Abstract
Exposure to Early Life Stress (ELS) is associated with behavioral-related alterations, increases in body mass index and higher systolic blood pressure in humans. Postnatal maternal separation and early weaning (MSEW) is a mouse model of neglect characterized by a long-term dysregulation of the neuroendocrine system.
Objectives
Given the contribution of adrenal-derived hormones to the development of obesity, we hypothesized that exposure to MSEW could contribute to worsen the cardiometabolic function in response to chronic high fat diet (HF) feeding by promoting adipose tissue expansion and insulin resistance.
Subjects
MSEW was performed in C57BL/6 mice from postnatal days 2–16 and weaned at postnatal day 17. Undisturbed litters weaned at postnatal day 21 served as the control (C) group. At the weaning day, mice were placed on a low fat diet (LF) or HF for 16 weeks.
Results
When fed a LF, male and female mice exposed to MSEW display similar body weight but increased fat mass compared to controls. However, when fed a HF, only female MSEW mice display increased body weight, fat mass and adipocyte hypertrophy compared with controls. Also, female MSEW mice display evidence of an early onset of cardiometabolic risk factors, including hyperinsulinemia, glucose intolerance and hypercholesterolemia. Yet, both male and female MSEW mice fed a HF show increased blood pressure compared with controls.
Conclusions
This study shows that MSEW promotes a sex-specific dysregulation of the adipose tissue expansion and glucose homeostasis that precedes the development of obesity-induced hypertension.
Keywords: maternal separation, obesity, hypertension, adipose tissue, sex-differences
Introduction
The prevalence of obesity has increased to epidemic proportions and has become a major heath issue worldwide in children and adults1–3. Obesity induces hypertension, a major risk factor for cardiovascular disease (CVD) that affects more than 70 million Americans 4 In addition, metabolic syndrome, also known as syndrome X, refers to a cluster of metabolic conditions that can lead to heart disease. The National Cholesterol Education Program’s Adult Treatment Panel III report (ATP III) identified six components of the metabolic syndrome that relate to CVD: abdominal obesity, insulin resistance with/without glucose intolerance, atherogenic dyslipidemia, proinflammatory/prothrombotic states and increased blood pressure5.
To reduce the burden of uncontrolled hypertension morbidity and mortality, it is critical to assess the contribution of less-studied modifiable risk factors. During early life, approximately one third of the population is exposed to a wide range of unpredictable environments, including physical or sexual abuse, neglect, loss of a parent or caregiver, or a natural disaster. Child neglect is the most prevalent form of child maltreatment in the United States, and poses a serious public health concern6. Child neglect is frequently defined as the failure of a parent or caregiver to provide proper nutrition, clothing, shelter, medical care, or supervision to the degree that the child’s health is at risk. Children who survive such episodes experience long-lasting psychological and behavioral problems7.
Epidemiological studies identified the exposure to all these forms of early life stress (ELS) as an independent risk factor for type 2 diabetes and ischemic heart disease, in part by increasing body mass index (BMI) and systolic blood pressure (SBP) 8–11. Findings from the Georgia Stress and Heart Study (GSH) including 213 African Americans and 181 European Americans, revealed that individuals exposed to multiple ELS factors display a greater increase in blood pressure (BP) levels compared to control individuals in a 23-year follow up period 12,13. Furthermore, the 1958 British Birth Cohort Study including 6,714 members, revealed that metabolic risk was higher among people with psychological distress in childhood only and persist across the life course 14 More recent studies highlighted that when the environment is harsh and unpredictable, a fast life history strategy is adaptive. That is, in these environments, somatic efforts are unsuitable. However, fast life-history strategies has been linked to impulsivity and a focus on short-term goals as a causal factor for high obesity risk 15. A role for maternal care during early life has been closely observed. In fact, poor quality of the early maternal-child relationship was associated with a higher prevalence of adolescent obesity16. For instance, in a 3.5-year prospective study conducted in 6-month old babies, a significant association was demonstrated between low maternal sensitivity (i.e. timely and appropriate response on the part of the mother towards the cues of her infant) and high body mass indices.17
Although the prevalence of hypertension is higher in men than in age-matched, pre-menopausal women 18, population-based studies during the last decade show that obesity affects women more than age-matched men, also identified as the cause of the 3-fold increase in the risk for hypertension in premenopausal women13–15. Also, stress-related disorders (e.g., depression and anxiety), obesity and hypertension are comorbidities observed more frequently in women beginning in adolescence 19–22. Importantly, a growing body of evidence suggests that long-term effects of ELS may impact women’s susceptibility to gain weight and develop insulin resistance in a greater fashion than men 8,23. Yet, an established experimental paradigm to determine the sex-specific effects of ELS on the origins of obesity-induced hypertension is missing, demanding the validation of models to decipher the causative mechanisms of this relationship.
A widely-used animal model for the investigation of molecular and behavioral responses to ELS is the maternal separation paradigm. Developed as a rat model, pups are subjected to daily maternal separation (180 min=MS180) from postnatal days 2–14 24–27. Efforts have been made to adapt the paradigm to mice in order to study in-depth molecular pathways using transgenic approaches. However, attempts to replicate the MS180 phenotype in mice have displayed mixed results 28–33. The lack of consistency with regard to maternal separation in mice seems to be driven by at least two components: both the duration of separation and the quality of maternal care provided to the pups upon being returned to the nest. For these reasons, Simens’ group developed a model called Maternal Separation with Early Weaning (MSEW), which includes separation periods that occur over a broad range of postnatal ages and an additional component of early weaning 34 As developed, MSEW is considered a mouse model of neglect that has been associated with the overactivation of the hypothalamic-pituitary-adrenal (HPA) axis, changes in the brain architecture and misprogramming of the vascular function in mice fed a standard chow diet 34–37. To date, this paradigm may serve as a unique tool to decipher the link between ELS and CVD risk. In this study, we combine MSEW with an obesogenic diet starting at weaning to model the interplay between these two environmental stressors. Therefore, we hypothesized that MSEW will worsen the development of diet-induced obesity and hypertension via increases in: 1) fat mass and adipocyte hypertrophy; 2) cardiometabolic risk factors (e.g. plasma insulin, leptin, lipids and glucose intolerance); and 3) blood pressure and heart rate after 16 weeks of high fat diet (HF) feeding. Furthermore, we performed this study in male and female mice to investigate a potential sex-specific cardiometabolic susceptibility secondary to MSEW exposure.
Methods
Animal model
All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and approved and monitored by the Institutional Animal Care and Use Committee at the University of Kentucky. Mice were given ad libitum access to food and water and housed in a pathogen-free environment with constant temperature and humidity with a 14:10 hour light: dark cycle38. Maternal separation with early weaning (MSEW) protocol was performed in C57BL/6 mice as follows: pups were separated from their mothers by transferring the pups to a clean cage in an incubator (30 ± 1° C) for 4 hours from postnatal day 2 to day 5 and for 8 hours from postnatal day 6 to 16 of life with early weaning at postnatal day 17. Normally reared, non-handled litters remained with their mother and served as the control group and were weaned at postnatal day 21. For the LF-fed groups, we used 9 control litters and 11 MSEW litters. For the HF-fed groups, we used 12 control litters and 14 MSEW litters. Litters were included when size ranged from 6 to 8 pups and a different diet was randomly assigned.
Experimental Design
Dams were fed a regular chow diet (Teklad 8604, Madison, WI). Upon weaning, offspring were fed a LF (10% kcal from fat, F6256, BioServ, Frenchtown, NJ) or a HF (60% kcal from fat, F2685, BioServ, Frenchtown, NJ) for 16 weeks. All the experiments were conducted in both male and female mice. Body weight was measured weekly and body composition was measured monthly by an Echo Magnetic resonance imaging system (Echo-MRI; Echo Medical Systems, Houston, TX). At week 13, mice were fasted for 16 hours to perform an oral glucose tolerance test (OGTT). After an oral gavage of D-glucose (2 g/kg body weight)39, blood glucose measurements were taken via tail prick at 0, 15, 30, 60, and 120 minutes using a glucometer (Accu-check, Roche, Germany). Area under the curve was calculated using GraphPad Prism version 5.01 for Windows (GraphPad Software, San Diego, CA). At weeks 7 and 14, food intake was measured in individually-housed mice for 5 consecutive days. By the end of week 14, a subset of mice was implanted with radiotelemetry for the monitoring of blood pressure, heart rate and locomotor activity. At the end of the study, animals were anesthetized with ketamine/xylazine (100/10 mg/kg, IP) for exsanguination by cardiac puncture and tissue harvesting. Liver and white adipose tissue (WAT) were collected and snap frozen for mRNA quantification or fixed in 10% buffered formalin for histological analysis.
