Abstract
Background
We have previously reported anticancer activities of Melicope ptelefolia (MP) leaf extracts on four different cancer cell lines. However, the underlying mechanisms of actions have yet to be deciphered. In the present study, the anticancer activity of MP hexane extract (MP-HX) on colorectal (HCT116) and hepatocellular carcinoma (HepG2) cell lines was characterized through microarray gene expression profiling.
Methods
HCT116 and HepG2 cells were treated with MP-HX for 24 hr. Total RNA was extracted from the cells and used for transcriptome profiling using Applied Biosystem GeneChip™ Human Gene 2.0 ST Array. Gene expression data was analysed using an Applied Biosystems Expression Console and Transcriptome Analysis Console software. Pathway enrichment analyses was performed using Ingenuity Pathway Analysis (IPA) software. The microarray data was validated by profiling the expression of 17 genes through quantitative reverse transcription PCR (RT-qPCR).
Results
MP-HX induced differential expression of 1,290 and 1,325 genes in HCT116 and HepG2 cells, respectively (microarray data fold change, MA_FC ≥ ±2.0). The direction of gene expression change for the 17 genes assayed through RT-qPCR agree with the microarray data. In both cell lines, MP-HX modulated the expression of many genes in directions that support antiproliferative activity. IPA software analyses revealed MP-HX modulated canonical pathways, networks and biological processes that are associated with cell cycle, DNA replication, cellular growth and cell proliferation. In both cell lines, upregulation of genes which promote apoptosis, cell cycle arrest and growth inhibition were observed, while genes that are typically overexpressed in diverse human cancers or those that promoted cell cycle progression, DNA replication and cellular proliferation were downregulated. Some of the genes upregulated by MP-HX include pro-apoptotic genes (DDIT3, BBC3, JUN), cell cycle arresting (CDKN1A, CDKN2B), growth arrest/repair (TP53, GADD45A) and metastasis suppression (NDRG1). MP-HX downregulated the expression of genes that could promote anti-apoptotic effect, cell cycle progression, tumor development and progression, which include BIRC5, CCNA2, CCNB1, CCNB2, CCNE2, CDK1/2/6, GINS2, HELLS, MCM2/10 PLK1, RRM2 and SKP2. It is interesting to note that all six top-ranked genes proposed to be cancer-associated (PLK1, MCM2, MCM3, MCM7, MCM10 and SKP2) were downregulated by MP-HX in both cell lines.
Discussion
The present study showed that the anticancer activities of MP-HX are exerted through its actions on genes regulating apoptosis, cell proliferation, DNA replication and cell cycle progression. These findings further project the potential use of MP as a nutraceutical agent for cancer therapeutics.
Keywords: Melicope ptelefolia, HepG2, Hepatocellular carcinoma, Colorectal cancer, Ingenuity pathway analysis, Microarray, HCT116, Transcriptome profiling, Cancer
Introduction
Cancer is recognized by distinct abnormalities or dysregulations of genes, which cause sustained proliferative signaling, evasion of growth suppressors, activation of invasion and metastasis, replicative immortality, induction of angiogenesis and resisting cell death (Hanahan & Weinberg, 2011). An effective cancer drug should ideally be able to overcome most of these dysregulations and achieve the desired therapeutic effect with minimal side effects (Pérez-Herrero & Fernández-Medarde, 2015).
The plant kingdom provides a plethora of novel molecules for numerous therapeutic uses, which include treatment and prevention of chronic diseases including cancer. The study of plant extracts bioactivity is an important step for drug discovery and development, as it could lead to isolation of novel bioactive phytochemical(s) with therapeutic and medicinal properties (Singh et al., 2016). Some examples of anticancer drugs isolated from the plant kingdom include colchicine, podophyllotoxin, vincristine, vinblastine and taxol (Singh et al., 2016). Vinblastine is an antimitotic agent that is clinically indicated in the treatment of patients with Hodgkin’s and non-Hodgkin’s lymphomas, breast cancer, Kaposi’s sarcoma, renal cell cancer, and testicular cancer (Prendergast & Jaffee, 2013).
Melicope ptelefolia (MP) is a well-known herb in several Asian countries, including Malaysia, Indonesia, Thailand and Vietnam. In Malaysia, MP is locally known as “tenggek burung” and commonly used in a vegetable salad. MP has been used as a traditional medicine in Malaysia to treat several illnesses including high blood pressure, fatigue and erectile dysfunction (Aman, 2006). We have recently reported the anticancer and apoptosis induction activities of MP on colorectal, breast and liver cancer cell lines. The hexane leaf extract (MP-HX) appeared to show the most notable anti-proliferative activity against the four cancer cell lines tested (Kabir et al., 2017). However, the underlying molecular mechanisms involved have yet to be fully elucidated. The aim of the present study was to characterize anticancer activity of MP-HX on colorectal HCT116 and hepatocellular HepG2 carcinoma cell lines through microarray gene expression profiling.
Materials and Methods
Extract preparation
Fresh, healthy and young MP leaves were purchased from the local wet market and processed on the same day. The sample identity was authenticated by a plant taxonomist at the University of Malaya herbarium, Dr. Sugumaran Manickam. A voucher specimen was also deposited at the herbarium, with a registration number KLU 49190. The leaves were washed with distilled water and air dried for 3 days at room temperature. Sample drying was completed by incubating the leaves in an oven at 40 °C for 24 h. The dried leaves were then powdered using a table blender and stored at –20 °C until further analysis. MP-HX extract preparation was initiated by mixing fifty grams of the powdered leaves with 500 mL of hexane (1:10 ratio of sample weight to solvent volume). The mixture was constantly shaken (150 rpm) for 6 h at 37 °C using Innova 4300 Incubator Shaker (New Brunswick Scientific). The mixture was centrifuged at 1,500 rpm for 10 min, after which the supernatant was collected and filtered using a Whatman filter paper (No. 4). The residues were extracted again with the same solvent twice. The hexane solvent collected (∼1,500 mL) was evaporated at 40 °C using a rotary evaporator (Buchi Rotavapor R-215). The dried extract was dissolved in 10% dimethyl sulfoxide (DMSO) at 2 mg/mL and stored at –20 °C.
Cell culture
Human colorectal (HCT116) and hepatocellular (HepG2) carcinoma cell lines were purchased from American Type Culture Collection (ATCC) and were cultured in Dulbecco’s modified minimum essential media (DMEM) (Catalogue No. 08458-45, Nacalai Tesque), supplemented with 10% FBS (Catalogue No. 10270, Gibco), 100 U/mL penicillin and 100 µg/mL streptomycin (09367-34, Nacalai Tesque). Cells were cultured in a 37 °C incubator with 5% CO2.
Summary of study workflow
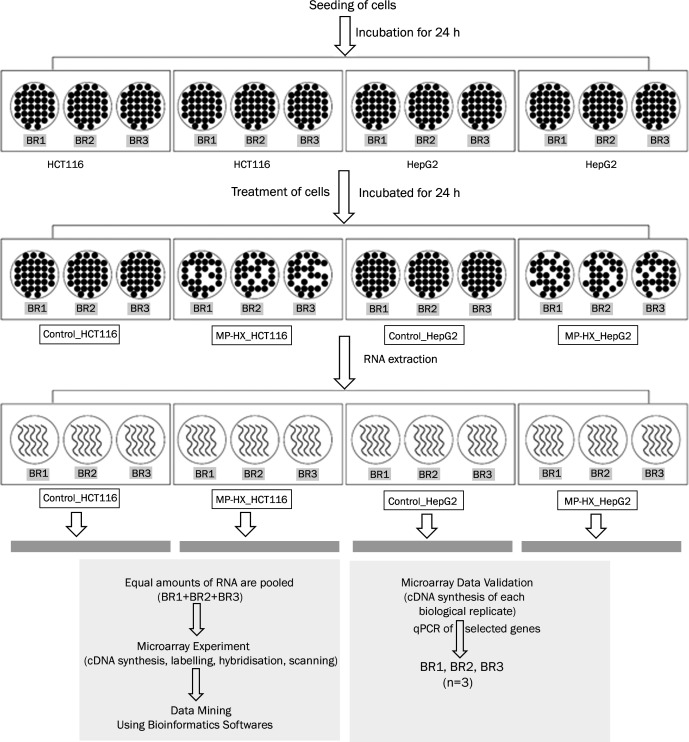
The workflow employed for the present study is summarized in Fig. 1. The workflow involves seeding of HCT116 and HepG2 cells, treatment of the cells with either MP-HX or 0.39% DMSO (vehicle control), extraction of RNA, cDNA synthesis & labelling, microarray hybridization and scanning, validation of microarray data through quantitative reverse transcription PCR (RT-qPCR) and bioinformatics analysis of the dataset. The experimental protocols are detailed in the next sections.
Figure 1. Workflow scheme for transcriptome profiling of HCT116 and HepG2 cells treated with MP-HX.
The figure shows the workflow summary employed for the present study. BR, biological replicate.
RNA extraction
For RNA extraction, the cells were seeded in 6 wells plate and incubated for 24 h. Following that, the cells were treated for 24 h with MP-HX at ∼IC50 concentration of the cell lines, which was 60 µg/mL for HCT116 and 78 µg/mL for HepG2 (Kabir et al., 2017). The control cells were treated with the vehicle (DMSO) used to dissolve MP-HX, and the final concentration of DMSO was 0.39 % v/v. After 24-hour treatment, total RNA from HCT116 and HepG2 cells were extracted and purified using PureLink RNA mini kit (Catalogue No. 12183018A; Invitrogen, Carlsbad, CA, USA), according to manufacturer’s instructions. RNA quantity and purity were verified using Nanodrop™ 2000 (Thermo Fisher Scientific, Waltham, MA, USA), by measuring absorbance ratios at 260/280 and 260/230. The RNA quality and integrity were also verified through agarose gel electrophoresis. Only RNA of good quality and integrity with an absorbance ratio of at least 2.0 was used for microarray and RT-qPCR assays.
Microarray gene expression profiling
Microarray profiling was performed using Human Gene (HuGene) 2.0 ST array (Catalogue No. 902499; Applied Biosystems, Foster City, CA, USA). Briefly, equal amounts of total RNA from three biological replicates of an assay point were pooled to get a minimum of 500 ng total RNA (Ruiz-Laguna et al., 2016). The RNA was then reverse transcribed to synthesize complementary RNA (cRNA) using GeneChip™ WT Plus kit (Catalogue No. 902280; Applied Biosystems). The purified cRNA was quantified using Nanodrop™ 2000. Following this, cDNA was synthesized from 15 µg of cRNA. The purified cDNA (5.5 µg) was fragmented, labelled and hybridized onto GeneChip™ Human Gene 2.0 ST array, according to manufacturer’s guidelines. After 16 h of hybridisation in GeneChip™ Hybridization Oven 645, the arrays were washed and stained using GeneChip™ Expression, Wash and Stain kit (Catalogue No. 900720; Applied Biosystems) on GeneChip™ Fluidics Station 450. The stained arrays were scanned using GeneChip™ Scanner 3000 7G. Raw data (CEL files) was generated using GeneChip Operating Software (GCOS). The CEL files were analyzed by Expression Console software (version 1.4) (Applied Biosystems), to ensure the quality control parameters and the data produced by the arrays were acceptable. Gene expression data was analyzed using Transcriptome Analysis Console (TAC) software (version 3.1) (Applied Biosystems). The genes were filtered using microarray fold change (MA_FC) value of ±2.00. The list of genes (MA_FC ≥ ±2.00) was also analyzed using Ingenuity Pathway Analysis (IPA) (http://www.ingenuity.com) software to identify the canonical pathways, biological functions, gene networks and key processes that were enriched/modulated by MP-HX in HCT116 and HepG2 cell lines. The raw microarray data files were submitted to Gene Expression Omnibus (GEO) repository (accession number GSE114743).
Quantitative reverse transcription PCR (RT-qPCR)
Each data point presented for the quantitative PCR assay was derived from three biological replicates (BR). Total RNA from each BR (Fig. 1) was reversed transcribed and the cDNA from each BR was used as a template for the qPCR. One microgram of total RNA was converted to cDNA using Maxima First Strand cDNA synthesis kit (Catalogue No. K1641; Thermo Fisher Scientific). The RT-qPCR was performed using Luminaris color HiGreeen High ROX qPCR master mix (Catalogue No. K0362; Thermo Fisher Scientific) on StepOne Plus PCR thermocycler (Applied Biosystems). Seventeen genes were selected to validate the microarray data, comprising of nine upregulated genes (BBC3, CDKN1A, CDKN2B, DDIT3, GABARAPL1, GADD45A, JUN, NDRG1, TP53) and eight downregulated genes (CCNA2, GINS2, HELLS, MCM2, MCM10, PLK1, RRM2 and SKP2). The primers used in the assay are listed in Table 1. The thermocycling condition for the real-time PCR was 50 °C for 2 min, 95 °C for 10 min, followed by 40 cycles of 95 °C for 15s, 60 °C for 30s (except for MCM2 and CDKN2B, 63 °C) and extension at 72 °C for 30s. RPS29 was selected as the internal control gene to normalize the gene expression data (De Jonge et al., 2007). Relative quantification of the target genes was calculated by comparative 2−ΔΔCT method (Livak & Schmittgen, 2001).
