Abstract
BACKGROUND
Hospitalized cancer patients are nearly 10 times more likely to develop sepsis when compared to patients with no cancer history. We compared the risk of sepsis between cancer survivors and no cancer history participants, and whether race was an effect modifier.
METHODS
We performed a prospective analysis of data from the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort. We categorized participants as “cancer survivors” or “no cancer history” derived from self-reported responses of being diagnosed with any cancer, excluding non-melanoma skin cancer. We defined sepsis as hospitalization for a serious infection with ≥2 systemic inflammatory response syndrome criteria. We performed Cox proportional hazard models to examine the risk of sepsis after cancer (adjusted for sociodemographics, health behaviors, and comorbidities), and stratified by race.
RESULTS
Among 29,693 eligible participants, 2959 (9.97%) were cancer survivors, and 26,734 (90.03%) were no cancer history participants. Among 1393 sepsis events, the risk of sepsis was higher for cancer survivors (adjusted HR: 2.61, 95% CI: 2.29 – 2.98) when compared to no cancer history participants. Risk of sepsis after cancer survivorship was similar for Black and White participants (p value for race and cancer interaction = 0.63).
CONCLUSION
In this prospective cohort of community-dwelling adults we observed that cancer survivors had more than a 2.5-fold increased risk of sepsis. Public health efforts should attempt to mitigate sepsis risk by awareness and appropriate treatment (e.g., antibiotic administration) to cancer survivors with suspected infection regardless of the number of years since cancer remission.
Keywords: Cancer Survivors, infection, sepsis, Community-dwelling, survival analysis, racial disparities
INTRODUCTION
Sepsis, characterized by a systemic inflammatory response to an infection, is a major public health problem responsible for more than 200,000 deaths and 750,000 hospitalizations annually in the United States (US) (1–3). Among hospitalized patients, cancer patients are nearly ten times more likely to develop sepsis when compared with no cancer history patients (4). A diagnosis of sepsis among cancer patients has been shown to increase the risk of mortality up to 2 to 3- fold, making sepsis a significant but modifiable threat to cancer survivorship (4–7). Treatment modalities for cancer have improved in the past decades, with average 5-year survival approaching 70% (8, 9); however, there are disparities in survival rates by race and socio-economic status, a trend that mirrors disparities in sepsis rates among US adults (4, 10–27).
Cancer and sepsis have a physiologically plausible association, as infections are common complications among cancer patients receiving major cancer surgeries or treatment within the hospital setting (6). However, there exists limited epidemiologic evidence to support the mechanism of cancer survivors having long-term increased risk of sepsis years following their perspective cancers. Further, there is very limited evidence for the association between cancer and sepsis among a longitudinal cohort of participants considered community dwelling at baseline. Little attention has focused on risk of infection among cancer survivors living in communities as past research has focused exclusively among hospitalized cancer patients receiving surgical treatment and/or emergency care. An important and inherent limitation of prior studies was the use of hospital discharge data to identify sepsis events and the assessment of hospital-acquired rather than community acquired sepsis (4–6). First, in hospital discharge datasets, it is difficult disentangle early (community-acquired) sepsis from later (hospital-acquired) sepsis as a result of limited availability of present-on-admission status of the sepsis diagnosis (28). Secondly, there is often insufficient information about confounders on the association of cancer and sepsis including initial clinical presentation; chronic comorbidities such as diabetes and obesity; personal characteristics such as income, education, and body mass index; and behavioral characteristics such as smoking, alcohol use, and diet.
Modern cancer treatment and therapies have improved cancer survivorship over the past few decades, but there are inherent properties of cancer and effects of treatment that may cause cancer survivors to have reduced immune function and affect overall quality of life when compared with the general population. To date, there is limited knowledge on the risk of sepsis among cancer survivors as compared with participants with no cancer within a well-defined longitudinal cohort of community-dwelling adults (4–6). Therefore, the objective of this study was to determine the risk of sepsis after cancer compared with no cancer history participants in the REGARDS cohort. Additionally, because there is conflicting evidence on racial differences in sepsis we aimed to determine whether risk of sepsis after cancer was modified by race (4–6, 24–26, 29–33). We hypothesize that, within the REGARDS cohort, sepsis risk will be higher among cancer survivors when compared to those without a history of cancer and that there will be racial differences in the risk of sepsis after cancer.
METHODS
Study Design & Data Source
We performed a retrospective analysis of prospectively collected data using REGARDS, a cohort of community-dwelling adults in the US (34). The REGARDS cohort was designed to examine risk factors for racial and geographic differences in excess stroke deaths observed among Blacks and those living in the southeastern US. The REGARDS cohort includes 30,239 participants aged ≥ 45 years at baseline. The cohort is 45% male, 41% black race, and 69% >60 years old. REGARDS recruited participants between January 2003 and October 2007. At six-month intervals, REGARDS investigators have contacted the participants by telephone to identify any hospitalizations experienced by the participant in the previous six months. Further details related to REGARDS study methods are described elsewhere (34). While the objective of REGARDS was to identify and characterize stroke events, the population of REGARDS included community-dwelling adults at healthy baseline. The REGARDS-sepsis ancillary study used the infrastructure of the parent REGARDS study to independently identify sepsis hospitalizations. Thus, the REGARDS cohort provides a unique opportunity to examine cancer survivors living in communities at baseline and future risk of community-acquired sepsis.
Primary Exposure of Interest – Cancer Survivors
Our primary cancer exposure was defined as cancer survivorship at baseline (i.e., participants that reported a history of cancer at baseline). We classified those with a history of cancer as “cancer survivors” and those without cancer as “no cancer history.” REGARDS investigators identified participants with self-reported history of cancer during baseline interview using the following baseline question: “Have you ever been diagnosed with cancer?” If the participant answered “yes”, then they were asked the following follow-up question regarding the date of their last treatment: “Have you been treated with chemotherapy or radiation in the past two years?” If the participant had been treated within past two years, they were excluded from participation in the study. Due to the focus on community-dwelling participants, REGARDS investigators excluded participants receiving treatment for cancer within past two years in order to study participants considered “healthy” at baseline. Therefore, participants defined as cancer survivors at baseline were those that had cancer remission for at least two years before entrance into REGARDS cohort. Further, our study excluded participants that may be undergoing cancer treatment in the hospital, leading to an increased risk of sepsis. Self-reported history of cancer in prospective cohort studies have been previously shown to have sensitivity values of 0.90 and positive predictive values of 0.75 (35).
Primary Outcome of Interest – Community-Acquired Sepsis
The primary outcome of this study was first sepsis events. Our analysis focused on community-acquired sepsis; therefore, we assessed vital signs and laboratory findings within the first 28-hours of hospitalization to include ED care and up to one full day of inpatient care. We included hospitalization events reported from January 1, 2003 through December 31, 2012.
In order to identify infection events among REGARDS participants, two trained abstractors independently reviewed all relevant medical records to confirm the presence of a serious infection and its pertinence to the hospitalization. Our trained abstractors reviewed emergency department physician and nursing notes, hospital admission notes, initial laboratory test and vital signs, and the discharge summary. We then identified all serious infections (i.e., all hospitalizations with a bacterial, fungal, or viral infectious process) based on the taxonomy of Angus et al (2001) for identifying severe sepsis (1). We did not use laboratory, microbiological, or radiographic information for defining serious infections.
We defined a sepsis event as a hospital admission for serious infection with the presence of at least two Systemic Inflammatory Response Syndrome (SIRS) criteria, including heart rate >90 beats/minute, fever (temperature >38.3°C or <36°C), tachypnea (>20 breaths/min) or PCO2<32 mmHg, and leukocytosis (white blood cells >12,000 or <4,000 cells/mm3 or >10% band forms) (1).
