Highlights
-
•
Natural history of biliary cancers metastatic to bone
-
•
The role of skeletal events in patients with biliary cancer
-
•
Biliary cancer and bone metastases: role of bisphosphonates
1. Introduction
Biliary cancers are a heterogeneous group of highly malignant tumors comprising the intrahepatic cholangiocarcinoma (ICCA), extrahepatic perihilar cholangiocarcinoma (PCCA), extrahepatic distal cholangiocarcinoma (DCCA) and gallbladder cancer (GBC) [1]. These malignancies commonly share a dismal prognosis due to late diagnosis and poor treatment options. The skeleton is the third site of metastasis (following the liver and the lung) from solid tumors, especially lung cancer, breast and prostate cancers. Studies carried out on this type of tumors have shown that the presence of bone metastases may worsen the prognosis and impair the patients’ quality of life [2], [3], [4]. However, the possibility to offer different schedules of chemotherapy and the improvement of supportive care have allowed an increase of life expectancy with a consequent increased risk to develop metastases in uncommon site for the natural history of biliary tract cancers. In literature, data concerning the natural history of bone metastases from biliary cancers are lacking: to our knowledge, this issue has been discussed only in small series or case reports and never investigated on a large cohort of patients [5], [6], [7], [8]. Therefore, we planned a multicenter retrospective survey aiming to explore the prognostic impact of skeletal involvement in patients with biliary cancers, evaluating clinical features, incidence and type of skeletal related events and the potential clinical impact of treatment with bisphosphonates.
2. Patients and methods
2.1. Ethic statement
The Ethics Committee of the coordination center approved this multicenter observational study and determined that the written consent was not necessary since all patients were dead at the time of the survey. All methods are in accordance with relevant guidelines and regulations.
2.2. Study design
This retrospective, observational study conducted in eleven Italian cancer centers, enrolled 137 patients dead for biliary cancer with bone metastases. The data collected related to patients of all ages with a histological diagnosis of biliary cancer with bone metastases, treated in the period from 2003 to 2014. At least two of the following criteria were to be met to detect bone metastases: the presence reported by the physician, the use of palliative bone radiotherapy and the recognition of bone metastases by imaging tool (standard x-ray, computed tomography scans or magnetic resonance imaging of the skeleton). All patients had received best treatment according to single center clinical practice experience.
We excluded patients enrolled in clinical trials or dead for other causes unrelated to cancer.
We collected data regarding clinical and epidemiological characteristics, including age, specific cancer risk factors, histological type, site of cancer in biliary tree; number, sites, type and time of onset of bone metastases; number, type and time of occurrence of skeletal related events (SREs); treatment with bisphosphonates or radiation therapy; overall survival from biliary tract cancer diagnosis; overall survival from bone metastasis diagnosis; time to first SRE.
We performed descriptive and survival analysis for each of these parameters to identify their prognostic role.
2.3. Statistical analysis
Data were collected through descriptive statistics. The Kaplan–Meier method was used to estimate the survival intervals. Survival differences between specified groups were compared using the log-rank test [9], [10]. All the variables found to be significant in the univariate model were considered for multivariate survival analysis and assessed by application of the Cox proportional hazards model.
All significance levels were set at a 0.05 value. SPSS software (version 19.00, SPSS, Chicago) was used for all statistical analysis. Propensity score analysis was performed in R (v3.1.1) using nearest neighbor matching with R package “MatchIt” .
