Abstract
Childhood lead exposure has been correlated to acts of delinquency and criminal behavior; however, little research has been conducted to examine its potential long term influence on behavioral factors such as personality, specifically psychopathic personality. Neuroimaging studies have demonstrated that the effects of childhood lead exposure persist into adulthood, with structural abnormalities found in gray and white matter regions involved in behavioral decision making. The current study examined whether measurements of adult psychopathy were associated with neuroanatomical differences in structural brain volumes for a longitudinal cohort with measured childhood lead exposure. We hypothesized that increased total psychopathy scores and increased blood lead concentration at 78 months of age (PbB78) would be inversely associated with volumetric measures of gray and white matter brain structures responsible for executive and emotional processing. Analyses did not display a direct effect between total psychopathy score and gray matter volume; however, reduced white matter volume in the cerebellum and brain stem in relation to increased total psychopathy scores was observed. An interaction between sex and total psychopathy score was also detected. Females displayed increased gray matter volume in the frontal, temporal, and parietal lobes associated with increased total psychopathy score, but did not display any white matter volume differences. Males primarily displayed reductions in frontal gray and white matter brain volume in relation to increased total psychopathy scores. Additionally, reduced gray and white matter volume was associated with increased blood lead levels in the frontal lobes; reduced white matter volume was also observed in the parietal and temporal lobes. Females demonstrated gray and white matter volume loss associated with increased PbB78 values in the right temporal lobe, as well as reduced gray matter volume in the frontal lobe. Males displayed reduced white matter volumes associated with increased PbB78 values in the frontal, temporal, and parietal lobes. Comparison of the two primary models revealed a volumetric decrease in the white matter of the left prefrontal cortex associated with increased total psychopathy scores and increased blood lead concentration in males. The results of this study suggested that increased psychopathy scores in this cohort may be attributable to the neuroanatomical abnormalities observed and that childhood lead exposure may be influential to these outcomes.
Keywords: Voxel Based Morphometry, Psychopathy, Magnetic Resonance Imaging, Childhood Lead Exposure
1.0 Introduction
The influence of childhood lead exposure on behavior has been well documented (Bellinger et al., 1994, Mendelsohn et al., 1998); however, less is known about how childhood lead exposure impacts the development of personality disorders such as psychopathy. Several studies have reported associations between childhood lead exposure and delinquent and violent behavior (Denno, 1990, Needleman et al., 2002, Pihl and Ervin, 1990, Sciarillo et al., 1992, Stretesky and Lynch, 2001, Stretesky and Lynch, 2004, Marcus et al., 2010, Needleman et al., 1996). Specifically within the Cincinnati Lead Study (CLS) cohort, Dietrich et al. (2001) observed increased rates of juvenile delinquency associated with pre- and post-natal blood lead levels. The CLS has also documented a prospective association between arrests in adulthood and early lead exposure (Wright et al., 2008). For males especially, a history of lead exposure has been among the most significant predictors of adolescent delinquency and adult criminality (Denno, 1990), and more recently psychopathy (Wright et al., 2009).
Psychopathy is characterized by a pattern of personality traits such as a lack of empathy, egocentrism, and grandiosity, and behaviors including excessive deceit, aggression, and a parasitic lifesyle (Cleckley, 1955, Hare and Neumann, 2008). It is also thought to be influenced by deficits in executive functioning, emotional processing, and moral reasoning (Hyde et al., 2014, Sethi et al., 2015, Harenski et al., 2010). Although psychopathy occurs in both sexes, it occurs most frequently in males (Grann, 2000, Vitale et al., 2002). Functional and structural imaging studies conducted in populations with psychopathic personalities have repeatedly found brain abnormalities in the frontal and temporal lobes, especially structures involved with the limbic system (Craig et al., 2009, Motzkin et al., 2011, Juarez et al., 2013, Yang et al., 2009, Yang et al., 2005), providing a neurobiological basis for psychopathy. Etiologically, psychopathy has been linked to genetics (Bezdjian et al., 2011, Baker et al., 2007, Larsson et al., 2006) and to a lesser degree, socialization factors (Gao et al., 2010, Daversa, 2010). That said, exposure to environmental toxicants early in life may be important to the development of psychopathy (Raine, 2013, Glenn and Raine, 2014). Psychopathy includes not only a series of personality traits, but also behaviors such as aggression, juvenile delinquency, and criminal conduct that prior research has shown to be connected with exposure to neurotoxicants such as lead (Pb). Given the empirical overlap between psychopathy and misconduct (DeLisi et al., 2017), early lead exposure may be implicated in both.
We previously reported findings from the CLS cohort showing structural and organizational changes in brain regions associated with compromised cognitive functioning and behavior. Cecil et al. (2008) and Brubaker et al. (2010) employed voxel based morphometry (VBM) approaches to allow voxel by voxel comparisons between high-resolution anatomical magnetic resonance imaging (MRI) brain scans obtained at approximately 20 years of age and their blood lead concentrations obtained regularly from the age of 3 months to 78 months. Reduced gray matter volumes in the prefrontal cortex, anterior cingulate and other regions associated with both mean childhood and annual blood lead concentrations were found. Greater reductions in brain volume, especially within the frontal lobe, were noted in males. Additionally, Brubaker et al. (2009) reported altered patterns of myelination revealed by diffusion tensor imaging in association with childhood blood lead levels for numerous white matter tracts including limbic tracts such as the cingulum, uncinate, and stria terminalis.
Though several neuroimaging studies have been conducted on populations with antisocial personality disorder and psychopathic personalities (Yang and Raine, 2009), no studies examining brain abnormalities related to psychopathy have been conducted in a population with known previous lead exposure.
The current study was a cross-sectional analysis utilizing data from the CLS. Looking at measures of regional brain volumes, we hypothesized that increased psychopathy scores would be inversely associated with gray and white matter volumes in regions and structures responsible for executive and emotional processing, specifically limbic and prefrontal regions including the anterior cingulate and ventromedial prefrontal cortex (VMPFC). Additionally, we hypothesized that similar differences in regional brain volumes would be observed in relation to measured childhood blood lead levels. We also explored sex interactions and differences in our analyses to illustrate regional differences given the higher rates of psychopathy in males (Forth et al., 1996, Levenson et al., 1995), the recognition of greater vulnerability of males to adverse neurobehavioral effects of lead exposure (Bellinger et al., 1990), and the distinct sex differences observed previously in neuroimaging studies of lead exposure (Brubaker et al., 2010, Cecil et al., 2008).
2.0 Methods
2.1 Participants
Participants provided informed written consent prior to participation. This protocol was reviewed and approved by the institutional review boards at the University of Cincinnati College of Medicine and Cincinnati Children’s Hospital Medical Center.
Pregnant women recruited from inner city neighborhoods historically displaying an elevated incidence of childhood lead poisoning were enrolled in the CLS between 1979 and 1984 (Bornschein et al., 1985). A total of 376 newborns were recruited into the study at birth, of which 305 received follow up examinations at ages 3 and 6 months. Blood lead assessments commenced prenatally followed by quarterly measurements through age 60 months and semiannually between ages 60 and 78 months. Blood lead concentrations were calculated via anodic stripping voltammetry. Please see Roda et al. (1988) and Dietrich et al. (1987) for specific details regarding anodic stripping voltammetry and blood lead level calculation for the CLS. Two hundred fifty adult CLS participants between the ages of 19 and 24 years received follow-up visits through at least age 78 months and were eligible for additional research studies, including criminal activity and behavioral analyses (Wright et al., 2008). For a subsequent phase of the study between 2008 and 2012, 174 CLS cohort participants were recruited to receive high-resolution anatomical neuroimaging. We prioritized recruitment with the oldest participants first, targeting a recruitment age of 26 years. Due to contraindications to imaging, 16 were unable to be scanned. Three additional participants (2 female, 1 male) were excluded due to MRI image reconstruction issues or artifacts. The images from the remaining 155 (90 Female, 65 Male) CLS participants were utilized in the current study. Participant characteristics for this study are shown in Table 1.
Table 1.
Characteristics for Participants and their Mothers
| Characteristics | Cohort (N =155) |
Range | Female (N = 90) |
Male (N = 65) |
Philips Scanner (N = 123) |
Siemens Scanner (N = 32) |
|---|---|---|---|---|---|---|
| African-American | 144 (~93%) | 83 (~92%) | 61 (~94%) | 115 (~93%) | 29 (~91%) | |
| PbB78 (ug/dL) | 7.99 (±4.17) | 1.8–24.75 | 7.5 (±3.95) | 8.66 (±4.4) | 7.82 (±7.82) | 8.65 (±4.03) |
| Total PPI Score (Raw) | 365.4 (±30.8) | 294–493 | 355.95 (±28.3) | 378.53 (±26.55) | 366.57 (±28.84) | 360.97 (±37.78) |
| Age3T (yrs) | 26.8 (±1.08) | 25.05–30.58 | 26.69 (±1.08) | 26.99 (±1.05) | 26.82 (±1.17) | 26.79 (±0.61) |
| BW (grams)‡ | 3121.25 (±492.7) | 1814–4400 | 3081.1 (±432.55) | 3176.82 (±564.43) | 3163.54 (±486.4) | 2958.69 (±490.6) |
| GEST (wks)‡ | 39.47 (±1.8) | 35–43 | 39.43 (±1.74) | 39.52 (±1.88) | 39.63 (±1.7) | 38.8 (±2.05) |
| Educational Attainment† | 11.9 (±1.77) | 7–16 | 12.19 (±1.88) | 11.6 (±1.57) | 11.9 (±1.77) | 11.94 (±1.83) |
| HOME | 31.17 (±5.5) | 17–43 | 31.2 (±5.77) | 31.16 (±5.12) | 30.9 (±5.17) | 32.19 (±6.54) |
| Mean SES | 18.4 (±4.4) | 8–33.63 | 18.23 (±4.2) | 18.64 (±4.61) | 18.23 (±4.3) | 19.18 (±4.61) |
| FSIQ at 7 yrs† | 85.8 (±11.66) | 50–116 | 87.38 (±11.32) | 83.57 (±11.85) | 85.98 (±11.33) | 85.04 (±13.02) |
| Maternal IQ2 | 75.6 (±9.09) | 58–100 | 75.64 (±9.06) | 74.12 (±8.7) | 75.06 (±8.75) | 74.75 (±9.66) |
| Participant Marijuana Use† | 82 Yes (~53%) | 45 Yes (50%) | 37 Yes (~57%) | 70 Yes (~57%) | 12 Yes (~38%) | |
| Maternal Cigarette Use‡ | 76 Yes (~49%) | 45 Yes (50%) | 31 Yes (~48%) | 56 Yes (~46%) | 20 Yes (~63%) | |
| Maternal Alcohol Use | 21 Yes(~14%) | 13 Yes(~14%) | 8 Yes(~12%) | 15 Yes(~12%) | 6 Yes (~19%) | |
| Maternal Marijuana Use | 16 Yes (~10%) | 9 Yes (10%) | 7 Yes (~11%) | 13 Yes (~11%) | 3 Yes (~9%) | |
| Maternal Narcotic Use | 2 Yes (~1 %) | 2 Yes (~2%) | 0 Yes (0%) | 1 Yes (~1%) | 1 Yes (~3%) |
Data presented as mean (± S.D.) or n (%)
Abbreviations: Age3T Age at Imaging; PbB78 Blood lead concentration at age 78 months; BW Birth Weight; FSIQ Full Scale Intelligence Quotient; GEST Participant Gestational Age at Birth; HOME Childhood HOME Inventory Score; SES Hollingshead Socioeconomic Status; Total PPI Total raw score on Psychopathic Personality Inventory
Denotes that differences between female and male participants are statistically significant (P < 0.05)
Denotes that differences between participants on Philips and Siemens scanners are statistically significant (P < 0.05)
2.2 Psychopathic Personality Inventory
Adult CLS participants were administered the Psychopathic Personality Inventory (PPI) (Lilienfeld and Andrews, 1996) via computer as described by Wright et al. (2009). The PPI is a 187 question self-reporting survey designed to delineate and assess the fundamental personality traits of psychopathy in a non-prison population. The total PPI score functions as a measure of overall psychopathy with elevated scores suggesting a greater likelihood of psychopathic personality traits being present. The PPI provided a reliable assessment of psychopathy and has been validated over several different populations (Edens et al., 2001, Lilienfeld, 1998, Lilienfeld and Andrews, 1996, Poythress et al., 1998); additionally, the full length version of the PPI has demonstrated greater construct validity over the short-form version (Kastner et al., 2012). It is important to note that the measurement of psychopathic personality traits in relatively modest manifestations for non-incarcerated "healthy" samples remains debated, as there is low to moderate convergence between measures like PPI and Psychopathy Check List Revised (PCL-R) (Malterer et al., 2010). For the purposes of this study, total PPI score was the primary variable used. Chapman et al. (2003) suggests that total PPI score is a valid representation of psychopathy in a psychometric analysis of the PPI. Furthermore, Total PPI scores demonstrated high internal consistency ratings with the other PPI subscales (Poythress et al., 1998) and correlate well with other personality tests that measure and assess psychopathic personality characteristics (Malterer et al., 2010, Ross et al., 2009, Edens et al., 2001).
2.3 Lead (Pb) Exposure
For this study, blood lead concentrations obtained at age 78 months (PbB78) were employed in our models. This value was selected over other time points for blood lead concentrations because later childhood blood lead levels are thought to better reflect body-lead accumulation and demonstrate greater associations with neuropsychological and behavioral measures (Baghurst et al., 1992, Wasserman et al., 1997, Wright et al., 2009, Wright et al., 2008, Hornung et al., 2009, Ris et al., 2004). Although blood lead levels do not typically display a normal distribution within the general population, it has been reported that low to moderate values perform as well in linear models as they do in other statistical models (National Research Council, 1993), so blood lead levels were retained in micrograms per deciliter (µg/dL).
2.4 Image Acquisition
Imaging for this study was first initiated on a 3T Siemens Trio scanner (Siemens, Erlangen, Germany) and shortly thereafter switched to a 3T Philips Achieva scanner (Philips Medical Systems, Cleveland, OH) using an 8-channel head coil. The usage of two scanners was due to the institutional decision to remove the 3T Siemens Trio scanner and replace it with a 3T Philips Achieva during the study enrollment period. The oldest cohort members were contacted first, with participants consecutively enrolled with the first (N= 32) participants scanned on the Siemens 3T Trio scanner, and all subsequent (N=123) participants scanned on the Philips 3T Achieva scanner.
For the 3T Siemens Trio, three-dimensional (3D) T1-weighted images were obtained using a magnetization-prepared rapid acquisition of gradient echo (MPRAGE) pulse sequence with the following parameters: repetition time (TR) = 2000 milliseconds (ms); echo time (TE) = 2.93 ms; inversion delay time (TI) = 1100 ms; flip angle = 12°. For the 3T Philips Achieva, a single-shot Turbo Field Echo (TFE) pulse sequence was used with the following parameters: TR = 8.1 ms; TE = 3.7 ms; TI = 889.7 ms; flip angle = 8°.
