Abstract
Introduction
The benefits of long-term noninvasive ventilation (NIV) in stable COPD with chronic hypercapnic respiratory failure (CHRF) have been debated for many years due to the conflicting results observed in these patients.
Materials and methods
We investigated the effects of domiciliary NIV in stable hypercapnic COPD patients for a period of 1 year using COPD Assessment Test (CAT), BODE Index, and the number of acute exacerbations. NIV was administered in 57 stable COPD patients with CHRF in the spontaneous/timed mode. Spirometry, 6 minute walk test, Medical Research Council dyspnea scale, arterial blood gases, number of acute exacerbations, BODE Index, and CAT were assessed. Study participants were reassessed in the 1st, 6th, and 12th months after the initial evaluation.
Results
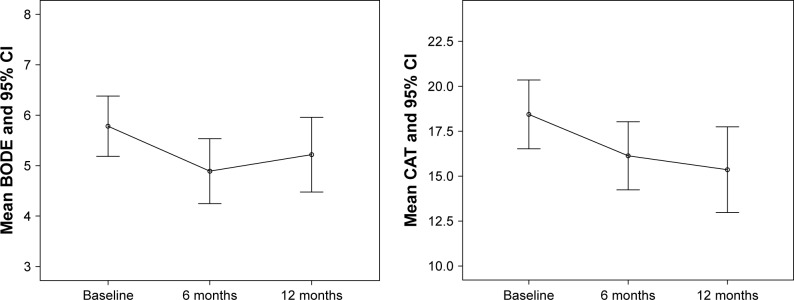
There was a significant improvement in COPD exacerbations (p<0.001), CAT (p<0.001), PO2 (p<0.001), PCO2 (p<0.001), and Medical Research Council dyspnea scale (p<0.001) in 1 year of follow-up. BODE Index was improved in the first 6 months (5.8±2.2 vs 4.8±2.4, p<0.001), but the improvement was not maintained.
Conclusion
In conclusion, domiciliary NIV in stable COPD patients with CHRF has beneficial effect on CAT, arterial blood gases, and number of acute exacerbations in a year of NIV use at home. A significant improvement in BODE Index from baseline to 12 months was found in patients aged >70 years, while for those aged <70, the improvement was not maintained after the sixth month.
Keywords: hypercapnic COPD, domiciliary, improvement, BODE Index, CAT
Introduction
COPD is currently one of the leading causes of morbidity and mortality worldwide in the adult population and will be the third most frequent cause of death in the world by the year 2030.1 COPD is a chronic, progressive disease and many patients, especially those at stage III and IV,2 will eventually develop chronic hypercapnic respiratory failure (CHRF). These patients are more likely to develop more often exacerbations, worsening their prognosis.3,4
Short-term noninvasive ventilation (NIV) has become an accepted management approach for patients with acute respiratory failure.5 It has been shown that NIV in acute settings can prevent intubation and invasive ventilation, while it can reduce hospital mortality.6,7 Nevertheless, the role of NIV in long-term management of stable COPD with CHRF is still debated.8–10 There are a number of clinical trials that have reported improvement in blood gasses,3,11–13 functional status reporting both forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1),3,14,15 and an improvement of survival in stable COPD,16,17 but at the cost of worsening of quality of life.16 On the other hand, there are several clinical trials that have reported an improvement of quality of life.3,13,15,18–21 Therefore, it is necessary to select subgroups of COPD patients, who will respond to the use of NIV. The difficulty in finding these subgroups probably lies on the fact that COPD is a systematic disease, and therefore, many parameters must be taken into consideration.
The necessity to understand a complicated disease such as COPD gave birth to a multidimensional predictor factor known as BODE Index. BODE Index was reported to be superior to FEV1 in reflecting the severity of COPD and effective in predicting the mortality in patients with COPD.22 Furthermore, the recently developed COPD Assessment Test (CAT) is a potentially useful instrument to assess the efficacy of treatments following COPD exacerbations23 and to quantify the impact of COPD in routine practice.24
The purpose of our study is to investigate the influence of NIV on BODE Index, CAT, and the number of acute exacerbations in COPD patients with CHRF in long-term NIV use at home. Our secondary target is to define in advance the characteristics of patients who will respond to the use of NIV at home.
Materials and methods
Seventy-eight COPD patients from the outpatient clinic of a tertiary university hospital, while in stable conditions, were enrolled in the trial. COPD was diagnosed according to the American Thoracic Society (ATS)/European Respiratory Society (ERS) guidelines,25 smoking history of >20 pack-years, FEV1 <50% predicted, and FEV1/FVC <70% on stable conditions.
Patients’ inclusion criteria were: age ≤75 years, ex-smokers (≥20 pack-years), and CHRF constant for at least 4 weeks (PO2 <60 mmHg, PCO2 >50 mmHg, and pH >7.35 while breathing room air). All patients received long-acting bronchodilators. Some also received inhaled corticosteroids. All patients received long-term oxygen therapy. Our patients’ therapy was in accordance with ATS/ERS guidelines.25 During enrollment, all patients were free of exacerbations for the last 4 weeks and domiciliary NIV was offered to all our patients who agreed to receive it as an additional therapy. The study protocol was approved by the ethics committee of the University Hospital of Larissa, and written informed consent was obtained from all study participants.
