Abstract
A robust and selective late-stage deuteration methodology was applied to 13C-enriched amino and alpha hydroxy acids to increase spin-lattice relaxation constant T1 for hyperpolarized 13C magnetic resonance imaging. For the five substrates with 13C-labeling on the C1-position ([1-13C]alanine, [1-13C]serine, [1-13C]lactate, [1-13C]glycine, and [1-13C]valine), significant increase of their T1 was observed at 3T with deuterium labeling (+26%, 22%, +16%, +25% and +29%, respectively). Remarkably, in the case of [2-13C]alanine, [2-13C]serine and [2-13C]lactate, deuterium labeling led to a greater than four fold increase in T1. [1-13C,2-2H]alanine, produced using this method, was applied to in vitro enzyme assays with alanine aminotransferase, demonstrating a kinetic isotope effect.
Table of Contents Entry
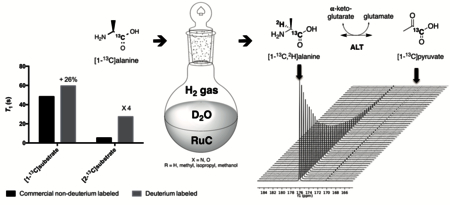
Magnetic resonance imaging employing hyperpolarized substrates (HP MRI) has recently emerged as a powerful tool for studying metabolism in cells, animal models and patients.1–9 Polarization of substrates can be realized through a variety of mechanisms including dissolution dynamic nuclear polarization (DNP),10 parahydrogen induced polarization (PHIP),11,12 or signal amplification by reversible exchange (SABRE).13 While these are versatile methods that allow for real time imaging of metabolism, the short lifetime of the hyperpolarized signal, which decays exponentially based upon the spin lattice relaxation time T1, remains one of the key limiting factors in the implementation of this technology. The most widely used HP 13C probe is [1-13C]pyruvate, a key metabolic intermediate, which has a T1 of 67 s at 3T. However, other 13C nuclei, especially those with directly attached protons, are not feasible HP 13C probes due to very short T1’s (less than 5s).14 One approach to increase T1 is the substitution of 1H with 2H (or D), a quadrupolar nucleus with a gyromagnetic ratio γ about 6.5-fold smaller than the one for 1H.15–29 This use of deuterated substrates has proved particularly fruitful in the case of SABRE13 and PHIP11,12 methods. This approach is effective when dipolar 13C-1H coupling contributes substantially to T1 relaxation. Fortunately, in the case of pyruvate, the incorporation of deuterium is straightforward owing to lack of stereocenters.30 However, the synthesis of multiply labelled molecules containing stereocenters including both 13C and 2H is generally both expensive and time consuming, and most isotopically enriched molecules require multi-step syntheses. Therefore, a robust method for incorporation of deuterium in the final step of synthesis would be generally valuable in the field of HP MRI.
For the synthesis of deuterated molecules, late-stage isotopic exchange has several advantages over a synthetic pathway from enriched building blocks. Numerous methods based on homogeneous or heterogeneous catalysts for H/D exchange have already been described, but the development of a deuteration methodology with mild reaction conditions, high selectivity and deuterium incorporation is still a challenge.31,32 In order to develop [13C,2H]labelled probes for HP MRI, we considered the regioselective deuteration, at the α-position of aliphatic alcohols and sugars, developed by Sajiki et al., as a straightforward way to the deuterium labelling of 13C-substrates with attached O or N.33,34 In this manuscript, we report the application of this methodology to a variety of 13C-enriched compounds, enabling high incorporation yields with retention of configuration, and demonstrate a significant increase in T1 of the resulting deuterated substrates. One of the probes, [1-13C,2-2H]alanine, was studied in an in vitro enzymatic assay with alanine aminotransferase (ALT), revealing a deuterium kinetic isotope effect.