International consensus conferences (“Sepsis-3”) have proposed new definitions for sepsis (36). Because of its common use in prior sepsis epidemiology studies, we used the SIRS-based sepsis definition as the primary analysis. However, in a secondary analysis, we repeated the analysis using the Sepsis-3 definition of sepsis as the presence of a serious infection plus a sequential organ failure assessment (SOFA) score ≥2 (36).
Participant Characteristics
Baseline demographic variables used in the analysis included self-reported age, race, sex, household income, education, and geographic region. Health behaviors included tobacco, alcohol use, and physical activity. We defined alcohol use as moderate (one drink per day for women or two drinks per day for men) and heavy alcohol use (>1 drink per day for women and >2 drinks per day for men), per the National Institute on Alcohol Abuse and Alcoholism classification (37). Baseline medical conditions included self-reported history of atrial fibrillation, chronic lung disease, coronary artery disease, deep vein thrombosis, diabetes, dyslipidemia, hypertension, myocardial infarction, obesity, peripheral artery disease, and stroke. We additionally created an individual level comorbidity score based on the sum of total number of baseline medical conditions. Those with missing information for an individual medical conditions were included as having no presence of a medical condition. Biomarkers included in this analysis were high sensitivity C-reactive protein (indicator of systemic inflammation), albumin-creatinine ratio (ACR) (indicator of kidney function), and cystatin-C (indicator of kidney function and cardiovascular disease). Baseline medication use that was analyzed in this study included aspirin, statin, and chronic steroid usage. We have provided detailed information regarding participant characteristics in Supplemental Table 1.
Hospital Course and Presentation Variables
We defined hospital course as information among sepsis events during hospital stay. Hospital course variables included infection type, presence of severe sepsis, SOFA for respiratory, renal hepatic, cardiovascular, hematologic, and neurologic systems, Mortality in Emergency Department Sepsis (MEDS) score, intensive care unit (ICU) admission, in-hospital sepsis mortality, 30-day sepsis case fatality, hospital length of stay (LOS) in days. Based on medical record and/or death certificate, we defined 30-day sepsis case fatality as death attributed to sepsis or death within 30-days after hospital discharge of a physician-adjudicated sepsis event. We calculated the hospital length of stay (LOS) for each sepsis event as the difference between the sepsis hospitalization date and hospitalization discharge date.
Statistical Analysis
We compared baseline characteristics, infection types, and hospital characteristics between cancer survivors and those with no cancer history using Chi-square tests for categorical characteristics, Analysis of Variance (ANOVA) for normal continuous variables, and Wilcoxon rank-sum test for non-normal continuous variables. We tested differences in the survival function of sepsis between cancer survivors and no cancer history participants using the log-rank test. We estimated the mean survival times using the product-limit method of the Kaplan-Meier survival estimator. To estimate the relative rates of first-sepsis between cancer survivors and no cancer history participants, we fit a series of Cox proportional hazards models with time-to-first-sepsis as endpoints. We censored individuals at the time of their event, death, or end of follow-up (December 31, 2012). We sequentially adjusted our models for 1) sociodemographics, 2) health behaviors, and 3) chronic medical conditions, biomarkers, and baseline medication use. A priori we decided to examine race as an effect modifier, and thus we stratified analysis examining the association between cancer and sepsis by race. We additionally assessed statistical interaction between race and cancer in our Cox proportional hazard model to determine significance of race effect modification.
In a secondary analysis, we used the Fine & Gray model to examine all-cause mortality as a potential competing risk for sepsis events (38). In short, we employed the Fine & Gray method to estimate the subdistribution hazard of sepsis, the marginal failure probability, accounting for all-cause mortality using a proportional hazards model (38). Our study utilizes the SIRS-based sepsis definition in primary analysis due to the common use in our prior REGARDS-sepsis investigations. However, we additionally examined the association between cancer survivorship and risk of sepsis-SOFA (presence of a serious infection plus a SOFA score ≥2) using Cox proportional hazards model based on newer “Sepsis-3” definitions. Our study focused on examining cancer survivors that were no longer undergoing treatment (i.e., radiation and/or chemotherapy) or with underlying and unknown cancer; therefore, we additionally examined the association between cancer and sepsis excluding participants that died from a cancer-related death within three years of follow-up time. We used SAS version 9.4 and Stata version 13 for all analyses. We considered p-values ≤ 0.05 statistically significant. We presented the estimates from our Cox models as hazard ratios (HRs) and associated 95% confidence intervals (CIs). We additionally presented results from our competing risks analysis as subdistribution hazard ratios (SHR) and associated 95% CIs.
Ethical Statement
The institutional review board of all participating universities approved the study and all participants provided written informed consent.
RESULTS
Among 30,239 REGARDS participants, 546 were excluded due to missing cancer status at baseline and follow-up information regarding sepsis, resulting in 29,693 for analysis. A flowchart depicting the exclusion of participants from baseline is presented in Figure 1 (Figure 1: Flowchart of REGARDS study participants used in study analysis). Among the study participants 2959 (9.97%) were categorized as cancer survivors at baseline and 26,734 (90.03%) with no cancer history.
Figure 1.
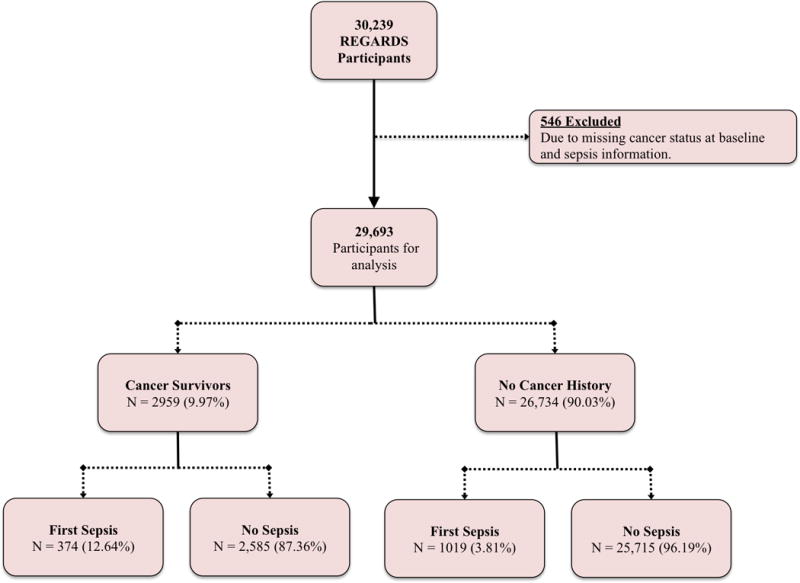
Flowchart of REGARDS study participants used in study analysis.
In general, when compared with no cancer history participants, cancer survivors were older (69.66 vs. 64.37 years, p value <0.01), more likely to be male (57.01% vs. 43.58%, p value <0.01), more likely to identify as White race (69.45% vs. 57.69%, p value <0.01), had slightly higher college educational attainment (36.90% vs. 34.63%, p value = 0.02), and had slightly higher proportion of individuals earning less than $20,000 in annual income (18.42% vs. 17.95%, p value <0.01) (Table 1). Cancer survivors were more likely to reside in the Stroke Belt (36.09% vs. 34.48%, p value <0.01) and be past tobacco users (49.37% vs. 39.26%, p value <0.01). In addition, cancer survivors had a greater prevalence of atrial fibrillation (11.56% vs. 8.48%), chronic lung disease (10.88% vs. 9.01), coronary artery disease (23.91% vs. 17.30%), deep vein thrombosis (7.78% vs. 4.98%), hypertension (63.35% vs. 58.79), myocardial infarction (17.06% vs. 12.30%), and stroke (8.82% vs. 6.14%) when compared with participants with no cancer history (p values <0.01). Cancer survivors had higher baseline levels of ACR (7.84 vs. 7.38, p value <0.01) and Cystatin-C (0.98 vs. 0.94, p values < 0.01) and were more likely to be chronic aspirin users at baseline (47.51% vs. 42.92%, p <0.01).