Patients’ exclusion criterion was presence of other severe comorbidities that could interfere with the outcome of our study, such as bronchiectasis, asthma, post-tuberculosis sequelae, rib and thoracic cage deformities, neuromuscular disorders, lung cancer, and chronic heart failure. To rule out the coexistence of moderate and severe obstructive sleep apnea, patients were screened with Epworth Sleepiness Scale (ESS) and polysomnography (PSG) study (Alice 4 Diagnostic Device OBS/G7829, Respironics). In fact, patients were excluded from the study when the PSG study gave a result of apnea-hypopnea index (AHI) >15/hour or when AHI was >5/hour in combination with ESS >12. We also excluded patients with a body mass index (BMI) >35 kg/m2 in order to exclude the coexistence of obesity – hypoventilation syndrome.3 We also excluded patients with BMI <19 kg/m2 as they were small in number, had more than three exacerbations with long hospitalization period time in 1 year, and thus, their period of stable condition and domiciliary NIV use was really short. Probably these patients must be followed up for longer than a year to have more reliable results.
Patients were hospitalized for 2–3 days during the initial application of NIV, in order to ensure the optimal compliance of the patients. NIV was administered in the spontaneous/timed mode via a full-face mask using a bi-level positive airway pressure system (VPAP III ST; ResMed, Sydney, Australia). This mode was chosen for the ability to set inspiratory and expiratory time and has been proven effective in COPD patients.17 Supplement oxygen was added in order to obtain 88%–92% saturation. Inspiratory positive airway pressure and expiratory positive airway pressure were adjusted according to the patients’ comfort and synchrony with the ventilator. Twenty-four hours before patients’ return at home, patients and their relatives were trained in the hospital in order to be able to use the ventilator properly. Technically skilled personnel installed the ventilator at patients’ homes and provided full technical support when required.
Home NIV was offered eventually to 65 patients. Patients were reassessed after 1 month to establish their compliance. In fact, 1 month later, eight of our patients were excluded from our study because of their poor compliance with the ventilator (intermittent use, use <4 hours/day). Patients were examined at M6 (6 months after the initial NIV application) and at M12 (a year after the initial NIV application) with new spirometry test, blood gasses analysis, BODE Index, CAT, and the number of acute exacerbations.
Physiological measurements and questionnaires
Spirometry was performed with a dry spirometer (KoKo Legend; Ferraris Respiratory, Louisville, CO, USA) according to the ATS guidelines.26 Arterial blood gases were measured with the patient at rest, patient in sitting position, while breathing room air, and before submitting our patient to 6 minute walk test (6MWT). Dyspnea was assessed with the Medical Research Council (MRC) dyspnea scale.27 Subjective daytime sleepiness was evaluated with the ESS28 that has been validated for the Greek language.29 CAT is a short, simple questionnaire that is easily completed by the patient and is used for assessing and monitoring COPD;24 it has been also validated for the Greek language. BODE Index, a composite marker of disease taking into consideration the systemic nature of COPD,30 was evaluated at any appointment for every patient.
Study protocol
Patients enrolled in our study were re-evaluated in the NIV outpatient clinic on the 1st, 6th, and 12th months after the initial evaluation. If the patient had an exacerbation at any of these time points, measurements were obtained 1 month after the event. As a COPD exacerbation, we define an event in the natural course of the disease characterized by a change in the patient’s baseline dyspnea, cough, and/or sputum that is beyond normal day-on-day variations, is acute in onset, and may warrant a change in regular medication.31
In every appointment, the settings and the hours of NIV use (as obtained from the machine’s counter) were evaluated, in order to determine proper NIV use. During the follow-up period, patients underwent adjustments in NIV masks or ventilator settings as needed, in order to maintain patient–ventilator synchrony and optimize gas exchange and functional status. In order to be considered compliant with the device, each patient had to use the ventilator for at least 6 hours daily and with no more than 10 L/minute air leaks.
Statistical analysis
Normally distributed variables are expressed as mean (SD), while variables with skewed distribution are expressed as median (interquartile range). Qualitative variables are expressed as absolute and relative frequencies. Repeated measurements analysis of variance was used to evaluate the changes observed in all study parameters over the follow-up period in the total sample and among different groups. Log transformations were used in case of skewed distribution and effect sizes were also calculated. Bonferroni correction was used in case of multiple testing in order to control for type I error. Pearson’s or Spearman’s correlations coefficients were used to explore the association of two continuous variables. All reported p-values are two-tailed. Statistical significance was set at p <0.05, and analyses were conducted using SPSS statistical software (version 19.0).
Results
Seventy-eight COPD patients from the outpatient clinic of a tertiary university hospital, while in stable conditions, were enrolled in the trial. Eight patients were excluded from the study when the PSG study gave a result of AHI >15/hour. Five patients were excluded because of their low BMI. Home NIV was offered to 65 patients. One month later, eight of our patients were excluded from our study because of their poor compliance with the ventilator (intermittent use, use <4 hours/day).
Eventually, our sample consisted of 57 participants (91.2% men and 8.8% women) with mean age 68.8 years (SD=8.0 years). Sample characteristics at baseline measures are shown in Table 1.
Table 1.