Histological analysis of adipose tissue morphology
The gonadal (epididymal or periovaric) WAT depots were collected in 10% buffered formalin, fixed for 24 hours, and paraffin-embedded. Following, 5-µm sections were cut and stained with hematoxylin and eosin. Images of slides were obtained at ×10 magnification. Using the “detect edges,” image threshold, and object count features of NIS Elements software (Nikon Instruments, Inc., Tokyo, Japan), the area of each adipocyte and cell number within a 1500 × 1500 µm2 measurement frame were quantified. Adipocyte size and number were quantified on two measurement frames within each section of adipose tissue (n=3 sections/mice) from mice in each group. The blinded quantification of the adipocyte cross-sectional size was expressed as ratio of the MSEW /Control.
Quantitative real time PCR
RNA was extracted from dissected gonadal WAT and liver using Aurum total RNA mini kit (Bio-Rad, Hercules, CA) as previously described 40. Briefly, total mRNA was extracted from tissues using the TRIZOL reagent (Invitrogen Life Technologies, Carlsbad, CA) according to the manufacturer’s protocol. The mRNA concentrations were quantified via the NanoDrop 2000 spectrophotometer (Applied Biosystems, Waltham, MA) and reverse transcription was performed using the iScript cDNA Synthesis kit (Bio-Rad). The levels of mRNA expression were assessed by quantitative real-time PCR using a CFX 96 Touch Detection System (Bio-Rad) and iTaq Univer SYBR Green supermix (Bio-Rad). Data analysis was performed using the relative quantification method (2−ΔΔCt), in which relative mRNA expression for target mRNAs was compared to a constitutively expressed gene (i.e., 18S) within mRNA samples from tissues. Primer sequences for SYBR Green chemistry were designed using the Primer Express Software 3.0 for RT-PCR (Applied Biosystems).
Plasma risk factors for obesity and insulin resistance
Plasma was collected using EDTA 7.5% solution. Leptin, insulin, corticosterone and aldosterone EIA kits (Cayman, Ann Arbor, MI) and adiponectin (Invitrogen, Waltham, MA) were performed following manufacturer’s specifications. Serum cholesterol and plasma triglyceride were determined by an enzymatic kit (Wako Diagnostics, Richmond, VA).
Blood pressure measurement using radiotelemetry
Blood pressure was measured by radiotelemetry during week 16 as previously described 41. Briefly, anesthetized (isoflurane) male and female mice fed a HF were implanted with carotid artery catheters and telemetry devices (Data Sciences, Inc., St. Paul, MN) during week 15 of HF feeding, allowed 10 days to recover. Then, mean arterial pressure (MAP), heart rate (HR) and locomotor activity within the cage were continuously recorded during 3 consecutive days using the Dataquest ART Acquisition program (Data Sciences International, St. Paul, MN) and expressed in 24 hour averages.
Statistical Analysis
Prior to analysis, all observations were averaged within litters, and analyses were performed with litters as experimental units. All data are presented as mean±SEM. Differences in means among groups and treatments were compared by 2-way ANOVA to assess the effect of diet and separation in each sex. To adjust for the false positive rate due to multiple testing we have used Benjamini-Hochberg adjustment. Fisher’s LSD post-hoc tests were calculated in all significant ANOVAs.
Results
Supplementary information is available at IJO’s website at the end of the article and before the references.
Effect of MSEW on body composition, organ weight and food intake
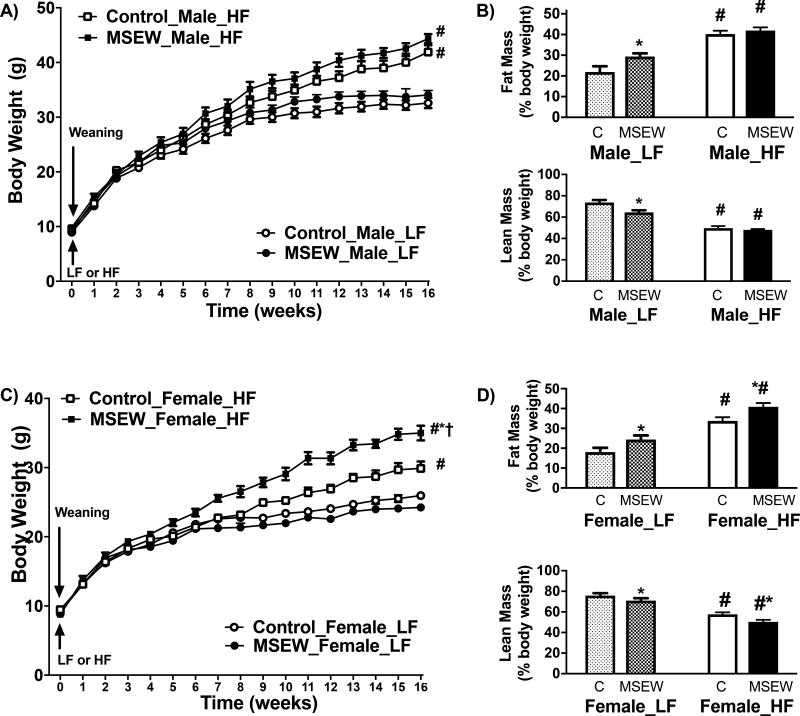
Body weight was not different in MSEW and control weanlings at postnatal day 21 (Fig. S1). Likewise, adult male and female MSEW mice fed a LF displayed comparable body weight (Fig. 1) and tissue weights (Table S1) but increased adiposity (Fig. 1B and 1D) after a 16-week period. In HF-fed males, body weight (Fig. 1A), fat mass (Fig. 1B, top) and lean mass (Fig. 1B, bottom) percentages were not statistically different between MSEW and control mice. However, HF-fed female MSEW mice showed increased body weight (Fig. 1C), fat mass (Fig. 1D, top) and reduced lean mass (Fig. 1D, bottom) compared to controls. In addition, HF diet also increased liver, gWAT and scWAT depots weights in female MSEW compared to controls (Table S1). These increases in body weight and adiposity in female MSEW mice seem independent of significant variations in food intake (Table S2).
Figure 1.
Effect of MSEW in body weight (BW) and body composition after 16 weeks on LF or HF diet initiated at weaning: A) BW and B) body composition in male mice; C) BW and D) body composition in female mice. MSEW male LF (n=9), control male LF (n=9), MSEW male HF (n=13), control male HF (n=11), MSEW female LF (n=11), control female LF (n=9), MSEW female HF (n=14), control female HF (n=12).
Test between subjects Body weight
Male mice: Diet= F ratio (107), Prob>F (<0.0001); Separation= F ratio (3.9), Prob>F (0.053); Diet*Separation= F ratio (0.36), Pro>F (0.54). Female mice: Diet= F ratio (84), Prob>F (<0.0001); Separation= F ratio (5.8), Prob>F (<0.002); Diet*Separation= F ratio (7.4), Pro>F (<0.01). * P separation, # P diet, †P interaction.
Test between subjects % fat mass
Male mice: Diet= F ratio (101), Prob>F (<0.0001); Separation= F ratio (12.8), Prob>F (0.001); Diet*Separation= F ratio (6.6), Pro>F (0.01). Female mice: Diet= F ratio (64), Prob>F (<0.0001); Separation= F ratio (11.2), Prob>F (<0.002); Diet*Separation= F ratio (0.03), Pro>F (0.85).