Table 1. Oligonucleotide sequences used for real-time qPCR assay.
| Gene name | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| BBC3 | cccgtgaagagcaaatgag | accccctgatgaaggtgag |
| CDKN1A | gaccatgtggacctgtcac | ggcttcctcttggagaagatc |
| CDKN2B | atcccaacggagtcaaccg | agtctcagacaggcttgcagg |
| DDIT3 | tcaccacacctgaaagcag | gagccgttcattctcttcag |
| GABARAPL1 | gaccatccctttgagtatcgg | gaagaataaggcgtcctcaggtc |
| GADD45A | gttttgctgcgagaacgac | gaacccattgatccatgtag |
| JUN | ttctatgacgatgccctcaacgcctc | gaagccctcctgctcatctgtcacgttc |
| NDRG1 | gcagagtaacgtggaagtggtcc | acggcatccactgcaggc |
| TP53 | tgactgtaccaccatccactacaac | ttgcggagattctcttcctctg |
| CCNA2 | cagaaaaccattggtccctc | cactcactggcttttcatcttc |
| GINS2 | gccgagaaggagctggttac | gtttcaggttaatcgccagc |
| HELLS | gcttgatgggtccatgtctt | gacatctatcctgggcctga |
| MCM2 | aagtgcaatttcgtcctgggtc | gcaaccttgttgtccttcttgg |
| MCM10 | gcaaaaatcccctgtagagaag | tccccacaatttgacctctag |
| PLK1 | acttcgtgttcgtggtgttgg | gcttgaggtctcgatgaataac |
| RRM2 | gcgatttagccaagaagttcag | cccagtctgccttcttcttg |
| SKP2 | cgctgcccacgatcatttat | ggagattctttctgtagccgct |
| RPS29 | gcactgctgagagcaagatg | ataggcagtgccaaggaaga |
Statistical analysis
Statistical comparisons were carried out by independent t-test using SPSS statistical software version 22.0 (SPSS Inc., Chicago, IL, USA). Values are shown as mean ±SD. The acceptable level for statistical significance was P < 0.05.
Results
Microarray data validation by RT-qPCR
The validation was performed by measuring the expression of 17 different genes (nine upregulated, eight downregulated) through RT-qPCR. These genes were selected based on their important roles in processes such as cell cycle progression, cellular proliferation and programmed cell death. The selected genes had microarray fold change (MA_FC) value of ≥ ± 1.50, except TP53, which had MA_FC of approximately +1.30. Their differential expression likely made significant contribution towards MP-HX anticancer activity. A comparison of FC values between microarray (MA_FC) and RT-qPCR (qPCR_FC) techniques are shown in Table 2 and Fig. S1. The result showed that the direction of gene expression changes as revealed by RT-qPCR assay (17/17) was in agreement with the microarray data.
Table 2. Comparison of gene expression fold changes (FCs) obtained from microarray (MA_FC) and real-time qPCR (qPCR_FC) assays.
The table shows gene expression FCs obtained by MA and qPCR assays. For qPCR assay, the data were normalized to RPS29 expression and each value represents mean qPCR_FC ± SD (n = 3). A positive FC value indicates upregulation, while negative FC value indicates downregulation.
| Cell line | Gene symbol | Gene name | MA_FC | qPCR_FC |
|---|---|---|---|---|
| HCT116 | 1. BBC3 | BCL2 binding component 3 | +2.93 | +4.48 ± 0.69 |
| 2. CDKN1A | Cyclin-dependent kinase inhibitor 1A | +2.36 | +4.44 ± 0.33 | |
| 3. DDIT3 | DNA-damage-inducible transcript 3 | +8.36 | +12.29 ± 0.15 | |
| 4. GADD45A | Growth arrest and DNA-damage-inducible, alpha | +3.23 | +5.49 ± 0.49 | |
| 5. NDRG1 | N-myc downstream regulated 1 | +3.16 | +6.52 ± 0.83 | |
| 6. GABARAPL1 | GABA(A) receptor-associated protein like 1 | +3.74 | +8.35 ± 0.63 | |
| 7. JUN | Jun proto-oncogene | +4.53 | +9.45 ± 0.86 | |
| 8. CDKN2B | Cyclin-dependent kinase inhibitor 2B | +3.95 | +9.82 ± 0.21 | |
| 9. TP53 | Tumor protein p53 | +1.38 | +2.01 ± 0.04 | |
| 10. CCNA2 | Cyclin A2 | −4.21 | −18.89 ± 1.92 | |
| 11. GINS2 | GINS complex subunit 2 (Psf2 homolog) | −7.03 | −6.73 ± 0.10 | |
| 12. HELLS | Helicase, lymphoid-specific | −6.15 | −6.43 ± 0.27 | |
| 13. RRM2 | Ribonucleotide reductase M2 | −3.80 | −7.45 ± 1.05 | |
| 14. PLK1 | Polo-like kinase 1 | −4.60 | −19.52 ± 0.87 | |
| 15. SKP2 | S-phase kinase-associated protein 2 | −4.86 | −10.90 ± 0.17 | |
| 16. MCM2 | Minichromosome maintenance complex component 2 | −4.70 | −32.97 ± 2.84 | |
| 17. MCM10 | Minichromosome maintenance 10 replication initiation factor | −5.86 | −23.99 ± 3.78 | |
| HepG2 | 1. BBC3 | BCL2 binding component 3 | +2.71 | +2.68 ± 0.38 |
| 2. CDKN1A | Cyclin-dependent kinase inhibitor 1A | +2.31 | +2.03 ± 0.14 | |
| 3. DDIT3 | DNA-damage-inducible transcript 3 | +5.21 | +7.69 ± 0.24 | |
| 4. GADD45A | Growth arrest and DNA-damage-inducible, alpha | +2.48 | +2.12 ± 0.07 | |
| 5. NDRG1 | N-myc downstream regulated 1 | +2.29 | +5.69 ± 1.70 | |
| 6. GABARAPL1 | GABA(A) receptor-associated protein like 1 | +4.87 | +5.63 ± 0.14 | |
| 7. JUN | Jun proto-oncogene | +5.06 | +6.00 ± 1.04 | |
| 8. CDKN2B | Cyclin-dependent kinase inhibitor 2B | +2.30 | +3.52 ± 0.59 | |
| 9. TP53 | Tumor protein p53 | +1.34 | +1.41 ± 0.05 | |
| 10. CCNA2 | Cyclin A2 | −2.66 | −6.18 ± 0.52 | |
| 11. GINS2 | GINS complex subunit 2 (Psf2 homolog) | −2.37 | −2.19 ± 0.07 | |
| 12. HELLS | Helicase, lymphoid-specific | −4.03 | −3.01 ± 0.14 | |
| 13. RRM2 | Ribonucleotide reductase M2 | −2.19 | −3.83 ± 0.73 | |
| 14. PLK1 | Polo-like kinase 1 | −1.87 | −14.94 ± 2.33 | |
| 15. SKP2 | S-phase kinase-associated protein 2 | −3.65 | −28.53 ± 3.72 | |
| 16. MCM2 | Minichromosome maintenance complex component 2 | −1.59 | −12.90 ± 2.53 | |
| 17. MCM10 | Minichromosome maintenance 10 replication initiation factor | −4.04 | −21.03 ± 1.00 |
Analysis of microarray data using Transcriptome Analysis Console (TAC)
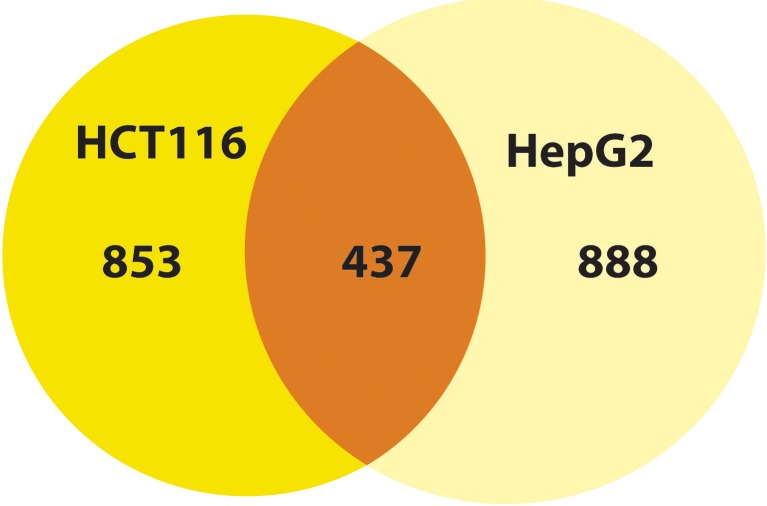
Using a cut-off fold change (MA_FC) value of ±2.00, 1,290 and 1,325 genes were noted to be differentially regulated in HCT116 and HepG2 cells, respectively. Among these genes, 437 were differentially regulated in both cell lines (Fig. 2). Subjecting the genes to TAC software analysis revealed a total of 130 and 164 Wikipathways (WPs) that were significantly regulated (p < 0.05) in HCT116 and HepG2 cells, respectively (Data S1). TAC ranked the WPs based on significance score.
Figure 2. MP-HX treatment induced differential expression of genes inHCT116 and HepG2 cells.
The Venn diagram shows the number of differentially expressed genes induced by MP-HX in HCT116 and HepG2 cells (MA_FC ≥ ±2.00).
Among the WPs that were significantly regulated, “Retinoblastoma (RB) in cancer” (RIC-WP) ranked the highest in HCT116 and HepG2 cells, with significant scores of 36.09 and 22.82, respectively. In both cell lines, almost all of RIC-WP genes which can promote cell cycle progression and proliferation were downregulated. This pathway interacted with 45 genes (one upregulated, 44 downregulated) in HCT116 cells, and 35 genes (four upregulated, 31 downregulated) in HepG2 cells (Figs. S2 and S3). These figures showed that MP-HX treatment induced downregulation of E2F, CDK1, CDK2, CDK6, CCNA2, CCNB1, CCNB2, CCNE1 and CCNE2 in both cell lines.
The ‘G1 to S cell cycle control’ Wikipathway (G1SCC-WP) had significant scores of 18.42 in HCT116 and 10.58 in HepG2 cells (Figs. S4 and S5). Twenty-seven genes in G1SCC-WP were modulated in HCT116 cells, of which 23 were downregulated. In HepG2 cells, 20 genes were modulated, of which 15 were downregulated. The G1/S cell cycle transition is activated by cyclins, CDKs and E2F proteins. This transition can be inhibited by CDK inhibitors. From the figures, many genes in G1SCC-WP which can promote proliferation were downregulated in both cell lines (CDC25A, CCNE2, PCNA, MCM3/5/6, POLE2, POLA2, CDK6, PRIM1, CDK1, CCNB1). In contrast, MP-HX upregulated GADD45A, CDK inhibitors p15 (CDKN2B) and p21 (CDKN1A) in both cell lines (Figs. S4 and S5).
The ‘Cell Cycle’ WikiPathway (CC-WP) (Figs. S6 and S7) had significant scores of 17.05 in HCT116 and 7.93 in HepG2. In HCT116, 31 genes were modulated, and they were all downregulated. In contrast, HepG2 cells showed modulation of 21 genes, of which 19 were downregulated and only two were upregulated. The dataset showed that MP-HX downregulated the expression of many cell cycle promoting genes in CC-WP in both cell lines, and these include CCNA2, CCNB1, CCNB2, CCNE2, CDK1, CDK2 and CDK6 (Figs. S6 and S7). MP-HX also downregulated MCM2 and PLK1 expression in HCT116, while MCM10 was downregulated in both cell lines.
The “DNA damage response” WikiPathway (DDR-WP) was also modulated by MP-HX, with significance score values of 6.67 and 6.38 in HCT116 and HepG2, respectively. In HCT116, four genes were upregulated and 11 genes were downregulated (Fig. S8). In HepG2, six genes were upregulated and nine genes were downregulated (Fig. S9). MP-HX significantly downregulated CHEK1, RAD51, CDC25A, cyclins and CDKs expression, while CDKN1A, BBC3 and GADD45A were upregulated in both cell lines.
The “Apoptosis” Wikipathway (AP-WP) is was modulated by MP-HX with significance scores of 3.95 and 3.16 in HCT116 and HepG2 cells, respectively (Figs. S10 and S11). In HCT116, 10 genes were upregulated and three genes were downregulated. In HepG2, 12 genes were modulated and seven of them were upregulated. MP-HX upregulated the expression of BBC3 and JUN while it downregulated the expression of HELLS in both cell lines. In HepG2 cells, BAK1 and PMAIP1 were also upregulated by MP-HX. On the other hand, MP-HX downregulated BIRC5 expression in HCT116.