3. Results
3.1. Patients and tumors characteristics
The median age of all patients was 64 years (34–84 years). Seventy-three patients were men (53%), sixty-four women (47%). The most frequent cancer was the ICCA (88 patients, 64%), followed by GBC (19 patients, 14%), ECCA (18 patients, 13%) and unknown site tumors (12 patients, 9%). Most patients had metastatic disease at diagnosis (94 patients, 68.6%); in the remaining cases the disease was diagnosed at stage III in 12% of patients, stage II in 13% and 2% in I stage. 35% of patients had bone metastases at the time of cancer diagnosis. The most frequently used first-line regimens for metastatic disease were cisplatin plus gemcitabine (24 patients, 17.5%), and gemcitabine plus oxaliplatin (39 patients, 28.5%). Sixty-six patients (42%) were treated with other chemotherapy regimens (48.2%) and only 8 patients (6%) received supportive care alone due to poor clinical conditions or refusal. Eighty patients underwent initial surgical treatment (58%), but only 35 of them had no-residual surgery (R0). Eight patients (6%) received other loco-regional treatments (thermoablation in 3 cases, chemoembolization in 4 cases, yttrium-90 treatment in one case) [Table 1].
Table 1.
Descriptive analysis: patients and tumors characteristics.
| Patients and tumors characteristics | N° patients (%) |
|---|---|
| Risk factors | |
| • No risk factor | 69 (50.4) |
| • Alcoholic cirrhosis | 6 (4.4) |
| • Biliary lithiasis | 15 (11) |
| • Viral hepatitis | 24 (17.6) |
| • Cholangitis | 6 (4,4) |
| • Asbestos exposure | 4 (3) |
| • Smoke | 10 (7.3) |
| • Smoke and viral hepatitis | 1 (0.7) |
| • Smoke and alcoholic cirrhosis | 2 (1.4) |
| Primary site | |
| • ICCA | 88 (64) |
| • ECCA | 18 (13) |
| • GBC | 19 (14) |
| • Unknown site | 12 (9) |
| First-line chemotherapy | |
| • Cisplatin plus gemcitabine | 24 (17.5) |
| • Oxaliplatin plus gemcitabine | 39 (28,5) |
| • Others | 66 (48) |
| • Unknown | 8 (6) |
| Surgery | |
| • No surgery | 80 (58.4) |
| • R0 | 35 (25.5) |
| • R1 | 14 (10.2) |
| • R2 | 5 (3.6) |
| • Unknown | 2 (2.3 ) |
| Loco-regional treatments | |
| • No treatments | 128 (93.4) |
| • Thermo-ablation | 3 (2,2) |
| • Chemoembolization | 4 (3) |
| • Yttrium-90 | 1 (0.7) |
3.2. Bone metastases and skeletal related events
At the diagnosis of bone metastases, 36 patients had an Eastern Cooperative Oncology Group Performance Status (ECOG PS) 0 (26.8%), 51 patients ECOG PS1 (37.6%), 35 patients ECOG PS 2 (25.5%), 13 patients ECOG PS 3 (9.5%) and one patient ECOG PS 4 (0.6%). Lytic metastases occurred in 79 patients (57.5%), osteoblastic and mixed lesions in 23 cases (17%) and in 28 cases (20.5%), respectively. At the time of skeletal involvement, 26 patients had a single bone metastasis (19%), 53 patients (39%) two or three metastases, 58 patients (42%) more than three metastases. Only in seven cases (5%) the appearance of bone metastasis preceded visceral metastases; in 35% of cases (48 patients) the diagnosis of bone metastases was synchronous with visceral metastases and in 60% of cases (82 patients) was subsequent. The spine was the most affected site (102 patients, 74.5%), followed by pelvic bones (59 patients, 43%), long bones (43 patients, 31%) and other sites (19 patients, 14%). Eighty-one patients did not develop any SRE (59%). Among the 56 patients who experienced at least one SRE (41%), 19 patients of them had multiple SREs (sixteen patients had 2 SREs, three patients had 3 SREs). The most common SRE was the need of radiotherapy (44 cases, 58.6%), followed by fractures (12 cases, 16%), orthopedic surgery (9 cases, 12%), spinal compression (6 cases, 8%), and hypercalcemia (4 cases, 5.4%). Sixty-seven patients (49%) were treated with bisphosphonates, all but one with zoledronic acid [Table 2].
Table 2.