Effect of MSEW on adipose tissue morphology
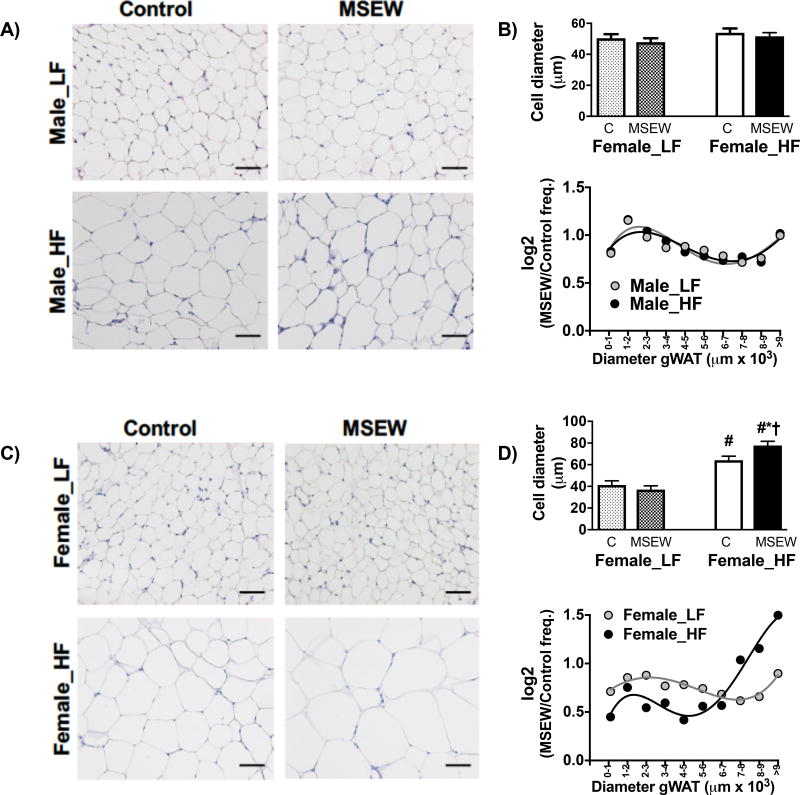
Male mice showed no influence of MSEW on gWAT morphology (Fig. 2A), as adipocyte diameter and area (Fig. 2B, top) were similar between groups. The log (MSEW/ control frequency) ratio revealed that male mice have a similar adipocyte diameter regardless of separation or diet factors (Fig. 2B, bottom). However, adipocyte size was increased in female MSEW mice fed a HF (Fig. 2C). Specifically, female MSEW mice displayed hypertrophy of adipocytes, increases in cell diameter and reduced cell number compared to control mice (Fig. 2D, top). Also, MSEW displayed increased frequency of the larger diameter adipocytes (Fig. 2D, bottom). Yet, scWAT adipocytes area and diameter were similar in MSEW and control female mice fed a HF (Fig. S2); however, adipocytes from 3000–5000 µm area were bigger in MSEW mice compared to controls. This data suggests that gWAT expansion is an important contributor for the greater fat mass observed in HF-fed female MSEW mice. Because of these changes in adipocyte morphology observed in female mice, we analyzed body fat distribution by MRI (Fig. S3). Female MSEW and control mice displayed similar subcutaneous adipose tissue (SAT) volume (Fig. S3C); however, visceral adipose tissue (VAT) was increased in female MSEW fed compared to controls regardless of diet (Fig. S3D).
Figure 2.
Effect of MSEW on white adipose tissue morphology after 16 weeks on LF or HF diet initiated at weaning: A) adipocyte morphology and B) adipocyte diameter histogram in male mice; C) adipocyte morphology and B) adipocyte diameter histogram in female mice. N=4–5 per group. * P separation, # P diet, †P interaction.
Test between subjects adipocyte hypertrophy:
Male mice: Diet= Prob>F (NS); Separation= Prob>F (NS); Diet*Separation= Pro>F (NS). Female mice: Diet= F ratio (71), Prob>F (<0.0001); Separation= F ratio (1.6), Prob>F (0.22); Diet*Separation= F ratio (5.7), Pro>F (0.03).
Effect of MSEW on adipose tissue gene expression
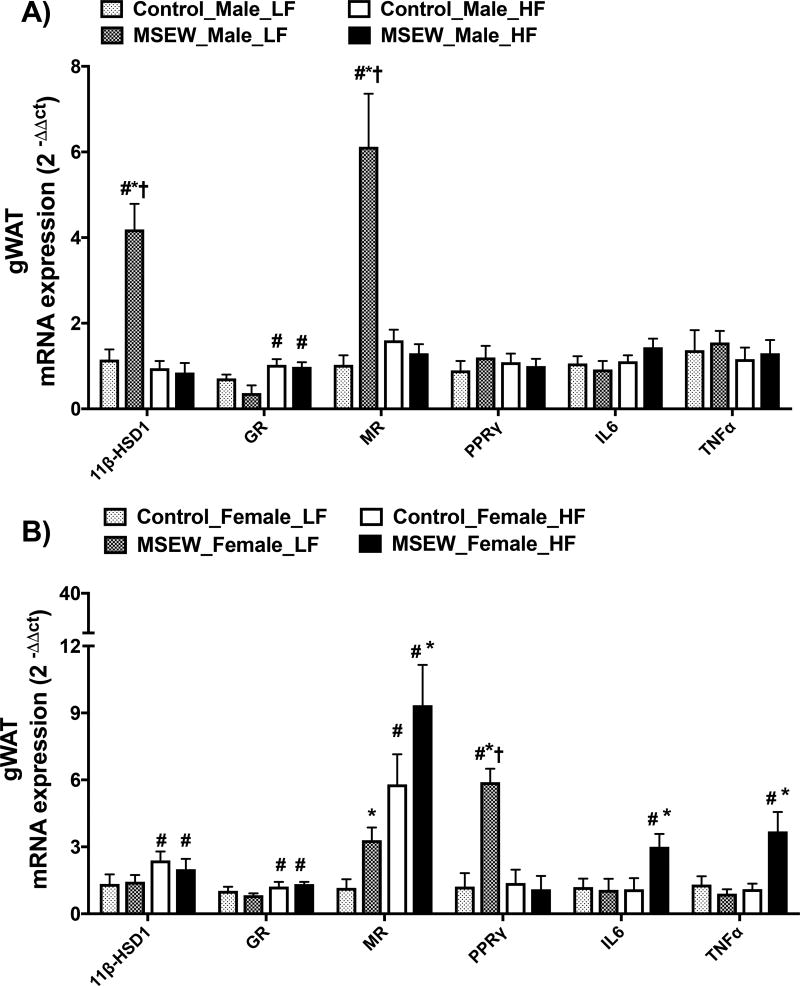
MSEW increased 11βHSD-1 mRNA abundance in gWAT only in male mice fed a LF (Fig. 3A). Exposure to MSEW did not influence GR mRNA expression in mice fed a LF, while increased it in all groups of HF-fed mice (Fig. 3B). Conversely, MR expression was increased in male and female MSEW mice fed a LF; however, only female MSEW mice fed a HF showed further increases in MR expression compared with controls. We also determined the mRNA abundance of PPARg, a master regulator of adipocyte differentiation, which was increased only in LF-fed female MSEW mice compared with controls but not in HF-fed mice. Finally, no significant effects of separation or diet were found in IL-6 and TNF-α mRNA expression in male mice, while MSEW increased these inflammatory markers only in female mice fed a HF. The mRNA levels of the housekeeper gene 18S in all groups are shown in Table S3, and the sequence for RT-PCR primers is listed in Table S4.
Figure 3.
Effect of MSEW on gonadal WAT (gWAT) mRNA expression after 16 weeks on LF or HF initiated at weaning in A) male and B) female mice. N=5–8 per group.
* P separation, # P diet, †P interaction.
Test between subjects gWAT 11bHSD1 gene expression
Male mice: Diet= F ratio (26), Prob>F (<0.0001); Separation= F ratio (17.7), Prob>F (0.0004); Diet*Separation= F ratio (21), Pro>F (0.0002). Female mice: Diet= F ratio (5), Prob>F (<0.03); Separation= F ratio (0.33), Prob>F (0.57); Diet*Separation= F ratio (0.15), Pro>F (0.69).
Test between subjects gWAT MR gene expression
Male mice: Diet= F ratio (13), Prob>F (0.002); Separation= F ratio (16.4), Prob>F (0.0007); Diet*Separation= F ratio (21), Pro>F (0.0002). Female mice: Diet= F ratio (36), Prob>F (<0.0001); Separation= F ratio (13.5), Prob>F (<0.001); Diet*Separation= F ratio (0.87), Pro>F (0.35).
