Abstract
Cell transplantation is promising for regenerative medicine. A combination of a three-dimensional spheroid culture system with gene transfection was developed to enhance the therapeutic effects of mesenchymal stem cell (MSC) transplantation. The spheroid cell culture system is based on micropatterned substrates composed of a regular array of 100-μm-diameter cell-adhesion areas coated with a temperature-responsive polymer, poly (N-isopropylacrylamide-co-methacrylic acid), which allows for spheroid detachment by simply cooling the plates. In this study, MSC spheroids were transfected with plasmid DNA encoding runt-related transcription factor 2 (Runx2) and tested for their ability to enhance bone regeneration. In vitro analyses revealed that osteogenic differentiation of the MSCs was enhanced by forming spheroids and was further promoted by Runx2 expression. The enhanced osteogenic differentiation was maintained under pathological conditions, such as hypoxia and inflammation. Transplanting Runx2-transfected MSC spheroids into bone defects on rat femurs induced significantly faster bone regeneration compared with nontransfected MSC spheroids or genetically modified MSCs from conventional monolayer culture. MSC migration into the bone defect area was enhanced by upregulation of cell-migration-related genes. In conclusion, genetically modified MSC spheroids are effective for enhancing bone regeneration, providing a promising option for cell transplantation therapy in the fields of regenerative medicine.
Keywords: mesenchymal stem cell, spheroid, bone regeneration, cell transplantation, genetic modification, regenerative medicine
Graphical Abstract
Introduction
Cell transplantation is widely used in the field of regenerative medicine. Among the various cell sources available, mesenchymal stem cells (MSCs) attract much attention because of their capacity for differentiation into mesodermal tissues, including those of the musculoskeletal system, blood vessels, and lymphatic and fibrous tissue.1, 2, 3 In addition, MSCs can exert a paracrine effect by producing various secretory factors that can affect the neighboring cells and enhance new tissue formation.4
For enhancing the therapeutic effects of MSC transplantation, we established a three-dimensional (3D) spheroid culture system. 3D cell culture maintains cell-to-cell interactions similarly to in vivo conditions, which can improve cell survival and function.5, 6 Our system is based on micropatterned plates composed of a regular array of 100-μm-diameter cell-adhesion areas surrounded by non-adhesive polyethylene glycol (PEG)-coated areas.7 The cell adhesion areas are coated with a temperature-responsive polymer, poly (N-isopropylacrylamide-co-methacrylic acid) (PIPAAm), which allows for detachment of the spheroids while maintaining spheroid architecture, using a simple procedure of cooling the plates to below 25°C. Compared with other conventional methods for spheroid formation, this system has the advantage of allowing for collection of equal-diameter spheroids at the same time.8, 9
To further improve the cell’s regenerative capacity, we combined the use of the spheroid culture system with gene introduction. Although lipid-based transfection reagents are likely to induce disruption of spheroids, we found that our original polymer-based gene carrier, poly (N’-[N-(2-aminoethyl)-2-aminoethyl] aspartamide) (PAsp[DET]), enabled gene introduction without disrupting the spheroid structure.10 Using this combined system for transplantation of genetically modified spheroid cells, we achieved preservation of hepatocyte function for more than 1 month after transplantation into the body. In addition, transplantation of brain-derived neurotrophic factor (BDNF)-expressing MSC spheroids promoted excellent motor function recovery in a mouse model of spinal cord injury (SCI), along with a high preservation of myelinated axons in the SCI region.9
The purpose of this study is to apply our combined system involving genetically modified MSC spheroids for bone regeneration and to perform in-depth analysis of the mechanisms that could affect the therapeutic outcomes. In the case of SCI treatment, as reported previously, it is strongly suggested that transplanted MSCs affect host cells via many secretory growth factors, as well as by enhanced BDNF secretion, and that these therapeutic molecules synergistically exert neuroprotective effects on damaged axons in the acute phase of SCI.9 In contrast, bone regeneration would involve longer term processes. We hypothesized that MSCs would be involved in bone matrix production rather than paracrine effects and that cell survival and localization after transplantation would be closely correlated with the outcome of bone regeneration.
In this study, we used MSC spheroids for a bone defect model of the rat femur. The genetic modification was performed using a transcriptional factor, runt-related transcription factor 2 (Runx2).11, 12, 13, 14 This is a strong activator of osteogenic differentiation and osteogenesis. We focused on the cell behavior after transplantation, including cell differentiation and migration into the site of the bone defect. The genetically modified MSC spheroids induced greater bone regeneration, osteogenic differentiation, and chemotactic activity than the controls, including nontransfected MSC spheroids or genetically modified MSCs from conventional monolayer culture.
Results
Efficacy of Runx2-Transfected MSC Spheroids In Vitro
MSC spheroids were prepared on the micropatterned substrate, on which the cells formed spheroids with 100-μm diameter. We previously reported that differentiation-inducing stimulants are more effective at converting MSCs into mature osteoblasts in spheroid culture than in monolayer culture.15 To confirm that gene introduction would be effective for differentiation, we evaluated the osteogenic markers by measuring alkaline phosphatase (ALP) levels using a cellular enzyme assay based on the conversion of para-nitrophenylphosphate (p-NPP) to para-nitrophenol and osteocalcin expression levels by real-time qPCR after transfection of Runx2-encoded plasmid DNA (pDNA) into MSC spheroids or MSCs from monolayer culture.
