Abstract
Lymphatic filariasis (LF) affects 120 million people around the world and another 856 million people are at risk of acquiring the infection. Mass Drug Administration (MDA) spearheaded by the World Health Organization is the only current strategy to control this infection. Recent reports suggest that despite several rounds of MDA, elimination has not been achieved and there is a need for more stringent control strategies for control of LF. An effective prophylactic vaccine combined with MDA has significant potential. Initial trials using a prophylactic trivalent recombinant Brugia malayi heat shock protein 12.6, abundant larval transcript 2 and tetraspanin large extra cellular loop (rBmHAT) vaccine developed in our laboratory conferred only 35% protection in macaques. Therefore, the focus of the present study was to improve the current vaccine formulation to obtain better protection in non-human primates. We made two modifications to the current formulation: (i) the addition of another antigen, thioredoxin peroxide (TPX-2) to make it a tetravalent vaccine (rBmHAXT) and (ii) the inclusion of an adjuvant; AL019 (alum plus glucopyranosyl lipid adjuvant-stable emulsion) that is known to promote a balanced Th1/Th2 response. A double-blinded vaccination trial was performed with 40 macaques that were divided into three treatment groups and one control group (n= 10/group). Vaccinated animals received 4 immunizations at month intervals with 150 μg/ml of rBmHAT plus alum, rBmHAT plus AL019 or rBmHAXT plus AL019. Control animals received AL019 only. All vaccinated macaques developed significant (P≤0.003) titers of antigen-specific IgG antibodies (1:20,000) compared with the controls. One month after the last dose, all macaques were challenged s.c. with 130–180 B. malayi L3s. Our results showed that seven out of 10 (70%) of macaques given the improved rBmHAXT vaccine did not develop the infection compared with AL019 controls, of which seven out of 10 macaques developed the infection. Titers of antigen-specific IgG1 and IgG2 antibodies were significantly (P≤0.01) higher in vaccinated animals and there was an increase in the percentage of IL-4 and IFN-γ secreting antigen-responding memory T cells. These studies demonstrated that the improved formulation (rBmHAXT plus AL019) is a promising vaccine candidate against human lymphatic filariasis.
Keywords: Lymphatic filariasis, Vaccine, Non-human primates, Adjuvant, TLR-4 agonist, Multivalent vaccine
Graphical Abstract
1. Introduction
Lymphatic filariasis (LF) is a chronic tropical filarial parasitic infection caused by Wuchereria bancrofti, Brugia malayi and Brugia timori and is transmitted by mosquitoes. The disease is characterized by severe physical disability and morbidity in infected individuals (Brady and Global Alliance to Eliminate Lymphatic Filariasis, 2014). Significant progress has been made in the last decade to interrupt the transmission of the disease by administering a selected combination of three drugs annually to all the individuals living in an endemic area (mass drug administration, MDA) (Brady and Global Alliance to Eliminate Lymphatic, 2014; Ramaiah and Ottesen, 2014; Bhattacharjee, 2016). Although this MDA approach is highly effective in reducing the transmission of LF infection in most countries, there are several reports of non-compliance by the person being treated, leading to reemergence of the disease in a few parts of the world (Das et al., 2002; Anil, 2012; Lima et al., 2012; Nujum et al., 2012; Krentel et al., 2013; Sunish et al., 2014; Bhattacharjee, 2016; NVBDCP., 2016; WHO, 2016; Dyson et al., 2017). These findings brought to light the critical need for a more sustainable approach such as a prophylactic vaccine together with MDA to interrupt the transmission and control of LF infection in endemic areas (Ramaswamy 2016). Our laboratory and others have identified and characterized several potential candidate vaccine antigens of LF and evaluated their vaccine potential in rodent models (Denham, 1980; Dissanayake et al., 1995; Gregory et al., 1997; Anand et al., 2008, 2011; Gnanasekar et al., 2008; Vedi et al., 2008; Veerapathran et al., 2009; Kalyanasundaram and Balumuri, 2011; Babayan et al., 2012; Dakshinamoorthy et al., 2012; Anugraha et al., 2013; Dakshinamoorthy et al., 2013a; Gomase et al., 2013; Arumugam et al., 2014; Gupta et al., 2016). Among the various antigens that we characterized, four antigens, abundant larval transcript-2 (ALT-2) (Anand et al., 2008; Kalyanasundaram and Balumuri, 2011; Madhumathi et al., 2017), heat shock protein (HSP) 12.6 (Dakshinamoorthy et al., 2012), thioredoxin peroxidase-2 (TPX-2) (Anand et al., 2008; Anugraha et al., 2013) and tetraspanin large extracellular loop (TSP-LEL) (Gnanasekar et al., 2008; Dakshinamoorthy et al., 2013a) gave excellent protection in rodent models. Subsequently, we showed that combining three of these antigens as a multivalent fusion protein, rBmHAT (recombinant B. malayi HSP12.6, ALT-2 and TSP-LEL) gave close to sterile immunity in mouse and jird models (Dakshinamoorthy and Kalyanasundaram, 2013; Dakshinamoorthy et al., 2013a). Based on these promising results in rodents, we performed a vaccination trial in rhesus macaques with rBmHAT and alum adjuvant (Dakshinamoorthy et al., 2014). Unfortunately, however, we only obtained approximately 35% protection against challenge infections in macaques and the immune response elicited was predominantly IgG1/IL-10 driven due to the alum adjuvant. Subsequent vaccination trials with AL019 in a mouse model showed that AL019 (alum plus GLA, a synthetic TLR4 agonist) is a better adjuvant for rBmHAT than alum (Dakshinamoorthy and Kalyanasundaram, 2013; Chauhan et al., 2017). Protective responses in humans and rodents correlated with a balanced Th1/Th2 response and AL019 was shown to promote a balanced Th1/Th2 response (Dakshinamoorthy and Kalyanasundaram, 2013; Dakshinamoorthy et al., 2013a). Therefore, we decided to evaluate the potential of AL019 as an adjuvant, for vaccination in rhesus macaque in this study. In an attempt to improve the vaccine antigen formulation, we included TPX2 as the fourth antigen to the trivalent rBmHAT to make a tetravalent (rBmHAXT) vaccine construct. Thus, the major aim of this study was to evaluate the vaccine potential of rBmHAXT together with AL019 in the rhesus macaque model, to determine whether the improved vaccine formulation gave better protection in rhesus macaques against challenge infections with B. malayi infective larvae and assess the immunological correlates of protection.
2. Materials and methods
2.1. Ethics statement
Use of macaques and the experimental procedures performed in this study were reviewed and approved by the The Institutional Animal Care and Use Committee (IACUC) committee at Bioqual Inc, Rockville, MA, USA and by the University of Illinois College of Medicine at Rockford, USA. Humane use of animals was performed in this study according to the guidelines for the care and use of laboratory animals and with the rules formulated under the Animal Welfare Act by the U.S. Department of Agriculture.
2.2. Non-human primates
Forty male or female disease-free rhesus macaques (3 to 5 years old) were purchased from PrimGen (Hines, IL, USA) and housed at the facility of Bioqual at Rockville, MD, USA. All the procedures for maintenance of the animals were as described previously (Dakshinamoorthy et al., 2013a). All animals were screened for the absence of filarial infections prior to enrolling them in the study by analyzing the blood for the presence of microfilarial Hha-1 by PCR (Hoti et al., 2003; Rao et al., 2006); and serum for the presence of antibodies against rBmSXP-1 (Vasuki et al., 2003; Abdul Rahman et al., 2007), and rBmHAXT proteins were analyzed using an ELISA. Animals that were positive for any of the proteins were not enrolled in the study.
2.3. Parasites
Brugia malayi infective L3s were obtained from the National Institute of Allergy and Infectious Diseases/National Institute of Health (NIAID/NIH), USA, Filariasis Research Reagent Resource Center (University of Georgia, Athens, GA, USA) under an NIAID supply contract AI#30022
2.4. Adjuvants
Two different adjuvants were compared in this study. Alum (AL007) and Alum plus a synthetic TLR4 agonist GLA (AL019) purchased from the Infectious Disease Research Institute, Seattle, WA, USA.
2.5. Cloning and expression of multivalent recombinant proteins
rBmHAT protein was expressed in the Escherichia coli BL21 strain (DE3), purified and analyzed as described previously (Dakshinamoorthy et al., 2014). The coding sequence (CDS) of multivalent fusion protein rBmHAT (consisting of bmhsp 12.6, bmalt-2 and bmtsp) and rBmHAXT (consisting of bmhsp 12.6, bmalt-2, bmtpx2 and bmtsp) were synthesized at GenScript (Piscataway, NJ, USA). The sequences were provided in a pUC 51 vector. Both CDS were PCR amplified using the same gene-specific primers (Forward primer: 5’ CGGGATCCATGGAAGAAAAGGTAGTG 3’ & Reverse primer: 5’ CGGAATTCTCAATCTTTTTGAGATGAAT 3’) with restriction sites for BamHI and EcoRI, and cloned into the expression vector pRSETA (Invitrogen, Carlsbad, CA, USA) with the 6X Histidine tag. The ligated constructs for both bmhat and bmhaxt were further transformed into the expression strain of E. coli BL21 (DE3). Expression of recombinant proteins was induced with 1 mM Isopropyl β-D-1-thiogalactopyranoside (IPTG). The recombinant proteins were purified using Nickel affinity column chromatography (GE Healthcare Life Sciences, Pittsburg, PA, USA) and the purity of the recombinant proteins was confirmed in a 12% SDS PAGE gel and by western blot using anti-penta His antibodies (Qiagen, Velencia, CA, USA). Endotoxin in the final purified recombinant protein was removed using an endotoxin removal column (Thermo Fisher Scientific, Rockford, IL, USA). The final concentrations of rBmHAT and rBmHAXT proteins were determined by Bradford assays (BioRad Laboratories, Hercules, CA, USA ).
