Abstract
Prism adaptation is a well-known model to study sensorimotor adaptive processes. It has been shown that following prism exposure, after-effects are not only restricted to the sensorimotor level but extend as well to spatial cognition. The main purpose of the present study was to investigate in healthy individuals whether expansion to spatial cognition is restricted to adaptive processes peculiar to prism adaptation or whether it occurs as well following other forms of adaptive process such as adaptation to a novel dynamic environment during pointing movements. Representational after-effects were assessed by the perceptual line bisection task before and after adaptation to a leftward or a rightward force field. The main results showed that adaptation developed at sensorimotor level but did not influence space representation. Our results have therefore a strong methodological impact for prospective investigations focusing on sensorimotor plasticity while sparing space cognition. These methodological considerations will be particulary relevant when addressing sensorimotor plasticity in patients with specific representational feature to preserve. The discussion highlights the differences between prism and dynamic adaptation that could explain the lack of after-effect on space representation following force field adaptation.
Introduction
Graceful motor actions rely on successful interaction between our body and the environment. When one moves an object, the brain takes into account the physics of the task to adjust the motor commands sent to the arm and the hand. Handing a book to someone is a smooth and effortlessly process. In unfamiliar circumstances, however, early movements can exhibit large errors, because the consequences are very different from what was initially planned. In the latter case, this discrepancy – an error signal – is used by the brain to drive adaptation such that smooth movements are eventually restored. This generic mechanism has been generalized to many types of actions1–3.
One of the classical and oldest models to study sensorimotor adaptive processes is prism adaptation. Participants wear prisms that deviate the visual field laterally while they point to visual targets4. Initially, subjects make pointing errors in the direction of the optical deviation. On the basis of these error signals, subjects gradually improve their performance until they achieve an accurate behavior. When the prisms are removed, the sensorimotor correlations revert to an inappropriate state and the pointing movements are shifted in the direction opposite to the prismatic shift. The sensorimotor after-effects can be explained by proprioceptive, visual and motor control changes5.
The interest taken in prism adaptation was considerably increased since the demonstration that after-effects are not restricted to the sensorimotor level but that they extend as well to cross-modal processes that are not involed in the visuo-motor perturbation. Indeed, cross-modal transfers of prism adaptation have been shown in non-visual tasks as diverse as haptic task6, tactile extinction7 and tactile detection thresholds8, auditory perception9, pain perception10 and even on thermoregulation11. Prism adaptation acts also on spatial cognition. The term ‘cognition’ is not picked up randomly: it refers to the fact effects are not bound to the usual framework of compensatory sensorimotor after-effects but also involve mental abilities such as judgement, comparison or mental representation of space. It is worth underlying that in healthy individuals, cognitive after-effects occur following adaptation to a leftward optical deviatio/n. For example only adaptation to leftward optical deviation turns representational pseudoneglect (leftward bias in midline judgments corresponding to a mental over-representation of the left part of space) into neglect-like behavior (rightward bias in midline judgments corresponding to a mental over-representation of the right part of space). Neglect simulation was not only described in peripersonal (i.e. within arm-reach12,13), extrapersonal (i.e. beyond arm-reach14,15) and bodily space representation16 but also in mental numbers17 and letters scales18. The influence of prism adaptation extends also to spatial attention19, hierarchical processing20,21 and spatial remapping22,23.
Here, we set out to investigate whether the asymmetrical expansion of sensorimotor after-effects induced by prisms to spatial cognition is a phenomenon specific to prism adaptation or whether it occurs as well following other forms of adaptive processes such as when we adapt pointing movements to a novel dynamic environment producing proprioceptive changes24. If neglect-like cognitive after-effects in healthy individuals depend on the occurrence of sensorimotor effects, then we should observe space representational biases only after dynamic adaptation to a leftward force field that requires a compensatory rightward motor command. In contrast, if the generalization of adaptation to spatial cognition depends on adaptive processes peculiar to prism adaptation, after-effects of adaptation to force field should not interfere with space representation.
Results
In the present study we investigated both sensorimotor and cognitive after-effects following adaptation to velocity-dependent forces perpendicular to vertical arm movements. Cognitive after-effects on spatial representation were assessed with the classical perceptual line bisection task25,26.
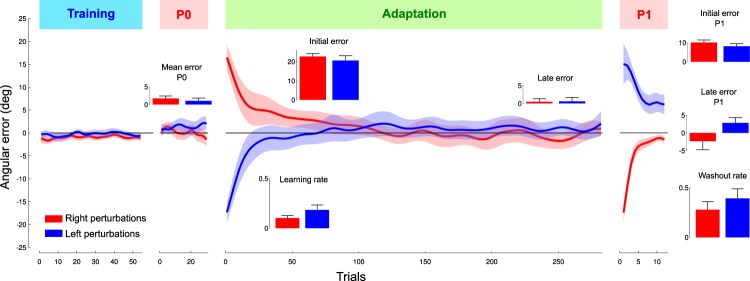
Sensorimotor adaptation to force field
Participants reached toward 3 targets vertically aligned but displayed at three distances from the start position. We formed two groups (see Fig. 1, GLeft and GRight) depending on the direction of force field perturbation (Left vs. Right). Overall, participants achieved peak velocities in line with instructions (mean ± SD, 70.7 ± 2.8, 78.6 ± 4.3 and 88.7 ± 7.2 cm/s for the near, medium and far targets, respectively). During the training session (Figs 1 “Training” and 2 “Training”) and for each group separately, we did not observe significant effects of trials or targets on angular errors 150 ms after movement onset (Trials: GLeft, F53,711 = 1.0, p = 0.447; GRigh, F53,757 = 0.8, p = 0.818 and Targets: GLeft, F2,54 = 0.5, p = 0.634; GRight, F2,54 = 0.1, p = 0.871). A direct comparison between these two groups yielded no difference either (independent t-test, t37 = −0.2, p = 0.407).
Figure 1.