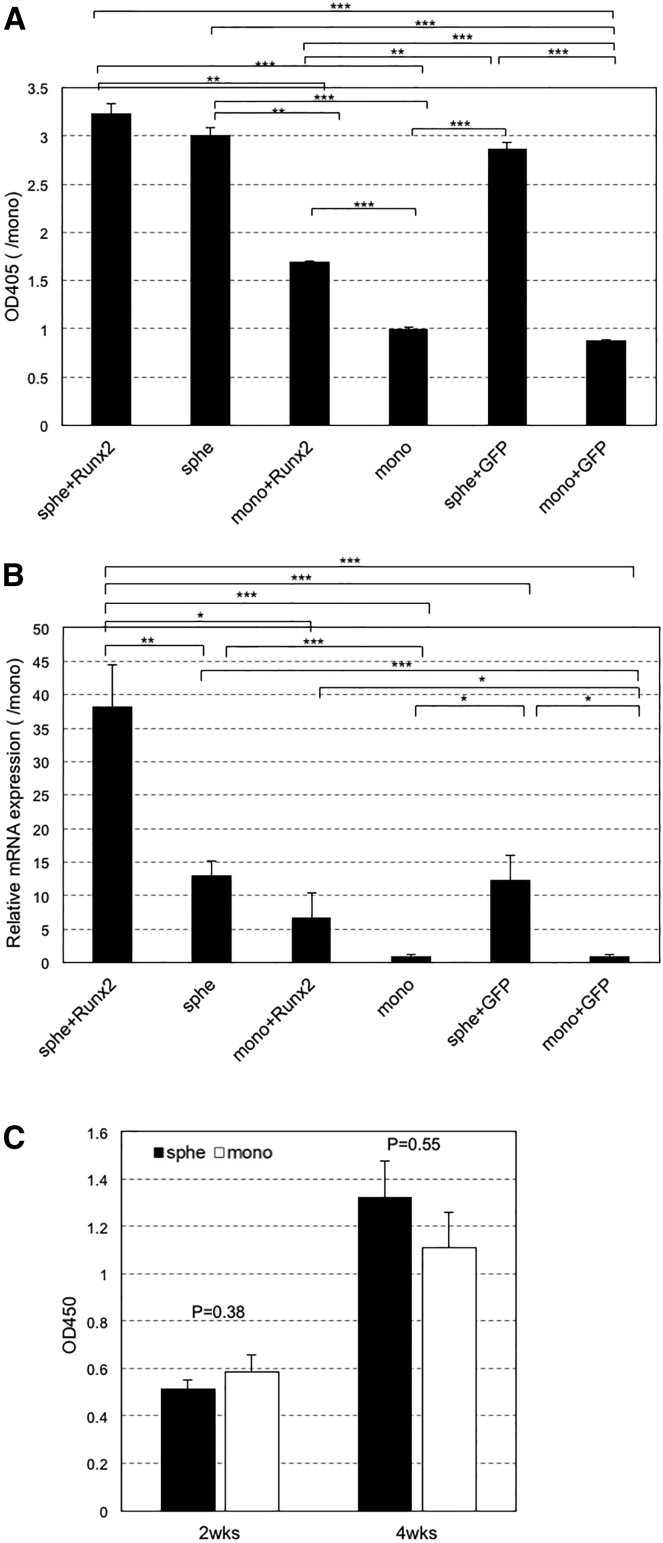
The ALP and osteocalcin levels in MSC spheroids are both significantly higher than those in MSCs from monolayer culture (Figure 1). This is consistent with a previous study indicating that spheroid formation augments the capacity of MSCs for osteogenic differentiation.15 Runx2 introduction led to a further increase in both levels compared to their nontransfected counterparts. This increase was not induced after GFP introduction. However, a significant difference was found only between osteocalcin expression in MSC spheroids with Runx2 introduction and those without and between ALP in MSCs from the monolayer with Runx2 introduction and those without. Nevertheless, these data strongly suggest that spheroid formation and Runx2 transfection can interdependently enhance the capacity of MSCs for osteogenic differentiation. Besides, the ALP and osteocalcin levels in MSC spheroids are both significantly higher than those in MSCs from monolayer culture. The high expression of osteogenic markers in spheroids was thought to be caused by not only an enhanced capacity of MSCs for osteogenic differentiation but also improved cell proliferation in spheroid culture compared to monolayer. Therefore, cell proliferation in spheroid and monolayer cultures was evaluated, but no significant difference in cell proliferation between spheroid and monolayer was found (Figure 1C). This result strongly suggested that spheroid formation can enhance the capacity of MSCs for osteogenic differentiation.
Figure 1.
Evaluation of Osteogenic Differentiation after Introduction of Runx2-Encoded pDNA into MSC Spheroids or MSCs from Monolayer Culture
Twenty-four hours after Runx2 and GFP introduction, MSC spheroids or MSCs from monolayer culture were cultured with osteogenic medium and osteogenic differentiation was evaluated by osteogenic markers (alkaline phosphatase [ALP] and osteocalcin). (A) ALP activity was measured using a cellular enzyme assay after 14 days of osteogenic differentiation. ALP activity was shown by measuring the absorbance at 405 nm. (B) Osteocalcin expression levels were measured by real-time qPCR after 28 days of osteogenic differentiation. (C) Cell proliferation of MSC spheroids and MSCs from monolayer culture at 14 or 28 days after osteogenic differentiation was evaluated with (CCK)-8 solution. Cell proliferation was shown by measuring the absorbance at 450 nm. Data are presented as the mean ± SEM (n = 7, A; n = 5, B; n = 8 [2 weeks] and n = 12 [4 weeks], C). Asterisks indicate significant differences (*p < 0.05; **p < 0.01; ***p < 0.001) determined by two-tailed Student’s t test.
Bone Regeneration Induced by MSC Transplantation
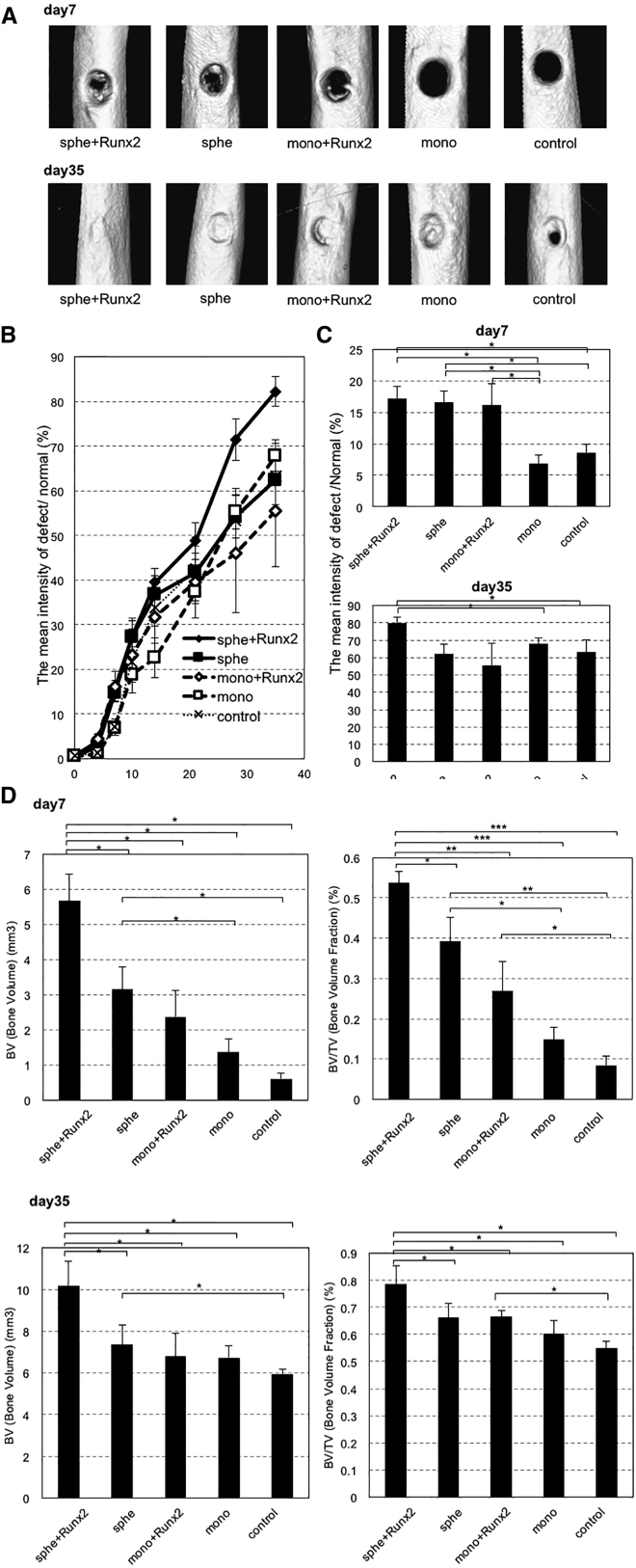
MSC spheroids, with or without Runx2 transfection, were used for treating bone defects. Spheroids were collected from micropatterned substrates and seeded in collagen-based scaffolds and placed on the bone defect that was made on rat femur (Figure 2). MSCs from monolayer culture, with or without Runx2 transfection, were used as controls. The new bone formation in the bone defect was evaluated using quantitative micro-computed tomography (μCT) over time. Figure 3A shows representative three-dimensional images of the femur on day 7 and day 35, in which bone regeneration appears to be promoted in the defect receiving transplantation of Runx2-transfected MSC spheroids. On the basis of CT images, the mean bone intensity of the defect area was quantified to calculate the percent of the intensity compared to the intensity of normal cortex over 35 days (Figures 3B and 3C). Bone regeneration seems to be promoted in Runx2-transfected MSC spheroids over the course of the observation. To perform a more detailed analysis of bone regeneration, bone volume (BV) (mm3) and bone volume fraction (BV/TV) (%) were evaluated as indicators of new bone formation (Figure 3D). On day 7, a significant increase in BV was observed in Runx2-transfected MSC spheroids compared to nontransfected MSC spheroids, MSCs from monolayer culture (with or without Runx2), and control. Similarly, BV/TV showed significant increases in Runx2-transfected MSC spheroids group compared to other groups. In contrast, significant increases in BV and BV/TV were not observed in MSCs from monolayer culture with Runx2 compared to those without Runx2. In addition, BV and BV/TV increased significantly in MSC spheroids compared to MSC monolayer, regardless of transfection. These results suggest that, consistently with the in vitro results of osteogenic differentiation of MSCs (Figure 1), spheroid formation and Runx2 transfection interdependently enhanced the capacity of transplanted cells for bone regeneration. In contrast, on day 35, a significantly higher value of BV and BV/TV was observed in Runx2-transfected MSC spheroids compared to nontransfected MSC spheroids, MSCs from monolayer culture (with or without Runx2), and control. However, there were almost no significant differences among the groups except Runx2-transfected MSC spheroids, although all groups showed remarkable bone regeneration with the increase in the BV and BV/TV values over time.