2.5 Data Processing
Voxel Based Morphometry was used to assess brain volume changes (BVC) in relation to Total PPI score and blood lead concentrations obtained at 78 months of age. VBM is a technique often used with high resolution MRI anatomical data to compare voxel level measurements of tissue concentration (gray and white brain matter for this study) within or between multiple groups or subjects. In brief, brains are typically fitted into the same stereotactic space through a process referred to as “normalization.” Normalized brains are then segmented into tissue classes (gray matter, white matter, cerebrospinal fluid) and smoothed. Finally, statistical analyses can be conducted on the smoothed tissue classes. Analyses can focus on individual voxels or on clusters of contiguous voxels. [Please see the following for a more detailed description of VBM: Ashburner and Friston (2000); Ashburner and Friston (2001); Good et al. (2001); Mechelli et al. (2005)]
T1-weighted anatomical images were reconstructed using Cincinnati Children’s Image Processing Software (Schmithorst et al., 2000) running in IDL 8.1 (Exelis Visual Information Solutions, Boulder CO). 3D images were imported into Statistical Parametric Mapping 8 (SPM8) version 4290 (SPM8; Wellcome Department of Cognitive Neurology, London) running in Matlab 7.13.0.564 (The Mathworks, Inc., Natick, MA) where they were visually inspected for artifacts and other scan abnormalities. Images were manually reoriented with the origin voxel [0, 0, 0] being set medially on the anterior commissure with the posterior commissure in the same plane. Images free from artifacts or other abnormalities were then processed using the VBM8 toolbar version 369 (Kurth et al., 2010) for SPM8.
Instead of a single volume number for each tissue class (gray matter, white matter, cerebrospinal fluid (CSF)) for the whole brain or a region, the VBM approach essentially quantifies and compares volume in high-resolution volume elements (voxels) across the images for all the individuals in the cohort. Anatomical T1 images were first segmented into tissues classes (gray matter, white matter, and cerebral spinal fluid) using a unified segmentation model in SPM8 (Ashburner and Friston, 2005). This model is based upon an adaptive Maximum A Posteriori (MAP) method that does not require prior tissue-probability data (Rajapakse et al., 1997). The segmentation procedure also implemented a Partial Volume Estimation (PVE) to improve segmentation accuracy by estimating the proportion of each tissue class contained in every voxel (Tohka et al., 2004). Noise reduction was also incorporated into image preprocessing via two de-noising methods. First, a spatially adaptive nonlocal means (SANLM) filter was utilized to reduce noise and maintain edges (Manjon et al., 2010). Second, the application of a Markov Random Field (MRF) model reduced noise by assimilating spatial data from neighboring voxels into the segmentation estimates (Cuadra et al., 2005, Rajapakse et al., 1997). Tissue classes were then registered and normalized to a template from the IXI-database (http://www.braindevelopment.org) in MNI space utilizing the DARTEL toolbox (Ashburner, 2007). Normalized images were modulated by multiplying voxels by non-linear components, correcting for differences in subject brain sizes. An 8 mm full-width half-maximum (FWHM) Gaussian Kernel was used to smooth modulated gray and white matter images to improve the signal to noise ratio, help normalize the distribution of the data, and minimize the impact of registration errors (Scouten et al., 2006, Kiebel et al., 1999).
2.6 Statistical Analyses
2.6.1 Approach
Our statistical analyses were conducted in multiple steps. First the data were examined for completeness, any missing data points were estimated using multiple imputation. All participant characteristic variables were then compared to the CLS birth cohort. Additional analyses were conducted comparing the characteristics of female against males, and those from the Siemens scanner against those from Philips scanner. We then conducted analyses examining the relationship between Total PPI, PbB78, and sex. Finally, we used VBM to look for brain volume changes in relation to Total PPI, PbB78, and sex. Our statistical approach for VBM was conducted in three steps: 1) To assess BVC associated with total PPI score 2) To assess BVC associated with blood lead concentrations obtained at 78 months of age; 3) Perform conjunction analyses of steps 1) and 2) to examine the relationship between BVC associated with total PPI score and PbB78. BVC represented four imaging outcomes: gray matter decrease, gray matter increase, white matter decrease or white matter increase. Additionally, an evaluation of confounding variables was performed and adjusted for in the final models.
2.6.2 Software
Demographic characteristics, imputations of missing demographic variables, assessment of collinearity, and PPI performance measures were analyzed using SAS 9.3 (SAS Institute Inc., Cary, NC). R (version 3.4.3; R Core Team (2017)) was used to estimate the significance of missing variables. For VBM, the spatial and quantitative aspects of the MRI data required using specific image analysis software SPM8 and AFNI (version 16.3.13; https:/afni.nimh.nih.gov) for analyses.
2.6.3 Participant Characteristics
Comparisons between participant characteristics were made in SAS using a Chi-Square test for categorical variables, and a one-way ANOVA for all other variables. Demographic differences were examined between the original birth cohort and the cohort described for this analysis to evaluate for any systemic biases in sample attrition. We also evaluated the demographic data for differences due to sex and to the scanner used for their imaging acquisition. The relationship between PbB78, total PPI score, and relevant covariates was established using a general linear model in SAS. Collinearity between relevant covariates was also tested for but not observed.
2.6.4 Model Framework
The individual preprocessed gray and white matter images were analyzed within the framework of a general linear model (GLM) using SPM8. An initial factorial model was constructed with brain volume (gray matter or white matter) as the dependent variable and either Total PPI or PbB78 as the independent variable. Sex was included as a factor. BVC was examined for each independent variable, and for an interaction with sex. After preliminary analyses, relevant covariates were selected for each model for further analysis (see section 2.6.8). Final models were constructed with a full-factorial design using participant sex as a factor with relevant covariates included in the final models.
2.6.5 Image Comparisons
Within SPM8, absolute threshold masking was applied to eliminate voxels with a value less than 0.1 to reduce edge effects between tissue classes, and explicit binary masks were generated and applied to limit the analysis to voxels within gray matter or white matter tissue respectively. Monte Carlo simulations were conducted a priori for gray matter and white matter analyses using the 3dClustSim function in the AFNI software package to establish minimum cluster size thresholds. This version of AFNI addressed previous concerns over the validity of the cluster simulation procedure. We selected an uncorrected voxel level P-value = 0.005 to establish the minimum cluster size for the cohort; 10,000 simulations were conducted for each tissue class yielding a minimum cluster threshold corrected for multiple comparisons at P ≤ 0.05. The full width half maximum (FWHM) was estimated using the spm_est_smoothness function in SPM8. The minimum cluster threshold was 1377 contiguous voxels for gray matter, and 1461 voxels for white matter.
2.6.6 Conjunction Analysis
In the event that the VBM analyses demonstrated that the same brain regions were associated with total PPI score and PbB78 values, a conjunction analysis was performed to examine the relationship between brain volume, total PPI, and PbB78. As stated in Friston et al. (1999), a conjunction is “the joint refutation of multiple null hypotheses,” which allows for an inference to be made regarding multiple effects. To test this relationship, we conducted a conjunction analysis of the global null hypothesis (H0 = There are no activations) using a family-wise error rate (FWE) correction of 0.05 at the voxel level. As noted in Friston et al. (2005), we acknowledge that a significant conjunction does not imply that all contrasts were individually significant, but that the constrasts were “consistently high and jointly significant.”
2.6.7 Visualization
For visualization of all VBM results, an overlay of significant association clusters for each analysis was created in SPM8 and superimposed upon a composite IXI-database template. Identification of brain regions required the use of the xjView and several atlases (Haines, 2008, Nolte and Angevine, 2007, Oishi, 2011). Masks for intersecting spatial regions from the primary models (BVC and PbB78, BVC and PPI) were generated using the xjView 8.14 (http://www.alivelearn.net/xjview) plugin for SPM8. For reporting a finding from the conjunction analysis, the association cluster was required to be present on both of the primary models (BVC with PbB78, BVC with PPI).
2.6.8 Covariate Selection for Primary Models
Covariates for each of the primary models (BVC and PPI, BVC and PbB78) were chosen using a selection method adopted from Yuan et al. (2006). In brief, variables found to cause a 10% or greater change in beta values for at least 20% of the significantly associated voxels when compared to the initial analysis with the independent variable (either Total PPI score or PbB78) were included using SPM8. This method has been used by Yuan et al. (2007), Cecil et al. (2008), and Brubaker et al. (2010). Variables considered included sex, race, age at time of imaging, gestational age at birth, weight at birth, maternal IQ (Silverstein, 1985, Wechsler, 1981), participant full-scale IQ (Wechsler, 1991), childhood total HOME inventory score (Bradley and Caldwell, 1977, Bradley and Caldwell, 1979, Caldwell and Bradley, 1978), highest level of educational attainment for participant, mean childhood Hollingshead socioeconomic status (SES) score (Cirino et al., 2002), adult marijuana usage based upon urine screen at time of imaging, maternal prenatal alcohol use, maternal prenatal cigarette use, maternal narcotic use, and maternal prenatal marijuana use. For Total PPI, age at scan and PbB78 were included in the gray model analysis, and PbB78 was included in the white matter analyses. Age, birth weight, FSIQ, and maternal IQ were included as covariates for the gray matter analysis for PbB78. Age and FSIQ were included as covariates in the white matter analysis for PbB78. Sex was found to be significantly associated and was accounted for in all analyses as a factor instead of a covariate. Additionally, all analyses included a covariate to account for two scanners being used for data collection.
2.6.9 Missing Data Points
Several variables in the CLS data set contained missing data points, we therefore used multiple imputation to estimate these missing data points rather than eliminate participants with missing data (Supplemental Table 1). A requirement for conducting multiple imputation is that it must be assumed that the data are missing completely at random (MCAR) or at least missing at random (MAR). To test this assumption, we used Little’s MCAR test (Little, 1988) to estimate the probability that the data are missing completely at random. The LittleMCAR program within the BaylorEdPsych package (Beaujean, 2012) was used to conduct Little’s Chi-Squared test for MCAR using R. The rate of missing variables was not predicted by any variables in the imputation analysis and data points were assumed to be missing completely at random [X2 (191, N = 155) = 205.2959, P = 0.2272727)]. Missing data points for independent variables and covariates were generated using a multivariate normal distribution multiple imputation model in SAS. Parameter estimates for the data set with missing data points were calculated using a GLM. Ten data sets were imputed using the PROC MI procedure. Parameter estimates for each imputed data set were generated via a GLM and a pooled analysis was conducted to estimate the standard error for the imputed data set and compared to the initial estimate. An ANOVA was used to compare variable means before and after imputation, no statistically significant differences were found.
3.0 Results
3.1 Population Characteristic Comparisons
The participants in the current study demonstrated an increased percentage of female [X2 (1, N = 539) = 4.32, P = 0.0377] and African American [X2 (1, N = 539) = 8.08, P = 0.0045] when compared to the original CLS birth cohort (Table 1). All other characteristics remained statistically insignificant between cohorts. When characteristics were compared by sex, males displayed higher total PPI scores than females [F (1, 153) = 23.14, p < 0.001], lower FSIQ [F (1, 153) = 4.12, P = 0.0441], and a lower level of educational attainment [F (1, 153) = 4.3, P = 0.0397]. When characteristics were compared by scanner, differences in birth weight [F (1,153) = 4.49, P = 0.0357], gestational age [F (1, 153) = 5.25, P = 0.0233], and maternal cigarette use during pregnancy [F (1, 153) = 7.94, P = 0.0055] were observed.
The total PPI score for our cohort (M=365.4 [SD=30.8]) resembled the score reported by Sellborn et al. (2007) in a community cohort of 95 (45 female) single, undergraduate students (M=367.64 [SD=43.4]). For males, Malterer et al. (2010) reported two samples of incarcerated males with mean scores (one with M=380.24 [SD= 41.61] and the other M=386.74 [SD=40.33]) and ages similar to the males of the CLS (M=378.53 [SD=26.55]).
3.2 Total PPI score and PbB78
The analysis between total PPI score and PbB78 for the current CLS cohort demonstrated a significant direct association [F (1, 153) = 7.72, P = 0.0062, R2 = 0.048, BetaPbB78 = 0.219 (t = 2.78, P = 0.0062)]. An interaction between sex and PbB78 was also observed [F (1, 152) = 12.25, P = 0.001]. For females, the association was F (1, 88) = 2.32, P = 0.1313, R2 = 0.026, BetaPbB78 = 0.160 (t = 1.52, P = 0.1313), the male association showed F (1, 63) = 3.24, P = 0.0768, R2 = 0.049, BetaPbB78 = 0.22108 (t = 1.80, P = 0.0768).
3.3 Summary of Brain Volume Changes
A composite summary table qualitatively describes the covariate adjusted BVC from each of the examined models for the CLS (Table 2). Specific details for the results are described below.
Table 2.