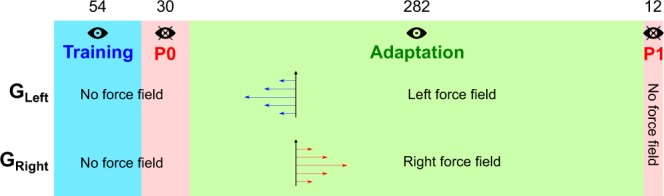
Experimental procedures. The four rectangles correspond to the four experimental sequences. Number of trials is reported above each sequence. Each group (GLeft, N = 20 and GRight, N = 19) were subjected to different force field perturbation directions as depicted by the blue and red horizontal arrows in the “Adaptation” sequence. An “eye” icon corresponds to the presence of visual feedback information about the cursor trajectory. “P0” and “P1” stand for “Probe before adaptation” and “Probe after adaptation”, respectively.
Figure 2.
Angular errors 150 ms after movement onset during the four sequences of the “Adaption” phase for the group who experienced rightward (red) or leftward perturbation (blue). (Training) Baseline trials show that errors fluctuate around zero. (P0) First open-loop sequence. The inset presents the average of the errors for the block. (Adaptation) Errors decrease from positive (right group, red) or negative (left group, blue) values to close to zero. Initial error and late error insets report the average initial and final errors, respectively. Learning rates calculated for each subject on the exponential fit are shown below the x-axis in panel P1. (P1) Second open-loop sequence. Initial errors highlight after-effects in the opposite direction than initial errors in the “Adaptation” sequence. Note that late errors did not vanish completely. Washout rates are also reported for the sake of comparability. Error bars and shaded areas correspond to SE.
Participants then performed 30 trials without any visual feedback about trajectory (Figs 1 “P0” and 2 “P0”). This short block allowed us to assess open loop performance. Again, we failed to find any difference across trials (GLeft, F29,421 = 0.6, p = 0.940; GRight, F29,435 = 1, p = 0.527) or targets (GLeft, F2,57 = 0.3, p = 0.750; GRight, F2,54 = 0, p = 0.984) on the initial angular error and both groups were not different (independent t-test, t37 = −0.6, p = 0.271).
We then exposed participants to a large block (282 trials) during which they underwent a leftward (GLeft) or rightward (GRight) velocity-dependent force field that perturbed movement trajectory (Figs 1 “Adaptation” and 2 “Adaptation”). Initially and expectedly, both groups made large errors in the direction of the perturbation (first three trials, GLeft: −22.7 ± 5.7 deg; GRight: 20.6 ± 8.9 deg) that vanished after a brief exposure period (average for the last 30 trials, GLeft: 0.5 ± 3.8 deg; GRight: 0.6 ± 4.8 deg). Both groups exhibited the same behavior in terms of (unsigned) initial and (signed) final absolute errors (independent t-test, initial error t37 = 0.7, p = 0.237, final: t37 = 0, p = 0.469) and adaptation rates (mean = 0.16; t37 = 0.6, p = 0.286).
Participants ended this session with a second 12-trial block without any visual feedback about performance (Figs 1 “P1” and 2 “P1”). Since the perturbation was absent in that last block, the initial errors reflected feedforward behavior. Both groups made large errors in the opposite direction of the perturbation (first three trials, GLeft: 10.1 ± 5.6 deg; GRight: −7.9 ± 5.9 deg) that decreased over trials. Groups had similar performances for initial and final errors (independent t-test, t < 0.7, p > 0.267) and washout rate (mean = 0.37, t37 = −0.6, p = 0.267). Importantly, we found significantly larger errors at the end of the P1 sequence compared to baseline errors in the P0 sequence in the GLeft (paired t-test, t19 = 3.2, p = 0.005, ) and GRight groups (paired t-test, t18 = 2.9, p = 0.010, ). In other words, participants of either group were not fully de-adapted at completion of the washout sequence and hence before entering the second bisection test.
Baseline space representation assessed by the first bisection task
Participants always provided judgments of the location of the tick within 3000 ms after stimulus presentation. However, this timing was influenced by two experimental factors. On the one hand, response time was modulated by tick offset following a bell-shaped curve. Timing increased from low values (~1300 ms) for large offsets to much longer latencies (~2600 ms) when the offset was nearer midline and therefore harder to judge. On the other hand, participants were on average 15% faster to react during the second session (Bisection-Post). A 2-way repeated measure ANOVA confirmed these main effects (Offset: F16,1292 = 21.2, p < 0.001, ; Session: F1,1292 = 35.7, p < 0.001, ) without interaction (F16,1292 = 0.6, p = 0.853).
We estimated, for each participant, the threshold that corresponded to the offset for which the probability of correct response was 50% (see Methods). We did not find any difference between groups (independent t-test, t37 = −0.5, p = 0.323). Furthermore, the thresholds in this initial Bisection-Pre session were not different from an offset of 0 mm (mean = −0.04 mm, SD = 0.29 mm, independent t-test against zero: t76 = −0.8, p = 0.225). Both groups had comparable performance before entering the Adaptation session and estimated mid-segment slightly on the left.
Effects of sensorimotor adaptation on space representation
We now ask the question as to whether the point of subjective equality was influenced by sensorimotor adaptation induced by the force field. We failed to report any difference between thresholds before and after the adaptation phase (Bisection-Pre vs. Bisection-Post, F1,44 = 0, p = 0.926) or between groups (GLeft vs. GRight, F1,44 = 0.2, p = 0.648). Furthermore, the ANOVA did not report any interaction between group and the bisection task on these thresholds (F1,44 = 0, p = 0.820).
