Abstract
Nonalcoholic fatty liver disease (NAFLD) is now the most common cause of chronic liver disease worldwide, and the progressive form of this condition, nonalcoholic steatohepatitis (NASH), has become one of the leading indications for liver transplant. Despite intensive investigations, there are currently no FDA approved therapies for treating NASH. A major barrier for drug development in NASH is that treatment response assessment continues to require liver biopsy, which is invasive and interpreted subjectively. Therefore, there is a major unmet need for developing non-invasive, objective and quantitative biomarkers for diagnosis and assessment of treatment response. Emerging data support the use of magnetic resonance imaging derived proton density fat fraction (MRI-PDFF) as a non-invasive, quantitative, and accurate measure of liver fat content to assess treatment response in early-phase of NASH trials. In this review, we will discuss the role and utility, including potential sample-size reduction, of using MRI-PDFF as a quantitative and non-invasive imaging-based biomarker in early-phase NASH trials.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is now the most common cause of chronic liver disease worldwide (1–3). NAFLD can be broadly classified into two categories: nonalcoholic fatty liver (NAFL), which is thought to have a minimal risk of progression to cirrhosis, and nonalcoholic steatohepatitis (NASH), the more progressive form of NAFLD, which is thought to have a significantly increased risk of progression to cirrhosis (4). Over the past two decades, NASH-related cirrhosis has become the second leading indication for liver transplants in the United States (5). For these reasons pharmacological therapy for NASH is urgently needed. Despite intensive investigations, there are currently no FDA approved therapies for treating NASH (6).
Need for non-invasive assessment in treatment trials
Currently, therapeutic trials in NASH require liver biopsy to establish an initial diagnosis of NASH and to document treatment response. However, it is an expensive and invasive procedure that carries potential risks (abdominal pain, bleeding, death) and is consequently disfavored by many providers and patients (7). Moreover, interpretation and scoring of biopsy are characterized by significant inter- and intra-observer variability (7, 8) and biopsy assesses only a small liver sample, approximately 1/50,000th of the liver. Given the known spatial heterogeneity of diffuse liver disease, limited sampling of the liver can lead to meaningful errors in determining diagnosis, disease stage and longitudinal evolution (9, 10). These limitations directly impact clinical trials design as the diagnostic accuracy, reliability, and response to treatment end-points are key determinants of trial size requirements, feasibility, and costs. Furthermore, extending clinical trial findings to clinical routine practice remains a major obstacle due to barriers in obtaining repeated liver biopsies for treatment monitoring and patient follow-up. Therefore, non-invasive, reliable, accurate, safe and quantitative biomarkers are needed as an alternative to liver biopsy in clinical trials and to extend clinical trial practice to routine practice. Although histologic endpoint remain necessary in NASH clinical trials, emerging data support the use of magnetic resonance imaging derived proton-density-fat-fraction (MRI-PDFF) for treatment response assessment in early-phase trials in NASH for drugs which have an anti-steatotic mechanism of action.
MRI-PDFF
MRI-PDFF is a quantitative imaging biomarker that enables accurate, repeatable and reproducible quantitative assessment of liver fat over the entire liver (9, 11–14). Thus, MRI-PDFF is emerging as one of the leading non-invasive quantitative biomarkers suitable as a surrogate to liver biopsy for assessing treatment response in a subset of NASH trials.
In this review article, we will summarize the currently available evidence regarding the benefits of using MRI-PDFF in NASH patients and the advantages of this imaging technique compared to other methods especially when this imaging is used as an endpoint in NASH trials.
MRI-PDFF Methodology: How does MRI-PDFF quantify liver fat?
Chemical-Shift Encoded MRI and Proton Density Fat Fraction
MRI is sensitive to the signal from protons in mobile, unbound molecules such as water and triglycerides. Serendipitously, the signal from protons bound in structures such as the lipid bilayer of cells (including cholesterols, sphingolipids, and phospholipids) are invisible using conventional MRI.
Further, differential electronic shielding of protons in water and triglycerides leads to differences in the MR resonant frequency of different proton groups. This “chemical shift” between water and fat proton signals can be exploited by emerging chemical shift encoded MRI (CSE-MRI) methods to quantify the relative amount of water and fat signal arising from the tissue (15).
When all confounding factors such as T1 (16) and T2* (17–19), complex spectral characteristics of triglycerides (18–20), the noise behavior of MRI (16) and MRI system instabilities (21, 22) have been addressed, CSE-MRI methods can accurately measure the “proton density fat fraction” (PDFF) (23).
