Abstract
Gene therapy for the treatment of genetic disorders has demonstrated considerable therapeutic success in clinical trials. Among the most effective and commonly used gene delivery vectors are those based on adeno-associated virus (AAV). Despite these advances in clinical gene therapy, further improvements in AAV vector properties such as rapid intracellular processing and transgene expression, targeted transduction of therapeutically relevant cell types, and longevity of transgene expression, will render extension of such successes to many other human diseases. Engineering of AAV capsids continues to evolve the specificity and efficiency of AAV-mediated gene transfer. Here, we describe a triple AAV6 mutant, termed AAV6.2FF, containing F129L, Y445F, and Y731F mutations. AAV6.2FF yielded 10-fold greater transgene expression in lung than AAV6 after 21 days. Additionally, this novel capsid demonstrated 101-fold and 49-fold increased transgene expression in the muscle and lungs, respectively, 24 hr post vector delivery when compared with the parental AAV6. Furthermore, AAV6.2FF retains heparin sulfate binding capacity and displays a 10-fold increase in resistance to pooled immunoglobulin neutralization in vitro. The rapid and potent expression mediated by AAV6.2FF is ideally suited to applications such as vectored immunoprophylaxis, in which rapid transgene expression is vital for use during an outbreak response scenario.
Keywords: AAV, AAV capsid, gene therapy, lung transduction, muscle transduction, vectored immunoprophylaxis
Introduction
Adeno-associated virus (AAV) is widely regarded as a safe and effective method of gene transfer to a variety of tissues. The in vitro and in vivo transduction profiles have been well characterized for many AAV serotypes.1, 2 Engineering of AAV capsids by rational design or directed evolution can produce capsid variants with desirable characteristics including altered tissue tropism, enhanced transgene expression in target cells, or the introduction of binding domains to aid in purification, to name a few. A prime example, AAV-DJ, is a product of AAV2 and AAV8 capsid shuffling resulting in a hybrid capsid with beneficial properties of both capsids: heparin binding capacity and in vitro transduction capacities from AAV2 and potent in vivo liver transduction from AAV8.3, 4
Alternatively, single-point mutations in an AAV capsid can also yield desirable modifications. AAV6.2, an AAV6 F129L point mutant, was demonstrated by Limberis et al.5 to be 2-fold more efficient at transducing the nose, airways, and alveolar type II cells of mice than AAV6. Similarly, when delivered intravenously to mice, AAV6.2 mediated 2-fold greater serum concentrations of human alpha-1 antitrypsin (hA1AT) than AAV6.6 Moreover, intramuscular administration of the same AAV6.2-hA1AT vector mediated higher serum levels of hA1AT than AAV6 or AAV9.6 Interestingly, F129L is a naturally occurring singleton residue in the majority of over 100 known primate AAV capsid sequences. In fact, AAV5 and AAV6 are the only serotypes that encode a phenylalanine instead of a leucine at this position.6
AAV capsids are prone to phosphorylation of tyrosine residues by epidermal growth factor receptor protein tyrosine kinase (EGFR-PTK), leading to alternative cellular trafficking, ubiquitination, and degradation.7, 8 Mutation of various surface-exposed tyrosine residues on AAV capsids has been shown to obstruct ubiquitin-mediated degradation of intracellular vector, thereby leading to more robust transgene expression.9 Tyrosine-to-phenylalanine mutations introduced at positions 444 and 730 in the AAV2 capsid yielded 9- and 11-fold greater transgene expression in vitro and 13- and 29-fold greater hepatocyte transduction in mice, respectively.9 A double AAV2 Y444F+Y730F mutant generated significantly greater hepatocyte transduction in vivo than either of the singleton mutants.10 Similar single-tyrosine mutations have been introduced into corresponding positions in AAV6 (Y445F, Y731F), AAV8 (Y447F, Y733F), and AAV9 (Y731F, Y446F) capsids with success in transducing various tissues; however, to our knowledge, an AAV6 capsid containing Y445F+Y731F has not yet been investigated.9, 10 Alternatively, AAV1 Y445F+Y731F resulted in decreased transgene output in vitro, demonstrating that the benefit of mutating surface-exposed tyrosine residues is capsid specific.11
Here, we have engineered a triple-mutant AAV6 capsid, termed AAV6.2FF, encoding F129L, Y445F, and Y731F point mutations, which we demonstrate to be superior to the parental capsid in terms of muscle and airway transgene expression kinetics in a mouse model.