Table 1.
Comparison of demographic, substance use, and comorbidity characteristics by cancer survivorship status. Among 29,693 REGARDS participants.
| Cancer Survivors (N = 2959) |
No Cancer History (N = 26734) |
||
|---|---|---|---|
|
| |||
| N (%) Mean (SD)a |
N (%) Mean (SD)a |
p valueb | |
| Agea | 69.66 (8.67) | 64.37 (9.36) | <0.01 |
| Sex | |||
| Male | 1687 (57.01) | 11650 (43.58) | <0.01 |
| Female | 1272 (42.99) | 15084 (56.42) | |
| Race | |||
| Black | 904 (30.55) | 11312 (42.31) | <0.01 |
| White | 2055 (69.45) | 15422 (57.69) | |
| Education | |||
| Less than high school | 389 (13.16) | 3319 (12.42) | 0.02 |
| High School Graduate | 710 (24.01) | 6958 (26.05) | |
| Some College | 767 (25.94) | 7184 (26.89) | |
| College or higher | 1091 (36.90) | 9252 (34.63) | |
| Income ≤ $20 000 | 545 (18.42) | 4799 (17.95) | <0.01 |
| Geographic Region | |||
| Stroke Belt | 1068 (36.09) | 9218 (34.48) | <0.01 |
| Stroke Buckle | 466 (15.75) | 5750 (21.51) | |
| Non-Stroke Belt | 1425 (48.16) | 11766 (43.99) | |
| Tobacco Use | |||
| Never | 1171 (39.74) | 12212 (45.85) | <0.01 |
| Past | 1455 (49.37) | 10457 (39.26) | |
| Current | 321 (10.89) | 3963 (14.88) | |
| Alcohol Use | |||
| None | 1764 (61.04) | 16479 (62.84) | 0.02 |
| Moderate | 1023 (35.40) | 8670 (33.06) | |
| Heavy | 103 (3.56) | 1074 (4.10) | |
| Exercise Activity | |||
| None | 1080 (37.25) | 8986 (34.10) | <0.01 |
| 1-3 Times per week | 974 (33.60) | 9562 (36.29) | |
| 4+ Times per week | 845 (29.15) | 7804 (29.61) | |
| Baseline Medical Condition | |||
| Atrial fibrillation | 335 (11.56) | 2214 (8.48) | <0.01 |
| Chronic lung disease | 322 (10.88) | 2410 (9.01) | <0.01 |
| Coronary artery disease | 694 (23.91) | 4539 (17.30) | <0.01 |
| Chronic kidney disease | 345 (11.66) | 2911 (10.89) | 0.20 |
| Deep vein thrombosis | 230 (7.78) | 1325 (4.98) | <0.01 |
| Diabetes | 684 (23.16) | 6021 (22.60) | 0.49 |
| Dyslipidemia | 1745 (61.55) | 15219 (59.10) | <0.01 |
| Hypertension | 1870 (63.35) | 15678 (58.79) | <0.01 |
| Obesity | 1510 (51.10) | 14357 (53.79) | <0.01 |
| Peripheral artery disease | 85 (2.87) | 578 (2.16) | 0.01 |
| Stroke | 260 (8.82) | 1636 (6.14) | <0.01 |
| Comorbidity Score, Mean (SD)a | 2.27 (1.58) | 1.98 (1.48) | <0.01 |
| Biomarkers, Median (P25, P75)c | |||
| hs-CRP | 2.14 (0.96 – 4.85) | 2.22 (0.96 – 5.06) | 0.57 |
| ACR | 7.84 (4.84 – 18.97) | 7.38 (4.63 – 15.79) | <0.01 |
| Cystatin-C | 0.98 (0.85 – 1.18) | 0.94 (0.83 – 1.11) | <0.01 |
| Baseline Medication Use | |||
| Aspirin | 1405 (47.51) | 11,467 (42.92) | <0.01 |
| Statins | 969 (32.75) | 8391 (31.39) | 0.13 |
| Steroids | 119 (4.02) | 920 (3.44) | 0.10 |
Presented as Mean (Standard deviation).
Estimated using χ2 test, ANOVA, Wilcoxon rank sums test as appropriate.
Presented as Median (25th percentile to 75th percentile).
hs-CRP = high sensitivity C-reactive protein, ACR = Albumin-Creatinine Ratio
Comorbidity score is total of comorbidities, presented as mean and standard deviation (SD).
Among the REGARDS participants, there were a total of 1393 first sepsis hospitalizations with the majority being attributed to lung (48.46%), kidney (17.09%), and abdominal infections (15.08%, Table 2). There was a total 374 (12.64%) sepsis events among cancer survivors compared with 1019 (3.81%) sepsis events among participants with no cancer history. Among those that developed sepsis, there were no differences in sepsis infection types, ICU admission rates, and sepsis 30-Day case fatality between cancer survivors and participants with no cancer history. However, cancer survivors that developed sepsis were more likely to have SOFA scores greater than or equal to 2 (24.60% vs. 18.65%, p value = 0.03), higher distribution MEDS scores (p value <0.01), higher rates of sepsis-related hospital deaths (8.29% vs. 3.93%, p value <0.01), and longer hospital length of stay (Median in days: 5 vs. 4, p value <0.01) when compared with sepsis cases among the no cancer history participants.
Table 2.
Infection types, hospital characteristics, and outcomes among 1393 first sepsis hospitalizations.
| Variable | All Participants (N = 1393) |
Cancer Survivors (N = 374) |
No Cancer History (N = 1019) |
p valuea |
|---|---|---|---|---|
| Infection Type (%)b | ||||
| Lung | 675 (48.46) | 182 (48.66) | 493 (48.38) | 0.89 |
| Kidney | 238 (17.09) | 62 (16.58) | 176 (17.27) | |
| Abdominal | 210 (15.08) | 61 (16.31) | 149 (14.62) | |
| Skin | 109 (7.82) | 26 (6.95) | 83 (8.15) | |
| Sepsis | 93 (6.68) | 27 (7.22) | 66 (6.48) | |
| Other | 68 (4.88) | 16 (4.28) | 52 (5.10) | |
| Hospital Characteristics | ||||
| SOFA Score (%)b | ||||
| 0 | 971 (69.71) | 241 (64.44) | 730 (71.64) | 0.03 |
| 1 | 140 (10.05) | 41 (10.96) | 99 (9.72) | |
| ≥2 | 282 (20.24) | 92 (24.60) | 190 (18.65) | |
| MEDS Scorec | 3 (3 – 3) | 3 (3 – 9) | 3 (3 – 3) | <0.01 |
| ICU Admission (%)b | 67 (4.81) | 23 (6.15) | 44 (4.32) | 0.16 |
| Outcomes | ||||
| Hospital death (%)b | 71 (5.10) | 31 (8.29) | 40 (3.93) | <0.01 |
| Hospital LOSc | 5 (2 – 8) | 5 (3 – 8) | 4 (2 – 8) | <0.01 |
| 30-day Fatalityd (%)b | 167 (11.99) | 47 (12.57) | 120 (11.78) | 0.69 |
Significance for comparison between cancer survivors and no history of cancer participants with sepsis; determined using Chi-square or Wilcoxon Rank-Sum test.
Presented as N (column percentage).
Presented as Median (25th percentile to 75th percentile).