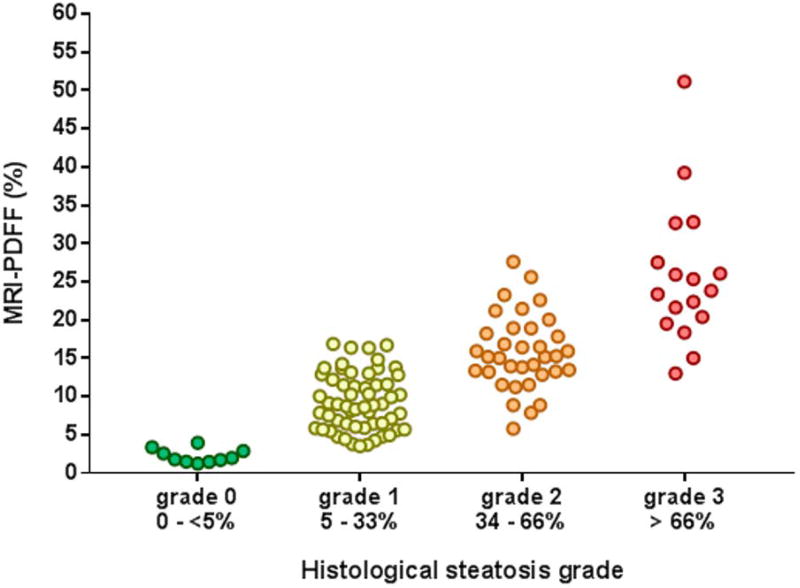
PDFF is defined as the ratio of the density of mobile protons from triglycerides and the total density of protons from mobile triglycerides and mobile water. It is expressed as an absolute percentage (%) and ranges from 0–100%. PDFF is a fundamental property of tissue that reflects the concentration of mobile triglycerides within that tissue. Although PDFF correlates closely with chemically determined tissue triglyceride concentration (24), PDFF and triglyceride concentration are not equivalent. Chemical-assay measurements of triglyceride include MR invisible chemical species that do not contribute to PDFF estimation. Similarly, PDFF is correlated with histological assessment of hepatic steatosis, which is expressed as the percentage of cells containing intracellular droplets of fat (25). Although closely correlated, these two metrics, both expressed as a percentage, are fundamentally different and not equivalent metrics of tissue fat content Figure 1.
Figure 1. Correlation between MRI-PDFF and percentage of hepatocytes with steatosis by histology.
Correlation between MRI-PDFF and histologic steatosis grade classified by the percentage of hepatocyte with steatosis using (25) in individuals with biopsy-proven NAFLD (60).
Types of MRI-PDFF Strategies
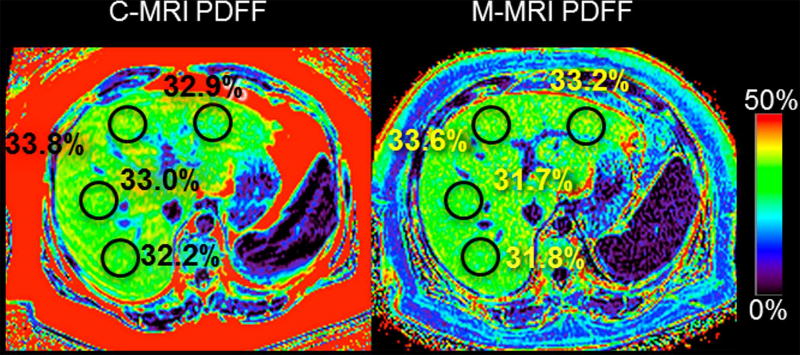
Two primary CSE-MRI strategies have emerged, known as “complex” CSE-MRI and “magnitude” CSE-MRI. Complex-based methods utilize both the phase and the magnitude of the MRI signal and can fully separate the water and fat proton signals. The advantages of complex CSE-MRI include a full PDFF range from 0–100% fat concentration and improved signal to noise ratio (SNR) performance. Magnitude based methods utilize only the magnitude of the MRI signal, and thus are limited to a dynamic range of 0–50%, and lower SNR performance. However, magnitude based methods are less sensitive to system instabilities. Both methods provide highly accurate and precise estimates of liver fat concentration, which is almost always less than 50%, within the dynamic range of magnitude CSE-MRI. Both methods can rapidly assess PDFF over the entire liver in a short breath-hold (~20s). PDFF maps are automatically reconstructed without user input or post-processing – a major advantage over MR spectroscopy based methods Figure 2.
Figure 2. MRI-PDFF assessment and quantification of hepatic steatosis.
Example PDFF maps using complex MRI (C-MRI, left) and magnitude MRI (M-MRI, right) both show elevated PDFF in the liver (~32%). M-MRI is limited to a dynamic range of 0–50% unlike C-MRI which has a full dynamic range from 0–100%.
The data validating the use of advanced CSE-MRI methods to quantify PDFF is extensive. These include phantom studies (26, 27), animal studies (28, 29), ex vivo human liver tissue (24), numerous studies comparing CSE-MRI to MRS (12–14, 30–43), as well as liver biopsy as the reference standard (44, 45). A comprehensive review of the technical details of CSE-MRI and data validating MRI-PDFF is beyond the scope and purpose of this review.
When to use MRI-PDFF as an endpoint in NASH trials?
MRI-PDFF is accurate, precise, and reliable, with excellent inter and intra-rater agreement (11, 32, 37, 46, 47). Furthermore, it should be noted that complex MRI methods have been successfully implemented, are FDA approved, and commercially available on the three major MRI vendors, GE Healthcare, Siemens and Philips, ensuring eventual wide-spread availability. In addition, it has been successfully applied in the setting of several clinical trials (32, 37, 48, 49).
Although MRI-PDFF is a useful tool, it is best suited for the following scenarios as a treatment endpoint in NASH trials.
When the drug or intervention has a high likelihood of an anti-steatotic effect.
Typically in an early phase trial to see if there is a significant reduction in liver fat content along with collateral improvement in an another biomarker (e.g. serum ALT or a plasma based biomarker) before moving on to a larger study using a biopsy-based endpoint.
Interventions that are associated with weight loss would also benefit in quantifying the relative reduction in liver fat content after intervention from baseline. This would help with sample-size assessment for a larger phase 2B or phase 3 trial with liver histology as an endpoint.