2.6. Immunization of macaques
This was a double-blinded vaccination trial. A total of 40 macaques were randomly divided into three treatment groups and one control group with 10 macaques per group. All the treated animals received four doses of 150 μg of the vaccine antigen and 2 mg of the adjuvant on days 0, 28, 56 and 84. Treatment group 1 received rBmHAT plus alum, treatment group 2 received rBmHAT plus AL019 and treatment group 3 received rBmHAXT plus AL019. Control animals received AL019 adjuvant only. The injection sites were monitored daily for any adverse reactions (redness, swelling, etc.) for up to 7 days post immunization. Blood samples were collected prior to each immunization and before challenge.
2.7. Cell Counts, serum chemistry and completebBlood count (CBC) analysis
CBC and serum chemistries were analyzed using commercial automated hematology and serum chemistry analyzers by IDEXX Laboratories (Westbrook, ME, USA). Samples collected prior to the initiation of the study served as a normal reference baseline for each animal.
2.8. Analysis of the levels of antigen-specific antibodies in the sera of macaques
Levels of rBmHAT, rBmHAXT, rBmHSP, rBmALT-2, rBmTPX, or rBmTSP specific total IgG, IgG1, IgG2, IgG3, IgM and IgE antibodies were determined in the serum of each rhesus macaque using an indirect ELISA as described previously (Dakshinamoorthy et al., 2014).
2.9. Antibody-Dependent Cell-mediated Cytotoxicity (ADCC) assay
The ADCC assay was performed as described previously (Dakshinamoorthy et al., 2014). Approximately 10 live B. malayi L3s were incubated at 37°C with 5% CO2 in triplicate wells together with 2×105 peripheral blood mononuclear cells (PBMCs) and 50 μl of sera samples. After 72 h incubation, viability of the B. malayi L3s was determined. The percentage of larval death was expressed as the ratio of the number of dead L3s to the total number recovered from each well multiplied by 100.
We also collected the culture supernatant from the ADCC assay to determine the level of myeloperoxidase (MPO) activity using a kit purchased from Biovision (Milpitas, CA, USA) and the values are expressed as mU/min per ml of culture supernatant.
2.10. Antigen-specific proliferation of peripheral blood mononuclear cells (PBMC)
PBMCs (1×107 cells /well in 1 ml of complete RPMI 1640 medium) were incubated at 37°C in the dark for 15 min with 5 mM of carboxyfluorescein diacetate succinimidyl ester (CFSE). Following incubation, cells were washed, incubated for an additional 30 min at 37°C and plated at 2 ×106 cells/well in 1 ml of complete RPMI 1640 medium in a 24-well tissue culture plate. Medium (500 μl) was removed the next day and rBmHAXT at 1 μg in 500 μl of complete RPMI medium was added to the wells. Cells incubated with RPMI medium alone served as negative controls and concanavalin A (1 mg/ well) stimulated cells served as positive controls. Cells were harvested on day 5 after culture, labeled with anti-CD3-APC antibody, fixed in 4% paraformaldehyde for 10 min at room temperature and data were acquired on a BD FACS Calibur flow cytometer and analyzed using ModFit LT software (Verity Software House, Topsham, ME, USA).
Parallel cultures of cells incubated for 3 days were harvested, paraformaldehyde fixed and labeled with combinations of anti-CD3-APC (allophycocyanin), anti-CD4-FITC (fluorescein isothiocyanate), anti-CD8-PE (phycoerythrin), anti-CD28-PE, or anti-CCR7-FITC Intracellular IFN-γ and IL-4 were determined after fixing and permeabilization. Data was acquired on a BD FACS Calibur flow cytometer and analyzed using Flow Jo v10.1 (FlowJo, LLC, Ashland, OR, USA).
Secreted levels of cytokines (Granulocyte-macrophage colony-stimulating factor (GM-CSF), Interferon (IFN)-γ, IL-12p70, IL-1β, IL-4, IL-5, IL-6, IL-15, IL-16 and Tumor Necrosis Factor (TNF)-α) in the cell culture supernatants were measured using an antibody-based Rhesus Cytokine Quantibody Array GSI (RayBiotech, Inc., Norcross, GA, USA) according to the manufacturer’s protocol. The intensity of the fluorescence signals from the slide arrays was scanned and data analyzed after subtracting the background signals and normalization to positive controls.
2.11. Challenge with Brugia malayi infective L3
One month after the last immunization, all macaques were challenged s.c. with 130–180 B. malayi L3s. All the larvae were examined microscopically for their viability, counted and only the viable larvae were used for challenge. Following challenge, all the macaques were monitored routinely for any possible alterations in the clinical biochemistry panel (serum chemistry, hematology, complete blood count analysis and CD4+/CD8+ T-cell counts), physical parameters (signs of adverse reactions at the site of challenge, body weight, body temperature, body condition, lymphoedema and lymph node measurements) and behavioral patterns.
2.12. Confirming the establishment of challenge infection in macaques
It is difficult to count the number of adult worms established in each macaque. However, several pieces of evidence were used to confirm the presence of active infection in macaques.
2.12.1. Microscopic analyses
In weeks 5, 10, 15 and 18 post challenge, 10 ml of blood were collected from each macaque between 18:00 and 22:00 h, and screened for the presence of microfilariae using a modified Knott technique (Dakshinamoorthy et al., 2014).
2.12.2. Hha-1 PCR analyses
DNA isolated from 200 μl of blood samples using the Gen Elute blood genomic DNA kit (Sigma-Aldrich) were PCR amplified for Hha-1 tandem repeat genes as described previously (Hoti et al., 2001) and the amplified PCR products were sequenced to confirm the Hha-1 sequence.
2.12.3. ELISA to determine the titer of anti-rBmSXP-1 antibodies in the sera
BmSXP-1 is a highly sensitive and specific diagnostic antigen for B. malayi infections (Vasuki et al., 2003; Abdul Rahman et al., 2007). Typically, a single sex infection will not have any microfilariae (Mf) in the peripheral circulation. Thus, microscopy and PCR approaches may not detect these dormant infections. However, the presence of active worms in infected patients or animals can be confirmed by determining the titer of IgG4 antibodies against the BmSXP-1 antigen. We used an ELISA to determine the titer of these antibodies in the sera of macaques. Since there was no reliable commercial anti-macaque IgG4 antibodies (we screened several human antibodies however none of these antibodies cross-reacted), we used a titer of anti-rBmSXP-1 IgG antibodies as a marker to detect dormant infections.
2.13. Lymphoscintigraphy analysis
The lymphoscintigraphic analysis was carried out as described previously (Dakshinamoorthy et al., 2014).
2.14. Statistical analysis
Data are presented as the mean ± S.D. Statistical significance of mean differences among different sample groups was analyzed using a non-parametric Kruskal-Wallis test followed by Bonferroni correction for multiple tests using SPSS software (v24.0, IBM, NY, USA). The significance level was defined as P<0.05.
To analyze the vaccine-induced protection, a Chi-square test was used to compare the proportions across the groups and a Fisher’s exact test was used where appropriate. Odds ratios (OR) were calculated to determine the differences between groups.
3. Results
3.1. Immunization with the vaccine candidates generated high titers of antigen-specific IgG antibodies and their isotypes.
The molecular mass of rBmHAT is approximately 39 kDa and rBmHAXT is approximately 60 kDa. We were able to express both the proteins to over 95% purity with endotoxin levels < 10 EU/μg of the protein (data not shown). Immunization of macaques with the purified proteins generated high titers of antigen-specific IgG antibodies (Fig. 1 A). The IgG antibodies were specific to each component of the fusion protein (Fig. 1B-E). The maximum IgG antibody titer was achieved after the second immunization dose (Table 1), suggesting that two doses of vaccine is sufficient to achieve maximum antibody titer. IgG1 and IgG2 were the most predominant isotype of IgG antibodies in the sera of all immunized animals (Fig. 2). Levels of IgG3 antibodies were significantly elevated in the sera of the rBmHAXT plus AL019 immunized group compared with the control animals (Fig. 2). IgM and IgE antibodies were not significantly different from controls in the sera of vaccinated animals.
Fig. 1.