Defined as death attributed to sepsis or death within 30-days after hospital discharge of a physician-adjudicated sepsis event.
SOFA=Sequential Organ Failure Assessment Score. MEDS=Mortality in Emergency Department Sepsis Score. ICU=Intensive Care Unit. LOS=Length of Stay (in days)
The risk of sepsis was higher for cancer survivors when compared with participants with no cancer history (Crude HR: 3.22, 95% CI: 2.86 – 3.63, Table 3, Figure 2: Kaplan Meier survival curve for time to sepsis event by cancer status). Cancer survivors were at higher risk for sepsis even after adjustment for age, sex, race, geographic region, education, and income (Model 1 HR: 2.73, 95% CI: 2.41 – 3.08), additional adjustment for tobacco use and alcohol use (Model 2 HR: 2.70, 95% CI: 2.39 – 3.06), and further adjustments for baseline chronic medical conditions, medications, and biomarkers (Model 3 HR: 2.61, 95% CI: 2.29 – 2.98).
Table 3.
Hazard ratiosa (HR) and Subdistribution HR (SHR) for the association between cancer and first sepsis events. Among 1393 Sepsis Events
| HR or SHR (95% CI) |
||||||
|---|---|---|---|---|---|---|
| No. Events (%)‡ |
Mean Survival Timec (95% CI) |
Crude | Model 1 | Model 2 | Model 3 | |
| Risk of First Sepsis | ||||||
|
| ||||||
| No Cancer History | 1019 (3.81) | 9.19 (9.17 – 9.20) | Ref | Ref | Ref | Ref |
| Cancer Survivors | 374 (12.64) | 8.56 (8.49 – 8.64) | 3.22 (2.86 – 3.63) |
2.73 (2.41 – 3.08) |
2.70 (2.39 – 3.06) |
2.61 (2.29 – 2.98) |
|
| ||||||
| All-Cause Mortality Competing Risk (Subdistribution HR)d | ||||||
|
| ||||||
| No Cancer History | 1019 (3.81) | – | Ref | Ref | Ref | Ref |
| Cancer Survivors | 374 (12.64) | – | 3.22 (2.86 – 3.62) |
2.72 (2.41 – 3.08) |
2.70 (2.38 – 3.06) |
2.61 (2.29 – 2.99) |
|
| ||||||
| Risk of First Sepsis-SOFAe | ||||||
|
| ||||||
| No Cancer History | 190 (0.71) | 8.82 (8.82 – 8.83) | Ref | Ref | Ref | Ref |
| Cancer Survivors | 92 (3.11) | 9.16 (9.12 – 9.20) | 4.08 (3.18 – 5.24) |
3.02 (2.34 – 3.91) |
2.99 (2.30 – 3.89) |
2.84 (2.15 – 3.76) |
|
| ||||||
| Risk of First Sepsis (Excluding cancer deaths within 3 years of follow-up)f | ||||||
|
| ||||||
| No Cancer History | 1014 (3.81) | 9.19 (9.17 – 9.20) | Ref | Ref | Ref | Ref |
| Cancer Survivors | 369 (12.70) | 8.57 (8.50 – 8.65) | 3.21 (2.84 – 3.61) |
2.72 (2.41 – 3.08) |
2.70 (2.38 – 3.06) |
2.60 (2.28 – 2.97) |
Estimated from Cox proportional hazard models.
Row percentage, proportion of participants experiencing a sepsis event.
Mean survival time in years.
Estimated using the Fine & Gray method of Cox proportional hazard models. Analysis includes 1393 sepsis events and 3483 competing deaths.
Among 282 sepsis-SOFA events defined as infection + sequential organ failure assessment (SOFA) score ≥2.
Among 1383 sepsis events, excluding participants that died from cancer-deaths within 3 years of study follow-up.
Model 1: adjusted for age, sex, race, geographic region, education level, and income.
Model 2: additionally adjusted for tobacco and alcohol use.
Model 3: additionally adjusted for baseline comorbidity score, aspirin use, albumin-creatinine ratio, and Cystatin-C.
Figure 2.
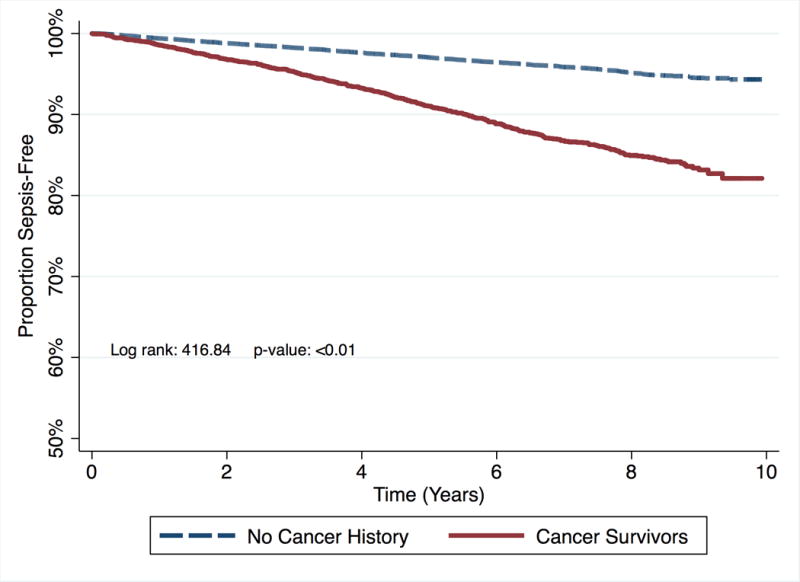
Kaplan Meier survival curve for time to sepsis event by cancer status.
Secondary Analysis
Cancer remained associated with increased risk of sepsis when considering deaths as a competing risk even after adjustment for sociodemographics (Model 1 SHR: 2.72, 95% CI: 2.41 – 3.08), health behaviors (Model 2 SHR: 2.70, 95% CI: 2.38 – 3.06), and baseline comorbidities, medications, and biomarkers (Model 3 SHR: 2.61, 95% CI: 2.29 – 2.99). Similarly, when defining sepsis using the sepsis-SOFA criteria, cancer survivors were at higher risk of sepsis when compared with no cancer history participants (Crude HR: 4.08, 95% CI: 3.18 – 5.24). This association remained significant after adjustments for sociodemographics (Model 1 HR: 3.02, 95% CI: 2.34 – 3.91), health behaviors (Model 2 HR: 2.99, 95% CI: 2.30 – 3.89), comorbidities, medications, and biomarkers (Model 3 HR: 2.84, 95% CI: 2.15 – 3.76).
In a separate analysis, we excluded individuals that experienced a cancer death within 3 years of study follow-up. As a result, we excluded 145 (0.54%) of participants with no cancer history, and 54 (1.82%) of cancer survivors. Cancer remained associated with increased risk of sepsis (Crude HR: 3.21, 95% CI: 2.84 – 3.61) even after adjustments for sociodemographics (Model 1 HR: 2.72, 95% CI: 2.41 – 3.08), health behaviors (Model 2 HR: 2.70, 95% CI: 2.38 – 3.06), comorbidities, medications, and biomarkers (Model 3 HR: 2.60, 95% CI: 2.28 – 2.97).
Effect Modification by Race Analysis
Race did not modify the effect of cancer survivorship on subsequent risk of sepsis (p value for the interaction of race and cancer = 0.63, Table 4). Among Black participants, the risk of sepsis for cancer survivors was higher for cancer survivors (Model 3 HR: 3.10, 95% CI: 2.42 – 3.97, Figure 3A: Kaplan Meier survival curve for time to sepsis event by cancer status for Blacks) than for participants with no cancer history. Among White participants the risk of sepsis was higher for cancer survivors (Model 3 HR: 2.45, 95% CI: 2.10 – 2.86, Figure 3B: Kaplan Meier survival curve for time to sepsis event by cancer status for Whites) than for participants with no cancer history.
