression of 214 BnRLK genes was altered after inoculation with S. sclerotiorum, with 158 genes being down-regulated and 56 genes being up-regulated (Table S6). Nucleotide binding site leucine-rich repeat (NBS-LRR) genes, characterized as R genes, have been found to be involved in plant-pathogen interactions48. In our study, the expression of 29 NBS-LRR genes was altered after inoculation with S. sclerotiorum, with 21 genes being down-regulated and 8 genes being up-regulated (Table S6).
Signal transduction is involved in B. napus-S. sclerotiorum interactions
Mitogen-activated protein kinase (MAPK) cascades, which consist of the MAPKKK-MAPKK-MAPK module, have been characterized in the response to pathogen infection49. In the current study, 28 MAPKKK, one MAPKK, and five MAPK genes were induced at 48 hours after inoculation with S. sclerotiorum. Among these, 21 MAPKKK and five MAPK genes were down-regulated, and one MAPKK (BnaC02g22230D) gene was up-regulated by more than 16-fold at 48 hpi with S. sclerotiorum (Table S7). G-proteins transduce detection signals from RLKs50. In our study, seven G-protein transcripts were up-regulated at 48 hpi with S. sclerotiorum (Table S7). Calcium (Ca2+), acting as an essential second messenger, is involved in the plant’s response to abiotic and biotic stresses51. In our study, 91 calcium-related genes, such as calcium-binding EF-hand family proteins, calcium-dependent protein kinase (CDPK), and calmodulin binding protein (CAM), showed differential expression (most of them were down-regulated) (Table S7). In another gene family, MILDEW RESISTANCE LOCUS O (MLO), MLO2, MLO8, and MLO12 were down regulated, while three copies of MLO6 were up regulated in the S. sclerotiorum-inoculated samples. (Table S7).
Transcriptional regulation
TFs play crucial roles in response to pathogen attack52, and 602 TFs belonging to 39 families were identified in this study (Fig. 1E). In total, 51 WRKY genes were differently expressed after S. sclerotiorum infection. Among these, 18 genes (one WRKY3, three WRKY6s, one WRKY 14, two WRKY28s, two WRKY29s, two WRKY42s, one WRKY57, one WRKY61, one WRKY72, and four WRKY75s) were up-regulated (Table S8). MYB TFs, which comprise a large TF family, have central roles in pathogen responses53. In this study, 67 MYB TF transcripts were differentially expressed at 48 hpi. Of them, 25 MYBs genes were up-regulated in the S. sclerotiorum-inoculated plants (Table S8).
Hormone regulation
Besides their functions in plant growth and development, plant hormone-regulated genes have important roles in plant biotic stress response54. The biosynthesis or signal transduction of hormones, such as CK, IAA, BR, SA, JA, ET, ABA, and GA, were affected at 48 hpi with S. sclerotiorum (Fig. 1F). As a positive regulator, JA plays important roles in plant disease resistance55. In this study, several transcripts encoding proteins involved in JA biosynthesis, including jasmonic acid carboxyl methyltransferase (JMT), allene oxide synthase (AOS), allene oxide cyclase 2 (AOC2), fatty acid desaturase (FAD), and lipoxygenase (LOX) family proteins, were differently expressed in the S. sclerotiorum infected samples (Table S9). Five transcripts encoding jasmonate-zim-domain protein 3 (JAZ3), which negatively regulates JA transcriptional activity, were down-regulated at 48 hpi with S. sclerotiorum (Table S9). ET, which is involved in the regulation of growth and development, acts synergistically with JA in responses to environmental stresses54. In our study, key enzymes involved in ET biosynthesis, including 1-aminocyclopropane-1-carboxylatesynthase (ACS) and 1-aminocyclopropane-1-carboxylateoxidase (ACO), were up-regulated in the S. sclerotiorum-inoculated samples, except for one copy (BnaC02g15560D) of ACS12. Moreover, ET signaling pathway genes, including ethylene response sensor (ERS), ethylene response factor (ERF), and octadecanoid-responsive Arabidopsis AP2/ERF 59 (ORA59), and their regulated genes, such as pathogenesis-related 4 (PR4) and plant defensin 1.2 (PDF1.2), were detected in our study (Table S9).
PR proteins
Pathogenesis-related genes (PRs) play central roles in disease resistance56. In our study, the expression of 19 genes encoding pathogenesis-related 4 (PR4), pathogenesis-related family protein, pathogenesis-related gene 1 (PR1), and pathogenesis-related thaumatin superfamily proteins, was altered. Of them, eight genes encoding pathogenesis-related thaumatin superfamily proteins were down-regulated and the rest were up-regulated in the S. sclerotiorum-inoculated samples (Table S10). Chitinase degrades chitin, which is the primary structural component of fungal cell walls57. Seventeen genes encoding chitinase proteins were induced after S. sclerotiorum infection (Table S10). Among these, two genes (BnaC07g30330D and BnaA06g26630D) encoding chitinase A (CHIA) and one gene (BnaA05g03440D) encoding a chitinase family protein were down-regulated at 48 hpi, while the other 14 genes encoding chitinase family proteins were up-regulated.