We adopted a more sensitive approach to try and detect any difference between these thresholds. For every participant, we fitted a sigmoid function and regressed the offset that corresponded to chance level to measure the subjective estimation of the line center. Fig. 3A depicts the fit for all participants together, before and after the Adaptation phase. Note that the red and blue curves are nearly superimposed. We defined a new statistics as the difference between the thresholds calculated during Bisection-Pre and Bisection-Post phases and we averaged these values across participants. We then ran a bootstrap procedure (50,000 repetitions) to approximate the population (Fig. 3B). We could not reject the null hypothesis (no difference between the means, Fig. 3B, green cursor). Indeed, the true mean (Fig. 3B, yellow cursor, 0.01) is well contained within the 95%-confidence interval (Fig. 3B, grey cursors, [−0.13 to 0.15], p = 0.926). In sum, these analyses show that sensorimotor adaptation to a force-field did not influence the perceptual thresholds in the line bisection task.
Figure 3.
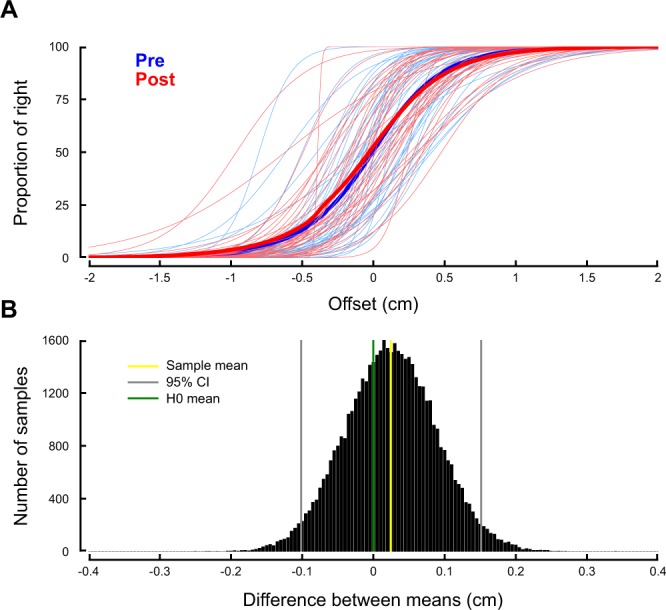
No difference between bisection thresholds before and after force field adaptation. (A) Solid lines: mean sigmoidal fit across participants before (blue) and after adaptation (red). The thin lines correspond to individual participant fits. (B) Bootstrapped population (50,000 repetitions) of the difference between thresholds before and after adaptation. The estimated population mean (yellow cursor) is not different from 0 (green cursor). The 95%-confidence interval is enclosed within the vertical grey cursors.
Perceptual thresholds and washout rates
Why did we fail to highlight an effect of a strong force field adaptation task on the perceptual thresholds? One reason may be because participants partially de-adapted during the P1-sequence. Indeed, there were reminiscent errors at the end of P1 compared to P0. In other words, only a few trials without perturbation and without visual feedback of the performance were sufficient to significantly decrease the error (see Fig. 2 “P1”).
However, all participants did not de-adapt the same way. We pushed our investigations one step further and coupled data from the three experimental phases. We reasoned that the slower a participant de-adapt, the stronger the influence on her/his perceptual judgment on the second bisection task. To quantify this, we correlated the absolute difference between thresholds after and before the adaptation session (|Tpost − Tpre|) and the washout rate (parameter a2 calculated in the P1 sequence). The spearman correlation yielded no significant relationship between a shift in threshold and the washout rate (r = 0.17, p = 0.309). However similar in many ways, the two groups were exposed to left and right dynamic perturbations. The same correlation calculated on each group separately provided no significant outcome either (GLeft: r = 0.02, p = 0.938; GRight: r = 0.26, p = 0.259). Therefore, we cannot conclude that a reminiscent adapted state led to a shift in thresholds nor that there was an influence of direction of perturbation on these perceptual thresholds.
The passage of time is known to reinforce internal motor memories27 and after-effects have been shown to emerge some time after being exposed to perturbations28. We conducted the same analysis but we considered separately the first half (68 trials) and the second half of trials during Bisection-Post. We experimentally designed the Bisection-Post block in such a way to ensure balanced distributions of offsets. Our analysis could not highlight any significant correlation in the short term Bisection-Post phase (GLeft: r = 0.45, p = 0.059; GRight: r = 0.07, p = 0.772) or in the long term Bisection-Post phase (GLeft: r = 0.04, p = 0.887; GRight: r = 0.39, p = 0.088). In other words, these results show that the adapted state has no significant influence on perceptual thresholds.
Discussion and Conclusion
The main objective of the present experiment was to investigate whether adaptation to a dynamic perturbation transfers to space representation in healthy individuals. We failed to show that adaptation developed at sensorimotor level influenced after-effects in space representation.
Replication of sensorimotor adaptation and sensitive line bisection conditions
Robotic devices are commonly used to highlight sensorimotor adaptation by perturbing motor tasks using different forms of dynamics such as elastic29,30, viscous3, inertial31 or composite force fields32. Here, participants exhibited the classical adaptive behaviour to novel dynamic environments: large initial errors in the direction of the perturbation and then large errors in the opposite direction of the expected perturbation, when it is removed unbeknownst to the participants. Here, we set high viscous gains and asked participants to perform reaching movements of large amplitudes. The combined effects of high velocity toward the target and large viscous gain put us in a position to amplify adaptation. Even if the amplitude of after-effects decreased over trials in the open-loop reaching following adaptation (“P1” period), participants were still partially adapted at completion of the washout sequence. Participants were therefore in a state in which their internal model was still partially adjusted to the task dynamics. This experimental paradigm leaves us on sound ground to test whether this dynamic sensorimotor adaptation interferes with space representation.
We took the precaution to assess pseudoneglect by means of the perceptual judgment version of the line bisection task (landmark test) to prevent any active or passive movement of the participant. Furthermore we used long segments (400 mm) because they can capture subtle representational effects33. The experimental conditions are therefore appropriate to assess if a strong sensorimotor adaptation to force fields influences space representation.
Sensorimotor adaptation and space representation: two independent processes?
