Abstract
Introduction
Since 2006, human papillomavirus vaccine has been recommended for young females in the U.S. This study aimed to compare cervical cancer incidence among young women before and after the human papillomavirus vaccine was introduced.
Methods
This cross-sectional study used data from the National Program for Cancer Registries and Surveillance, Epidemiology, and End Results Incidence–U.S. Cancer Statistics 2001–2014 database for U.S. females aged 15–34 years. This study compared the 4-year average annual incidence of invasive cervical cancer in the 4 years before human papillomavirus vaccine was introduced (2003–2006) and the 4 most recent years in the vaccine era (2011–2014). Joinpoint regression models of cervical incidence from 2001 to 2014 were fitted to identify the discrete joints (year) that represent statistically significant changes in the direction of the trend after the introduction of human papillomavirus vaccination in 2006. Data were collected in 2001–2014, released, and analyzed in 2017.
Results
The 4-year average annual incidence rates for cervical cancer in 2011–2014 were 29% lower than that in 2003–2006 (6.0 vs 8.4 per 1,000,000 people, rate ratio=0.71, 95% CI=0.64, 0.80) among females aged 15–24 years, and 13.0% lower among females aged 25–34 years. Joinpoint analyses of cervical cancer incidence among females aged 15–24 years revealed a significant joint at 2009 for both squamous cell carcinoma and non–squamous cell carcinoma. Among females aged 25–34 years, there was no significant decrease in cervical cancer incidence after 2006.
Conclusions
A significant decrease in the incidence of cervical cancer among young females after the introduction of human papillomavirus vaccine may indicate early effects of human papillomavirus vaccination.
INTRODUCTION
Cervical cancers are caused by human papillomavirus (HPV) infections.1 HPV vaccination was introduced in the U.S. in 2006. Currently, there are three types of HPV vaccines available: bivalent,2 quadrivalent,3 and nonavalent.4 The bivalent and quadrivalent vaccines protect against HPV 16 and 18, which are responsible for approximately 70% of cervical cancers.1 The nonavalent vaccine covers an additional five oncogenic types responsible for another 20% of cervical cancers.5 HPV vaccination is recommended for girls aged 11–12 years, with catchup vaccination up to 26 years. Nationwide in 2015, a total of 63% of girls aged 13–17 years had received at least one dose of the HPV vaccine.6
Prior studies on the HPV vaccine have investigated the vaccine’s impact on the prevalence of the virus and high-grade cervical lesions.7–11 To date, no study has examined trends in cervical cancer incidence before and after the HPV vaccination was introduced among young females who may have been vaccinated. An ecologic study comparing cervical cancer incidence among young females before and after HPV vaccine introduction provides insight into whether HPV vaccination has contributed to potential changes in cervical cancer incidence, particularly among younger girls who may not routinely undergo screening to detect cervical lesions.12–14 The objective of this study is to compare cervical cancer incidence by histology between the pre-vaccine and vaccine eras among young women (aged 15–24 years and 25–34 years) using data from U.S. Cancer Statistics (USCS), the combined data from the Centers for Disease Control and Prevention (CDC’s) National Program for Cancer Registries (NPCR) and the National Cancer Institute’s (NCI’s) Surveillance, Epidemiology, and End Results (SEER) Program. To explore the relationship between HPV vaccination and changes in cervical cancer incidence, as secondary aims, this study also assesses HPV vaccine uptake, HPV infections, and changes in cervical cancer screening guidelines.
METHODS
Study Sample
This study included data on young females (aged 15–34 years) from USCS.15 USCS 2001–2014 database combines data from NPCR and SEER, including cancer incidence and population data for all 50 states, and the District of Columbia. Hospitals, physicians, and laboratories across the nation report data on demographic characteristics and tumor characteristics to central cancer registries supported by CDC and NCI. The NPCR and SEER Incidence–USCS Public Use Database (2001–2014 database) covered essentially all of the young female population (aged 15–34 years) between 2001 and 2014 in the U.S. (Puerto Rico not included). This study was not considered human subjects research by the IRB at The University of Texas Medical Branch, Galveston, TX.