Summary of Brain Volume Changes
| Model | All (N = 155) | Female (N = 90) | Male (N = 65) |
|---|---|---|---|
| Total PPI | |||
| Gray Matter | Interaction between TPPI score and Sex | GM increase associated with increased Total PPI score | GM decrease associated with increased Total PPI score |
| White Matter | WM decrease associated with increased Total PPI score and interaction between TPPI score and Sex | N/S | WM decrease associated with increased Total PPI score |
| PbB78 | |||
| Gray Matter | GM decrease associated with increased PbB78 | GM decrease associated with increased PbB78 | N/A |
| White Matter | WM decrease associated with increased PbB78 | WM decrease associated with increased PbB78 | WM decrease associated with increased PbB78 |
| Conjunction | |||
| Gray Matter | N/A | N/A | N/A |
| White Matter | N/A | N/A | Overlap between WM decrease, increased PbB78, and increased Total PPI scores |
Abbreviations: PbB78 Blood lead at age 78 months; GM Gray matter; Total PPI Total psychopathic personality inventory score; WM White matter
3.3.1 VBM Analyses – BVC and PPI Relationship
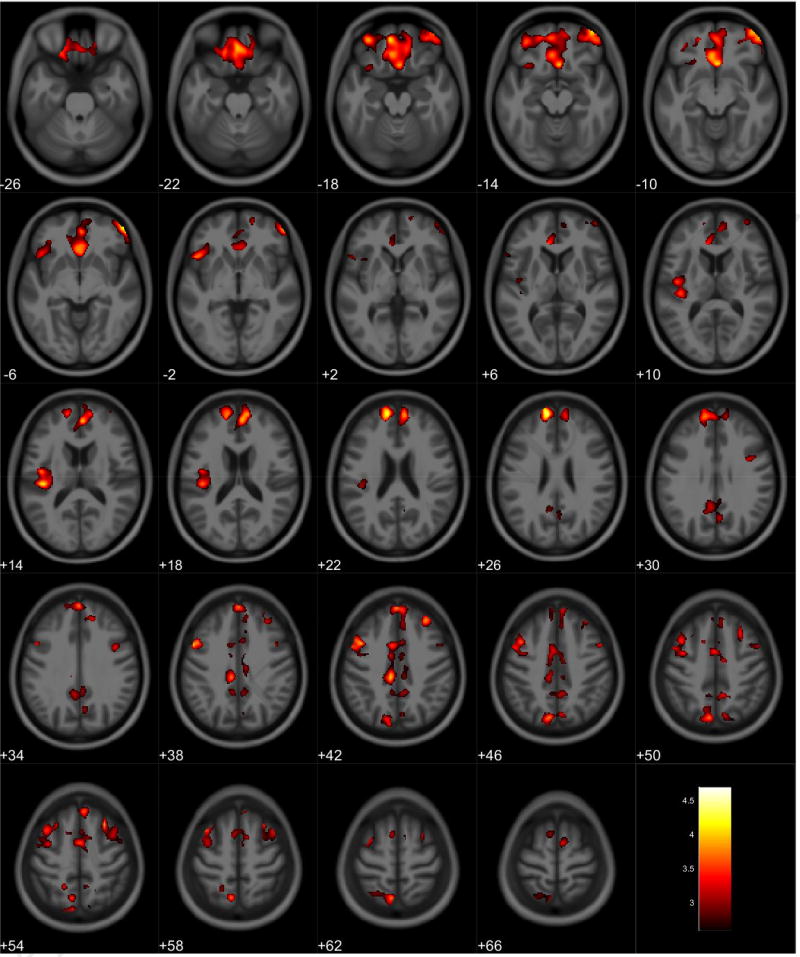
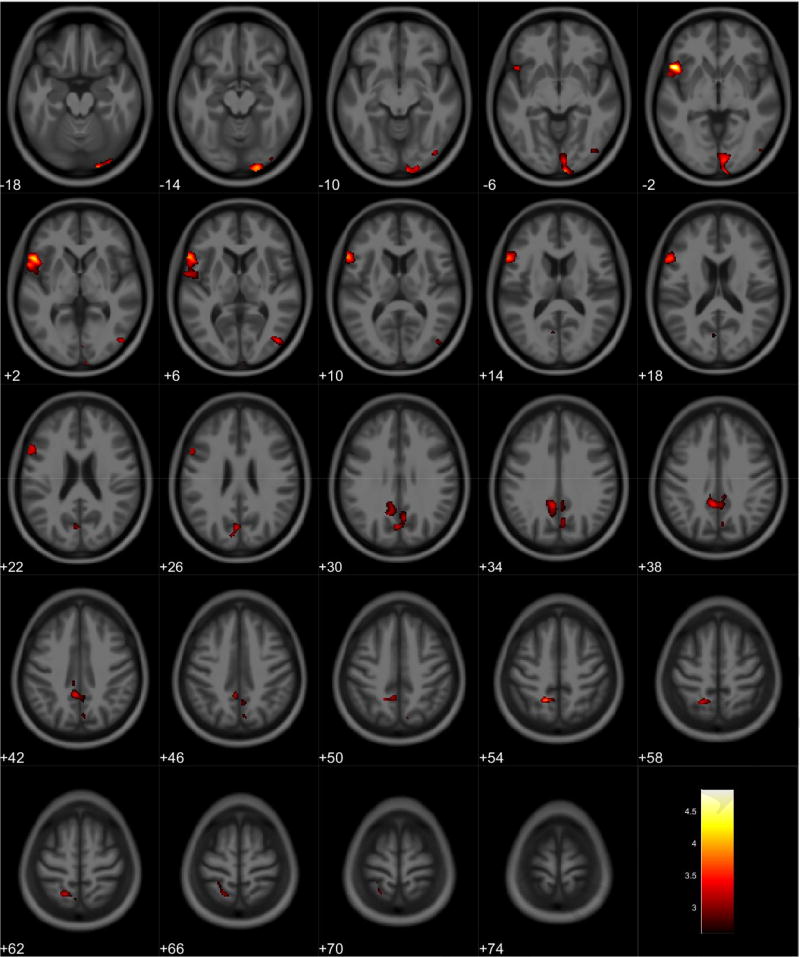
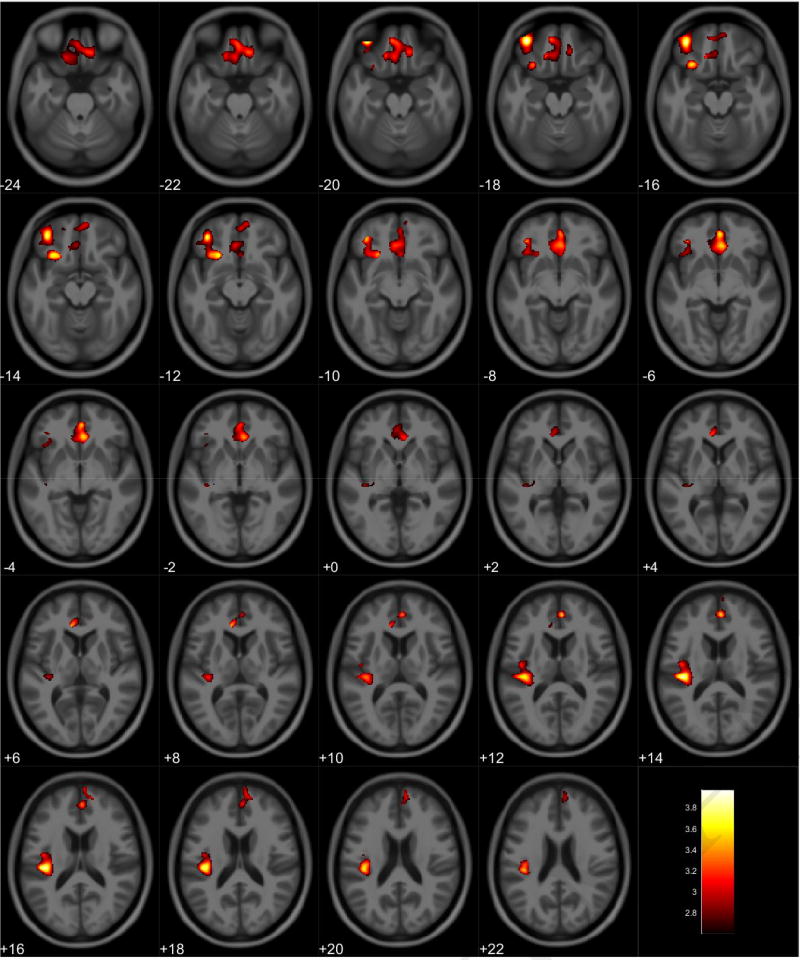
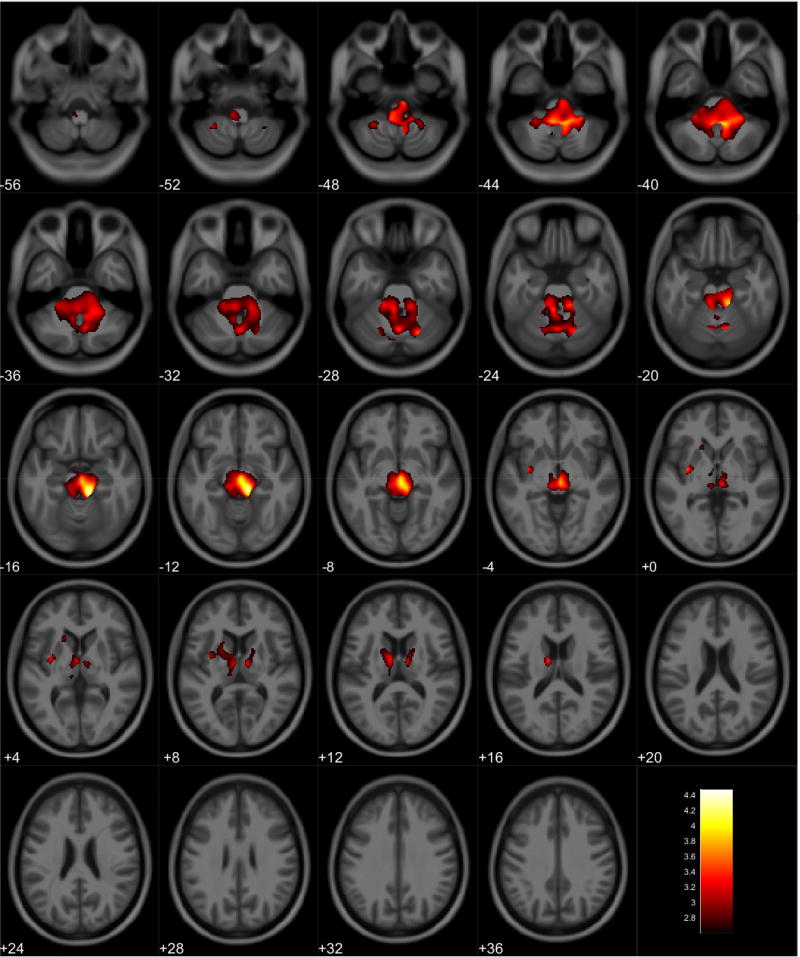
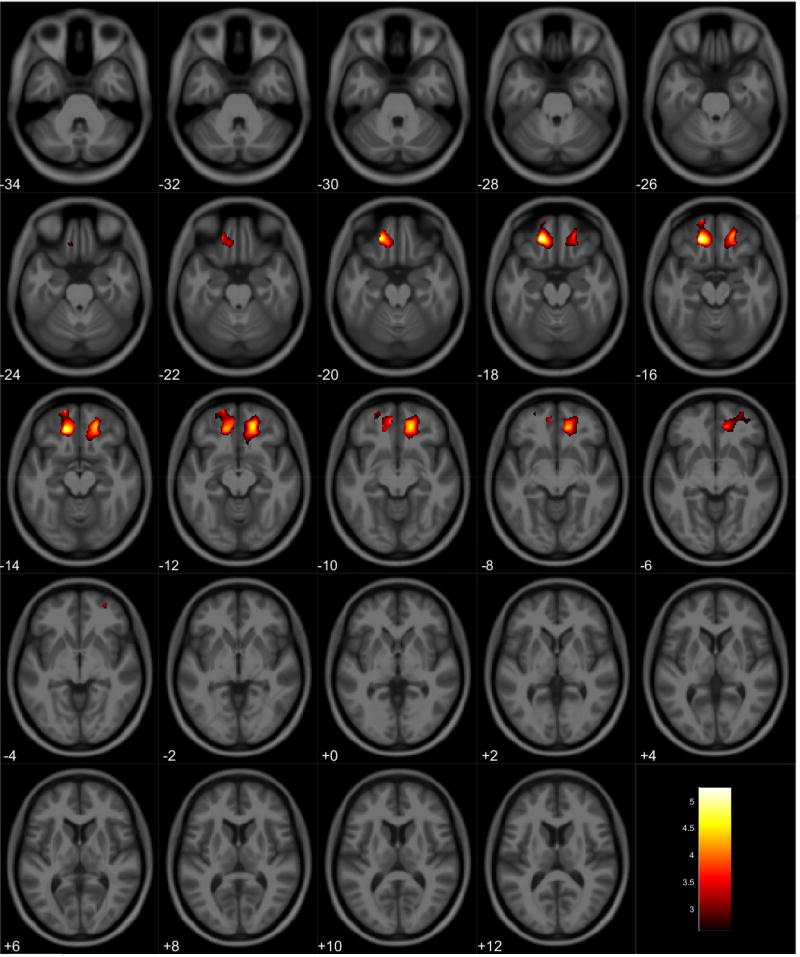
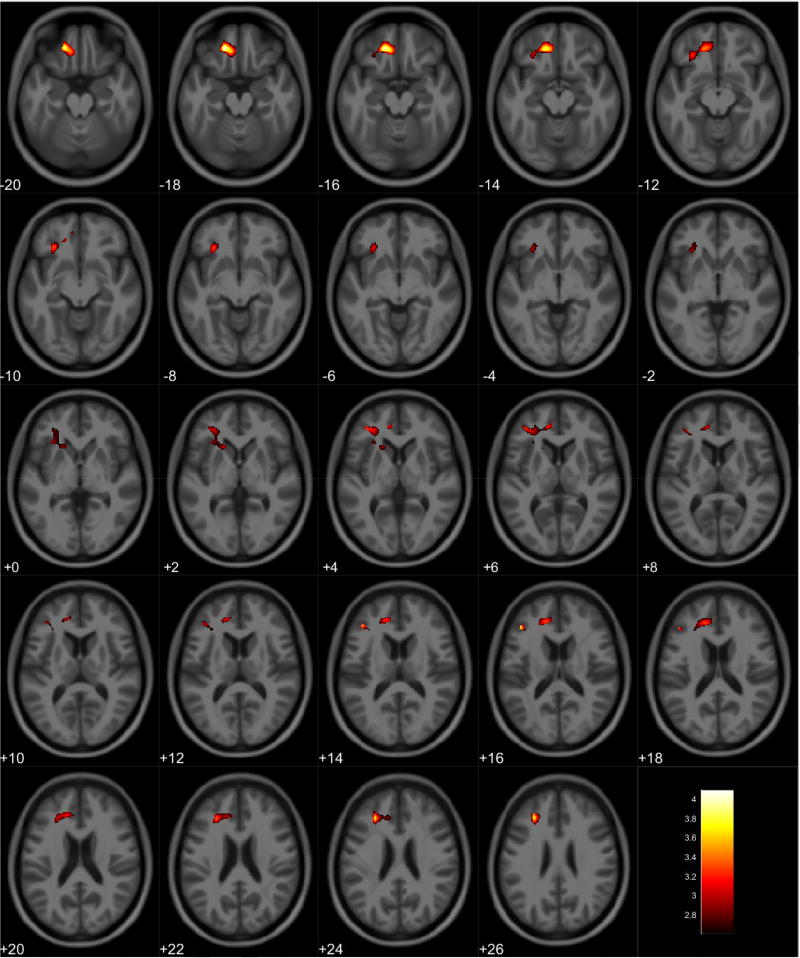
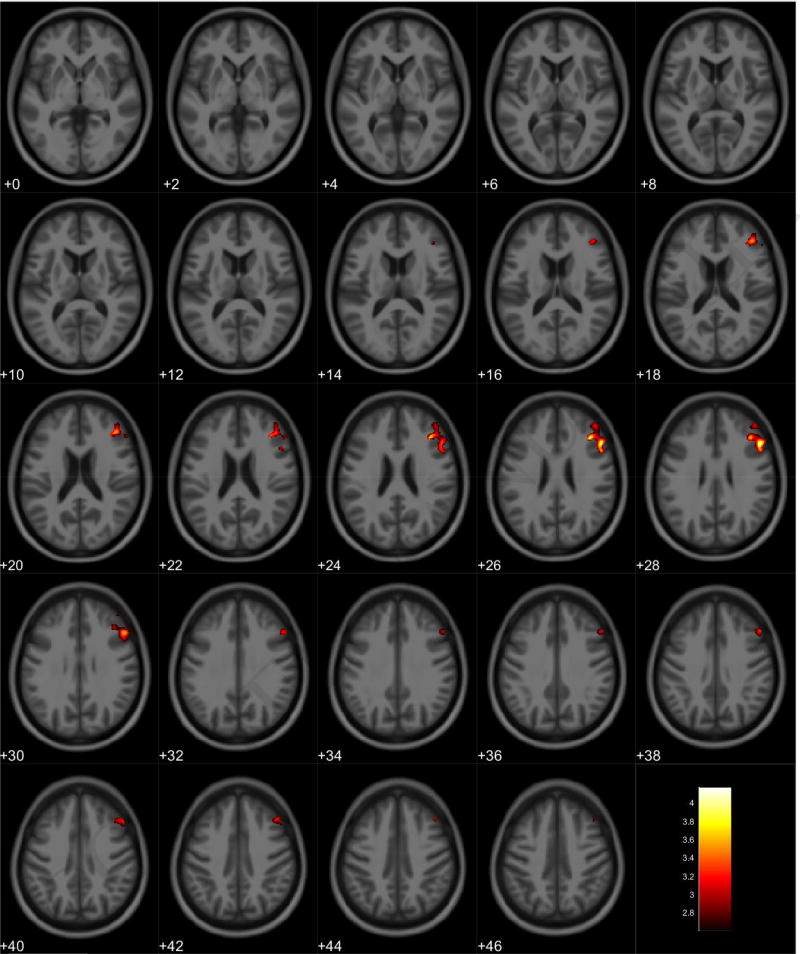
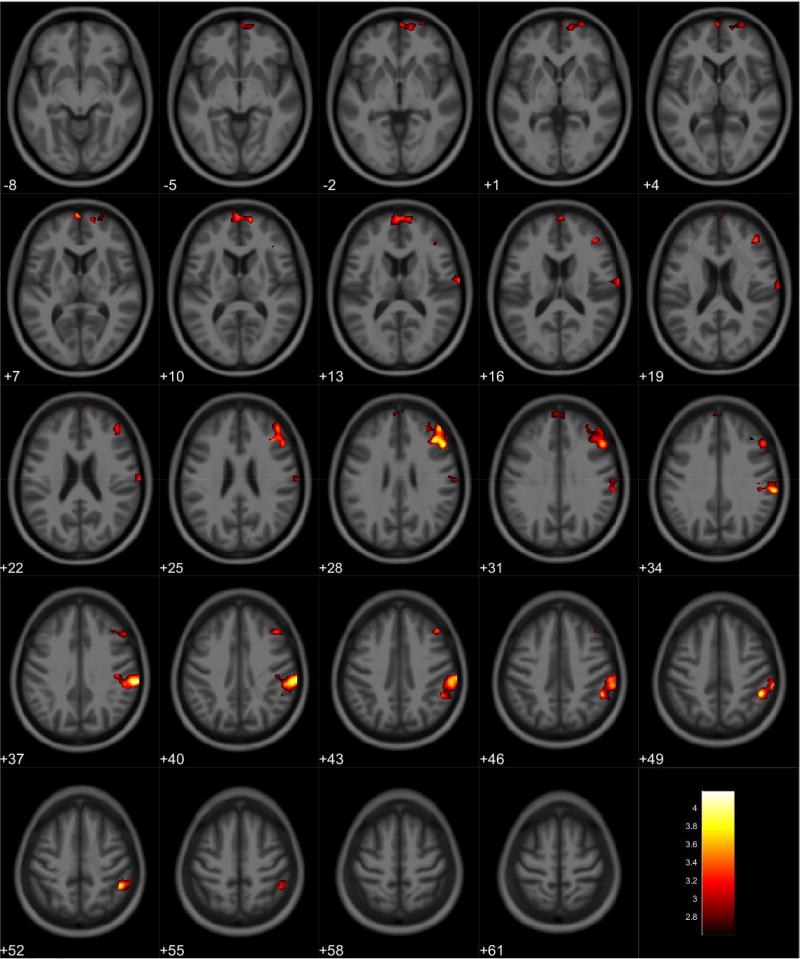
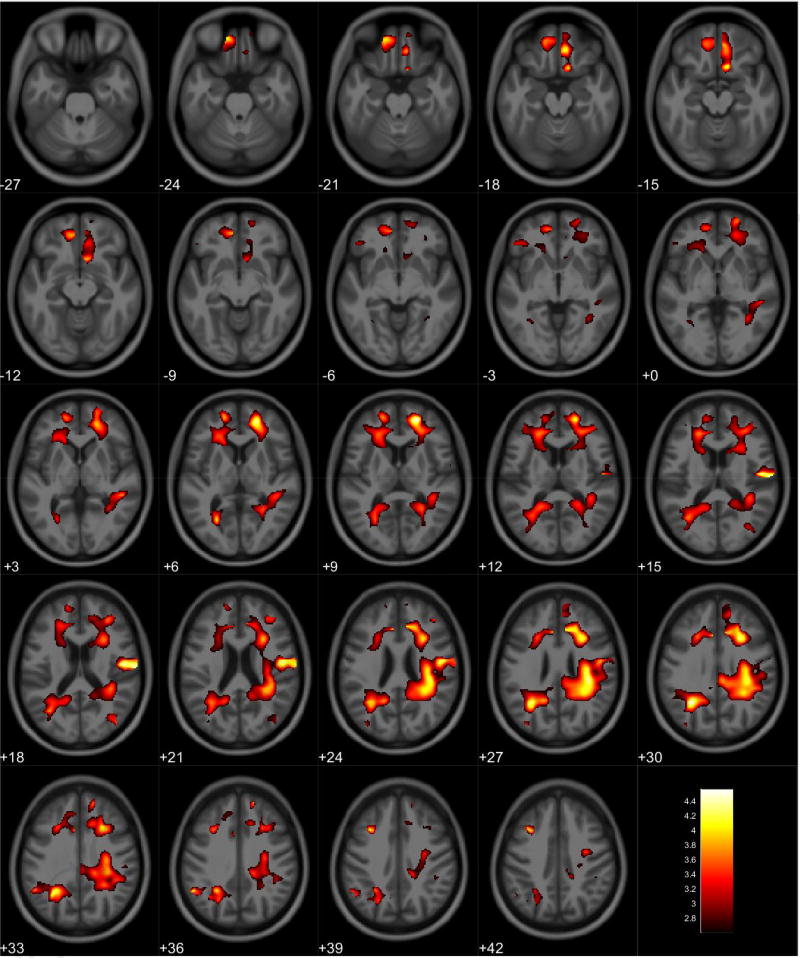
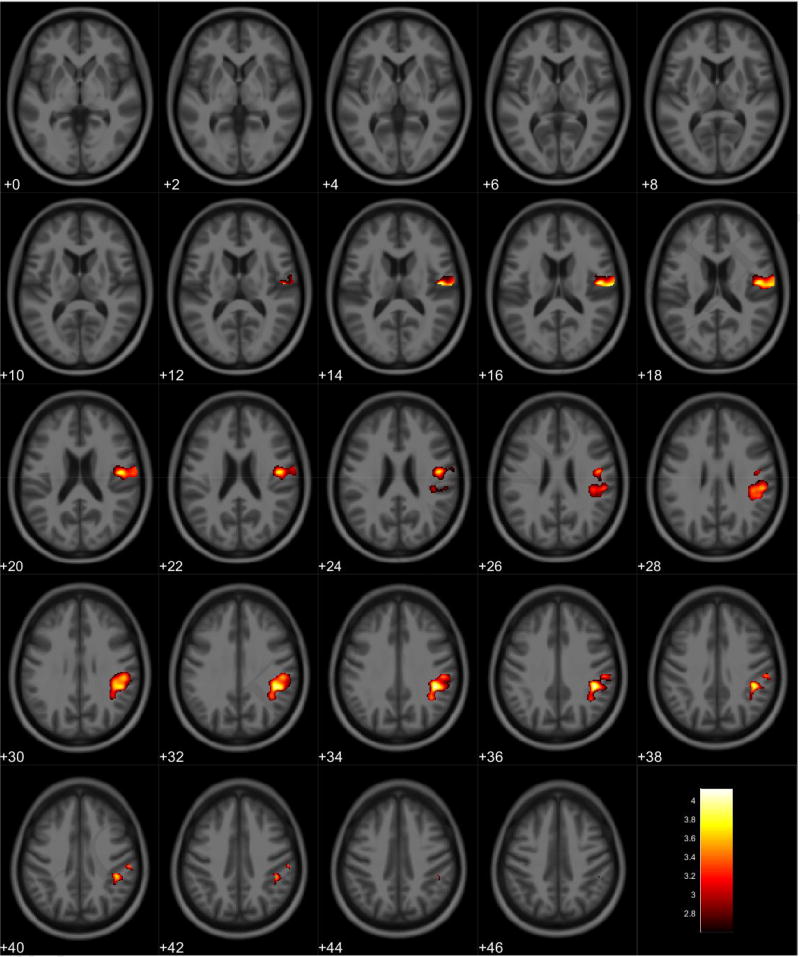
VBM analyses showed no volumetric differences in gray matter volume associated with total PPI score; however, there was a significant interaction between sex and total PPI score. Seven clusters were observed demonstrating regions where males displayed reduced gray matter volumes compared to females in relation to total PPI score. These regions were primarily located in the frontal and temporal lobes (Table 3, Figure 1). Further investigation of the interaction established that females showed increased gray matter volume in relation to increased total PPI scores in the frontal, temporal, and parietal lobes (Table 4, Figure 2). In contrast, males displayed reduced gray matter volume in relation to increased total PPI scores, primarily in the prefrontal cortex and anterior cingulate (Table 5, Figure 3). For the white matter analysis, an inverse association between white matter volume and total PPI score was observed bilaterally in the cerebellum, brain stem, and lower portion of the thalamus. This association also extended to the white matter near the basal ganglia, primarily the caudate and lentiform nucleus (Table 6, Figure 4). Similar to gray matter, and interaction between sex and total PPI score was also observed in the white matter volume, with males displaying reduced white matter volumes compared to females in relation to total PPI score. This difference was observed bilaterally in the white matter of the prefrontal cortex (Table 7, Figure 5). Post-hoc analyses revealed no volumetric differences in female white matter. In contrast, males displayed an inverse association between total PPI score and white matter volume in the left prefrontal white matter (Table 8, Figure 6).
Table 3.
Regional Interactions in Gray Matter Volume between Total PPI Scores and Sex
| Anatomical Region | Cluster Size* (Voxels) |
MNI Coordinates (X, Y, Z) |
Peak Voxel F | Peak Voxel T |
|---|---|---|---|---|
| Medial Frontal Gyrus | 13379 | −11, 56, 25 | 20.02 | 4.47 |
| Superior Frontal Gyrus | ||||
| Inferior Frontal Gyrus | ||||
| Anterior Cingulate | ||||
| Orbital Gyrus | ||||
| R Middle Frontal Gyrus | 1863 | 50, 51, −6 | 17.48 | 4.18 |
| R Inferior Frontal Gyrus | ||||
| L Insula | 1514 | −42, −24, 15 | 16.73 | 4.09 |
| L Heschl’s Gyrus | ||||
| Precuneus | 3240 | −8, −73, 48 | 14.52 | 3.81 |
| Cingulate Gyrus | ||||
| Superior Parietal Lobule | ||||
| R Middle Frontal Gyrus | 1557 | 29, 21, 55 | 15.57 | 3.95 |
| R Superior Frontal Gyrus | ||||
| Cingulate Gyrus | 3224 | 15, 0, 73 | 21.99 | 4.69 |
| Superior Frontal Gyrus | ||||
| Medial Frontal Gyrus | ||||
| Middle Frontal Gyrus | 1922 | −41, 11, 42 | 15.74 | 3.97 |
| Inferior Frontal Gyrus | ||||
| Superior Frontal Gyrus |
Clusters corrected for multiple comparisons at P ≤ 0.05
MNI Montreal Neurological Institute
Figure 1.
Regional gray matter volume interaction between sex and total PPI. All clusters presented have been corrected for multiple comparisons (P ≤ 0.05). Slice numbers represent distance from origin (0, 0, 0), which was set at the anterior commissure. Color bar represents voxel level T-statistic. Orientation: Left = Left, Right = Right. Total PPI total psychopathy inventory score
Table 4.
Regional Gray Matter Volumetric Increases Associated with Increased Total PPI Scores for Female Participants
| Anatomical Region | Cluster Size* (Voxels) |