When the drug or intervention has a strong likelihood of pro-steatotic effect, it would be useful to include MRI-PDFF as an assessment of drug toxicity to quantify and assess the likelihood of harm to the liver (e.g. basal insulin have been shown to increase liver fat content)
The role and utility of MRI-PDFF can be illustrated using the example of a trial that was designed to assess the efficacy of colesevelam versus placebo in the treatment of NASH. In this trial, 50 biopsy-proven NASH patients were randomized to either colesevelam or placebo for 24 weeks (37). Based upon strong anti-steatotic from pre-clinical data in animal models, the primary hypothesis was that colesevelam would significantly reduce liver fat compared to placebo. Therefore, MRI-PDFF was an appropriate endpoint for assessment of treatment response in this clinical setting. Contrary to the study hypothesis, colesevelam was found to increase hepatic MRI-PDFF. This small but real increase in liver PDFF by colesevelam was only detectable with MRI-PDFF and not appreciable on liver histology assessment, due to the lower sensitivity of liver biopsy compared to CSE-MRI to detect small but real longitudinal changes. This example illustrates the utility of MRI-PDFF to detect small modification in liver fat content in the setting of clinical trial and further studies are needed to determine how well an improvement in hepatic steatosis correlates with resolution of NASH when a drug has different degree of anti-steatotic activity. It is also important to understand the challenges and limitations of using MRI-PDFF as a biomarker in early phase clinical trials.
When the drug or intervention has no or low likelihood of an anti-steatotic effect, MRI-PDFF is unlikely to be useful for the assessment of treatment response in NASH. Under such circumstances, alternative modalities and biomarkers should be considered.
Although MRI-PDFF is suitable as a steatosis marker, it does not assess NASH, fibrosis, inflammation, or other potential endpoints of interest
We anticipate that over the next several years, clinical trials will transition from magnitude-based to complex-based MRI-PDFF sequences. This will likely alleviate some but not all the quality control procedures currently needed. All of the commercially available MRI-PDFF methods are complex based, and as they are disseminated will become increasingly available.
MRI-PDFF may not be feasible in a small minority of patients including claustrophobic patients, patients too large to fit into the MRI scanner, and patients with contraindications to MRI such as certain metallic implants. MRI-PDFF does not require the administration of gadolinium based contrast agents.
MRI-PDFF sequences have relatively low accuracy for the detection of incidental but potentially important abnormalities such as liver tumors. Patients should be counseled that the MRI-PDFF is designed to measure liver fat, and that incidental abnormalities are not reliably excluded. However, it should be noted that it is very straightforward to include MRI-PDFF as part of a standard liver MRI protocol designed to detect and characterize liver tumors and other abnormalities.
Benefits of using MRI-PDFF
MRI-PDFF versus MRS-PDFF
Advanced MRS is considered the most accurate method for measuring PDFF. However, MRS has limited availability, is not fully supported by the system software on clinical MR scanners, usually needs to be run on special research modes, requires technical expertise for its acquisition and analysis, and only measures PDFF in one or a limited number of tissue voxels. The latter limitation introduces sampling variability, especially in longitudinal studies, because the voxel placement is difficult to replicate exactly with existing technology. Since MRI-PDFF and MRS-PDFF agree closely (13, 26, 32, 33, 37, 50), MRI-PDFF is generally preferred in clinical trials due to its greater practicality and lower sampling variability.
MRI-PDFF versus liver tissue
Historically, liver biopsy with histology scoring was the reference standard for hepatic steatosis. Due to its sampling variability and relatively broad grading categories, biopsy is insensitive to small but real changes in liver fat content. A major problem with using liver tissue as an endpoint in clinical trials is that true reductions (or progressions) in steatosis may be missed. Due to this problem as well as other limitations mentioned earlier, liver biopsy is not recommended as an endpoint in clinical trials if the primary outcome measure is steatosis reduction.
MRI-PDFF versus CT-attenuation; versus ultrasound-quantitative; versus ultrasound-qualitative; versus CAP
The attenuation X-rays passing through fat is lower than water, therefore livers with hepatic steatosis have lower attenuation on computed tomography (CT), an X-ray based imaging method (51, 52). Recently, Kramer et al. directly examined the relationship between PDFF and CT in patients undergoing same day CSE-MRI and non-contrast CT (53). Excellent linear correlation between PDFF and CT attenuation was observed (r2=0.86), providing, for the first time, a direct calibration between PDFF and CT attenuation. However, while the correlation of PDFF and CT attenuation was strong overall, there was no meaningful correlation of PDFF and CT attenuation (r2=0.04) at low levels of liver fat (PDFF < 6%). Thus, CT may be a useful biomarker to detect and quantify moderate to severe hepatic steatosis but its utility is limited, particularly at low fat concentrations that are likely the most clinically relevant (41, 54). Finally, the need for ionizing radiation makes CT less attractive, particularly in children, when alternative non-invasive imaging methods such as MRI are readily available.