Measures
The information collected about each incident of cancer diagnosis included demographic characteristics, date of diagnosis, and cancer histology. Patients were divided into the following groups according to age: 15–19, 20–24, 25–29, and 30–34. Histology of invasive cervical cancer was classified using the International Classification of Disease for Oncology, 3rd edition (ICD-O-3), topography, and morphology codes. Cervical cancer was stratified by histology, as adenocarcinoma may progress from a normal Pap smear to invasive cancer faster than squamous cell cancer.16 Thus, changes in the incidence of these two cancers may differ. Invasive cervical cancers were grouped into squamous cell carcinoma (SCC) and non-SCC. SCC was defined as ICD-O-3 codes 8050–8084. Non-SCC was defined as all other types excluding SCC, and was mainly composed of adenocarcinoma (8140–8149, 8160–8162, 8190–8221, 8260–8337, 8350–8551, and 8570–8576). Hispanic ethnicity for all cancer cases was identified by the North American Association of Central Cancer Registries National Hispanic/Latino Identification Algorithm.17
Statistical Analysis
All data analyses were carried out in 2017 using the SEER*Stat statistical software package, version 8.3.4 or SAS software, version 9.4. Statistical significance was determined as two-sided p-values<0.05. Cervical cancer incidence rates were calculated as cases per 1,000,000 people and age-adjusted to the 2000 U.S. standard population. CIs were calculated using the Tiwari method.18 The 4-year average annual incidence rates were calculated for 4 years before the introduction of HPV vaccination (2003–2006) and the 4 most recent years in the vaccine era (2011–2014). Differences in age-adjusted rates were evaluated using rate ratio (RR) and the corresponding 95% CI. Considering lag time between the introduction of HPV vaccination in 2006 and the subsequent impact on cervical cancer incidence, joinpoint regression models19 were fitted to identify the joinpoints (year) when annual percentage changes (APC) changed significantly, using the NCI’s Joinpoint Regression Analysis program, version 4.5.0.20 APC statistics were used to characterize the magnitude and direction of trends. APC was calculated as (exp[β]−1) × 100, where the regression coefficient (β) was estimated by fitting a least squares regression line to the natural logarithm of the rates, using the calendar year as a regressor variable. Joinpoint regression uses least squares regression to fit line segments to the natural log of the age-standardized incidence rates, joined at discrete points that represent statistically significant changes in direction of the trend.20 Subgroup analyses were performed in age groups, races/ethnicities, and cervical cancer histologic subtypes (SCC and non-SCC).
This study also examined the trends in HPV vaccine coverage among girls aged 13–17 years using data from the National Immunization Survey–Teen 2008–2014, and among women aged 18–24 or 25–34 years using data from National Health Interview Survey 2008–2015, the trends in prevalence of high-risk vaccine type HPV (16 and 18) among females aged 18–24 or 25–34 years using test results of HPV DNA from vaginal swabs in the National Health and Nutrition Examination Survey 2003–2014, and changes in cervical cancer screening recommendations from major national guidelines during the study period in the U.S. Details of National Immunization Survey–Teen, National Health Interview Survey, and National Health and Nutrition Examination Survey are available at each study’s websites at the CDC.21 As HPV vaccination data from National Immunization Survey–Teen and National Health Interview Survey were recall data, the estimates were plotted 1 year prior to the interview date.
RESULTS
In USCS, there were 25,427 cases of invasive cervical cancer among young females aged 15–34 years during 2001–2014 (Appendix Table 1). During the pre-vaccine era (2001–2006), there were 1,056 cases among females aged 15–24 years and 10,498 cases among women aged 25–34 years for any histologic type. During the vaccine era (2007–2014), there were 1,171 cases in females aged 15–24 years and 12,702 cases in women aged 25–34 years for any histologic type. Comparing the pre-vaccine era (2003–2006) to the vaccine era (2011–2014), the 4-year average annual incidence rate decreased by 29%, from 8.4 to 6.0 per 1,000,000 people among females aged 15–24 years (RR=0.71, 95% CI=0.64, 0.80; Table 1) and by 13%, from 87.8 to 76.1 per 1,000,000 among women aged 25–34 years (RR=0.87, 95% CI=0.84, 0.90; Table 2). Among females aged 15–24 years and women aged 25–34 years, the average annual rates in 2011–2014 were significantly lower than that in 2003–2006 for both SCC and non-SCC. Among non-Hispanic white females aged 15–24 years, the annual incidence rate decreased by 24% from 4.6 to 3.5 per 1,000,000 people for SCC (RR=0.76, 95% CI=0.62, 0.92), and by 26% from 3.6 to 2.6 per 1,000,000 for non-SCC (RR=0.74, 95% CI=0.59, 0.93). Among non-Hispanic white women aged 25–34 years, the annual incidence rate decreased by 17% from 57.6 to 48.1 per 1,000,000 for SCC (RR=0.83, 95% CI=0.79, 0.88), and by 13% from 34.1 to 29.6 per 1,000,000 for non-SCC (RR=0.87, 95% CI=0.81, 0.93).