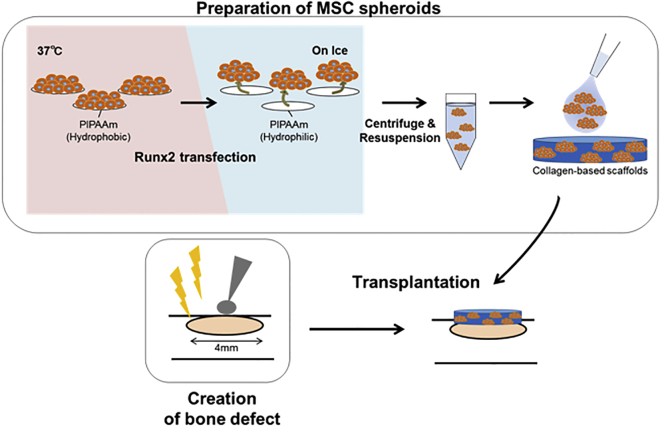
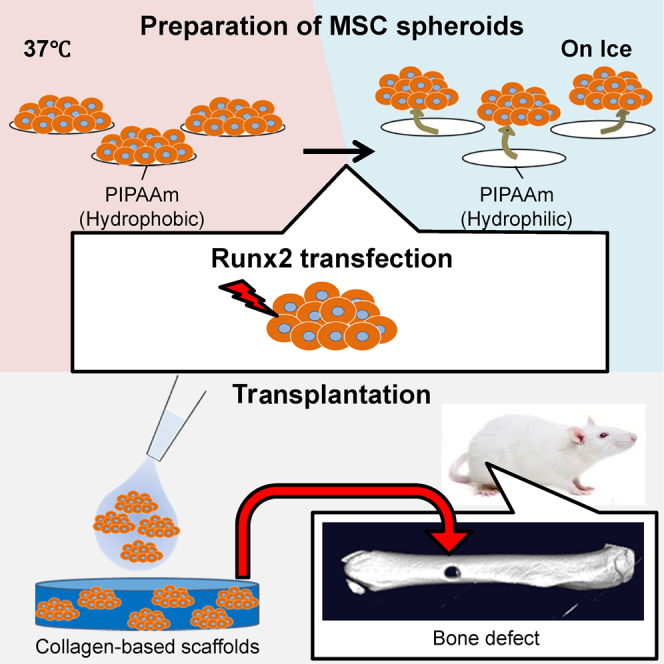
Figure 2.
Procedure to Make MSC Spheroids and Transplant Them to Bone Defects
MSC spheroids were prepared by culturing MSCs on specially coated thermosensitive plates. Spheroids were recovered by placing the plates on ice, which induces a phase transition to convert the PIPAAm polymer from a hydrophobic state to a hydrophilic state, releasing the spheroids into the media. Spheroids were then concentrated by centrifugation. The supernatant was removed by aspiration, and the cell pellets were resuspended in 50 μL PBS. MSC spheroids were then added to 4-mm-diameter collagen-based scaffolds, and the scaffolds were placed on the bone defect site.
Figure 3.
Evaluation of Bone Regeneration in Bone Defect on Rat Femur
After transplantation of MSC spheroids and MSCs from monolayer culture with or without Runx2, the defect area was imaged using micro-CT. (A) Representative images of micro-CT on day 7 and day 35 are shown. (B) Quantification of bone regeneration in bone defects from day 0 to day 35 is shown, represented by the percent of the intensity compared to normal cortex. (C) Extracted data of day 7 and 35 from (B) are shown. (D) Analysis of bone volume (mm3) and bone volume fraction (%) at day 7 and day 35 is shown.
Data are presented as the mean ± SEM (n = 4). Asterisks indicate significant differences (*p < 0.05; **p < 0.01; ***p < 0.001) determined by two-tailed Student’s t test.
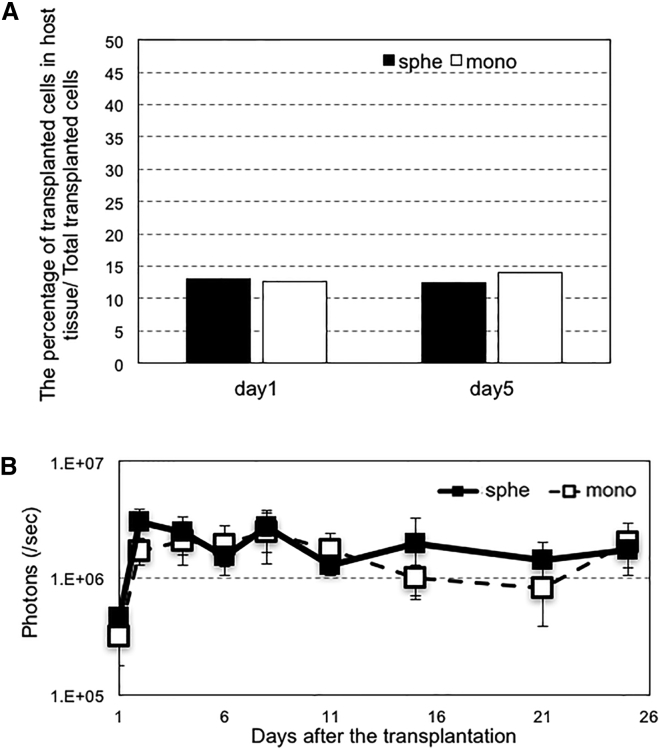
For in-depth analysis of the behavior of transplanted cells in the bone defect, cell survival was evaluated using real-time qPCR of SRY gene, a Y-chromosome-specific gene. The MSCs to be used for transplantation were collected from male rats and were transplanted into the femur bone defect of female rats. The survival rates of the transplanted cells were analyzed by distinguishing them from the host cells. Contrary to expectations, there were no significant differences between the survival rates of MSC spheroids and MSCs from monolayer culture (Figure 4A). In addition, we evaluated the levels of transgene expression in MSCs after transplantation. MSCs (spheroids or monolayer) were prepared with transfection of luciferase-encoded pDNA and transplanted subcutaneously into nude mice, and luciferase expression level in host tissue was measured using the IVIS imaging system. We did not find a significant difference between the luciferase expression levels of the two groups (Figure 4B). Therefore, we concluded that the enhanced induction of bone regeneration by Runx2-transfected MSC spheroids (Figure 3) would not be due to the increased cell survival or transgene expression levels after transplantation.