Results
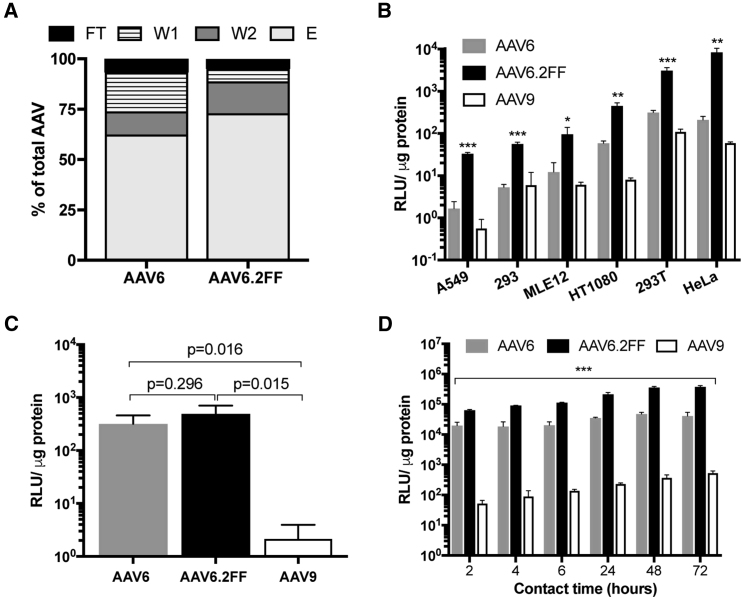
F129L, Y445F, and Y731F Mutations Do Not Impede Heparin Sulfate Binding Capacity
The ability of AAV6 to bind heparin sulfate is a favorable characteristic that enables heparin chromatography purification methods; therefore, we sought to determine whether AAV6.2FF retains heparin-binding capacity. In a heparin-binding assay, 62.4% of the input AAV6 vector was found in the elution fraction compared with 72.9% for AAV6.2FF (Figure 1A). Furthermore, less AAV6.2FF vector was located in the flow-through (FT) and wash (W) fractions than AAV6 (AAV6: FT, 6.7%, W1, 19.3%, W2, 11.4%; AAV6.FF: FT, 4.9%, W1, 6.1%, W2, 15.8%), indicating the AAV6.2FF mutations do not negatively impact heparin binding.
Figure 1.
Heparin Binding and Transduction Profiles of AAV6 and AAV6.2FF In Vitro
(A) Distribution of AAV vector genomes following heparin binding assay. (B) AAV6-, AAV6.2FF-, or AAV9-Luciferase were added to cells at an MOI of 2,000 and incubated for 72 hr prior to luciferase quantification to determine transduction efficiency. Binding (C) and internalization (D) of AAV vectors in HeLa cells. AAV6.2FF transduction was significantly greater than AAV6 and AAV9 over all time points. All experiments were conducted in triplicate. Values are expressed as means ± SD. Multiple t tests compared AAV6.2FF-mediated luciferase expression with AAV6 and AAV9. *p < 0.05; **p < 0.01; ***p < 0.001. E, elution; FT, flow-through; W1, wash 1; W2, wash 2.
AAV6.2FF Mediates Enhanced Transduction In Vitro
AAV transduction of a panel of cell lines, including human and murine lung epithelial cells, resulted in significantly increased transgene expression from AAV6.2FF compared with AAV6 and AAV9 (Figure 1B). While AAV9 is known to be relatively poor at transducing cells in culture,2 AAV6 is considered one of the better serotypes for in vitro use2; however, AAV6.2FF outperformed AAV6, mediating between a 7- and 39-fold increase in transgene expression, depending on the cell line.
AAV cell binding assays demonstrated similar binding properties for both AAV6 and AAV6.2FF (Figure 1C); however, internalization assays exposed variation in AAV6 and AAV6.2FF luciferase expression (Figure 1D). After 2 hr of contact time, there was a 3.2-fold increase in AAV6.2FF-mediated luciferase expression compared with AAV6, and this trend steadily increased with longer contact times to a 9.1-fold difference at 72 hr. These results indicate that the mechanism of improved AAV6.2FF transduction efficiency is not due to cell binding properties, but rather occurs post-internalization, potentially as increased efficiency in vector trafficking to the nucleus because of reduced capsid ubiquitination at exposed tyrosine residues.
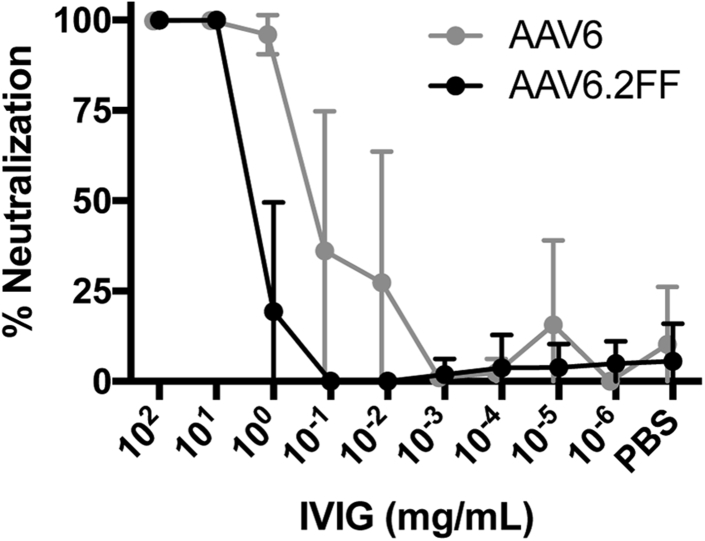
Mutation of Surface-Exposed Tyrosine Residues Reduces IVIG Neutralization
Unexpectedly, the three mutations that generate the AAV6.2FF capsid conferred a 10-fold increase in resistance to pooled intravenous immunoglobulin (IVIG) neutralization. 1 mg/mL IVIG was able to neutralize 97% of AAV6; however, the same concentration was only able to neutralize 20% AAV6.2FF (Figure 2). AAV6.2FF required a minimum of 10 mg/mL to neutralize 100% of the vector.