Secondary metabolism
Secondary metabolites play crucial roles in plant defense against pathogen attack58. Genes involved in the biosynthesis of phenylpropanoids, flavonoids, isoprenoids, glucosinolates, and wax were differentially expressed after S. sclerotiorum infection in our study.
Phenylpropanoid is one of the most important secondary metabolites involved in stress-defense59. Fourty-three genes encoding components of phenylpropanoid metabolism were detected and 12 of them were up-regulated at 48 hpi with S. sclerotiorum. In addition, 21 genes involved in lignin formation (including cinnamicacid 3-hydroxylase (C3H), cinnamicacid 4-hydroxylase (C4H), cynnamoyl-CoA reductase (4CL), cinnamyl alcohol dehydrogenase (CAD), caffeoyl-CoA O-Methyltransferase (CCoAOMT), cinnamoyl-CoA reductase1 (CCR1), caffeic acid O-methyltransferase (COMT), ferulate 5-hydroxylase (F5H), and shikimate quinatehydroxy cinnamoyl transferase (HCT)) were differently expressed in our study (Table S11).
Owning to their high antioxidant capacity, flavonoids have been used in transgenic engineering to increase resistance to pathogen attack60. Fourty-two key genes encoding proteins related to flavonoid biosynthesis, including flavonol synthase, chalcone synthase, flavonoid 3″-monooxygenase, chalcone isomerase, anthocyanin 5-aromatic acyltransferase, and dihydro flavonol reductase (DFR), were differentially expressed in the S. sclerotiorum-infected samples. Ten out of 14 genes involved in anthocyanin biosynthesis were up-regulated in the S. sclerotiorum-infected samples, and two genes, BnaA04g03320D and BnaC04g25200D, were jasmonate-regulated gene 21 (JRG21) genes with a 5.65 and 9.35 log2-fold increase, respectively. Interestingly, all seven chalcones synthesis genes and all eight dihydroflavonols genes, including TRANSPARENT TESTA, DFR, and F3H, were down-regulated at 48 h after S. sclerotiorum infection. Furthermore, six out of 13 genes associated with flavonol biosynthesis were increased in the S. sclerotiorum-inoculated plants (Table S11).
Isoprenoids, which are involved in plant growth and development, have specialized functions in response to pathogen attack61. Thirty-five genes related to the metabolism of carotenoids, terpenoids, and tocopherol biosynthesis were differentially expressed at 48 hpi with S. sclerotiorum. Most of them, including terpene synthase 21 (TPS21), LUTEIN DEFICIENT 2 (LUT2), and zeta-carotene desaturase (ZDS), were down-regulated in the S. sclerotiorum-inoculated plants. Three copies of phytoene desaturation 1 (PDS1), involved in tocopherol biosynthesis, had a 3.05 to 4.32 log2-fold increase (Table S11).
Genes related to glucosinolate synthesis, another secondary metabolite, were found to be differentially expressed in our study. Two genes involved in glucosinolate synthesis were induced while 19 genes were suppressed at 48 hpi with S. sclerotiorum (Table S11). The two up-regulated genes, BnaC03g30410D and BnaCnng43090D, encoding AOP1 and SUR1, respectively, are responsible for the synthesis of indole glucosinolates62.
Transport
Altered expression was observed in genes involved in transport pathways, such as ABC transporters, major intrinsic proteins (MIPs), and amino acid transporters/permeases at 48 hpi with S. sclerotiorum.
Multidrug transporters, comprised of MATE efflux proteins and ABC transporters, remove noxious compounds from cells63. Fifty-five multidrug transporter genes were found to have altered expression in this study, including 26 with increased abundance after S. sclerotiorum infection. Of the multidrug transporter genes, seven MATE efflux family proteins, nine multidrug resistance-associated proteins (MRPs), eight pleiotropic drug resistance proteins (PDR), nine ATP-binding cassette proteins (ABC), two ABC transporter family proteins, and 11 ABC-2-type transporter family proteins aid in secondary metabolite transport to resist pathogens (Table S12).
MIPs, including NOD26-like intrinsic protein (NIPs), plasma membrane intrinsic proteins (PIPs), and tonoplast intrinsic proteins (TIPs), play crucial roles in water and solute transport and plant stress responses64. Seven NIPs, 16 PIPs, and seven TIPs were differently expressed at 48 hpi with S. sclerotiorum (Table S12), with most of these genes being down-regulated. These expression changes may be regulated by the pathogen to improve invasion into plant tissues65; alternatively, these membrane proteins could promote the conduction of hydrogen peroxide (H2O2) to support defense signaling66.
In our study, 28 differentially expressed amino acid transporter genes were detected, including amino acid permeases (AAPs), bidirectional amino acid transporter 1 (BAT1), cationic amino acid transporter (CAT), and transmembrane amino acid transporter family proteins (Table S12). Two transcripts encoding AAP3 (BnaC06g38080D and BnaA07g33510D) were up-regulated at 48 hpi with S. sclerotiorum.