Initially, we evaluated the performance of the labelling methodology with a variety of labelled substrates including α-amino and hydroxy acids. We performed the one-step deuterium labelling reaction on position C2 of several commercial 13C-labeled substrates (Scheme 1, Table 1). Reactions were incubated in D2O in the presence of RuC 5% (40 wt%), under H2, overnight, at 80°C (Table S1, ESI†). Efficient deuterium incorporation on position C2 (95–97 %) was observed for aliphatic amino acids [1-13C,2-2H] and [2-13C,2-2H]alanine (1 and 6), [1-13C,2-2H2]glycine 4 and [1-13C,2-2H]valine 5, with enantiomeric excesses greater than 99%. Isotopic enrichments on position C2 of [1-13C,2-2H] and [2-13C,2-2H]sodium lactate (3 and 8) were 97% and 98%, respectively, with lower enantiomeric excesses (86 and 94%). Moderate chemical yield on [1-13C,2-2H] valine 5, 53%, may be due to its lower solubility in D2O. Enantiomeric excess was 98% for both [1-13C,2-2H] and [2-13C,2-2H]serine (2 and 7) whereas chemical yields were 78% and 77%, respectively. Their lower isotopic enrichments on position C2 (52 and 90%) may be due to the additional deuterium labelling on their position C3. In a few cases, side reactions were encountered which led to decomposition of the desired product (ESI†). Taken together with prior reports,27,35 our data indicate that this is a versatile method for deuterium incorporation in biologically relevant molecules.
Scheme 1:
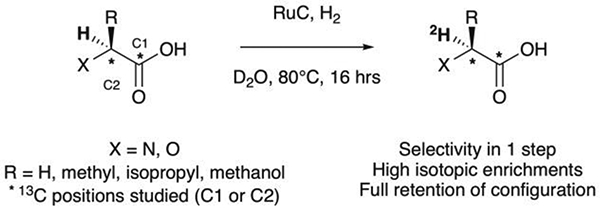
Regioselective catalytic deuterium labelling via 1H/2H exchange using ruthenium on carbon (RuC).
Table 1:
Structures of 13C-enriched molecules after deuterium enrichment. The bracketed number indicates the isotopic enrichment determined by 1H,13C NMR and HRMS (analyses described in the ESI†). ee: enantiomeric excess.
| Molecule | Structure | Chemical yield | ee |
|---|---|---|---|
|
13C on position C1 and 2H on position C2 | |||
| [1-13C,2-2H]alanine 1 | 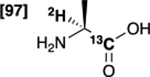 |
99 % | 99 % |
| [1-13C,2,3-2H3]serine 2 | 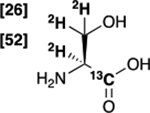 |
78 % | 98 % |
| [1-13C,2-2H]lactate 3 | 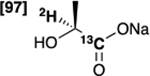 |
98 % | 86 % |
| [1-13C,2-2H2]glycine 4 | 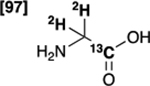 |
79 % | - |
| [1-13C,2-2H]valine 5 | 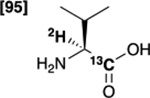 |
53 % | 99 % |
|
13C on position C2 and 2H on position C2 | |||
| [2-13C,2-2H]alanine 6 | 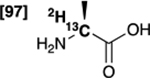 |
89 % | 99 % |
| [2-13C,2,3-2H3]serine 7 | 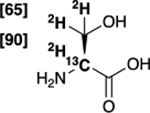 |
77 % | 98 % |
| [2-13C,2-2H]lactate 8 | 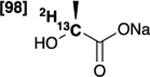 |
99 % | 94 % |
In order to determine the impact of deuterium incorporation on T1, we then prepared the labelled substrates for hyperpolarization. Solutions of 4 to 6 M substrate with 1 to 1.2 equivalents NaOH and 23 to 24 mM free radical (OX063) were prepared for hyperpolarization using DNP.36 Following polarization, T1 measurements were performed on a 3T preclinical MR scanner. Deuterium substitution at the C2 position yielded significant improvements of the T1 with 13C at the C1 position, ranging from 16–29% (Figure 1). The relatively modest improvement in T1 yielded larger signal gains at later time points. For