IPA analysis - Top canonical pathways modulated by MP-HX in HCT116 and HepG2 Cells
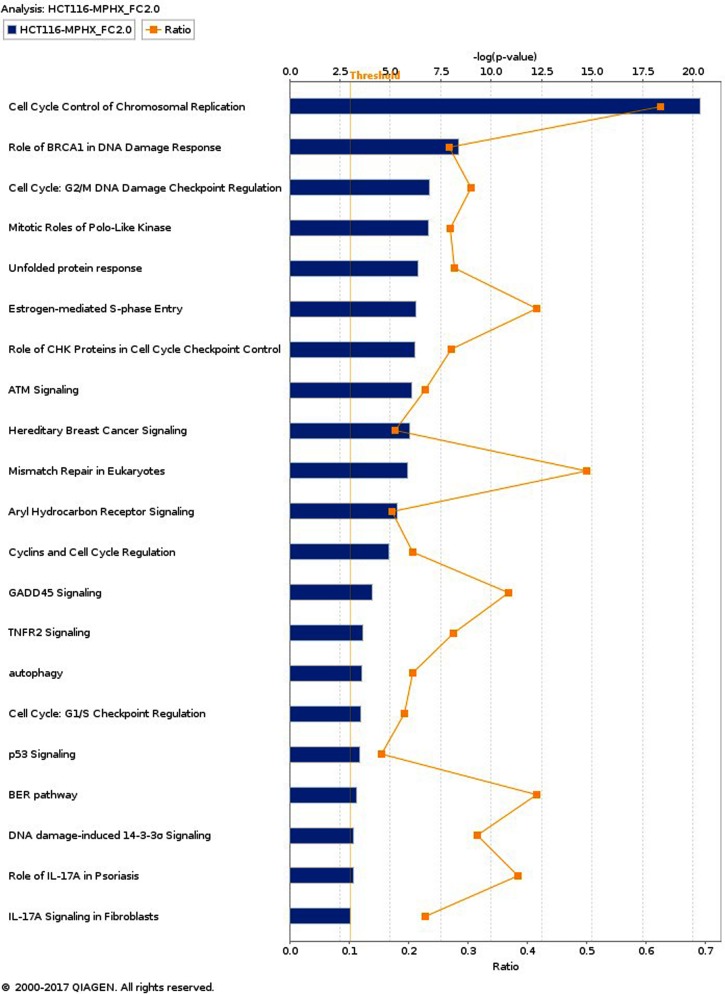
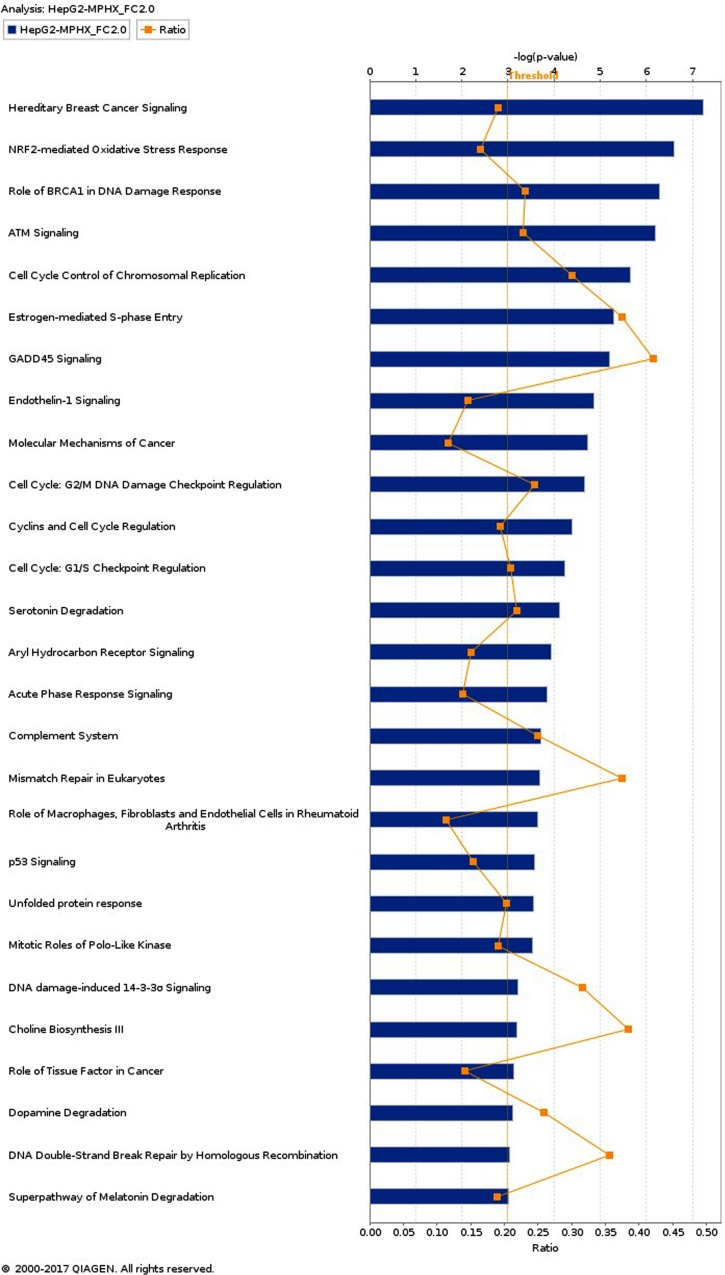
IPA enriched 55 and 135 canonical pathways (p < 0.05) in HCT116 and HepG2, respectively. Canonical pathways (CPs) with -log (p-value) of 3.0 and above are shown in Figs. 3 and 4. In HCT116, top most significant CPs include “cell cycle control of chromosomal replication”, “role of BRCA1 in DNA damage response”, “cell cycle: G2/M DNA damage checkpoint regulation”, “mitotic roles of polo-like kinase”, “unfolded protein response”, and “estrogen mediated S-phase entry” (Fig. 3). In HepG2, top CPs were “hereditary breast cancer signaling”, “NRF2- mediated oxidative stress response”, “role of BRCA1 in DNA damage response”, “ATM signaling”, “cell cycle control of chromosomal replication”, “estrogen mediated S-phase entry” and “GADD45 signaling” (Fig. 4).
Figure 3. Top canonical pathways (CPs) enriched in HCT116 cells after MP-HX treatment.
IPA software ranked the top CPs based on -log (p-value). Ratio value denotes the number of molecules in the dataset divided by the total number of molecules associated with the pathway. Data were analyzed through the use of IPA (QIAGEN Inc., https://www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis).
Figure 4. Top canonical pathways (CPs) enriched in HepG2 cells after MP-HX treatment.
IPA software ranked the top CPs based on -log (p-value). Ratio value denotes the number of molecules in the dataset divided by the total number of molecules associated with the pathway. Data were analyzed through the use of IPA (QIAGEN Inc., https://www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis).
A comparison of over-represented CPs in HCT116 and HepG2 are shown in Table 3. The CPs are ranked according to their respective -log (p-value). Based on the list, HCT116 showed a higher –log (p-value) for most of the CPs. In HepG2, only three CPs showed higher –log (p-value) than that of HCT116, and these include “hereditary breast cancer signaling”, “ATM signaling” and “NRF2-mediated oxidative stress response”. These observations could mean that although MP-HX may have exerted a similar anticancer effect in both cell lines, a subset of different effect or mechanism of action may have also occurred between the cell lines.
Table 3. Modulation of canonical pathways (CPs) in HCT116 and HepG2 by MP-HX.
The table shows the top canonical pathways modulated by MP-HX in the cell lines. They were ranked by IPA software based on −log (p-value).
| Canonical pathway | −log (p-value) | |
|---|---|---|
| HCT116 | HepG2 | |
| 1. Cell Cycle Control of Chromosomal Replication | 20.34 | 5.67 |
| 2. Role of BRCA1 in DNA Damage Response | 8.36 | 6.28 |
| 3. Hereditary Breast Cancer Signaling | 5.95 | 7.25 |
| 4. ATM Signaling | 6.08 | 6.19 |
| 5. Cell Cycle: G2/M DNA Damage Checkpoint Regulation | 6.96 | 4.66 |
| 6. Estrogen-mediated S-phase Entry | 6.25 | 5.29 |
| 7. Mitotic Roles of Polo-Like Kinase | 6.91 | 3.54 |
| 8. Unfolded protein response | 6.35 | 3.55 |
| 9. NRF2-mediated Oxidative Stress Response | 2.96 | 6.61 |
| 10. Mismatch Repair in Eukaryotes | 5.84 | 3.68 |
IPA analysis - Diseases and Functions Modulated by MP-HX in HCT116 and HepG2 Cells
The top category of diseases and functions modulated in HCT116 and HepG2 cells by MP-HX include “cancer”, “organismal injury and abnormalities”, “gastrointestinal disease”, “cell death and survival”, “cell cycle”, “cellular assembly and organization”, “DNA replication, recombination and repair”, “cellular development” and “cellular growth and proliferation” (Figs. S12 and S13). In both HCT116 and HepG2 cells, the predicted activation z-scores appeared supportive of MP-HX activity, as the negative values are suggestive of inhibition of processes associated with cell growth, cell proliferation and cell cycle (Table 4).
Table 4. Modulation of diseases and biological functions by MP-HX in HCT116 and HepG2 cells.
The table shows the enriched terms corresponding to diseases or biological functions, along with the number of associated genes in the experimental dataset, p-value and activation z-score. The z-score is calculated by the IPA software which predicts whether a specific disease or bio-function is increased (positive z-score) or decreased (negative z-score) based on the experimental dataset.
| Cell line | Categories | Diseases or biological functions | p-value | z-score | Number of genes |
|---|---|---|---|---|---|
| HCT116 | Cellular development; cellular growth and proliferation | Cell proliferation of tumor cell lines | 6.26E−16 | −2.60 | 218 |
| DNA replication, recombination, and repair | DNA replication | 3.48E−13 | −2.07 | 27 | |
| Cell cycle | S-phase | 1.37E−11 | −3.47 | 40 | |
| Cell cycle | M-phase of tumor cell lines | 5.02E−11 | −2.00 | 28 | |
| Cell cycle; DNA replication, recombination, and repair | Checkpoint control | 6.93E−09 | −2.55 | 15 | |
| Cell death and survival | Cell death of cervical cancer cell lines | 7.07E−08 | +2.66 | 60 | |
| Cell death and survival | Apoptosis of cervical cancer cell lines | 1.71E−06 | +3.37 | 47 | |
| Cell cycle | G1-phase of tumor cell lines | 4.41E−06 | +2.39 | 35 | |
| Cell cycle | Entry into interphase | 7.87E−06 | −2.30 | 17 | |
| DNA replication, recombination, and repair | DNA damage | 9.03E−06 | +2.42 | 24 | |
| Cell cycle | Entry into S-phase | 1.42E−05 | −2.57 | 16 | |
| Cell death and survival | Cell viability of cervical cancer cell lines | 3.01E−05 | −2.18 | 34 | |
| Cellular development; cellular growth and proliferation | Cell proliferation of breast cancer cell lines | 3.58E−05 | −2.94 | 61 | |
| HepG2 | Cell death and survival | Apoptosis of cervical cancer cell lines | 1.48E−08 | +2.34 | 51 |
| Cell death and survival | Cell viability of cervical cancer cell lines | 4.67E−05 | −2.01 | 33 | |
| Cell cycle | G1-phase of tumor cell lines | 1.96E−04 | +2.01 | 30 | |
| Cell cycle | M-phase of tumor cell lines | 2.17E−04 | −2.34 | 17 | |
| Cell cycle; DNA replication, recombination, and repair | Checkpoint control | 5.56E−04 | −2.38 | 9 | |
| Cell death and survival; cellular development | Self-renewal of tumor cell lines | 9.53E−04 | −2.20 | 5 |
IPA analysis - Upstream Regulators Prediction in HCT116 and HepG2 Cells
IPA upstream regulator analysis (URA) examines how many known targets of each transcriptional regulator (TR) are present in the dataset and compares their direction of change to what is expected from the literature to predict the likely relevant TRs. If the observed direction of change is mostly consistent with a particular activation state of the TR (“activated” or “inhibited”), then a prediction is computed about that activation state (z-score). This can provide insights on the underlying mechanism for the biological activities in the tissue/cells being studied.
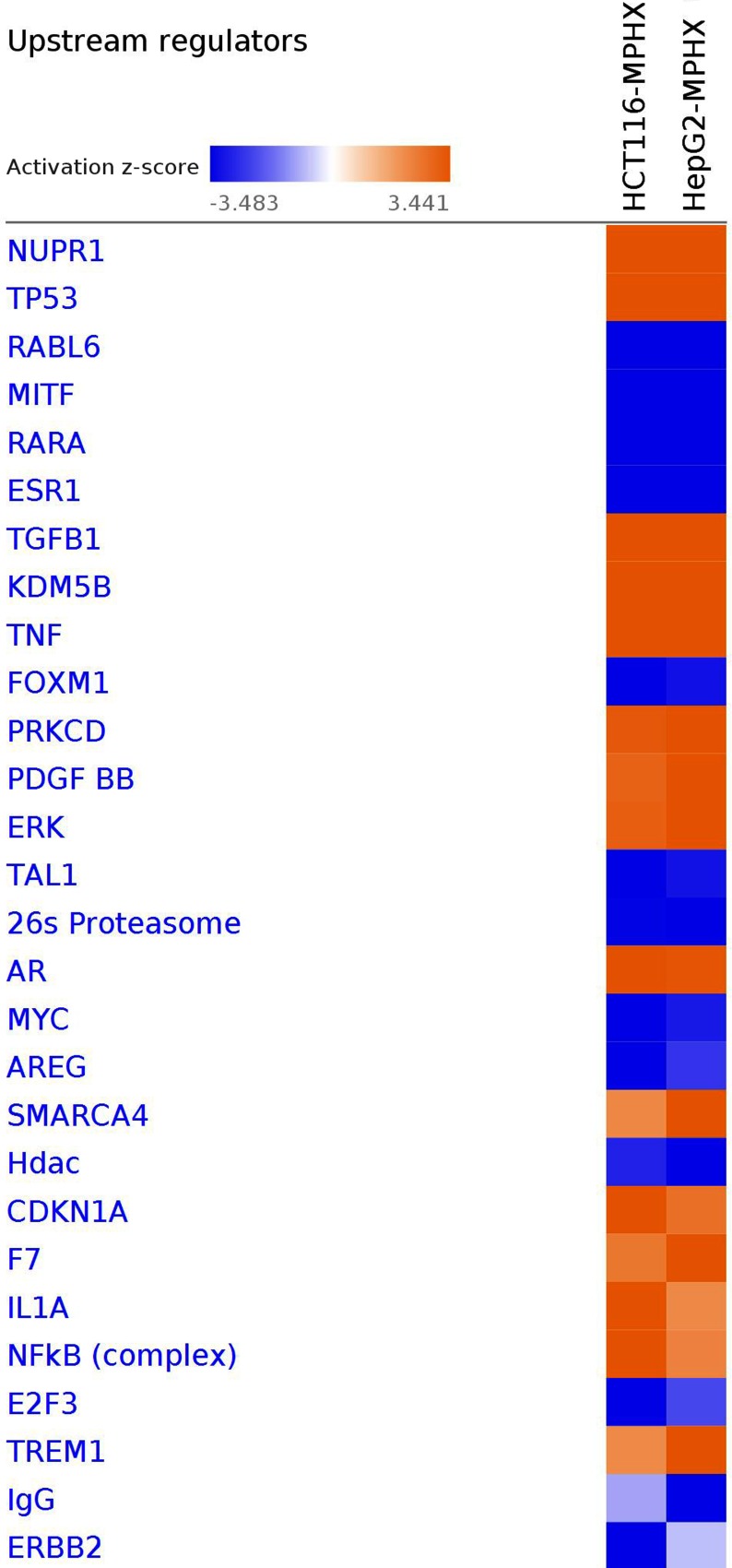
A comparison of top upstream regulators modulated in HCT116 and HepG2 cells is depicted in Fig. 5. The heatmap shows that most of the upstream regulators were modulated in the same direction in both cell lines, except IgG and ERBB2. There were only slight differences in the activation z-scores of AREG, SMARCA4, HDAC, CDKN1A, F7, IL1A, NFKB, E2F3 and TREM1 between the HCT116 and HepG2 cells. This observation suggests MP-HX exerted its effect by modulating a similar set of upstream regulators in both cell lines. The top upstream regulators that were predicted to be modulated by MP-HX in HCT116 and HepG2 cells are also listed in Table 5. NUPR1 was the most significant upstream regulator in HCT116 and HepG2 with activation z-scores of +10.94 and +12.21, respectively. IPA also ranked TP53 as the second most significant upstream regulator in both cell lines, with activation z-scores of +7.16 and +6.22 in HCT116 and HepG2 cells, respectively. Another upstream regulator predicted by IPA was RABL6 (RAB, member RAS oncogene family like 6), which had activation z-scores of −6.25 and −4.80 in HCT116 and HepG2 cells, respectively.