Table 4.
Hazard ratios (HRs) and 95% confidence intervals for the association between cancer survivors and sepsis, stratified by race.
| HR (95% CI)
|
||
|---|---|---|
| Black (N = 12,216) |
White (N = 17,477) |
|
| Cancer Survivors | ||
| No Events (%)a | 101 (11.17) | 273 (13.28) |
| Mean Survival Time (95% CI) | 8.13 (8.01 – 8.25) | 8.53 (8.43 – 8.62) |
|
| ||
| No Cancer History (Referent) | ||
| No Events (%)a | 371 (3.28) | 648 (4.20) |
| Mean Survival Time (95% CI) | 9.21 (9.19 – 9.24) | 8.82 (8.80 – 8.84) |
|
| ||
| Crude | 3.29 (2.64 – 4.10) | 3.11 (2.70 – 3.58) |
| Model 1 | 3.06 (2.43 – 3.84) | 2.60 (2.25 – 3.01) |
| Model 2 | 2.94 (2.33 – 3.71) | 2.61 (2.25 – 3.02) |
| Model 3 | 3.10 (2.42 – 3.97) | 2.45 (2.10 – 2.86) |
Model 1: adjusted for age, sex, geographic region, education level, and income.
Model 2: additionally adjusted for tobacco and alcohol use.
Model 3: additionally adjusted for baseline comorbidity score, aspirin use, albumin-creatinine ratio, and Cystatin-C.
Proportion among cancer group by race/ethnicity to have incident sepsis event.
p-value for race-cancer history interaction = 0.63
Figure 3.
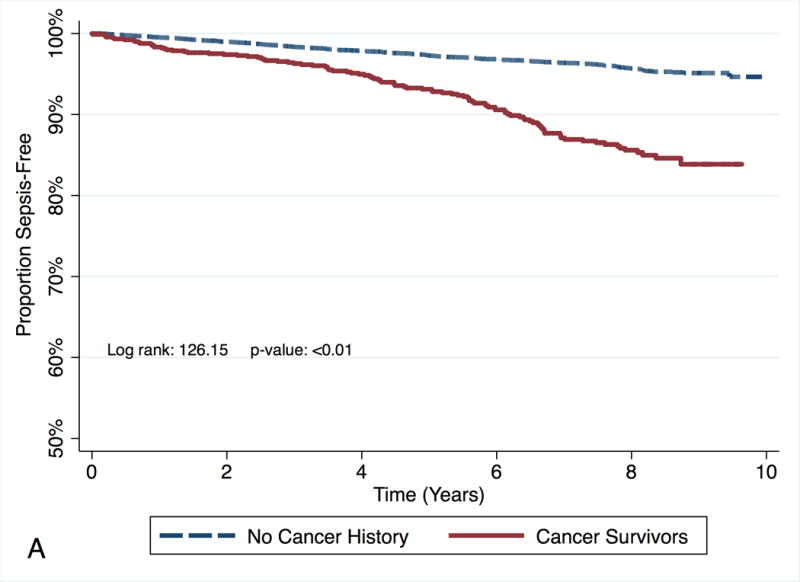
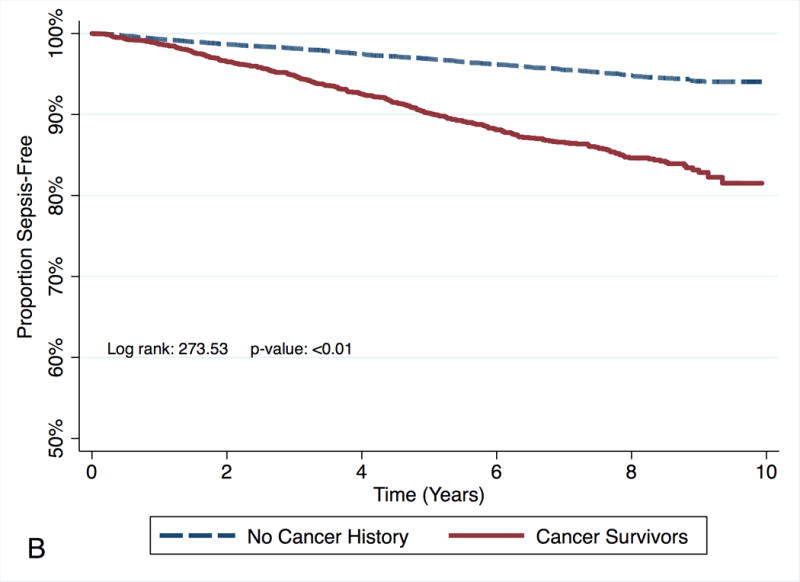
Kaplan Meier survival curve for time to sepsis event by cancer status for each race/ethnicity. Figure in panel A represents the survival for Blacks. Figure in panel B represents the survival for Whites.
DISCUSSION
The current analysis among a prospective cohort of community-dwelling participants afforded two major contributions to the current literature gap; the opportunity to examine long-term risk for sepsis between cancer survivors and no cancer history participants and to examine whether these differences were modified by race/ethnicity. Our research hypothesis was derived from the biological plausible connection between cancer survivorship and sepsis. Possible physiological mechanisms that could explain the association between cancer survivorship and long-term risk of sepsis are: 1) the underlying malignancy causing an increase in circulating cytokines and thus causing a chronic inflammatory state (39–43), and/or 2) degradation of healthy cells due to cancer treatment and therapy, both of which may lead cancer survivors to having a more immune-compromised physiology at baseline which would in turn lead to increased risk for infection and sepsis (44). Using the large population-based study of community-dwelling adults from REGARDS cohort, we observed that cancer survivors were at more than a two-fold greater risk of sepsis when compared with community-dwelling REGARDS participants with no cancer history. Further, this association remained even after several adjustments for confounders, secondary analyses that excluded participants with a cancer death within three years of study follow-up, and accounting for all-cause mortality as competing risk. We observed no racial differences in risk and incidence rates of sepsis after cancer.
To date, this is the first study to utilize a cohort of community-dwelling adults to examine the association between cancer survivorship and future risk of sepsis. Only a limited number of studies have investigated an association between cancer and sepsis. Moreover, prior studies that have examined this association have been based on data collected during hospitalization (i.e., cross-sectional), after major cancer surgical procedures, or only among cancer populations (45–50). For instance, one of the first studies to examine the association between cancer and sepsis utilized hospital discharge data from six US states (Florida, Massachusetts, New Jersey, New York, Virginia, and Washington) linked with SEER cancer prevalence data (50). In this study, Williams et al (2004) reported that sepsis was a very common complication among cancer patients, being responsible for more than 126,000 sepsis cases per year and that when compared to the overall population cancer patients were at nearly a 4-fold increased risk of developing sepsis (50). In another study, Danai et al (2006) examined the prevalence of sepsis among hospitalized patients with a history of cancer complemented with data from the SEER with hospital discharge data from the National Hospital Discharge Survey (NHDS) spanning over 20 years from 1979 through 2001 (4). The authors reported that, compared to no cancer history patients, cancer patients had nearly a 10-fold increased risk of having sepsis (4). The results of prior studies indicate that cancer patients hospitalized or undergoing surgery are at an increased risk of sepsis (45–50). Similarly, the results of our study illuminate that cancer survivors of any type are at more than a two-fold increased risk of sepsis when compared with other community dwelling adults. The results of our study suggest that even after surviving cancer for greater than two years, cancer survivors remain at increased risk of community-acquired sepsis. Among patients hospitalized for infection, cancer survivors represent a very pertinent population for mitigation, infection prevention, and early detection and treatment of infection with necessary antibiotics. We also understand that cancer is a heterogeneous disease that has many underlying risk factors such as obesity, diet, exercise, and genetics. While we were unable to examine the risk of sepsis by specific cancers in the current study, we elucidated that cancer survivors considered to be healthy enough to be considered community-dwelling and participate in a longitudinal prospective cohort study were still at nearly a 2.5 to 3-fold increased risk of sepsis infection regardless of the cancer type, race, and accounting for multiple risk factors such as obesity, health behaviors, and comorbidities. Thus, cancer is a very pertinent risk factor in the treatment of sepsis and expeditious and urgent care should be taken when dealing treating patients with a history of cancer.