Figure 4.
Analyses on Cell Behavior after Transplantation
(A) The percentage of transplanted MSCs (spheroids or monolayer) was measured by real-time qPCR. MSCs from male rats were transplanted into bone defects on femurs of female rats. At 1 day or 5 days after transplantation, the collagen scaffolds were removed from the transplantation site, and total DNA was extracted from the scaffold. The number of SRY gene copies was evaluated with real-time qPCR. The percentage of surviving cells was calculated by assuming that the initial 2,400,000 transplanted cells were 100%. Data are presented as the mean ± SEM (n = 5). (B) Luciferase expression in host tissue after transplantation was evaluated by quantification of luminescence intensity. MSCs in spheroid or monolayer culture were transfected with luciferase expressing plasmid DNA. At 24 hours post-transfection, MSCs were added to collagen scaffolds and transplanted subcutaneously into nude mice. The level of luciferase expression was measured with IVISTM imaging system. Data are presented as the mean ± SEM (n = 8).
Cell Migration into the Bone Defect Area
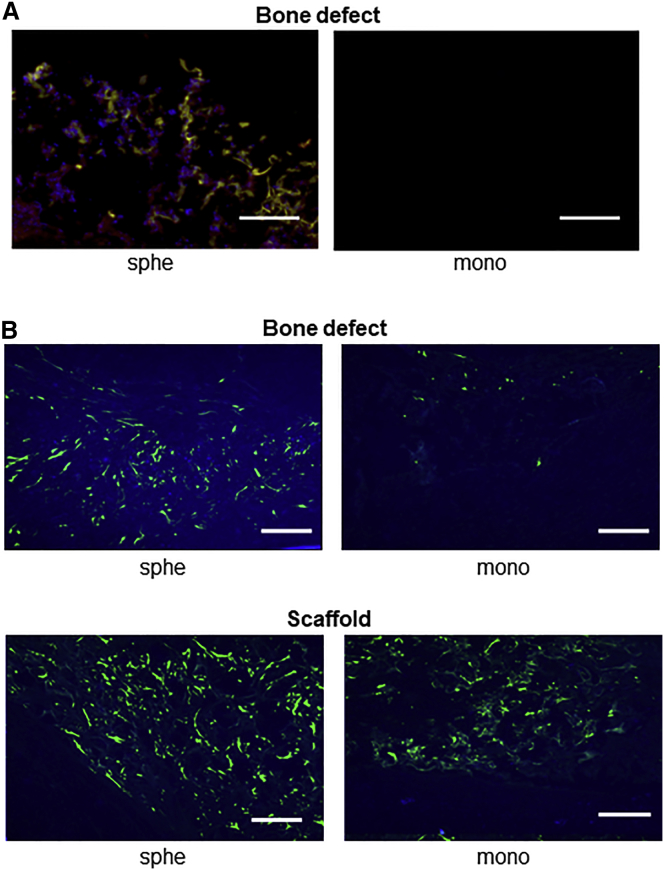
Another possibility is that cell migration is enhanced by the spheroid formation.16 We evaluated the migration of GFP-expressing MSCs (spheroids or monolayer), followed by immunohistological examination of the bone defect area. At 14 days after transplantation, sections of the bone defect were stained with anti-GFP antibody as well as anti-type 1 collagen antibody to confirm new bone formation. Many GFP-positive MSCs were found in the area stained by anti-type 1 collagen antibody (Figure 5A) in mice that received spheroid MSCs. There were almost no GFP-positive cells in the bone defect after the transplantation of MSCs from monolayer culture, strongly suggesting that the enhanced migration of MSC spheroids from the scaffold to the bone defect is correlated with the enhanced new bone formation in the bone defect. When we examined sections of both the bone defect area and the scaffolds 5 days after the transplantation of MSC spheroids, there were a considerable number of GFP-positive cells in both the bone defect area and scaffolds. In contrast, when using MSCs from the monolayer, the number of GFP-positive cells in the bone defect area was much lower than that in the scaffold (Figure 5B). Considering that cell survival was similar between MSC spheroids and MSCs from monolayer culture (Figure 4), it is reasonable to assume that the difference in the cell number in the bone defect area would be due to the difference in cell migration from the scaffold. Indeed, analyses of the expression levels of cell-migration-related genes, CXCR4 and integrin-α2, revealed that both genes showed significantly higher values in MSC spheroids than in MSCs from monolayer culture (Figure 6). The upregulation of these genes in spheroid cells was previously reported, and the current results were consistent.17, 18
Figure 5.
Immunofluorescent Staining of GFP
MSCs were taken from GFP-expressing transgenic rat and transplanted into non-GFP rats to analyze the movement of transplanted cells in the host tissue. (A) Double staining of GFP and type 1 collagen at 14 days after cell transplantation is shown. Frozen sections of bone defect area were stained using the Zenon technique (blue, nucleus; green, GFP-expressing MSCs; red, type 1 collagen) and observed by fluorescence microscope. a, spheroid; b, monolayer. Scale bars represent 10 μm. (B) GFP staining at 5 days after cell transplantation is shown. Frozen section of scaffold and bone defect area were stained for GFP, respectively, and observed by fluorescence microscope. a, bone defect area of spheroid; b, bone defect area of monolayer; c, scaffold of spheroid; d, scaffold of monolayer. Scale bars represent 50 μm.
Figure 6.