MNI Coordinates (X, Y, Z) |
Peak Voxel F | Peak Voxel T |
|---|---|---|---|---|
| Lingual Gyrus | 1391 | 20, −97, −15 | 16.18 | 4.02 |
| Inferior Occipital Gyrus | ||||
| Middle Occipital Gyrus | ||||
| L Inferior Frontal Gyrus | 1890 | −50, 17, −2 | 23.46 | 4.84 |
| L Superior Temporal Gyrus | ||||
| Posterior Cingulate | 2315 | −15, −51, 53 | 15.70 | 3.96 |
| Precuneus | ||||
| Cuneus |
Clusters corrected for multiple comparisons at P ≤ 0.05
MNI Montreal Neurological Institute
Figure 2.
Regional gray matter volume increase in females. Overlay of significant clusters representing a volumetric increase associated with increased Total PPI scores for female subjects (N = 90). All clusters presented have been corrected for multiple comparisons (P ≤ 0.05). Slice numbers represent distance from origin (0, 0, 0), which was set at the anterior commissure. Color bar represents voxel level T-statistic. Orientation: Left = Left, Right = Right. Total PPI Total psychopathic personality inventory score.
Table 5.
Regional Gray Matter Volumetric Decrease Associated with Increased Total PPI Scores for Male Participants
| Anatomical Region | Cluster Size* (Voxels) |
MNI Coordinates (X, Y, Z) |
Peak Voxel F | Peak Voxel T |
|---|---|---|---|---|
| Anterior Cingulate | 4479 | 3, 44, −6 | 13.15 | 3.63 |
| Medial Frontal Gyrus | ||||
| Rectal Gyrus | ||||
| Orbital Gyrus | ||||
| Inferior Frontal Gyrus | 1777 | −36, 47, −18 | 15.76 | 3.97 |
| Middle Frontal Gyrus | ||||
| Orbital Gyrus | ||||
| Insula | 1581 | −39, −25, 15 | 15.38 | 3.92 |
| Heschl’s Gyrus | ||||
| Rolandic Operculum |
Clusters corrected for multiple comparisons at P ≤ 0.05
MNI Montreal Neurological Institute
Figure 3.
Regional gray matter volume decrease in males. Overlay of significant clusters representing a volumetric decrease associated with increased Total PPI scores for male subjects (N = 65). All clusters presented have been corrected for multiple comparisons (P ≤ 0.05). Slice numbers represent distance from origin (0, 0, 0), which was set at the anterior commissure. Color bar represents voxel level T-statistic. Orientation: Left = Left, Right = Right. Total PPI Total psychopathic personality inventory score.
Table 6.
Regional White Matter Volumetric Reductions Associated with Increased Total PPI Scores for All Participants
| Anatomical Region | Cluster Size* (Voxels) |
MNI Coordinates (X, Y, Z) |
Peak Voxel F | Peak Voxel T |
|---|---|---|---|---|
| Cerebellum | 19135 | 12, −30, −15 | 19.25 | 4.25 |
| Brainstem | ||||
| Pons | ||||
| Midbrain | ||||
| Thalamus | ||||
| Basal Ganglia | ||||
| Hippocampus |
Clusters corrected for multiple comparisons at P ≤ 0.05
MNI Montreal Neurological Institute
Figure 4.
Regional white matter volume decrease for all participants. Overlay of significant clusters representing a volumetric decrease associated with increased Total PPI scores across all subjects (N = 155). All clusters presented have been corrected for multiple comparisons (P ≤ 0.05). Slice numbers represent distance from origin (0, 0, 0), which was set at the anterior commissure. Color bar represents voxel level T-statistic. Orientation: Left = Left, Right = Right. Total PPI Total psychopathic personality inventory score.
Table 7.
Regional Interactions in White Matter Volume between Total PPI Scores and Sex
| Anatomical Region | Cluster Size* (Voxels) |
MNI Coordinates (X, Y, Z) |
Peak Voxel F | Peak Voxel T |
|---|---|---|---|---|
| L Medial Frontal Gyrus | 1467 | −17, 38, −17 | 27.61 | 5.25 |
| L Superior Frontal Gyrus | ||||
| L Middle Frontal Gyrus | ||||
| L Inferior Frontal Gyrus | ||||
| L Orbital Gyrus | ||||
| R Medial Frontal Gyrus | 1466 | 17, 42, −12 | 20.93 | 4.57 |
| R Superior Frontal Gyrus | ||||
| R Middle Frontal Gyrus | ||||
| R Inferior Frontal Gyrus | ||||
| R Orbital Gyrus |
Clusters corrected for multiple comparisons at P ≤ 0.05
MNI Montreal Neurological Institute
Figure 5.
Regional white matter volume interaction between sex and total psychopathy score. All clusters presented have been corrected for multiple comparisons (P ≤ 0.05). Slice numbers represent distance from origin (0, 0, 0), which was set at the anterior commissure. Color bar represents voxel level T-statistic. Orientation: Left = Left, Right = Right. Total PPI total psychopathy inventory score
Table 8.
Regional White Matter Volumetric Decreases Associated with Increased Total PPI Scores for Male Participants
| Anatomical Region | Cluster Size* (Voxels) |
MNI Coordinates (X, Y, Z) |
Peak Voxel F | Peak Voxel T |
|---|---|---|---|---|
| L Inferior Frontal Gyrus | 2538 | −17, 39, −18 | 16.78 | 4.10 |
| L Middle Frontal Gyrus | ||||
| L Anterior Cingulate | ||||
| L Medial Frontal Gyrus | ||||
| L Orbital Gyrus |
Clusters corrected for multiple comparisons at P ≤ 0.05
MNI Montreal Neurological Institute
Figure 6.
