Abstract
Cholinergic signaling plays a key role in regulating striatal function. The principal source of acetylcholine in the striatum are the cholinergic interneurons which, although low in number, densely arborize to modulate striatal neurotransmission. This modulation occurs via strategically positioned nicotinic and muscarinic acetylcholine receptors that influence striatal dopamine, GABA and other neurotransmitter release. Cholinergic interneurons integrate multiple striatal synaptic inputs and outputs to regulate motor activity under normal physiological conditions. Consequently, an imbalance between these systems is associated with basal ganglia disorders. Here, we provide an overview of how striatal cholinergic interneurons modulate striatal activity under normal and pathological conditions. Numerous studies show that nigrostriatal damage such as that occurs with Parkinson’s disease affects cholinergic receptor-mediated striatal activity. This altered cholinergic signaling is an important contributor to Parkinson’s disease as well as to the dyskinesias that develop with L-dopa therapy, the gold standard for treatment. Indeed, multiple preclinical studies show that cholinergic receptor drugs may be beneficial for the treatment of L-dopa induced dyskinesias. In this review, we discuss the evidence indicating that therapeutic modulation of the cholinergic system, particularly targeting of nicotinic cholinergic receptors, may offer a novel approach to manage this debilitating side effect of dopamine replacement therapy for Parkinson’s disease.
Keywords: Acetylcholine, dyskinesia, Parkinson’s disease, striatum, cholinergic receptor
1. Striatal cholinergic transmission modulates striatal output
Normal striatal function is dependent in part on an equilibrium between the midbrain dopaminergic (DAergic) and striatal cholinergic systems (Lim et al., 2014). The striatum has the highest density of cholinergic markers and acetylcholine levels in the brain (Macintosh, 1941; Graybiel, 1990; Mesulam et al., 1992; Contant et al., 1996). The two sources of acetylcholine in the striatum are the pedunculopontine nucleus-laterodorsal tegmental complex (Dautan et al., 2014) and striatal cholinergic interneurons (ChIs) (Woolf and Butcher, 1981; Graybiel, 1990), the latter of which is the focus of this review. Cholinergic interneurons comprise 1–2% of striatal neurons (Zhou et al., 2001; Bohnen and Albin, 2011; Lenz and Lobo, 2013). Although low in number, ChIs have widespread dendritic and axonal arbors, with a preferential distribution in the matrix area along the patches border (Bolam et al., 1984; Smith and Bolam, 1990; Wilson et al., 1990; van Vulpen and van der Kooy, 1998). Thus, ChIs have the ability to integrate the majority of striatal synaptic inputs as well as its output via medium spiny neurons (MSNs), highlighting the importance of cholinergic transmission to striatal function.
Cholinergic interneurons show a range of spontaneous tonic firing activity which can vary from irregular single spiking to rhythmic bursting (Bolam et al., 1984; Wilson et al., 1990; Kawaguchi, 1993; Aosaki et al., 1995; Bennett and Wilson, 1998; Bennett et al., 2000; Zhou et al., 2002; Goldberg and Wilson, 2005; Wilson and Goldberg, 2006; Goldberg et al., 2009). This activity is highly regulated not only via their intrinsic properties but also by the neuromodulatory control exerted on ChIs by DAergic, serotonergic, noradrenergic, and GABAergic afferents as well as cortical and thalamic glutamatergic inputs (Lim et al., 2014). Indeed, ChIs express a plethora of receptors for these systems (Lim et al., 2014). Importantly, the pattern of ChI firing maintains a background tone of acetylcholine that gates striatal output by modulating MSN activity (Calabresi et al., 1998; Galarraga et al., 1999; Calabresi et al., 2000; Koos and Tepper, 2002; Zhou et al., 2002; Pakhotin and Bracci, 2007; Carrillo-Reid et al., 2009; Goldberg et al., 2012; Mamaligas and Ford, 2016). This modulation of MSN output can occur directly or indirectly via muscarinic acetylcholine receptors (mAChRs) and nicotinic acetylcholine receptors (nAChRs).
a. Direct cholinergic modulation of striatal MSN output via mAChRs
Acetylcholine directly modulates striatal output primarily by acting on mAChRs present on MSNs as the evidence indicating that nAChRs are expressed on MSNs is limited (Matsubayashi et al., 2001; Xiao et al., 2009; Goldberg et al., 2012; Luo et al., 2013). Activation of metabotropic mAChRs exerts a long-term modulatory role on striatal function. These receptors are divided into two classes depending on the intracellular signaling cascades through which they act. Excitatory mAChRs include the M1, M3 and M5 subtypes which are couple to Gq/11 and induce activation of phospholipase C. Inhibitory receptors include M2 and M4, which are coupled to Gi/o proteins and decrease adenylyl cyclase activity. Although all five mAChR subtypes are expressed in the striatum (Table 1), direct modulation of MSN activity by acetylcholine occurs via M1 and M4 mAChRs (Perez-Rosello et al., 2005; Ding et al., 2006; Wang et al., 2006; Ding et al., 2010; Goldberg et al., 2012; Kuroiwa et al., 2012; Hernandez-Flores et al., 2015). M1 mAChRs are robustly expressed on both direct (D1 DA receptor-expressing) and indirect (D2 DA receptor expressing) pathway MSNs (Hersch et al., 1994). Activation of these receptors depresses K+-currents and enhance Na+-currents such that their activation increases MSN excitability (Galarraga et al., 1999; Perez-Rosello et al., 2005; Xiang et al., 2012; Shen et al., 2015; Lv et al., 2017). By contrast, M4 mAChRs are preferentially expressed on D1 DA receptor-expressing MSNs (D1 MSNs), where they decrease excitability upon activation (Bernard et al., 1992; Hersch et al., 1994; Howe and Surmeier, 1995; Jeon et al., 2010; Shen et al., 2015). The concerted action of acetylcholine at M1 and M4 mAChRs potently controls MSN activity. For instance, studies have shown that ChIs participate in long-term potentiation (LTP) induction at MSNs via a direct action on M1 mAChRs (Wang et al., 2006). In addition, striatal ChI activation via thalamic inputs interrupts cortical signaling to MSNs and subsequently enhances D2 DA receptor expressing MSN (D2 MSN) activity via M1 receptors, which is thought to contribute to attentional shift and interruption of ongoing motor activity (Ding et al., 2010). The effect of M4 mAChR on MSN activity has been less studied; nevertheless, recent work shows that activation of these receptors enhances Ca2+-currents and facilitates direct pathway long-term depotentiation (LTD) (Hernandez-Flores et al., 2015; Shen et al., 2015). In fact, M4 mAChRs are becoming increasingly viewed as important regulators of D1 pathway activity and motor function (Jeon et al., 2010; Hernandez-Flores et al., 2015; Shen et al., 2015; Ztaou et al., 2016).
Table 1.
Striatal nAChR and mAChR subtypes
b. Indirect cholinergic modulation of MSN output via control of DA release
Multiple studies have shown that acetylcholine-mediated activation of nAChRs is a primary facilitator of striatal dopamine release (Grady et al., 1992; Marshall et al., 1997; Wonnacott et al., 2000; Zhou et al., 2001; Champtiaux et al., 2003; Rice and Cragg, 2004; Salminen et al., 2004; Zhang and Sulzer, 2004; Exley and Cragg, 2008; Perez et al., 2008; Zhang et al., 2009; Drenan et al., 2010; Perez et al., 2010; Threlfell et al., 2010; Quik et al., 2011; Quik and Wonnacott, 2011; Threlfell et al., 2012; Zhang et al., 2012; Exley et al., 2013; Koranda et al., 2014; Kosillo et al., 2016). nAChRs are pentameric ligand-gated ion channels of which there are multiple subtypes comprised of either α subunits or a combination of α and β subunits (Albuquerque et al., 2009; Millar and Gotti, 2009; Quik and Wonnacott, 2011). Acute agonist activation of these receptors induces rapid depolarization and Ca2+ entry that lead to neurotransmitter release, while prolonged exposure to agonist can activate Ca2+ dependent signaling cascades and induce nAChR desensitization to ultimately reduce neurotransmitter release (Dajas-Bailador and Wonnacott, 2004; Garcia-Montes et al., 2012; Perez et al., 2012; Quik et al., 2012b; Bordia et al., 2013; Exley et al., 2013; Perez et al., 2013). Extensive studies show that the main functionally active nAChRs in the striatum are the β2* nAChRs, which include the α6β2* and α4β2* receptor subtypes (the asterisks indicate the possible presence of other nAChR subunits in the receptor complex), with a minor population of the α7 nAChR subtype (Champtiaux et al., 2003; Quik and Wonnacott, 2011) (Table 1). α6β2* nAChRs are highly localized to dopaminergic terminals projecting from the substantia nigra while α4β2* nAChRs are abundantly expressed on DA terminals, GABAergic interneurons and serotonergic afferents (Takahashi et al., 1998; Livingstone and Wonnacott, 2009; Xiao et al., 2009; Quik et al., 2011; Luo et al., 2013) (Table 1). By contrast, α7 nAChRs appear to be primarily localized on glutamatergic afferents (Kaiser and Wonnacott, 2000; Marchi et al., 2002; Livingstone and Wonnacott, 2009; Quik et al., 2015b) (Table 1). Thus, nAChRs can indirectly modulate MSN activity not only by regulating striatal DA release but also GABA and glutamate release. Additionally, α4β2* and α7 nAChRs are widely expressed on other neuronal circuits and connections of the basal ganglia such as the cortical and thalamic regions where they can also regulate GABA and glutamate transmission, respectively, to ultimately influence dopaminergic transmission.