Table 1.
Age-Adjusted 4-Year Average Annual Incidence Rate of Invasive Cervical Cancer Among Women 15–24 Years
| Race/ethnicity and histopathology | Incidence rate (95% CI) | RR (95% CI) | |
|---|---|---|---|
| 2003–2006 | 2011–2014 | ||
| Histology | |||
| All cases | 8.4 (7.8, 9.0) | 6.0 (5.5, 6.5) | 0.71 (0.64, 0.80) |
| SCC | 4.9 (4.5, 5.4) | 3.5 (3.1, 3.9) | 0.71 (0.61, 0.83) |
| Non-SCC | 3.5 (3.0, 3.9) | 2.5 (2.0, 2.8) | 0.71 (0.59, 0.85) |
| Race/Ethnicity | |||
| Hispanic | |||
| All cases | 9.1 (7.6, 10.8) | 5.8 (4.8, 7.1) | 0.64 (0.49, 0.84) |
| SCC | 5.4 (4.3, 6.7) | 3.7 (2.8, 4.7) | 0.68 (0.48, 0.96) |
| Non-SCC | 3.7 (2.8, 4.8) | 2.2 (1.5, 3.0) | 0.59 (0.38, 0.91) |
| Non-Hispanic white | |||
| All cases | 8.2 (7.4, 9.0) | 6.1 (5.5, 6.9) | 0.75 (0.65, 0.87) |
| SCC | 4.6 (4.1, 5.2) | 3.5 (3.0, 4.0) | 0.76 (0.62, 0.92) |
| Non-SCC | 3.6 (3.1, 4.1) | 2.6 (2.2, 3.1) | 0.74 (0.59, 0.93) |
| Non-Hispanic black | |||
| All cases | 8.4 (6.9, 10.2) | 6.5 (5.2, 7.9) | 0.77 (0.57, 1.03) |
| SCC | 5.4 (4.2, 6.9) | 3.8 (2.9, 5.0) | 0.70 (0.48, 1.02) |
| Non-SCC | 3.0 (2.1, 4.1) | 2.7 (1.9, 3.7) | 0.89 (0.55, 1.44) |
Notes: Boldface indicates statistical significance (p<0.05). Data were from U.S. Cancer Statistics (USCS), the combined data from the Centers for Disease Control and Prevention (CDC’s) National Program for Cancer Registries (NPCR) and the National Cancer Institute’s (NCI’s) Surveillance, Epidemiology, and End Results (SEER) Program. USCS Public Use Database (2001–2014 database) covered essentially all young female population (15–34 years old) between 2001 and 2014 in the U.S. (Puerto Rico not included). Rates are per 1,000,000 and age-adjusted to the 2000 U.S. Standard Population standard. CIs are 95% for rates (Tiwari method). Differences in age-adjusted 4-year average annual rates between 2011–2014 and 2003–2006 were evaluated using RR and the corresponding 95% CI.
RR, rate ratio; SCC, squamous cell carcinoma.
Table 2.