Descriptive analysis: bone metastases and skeletal related events.
| Bone metastases and SREs | N° patients (%) |
|---|---|
| Type | |
| • Lytic | 79 (57.5) |
| • Osteoblastic | 23 (17) |
| • Mixed | 28 (20) |
| • Unknown | 7 (5) |
| Number of lesions | |
| • 1 | 26 (19) |
| • 2–3 | 53 (39) |
| • > 3 | 58 (42) |
| Site | |
| • Spine | 102 (74.5) |
| • Pelvic bones | 59 (43) |
| • Long bones | 43 (31) |
| • Other sites | 19 (14) |
| Number of SRE | |
| • 0 | 81 (59) |
| • At least 1 | 56(40) |
| • 2 | 16 (11.7) |
| • 3 | 3 (2,2) |
| SRE type | |
| • Hypercalcemia | 4 (5.4) |
| • Fractures | 12 (16) |
| • Spinal compression | 6 (8) |
| • Need of radiotherapy | 44 (58.6) |
| • Orthopedic surgery | 9 (12) |
| Bisphosphonates treatment | |
| • Yes | 67 (49) |
| • No | 70 (51) |
Thirty patients treated with bisphosphonates had at least one SRE (45%), twelve of these had more than one SRE. Of the 70 patients who were not treated with bisphosphonates, 27 (38%) had at least one SRE, seven patients developed more than one SRE. In both groups, bone metastases were predominantly osteolytic (in 41 cases in patients treated with bisphosphonates and in 38 cases in untreated patients). The need for radiotherapy is the first SRE in both groups (21 cases in the group of patients treated with bisphosphonates and 23 in untreated patients).
Table 3 displays the results obtained distinguishing the different types of primitive biliary tumors (ICCA, ECCA, GBC). Number, type and sites of bone metastases were similar across the three anatomic subgroups. The same applies to the number of SREs and type of the first SRE.
Table 3.
Descriptive analysis: bone metastases and skeletal related events based on the type of primary tumor.
| Bone metastases and SREs | N° events (%) |
||
|---|---|---|---|
| ICCA | ECCA | GBC | |
| Type Bone Metastases | |||
| • Lytic | 55 (62) | 10 (11,5) | 9 (13) |
| • Osteoblastic | 14 (16) | 3 (3,4) | 4 (5,8) |
| • Mixed | 16 (18) | 5 (5,7) | 5 (7,2) |
| • Unknown | 3 (3,4) | 0 | 1 (1,4) |
| Number of lesions | |||
| • 1 | 15 (17) | 6 (7) | 4 (5,8) |
| • 2–3 | 40 (45) | 4 (4,6) | 6 (8,7) |
| • > 3 | 33 (37) | 8 (9) | 9 (13) |
| Site of metastases | |||
| • Spine | 63 (71) | 14 (16) | 14 (20) |
| • Pelvic bones | 34 (38) | 7 (8) | 10 (14,5) |
| • Long bones | 27 (30) | 5 (5,7) | 8 (11,6) |
| • Other sites | 11 (12) | 4 (4,6) | 3 (4,3) |
| Number of SRE | |||
| • 0 | 50 (56) | 11 (12) | 13 (19) |
| • 1 | 26 (30) | 4 (4,6) | 5 (7,2) |
| • 2 | 11 (12) | 2 (2,3) | 1 (1,4) |
| • 3 | 1 (1) | 1 (1) | 0 |
| First SRE | |||
| • Hypercalcemia | 4 (4,5) | 0 | 0 |
| • Fractures | 6 (7) | 1 (1,1) | 1 (1,4) |
| • Spinal compression | 3 (3,4) | 0 | 1 (1,4) |
| • Need of radiotherapy | 21 (24) | 5 (5,7) | 2 (3) |
| • Orthopedic surgery | 3 (3,4) | 1 (1) | 0 |
3.3. Survival outcomes and time to first SRE
The median overall survival (OS) from biliary cancer diagnosis was 16.5 months (95%CI: 14.12–18.87). This outcome showed a statistically significant correlation in univariate analysis with disease stage and tumor site (p = 0.001 and p = 0.016, respectively). Patients with GBC had shorter survival (10.9 months) compared to patients with ECCA or ICCA (22.1 months and 16.3 months, respectively). In the multivariate analysis, the primary tumor site lost its statistical significance, although maintains a significance trend.