Figure 2.
IVIG Neutralization of AAV In Vitro
AAV vectors were incubated with 10-fold dilutions of IVIG for 1 hr at 37°C prior to adding to HeLa cells. 72 hr later, luciferase expression was quantified and the data expressed as percent AAV neutralization compared with control virus incubated with PBS only (n = 6). Values are expressed as mean ± SD.
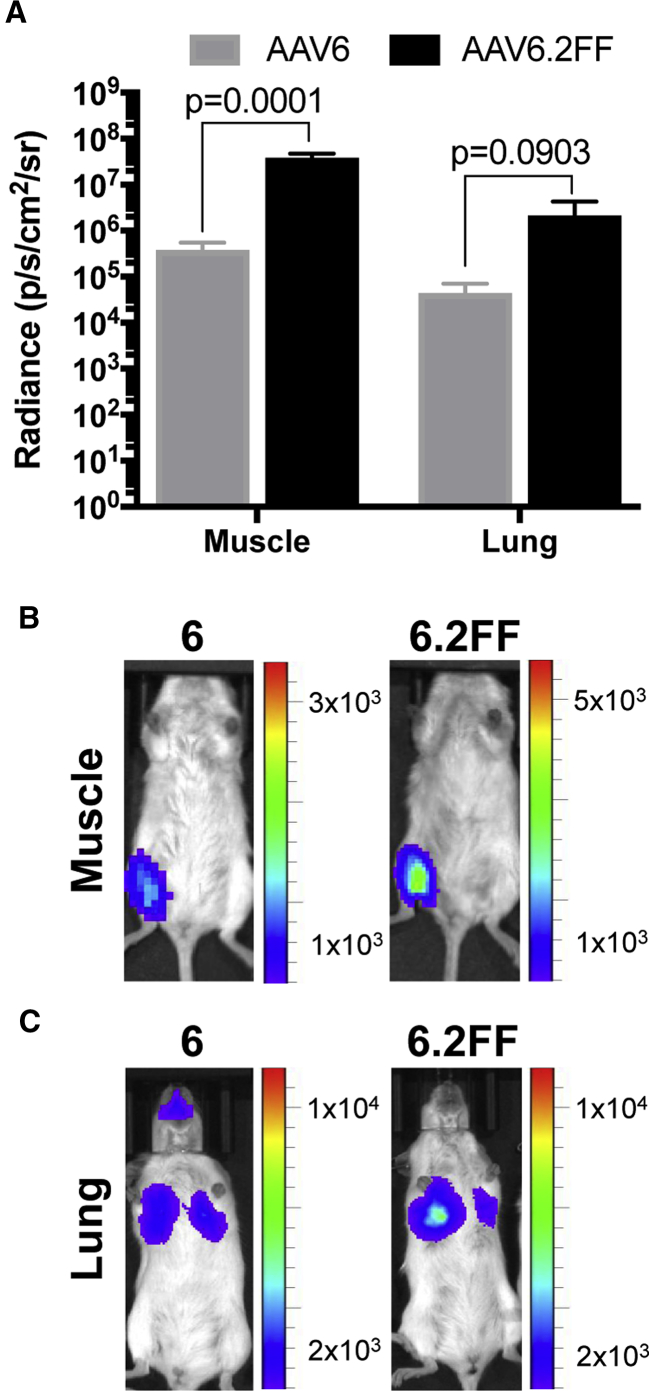
AAV6.2FF Displays Rapid Early Expression Kinetics in the Muscle and Lung of Mice
Most AAV capsids reach peak transgene expression between 14 and 28 days post-delivery; however, little is known about the early transgene expression kinetics of various capsids. AAV6 and AAV6.2FF vectors expressing firefly luciferase delivered to the muscle or lungs of mice were compared 24 hr following AAV administration using an in vivo imaging system (IVIS). Remarkably, in both tissues, AAV6.2FF produced significantly more transgene expression than AAV6 after only 24 hr (Figure 3A). In the muscle, AAV6.2FF yielded 101-fold greater radiance than AAV6 (Figure 3B), while in the lung there was a 49-fold difference favoring AAV6.2FF (Figure 3C).