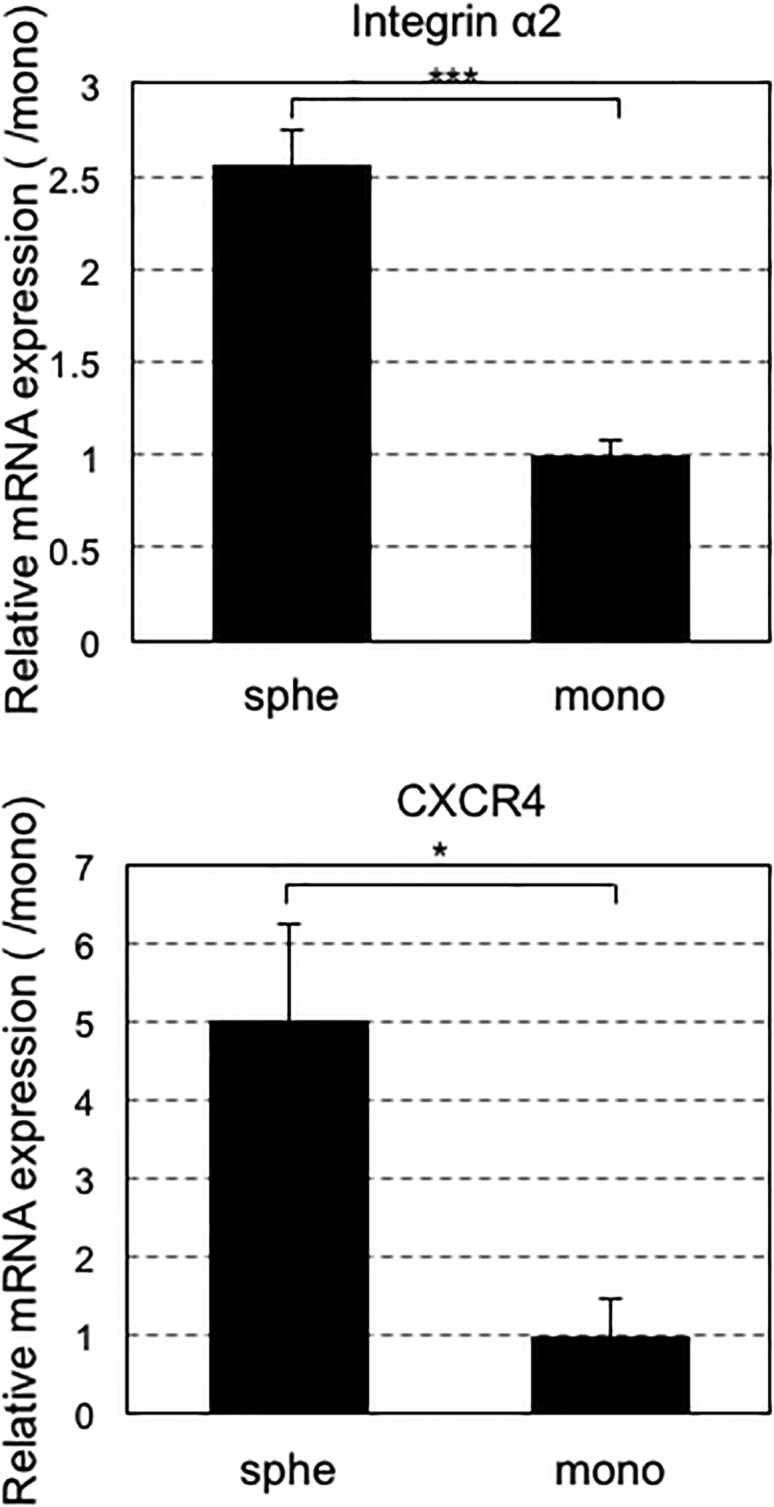
Expression Levels of Cell-Migration-Related Genes
Expression levels of CXCR4 and integrinα2 evaluated by real-time qPCR after 3-day incubation of MSCs after forming spheroids or in monolayer. Data are presented as the mean ± SEM (n = 5). Asterisks indicate significant differences (*p < 0.05; ***p < 0.001) determined by two-tailed Student’s t test.
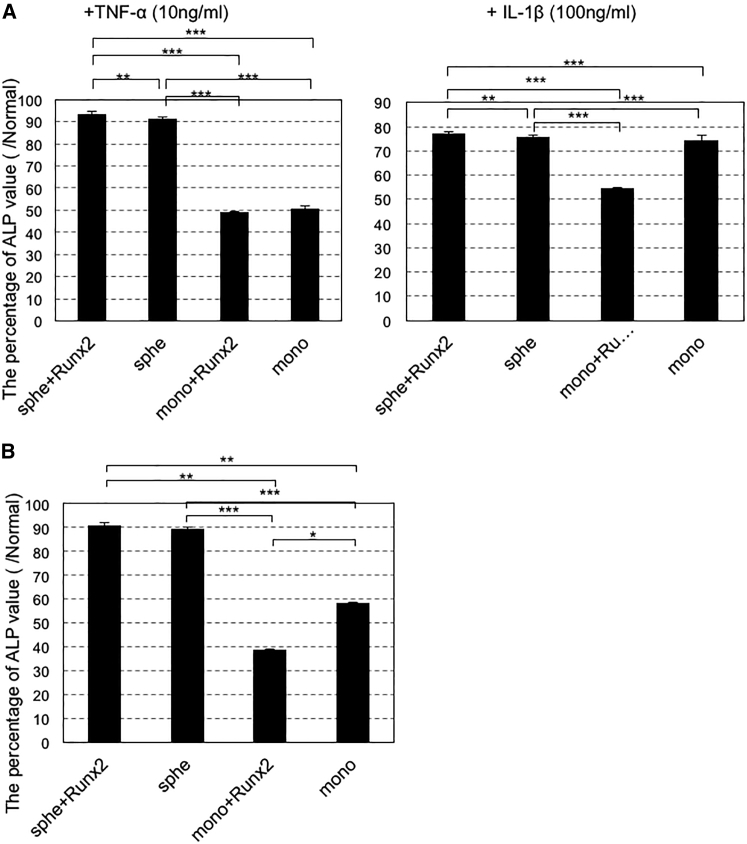
Effect of Pathological Conditions on Transplanted Cells
We evaluated the effect of spheroid formation and/or Runx2 gene introduction on the tolerance to pathological conditions, such as hypoxia and inflammation. The conditions were mimicked by adding deferoxiamine (DFx) or inflammatory cytokines (tumor necrosis factor alpha [TNF-α] or interleukin-1β [IL-1β]) into the culture medium (see Materials and Methods). For both hypoxia and inflammation, MSC spheroids showed greater levels of ALP activity than did MSCs from monolayer culture (Figure 7). In contrast, the effect of Runx2 gene introduction was unclear. Thus, tolerance to pathological conditions was likely enhanced because of maintenance of cell-to-cell interaction by spheroid formation.
Figure 7.
Effects of Spheroid Formation and Runx2 Gene Introduction for Enhancing Tolerance to Pathological Conditions
Twenty-four hours after Runx2 introduction, MSC spheroids or MSCs from monolayer culture were cultured with osteogenic medium. ALP activity was measured using a cellular enzyme assay 14 days after osteogenic differentiation. (A) TNF-α (10 ng/mL), IL-1β (100 ng/mL), and (B) deferoxiamine (DFx) (10 μM) were added to the medium to mimic pathological conditions. ALP activity is shown as percent of controls without TNF-α, IL-1β, or DFx. The data are presented as the mean ± SEM (n = 4, A; n = 5, B). Asterisks indicate significant differences (*p < 0.05; **p < 0.01; ***p < 0.001) determined by two-tailed Student’s t test.
Discussion
This study shows the usefulness of transplantation of genetically modified MSC spheroids for bone regeneration. The combination of two technologies for spheroid formation, that is, use of micropatterned culture plates and Runx2 gene introduction using polymer-based gene carriers, remarkably enhanced the therapeutic effects and bone regeneration after MSC transplantation.
The increase in osteogenesis is thought to be a result of interdependent contribution of multiple factors. The first is enhanced osteogenic differentiation of MSCs in the spheroids, in which the sensitivity to various differentiation stimuli increases. In addition, osteogenic differentiation was further promoted by introduction of the Runx2 gene. Eventually, the greatest increase in bone regeneration in the bone defect area was observed after transplantation of the Runx2-transfected MSC spheroids.