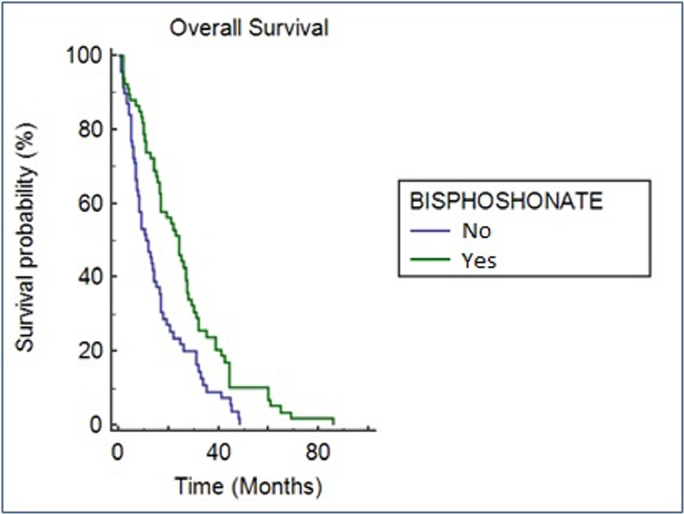
The median OS from bone metastases diagnosis in the whole population was 6 months (95% CI: 4.9–7.1). Patients with a better ECOG PS at the time of bone lesion diagnosis and treated with bisphosphonates had significantly longer OS. We recorded median OS (mOS) ranging from 10.9 months in patients with ECOG PS 0 to 2 months in patients with ECOG PS 3 (p = < 0.001). Similarly, patients treated with bisphosphonates had a longer mOS than those who were not exposed to bisphosphonates: 8 months (95% CI: 6.26–9.74) versus 4 months (95% CI: 3.00–4.99, p = 0.001). This data was confirmed in multivariate analysis (p = 0.001) [Table 4, Fig. 1]. Radiological parameters such as type, number and site of metastases did not shown any correlation with the survival outcomes. The median time to first SRE by the diagnosis of bone metastases in patients who experienced at least one SRE was 1.2 months (95% CI: 1.00–2.00). In patients treated with bisphosphonates, the median time to first SRE is 2 months compared to 1 month in untreated patients (p = 0,042; 95% Cl: 0.60–3.40).
Table 4.
OS from diagnosis of bone metastases: univariate.
| UNIVARIATE ANALYSIS | |||
|---|---|---|---|
| PARAMETER | OS (months) | P value | 95% CI |
| ECOG PS | |||
| • 0 | 10.9 | 7,23 – 14,56 | |
| • 1 | 6 | 4,53 – 7,46 | |
| • 2 | 4 | 2,31 – 5,68 | |
| • 3 | 2 | 1,54 – 2,45 | |
| • 4 | 1 | ||
| 0.001 | |||
| BONE METASTASE TYPE | |||
| • Mixed | 7 | 4,37 – 9,62 | |
| • Osteoblastic | 5 | 2,78 – 7,21 | |
| • Osteolytic | 5,3 | 3,17 – 7,42 | |
| 0.762 | |||
| NUMBER OF BONE METASTASES | |||
| • 1 | 5 | 3,44 – 6,56 | |
| • 2–3 | 7 | 4,88 – 9,11 | |
| • > 3 | 6 | 3,70 – 8,29 | |
| 0.810 | |||
| SITES OF BONE METASTASES | |||
| • Axial | 6 | 0.232 | 4,57 – 7,42 |
| • Long bones | 6,3 | 0.413 | 4,71 – 7,88 |
| • Pelvic bones | 7 | 0.373 | 4,89 – 9,10 |
| • Other sites | 6 | 0.579 | 2,72 – 9,27 |
| VITAMIN D SUPPLEMENTATION | |||
| • Yes | 10 | 5,78 – 14,21 | |
| • No | 5,3 | 3,57 – 7,02 | |
| 0.185 | |||
| BONE METASTASES ONSET | |||
| • After visceral metastases | 5,1 | 3,90 – 6,30 | |
| • Before visceral metastases | 4 | 0,40 – 7,59 | |
| • Synchronous | 6,3 | 4,06 – 8,53 | |
| 0.950 | |||
| BISPHOSPHONATES | |||
| • Yes | 8 | 6,25 – 9,74 | |
| • No | 4 | 3,00 – 4,99 | |
| 0.001 | |||
| AGE (yy) | |||
| • < 64 | 4 | 0.118 | 2,850- 5,150 |
| • > / = 65 | 5.4 | 3,962 −6,838 | |
Figure. 1.