Sample characteristics at baseline
| n (%) | |
|---|---|
| Sex | |
| Men | 52 (91.2) |
| Women | 5 (8.8) |
| Age, years, mean (SD) | 68.8 (8.0) |
| BMI, kg/m2, mean (SD) | 28.5 (5.4) |
| BMI | |
| Normal | 14 (25.0) |
| Overweight | 18 (31.6) |
| Obese | 25 (43.9) |
| AHI, number of apnea-hypopnea/hour, mean (SD) | 8.5 (4.6) |
| S1, minutes, mean (SD) | 6.7 (4.3) |
| S2, minutes, mean (SD) | 62.2 (17.1) |
| S3+S4, minutes, mean (SD) | 14.4 (13.6) |
| REM, minutes, mean (SD) | 12.3 (7.6) |
| SAT <90%, mean (SD) | 71.7 (24) |
| SAT <80%, median (IQR) | 4.1 (1.3–8.9) |
| Mean saturation, mean (SD) | 86.2 (2.7) |
| Min saturation, mean (SD) | 74.7 (6.1) |
| OD, number of oxygen desaturation/hour, median (IQR) | 10 (4.8–17) |
Abbreviations: AHI, Apnea-Hypopnea Index; BMI, body mass index; IQR, interquartile range; OD, oxygen desaturation; REM, rapid eye movement; SAT, saturation.
The mean inspiratory positive airway pressure setting was 17.04±2.81 cmH2O with a range 16–20 cmH2O, while the expiratory positive airway pressure setting was 5.7±1.62 cmH2O with a range 5–8 cmH2O. Patients made use of the device for 8.71±1.43/24 hours. All of our patients needed supplemental oxygen with the device with a range 2–4 L/min.
In Table 2, we can see the changes in study parameters through the follow-up period.
Table 2.
Changes in study parameters through the follow-up period
| Baseline | 6 months | 12 months | Effect size | p-value** baseline vs 6 months | p-value** 6 vs 12 months | p-value** baseline vs 12 months | |
|---|---|---|---|---|---|---|---|
| PO2, median (IQR) | 58.0 (54.0–59.0) | 62 (58–68) | 63 (58–67) | 0.39 | <0.001 | 0.919 | <0.001 |
| PCO2, median (IQR) | 52.9 (50.0–55.0) | 46 (43–50) | 45 (43–48) | 0.67 | <0.001 | 0.663 | <0.001 |
| pH, median (IQR) | 7.41 (7.39–7.41) | 7.42 (7.40–7.43) | 7.41 (7.40–7.42) | 0.25 | <0.001 | 0.266 | 0.021 |
| FEV1, median (IQR) | 30.0 (27.0–46.0) | 38 (25–52) | 33 (26–50) | 0.15 | 0.013 | 0.439 | 0.652 |
| FVC, mean (SD) | 49.6 (15.1) | 53.5 (17.7) | 54.6 (16.5) | 0.14 | 0.071 | 1.000 | 0.028 |
| FEV1/FVC, mean (SD) | 56.0 (9.9) | 55.1 (11.5) | 53.6 (11.5) | 0.15 | 1.000 | 0.180 | 0.115 |
| MRC, mean (SD) | 2.79 (0.92) | 2.51 (0.98) | 2.50 (1.07) | 0.11 | 0.009 | 0.672 | <0.001 |
| BMI, mean (SD) | 28.5 (5.4) | 29.3 (5.6) | 28.9 (5.6) | 0.07 | 0.062 | 0.760 | 1.000 |
| 6MWT, mean (SD) | 247.5 (106.8) | 268.9 (114.6) | 254.2 (134.9) | 0.11 | 0.049 | 0.420 | 1.000 |
| BODE, mean (SD) | 5.8 (2.2) | 4.8 (2.4) | 5.2 (2.7) | 0.32 | <0.001 | 0.143 | 0.113 |
| EXACERB, median (IQR) | 1 (1–2) | 0 (0–1) | 0 (0–1) | 0.39 | <0.001 | 0.196 | <0.001 |
| CAT, mean (SD) | 18.7 (6.9) | 16.2 (6.8) | 15.5 (8.4) | 0.22 | <0.001 | 0.499 | 0.001 |
Notes: Repeated measurements ANOVA. Effects reported include differences between the groups in the degree of change over the follow-up period.
Pairwise comparisons after Bonferroni correction. Log transformations were used in analysis. Bold values are statistically significant.
Abbreviations: 6MWT, 6 minute walk test; ANOVA, analysis of variance; CAT, COPD Assessment Test; EXACERB, exacerbations; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; IQR, interquartile range; MRC, Medical Research Council.
From baseline to 12 months, there was a significant improvement in COPD number of acute exacerbations (1.46±1.03 vs 0.7±1.07, p<0.001, d: 0.39) and CAT (18.7±6.9 vs 15.5±8.4, p<0.001, d: 0.22). BODE Index showed an improvement in the first 6 months (5.8±2.2 vs 4.8±2.4, p<0.001, d: 0.32), which was not maintained. Figure 1 shows the mean BODE Index and CAT levels during the follow-up period.
Figure 1.
Mean BODE and CAT levels during follow-up.
Abbreviation: CAT, COPD Assessment Test.
BODE Index’s parameters, FEV1 (35.5±13.6 vs 39.2±15.5, p: 0.013, d: 0.15), 6MWT (247.5±106.8 vs 268.9±114.6, p: 0.049, d: 0.11), and MRC (2.79±0.92 vs 2.51±0.98, p: 0.009, d: 0.11) improved during the first 6 months and only MRC (2.79±0.92 vs 2.50±1.7, p<0.001, d: 0.11) continued its improvement in the entire follow-up period.