Sonographic image brightness relates to the backscatter and attenuation of the ultrasound wave. Compared to lean liver tissue, fatty tissue scatters and attenuates sound waves. Hence, mildly fatty liver appears bright due to backscattered signals returning to the transducer. As the amount of liver fat increases, the ultrasound wave becomes attenuated. Radiologists assess these changes (brightening of the liver in the near field, darkening of the liver in the far field, blurring of vessels) qualitatively to determine the presence of steatosis. Although qualitative assessment for liver fat may be useful clinically, subjectivity and imprecision render this approach unsuitable for measuring steatosis and its longitudinal change in clinical trials. To provide an objective sonographic assessment of hepatic steatosis, new methods are being developed to quantify the degree of backscatter (55, 56) as potential biomarkers of hepatic steatosis. Among them, the controlled attenuation parameter (CAP), measured by Fibroscan (Echosens) allows a rapid assessment and is reasonably accurate for diagnosing the presence of steatosis (57, 58). However CAP is limited by high failure rates in obesity, lack of exact anatomic localization, and low accuracy for quantifying the amount of steatosis (59). The latter two factors make CAP unsuitable for use as an endpoint in clinical trials due to measurement imprecision and inability to monitor treatment response (60). Other quantitative ultrasound methods are investigational and not ready for use in clinical trials.
Correlation between change in steatosis vs change MRI-PDFF and between change in MRS-PDFF vs change in MRI-PDFF
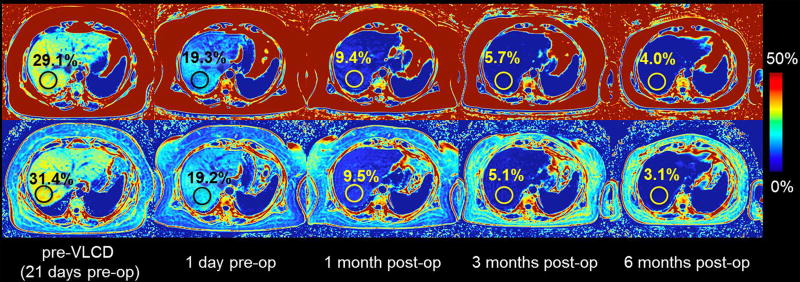
Two recent studies, one in adults (32) and one in children (42) with known or suspected NAFLD, have shown that longitudinal change in MRI-PDFF agrees closely with longitudinal change in MRS-PDFF (with correlation coefficients of 0.96 to 0.99 and 0.986, respectively), when the MRI and MRS measurements at each time points are meticulously co-localized. Longitudinal change in MRI-PDFF after weight loss surgery is shown in Figure 3. These data provide further validation for the use of MRI-PDFF in longitudinal clinical studies that will help to determine the prognostic significance of the severity and change of steatosis.
Figure 3. Longitudinal changes in MRI-PDFF after weight loss surgery.
PDFF is shown in a patient before (pre-op) and after (post-op) weight loss surgery. Images from left to right represent PDFF before a very low caloric diet (pre-VLCD) pre-op and the longitudinal follow-up showing a decrease in PDFF.
How to use MRI-PDFF as an endpoint in NASH trials?
Site selection, qualification, training, and technical support
Although now commercialized by the three major vendors, complex-based MRI-PDFF sequences are generally available only on the latest-generation scanners. Even if available, the sequences may require the purchase of a license to enable their use. Therefore, a substantial proportion of sites participating in clinical trials currently or in the next few years are unlikely to have access to these advanced sequences. Until now, therefore, most clinical trials have used magnitude-based MRI-PDFF sequences. These usually can be implemented by using commercial sequences developed for other purposes and then modifying the acquisition parameters as needed, often under the guidance of a central radiology coordinating center.
Site selection for clinical trials is usually based on hepatology expertise and enrollment capacity, not imaging capability. Hence, the radiology coordinating center must determine the relevant technical capabilities of each site, including field strength, scanner manufacturer, and scanner software. Based on this information, a standardized protocol is developed that is both feasible at every site and adequate for reliable PDFF estimation. A detailed MRI procedure manual is given to the sites, which usually provides all the necessary training. If needed, additional questions can be answered by email or teleconference. Site qualification is done in parallel with training and begins with a review of the site’s technical qualifications. The ability to perform the exact protocol is then confirmed by scanning a phantom (an inanimate object such as a bottle of water) or a human volunteer. Images are sent to coordinating center, which verifies that all parameters are within the allowable range. Additionally, the coordinating center provides as-needed technical support if sites are unable to acquire images using the allowed parameters or diverge from the protocol at any point in the study.
Quality control: acquisition, intake, analysis, reporting
Quality control (QC) is essential. The technologist performing the study at each site is responsible for verifying MRI safety for each patient, positioning the patient correctly, adhering to the research protocol, checking images as they are collected, and repeating any images that are degraded by a correctable error such as sudden motion from a cough. The coordinating center is responsible for intake QC (verifying all parameters are within range, the appropriate anatomy was covered, images are of adequate quality for analysis), analysis QC (verifying that the analysis described below follows a standard operating procedure, that the exact locations of the regions of interest are recorded, and that the values taken from the images are within expected range or rechecked), and reporting QC (ensuring that the values recorded form the images match the values inputted into the database and that the final imaging database is complete)
Analysis: co-localization, number of ROIs, size of ROIs
Once PDFF maps have been acquired, analysis of these maps must be performed to derive a single PDFF estimate for that MRI exam. There are many possible approaches and currently there is no official consensus on how to analyze PDFF maps. Region of interest (ROI)-based methods that measure the average PDFF value in a region of the liver are generally used, and standardized approaches are emerging. Standardized approaches that have excellent intra- and inter-reader variability are preferred, although they may be labor intensive. Whole-liver segmentation that avoids large blood vessels, bile ducts, liver lesions and image artifacts would likely provide the best intra- and inter-reader variability, but are not practical and probably not necessary. The use of multiple large ROI’s is usually sufficient to provide adequate sampling of the liver. Hines et al first proposed a sampling strategy that placed one ROI per Couinaud segment (61), while other groups have described the use of four ROI paradigms, with one ROI in the anterior, posterior, medial and lateral segments (62). More recently, Campo et al rigorously evaluated the effects of the number of ROI’s used, ROI size, and ROI location on the intra- and inter-reader variability of PDFF measurements, and evaluated the time burden required for different ROI strategies. Based on the results of this study, we conclude that the use of large ROI’s (≥4cm2) with either a 4-ROI paradigm (anterior, posterior, medial, and lateral) or 9-ROI paradigm (Couinaud segments) are preferred to provide the best intra- and inter-reader variability with an analysis time of approximately 1–2.5 minutes by an experienced user (63)
Longitudinal changes: what is the quantitative change in liver fat that is associated with improvement in liver histology?