In spite of both favourable sensorimotor and cognitive experimental conditions, we failed to observe obvious cognitive after-effects following adaptation to a strong dynamic perturbation. Sensorimotor adaptation to both leftward and rightward deviating prisms share a similar effect as force field adaptation in that significant sensorimotor after-effects are observed once the perturbation is removed. However, a peculiar difference resides in the fact that prism adaptation – when the optical deviation is at least 10 deg34 – transfers to more cognitive dimensions such as mental scales or spatial attention and untrained sensory domains (see Introduction). Furthermore, this effect is asymmetrical: only leftward deviating prisms induce a rightward shift in the perception of segment midline12,16,19,35. What fundamental differences between these two types of adaptation are responsible for this lack of cognitive effect?
For years, prism adaptation and the reason why after-effects are observed have been described through a dual-process framework. Initially, a rapid process occurs (referred to as ‘calibration’) and allows to reduce the error by using feedback (proprioceptive and/or visual) to better characterize what is going on. This process is responsible for a quick decline of the initial error. In parallel, a slow process (referred to as ‘realignment’) brings back the different reference frames in congruence. Indeed, under prismatic shift, the visual/motor reference frame is affected while the proprioceptive/motor reference frame is not. The first process is cognitively demanding and contributes little to prism after-effects. The second, more automatic, process develops gradually and is thought to be mainly responsible for prism after-effects. An inescapable experimental support to the distinction between fast and slow processes was provided by a detailed analysis of arm movement trajectories36. The two compensatory processes could be distinguished within a single reaching movement. The fast strategic compensation of the optical shift consists of trial-by-trial updating of the planned movement direction on the basis of the error experienced during the previous trial. This compensation is a strategic demanding process that drives error correction early during prism exposure and does not generate after-effects. In contrast, the transformation of spatial maps (visual, proprioceptive and motor) to bring origins of coordinate systems into correspondence is an automatic process that develops more gradually during prism exposure and correlates with after-effects measured at the end of the experiment. These two processes operate relatively independently from one another37.
More recently though, this two-timescale process was challenged. Indeed, a third ultra-slow process was needed to explain the immediate and long-term retention of prism after-effects when prismatic exposure reached 500 trials38. More generally, there may be a continuum of learning processes that are combined according to a number of contextual factors. In support of this view, different timescales in neural network dynamics have been shown in typical adaptation paradigms39,40 and models41. Furthermore, in other motor adaptation paradigms (visuomotor rotations or force field adaptation), the retention of after-effects also depends on the experimental context, such as if we learn using error-based mechanisms or use-dependent mechanisms42.
Even if none of these processes explain why prism adaptation generates cognitive after-effects while force field adaptation does not, we may hypothesise that the main difference between prism and force field adaptation may lie in the relative contribution of the fast and slow processes. Indeed, it has been shown that the occurrence of cognitive after-effects depends on the development of the slow process13. In prism adaptation, the slow process may dominate and be responsible for strong, persistent and generalized sensorimotor and cognitive after-effects23. In force field adaptation, the slow process may be minor and responsible for short-lasting and poorly generalized sensorimotor after-effects and non-significant cognitive after-effects. Indeed, adaptation to force field is not only spatially highly contextual but is also restricted to the exposure during training43–45.
From a neurophysiological point of view, common neural substrate for both adaptation to force field and to prismatic shifts involved neural structures well-known to be implicated in sensorimotor processes such as the cerebellum and the motor cortex46–48. Nevertheless, the impressive generalization of prism adaptation to high-order processes are mediated through the modulation of cerebral areas involved in spatial cognition via a bottom-up cerebello-cortical network involving temporal cortex49 or posterior parietal cortex (inferior parietal lobule)28,50,51. It is likely that the slow process developed during dynamic perturbation is not strong enough to mobilize such a bottom-up cerebello-cortical network underpinning cognitive processes.
The methodological interest of adaptation to force field
The most important result of our work was to show that after-effects of force field adaptation are confined to the sensorimotor level. The lack of representationnal after-effects has strong methodological implications. Indeed, adaptation to a force field reveals to be an appropriate tool to investigate specifically sensorimotor plasticity without producing any change in space representation in the specific conditions of this work. Hence, force field adaptation will be particularly appropriate to study sensorimotor plasticity in individuals who exhibit an inherent bias in space representation while preserving it from any change. For example, force field adaptation paradigms could be advised to explore sensorimotor plasticity in patients with schizophrenia who show a hyperpseudoneglect52 or in children with dyslexia who exhibit an inverse pseudoneglect53.
Here we show the main divergence between adaptation to force field and prism adaptation that could explain the lack of representational after-effects following a dynamic perturbation. This result has a strong methodological implication for all future investigations of sensorimotor plasticity while sparing space cognition. Follow-up experiments testing participants under different experimental procedures classically used to investigate sensorimotor plasticity (e.g visuomotor rotations) will allow to enrich the set of experimental procedures that do not alter space representational.
Materials and Methods
Participants
Thirty-nine self-reported right-handed adults (21 females, 18 males, mean age = 23.8, SD = 5 years) participated voluntarily in the experiment. All subjects were healthy, without neuromuscular disease and with normal or corrected to normal vision. All the participants gave their informed consent prior to their inclusion in the study, which was carried out in agreement with legal requirements and international norms (Declaration of Helsinki, 1964). The procedures were approved by the local ethics committee of Université de Bourgogne-Franche Comté. All participants were naïve as to the purpose of the experiments, were debriefed after the experimental sessions and received a USB stick for their participation.
Experimental procedure and apparatus
The experiment consisted in three separate phases (Bisection-Pre, Adaptation and Bisection-Post), described below. To assess space representation, we adopted the gold standard line bisection task because it is extensively used to explore peripersonnal space representation. The test has the advantage of being simple, quantitative, and reproducible54.