In our study, an improvement of PO2 (56±2.9 vs 62.9±8.41, p<0.001, d: 0.39), PCO2 (52.9±3.9 vs 45.8±4.6, p<0.001, d: 0.67), and FVC (49.6±15.1 vs 54.6±16.5, p: 0.028, d: 0.14) was found in the entire follow-up period. Indeed, patients with higher levels of PCO2 at baseline were found to have a greater improvement of CAT during 1 year of follow-up (p: 0.039). An interaction between age and BODE Index changes was also found. A significant improvement of BODE Index from baseline to 6 months (p=0.039) maintained to 12 months was found for patients aged >70 years, while for those aged <70, the improvement was not maintained after the sixth month (Figure 2).
Figure 2.
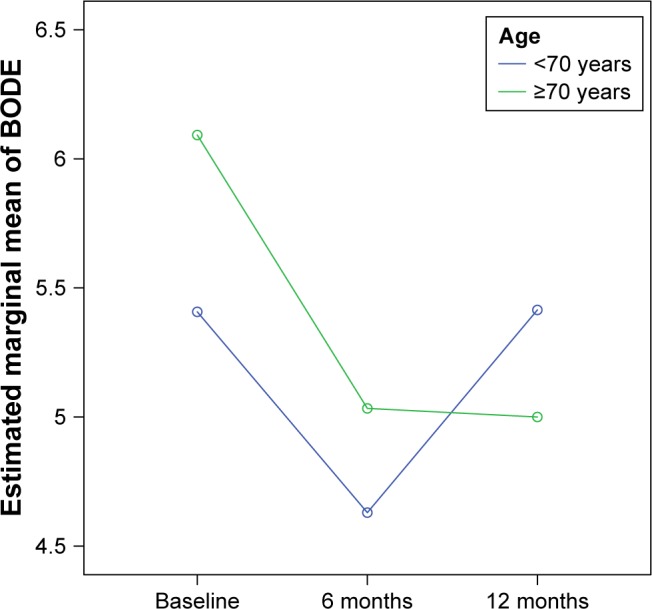
BODE Index changes during follow-up, according to age groups.
Another finding was the improvement of PO2 from baseline to the first 6 months for overweight and obese COPD patients (p: 0.002), which was maintained for the following 6 months (Figure 3).
Figure 3.
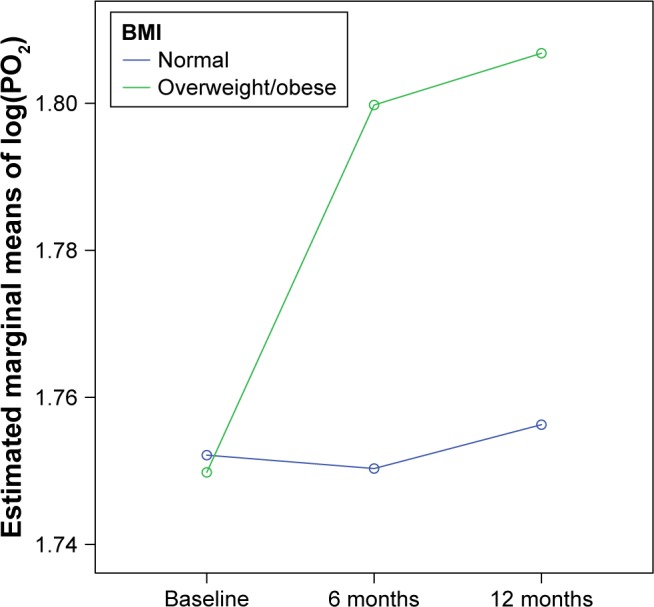
PO2 changes during the follow-up, according to BMI status.
Abbreviation: BMI, body mass index.
Concerning PSG’s baseline results, patients with higher rates of hypoxemia during sleep (minutes of SatO2 <90% and SatO2 <80%) were associated with a greater decrease in the number of acute exacerbations for the entire follow-up period (p<0.01).
Discussion
In this study, we evaluated the effect of domiciliary NIV in stable COPD patients with CHRF on CAT, BODE Index, and acute exacerbations. We found an improvement in the number of acute exacerbations, CAT, and blood gases over time. BODE Index was improved in the first 6 months, but was not maintained for the entire monitoring time. On subsequent analysis of BODE Index parameters, we found that there was a statistically significant improvement in FEV1, MRC, and 6MWT measurements in the first 6 months. Of these three parameters, MRC was the one that maintained the improvement during the entire follow-up period. There was no change in BMI, which is the fourth parameter of BODE Index.
The role of NIV in the acute exacerbation of COPD is well established,11,32–34 but the role of domiciliary nocturnal NIV in stable COPD is still controversial. Despite the fact that meta-analysis and clinical guidelines do not recommend the routine use of domiciliary NIV for patients diagnosed with severe stable COPD and CHRF, it is a common practice followed in some countries. Recurrent exacerbations and failed weaning from in-hospital NIV were the main reasons for its prescription.35 Although COPD is mainly characterized by the presence of airflow obstruction, several systemic manifestations that accompany this disease affect the progression of COPD and may contribute to the differences in the response of NIV.