In order for MRI-PDFF to be more widely acceptable for the assessment of treatment response in NASH, one of the key data that are needed are the amount of liver fat decline that is clinical meaningful. Long-term studies are needed to assess whether a sustained and significant reduction in liver fat will lead to improvement in fibrosis, reduction in the risk to progression to cirrhosis and reduction in the risk of death from liver disease. This may be a task that is not likely to be achieved in the near future. Therefore, investigators have initiated seminal studies that aim to solve this puzzle by answering questions that can be answered in the near future. One such attempt was done by Patel et al using paired MRI-PDFF and liver histology data from two high quality randomized trials. Utilizing paired MRI-PDFF and liver histology data, Patel et al. demonstrated that a relative reduction of 29% in liver fat on MRI-PDFF is associated with a histologic response in NASH (defined as a 2-point improvement in NAFLD activity Score) (64). Although these preliminary data need to be confirmed in larger cohort, they are now being used to design future NASH clinical trials using the change in hepatic fat quantified by MRI-PDFF as a treatment endpoint. Future studies are needed to assess whether there is a tipping point for liver fat reduction that if sustained over a 6 month period would be associated with either resolution of NASH or improvement in one stage of fibrosis.
CONCLUSIONS
Non-invasive, quantitative, precise and reproducible, MRI-PDFF is emerging as a useful biomarker to assess treatment response in the setting of early phase clinical trials in NASH. It is suitable for quantifying liver fat content, however, it does not assess NASH, fibrosis, inflammation, or other potential endpoints of interest. Therefore, multi-modality assessment of treatment response is needed to examine the treatment response as MRI-PDFF is restricted to the liver fat domain alone. However, if there is a large enough quantitative decline in liver fat content it may also be associated with other collateral benefits such as improvement in inflammation. Emerging data suggests that a relative decline of 30% or more may be clinically meaningful but remains to be validated in larger studies. We anticipate that over the next several years, clinical trials will transition from magnitude-based to complex-based MRI-PDFF sequences. This will likely alleviate some but not all the quality control procedures that are currently needed, and help standardize liver fat quantification. Further research is being conducted to develop an MRI-based package including MRI-PDFF, two-dimensional magnetic resonance elastography (2D MRE) and three-dimensional (3D) MRE and other MR based biomarkers to have a comprehensive liver disease assessment. The future is extremely bright for non-invasive assessment of treatment response using imaging modalities with an exponential increase in innovative applications of MR-based modalities. Such novel quantitative imaging modalities will likely continue to transform clinical trial design in the years to come.
Acknowledgments
Grant support: RL is supported in part by the American Gastroenterological Association (AGA) Foundation – Sucampo – ASP Designated Research Award in Geriatric Gastroenterology and by a T. Franklin Williams Scholarship Award; Funding provided by: Atlantic Philanthropies, Inc, the John A. Hartford Foundation, OM, the Association of Specialty Professors, and the American Gastroenterological Association and grant K23-DK090303. The authors also wish to acknowledge support from the National Institute of Health (NIH) (UL1TR00427, R01DK106419, R01 DK083380, R01 DK088925, R01 DK100651 and K24 DK102595), as well GE Healthcare who provides research support to the UW-Madison and UCSD. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- AUROC
area under the receiver operator characteristic curve
- BMI
body mass index
- CAP
controlled attenuation parameter
- CI
confidence interval
- CSE-MRI
chemical shift encoded magnetic resonance imaging
- CT
computerized tomography
- DECT
dual-energy computerized tomography
- FDA
U.S Food and Drug administration
- HCC
hepatocellular carcinoma
- MRI
magnetic resonance imaging
- MRI-PDFF
magnetic resonance imaging proton density fat fraction
- MRS
magnetic resonance spectroscopy
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- TE
transient elastography
- VCTE
vibration-controlled transient elastography
- ROI
region of interest
Footnotes
Role of study sponsor: The study sponsor(s) had no role in the study design, collection, analysis, interpretation of the data, and/or drafting of the manuscript. All authors report that no conflicts of interest exist.
Conflict of interests: Dr. Sirlin consults, advises, and is on the speakers’ bureau for Bayer. Drs. Loomba and Sirlin received grants from GE Healthcare and Siemens Inc. Dr. Reeder reports that the University of Wisconsin receives research support from GE Healthcare and Bracco Diagnostics. Dr. Reeder consults for Parexel International and is a founder of Calimetrix, LLC. Dr. Caussy reports no other conflict of interests.
Author contributions:
Cyrielle Caussy: Drafting of the manuscript, approved final submission.
Scott B. Reeder: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, obtained funding, study supervision, approved final submission
Claude B. Sirlin: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, obtained funding, study supervision, approved final submission
Rohit Loomba: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, obtained funding, study supervision, approved final submission
All authors approved the final version of this article.