Figure 3.
Comparison of AAV6- and AAV6.2FF-Mediated Transgene Expression 24 hr Post-AAV Delivery
Albino C57BL/6 mice (n = 4 mice/group) received 1 × 1011 vg by intramuscular injection or intranasal delivery. (A) Quantification of luciferase expression in the muscle and lung 24 hr following intramuscular or intranasal delivery, respectively (n = 4/group). Data are expressed as mean ± SD. Multiple t tests compared AAV6.2FF-mediated luciferase expression with AAV6. Representative images illustrating the pattern and intensity of luciferase expression in the muscle (B) and the lungs (C).
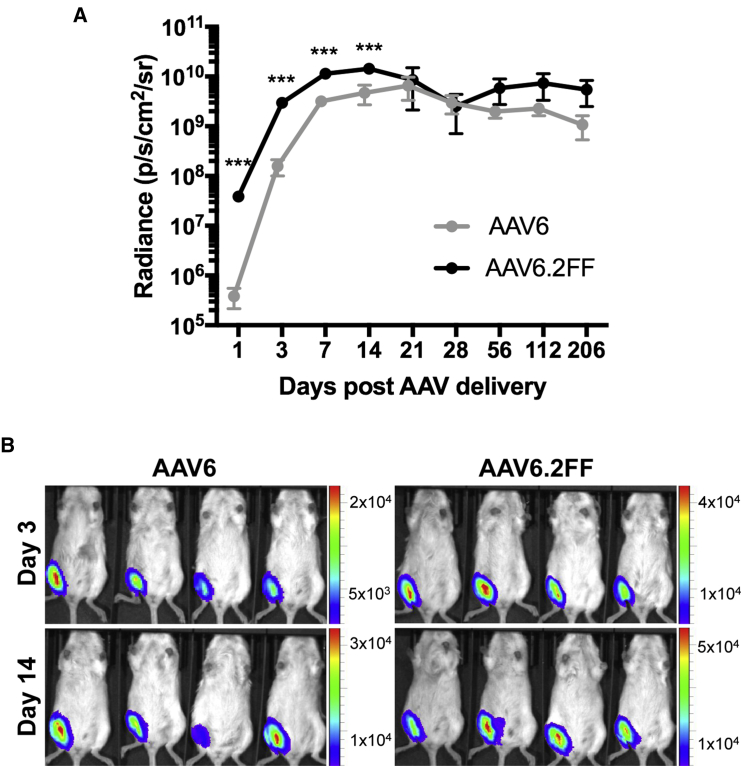
Long-Term AAV6- and AAV6.2FF-Mediated Expression in the Muscle Is Equivalent
In addition to the 24-hr time point, we also quantified luciferase expression from the muscle on days 3, 7, 14, 21, 28, 56, 112, and 205 post-AAV delivery (Figure 4A). AAV6.2FF-mediated transgene expression was significantly greater than AAV6 on days 3, 7, and 14, with 18.9-fold, 3.5-fold, and 3.0-fold greater signal, respectively (Figure 4B). Beyond 2 wk, there is an insignificant difference in the luciferase expression produced by the AAV6 and AAV6.2FF vectors; however, the luciferase signal from both groups plateaued at a high magnitude and has not begun to decline 16 weeks post-injection.
Figure 4.
Intramuscular Transgene Expression Kinetics of AAV6 and AAV6.2FF
Albino C57BL/6 mice (n = 4/ group) were injected with 1 × 1011 vg of AAV6- or AAV6.2FF-Luciferase. (A) Quantification of luciferase signal on days 1, 3, 7, 14, 21, 28, 56, 112, and 205 post-AAV delivery from the ventral view. Values are expressed as means ± SD. Paired t tests were used to calculate significance; ***p < 0.001. (B) Representative images demonstrating luciferase expression on days 3 and 14 post-AAV delivery to the gastrocnemius muscle.