Well-differentiated MSCs can perform multiple functions, including production of bone matrix in the bone defect area and providing a paracrine effect by acting on host cells. Regarding the latter, we recently obtained early motor function recovery in a mouse spinal cord injury model by transplantation of genetically modified MSC spheroids into the injured region. The secretion of various growth and trophic factors, such as hepatocyte growth factor and leukemia inhibitory factor, had a neuroprotective effect on the damaged neural cells.9 It is reasonable to assume that the enhanced secretion of these factors may stimulate the migration of the host stem cells to the site of osteogenesis and promote bone formation.
Furthermore, the study shows that MSCs in the spheroids have enhanced chemotaxis-related gene expression that is consistent with the high capacity to migrate to the bone defect area. These features are thought to significantly contribute to both increased bone matrix production by the transplanted cells themselves and enhancement of the paracrine effect at the bone defect area. Although it is difficult to strictly determine how much each of these two factors contributed to bone formation, and the detailed mechanism in the long-term benefits by the spheroids is not fully elucidated, it is strongly suggested that these factors acted interdependently to promote bone formation at the bone defect area.
The problems faced during cell transplantation therapy are the low survival rate of transplanted cells and the difficulty in maintaining the cell function.2, 6, 19 The combined system of spheroid formation and gene introduction has the potential of overcoming these problems. Among the three important elements of regenerative medicine, that is, cell source, signal control, and scaffolds, this system can effectively control the first two simultaneously, providing high practical value for clinical use.
In conclusion, bone formation could be promoted in the rat bone defect model by transplanting genetically modified MSC spheroids with the Runx2 gene, as compared to that in controls with nontransfected MSC spheroids or genetically modified MSCs obtained from conventional monolayer culture. The enhanced osteogenic differentiation and chemotactic activity of the MSCs contributed to enhanced bone regeneration. Thus, this system provides a promising option for cell transplantation therapy in various fields of regenerative medicine.
Materials and Methods
Materials
pcDEF3 pDNA expressing Flag-tagged mouse Runx2 were generous gifts from K. Miyazono (The University of Tokyo, Tokyo, Japan). For constructing GFP or luciferase-expressing plasmid DNA (pDNA), the protein-expressing segment of the pAcGFP or pGL4.13 plasmid (Promega, Madison, WI, USA) was cloned into the pCAG-GS plasmid (Riken, Tokyo, Japan) to obtain expression under the CAG promoter/enhancer. These pDNAs were amplified in competent DH5α Escherichia coli and purified using a NucleoBond Xtra Maxi Plus kit (Takara Bio, Shiga, Japan).
Animals
BALB/c nude mice (female mice; age, 7 weeks) and F344 rats (male rats: ages, 5 weeks and 9 weeks; female rats: age, 7 weeks) were purchased from Charles River Laboratories (Yokohama, Japan). Transgenic Sprague-Dawley rats (male rats; age, 5 weeks) expressing EGFP in all tissues under the control of CAG promoter/enhancer (EGFPe Sprague-Dawley rats) were purchased from Japan SLC (Shizuoka, Japan). All animal studies were conducted with the approval of the Animal Care and Use Committee of the University of Tokyo, Tokyo, Japan.
Preparation of MSC Spheroids
For in vivo analyses, thermoresponsive micropatterned plates that were constructed on thermoresponsive culture plates (Upcell; CellSeed, Tokyo, Japan) were used.8, 20 A micropatterned plate with the same construction but without thermoresponsiveness (Cell-able; Toyo Gosei, Tokyo, Japan) was used for in vitro analyses. Human MSCs (hMSCs) were purchased from Lonza (Allendale, NJ, USA) and cultured with MSCGM-CD BulletKit MSC culture medium (Lonza, Basel, Switzerland). To obtain rat MSCs (rMSCs), F344 rats (male rats; age, 5 weeks) and transgenic Sprague-Dawley rats (male rats; age, 5 weeks) expressing EGFP in all tissues were anesthetized with 1 mL of 5× diluted somnopentyl (200 μL somnopentyl + 800 μL normal saline). The bone marrow was collected from the femurs and tibias by inserting a 22G needle into the shaft of the bone and flushing it out with 10 mL of PBS. The cells were collected by filtration through a 100-μm nylon mesh (BD Falcon, Bedford, MA) and seeded onto 10-cm culture dishes using DMEM (Sigma-Aldrich, Saint Louis, MO, USA) containing 10% fetal bovine serum (FBS) (Life Technologies Japan, Tokyo, Japan) and 1% penicillin/streptomycin (Sigma-Aldrich). MSCs from passage five were seeded onto micropatterned plates for spheroid culture and onto conventional plates for monolayer culture at 400,000 cells/well in 12-well plates and 40,000 cells/well in 96-well plates, respectively.
Transfection
PAsp(DET) homopolymer was synthesized as previously reported; the polymerization degree of the PAsp(DET) segment was found to be 57 by 1H-NMR.21, 22, 23 For gene transfection, PAsp(DET) polymer and pDNA were separately suspended in 10 mM HEPES buffer (pH 7.3) and mixed at total amino groups in polymer (N)/total phosphate groups in pDNA (P) ratio of 5. In the 12-well plates, 10 μg pDNA was added to 1 mL of culture medium for each well. The transfection was performed 2 days after seeding of MSCs onto the plates.
Evaluation of Osteogenic Differentiation
hMSCs were used in in vitro experiments by considering the future clinical application using hMSCs. hMSCs were seeded onto micropatterned plates for spheroid culture and onto conventional plates for monolayer culture at 400,000 cells/well in 12-well plates and 40,000 cells/well in 96-well plates, respectively. At twenty-four hours after Runx2 and GFP transfection, the MSCs were cultured in osteogenic medium (50 μg/mL ascorbic acid phosphate [AsAP], 10 mM β-glycerophosphate [β-GP], and 0.1 μM dexamethasone [Dex], DMEM [high glucose]-containing 10% FBS, and 1% penicillin/streptomycin). At 14 days after incubation with osteogenic medium, the ALP activity of MSCs in the 96-well plates was assayed using the TRACP&ALP Assay Kit (Takara Bio) according to the manufacturer’s protocol. The kit uses p-NPP as a phosphatase substrate, which turns yellow when dephosphorylated by ALP. ALP activity was measured by the absorbance at 405 nm after color formation. At 28 days after incubation with osteogenic medium, total mRNA was extracted from the MSCs in the 12-well plates using RNeasy Mini Kits (QIAGEN), according to the manufacturer’s protocol. Then, osteogenic differentiation was evaluated by real-time qPCR for osteocalcin.
Cell Proliferation
hMSCs were seeded on micropatterned plate or conventional monolayer culture plate at 40,000 cells/well in 96-well plates and cultured with osteogenic medium for 14 or 28 days. 10 μL (CCK)-8 solution (Dojindo Laboratories, Kumamoto, Japan) was added into each well, and the absorbance at a wavelength of 450 nm was detected after two hours incubation.