Age-Adjusted 4-Year Average Annual Incidence Rate of Invasive Cervical Cancer Among Women Aged 25–34 Years
| Race/ethnicity and histopathology | Incidence rate (95% CI) | RR (95% CI) | |
|---|---|---|---|
| 2003–2006 | 2011–2014 | ||
| Histology | |||
| All cases | 87.8 (85.8, 90) | 76.1 (74.2, 78) | 0.87 (0.84, 0.90) |
| SCC | 58.9 (57.2, 60.6) | 50.0 (48.5, 51.6) | 0.85 (0.81, 0.89) |
| Non-SCC | 28.9 (27.8, 30.2) | 26.0 (24.9, 27.1) | 0.90 (0.85, 0.95) |
| Race/Ethnicity | |||
| Hispanic | |||
| All cases | 94.0 (88.9, 99.3) | 84.1 (79.7, 88.6) | 0.89 (0.83, 0.97) |
| SCC | 68.7 (64.4, 73.2) | 57.7 (54.0, 61.5) | 0.84 (0.77, 0.92) |
| Non-SCC | 25.3 (22.7, 28.1) | 26.4 (23.9, 29.0) | 1.04 (0.90, 1.20) |
| Non-Hispanic white | |||
| All cases | 91.8 (89.0, 94.5) | 77.7 (75.2, 80.2) | 0.85 (0.81, 0.88) |
| SCC | 57.6 (55.5, 59.9) | 48.1 (46.1, 50.1) | 0.83 (0.79, 0.88) |
| Non-SCC | 34.1 (32.5, 35.8) | 29.6 (28.1, 31.2) | 0.87 (0.81, 0.93) |
| Non-Hispanic black | |||
| All cases | 76.1 (70.9, 81.5) | 69.5 (64.8, 74.5) | 0.91 (0.83, 1.01) |
| SCC | 60.4 (55.8, 65.2) | 55.7 (51.4, 60.1) | 0.92 (0.83, 1.03) |
| Non-SCC | 15.7 (13.4, 18.3) | 13.8 (11.8, 16.1) | 0.88 (0.70, 1.10) |
Notes: Boldface indicates statistical significance (p<0.05). Data were from U.S. Cancer Statistics (USCS), the combined data from the Centers for Disease Control and Prevention (CDC’s) National Program for Cancer Registries (NPCR) and the National Cancer Institute’s (NCI’s) Surveillance, Epidemiology, and End Results (SEER) Program. USCS Public Use Database (2001–2014 database) covered essentially all young female population (15–34 years old) between 2001 and 2014 in the U.S. (Puerto Rico not included). Rates are per 1,000,000 and age-adjusted to the 2000 U.S. Standard Population standard. CIs are 95% for rates (Tiwari method). Differences in age-adjusted 4-year average annual rates between 2011–2014 and 2003–2006 were evaluated using RR and the corresponding 95% CI.
RR, rate ratio; SCC, squamous cell carcinoma.
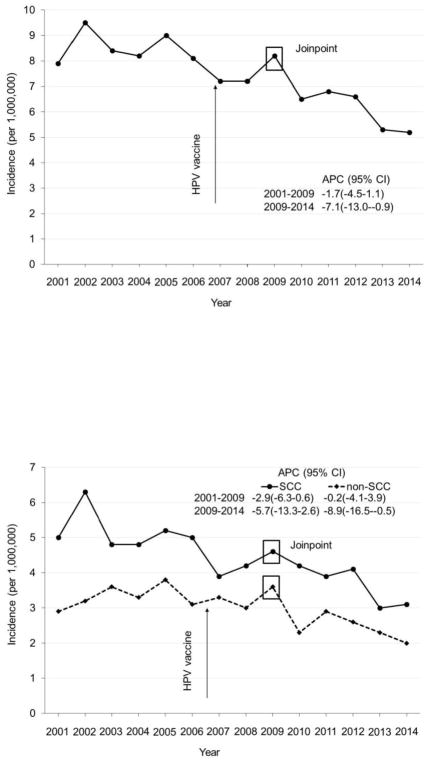
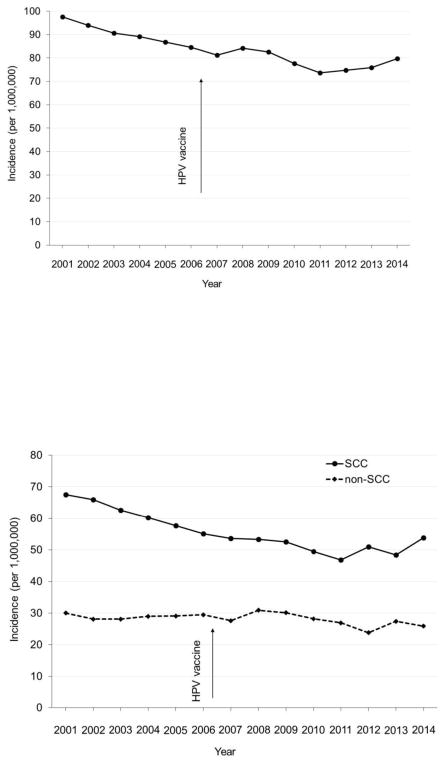