During the first, Bisection-Pre phase, participants faced a high resolution LCD screen (27″ 1440 × 2560) in a dimly illuminated room. Participants were asked to perform a bisection segment test26. A total of 130 horizontal green segments (length: 400 mm, thickness: 2 mm) were sequentially displayed on the screen together with a perpendicular green tick (height: 30 mm, thickness: 2 mm). Participants were requested to judge (forced-choice) whether pre-transected lines were bisected to the left or to the right of their centre. The response was given verbally. The experimenter manually recorded responses by hitting the appropriate key on a standard computer keyboard (‘L’ for left and ‘R’ for right) to limit any motor action for the participant. Reaction time was recorded as the time between stimulus onset and key strike by the experimenter. No response provided before a 20-s timeout was considered as missing information. Ticks offsets were not uniformly distributed but followed a Gaussian shape in order to increase sampling around the Euclidian center and, hence, enhance sensitivity of subsequent data analysis (Table 1).
Table 1.
Distribution of perpendicular ticks offsets with respect to the Euclidian center of the segment (0 mm).
| Offset (mm) | Total | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −40 | −20 | −14 | −9 | −6 | −4 | −2 | −1 | 0 | 1 | 2 | 4 | 6 | 9 | 14 | 20 | 40 | ||
| BISECTION_PRE | 4 | 4 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 11 | 10 | 9 | 8 | 7 | 6 | 4 | 4 | 130 |
| BISECTION_POST (1st half) | 2 | 2 | 3 | 4 | 4 | 5 | 5 | 6 | 6 | 6 | 5 | 5 | 4 | 4 | 3 | 2 | 2 | 68 |
| BISECTION_POST (2nd half) | 2 | 2 | 3 | 4 | 4 | 5 | 5 | 6 | 6 | 6 | 5 | 5 | 4 | 4 | 3 | 2 | 2 | 68 |
The first bold row reports the offsets (mm). The second row (Bisection-Pre) reports the number of times a tick was presented with that offset in the 130-trial block. The last two identical rows present the offset distribution during the first (Bisection-Post, 1st half) and second half (Bisection-Post, 2nd half) of the 136-trial block. The distribution was Gaussian and centered on the veridical line center. Note that the largest offsets (±40 mm) corresponded to 10% of the length of the horizontal segment.
To prevent any cognitive strategy, participants were specifically told that ticks never occurred exactly on the middle of the segment and that their distribution was not systematically symmetric. Ticks were presented randomly and the computer screen went white for 1500 ms between each trial to reset the visual display and prevent the participant from using any cue between trials. At completion of this Bisection-Pre phase, the participant was blindfolded (to avoid any movement involving visuo-manual coordination) and her/his stool was gently rotated 90-deg rightward in order to use a virtual environment. The experimenter assisted the participant in this maneuver to limit unnecessary movements.
During the Adaptation phase, participants faced a virtual environment equipped with a haptic device (Phantom 3.0, SensAble Technologies, USA) with the head on a chin rest. Participants looked into two mirrors that were mounted at 90 degrees to each other, such that they viewed one LCD screen with the right eye and one LCD screen with the left eye. This stereo display was calibrated such that the physical location of the robotic arm was consistent with the visual disparity information. Participants performed reaching movements with the right hand from the same starting position toward one of 3 targets situated 22, 27 or 32 cm above the starting position. Reaching to multiple targets concurrently allows slowing down learning rates and therefore increasing sensitivity to capture these parameters. Indeed, in a Bayesian framework, learning rates are up/down-regulated as a function of uncertainty in beliefs, which is underlined by the generalization across multiple targets55. Note that unlike in other similar experiments, we used three targets aligned vertically in order to induce only pure horizontal perturbations (see below). Movements were performed in the natural reaching space in an upward direction, involving shoulder and elbow movements, with the elbow pointing downwards. The 3d position of the robot handle was mapped in real time to a grey cursor (diameter: 3 mm). A trial started when the cursor was positioned inside a starting green sphere (diameter: 6 mm). Then, upon appearance of a white target (diameter: 6 mm), the participant performed a reaching movement to the target. The trial ended when the Euclidian distance between final cursor position and the target was below 4 mm and the cursor velocity fell below 1.5 cm/s for at least 40 ms. We instructed and trained participants to reach the target with a peak velocity (cm/s) within 65–75, 75–85 or 85–95 for the near, medium and far targets, respectively. After each trial, the target turned red or blue if movement was too fast or too slow respectively according to the speed criteria. The robot then gently pushed the hand back toward the starting position to prevent participants from planning and performing active movements and therefore, de-adapting.
After briefing, participants were familiarized with a practice block of 54 trials to the three targets. Cursor trajectories were displayed. These data were not included in the analysis. During the experimental session, participants performed 378 trials in the following order (Fig. 1). The training block consisted of 54 reaching movements (18 to each target) to randomly selected targets (Fig. 1, blue “Training” sequence) with full visual feedback of the cursor trajectory. Then, a smaller 30-trial probing block was presented in the same condition except that visual feedback of the cursor trajectory was withdrawn (Fig. 1, pink “P0” sequence). This sequence allowed us to measure initial feedforward performance in the task. A strong velocity-dependent perturbation was then introduced for the next 282 reaching movements (94 trials per target, Fig. 1 green “Adaptation” sequence). The robotic arm generated a force (Fx) in the horizontal direction (perpendicular to the movement trajectory) that was proportional to the forward velocity (Vy) of the hand following Fx = ±bVy, with b = 7 Ns/m. Participants used visual information about the cursor trajectory to compensate this perturbation over trials. Finally, a second probing block including 4 trials to each target (only 12 trials in total) was presented in the same condition as P0 (Fig. 1, pink “P1” sequence). This last sequence, without force field perturbation and without visual information, reliably quantified after-effects. We voluntarily limited the length of this last sequence to control that participants reached an adapted state while minimizing de-adaptation itself by measuring it. Indeed, complete de-adaptation occurs usually during the first 20 trials once the perturbation is removed42,56.