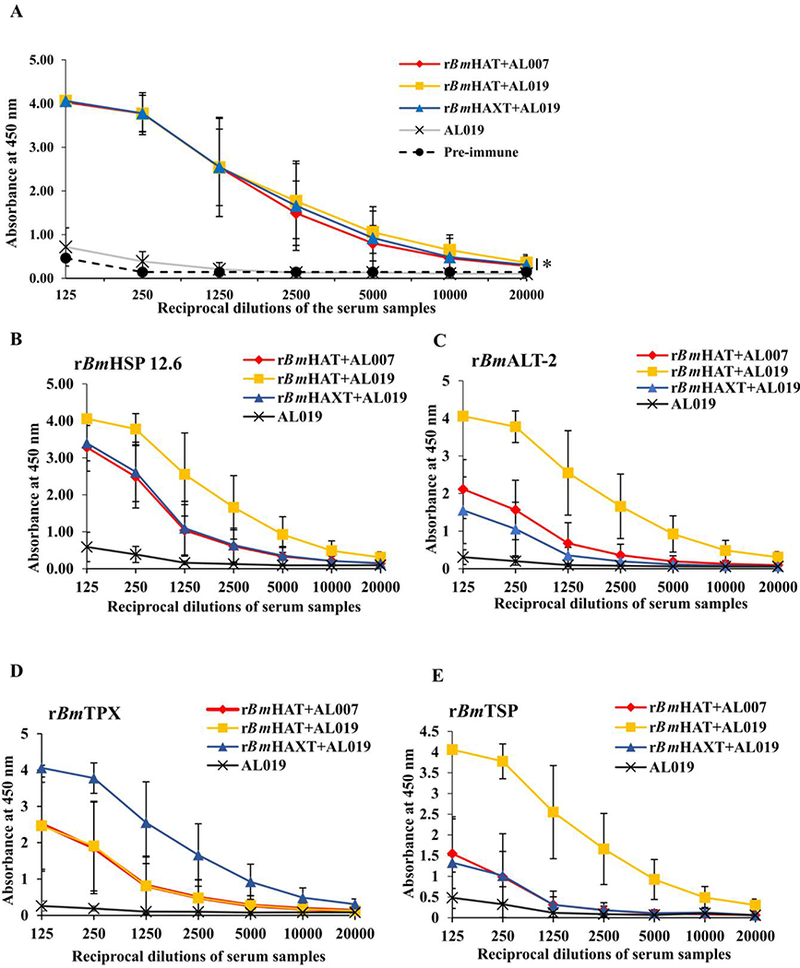
Titer of antigen-specific total IgG antibodies against recombinant Brugia malayi (rBm)HAXT, rBmHAT, rBmHAP 12.6, rBmALT-2, rBmTPX and rBmTSP antigens in the sera of macaques. Macaques were immunized (s.c.) with four doses (150 μg each dose) of respective protein in AL007 (alum) or A1019 (alum plus glucopyranosyl lipid adjuvant-stable emulsion) as adjuvant on days 0, 28, 56 and 84. Blood samples were collected prior to each immunization and the levels of antigen-specific total IgG antibodies were analyzed in all the groups of immunized animals using sera after the fourth immunization. (A) Titer of anti-rBmHAXT (rBm HSP12.6+ALT-2+TPX+TSPLEL) IgG antibodies. (B-E) Titer of IgG antibodies against each component antigen of the vaccine formulation. The variables are mean ± S.D. n=10 macaques per group. *P≤0.003 in comparison with the AL019 group as analyzed by a Kruskal-Wallis test followed by Bonferroni correction for multiple tests.
Table 1.
Titer of antigen-specific IgG antibodies in the sera of rBmHAXT or rBmHAT vaccinated macaques. Macaques were immunized (s/c) with four doses (150 μg each dose) of respective protein (rBmHAXT/rBmHAT) in AL007 (alum adjuvant) or Al019 (alum plus glucopyranosyl lipid adjuvant-stable emulsion) as adjuvant on days 0, 28, 56 and 84. Blood samples were collected prior to each immunization and the levels of antigen-specific total IgG antibodies were analyzed in all the groups of immunized animals. The variables are mean of each group. n=10 macaques per group.
| Macaque groups (n=10) |
Immunizations |
|||
|---|---|---|---|---|
| First | Second | Third | Fourth | |
| rBmHAT+AL007 | 1:1250 | 1:20,000 | 1:20,000 | 1:20,000 |
| rBmHAT+AL019 | 1:10,000 | 1:20,000 | 1:20,000 | 1:20,000 |
| rBmHAXT+AL019 | 1:1250 | 1:20,000 | 1:20,000 | 1:20,000 |
| AL019 | 1:125 | 1:125 | 1:125 | 1:125 |
rBmHAT, recombinant Brugia malayi HSP12.6+ALT-2+TSPLEL); rBmHAXT, recombinant B. malayi HSP12.6+ALT-2+TPX+TSPLEL.
Fig. 2.
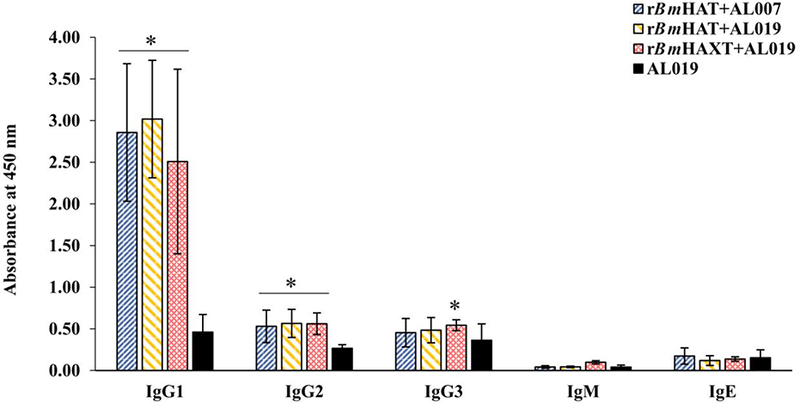
Levels of antigen-specific IgG1, IgG2, IgG3, IgM and IgE antibody isotypes in the sera of macaques. The levels of antigen-specific IgG1, IgG2, IgG3, IgM and IgE antibody isotypes were measured using an ELISA. The variables are mean ± S.D. n=10 macaques per group *P≤0.01 in comparison with the AL019 (alum plus glucopyranosyl lipid adjuvant-stable emulsion) group as analyzed by a Kruskal-Wallis test followed by Bonferroni correction for multiple tests.
3.2. Antigen responding memory cells were present in the blood of vaccinated macaques
The proliferation index of PBMCs from vaccinated animals was high in response to the respective antigens compared with controls (Fig. 3A). Cells from the control group replicated only once during the 5 days in culture compared with the vaccinated group where the cells divided up to eight generations (Fig. 3B). Evaluation of the memory cell population within the dividing antigen-responding CD4+ and CD8+ T cells (Fig. 4) showed that both CD28+CCR7-effector memory T cells (TEM) and CD28+ CCR7+ central memory T cells (TCM) were selectively expanded in rBmHAXT plus AL019 and rBmHAT plus AL007 immunized macaques (Fig. 4B). The TEM cells were predominantly positive for intracellular IFN-γ and TCM cells predominantly positive for intracellular IL-4 (Fig. 4D). Analysis of the culture supernatants of the PBMCs showed marked increases in the secreted levels of cytokines (GM-CSF, IFN-γ, IL-12p70, IL-1β, IL-4, IL-5, IL-6, IL-15, IL-16 and TNF-α) compared with AL019 controls (Fig. 5). PBMCs from rBmHAXT+AL0 l9 vaccinated animals secreted nearly 10-fold higher levels of IFN-γ compared with AL019 controls (Fig. 5).
Fig. 3.