Figure 5. IPA software prediction of upstream regulators and their activation state in HCT116 and HepG2 cells after MP-HX treatment.
IPA predicts modulation of regulators and ranked them based on the activation z-score. A positive score denotes activation, while a negative score denotes inhibition. The heatmap color intensity depicts relative value of activation z-score for the corresponding cell line. Upstream regulators were filtered based on -log (p-value) of 3.0 and activation z-score of 3.5. Orange color indicates activation whereas blue color indicates inhibition.
Table 5. IPA software prediction of upstream regulators in HCT116 and HepG2 cells that were treated with MP-HX.
IPA predicts modulation of upstream regulators and ranked them based on the activation z-score. A positive score denotes activation, while a negative score denotes inhibition.
| Cell line | Activation/Inhibition | UR | MA_FC | z-score | Overlap p-value |
|---|---|---|---|---|---|
| HCT116 | Activated | NUPR1 | +1.27 | +10.94 | 8.34E−57 |
| TP53 | +1.38 | +7.16 | 3.07E−32 | ||
| TNF | +1.07 | +4.81 | 1.15E−12 | ||
| KDM5B | +2.44 | +4.79 | 9.05E−18 | ||
| IL1A | +2.74 | +3.86 | 1.39E−08 | ||
| NFkB (complex) | +3.65 | 2.24E−12 | |||
| AR | +1.14 | +3.61 | 4.8E−15 | ||
| CDKN1A | +2.36 | +3.59 | 3.36E−10 | ||
| TGFB1 | −1.20 | +3.54 | 2.48E−21 | ||
| E2F6 | +1.27 | +3.46 | 1.01E−10 | ||
| Inhibited | RABL6 | −1.28 | −6.25 | 9.25E−37 | |
| MITF | +1.12 | −5.62 | 3.56E−26 | ||
| RARA | −1.24 | −5.10 | 4.43E−11 | ||
| ESR1 | −1.16 | −4.91 | 9.74E−17 | ||
| FOXM1 | −2.96 | −4.89 | 2.09E−21 | ||
| TAL1 | −1.21 | −4.15 | 6.83E−08 | ||
| E2F3 | −1.35 | −3.71 | 1.99E−14 | ||
| MYC | −1.40 | −3.67 | 3.23E−14 | ||
| ERBB2 | −1.12 | −3.54 | 7.77E−57 | ||
| E2f | −3.46 | 4.87E−19 | |||
| HepG2 | Activated | NUPR1 | +2.40 | +12.21 | 1.95E−73 |
| TP53 | +1.34 | +6.22 | 2.45E−27 | ||
| TGFB1 | +2.29 | +5.37 | 3.98E−17 | ||
| PDGF BB | +4.81 | 2.03E−14 | |||
| PRKCD | +1.63 | +4.74 | 9.17E−11 | ||
| ERK | +4.68 | 3.94E−10 | |||
| SMARCA4 | +1.06 | +4.26 | 2.52E−10 | ||
| TREM1 | +8.43 | +3.75 | 1.56E−08 | ||
| TGM2 | −1.34 | +3.72 | 0.0044 | ||
| KDM5B | +1.08 | +3.54 | 1.54E−13 | ||
| Inhibited | RABL6 | −1.11 | −4.80 | 1.15E−24 | |
| RARA | +1.09 | −4.40 | 1.22E−10 | ||
| MITF | +1.24 | −4.19 | 7.93E−11 | ||
| ESR1 | +1.08 | −4.04 | 5.29E−17 | ||
| IgG | −3.84 | 6.49E−07 | |||
| 26s Proteasome | −3.64 | 5.77E−14 | |||
| Hdac | −3.57 | 2.64E−08 | |||
| KIAA1524 | −2.21 | −3.26 | 7.02E−04 | ||
| FOXM1 | −1.87 | −3.20 | 6.48E−13 | ||
| TAL1 | −1.22 | −3.15 | 1.66E−06 |
IPA analysis—Networks Enriched in HCT116 and HepG2 by MP-HX
IPA network analysis display the interaction between molecules present in the dataset which reflects significance of biological function. The IPA software ranked top networks enriched in the microarray dataset based on ‘network score’ Table 6. This analysis considers the number of network eligible molecules in the network, network size, total number of network eligible molecules analyzed and total number of network eligible molecules in the Ingenuity® Knowledge Base that could be included in the network. The network score is calculated based on right tailed Fisher’s exact test and the presented network score is negative logarithm of Fisher’s exact p-value. The higher the score, the more significant is the interaction between network eligible molecules. The full list of molecules in the network indicated is listed in Data S2.
Table 6. Modulation of networks in HCT116 and HepG2 cells by MP-HX.
IPA software ranked top networks enriched in the microarray dataset based on the network score (NS).
| Cell line | NI | Score | Focus mlecules | Top disease and functions |
|---|---|---|---|---|
| HCT116 | 1 | 124 | 124 | Cell Cycle, Cellular Assembly and Organization, DNA Replication, Recombination, and Repair |
| 2 | 114 | 119 | Cellular Compromise, Cellular Function and Maintenance, Cancer | |
| 3 | 73 | 94 | Cell Death and Survival, Inflammatory Response, Cellular Development | |
| 4 | 66 | 84 | Cell Cycle, DNA Replication, Recombination, and Repair, Cell Death and Survival | |
| 5 | 57 | 83 | Cancer, Organismal Injury and Abnormalities, Respiratory Disease | |
| HepG2 | 1 | 122 | 122 | Cell Cycle, Cellular Assembly and Organization, DNA Replication, Recombination, and Repair |
| 2 | 110 | 116 | Cell Death and Survival, Cell Morphology, Cellular Function and Maintenance | |
| 3 | 68 | 90 | Cell Death and Survival, Organismal Injury and Abnormalities, Cell Cycle | |
| 4 | 61 | 85 | Cell Cycle, Cancer, Organismal Injury and Abnormalities | |
| 5 | 57 | 82 | Cell Cycle, Cellular Assembly and Organization, DNA Replication, Recombination, and Repair |
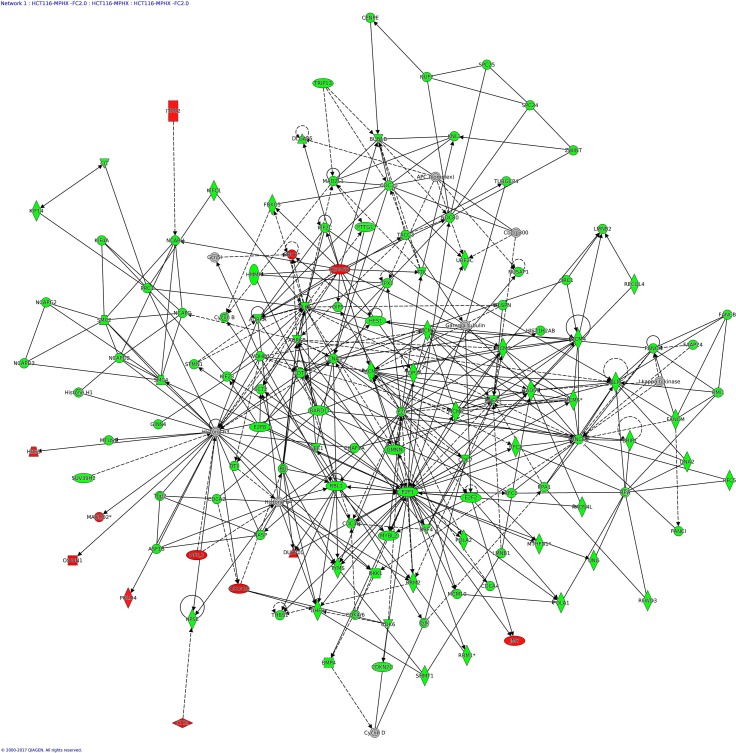
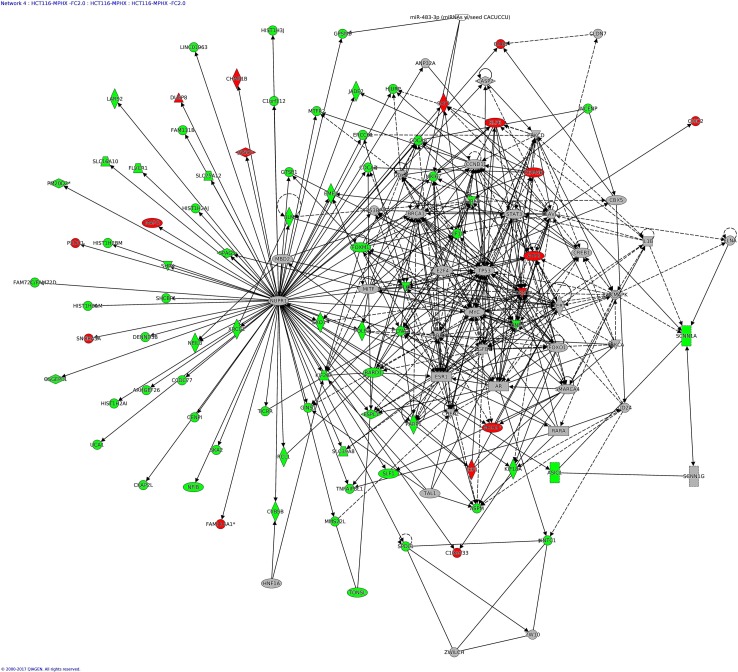
The top network (Network 1) in HCT116 had a network score (NS) of 124, and contains 124 focus molecules which include AURKA, AURKB, BBC3, CCNA2, CDK6, CDKN2B, E2F1, MCM2, MCM10, PLK1, POLA1, RAD51, RFC3, RRM2, TOP2A, TYMS (Fig. 6). Many of the focus molecules in this network were downregulated. Network 2 (NS = 73) in HCT116 has 119 focus molecules which include BIRF5, CCNB1, CDC25A, CDK2, CDKN1A, DDIT3, GADD45A, HMOX1, JUN, NDRG1, PCNA, while network 4 (NS = 66) has 84 focus molecules which include AURKB, CCNA2, CDKN1A, GINS1, KIF11, MXD1, PLK1, POLE2, TP53. Although NUPR1 was not considered as a focus molecule in network 4 (due to its MA_FC value of +1.27), IPA network analysis nevertheless indicated NUPR1 interacted with many other focus molecules in the network, and most of them were downregulated (Fig. 7), an observation similar to that seen in HepG2 (Fig. 8) (see below).
Figure 6. IPA network analysis: annotated interactions between genes in HCT116 cells treated with MP-HX.
The figure shows network 1 in HCT116, containing 124 focus molecules. Most of the focus molecules in this network were downregulated by MP-HX. Top diseases and functions associated with this network include processes related to cell proliferation, cell cycle and DNA replication. Molecules in the network are colored red (MA_FC ≥ + 2.0), green (MA_FC ≥ − 2.0), or grey (MA_FC < ± 2). Data were analyzed through the use of IPA (QIAGEN Inc., https://www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis).
Figure 7. IPA network analysis: annotated interactions between genes in HCT116 cells treated with MP-HX.
The figure shows network 4 in HCT116, containing 84 focus molecules. NUPR1 shows interaction with many other focus molecules that show expression downregulation (MA_FC ≥ − 2.0). Top diseases and functions associated with this network include processes related to cell cycle regulation, DNA replication and cell death/survival. Molecules in the network are colored red (MA_FC ≥ + 2.0), green (MA_FC ≥ − 2.0), or grey (MA_FC < ± 2). Data were analyzed through the use of IPA (QIAGEN Inc., https://www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis).