example, in the case of [1-13C]alanine, deuteration yielded an increase in signal to noise ratio of 60% at 90s after the start of the experiment (Figure S68c, ESI†). Remarkably, in the case of [2-13C,2-2H]alanine 6, [2-13C,2,3-2H]serine 7 and [2-13C,2-2H]lactate 8, deuterium labelling led to a greater than four-fold increase in T1. Due to rapid signal decay on [2-13C]alanine, [2-13C]serine and [2-13C]lactate, their T1 could not be measured using a hyperpolarized method36 and were instead assayed using an inversion recovery-sequence. Part of the reason why the T1 gains due to deuteration are relatively limited is because of chemical shift anisotropy (CSA) which is likely the dominant relaxation mechanism at 3T.37,38 Therefore, at 1.5T, there could be further improvements in T1 prolongation.39
Figure 1:
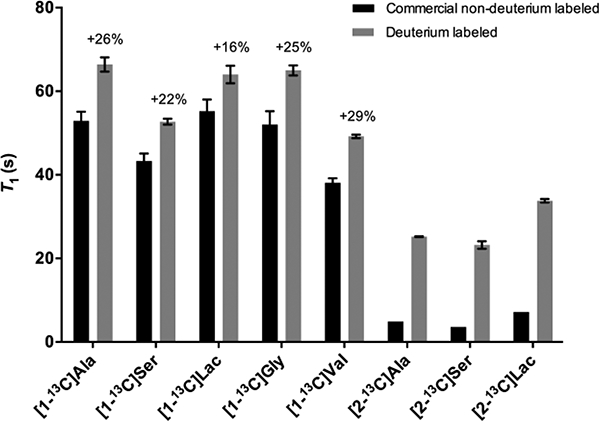
T1 relaxation times at 3T for proton and deuterium-labelled 13C-substrates (n = 3, ± s.d., p < 0.02). Due to very low polarization for commercial non-deuterium labeled [2–13C]alanine, [2–13C]serine and [2–13C]lactate, T1 could not be evaluated using hyperpolarized methods, and inversion-recovery was used at 11.7T: T1 = 4.9 s, 3.6 s and 7.2 s, respectively.
We then evaluated the T1 of one of our substrates, [1-13C,2-2H]alanine 1, in an in vivo experiment in a mouse model and compared its properties with those of [1-13C]alanine. MR measurements where performed on a preclinical 3T scanner (Figure S71, ESI†). 300 μL of 80 mM solutions of hyperpolarized [1-13C]alanine and [1-13C,2-2H]alanine 1 were injected intravenously immediately followed by dynamic acquisition of 13C MRS spectra. As expected, based on the in vitro studies, we found an increase in the apparent in vivo T1 at 3T, from 32 s, for [1-13C]alanine, to 42 s, for [1-13C,2-2H]alanine 1.
As a demonstration of the utility of the deuteration method, we next applied the labelled alanine probes in an in vitro enzyme assay using alanine transaminase (ALT). ALT is an abundant enzyme and a biomarker for liver disease, which converts alanine and α-ketoglutarate to pyruvate and glutamate, respectively (Figure 2a). Previous reports have studied this enzyme both in vitro and in vivo using hyperpolarized 13C methods.36,40,41 Therefore, we developed an assay for the detection of 13C pyruvate production by incubation of polarized [1-13C]alanine or [1-13C,2-2H]alanine 1 with α-ketoglutarate, glutamate and ALT based on prior reports.42 As expected, 13C pyruvate was rapidly formed during the time course of the hyperpolarized experiment (Figures 2b-d). Furthermore, the initial rate of pyruvate signal growth, which approximates the forward conversion rate, was about 2.42-fold lower for the [1-13C,2-2H]alanine 1 as compared with the [1-13C]alanine (n = 3 each, p < 0.002, neutral pH). This agrees closely with the previously reported kinetic isotope effect of 2.3.42 In order to confirm these findings, we fit the dynamic alanine and pyruvate MRS data to a kinetic model accounting for HP signal exchange between protonated and deuterated [1-13C]alanine and [1-13C]pyruvate pools as well as signal loss due to RF sampling and T1 loss (Fig. S72, ESI†).43 We thus obtained pseudo-first order rate constants of (1.87 ± 0.174) x 10−3 s−1 (n = 3) and (0.736 ± 0.015) × 10−3 s−1 (n = 3) for protonated and deuterated alanine, respectively. This difference in kinetic rates suggested a kinetic isotope effect of 2.53, in close agreement with our previous analysis and with the literature.42