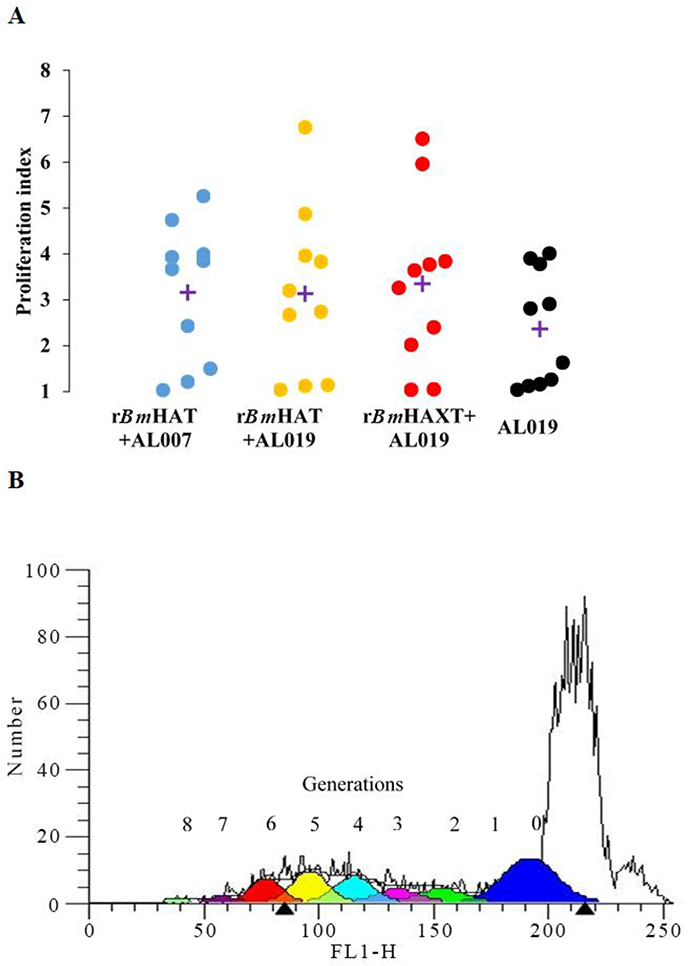
Proliferative response of antigen-responding peripheral blood mononuclear cells (PBMCs)) in the blood of vaccinated macaques. (A) PBMCs (1×106) were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) and stimulated with 1 ug/ml of rBmHAT or rBmHAXT (recombinant Brugia malayi HSP12.6+ALT-2+TPX+TSPLEL) or ConA for 5 days. Dividing cells were counted in a flow cytometer. Each data point represents a proliferation index. ‘+’indicates the mean proliferation index for each group. (B) Representative flow cytometer data from the experimental group. Up to eight generations of dividing cells were present in the PBMC cultures that were stimulated with rBmHAXT. n=10 macaques per group. AL007, alum; AL019, alum plus glucopyranosyl lipid adjuvant-stable emulsion; FL1-H, fluorescence relative intensity.
Fig. 4.
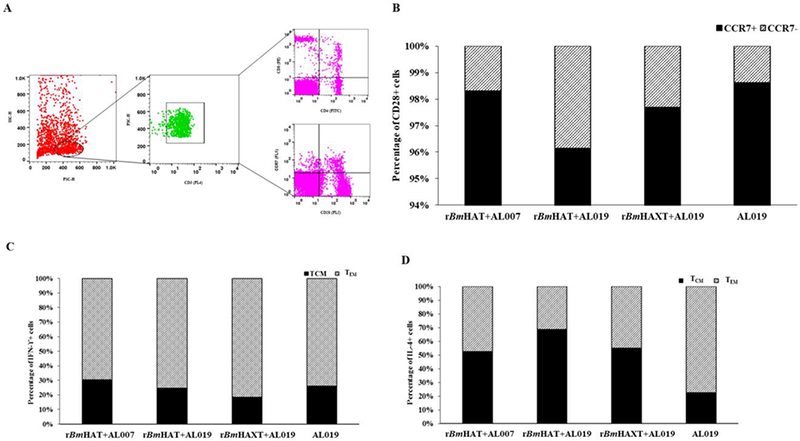
T cell population and their cytokine profile in the peripheral blood mononuclear cells (PBMCs) from rBmHAT and rBmHAXT (recombinant Brugia malayi HSP/ALT-2/TPX/TSPLEL) vaccinated macaques. PBMCs (1×107) were incubated with rBmHAT or rBmHAXTfor 3 days. Following incubation, cells were stained with fluorescent antibodies and processed for flow cytometry. Cells were initially gated for CD3 and the population of CD4, CD8 and CD28 positive cells was identified as indicated in (A). The CD28 positive cells were then further gated to determine the population of CCR7 positive cells with intracellular IFN-γ or IL-4. (B) Percentage of CCR7+ and CCR7− cells among CD28+ cell populations. (C) Percentage of IFN-γ+ and (D) IL-4+ cells among CCR7-CD28+ cell populations. The values represent percentage of cell population in each group (n = 10 macaques per group). (SC-H, side scatter-height; FSC-H, forward scatter-height; PE, phycoerythrin; TCM, T central memory cells; TEM, T effector memory cells; Interferon IFN)-γ; Interleukin (IL)-4; CD, cluster of differentiation; CCR, C-C chemokine receptor type.
Fig. 5.
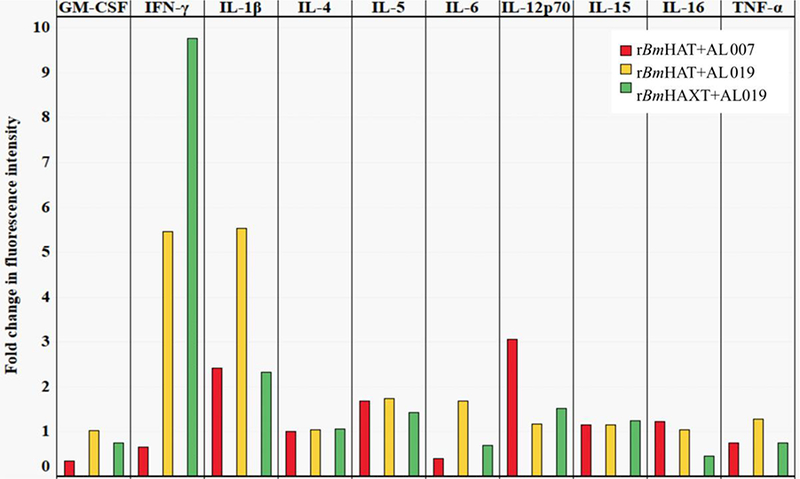
Secreted levels of cytokines in the culture supernatants of peripheral blood mononuclear cells. (PBMCs) (1×106) were cultured with rBmHAT and rBmHAXT (recombinant Brugia malayi HSP/ALT-2/TPX/TSPLEL) for 5 days. Secreted levels of cytokines in the culture supernatants were determined using an antibody slide array. Median fluorescence intensities were calculated after adjusting the background fluorescence values and normalizing the data with intensity of fluorescence in the positive control. The values represent fold changes in the mean fluorescence intensity relative to the AL019 (alum plus glucopyranosyl lipid adjuvant-stable emulsion) control group (n=10 per group). GM-CSF, Granulocyte-macrophage colony-stimulating factor.
3.3. Immunization with rBmHAXT conferred maximum protection
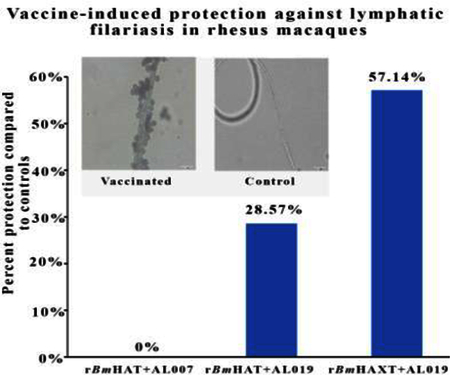
Ten weeks after challenge with B. malayi L3s, seven out of 10 control animals (70%) showed Mf in their peripheral blood and they continued to be positive for Mf until completion of the experiment (18 weeks post challenge). However, only three out of 10 macaques (30%) in the rBmHAXT plus AL019 group showed Mf in their blood ( Fig. 6). There was significant reduction in the Mf counts in the peripheral blood of vaccinated animals (Fig. 7A). These findings were further confirmed by PCR analyses of the blood samples for the presence of B. malayi-specific Hha-1 (Fig. 7B) and by an ELISA for IgG antibodies against the SXP-1 antigen (Fig. 7C). Repeated examination of the blood did not show any evidence of infection challenge in any of the negative animals until completion of the experiment (18 weeks post challenge) suggesting that immunization with rBmHAXT plus AL019 conferred 57.14% protection after adjusting for the 30% of amicrofilaremic macaques in the control group (P=0.073, OR=0.18; Fig. 7). Five out of 10 animals in the rBmHAT plus AL019 group (P=0.649, OR =0.42) and seven out of 10 animals in the rBmHAT plus AL007 group (P=1.0, OR =1.0) were positive for Mf. Statistical correlation between different groups showed that rBmHAXT plus AL019 conferred better protection than other vaccinated groups and the AL019 control (rBmHAXT+AL019 versus AL019 control - P=0.073; rBmHAXT+AL019 versus rBmHAT+AL019 - P=0.649; rBmHAXT+AL019 versus rBmHAT+AL007 - P=0.073 and rBmHAT+AL019 versus rBmHAT+AL007 - P=0.649). Thus, based on the OR, the odds that the AL019 control group would be positive for Mf were 2.33 times higher than the odds that the rBmHAT+AL019 group would be Mf-positive. The odds that the AL019 control group would be positive for Mf were 5.43 times higher than the odds that the rBmHAXT+AL019 group would be Mf-positive. The odds that the rBmHAT+AL019 group would be positive for Mf were 1.95 times higher than the odds that the rBmHAXT+AL019 group would be Mf-positive. These statistical analyses suggest that protection conferred by the rBmHAXT+AL019 immunization was significantly better than the rBmHAT+AL007 or rBmHAT+AL019 immunizations.
Fig. 6.