In the current investigation we observed no evidence of racial differences in sepsis incidence after cancer survival. In contrast, several studies have suggested that Blacks suffer poorer outcomes in cancer survival due to delays, receipt, and adherence to cancer treatment (51–64). For example, Akinyemiju et al (2015) performed a cross-sectional analysis among 71,156 women from the NIS and reported that Black women were 23% more likely to have metastatic breast cancer, but 6% less likely to receive mastectomy after breast cancer diagnosis (55). Likewise, much research has shown that Blacks have a higher risk of sepsis when compared to their White counterparts (24–26, 29–33, 65, 66). However, we note that in our prior REGARDS investigation we observed Blacks were 35% less likely to have an infection and there was no difference in odds of sepsis when compared to White participants (28). Furthermore, there is little research that has investigated racial differences in the association between cancer and sepsis. Sammon et al (2015) reported that after MCS, Black patients were 35% more likely to have sepsis when compared to White patients (5). While the findings from Sammon et al (2015) differ from our study results, these differences are likely attributed to dissimilarity in the identification of sepsis events, use of cross-sectional data, and use of cancer patients undergoing surgery. Nevertheless, the results from our study suggest that race may not play an important role in the prediction of sepsis after cancer.
Lastly, our current analysis differed much from prior studies using administrative codes for investigating risk of sepsis because we defined sepsis from physician-adjudicated medical chart review within a prospective cohort of community dwelling adults. Moreover, we were able to identify community-acquired sepsis based on limiting sepsis events to those within the first 28-hours. Community-acquired sepsis, or sepsis events occurring within first day of hospitalization is a condition indicative of patients presenting to the hospital from communities with very severe infections. Thus, our observed near three-fold increased risk of sepsis comparing cancer survivors with no cancer history participants indicates that cancer survivors are not just getting sick while undergoing cancer treatment, but rather there are underlying pathophysiologic differences comparing healthy participants to cancer survivors. In addition, cancer survivors may be at more of an acute risk of death from community-acquired sepsis as indicative by the fact that cancer survivors had higher sepsis related mortality (in-hospital mortality) but similar 30-day sepsis case fatality when compared to no cancer history participants. This is likely a representation of more severe and higher acuity sepsis infection (which can also be observed when comparing SOFA and MEDS scores between cancer survivors and no cancer history participants) among cancer survivors when compared to no cancer participants that subsides over time.
Limitations
While this study has several strengths, there are a few limitations that should be considered when interpreting the aforementioned results. First, the REGARDS cohort was originally designed to investigate risk factors for stroke, and was not a surveillance study for identifying both cancer survivors and sepsis events. As a result many of the available covariates including comorbidities, medications, and biomarkers are potentially associated with cerebrovascular disease. Furthermore, the original focus on stroke incidence may lead to misclassification, and it is possible that we underestimated the true number of cancer survivors and sepsis events. Thus, if we under-detected the true number of cancer survivors (and cancer survivors had much higher risk of sepsis risk) we may have underestimated the risk of sepsis among cancer survivors. On the other hand, if we under-detected the true number of sepsis events (and cancer survivors still had much higher risk of sepsis) our observed effect sizes may still be underestimates of the true association between cancer survivorship and sepsis risk. Nonetheless, there is likely minimal bias in the identification of sepsis because events were physician-adjudicated with an inter-rater agreement of 0.90. Further, self-reported history of cancer has been shown to have high sensitivity values that range from 0.79 to 0.90 for recall of cancer site and year of diagnosis (35). However, there remains considerable variability in the sensitivity of self-reported cancer history by site as major cancers such as lung (0.90), breast (0.91), and prostate (0.90) have excellent sensitivity; and less common cancers such as rectal (0.16) and melanoma (0.53) have generally poor sensitivity (35). Additionally, we were unable to account for cancer as a time-varying exposure (i.e., no cancer history participants could develop cancer over study follow-up). As a result, it is likely that we underestimated the true effect of the association between cancer survivorship and sepsis due to this misclassification resulting in cancer survivors being in the non-exposed (i.e., no cancer history) participant category. We additionally performed several secondary analyses to account for all-cause mortality and death attributed to cancer within 3 years of follow-up. These analyses possibly accounted for participants that with unknown cancers during baseline (including those individuals that were misclassified as no cancer history participants), and participants with higher cancer morbidity and lower cancer survival. Lastly, we could not assess the specific cancer types and sites and their respective associations with risk of sepsis, and it is possible that the observed risk of sepsis varies by cancer type and site.
CONCLUSION
In this large community-dwelling cohort of Black and White adults, cancer survivors are at more than a two-fold increased risk of developing sepsis. Public health and acute care initiatives should focus on identifying patients with any history of cancer, regardless of time in remission, in order to mitigate the increased sepsis risk observed in this specific population. Future studies should further investigate the risk of sepsis among cancer survivors and additionally examine whether there are differences by cancer subtype.