To our knowledge, our study is the first study that used the BODE Index and CAT test in order to study NIV in stable hypercapnic COPD patients. Our findings are consistent with the literature. There are four short-term follow-up studies36–39 with a duration of 8 weeks to 3 months and three long-term studies3,12,18 with a duration of 6 months to 2 years, demonstrating no significant differences in FEV1 between NIV and standard care groups, while in three long-term follow-up studies with a duration of 1–2 years, limited effects on FEV1 have been demonstrated.21,40,41 In our study, other than FEV1, we found that FVC was also improved (p: 0.028) in the 1-year follow-up period. FVC improvement reflects an amelioration of lung hyperinflation. Theoretically, multiple mechanisms could attribute to a positive effect of domiciliary NIV. One of these mechanisms, the reduction of airway wall edema, could improve both FVC and FEV1, thus improving COPD patients’ lung function. But it is well known that COPD is a progressive disease and stable COPD patients are not a homogeneous population. This could be the cause of the non-maintenance of FEV1 improvement in the first 6 months.
As for the effect on exercise capacity, there are eight long-term follow-up studies3,5,18,20,21,40,42,43 with duration of 6 months to 2 years demonstrating moderate effect of treatment on 6MWT. Patients who participated in our study showed statistically significant improvement of 6MWT in the first 6 months of their follow-up, which was not maintained. NIV relieves fatiguing respiratory muscles by reducing the degree of hyperinflation, providing patients a better exercise capacity. The insignificant improvement over time could be attributed to the fact that our patients did not follow a rehabilitation program in order to maintain their improvement.44
The only parameter of BODE Index that presents a statistically significant improvement after 1 year of domiciliary NIV use is MRC. In fact, four studies3,12,21,40 demonstrated that significant improvements in dyspnea occurred in the NIV group. A plausible explanation of MRC amelioration could be attributed to the reduction of end-expiratory volume and, hence, the degree of hyperinflation.45–47
An important finding is that domiciliary NIV in stable COPD patients reduces the number of acute exacerbations in 1-year follow-up period. Our findings are in agreement with a recent study according to which long-term NIV seems effective in reducing recurrent acute exacerbations and readmissions in a highly selected group of severe COPD patients with frequent exacerbations.48 A possible mechanism could be the mobilization of sputum. Positive end-expiratory pressure is known to help mobilize mucus,49 reducing the risk of airway infections, which are the most important cause of COPD exacerbations. Symptom management and prevention of acute exacerbations are important in preventing COPD progression. A remarkable observation of PSG’s baseline results was that patients with higher rates of hypoxemia during sleep (minutes of SatO2 <90% and SatO2 <80%) were associated with a greater decrease in the number of acute exacerbations for the entire follow-up period. Probably, the second mechanism responsible for this is the improvement of sleep hypoventilation. Another possible mechanism ameliorating the number of exacerbations is the ventilation–perfusion (V/Q) matching. De Backer et al5 indicated that in NIV-treated patients who showed improvement in their blood gases, mass flow was redistributed toward areas with better perfusion. COPD patients who are treated with indicated standard treatment in combination with domiciliary NIV do not deteriorate quickly. Recurrent type II respiratory failure occurs in over 30% and readmission at 1 year in 60% of those who require NIV acutely in hospital.50 A more recent study has shown that patients who require mechanical ventilation due to an acute exacerbation and remain hypercapnic thereafter may benefit from long-term NIV.42
In our patients, there was a marked reduction of hypercapnia and a significant increase in diurnal hypoxemia. Patients with COPD are likely to develop nocturnal hypoventilation, especially during rapid eye movement sleep when the upper airway tone and accessory muscle activity is impaired.51 In two studies, 43% of COPD patients had a >10 mmHg increase in PCO2 during night, resulting in progressive resetting of respiratory control to higher PCO2 value.52,53 But worsening of V/Q matching is probably the leading mechanism for the occurrence of hypoxemia by the enlargement of physiological dead space and increase of wasted ventilation54 leading to diurnal hypercapnic respiratory failure eventually. Kohnlein et al20 suggest that when NIV is applied, it effectively reduces PCO2 in stable COPD patients and on the other hand, improves PO2. In fact, in our study, it was noticed that overweight patients showed a significant improvement in PO2 compared to those with normal weight. The answer is probably the removal of nocturnal hypoventilation and the redistribution of blood volume in the lung areas that are most ventilated.
Another important finding is the significant improvement of CAT for the entire monitoring time. To our knowledge, there are no studies using CAT in stable COPD patients using domiciliary NIV. However, evidence has shown CAT’s good internal consistency and retest reliability,24,55 and that it is suitable for routine clinical use for stable COPD.33,56–59 In our study, patients perceived a significant improvement of their clinical symptoms, such as cough, sputum, and anxiety, and improvement in their sleep as reflected in the CAT’s results. Also, an interaction between baseline PCO2 and CAT was found. Specifically, patients with higher PCO2 at baseline showed greater improvement in CAT. The most plausible explanation is the positive effect of NIV in nocturnal hypoventilation and the V/Q matching leading to better blood gas exchange. Furthermore, a reduction of hypercapnia leads to an improvement of the sensitivity of the respiratory center to carbon dioxide.60–62 Patients are more alert, more mobilized, and more confident about themselves.
Additionally, in subgroup analysis, it was found that patients of age ≥70 years showed a steady improvement in BODE Index for the entire follow-up period. Our findings are in accordance with an earlier study,63 which showed that COPD patients over 75 years of age using NIV at home improved their respiratory function and blood gases. This could be explained not only by the better compliance of the elderly patients, but also by the greater hypoventilation in the context of greater respiratory muscle hypotonia. So, the NIV benefits are more apparent in the elderly.