Other genes
Differential expression of numerous other gene groups involved in the response to pathogen attack were identified in our study. Many cytochrome family protein (CYP) P450 genes, a large group of genes involved in diverse metabolic processes, were up-regulated (about 50%) at 48 hpi with S. sclerotiorum (Table S13). Many UDP-glucosyl transferase family proteins (UGTs), which transfer UDP-glucose to diverse secondary metabolites or hormones, were up-regulated at 48 hpi with S. sclerotiorum. One transcript, BnaA01g03020D encoding UGT73B3, which is involved in abscisic acid glucosyltransferase, was the most up-regulated of the UGTs with a 6.59 log 2-fold change (Table S13). Groups of genes involved in modifying or degrading other proteins were found to be differently expressed. These transcripts encode enzymes including 11 aspartate proteases, 20 cysteine proteases, 16 serine proteases, 14 subtilases, and 168 ubiquitin genes. Most of these genes were down-regulated in the S. sclerotiorum-infected samples (Table S13). Finally, DEGs encoding enzymes involved in lipid metabolism were also observed. For example, seven transcripts encoding alpha beta hydrolases were up-regulated while all five SHAVEN 3 (SHV3) genes were down-regulated at 48 hpi with S. sclerotiorum (Table S13).
As post-transcriptional gene regulators, miRNAs are involved in plant development and various stress responses, including plant–fungus interactions, by suppressing corresponding target genes67. Using next generation sequencing technologies, several fungi-responsive miRNAs were identified in A. thaliana68, Paulownia69, wheat70, and oilseed rape41. However, only several studies to date have reported using deep sequencing approaches to identify miRNAs and their expression in B. napus in response to S. sclerotiorum infection. What noteworthy is that 280 B. napus miRNA candidates small RNAs from both normal and S. sclerotiorum inoculated leaves were identified using high-throughput deep sequencing technologies and over-expression of AGO1 and AGO2 could decrease resistance to S. sclerotiorum in oilseed rape71. In the current study, sRNA-seq approach was used to identify the miRNAs responsive to S. sclerotiorum infection and degradome sequencing was performed to identify the targets of corresponding miRNAs. In our study, 77 known and 176 novel miRNAs were detected (Tables 2, S4), and 10 known and 41 novel miRNAs were considered as differentially expressed miRNAs after S. sclerotiorum infection (Fig. 8B,C).
In plants, miRNAs play key roles in many biological processes and in the response to various stresses by regulating their corresponding target genes29. Many of the target genes of miRNAs are TFs72. In our degradome sequencing data, miRNA393, an important miRNA which is involved in many processes, cleaved seven genes encoding auxin signaling F-box 2/3 (AFB2/3), TRANSPORT INHIBITOR RESPONSE 1 (TIR1), and basic helix-loop-helix (bHLH) DNA-binding superfamily proteins with diverse functions. A previous study on the interactions between auxin signaling and miR393 showed that over-expression of miR393 in A. thaliana lines resulted in greater susceptibility to necrotrophic pathogens, and that auxin signaling repressed SA levels and signaling29. Furthermore, higher glucosinolate contents and lower camalexin levels were also detected in the miR393 over-expression lines31. In B. napus–V. longisporum interactions, modulation of the miR168-Argonaute 1 (AGO1) interaction played key regulatory roles41. Unfortunately, miR168 was not differentially expressed in our study, and its target, AGO1, was not detected. bna-miR6028, which targets receptor like protein 27 (RLP27) and jasmonate-zim-domain protein 10, which are responsive to stress and wounding73, was significantly down-regulated at 48 hpi with S. sclerotiorum. The target genes of bna-miR164 and bna-miR395, which encode NAC proteins and AOC2, respectively, were responsive to oxidative stress74, cold75, and salt stress76. Taken together, the results illustrated that cross-talk exists between miRNAs and target gene pathways, thereby regulating the response to fungal pathogens and other stresses in B. napus.
Materials and Methods
Fungal and plant materials
A strain of S. sclerotiorum 1980 was provided by Dr. Jiaqin Mei, Chongqing Engineering Research Center for Rapeseed, College of Agronomy and Biotechnology, Southwest University, and was cultured on potato dextrose agar media (PDA, Becton Dickinson, Sparks, MD, USA) at 22 °C in darkness. Five relatively resistant B. napus lines used in this experiment were identified and selected in our previous study10. Three plants per line and two sites per plant were used to detect the response to S. sclerotiorum infection and bulked for mRNA, sRNA and degradome analysis. Pathogen inoculation and tissue harvest were conducted following the procedures described in our previous study10. For verifying its accuracy, ZS11 was used to detect genes response to S. sclerotiorum infection. Stem tissues from inoculated (48 hpi) and control plants were obtained, frozen immediately in liquid nitrogen, and stored at −80 °C.