Supplementary Material
Acknowledgments
FINANCIAL SUPPORT
This work was supported by award {grant number R01-NR012726} from the National Institute for Nursing Research, {grant number UL1-RR025777} from the National Center for Research Resources, as well as by grants from the Center for Clinical and Translational Science and the Lister Hill Center for Health Policy of the University of Alabama at Birmingham. The parent REGARDS study was supported by cooperative agreement {grant number U01-NS041588} from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. Representatives of the funding agencies have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org and http://www.regardssepsis.org. Dr. Moore received grant support from {grant R25 CA47888}, the Cancer Prevention and Control Training Program grant, funded by the National Cancer Institute, National Institutes of Health. Dr. Moore was additionally supported by grant T32190194 (Colditz) and by the foundation for Barnes Jewish Hospital and by Siteman Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
CONFLICT OF INTERESTS: The authors declare no potential conflicts of interest.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Critical Care Medicine. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. New England Journal of Medicine. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Wang HE, Shapiro NI, Angus DC, Yealy DM. National estimates of severe sepsis in United States emergency departments. Critical Care Medicine. 2007;35:1928–36. doi: 10.1097/01.CCM.0000277043.85378.C1. [DOI] [PubMed] [Google Scholar]
- 4.Danai PA, Moss M, Mannino DM, Martin GS. The epidemiology of sepsis in patients with malignancy. Chest. 2006;129:1432–40. doi: 10.1378/chest.129.6.1432. [DOI] [PubMed] [Google Scholar]
- 5.Sammon JD, Klett DE, Sood A, Olugbade K, Schmid M, Kim SP, Menon M, Trinh Q. Sepsis after major cancer surgery. Journal of Surgical Research. 2015;193:788–94. doi: 10.1016/j.jss.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 6.Williams MD, Braun LA, Cooper LM, Johnston J, Weiss RV, Qualy RL, Linde-Zwirble W. Hospitalized cancer patients with severe sepsis: analysis of incidence, mortality, and associated costs of care. Crit Care. 2004;8:R291–8. doi: 10.1186/cc2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Remick DG. Pathophysiology of sepsis. The American journal of pathology. 2007;170:1435–44. doi: 10.2353/ajpath.2007.060872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gloeckler Ries LA, Reichman ME, Lewis DR, Hankey BF, Edwards BK. Cancer survival and incidence from the Surveillance, Epidemiology, and End Results (SEER) program. The oncologist. 2003;8:541–52. doi: 10.1634/theoncologist.8-6-541. [DOI] [PubMed] [Google Scholar]
- 9.Welch HG, Schwartz LM, Woloshin S. Are increasing 5-year survival rates evidence of success against cancer? JAMA. 2000;283:2975–8. doi: 10.1001/jama.283.22.2975. [DOI] [PubMed] [Google Scholar]
- 10.Aizer AA, Wilhite TJ, Chen MH, Graham PL, Choueiri TK, Hoffman KE, Martin NE, Trinh QD, Hu JC, Nguyen PL. Lack of reduction in racial disparities in cancer-specific mortality over a 20-year period. Cancer. 2014;120:1532–9. doi: 10.1002/cncr.28617. [DOI] [PubMed] [Google Scholar]
- 11.Akinyemiju TF, Soliman AS, Yassine M, Banerjee M, Schwartz K, Merajver S. Healthcare access and mammography screening in Michigan: a multilevel cross-sectional study. International journal for equity in health. 2012;11:16. doi: 10.1186/1475-9276-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akinyemiju TF, Soliman AS, Johnson NJ, Altekruse SF, Welch K, Banerjee M, Schwartz K, Merajver S. Individual and neighborhood socioeconomic status and healthcare resources in relation to black-white breast cancer survival disparities. J Cancer Epidemiol. 2013;2013:490472. doi: 10.1155/2013/490472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akinyemiju TF, Soliman AS, Copeland G, Banerjee M, Schwartz K, Merajver SD. Trends in breast cancer stage and mortality in Michigan (1992-2009) by race, socioeconomic status, and area healthcare resources. PLoS One. 2013;8:e61879. doi: 10.1371/journal.pone.0061879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akinyemiju TF, Vin-Raviv N, Chavez-Yenter D, Zhao X, Budhwani H. Race/ethnicity and socio-economic differences in breast cancer surgery outcomes. Cancer epidemiology. 2015;39:745–51. doi: 10.1016/j.canep.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94:490–6. doi: 10.1093/jnci/94.7.490. [DOI] [PubMed] [Google Scholar]
- 16.Cross CK, Harris J, Recht A. Race, socioeconomic status, and breast carcinoma in the U.S: what have we learned from clinical studies. Cancer. 2002;95:1988–99. doi: 10.1002/cncr.10830. [DOI] [PubMed] [Google Scholar]
- 17.Dai D. Black residential segregation, disparities in spatial access to health care facilities, and late-stage breast cancer diagnosis in metropolitan Detroit. Health & Place. 2010;16:1038–52. doi: 10.1016/j.healthplace.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 18.El-Tamer MB, Homel P, Wait RB. Is race a poor prognostic factor in breast cancer? J Am Coll Surg. 1999;189:41–5. doi: 10.1016/s1072-7515(99)00055-1. [DOI] [PubMed] [Google Scholar]
- 19.Gwyn K, Bondy ML, Cohen DS, Lund MJ, Liff JM, Flagg EW, Brinton LA, Eley JW, Coates RJ. Racial differences in diagnosis, treatment, and clinical delays in a population-based study of patients with newly diagnosed breast carcinoma. Cancer. 2004;100:1595–604. doi: 10.1002/cncr.20169. [DOI] [PubMed] [Google Scholar]
- 20.Kim SH, Ferrante J, Won BR, Hameed M. Barriers to adequate follow-up during adjuvant therapy may be important factors in the worse outcome for Black women after breast cancer treatment. World Journal of Surgical Oncology. 2008;6:10. doi: 10.1186/1477-7819-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laiyemo AO. Reducing racial disparity in colorectal cancer burden. Dig Dis Sci. 2014;59:2025–7. doi: 10.1007/s10620-014-3238-8. [DOI] [PubMed] [Google Scholar]
- 22.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med. 2003;163:49–56. doi: 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- 23.Sheppard VB, Oppong BA, Hampton R, Snead F, Horton S, Hirpa F, Brathwaite EJ, Makambi K, Onyewu S, Boisvert M, Willey S. Disparities in breast cancer surgery delay: the lingering effect of race. Annals of surgical oncology. 2015;22:2902–11. doi: 10.1245/s10434-015-4397-3. [DOI] [PubMed] [Google Scholar]
- 24.Barnato AE, Alexander SL, Linde-Zwirble WT, Angus DC. Racial variation in the incidence, care, and outcomes or severe sepsis: analysis of population, patient, and hospital characteristics. Am J Respir Crit Care Med. 2008;177 doi: 10.1164/rccm.200703-480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 26.Mayr FB, Yende S, Linde-Zwirble WT, Peck-Pamler OM, Weissfeld LA, Angus DC. Infection rate and acute organ dysfunction risk as explanations for racial differences in severe sepsis. JAMA. 2010;303:2495–503. doi: 10.1001/jama.2010.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence. 2014;5:4–11. doi: 10.4161/viru.27372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore JX, Donnelly JP, Griffin R, Safford MM, Howard G, Baddley J, Wang HE. Black-white racial disparities in sepsis: a prospective analysis of the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort. Critical Care. 2015;19:10. doi: 10.1186/s13054-015-0992-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baine W, Yu W, Summe JP. The epidemiology of hospitalization of elderly Americans for septicemia or bacteremia in 1991-1998. Application of Medicare claims data. Ann Epidemiol. 2001;11:118–26. doi: 10.1016/s1047-2797(00)00184-8. [DOI] [PubMed] [Google Scholar]
- 30.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Occurrence and outcomes of sepsis: influence of race. Critical Care Medicine. 2007;35:763–8. doi: 10.1097/01.CCM.0000256726.80998.BF. [DOI] [PubMed] [Google Scholar]
- 31.Esper AM, Moss M, Lewis CA, Nisbet R, Mannino DM, Martin GS. The role of infection and comorbidity: Factors that influence disparities in sepsis. Critical Care Medicine. 2006;34:2576–82. doi: 10.1097/01.CCM.0000239114.50519.0E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McBean M, Rajamani S. Increasing rates of hospitalization due to septicimia in the US elderly population, 1986-1997. Journal of Infectious Diseases. 2001;183:596–603. doi: 10.1086/318526. [DOI] [PubMed] [Google Scholar]