AAV6.2FF Is Highly Efficient at Transducing the Lung Parenchyma of Mice
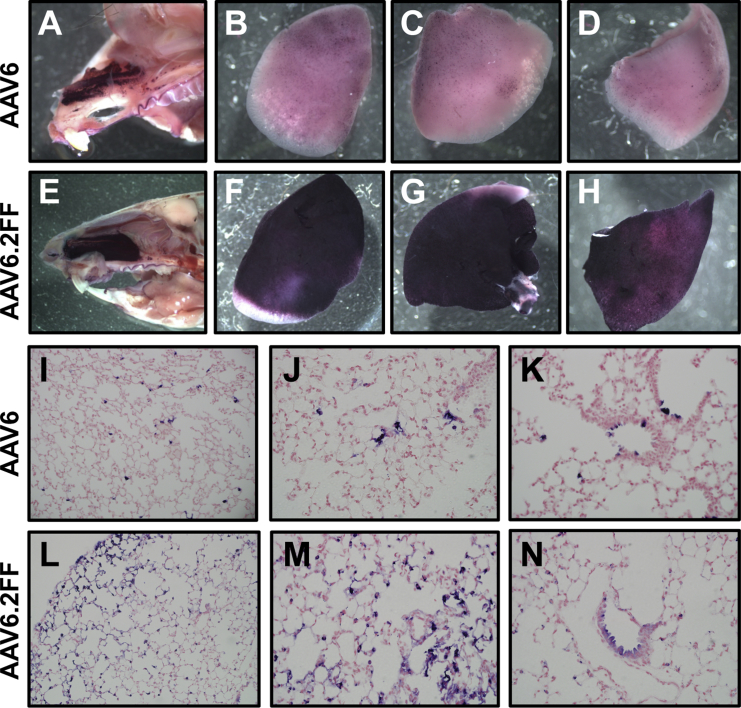
Intranasal delivery of AAV6 and AAV6.2FF vectors expressing a heat-stable alkaline phosphatase (AP) reporter gene demonstrated potent transduction of mouse lungs by AAV6.2FF. Although the overall AP staining distribution was consistent between the two vectors, there is a visible difference in the extent of transduction in the nasal cavity and lung lobes when observed grossly (Figures 5A–5H). Microscopic analysis of the lung tissue revealed that both vectors predominantly transduced cells of the distal lung; however, a greater proportion of cells was transduced with the AAV6.2FF vector compared with AAV6 (Figures 5I–5N). Additionally, both vectors were able to transduce epithelial cells of the proximal airway (Figures 5K and 5N).
Figure 5.
AP Staining of AAV6- and AAV6.2FF-Transduced Nose and Lungs
1 × 1011 vg of AAV6- or AAV6.2FF-AP was delivered intranasally to C57BL/6 mice (n = 4/group), and tissues were harvested and stained after 3 wk. Representative images of (A and E) the nose and (B–D, F–H) lung lobes for both vectors are shown. Lung sections were counterstained with nuclear fast red, and transduced cells expressing AP appear purple. Representative sections of transduced lung are shown at (I and L) ×20 and (J, K, M, and N) ×40 original magnification for AAV6 and AAV6.2FF.
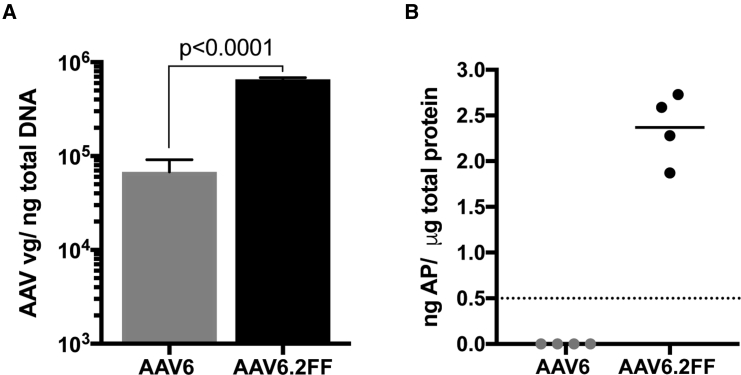
Quantification of the AAV vector genome copy numbers present in lung tissue 3 wk post-vector delivery demonstrates AAV6.2FF transduced the mouse lung 9.6-fold more efficiently than AAV6 (Figure 6A). A secondary quantitative analysis of AP enzymatic activity showed AAV6.2FF transduction yielded a mean of 2.36 ng of AP per microgram of total lung homogenate, while the lungs transduced by AAV6 contained concentrations of AP below the level of detection (Figure 6B).
Figure 6.
Quantification of AAV Vector Genomes in Transduced Lung Tissue
(A) TaqMan qPCR quantification of AAV genome copy number per nanogram of total DNA extracted from paraffin-embedded lung tissue. Values are expressed as means ± SD. Differences in AAV vg copy number between AAV6 and AAV6.2FF were determined using a Student’s t test. (B) Quantification of AP enzymatic activity in unfixed lung tissue. Dashed line indicates the threshold of detection for the assay.
Discussion
Similar to AAV6.2, AAV6.2FF maintains the heparin sulfate binding properties of AAV6, which is ideal for large-scale production of AAV using heparin column chromatography, as ultracentrifugation is impractical for large volumes.6, 12 AAV6 heparin binding is conferred by a single lysine at position 531, and replacing this residue with a glutamate (K531E) abolishes heparin binding capacity,13 indicating that F129L, Y445F, or Y731F mutations do not hamper K531 interaction with heparin sulfate.
AAV can be difficult to work with in cell culture, sometimes requiring large MOIs. AAV6 is one of the most amenable serotypes to in vitro transduction; however, the mutations introduced to produce AAV6.2FF further improve the in vitro transducing property of this capsid. AAV6.2FF mediated the strongest luciferase expression in all cell lines tested, making it a useful tool for cell culture experiments.