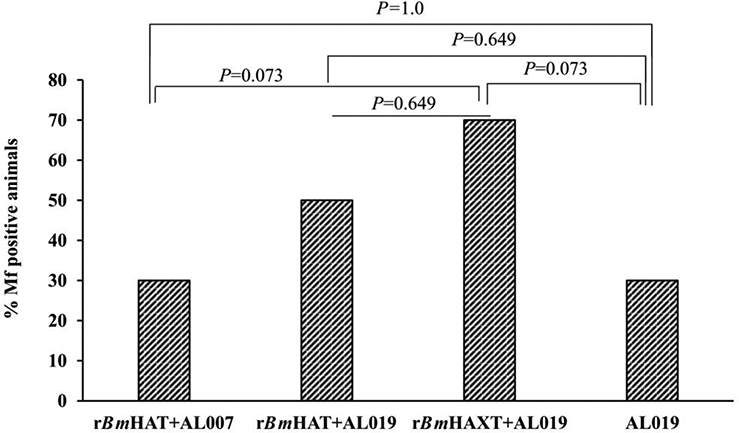
Vaccine-induced protection against Brugia malayi infection in macaques. All animals (vaccinated and control) were challenged with 130–180 L3s of Brugia malayi 1 month after the last immunization. In weeks 5, 10, 15 and 18 post challenge, 10 ml of blood were collected from each macaque between 18:00 and 22:00 h and screened for the presence of microfilariae using a modified Knott technique (Dakshinamoorthjy et al., 2014) and analysed by PCR for the Hha-1 gene (Hoti et al., 2001). Absence of infection in microfilaria (Mf)-negative animals was further confirmed by SXP-1 (B. malayi diagnostic antigen) ELISA (Abdul Rehman et al., 2007). Results show that rBmHAXT+AL019 (alum plus glucopyranosyl lipid adjuvant-stable emulsion) is a better vaccine formulation than the other formulations tested. (n=10 per group). Chi-square test and Fisher’s exact test were used to compare the proportions across the groups.
Fig. 7.
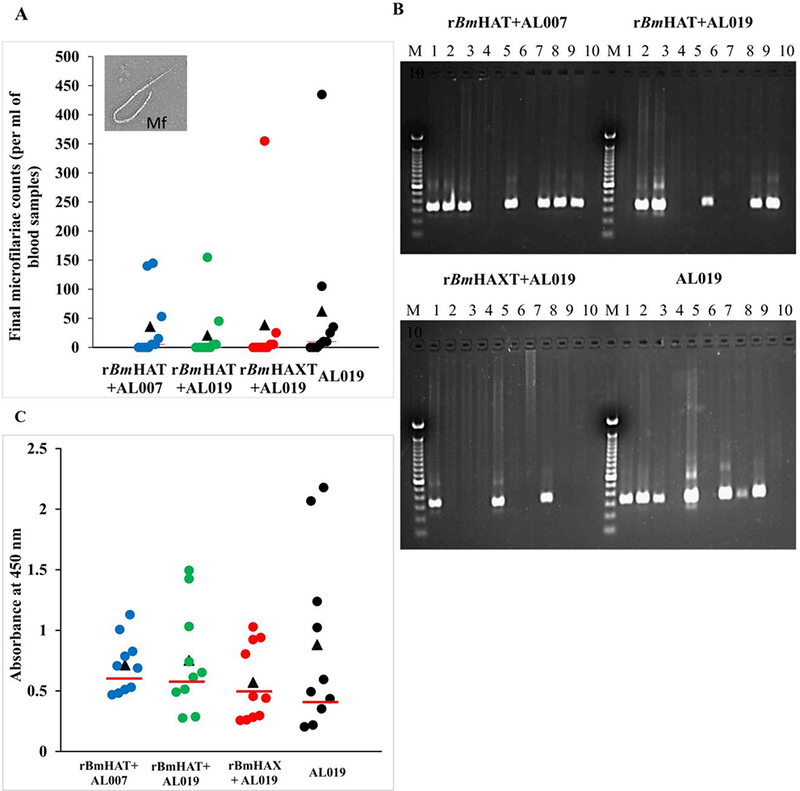
Screening macaques for the presence of filarial infection. One month after the last dose of the vaccine, all macaques were challenged s.c. with 130–180 Brugia malayi L3s. Blood was collected from all the animals between 18:00 and 22:00 h in weeks 5, 10, 15 and 18 post challenge and were screened for the presence of microfilariae using the Knott’s technique. (A) Microfilaria (Mf) counts in per ml of final blood collection samples. (B) DNA was isolated from the blood samples and analyzed by PCR to detect the Mf-specific Hha-1 gene in a 1% agarose gel. Lanes: M, 100 bp DNA ladder; lanes 1 to 10, Hha-1 PCR amplified sample from each macaque. (C) An ELISA was performed to detect the levels of anti-rBmSXP-1 (recombinant B. malayi diagnostic antigen) IgG antibodies in the sera of animals. Each data point represents an animal within the group and the bar indicates the average value of Mf-negative animals. Values above the bar were considered positive and the values below the bar were considered negative. (n=10 per group). AL007, alum; AL019, alum plus glucopyranosyl lipid adjuvant-stable emulsion; rBmHAXT, recombinant B. malyi HSP/ALT-2/TPX/TSPLEL.
We also performed an ADCC assay as an in vitro surrogate to determine vaccine-induced protection. These assays also confirmed that sera samples from macaques vaccinated with rBmHAXT plus AL019 was more efficient in killing the B. malayi L3s in vitro compared with the sera samples from the other two vaccinated groups (Fig. 8A). Several cells were found attached to the dead larvae (Fig. 8B). In fact, many of the dead larvae were completely covered by cells within 24 h after incubation. In this study we did not identify the morphology of the cells. However, previous studies suggested that the bound cells are predominantly monocytes. To determine the activation status of these cells, we measured levels of MPOs in the culture supernatants from these ADCC assays. Our results showed increases in the secreted levels of MPO (2.08 to 2.78 mmol/min/ml) in the wells containing sera from vaccinated animals and PBMCs (Fig. 9) compared with the wells containing sera from control animals (1.99 mmol/min/ml), suggesting activation of MPO producing cells.
Fig. 8.
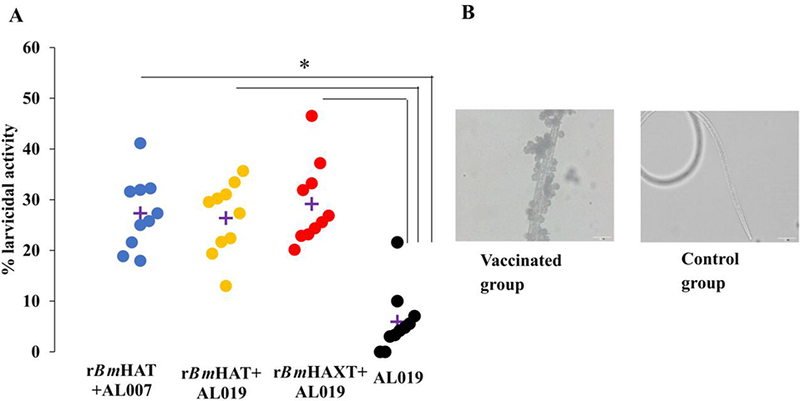
Antibody-dependent cell mediated cytotoxicity (ADCC) assay. Approximately 10 Brugia malayi larvae were incubated for 72 h at 37°C with 2×105 peripheral blood mononuclear cells (PBMCs) and 50 μl of sera samples from each macaque. Larval death in each well was monitored under a light microscope. (A) Each data point indicates the percent larval death using a serum sample from one animal. ‘+’ indicates the average percent larvicidal activity for that group. (B) Note the large number of cells attached to the dead larva when a serum sample from a vaccinated macaque was used. No cells were found attached to the live larvae when serum sample from control macaque were used. n=10 macaques per group. *P≤0.005 compared with the AL019 (alum plus glucopyranosyl lipid adjuvant-stable emulsion) group. Statistical analysis was performed by a Kruskal-Wallis test followed by Bonferroni correction for multiple tests. rBmHAXT, recombinant B. malyi HSP/ALT-2/TPX/TSPLEL.
Fig. 9.
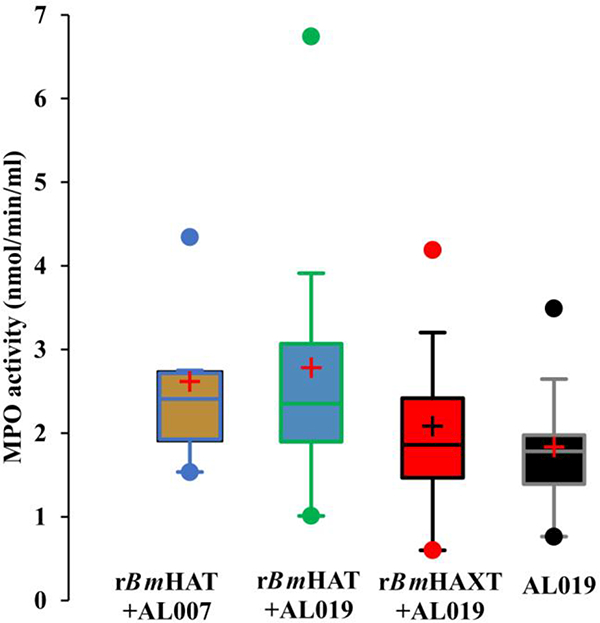
Myeloperoxidase (MPO) activity in the culture supernatants of the cells from the antibody dependent cell mediated cytotoxicity (ADCC) assay. Culture supernatants from the ADCC assay were analyzed for the MPO activity using a colorimetric assay. Data (mean± S.D.) are presented in box plots showing the upper (75%) and lower (25%) quartiles, with the horizontal line as the median and the whiskers as the maximum and minimum values observed, ‘+’ indicates mean myeloperoxidase activity per ml in the culture supernatant (n=10 per group). rBmHAXT, recombinant B. malyi HSP/ALT-2/TPX/TSPLEL.