Figure 2:
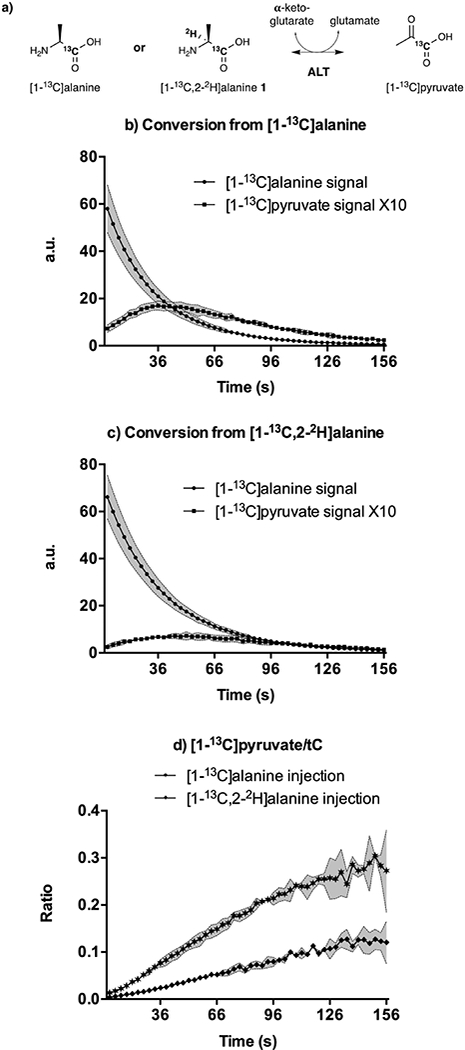
Influence of deuterium labelling on [1–13C]pyruvate formation after conversion from [1–13C]alanine and from [1–13C,2–2H]alanine 1 in solution in the presence of ALT enzyme. a) Metabolic pathways of hyperpolarized [1–13C]alanine and [1–13C,2–2H]alanine 1 via ALT. b) and c) Time courses of integrated spectra showing the evolution of HP [1–13C]alanine,[1–13C,2–2H]alanine 1 and their metabolite [1–13C]pyruvate (normalized peak integrations) (n = 3, ± s.d.). For clarity, pyruvate integrals were ten-fold upscaled. Shaded areas denote the experimental error bars. Spectral acquisition started 11s (b) and 10 s (c) after incubation of the HP probe and the enzyme solution in an NMR tube. d) Measurements of [1–13C]pyruvate/total 13C-labeled signals (tC) ratios (n = 3, ± s.d.).
In summary, these data indicate that the RuC labelling method represents a versatile method for high-yield deuteration of 13C labelled substrates, ideal for application to hyperpolarized 13C MRI. When the deuterium was incorporated adjacent to a 13C-enriched carbonyl, the effect on T1 prolongation was moderate, ranging from 16–29%. In contrast, when applied to 13C nuclei with directly attached protons ([2-13C,2-2H]alanine 6, [2-13C,2,3-2H]serine 7 and [2-13C,2-2H]lactate 8), an approximately 4-fold increase in T1 was observed. To further study the behavior of doubly-enriched substrates, we applied [1-13C]alanine and [1-13C,2-2H]alanine 1 to an in vitro enzyme assay with purified ALT enzyme, demonstrating a kinetic isotope effect, in agreement with prior reports. We anticipate that this versatile method will find application to a variety of substrates for hyperpolarized 13C MRI.
Supplementary Material
Footnotes
Conflicts of interest
There are no conflicts to declare. C.T. acknowledges support from US DOD Prostate Cancer Research Program-Early Investigator Research Award (PC161000). R.R.F. acknowledges support from US DOD Physician Research Training Grant (PC150932) and Prostate Cancer Foundation Young Investigator Award. D.B.V. and S.M.R. acknowledge support from NIH grants P41EB013598, R01CA172845 and R01CA197254.
Electronic Supplementary Information (ESI) available: Reagents and procedures for deuteration reaction, deuterium incorporation quantification, characterization for compounds 1 to 8, experimental details for T1 measurements in solution, in vivo and in vitro enzyme experiments. See DOI: 10.1039/x0xx00000x
Notes and references
‡ C.T. carried out the experiments and wrote the manuscript with support from D.E.K. R.R.F. and D.M.W. designed and directed the project. H.V.B, D.B.V., S.M.R., R.S. and J.K. helped supervise the project and helped edit the manuscript. C.V.M., J.Y., S.W. J.E.B., S.S. and R.B. helped with characterization of obtained compounds, T1 measurements and in vivo experiments. C.N. and S.M.R. provided critical feedback and helped shape the research, notably for the study of [2-13C,2-2H]enriched substrates.