The 378 trials were equally distributed between 9 blocks of 42 trials to limit fatigue. We designed the blocks such that a change of condition never occurred between blocks but within blocks. The perturbation was a rightward (positive) force field for 19 participants (Fig. 1, GRight) and a leftward (negative) force field for 20 participants (Fig. 1, GLeft). At completion of this second sequence with the robotic device, the experimenter assisted the participant to bring her/him back in the position s/he had during the Bisection-Pre phase. Again, the participant was blindfolded during the 90 deg leftward rotation maneuver.
The apparatus, instructions and task in the last phase (Bisection-Post) were identical to those used in the Bisection-Pre phase except for the number of segments presented on the screen. In this third phase, a total of 136 horizontal green segments were displayed on the screen (there were 130 segments during Bisection-Pre). This intentional 6-trial difference was due to the fact we partitioned the distribution of tick offsets equally between the first and second halves of the block (Table 1). This design allowed to specifically assess representational after-effects in the early period (from trial 1 to trial 68) and late period (from trial 69 to trial 136) following sensorimotor adaptation to force field.
Data processing and analysis
In the Bisection-Pre and Bisection-Post phases, we recorded participants’ verbal responses for every trial (“Is the tick positioned to the right or left of the veridical segment midline?”). The time at which the experimenter hit the ‘L’ or ‘R’ stroke allowed us to have an estimate of participant’s reaction times. For each phase, we calculated the proportion of ‘RIGHT’ responses in function of the offset. This S-shape function saturated at 0% for large negative (left) offsets and 100% for large positive offsets (right). Indeed, the further the offsets from the segment midline, the lower the probability to make erroneous judgments.
To quantify the offset that corresponded to chance level (50%), i.e. the subjective perceptual estimation of the line center, we fitted logistic functions separately through Bisection-Pre, Bisection-Post, Bisection-Post 1st half and Bisection-Post 2nd half data sets, and for each participant separately (r2 = 0.96 on average), , where a1 and a2 were regressed (function nlinfit of Matlab) and t correspond to the offset. The threshold that corresponds to f = 50 was calculated according to . We used two tailed paired t-tests and bootstrapping methods to compare thresholds between Bisection-Pre and Bisection-Post and between the two halves of the Bisection-Post phase.
During the Adaptation phase, cursor positions were recorded with a sampling rate of 500 Hz. Movement start was detected when movement velocity exceeded 3 cm/s for at least 100 ms. Direction error of each movement was defined as the angle between straight ahead and the segment connecting the start position to the position of the cursor 150 ms after movement onset. Such delay reflects mostly the predictive component, i.e. before any feedback could be used to correct the movement57,58.
We quantified the amount of adaptation by looking at the difference between angular errors in P0 and P1, recorded in the same conditions. We calculated this index for each participant. To quantify adaptation rate, we fitted a power model to the initial angle error, , and regressed the 3 free parameters a1, a2 and a3. The first, second and third parameters quantify amplitude of adaptation, adaptation rate and final error, respectively.
Sample size was fixed at 15+ participants per group before the start of the experiment. Quantile-quantile plots were used to assess normality of the data before using parametric statistical tests. Variables of interest were submitted to different statistical models (repeated measures ANOVAs) according to the effects analyzed (see Results). Independent t-tests were conducted to compare data between both groups (of unequal size) and paired t-tests were used to compare different conditions within groups. We also report partial eta-squared for significant results to account for effect size. Data processing and statistical analyses were done using Matlab (The Mathworks, Chicago, IL).
Acknowledgements
This research was supported by the << Institut National de la Santé et de la Recherche Médicale >> (INSERM), the << Conseil Général de Bourgogne >> (France) and the << Fonds Européen de Développement Régional >> (FEDER).
Author Contributions
O.W. and C.M. have designed the experimental protocol, analysed the data and written the manuscript. L.B. has collected the data and contributed to the statistical analysis. S.A. collected the supplementary data for the revision of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shadmehr R. Learning to Predict and Control the Physics of Our Movements. J. Neurosci. 2017;37:1663–1671. doi: 10.1523/JNEUROSCI.1675-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gauthier GM, Blouin J, Bourdin C, Vercher JL. Adaptive control: A review of the ability to acquire and maintain high sensorimotor performance. Comput. Biol. Med. 2007;37:989–1000. doi: 10.1016/j.compbiomed.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 3.Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J. Neurosci. 1994;14:3208–3224. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Held R, Freedman SJ. Plasticity in human sensorimotor control. Science (80-.). 1963;142:455–462. doi: 10.1126/science.142.3591.455. [DOI] [PubMed] [Google Scholar]
- 5.Kornheiser AS. Adaptation to laterally displaced vision: a review. Psychol. Bull. 1976;83:783–816. doi: 10.1037/0033-2909.83.5.783. [DOI] [PubMed] [Google Scholar]