References
- 1.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 4.Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643–654. e641–649. doi: 10.1016/j.cgh.2014.04.014. quiz e639-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 6.Rotman Y, Sanyal AJ. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut. 2016 doi: 10.1136/gutjnl-2016-312431. [DOI] [PubMed] [Google Scholar]
- 7.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 8.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD, American Association for the Study of Liver D Liver biopsy. Hepatology. 2009;49:1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 9.Bonekamp S, Tang A, Mashhood A, Wolfson T, Changchien C, Middleton MS, Clark L, et al. Spatial distribution of MRI-Determined hepatic proton density fat fraction in adults with nonalcoholic fatty liver disease. J Magn Reson Imaging. 2014;39:1525–1532. doi: 10.1002/jmri.24321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 11.Noureddin M, Lam J, Peterson MR, Middleton M, Hamilton G, Le TA, Bettencourt R, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology. 2013;58:1930–1940. doi: 10.1002/hep.26455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Negrete LM, Middleton MS, Clark L, Wolfson T, Gamst AC, Lam J, Changchien C, et al. Inter-examination precision of magnitude-based MRI for estimation of segmental hepatic proton density fat fraction in obese subjects. J Magn Reson Imaging. 2014;39:1265–1271. doi: 10.1002/jmri.24284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yokoo T, Shiehmorteza M, Hamilton G, Wolfson T, Schroeder ME, Middleton MS, Bydder M, et al. Estimation of hepatic proton-density fat fraction by using MR imaging at 3.0 T. Radiology. 2011;258:749–759. doi: 10.1148/radiol.10100659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang GH, Cruite I, Shiehmorteza M, Wolfson T, Gamst AC, Hamilton G, Bydder M, et al. Reproducibility of MRI-determined proton density fat fraction across two different MR scanner platforms. J Magn Reson Imaging. 2011;34:928–934. doi: 10.1002/jmri.22701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging. 2011;34:729–749. doi: 10.1002/jmri.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu CY, McKenzie CA, Yu H, Brittain JH, Reeder SB. Fat quantification with IDEAL gradient echo imaging: correction of bias from T(1) and noise. Magn Reson Med. 2007;58:354–364. doi: 10.1002/mrm.21301. [DOI] [PubMed] [Google Scholar]
- 17.Yu H, McKenzie CA, Shimakawa A, Vu AT, Brau AC, Beatty PJ, Pineda AR, et al. Multiecho reconstruction for simultaneous water-fat decomposition and T2* estimation. J Magn Reson Imaging. 2007;26:1153–1161. doi: 10.1002/jmri.21090. [DOI] [PubMed] [Google Scholar]
- 18.Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, Reeder SB. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med. 2008;60:1122–1134. doi: 10.1002/mrm.21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bydder M, Yokoo T, Hamilton G, Middleton MS, Chavez AD, Schwimmer JB, Lavine JE, et al. Relaxation effects in the quantification of fat using gradient echo imaging. Magn Reson Imaging. 2008;26:347–359. doi: 10.1016/j.mri.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton G, Middleton MS, Hooker JC, Haufe WM, Forbang NI, Allison MA, Loomba R, et al. In vivo breath-hold (1) H MRS simultaneous estimation of liver proton density fat fraction, and T1 and T2 of water and fat, with a multi-TR, multi-TE sequence. J Magn Reson Imaging. 2015;42:1538–1543. doi: 10.1002/jmri.24946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu H, Shimakawa A, Hines CD, McKenzie CA, Hamilton G, Sirlin CB, Brittain JH, et al. Combination of complex-based and magnitude-based multiecho water-fat separation for accurate quantification of fat-fraction. Magn Reson Med. 2011;66:199–206. doi: 10.1002/mrm.22840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernando D, Hines CD, Yu H, Reeder SB. Addressing phase errors in fat-water imaging using a mixed magnitude/complex fitting method. Magn Reson Med. 2012;67:638–644. doi: 10.1002/mrm.23044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reeder SB, Hu HH, Sirlin CB. Proton density fat-fraction: a standardized MR-based biomarker of tissue fat concentration. J Magn Reson Imaging. 2012;36:1011–1014. doi: 10.1002/jmri.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bannas P, Kramer H, Hernando D, Agni R, Cunningham AM, Mandal R, Motosugi U, et al. Quantitative magnetic resonance imaging of hepatic steatosis: Validation in ex vivo human livers. Hepatology. 2015;62:1444–1455. doi: 10.1002/hep.28012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 26.Hines CD, Yu H, Shimakawa A, McKenzie CA, Brittain JH, Reeder SB. T1 independent, T2* corrected MRI with accurate spectral modeling for quantification of fat: validation in a fat-water-SPIO phantom. J Magn Reson Imaging. 2009;30:1215–1222. doi: 10.1002/jmri.21957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernando D, Sharma SD, Aliyari Ghasabeh M, Alvis BD, Arora SS, Hamilton G, Pan L, et al. Multisite, multivendor validation of the accuracy and reproducibility of proton-density fat-fraction quantification at 1.5T and 3T using a fat-water phantom. Magn Reson Med. 2016 doi: 10.1002/mrm.26228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hines CD, Yu H, Shimakawa A, McKenzie CA, Warner TF, Brittain JH, Reeder SB. Quantification of hepatic steatosis with 3-T MR imaging: validation in ob/ob mice. Radiology. 