Striatal dopamine release is also facilitated by activation of M4 and M5 mAChRs, inhibited by M3 mAChRs and unaffected by M1 and M2 mAChRs (Zhang et al., 2002). Although it has generally been thought that mAChRs are not present on striatal dopaminergic terminals, some studies support a role for the M3 and M5 mAChRs subtypes to directly modulate DA release (Zhang et al., 2002; Kuroiwa et al., 2012; Foster et al., 2014) (Table 1). M2/M4 mAChRs are present in cholinergic interneurons, where they can inhibit neuron activity and control acetylcholine release; thereby, indirectly influencing nAChR-mediated DA release (Bernard et al., 1992; Hersch et al., 1994; Yan and Surmeier, 1996; Threlfell et al., 2010; Foster et al., 2016) (Table 1). mAChR activation however can also lead to sustained DA release inhibition independent of nAChR signaling. This effect appears to be mediated via activation of M4 mAChRs on D1 receptor-expressing MSNs that induces the release of endocannabinoids and activation of CB2 cannabinoid receptor signaling (Foster et al., 2016).
The ability of nAChRs and mAChRs to regulate striatal DA release makes the cholinergic system a key player in modulating the output of D1 and D2 MSNs and basal ganglia function. Based on this, preclinical studies have been carried out to elucidate the alterations in nAChR- and mAChR-mediated signaling that occur with nigrostriatal DAergic damage, which underlie movement disorders such as L-dopa-induced dyskinesias (LIDs).
2. Alterations in striatal cholinergic signaling with nigrostriatal DAergic damage
The loss of nigral DA neuron innervation to the striatum in Parkinson’s disease (PD) leads to a hypercholinergic tone in the basal ganglia (Fino et al., 2007; Salin et al., 2009; Tubert et al., 2016). This increase in cholinergic signaling occurs without changes in the tonic firing of ChIs (Ding et al., 2006). However, ChI activity becomes highly synchronized (Raz et al., 1996). There is also a shift toward greater acetylcholine innervation of D2 MSNs than D1 MSNs (Salin et al., 2009), which may contribute to the selective pruning of spines and glutamatergic synapses in D2 MSNs observed after DA depletion (Shen et al., 2007). More recent studies show that optogenetic ChI silencing potentiated excitatory postsynaptic currents in D1 and D2 MSNs and decreased excitability more in D1 than D2 MSNs while alleviating motor symptoms in animal models of DA depletion (Maurice et al., 2015). Additional work now suggests that a reduction in NMDA/AMPA ratio at glutamatergic parafascicular synapses to ChIs after DA depletion also plays a primary role in disrupting the balance between D1 and D2 MSNs (Aceves Buendia et al., 2017). These changes in striatal cholinergic signaling with DA depletion certainly not only contribute to PD pathology but also to the appearance of LIDs, a debilitating side effect of DA replacement therapy in PD.
a. Alterations in nAChR-mediated function contribute to changes in dopaminergic transmission in DA depletion models
Under normal physiological conditions, activation of nAChRs promotes striatal DA release as these receptors are strategically expressed on DA neurons and terminals (Quik and Wonnacott, 2011). Nigrostriatal damage alters nAChR expression and its contribution to the release process. Receptor studies show decreases in α4β2* and α6β2* nAChRs in animals lesioned with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 6-hydroxydopamine, with nigrostriatal damage inducing a greater decrease in the α6β2* than the α4β2* nAChR subtype (Quik et al., 2003; McCallum et al., 2005; Bordia et al., 2007; Perez et al., 2010; Perez and Quik, 2011; Quik and Wonnacott, 2011). Further studies using striatal synaptosomal preparations and tissue slices from lesioned animals also revealed a decline in DA release mediated via these receptor subtypes (McCallum et al., 2005; McCallum et al., 2006; Quik et al., 2006; Perez et al., 2010; Quik and Wonnacott, 2011). In contrast, although no alterations in α7 receptor expression or its mediation of striatal DA release have been found thus far, the dense expression of this receptor subtype in neuronal circuits that regulate striatal function suggests their involvement in PD (Quik et al., 2015b).
Work using cyclic voltammetry in striatal slices has shown that the control exerted by α4β2* and α6β2* nAChRs on DA release is dependent on DA neuron firing frequency (Zhou et al., 2001; Rice and Cragg, 2004; Zhang and Sulzer, 2004; Exley and Cragg, 2008; Zhang et al., 2009; Perez et al., 2010; Perez and Quik, 2011). Interestingly, studies in 6-hydroxydopamine-lesioned rodents show a dysregulation of DA release after nigrostriatal damage such that release is less sensitive to DA neuron activity (Perez et al., 2010; Jennings et al., 2015). Importantly, these impairments in DA release appear to be exacerbated by a loss of nAChR activation. Paradoxically, pre-clinical and clinical studies to date do not indicate that nAChR drugs consistently ease the motor deficits typically associated with nigrostriatal damage despite their ability to sensitize DA receptor-mediated responses (Gregorio et al., 2009; Quik et al., 2015a); However, multiple evidence outlined later in this review suggest that nAChR drugs may ameliorate the dopaminergic imbalance contributing to the development of LIDs in PD patients.
b. Changes in mAChR-mediated transmission after nigrostriatal DAergic damage
Muscarinic antagonists were among the first treatments for Parkinson’s disease, although their use is limited by poor selectivity and side effects (Duvoisin, 1967; Katzenschlager et al., 2003). Striatal DA depletion provokes a reduction in the efficacy of M4 auto-receptors on ChIs resulting in the self-disinhibition of acetylcholine release that contributes to the hypercholinergic tone observed with DA depletion (Ding et al., 2006). In addition, dysregulated striatal cholinergic transmission via M1 and M4 mAChRs has directly been shown to contribute to the motor impairments observed with dopamine depletion (Ztaou et al., 2016). These finding together with the recent development of drugs interacting with allosteric sites in mAChRs have led to interest in these receptors as novel therapeutics for Parkinson’s disease and other disorders of the basal ganglia (Jeon et al., 2010; Hernandez-Flores et al., 2015; Shen et al., 2015; Ztaou et al., 2016).
3. Role for the cholinergic system in LIDs
A motor complication commonly observed in PD patients arises from the use of the DA precursor L-dopa, which is the main therapeutic agent used to treat the motor deficits associated with the disease. L-dopa enhances synaptic DA transmission and thus alleviates the dopaminergic deficit that arises with nigrostriatal damage. Although it still remains the most effective treatment for the motor symptoms of PD, long-term L-dopa use leads to the development of fluctuations in motor response (Quinn et al., 1982; Quinn, 1998). This side effect includes unpredictable changes in mobility, a decrease in the duration of L-dopa action and LIDs (Huot et al., 2013; Heumann et al., 2014; Bastide et al., 2015). LIDs are abnormal involuntary movements that develop in the majority of patients within the first decade of treatment and can be very debilitating (Ahlskog and Muenter, 2001; Huot et al., 2013; Schaeffer et al., 2014). Although both amantadine and deep brain stimulation help reduce LIDs, both of these approaches are associated with complications that limit their usefulness (Brotchie, 2010; Tambasco et al., 2012; Heumann et al., 2014; Merola et al., 2014; Rizzone et al., 2014; Schaeffer et al., 2014). Thus, there is a need for novel anti-dyskinetic drugs. To achieve this, studies in rodent and primate models of LIDs have been widely used to broaden our understanding of the pathophysiology related to LIDs and facilitate preclinical testing of novel therapeutics targets. We will focus on preclinical studies highlighting the ability of cholinergic drugs to decrease LIDs in rodent and primate models.
LIDs are unquestionably linked to aberrant striatal MSN output arising from alterations in D1 and D2 DA receptor signaling due to nigrostriatal damage and chronic L-dopa exposure (Huot et al., 2013; Cenci, 2014; Bastide et al., 2015; Suarez et al., 2016). Multiple evidence supports a stronger role for D1 MSNs in LIDs as studies show that D1 receptor agonists promote dyskinesias and D1 receptor antagonists reduce them (Grondin et al., 1999; Taylor et al., 2005; Delfino et al., 2007; Westin et al., 2007). Additionally, genetically modified mice lacking D1 receptors do not develop LIDs and deletion of D3 receptors decreases LIDs via targeting of D1 receptor-mediated signaling (Darmopil et al., 2009; Solis et al., 2015). Accordingly, optogenetic stimulation of D1 MSN induces LIDs while inhibition of D1 MSN activity via chemical ablation or chemogenetic approaches reduces them (Revy et al., 2014; Engeln et al., 2016; Alcacer et al., 2017; Perez et al., 2017). Importantly, most molecular markers associated with LIDs are expressed in D1 MSNs (Pavon et al., 2006). By contrast, the contribution of D2 MSNs to LIDs is less clear. D2 receptor agonists produce only mild or no dyskinesias in L-dopa-naïve lesioned rodents. However, their administration leads to more severe dyskinesia in L-dopa-primed animals as alterations in D2 receptor-mediated signaling contribute to the D1 MSN modulation of LIDs (Grondin et al., 1999; Delfino et al., 2007; Gold et al., 2007; Larramendy et al., 2008). In addition, D2 receptor antagonists attenuate LIDs (Taylor et al., 2005; Lindgren et al., 2009). Thus, although D1 MSNs are primary players in LIDs development, a role for D2 MSNs cannot be disregarded.