3.4. Mf-negative vaccinated animals did not develop lymphatic pathology
Adult filarial parasites living within the lymphatic vessels cause severe inflammation leading to lymphedema and blockage of lymph flow. The presence of lymphatic blockage in this study was determined by lymphoscintigraphy. Lymph flow was compared between the right and left legs of the same animal. Challenge infections were given on the right leg. Our results show that at week 16 post challenge, there was a significant reduction in the lymph flow in the right leg of all Mf-positive animals compared with their left leg (Fig. 10), suggesting lymph blockage. Lymph flow did not show any significant difference between the right and left leg in any Mf-negative animals in the vaccinated group (Fig. 10). These findings thus suggested that lymphatic pathology was minimal or was absent in vaccinated and challenged macaques that did not show Mf in their peripheral blood.
Fig. 10.
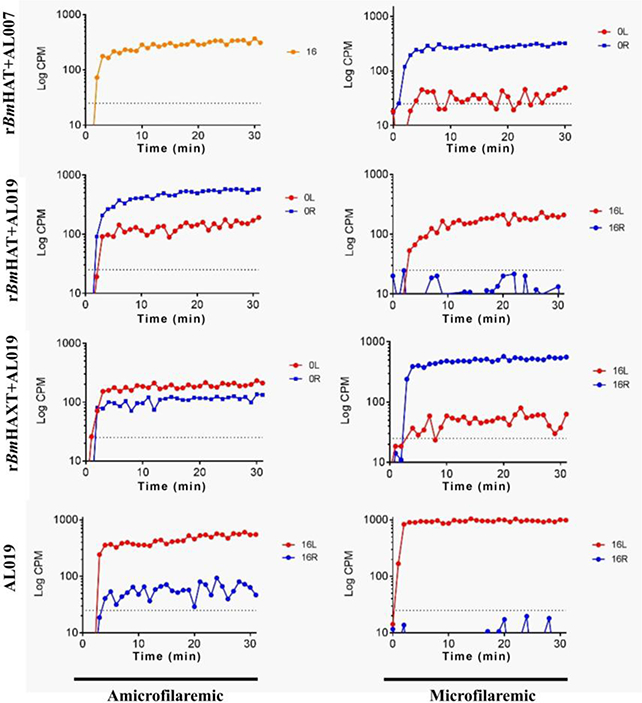
Representative data from the lymphoscintigraphy analyses of macaques 16 weeks post challenge with Brugia malayi L3s. Lymphoscintigraphy analysis was performed in all the groups of macaques on weeks 12 and 16 post challenge to assess the lymph flow and associated lymphatic pathology. All macaques were challenged on the right thigh. Data was compared between the left and right thighs of the same animal. Representative data are shown for one animal from each group at 16 weeks post challenge. In the AL019 (alum plus glucopyranosyl lipid adjuvant-stable emulsion) control animal, lymph flow in the right leg was significantly blocked. However, in all vaccinated animals, the lymph flow appeared to be comparable between the left and right legs. Lymph flow was least affected in the rBmHAXT+AL019 vaccinated animals. rBmHAXT, recombinant B. malyi HSP/ALT-2/TPX/TSPLEL; CPM, counts per minute.
4. Discussion
Several recent reports suggest that despite significant progress made towards elimination of LF from endemic regions using the MDA approach, active transmission of LF continues at low levels (Drexler et al., 2012; Cano et al., 2014; Sunish et al., 2014; Ramaswamy, 2016; Sangshetti et al., 2017). Human subject non-compliance appears to be one of the major factors hindering the success of the elimination strategy based on MDA only (Nujum et al., 2012; Krentel et al., 2013). Another issue with MDA only approach is that the drug used in the mass control (Diethylcarbamazine (DEC) and albendazole) has very little effect on the adult parasites living inside the lymphatic vessels (Critchley et al., 2005; Sangshetti et al., 2017) that continue to cause lymphatic damage and pathology (Bennuru and Nutman, 2009; Babayan et al., 2012). These adult parasites can also produce Mf that will start appearing in the circulation as the effects of the drug wane. Since mature adult female parasites can live in the host for approximately 10 years producing Mf, annual MDA treatment is critical to clear Mf from the circulation (Brady and Global Alliance to Eliminate Lymphatic, 2014; Ramaiah and Ottesen, 2014). The 10 years of control strategy proved that MDA treatment alone is not sufficient as a sustainable approach to eliminate LF (Ramaswamy, 2016; Sangshetti et al., 2017). There is a need for other sustainable approaches such as an effective prophylactic vaccine to supplement the MDA approach. A prophylactic vaccine has the potential to protect the at-risk population from getting the infection and possibly boost immunity by natural exposure to a low-level infection in endemic areas. Our group and others have been working during the last decade to develop an effective prophylactic vaccine against LF (Denham, 1980; Dissanayake et al., 1995; Gregory et al., 1997; Anand et al., 2008; Gnanasekar et al., 2008; Vedi et al., 2008; Veerapathran et al., 2009; Anand et al., 2011; Kalyanasundaram and Balumuri, 2011; Babayan et al., 2012; Dakshinamoorthy et al., 2012; Anugraha et al., 2013; Dakshinamoorthy et al., 2013a; Gomase et al., 2013; Arumugam et al., 2014; Gupta et al., 2016). A multivalent rBmHAT vaccine developed in our laboratory showed significant protection against challenge infections in rodent models (Dakshinamoorthy and Kalyanasundaram, 2013; Dakshinamoorthy et al., 2013a). However, when evaluated in a non-human primate model, the vaccine was only partially effective (Dakshinamoorthy et al., 2014). This encouraged us to improve the current vaccine formulation and test it again in the non-human primate model. Results presented in this double blinded study show that the new tetravalent vaccine formulation, rBmHAXT, conferred 57.14% protection against challenge infections in rhesus macaques and prevented the development of lymphatic pathology, which is a typical clinical feature of chronic LF infection. According to the WHO, a vaccine that can lower helminth parasite burden by 50% will be effective in lowering morbidity and mortality and in reducing disease transmission (Ahmad et al., 2011). Therefore, the 57% protection conferred by the improved vaccine formulation meets the WHO recommendation and thus has great potential for moving this vaccine to testing in human clinical trials.
In addition to improving the vaccine antigen composition, we compared two different adjuvant formulations in this study to determine their ability to promote vaccine-induced protection. Previous vaccination trials in the macaque showed that when alum was used as the adjuvant, the immune response generated was predominantly Th2 (IL-4/IgG1) biased and the protection was weak (Dakshinamoorthy et al., 2013a). Protective immune responses in the rodent model and in the human correlated with the development of antigen-specific IFN-γ/IgG2 (Th1) and IL-4/IgGl (Th2) responses (Dakshinamoorthy and Kalyanasundaram, 2013; Dakshinamoorthy et al., 2013a). Therefore, in this study we used AL019 adjuvant that is known to promote both Th1 and Th2 responses (Didierlaurent et al., 2009; Fox et al., 2010; Dakshinamoorthy and Kalyanasundaram, 2013; Baldwin et al., 2016; Carter et al., 2016). When AL019 was used as the adjuvant for rBmHAXT in this study, antigen-specific IgG1 and IgG2 antibodies were produced and the PBMCs from the rhesus macaque also secreted Th1/Th2 cytokines. The balanced Th1/Th2 responses elicited following immunization with rBmHAXT plus AL019 also correlated with high protection, suggesting that the improved formulation is significantly better than the original formulation.