2010;254:119–128. doi: 10.1148/radiol.09090131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hines CD, Agni R, Roen C, Rowland I, Hernando D, Bultman E, Horng D, et al. Validation of MRI biomarkers of hepatic steatosis in the presence of iron overload in the ob/ob mouse. J Magn Reson Imaging. 2012;35:844–851. doi: 10.1002/jmri.22890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Artz NS, Haufe WM, Hooker CA, Hamilton G, Wolfson T, Campos GM, Gamst AC, et al. Reproducibility of MR-based liver fat quantification across field strength: Same-day comparison between 1.5T and 3T in obese subjects. J Magn Reson Imaging. 2015;42:811–817. doi: 10.1002/jmri.24842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bashir MR, Zhong X, Nickel MD, Fananapazir G, Kannengiesser SA, Kiefer B, Dale BM. Quantification of hepatic steatosis with a multistep adaptive fitting MRI approach: prospective validation against MR spectroscopy. AJR Am J Roentgenol. 2015;204:297–306. doi: 10.2214/AJR.14.12457. [DOI] [PubMed] [Google Scholar]
- 32.Cui J, Philo L, Nguyen P, Hofflich H, Hernandez C, Bettencourt R, Richards L, et al. Sitagliptin vs. placebo for non-alcoholic fatty liver disease: A randomized controlled trial. J Hepatol. 2016;65:369–376. doi: 10.1016/j.jhep.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meisamy S, Hines CD, Hamilton G, Sirlin CB, McKenzie CA, Yu H, Brittain JH, et al. Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology. 2011;258:767–775. doi: 10.1148/radiol.10100708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson BL, Schroeder ME, Wolfson T, Gamst AC, Hamilton G, Shiehmorteza M, Loomba R, et al. Effect of flip angle on the accuracy and repeatability of hepatic proton density fat fraction estimation by complex data-based, T1-independent, T2*-corrected, spectrum-modeled MRI. J Magn Reson Imaging. 2014;39:440–447. doi: 10.1002/jmri.24153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim KY, Song JS, Kannengiesser S, Han YM. Hepatic fat quantification using the proton density fat fraction (PDFF): utility of free-drawn-PDFF with a large coverage area. Radiol Med. 2015;120:1083–1093. doi: 10.1007/s11547-015-0545-x. [DOI] [PubMed] [Google Scholar]
- 36.Kuhn JP, Hernando D, Mensel B, Kruger PC, Ittermann T, Mayerle J, Hosten N, et al. Quantitative chemical shift-encoded MRI is an accurate method to quantify hepatic steatosis. J Magn Reson Imaging. 2014;39:1494–1501. doi: 10.1002/jmri.24289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le TA, Chen J, Changchien C, Peterson MR, Kono Y, Patton H, Cohen BL, et al. Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology. 2012;56:922–932. doi: 10.1002/hep.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levin YS, Yokoo T, Wolfson T, Gamst AC, Collins J, Achmad EA, Hamilton G, et al. Effect of echo-sampling strategy on the accuracy of out-of-phase and in-phase multiecho gradient-echo MRI hepatic fat fraction estimation. J Magn Reson Imaging. 2014;39:567–575. doi: 10.1002/jmri.24193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mashhood A, Railkar R, Yokoo T, Levin Y, Clark L, Fox-Bosetti S, Middleton MS, et al. Reproducibility of hepatic fat fraction measurement by magnetic resonance imaging. J Magn Reson Imaging. 2013;37:1359–1370. doi: 10.1002/jmri.23928. [DOI] [PubMed] [Google Scholar]
- 40.Motosugi U, Hernando D, Bannas P, Holmes JH, Wang K, Shimakawa A, Iwadate Y, et al. Quantification of liver fat with respiratory-gated quantitative chemical shift encoded MRI. J Magn Reson Imaging. 2015;42:1241–1248. doi: 10.1002/jmri.24896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rehm JL, Wolfgram PM, Hernando D, Eickhoff JC, Allen DB, Reeder SB. Proton density fat-fraction is an accurate biomarker of hepatic steatosis in adolescent girls and young women. Eur Radiol. 2015;25:2921–2930. doi: 10.1007/s00330-015-3724-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tyagi A, Yeganeh O, Levin Y, Hooker JC, Hamilton GC, Wolfson T, Gamst A, et al. Intra- and inter-examination repeatability of magnetic resonance spectroscopy, magnitude-based MRI, and complex-based MRI for estimation of hepatic proton density fat fraction in overweight and obese children and adults. Abdom Imaging. 2015;40:3070–3077. doi: 10.1007/s00261-015-0542-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zand KA, Shah A, Heba E, Wolfson T, Hamilton G, Lam J, Chen J, et al. Accuracy of multiecho magnitude-based MRI (M-MRI) for estimation of hepatic proton density fat fraction (PDFF) in children. J Magn Reson Imaging. 2015;42:1223–1232. doi: 10.1002/jmri.24888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang A, Tan J, Sun M, Hamilton G, Bydder M, Wolfson T, Gamst AC, et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology. 2013;267:422–431. doi: 10.1148/radiol.12120896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Idilman IS, Aniktar H, Idilman R, Kabacam G, Savas B, Elhan A, Celik A, et al. Hepatic steatosis: quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology. 2013;267:767–775. doi: 10.1148/radiol.13121360. [DOI] [PubMed] [Google Scholar]