In addition, multiple other neurotransmitter systems contribute to the changes that lead to LIDs development, including the cholinergic system (Quik and Wonnacott, 2011; Huot et al., 2013; Bastide et al., 2015). Enhanced cholinergic tone and its facilitation of LTP with DAergic loss is thought to be a contributing factor to LIDs development (Fino et al., 2007; Salin et al., 2009; Cenci and Konradi, 2010; Nishijima et al., 2014; Tubert et al., 2016). Work by Ding and colleagues showed that while acute L-dopa administration increases ERK phosphorylation in MSNs, repeated exposure leads to a shift of ERK activation from MSNs to ChIs (Ding et al., 2011). This enhanced ERK activation leads to higher basal firing and potentiated excitatory responses to DA in ChIs concomitant with LIDs expression (Ding et al., 2011). Interestingly, pharmacological reduction of striatal cholinergic tone and ablation of striatal ChIs have both been shown to decrease LIDs (Ding et al., 2011; Won et al., 2014). In addition, our recent optogenetic studies show that stimulation of ChIs modulates LIDs (Bordia et al., 2016) as discussed later in section 3c. Thus, converging evidence supports the idea that enhanced cholinergic activity contributes to LIDs expression. As such, it has become increasingly important to understand the involvement of nAChR- and mAChR-mediated transmission in LIDs with the aim of uncovering novel targets for treatment.
a. nAChR drugs as therapeutic targets to ameliorate LIDs
Alterations in the nicotinic cholinergic system are implicated in the pathological events leading to LIDs (Quik and Wonnacott, 2011; Huot et al., 2013; Schaeffer et al., 2014; Bastide et al., 2015; Perez, 2015; Quik et al., 2015a). The first evidence for this came from studies showing that long-term nicotine treatment decreased LIDs by ~50% in a cohort of MPTP-treated primates (Quik et al., 2007) (Table 2). Additional studies in mice, rats and primates have since shown that route of administration and treatment regimen (pre- vs post-treatment) do not affect nicotine’s antidyskinetic effect (Bordia et al., 2008; Bordia et al., 2010; Huang et al., 2011a; Huang et al., 2011b; Quik et al., 2012a; Bordia et al., 2013; Quik et al., 2013a; Quik et al., 2013b; Quik et al., 2013c; Quik et al., 2013d; Bordia et al., 2015). Further experiments with general nAChR agonists have yielded a similar reduction in LIDs as nicotine treatment indicating that nAChRs mediate nicotine’s effect (Huang et al., 2011a; Zhang et al., 2013) (Table 2). Interestingly, treatment with the general nAChR antagonist mecamylamine also decreased LIDs to a similar extent as that observed with nAChR agonists (Bordia et al., 2010) (Table 2). This suggests that long-term treatment with nAChR agonists exerts its antidyskinetic effect via a receptor desensitization block. Altogether, the preclinical evidence thus far indicates that nAChR compounds can interfere with LIDs development and reduce existing LIDs. In fact, a small phase I/II clinical trial by Neuraltus Inc. has demonstrated the potential of oral nicotine treatment as a safe and well-tolerated approach to improve dyskinesias in PD patients with LIDs (https://www.prnewswire.com/news-releases/neuraltus-pharmaceuticals-reports-clinical-results-from-phase-12-np002-study-in-the-treatment-of-dyskinesias-resulting-from-levodopa-therapy-for-parkinsons-disease-111255279.html).
Table 2.
Nicotinic and muscarinic cholinergic receptor drugs decrease LIDs in parkinsonian rats, mice or monkeys
| Receptor subtype | Receptor subtype | Drug | Decline in LIDs | Reference |
|---|---|---|---|---|
| nAChRs | Nonselective agonist | Nicotine | ~35–60% | (Quik et al., 2007; Bordia et al., 2008; Bordia et al., 2010; Quik et al., 2013d; Bordia et al., 2016) |
| Varenicline | ~10–50% | (Huang et al., 2011a; Zhang et al., 2013) | ||
|
| ||||
| β2* selective agonist | ABT-089 | ~50% | (Zhang et al., 2014a) | |
| ABT-894 | ~60% | (Zhang et al., 2014a) | ||
| AZD1446 | ~30% | (Mather et al., 2014) | ||
| Sazetidine | ~23% | (Quik et al., 2013a) | ||
| TC2696 | ~30% | (Quik et al., 2013a) | ||
| TC-8831 | ~25–50% | (Johnston et al., 2013; Quik et al., 2013a; Zhang et al., 2013) | ||
| TC-10600 | ~30% | (Quik et al., 2013a) | ||
|
| ||||
| α7 selective agonist | ABT-107 | ~60% | (Zhang et al., 2014b) | |
| ABT-126 | ~60% | (Zhang et al., 2015) | ||
| AQW051 | ~60% | (Di Paolo et al., 2014) | ||
|
| ||||
| β2* nonselective antagonist | Mecamylamine | (Bordia et al., 2010; Bordia et al., 2016) | ||
|
| ||||
| mAChRs | Nonselective antagonist | Dicyclomine | 60% | (Ding et al., 2011) |
| Atropine | No effect | (Bordia et al., 2016) | ||
|
| ||||
| M4 PAM | VU0467154, VU0476406 | 20–65% | (Shen et al., 2015) | |
Several nAChR subtypes appear to be involved in the antidyskinetic effect of nicotine. Studies in 6-OHDA-lesioned genetically modified mice have shown that nicotine reduces LIDs via β2* and α7 nAChRs. Specifically, it appears that β2* nAChRs are required for the appearance of LIDs as well as for the antidyskinetic effect of nicotine, with the relevant receptors being the α4β2* and α6β2* nAChR subtypes (Huang et al., 2011b; Quik et al., 2012a; Bordia et al., 2015). By contrast, studies with α7 nAChR null mice show that these receptors partly suppress the occurrence of LIDs (Quik et al., 2013b). Altogether, these findings suggested that nAChR subtype selective drugs may be beneficial therapeutic agents for LIDs management.
Studies to determine the usefulness of nAChR drugs to reduce LIDs focused on β2* nAChRs as these are the main subtype expressed in the nigrostriatal pathway. β2* nAChR agonists such as A-85380, sazetidine, TC-2696, TI-10165, TC-8831, TC-10600, ABT-089 and ABT-894 decreased LIDs by 20–60% in dyskinetic rats and primates (Huang et al., 2011a; Bordia et al., 2013; Johnston et al., 2013; Quik et al., 2013a; Zhang et al., 2013; Quik et al., 2014; Zhang et al., 2014a) (Table 2). These drugs decreased LIDs severity in most dyskinetic animals without worsening parkinsonism. Importantly, no tolerance developed with any of the doses tested. Thus, drugs targeting the β2* nAChR subtype alone appear to be a good therapeutic approach to decrease LIDs.
As mentioned earlier, nicotine also exerts its antidyskinetic effect via α7 nAChRs. Although this receptor subtype is not densely expressed in the basal ganglia, it is widely expressed on other neuronal circuits that regulate basal ganglia function (Quik et al., 2015b). Therefore, studies were carried out in monkeys to test the ability of the α7 nAChR agonists AQW051, ABT-107 and ABT-126 to modulate LIDs expression (Di Paolo et al., 2014; Zhang et al., 2014b; Quik et al., 2015b; Zhang et al., 2015) (Table 2). Interestingly, ABT-107 and ABT-126 decreased LIDs to the same extent as β2* nAChR agonists in moderately lesioned animals (Table 2). In addition, co-administration of α7 and β2* nAChR agonists did not increase the extent by which either type of drug alone decreases LIDs suggesting they exert their therapeutic effect through a common mechanism of action. Conversely, only α7 nAChR drug treatment ameliorated LIDs in severely-lesioned animals (Zhang et al., 2015). This is probably because β2* nAChRs are markedly reduced with severe nigrostriatal damage while α7 nAChR expression is less affected due their neuronal localization.
Overall, the evidence thus far indicates that β2* and α7 nAChR agonists are effective to alleviate LIDs. Thus, both classes of drugs may be promising antidyskinetic agents to test in the clinical setting, with β2* nAChR drugs likely being more effective in the earlier stages of the disease.
b. Involvement of mAChRs in LIDs
Non selective muscarinic receptor antagonism has been shown to decrease (Ding et al., 2011) or not affect (Bordia et al., 2016) LIDs expression (Table 2). This discrepancy may be due to differences in drug dosing or timing. This possibility is supported by studies showing that the order of mAChR and DA receptor activation influences MSN activity (Hernandez-Flores et al., 2015) which could in turn affect behavioral outcome. The non-selective nature of the antagonists used in these studies may also contribute to their discrepant results. In fact, more recent studies with selective antagonist show that enhanced M4 mAChR signaling via positive allosteric modulators reduced LTP induction in D1 MSNs and decreased dyskinesias (Shen et al., 2015) (Table 2). Importantly, the actions of M4 mAChRs may be additive to enhance D1 MSN excitability or conversely oppose it depending on whether they are activated before or after D1 receptors (Hernandez-Flores et al., 2015). This observation may explain why a decrease in LIDs is reported with M4 PAMs (Shen et al., 2015) as well as with muscarinic receptor blockade (Ding et al., 2011; Won et al., 2014).
c. Optogenetic and pharmacogenetic evidence demonstrating a role for striatal ChIs and cholinergic receptors in LIDs
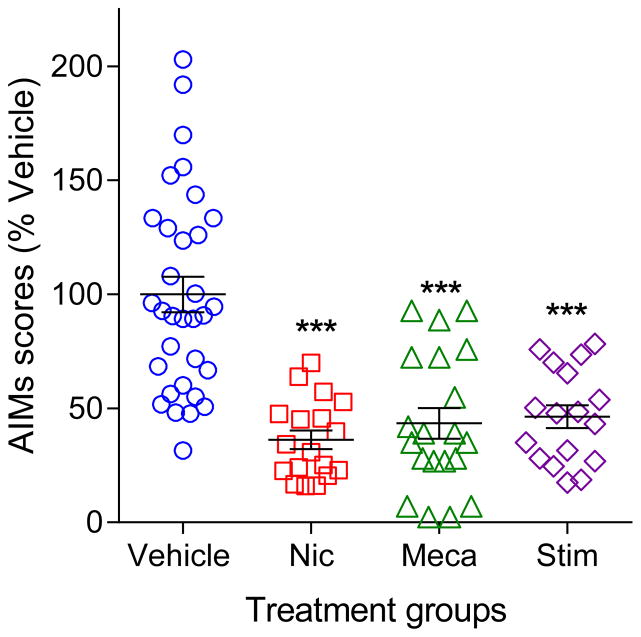
The advent of optogenetics and chemogenetics has broadened our understanding of the role of cholinergic interneurons in striatal function. For instance, studies have shown that synchronous activation of ChIs modulates DA release primarily via nAChRs and drives GABA release from DA terminals (Cachope et al., 2012; Threlfell et al., 2012; Nelson et al., 2014a; Nelson et al., 2014b; Wang et al., 2014). In addition, striatal ChI firing drives spontaneous muscarinic activation in MSNs (Mamaligas and Ford, 2016). With respect to LIDs, our ongoing work using ChAT-cre mice expressing channelrhodopsin in striatal ChIs shows that selective striatal cholinergic activation regulates LIDs expression in L-dopa-treated 6-OHDA lesioned mice (Bordia et al., 2016). Specifically, prolonged optical ChI stimulation decreased LIDs to a similar extent as that previously observed with nicotine and nAChR antagonist treatment (Fig. 1) (Bordia et al., 2008; Bordia et al., 2010; Bordia et al., 2016). This observation supports the hypothesis that striatal nAChR desensitization underlies nicotine’s antidyskinetic effect as prolonged ChI stimulation may result in a relatively large release of acetylcholine that induces nAChR desensitization similar to that observed with nicotine exposure (Mamaligas et al., 2016). Importantly, the effect of optical stimulation was prevented by pre-treatment with the general nAChR antagonist mecamylamine, further implicating an involvement for nAChRs (Bordia et al., 2016). In addition, the decrease in LIDs with optical stimulation was associated with an increase in c-Fos+ ChAT neurons but decreases in c-Fos+ non-ChAT neurons, indicating a role for this immediate early gene (Bordia et al., 2016).