Bone Defect and MSC Transplantation
F344 rats (male rats; age, 9 weeks) were anesthetized with 3% isoflurane (Abbott Japan, Tokyo, Japan). The surgical site on the thigh (around the femur) was shaved. The skin, subcutaneous layer, and fascia were incised, and the biceps femoris muscles were separated. The femur was exposed, with the periosteum being preserved. A 4-mm-diameter monocortical defect was created at the center of the femur using round dental steel bars. Immediately after creating the bone defect, rMSCs derived from F344 rat were transplanted, as described below, after washing with saline. MSC spheroids were recovered by placing the plates on ice twenty-four hours after gene transfection. The recovered spheroids were concentrated by centrifugation at 100 g for 3 min. Monolayer culture MSCs were recovered by trypsinization. MSCs were added to collagen-based scaffolds (Integra; Integra Life Science, NJ, USA) of 4 mm in diameter, which were then placed in the bone defect site; 2,400,000 cells were transplanted per bone defect. The scaffold was composed of collagen-GAG matrix with three-dimensional porous structure. It helps the cells to invade and migrate into the scaffold. Then, the muscles over the femur and skin were closed with 4e0 nylon sutures. We used inbred rat in vivo experiments to avoid transplantation immunity.
Micro-CT Analysis
Micro-CT scanning of the bone defect sites was performed using the microfocus X-ray computed tomography system CosmoScan FX (Rigaku, Tokyo, Japan) under the following conditions: tube voltage, 90 kV and tube current, 110 mA. The three-dimensional construction software was used for quantitative analysis. The mean bone intensity of the defect area was quantified by measuring CT values in the bone defect area in a time-dependent manner. We measured the mean CT values in the bone defect area. The percentage of bone regeneration was calculated with the mean CT values of bone defect area when the CT value of background was assumed 0 and the CT value of bone was assumed 100. BV and BV/TV were analyzed by a three-dimensional segmentation algorithm using the imaging analysis software ImageJ (NIH, MD, USA). The micro-CT datasets were transferred into a three-dimensional structure, and BoneJ plugin was used for three-dimensional imaging and quantification of the BV and BV/TV in the bone defect area.
Quantification of Transplanted Cells in Host Tissue
Implanted collagen scaffolds were removed from bone defects at twenty-four hours or at 5 days after rMSCs derived from F344 rat transplantation into F344 rats (female rats; age, 6 weeks). The total DNA of transplanted cells that remained in the scaffold was extracted from the transplantation site using DNeasy Blood and Tissue Kits (QIAGEN), according to the manufacturer’s protocol. Real-time qPCR was performed in 96-well PCR plates containing the 10 μL of FastStart Universal SYBR Green Master (ROX) mix (Roche, Basel, Switzerland), 0.6 μL of each primer (10 μM), 2 μL of cDNA, and 6.8 μL of sterile water. Real-time PCR reactions in a ABI Prism 7500 Sequence Detector (Applied Biosystems, Foster City, CA, USA) were initiated by heating to 95°C for 10 min, followed by 40 cycles of 95°C (10 s) and 60°C (40 s). The relative quantification of gene expression was performed using the standard curve method. The standard curve for the number of cells that expressed SRY gene was constructed. Because MSCs from male rats were transplanted into female mice, the number of transplanted cells in the host tissue was proportional to the copy number of SRY genes on Y chromosomes, which was amplified using the following primer pair: forward, 5′-CATCGAAGGGTTAAAGTGCCA-3′ and reverse, 5′-ATAGTGTGTAGGTTGTTGTCC-3′.
In Vivo Measurement of Luciferase Expression
rMSCs derived from F344 rat in either spheroid or monolayer culture were transfected with luciferase-expressing plasmid DNA. At twenty-four hours post-transfection, MSCs were added to 4-mm collagen scaffolds at 2,400,000 cells each. MSC scaffolds were then subcutaneously transplanted into BALB/c nude mice. In vivo luciferase expression after transplantation was measured using an IVIS Imaging System (Xenogen, Alameda, CA, USA) after intravenous injection of d-luciferin (150 mg/kg; Sumisho Pharmaceuticals International, Tokyo, Japan).
Histological Analyses
At 5 or 14 days after transplantation of EGFP-expressing MSCs derived from Sprague-Dawley rat, the tissues of the bone defect site, including the scaffold and neighboring tissues, were excised en bloc. The tissues were fixed by overnight incubation in 4% paraformaldehyde at 4°C, decalcified, and frozen in optimal cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA, USA). Transverse 10-μm-thick sections were prepared. For samples from day 5 after transplantation, cells expressing GFP were immunostained by incubation with anti-GFP rabbit immunoglobulin G (IgG) antibody (1:300 dilution; Invitrogen, Carlsbad, CA, USA) and with Alexa 488 goat anti-rabbit IgG secondary antibody (1:300 dilution; Invitrogen). For samples from day 14 after transplantation, cells expressing GFP were immunostained by incubation with anti-GFP rabbit IgG antibody (1:300 dilution; Invitrogen) and with Zenon Alexa 488 rabbit IgG labeling antibody (Invitrogen), and collagen type I was immunostained using anti-collagen-I rabbit antibody (1:100 dilution; Cosmo Bio, Tokyo, Japan) and Zenon Alexa Fluor 647 rabbit IgG labeling antibody (Invitrogen). The nuclei were stained with Hoechst 33342 (1:100 dilution; Dojindo, Kumamoto, Japan). The sections were observed under an Axiovert 200 fluorescence microscope (Carl Zeiss, Jena, Germany).
Analyses of Cell-Migration-Related Gene Expression
hMSCs were seeded onto micropatterned plates for spheroid culture and onto conventional plates for monolayer culture, at 400,000 cells/well in 12-well plates. At 3 days after incubation, total mRNA was extracted using RNeasy Mini Kits (QIAGEN), according to the manufacturer’s protocol. Gene expression was evaluated by real-time qPCR.
Gene Expression Analysis with Real-Time qPCR
Quantitative relative gene expression was analyzed by real-time qPCR with an ABI Prism 7500 Sequence Detector (Applied Biosystems) and TaqMan Gene Expression Assays (Applied Biosystems; Hs01587814_g1 for osteocalcin, Hs00607978_s1 for C-X-C chemokine receptor type 4 [CXCR4], Hs00158127_m1 for integrin-α2, and Hs01060665_g1 for β-actin). For quantitative analysis, 2 μL cDNA was used for each reaction. Data were analyzed according to the ΔΔCt method, as per manufacturer’s instructions (Applied Biosystems).
Evaluation of ALP Activity under Pathological Conditions
hMSCs were seeded onto the micropatterned plates for spheroid culture and onto conventional plates for monolayer culture, at 40,000 cells/well in 96-well plates. After Runx2 transfection, MSCs were cultured for twenty-four hours in osteogenic medium containing 10 ng/mL TNF-α (Wako Pure Chemicals Industries, Osaka, Japan) and 100 ng/mL IL-1β (Wako) for mimicking inflammatory conditions and in 10 μM DFx (Sigma-Aldrich) for mimicking ischemic conditions. After 14-day incubation, ALP activity was assayed using the TRACP&ALP Assay Kit (Takara Bio) according to the manufacturer’s protocol.
Author Contributions
Conceptualization, K.I.; Methodology, K.Y., S.U., and K.I.; Investigation, K.Y. and S.U.; Resources, S.O.; Writing – Original Draft, K.Y. and K.I.; Writing – Review & Editing, S.U., S.O., and K.K.; Funding Acquisition, K.I. and K.K.
Conflicts of Interest
We declare no competing financial interest.