- 6.Girardi M, McIntosh RD, Michel C, Vallar G, Rossetti Y. Sensorimotor effects on central space representation: Prism adaptation influences haptic and visual representations in normal subjects. Neuropsychologia. 2004;42:1477–1487. doi: 10.1016/j.neuropsychologia.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Maravita A, et al. Prism adaptation can improve contralesional tactile perception in neglect. Neurology. 2003;60:1829–1831. doi: 10.1212/WNL.60.11.1829. [DOI] [PubMed] [Google Scholar]
- 8.Dijkerman HC, Webeling M, Ter Wal JM, Groet E, Van Zandvoort MJE. A long-lasting improvement of somatosensory function after prism adaptation, a case study. Neuropsychologia. 2004;42:1697–1702. doi: 10.1016/j.neuropsychologia.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Jacquin-Courtois S, et al. Effect of prism adaptation on left dichotic listening deficit in neglect patients: Glasses to hear better? Brain. 2010;133:895–908. doi: 10.1093/brain/awp327. [DOI] [PubMed] [Google Scholar]
- 10.Sumitani M, et al. Prism adaptation to optical deviation alleviates pathologic pain. Neurology. 2007;68:128–133. doi: 10.1212/01.wnl.0000250242.99683.57. [DOI] [PubMed] [Google Scholar]
- 11.Calzolari E, Gallace A, Moseley GL, Vallar G. Effect of prism adaptation on thermoregulatory control in humans. Behav. Brain Res. 2016;296:339–350. doi: 10.1016/j.bbr.2015.08.036. [DOI] [PubMed] [Google Scholar]
- 12.Colent C, Pisella L, Rossetti Y, Bernieri C, Rode G. Cognitive bias induced by visuo-motor adaptation to prisms: a simulation of unilateral neglect in normal individuals? Neuroreport. 2000;11:1899–1902. doi: 10.1097/00001756-200006260-00019. [DOI] [PubMed] [Google Scholar]
- 13.Michel C, et al. Simulating unilateral neglect in normals using prism adaptation: Implications for theory. Neuropsychologia. 2003;41:25–39. doi: 10.1016/S0028-3932(02)00135-5. [DOI] [PubMed] [Google Scholar]
- 14.Berberovic N, Mattingley JB. Effects of prismatic adaptation on judgements of spatial extent in peripersonal and extrapersonal space. Neuropsychologia. 2003;41:493–503. doi: 10.1016/S0028-3932(02)00090-8. [DOI] [PubMed] [Google Scholar]
- 15.Michel C, Vernet P, Courtine G, Ballay Y, Pozzo T. Asymmetrical after-effects of prism adaptation during goal oriented locomotion. Exp. Brain Res. 2008;185:259–268. doi: 10.1007/s00221-007-1152-4. [DOI] [PubMed] [Google Scholar]
- 16.Michel C, Rossetti Y, Rode G, Tilikete C. After-effects of visuo-manual adaptation to prisms on body posture in normal subjects. Exp. Brain Res. 2003;148:219–226. doi: 10.1007/s00221-002-1294-3. [DOI] [PubMed] [Google Scholar]
- 17.Loftus AM, Nicholls MER, Mattingley JB, Bradshaw JL. Left to right: Representational biases for numbers and the effect of visuomotor adaptation. Cognition. 2008;107:1048–1058. doi: 10.1016/j.cognition.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Nicholls MER, Kamer A, Loftus AM. Pseudoneglect for mental alphabet lines is affected by prismatic adaptation. Exp. Brain Res. 2008;191:109–115. doi: 10.1007/s00221-008-1502-x. [DOI] [PubMed] [Google Scholar]
- 19.Loftus AM, Vijayakumar N, Nicholls MER. Prism adaptation overcomes pseudoneglect for the greyscales task. Cortex. 2009;45:537–543. doi: 10.1016/j.cortex.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Bultitude JH, Woods JM. Adaptation to leftward-shifting prisms reduces the global processing bias of healthy individuals. Neuropsychologia. 2010;48:1750–1756. doi: 10.1016/j.neuropsychologia.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 21.Reed SA, Dassonville P. Adaptation to leftward-shifting prisms enhances local processing in healthy individuals. Neuropsychologia. 2014;56:418–427. doi: 10.1016/j.neuropsychologia.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bultitude JH, Van der Stigchel S, Nijboer TCW. Prism adaptation alters spatial remapping in healthy individuals: Evidence from double-step saccades. Cortex. 2013;49:759–770. doi: 10.1016/j.cortex.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Michel, C. Beyond the sensorimotor plasticity: Cognitive Expansion of Prism Adaptation in Healthy Individuals. Frontiers in Psychology6 (2016). [DOI] [PMC free article] [PubMed]
- 24.Ostry DJ, Darainy M, Mattar AAG, Wong J, Gribble PL. Somatosensory plasticity and motor learning. J. Neurosci. 2010;30:5384–93. doi: 10.1523/JNEUROSCI.4571-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jewell G, McCourt ME. Pseudoneglect: A review and meta-analysis of performance factors in line bisection tasks. Neuropsychologia. 2000;38:93–110. doi: 10.1016/S0028-3932(99)00045-7. [DOI] [PubMed] [Google Scholar]
- 26.Milner AD, Brechmann M, Pagliarini L. To halve and to halve not: An analysis of line bisection judgements in normal subjects. Neuropsychologia. 1992;30:515–526. doi: 10.1016/0028-3932(92)90055-Q. [DOI] [PubMed] [Google Scholar]
- 27.Shadmehr R, Brashers-Krug T. Functional stages in the formation of human long-term motor memory. J. Neurosci. 1997;17:409–419. doi: 10.1523/JNEUROSCI.17-01-00409.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schintu S, et al. Prism adaptation in the healthy brain: The shift in line bisection judgments is long lasting and fluctuates. Neuropsychologia. 2014;53:165–170. doi: 10.1016/j.neuropsychologia.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Descoins M, Danion F, Bootsma RJ, Fre DÆ. Predictive control of grip force when moving object with an elastic load applied on the arm. Exp. Brain Res. 2006;172:331–342. doi: 10.1007/s00221-005-0340-3. [DOI] [PubMed] [Google Scholar]
- 30.White, O., Karniel, A., Leib, R., Papaxanthis, C. & Nisky, I. Smart switching in feedforward control of grip force during manipulation of elastic objects. bioRxiv (2017).