Test between subjects gWAT PPARg gene expression
Male mice: Diet= Prob>F (NS); Separation= Prob>F (NS); Diet*Separation= Pro>F (NS). Female mice: Diet= F ratio (5.5), Prob>F (0.02); Separation= F ratio (5), Prob>F (0.03); Diet*Separation= F ratio (6.3), Pro>F (0.02).
Effect of MSEW on plasma risk factors for obesity and insulin resistance
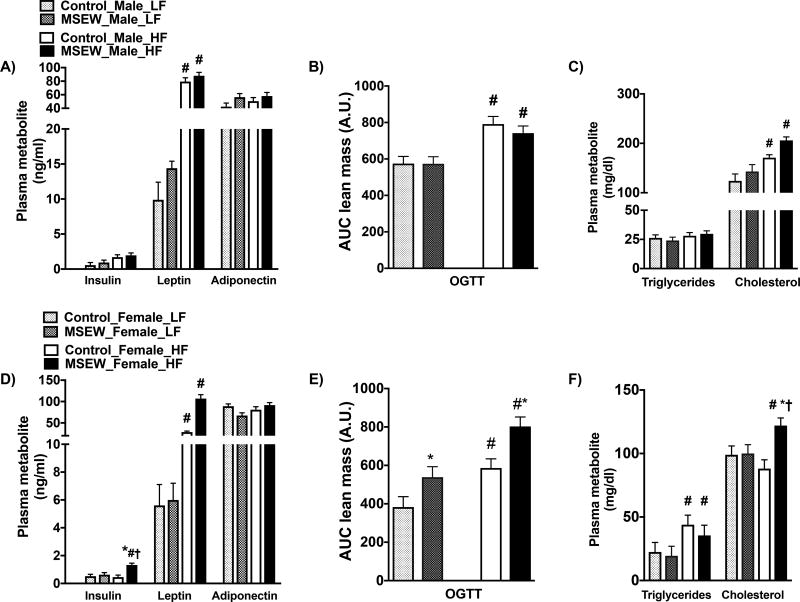
Overall, MSEW did not influence the plasma metabolic profile in mice fed a LF diet. While HF-fed male MSEW and control mice showed similar levels of plasma insulin (Fig. 4A), female MSEW mice displayed hyperinsulinemia compared with controls (Fig. 4D). HF induced comparable increases in plasma leptin in both groups and did not affect adiponectin. Furthermore, we determined the effect of MSEW on the glucose disposal.
Figure 4.
Effect of MSEW on the plasma metabolic profile after 16 weeks on HF initiated at weaning in males and female mice. Insulin, leptin and adiponectin in A) male and D) female mice. Oral glucose tolerance test (OGTT) in B) male and E) female mice. Plasma triglycerides and cholesterol in C) male and F) female mice. N=6–12 per group.
* P separation, # P diet, †P interaction.
Test between subjects insulin
Male mice: Diet= Prob>F (NS); Separation= Prob>F (NS); Diet*Separation= Pro>F (NS). Female mice: Diet= F ratio (5.2), Prob>F (<0.03); Separation= F ratio (13.5), Prob>F (<0.01); Diet*Separation= F ratio (0.03), Pro>F (0.85).
Test between subjects OGTT
Male mice: Diet= F ratio (23), Prob>F (<0.0001); Separation= F ratio (0.40), Prob>F (0.53); Diet*Separation= F ratio (0.37), Pro>F (0.55). Female mice: Diet= F ratio (20), Prob>F (<0.0001); Separation= F ratio (12.8), Prob>F (<0.001); Diet*Separation= F ratio (0.33), Pro>F (0.56).
Test between subjects Cholesterol
Male mice: Diet= F ratio (17), Prob>F (0.0003); Separation= F ratio (4.2), Prob>F (0.051); Diet*Separation= F ratio (0.38), Pro>F (0.54). Female mice: Diet= F ratio (0.64), Prob>F (0.43); Separation= F ratio (6.6), Prob>F (<0.02); Diet*Separation= F ratio (5.5), Pro>F (0.03).
Fasting glucose was not different between groups, though there was a trend for elevated fasting glucose with HF in both male and female MSEW mice (Table S5). OGTT was increased similarly in male MSEW and control mice fed a HF (Fig. 4B). However, female MSEW showed greater impairments in glucose tolerance in both LF and HF compared with control female mice (Fig. 4E). The atherogenic profile revealed that HF induced similar increases in plasma cholesterol in MSEW and control males (Fig.5C). However, in females, HF increased triglycerides similarly in both groups but significantly increased cholesterol levels in MSEW compared with controls (Fig. 4F).
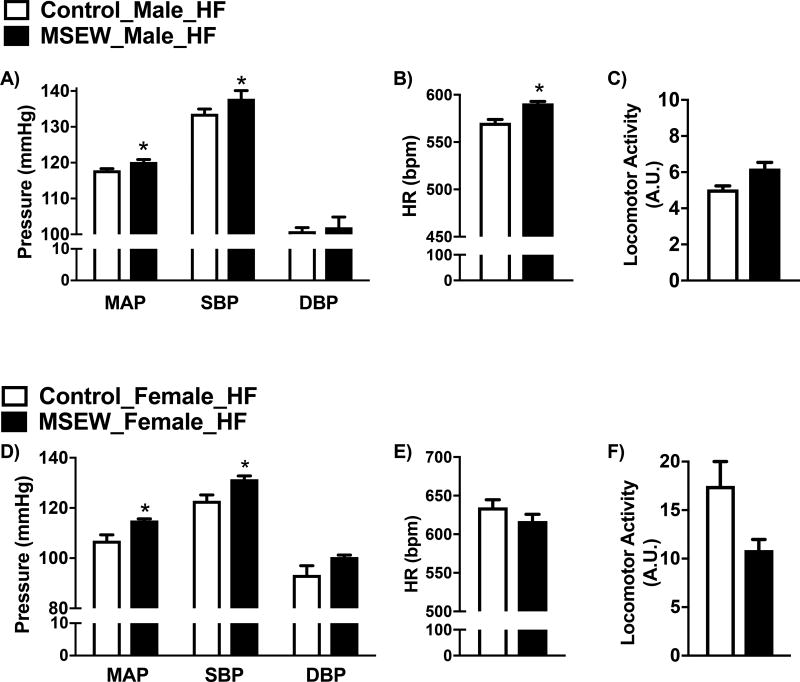
Figure 5.
Effect of MSEW on 24hr hemodynamic parameters after 16 weeks on HF initiated at weaning. A) mean arterial pressure (MAP), systolic blood pressure (SBP) and diastolic blood pressure (DBP); B) heart rate (HR) and C) locomotor activity in male mice. D) mean arterial pressure (MAP), systolic blood pressure (SBP) and diastolic blood pressure (DBP), E) heart rate (HR) and F) locomotor activity in female mice. N=4–5 per group. * P <0.05 vs. control.
Effect of MSEW on adrenal cortex-derived hormones
Table 1 shows similar plasma levels of corticosterone and aldosterone in MSEW and control mice fed a LF diet. HF-induced increases in these hormones in both sexes, although there were no differences between MSEW and control mice.
Table 1.
Adrenal-derived hormones in male and female mice fed 16 weeks on a LF or HF diet. N=6–9 per group. # P <0.05 vs. LF.
| Control LF |
MSEW LF |
Control HF |
MSEW HF |
||
|---|---|---|---|---|---|
| Corticosterone (ng/ml) | Male | 53±12 | 65±8 | 82.1±22.0 | 114.6±31.1# |
| Female | 49±9 | 42±11 | 72.9±15.9 | 139.4±28.5# | |
| Aldosterone (ng/ml) | Male | 8.9±2.5 | 11.9±2.3 | 129.1±25.11 # | 132.0±21.5# |
| Female | 63.9±27 | 101.8±21 | 333.7±70.7# | 447.6±65.7# |
Effect of MSEW on obesity-induced increases in blood pressure
MSEW increased 24 hour-MAP and SBP in male and female mice fed a HF (Fig. 5A and 5D). No differences were observed in DBP due to separation in either sexes. MSEW increased HR only in male mice (Fig. 5B and 5E), while no significant differences in either sex were observed in locomotor activity (Fig. 5C and 5F) compared with controls.