RNA isolation and high-throughput sequencing
Total RNA was extracted from stems of each treatment (inoculated and mock-inoculated controls) using TRIZOL reagent (TianGen, China) according to the manufacturer’s instructions. At least five plants per treatment and two sites per plant (10 sites) were mixed for each sample. DNA contamination was removed from the total RNA using RNA-free DNase I (Promega). A Qubit Fluorometer was used to determine the RNA quantity and quantity and an Agilent 2100 Bioanalyzer was used to detect the RNA purity and integrity. Two RNA samples were sent to Beijing Biomarker Technologies Co., Ltd. (Beijing, China) for mRNA, sRNA, and degradome sequencing using an Illumina HiSeqTM 2500. The original mRNA, sRNA, and degradome sequencing data were deposited in the NCBI Sequence Read Archive with accession nos. SRP096626, SRP096847, and SRP0596850, respectively.
mRNA sequencing data analysis
After removal of the adapter sequences and low quality sequences, clean reads were mapped to the B. napus reference genome (http://www.genoscope.cns.fr/brassicanapus/) and were then assembled using TopHat 2.0.0 and DEGseq. Gene expression levels were estimated using the FPKM (fragments per kilobase of exon per million mapped fragments) values and DEGs were identified with Cuffdiff using the following two criteria: (i) false discovery rate (FDR) p-value correction of <0.05 and (ii) |log2 (fold change)| >1.
To screen for possible TF and plant hormone genes, the DEGs were aligned to known TF and plant hormone genes, which were downloaded from The Plant Transcription Factor Database (http://planttfdb.cbi.pku.edu.cn/index.php) and the Arabidopsis Hormone Database 2.0 (http://ahd.cbi.pku.edu.cn/), respectively.
Identification of known and novel miRNAs in B. napus
The clean reads were obtained after removing low-quality reads, contaminants, and adaptors using the SOAPnuke software (http://soap.genomics.org.cn/)42. The length distribution of the clean reads was categorized and then the clean reads were immediately used to search GenBank and the Rfam 11.0 database (http://rfam.sanger.ac.uk/) to annotate the noncoding RNAs. Subsequently, the sRNAs were aligned to miRNA precursors of rapeseed in miRBase 21.0 to obtain known miRNAs based on the criteria of our previous publication25. The remaining unannotated reads were used to predict novel miRNAs using criteria described before77.
Analysis of differential expression conserved and novel miRNAs and target prediction
The expression of each miRNA was normalized using the formula: normalized expression = actual miRNA count/total count of clean reads (×1,000,000). The fold change of each miRNA after inoculation was calculated as (fold change = log2 (miRNAT48/miRNACK)). miRNAs with fold changes ≥1 or ≤−1 and with P ≤ 0.01 were regarded as up-regulated or down-regulated, respectively. The TargetFinder software was used for target prediction of significantly differentially expressed miRNAs in response to S. sclerotiorum infection using the previously described scoring system and criteria25.
Degradome sequencing data analysis
For degradome sequencing, two library constructions were performed on an Illumina HiSeq2500 sequencing system according to the manufacturer’s instructions. Clean reads were filtered from the raw data and sRNAs were aligned to GenBank and Rfam 11.0 databases for annotation of the cleaved target genes. Furthermore, CleaveLand v3.0.1 (August 26, 2011)78 was used to predict the miRNA cleavage sites and T-plot figures were drawn.
Functional annotation of DEGs and target genes
For annotation of gene function, GOatools (https://github.com/tanghaibao/GOatools) was used to analyze Gene Ontology (GO) term enrichment for the DEGs and the predicted targets identified above, and enriched terms, in which the FDR was less than 0.01, were displayed using the online tool WEGO (Web Gene Ontology annotations Plot, http://wego.genomics.org.cn). The KOBAS2.0 website (http://kobas.cbi.pku.edu.cn/home.do) was used to analyze the enrichment of the DEGs in the KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway and MapMan software was used to analyze the metabolic pathways and functional classifications of the DEGs.
Validation of sequencing data by qRT-PCR
To validate the reliability of the sequencing data, 55 and 15 randomly selected DEGs and significantly differentially expressed miRNAs, respectively, were selected for qRT-PCR analysis. Total RNA was used for library construction and the qRT-PCR procedures for the DEGs and miRNAs were described in previous papers10,25. Actin7 and U6 snRNA of B. napus were used as the references for genes and miRNAs, respectively. All primers used for qRT-PCR are listed in Table S1. Three technical replicates were performed for each reaction.
Electronic supplementary material
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31371655), the Chongqing Science and Technology Commission (cstc2016shmszx80083), and 973 Program (2015CB150201).