Conclusion
The present study demonstrates a positive effect of NIV on CAT, blood gases, dyspnea, and number of exacerbations in a year of domiciliary NIV use. No change in BODE Index does not necessarily mean failure of NIV, as COPD is a progressive disease. Use of NIV improves FEV1, MRC, and 6MWT, although it was not statistically significant in 1-year follow-up, which could contribute to slowing the progress of COPD. The significant compliance of our patients in domiciliary NIV, the hours of NIV use, as well as the personalized ventilator settings are the reasons for the outcomes of the study. Perhaps it would be appropriate for COPD patients who are overweight with frequent exacerbations and without showing clinical symptoms of sleep apnea to undergo nocturnal oximetry to detect patients with nocturnal hypoventilation and predisposition to hypercapnia, in order to make timely use of domiciliary NIV, prevent COPD progression, and avoid serious socioeconomic consequences.
Acknowledgments
The authors are grateful to the nurse and technical staff of the Sleep Study Laboratory of University Hospital of Larissa and the nurse staff of Outpatient Clinic of University Hospital of Larisa for their help and support in patients’ management.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349(9061):1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 2.Mannino DM, Doherty DE, Sonia Buist A. Global Initiative on Obstructive Lung Disease (GOLD) classification of lung disease and mortality: findings from the Atherosclerosis Risk in Communities (ARIC) study. Respir Med. 2006;100(1):115–122. doi: 10.1016/j.rmed.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 3.Clini E, Sturani C, Rossi A, et al. Rehabilitation and Chronic Care Study Group, Italian Association of Hospital Pulmonologists (AIPO) The Italian multicentre study on noninvasive ventilation in chronic obstructive pulmonary disease patients. Eur Respir J. 2002;20(3):529–538. doi: 10.1183/09031936.02.02162001. [DOI] [PubMed] [Google Scholar]
- 4.Costello R, Deegan P, Fitzpatrick M, McNicholas WT. Reversible hypercapnia in chronic obstructive pulmonary disease: a distinct pattern of respiratory failure with a favorable prognosis. Am J Med. 1997;102(3):239–244. doi: 10.1016/S0002-9343(97)00017-X. [DOI] [PubMed] [Google Scholar]
- 5.De Backer L, Vos W, Dieriks B, et al. The effects of long-term noninvasive ventilation in hypercapnic COPD patients: a randomized controlled pilot study. Int J Chron Obstruct Pulmon Dis. 2011;6:615–624. doi: 10.2147/COPD.S22823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plant PK, Elliott MW. Chronic obstructive pulmonary disease * 9: management of ventilatory failure in COPD. Thorax. 2003;58(6):537–542. doi: 10.1136/thorax.58.6.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasche K, Hader C, Leidag M, Duchna HW, Orth M. Non-invasive ventilation in chronic obstructive pulmonary disease. J Physiol Pharmacol. 2004;55(Suppl 3):115–119. [PubMed] [Google Scholar]
- 8.Simonds AK. Long-term ventilation in obstructive ventilatory disorders. Respir Care Clin N Am. 2002;8(4):533–544. doi: 10.1016/s1078-5337(02)00024-2. [DOI] [PubMed] [Google Scholar]
- 9.Simonds AK. Home ventilation. Eur Respir J Suppl. 2003;47:38S–46S. doi: 10.1183/09031936.03.00029803. [DOI] [PubMed] [Google Scholar]
- 10.Tuggey JM, Plant PK, Elliott MW. Domiciliary non-invasive ventilation for recurrent acidotic exacerbations of COPD: an economic analysis. Thorax. 2003;58(10):867–871. doi: 10.1136/thorax.58.10.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung AP, Chan VL, Liong JT, et al. A pilot trial of non-invasive home ventilation after acidotic respiratory failure in chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2010;14(5):642–649. [PubMed] [Google Scholar]
- 12.Casanova C, Celli BR, Tost L, et al. Long-term controlled trial of nocturnal nasal positive pressure ventilation in patients with severe COPD. Chest. 2000;118(6):1582–1590. doi: 10.1378/chest.118.6.1582. [DOI] [PubMed] [Google Scholar]
- 13.Dreher M, Storre JH, Schmoor C, Windisch W. High-intensity versus low-intensity non-invasive ventilation in patients with stable hypercapnic COPD: a randomised crossover trial. Thorax. 2010;65(4):303–308. doi: 10.1136/thx.2009.124263. [DOI] [PubMed] [Google Scholar]
- 14.Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333(13):817–822. doi: 10.1056/NEJM199509283331301. [DOI] [PubMed] [Google Scholar]
- 15.Tsolaki V, Pastaka C, Karetsi E, et al. One-year non-invasive ventilation in chronic hypercapnic COPD: effect on quality of life. Respir Med. 2008;102(6):904–911. doi: 10.1016/j.rmed.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 16.McEvoy RD, Pierce RJ, Hillman D, et al. Australian trial of non-invasive Ventilation in Chronic Airflow Limitation (AVCAL) Study Group Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax. 2009;64(7):561–566. doi: 10.1136/thx.2008.108274. [DOI] [PubMed] [Google Scholar]