Regional white matter volume decrease in males. Overlay of significant clusters representing a volumetric decrease associated with increased Total PPI scores for male subjects (N = 65). All clusters presented have been corrected for multiple comparisons (P ≤ 0.05). Slice numbers represent distance from origin (0, 0, 0), which was set at the anterior commissure. Color bar represents voxel level T-statistic. Orientation: Left = Left, Right = Right. Total PPI Total psychopathic personality inventory score.
3.3.2 VBM Analyses – BVC and PbB78 Relationship
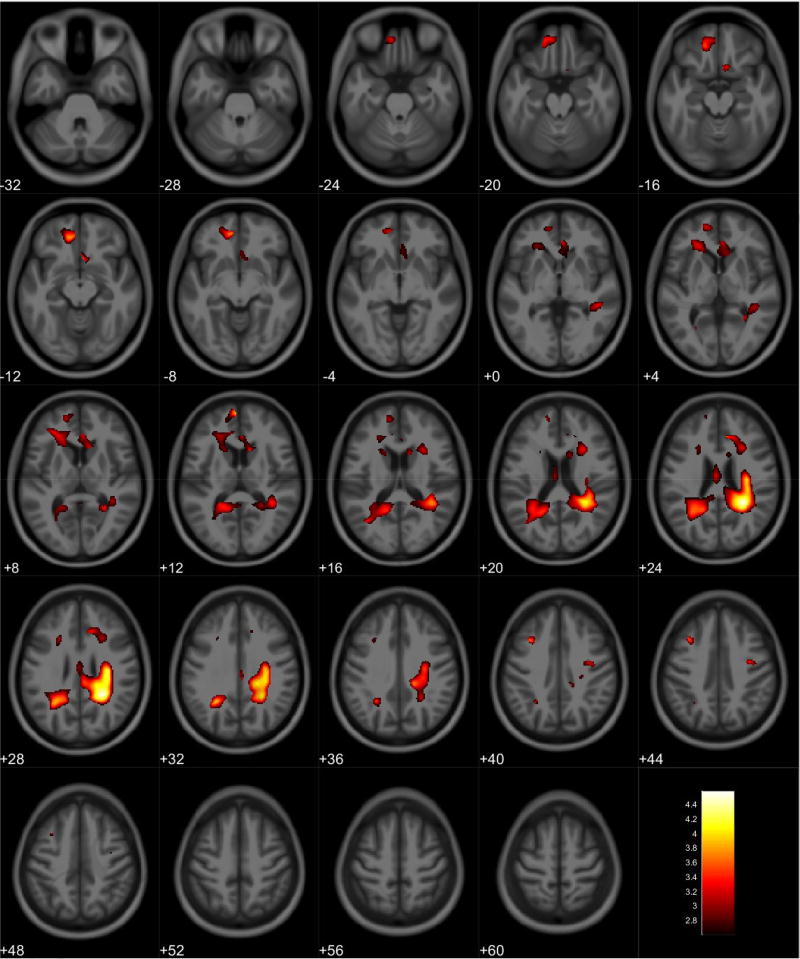
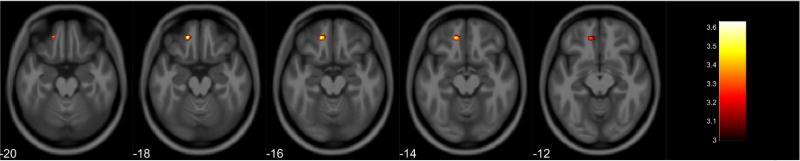
Gray matter volume loss associated with PbB78 values was observed in the right frontal lobe, primarily in the middle frontal gyrus (Table 9, Figure 7). A post-hoc analysis showed reduced gray matter volume in the frontal and parietal lobes of females in relation to increased PbB78 values (Table 10, Figure 8). Males did not display any volumetric differences in gray matter associated with PbB78. In white matter, an inverse association was observed with PbB78 in frontal, limbic, temporal, and parietal lobes (Table 11, Figure 9). Post-hoc analyses showed a white matter decrease primarily in the right parietal lobe associated with increased PbB78 values in females (Table 12, Figure 10). Males displayed reduced white matter volumes in the frontal, limbic, temporal, and parietal lobes in relations to increased PbB78 values (Table 13, Figure 11).
Table 9.
Regional Gray Matter Volumetric Decrease Associated with Increased PbB78 Values for All Participants
| Anatomical Region | Cluster Size* (Voxels) |
MNI Coordinates (X, Y, Z) |
Peak Voxel F | Peak Voxel T |
|---|---|---|---|---|
| R Inferior Frontal Gyrus | 1385 | 51, 23, 27 | 17.40 | 4.17 |
| R Middle Frontal Gyrus |
Clusters corrected for multiple comparisons at P ≤ 0.05
MNI Montreal Neurological Institute
Figure 7.
Regional gray matter volume decrease for all participants. Overlay of significant clusters representing a volumetric decrease associated with increased PbB78 scores across all subjects (N = 155). All clusters presented have been corrected for multiple comparisons (P ≤ 0.05). Slice numbers represent distance from origin (0, 0, 0), which was set at the anterior commissure. Color bar represents voxel level T-statistic. Orientation: Left = Left, Right = Right. PbB78 Blood lead at age 78 months.
Table 10.
Regional Gray Matter Volumetric Decrease Associated with Increased PbB78 Values for Female Participants
| Anatomical Region | Cluster Size* (Voxels) |
MNI Coordinates (X, Y, Z) |
Peak Voxel F | Peak Voxel T |
|---|---|---|---|---|
| Medial Frontal Gyrus | 1395 | −2, 66, 6 | 12.75 | 3.57 |
| Superior Frontal Gyrus | ||||
| Orbital Gyrus | ||||
| R Supramarginal Gyrus | 2509 | 63, −28, 37 | 16.98 | 4.12 |
| R Inferior Parietal Lobule | ||||
| R Postcentral gyrus | ||||
| R Precentral Gyrus | ||||
| R Middle Frontal Gyrus | 1694 | 39, 27, 27 | 17.49 | 4.18 |
| R Inferior Frontal Gyrus |
Clusters corrected for multiple comparisons at P ≤ 0.05
MNI Montreal Neurological Institute
Figure 8.
Regional gray matter volume decrease in females. Overlay of significant clusters representing a volumetric decrease associated with increased PbB78 scores for female subjects (N = 90). All clusters presented have been corrected for multiple comparisons (P ≤ 0.05). Slice numbers represent distance from origin (0, 0, 0), which was set at the anterior commissure. Color bar represents voxel level T-statistic. Orientation: Left = Left, Right = Right. PbB78 Blood lead at age 78 months.
Table 11.
Regional White Matter Volumetric Decrease Associated with Increased PbB78 Values for All Participants
| Anatomical Region | Cluster Size* (Voxels) |
MNI Coordinates (X, Y, Z) |
Peak Voxel F | Peak Voxel T |
|---|---|---|---|---|
| Medial Frontal Gyrus | 6828 | −15, 48, −23 | 17.76 | 4.21 |
| Anterior Cingulate | ||||
| Middle Frontal Gyrus | ||||
| Orbital Gyrus | ||||
| Superior Frontal Gyrus | ||||
| R Medial Frontal Gyrus | 20094 | 20, 50, 9 | 20.75 | 4.56 |
| R Cingulate Gyrus | ||||
| R Inferior Parietal Lobule | ||||
| R Rolandic Operculum | ||||
| R Superior Frontal Gyrus | ||||
| R Middle Frontal Gyrus | ||||
| R Insula | ||||
| R Superior Temporal Gyrus | ||||
| R Middle Temporal Gyrus | ||||
| L Precuneus | 5509 | −30, −55, 30 | 20.86 | 4.57 |
| L Supramarginal Gyrus | ||||
| L Superior Temporal Gyrus | ||||
| L Middle Temporal Gyrus | ||||
| L Posterior Cingulate Gyrus | ||||
| L Inferior Parietal Lobule |
Clusters corrected for multiple comparisons at P ≤ 0.05
MNI Montreal Neurological Institute
Figure 9.
Regional white matter volume decrease for all participants. Overlay of significant clusters representing a volumetric decrease associated with increased PbB78 scores across all subjects (N = 155). All clusters presented have been corrected for multiple comparisons (P ≤ 0.05). Slice numbers represent distance from origin (0, 0, 0), which was set at the anterior commissure. Color bar represents voxel level T-statistic. Orientation: Left = Left, Right = Right. PbB78 Blood lead at age 78 months.
Table 12.
Regional White Matter Volumetric Decrease Associated with Increased PbB78 Values for Female Participants
| Anatomical Region | Cluster Size* (Voxels) |
MNI Coordinates (X, Y, Z) |
Peak Voxel F | Peak Voxel T |
|---|---|---|---|---|
| R Inferior Parietal Lobule | 3132 | 42, −37, 34 | 17.02 | 4.13 |
| R Supramarginal Gyrus | ||||
| R Postcentral Gyrus | ||||
| R Precentral Gyrus |
Clusters corrected for multiple comparisons at P ≤ 0.05
MNI Montreal Neurological Institute
Figure 10.
Regional white matter volume decrease in females. Overlay of significant clusters representing a volumetric decrease associated with increased PbB78 scores for female subjects (N = 90). All clusters presented have been corrected for multiple comparisons (P ≤ 0.05). Slice numbers represent distance from origin (0, 0, 0), which was set at the anterior commissure. Color bar represents voxel level T-statistic. Orientation: Left = Left, Right = Right. PbB78 Blood lead at age 78 months.
Table 13.
Regional White Matter Volumetric Decrease Associated with Increased PbB78 Values for Male Participants
| Anatomical Region | Cluster Size* (Voxels) |
MNI Coordinates (X, Y, Z) |
Peak Voxel F | Peak Voxel T |
|---|---|---|---|---|
| L Medial Frontal Gyrus | 1717 | −9, 42, −11 | 15.08 | 3.88 |
| L Orbital Gyrus | ||||
| L Superior Frontal Gyrus | ||||
| L Middle Frontal Gyrus | ||||
| L Anterior Cingulate | ||||
| R Anterior Cingulate | 2388 | 9, 30, 25 | 13.08 | 3.62 |
| R Corpus Callosum | ||||
| R Cingulate Gyrus | 7642 | 27, −43, 25 | 21.02 | 4.59 |
| R Superior Temporal Gyrus | ||||
| R Precuneus | ||||
| R Inferior Parietal Lobule | ||||
| P Precentral Gyrus | ||||
| L Middle Frontal Gyrus | 1687 | −33, 18, 40 | 13.82 | 3.72 |
| L Anterior Cingulate | ||||
| L Precuneus | 3650 | −27, −52, 30 | 15.59 | 3.95 |
| L Cingulate Gyrus | ||||
| L Middle Temporal Gyrus | ||||
| Corpus Callosum |
Clusters corrected for multiple comparisons at P ≤ 0.05
MNI Montreal Neurological Institute
Figure 11.
Regional white matter volume decrease in males. Overlay of significant clusters representing a volumetric decrease associated with increased PbB78 scores for male subjects (N = 65). All clusters presented have been corrected for multiple comparisons (P ≤ 0.05). Slice numbers represent distance from origin (0, 0, 0), which was set at the anterior commissure. Color bar represents voxel level T-statistic. Orientation: Left = Left, Right = Right. PbB78 Blood lead at age 78 months.
3.3.3 Conjunction Analysis
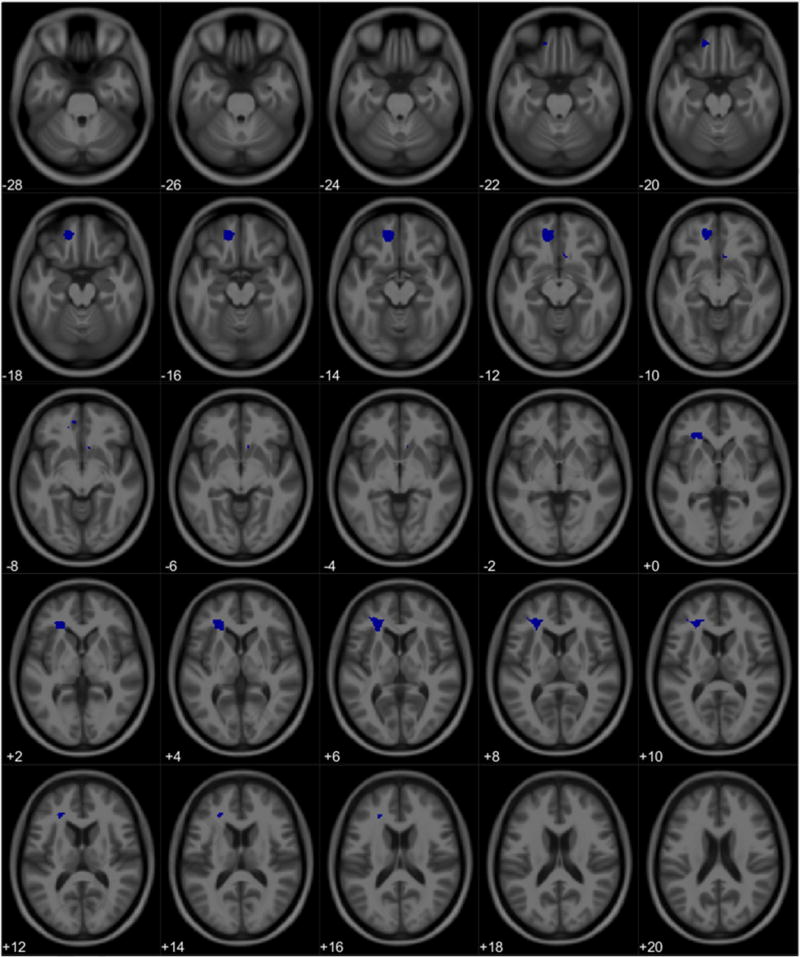
For the cohort, there were no intersections of significant volume association clusters in gray or white matter between total PPI scores and PbB78. Likewise, there were no overlapping clusters between total PPI score and PbB78 in female gray matter or white matter. However, males did show an intersection of volume association clusters between total PPI score and PbB78 in white matter. The formal conjunction analysis suggested this region as containing voxels associated with increased total PPI score and PbB78 values. Specifically, there was an intersection representing a volumetric decrease associated with increased total PPI scores and PbB78 values primarily in the left orbitofrontal white matter (Tables 14, Figures 12 & 13). No overlap of association clusters was observed in male gray matter.
Table 14.
White Matter Regional Overlap Between Total PPI Score and PbB78 in Males
| Anatomical Region | Cluster Size (Voxels) |
MNI Coordinates (X, Y, Z) |
Peak Voxel T |
|---|---|---|---|
| Regional Overlap | |||
| L Orbital Gyrus | 603 | −17, 41, −23 | |
| L Medial Frontal Gyrus | |||
| L Rectal Gyrus | |||
| L Superior Frontal Gyrus | |||
| R Anterior Cingulate | 65 | 11, 18, −14 | |
| L Anterior Corona Radiata | 678 | −21, 30, −3 | |
| Conjunction Analysis* | |||
| L Orbital Gyrus | 142 | −17, 41, −17 | 3.63 |
| L Superior Frontal Gyrus |
Clusters corrected for multiple comparisons at P ≤ 0.05
MNI Montreal Neurological Institute
Figure 12.
Regional overlap of white matter volume loss between Total PPI scores and PbB78 in males. Clusters represent neuroanatomical overlap of white matter loss associated with increasing Total PPI scores and white matter loss associated with increasing PbB78 values.
Figure 13.