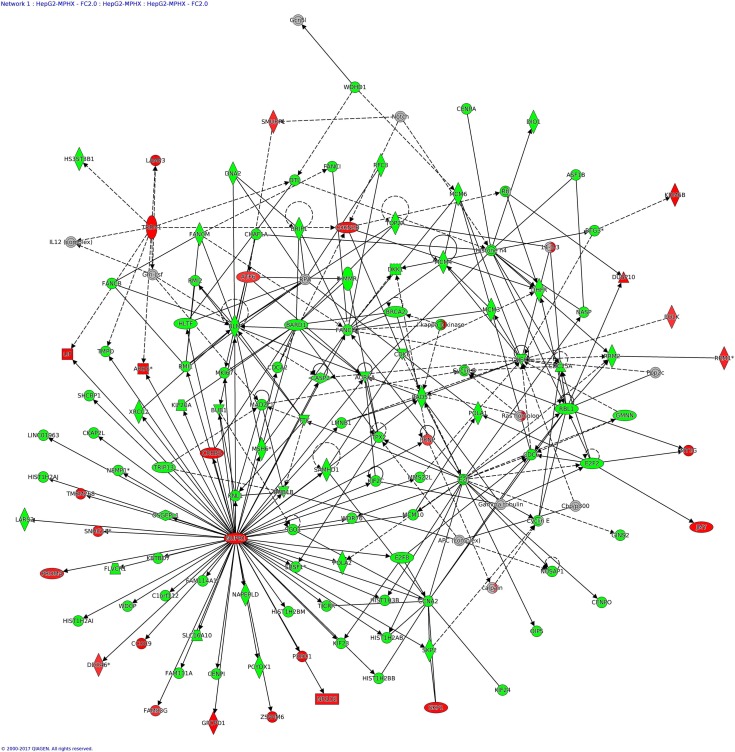
Figure 8. IPA network analysis: annotated interactions between genes in HepG2 cells treated with MP-HX.
The figure shows network 1 in HepG2 cells, containing 122 focus molecules. NUPR1 (MA_FC +2.40) shows interaction with many other focus molecules that show expression downregulation (MA_FC ≥ − 2.0). Top diseases and functions associated with this network include processes related to cell cycle, cancer and DNA replication. Molecules in the network are colored red (MA_FC ≥ + 2.0), green (MA_FC ≥ − 2.0), or grey (MA_FC < ±2). Data were analyzed through the use of IPA (QIAGEN Inc., https://www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis).
In HepG2, the top network (NS = 122) has 122 focus molecules which include AURKA, BUB1, CCNA2, CDK6, CDKN2B, CHEK1, GINS2, GMNN, MCM3, MCM10, NUPR1, POLA2, RAD51, RFC3, RRM2, SKP2, TOP2A (Fig. 8). The network figure shows NUPR1 (MA_FC +2.40) interacted with many other focus molecules, and many of them were downregulated. Identification of NUPR1 as a focus molecule here is supportive of the earlier finding, i.e., upstream regulator analysis, where NUPR1 was predicted by IPA to be the top upstream regulator in both HepG2 and HCT116 cells. Network 2 (NS = 110) in HepG2 has 116 focus molecules which include BBC3, DDIT4, ERN1, GABARAPL1, NDRG1, while network 3 (NS = 68) has 90 focus molecules which include BAK1, CCNB1, CDK1, CDKN1A, DDIT3, DUSP1, GADD45B, HMOX1, JUN, PCNA.
Discussion
In the present study, the anticancer activity of MP-HX against HepG2 and HCT116 cells was characterised through microarray gene expression profiling followed by bioinformatics data analysis using TAC and IPA software. The microarray data was validated by performing RT-qPCR assay on selected genes that were differentially regulated. The direction of gene expression changes for the 17 genes assayed through RT-qPCR was found to be consistent with the microarray data.
TAC software analysis revealed that MP-HX significantly regulated many Wikipathways (WP) associated with cell cycle, cell proliferation and cell death. The pathways that were modulated include retinoblastoma in cancer (RIC), G1 to S cell cycle control (G1SCC), cell cycle (CC), DNA damage response (DDR) and apoptosis (AP).
The “Retinoblastoma in cancer Wikipathway” (RIC-WP) was the top pathway that was significantly regulated in both cell lines (Figs. S2 and S3). The RIC-WP mainly consists of Rb family protein pRb (also known as RB1), cyclin dependent kinases (CDKs), cyclin dependent kinase inhibitors and E2F proteins. The pRb protein acts as a tumor suppressor by inhibiting the function of E2F transcription factors, leading to inhibition of cell cycle progression (Giacinti & Giordano, 2006). The presence of CDKs in the G1 phase of the cell cycle inactivates pRb through phosphorylation, allowing the cells to enter S-phase and initiate DNA replication (Henley & Dick, 2012). The phosphorylation of pRb by CDKs are prevented by CDK inhibitors INK4 (p15, p16, p18, and p19) and Cip/Kip (p21, p27 and p57) (Asghar et al., 2015). In both cell lines, the concomitant decrease in the expression of E2F, CDK1, CDK2, CDK6, CCNA2, CCNB1, CCNB2, CCNE1 and CCNE2 as observed in the dataset may have conferred a synergistic effect, leading to MP-HX antiproliferative activity.
CDKN1A (p21) is an inhibitor of CDK in RIC-WP. CDKN1A expression was upregulated in both HCT116 (qPCR_FC +4.44) and HepG2 (qPCR_FC +2.03). CDKN1A is a sensor and effector of multiple anti-proliferative signals and capable of inducing cell cycle arrest (Abbas & Dutta, 2009). MP-HX also induced downregulation of E2F and CDKs expression in both HepG2 and HCT116 (Figs. S2 and S3). These facts are in accordance with CDKN1A upregulation as noted above, and these events are likely to contribute to the inhibition of G1/S transition, leading to anti-proliferative effect by MP-HX in both cell lines.
Cyclin A2 (CCNA2) is a component of RIC-WP. CCNA2 was downregulated by MP-HX in both HCT116 (qPCR_FC −18.89) and HepG2 (qPCR_FC −6.18) (Figs. S2 and S3). This gene is widely expressed in many tissues with important roles in mediating G1-S and G2/M transitions (Hochegger, Takeda & Hunt, 2008). Cyclin A2 promotes DNA replication during S-phase by activating CDK2. It also activates CDK1 to induce mitotic entry (Wolgemuth, 2008; Hochegger, Takeda & Hunt, 2008). CCNA2 overexpression has been reported in numerous types of cancers (Uhlen et al., 2010).
Ribonucleotide Reductase Regulatory Subunit-M2 (RRM2) is another component of RIC-WP that was downregulated by MP-HX in both HCT116 (qPCR_FC −7.45) and HepG2 (qPCR_FC −6.18). RRM2 is a subunit of ribonucleotide reductase (RNR). It catalyzes conversion of NDP to dNDP, which is pivotal for the supply of nucleotides for DNA synthesis and cell proliferation. RRM2 can be classified as an oncogene as its overexpression in transgenic mice promoted tumour development (Furuta et al., 2010). RRM2 overexpression in epithelial ovarian carcinoma (EOC) cases predicts a shorter overall survival. Knockdown of RRM2 in EOC cell lines inhibited its proliferation and triggers cellular senescence (Aird et al., 2014).
MP-HX also modulated “G1 to S cell cycle control” Wikipathway (G1SCC-WP) in both cell lines (Figs. S4 and S5). Cyclin Dependent Kinase Inhibitor 2B (CDKN2B) is a component of G1SCC-WP that was upregulated after treatment with MP-HX in HCT116 (qPCR_FC +9.82) and HepG2 (qPCR_FC +3.52). CDKN2B encodes a cyclin-dependent kinase inhibitor, which can form a complex with CDK4 or CDK6, and prevents the activation of the CDK kinases (Santo, Siu & Raje, 2015). CDKN2B is capable of inhibiting CDK4/6 activity by preventing them from hyperphosphorylating RB proteins, leading to G1 cell cycle arrest (Kim & Sharpless, 2006). CDKN2B was reported to be deleted in a wide variety of human tumors (Kim & Sharpless, 2006). Inactivation of this gene promoted carcinogenesis in hepatocellular and colorectal carcinoma (Ren et al., 2015; Ishiguro et al., 2006).
GADD45A is another component gene in G1SCC-WP that was upregulated by MP-HX in HCT116 (qPCR_FC +5.49) and HepG2 (qPCR_FC +2.12). This gene is a member of growth arrest and DNA damage family of proteins (GADD). GADD45A upregulation is often induced by DNA damage and other stress signals associated with growth arrest and apoptosis (Salvador, Brown-Clay & Fornace Jr, 2013). GADD45A interactions with CDK1, cyclin B1 and p21 can inhibit cell cycle progression and induce apoptotic cell death through JNK/p38 pathway (Zhan, 2005). During G2/M checkpoint, GADD45A can arrest cell cycle progression by inhibiting cyclin-B1 and CDC2 interaction, preventing entry into M-phase. Repression of GADD45A expression alleviated G2/M arrest (Rosemary Siafakas & Richardson, 2009).
The “Cell cycle Wikipathway” (CC-WP) was also significantly modulated by MP-HX treatment in both cell lines (Figs. S6 and S7). The CC-WP member genes include cyclins and CDKs proteins, which are responsible for the promotion of cell cycle progression. These proteins are frequently overexpressed in numerous types of cancer (Asghar et al., 2015). In both cell lines, MP-HX downregulated many of the cyclins and CDKs genes, including CCNA2, CCNB1, CCNB2, CCNE2, CDK1, 2 and 6. Downregulation of these genes likely contributed to MP-HX inhibition of cell cycle progression in the cancer cells. Many anticancer drugs have been reported to exert anticancer effect by arresting cell cycle progression at G1/S and G2/M transition points, and this is achieved through the inhibition of key proteins that promote cell cycle progression (Santo, Siu & Raje, 2015; Dominguez-Brauer et al., 2015).
The CC-WP also include minichromosome maintenance (MCM), S-phase kinase-associated protein 2 (SKP2) and Polo-like kinase 1 (PLK1) genes. These genes were also downregulated by MP-HX in both cell lines. The MCM family of proteins play important roles in replication, transcription, checkpoint response and cancer progression (Forsburg, 2004; Deegan & Diffley, 2016). MCM2 was markedly downregulated by MP-HX treatment in HCT116 (qPCR_FC −32.97) and HepG2 (qPCR_FC −12.90). MCM2 is a component member of Mcm2-7 complex. The complex unwinds parental DNA to form single-stranded DNA template during replication (Simon & Schwacha, 2014). MCM2 overexpression has been reported in colorectal, glioma and oral squamous cell carcinomas (Guzińska-Ustymowicz et al., 2009; Razavi et al., 2015; Hua et al., 2014). MCM10 was also significantly downregulated by MP-HX treatment in HCT116 (qPCR_FC −23.99) and HepG2 (qPCR_FC −21.03). MCM10 is thought to be essential for activating MCM2-7 helicase activity, since the unwinding of origins of replication is defective in the absence of MCM10 (Deegan & Diffley, 2016). MCM10 and MCM2 overexpression have been reported in urothelial and cervical cancers (Li et al., 2016a; Das et al., 2013). Of note, MP-HX also downregulated other MCMs, which include MCM3 (MA_FC −4.15, −2.90) and MCM7 (MA_FC −2.79, −1.53) in HCT116 and HepG2, respectively.
SKP2 is a component of ubiquitin–proteasome system (UPS), which plays vital roles in regulating the degradation of various cellular proteins (Wang et al., 2012). This gene was downregulated in both HCT116 (qPCR_FC −10.90) and HepG2 (qPCR_FC -28.53). SKP2 is a subunit in Skp1–Cullin1–F-box (SCF) E3 ligase complex. Skp2 is an F-box protein of the SCF complex SCFskp2 E3 ligase, which targets many tumor suppressor proteins (eg. p27, p21, p57, TOB1, FOXO1) for degradation. For this reason, it is not surprising that overexpression of SKP2 is observed in diverse type of human cancers (Wang et al., 2012).
Polo-like kinase 1 (PLK1) is a member of polo-like kinase family. PLK1 was also downregulated in HCT116 (qPCR_FC -19.52) and HepG2 (qPCR_FC -14.94). PLK1 roles include regulation of mitotic entry, centrosome separation and maturation, kinetochore attachment and cytokinesis (Schmucker & Sumara, 2014). PLK1 was reported to be overexpressed in various cancers and thought to confer tolerance of cells to cancer associated cellular stress (Strebhardt, 2010). Inhibition of PLK1 was also reported to induce anticancer effect in hepatocellular carcinoma and retinoblastoma cell lines (Xu et al., 2017; Schwermer et al., 2017). Up to this point, it is interesting to note that in both cell lines, MP-HX downregulated all six top-ranked genes (PLK1, MCM2, MCM3, MCM7, MCM10 and SKP2) that were proposed to be cancer-associated (Wu, Zhu & Zhang, 2012).
MP-HX also modulated many components genes of “DNA damage response Wikipathway” (DDR-WP) in both cell lines. The expression of genes in DDR-WP can be induced by genotoxic agents or environmental stress such as UV and ionizing radiation, and the response could lead to induction of cell cycle arrest and mobilisation of DNA repair mechanisms to circumvent DNA damage (O’Connor, 2015). In both HCT116 and HepG2, MP-HX downregulated some of the genes in DDR-WP, which include CHEK1, RAD51, CDC25A, cyclins and CDKs, while CDKN1A, BBC3 and GADD45A expression were upregulated. With regards to their functions, CHEK1, RAD51 and CDC25A have been suggested to promote tumor development, while GADD45A can act as a tumor suppressor (Hosoya & Miyagawa, 2014; Broustas & Lieberman, 2014). CDKN1A, as discussed earlier, can induce cell cycle arrest through inhibition of cyclins and CDKs. BBC3 is a pro-apoptotic protein which is capable of suppressing cancer development (Yu & Zhang, 2008), and a more detailed description of its role is discussed below.