- 46.Permutt Z, Le TA, Peterson MR, Seki E, Brenner DA, Sirlin C, Loomba R. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease - MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther. 2012;36:22–29. doi: 10.1111/j.1365-2036.2012.05121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dulai PS, Sirlin CB, Loomba R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: Clinical trials to clinical practice. J Hepatol. 2016;65:1006–1016. doi: 10.1016/j.jhep.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Middleton MS, Heba ER, Hooker CA, Bashir MR, Fowler KJ, Sandrasegaran K, Brunt EM, et al. Agreement Between Magnetic Resonance Imaging Proton Density Fat Fraction Measurements and Pathologist-assigned Steatosis Grades of Liver Biopsies from Adults with Nonalcoholic Steatohepatitis. Gastroenterology. 2017 doi: 10.1053/j.gastro.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loomba R, Sirlin CB, Ang B, Bettencourt R, Jain R, Salotti J, Soaft L, et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial) Hepatology. 2015;61:1239–1250. doi: 10.1002/hep.27647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yokoo T, Bydder M, Hamilton G, Middleton MS, Gamst AC, Wolfson T, Hassanein T, et al. Nonalcoholic fatty liver disease: diagnostic and fat-grading accuracy of low-flip-angle multiecho gradient-recalled-echo MR imaging at 1.5 T. Radiology. 2009;251:67–76. doi: 10.1148/radiol.2511080666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pickhardt PJ, Hahn L, Munoz del Rio A, Park SH, Reeder SB, Said A. Natural history of hepatic steatosis: observed outcomes for subsequent liver and cardiovascular complications. AJR Am J Roentgenol. 2014;202:752–758. doi: 10.2214/AJR.13.11367. [DOI] [PubMed] [Google Scholar]
- 52.Jehangir M, Nazir R, Jang A, Rana A, Rafique S, Dar FS. Macrovesicular steatosis in living related liver donors: correlation of biopsy findings with CT liver attenuation index and body mass index. Clin Transplant. 2016;30:1016–1020. doi: 10.1111/ctr.12782. [DOI] [PubMed] [Google Scholar]
- 53.Kramer H, Pickhardt PJ, Kliewer MA, Hernando D, Chen GH, Zagzebski JA, Reeder SB. Accuracy of Liver Fat Quantification With Advanced CT, MRI, and Ultrasound Techniques: Prospective Comparison With MR Spectroscopy. AJR Am J Roentgenol. 2017;208:92–100. doi: 10.2214/AJR.16.16565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang A, Desai A, Hamilton G, Wolfson T, Gamst A, Lam J, Clark L, et al. Accuracy of MR imaging-estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology. 2015;274:416–425. doi: 10.1148/radiol.14140754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin SC, Heba E, Wolfson T, Ang B, Gamst A, Han A, Erdman JW, Jr, et al. Noninvasive Diagnosis of Nonalcoholic Fatty Liver Disease and Quantification of Liver Fat Using a New Quantitative Ultrasound Technique. Clin Gastroenterol Hepatol. 2015;13:1337–1345. e1336. doi: 10.1016/j.cgh.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, Sandrin L, et al. Controlled attenuation parameter (CAP): a novel VCTE guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825–1835. doi: 10.1016/j.ultrasmedbio.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 57.Karlas T, Petroff D, Garnov N, Bohm S, Tenckhoff H, Wittekind C, Wiese M, et al. Non-invasive assessment of hepatic steatosis in patients with NAFLD using controlled attenuation parameter and 1H-MR spectroscopy. PLoS One. 2014;9:e91987. doi: 10.1371/journal.pone.0091987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Caussy C, Alquiraish MH, Nguyen P, Hernandez C, Cepin S, Fortney LE, Ajmera V, et al. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology. 2017 doi: 10.1002/hep.29639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Ledinghen V, Vergniol J, Capdepont M, Chermak F, Hiriart JB, Cassinotto C, Merrouche W, et al. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol. 2014;60:1026–1031. doi: 10.1016/j.jhep.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 60.Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L, Hooker J, et al. Magnetic Resonance Elastography vs Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients With Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology. 2017;152:598–607.e592. doi: 10.1053/j.gastro.2016.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hines CD, Frydrychowicz A, Hamilton G, Tudorascu DL, Vigen KK, Yu H, McKenzie CA, et al. T(1) independent, T(2) (*) corrected chemical shift based fat-water separation with multi-peak fat spectral modeling is an accurate and precise measure of hepatic steatosis. J Magn Reson Imaging. 2011;33:873–881. doi: 10.1002/jmri.22514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sofue K, Mileto A, Dale BM, Zhong X, Bashir MR. Interexamination repeatability and spatial heterogeneity of liver iron and fat quantification using MRI-based multistep adaptive fitting algorithm. J Magn Reson Imaging. 2015;42:1281–1290. doi: 10.1002/jmri.24922. [DOI] [PubMed] [Google Scholar]
- 63.Campo CA, Hernando D, Schubert T, Bookwalter CA, Pay AJV, Reeder SB. Standardized Approach for ROI-Based Measurements of Proton Density Fat Fraction and R2* in the Liver. AJR Am J Roentgenol. 2017:1–12. doi: 10.2214/AJR.17.17812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patel J, Bettencourt R, Cui J, Salotti J, Hooker J, Bhatt A, Hernandez C, et al. Association of noninvasive quantitative decline in liver fat content on MRI with histologic response in nonalcoholic steatohepatitis. Therap Adv Gastroenterol. 2016;9:692–701. doi: 10.1177/1756283X16656735. [DOI] [PMC free article] [PubMed] [Google Scholar]