Significant voxels in a conjunction analysis for white matter loss between Total PPI scores and PbB78 values. Slice numbers represent distance from origin (0, 0, 0), which was set at the anterior commissure. Color bar represents voxel level T-statistic. Orientation: Left = Left, Right = Right. PbB78 Blood lead at age 78 months; Total PPI Total psychopathic personality inventory score.
4.0 Discussion
The purpose of this study was to examine brain volume changes in relation to measures of psychopathy, and to determine whether those changes may be related to childhood lead exposure. For the relationship between BVC and psychopathy, the results demonstrated a significant volumetric reduction primarily in cerebellar white matter associated with elevated total PPI scores in a longitudinal cohort with documented childhood lead exposure. These results appeared to be primarily influenced by males who displayed volumetric reductions in frontal gray and white matter. Furthermore, males displayed an overlap in frontal white matter associated with total PPI score and PbB78. In contrast, females only displayed a gray matter increase associated with total PPI score. For the relationship between BVC and blood lead concentrations obtained at 78 months of age, we found significant volumetric reductions in gray and white matter inversely associated with PbB78 in several regions, including the white matter of the pre- and post-central gyri, the frontal lobe, and the cingulate. The white matter decrease in the pre- and post-central gyri are observed in females and males, though the volume loss for females appeared more laterally while the male white matter decrease appeared more medially. Furthermore, only males displayed the white matter decrease in the frontal lobe.
For the Total PPI analyses, the results in the cerebellar white matter were unexpected. Initially thought to be primarily involved with the modulation of motor behavior (Holmes, 1939, Llinas and Welsh, 1993), more recent studies have demonstrated that there is a wide reaching cerebellar network involved with many different cognitive processes (Schmahmann, 2004, Allen et al., 1997, Mogenson et al., 1980, Schmahmann, 1991, Stoodley and Schmahmann, 2009, Strick et al., 2009). Implications with executive and emotional processing have been observed (Schmahmann, 2004, Baumann and Mattingley, 2012, Rosenbloom et al., 2012, Schmahmann and Sherman, 1998) as well as involvement in working memory (Ding et al., 2012). Though not prominently associated with psychopathy, there is some precedent for cerebellar involvement. Reduced cerebellar gray matter was observed in a cohort of males with elevated psychopathy scores in a youth correction facility (Ermer et al., 2013). Studies of bipolar disorder and PTSD, respectively, have observed decreased cerebellar volumes (Baldacara et al., 2011a, Baldacara et al., 2011b) establishing a connection between cerebellar volume and mental health disorders. fMRI studies demonstrated differences in cerebellar activity in relation to psychopathy (Deeley et al., 2006, Muller et al., 2003) implying that the cerebellum holds some functional role for the regulation of behavior. Previous CLS studies observed some volume loss in the cerebellum (Brubaker et al., 2010, Cecil et al., 2008). Furthermore, Cecil et al. (2011) reported abnormalities in cerebellar metabolites associated with childhood lead exposure, which may be indicative of cellular damage. These findings suggested that cerebellar involvement in the presentation of psychopathic traits within this cohort is plausible. The cerebellum’s role in regulating and modulating behavior suggest that damage would result in deficits regarding behavioral inhibitions (Schmahmann, 2004, Schmahmann and Sherman, 1998). The involvement of emotional processing may be a contributing factor to the lack of empathy observed in psychopathy.
When examined by sex, males displayed volumetric reductions primarily in frontal gray and white matter regions. Most notably, males displayed a reduction in gray and white matter in the VMPFC and anterior cingulate associated with increased total PPI scores. There is strong evidence suggesting that the VMPFC is heavily involved in decision making (Bechara et al., 2000b, Bechara et al., 1999, Hampton et al., 2006, Fellows and Farah, 2007) and the regulation of the fear response (Quirk et al., 2000). Finger et al. (2008) reports VMPFC dysfunction in an fMRI study with children who display psychopathic traits, while Raine et al. (2011) found smaller frontal gray matter volumes in antisocial personality disorder; specifically that males showed smaller frontal volumes than females, suggesting that males with antisocial personality disorder may be more susceptible to volumetric effects. Similarly, the anterior cingulate appears to have an important role in regulating conflict and control (Kerns et al., 2004, MacDonald et al., 2000), as well as involvement in cognitive and emotional processing (Lane et al., 1998). Kiehl et al. (2001) and Marsh et al. (2013) found reduced fMRI activity in the anterior cingulate in criminal psychopaths and youths with psychopathic personality traits respectively when compared to controls. Birbaumer et al. (2005) observed that psychopaths displayed no reactivity in the prefrontal limbic system when compared to controls in a fear-conditioning task, suggesting a disconnection or disengagement of limbic system circuitry in psychopathy. Though there is some evidence for volumetric reductions in the anterior cingulate in psychopathy (Boccardi et al., 2011), there is also evidence against any differences (Ermer et al., 2013, Glenn et al., 2010b). Our results are also similar to the sex differences reported in Cecil et al. (2008) and Brubaker et al. (2010) which found that the majority of volume loss associated with childhood lead exposure was found in male participants, was most heavily associated with later childhood lead levels, and was observed primarily in the frontal lobe.
In contrast, females display a regional volumetric gray matter increase associated with total PPI score in the frontal, temporal, and occipital lobes. There is evidence for reduced gray matter in the fronto-temporal region related to psychopathy (de Oliveira-Souza et al., 2008, Ermer et al., 2012). Cope et al. (2014) reported decreases in limbic and paralimbic gray matter in females; however, there is not much evidence for volumetric increases, especially in females. Raine et al. (2003) reports increased corpus callosum volumes in individuals with psychopathic personalities. Similarly, Glenn et al. (2010a) observed increased volumes in striatal structures associated with psychopathy. Tiihonen et al. (2008) also observed increased volumes in the cerebellum and in the parietal and occipital lobes, but this was seen in male violent offenders.
The sex differences observed in this study are similar to those reported in both psychopathy and lead exposure studies. Males are traditionally reported to score higher on psychopathy inventories (Levenson et al., 1995, Forth et al., 1996). However, there is emerging evidence that females score higher on secondary psychopathic traits (Grann, 2000, Lilienfeld and Hess, 2001) and that the factor structure in psychopathy tests may not be adequate to elucidate sex differences (Dolan and Vollm, 2009). Regarding lead exposure, males often display greater sensitivity to lead exposure than females in many different ways including general intelligence, neurobehavioral performance in areas of attention, visuoconstruction, planning, rule learning and reversal (Ris et al., 2004, Bellinger et al., 1990, Dietrich et al., 1987, Pocock et al., 1987, Leasure et al., 2008, Froehlich et al., 2007). Furthermore, the effects of childhood lead exposure on brain volume are significantly more pronounced in males (Brubaker et al., 2010, Cecil et al., 2008). Though there is no single explanation for these sex differences in the outcomes of lead exposure, it has been shown that sex hormones such as estradiol may exhibit neuroprotective properties and reduce the impact of lead toxicity in females (Chetty et al., 2007, Ronis et al., 1998). Greater expression of the mitochondrial enzyme paraoxonase-2 (PON2) in females may protect cells from oxidative stress-mediated toxicity (Giordano 2013, Costa 2014).
The relationship observed between total PPI score and childhood lead exposure was reanalyzed with the data collected in Wright et al. (2009) for a smaller CLS subcohort who also had the MRI evaluation and included all of the subscales, as the previous report excluded two sub-scales (Coldheartedness and Stress Immunity). The results followed similarly reported relationships between lead exposure and behavior (Dietrich et al., 2001, Wright et al., 2008, Needleman et al., 1996). For the conjunction analyses, one region intersecting within both primary models (BVC and PbB78, BVC and PPI) was found in the white matter of the left orbitofrontal cortex (OFC) of males (Figure 5). The OFC has a significant role in emotional processing, decision-making, and risk/reward relationships (Bechara, 2004, Rolls, 2004, Gottfried et al., 2003, Bechara et al., 2000a, Winstanley et al., 2004). It has been proposed that OFC dysfunction plays a significant role in the behavioral abnormalities underlying psychopathy (Blair, 2004, Blair, 2007, Mitchell et al., 2002). Decreased OFC activity has been reported in studies of psychopathy (Lapierre et al., 1995, Veit et al., 2002), as have reduced OFC volumes (Yang et al., 2010, Yang et al., 2005). As noted earlier, Cecil et al. (2008) and Brubaker et al. (2010) reported reduced prefrontal brain volumes associated with childhood lead exposure, noting that the majority of volume loss was observed in males. Likewise, Raine et al. (2011) showed that male psychopaths displayed more OFC volume loss compared to healthy control and females. Furthermore, Wright et al. (2008) observed a direct correlation between criminal arrests and childhood blood lead levels within the CLS, which was greatest in male participants. Put together, these results suggest that at least in regards to males, there may be a direct relationship with between childhood lead exposure - related brain damage and the incidence of psychopathic personality traits.
Though this study utilized a relatively large sample size for MRI (N = 155), the results are not easily generalizable due to the CLS sampling from a predominantly African-American, urban population. Scanner bias is another area of concern due to the use of two different scanners during the study. To minimize the impact of this, we added an additional covariate into each analysis to account for scanner differences, which has been shown in the literature to be an effective option (Pardoe et al., 2008, Stonnington et al., 2008, Teipel et al., 2010). We also must note that though the results of the VBM analysis with PbB78 presented similar regional volumetric decreases as reported in Cecil et al. (2008), our results were primarily confined to white matter opposed to gray matter. This may be the result of changes in SPM software over time to minimize the impact of partial volume effects, or could suggest changes in the brain have occurred. We also recognize there is some debate regarding the use of total PPI score alone. Several studies examining the validity of the PPI suggest exploring factors and all facets of the PPI is a more adequate approach (Patrick et al., 2006, Benning et al., 2003).
5.0 Conclusion
Our results showed a relationship between measures of psychopathy and brain volume, which displayed overlap with previous research connecting childhood lead exposure with behavioral and neurological deficits. Furthermore, our research demonstrates that males display more volumetric effects related to psychopathy than females, and that males displayed an association between region brain volume, psychopathy, and childhood lead exposure. Although our results do not causally demonstrate that the observed relationship between regional frontal brain volume and psychopathy, they do strengthen the argument that there is a connection between these childhood lead exposure and psychopathy. Future studies are needed to improve our understanding of how childhood exposure to environmental toxicants such as lead can result in neuroanatomical differences that may impact personality and future behavior.
Supplementary Material
HIGHLIGHTS.
Regional brain volume loss associated with measures of Psychopathy
Brain volume loss associated with Psychopathy appears primarily in males
Association between brain volume loss, Psychopathy, and lead exposure in males
Acknowledgments
Funding
This work was supported by funding from the National Institute of Environmental Health Sciences with R01 ES015559 and ES027224 and the National Center for Advancing Translational Sciences with CTSA UL1 RR026314.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Allen G, Buxton RB, Wong EC, Courchesne E. Attentional Activation of the Cerebellum Independent of Motor Involvement. Science. 1997;275:1940–1943. doi: 10.1126/science.275.5308.1940. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A Fast Diffeomorphic Image Registration Algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Why voxel-based morphometry should be used. Neuroimage. 2001;14:1238–1243. doi: 10.1006/nimg.2001.0961. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baghurst PA, Mcmichael AJ, Wigg NR, Vimpani GV, Robertson EF, Roberts RJ, Tong SL. Environmental Exposure to Lead and Children's Intelligence at the Age of Seven Years. The Port Pirie Cohort Study. The New England Journal of Medicine. 1992;327:1279–84. doi: 10.1056/NEJM199210293271805. [DOI] [PubMed] [Google Scholar]
- Baker LA, Jacobson KC, Raine A, Lozano DI, Bezdjian S. Genetic and Environmental Bases of Childhood Antisocial Behavior: A Multi-Informant Twin Study. Journal of Abnormal Psychology. 2007;116:219–35. doi: 10.1037/0021-843X.116.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldacara L, Jackowski AP, Schoedl A, Pupo M, Andreoli SB, Mello MF, Lacerda ALT, Mari JJ, Bressan RA. Reduced Cerebellar Left Hemisphere and Vermal Volume in Adults with PTSD from a Community Sample. Journal of Psychiatric Research. 2011a;45:1627–1633. doi: 10.1016/j.jpsychires.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Baldacara L, Nery-Fernandes F, Rocha M, Quarantini LC, Rocha GGL, Guimaraes JL, Araujo C, Oliveira I, Miranda-Scippa A, Jackowski A. Is Cerebellar Volume Related to Bipolar Disorder? Journal of Affective Disorders. 2011b;135:305–309. doi: 10.1016/j.jad.2011.06.059. [DOI] [PubMed] [Google Scholar]
- Baumann O, Mattingley JB. Functional Topography of Primary Emotion Processing in the Human Cerebellum. Neuroimage. 2012;61:805–11. doi: 10.1016/j.neuroimage.2012.03.044. [DOI] [PubMed] [Google Scholar]
- Beaujean AA. R Package for Baylor University Educational Psychology Quantitative Courses 2012 [Google Scholar]
- Bechara A. The Role of Emotion in Decision-Making: Evidence from Neurological Patients with Orbitofrontal Damage. Brain and Cognition. 2004;55:30–40. doi: 10.1016/j.bandc.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000a;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different Contributions of the Human Amygdala and Ventromedial Prefrontal Cortex to Decision-Making. Journal of Neuroscience. 1999;19:5473–81. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H. Characterization of the Decision-Making Deficit of Patients with Ventromedial Prefrontal Cortex Lesions. Brain. 2000b;123(11):2189–202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Bellinger D, Leviton A, Allred E, Rabinowitz M. Pre-and postnatal lead exposure and behavior problems in school-aged children. Environmental research. 1994;66:12–30. doi: 10.1006/enrs.1994.1041. [DOI] [PubMed] [Google Scholar]