MP-HX treatment also modulated “Apoptosis Wikipathway” (AP-WP) in both cell lines. Apoptosis is a form of programmed cell death, characterized by chromatin condensation, cytoplasmic shrinkage, membrane blebbing and nuclear fragmentation (Wong, 2011). Many type of cancers are known to evade apoptosis for their survival (Hanahan & Weinberg, 2011). Apoptotic signaling is modulated by BCL-2 anti-apoptotic and pro-apoptotic family proteins, inhibitor of apoptosis proteins (IAP), p53 tumor suppressor, caspases and death receptor signaling (Wong, 2011). The Bcl-2 family of proteins is one of the major regulators of apoptosis, namely the intrinsic pathway of apoptosis. This family contains pro-apoptotic and anti-apoptotic (or pro-survival) protein members (Delbridge & Strasser, 2015). An imbalance in the amount of BCL-2 family of proteins, overexpression of IAP proteins, inhibition of caspases, reduced p53 function and impaired death receptor signaling are responsible for the development of cancer and chemoresistance (Pistritto et al., 2016). Therefore, targeting proteins which modulate apoptosis is of key interest in cancer therapy (Goldar et al., 2015). Apoptosis modulating drugs may inhibit BCL-2 anti-apoptotic proteins, IAP proteins or activate BCL-2 pro-apoptotic proteins, caspase enzymes and p53 functions to confer anticancer effect (Wong, 2011).
MP-HX treatment induced upregulation of BBC3 in HCT116 (qPCR_FC +4.48) and HepG2 (qPCR_FC +2.68). BBC3 is a pro-apoptotic gene of the BCL-2 family. At the same time, MP-HX treatment induced downregulation of BIRC5, which is an inhibitor of apoptosis (IAP) protein, in HCT116 (MA_FC −3.44) and HepG2 (MA_FC −1.82). BBC3 is known to facilitate p53-dependent or independent apoptosis (Yu & Zhang, 2008) while BIRC5 was reported to be overexpressed in various type of cancers (Kelly et al., 2011). BBC3 antagonizes anti-apoptotic members of Bcl-2 to promote cell death. BBC3 colocalizes in mitochondrial membrane to inhibit anti-apoptotic Bcl-2 family members, while activating pro-apoptotic Bax/Bak, causing outer mitochondrial membrane permeabilization and leading the initiation of mitochondrial pathway of apoptosis. Inhibition or deletion of BBC3 impairs apoptosis and contributing to the development of cancer and chemo-resistance (Hikisz & Kilianska, 2012).
MP-HX also induced the upregulation of another AP-WP member, PMAIP1 (NOXA) in HCT116 (MA_FC +1.69) and HepG2 (MA_FC +3.10). In HepG2 cells, MP-HX upregulated pro-apoptotic gene BAK1 (MA_FC +2.14), another member of AP-WP. NOXA is capable of inactivating anti-apoptotic members of BCL-2 family, allowing BAK and BAX to interact with pro-apoptotic members of BCL-2 and leading to apoptosis (Hata, Engelman & Faber, 2015; Albert, Brinkmann & Kashkar, 2014).
Lymphoid specific helicase (HELLS) is another component member of AP-WP that was downregulated by MP-HX in both HCT116 (qPCR_FC −6.43) and HepG2 (qPCR_FC −3.01). HELLS demonstrated diverse functions, which include epigenetics regulation of development, co-activator of E2F to stimulate cell growth and DNA repair (Mjelle et al., 2015). HELLS can promote cancer, as it enhances growth, migration, and invasion of nasopharyngeal carcinoma cell lines (He et al., 2016). Overexpression of HELLS was also reported in prostate and oropharyngeal squamous cell carcinomas (Von Eyss et al., 2012); (Janus et al., 2011). Increased HELLS expression was associated with poor prognosis in patients with renal cell carcinoma (Chen et al., 2017).
JUN (cJUN) is another component member of AP-WP that was upregulated by MP-HX in HCT116 (qPCR_FC +9.45) and HepG2(qPCR_FC +6.00). JUN proto-oncogene is a component of the AP-1 transcription factor that demonstrated cancer promoting, as well as apoptotic induction activities (Eferl et al., 2003; Fan & Chambers, 2001; Dhanasekaran & Reddy, 2008; Bossy-Wetzel, Bakiri & Yaniv, 1997). In glioma cells treated with temozolomide and nimustine, apoptosis was achieved through c-JUN mediated activation of BIM (BCL-2 interacting mediator of cell death) (Tomicic et al., 2015). The anticancer activity of tylophorine, an alkaloid from Tylophora indica was also demonstrated to be mediated by c-JUN in HONE-1, NUGC-3 and HepG2 carcinoma cells. The upregulation of c-JUN by tylophorine resulted to cyclin A2 transcriptional repression, leading to G1 cell cycle arrest (Yang et al., 2013). The induction of pro-apoptotic and downregulation of anti-apoptotic genes suggests that MP-HX promoted network signaling or transcriptome profile favoring apoptosis induction. This finding is consistent with our previous report, which demonstrated apoptosis induction of MP-HX on four different cancer cell lines (Kabir et al., 2017).
IPA software analysis also revealed that MP-HX modulated many canonical pathways, biological functions and gene networks linked to cell cycle, cell proliferation, DNA replication, DNA damage, cell death and apoptosis in both HepG2 and HCT116 cell lines. IPA ranked nuclear protein 1 (NUPR1) as the top upstream regulator that was activated in both cell lines by MP-HX treatment. The microarray data also showed that MP-HX upregulated NUPR1 expression in both HCT116 (MA_FC +1.27) and HepG2 (MA_FC +2.40) cell lines. NUPR1 is a transcription factor that has been reported to show tumor inhibiting and promoting activities. NUPR1 was able to inhibit tumor progression in brain and prostate cancers, as well as being overexpressed in several other cancers (Goruppi & Iovanna, 2010). A recent study reported NUPR1 acted as a negative regulator of tumor repopulating cells (TRCs) growth (Jia et al., 2016). The above observations suggest NUPR1 had a significant role in mediating MP-HX anticancer activity. Future study could investigate this hypothesis by silencing NUPR1 expression in MP-HX treated cells.
IPA analysis also predicted tumor protein 53 (TP53) as another top upstream regulator that was activated in both cell lines. TP53 is a classical tumor suppressor gene which is frequently mutated in cancers (Leroy et al., 2014). TP53 and its target genes are renowned for their involvement in cell cycle arrest, DNA repair and apoptosis (Fischer, 2017). IPA also predicted RABL6 (RAB, member RAS oncogene family like 6) as another top upstream regulator, with activation z-scores that are suggestive of its inhibition in both cell lines. The microarray data showed that RABL6 was mildly downregulated in HepG2 and HCT116 (MA_FC of −1.11 and −1.28, respectively). RABL6 has been reported to promote proliferation of osteosarcoma and pancreatic neuroendocrine tumor through inhibition of retinoblastoma 1 (pRb) (Tang et al., 2016; Hagen et al., 2014). RABL6 also enhanced MDM2-induced TP53 degradation (Lui et al., 2013). These highlight RABL6 tumor promoting properties. The activation or inhibition of the above transcriptional regulators in both cell lines likely played significant roles in modulating the antiproliferative effect induced by MP-HX, and a more detailed investigation on their contributions could be explored in future studies.
IPA software network analysis suggested N-myc Downstream Regulated 1 (NDRG1) as a focus molecule in both HCT116 (network 2) and HepG2 (network 2) cells. NDRG1 expression was upregulated by MP-HX in both HCT116 (qPCR_FC +6.52) and HepG2 (qPCR_FC +5.69) cells. NDRG1 demonstrated anti-oncogenic function and was proposed to be a metastasis suppressor in diverse type of cancers (Fang et al., 2014). NDRG1 is capable of blocking metastasis by activating cell adhesion molecules (E-cadherin, β-catenin), and inhibiting snail/slug, Wnt signalling and ROCK1/pMLC2 pathways (Bae et al., 2013). GINS complex subunit 2 (GINS2) is another focus molecule highlighted by IPA network analysis. GINS2 is a component member of network 1 in HepG2 and network 5 in HCT116 cells, and its expression was downregulated in both HCT116 (qPCR_FC −6.73) and HepG2 (qPCR_FC −2.19) cell lines. GINS2 is a component of ‘GINS complex’, a tetrameric complex with crucial roles in the initiation of DNA replication, including correct assembly and maintenance of DNA replication forks and its progression during replication (Gambus et al., 2006). GINS2 upregulation was associated with poor prognosis and reduced survival in early-stage cervical cancer (Ouyang et al., 2017). In breast cancer patients, elevated GINS2 transcript level was associated with poor relapse-free and distant metastasis-free survival (Zheng et al., 2014).
The present study shows that MP-HX exert anticancer activity by inducing gene expression changes in HCT116 and HepG2 cells that can promote antiproliferative or anticancer effect. Phytochemical compounds with anticancer properties which have been identified in MP include β-sitosterol, lupeol, oleanolic acid, kokusaginine and genistein (Baskar et al., 2010; Rauth et al., 2016; Shaari et al., 2011; Tiwari, Brunton & CS, 2013; Li et al., 2016b; Molnar et al., 2013). Further studies are warranted to identify the exact constituent(s) that is/are responsible for MP anticancer activity.
Conclusions
The present study showed that MP-HX conferred its anticancer activity by modulating the expression of various key genes regulating DNA replication, cell cycle progression, cell growth/proliferation and programmed cell death in a direction favoring anticancer effect. The transcriptome profiles induced by MP-HX include upregulation of pro-apoptotic, cell cycle arresting and metastasis suppression genes and downregulation of genes that promote anti-apoptotic effect, cell cycle progression, tumor development and progression. The ability of MP-HX to downregulate all six top-ranked genes that were proposed to be cancer-associated is also supportive of its anticancer activity (Wu, Zhu & Zhang, 2012). The findings in the present study provide novel insights on the anticancer activity of MP and further project its potential use as a nutraceutical agent for cancer therapeutics.
Supplemental Information
The bar chart shows gene expression fold changes (FC) obtained from microarray and real-time PCR assays. The data for each assay was derived from three biological replicates. For each qPCR assay, the data was normalized to RPS29 expression and the value represent mean qPCR_FC ±SD (n = 3).
The figure shows component genes of RIC-WP that were differentially regulated by MP-HX in HCT116 cells. The genes in the pathway are colored red (MA_FC ≥ + 2.0), or green (MA_FC ≥ − 2.0), or depicted as grey hashed boxes (MA_FC < ±2).
The figure shows component genes of RIC-WP that were differentially regulated by MP-HX in HepG2 cells. The genes in the pathway are colored red (MA_FC ≥ + 2.0), or green (MA_FC ≥ − 2.0), or depicted as grey hashed boxes (MA_FC < ±2).
The figure shows component genes in G1SCC-WP that were differentially regulated by MP-HX treatment in HCT116 cells. The genes in the pathway are colored red (MA_FC ≥ + 2.0), or green (MA_FC ≥ − 2.0), or depicted as grey hashed boxes (MA_FC < ±2).
The figure shows component genes of G1SCC-WP that were differentially regulated by MP-HX in HepG2 cells.The genes in the pathway are colored red (MA_FC ≥ + 2.0), or green (MA_FC ≥ − 2.0), or depicted as grey hashed boxes (MA_FC < ±2).
The figure shows component genes of CC-WP that were differentially regulated by MP-HX in HCT116 cells. The genes in the pathway are colored red (MA_FC ≥ + 2.0), or green (MA_FC ≥ − 2.0), or depicted as grey hashed boxes (MA_FC < ±2).
The figure shows component genes of CC-WP that were differentially regulated by MP-HX in HepG2 cells. The genes in the pathway are colored red (MA_FC ≥ + 2.0), or green (MA_FC ≥ − 2.0), or depicted as grey hashed boxes (MA_FC < ±2).
The figure shows component genes in DDR-WP that were differentially regulated by MP-HX in HCT116 cells. The genes in the pathway are colored red (MA_FC ≥ + 2.0), or green (MA_FC ≥ − 2.0), or depicted as grey hashed boxes (MA_FC < ±2).
The figure shows component genes in DDR-WP that were differentially regulated by MP-HX in HepG2 cells. The genes in the pathway are colored red (MA_FC ≥ + 2.0), or green (MA_FC ≥ − 2.0), or depicted as grey hashed boxes (MA_FC < ±2).
The figure shows component genes in AP-WP that were differentially regulated by MP-HX in HCT116 cells. The genes in the pathway are colored red (MA_FC ≥ + 2.0), or green (MA_FC ≥ − 2.0), or depicted as grey hashed boxes (MA_FC < ±2).
The figure shows component genes in AP-WP that were differentially regulated (MA_FC ≥ ±2.00) by MP-HX in HepG2 cells. The genes in the pathway are colored red (MA_FC ≥ + 2.0), or green (MA_FC ≥ − 2.0), or grey (MA_FC < ±2).
The figure shows top category of diseases and biological functions that were modulated by MP-HX (FC ≥ ±2.0) in HCT116 cells, and they were ranked by IPA software based on -log (p-value) ≥5.0.