Author Contributions
Conceived and designed the experiments: L.L. Performed the experiments: H.J., J.M., L.W. Analyzed the data: L.W., A.Z., P.L., B.Y., H.J. Contributed reagents/materials/analysis tools: X.X., J.L., J.M. Wrote the paper: H.J.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-29365-y.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liu, L. Z. et al. A High-Density SNP Map for Accurate Mapping of Seed Fibre QTL in Brassica napus L. Plos One8, 10.1371/journal.pone.0083052 (2013). [DOI] [PMC free article] [PubMed]
- 2.Bolton MD, Thomma BP, Nelson BD. Sclerotinia sclerotiorum (Lib.) de Bary: biology and molecular traits of a cosmopolitan pathogen. Mol Plant Pathol. 2006;7:1–16. doi: 10.1111/j.1364-3703.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- 3.Garg H, Li H, Sivasithamparam K, Kuo J, Barbetti MJ. The infection processes of Sclerotinia sclerotiorum in cotyledon tissue of a resistant and a susceptible genotype of Brassica napus. Ann Bot. 2010;106:897–908. doi: 10.1093/aob/mcq196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behla RS, Fernando WGD, Li G. Identification of quantitative trait loci for resistance against sclerotinia stem rot in Brassica napus. Can J Plant Pathol. 2009;31:477–478. [Google Scholar]
- 5.Wei DY, et al. Quantitative trait loci analyses for resistance to Sclerotinia sclerotiorum and flowering time in Brassica napus. Mol Breeding. 2014;34:1797–1804. doi: 10.1007/s11032-014-0139-7. [DOI] [Google Scholar]
- 6.Wu, J. et al. Identification of QTLs for Resistance to Sclerotinia Stem Rot and BnaC.IGMT5.a as a Candidate Gene of the Major Resistant QTL SRC6 in Brassica napus. Plos One8, 10.1371/journal.pone.0067740 (2013). [DOI] [PMC free article] [PubMed]
- 7.Yin XR, et al. Mapping of QTLs detected in a Brassica napus DH population for resistance to Sclerotinia sclerotiorum in multiple environments. Euphytica. 2010;173:25–35. doi: 10.1007/s10681-009-0095-1. [DOI] [Google Scholar]
- 8.Zhao JW, Meng JL. Genetic analysis of loci associated with partial resistance to Sclerotinia sclerotiorum in rapeseed (Brassica napus L.) Theor Appl Genet. 2003;106:759–764. doi: 10.1007/s00122-002-1171-2. [DOI] [PubMed] [Google Scholar]
- 9.Zhao JW, et al. Quantitative trait loci for resistance to Sclerotinia sclerotiorum and its association with a homeologous non-reciprocal transposition in Brassica napus L. Theor Appl Genet. 2006;112:509–516. doi: 10.1007/s00122-005-0154-5. [DOI] [PubMed] [Google Scholar]
- 10.Wei L, et al. Genome-wide association analysis and differential expression analysis of resistance to Sclerotinia stem rot in Brassica napus. Plant Biotechnol J. 2016;14:1368–1380. doi: 10.1111/pbi.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gyawali, S. et al. Microsatellite markers used for genome-wide association mapping of partial resistance to Sclerotinia sclerotiorum in a world collection of Brassica napus. Mol Breeding36, 10.1007/s11032-016-0496-5 (2016). [DOI] [PMC free article] [PubMed]
- 12.Wang Z, et al. Overexpression of Brassica napus MPK4 Enhances Resistance to Sclerotinia sclerotiorum in Oilseed Rape. Mol Plant Microbe In. 2009;22:235–244. doi: 10.1094/MPMI-22-3-0235. [DOI] [PubMed] [Google Scholar]
- 13.Chen XT, et al. Overexpression of AtWRKY28 and AtWRKY75 in Arabidopsis enhances resistance to oxalic acid and Sclerotinia sclerotiorum. Plant Cell Rep. 2013;32:1589–1599. doi: 10.1007/s00299-013-1469-3. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, et al. Overexpression of BnWRKY33 in oilseed rape enhances resistance to Sclerotinia sclerotiorum. Molecular Plant Pathology. 2014;15:677–689. doi: 10.1111/mpp.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rietz S, Bernsdorff FEM, Cai DG. Members of the germin-like protein family in Brassica napus are candidates for the initiation of an oxidative burst that impedes pathogenesis of Sclerotinia sclerotiorum. J Exp Bot. 2012;63:5507–5519. doi: 10.1093/jxb/ers203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Novakova M, Sasek V, Dobrev PI, Valentova O, Burketova L. Plant hormones in defense response of Brassica napus to Sclerotinia sclerotiorum - reassessing the role of salicylic acid in the interaction with a necrotroph. Plant Physiol Biochem. 2014;80:308–317. doi: 10.1016/j.plaphy.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Zhao JW, et al. Patterns of differential gene expression in Brassica napus cultivars infected with Sclerotinia sclerotiorum. Molecular Plant Pathology. 2009;10:635–649. doi: 10.1111/j.1364-3703.2009.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawahara, Y. et al. Simultaneous RNA-Seq Analysis of a Mixed Transcriptome of Rice and Blast Fungus Interaction. Plos One7, 10.1371/journal.pone.0049423 (2012). [DOI] [PMC free article] [PubMed]