OS from bone metastases diagnosis in patients treated with bisphosphonates or untreated. Kaplan-Meier survival analysis.
After testing the proportional hazards assumption for a Cox regression model fit we excluded ECOG PS as this variable did not satisfy the proportional hazards assumption [Supplementary Table A-B].
Cox regression analysis confirmed that Bisphosphonate treatment was significantly associated with better prognosis in term of OS [Table 5].
Table 5.
OS from diagnosis of bone metastases: multivariate analysis.
| P VALUE | HR | 95% C.I |
||
|---|---|---|---|---|
| LOWER | UPPER | |||
| BISPHOSHONATE | 0,006 | 0,506 | 0,312 | 0,821 |
| BONE METASTASIS TYPE | ||||
| Mixed | 0,919 | 1,113 | 0,139 | 8,906 |
| Osteoblastic | 0,417 | 0,801 | 0,469 | 1,368 |
| Osteolytic | 0,634 | 0,870 | 0,491 | 1,541 |
| NUMBER OF BONE METASTASES | 0,842 | 0,929 | 0,452 | 1,911 |
| SITES OF BONE METASTASES | ||||
| Axial | 0,152 | 1,478 | 0,864 | 2,528 |
| Long bones | 0,772 | 1,077 | 0,649 | 1,786 |
| Pelvic bones | 0,250 | 1,274 | 0,842 | 1,929 |
| Other site | 0,504 | 0,824 | 0,467 | 1,452 |
| VITAMIN D SUPPLEMENTATION | 0,746 | 0,907 | 0,502 | 1,636 |
| BONE METASTASES ONSET (VS SYNCHRONOUS) | ||||
| After visceral metastases | 0,345 | 1,229 | 0,801 | 1,885 |
| Before visceral metastases | 0,562 | 0,724 | 0,243 | 2,157 |
| AGE | 0,178 | 0,987 | 0,969 | 1,005 |
4. Discussion
This is the first study that explored the natural history of bone metastases in patients with biliary cancer, investigating multiple parameters that may affect the onset of SRE and overall survival.
The Performance Status is the only parameter that significantly correlates both with OS from diagnosis of bone metastases and with the time to first SRE. It seems to be the most important prognostic factor: bone metastatic biliary tract cancer patients with a PS ECOG > 2 showed a significantly lower survival than those with better performance status. Similarly, the PS appears to be a “frailty indicator” because patients with poor PS were at increased risk of developing SREs. The descriptive analysis provided data never before achieved in such a large number of patients: bone metastases from biliary cancers are more frequently osteolytic (but osteoblastic ones are not a rare event) and often associated with at least one SRE. The spine is the most involved site and the need of radiotherapy is the most common SRE.
We believe that this study may contribute to increase our knowledge on the prognostic impact of metastatic bone disease in biliary cancers. In particular, the skeletal involvement seems to be associated with a worse prognosis and a deterioration in quality of life. The high percentage of cases with multiple lesions at the time of diagnosis of bone metastases and the high number of patients with at least one SRE (especially need of radiation therapy), suggests that most patients show a low quality of life as well as a poor prognosis. In fact, although not further investigated in our study, pain was the most common symptom.