- 33.Richardus JH, Kunst AE. Black-white differences in infectious disease mortality in the United States. Am J Public Health. 2001;91:1251–3. doi: 10.2105/ajph.91.8.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The Reasons for Geographic and Racial Differences in Stroke Study: Objectives and Design. Neuroepidemiology. 2005;25:135–43. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 35.Bergmann MM, Calle EE, Mervis CA, Miracle-McMahill HL, Thun MJ, Heath CW. Validity of self-reported cancers in a prospective cohort study in comparison with data from state cancer registries. Am J Epidemiol. 1998;147:556–62. doi: 10.1093/oxfordjournals.aje.a009487. [DOI] [PubMed] [Google Scholar]
- 36.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA : the journal of the American Medical Association. 2016;315:801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willenbring ML, Massey SH, Gardner MB. Helping patients who drink too much: an evidence-based guide for primary care clinicians. American family physician. 2009;80:44–50. [PubMed] [Google Scholar]
- 38.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 39.Pine SR, Mechanic LE, Enewold L, Chaturvedi AK, Katki HA, Zheng YL, et al. Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. Journal of the National Cancer Institute. 2011;103:1112–22. doi: 10.1093/jnci/djr216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kakourou A, Koutsioumpa C, Lopez DS, Hoffman-Bolton J, Bradwin G, Rifai N, et al. Interleukin-6 and risk of colorectal cancer: results from the CLUE II cohort and a meta-analysis of prospective studies. Cancer causes & control : CCC. 2015;26:1449–60. doi: 10.1007/s10552-015-0641-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsilidis KK, Branchini C, Guallar E, Helzlsouer KJ, Erlinger TP, Platz EA. C-reactive protein and colorectal cancer risk: a systematic review of prospective studies. International journal of cancer. 2008;123:1133–40. doi: 10.1002/ijc.23606. [DOI] [PubMed] [Google Scholar]
- 42.Izano M, Wei EK, Tai C, Swede H, Gregorich S, Harris TB, et al. Chronic inflammation and risk of colorectal and other obesity-related cancers: The health, aging and body composition study. International journal of cancer. 2016;138:1118–28. doi: 10.1002/ijc.29868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Il’yasova D, Colbert LH, Harris TB, Newman AB, Bauer DC, Satterfield S, et al. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2005;14:2413–8. doi: 10.1158/1055-9965.EPI-05-0316. [DOI] [PubMed] [Google Scholar]
- 44.Wang HE, Shapiro NI, Griffin R, Safford MM, Judd S, Howard G. Inflammatory and endothelial activation biomarkers and risk of sepsis: a nested case-control study. Journal of critical care. 2013;28:549–55. doi: 10.1016/j.jcrc.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Danai PA, Moss M, Mannino DM, Martin GS. The epidemiology of sepsis in patients with malignancy. Chest. 2006;129:1432–40. doi: 10.1378/chest.129.6.1432. [DOI] [PubMed] [Google Scholar]
- 46.Rosolem MM, Rabello LS, Lisboa T, Caruso P, Costa RT, Leal JV, et al. Critically ill patients with cancer and sepsis: clinical course and prognostic factors. J Crit Care. 2012;27:301–7. doi: 10.1016/j.jcrc.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 47.Sammon JD, Klett DE, Sood A, Olugbade K, Jr, Schmid M, Kim SP, et al. Sepsis after major cancer surgery. The Journal of surgical research. 2015;193:788–94. doi: 10.1016/j.jss.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 48.Thirumala R, Ramaswamy M, Chawla S. Diagnosis and management of infectious complications in critically ill patients with cancer. Critical care clinics. 2010;26:59–91. doi: 10.1016/j.ccc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 49.Torres VB, Azevedo LC, Silva UV, Caruso P, Torelly AP, Silva E, et al. Sepsis-Associated Outcomes in Critically Ill Patients with Malignancies. Ann Am Thorac Soc. 2015;12:1185–92. doi: 10.1513/AnnalsATS.201501-046OC. [DOI] [PubMed] [Google Scholar]
- 50.Williams MD, Braun LA, Cooper LM, Johnston J, Weiss RV, Qualy RL, et al. Hospitalized cancer patients with severe sepsis: analysis of incidence, mortality, and associated costs of care. Crit Care. 2004;8:R291–8. doi: 10.1186/cc2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, et al. Annual Report to the Nation on the Status of Cancer, 1975–2014, Featuring Survival. Journal of the National Cancer Institute. 2017;109 doi: 10.1093/jnci/djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akinyemiju T, Meng Q, Vin-Raviv N. Race/ethnicity and socio-economic differences in colorectal cancer surgery outcomes: analysis of the nationwide inpatient sample. BMC cancer. 2016;16:715. doi: 10.1186/s12885-016-2738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akinyemiju T, Moore JX, Ojesina AI, Waterbor JW, Altekruse SF. Racial disparities in individual breast cancer outcomes by hormone-receptor subtype, area-level socio-economic status and healthcare resources. Breast cancer research and treatment. 2016;157:575–86. doi: 10.1007/s10549-016-3840-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akinyemiju TF, Soliman AS, Johnson NJ, Altekruse SF, Welch K, Banerjee M, et al. Individual and neighborhood socioeconomic status and healthcare resources in relation to black-white breast cancer survival disparities. Journal of cancer epidemiology. 2013;2013:490472. doi: 10.1155/2013/490472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akinyemiju TF, Vin-Raviv N, Chavez-Yenter D, Zhao X, Budhwani H. Race/ethnicity and socio-economic differences in breast cancer surgery outcomes. Cancer epidemiology. 2015;39:745–51. doi: 10.1016/j.canep.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 56.Damle RN, Flahive JM, Davids JS, Maykel JA, Sturrock PR, Alavi K. Examination of Racial Disparities in the Receipt of Minimally Invasive Surgery Among a National Cohort of Adult Patients Undergoing Colorectal Surgery. Diseases of the colon and rectum. 2016;59:1055–62. doi: 10.1097/DCR.0000000000000692. [DOI] [PubMed] [Google Scholar]
- 57.Fedewa SA, Edge SB, Stewart AK, Halpern MT, Marlow NM, Ward EM. Race and ethnicity are associated with delays in breast cancer treatment (2003-2006) Journal of health care for the poor and underserved. 2011;22:128–41. doi: 10.1353/hpu.2011.0006. [DOI] [PubMed] [Google Scholar]
- 58.Fedewa SA, Ward EM, Stewart AK, Edge SB. Delays in adjuvant chemotherapy treatment among patients with breast cancer are more likely in African American and Hispanic populations: a national cohort study 2004-2006. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:4135–41. doi: 10.1200/JCO.2009.27.2427. [DOI] [PubMed] [Google Scholar]
- 59.Freedman RA, Virgo KS, He Y, Pavluck AL, Winer EP, Ward EM, et al. The association of race/ethnicity, insurance status, and socioeconomic factors with breast cancer care. Cancer. 2011;117:180–9. doi: 10.1002/cncr.25542. [DOI] [PubMed] [Google Scholar]
- 60.Hershman DL, Tsui J, Wright JD, Coromilas EJ, Tsai WY, Neugut AI. Household net worth, racial disparities, and hormonal therapy adherence among women with early-stage breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:1053–9. doi: 10.1200/JCO.2014.58.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmid M, Meyer CP, Reznor G, Choueiri TK, Hanske J, Sammon JD, et al. Racial Differences in the Surgical Care of Medicare Beneficiaries With Localized Prostate Cancer. JAMA oncology. 2016;2:85–93. doi: 10.1001/jamaoncol.2015.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. Journal of the National Cancer Institute. 2002;94:334–57. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 63.Sheppard VB, Oppong BA, Hampton R, Snead F, Horton S, Hirpa F, et al. Disparities in breast cancer surgery delay: the lingering effect of race. Annals of surgical oncology. 2015;22:2902–11. doi: 10.1245/s10434-015-4397-3. [DOI] [PubMed] [Google Scholar]
- 64.Wang EH, Yu JB, Abouassally R, Meropol NJ, Cooper G, Shah ND, et al. Disparities in Treatment of Patients With High-risk Prostate Cancer: Results From a Population-based Cohort. Urology. 2016;95:88–94. doi: 10.1016/j.urology.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 65.Moore JX, Donnelly JP, Griffin R, Safford MM, Howard G, Baddley J, et al. Black-white racial disparities in sepsis: a prospective analysis of the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort. Crit Care. 2015;19:279. doi: 10.1186/s13054-015-0992-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Occurrence and outcomes of sepsis: influence of race. Crit Care Med. 2007;35:763–8. doi: 10.1097/01.CCM.0000256726.80998.BF. [DOI] [PubMed] [Google Scholar]
- 67.National Institute on Alcohol Abuse and Alcoholism. Helping Patients Who Drink Too Much, a Clinician’s Guide. 2005 [cited 2012 February 13]; Available from: http://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/guide.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