Joinpoint analyses of cervical cancer incidence among young females aged 15–24 years revealed a significant joint at 2009 and a decrease in cervical cancer incidence in 2009 and after (Figure 1A). Cervical cancer incidence among young females aged 15–24 years was stable during 2001–2009 from 7.9 per 1,000,000 in 2001 to 8.2 per 1,000,000 in 2009, but decreased from 8.2 per 1,000,000 in 2009 to 5.2 per 1,000,000 in 2014 (APC= −7.1, 95% CI= −13.0, −0.9). Among young females aged 15–24 years, joinpoint analyses also revealed a significant joint at 2009 and a decrease in cervical cancer incidence in 2009 and after, for both SCC and non-SCC (Figure 1B). Among women aged 25–34 years, joinpoint analyses of cervical cancer incidence revealed no significant joint and there was no significant decrease in cervical cancer incidence after the introduction of HPV vaccination in 2006 (Figure 2).
Figure 1.
Cervical cancer incidence among women aged 15–24 years, USCS 2001–2014. (A) Any histology. (B) SCC and non-SCC.
Notes: Rates are per 1,000,000 and age-adjusted to the 2000 U.S. Standard Population standard. APC, annual percentage change; USCS, U.S. Cancer Statistics; SCC, squamous cell carcinoma.
Figure 2.
Age-adjusted incidence rate of invasive cervical cancer by histology in women (25–34 years), USCS 2001–2014. (A) Any histology. (B) SCC and non-SCC.
Notes: Rates are per 1,000,000 and age-adjusted to the 2000 U.S. Standard Population standard. USCS, U.S. Cancer Statistics; SCC, squamous cell carcinoma.
From 2007 to 2013, HPV vaccine coverage increased significantly from 28.9% to 55.3% among girls aged 13–17 years (p<0.001; Appendix Figure 1). HPV vaccine coverage also increased significantly among U.S. women aged 18–24 or 25–34 years (Appendix Figure 2). Prevalence of high-risk vaccine type HPV decreased by 65.4% from 14.1% in 2005–2006 to 4.9% in 2013–2014 among women aged 18–24 years, and decreased by 39.8% from 8.3% in 2005–2006 to 5.0% in 2013–2014 among women aged 25–34 years (p<0.001 for both trends; Appendix Figure 3). Major changes in cervical cancer screening recommendations occurred in 2002 by American Cancer Society and in 2003 by American College of Obstetricians and Gynecologists regarding the screening interval (extended the length of time) and liquid-based cytology (Appendix Table 2). In 2009, American College of Obstetricians and Gynecologists started to recommend no screening among young females aged <21 years, and a longer screening interval and the combination of cytology plus HPV DNA testing for women aged ≥30 years. In 2012, both the American Cancer Society and U.S. Preventive Services Task Force issued similar recommendations.