- 17.Windisch W, Kostic S, Dreher M, Virchow JC, Jr, Sorichter S. Outcome of patients with stable COPD receiving controlled noninvasive positive pressure ventilation aimed at a maximal reduction of Pa(CO2) Chest. 2005;128(2):657–662. doi: 10.1378/chest.128.2.657. [DOI] [PubMed] [Google Scholar]
- 18.Bhatt SP, Peterson MW, Wilson JS, Durairaj L. Noninvasive positive pressure ventilation in subjects with stable COPD: a randomized trial. Int J Chron Obstruct Pulmon Dis. 2013;8:581–589. doi: 10.2147/COPD.S53619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duiverman ML, Wempe JB, Bladder G, et al. Nocturnal non-invasive ventilation in addition to rehabilitation in hypercapnic patients with COPD. Thorax. 2008;63(12):1052–1057. doi: 10.1136/thx.2008.099044. [DOI] [PubMed] [Google Scholar]
- 20.Kohnlein T, Windisch W, Kohler D, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med. 2014;2(9):698–705. doi: 10.1016/S2213-2600(14)70153-5. [DOI] [PubMed] [Google Scholar]
- 21.Xiang PC, Zhang X, Yang JN, et al. The efficacy and safety of long term home noninvasive positive pressure ventilation in patients with stable severe chronic obstructive pulmonary disease. Zhonghua Jie He He Hu Xi Za Zhi. 2007;30(10):746–750. [PubMed] [Google Scholar]
- 22.Celli BR. Change in the BODE index reflects disease modification in COPD: lessons from lung volume reduction surgery. Chest. 2006;129(4):835–836. doi: 10.1378/chest.129.4.835. [DOI] [PubMed] [Google Scholar]
- 23.Tu YH, Zhang Y, Fei GH. Utility of the CAT in the therapy assessment of COPD exacerbations in China. BMC Pulm Med. 2014;14:42. doi: 10.1186/1471-2466-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 25.Celli BR, MacNee W, ATS/ERS Task Force Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 26.Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 27.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–586. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 28.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 29.Tsara V, Serasli E, Amfilochiou A, Constantinidis T, Christaki P. Greek version of the Epworth Sleepiness Scale. Sleep Breath. 2004;8(2):91–95. doi: 10.1007/s11325-004-0091-6. [DOI] [PubMed] [Google Scholar]
- 30.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 31.Burge S, Wedzicha JA. COPD exacerbations: definitions and classifications. Eur Respir J Suppl. 2003;41:46S–53S. doi: 10.1183/09031936.03.00078002. [DOI] [PubMed] [Google Scholar]
- 32.Kramer N, Meyer TJ, Meharg J, Cece RD, Hill NS. Randomized, prospective trial of noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med. 1995;151(6):1799–1806. doi: 10.1164/ajrccm.151.6.7767523. [DOI] [PubMed] [Google Scholar]
- 33.Lightowler JV, Wedzicha JA, Elliott MW, Ram FS. Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: cochrane systematic review and meta-analysis. BMJ. 2003;326(7382):185. doi: 10.1136/bmj.326.7382.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thys F, Roeseler J, Reynaert M, Liistro G, Rodenstein DO. Noninvasive ventilation for acute respiratory failure: a prospective randomised placebo-controlled trial. Eur Respir J. 2002;20(3):545–555. doi: 10.1183/09031936.02.00287402. [DOI] [PubMed] [Google Scholar]
- 35.Crimi C, Noto A, Princi P, et al. Domiciliary non-invasive ventilation in COPD: an international survey of indications and practices. COPD. 2016;13(4):483–490. doi: 10.3109/15412555.2015.1108960. [DOI] [PubMed] [Google Scholar]
- 36.Garrod R, Mikelsons C, Paul EA, Wedzicha JA. Randomized controlled trial of domiciliary noninvasive positive pressure ventilation and physical training in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1335–1341. doi: 10.1164/ajrccm.162.4.9912029. [DOI] [PubMed] [Google Scholar]
- 37.Gay PC, Hubmayr RD, Stroetz RW. Efficacy of nocturnal nasal ventilation in stable, severe chronic obstructive pulmonary disease during a 3-month controlled trial. Mayo Clin Proc. 1996;71(6):533–542. doi: 10.4065/71.6.533. [DOI] [PubMed] [Google Scholar]
- 38.Meecham Jones DJ, Paul EA, Jones PW, Wedzicha JA. Nasal pressure support ventilation plus oxygen compared with oxygen therapy alone in hypercapnic COPD. Am J Respir Crit Care Med. 1995;152(2):538–544. doi: 10.1164/ajrccm.152.2.7633704. [DOI] [PubMed] [Google Scholar]
- 39.Strumpf DA, Millman RP, Carlisle CC, et al. Nocturnal positive-pressure ventilation via nasal mask in patients with severe chronic obstructive pulmonary disease. Am Rev Respir Dis. 1991;144(6):1234–1239. doi: 10.1164/ajrccm/144.6.1234. [DOI] [PubMed] [Google Scholar]
- 40.Duiverman ML, Wempe JB, Bladder G, et al. Two-year home-based nocturnal noninvasive ventilation added to rehabilitation in chronic obstructive pulmonary disease patients: a randomized controlled trial. Respir Res. 2011;12:112. doi: 10.1186/1465-9921-12-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Struik FM, Sprooten RT, Kerstjens HA, et al. Nocturnal non-invasive ventilation in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure: a randomised, controlled, parallel-group study. Thorax. 2014;69(9):826–834. doi: 10.1136/thoraxjnl-2014-205126. [DOI] [PubMed] [Google Scholar]