The figure shows top category of diseases and biological functions that were modulated by MP-HX (FC ≥ ±2.0) in HepG2 cells and they were ranked by IPA software based on -log (p-value) ≥5.0.
Funding Statement
This study was funded by University of Malaya Research Grant PG018-2016A and Malaysian Ministry of Higher Education Research Fund HIR-MOHE H-20001-00-E000009. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Mohammad Faujul Kabir performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Johari Mohd Ali conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Onn Haji Hashim analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
Raw data has been uploaded into Gene Expression Omnibus (GEO) repository with the accession number given as GSE114743.
References
- Abbas & Dutta (2009).Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nature Reviews Cancer. 2009;9:400–414. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aird et al. (2014).Aird KM, Li H, Xin F, Konstantinopoulos PA, Zhang R. Identification of ribonucleotide reductase M2 as a potential target for pro-senescence therapy in epithelial ovarian cancer. Cell Cycle. 2014;13:199–207. doi: 10.4161/cc.26953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert, Brinkmann & Kashkar (2014).Albert M-C, Brinkmann K, Kashkar H. Noxa and cancer therapy. Molecular & Cellular Oncology. 2014;1:e29906. doi: 10.4161/mco.29906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aman (2006).Aman R. Tumbuhan liar berkhasiat ubatan. Dewan Bahasa dan Pustaka; Kuala Lumpur: 2006. [Google Scholar]
- Asghar et al. (2015).Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nature Reviews Drug Discovery. 2015;14:130–146. doi: 10.1038/nrd4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae et al. (2013).Bae DH, Jansson PJ, Huang ML, Kovacevic Z, Kalinowski D, Lee CS, Sahni S, Richardson DR. The role of NDRG1 in the pathology and potential treatment of human cancers. Journal of Clinical Pathology. 2013;66:911–917. doi: 10.1136/jclinpath-2013-201692. [DOI] [PubMed] [Google Scholar]
- Baskar et al. (2010).Baskar AA, Ignacimuthu S, Paulraj GM, Al Numair KS. Chemopreventive potential of β-Sitosterol in experimental colon cancer model—an in vitro and in vivo study. BMC Complementary and Alternative Medicine. 2010;10:24–24. doi: 10.1186/1472-6882-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossy-Wetzel, Bakiri & Yaniv (1997).Bossy-Wetzel E, Bakiri L, Yaniv M. Induction of apoptosis by the transcription factor c-Jun. EMBO Journal. 1997;16:1695–1709. doi: 10.1093/emboj/16.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broustas & Lieberman (2014).Broustas CG, Lieberman HB. DNA damage response genes and the development of cancer metastasis. Radiation Research. 2014;181:111–130. doi: 10.1667/RR13515.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2017).Chen D, Maruschke M, Hakenberg O, Zimmermann W, Stief CG, Buchner A. TOP2A, HELLS, ATAD2, and TET3 are novel prognostic markers in renal cell carcinoma. Urology. 2017;102:265.e1–265.e7. doi: 10.1016/j.urology.2016.12.050. [DOI] [PubMed] [Google Scholar]
- Das et al. (2013).Das M, Prasad SB, Yadav SS, Govardhan HB, Pandey LK, Singh S, Pradhan S, Narayan G. Over expression of minichromosome maintenance genes is clinically correlated to cervical carcinogenesis. PLOS ONE. 2013;8:e69607. doi: 10.1371/journal.pone.0069607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jonge et al. (2007).De Jonge HJM, Fehrmann RSN, De Bont ESJM, Hofstra RMW, Gerbens F, Kamps WA, De Vries EGE, Van der Zee AGJ, Te Meerman GJ, Ter Elst A. Evidence based selection of housekeeping genes. PLOS ONE. 2007;2:e898. doi: 10.1371/journal.pone.0000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deegan & Diffley (2016).Deegan TD, Diffley JF. MCM: one ring to rule them all. Current Opinion in Structural Biology. 2016;37:145–151. doi: 10.1016/j.sbi.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Delbridge & Strasser (2015).Delbridge AR, Strasser A. The BCL-2 protein family, BH3-mimetics and cancer therapy. Cell Death and Differentiation. 2015;22:1071–1080. doi: 10.1038/cdd.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanasekaran & Reddy (2008).Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Brauer et al. (2015).Dominguez-Brauer C, Thu KL, Mason JM, Blaser H, Bray MR, Mak TW. Targeting mitosis in cancer: emerging strategies. Molecular Cell. 2015;60:524–536. doi: 10.1016/j.molcel.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Eferl et al. (2003).Eferl R, Ricci R, Kenner L, Zenz R, David JP, Rath M, Wagner EF. Liver tumor development: c-Jun antagonizes the proapoptotic activity of p53. Cell. 2003;112:181–192. doi: 10.1016/S0092-8674(03)00042-4. [DOI] [PubMed] [Google Scholar]
- Fan & Chambers (2001).Fan M, Chambers TC. Role of mitogen-activated protein kinases in the response of tumor cells to chemotherapy. Drug Resistance Updates. 2001;4:253–267. doi: 10.1054/drup.2001.0214. [DOI] [PubMed] [Google Scholar]
- Fang et al. (2014).Fang BA, Kovacevic Z, Park KC, Kalinowski DS, Jansson PJ, Lane DJ, Sahni S, Richardson DR. Molecular functions of the iron-regulated metastasis suppressor, NDRG1, and its potential as a molecular target for cancer therapy. Biochimica et Biophysica Acta/General Subjects. 2014;1845:1–19. doi: 10.1016/j.bbcan.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Fischer (2017).Fischer M. Census and evaluation of p53 target genes. Oncogene. 2017;36:3943–3956. doi: 10.1038/onc.2016.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg (2004).Forsburg SL. Eukaryotic MCM proteins: beyond replication initiation. Microbiology and Molecular Biology Reviews. 2004;68:109–131. doi: 10.1128/mmbr.68.1.109-131.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta et al. (2010).Furuta E, Okuda H, Kobayashi A, Watabe K. Metabolic genes in cancer: their roles in tumor progression and clinical implications. Biochimica et Biophysica Acta/General Subjects. 2010;1805:141–152. doi: 10.1016/j.bbcan.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambus et al. (2006).Gambus A, Jones RC, Sanchez-Diaz A, Kanemaki M, Van Deursen F, Edmondson RD, Labib K. GINS maintains association of Cdc45 with MCM in replisome progression complexes at eukaryotic DNA replication forks. Nature Cell Biology. 2006;8:358–366. doi: 10.1038/ncb1382. [DOI] [PubMed] [Google Scholar]
- Giacinti & Giordano (2006).Giacinti C, Giordano A. RB and cell cycle progression. Oncogene. 2006;25:5220–5227. doi: 10.1038/sj.onc.1209615. [DOI] [PubMed] [Google Scholar]
- Goldar et al. (2015).Goldar S, Khaniani MS, Derakhshan SM, Baradaran B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pacific Journal of Cancer Prevention. 2015;16:2129–2144. doi: 10.7314/apjcp.2015.16.6.2129. [DOI] [PubMed] [Google Scholar]
- Goruppi & Iovanna (2010).Goruppi S, Iovanna JL. Stress-inducible protein p8 is involved in several physiological and pathological processes. Journal of Biological Chemistry. 2010;285:1577–1581. doi: 10.1074/jbc.R109.080887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzińska-Ustymowicz et al. (2009).Guzińska-Ustymowicz K, Pryczynicz A, Kemona A, Czyzewska J. Correlation between proliferation markers: PCNA, Ki-67, MCM-2 and antiapoptotic protein Bcl-2 in colorectal cancer. Anticancer Research. 2009;29:3049–3052. [PubMed] [Google Scholar]
- Hagen et al. (2014).Hagen J, Muniz VP, Falls KC, Reed SM, Taghiyev AF, Quelle FW, Gourronc FA, Klingelhutz AJ, Major HJ, Askeland RW, Sherman SK, O’Dorisio TM, Bellizzi AM, Howe JR, Darbro BW, Quelle DE. RABL6A promotes G1-S phase progression and pancreatic neuroendocrine tumor cell proliferation in an Rb1-dependent manner. Cancer Research. 2014;74:6661–6670. doi: 10.1158/0008-5472.CAN-13-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan & Weinberg (2011).Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hata, Engelman & Faber (2015).Hata AN, Engelman JA, Faber AC. The BCL2 family: key mediators of the apoptotic response to targeted anticancer therapeutics. Cancer Discovery. 2015;5:475–487. doi: 10.1158/2159-8290.CD-15-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He et al. (2016).He X, Yan B, Liu S, Jia J, Lai W, Xin X, Tang CE, Luo D, Tan T, Jiang Y, Shi Y, Liu Y, Xiao D, Chen L, Liu S, Mao C, Yin G, Cheng Y, Fan J, Cao Y, Muegge K, Tao Y. Chromatin remodeling factor LSH drives cancer progression by suppressing the activity of fumarate hydratase. Cancer Research. 2016;76:5743–5755. doi: 10.1158/0008-5472.CAN-16-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley & Dick (2012).Henley SA, Dick FA. The retinoblastoma family of proteins and their regulatory functions in the mammalian cell division cycle. Cell Division. 2012;7 doi: 10.1186/1747-1028-7-10. Article 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikisz & Kilianska (2012).Hikisz P, Kilianska ZM. PUMA, a critical mediator of cell death—one decade on from its discovery. Cellular & Molecular Biology Letters. 2012;17:646–669. doi: 10.2478/s11658-012-0032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochegger, Takeda & Hunt (2008).Hochegger H, Takeda S, Hunt T. Cyclin-dependent kinases and cell-cycle transitions: does one fit all? Nature Reviews Molecular Cell Biology. 2008;9:910–916. doi: 10.1038/nrm2510. [DOI] [PubMed] [Google Scholar]
- Hosoya & Miyagawa (2014).Hosoya N, Miyagawa K. Targeting DNA damage response in cancer therapy. Cancer Science. 2014;105:370–388. doi: 10.1111/cas.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua et al. (2014).Hua C, Zhao G, Li Y, Bie L. Minichromosome Maintenance (MCM) Family as potential diagnostic and prognostic tumor markers for human gliomas. BMC Cancer. 2014;14:526. doi: 10.1186/1471-2407-14-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro et al. (2006).Ishiguro A, Takahata T, Saito M, Yoshiya G, Tamura Y, Sasaki M, Munakata A. Influence of methylated p15 and p16 genes on clinicopathological features in colorectal cancer. Journal of Gastroenterology and Hepatology. 2006;21:1334–1339. doi: 10.1111/j.1440-1746.2006.04137.x. [DOI] [PubMed] [Google Scholar]
- Janus et al. (2011).Janus JR, Laborde RR, Greenberg AJ, Wang VW, Wei W, Trier A, Olsen SM, Moore EJ, Olsen KD, Smith DI. Linking expression of FOXM1, CEP55 and HELLS to tumorigenesis in oropharyngeal squamous cell carcinoma. Laryngoscope. 2011;121:2598–2603. doi: 10.1002/lary.22379. [DOI] [PubMed] [Google Scholar]
- Jia et al. (2016).Jia Q, Zhou W, Yao W, Yang F, Zhang S, Singh R, Chen J, Chen JJ, Zhang Y, Wei F, Zhang Y, Jia H, Wang N. Downregulation of YAP-dependent Nupr1 promotes tumor-repopulating cell growth in soft matrices. Oncogenesis. 2016;5:e220. doi: 10.1038/oncsis.2016.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir et al. (2017).Kabir MF, Mohd Ali J, Abolmaesoomi M, Hashim OH. Melicope ptelefolia leaf extracts exhibit antioxidant activity and exert anti-proliferative effect with apoptosis induction on four different cancer cell lines. BMC Complementary and Alternative Medicine. 2017;17:252. doi: 10.1186/s12906-017-1761-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly et al. (2011).Kelly RJ, Lopez-Chavez A, Citrin D, Janik JE, Morris JC. Impacting tumor cell-fate by targeting the inhibitor of apoptosis protein survivin. Molecular Cancer. 2011;10 doi: 10.1186/1476-4598-10-35. Article 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim & Sharpless (2006).Kim WY, Sharpless NE. The regulation of INK4/ARF in cancer and aging. Cell. 2006;127:265–275. doi: 10.1016/j.cell.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Leroy et al. (2014).Leroy B, Girard L, Hollestelle A, Minna JD, Gazdar AF, Soussi T. Analysis of TP53 mutation status in human cancer cell lines: a reassessment. Human Mutation. 2014;35:756–765. doi: 10.1002/humu.22556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2016a).Li W-M, Huang C-N, Ke H-L, Li C-C, Wei Y-C, Yeh H-C, Chang L-L, Huang C-H, Liang P-I, Yeh B-W, Chan T-C, Li C-F, Wu W-J. MCM10 overexpression implicates adverse prognosis in urothelial carcinoma. Oncotarget. 2016a;7:77777–77792. doi: 10.18632/oncotarget.12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2016b).Li X, Song Y, Zhang P, Zhu H, Chen L, Xiao Y, Xing Y. Oleanolic acid inhibits cell survival and proliferation of prostate cancer cells in vitro and in vivo through the PI3K/Akt pathway. Tumor Biology. 2016b;37(6):7599–7613. doi: 10.1007/s13277-015-4655-9. [DOI] [PubMed] [Google Scholar]