DISCUSSION
This study reveals that MSEW, a mouse model of neglect, heightens obesity-induced hypertension in male and female mice fed a HF since weanlings. Despite significant increases in blood pressure in both sexes, hypertension was accompanied by increased HR only in male mice exposed to MSEW. Conversely, HF induced greater percentages of fat mass, adipocyte hypertrophy, insulin resistance and hypercholesterolemia in female MSEW mice compared to female controls. Taken together, our study highlights that MSEW serves as a unique model to decipher sex-specific mechanisms linking ELS and cardiovascular risk in the onset of metabolic disease.
ELS is defined by exposure to a wide range of adverse childhood experiences such as abuse, neglect or loss during the first decade of life that imparts negative effects on health outcomes. Specifically, epidemiological studies indicate that adults exposed to ELS develop several risk factors for cardiovascular and metabolic disease including elevated systolic blood pressure (SBP), BMI, waist circumference, and resting HR 11,13,14,42. Moreover, ELS has a positive correlation with the clustering of metabolic risk biomarkers such as higher circulating levels of leptin and the acute phase proinflammatory mediator, C reactive protein (CRP), as well as elevations in plasma glucose and insulin 43–45. Among the mechanisms postulated, it was proposed that children that underwent ELS are at greater risk for negative affect and internalizing disorders such as depression and anxiety 46–48, which may contribute to behavioral eating disorders including unhealthy dietary choices 49,50,51. These alterations have been often linked to the early life dysregulation of the sympathetic nerve activity (SNA) and/or the HPA axis 52–54. Despite most of these studies have not been designed to test sex-specific effects on the cardiovascular and metabolic risk, a growing body of evidence shows that long-term effects of ELS may impact women’s susceptibility to gain weight and develop insulin resistance at a greater fashion 23,55–57; however, the potential origins for this predisposition remain unclear.
Maternal separation has been shown to induce detrimental effects on behavioral and neuroendocrine responses that mimic several aspects of ELS-induced derangements in humans24–27. Efforts have been made to adapt the paradigm to mice given the advances in murine studies since the C57Bl/6 mouse genome has been sequenced and satisfactorily annotated. However, anxiety and stress response in maternal separation in mice has been reported to be increased 28,29, decreased 30 or unchanged 31. A more comprehensive study in mice revealed that levels of maternal care (i.e. licking, nursing, covering, pup handling, and nesting) increase as a function of MS180 immediately after daily maternal separation, but long-term care remains unchanged, showing that C57Bl/6 mice are resilient to maternal separation, exhibiting subtle decreases in anxiety and unchanged stress-induced corticosterone response as adults, irrespective of separation duration58. Hence, the MSEW model has been generated with the goal to replicate a consistent phenotype. As adult, mice exposed to MSEW display alterations across several behavioral domains, increases in hyperactivity, anxiety, and depressive- like behavior 34,35,37.
To note, studies using maternal separation paradigms have been strongly focused on the behavioral aspects of the phenotype, reporting the absence in changes of pup mortality or morbidity, weight, or metabolic derangements. Accordingly, we found that neonates exposed to MSEW show normal BW compared with control mice at postnatal day 21. Thus, MSEW does not induce a catch-up growth effect in the pups and weanlings, which is a hallmark of low birth weight models associated with greater cardiovascular and metabolic risk later in life 59–61. Nevertheless, the long-term consequences in the cardiometabolic responses have been disregarded. We speculate that MSEW could not be restricted to psychological/psychosocial stress only, and may contribute with early metabolic disturbances that predispose to the development of obesity. Yet, the molecular mechanisms involved in such hypotheses have not been rigorously tested. Therefore, we cannot rule out the contribution of prolonged periods of food constraint with endocrine and metabolic alterations influencing the adipose tissue plasticity and glucose homeostasis during early life. While there is no direct evidence to support this association, the assessment of the body composition and metabolic function in adult mice exposed to models of maternal separation involving shorter periods of time, such as MS180, could be helpful to tease out the impact of the prolonged separation procedures combined with early weaning per se.
To investigate whether postnatal MSEW worsens the cardiometabolic function, we subjected these mice to two dietary conditions. First, we determined the long-term effect of MSEW on body composition and metabolic responses in mice fed a LF. MSEW did not influence BW, organ weight and adipose tissue morphology; however, MSEW increased the percentage of fat mass in both male and females. This data suggest that increased adiposity may predispose these mice to develop obesity-hypertension later in life. Thus, investigating the effect of stress (or glucocorticoids) early on adipocyte differentiation may provide some insights on the mechanisms by which MSEW increases fat mass in these mice. Second, we challenged MSEW to a secondary stressor (or “second hit”) by feeding them a HF starting at weaning. HF increased BP in male and female exposed to MSEW; however, adipocyte hypertrophy, impaired glucose disposal, and hypercholesterolemia were heightened only in female MSEW mice compared to controls. Further, LF-fed female MSEW mice showed an early onset of cardiometabolic risk including greater visceral fat deposition and impaired glucose tolerance. Taken together, these data indicate that the obesogenic diet induces a metabolic compromise in female MSEW mice that could play an important role in the lack of protective factors against cardiovascular risk.
Increased plasma glucocorticoids are a hallmark of obesity-associated metabolic derangements 62. However, despite the effect of HF, MSEW did not increase plasma corticosterone. Circulating glucocorticoids are tightly controlled by the activation of the HPA axis; however, tissue-specific availability is regulated by the receptor expression, affinity, and alterations in glucocorticoid metabolism and clearance 63–65. Intracellular levels of glucocorticoids are influenced by the enzyme 11β-HSD1, which converts inactive cortisone to its active form corticosterone 66. Thus, at the tissue level, the relative contribution of glucocorticoids and mineralocorticoid are influenced by the relative abundance of MR and GR, circulating levels of glucocorticoids and aldosterone, and local conversion of cortisone-cortisol by HSD1 and HSD2 67. Dasarzens and Farasse have reported that the adipocyte-specific deletion of GR did not affect body weight gain or adipose tissue differentiation and distribution; however, the lack of GR in adipocyte was associated with higher pro-inflammatory genes. They also showed that adipose tissue GR ablation did not correlate with insulin resistance or dyslipidemia, but was associated with disturbed glucose tolerance, supporting the notion that GR deletion may impair adipose tissue function but not its expansion during a high calorie diet 68. Recent studies demonstrate that MR (NR3C2) is expressed in adipose tissue and is implicated in both adipogenesis and adipose endocrine function 65,69. Since the MR binds to cortisol with 10-fold higher affinity than GR 70, high local concentration of cortisol within adipose tissue may activate both GR and MR. MR activation increases proinflammatory adipokine expression such as interleukin-6 (IL-6) 71. Conversely, MR blockade reduces inflammation in adipose tissue and increases the expression of adiponectin in heart and adipose tissue of obese, diabetic mice 72. Therefore, increased fat mass in LF-fed MSEW mice could be linked with a greater effect of mineralocorticoids at the tissue level secondary to excess of glucocorticoids. Interestingly, HF-induced MR mRNA upregulation was further augmented only in tissue from female MSEW mice, suggesting that the local actions of mineralocorticoids could be implicated in the mechanism by which MSEW promotes adipose tissue expansion in a sex-specific manner.
Given the important contribution of the liver to the glucocorticoid metabolism 73, we also determined the effect of MSEW on the hepatic expression of the enzyme 11β-HSD1. While HF-fed female MSEW mice showed a greater hepatic pro-adipogenic capacity (Fig. S4), normal hepatic gene expression in mice fed a LF supports the concept that increased adiposity could precede the metabolic derangements in these mice. Taken together, our study is consistent with the idea that MSEW-induced HPA dysregulation at tissue level could play an important role in promoting adiposity and metabolic dysfunction, particularly in female mice fed a HF.