- 19.Camilios-Neto, D. et al. Dual RNA-seq transcriptional analysis of wheat roots colonized by Azospirillum brasilense reveals up-regulation of nutrient acquisition and cell cycle genes. Bmc Genomics15, 10.1186/1471-2164-15-378 (2014). [DOI] [PMC free article] [PubMed]
- 20.Yazawa, T., Kawahigashi, H., Matsumoto, T. & Mizuno, H. Simultaneous Transcriptome Analysis of Sorghum and Bipolaris sorghicola by Using RNA-seq in Combination with De Novo Transcriptome Assembly. Plos One 8, 10.1371/journal.pone.0062460 (2013). [DOI] [PMC free article] [PubMed]
- 21.Que Y, Su Y, Guo J, Wu Q, Xu L. A global view of transcriptome dynamics during Sporisorium scitamineum challenge in sugarcane by RNA-Seq. Plos One. 2014;9:e106476. doi: 10.1371/journal.pone.0106476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adhikari, B. N. et al. Expression Profiling of Cucumis sativus in Response to Infection by Pseudoperonospora cubensis. Plos One7, 10.1371/journal.pone.0034954 (2012). [DOI] [PMC free article] [PubMed]
- 23.Joshi RK, Megha S, Rahman MH, Basu U, Kav NNV. A global study of transcriptome dynamics in canola (Brassica napus L.) responsive to Sclerotinia sclerotiorum infection using RNA-Seq. Gene. 2016;590:57–67. doi: 10.1016/j.gene.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Wu, J. et al. Comparative transcriptomic analysis uncovers the complex genetic network for resistance to Sclerotinia sclerotiorum in Brassica napus. Sci Rep-Uk6, 10.1038/Srep19007 (2016). [DOI] [PMC free article] [PubMed]
- 25.Jian, H. J. et al. Identification of Rapeseed MicroRNAs Involved in Early Stage Seed Germination under Salt and Drought Stresses. Front Plant Sci7, 10.3389/Fpls.2016.00658 (2016). [DOI] [PMC free article] [PubMed]
- 26.Achard P, Herr A, Baulcombe DC, Harberd NP. Modulation of floral development by a gibberellin-regulated microRNA. Development. 2004;131:3357–3365. doi: 10.1242/dev.01206. [DOI] [PubMed] [Google Scholar]
- 27.Guo HS, Xie Q, Fei JF, Chua NH. MicroRNA directs mRNA cleavage of the transcription factor NAC1 to downregulate auxin signals for Arabidopsis lateral root development. Plant Cell. 2005;17:1376–1386. doi: 10.1105/tpc.105.030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Q, et al. Expression analysis of phytohormone-regulated microRNAs in rice, implying their regulation roles in plant hormone signaling. Febs Lett. 2009;583:723–728. doi: 10.1016/j.febslet.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 29.Navarro L, et al. A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science. 2006;312:436–439. doi: 10.1126/science.1126088. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan CS, Ganem D. MicroRNAs and viral infection. Mol Cell. 2005;20:3–7. doi: 10.1016/j.molcel.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Robert-Seilaniantz A, et al. The microRNA miR393 re-directs secondary metabolite biosynthesis away from camalexin and towards glucosinolates. Plant J. 2011;67:218–231. doi: 10.1111/j.1365-313X.2011.04591.x. [DOI] [PubMed] [Google Scholar]
- 32.He XF, Fang YY, Feng L, Guo HS. Characterization of conserved and novel microRNAs and their targets, including a TuMV-induced TIR-NBS-LRR class R gene-derived novel miRNA in Brassica. Febs Lett. 2008;582:2445–2452. doi: 10.1016/j.febslet.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Hewezi T, Maier TR, Nettleton D, Baum TJ. The Arabidopsis MicroRNA396-GRF1/GRF3 Regulatory Module Acts as a Developmental Regulator in the Reprogramming of Root Cells during Cyst Nematode Infection. Plant Physiol. 2012;159:321–335. doi: 10.1104/pp.112.193649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li F, et al. MicroRNA regulation of plant innate immune receptors. P Natl Acad Sci USA. 2012;109:1790–1795. doi: 10.1073/pnas.1118282109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie FL, et al. Computational identification of novel microRNAs and targets in Brassica napus. Febs Lett. 2007;581:1464–1474. doi: 10.1016/j.febslet.2007.02.074. [DOI] [PubMed] [Google Scholar]
- 36.Huang SQ, et al. A set of miRNAs from Brassica napus in response to sulphate deficiency and cadmium stress. Plant Biotechnology Journal. 2010;8:887–899. doi: 10.1111/j.1467-7652.2010.00517.x. [DOI] [PubMed] [Google Scholar]
- 37.Korbes, A. P. et al. Identifying Conserved and Novel MicroRNAs in Developing Seeds of Brassica napus Using Deep Sequencing. Plos One7, 10.1371/journal.pone.0050663 (2012). [DOI] [PMC free article] [PubMed]
- 38.Xu, M. Y. et al. Identification of miRNAs and their targets from Brassica napus by high-throughput sequencing and degradome analysis. Bmc Genomics13, 10.1186/1471-2164-13-421 (2012). [DOI] [PMC free article] [PubMed]