Interestingly, the use of bisphosphonates was associated with longer survival and significantly delayed the onset of the first SRE. At first glance, these data seem to be vitiated by a selection bias, assuming that only patients with good prognosis are treated with bisphosphonates. We therefore considered four major clinical-pathological prognostic parameters both in patients treated with bisphosphonates than in those not treated (ECOG PS, site of bone metastases, number of bone metastases, bone metastases onset). Data obtained were similar in the two groups [Table 6].
Table 6.
Clinical and prognostic parameters in patients treated or not with bisphosphonates.
| BISPHOSPHONATES TREATMENT |
||
|---|---|---|
| NO BPs (%) | YES BPs (%) | |
| ECOG PS | ||
| • 0 | 27,1 | 25,8 |
| • 1 | 31,4 | 43,9 |
| • 2 | 30 | 21,2 |
| • 3–4 | 12,5 | 9,1 |
| SITE PRIMARY CANCER | ||
| • ICCA | 67 | 62,1 |
| • ECCA | 10 | 16,7 |
| • GBC | 12,9 | 15,2 |
| • NA | 10 | 6,1 |
| BONE METASTASES ONSET | ||
| • After visceral metastases | 58,6 | 62,1 |
| • Before visceral metastases | 5,7 | 4,5 |
| • Synchronous | 35,7 | 33,3 |
| NUMBER BONE METASTASES | ||
| • 1 | 20 | 16,7 |
| • 2–3 | 35,7 | 42,4 |
| • > 3 | 44,3 | 40,9 |
Anyway in a subsequent propensity score analysis, only presence of metastases in other sites than axial skeleton, long bone and pelvic bones demonstrated to have an influence on the decision to use bisphosphonate [Supplementary table C]. Although bisphosphonates’ treatment is indicated in the presence of bone metastases regardless of the skeletal sites involved, it is known that the involvement of other sites is usually associated with a widespread skeletal involvement. In addition, unusual bone metastases are often not susceptible to targeted treatments such as radiotherapy.
Limitations of this study include its retrospective design and the heterogeneity of the population under analysis (primary sites of biliary cancer, multiple treatments considered).
Similar studies investigated the impact of bone metastases and bisphosphonates in patients with solid tumors such as lung cancer, breast cancer, prostate and gastrointestinal cancers. The presence of bone metastases seems to be an independent predictor of poor prognosis. Instead, the role of bisphosphonates is validated for many tumors, but never adequately investigated in biliary cancers.
In lung cancer, bone metastases worsen the quality of life and appear to be associated with worse prognosis. The use of bisphosphonates in these patients delay the first related skeletal event and appears to be associated with an increase in survival [2]. For the same reasons, a rich literature and major international guidelines sanction bisphosphonates as a valid and useful support for patients with bone metastases from breast or prostatic cancers [11], [12], [13], [14]. Also in gastrointestinal and kidney cancers, zoledronic acid treatment shown significant clinical benefit in patients with bone metastases, although further investigations in these populations of patients are warranted [15], [16], [17], [18].
Likewise, our survey identified patients with biliary cancers as a population whose prognosis and quality of life are deteriorated by skeletal involvement and therefore susceptible to bisphosphonate treatment.
This study has enhanced the knowledge about the natural history of bone metastases in biliary cancers, providing some parameters that correlate with survival and quality of life of the patients. These findings may help the clinicians in clinical choices, but perspective studies are needed to validate the survival impact of bisphosphonates.
Competing financial interests
The authors declare no competing financial interests.
Author contribution statement
M.R. and D.S. wrote the main manuscript. F.P performed statistical analysis. M.R. and F.P. prepared tables and figures. G. B., G. A., S.C., F.L., S.L., L.F., M.S., N.S., S.B., B.V., A.P., G.F., M.C., L.F., V.D., R.I., R.F., E.V., C.V., L.F., O.B., M.R., M.A. and G.T. reviewed the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jbo.2017.11.006.