- 42.Funk GC, Breyer MK, Burghuber OC, et al. Long-term non-invasive ventilation in COPD after acute-on-chronic respiratory failure. Respir Med. 2011;105(3):427–434. doi: 10.1016/j.rmed.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 43.Chiang LL, Liu CY, Ho SC, et al. Efficacy of nocturnal nasal positive pressure ventilation in hypercapnic patients with severe obstructive lung diseases. Chang Gung Med J. 2004;27(2):98–106. [PubMed] [Google Scholar]
- 44.Ambrosino N, Cigni P. Non invasive ventilation as an additional tool for exercise training. Multidiscip Respir Med. 2015;10(1):14. doi: 10.1186/s40248-015-0008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kolodziej MA, Jensen L, Rowe B, Sin D. Systematic review of noninvasive positive pressure ventilation in severe stable COPD. Eur Respir J. 2007;30(2):293–306. doi: 10.1183/09031936.00145106. [DOI] [PubMed] [Google Scholar]
- 46.Budweiser S, Heinemann F, Fischer W, Dobroschke J, Pfeifer M. Long-term reduction of hyperinflation in stable COPD by non-invasive nocturnal home ventilation. Respir Med. 2005;99(8):976–984. doi: 10.1016/j.rmed.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Pessoa IM, Costa D, Velloso M, Mancuzo E, Reis MA, Parreira VF. Effects of noninvasive ventilation on dynamic hiperinflation of patients with COPD during activities of daily living with upper limbs. Rev Bras Fisioter. 2012;16(1):61–67. [PubMed] [Google Scholar]
- 48.Ankjaergaard KL, Maibom SL, Wilcke JT. Long-term non-invasive ventilation reduces readmissions in COPD patients with two or more episodes of acute hypercapnic respiratory failure. Eur Clin Respir J. 2016;3:28303. doi: 10.3402/ecrj.v3.28303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang CY, Taylor NF, Blackstock FC. Chest physiotherapy for patients admitted to hospital with an acute exacerbation of chronic obstructive pulmonary disease (COPD): a systematic review. Physiotherapy. 2010;96(1):1–13. doi: 10.1016/j.physio.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 50.Chung LP, Winship P, Phung S, Lake F, Waterer G. Five-year outcome in COPD patients after their first episode of acute exacerbation treated with non-invasive ventilation. Respirology. 2010;15(7):1084–1091. doi: 10.1111/j.1440-1843.2010.01795.x. [DOI] [PubMed] [Google Scholar]
- 51.Weitzenblum E, Chaouat A. Sleep and chronic obstructive pulmonary disease. Sleep Med Rev. 2004;8(4):281–294. doi: 10.1016/j.smrv.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 52.Becker HF, Piper AJ, Flynn WE, et al. Breathing during sleep in patients with nocturnal desaturation. Am J Respir Crit Care Med. 1999;159(1):112–118. doi: 10.1164/ajrccm.159.1.9803037. [DOI] [PubMed] [Google Scholar]
- 53.O’Donoghue FJ, Catcheside PG, Ellis EE, et al. Australian trial of Noninvasive Ventilation in Chronic Airflow Limitation investigators Sleep hypoventilation in hypercapnic chronic obstructive pulmonary disease: prevalence and associated factors. Eur Respir J. 2003;21(6):977–984. doi: 10.1183/09031936.03.00066802. [DOI] [PubMed] [Google Scholar]
- 54.Calverley PM. Respiratory failure in chronic obstructive pulmonary disease. Eur Respir J Suppl. 2003;47:26S–30S. doi: 10.1183/09031936.03.00030103. [DOI] [PubMed] [Google Scholar]
- 55.Jones PW, Brusselle G, Dal Negro RW, et al. Properties of the COPD assessment test in a cross-sectional European study. Eur Respir J. 2011;38(1):29–35. doi: 10.1183/09031936.00177210. [DOI] [PubMed] [Google Scholar]
- 56.Jones PW, Harding G, Wiklund I, et al. Tests of the responsiveness of the COPD assessment test following acute exacerbation and pulmonary rehabilitation. Chest. 2012;142(1):134–140. doi: 10.1378/chest.11-0309. [DOI] [PubMed] [Google Scholar]
- 57.Marchand E, Maury G. Evaluation of the COPD Assessment Test in patients with stable COPD. Rev Mal Respir. 2012;29(3):391–397. doi: 10.1016/j.rmr.2011.09.043. French. [DOI] [PubMed] [Google Scholar]
- 58.Kelly JL, Bamsey O, Smith C, et al. Health status assessment in routine clinical practice: the chronic obstructive pulmonary disease assessment test score in outpatients. Respiration. 2012;84(3):193–199. doi: 10.1159/000336549. [DOI] [PubMed] [Google Scholar]
- 59.British Thoracic Society Standards of Care Committee Non-invasive ventilation in acute respiratory failure. Thorax. 2002;57(3):192–211. doi: 10.1136/thorax.57.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nickol AH, Hart N, Hopkinson NS, et al. Mechanisms of improvement of respiratory failure in patients with COPD treated with NIV. Int J Chron Obstruct Pulmon Dis. 2008;3(3):453–462. doi: 10.2147/copd.s2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nishimura M, Makita H, Nagai K, et al. Hokkaido COPD Cohort Study Investigators Annual change in pulmonary function and clinical phenotype in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185(1):44–52. doi: 10.1164/rccm.201106-0992OC. [DOI] [PubMed] [Google Scholar]
- 62.Hurst JR, Vestbo J, Anzueto A, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 63.Comer DM, Oakes A, Mukherjee R. Domiciliary non-invasive ventilation in the elderly. Effective, tolerated and justified. Ulster Med J. 2015;84(1):22–25. [PMC free article] [PubMed] [Google Scholar]