It has been shown that diet-induced obesity starting at weaning upregulates systemic and adipose tissue glucocorticoid metabolism 74; however, a handful of studies have challenged maternally separated rodents to a obesogenic diet 75–77. Recently, our laboratory has shown that female rats exposed to maternal separation (3 hours a day, postnatal days 2–14) respond to a chronic HF by increasing body weight and fat mass compared with their control littermates 40. Nonetheless, this increase in adiposity was not associated with elevated blood pressure 40. Elevated levels of glucocorticoids during the postnatal “stress hyporesponsive period”, which takes place during the first two weeks in life in rodents, has been proposed as causal factor for the long-lasting increased HPA axis sensitivity to secondary stressors 24,36,78. Interestingly, we showed that the pharmacological inhibition of the corticosterone synthase during postnatal life abrogated the heightened effects of diet-induced obesity in female rats, suggesting that postnatal exposure to corticosterone appears to be a key event associated with the programming of the adipose tissue plasticity in females 40.
In summary, we have shown that MSEW serves as a unique approach for the study of the ELS-induced sexual dimorphism on the cardiovascular system, particularly in the onset of metabolic disease. Our previous work in a rat model of maternal separation 40 and the present study performed in a mouse model of neglect, demonstrate that female rodents exposed to postnatal stress display a lack of protective factors against diet-induced obesity, which may predispose them to develop worsened cardiovascular outcomes. As women show greater incidence of depression, stress and cardiovascular risk comorbidities 19,21,79, early life-induced alterations in the neuroendocrine mechanisms interplaying with cardiovascular pathways may place females at higher risk for these comorbidities during adult life. Our study contributes to the advancement of the Developmental Origins of Health and Disease (DOHaD) field by validating a paradigm linking ELS and cardiovascular risk, with the primary goal to provoke further clinical and experimental studies on this important translational research topic. Thus, interventions aimed at improving the quality of maternal-child interactions should consider assessing effects on children’s weight and examining potential mechanisms involving stress response and emotion regulation.
Supplementary Material
Acknowledgments
We gratefully acknowledge the outstanding technical support Timothy Mahanes and Sara Tenlep. We thank Wendy Katz for the assistance with tissue embedding and stain techniques.
SOURCES OF FUNDING
This study was supported by funds from the NIH National Heart, Lung, and Blood Institute to A.S.L (R00 HL111354), start-up funds from the University of Kentucky to A.S.L, and the pilot project from the University of Kentucky Center of Research in Obesity and Cardiovascular Disease COBRE P20 GM103527-06 to A.S.L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication. Springer Nature are providing this early version of the manuscript as a service to our customers. The manuscript will undergo copyediting, typesetting and a proof review before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers apply.
DISCLOSURES
None
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–14. doi: 10.1001/jama.2014.732. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24570244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organisation. Obesity and overweight. Fact sheet N°311. Fact sheet N. 2013:8–11. Available from: http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:Media+centre+Obesity+and+overweight#5.
- 3.Centers for Disease Control and Prevention. Adult Obesity Facts. DNPAO Data Stat. 2015 Available from: http://www.cdc.gov/obesity/data/adult.html.
- 4.Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011–2012. NCHS Data Brief. 2013:1–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24171916. [PubMed]
- 5.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and T of HBC in A (Adult TPI. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 6.U.S. Department of Health & Human Services. Child Maltreatment 2012. 2013 Available from: http://www.acf.hhs.gov/programs/cb/research-data-technology/statistics-research/child-maltreatment.
- 7.Child Welfare Information Gateway. Definitions of Child Abuse and Neglect. State Statut. 2011;2010:92. [Google Scholar]
- 8.Liu H, Umberson D. Gender, stress in childhood and adulthood, and trajectories of change in body mass. Soc Sci Med. 2015;139:61–9. doi: 10.1016/j.socscimed.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alciati A, Gesuele F, Casazza G, Foschi D. The relationship between childhood parental loss and metabolic syndrome in obese subjects. Stress Health. 2013;29:5–13. doi: 10.1002/smi.1435. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22190357. [DOI] [PubMed] [Google Scholar]
- 10.Slopen N, Kubzansky LD, McLaughlin KA, Koenen KC. Childhood adversity and inflammatory processes in youth: A prospective study. Psychoneuroendocrinology. 2013;38:188–200. doi: 10.1016/j.psyneuen.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pretty C, O’Leary DD, Cairney J, Wade TJ. Adverse childhood experiences and the cardiovascular health of children: a cross-sectional study. BMC Pediatr. 2013;13:208. doi: 10.1186/1471-2431-13-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su S, Wang X, Pollock JS, Treiber FA, Xu X, Snieder H, et al. Adverse childhood experiences and blood pressure trajectories from childhood to young adulthood: the Georgia stress and Heart study. Circulation. 2015;131:1674–81. doi: 10.1161/CIRCULATIONAHA.114.013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su S, Wang X, Kapuku GK, Treiber FA, Pollock DM, Harshfield GA, et al. Adverse childhood experiences are associated with detrimental hemodynamics and elevated circulating endothelin-1 in adolescents and young adults. Hypertension. 2014;64:201–7. doi: 10.1161/HYPERTENSIONAHA.113.02755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winning A, Glymour MM, McCormick MC, Gilsanz P, Kubzansky LD. Psychological Distress Across the Life Course and Cardiometabolic Risk: Findings From the 1958 British Birth Cohort Study. J Am Coll Cardiol. 2015;66:1577–86. doi: 10.1016/j.jacc.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 15.Manera Jon K, Dittmannb Andrea, Meltzera Andrea L, J KM. Implications of life-history strategies for obesity. doi: 10.1073/pnas.1620482114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson SE, Gooze RA, Lemeshow S, Whitaker RC. Quality of Early Maternal-Child Relationship and Risk of Adolescent Obesity. Pediatrics. 2012;129:132–40. doi: 10.1542/peds.2011-0972. Available from: http://pediatrics.aappublications.org/content/129/1/132%5Cn http://pediatrics.aappublications.org/content/129/1/132.full.pdf%5Cn http://pediatrics.aappublications.org/content/129/1/132.short%5Cn www.ncbi.nlm.nih.gov/pubmed/22201144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wendland BE, Atkinson L, Steiner M, Fleming AS, Pencharz P, Moss E, et al. Low maternal sensitivity at 6 months of age predicts higher BMI in 48 month old girls but not boys. Appetite. 2014;82:97–102. doi: 10.1016/j.appet.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart Disease and Stroke Statistics--2015 Update: A Report From the American Heart Association. 2015 doi: 10.1161/CIR.0000000000000152. Available from: http://circ.ahajournals.org/cgi/doi/10.1161/CIR.0000000000000152. [DOI] [PubMed]
- 19.Orth-Gomér K, Schneiderman N, Wang HX, Walldin C, Blom M, Jernberg T. Stress reduction prolongs life in women with coronary disease: The Stockholm women’s intervention trial for coronary heart disease (SWITCHD) Circ Cardiovasc Qual Outcomes. 2009;2:25–32. doi: 10.1161/CIRCOUTCOMES.108.812859. [DOI] [PubMed] [Google Scholar]
- 20.Vaccarino V, Johnson BD, Sheps DS, Reis SE, Kelsey SF, Bittner V, et al. Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia. J Am Coll Cardiol. 2007;50:2044–50. doi: 10.1016/j.jacc.2007.07.069. [DOI] [PubMed] [Google Scholar]
- 21.Möller-Leimkühler AM. Higher comorbidity of depression and cardiovascular disease in women: a biopsychosocial perspective. World J Biol Psychiatry. 2010;11:922–33. doi: 10.3109/15622975.2010.523481. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20950120. [DOI] [PubMed] [Google Scholar]