Appendix. Supplementary materials
References
- 1.Edge SB. seventh ed. Springer; New York: 2009. American Joint Committee On Cancer: AJCC Cancer Staging Manual. [Google Scholar]
- 2.Santini D. Natural history of non-small-cell lung cancer with bone metastases. Sci. Rep. 2015;5:18670. doi: 10.1038/srep18670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colonna S., Werner L.T. Breast cancer bone metastases. Metastatic Bone Dis. 2016:45–54. [Google Scholar]
- 4.Bruce I. et al. The natural history, skeletal complications, and management of bone metastases in patients with prostate carcinoma. in: Cancer Wiley Online Library. Supplement June 15, 2000 / Volume 88 / Number 12 (2000). [DOI] [PubMed]
- 5.Takahashi I, Nakamura Y, Suzuki Y. Bone metastasis from cholangiocarcinoma showing unusual accumulation on bone scintigraphy and 67Ga scintigraphy. Br. J. Radiol. 1994 doi: 10.1259/0007-1285-67-795-303. [DOI] [PubMed] [Google Scholar]
- 6.Carlisle RT, Roberts CS. Pathologic fracture of the humerus due to metastatic cholangiocarcinoma. South. Med. J. 1999 doi: 10.1097/00007611-199912000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Kumar A, Bhargava SK, Upreti L, Kumar J. Disseminated osteoblastic skeletal metastases from carcinoma of gall bladder-a case report. Indian J. Radiol. 2003;13:37–39. [Google Scholar]
- 8.Singh S, Bhojwani R, Singh S, Bhatnagar A, Saran RK. Wiley Online Library; 2007. Skeletal Metastasis in Gall Bladder Cancer. Hpb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan E.L., Meier P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958;53:457–481. [Google Scholar]
- 10.Peto R. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analysis and examples. Br. J. Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hillner BE. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J. Clin. Oncol. 2003 Nov 1;21(21):4042–4057. doi: 10.1200/JCO.2003.08.017. Epub 2003 Sep 8. [DOI] [PubMed] [Google Scholar]
- 12.Khan MasoodA, Partin AlanW. Bisphosphonates in metastatic prostate cancer. Rev. Urol. 2003 Summer;5(3):204–206. [PMC free article] [PubMed] [Google Scholar]
- 13.Boissier S. Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Cancer Res. 2000 Jun 1;60(11):2949–2954. [PubMed] [Google Scholar]
- 14.Hillner BE. American society of clinical oncology guideline on the role of bisphosphonates in breast cancer. American society of clinical oncology bisphosphonates expert panel. J. Clin. Oncol. 2000 Mar;18(6):1378–1391. doi: 10.1200/JCO.2000.18.6.1378. [DOI] [PubMed] [Google Scholar]
- 15.Santini D. Natural history of bone metastasis in colorectal cancer: final results of a large Italian bone metastases study. Ann. Oncol. 2012 Aug;23(8):2072–2077. doi: 10.1093/annonc/mdr572. Epub 2012 Jan 4. [DOI] [PubMed] [Google Scholar]
- 16.Lipton A. Zoledronic acid delays the onset of skeletal-related events and progression of skeletal disease in patients with advanced renal cell carcinoma. Cancer. 2003 Sep 1;98(5):962–969. doi: 10.1002/cncr.11571. [DOI] [PubMed] [Google Scholar]
- 17.Silvestris N. Natural history of malignant bone disease in gastric cancer: final results of a multicenter bonemetastasis survey. PLoS One. 2013 Oct 28;8(10):e74402. doi: 10.1371/journal.pone.0074402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santoni M. Bone metastases in patients with metastatic renal cell carcinoma: are they always associated with poor prognosis? J. Exp. Clin. Cancer Res. 2015 Feb 5;34:10. doi: 10.1186/s13046-015-0122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.