Fig. 1.
Nicotine, mecamylamine and striatal cholinergic stimulation similarly decrease L-dopa-induced AIMs. Four groups of mice were rendered parkinsonian by unilateral intracranial injection of 6-OHDA and treated with L-dopa until stably dyskinetic as previously (Huang et al., 2011b; Quik et al., 2013a; Quik et al., 2013b; Bordia et al., 2015). Briefly, one group of mice received vehicle treatment while the mice in the nicotinic drug treatment groups were chronically exposed to nicotine (Nic, drinking water for at least 1 month) or injected with the nAChR blocker mecamylamine (Meca, sc, 30 min before L-dopa), and rated for L-dopa-induced AIMs. To directly asses the role for striatal cholinergic interneurons, ChAT-Cre mice expressing channelrhodopsin were optically stimulated (Stim) >20 ms pulses every 0.5 s for the whole 2 h of L-dopa time course. All three treatment conditions reduced AIMs to a similar extent. Prolonged cholinergic interneuron stimulation results in a relatively large release of acetylcholine that induces nAChR desensitization and a subsequent decrease in nAChR-mediated function similar to that observed with long-term nicotine exposure and mecamylamine. Thus. these combined data support the hypothesis that decreasing nAChR-mediated signaling alleviates LIDs.
In regard to the involvement of mAChRs in LIDs regulation, our optogenetic work thus far indicates that non-selective mAChR activation facilitates LIDs. This is based on our data showing that short optical stimulation of ChIs increases LIDs via an interaction at mAChRs (Bordia et al., 2016). Interestingly, short pulse durations enhanced LIDs in L-dopa-primed dyskinetic as well as non-dyskinetic mice (Bordia et al., 2016). Recent pharmacogenetic studies in lesioned ChAT-Cre rats expressing excitatory designer receptors exclusively activated by designer drugs (DREADD) have also shown that striatal ChI activation potentiates the therapeutic effect of L-dopa. However, it aggravates LIDs and D2 agonist-induced dyskinesias without affecting D1 agonist-induced dyskinesias (Aldrin-Kirk et al., 2018). The authors conclude the increase in LIDs with ChI activation arises from muscarinic signaling in MSNs and pre-synaptic glutamatergic terminals (Aldrin-Kirk et al., 2018).
Overall, these data provide further evidence for the importance of striatal ChIs in LIDs development and expression. Further studies of how the nicotinic cholinergic system, and specifically nAChR agonist treatment, modulates ChI activity and cholinergic receptor function are critical for the development of novel therapies for PD management.
4. Concluding remarks
Striatal ChIs regulate striatal activity and play an essential role in basal ganglia function. Thus, advancing our understanding of the alterations that occur in cholinergic signaling in disorders such as LIDs is critical for the development of novel tools that will allow for pharmacological manipulation of cholinergic transmission. Specifically, drugs targeting various nAChR subtypes such the β2* and α7 nAChRs as well as M4 mAChRs appear to be effective interventions to regulate cholinergic transmission and provide some relief for basal ganglia disorders such as dyskinesias.
Acknowledgments
Funding provided by NIH grant R56NS095965-01A1
References
- Aceves Buendia JJ, Tiroshi L, Chiu WH, Goldberg JA. Selective remodeling of glutamatergic transmission to striatal cholinergic interneurons after dopamine depletion. The European journal of neuroscience. 2017 doi: 10.1111/ejn.13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16:448–458. doi: 10.1002/mds.1090. [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiological reviews. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcacer C, Andreoli L, Sebastianutto I, Jakobsson J, Fieblinger T, Cenci MA. Chemogenetic stimulation of striatal projection neurons modulates responses to Parkinson’s disease therapy. J Clin Invest. 2017;127:720–734. doi: 10.1172/JCI90132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrin-Kirk P, Heuer A, Rylander Ottosson D, Davidsson M, Mattsson B, Bjorklund T. Chemogenetic modulation of cholinergic interneurons reveals their regulating role on the direct and indirect output pathways from the striatum. Neurobiol Dis. 2018;109:148–162. doi: 10.1016/j.nbd.2017.10.010. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Kimura M, Graybiel AM. Temporal and spatial characteristics of tonically active neurons of the primate’s striatum. J Neurophysiol. 1995;73:1234–1252. doi: 10.1152/jn.1995.73.3.1234. [DOI] [PubMed] [Google Scholar]
- Bastide MF, Meissner WG, Picconi B, Fasano S, Fernagut PO, Feyder M, Francardo V, Alcacer C, Ding Y, Brambilla R, Fisone G, Jon Stoessl A, Bourdenx M, Engeln M, Navailles S, De Deurwaerdere P, Ko WK, Simola N, Morelli M, Groc L, Rodriguez MC, Gurevich EV, Quik M, Morari M, Mellone M, Gardoni F, Tronci E, Guehl D, Tison F, Crossman AR, Kang UJ, Steece-Collier K, Fox S, Carta M, Angela Cenci M, Bezard E. Pathophysiology of L-dopa-induced motor and non-motor complications in Parkinson’s disease. Progress in neurobiology. 2015;132:96–168. doi: 10.1016/j.pneurobio.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Bennett BD, Callaway JC, Wilson CJ. Intrinsic membrane properties underlying spontaneous tonic firing in neostriatal cholinergic interneurons. J Neurosci. 2000;20:8493–8503. doi: 10.1523/JNEUROSCI.20-22-08493.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BD, Wilson CJ. Synaptic regulation of action potential timing in neostriatal cholinergic interneurons. J Neurosci. 1998;18:8539–8549. doi: 10.1523/JNEUROSCI.18-20-08539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard V, Normand E, Bloch B. Phenotypical characterization of the rat striatal neurons expressing muscarinic receptor genes. J Neurosci. 1992;12:3591–3600. doi: 10.1523/JNEUROSCI.12-09-03591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Albin RL. The cholinergic system and Parkinson disease. Behav Brain Res. 2011;221:564–573. doi: 10.1016/j.bbr.2009.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam JP, Wainer BH, Smith AD. Characterization of cholinergic neurons in the rat neostriatum. A combination of choline acetyltransferase immunocytochemistry, Golgi-impregnation and electron microscopy. Neuroscience. 1984;12:711–718. doi: 10.1016/0306-4522(84)90165-9. [DOI] [PubMed] [Google Scholar]
- Bordia T, Campos C, Huang L, Quik M. Continuous and intermittent nicotine treatment reduces L-3,4-dihydroxyphenylalanine (L-DOPA)-induced dyskinesias in a rat model of Parkinson’s disease. J Pharmacol Exp Ther. 2008;327:239–247. doi: 10.1124/jpet.108.140897. [DOI] [PubMed] [Google Scholar]
- Bordia T, Campos C, McIntosh JM, Quik M. Nicotinic receptor-mediated reduction in L-DOPA-induced dyskinesias may occur via desensitization. J Pharmacol Exp Ther. 2010;333:929–938. doi: 10.1124/jpet.109.162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordia T, Grady SR, McIntosh JM, Quik M. Nigrostriatal damage preferentially decreases a subpopulation of alpha6beta2* nAChRs in mouse, monkey, and Parkinson’s disease striatum. Mol Pharmacol. 2007;72:52–61. doi: 10.1124/mol.107.035998. [DOI] [PubMed] [Google Scholar]
- Bordia T, McGregor M, McIntosh JM, Drenan RM, Quik M. Evidence for a role for alpha6(*) nAChRs in l-dopa-induced dyskinesias using Parkinsonian alpha6(*) nAChR gain-of-function mice. Neuroscience. 2015;295:187–197. doi: 10.1016/j.neuroscience.2015.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordia T, McIntosh JM, Quik M. The nicotine-mediated decline in l-dopa-induced dyskinesias is associated with a decrease in striatal dopamine release. J Neurochem. 2013;125:291–302. doi: 10.1111/jnc.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordia T, Perez XA, Heiss J, Zhang D, Quik M. Optogenetic activation of striatal cholinergic interneurons regulates L-dopa-induced dyskinesias. Neurobiol Dis. 2016;91:47–58. doi: 10.1016/j.nbd.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotchie J. Antidyskinetic actions of amantadine in Parkinson’s disease: are benefits maintained in the long term? Expert Rev Neurother. 2010;10:871–873. doi: 10.1586/ern.10.70. [DOI] [PubMed] [Google Scholar]
- Cachope R, Mateo Y, Mathur BN, Irving J, Wang HL, Morales M, Lovinger DM, Cheer JF. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell reports. 2012;2:33–41. doi: 10.1016/j.celrep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Blockade of M2-like muscarinic receptors enhances long-term potentiation at corticostriatal synapses. The European journal of neuroscience. 1998;10:3020–3023. doi: 10.1111/j.1460-9568.1998.00348.x. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Acetylcholine-mediated modulation of striatal function. Trends Neurosci. 2000;23:120–126. doi: 10.1016/s0166-2236(99)01501-5. [DOI] [PubMed] [Google Scholar]