- 39.Zhao YT, et al. Small RNA Profiling in Two Brassica napus Cultivars Identifies MicroRNAs with Oil Production- and Development-Correlated Expression and New Small RNA Classes. Plant Physiol. 2012;158:813–823. doi: 10.1104/pp.111.187666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou ZS, Song JB, Yang ZM. Genome-wide identification of Brassica napus microRNAs and their targets in response to cadmium. J Exp Bot. 2012;63:4597–4613. doi: 10.1093/jxb/ers136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen D, et al. Identification and characterization of microRNAs in oilseed rape (Brassica napus) responsive to infection with the pathogenic fungus Verticillium longisporum using Brassica AA (Brassica rapa) and CC (Brassica oleracea) as reference genomes. New Phytol. 2014;204:577–594. doi: 10.1111/nph.12934. [DOI] [PubMed] [Google Scholar]
- 42.Li R, et al. SOAP2: an improved ultrafast tool for short read alignment. Bioinformatics. 2009;25:1966–1967. doi: 10.1093/bioinformatics/btp336. [DOI] [PubMed] [Google Scholar]
- 43.Addo-Quaye C, Miller W, Axtell MJ. CleaveLand: a pipeline for using degradome data to find cleaved small RNA targets. Bioinformatics. 2009;25:130–131. doi: 10.1093/bioinformatics/btn604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang B, Srivastava S, Deyholos MK, Kav NNV. Transcriptional profiling of canola (Brassica napus L.) responses to the fungal pathogen Sclerotinia sclerotiorum. Plant Sci. 2007;173:156–171. doi: 10.1016/j.plantsci.2007.04.012. [DOI] [Google Scholar]
- 45.Galindo-Gonzalezi, L. & Deyholos, M. K. RNA-seq Transcriptome Response of Flax (Linum usitatissimum L.) to the Pathogenic Fungus Fusarium oxysporum f. sp lini. Front Plant Sci 7, 10.3389/Fpls.2016.01766 (2016). [DOI] [PMC free article] [PubMed]
- 46.Li, C. Y. et al. Transcriptome profiling of resistant and susceptible Cavendish banana roots following inoculation with Fusarium oxysporum f. sp cubense tropical race 4. Bmc Genomics13 (2012). [DOI] [PMC free article] [PubMed]
- 47.Xing, M. M. et al. Transcriptome Profiling of Resistance to Fusarium oxysporum f. sp conglutinans in Cabbage (Brassica oleracea) Roots. Plos One11, 10.1371/journal.pone.0148048 (2016). [DOI] [PMC free article] [PubMed]
- 48.Mueth, N. A., Ramachandran, S. R. & Hulbert, S. H. Small RNAs from the wheat stripe rust fungus (Puccinia striiformis f.sp tritici). Bmc Genomics 16, 10.1186/S12864-015-1895-4 (2015). [DOI] [PMC free article] [PubMed]
- 49.Asai T, et al. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- 50.Liu JM, et al. Heterotrimeric G Proteins Serve as a Converging Point in Plant Defense Signaling Activated by Multiple Receptor-Like Kinases. Plant Physiol. 2013;161:2146–2158. doi: 10.1104/pp.112.212431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lecourieux D, Raneva R, Pugin A. Calcium in plant defence-signalling pathways. New Phytol. 2006;171:249–269. doi: 10.1111/j.1469-8137.2006.01777.x. [DOI] [PubMed] [Google Scholar]
- 52.Jalali BL, Bhargava S, Kamble A. Signal transduction and transcriptional regulation of plant defence responses. J Phytopathol. 2006;154:65–74. doi: 10.1111/j.1439-0434.2006.01073.x. [DOI] [Google Scholar]
- 53.Ambawat S, Sharma P, Yadav NR, Yadav RC. MYB transcription factor genes as regulators for plant responses: an overview. Physiol Mol Biol Pla. 2013;19:307–321. doi: 10.1007/s12298-013-0179-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pieterse CMJ, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SCM. Hormonal Modulation of Plant Immunity. Annu Rev Cell Dev Bi. 2012;28:489–521. doi: 10.1146/annurev-cellbio-092910-154055. [DOI] [PubMed] [Google Scholar]
- 55.Chung HS, et al. Regulation and function of Arabidopsis JASMONATE ZIM-domain genes in response to wounding and herbivory. Plant Physiol. 2008;146:952–964. doi: 10.1104/pp.107.115691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kitajima S, Sato F. Plant pathogenesis-related proteins: Molecular mechanisms of gene expression and protein function. J Biochem-Tokyo. 1999;125:1–8. doi: 10.1093/oxfordjournals.jbchem.a022244. [DOI] [PubMed] [Google Scholar]
- 57.Sels J, Mathys J, De Coninck BMA, Cammue BPA, De Bolle MFC. Plant pathogenesis-related (PR) proteins: A focus on PR peptides. Plant Physiol Bioch. 2008;46:941–950. doi: 10.1016/j.plaphy.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 58.Pusztahelyi, T., Holb, I. J. & Pocsi, I. Secondary metabolites in fungus-plant interactions. Front Plant Sci6, 10.3389/Fpls.2015.00573 (2015). [DOI] [PMC free article] [PubMed]