- 1.Goodson BM, J. Magn. Reson, 2002, 155, 157–216. [DOI] [PubMed] [Google Scholar]
- 2.Zacharias NM, Chan HR, Sailasuta N, Ross BD and Bhattacharya P, J. Am. Chem. Soc, 2012, 134, 934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chekmenev EY, Hövener J, Norton VA, Harris K, Batchelder LS, Bhattacharya P, Ross BD and Weitekamp DP, J. Am. Chem. Soc, 2008, 130, 4212–4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PEZ, Harzstark AL, Ferrone M, Van Criekinge M, Chang JW, Bok R, Park I, Reed G, Carvajal L, Small EJ, Munster P, Weinberg VK, Ardenkjaer-larsen JH, Chen AP, Hurd RE, Odegardstuen L, Robb FJ, Tropp J and Murray JA, Sci. Transl. Med, 2013, 5, 198ra108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golman K, Ardenkjaer-Larsen JH, Petersson JS, Mansson S and Leunbach I, Proc. Natl. Acad. Sci, 2003, 100, 10435–10439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golman K, in ‘t Zandt R and Thaning M, Proc. Natl. Acad. Sci, 2006, 103, 11270–11275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albers MJ, Bok R, Chen AP, Cunningham CH, Zierhut ML, Zhang VY, Kohler SJ, Tropp J, Hurd RE, Yen YF, Nelson SJ, Vigneron DB and Kurhanewicz J, Cancer Res, 2008, 68, 8607–8615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dafni H, Larson PEZ, Hu S, Yoshihara HAI, Ward CS, Venkatesh HS, Wang C, Zhang X, Vigneron DB and Ronen SM, Cancer Res, 2010, 70, 7400–7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Day SE, Kettunen MI, Gallagher FA, Hu D-E, Lerche M, Wolber J, Golman K, Ardenkjaer-Larsen JH and Brindle KM, Nat. Med, 2007, 13, 1382–1387. [DOI] [PubMed] [Google Scholar]
- 10.Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M and Golman K, Proc. Natl. Acad. Sci, 2003, 100, 10158–10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhattacharya P, Chekmenev EY, Reynolds WF, Wagner S, Zacharias N, Chan HR, Bünger R and Ross BD, NMR Biomed, 2011, 24, 1023–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coffey AM, Shchepin RV, Truong ML, Wilkens K, Pham W and Chekmenev EY, Anal. Chem, 2016, 88, 8279–8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rayner PJ, Burns MJ, Olaru AM, Norcott P, Fekete M, Green GGR, Highton LAR, Mewis RE and Duckett SB, Proc. Natl. Acad. Sci. U. S. A, 2017, 114, E3188–E3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keshari KR and Wilson DM, Chem. Soc. Rev, 2014, 43, 1627–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hundshammer C, Düwel S, Köcher SS, Gersch M, Feuerecker B, Scheurer C, Haase A, Glaser SJ, Schwaiger M and Schilling F, ChemPhysChem, 2017, 18, 2421. [DOI] [PubMed] [Google Scholar]
- 16.Allouche-Arnon H, Gamliel A, Barzilay CM, Nalbandian R, Gomori JM, Karlsson M, Lerche MH and Katz-Brull R, Contrast Media Mol. Imaging, 2011, 6, 139–147. [DOI] [PubMed] [Google Scholar]
- 17.Meier S, Karlsson M, Jensen PR, Lerche MH and Duus JØ, Mol. Biosyst, 2011, 7, 2834. [DOI] [PubMed] [Google Scholar]
- 18.Korenchan DE, Taglang C, von Morze C, Blecha JE, Gordon JW, Sriram R, Larson PEZ, Vigneron DB, VanBrocklin HF, Kurhanewicz J, Wilson DM and Flavell RR, Analyst, 2017, 142, 1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson DM, Hurd RE, Keshari K, Van Criekinge M, Chen AP, Nelson SJ, Vigneron DB and Kurhanewicz J, Proc Natl Acad Sci USA, 2009, 106, 5503–5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grant AK, Vinogradov E, Wang X, Lenkinski RE and Alsop DC, Magn. Reson. Med, 2011, 66, 746–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allouche-Arnon H, Wade T, Waldner LF, Miller VN, Gomori JM, Katz-Brull R and McKenzie CA, Contrast Media Mol. Imaging, 2013, 8, 72–82. [DOI] [PubMed] [Google Scholar]