- Livak & Schmittgen (2001).Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lui et al. (2013).Lui K, An J, Montalbano J, Shi J, Corcoran C, He Q, Sun H, Sheikh MS, Huang Y. Negative regulation of p53 by Ras superfamily protein RBEL1A. Journal of Cell Science. 2013;126:2436–2445. doi: 10.1242/jcs.118117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjelle et al. (2015).Mjelle R, Hegre SA, Aas PA, Slupphaug G, Drablos F, Saetrom P, Krokan HE. Cell cycle regulation of human DNA repair and chromatin remodeling genes. DNA Repair. 2015;30:53–67. doi: 10.1016/j.dnarep.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Molnar et al. (2013).Molnar J, Ocsovszki I, Puskas L, Ghane T, Hohmann J, Zupko I. Investigation of the antiproliferative action of the quinoline alkaloids kokusaginine and skimmianine on human cell lines. Current Signal Transduction Therapy. 2013;8:148–155. doi: 10.2174/15743624113086660006. [DOI] [Google Scholar]
- O’Connor (2015).O’Connor MJ. Targeting the DNA damage response in cancer. Molecular Cell. 2015;60:547–560. doi: 10.1016/j.molcel.2015.10.040. [DOI] [PubMed] [Google Scholar]
- Ouyang et al. (2017).Ouyang F, Liu J, Xia M, Lin C, Wu X, Ye L, Song L, Li J, Wang J, Guo P, He M. GINS2 is a novel prognostic biomarker and promotes tumor progression in early-stage cervical cancer. Oncology Reports. 2017;37:2652–2662. doi: 10.3892/or.2017.5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Herrero & Fernández-Medarde (2015).Pérez-Herrero E, Fernández-Medarde A. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. European Journal of Pharmaceutics and Biopharmaceutics. 2015;93:52–79. doi: 10.1016/j.ejpb.2015.03.018. [DOI] [PubMed] [Google Scholar]
- Pistritto et al. (2016).Pistritto G, Trisciuoglio D, Ceci C, Garufi A, D’Orazi G. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging. 2016;8:603–619. doi: 10.18632/aging.100934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast & Jaffee (2013).Prendergast GC, Jaffee EM. Cancer immunotherapy: immune suppression and tumor growth. Academic Press/Elsevier; Amsterdam: 2013. [Google Scholar]
- Rauth et al. (2016).Rauth S, Ray S, Bhattacharyya S, Mehrotra DG, Alam N, Mondal G, Nath P, Roy A, Biswas J, Murmu N. Lupeol evokes anticancer effects in oral squamous cell carcinoma by inhibiting oncogenic EGFR pathway. Molecular and Cellular Biochemistry. 2016;417(1–2):97–110. doi: 10.1007/s11010-016-2717-y. [DOI] [PubMed] [Google Scholar]
- Razavi et al. (2015).Razavi SM, Jafari M, Heidarpoor M, Khalesi S. Minichromosome maintenance-2 (MCM2) expression differentiates oral squamous cell carcinoma from pre-cancerous lesions. Malaysian Journal of Pathology. 2015;37:253–258. [PubMed] [Google Scholar]
- Ren et al. (2015).Ren W-H, Li Y-W, Li R, Feng H-B, Wu J-L, Wang H-R. P15 gene methylation in hepatocellular carcinomas: a systematic review and meta-analysis. International Journal of Clinical and Experimental Medicine. 2015;8:4762–4768. [PMC free article] [PubMed] [Google Scholar]
- Rosemary Siafakas & Richardson (2009).Rosemary Siafakas A, Richardson DR. Growth arrest and DNA damage-45 alpha (GADD45alpha) International Journal of Biochemistry and Cell Biology. 2009;41:986–989. doi: 10.1016/j.biocel.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Ruiz-Laguna et al. (2016).Ruiz-Laguna J, Velez JM, Pueyo C, Abril N. Global gene expression profiling using heterologous DNA microarrays to analyze alterations in the transcriptome of Mus spretus mice living in a heavily polluted environment. Environmental Science and Pollution Research International. 2016;23:5853–5867. doi: 10.1007/s11356-015-5824-5. [DOI] [PubMed] [Google Scholar]
- Salvador, Brown-Clay & Fornace Jr (2013).Salvador JM, Brown-Clay JD, Fornace Jr AJ. Gadd45 in stress signaling, cell cycle control, and apoptosis. Advances in Experimental Medicine and Biology. 2013;793:1–19. doi: 10.1007/978-1-4614-8289-5_1. [DOI] [PubMed] [Google Scholar]
- Santo, Siu & Raje (2015).Santo L, Siu KT, Raje N. Targeting cyclin-dependent kinases and cell cycle progression in human cancers. Seminars in Oncology. 2015;42:788–800. doi: 10.1053/j.seminoncol.2015.09.024. [DOI] [PubMed] [Google Scholar]
- Schmucker & Sumara (2014).Schmucker S, Sumara I. Molecular dynamics of PLK1 during mitosis. Molecular & Cellular Oncology. 2014;1:e954507. doi: 10.1080/23723548.2014.954507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwermer et al. (2017).Schwermer M, Dreesmann S, Eggert A, Althoff K, Steenpass L, Schramm A, Schulte JH, Temming P. Pharmaceutically inhibiting polo-like kinase 1 exerts a broad anti-tumour activity in retinoblastoma cell lines. Clinical & Experimental Ophthalmology. 2017;45:288–296. doi: 10.1111/ceo.12838. [DOI] [PubMed] [Google Scholar]
- Shaari et al. (2011).Shaari K, Zareen S, Akhtar MN, Lajis NH. Chemical constituents of melicope ptelefolia. Natural Product Communications. 2011;6:343–348. [PubMed] [Google Scholar]
- Simon & Schwacha (2014).Simon NE, Schwacha A. The Mcm2-7 replicative helicase: a promising chemotherapeutic target. BioMed Research International. 2014;2014 doi: 10.1155/2014/549719. Article 549719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh et al. (2016).Singh S, Sharma B, Kanwar SS, Kumar A. Lead phytochemicals for anticancer drug development. Frontiers in Plant Science. 2016;7 doi: 10.3389/fpls.2016.01667. Article 1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strebhardt (2010).Strebhardt K. Multifaceted polo-like kinases: drug targets and antitargets for cancer therapy. Nature Reviews Drug Discovery. 2010;9:643–660. doi: 10.1038/nrd3184. [DOI] [PubMed] [Google Scholar]
- Tang et al. (2016).Tang H, Ji F, Sun J, Xie Y, Xu Y, Yue H. RBEL1 is required for osteosarcoma cell proliferation via inhibiting retinoblastoma 1. Molecular Medicine Reports. 2016;13:1275–1280. doi: 10.3892/mmr.2015.4670. [DOI] [PubMed] [Google Scholar]
- Tiwari, Brunton & CS (2013).Tiwari BK, Brunton N, CS B. Handbook of plant food phytochemicals: sources, stability and extraction. Wiley-Blackwell; Hoboken: 2013. [Google Scholar]
- Tomicic et al. (2015).Tomicic MT, Meise R, Aasland D, Berte N, Kitzinger R, Kramer OH, Kaina B, Christmann M. Apoptosis induced by temozolomide and nimustine in glioblastoma cells is supported by JNK/c-Jun-mediated induction of the BH3-only protein BIM. Oncotarget. 2015;6:33755–33768. doi: 10.18632/oncotarget.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlen et al. (2010).Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, Zwahlen M, Kampf C, Wester K, Hober S, Wernerus H, Bjorling L, Ponten F. Towards a knowledge-based Human Protein Atlas. Nature Biotechnology. 2010;28:1248–1250. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- Von Eyss et al. (2012).Von Eyss B, Maaskola J, Memczak S, Mollmann K, Schuetz A, Loddenkemper C, Tanh MD, Otto A, Muegge K, Heinemann U, Rajewsky N, Ziebold U. The SNF2-like helicase HELLS mediates E2F3-dependent transcription and cellular transformation. EMBO Journal. 2012;31:972–985. doi: 10.1038/emboj.2011.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2012).Wang Z, Fukushima H, Inuzuka H, Wan L, Liu P, Gao D, Sarkar FH, Wei W. Skp2 is a promising therapeutic target in breast cancer. Frontiers in Oncology. 2012;1 doi: 10.3389/fonc.2011.00057. Article 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolgemuth (2008).Wolgemuth DJ. Function of cyclins in regulating the mitotic and meiotic cell cycles in male germ cells. Cell Cycle. 2008;7:3509–3513. doi: 10.4161/cc.7.22.6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong (2011).Wong RS. Apoptosis in cancer: from pathogenesis to treatment. Journal of Experimental & Clinical Cancer Research. 2011;30 doi: 10.1186/1756-9966-30-87. Article 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Zhu & Zhang (2012).Wu C, Zhu J, Zhang X. Integrating gene expression and protein-protein interaction network to prioritize cancer-associated genes. BMC Bioinformatics. 2012;13:182. doi: 10.1186/1471-2105-13-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. (2017).Xu L, Zhu Y, Shao J, Chen M, Yan H, Li G, Zhu Y, Xu Z, Yang B, Luo P, He Q. Dasatinib synergises with irinotecan to suppress hepatocellular carcinoma via inhibiting the protein synthesis of PLK1. British Journal of Cancer. 2017;116:1027–1036. doi: 10.1038/bjc.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al. (2013).Yang CW, Lee YZ, Hsu HY, Wu CM, Chang HY, Chao YS, Lee SJ. c-Jun-mediated anticancer mechanisms of tylophorine. Carcinogenesis. 2013;34:1304–1314. doi: 10.1093/carcin/bgt039. [DOI] [PubMed] [Google Scholar]
- Yu & Zhang (2008).Yu J, Zhang L. PUMA, a potent killer with or without p53. Oncogene. 2008;27(Suppl 1):S71–S83. doi: 10.1038/onc.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan (2005).Zhan Q. Gadd45a, a p53- and BRCA1-regulated stress protein, in cellular response to DNA damage. Mutation Research/DNA Repair. 2005;569:133–143. doi: 10.1016/j.mrfmmm.2004.06.055. [DOI] [PubMed] [Google Scholar]
- Zheng et al. (2014).Zheng M, Zhou Y, Yang X, Tang J, Wei D, Zhang Y, Jiang JL, Chen ZN, Zhu P. High GINS2 transcript level predicts poor prognosis and correlates with high histological grade and endocrine therapy resistance through mammary cancer stem cells in breast cancer patients. Breast Cancer Research and Treatment. 2014;148:423–436. doi: 10.1007/s10549-014-3172-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The bar chart shows gene expression fold changes (FC) obtained from microarray and real-time PCR assays. The data for each assay was derived from three biological replicates. For each qPCR assay, the data was normalized to RPS29 expression and the value represent mean qPCR_FC ±SD (n = 3).
The figure shows component genes of RIC-WP that were differentially regulated by MP-HX in HCT116 cells. The genes in the pathway are colored red (MA_FC ≥ + 2.0), or green (MA_FC ≥ − 2.0), or depicted as grey hashed boxes (MA_FC < ±2).
The figure shows component genes of RIC-WP that were differentially regulated by MP-HX in HepG2 cells. The genes in the pathway are colored red (MA_FC ≥ + 2.0), or green (MA_FC ≥ − 2.0), or depicted as grey hashed boxes (MA_FC < ±2).
The figure shows component genes in G1SCC-WP that were differentially regulated by MP-HX treatment in HCT116 cells. The genes in the pathway are colored red (MA_FC ≥ + 2.0), or green (MA_FC ≥ − 2.0), or depicted as grey hashed boxes (MA_FC < ±2).
The figure shows component genes of G1SCC-WP that were differentially regulated by MP-HX in HepG2 cells.The genes in the pathway are colored red (MA_FC ≥ + 2.0), or green (MA_FC ≥ − 2.0), or depicted as grey hashed boxes (MA_FC < ±2).
The figure shows component genes of CC-WP that were differentially regulated by MP-HX in HCT116 cells. The genes in the pathway are colored red (MA_FC ≥ + 2.0), or green (MA_FC ≥ − 2.0), or depicted as grey hashed boxes (MA_FC < ±2).
The figure shows component genes of CC-WP that were differentially regulated by MP-HX in HepG2 cells. The genes in the pathway are colored red (MA_FC ≥ + 2.0), or green (MA_FC ≥ − 2.0), or depicted as grey hashed boxes (MA_FC < ±2).
The figure shows component genes in DDR-WP that were differentially regulated by MP-HX in HCT116 cells. The genes in the pathway are colored red (MA_FC ≥ + 2.0), or green (MA_FC ≥ − 2.0), or depicted as grey hashed boxes (MA_FC < ±2).
The figure shows component genes in DDR-WP that were differentially regulated by MP-HX in HepG2 cells. The genes in the pathway are colored red (MA_FC ≥ + 2.0), or green (MA_FC ≥ − 2.0), or depicted as grey hashed boxes (MA_FC < ±2).
The figure shows component genes in AP-WP that were differentially regulated by MP-HX in HCT116 cells. The genes in the pathway are colored red (MA_FC ≥ + 2.0), or green (MA_FC ≥ − 2.0), or depicted as grey hashed boxes (MA_FC < ±2).
The figure shows component genes in AP-WP that were differentially regulated (MA_FC ≥ ±2.00) by MP-HX in HepG2 cells. The genes in the pathway are colored red (MA_FC ≥ + 2.0), or green (MA_FC ≥ − 2.0), or grey (MA_FC < ±2).
The figure shows top category of diseases and biological functions that were modulated by MP-HX (FC ≥ ±2.0) in HCT116 cells, and they were ranked by IPA software based on -log (p-value) ≥5.0.
The figure shows top category of diseases and biological functions that were modulated by MP-HX (FC ≥ ±2.0) in HepG2 cells and they were ranked by IPA software based on -log (p-value) ≥5.0.
Data Availability Statement
The following information was supplied regarding data availability:
Raw data has been uploaded into Gene Expression Omnibus (GEO) repository with the accession number given as GSE114743.