- 22.Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BWJH, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67:220–9. doi: 10.1001/archgenpsychiatry.2010.2. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20194822. [DOI] [PubMed] [Google Scholar]
- 23.Bleil ME, Appelhans BM, Latham MD, Irving MA, Gregorich SE, Adler NE, et al. Neighborhood socioeconomic status during childhood versus puberty in relation to endogenous sex hormone levels in adult women. Nurs Res. 2015;64:211–20. doi: 10.1097/NNR.0000000000000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lajud N, Roque A, Cajero M, Gutierrez-Ospina G, Torner L. Periodic maternal separation decreases hippocampal neurogenesis without affecting basal corticosterone during the stress hyporesponsive period, but alters HPA axis and coping behavior in adulthood. Psychoneuroendocrinology. 2012;37:410–20. doi: 10.1016/j.psyneuen.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Huot RL, Gonzalez ME, Ladd CO, Thrivikraman KV, Plotsky PM. Foster litters prevent hypothalamic-pituitary-adrenal axis sensitization mediated by neonatal maternal separation. Psychoneuroendocrinology. 2004;29:279–89. doi: 10.1016/s0306-4530(03)00028-3. [DOI] [PubMed] [Google Scholar]
- 26.Ladd CO, Thrivikraman KV, Huot RL, Plotsky PM. Differential neuroendocrine responses to chronic variable stress in adult Long Evans rats exposed to handling-maternal separation as neonates. Psychoneuroendocrinology. 2005;30:520–33. doi: 10.1016/j.psyneuen.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM. Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur J Neurosci. 2007;25:3091–8. doi: 10.1111/j.1460-9568.2007.05522.x. [DOI] [PubMed] [Google Scholar]
- 28.Bhansali P, Dunning J, Singer SE, David L, Schmauss C. Early Life Stress Alters Adult Serotonin 2C Receptor Pre-mRNA Editing and Expression of the Subunit of the Heterotrimeric G-Protein Gq. J Neurosci. 2007;27:1467–73. doi: 10.1523/JNEUROSCI.4632-06.2007. Available from: http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.4632-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veenema AH, Bredewold R, Neumann ID. Opposite effects of maternal separation on intermale and maternal aggression in C57BL/6 mice: Link to hypothalamic vasopressin and oxytocin immunoreactivity. Psychoneuroendocrinology. 2007;32:437–50. doi: 10.1016/j.psyneuen.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Savignac HM, Dinan TG, Cryan JF. Resistance to Early-Life Stress in Mice: Effects of Genetic Background and Stress Duration. Front Behav Neurosci. 2011:5. doi: 10.3389/fnbeh.2011.00013. Available from: http://journal.frontiersin.org/article/10.3389/fnbeh.2011.00013/abstract. [DOI] [PMC free article] [PubMed]
- 31.Wang L, Jiao J, Dulawa SC. Infant maternal separation impairs adult cognitive performance in BALB/cJ mice. Psychopharmacology (Berl) 2011;216:207–18. doi: 10.1007/s00213-011-2209-4. [DOI] [PubMed] [Google Scholar]
- 32.Fabricius K, Wörtwein G, Pakkenberg B. The impact of maternal separation on adult mouse behaviour and on the total neuron number in the mouse hippocampus. Brain Struct Funct. 2008;212:403–16. doi: 10.1007/s00429-007-0169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liebl C, Panhuysen M, Pütz B, Trümbach D, Wurst W, Deussing JMJM, et al. Gene expression profiling following maternal deprivation: Involvement of the brain Renin-Angiotensin system. Front Mol Neurosci. 2009;2:1. doi: 10.3389/neuro.02.001.2009. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19506703%5Cn http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2691150&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.George ED, Bordner KA, Elwafi HM, Simen AA. Maternal separation with early weaning: a novel mouse model of early life neglect. BMC Neurosci. 2010;11:123. doi: 10.1186/1471-2202-11-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ho DH, Burch ML, Musall B, Musall JB, Hyndman KA, Pollock JS. Early-life stress in male mice induces superoxide production and endothelial dysfunction in adulthood. Am J Physiol - Hear Circ Physiol. 2016 doi: 10.1152/ajpheart.00016.2016. ajpheart.00016.2016. Available from: http://ajpheart.physiology.org/lookup/doi/10.1152/ajpheart.00016.2016. [DOI] [PMC free article] [PubMed]
- 36.Schmidt M, Levine S, Oitzl MS, Van Der Mark M, Müller MB, Holsboer F, et al. Glucocorticoid receptor blockade disinhibits pituitary-adrenal activity during the stress hyporesponsive period of the mouse. Endocrinology. 2005;146:1458–64. doi: 10.1210/en.2004-1042. [DOI] [PubMed] [Google Scholar]
- 37.Carlyle BC, Duque A, Kitchen RR, Bordner KA, Coman D, Doolittle E, et al. Maternal separation with early weaning: A rodent model providing novel insights into neglect associated developmental deficits. Dev Psychopathol. 2012;24:1401–16. doi: 10.1017/S095457941200079X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Platt KM, Charnigo RJ, Kincer JF, Dickens BJ, Pearson KJ. Controlled exercise is a safe pregnancy intervention in mice. J Am Assoc Lab Anim Sci. 2013;52:524–30. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3784655&tool=pmcentrez&rendertype=abstract. [PMC free article] [PubMed] [Google Scholar]
- 39.Carter LG, Qi NR, De Cabo R, Pearson KJ. Maternal Exercise Improves Insulin Sensitivity in Mature Rat Offspring. Med Sci Sport Exerc. 2013;45:832–40. doi: 10.1249/MSS.0b013e31827de953. Available from: http://search.ebscohost.com/login.aspx?direct=true&db=sph&AN=87099299&site=ehost-live. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy MO, Herald JB, Wills CT, Unfried SG, Cohn DM, Loria AS. Postnatal treatment with metyrapone attenuates the effects of diet-induced obesity in female rats exposed to early-life stress. Am J Physiol - Endocrinol Metab. 2017;312:E98–108. doi: 10.1152/ajpendo.00308.2016. Available from: http://ajpendo.physiology.org/lookup/doi/10.1152/ajpendo.00308.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Shoemaker R, Thatcher SE, Batifoulier-Yiannikouris F, English VL, Cassis LA. Administration of 17β-estradiol to ovariectomized obese female mice reverses obesity-hypertension through an ACE2-dependent mechanism. Am J Physiol Endocrinol Metab. 2015;308:E1066–75. doi: 10.1152/ajpendo.00030.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci U S A. 2007;104:1319–24. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joung KE, Park K-H, Zaichenko L, Sahin-Efe A, Thakkar B, Brinkoetter M, et al. Early life adversity is associated with elevated levels of circulating leptin, irisin, and decreased levels of adiponectin in midlife adults. J Clin Endocrinol Metab. 2014;99:E1055–60. doi: 10.1210/jc.2013-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slopen N, Koenen KC, Kubzansky LD. Childhood adversity and immune and inflammatory biomarkers associated with cardiovascular risk in youth: A systematic review. 2012 doi: 10.1016/j.bbi.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, et al. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med. 2009;163:1135–43. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McLaughlin Ka, Greif Green J, Gruber MJ, Sampson Na, Zaslavsky AM, Kessler RC. Childhood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Arch Gen Psychiatry. 2012;69:1151–60. doi: 10.1001/archgenpsychiatry.2011.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moylan CA, Herrenkohl TI, Sousa C, Tajima EA, Herrenkohl RC, Russo MJ. The Effects of Child Abuse and Exposure to Domestic Violence on Adolescent Internalizing and Externalizing Behavior Problems. J Fam Violence. 2010;25:53–63. doi: 10.1007/s10896-009-9269-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20495613%5Cn http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC2872483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Midei AJ, Matthews KA. Interpersonal violence in childhood as a risk factor for obesity: A systematic review of the literature and proposed pathways. Obes Rev. 2011;12 doi: 10.1111/j.1467-789X.2010.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dallman MF, Pecoraro N, Akana SF, la Fleur SE, Gomez F, Houshyar H, et al. Chronic stress and obesity: A new view of “comfort food”. Proc Natl Acad Sci. 2003;100:11696–701. doi: 10.1073/pnas.1934666100. Available from: http://www.pnas.org/cgi/doi/10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lohman BJ, Stewart S, Gundersen C, Garasky S, Eisenmann JC. Adolescent Overweight and Obesity: Links to Food Insecurity and Individual, Maternal, and Family Stressors. J Adolesc Heal. 2009;45:230–7. doi: 10.1016/j.jadohealth.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 51.Chilton MM, Rabinowich JR, Woolf NH. Very low food security in the USA is linked with exposure to violence. Public Health Nutr. 2013:1–10. doi: 10.1017/S1368980013000281. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23432921. [DOI] [PMC free article] [PubMed]