- 59.Ferrer JL, Austin MB, Stewart C, Noe JP. Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiol Bioch. 2008;46:356–370. doi: 10.1016/j.plaphy.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lorenc-Kukula K, et al. Engineering Flax with the GT Family 1 Solanum sogarandinum Glycosyltransferase SsGT1 Confers Increased Resistance to Fusarium Infection. J Agr Food Chem. 2009;57:6698–6705. doi: 10.1021/jf900833k. [DOI] [PubMed] [Google Scholar]
- 61.Tholl D. Biosynthesis and Biological Functions of Terpenoids in Plants. Adv Biochem Eng Biot. 2015;148:63–106. doi: 10.1007/10_2014_295. [DOI] [PubMed] [Google Scholar]
- 62.Mikkelsen MD, Naur P, Halkier BA. Arabidopsis mutants in the C-S lyase of glucosinolate biosynthesis establish a critical role for indole-3-acetaldoxime in auxin homeostasis. Plant J. 2004;37:770–777. doi: 10.1111/j.1365-313X.2004.02002.x. [DOI] [PubMed] [Google Scholar]
- 63.Kosaka A, Manickavelu A, Kajihara D, Nakagawa H, Ban T. Altered Gene Expression Profiles of Wheat Genotypes against Fusarium Head Blight. Toxins. 2015;7:604–620. doi: 10.3390/toxins7020604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Afzal, Z., Howton, T. C., Sun, Y. L. & Mukhtar, M. S. The Roles of Aquaporins in Plant Stress Responses. J Dev Biol 4, 10.3390/Jdb4010009 (2016). [DOI] [PMC free article] [PubMed]
- 65.Casassola A, et al. Changes in gene expression profiles as they relate to the adult plant leaf rust resistance in the wheat cv. Toropi. Physiol Mol Plant P. 2015;89:49–54. doi: 10.1016/j.pmpp.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dynowski M, Schaaf G, Loque D, Moran O, Ludewig U. Plant plasma membrane water channels conduct the signalling molecule H2O2. Biochem J. 2008;414:53–61. doi: 10.1042/BJ20080287. [DOI] [PubMed] [Google Scholar]
- 67.Sunkar R, Li YF, Jagadeeswaran G. Functions of microRNAs in plant stress responses. Trends Plant Sci. 2012;17:196–203. doi: 10.1016/j.tplants.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 68.Li Y, et al. Identification of MicroRNAs Involved in Pathogen-Associated Molecular Pattern-Triggered Plant Innate Immunity. Plant Physiol. 2010;152:2222–2231. doi: 10.1104/pp.109.151803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fan, G. Q. et al. Plant-Pathogen Interaction-Related MicroRNAs and Their Targets Provide Indicators of Phytoplasma Infection in Paulownia tomentosa x Paulownia fortunei. Plos One10, 10.1371/journal.pone.0140590 (2015). [DOI] [PMC free article] [PubMed]
- 70.Zhang, H. et al. Genome-wide identification and functional prediction of novel and fungi-responsive lincRNAs in Triticum aestivum. Bmc Genomics17, 10.1186/S12864-016-2570-0 (2016). [DOI] [PMC free article] [PubMed]
- 71.Cao JY, Xu YP, Zhao L, Li SS, Cai XZ. Tight regulation of the interaction between Brassica napus and Sclerotinia sclerotiorum at the microRNA level. Plant Mol Biol. 2016;92:39–55. doi: 10.1007/s11103-016-0494-3. [DOI] [PubMed] [Google Scholar]
- 72.Candar-Cakir B, Arican E, Zhang BH. Small RNA and degradome deep sequencing reveals drought-and tissue-specific micrornas and their important roles in drought-sensitive and drought-tolerant tomato genotypes. Plant Biotechnology Journal. 2016;14:1727–1746. doi: 10.1111/pbi.12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yan YX, et al. A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell. 2007;19:2470–2483. doi: 10.1105/tpc.107.050708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Woo HR, Kim JH, Nam HG, Lim PO. The delayed leaf senescence mutants of Arabidopsis, ore1, ore3, and ore9 are tolerant to oxidative stress. Plant Cell Physiol. 2004;45:923–932. doi: 10.1093/pcp/pch110. [DOI] [PubMed] [Google Scholar]
- 75.Goulas E, et al. The chloroplast lumen and stromal proteomes of Arabidopsis thaliana show differential sensitivity to short- and long-term exposure to low temperature. Plant J. 2006;47:720–734. doi: 10.1111/j.1365-313X.2006.02821.x. [DOI] [PubMed] [Google Scholar]
- 76.He XJ, et al. AtNAC2, a transcription factor downstream of ethylene and auxin signaling pathways, is involved in salt stress response and lateral root development. Plant J. 2005;44:903–916. doi: 10.1111/j.1365-313X.2005.02575.x. [DOI] [PubMed] [Google Scholar]
- 77.Wang, L. W., Liu, H. H., Li, D. T. & Chen, H. B. Identification and characterization of maize microRNAs involved in the very early stage of seed germination. Bmc Genomics 12, 10.1186/1471-2164-12-154 (2011). [DOI] [PMC free article] [PubMed]
- 78.Liu NA, et al. Small RNA and degradome profiling reveals a role for miRNAs and their targets in the developing fibers of Gossypium barbadense. Plant J. 2014;80:331–344. doi: 10.1111/tpj.12636. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.