Pre-existing immunity to AAV capsids has had detrimental outcomes for some past gene therapy clinical trials.14 Two separate studies reported the presence of anti-AAV6 neutralizing antibodies in 30%–46% of healthy individuals sampled.11, 13 The increased resistance to IVIG neutralization mediated by AAV6.2FF allows the generation of more effective gene therapies by evading pre-existing immunity while retaining other beneficial properties of AAV6. However, the mechanism of decreased IVIG neutralization against AAV6.2FF is interesting because there are only three amino acid differences between AAV6.2FF and the parental AAV6. F129L is not a surface-exposed residue and only becomes externally available during endosomal trafficking; therefore, its role in antibody neutralization is likely minimal.15 The surface-exposed tyrosine residues at positions 445 and 731 were replaced with phenylalanine, and although the chemical structures of these amino acids are similar, the mutations resulted in a beneficial reduction in IVIG neutralization.
AAV6.2FF mediated significantly greater transgene expression than AAV6 in the lungs and nasal cavities of mice 3 wk post-AAV administration. AAV6.2 was previously reported to generate an approximately 2-fold increase in transduction of alveolar cells compared with AAV6,5 whereas AAV6.2FF enhanced transduction 9.6 times over AAV6 in the lung. Conversely, AAV6.2FF did not yield a significant difference compared with AAV6 in the muscle because long-term expression of these vectors beyond 2 wk produced equivalent levels of transgene. However, at 24 hr post-vector administration, transgene expression was significantly stronger from AAV6.2FF in both the muscle and lungs with 101- and 49-fold greater expression, respectively. Therefore, we have engineered an AAV6 variant that enhances the magnitude of early transgene expression without negatively altering the long-term expression kinetics of AAV6. We hypothesize that this increase in 24-hr expression is due to a reduction of ubiquitin-mediated degradation of AAV6.2FF capsid protein due to the absence of tyrosine residues at positions 445 and 731. Consequently, AAV trafficking and entry to the nucleus is more efficient, resulting in greater transgene expression shortly after viral entry.9
AAV6.2FF was developed as a dual-purpose capsid for a vectored immunoprophylaxis (VIP) platform to enable muscular and respiratory antibody gene expression, in addition to use as a traditional gene transfer vector to the lung. Using the same AAV capsid for multiple applications will facilitate standardization of vector production both for research use and translation to the clinic. Although early and robust expression of transgenes may not be critical for some traditional gene therapy applications, this advancement may have implications for the use of VIP as a post-exposure treatment option during outbreaks of emerging infectious diseases.
Materials and Methods
Cell Culture and Plasmids
Cell lines were maintained in DMEM (HyClone) supplemented with L-glutamine (HyClone) and 7% fetal bovine serum (HyClone) at 37°C and 5% CO2. The pDGM6 plasmid contains the Rep and AAV6 Cap genes, which was modified to generate a pDGM6.2FF plasmid. The AAV genome plasmids contained firefly luciferase or human placental AP driven by the CASI promoter16 followed by an SV40 polyA signal and a Woodchuck hepatitis virus (WHP) posttranscriptional regulatory element (WPRE).
AAV Vector Production
AAV vectors were produced by transfection of HEK293 cells as described previously.17 AAV6 and AAV6.2FF vectors were purified using a GE HiTrap heparin column,18 while AAV9 was purified by iodixanol gradient.19 Vector titers were determined using a TaqMan qPCR assay.20
Heparin Binding Assay
Crude lysate from cells transfected with plasmids for production of AAV6 and AAV6.2FF pseudotyped vectors was clarified and 0.22 μm filtered prior to loading onto a 5-mL HiTrap heparin HP column (GE). The column was equilibrated with DMEM prior to loading the vector and subsequently washed first with 25 mL of HBSS without Mg2+/Ca2+ (HyClone), again with 25 mL of HBSS with Mg2+/Ca2+ and finally eluted with 10 mL of HBSS with Mg2+/Ca2+ supplemented with 300 mM NaCl. The proportion of AAV in each fraction was determined by TaqMan qPCR.21
AAV Transduction, Binding, and Internalization Assays
1 × 105 cells were seeded into 24-well plates and allowed to adhere overnight. AAV was added the next day at an MOI of 2,000 in triplicate. 72 hr following the addition of AAV vector, cell lysate was harvested for quantification of luciferase expression (Promega) and Bradford analysis to determine the total protein content (Bio-Rad). Transgene expression is reported as relative light units (RLUs) per microgram of total protein.
IVIG Neutralization Assay
Human IVIG (Privigen) was serially diluted in PBS and added to 1 × 108 vector genomes (vg) of AAV in an equal volume, for a total of 25 μL, and incubated for 1 hr at 37°C, then added to HeLa cells in triplicate. After 72 hr, cell lysate was harvested for luciferase quantification according to the manufacturer’s instructions (Promega).