- Bellinger D, Leviton A, Sloman J. Antecedents and Correlates of Improved Cognitive Performance in Children Exposed in Utero to Low Levels of Lead. Environmental Health Perspectives. 1990;89:5–11. doi: 10.1289/ehp.90895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning SD, Patrick CJ, Hicks BM, Blonigen DM, Krueger RF. Factor structure of the psychopathic personality inventory: validity and implications for clinical assessment. Psychological assessment. 2003;15:340. doi: 10.1037/1040-3590.15.3.340. [DOI] [PubMed] [Google Scholar]
- Bezdjian S, Raine A, Baker LA, Lynam DR. Psychopathic Personality in Children: Genetic and Environmental Contributions. Psychological Medicine. 2011;41:589–600. doi: 10.1017/S0033291710000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbaumer N, Veit R, Lotze M, Erb M, Hermann C, Grodd W, Flor H. Deficient Fear Conditioning in Psychopathy: A Functional Magnetic Resonance Imaging Study. Archives of General Psychiatry. 2005;62:799–805. doi: 10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- Blair RJ. The Roles of Orbital Frontal Cortex in the Modulation of Antisocial Behavior. Brain and Cognition. 2004;55:198–208. doi: 10.1016/S0278-2626(03)00276-8. [DOI] [PubMed] [Google Scholar]
- Blair RJ. Dysfunctions of Medial and Lateral Orbitofrontal Cortex in Psychopathy. Annals of the New York Academy of Sciences. 2007;1121:461–79. doi: 10.1196/annals.1401.017. [DOI] [PubMed] [Google Scholar]
- Boccardi M, Frisoni GB, Hare RD, Cavedo E, Najt P, Pievani M, Rasser PE, Laakso MP, Aronen HJ, Repo-Tiihonen E, Vaurio O, Thompson PM, Tiihonen J. Cortex and Amygdala Morphology in Psychopathy. Psychiatry Research: Neuroimaging. 2011;193:85–92. doi: 10.1016/j.pscychresns.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Bornschein RL, Hammond PB, Dietrich KN, Succop P, Krafft K, Clark S, Berger O, Pearson D, Que Hee S. The Cincinnati Prospective Study of Low-Level Lead Exposure and its Effects on Child Development: Protocol and Status Report. Environmental Research. 1985;38:4–18. doi: 10.1016/0013-9351(85)90067-2. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Caldwell BM. Home Observation for Measurement of the Environment: A Validation Study of Screening Efficiency. American Journal of Mental Deficiency. 1977;81:417–20. [PubMed] [Google Scholar]
- Bradley RH, Caldwell BM. Home Observation for Measurement of the Environment: A Revision of the Preschool Scale. American Journal of Mental Deficiency. 1979;84:235–44. [PubMed] [Google Scholar]
- Brubaker CJ, Dietrich KN, Lanphear BP, Cecil KM. The Influence of Age of Lead Exposure on Adult Gray Matter Volume. Neurotoxicology. 2010;31:259–266. doi: 10.1016/j.neuro.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker CJ, Schmithorst VJ, Haynes EN, Dietrich KN, Egelhoff JC, Lindquist DM, Lanphear BP, Cecil KM. Altered Myelination and Axonal Integrity in Adults with Childhood Lead Exposure: A Diffusion Tensor Imaging Study. Neurotoxicology. 2009;30:867–875. doi: 10.1016/j.neuro.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell BM, Bradley RH. Administration Manual: Home Observation for Measurement of the Environment. Little Rock, AR: University of Arkansas at Little Rock; 1978. [Google Scholar]
- Cecil KM, Brubaker CJ, Adler CM, Dietrich KN, Altaye M, Egelhoff JC, Wessel S, Elangovan I, Hornung R, Jarvis K, Lanphear BP. Decreased Brain Volume in Adults with Childhood Lead Exposure. Plos Medicine. 2008;5:741–750. doi: 10.1371/journal.pmed.0050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecil KM, Dietrich KN, Altaye M, Egelhoff JC, Lindquist DM, Brubaker CJ, Lanphear BP. Proton Magnetic Resonance Spectroscopy in Adults with Childhood Lead Exposure. Environmental Health Perspectives. 2011;119:403–408. doi: 10.1289/ehp.1002176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman AL, Gremore TM, Farmer RF. Psychometric analysis of the Psychopathic Personality Inventory (PPI) with female inmates. Journal of Personality Assessment. 2003;80:164–172. doi: 10.1207/S15327752JPA8002_05. [DOI] [PubMed] [Google Scholar]
- Chetty CS, Vemuri MC, Reddy GR, Suresh C. Protective Effect of 17-Beta-Estradiol in Human Neurocellular Models of Lead Exposure. Neurotoxicology. 2007;28:396–401. doi: 10.1016/j.neuro.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Cirino PT, Chin CE, Sevcik RA, Wolf M, Lovett M, Morris RD. Measuring Socioeconomic Status: Reliability and Preliminary Validity for Different Approaches. Assessment. 2002;9:145–55. doi: 10.1177/10791102009002005. [DOI] [PubMed] [Google Scholar]
- Cleckley HM. The Mask of Sanity: An Attempt to Clarify Some Issues About the So-Called Psychopathic Personality. St. Louis, MO: Mosby; 1955. [Google Scholar]
- Cope LM, Ermer E, Nyalakanti PK, Calhoun VD, Kiehl KA. Paralimbic Gray Matter Reductions in Incarcerated Adolescent Females with Psychopathic Traits. Journal of Abnormal Child Psychology. 2014;42:659–68. doi: 10.1007/s10802-013-9810-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig MC, Catani M, Deeley Q, Latham R, Daly E, Kanaan R, Picchioni M, Mcguire PK, Fahy T, Murphy DGM. Altered Connections on the Road to Psychopathy. Molecular Psychiatry. 2009;14:946–953. doi: 10.1038/mp.2009.40. [DOI] [PubMed] [Google Scholar]
- Cuadra MB, Cammoun L, Butz T, Cuisenaire O, Thiran JP. Comparison and Validation of Tissue Modelization and Statistical Classification Methods in T1-Weighted MR Brain Images. IEEE Transactions on Medical Imaging. 2005;24:1548–65. doi: 10.1109/TMI.2005.857652. [DOI] [PubMed] [Google Scholar]
- Daversa MT. Early Environmental Predictors of the Affective and Interpersonal Constructs of Psychopathy. International Journal of Offender Therapy and Comparative Criminology. 2010;54:6–21. doi: 10.1177/0306624X08328754. [DOI] [PubMed] [Google Scholar]
- de Oliveira-Souza R, Hare RD, Bramati IE, Garrido GJ, Ignacio FA, Tovar-Moll F, Moll J. Psychopathy as a Disorder of the Moral Brain: Fronto-Temporo-Limbic Grey Matter Reductions Demonstrated by Voxel-Based Morphometry. Neuroimage. 2008;40:1202–1213. doi: 10.1016/j.neuroimage.2007.12.054. [DOI] [PubMed] [Google Scholar]
- Deeley Q, Daly E, Surguladze S, Tunstall N, Mezey G, Beer D, Ambikapathy A, Robertson D, Giampietro V, Brammer MJ, Clarke A, Dowsett J, Fahy T, Phillips ML, Murphy DG. Facial Emotion Processing in Criminal Psychopathy - Preliminary Functional Magnetic Resonance Imaging Study. British Journal of Psychiatry. 2006;189:533–539. doi: 10.1192/bjp.bp.106.021410. [DOI] [PubMed] [Google Scholar]
- Delisi M, Alcala J, Kusow A, Hochstetler A, Heirigs MH, Caudill JW, Trulson CR, Baglivio MT. Adverse childhood experiences, commitment offense, and race/ethnicity: are the effects crime-, race-, and ethnicity-specific? International journal of environmental research and public health. 2017;14:331. doi: 10.3390/ijerph14030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denno DW. Biology and Violence: From Birth to Adulthood. Cambridge, England: Cambridge University; 1990. [Google Scholar]
- Dietrich KN, Krafft KM, Bornschein RL, Hammond PB, Berger O, Succop PA, Bier M. Low-Level Fetal Lead Exposure Effect on Neurobehavioral Development in Early Infancy. Pediatrics. 1987;80:721–30. [PubMed] [Google Scholar]
- Dietrich KN, Ris MD, Succop PA, Berger OG, Bornschein RL. Early Exposure to Lead and Juvenile Delinquency. Neurotoxicology and Teratology. 2001;23:511–518. doi: 10.1016/s0892-0362(01)00184-2. [DOI] [PubMed] [Google Scholar]
- Ding H, Qin W, Jiang TZ, Zhang YT, Yu CS. Volumetric Variation in Subregions of the Cerebellum Correlates with Working Memory Performance. Neuroscience Letters. 2012;508:47–51. doi: 10.1016/j.neulet.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Dolan M, Vollm B. Antisocial Personality Disorder and Psychopathy in Women: A Literature Review on the Reliability and Validity of Assessment Instruments. International Journal of Law and Psychiatry. 2009;32:2–9. doi: 10.1016/j.ijlp.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Edens JF, Poythress NG, Watkins MM. Further Validation of the Psychopathic Personality Inventory Among Offenders: Personality and Behavioral Correlates. Journal of Personality Disorders. 2001;15:403–15. doi: 10.1521/pedi.15.5.403.19202. [DOI] [PubMed] [Google Scholar]
- Ermer E, Cope LM, Nyalakanti PK, Calhoun VD, Kiehl KA. Aberrant Paralimbic Gray Matter in Criminal Psychopathy. Journal of Abnormal Psychology. 2012;121:649–58. doi: 10.1037/a0026371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermer E, Cope LM, Nyalakanti PK, Calhoun VD, Kiehl KA. Aberrant Paralimbic Gray Matter in Incarcerated Male Adolescents with Psychopathic Traits. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52:94–103 e3. doi: 10.1016/j.jaac.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. The Role of Ventromedial Prefrontal Cortex in Decision Making: Judgment Under Uncertainty or Dudgment Per Se? Cerebral Cortex. 2007;17:2669–74. doi: 10.1093/cercor/bhl176. [DOI] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Mitchell DG, Reid ME, Sims C, Budhani S, Kosson DS, Chen G, Towbin KE, Leibenluft E, Pine DS, Blair JR. Abnormal Ventromedial Prefrontal Cortex Function in Children with Psychopathic Traits During Reversal Learning. Archives of General Psychiatry. 2008;65:586–94. doi: 10.1001/archpsyc.65.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forth AE, Brown SL, Hart SD, Hare RD. The Assessment of Psychopathy in Male and Female Non-Criminals: Reliability and Validity. Personality and Individual Differences. 1996;20:531–543. [Google Scholar]
- Friston KJ, Holmes AP, Price C, Büchel C, Worsley K. Multisubject fMRI studies and conjunction analyses. Neuroimage. 1999;10:385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Penny WD, Glaser DE. Conjunction revisited. Neuroimage. 2005;25(3):661–667. doi: 10.1016/j.neuroimage.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Froehlich TE, Lanphear BP, Dietrich KN, Cory-Slechta DA, Wang N, Kahn RS. Interactive Effects of a DRD4 polymorphism, Lead, and Sex on Executive Functions in Children. Biological Psychiatry. 2007;62:243–9. doi: 10.1016/j.biopsych.2006.09.039. [DOI] [PubMed] [Google Scholar]
- Gao Y, Raine A, Chan F, Venables PH, Mednick SA. Early Maternal and Paternal Bonding, Childhood Physical Abuse and Adult Psychopathic Personality. Psychological Medicine. 2010;40:1007–1016. doi: 10.1017/S0033291709991279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn AL, Raine A. Psychopathy: An Introduction to Biological Findings and Their Implications. New York, NY: NYU Press; 2014. [Google Scholar]
- Glenn AL, Raine A, Yaralian PS, Yang Y. Increased Volume of the Striatum in Psychopathic Individuals. Biological Psychiatry. 2010a;67:52–8. doi: 10.1016/j.biopsych.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn AL, Yang YL, Raine A, Colletti P. No Volumetric Differences in the Anterior Cingulate of Psychopathic Individuals. Psychiatry Research: Neuroimaging. 2010b;183:140–143. doi: 10.1016/j.pscychresns.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O'Doherty J, Dolan RJ. Encoding Predictive Reward Value in Human Amygdala and Orbitofrontal Cortex. Science. 2003;301:1104–7. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Grann M. The PCL-R and gender. European Journal of Psychological Assessment. 2000;16:147–149. [Google Scholar]
- Haines D. Neuroanatomy: An Atlas of Structures, Sections, and Systems. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- Hampton AN, Bossaerts P, O'Doherty JP. The Role of the Ventromedial Prefrontal Cortex in Abstract State-Based Inference During Decision Making in Humans. Journal of Neuroscience. 2006;26:8360–7. doi: 10.1523/JNEUROSCI.1010-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare RD, Neumann CS. Psychopathy as a Clinical and Empirical Construct. Annual Review of Clinical Psychology. 2008;4:217–246. doi: 10.1146/annurev.clinpsy.3.022806.091452. [DOI] [PubMed] [Google Scholar]
- Harenski CL, Harenski KA, Shane MS, Kiehl KA. Aberrant Neural Processing of Moral Violations in Criminal Psychopaths. Journal of Abnormal Psychology. 2010;119:863–874. doi: 10.1037/a0020979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes G. The Cerebellum of Man. Brain. 1939;62:1–30. [Google Scholar]
- Hornung RW, Lanphear BP, Dietrich KN. Age of Greatest Susceptibility to Childhood Lead Exposure: A New Statistical Approach. Environmental Health Perspectives. 2009;117:1309–12. doi: 10.1289/ehp.0800426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde LW, Byrd AL, Votruba-Drzal E, Hariri AR, Manuck SB. Amygdala Reactivity and Negative Emotionality: Divergent Correlates of Antisocial Personality and Psychopathy Traits in a Community Sample. Journal of Abnormal Psychology. 2014;123:214–224. doi: 10.1037/a0035467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez M, Kiehl KA, Calhoun VD. Intrinsic Limbic and Paralimbic Networks are Associated with Criminal Psychopathy. Human Brain Mapping. 2013;34:1921–1930. doi: 10.1002/hbm.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner RM, Sellbom M, Lilienfeld SO. A comparison of the psychometric properties of the psychopathic personality inventory full-length and short-form versions. Psychological Assessment. 2012;24:261. doi: 10.1037/a0025832. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, Macdonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior Cingulate Conflict Monitoring and Adjustments in Control. Science. 2004;303:1023–6. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kiebel SJ, Poline JB, Friston KJ, Holmes AP, Worsley KJ. Robust Smoothness Estimation in Statistical Parametric Maps Using Standardized Residuals from the General Linear Model. Neuroimage. 1999;10:756–766. doi: 10.1006/nimg.1999.0508. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Mendrek A, Forster BB, Brink J, Liddle PF. Limbic Abnormalities in Affective Processing by Criminal Psychopaths as Revealed by Functional Magnetic Resonance Imaging. Biological Psychiatry. 2001;50:677–84. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- Kurth F, Luders E, Gase C. VBM8 Toolbox Manual. (369) 2010 [Google Scholar]
- Lane RD, Reiman EM, Axelrod B, Yun LS, Holmes A, Schwartz GE. Neural Correlates of Levels of Emotional Awareness. Evidence of an Interaction Between Emotion and Attention in the Anterior Cingulate Cortex. Journal of Cognitive Neuroscience. 1998;10:525–35. doi: 10.1162/089892998562924. [DOI] [PubMed] [Google Scholar]
- Lapierre D, Braun CM, Hodgins S. Ventral Frontal Deficits in Psychopathy: Neuropsychological Test Findings. Neuropsychologia. 1995;33:139–51. doi: 10.1016/0028-3932(94)00110-b. [DOI] [PubMed] [Google Scholar]
- Larsson H, Andershed H, Lichtenstein P. A Genetic Factor Explains Most of the Variation in the Psychopathic Personality. Journal of Abnormal Psychology. 2006;115:221–230. doi: 10.1037/0021-843X.115.2.221. [DOI] [PubMed] [Google Scholar]