- 31.Wang J, Sainburg RL. Interlimb transfer of novel inertial dynamics is asymmetrical. J. Neurophysiol. 2004;92:349–60. doi: 10.1152/jn.00960.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flanagan JR, Wing AM. The role of internal models in motion planning and control: evidence from grip force adjustments during movements of hand-held loads. J. Neurosci. 1997;17:1519–1528. doi: 10.1523/JNEUROSCI.17-04-01519.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCourt ME, Jewell G. Visuospatial attention in line bisection: stimulus modulation of pseudoneglect. Neuropsychologia. 1999;37:843–855. doi: 10.1016/S0028-3932(98)00140-7. [DOI] [PubMed] [Google Scholar]
- 34.Michel C, Cruz R. Prism adaptation power on spatial cognition: Adaptation to different optical deviations in healthy individuals. Neurosci. Lett. 2015;590:145–149. doi: 10.1016/j.neulet.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Goedert KM, Leblanc A, Tsai S-W, Barrett AM. Asymmetrical effects of adaptation to left- and right-shifting prisms depends on pre-existing attentional biases. J. Int. Neuropsychol. Soc. 2010;16:795–804. doi: 10.1017/S1355617710000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Shea J, et al. Kinematic markers dissociate error correction from sensorimotor realignment during prism adaptation. Neuropsychologia. 2014;55:15–24. doi: 10.1016/j.neuropsychologia.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 37.Petitet, P., O’Reilly, J. X. & O’Shea, J. Towards a neuro-computational account of prism adaptation. Neuropsychologia, 10.1016/j.neuropsychologia.2017.12.021 (2017). [DOI] [PMC free article] [PubMed]
- 38.Inoue M, et al. Three timescales in prism adaptation. J. Neurophysiol. 2015;113:328–338. doi: 10.1152/jn.00803.2013. [DOI] [PubMed] [Google Scholar]
- 39.Kim, S., Ogawa, K., Lv, J., Schweighofer, N. & Imamizu, H. Neural Substrates Related to Motor Memory with Multiple Timescales in Sensorimotor Adaptation. PLoS Biol. 13 (2015). [DOI] [PMC free article] [PubMed]
- 40.Spampinato D, Celnik P. Temporal dynamics of cerebellar and motor cortex physiological processes during motor skill learning. Sci. Rep. 2017;7:40715. doi: 10.1038/srep40715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaudhuri R, Knoblauch K, Gariel MA, Kennedy H, Wang XJ. A Large-Scale Circuit Mechanism for Hierarchical Dynamical Processing in the Primate Cortex. Neuron. 2015;88:419–431. doi: 10.1016/j.neuron.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diedrichsen J, White O, Newman D, Lally N. Use-dependent and error-based learning of motor behaviors. J. Neurosci. 2010;30:5159–5166. doi: 10.1523/JNEUROSCI.5406-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cothros N, Köhler S, Dickie EW, Mirsattari SM, Gribble PL. Proactive interference as a result of persisting neural representations of previously learned motor skills in primary motor cortex. J. Cogn. Neurosci. 2006;18:2167–76. doi: 10.1162/jocn.2006.18.12.2167. [DOI] [PubMed] [Google Scholar]
- 44.Kluzik J, Diedrichsen J, Shadmehr R, Bastian AJ. Reach adaptation: what determines whether we learn an internal model of the tool or adapt the model of our arm? J. Neurophysiol. 2008;100:1455–1464. doi: 10.1152/jn.90334.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mattar AAG, Ostry DJ. Modifiability of generalization in dynamics learning. J. Neurophysiol. 2007;98:3321–3329. doi: 10.1152/jn.00576.2007. [DOI] [PubMed] [Google Scholar]
- 46.Herzfeld DJ, Vaswani PA, Marko MK, Shadmehr R. A memory of errors in sensorimotor learning. Science (80-.). 2014;345:1349–53. doi: 10.1126/science.1253138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chapman HL, et al. Neural mechanisms underlying spatial realignment during adaptation to optical wedge prisms. Neuropsychologia. 2010;48:2595–2601. doi: 10.1016/j.neuropsychologia.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 48.Danckert J, Ferber S, Goodale MA. Direct effects of prismatic lenses on visuomotor control: An event-related functional MRI study. Eur. J. Neurosci. 2008;28:1696–1704. doi: 10.1111/j.1460-9568.2008.06460.x. [DOI] [PubMed] [Google Scholar]
- 49.Luauté J, et al. Functional anatomy of the therapeutic effects of prism adaptation on left neglect. Neurology. 2006;66:1859–1867. doi: 10.1212/01.wnl.0000219614.33171.01. [DOI] [PubMed] [Google Scholar]
- 50.Crottaz-Herbette S, Fornari E, Clarke S. Prismatic adaptation changes visuospatial representation in the inferior parietal lobule. J. Neurosci. 2014;34:11803–11. doi: 10.1523/JNEUROSCI.3184-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luauté J, et al. Dynamic changes in brain activity during prism adaptation. J. Neurosci. 2009;29:169–78. doi: 10.1523/JNEUROSCI.3054-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michel C, et al. Pseudoneglect in schizophrenia: A line bisection study with cueing. Cogn. Neuropsychiatry. 2007;12:222–234. doi: 10.1080/13546800601033266. [DOI] [PubMed] [Google Scholar]
- 53.Michel C, Bidot S, Bonnetblanc F, Quercia P. Left minineglect or inverse pseudoneglect in children with dyslexia? Neuroreport. 2011;22:93–96. doi: 10.1097/WNR.0b013e328342d2df. [DOI] [PubMed] [Google Scholar]
- 54.Halligan PW. Drawing attention to neglect: the contribution of line bisection. Psychologist. 1995;8:257–264. [Google Scholar]
- 55.Donchin O, Francis JT, Shadmehr R. Quantifying generalization from trial-by-trial behavior of adaptive systems that learn with basis functions: theory and experiments in human motor control. J. Neurosci. 2003;23:9032–45. doi: 10.1523/JNEUROSCI.23-27-09032.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Orban de Xivry, J.-J., Ahmadi-Pajouh, M. A., Harran, M. D., Salimpour, Y. & Shadmehr, R. Changes in corticospinal excitability during reach adaptation in force fields. J. Neurophysiol. 124–136, 10.1152/jn.00785.2012 (2012). [DOI] [PMC free article] [PubMed]
- 57.White, O. & Diedrichsen, J. Flexible Switching of Feedback Control Mechanisms Allows for Learning of Different Task Dynamics. PLoS One8 (2013). [DOI] [PMC free article] [PubMed]
- 58.Pruszynski JA, Kurtzer I, Scott SH. Rapid motor responses are appropriately tuned to the metrics of a visuospatial task. J. Neurophysiol. 2008;100:224–238. doi: 10.1152/jn.90262.2008. [DOI] [PubMed] [Google Scholar]