- Carrillo-Reid L, Tecuapetla F, Vautrelle N, Hernandez A, Vergara R, Galarraga E, Bargas J. Muscarinic enhancement of persistent sodium current synchronizes striatal medium spiny neurons. J Neurophysiol. 2009;102:682–690. doi: 10.1152/jn.00134.2009. [DOI] [PubMed] [Google Scholar]
- Cenci MA. Presynaptic Mechanisms of l-DOPA-Induced Dyskinesia: The Findings, the Debate, and the Therapeutic Implications. Front Neurol. 2014;5:242. doi: 10.3389/fneur.2014.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci MA, Konradi C. Maladaptive striatal plasticity in l-DOPA-induced dyskinesia. Prog Brain Res. 2010;183C:209–233. doi: 10.1016/S0079-6123(10)83011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Lena C, Clementi F, Moretti M, Rossi FM, Le Novere N, McIntosh JM, Gardier AM, Changeux JP. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci. 2003;23:7820–7829. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contant C, Umbriaco D, Garcia S, Watkins KC, Descarries L. Ultrastructural characterization of the acetylcholine innervation in adult rat neostriatum. Neuroscience. 1996;71:937–947. doi: 10.1016/0306-4522(95)00507-2. [DOI] [PubMed] [Google Scholar]
- Dajas-Bailador F, Wonnacott S. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol Sci. 2004;25:317–324. doi: 10.1016/j.tips.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Darmopil S, Martin AB, De Diego IR, Ares S, Moratalla R. Genetic inactivation of dopamine D1 but not D2 receptors inhibits L-DOPA-induced dyskinesia and histone activation. Biological psychiatry. 2009;66:603–613. doi: 10.1016/j.biopsych.2009.04.025. [DOI] [PubMed] [Google Scholar]
- Dautan D, Huerta-Ocampo I, Witten IB, Deisseroth K, Bolam JP, Gerdjikov T, Mena-Segovia J. A major external source of cholinergic innervation of the striatum and nucleus accumbens originates in the brainstem. J Neurosci. 2014;34:4509–4518. doi: 10.1523/JNEUROSCI.5071-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino M, Kalisch R, Czisch M, Larramendy C, Ricatti J, Taravini IR, Trenkwalder C, Murer MG, Auer DP, Gershanik OS. Mapping the effects of three dopamine agonists with different dyskinetogenic potential and receptor selectivity using pharmacological functional magnetic resonance imaging. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2007;32:1911–1921. doi: 10.1038/sj.npp.1301329. [DOI] [PubMed] [Google Scholar]
- Di Paolo T, Gregoire L, Feuerbach D, Elbast W, Weiss M, Gomez-Mancilla B. AQW051, a novel and selective nicotinic acetylcholine receptor alpha7 partial agonist, reduces l-Dopa-induced dyskinesias and extends the duration of l-Dopa effects in parkinsonian monkeys. Parkinsonism & related disorders. 2014;20:1119–1123. doi: 10.1016/j.parkreldis.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Ding J, Guzman JN, Tkatch T, Chen S, Goldberg JA, Ebert PJ, Levitt P, Wilson CJ, Hamm HE, Surmeier DJ. RGS4-dependent attenuation of M4 autoreceptor function in striatal cholinergic interneurons following dopamine depletion. Nature neuroscience. 2006;9:832–842. doi: 10.1038/nn1700. [DOI] [PubMed] [Google Scholar]
- Ding JB, Guzman JN, Peterson JD, Goldberg JA, Surmeier DJ. Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron. 2010;67:294–307. doi: 10.1016/j.neuron.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Won L, Britt JP, Lim SA, McGehee DS, Kang UJ. Enhanced striatal cholinergic neuronal activity mediates L-DOPA-induced dyskinesia in parkinsonian mice. Proc Natl Acad Sci U S A. 2011;108:340–345. doi: 10.1073/pnas.1006511108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenan RM, Grady SR, Steele AD, McKinney S, Patzlaff NE, McIntosh JM, Marks MJ, Miwa JM, Lester HA. Cholinergic modulation of locomotion and striatal dopamine release is mediated by alpha6alpha4* nicotinic acetylcholine receptors. J Neurosci. 2010;30:9877–9889. doi: 10.1523/JNEUROSCI.2056-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvoisin RC. Cholinergic-anticholinergic antagonism in parkinsonism. Arch Neurol. 1967;17:124–136. doi: 10.1001/archneur.1967.00470260014002. [DOI] [PubMed] [Google Scholar]
- Engeln M, Bastide MF, Toulme E, Dehay B, Bourdenx M, Doudnikoff E, Li Q, Gross CE, Boue-Grabot E, Pisani A, Bezard E, Fernagut PO. Selective Inactivation of Striatal FosB/DeltaFosB-Expressing Neurons Alleviates L-DOPA-Induced Dyskinesia. Biological psychiatry. 2016;79:354–361. doi: 10.1016/j.biopsych.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Exley R, Clements MA, Hartung H, McIntosh JM, Franklin M, Bermudez I, Cragg SJ. Striatal dopamine transmission is reduced after chronic nicotine with a decrease in alpha6-nicotinic receptor control in nucleus accumbens. The European journal of neuroscience. 2013 doi: 10.1111/ejn.12298. [DOI] [PubMed] [Google Scholar]
- Exley R, Cragg SJ. Presynaptic nicotinic receptors: a dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. Br J Pharmacol. 2008;153(Suppl 1):S283–297. doi: 10.1038/sj.bjp.0707510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Glowinski J, Venance L. Effects of acute dopamine depletion on the electrophysiological properties of striatal neurons. Neurosci Res. 2007;58:305–316. doi: 10.1016/j.neures.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Foster DJ, Gentry PR, Lizardi-Ortiz JE, Bridges TM, Wood MR, Niswender CM, Sulzer D, Lindsley CW, Xiang Z, Conn PJ. M5 receptor activation produces opposing physiological outcomes in dopamine neurons depending on the receptor’s location. J Neurosci. 2014;34:3253–3262. doi: 10.1523/JNEUROSCI.4896-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DJ, Wilson JM, Remke DH, Mahmood MS, Uddin MJ, Wess J, Patel S, Marnett LJ, Niswender CM, Jones CK, Xiang Z, Lindsley CW, Rook JM, Conn PJ. Antipsychotic-like Effects of M4 Positive Allosteric Modulators Are Mediated by CB2 Receptor-Dependent Inhibition of Dopamine Release. Neuron. 2016;91:1244–1252. doi: 10.1016/j.neuron.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galarraga E, Hernandez-Lopez S, Reyes A, Miranda I, Bermudez-Rattoni F, Vilchis C, Bargas J. Cholinergic modulation of neostriatal output: a functional antagonism between different types of muscarinic receptors. J Neurosci. 1999;19:3629–3638. doi: 10.1523/JNEUROSCI.19-09-03629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Montes JR, Boronat-Garcia A, Lopez-Colome AM, Bargas J, Guerra-Crespo M, Drucker-Colin R. Is nicotine protective against Parkinson’s disease? An experimental analysis. CNS Neurol Disord Drug Targets. 2012;11:897–906. doi: 10.2174/1871527311201070897. [DOI] [PubMed] [Google Scholar]
- Gold SJ, Hoang CV, Potts BW, Porras G, Pioli E, Kim KW, Nadjar A, Qin C, LaHoste GJ, Li Q, Bioulac BH, Waugh JL, Gurevich E, Neve RL, Bezard E. RGS9-2 negatively modulates L-3,4-dihydroxyphenylalanine-induced dyskinesia in experimental Parkinson’s disease. J Neurosci. 2007;27:14338–14348. doi: 10.1523/JNEUROSCI.4223-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JA, Ding JB, Surmeier DJ. Muscarinic modulation of striatal function and circuitry. Handb Exp Pharmacol. 2012:223–241. doi: 10.1007/978-3-642-23274-9_10. [DOI] [PubMed] [Google Scholar]
- Goldberg JA, Teagarden MA, Foehring RC, Wilson CJ. Nonequilibrium calcium dynamics regulate the autonomous firing pattern of rat striatal cholinergic interneurons. J Neurosci. 2009;29:8396–8407. doi: 10.1523/JNEUROSCI.5582-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JA, Wilson CJ. Control of spontaneous firing patterns by the selective coupling of calcium currents to calcium-activated potassium currents in striatal cholinergic interneurons. J Neurosci. 2005;25:10230–10238. doi: 10.1523/JNEUROSCI.2734-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady S, Marks MJ, Wonnacott S, Collins AC. Characterization of nicotinic receptor-mediated [3H]dopamine release from synaptosomes prepared from mouse striatum. J Neurochem. 1992;59:848–856. doi: 10.1111/j.1471-4159.1992.tb08322.x. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990;13:244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- Gregorio ML, Wietzikoski EC, Ferro MM, Silveira JL, Vital MA, Da Cunha C. Nicotine induces sensitization of turning behavior in 6-hydroxydopamine lesioned rats. Neurotox Res. 2009;15:359–366. doi: 10.1007/s12640-009-9041-1. [DOI] [PubMed] [Google Scholar]
- Grondin R, Goulet M, Morissette M, Bedard PJ, Di Paolo T. Dopamine D1 receptor mRNA and receptor levels in the striatum of MPTP monkeys chronically treated with SKF-82958. Eur J Pharmacol. 1999;378:259–263. doi: 10.1016/s0014-2999(99)00482-3. [DOI] [PubMed] [Google Scholar]
- Hernandez-Flores T, Hernandez-Gonzalez O, Perez-Ramirez MB, Lara-Gonzalez E, Arias-Garcia MA, Duhne M, Perez-Burgos A, Prieto GA, Figueroa A, Galarraga E, Bargas J. Modulation of direct pathway striatal projection neurons by muscarinic M(4)-type receptors. Neuropharmacology. 2015;89:232–244. doi: 10.1016/j.neuropharm.2014.09.028. [DOI] [PubMed] [Google Scholar]