Absence of infection in vaccinated animals was determined by (i) confirming the absence of Mf in the peripheral circulation by light microscopy and (ii) PCR analysis of the blood for the parasite-specific gene Hha-1, which is a definite method for confirming LF infection (Hoti et al., 2001; Vasuki et al., 2003). Additional confirmation included (iii) determining the titer of anti- BmSXP-1 antibodies in the sera using an ELISA (Abdul Rehman et al., 2007). BmSXP-1 is a diagnostic antigen that showed high sensitivity and specificity for the detection of LF infection in humans and in infected animals (Vasuki et al., 2003; Rao et al., 2006). We performed this ELISA because establishment of adult female or adult male worms (single sex infection) will not produce any Mf in the peripheral circulation and will be negative by microscopy or PCR. Such dormant single sex infections can be detected by measuring the titer of anti-BmSXP-1 antibodies antibodies in the sera. (iv) The fourth confirmatory data came from our lymphoscintigraphy studies, where the absence of lymphatic pathology correlated well with the absence of Mf in the circulation. In infected animals, the worms within the lymphatic vessels cause damage to the vessel wall, leading to decreased lymph flow that can be detected by lymphoscintigraphy (Dennis et al., 1998; Dakshinamoorthy et al., 2014). In our Mf-negative animals, especially from the rBmHAXT+AL019 vaccinated group, there was no change in the lymphatic flow, suggesting the absence of any adult worms in these animals. (v) Finally, we believe that the challenge infective larvae were killed by the protective antibodies and cells in vaccinated animals. We confirmed this using an in vitro ADCC assay that acts as a surrogate to demonstrate larval killing by protective antibodies and cells in vaccinated animals (Dakshinamoorthy et al., 2013b, 2014; Chauhan et al., 2017). Although protective antibodies were present in all the vaccinated animals, their ability to kill B. malayi L3s in vitro was different. rBmHAXT plus AL019 vaccination appeared to be slightly better than the other two vaccination protocols. More evidently, sera from Mf-negative animals in the rBmHAXT vaccinated group were more efficient in killing B. malayi L3s in the ADCC assay, confirming the presence of high levels of protective antibodies in these animals.
Analyses of the PBMCs confirmed the presence of antigen-responding T cells and these were predominantly central (TCM) and effector (TEM) memory cells. These antigen responding cells were predominantly positive for intracellular IFN-γ (TEM) and IL-4 (TCM)· Culture supernatants from the PBMCs of rBmHAXT+AL019 vaccinated animals secreted high levels of IFN-γ. This pattern of cytokine responses also correlated with higher protection and reduced pathology, suggesting that the improved formulation is significantly better than the original formulation in inducing balanced Th1/Th2 patterns of humoral and cellular responses.
One of the observations in our ADCC assay was the presence of large numbers of cells attached to the surface of dead larvae. In this study, we did not determine the phenotype or morphology of these attached cells. However, one of our previous studies using immune human sera suggested that the larval surface binding cells are predominantly macrophages (Ramaswamy, 2016). Therefore, in this study we wanted to see if these cells are activated upon binding to the larvae. We measured MPO as an indicator of activated cells. Our results showed that the MPO activity was significantly elevated when sera samples from rBmHAXT vaccinated animals were used compared with controls. The MPO activity was high when sera from Mf-ve rBmHAXT+AL019 vaccinated animals were used in the ADCC. We hypothesize that the protective antibodies in the sera of vaccinated animals bind to the larval surface (all four antigens used in the vaccine are expressed on the surface of the parasite) (Gnanasekar et al., 2004, 2008; Anand et al., 2008; Kalyanasundaram and Balumuri, 2011; Dakshinamoorthy et al., 2012, 2013a; Madhumathi et al., 2017) and the fragment crystallizable (Fc) regions of these antibodies bind macrophages and activate them. These activated macrophages secrete MPO as an effector molecule helping in the killing of the larvae. Further studies are needed to confirm the role of macrophages and their effector molecules in the killing of the infective larvae in vaccinated animals.
In conclusion, the results presented in this study show that immunization with rBmHAXT plus AL019 generated functional protective antibodies and memory T cells that conferred 57% protection against challenge infections in macaques. Based on the recommendation from the WHO that if a helminth vaccine can lower the parasite burden by 50%, it will be effective in lowering morbidity and mortality and in reducing disease transmission; the rBmHAXT vaccine is a significantly improved lymphatic filariasis vaccine formulation that is potentially ready to advance to testing in a human clinical trial.
Highlights.
A prophylactic vaccine (rBmHAXT) developed against lymphatic filariasis conferred 57.14% protection in rhesus macaques.
Immunization with rBmHAXT induced high titers of antigen-specific IgG1, IgG2 and IgG3 antibodies in rhesus macaques.
IL-4 and IFN-γ secreting antigen-specific memory T cells were generated in rBmHAXT-vaccinated rhesus macaques.
rBmHAXT vaccinated rhesus macaques had reduced lymphatic pathology.
Acknowledgements
This work was supported in part by the National Institutes of Health, National Institute of Allergy and Infectious Diseases, USA, grant R01AI072613 (RK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdul Rahman R, Hwen-Yee C, Noordin R, 2007. Pan LF-ELISA using BmR1 and BmSXP recombinant antigens for detection of lymphatic filariasis. Filaria J 6, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad G, Zhang W, Torben W, Ahrorov A, Damian RT, Wolf RF, White GL, Carey DW, Mwinzi PN, Ganley-Leal L, Kennedy RC, Siddiqui AA, 2011. Preclinical prophylactic efficacy testing of Sm-p80-based vaccine in a nonhuman primate model of Schistosoma mansoni infection and immunoglobulin G and E responses to Sm-p80 in human serum samples from an area where schistosomiasis is endemic. J Infect Dis 204, 1437–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand SB, Kodumudi KN, Reddy MV, Kaliraj P, 2011. A combination of two Brugia malayi filarial vaccine candidate antigens (BmALT-2 and BmVAH) enhances immune responses and protection in jirds. J Helminthol 85, 442–452. [DOI] [PubMed] [Google Scholar]
- Anand SB, Murugan V, Prabhu PR, Anandharaman V, Reddy MV, Kaliraj P, 2008. Comparison of immunogenicity, protective efficacy of single and cocktail DNA vaccine of Brugia malayi abundant larval transcript (ALT-2) and thioredoxin peroxidase (TPX) in mice. Acta Trop 107, 106–112. [DOI] [PubMed] [Google Scholar]
- Anil NS, 2012. Assessing Coverage of Mass Drug Administration against Lymphatic Filariasis in Gulbarga District, Karnataka. Int J Med Public Health 2, 25–28. [Google Scholar]
- Anugraha G, Jeyaprita PJ, Madhumathi J, Sheeba T, Kaliraj P, 2013. Immune responses of B. malayi thioredoxin (TRX) and venom allergen homologue (VAH) chimeric multiple antigen for lymphatic filariasis. Acta Parasitol 58, 468–477. [DOI] [PubMed] [Google Scholar]
- Arumugam S, Wei J, Ward D, Abraham D, Lustigman S, Zhan B, Klei TR, 2014. Vaccination with a genetically modified Brugia malayi cysteine protease inhibitor-2 reduces adult parasite numbers and affects the fertility of female worms following a subcutaneous challenge of Mongolian gerbils (Meriones unguiculatus) with B. malayi infective larvae. Int J Parasitol 44, 675–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babayan SA, Allen JE, Taylor DW, 2012. Future prospects and challenges of vaccines against filariasis. Parasite Immunol 34, 243–253. [DOI] [PubMed] [Google Scholar]
- Baldwin SL, Roeffen W, Singh SK, Tiendrebeogo RW, Christiansen M, Beebe E, Carter D, Fox CB, Howard RF, Reed SG, Sauerwein R, Theisen M, 2016. Synthetic TLR4 agonists enhance functional antibodies and CD4+ T-cell responses against the Plasmodium falciparum GMZ2.6C multi-stage vaccine antigen. Vaccine 34, 2207–2215. [DOI] [PubMed] [Google Scholar]
- Bennuru S, Nutman TB, 2009. Lymphatics in human lymphatic filariasis: in vitro models of parasite-induced lymphatic remodeling. Lymphat Res Biol 7, 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee J, 2016. Mass Drugs Administration in India - A Failure Story. Epidemiology (Sunnyvale) 6, 3. [Google Scholar]