In Vivo Imaging of Luciferase Expression
All animal experiments were approved by the Institutional Animal Care Committees of the Canadian Science Centre for Human and Animal Health, the University of Guelph, the Cincinnati Children’s Hospital Medical Center, and the National Microbiology Laboratory (NML). Albino C57BL/6 mice (Jackson Labs) received 1 × 1011 vg of AAV6-Luciferase or AAV6.2FF-Luciferase in the gastrocnemius muscle in a 40 μL injection. Luciferase expression was quantified on days 1, 3, 7, 14, 21, 28, 56, 112, and 205 post-AAV delivery using a Xenogen IVIS as previously described.22 Alternatively, for lung delivery, mice received the same total vector dose, but it was administered in two intranasal instillations of 40 μL each, given 20 min apart (20), and luciferase expression was quantified 24 hr later.
Lung AP Expression
C57BL/6 mice (Jackson Labs) were administered 1 × 1011 vg of AAV6-AP or AAV6.2FF-AP by a modified intranasal technique as described previously.23 Three weeks following AAV delivery, the lungs and nose were harvested and fixed in 2% paraformaldehyde for 2 or 16 hr, respectively. Tissues were washed three times in PBS and heat-inactivated for 1 hr at 65°C prior to overnight incubation in AP staining buffer (100 mM Tris [pH 8.5], 100 mM NaCl, 50 mM MgCl2) with 100× X-PHOS (10 mg/mL 5-bromo-4-chloro-3-indolyl phosphate; Sigma) and 100× nitro blue tetrazolium chloride (50 mg/mL; Invitrogen). Gross images were acquired prior to paraffin embedding and sectioning for histological staining. Transduction was quantified by analyzing the copy number of AAV vg per 100 ng of genomic DNA extracted from paraffin-embedded tissue by qPCR as previously described.23 Quantification of AP present in homogenized lung tissue was performed as previously reported.21
Statistical Analysis
GraphPad Prism 7 software was used for statistical analyses. Multiple t tests were used unless otherwise stated. Data are expressed as mean ± SD.
Author Contributions
L.P.v.L., J.P.B., and S.K.W. designed the experiments. L.P.v.L. and J.M.D. produced the vectors and completed in vitro and mouse experiments. J.P.B and S.A.B. oversaw the IVIS experiments, and T.N.R., K.L.F., D.L.S., and S.J.M executed these studies. L.P.v.L. and S.K.W. wrote the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
We thank Dr. Bernard Thebaud for supplying MLE-12 cells, Dr. David Russell for providing the pDGM6 plasmid, and all those who participated in the care of the mice at all institutions. This work was funded by Cystic Fibrosis Canada (grant numbers 2635 and 3017) and the Ontario Lung Association (grant number 052919).
References
- 1.Zincarelli C., Soltys S., Rengo G., Rabinowitz J.E. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol. Ther. 2008;16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- 2.Ellis B.L., Hirsch M.L., Barker J.C., Connelly J.P., Steininger R.J., 3rd, Porteus M.H. A survey of ex vivo/in vitro transduction efficiency of mammalian primary cells and cell lines with Nine natural adeno-associated virus (AAV1-9) and one engineered adeno-associated virus serotype. Virol. J. 2013;10:74. doi: 10.1186/1743-422X-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grimm D., Lee J.S., Wang L., Desai T., Akache B., Storm T.A., Kay M.A. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. J. Virol. 2008;82:5887–5911. doi: 10.1128/JVI.00254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lerch T.F., O’Donnell J.K., Meyer N.L., Xie Q., Taylor K.A., Stagg S.M., Chapman M.S. Structure of AAV-DJ, a retargeted gene therapy vector: cryo-electron microscopy at 4.5 Å resolution. Structure. 2012;20:1310–1320. doi: 10.1016/j.str.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Limberis M.P., Vandenberghe L.H., Zhang L., Pickles R.J., Wilson J.M. Transduction efficiencies of novel AAV vectors in mouse airway epithelium in vivo and human ciliated airway epithelium in vitro. Mol. Ther. 2009;17:294–301. doi: 10.1038/mt.2008.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vandenberghe L.H., Breous E., Nam H.J., Gao G., Xiao R., Sandhu A., Johnston J., Debyser Z., Agbandje-McKenna M., Wilson J.M. Naturally occurring singleton residues in AAV capsid impact vector performance and illustrate structural constraints. Gene Ther. 2009;16:1416–1428. doi: 10.1038/gt.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan Z., Zak R., Luxton G.W., Ritchie T.C., Bantel-Schaal U., Engelhardt J.F. Ubiquitination of both adeno-associated virus type 2 and 5 capsid proteins affects the transduction efficiency of recombinant vectors. J. Virol. 2002;76:2043–2053. doi: 10.1128/jvi.76.5.2043-2053.