- Hersch SM, Gutekunst CA, Rees HD, Heilman CJ, Levey AI. Distribution of m1-m4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype-specific antibodies. J Neurosci. 1994;14:3351–3363. doi: 10.1523/JNEUROSCI.14-05-03351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumann R, Moratalla R, Herrero MT, Chakrabarty K, Drucker-Colin R, Garcia-Montes JR, Simola N, Morelli M. Dyskinesia in Parkinson’s disease: mechanisms and current non-pharmacological interventions. J Neurochem. 2014;130:472–489. doi: 10.1111/jnc.12751. [DOI] [PubMed] [Google Scholar]
- Howe AR, Surmeier DJ. Muscarinic receptors modulate N-, P-, and L-type Ca2+ currents in rat striatal neurons through parallel pathways. J Neurosci. 1995;15:458–469. doi: 10.1523/JNEUROSCI.15-01-00458.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LZ, Campos C, Ly J, Ivy Carroll F, Quik M. Nicotinic receptor agonists decrease L-dopa-induced dyskinesias most effectively in partially lesioned parkinsonian rats. Neuropharmacology. 2011a;60:861–868. doi: 10.1016/j.neuropharm.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LZ, Grady SR, Quik M. Nicotine reduces L-DOPA-induced dyskinesias by acting at beta2* nicotinic receptors. J Pharmacol Exp Ther. 2011b;338:932–941. doi: 10.1124/jpet.111.182949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot P, Johnston TH, Koprich JB, Fox SH, Brotchie JM. The pharmacology of L-DOPA-induced dyskinesia in Parkinson’s disease. Pharmacol Rev. 2013;65:171–222. doi: 10.1124/pr.111.005678. [DOI] [PubMed] [Google Scholar]
- Jennings KA, Platt NJ, Cragg SJ. The impact of a parkinsonian lesion on dynamic striatal dopamine transmission depends on nicotinic receptor activation. Neurobiol Dis. 2015;82:262–268. doi: 10.1016/j.nbd.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon J, Dencker D, Wortwein G, Woldbye DP, Cui Y, Davis AA, Levey AI, Schutz G, Sager TN, Mork A, Li C, Deng CX, Fink-Jensen A, Wess J. A subpopulation of neuronal M4 muscarinic acetylcholine receptors plays a critical role in modulating dopamine-dependent behaviors. J Neurosci. 2010;30:2396–2405. doi: 10.1523/JNEUROSCI.3843-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston TH, Huot P, Fox SH, Koprich JB, Szeliga KT, James JW, Graef JD, Letchworth SR, Jordan KG, Hill MP, Brotchie JM. TC-8831, a nicotinic acetylcholine receptor agonist, reduces L-DOPA-induced dyskinesia in the MPTP macaque. Neuropharmacology. 2013;73:337–347. doi: 10.1016/j.neuropharm.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Kaiser S, Wonnacott S. alpha-bungarotoxin-sensitive nicotinic receptors indirectly modulate [(3)H]dopamine release in rat striatal slices via glutamate release. Mol Pharmacol. 2000;58:312–318. doi: 10.1124/mol.58.2.312. [DOI] [PubMed] [Google Scholar]
- Katzenschlager R, Sampaio C, Costa J, Lees A. Anticholinergics for symptomatic management of Parkinson’s disease. The Cochrane database of systematic reviews. 2003:CD003735. doi: 10.1002/14651858.CD003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci. 1993;13:4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koos T, Tepper JM. Dual cholinergic control of fast-spiking interneurons in the neostriatum. J Neurosci. 2002;22:529–535. doi: 10.1523/JNEUROSCI.22-02-00529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koranda JL, Cone JJ, McGehee DS, Roitman MF, Beeler JA, Zhuang X. Nicotinic receptors regulate the dynamic range of dopamine release in vivo. J Neurophysiol. 2014;111:103–111. doi: 10.1152/jn.00269.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosillo P, Zhang YF, Threlfell S, Cragg SJ. Cortical Control of Striatal Dopamine Transmission via Striatal Cholinergic Interneurons. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroiwa M, Hamada M, Hieda E, Shuto T, Sotogaku N, Flajolet M, Snyder GL, Hendrick JP, Fienberg A, Nishi A. Muscarinic receptors acting at pre- and post-synaptic sites differentially regulate dopamine/DARPP-32 signaling in striatonigral and striatopallidal neurons. Neuropharmacology. 2012;63:1248–1257. doi: 10.1016/j.neuropharm.2012.07.046. [DOI] [PubMed] [Google Scholar]
- Larramendy C, Taravini IR, Saborido MD, Ferrario JE, Murer MG, Gershanik OS. Cabergoline and pramipexole fail to modify already established dyskinesias in an animal model of parkinsonism. Behav Brain Res. 2008;194:44–51. doi: 10.1016/j.bbr.2008.06.021. [DOI] [PubMed] [Google Scholar]
- Lenz JD, Lobo MK. Optogenetic insights into striatal function and behavior. Behav Brain Res. 2013;255:44–54. doi: 10.1016/j.bbr.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Lim SA, Kang UJ, McGehee DS. Striatal cholinergic interneuron regulation and circuit effects. Front Synaptic Neurosci. 2014;6:22. doi: 10.3389/fnsyn.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren HS, Ohlin KE, Cenci MA. Differential involvement of D1 and D2 dopamine receptors in L-DOPA-induced angiogenic activity in a rat model of Parkinson’s disease. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2009;34:2477–2488. doi: 10.1038/npp.2009.74. [DOI] [PubMed] [Google Scholar]
- Livingstone PD, Wonnacott S. Nicotinic acetylcholine receptors and the ascending dopamine pathways. Biochem Pharmacol. 2009;78:744–755. doi: 10.1016/j.bcp.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Luo R, Janssen MJ, Partridge JG, Vicini S. Direct and GABA-mediated indirect effects of nicotinic ACh receptor agonists on striatal neurones. J Physiol. 2013;591:203–217. doi: 10.1113/jphysiol.2012.241786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv X, Dickerson JW, Rook JM, Lindsley CW, Conn PJ, Xiang Z. M1 muscarinic activation induces long-lasting increase in intrinsic excitability of striatal projection neurons. Neuropharmacology. 2017;118:209–222. doi: 10.1016/j.neuropharm.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintosh FC. The distribution of acetylcholine in the peripheral and the central nervous system. J Physiol. 1941;99:436–442. doi: 10.1113/jphysiol.1941.sp003913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamaligas AA, Cai Y, Ford CP. Nicotinic and opioid receptor regulation of striatal dopamine D2-receptor mediated transmission. Sci Rep. 2016;6:37834. doi: 10.1038/srep37834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamaligas AA, Ford CP. Spontaneous Synaptic Activation of Muscarinic Receptors by Striatal Cholinergic Neuron Firing. Neuron. 2016;91:574–586. doi: 10.1016/j.neuron.2016.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi M, Risso F, Viola C, Cavazzani P, Raiteri M. Direct evidence that release-stimulating alpha7* nicotinic cholinergic receptors are localized on human and rat brain glutamatergic axon terminals. J Neurochem. 2002;80:1071–1078. doi: 10.1046/j.0022-3042.2002.00805.x. [DOI] [PubMed] [Google Scholar]
- Marshall DL, Redfern PH, Wonnacott S. Presynaptic nicotinic modulation of dopamine release in the three ascending pathways studied by in vivo microdialysis: comparison of naive and chronic nicotine-treated rats. J Neurochem. 1997;68:1511–1519. doi: 10.1046/j.1471-4159.1997.68041511.x. [DOI] [PubMed] [Google Scholar]
- Mather J, Burdette Cebers, Posener Alexander, Leventer Ye, Poole Dunlop, Fox RAVENSCROFT, JOHNSTON HILL, BROTCHIE Potential of AZD1446, a novel nicotinic agonist, for the treatment of L-DOPA-induced dyskinesia in Parkinson’s disease. Society for Neuroscience Abstr. 2014;43:137.111/L131. [Google Scholar]
- Matsubayashi H, Amano T, Amano H, Sasa M. Excitation of rat striatal large neurons by dopamine and/or glutamate released from nerve terminals via presynaptic nicotinic receptor (A4beta2 type) stimulation. Jpn J Pharmacol. 2001;86:429–436. doi: 10.1254/jjp.86.429. [DOI] [PubMed] [Google Scholar]
- Maurice N, Liberge M, Jaouen F, Ztaou S, Hanini M, Camon J, Deisseroth K, Amalric M, Kerkerian-Le Goff L, Beurrier C. Striatal Cholinergic Interneurons Control Motor Behavior and Basal Ganglia Function in Experimental Parkinsonism. Cell reports. 2015;13:657–666. doi: 10.1016/j.celrep.2015.09.034. [DOI] [PubMed] [Google Scholar]
- McCallum SE, Parameswaran N, Bordia T, McIntosh JM, Grady SR, Quik M. Decrease in alpha3*/alpha6* nicotinic receptors but not nicotine-evoked dopamine release in monkey brain after nigrostriatal damage. Mol Pharmacol. 2005;68:737–746. doi: 10.1124/mol.105.012773. [DOI] [PubMed] [Google Scholar]
- McCallum SE, Parameswaran N, Perez XA, Bao S, McIntosh JM, Grady SR, Quik M. Compensation in pre-synaptic dopaminergic function following nigrostriatal damage in primates. J Neurochem. 2006;96:960–972. doi: 10.1111/j.1471-4159.2005.03610.x. [DOI] [PubMed] [Google Scholar]
- Merola A, Rizzi L, Zibetti M, Artusi CA, Montanaro E, Angrisano S, Lanotte M, Rizzone MG, Lopiano L. Medical therapy and subthalamic deep brain stimulation in advanced Parkinson’s disease: a different long-term outcome? J Neurol Neurosurg Psychiatry. 2014;85:552–559. doi: 10.1136/jnnp-2013-305271. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mash D, Hersh L, Bothwell M, Geula C. Cholinergic innervation of the human striatum, globus pallidus, subthalamic nucleus, substantia nigra, and red nucleus. J Comp Neurol. 1992;323:252–268. doi: 10.1002/cne.903230209. [DOI] [PubMed] [Google Scholar]
- Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2009;56:237–246. doi: 10.1016/j.neuropharm.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Nelson AB, Bussert TG, Kreitzer AC, Seal RP. Striatal Cholinergic Neurotransmission Requires VGLUT3. J Neurosci. 2014a;34:8772–8777. doi: 10.1523/JNEUROSCI.0901-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AB, Hammack N, Yang CF, Shah NM, Seal RP, Kreitzer AC. Striatal cholinergic interneurons Drive GABA release from dopamine terminals. Neuron. 2014b;82:63–70. doi: 10.1016/j.neuron.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima H, Suzuki S, Kon T, Funamizu Y, Ueno T, Haga R, Suzuki C, Arai A, Kimura T, Suzuki C, Meguro R, Miki Y, Yamada J, Migita K, Ichinohe N, Ueno S, Baba M, Tomiyama M. Morphologic changes of dendritic spines of striatal neurons in the levodopa-induced dyskinesia model. Mov Disord. 2014;29:336–343. doi: 10.1002/mds.25826. [DOI] [PubMed] [Google Scholar]
- Pakhotin P, Bracci E. Cholinergic interneurons control the excitatory input to the striatum. J Neurosci. 2007;27:391–400. doi: 10.1523/JNEUROSCI.3709-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavon N, Martin AB, Mendialdua A, Moratalla R. ERK phosphorylation and FosB expression are associated with L-DOPA-induced dyskinesia in hemiparkinsonian mice. Biological psychiatry. 2006;59:64–74. doi: 10.1016/j.biopsych.2005.05.044. [DOI] [PubMed] [Google Scholar]
- Perez-Rosello T, Figueroa A, Salgado H, Vilchis C, Tecuapetla F, Guzman JN, Galarraga E, Bargas J. Cholinergic control of firing pattern and neurotransmission in rat neostriatal projection neurons: role of CaV2.1 and CaV2.2 Ca2+ channels. J Neurophysiol. 2005;93:2507–2519. doi: 10.1152/jn.00853.2004. [DOI] [PubMed] [Google Scholar]
- Perez X, Ly J, McIntosh JM, Quik M. Chronic Nicotine Exposure Depresses Dopamine Release in Nonhuman Primate Nucleus Accumbens. J Pharmacol Exp Ther. 2012;342:335–344. doi: 10.1124/jpet.112.194084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez XA. Preclinical Evidence for a Role of the Nicotinic Cholinergic System in Parkinson’s Disease. Neuropsychol Rev. 2015;25:371–383. doi: 10.1007/s11065-015-9303-z. [DOI] [PubMed] [Google Scholar]
- Perez XA, Bordia T, McIntosh JM, Grady SR, Quik M. Long-term nicotine treatment differentially regulates striatal alpha6alpha4beta2* and alpha6(nonalpha4)beta2* nAChR expression and function. Mol Pharmacol. 2008;74:844–853. doi: 10.1124/mol.108.048843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez XA, Bordia T, McIntosh JM, Quik M. {Alpha}6{beta}2* and {alpha}4{beta}2* nicotinic receptors both regulate dopamine signaling with increased nigrostriatal damage: relevance to Parkinson’s disease. Mol Pharmacol. 2010;78:971–980. doi: 10.1124/mol.110.067561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez XA, McIntosh JM, Quik M. Long-term nicotine treatment down-regulates alpha6beta2* nicotinic receptor expression and function in nucleus accumbens. J Neurochem. 2013;127:762–771. doi: 10.1111/jnc.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez XA, Quik M. Focus on alpha4beta2* and alpha6beta2* nAChRs for Parkinson’s Disease Therapeutics. Mol Cell Pharmacol. 2011;3:1–6. [PMC free article] [PubMed] [Google Scholar]
- Perez XA, Zhang D, Bordia T, Quik M. Striatal D1 medium spiny neuron activation induces dyskinesias in parkinsonian mice. Mov Disord. 2017 doi: 10.1002/mds.26955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Bordia T, Zhang D, Perez XA. Nicotine and Nicotinic Receptor Drugs: Potential for Parkinson’s Disease and Drug-Induced Movement Disorders. Int Rev Neurobiol. 2015a;124:247–271. doi: 10.1016/bs.irn.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Quik M, Campos C, Bordia T, Strachan JP, Zhang J, McIntosh JM, Letchworth S, Jordan K. alpha4beta2 Nicotinic receptors play a role in the nAChR-mediated decline in L-dopa-induced dyskinesias in parkinsonian rats. Neuropharmacology. 2013a;71:191–203. doi: 10.1016/j.neuropharm.2013.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Campos C, Grady SR. Multiple CNS nicotinic receptors mediate L-dopa-induced dyskinesias: studies with parkinsonian nicotinic receptor knockout mice. Biochem Pharmacol. 2013b;86:1153–1162. doi: 10.1016/j.bcp.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Chen L, Parameswaran N, Xie X, Langston JW, McCallum SE. Chronic oral nicotine normalizes dopaminergic function and synaptic plasticity in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned primates. J Neurosci. 2006;26:4681–4689. doi: 10.1523/JNEUROSCI.0215-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Cox H, Parameswaran N, O’Leary K, Langston JW, Di Monte D. Nicotine reduces levodopa-induced dyskinesias in lesioned monkeys. Ann Neurol. 2007;62:588–596. doi: 10.1002/ana.21203. [DOI] [PubMed] [Google Scholar]
- Quik M, Mallela A, Chin M, McIntosh JM, Perez XA, Bordia T. Nicotine-mediated improvement in L-dopa-induced dyskinesias in MPTP-lesioned monkeys is dependent on dopamine nerve terminal function. Neurobiol Dis. 2013c;50:30–41. doi: 10.1016/j.nbd.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Mallela A, Ly J, Zhang D. Nicotine reduces established levodopa-induced dyskinesias in a monkey model of Parkinson’s disease. Mov Disord. 2013d;28:1398–1406. doi: 10.1002/mds.25594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Park KM, Hrachova M, Mallela A, Huang LZ, McIntosh JM, Grady SR. Role for alpha6 nicotinic receptors in l-dopa-induced dyskinesias in parkinsonian mice. Neuropharmacology. 2012a;63:450–459. doi: 10.1016/j.neuropharm.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Perez XA, Bordia T. Nicotine as a potential neuroprotective agent for Parkinson’s disease. Mov Disord. 2012b;27:947–957. doi: 10.1002/mds.25028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Perez XA, Grady SR. Role of alpha6 nicotinic receptors in CNS dopaminergic function: relevance to addiction and neurological disorders. Biochem Pharmacol. 2011;82:873–882. doi: 10.1016/j.bcp.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Sum JD, Whiteaker P, McCallum SE, Marks MJ, Musachio J, McIntosh JM, Collins AC, Grady SR. Differential declines in striatal nicotinic receptor subtype function after nigrostriatal damage in mice. Mol Pharmacol. 2003;63:1169–1179. doi: 10.1124/mol.63.5.1169. [DOI] [PubMed] [Google Scholar]
- Quik M, Wonnacott S. {alpha}6{beta}2* and {alpha}4{beta}2* Nicotinic Acetylcholine Receptors As Drug Targets for Parkinson’s Disease. Pharmacol Rev. 2011;63:938–966. doi: 10.1124/pr.110.003269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Zhang D, McGregor M, Bordia T. Alpha7 nicotinic receptors as therapeutic targets for Parkinson’s disease. Biochem Pharmacol. 2015b;97:399–407. doi: 10.1016/j.bcp.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Zhang D, Perez XA, Bordia T. Role for the nicotinic cholinergic system in movement disorders; therapeutic implications. Pharmacol Ther. 2014 doi: 10.1016/j.pharmthera.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn N, Marsden CD, Parkes JD. Complicated response fluctuations in Parkinson’s disease: response to intravenous infusion of levodopa. Lancet. 1982;2:412–415. doi: 10.1016/s0140-6736(82)90442-1. [DOI] [PubMed] [Google Scholar]
- Quinn NP. Classification of fluctuations in patients with Parkinson’s disease. Neurology. 1998;51:S25–29. doi: 10.1212/wnl.51.2_suppl_2.s25. [DOI] [PubMed] [Google Scholar]
- Raz A, Feingold A, Zelanskaya V, Vaadia E, Bergman H. Neuronal synchronization of tonically active neurons in the striatum of normal and parkinsonian primates. J Neurophysiol. 1996;76:2083–2088. doi: 10.1152/jn.1996.76.3.2083. [DOI] [PubMed] [Google Scholar]
- Revy D, Jaouen F, Salin P, Melon C, Chabbert D, Tafi E, Concetta L, Langa F, Amalric M, Kerkerian-Le Goff L, Marie H, Beurrier C. Cellular and Behavioral Outcomes of Dorsal Striatonigral Neuron Ablation: New Insights into Striatal Functions. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nature neuroscience. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Rizzone MG, Fasano A, Daniele A, Zibetti M, Merola A, Rizzi L, Piano C, Piccininni C, Romito LM, Lopiano L, Albanese A. Long-term outcome of subthalamic nucleus DBS in Parkinson’s disease: from the advanced phase towards the late stage of the disease? Parkinsonism & related disorders. 2014;20:376–381. doi: 10.1016/j.parkreldis.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Salin P, Lopez IP, Kachidian P, Barroso-Chinea P, Rico AJ, Gomez-Bautista V, Coulon P, Kerkerian-Le Goff L, Lanciego JL. Changes to interneuron-driven striatal microcircuits in a rat model of Parkinson’s disease. Neurobiol Dis. 2009;34:545–552. doi: 10.1016/j.nbd.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, Grady SR. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- Schaeffer E, Pilotto A, Berg D. Pharmacological strategies for the management of levodopa-induced dyskinesia in patients with Parkinson’s disease. CNS Drugs. 2014;28:1155–1184. doi: 10.1007/s40263-014-0205-z. [DOI] [PubMed] [Google Scholar]