Acknowledgments
We are grateful to Prof. Ung-il Chung and Fumiko Yano (The University of Tokyo) for scientific advice regarding bone metabolism. This work was financially supported in part by JSPS KAKENHI grants JP15H03017 and 16K15642 to K.I. and 15K20962 and 17K20086 to S.U. and the JSPS Core-to-Core Program, A. Advanced Research Networks from the Japan Society for the Promotion of Science (JSPS). This work was also supported by the Graduate Program for Leaders in Life Innovation, The University of Tokyo Life Innovation Leading Graduate School from The Ministry of Education, Culture, Sports, Science and Technology (MEXT). We thank S. Ogura, A. Miyoshi, T. Tamamoto, S. Suzuki, and K. Morii (The University of Tokyo) for technical assistance. We also thank Dr. Samuel Crowley (Tokyo Medical and Dental University) for English language editing.
References
- 1.Scadden D.T. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 2.Kelm J.M., Fussenegger M. Scaffold-free cell delivery for use in regenerative medicine. Adv. Drug Deliv. Rev. 2010;62:753–764. doi: 10.1016/j.addr.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Meyerrose T., Olson S., Pontow S., Kalomoiris S., Jung Y., Annett G., Bauer G., Nolta J.A. Mesenchymal stem cells for the sustained in vivo delivery of bioactive factors. Adv. Drug Deliv. Rev. 2010;62:1167–1174. doi: 10.1016/j.addr.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baraniak P.R., McDevitt T.C. Stem cell paracrine actions and tissue regeneration. Regen. Med. 2010;5:121–143. doi: 10.2217/rme.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zinchenko Y.S., Culberson C.R., Coger R.N. Contribution of non-parenchymal cells to the performance of micropatterned hepatocytes. Tissue Eng. 2006;12 doi: 10.1089/ten.2006.12.2241. 2241–1251. [DOI] [PubMed] [Google Scholar]
- 6.Robey T.E., Saiget M.K., Reinecke H., Murry C.E. Systems approaches to preventing transplanted cell death in cardiac repair. J. Mol. Cell. Cardiol. 2008;45:567–581. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otsuka H., Hirano A., Nagasaki Y., Okano T., Horiike Y., Kataoka K. Two-dimensional multiarray formation of hepatocyte spheroids on a microfabricated PEG-brush surface. ChemBioChem. 2004;5:850–855. doi: 10.1002/cbic.200300822. [DOI] [PubMed] [Google Scholar]
- 8.Uchida S., Itaka K., Nomoto T., Endo T., Matsumoto Y., Ishii T., Kataoka K. An injectable spheroid system with genetic modification for cell transplantation therapy. Biomaterials. 2014;35:2499–2506. doi: 10.1016/j.biomaterials.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Uchida S., Hayakawa K., Ogata T., Tanaka S., Kataoka K., Itaka K. Treatment of spinal cord injury by an advanced cell transplantation technology using brain-derived neurotrophic factor-transfected mesenchymal stem cell spheroids. Biomaterials. 2016;109:1–11. doi: 10.1016/j.biomaterials.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Endo T., Itaka K., Shioyama M., Uchida S., Kataoka K. Gene transfection to spheroid culture system on micropatterned culture plate by polyplex nanomicelle: a novel platform of genetically-modified cell transplantation. Drug Deliv. Transl. Res. 2012;2:398–405. doi: 10.1007/s13346-012-0091-1. [DOI] [PubMed] [Google Scholar]
- 11.Banerjee C., McCabe L.R., Choi J.Y., Hiebert S.W., Stein J.L., Stein G.S., Lian J.B. Runt homology domain proteins in osteoblast differentiation: AML3/CBFA1 is a major component of a bone-specific complex. J. Cell. Biochem. 1997;66:1–8. doi: 10.1002/(sici)1097-4644(19970701)66:1<1::aid-jcb1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 12.Komori T. Regulation of skeletal development by the Runx family of transcription factors. J. Cell. Biochem. 2005;95:445–453. doi: 10.1002/jcb.20420. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Z., Zhao M., Xiao G., Franceschi R.T. Gene transfer of the Runx2 transcription factor enhances osteogenic activity of bone marrow stromal cells in vitro and in vivo. Mol. Ther. 2005;12:247–253. doi: 10.1016/j.ymthe.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Liu T.M., Lee E.H. Transcriptional regulatory cascades in Runx2-dependent bone development. Tissue Eng. Part B Rev. 2013;19:254–263. doi: 10.1089/ten.teb.2012.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W., Itaka K., Ohba S., Nishiyama N., Chung U.I., Yamasaki Y., Kataoka K. 3D spheroid culture system on micropatterned substrates for improved differentiation efficiency of multipotent mesenchymal stem cells. Biomaterials. 2009;30:2705–2715. doi: 10.1016/j.biomaterials.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 16.Huang G.-S., Hsieh P.-S., Tseng C.-S., Hsu S. The substrate-dependent regeneration capacity of mesenchymal stem cell spheroids derived on various biomaterial surfaces. Biomater. Sci. 2014;2:1652–1660. doi: 10.1039/c4bm00053f. [DOI] [PubMed] [Google Scholar]
- 17.Potapova I.A., Gaudette G.R., Brink P.R., Robinson R.B., Rosen M.R., Cohen I.S., Doronin S.V. Mesenchymal stem cells support migration, extracellular matrix invasion, proliferation, and survival of endothelial cells in vitro. Stem Cells. 2007;25:1761–1768. doi: 10.1634/stemcells.2007-0022. [DOI] [PubMed] [Google Scholar]
- 18.Potapova I.A., Brink P.R., Cohen I.S., Doronin S.V. Culturing of human mesenchymal stem cells as three-dimensional aggregates induces functional expression of CXCR4 that regulates adhesion to endothelial cells. J. Biol. Chem. 2008;283:13100–13107. doi: 10.1074/jbc.M800184200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mothe A.J., Tator C.H. Review of transplantation of neural stem/progenitor cells for spinal cord injury. Int. J. Dev. Neurosci. 2013;31:701–713. doi: 10.1016/j.ijdevneu.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Itaka K., Uchida S., Matsui A., Yanagihara K., Ikegami M., Endo T., Ishii T., Kataoka K. Gene transfection toward spheroid cells on micropatterned culture plates for genetically-modified cell transplantation. J. Vis. Exp. 2015:e52384. doi: 10.3791/52384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanayama N., Fukushima S., Nishiyama N., Itaka K., Jang W.D., Miyata K., Yamasaki Y., Chung U.I., Kataoka K. A PEG-based biocompatible block catiomer with high buffering capacity for the construction of polyplex micelles showing efficient gene transfer toward primary cells. ChemMedChem. 2006;1:439–444. doi: 10.1002/cmdc.200600008. [DOI] [PubMed] [Google Scholar]
- 22.Miyata K., Oba M., Nakanishi M., Fukushima S., Yamasaki Y., Koyama H., Nishiyama N., Kataoka K. Polyplexes from poly(aspartamide) bearing 1,2-diaminoethane side chains induce pH-selective, endosomal membrane destabilization with amplified transfection and negligible cytotoxicity. J. Am. Chem. Soc. 2008;130:16287–16294. doi: 10.1021/ja804561g. [DOI] [PubMed] [Google Scholar]
- 23.Itaka K., Ishii T., Hasegawa Y., Kataoka K. Biodegradable polyamino acid-based polycations as safe and effective gene carrier minimizing cumulative toxicity. Biomaterials. 2010;31:3707–3714. doi: 10.1016/j.biomaterials.2009.11.072. [DOI] [PubMed] [Google Scholar]