- 52.McLaughlin KA, Sheridan MA, Alves S, Mendes WB. Child maltreatment and autonomic nervous system reactivity: identifying dysregulated stress reactivity patterns by using the biopsychosocial model of challenge and threat. Psychosom Med. 2014;76:538–46. doi: 10.1097/PSY.0000000000000098. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25170753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45. doi: 10.1038/nrn2639. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19401723%5Cn http://www.nature.com/nrn/journal/v10/n6/pdf/nrn2639.pdf%5Cn http://www.nature.com/doifinder/10.1038/nrn26:39. [DOI] [PubMed] [Google Scholar]
- 54.Luecken LJ, Kraft A, Appelhans BM, Enders C. Emotional and cardiovascular sensitization to daily stress following childhood parental loss. Dev Psychol. 2009;45:296–302. doi: 10.1037/a0013888. [DOI] [PubMed] [Google Scholar]
- 55.Liu H, Umberson D. Gender, stress in childhood and adulthood, and trajectories of change in body mass. Soc Sci Med. 2015;139:61–9. doi: 10.1016/j.socscimed.2015.06.026. Available from: http://www.sciencedirect.com/science/article/pii/S027795361530006X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cappelleri JC, Eckenrode J, Powers JL. The epidemiology of child abuse: Findings from the Second National Incidence and Prevalence Study of Child Abuse and Neglect. Am J Public Health. 1993;83:1622–4. doi: 10.2105/ajph.83.11.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kajantie E, Räikkönen K. Early life predictors of the physiological stress response later in life. Neurosci Biobehav Rev. 2010;35:23–32. doi: 10.1016/j.neubiorev.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 58.Own LS, Patel PD. Maternal behavior and offspring resiliency to maternal separation in c57bl/6 mice. Horm Behav. 2013;63:411–7. doi: 10.1016/j.yhbeh.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 59.Bol VV, Delattre a-I, Reusens B, Raes M, Remacle C. Forced catch-up growth after fetal protein restriction alters the adipose tissue gene expression program leading to obesity in adult mice. Am J Physiol Regul Integr Comp Physiol. 2009;297:R291–9. doi: 10.1152/ajpregu.90497.2008. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19458276. [DOI] [PubMed] [Google Scholar]
- 60.Bieswal F, Ahn M-T, Reusens B, Holvoet P, Raes M, Rees WD, et al. The importance of catch-up growth after early malnutrition for the programming of obesity in male rat. Obesity (Silver Spring) 2006;14:1330–43. doi: 10.1038/oby.2006.151. [DOI] [PubMed] [Google Scholar]
- 61.Ozanne SE, Hales CN. Lifespan: catch-up growth and obesity in male mice. Nature. 2004;427:411–2. doi: 10.1038/427411b. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14749819. [DOI] [PubMed] [Google Scholar]
- 62.McCormick CM, Mathews IZ. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol Biochem Behav. 2007;86:220–33. doi: 10.1016/j.pbb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 63.Wake DJ, Walker BR. Inhibition of 11beta-hydroxysteroid dehydrogenase type 1 in obesity. Endocrine. 2006;29:101–8. doi: 10.1385/ENDO:29:1:101. [DOI] [PubMed] [Google Scholar]
- 64.Abdallah BM, Beck-Nielsen H, Gaster M. Increased expression of 11beta-hydroxysteroid dehydrogenase type 1 in type 2 diabetic myotubes. Eur J Clin Invest. 2005;35:627–34. doi: 10.1111/j.1365-2362.2005.01552.x. [DOI] [PubMed] [Google Scholar]
- 65.Hirata A, Maeda N, Nakatsuji H, Hiuge-Shimizu A, Okada T, Funahashi T, et al. Contribution of glucocorticoid-mineralocorticoid receptor pathway on the obesity-related adipocyte dysfunction. Biochem Biophys Res Commun. 2012;419:182–7. doi: 10.1016/j.bbrc.2012.01.139. [DOI] [PubMed] [Google Scholar]
- 66.Masuzaki H, Flier JS. Tissue-specific glucocorticoid reactivating enzyme, 11 beta-hydroxysteroid dehydrogenase type 1 (11 beta-HSD1)--a promising drug target for the treatment of metabolic syndrome. Curr Drug Targets Immune Endocr Metabol Disord. 2003;3:255–62. doi: 10.2174/1568008033340135. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14683456. [DOI] [PubMed] [Google Scholar]
- 67.Lee MJ, Pramyothin P, Karastergiou K, Fried SK. Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity. Biochim. Biophys. Acta - Mol. Basis Dis. 2014;1842:473–81. doi: 10.1016/j.bbadis.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Desarzens S, Faresse N. Adipocyte glucocorticoid receptor has a minor contribution in adipose tissue growth. J Endocrinol. 2016;230:1–11. doi: 10.1530/JOE-16-0121. [DOI] [PubMed] [Google Scholar]
- 69.Marzolla V, Armani A, Zennaro MC, Cinti F, Mammi C, Fabbri A, et al. The role of the mineralocorticoid receptor in adipocyte biology and fat metabolism. Mol. Cell. Endocrinol. 2012;350:281–8. doi: 10.1016/j.mce.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 70.Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, et al. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987;237:268–75. doi: 10.1126/science.3037703. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3037703. [DOI] [PubMed] [Google Scholar]
- 71.Hoppmann J, Perwitz N, Meier B, Fasshauer M, Hadaschik D, Lehnert H, et al. The balance between gluco- and mineralo-corticoid action critically determines inflammatory adipocyte responses. J Endocrinol. 2010;204:153–64. doi: 10.1677/JOE-09-0292. [DOI] [PubMed] [Google Scholar]
- 72.Guo C, Ricchiuti V, Lian BQ, Yao TM, Coutinho P, Romero JR, et al. Mineralocorticoid receptor blockade reverses obesity-related changes in expression of adiponectin, peroxisome proliferator-activated receptor-γ, and proinflammatory adipokines. Circulation. 2008;117:2253–61. doi: 10.1161/CIRCULATIONAHA.107.748640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morgan SA, McCabe EL, Gathercole LL, Hassan-Smith ZK, Larner DP, Bujalska IJ, et al. 11 -HSD1 is the major regulator of the tissue-specific effects of circulating glucocorticoid excess. Proc Natl Acad Sci. 2014;111:E2482–91. doi: 10.1073/pnas.1323681111. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4066483&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boullu-Ciocca S, Achard V, Tassistro V, Dutour A, Grino M. Postnatal programming of glucocorticoid metabolism in rats modulates high-fat diet-induced regulation of visceral adipose tissue glucocorticoid exposure and sensitivity and adiponectin and proinflammatory adipokines gene expression in adulthood. Diabetes. 2008;57:669–77. doi: 10.2337/db07-1316. [DOI] [PubMed] [Google Scholar]
- 75.Bobrovskaya L, Maniam J, Ong LK, Dunkley PR, Morris MJ. Early life stress and post-weaning high fat diet alter tyrosine hydroxylase regulation and at1 receptor expression in the adrenal gland in a sex dependent manner. Neurochem Res. 2013;38:826–33. doi: 10.1007/s11064-013-0985-4. [DOI] [PubMed] [Google Scholar]
- 76.Murphy MO, Herald JB, Wills CT, Unfried SG, Cohn DM, Loria AS. Postnatal treatment with metyrapone attenuates the effects of diet-induced obesity in female rats exposed to early-life stress. doi: 10.1152/ajpendo.00308.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Paternain L, Martisova E, Milagro FI, Ramírez MJ, Martínez JA, Campión J. Postnatal maternal separation modifies the response to an obesogenic diet in adulthood in rats. Dis Model Mech. 2012;5:691–7. doi: 10.1242/dmm.009043. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3424467&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Biagini G, Pich EM, Carani C, Marrama P, Agnati LF. Postnatal maternal separation during the stress hyporesponsive period enhances the adrenocortical response to novelty in adult rats by affecting feedback regulation in the CA1 hippocampal field. Int J Dev Neurosci. 1998;16:187–97. doi: 10.1016/s0736-5748(98)00019-7. [DOI] [PubMed] [Google Scholar]
- 79.Räikkönen K, Matthews KA, Kuller LH. Depressive symptoms and stressful life events predict metabolic syndrome among middle-aged women: A comparison of World Health Organization, Adult Treatment Panel III, and International Diabetes Foundation definitions. Diabetes Care. 2007;30:872–7. doi: 10.2337/dc06-1857. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.