- Shen W, Plotkin JL, Francardo V, Ko WK, Xie Z, Li Q, Fieblinger T, Wess J, Neubig RR, Lindsley CW, Conn PJ, Greengard P, Bezard E, Cenci MA, Surmeier DJ. M4 Muscarinic Receptor Signaling Ameliorates Striatal Plasticity Deficits in Models of L-DOPA-Induced Dyskinesia. Neuron. 2015;88:762–773. doi: 10.1016/j.neuron.2015.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Tian X, Day M, Ulrich S, Tkatch T, Nathanson NM, Surmeier DJ. Cholinergic modulation of Kir2 channels selectively elevates dendritic excitability in striatopallidal neurons. Nature neuroscience. 2007;10:1458–1466. doi: 10.1038/nn1972. [DOI] [PubMed] [Google Scholar]
- Smith AD, Bolam JP. The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends Neurosci. 1990;13:259–265. doi: 10.1016/0166-2236(90)90106-k. [DOI] [PubMed] [Google Scholar]
- Solis O, Garcia-Montes JR, Gonzalez-Granillo A, Xu M, Moratalla R. Dopamine D3 Receptor Modulates l-DOPA-Induced Dyskinesia by Targeting D1 Receptor-Mediated Striatal Signaling. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez LM, Solis O, Aguado C, Lujan R, Moratalla R. L-DOPA Oppositely Regulates Synaptic Strength and Spine Morphology in D1 and D2 Striatal Projection Neurons in Dyskinesia. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Takada Y, Nagai N, Urano T, Takada A. Nicotine increases stress-induced serotonin release by stimulating nicotinic acetylcholine receptor in rat striatum. Synapse. 1998;28:212–219. doi: 10.1002/(SICI)1098-2396(199803)28:3<212::AID-SYN4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Tambasco N, Simoni S, Marsili E, Sacchini E, Murasecco D, Cardaioli G, Rossi A, Calabresi P. Clinical aspects and management of levodopa-induced dyskinesia. Parkinsons Dis. 2012;2012:745947. doi: 10.1155/2012/745947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Bishop C, Walker PD. Dopamine D1 and D2 receptor contributions to L-DOPA-induced dyskinesia in the dopamine-depleted rat. Pharmacology, biochemistry, and behavior. 2005;81:887–893. doi: 10.1016/j.pbb.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Threlfell S, Clements MA, Khodai T, Pienaar IS, Exley R, Wess J, Cragg SJ. Striatal muscarinic receptors promote activity dependence of dopamine transmission via distinct receptor subtypes on cholinergic interneurons in ventral versus dorsal striatum. J Neurosci. 2010;30:3398–3408. doi: 10.1523/JNEUROSCI.5620-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 2012;75:58–64. doi: 10.1016/j.neuron.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Tubert C, Taravini IR, Flores-Barrera E, Sanchez GM, Prost MA, Avale ME, Tseng KY, Rela L, Murer MG. Decrease of a Current Mediated by Kv1.3 Channels Causes Striatal Cholinergic Interneuron Hyperexcitability in Experimental Parkinsonism. Cell reports. 2016;16:2749–2762. doi: 10.1016/j.celrep.2016.08.016. [DOI] [PubMed] [Google Scholar]
- van Vulpen EH, van der Kooy D. Striatal cholinergic interneurons: birthdates predict compartmental localization. Brain Res Dev Brain Res. 1998;109:51–58. doi: 10.1016/s0165-3806(98)00012-1. [DOI] [PubMed] [Google Scholar]
- Wang L, Shang S, Kang X, Teng S, Zhu F, Liu B, Wu Q, Li M, Liu W, Xu H, Zhou L, Jiao R, Dou H, Zuo P, Zhang X, Zheng L, Wang S, Wang C, Zhou Z. Modulation of dopamine release in the striatum by physiologically relevant levels of nicotine. Nature communications. 2014;5:3925. doi: 10.1038/ncomms4925. [DOI] [PubMed] [Google Scholar]
- Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, Tkatch T, Lovinger DM, Surmeier DJ. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–452. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Westin JE, Vercammen L, Strome EM, Konradi C, Cenci MA. Spatiotemporal pattern of striatal ERK1/2 phosphorylation in a rat model of L-DOPA-induced dyskinesia and the role of dopamine D1 receptors. Biological psychiatry. 2007;62:800–810. doi: 10.1016/j.biopsych.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Chang HT, Kitai ST. Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J Neurosci. 1990;10:508–519. doi: 10.1523/JNEUROSCI.10-02-00508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Goldberg JA. Origin of the slow afterhyperpolarization and slow rhythmic bursting in striatal cholinergic interneurons. J Neurophysiol. 2006;95:196–204. doi: 10.1152/jn.00630.2005. [DOI] [PubMed] [Google Scholar]
- Won L, Ding Y, Singh P, Kang UJ. Striatal cholinergic cell ablation attenuates L-DOPA induced dyskinesia in Parkinsonian mice. J Neurosci. 2014;34:3090–3094. doi: 10.1523/JNEUROSCI.2888-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonnacott S, Kaiser S, Mogg A, Soliakov L, Jones IW. Presynaptic nicotinic receptors modulating dopamine release in the rat striatum. Eur J Pharmacol. 2000;393:51–58. doi: 10.1016/s0014-2999(00)00005-4. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. Cholinergic neurons in the caudate-putamen complex proper are intrinsically organized: a combined Evans blue and acetylcholinesterase analysis. Brain Res Bull. 1981;7:487–507. doi: 10.1016/0361-9230(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Thompson AD, Jones CK, Lindsley CW, Conn PJ. Roles of the M1 muscarinic acetylcholine receptor subtype in the regulation of basal ganglia function and implications for the treatment of Parkinson’s disease. J Pharmacol Exp Ther. 2012;340:595–603. doi: 10.1124/jpet.111.187856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Nashmi R, McKinney S, Cai H, McIntosh JM, Lester HA. Chronic nicotine selectively enhances alpha4beta2* nicotinic acetylcholine receptors in the nigrostriatal dopamine pathway. J Neurosci. 2009;29:12428–12439. doi: 10.1523/JNEUROSCI.2939-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Surmeier DJ. Muscarinic (m2/m4) receptors reduce N- and P-type Ca2+ currents in rat neostriatal cholinergic interneurons through a fast, membrane-delimited, G-protein pathway. J Neurosci. 1996;16:2592–2604. doi: 10.1523/JNEUROSCI.16-08-02592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Bordia T, McGregor M, McIntosh JM, Decker MW, Quik M. ABT-089 and ABT-894 reduce levodopa-induced dyskinesias in a monkey model of Parkinson’s disease. Mov Disord. 2014a;29:508–517. doi: 10.1002/mds.25817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Mallela A, Sohn D, Carroll FI, Bencherif M, Letchworth S, Quik M. Nicotinic receptor agonists reduce L-DOPA-induced dyskinesias in a monkey model of Parkinson’s disease. J Pharmacol Exp Ther. 2013;347:225–234. doi: 10.1124/jpet.113.207639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, McGregor M, Bordia T, Perez XA, McIntosh JM, Decker MW, Quik M. alpha7 nicotinic receptor agonists reduce levodopa-induced dyskinesias with severe nigrostriatal damage. Mov Disord. 2015;30:1901–1911. doi: 10.1002/mds.26453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, McGregor M, Decker MW, Quik M. The alpha7 nicotinic receptor agonist ABT-107 decreases L-Dopa-induced dyskinesias in parkinsonian monkeys. J Pharmacol Exp Ther. 2014b;351:25–32. doi: 10.1124/jpet.114.216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nature neuroscience. 2004;7:581–582. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- Zhang L, Dong Y, Doyon WM, Dani JA. Withdrawal from chronic nicotine exposure alters dopamine signaling dynamics in the nucleus accumbens. Biological psychiatry. 2012;71:184–191. doi: 10.1016/j.biopsych.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Doyon WM, Clark JJ, Phillips PE, Dani JA. Controls of tonic and phasic dopamine transmission in the dorsal and ventral striatum. Mol Pharmacol. 2009;76:396–404. doi: 10.1124/mol.109.056317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Yamada M, Gomeza J, Basile AS, Wess J. Multiple muscarinic acetylcholine receptor subtypes modulate striatal dopamine release, as studied with M1-M5 muscarinic receptor knock-out mice. J Neurosci. 2002;22:6347–6352. doi: 10.1523/JNEUROSCI.22-15-06347.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nature neuroscience. 2001;4:1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Wilson CJ, Dani JA. Cholinergic interneuron characteristics and nicotinic properties in the striatum. Journal of neurobiology. 2002;53:590–605. doi: 10.1002/neu.10150. [DOI] [PubMed] [Google Scholar]
- Ztaou S, Maurice N, Camon J, Guiraudie-Capraz G, Kerkerian-Le Goff L, Beurrier C, Liberge M, Amalric M. Involvement of Striatal Cholinergic Interneurons and M1 and M4 Muscarinic Receptors in Motor Symptoms of Parkinson’s Disease. J Neurosci. 2016;36:9161–9172. doi: 10.1523/JNEUROSCI.0873-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]