- 22.Barb AW, Hekmatyar SK, Glushka JN and Prestegard JH, J Magn Reson, 2013, 228, 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doura T, Hata R, Nonaka H, Ichikawa K and Sando S, Angew. Chemie - Int. Ed, 2012, 51, 10114–10117. [DOI] [PubMed] [Google Scholar]
- 24.Laustsen C, Pileio G, Tayler MCD, Brown LJ, Brown RCD, Levitt MH and Ardenkjaer-Larsen JH, Magn. Reson. Med, 2012, 68, 1262–1265. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy BWC, Kettunen MI, Hu DE and Brindle KM, J. Am. Chem. Soc, 2012, 134, 4969–4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Timm KN, Hartl J, Keller MA, Hu DE, Kettunen MI, Rodrigues TB, Ralser M and Brindle KM, Magn. Reson. Med, 2015, 74, 1543–1547. [DOI] [PubMed] [Google Scholar]
- 27.Mishkovsky M, Anderson B, Karlsson M, Lerche MH, Sherry AD, Gruetter R, Kovacs Z and Comment A, Sci. Rep, 2017, 7, 11719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nonaka H, Hata R, Doura T, Nishihara T, Kumagai K, Akakabe M, Tsuda M, Ichikawa K and Sando S, Nat. Commun, 2013, 4, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nonaka H, Hirano M, Imakura Y, Takakusagi Y, Ichikawa K and Sando S, Sci. Rep, 2017, 7, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shchepin RV, Coffey AM, Waddell KW and Chekmenev EY, Anal. Chem, 2014, 86, 5601–5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atzrodt J, Derdau V, Fey T and Zimmermann J, Angew. Chem. Int. Ed. Engl, 2007, 46, 7744–7765. [DOI] [PubMed] [Google Scholar]
- 32.Taglang C, Martínez-Prieto LM, Del Rosal I, Maron L, Poteau R, Philippot K, Chaudret B, Perato S, Sam Lone A, Puente C, Dugave C, Rousseau B and Pieters G, Angew. Chemie - Int. Ed, 2015, 54, 10474–10477. [DOI] [PubMed] [Google Scholar]
- 33.Maegawa T, Fujiwara Y, Inagaki Y, Monguchi Y and Sajiki H, Adv. Synth. Catal, 2008, 350, 2215–2218. [Google Scholar]
- 34.Sawama Y, Monguchi Y and Sajiki H, Synlett, 2012, 23, 959–972. [Google Scholar]
- 35.Michelotti A, Rodrigues F and Roche M, Org. Process Res. Dev, 2017, 21, 1741–1744. [Google Scholar]
- 36.Hu S, Zhu M, Yoshihara HAI, Wilson DM, Keshari KR, Shin P, Reed G, von Morze C, Bok R, Larson PEZ, Kurhanewicz J and Vigneron DB, Magn. Reson. Imaging, 2011, 29, 1035–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blicharski JS, Zeitschrift Fur Naturforsch, 1972, 27A, 1456–1458. [Google Scholar]
- 38.Anet FAL and O’leary DJ, Concepts Magn. Reson, 1992, 4, 35–52. [Google Scholar]
- 39.Wilson DM, Keshari KR, Larson PEZ, Chen AP, Hu S, Van Criekinge M, Bok R, Nelson SJ, MacDonald JM, Vigneron DB and Kurhanewicz J, J. Magn. Reson, 2010, 205, 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barb AW, Hekmatyar SK, Glushka JN and Prestegard JH, J Magn Reson, 2013, 228, 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park JM, Khemtong C, Liu S-C, Hurd RE and Spielman DM, Magn. Reson. Med, 2017, 77, 1741–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cooper AJL, J. Biol. Chem, 1976, 251, 1088–1096. [PubMed] [Google Scholar]
- 43.Chen HY, Larson PEZ, Bok RA, Von Morze C, Sriram R, Santos RD, Santos JD, Gordon JW, Bahrami N, Ferrone M, Kurhanewicz J and Vigneron DB, Cancer Res, 2017, 77, 3207–3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