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong L., Li B., Jayandharan G., Mah C.S., Govindasamy L., Agbandje-McKenna M., Herzog R.W., Weigel-Van Aken K.A., Hobbs J.A., Zolotukhin S. Tyrosine-phosphorylation of AAV2 vectors and its consequences on viral intracellular trafficking and transgene expression. Virology. 2008;381:194–202. doi: 10.1016/j.virol.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhong L., Li B., Mah C.S., Govindasamy L., Agbandje-McKenna M., Cooper M., Herzog R.W., Zolotukhin I., Warrington K.H., Jr., Weigel-Van Aken K.A. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc. Natl. Acad. Sci. USA. 2008;105:7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markusic D.M., Herzog R.W., Aslanidi G.V., Hoffman B.E., Li B., Li M., Jayandharan G.R., Ling C., Zolotukhin I., Ma W. High-efficiency transduction and correction of murine hemophilia B using AAV2 vectors devoid of multiple surface-exposed tyrosines. Mol. Ther. 2010;18:2048–2056. doi: 10.1038/mt.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuchs S.P., Martinez-Navio J.M., Gao G., Desrosiers R.C. Recombinant AAV vectors for enhanced expression of authentic IgG. PLoS ONE. 2016;11:e0158009. doi: 10.1371/journal.pone.0158009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiao C., Zhang W., Yuan Z., Shin J.H., Li J., Jayandharan G.R., Zhong L., Srivastava A., Xiao X., Duan D. Adeno-associated virus serotype 6 capsid tyrosine-to-phenylalanine mutations improve gene transfer to skeletal muscle. Hum. Gene Ther. 2010;21:1343–1348. doi: 10.1089/hum.2010.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Z., Asokan A., Grieger J.C., Govindasamy L., Agbandje-McKenna M., Samulski R.J. Single amino acid changes can influence titer, heparin binding, and tissue tropism in different adeno-associated virus serotypes. J. Virol. 2006;80:11393–11397. doi: 10.1128/JVI.01288-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mingozzi F., High K.A. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 2013;122:23–36. doi: 10.1182/blood-2013-01-306647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ng R., Govindasamy L., Gurda B.L., McKenna R., Kozyreva O.G., Samulski R.J., Parent K.N., Baker T.S., Agbandje-McKenna M. Structural characterization of the dual glycan binding adeno-associated virus serotype 6. J. Virol. 2010;84:12945–12957. doi: 10.1128/JVI.01235-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balazs A.B., Chen J., Hong C.M., Rao D.S., Yang L., Baltimore D. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2011;481:81–84. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halbert C.L., Allen J.M., Miller A.D. Efficient mouse airway transduction following recombination between AAV vectors carrying parts of a larger gene. Nat. Biotechnol. 2002;20:697–701. doi: 10.1038/nbt0702-697. [DOI] [PubMed] [Google Scholar]
- 18.Halbert C.L., Allen J.M., Chamberlain J.S. AAV6 vector production and purification for muscle gene therapy. Methods Mol. Biol. 2018;1687:257–266. doi: 10.1007/978-1-4939-7374-3_18. [DOI] [PubMed] [Google Scholar]
- 19.Khan I.F., Hirata R.K., Russell D.W. AAV-mediated gene targeting methods for human cells. Nat. Protoc. 2011;6:482–501. doi: 10.1038/nprot.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aurnhammer C., Haase M., Muether N., Hausl M., Rauschhuber C., Huber I., Nitschko H., Busch U., Sing A., Ehrhardt A., Baiker A. Universal real-time PCR for the detection and quantification of adeno-associated virus serotype 2-derived inverted terminal repeat sequences. Hum. Gene Ther. Methods. 2012;23:18–28. doi: 10.1089/hgtb.2011.034. [DOI] [PubMed] [Google Scholar]
- 21.Yu D.L., Linnerth-Petrik N.M., Halbert C.L., Walsh S.R., Miller A.D., Wootton S.K. Jaagsiekte sheep retrovirus and enzootic nasal tumor virus promoters drive gene expression in all airway epithelial cells of mice but only induce tumors in the alveolar region of the lungs. J. Virol. 2011;85:7535–7545. doi: 10.1128/JVI.00400-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Lieshout L.P., Soule G., Sorensen D., Frost K.L., He S., Tierney K., Safronetz D., Booth S.A., Kobinger G.P., Qiu X., Wootton S.K. Intramuscular adeno-associated virus-mediated expression of monoclonal antibodies provides 100% protection against Ebola virus infection in mice. J. Infect. Dis. 2018;217:916–925. doi: 10.1093/infdis/jix644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santry L.A., Ingrao J.C., Yu D.L., de Jong J.G., van Lieshout L.P., Wood G.A., Wootton S.K. AAV vector distribution in the mouse respiratory tract following four different methods of administration. BMC Biotechnol. 2017;17:43. doi: 10.1186/s12896-017-0365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]