- Leasure JL, Giddabasappa A, Chaney S, Johnson JE, Jr, Pothakos K, Lau YS, Fox DA. Low-Level Human Equivalent Gestational Lead Exposure Produces Sex-Specific Motor and Coordination Abnormalities and Late-Onset Obesity in Year-Old Mice. Environmental Health Perspectives. 2008;116:355–61. doi: 10.1289/ehp.10862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson MR, Kiehl KA, Fitzpatrick CM. Assessing Psychopathic Attributes in a Noninstitutionalized Population. Journal of Personality and Social Psychology. 1995;68:151–158. doi: 10.1037//0022-3514.68.1.151. [DOI] [PubMed] [Google Scholar]
- Lilienfeld SO. Methodological Advances and Developments in the Assessment of Psychopathy. Behaviour Research and Therapy. 1998;36:99–125. doi: 10.1016/s0005-7967(97)10021-3. [DOI] [PubMed] [Google Scholar]
- Lilienfeld SO, Andrews BP. Development and Preliminary Validation of a Self-Report Measure of Psychopathic Personality Traits in Noncriminal Populations. Journal of Personality Assessment. 1996;66:488–524. doi: 10.1207/s15327752jpa6603_3. [DOI] [PubMed] [Google Scholar]
- Lilienfeld SO, Hess TH. Psychopathic Personality Traits and Somatization: Sex Differences and the Mediating Role of Negative Emotionality. Journal of Psychopathology and Behavioral Assessment. 2001;23:11–24. [Google Scholar]
- Little RJ. A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association. 1988;83:1198–1202. [Google Scholar]
- Llinas R, Welsh JP. On the Cerebellum and Motor Learning. Current Opinion in Neurobiology. 1993;3:958–65. doi: 10.1016/0959-4388(93)90168-x. [DOI] [PubMed] [Google Scholar]
- Macdonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the Role of the Dorsolateral Prefrontal and Anterior Cingulate Cortex in Cognitive Control. Science. 2000;288:1835–8. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Malterer MB, Lilienfeld SO, Neumann CS, Newman JP. Concurrent Validity of the Psychopathic Personality Inventory with Offender and Community Samples. Assessment. 2010;17:3–15. doi: 10.1177/1073191109349743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjon JV, Coupe P, Marti-Bonmati L, Collins DL, Robles M. Adaptive Non-Local Means Denoising of MR Images with Spatially Varying Noise Levels. Journal of Magnetic Resonance Imaging. 2010;31:192–203. doi: 10.1002/jmri.22003. [DOI] [PubMed] [Google Scholar]
- Marcus DK, Fulton JJ, Clarke EJ. Lead and Conduct Problems: A Meta-Analysis. Journal of Clinical Child & Adolescent Psychology. 2010;39:234–41. doi: 10.1080/15374411003591455. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Fowler KA, Adalio CJ, Jurkowitz IT, Schechter JC, Pine DS, Decety J, Blair RJ. Empathic Responsiveness in Amygdala and Anterior Cingulate Cortex in Youths with Psychopathic Traits. Journal of Child Psychology and Psychiatry. 2013;54:900–10. doi: 10.1111/jcpp.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Friston KJ, Ashburner J. Voxel-based morphometry of the human brain: methods and applications. Current medical Imaging reviews. 2005;1:105–113. [Google Scholar]
- Mendelsohn AL, Dreyer BP, Fierman AH, Rosen CM, Legano LA, Kruger HA, Lim SW, Courtlandt CD. Low-level lead exposure and behavior in early childhood. Pediatrics. 1998;101:e10–e10. doi: 10.1542/peds.101.3.e10. [DOI] [PubMed] [Google Scholar]
- Mitchell DG, Colledge E, Leonard A, Blair RJ. Risky Decisions and Response Reversal: Is There Evidence of Orbitofrontal Cortex Dysfunction in Psychopathic Individuals? Neuropsychologia. 2002;40:2013–22. doi: 10.1016/s0028-3932(02)00056-8. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From Motivation to Action - Functional Interface Between the Limbic System and the Motor System. Progress in Neurobiology. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Motzkin JC, Newman JP, Kiehl KA, Koenigs M. Reduced Prefrontal Connectivity in Psychopathy. Journal of Neuroscience. 2011;31:17348–17357. doi: 10.1523/JNEUROSCI.4215-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JL, Sommer M, Wagner V, Lange K, Taschler H, Roder CH, Schuierer G, Klein HE, Hajak G. Abnormalities in Emotion Processing Within Cortical and Subcortical Regions in Criminal Psychopaths: Evidence from a Functional Magnetic Resonance Imaging Study Using Pictures with Emotional Content. Biological Psychiatry. 2003;54:152–162. doi: 10.1016/s0006-3223(02)01749-3. [DOI] [PubMed] [Google Scholar]
- National Research Council. Measuring lead exposure in infants, children, and other sensitive populations. National Academies Press; 1993. [PubMed] [Google Scholar]
- Needleman HL, Mcfarland C, Ness RB, Fienberg SE, Tobin MJ. Bone Lead Levels in Adjudicated Delinquents - A Case Control Study. Neurotoxicology and Teratology. 2002;24:711–717. doi: 10.1016/s0892-0362(02)00269-6. [DOI] [PubMed] [Google Scholar]
- Needleman HL, Riess JA, Tobin MJ, Biesecker GE, Greenhouse JB. Bone lead levels and delinquent behavior. JAMA. 1996;275:363–9. [PubMed] [Google Scholar]
- Nolte J, Angevine J. The Human Brain in Photographs and Diagrams. Philadelphia, PA: Mosby; 2007. [Google Scholar]
- Oishi K. MRI Atlas of Human White Matter. Amsterdam: Elsevier/Academic Press; 2011. [Google Scholar]
- Pardoe H, Pell GS, Abbott DF, Berg AT, Jackson GD. Multi-Site Voxel-Based Morphometry: Methods and a Feasibility Demonstration with Childhood Absence Epilepsy. Neuroimage. 2008;42:611–6. doi: 10.1016/j.neuroimage.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Edens JF, Poythress NG, Lilienfeld SO, Benning SD. Construct validity of the Psychopathic Personality Inventory two-factor model with offenders. Psychological assessment. 2006;18:204. doi: 10.1037/1040-3590.18.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihl RO, Ervin F. Lead and Cadmium Levels in Violent Criminals. Psychological Reports. 1990;66:839–844. doi: 10.2466/pr0.1990.66.3.839. [DOI] [PubMed] [Google Scholar]
- Pocock SJ, Ashby D, Smith MA. Lead Exposure and Children's Intellectual Performance. International Journal of Epidemiology. 1987;16:57–67. doi: 10.1093/ije/16.1.57. [DOI] [PubMed] [Google Scholar]
- Poythress NG, Edens JF, Lilienfeld SO. Criterion-Related Validity of the Psychopathic Personality Inventory in a Prison Sample. Psychological Assessment. 1998;10:426–430. [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The Role of Ventromedial Prefrontal Cortex in the Recovery of Extinguished Fear. Journal of Neuroscience. 2000;20:6225–31. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- Raine A. The Anatomy of Violence: The Biological Roots of Crime. New York: NY: Vintage; 2013. [Google Scholar]
- Raine A, Lencz T, Taylor K, Hellige JB, Bihrle S, Lacasse L, Lee M, Ishikawa S, Colletti P. Corpus Callosum Abnormalities in Psychopathic Antisocial Individuals. Archives of General Psychiatry. 2003;60:1134–42. doi: 10.1001/archpsyc.60.11.1134. [DOI] [PubMed] [Google Scholar]
- Raine A, Yang Y, Narr KL, Toga AW. Sex Differences in Orbitofrontal Gray as a Partial Explanation for Sex Differences in Antisocial Personality. Molecular Psychiatry. 2011;16:227–36. doi: 10.1038/mp.2009.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapakse JC, Giedd JN, Rapoport JL. Statistical Approach to Segmentation of Single-Channel Cerebral MR Images. IEEE Transactions on Medical Imaging. 1997;16:176–86. doi: 10.1109/42.563663. [DOI] [PubMed] [Google Scholar]
- Ris MD, Dietrich KN, Succop PA, Berger OG, Bornschein RL. Early Exposure to Lead and Neuropsychological Outcome in Adolescence. Journal of the International Neuropsychological Society. 2004;10:261–70. doi: 10.1017/S1355617704102154. [DOI] [PubMed] [Google Scholar]
- Roda SM, Greenland RD, Bornschein RL, Hammond PB. Anodic Stripping Voltammetry Procedure Modified for Improved Accuracy of Blood Lead Analysis. Clinical Chemistry. 1988;34:563–7. [PubMed] [Google Scholar]
- Rolls ET. The Functions of the Orbitofrontal Cortex. Brain and Cognition. 2004;55:11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Ronis MJ, Badger TM, Shema SJ, Roberson PK, Templer L, Ringer D, Thomas PE. Endocrine Mechanisms Underlying the Growth Effects of Developmental Lead Exposure in the Rat. Journal of Toxicology and Environmental Health Part A. 1998;54:101–20. doi: 10.1080/009841098158944. [DOI] [PubMed] [Google Scholar]
- Rosenbloom MH, Schmahmann JD, Price BH. The Functional Neuroanatomy of Decision-Making. The Journal of Neuropsychiatry and Clinical Neurosciences. 2012;24:266–77. doi: 10.1176/appi.neuropsych.11060139. [DOI] [PubMed] [Google Scholar]
- Ross SR, Benning SD, Patrick CJ, Thompson A, Thurston A. Factors of the Psychopathic Personality Inventory Criterion-Related Validity and Relationship to the BIS/BAS and Five-Factor Models of Personality. Assessment. 2009;16:71–87. doi: 10.1177/1073191108322207. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. An Emerging Concept - the Cerebellar Contribution to Higher Function. Archives of Neurology. 1991;48:1178–1187. doi: 10.1001/archneur.1991.00530230086029. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. Disorders of the Cerebellum: Ataxia, Dysmetria of Thought, and the Cerebellar Cognitive Affective Syndrome. Journal of Neuropsychiatry and Clinical Neurosciences. 2004;16:367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC. The Cerebellar Cognitive Affective Syndrome. Brain. 1998;121(Pt 4):561–79. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Schmithorst V, Holland SK, Dardzinski BJ. CCHIPS: Cincinnati Children's Hospital Imaging Processing Software. Imaging Research Center, Cincinnati Children’s Medical Center; Cincinnati OH: 2000. [Google Scholar]
- Sciarillo WG, Alexander G, Farrell KP. Lead-Exposure and Child-Behavior. American Journal of Public Health. 1992;82:1356–1360. doi: 10.2105/ajph.82.10.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scouten A, Papademetris X, Constable RT. Spatial Resolution, Signal-to-Noise Ratio, and Smoothing in Multi-Subject Functional MRI Studies. Neuroimage. 2006;30:787–793. doi: 10.1016/j.neuroimage.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Sethi A, Gregory S, Dell'Acqua P, Thomas EP, Simmons A, Murphy DGM, Hodgins S, Blackwood NJ, Craig MC. Emotional Detachment in Psychopathy: Involvement of Dorsal Default-Mode Connections. Cortex. 2015;62:11–19. doi: 10.1016/j.cortex.2014.07.018. [DOI] [PubMed] [Google Scholar]
- Silverstein AB. Two- and Four-Subtest Short Forms of the WAIS-R: A Closer Look at Validity and Reliability. Journal of Clinical Psychology. 1985;41:95–7. doi: 10.1002/1097-4679(198501)41:1<95::aid-jclp2270410116>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Stonnington CM, Tan G, Kloppel S, Chu C, Draganski B, Jack CR, Jr, Chen K, Ashburner J, Frackowiak RS. Interpreting Scan Data Acquired from Multiple Scanners: A Study with Alzheimer's Disease. Neuroimage. 2008;39:1180–5. doi: 10.1016/j.neuroimage.2007.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional Topography in the Human Cerebellum: A Meta-Analysis of Neuroimaging Studies. Neuroimage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Stretesky PB, Lynch MJ. The Relationship Between Lead Exposure and Homicide. Archives of Pediatrics & Adolescent Medicine. 2001;155:579–582. doi: 10.1001/archpedi.155.5.579. [DOI] [PubMed] [Google Scholar]
- Stretesky PB, Lynch MJ. The Relationship Between Lead and Crime. Journal of Health and Social Behavior. 2004;45:214–229. doi: 10.1177/002214650404500207. [DOI] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and Nonmotor Function. Annual Review of Neuroscience. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Ewers M, Wolf S, Jessen F, Kolsch H, Arlt S, Luckhaus C, Schonknecht P, Schmidtke K, Heuser I, Frolich L, Ende G, Pantel J, Wiltfang J, Rakebrandt F, Peters O, Born C, Kornhuber J, Hampel H. Multicentre Variability of MRI-Based Medial Temporal Lobe Volumetry in Alzheimer's Disease. Psychiatry Research: Neuroimaging. 2010;182:244–50. doi: 10.1016/j.pscychresns.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Rossi R, Laakso MP, Hodgins S, Testa C, Perez J, Repo-Tiihonen E, Vaurio O, Soininen H, Aronen HJ, Kononen M, Thompson PM, Frisoni GB. Brain Anatomy of Persistent Violent Offenders: More Rather than Less. Psychiatry Research: Neuroimaging. 2008;163:201–12. doi: 10.1016/j.pscychresns.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Tohka J, Zijdenbos A, Evans A. Fast and Robust Parameter Estimation for Statistical Partial Volume Models in Brain MRI. Neuroimage. 2004;23:84–97. doi: 10.1016/j.neuroimage.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Veit R, Flor H, Erb M, Hermann C, Lotze M, Grodd W, Birbaumer N. Brain Circuits Involved in Emotional Learning in Antisocial Behavior and Social Phobia in Humans. Neuroscience Letters. 2002;328:233–6. doi: 10.1016/s0304-3940(02)00519-0. [DOI] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Lolacono NJ, Factor-Litvak P, Kline JK, Popovac D, Morina N, Musabegovic A, Vrenezi N, Capuni-Paracka S, Lekic V, Preteni-Redjepi E, Hadzialjevic S, Slavkovich V, Graziano JH. Lead Exposure and Intelligence in 7-Year-Old Children: The Yugoslavia Prospective Study. Environmental Health Perspectives. 1997;105:956–62. doi: 10.1289/ehp.97105956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale for Adults, Revised. San Antonio, TX: Psychological Corporation; 1981. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children-III. San Antonio:TX: Psychological Corporation; 1991. [Google Scholar]
- Winstanley CA, Theobald DE, Cardinal RN, Robbins TW. Contrasting Roles of Basolateral Amygdala and Orbitofrontal Cortex in Impulsive Choice. Journal of Neuroscience. 2004;24:4718–22. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JP, Boisvert D, Vaske J. Blood Lead Levels in Early Childhood Predict Adulthood Psychopathy. Youth Violence and Juvenile Justice. 2009;7:208–222. [Google Scholar]
- Wright JP, Dietrich KN, Ris MD, Hornung RW, Wessel SD, Lanphear BP, Ho M, Rae MN. Association of Prenatal and Childhood Blood Lead Concentrations with Criminal Arrests in Early Adulthood. PLoS Medicine. 2008;5:e101. doi: 10.1371/journal.pmed.0050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Raine A. Prefrontal Structural and Functional Brain Imaging Findings in Antisocial, Violent, and Psychopathic Individuals: A Meta-Analysis. Psychiatry Research: Neuroimaging. 2009;174:81–8. doi: 10.1016/j.pscychresns.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Raine A, Colletti P, Toga AW, Narr KL. Abnormal Temporal and Prefrontal Cortical Gray Matter Thinning in Psychopaths. Molecular Psychiatry. 2009;14:561–562. doi: 10.1038/mp.2009.12. [DOI] [PubMed] [Google Scholar]
- Yang YL, Raine A, Colletti P, Toga AW, Narr KL. Morphological Alterations in the Prefrontal Cortex and the Amygdala in Unsuccessful Psychopaths. Journal of Abnormal Psychology. 2010;119:546–554. doi: 10.1037/a0019611. [DOI] [PubMed] [Google Scholar]
- Yang YL, Raine A, Lencz T, Bihrle S, Lacasse L, Colletti P. Volume Reduction in Prefrontal Gray Matter in Unsuccessful Criminal Psychopaths. Biological Psychiatry. 2005;57:1103–1108. doi: 10.1016/j.biopsych.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Yuan W, Holland SK, Cecil KM, Dietrich KN, Wessel SD, Altaye M, Hornung RW, Ris MD, Egelhoff JC, Lanphear BP. The Impact of Early Childhood Lead Exposure on Brain Organization: A Functional Magnetic Resonance Imaging Study of Language Function. Pediatrics. 2006;118:971–7. doi: 10.1542/peds.2006-0467. [DOI] [PubMed] [Google Scholar]
- Yuan W, Holland SK, Schmithorst VJ, Walz NC, Cecil KM, Jones BV, Karunanayaka P, Michaud L, Wade SL. Diffusion Tensor MR Imaging Reveals Persistent White Matter Alteration After Traumatic Brain Injury Experienced During Early Childhood. American Journal of Neuroradiology. 2007;28:1919–25. doi: 10.3174/ajnr.A0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.