- Brady M, Global Alliance to Eliminate Lymphatic, F., 2014. Seventh meeting of the Global Alliance to Eliminate Lymphatic Filariasis: reaching the vision by scaling up, scaling down, and reaching out. Parasit Vectors 7, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano J, Rebollo MP, Golding N, Pullan RL, Crellen T, Soler A, Kelly-Hope LA, Lindsay SW, Hay SI, Bockarie MJ, Brooker SJ, 2014. The global distribution and transmission limits of lymphatic filariasis: past and present. Parasites Vectors 7, 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter D, Fox CB, Day TA, Guderian JA, Liang H, Rolf T, Vergara J, Sagawa ZK, Ireton G, Orr MT, Desbien A, Duthie MS, Coler RN, Reed SG, 2016. A structure-function approach to optimizing TLR4 ligands for human vaccines. Clin Transl Immunology 5, e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan N, Banerjee P, Khatri VK, Canciamille A, Gilles J, Kalyanasundaram R, 2017. Improving the efficacy of a prophylactic vaccine formulation against lymphatic filariasis. Parasitol Res 116, 2821–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley J, Addiss D, Gamble C, Garner P, Gelband H, Ejere H 2005. International Filariasis Review Group. Albendazole for lymphatic filariasis. Cochrane Database Syst Rev 2005; 19, CD003753. [DOI] [PubMed] [Google Scholar]
- Dakshinamoorthy G, Samykutty AK, Munirathinam G, Shinde GB, Nutman T, Reddy MV, Kalyanasundaram R, 2012. Biochemical characterization and evaluation of a Brugia malayi small heat shock protein as a vaccine against lymphatic filariasis. PLoS One 7, e34077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakshinamoorthy G, Kalyanasundaram R, 2013. Evaluating the efficacy of rBmHATalphac as a multivalent vaccine against lymphatic filariasis in experimental animals and optimizing the adjuvant formulation. Vaccine 32, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakshinamoorthy G, Munirathinam G, Stoicescu K, Reddy MV, Kalyanasundaram R, 2013a. Large extracellular loop of tetraspanin as a potential vaccine candidate for filariasis. PLoS One 8, e77394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakshinamoorthy G, Samykutty AK, Munirathinam G, Reddy MV, Kalyanasundaram R, 2013b. Multivalent fusion protein vaccine for lymphatic filariasis. Vaccine 31, 1616–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakshinamoorthy G, von Gegerfelt A, Andersen H, Lewis M, Kalyanasundaram R, 2014. Evaluation of a multivalent vaccine against lymphatic filariasis in rhesus macaque model. PLoS One 9, e112982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das PK, Pani SP, Krishnamoorthy K, 2002. Prospects of elimination of lymphatic filariasis in India. ICMR Bull. 32, 1–14. [Google Scholar]
- Denham DA, 1980. Vaccination against filarial worms using radiation-attenuated vaccines. Int J Nucl Med Biol 7, 105–111. [DOI] [PubMed] [Google Scholar]
- Dennis VA, Lasater BL, Blanchard JL, Lowrie RC Jr., Campeau RJ, 1998. Histopathological, lymphoscintigraphical, and immunological changes in the inguinal lymph nodes of rhesus monkeys during the early course of infection with Brugia malayi. Exp Parasitol 89, 143–152. [DOI] [PubMed] [Google Scholar]
- Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, Kielland A, Vosters O, Vanderheyde N, Schiavetti F, Larocque D, Van Mechelen M, Garçon N, 2009. AS04, an Aluminum Salt- and TLR4 Agonist-Based Adjuvant System, Induces a Transient Localized Innate Immune Response Leading to Enhanced Adaptive Immunity. J Immunol 183, 6186. [DOI] [PubMed] [Google Scholar]
- Dissanayake S, Perler FB, Xu M, Southworth MW, Yee CK, Wang S, Dreyer G, Watawana L, Kurniawan L, Fuhrman JA, et al. , 1995. Differential recognition of microfilarial chitinase, a transmission-blocking vaccine candidate antigen, by sera from patients with Brugian and Bancroftian filariasis. Am J Trop Med Hyg 53, 289–294. [PubMed] [Google Scholar]
- Drexler N, Washington CH, Lovegrove M, Grady C, Milord MD, Streit T, Lammie P, 2012. Secondary mapping of lymphatic filariasis in Haiti-definition of transmission foci in low-prevalence settings. PLoS Negl Trop Dis 6, e1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson L, Stolk WA, Farrell SH, Hollingsworth TD, 2017. Measuring and modelling the effects of systematic non-adherence to mass drug administration. Epidemics 18, 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CB, Friede M, Reed SG, Ireton GC, 2010. Synthetic and natural TLR4 agonists as safe and effective vaccine adjuvants. Subcell Biochem 53, 303–321. [DOI] [PubMed] [Google Scholar]
- Gnanasekar M, Rao KVN, Mishra P, He YX, Nutman T, Kaliraj P, and Ramaswamy K, 2004. A novel phage display based screening to identify potential vaccine candidate antigens of the filarial parasite Brugia malayi. Infect Immun 72, 4707–4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnanasekar M, Anand SB, Ramaswamy K, 2008. Identification and cloning of a novel tetraspanin (TSP) homologue from Brugia malayi. DNA Seq 19, 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomase VS, Chitlange NR, Changbhale SS, Kale KV, 2013. Prediction of Brugia malayi antigenic peptides: candidates for synthetic vaccine design against lymphatic filariasis. Protein Pept Lett 20, 864–887. [DOI] [PubMed] [Google Scholar]
- Gregory WF, Blaxter ML, Maizels RM, 1997. Differentially expressed, abundant trans-spliced cDNAs from larval Brugia malayi. Mol Biochem Parasitol 87, 85–95. [DOI] [PubMed] [Google Scholar]
- Gupta J, Misra S, Misra-Bhattacharya S, 2016. Immunization with Brugia malayi Myosin as Heterologous DNA Prime Protein Boost Induces Protective Immunity against B. malayi Infection in Mastomys coucha. PLoS One 11, e0164991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoti SL, Subramaniyan K, Das PK, 2003. Detection of codon for amino acid 200 in isotype 1 beta-tubulin gene of Wuchereria bancrofti isolates, implicated in resistance to benzimidazoles in other nematodes. Acta Trop 88, 77–81. [DOI] [PubMed] [Google Scholar]
- Hoti SL, Vasuki V, Lizotte MW, Patra KP, Ravi G, Vanamail P, Manonmani A, Sabesan S, Krishnamoorthy K, Williams SA, 2001. Detection of Brugia malayi in laboratory and wild-caught Mansonioides mosquitoes (Diptera: Culicidae) using Hha I PCR assay. Bull Entomol Res 91, 87–92. [PubMed] [Google Scholar]
- Kalyanasundaram R, Balumuri P, 2011. Multivalent vaccine formulation with BmVAL-1 and BmALT-2 confer significant protection against challenge infections with Brugia malayi in mice and jirds. Res Rep Trop Med 2011, 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krentel A, Fischer PU, Weil GJ, 2013. A review of factors that influence individual compliance with mass drug administration for elimination of lymphatic filariasis. PLoS Negl Trop Dis 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima AW, Medeiros Z, Santos ZC, Costa GM, Braga C, 2012. Adverse reactions following mass drug administration with diethylcarbamazine in lymphatic filariasis endemic areas in the Northeast of Brazil. Rev Soc Bras Med Trop 45, 745–750. [DOI] [PubMed] [Google Scholar]
- Madhumathi J, Prince PR, Rao DN, Karande AA, Reddy MV, Kaliraj P, 2017. Epitope mapping of Brugia malayi ALT-2 and the development of a multi-epitope vaccine for lymphatic filariasis. J Helminthol 91, 43–54. [DOI] [PubMed] [Google Scholar]
- Nujum ZT, Remadevi S, Nirmala C, Rajmohanan K, Indu P, Nair SM, 2012. Factors determining noncompliance to mass drug administration for lymphatic filariasis elimination. Trop Parasitol 2, 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Vector Borne Disease Control Program (NVBDCP). National Roadmap for elimination of lymphatic filariasis (ELF). Delhi: Directorate of National Vector Borne Disease Control Program. Available at: http://nvbdcp.gov.in/Doc/National-Roadmap-ELF.pdf. Accessed on: June 8, 2017. [Google Scholar]
- Ramaiah KD, Ottesen EA, 2014. Progress and impact of 13 years of the global programme to eliminate lymphatic filariasis on reducing the burden of filarial disease. PLoS Negl Trop Dis 8, e3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanasundaram R, 2016. Lymphatic Filariasis: Current Status of Elimination Using Chemotherapy and the Need for a Vaccine In: Saxena A (ed) Communicable Diseases of the Developing World. Topics in Medicinal Chemistry, vol 29 Springer, Cham; Pp 97–124. [Google Scholar]
- Rao RU, Weil GJ, Fischer K, Supali T, Fischer P, 2006. Detection of Brugia parasite DNA in human blood by real-time PCR. J Clin Microbiol 44, 3887–3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangshetti JN, Shinde DB, Kulkarnia A, Arote R, 2017. Two decades of antifilarial drug discovery: a review. Royal Society of Chemistry 7, 20628–20666. [Google Scholar]
- Sunish IP, Munirathinam A, Kalimuthu M, Ashok Kumar V, Tyagi BK, 2014. Persistence of lymphatic filarial infection in the paediatric population of rural community, after six rounds of annual mass drug administrations. J Trop Pediatr 60, 245–248. [DOI] [PubMed] [Google Scholar]
- Vasuki V, Hoti SL, Sadanandane C, Jambulingam P, 2003. A simple and rapid DNA extraction method for the detection of Wuchereria bancrofti infection in the vector mosquito, Culex quinquefasciatus by Ssp I PCR assay. Acta Trop 86, 109–114. [DOI] [PubMed] [Google Scholar]
- Vedi S, Dangi A, Hajela K, Misra-Bhattacharya S, 2008. Vaccination with 73kDa recombinant heavy chain myosin generates high level of protection against Brugia malayi challenge in jird and mastomys models. Vaccine 26, 5997–6005. [DOI] [PubMed] [Google Scholar]
- Veerapathran A, Dakshinamoorthy G, Gnanasekar M, Reddy MV, Kalyanasundaram R, 2009. Evaluation of Wuchereria bancrofti GST as a vaccine candidate for lymphatic filariasis. PLoS Negl Trop Dis 3, e457. [DOI] [PMC free article] [PubMed] [Google Scholar]