DISCUSSION
This study compared cervical cancer incidence among young women before and after the introduction of HPV vaccine using newly released data from the NPCR and SEER Incidence–USCS database. This is the first time combined data from the CDC’s NPCR and NCI’s SEER Program have been made available in this format. The 2001–2014 USCS database includes high-quality population-based cancer incidence data on the entire U.S. population. This study found a significant decrease in average annual incidence rate in 2011–2014 compared with 2003–2006 among both young females aged 15–24 years and women aged 25–34 years for both SCC and non-SCC. A larger decrease in cervical cancer incidence was observed in the vaccine era among young females aged 15–24 years. Joinpoint analyses revealed a significant joint at 2009 for both SCC and non-SCC among young females aged 15–24 years. The HPV vaccine is recommended for females aged 9–26 years for the prevention of HPV infections and HPV-associated cancers.22 In young women aged less than 30 years, the mean time from normal Pap test to diagnosis of invasive cervical cancer can be only a few years.16 Because the HPV vaccine has been available since 2006, this means that it may be possible to see changes in the incidence of cervical cancer that are attributable to the introduction of HPV vaccination. These findings provide supporting evidence for the effectiveness of the HPV vaccine toward its primary goal of preventing cervical cancer.
The efficacy of HPV vaccines against vaccine-type HPV infections and high-grade cervical lesions has been demonstrated in clinical trials.2–4 The population-level impact following HPV vaccination programs has also been systematically reviewed.23 Previous comparisons of HPV vaccine types (HPV 6, 11, 16, and 18) in the pre- and post-vaccine periods and in vaccinated and unvaccinated women have revealed a decrease in HPV prevalence among younger females.8,11,24–28 For example, a recent analysis of the National Health and Nutrition Examination Survey data reported a vaccine effectiveness of 83%, and demonstrated that from the pre-vaccine era (2003–2006) to the vaccine era (2011–2014), vaccine-type prevalence decreased by 89% and 34% among vaccinated and unvaccinated females aged 14–24 years, respectively.26 The impact of HPV vaccination on high-grade cervical cancer lesions (cervical intraepithelial neoplasia grades 2 and 3 and adenocarcinoma in situ) has also been previously examined.7,29 A recent study investigated trends in high-grade cervical intraepithelial lesions among females who were likely to be impacted by the HPV vaccination between 2007 and 2014.10 It showed that high-grade lesions significantly declined annually by 8.3% among females aged 15–19 years and by 5.3% among women aged 20–24 years. The reduction in cervical cancer incidence among young women found in this study is likely due to the increased uptake of HPV vaccine and decreased prevalence of HPV 16 and 18. In this study, a more profound decrease in the prevalence of HPV 16 and 18 in young women aged 18–24 years compared with that in women aged 25–34 years can also partly explain the observed significant decrease in cervical incidence in females aged 15–24 years rather than in women aged 25–34 years. A lag time of 3 to 4 years from the introduction of HPV vaccine and the decrease in cervical cancer incidence among females aged 15–24 years also suggests plausible effects of HPV vaccination on cervical cancer incidence via reducing infection of high-risk vaccine type HPV (16 and 18) and high-grade cervical neoplasia. These findings are also consistent with the observed declines in cervical cancer precursors in countries with high HPV vaccine coverage, such as Australia and Denmark.9,30,31 A study on age groups with high HPV vaccine coverage in Denmark found a significant decrease in the incidence of cervical lesions,32 whereas another study showed that vaccinated young women aged 20–21 years had a greater reduction in diagnoses of cervical intraepithelial neoplasia grades 1, 2, and 3 than their unvaccinated peers in Scotland.31
Trends in cervical cancer incidence and mortality reduction in the U.S. are widely credited to regular Pap testing for precancerous lesions and subsequent follow-up for treatment.33,34 Another possible contributing factor to the reduction of cervical cancer incidence may be changes in high-risk sexual behaviors which results in reduction in HPV infection, the causal agent of cervical cancer. However, the trends in sexually transmitted diseases in the U.S. as reported by the CDC do not support the notion of a significant reduction in high-risk sexual behaviors among young adults.35 The observed decreasing trend in HPV prevalence among young females in the U.S. are most likely due to the introduction of HPV vaccination in 2006.8,11,24 National guidelines currently recommend that women undergo Pap testing at 3-year intervals starting at age 21 years and co-testing with HPV DNA testing starting at age 30 years, regardless of HPV vaccination status.12–14 Screening recommendations have changed several times during the study period: the screening interval is increased and screening is not recommended for young females aged less than 21 years.13,14,36,37 However, the increased screening interval and postponed screening start age will reduce the chance of detection of precancerous lesions among young females and subsequent preventive procedures, which may slightly increase the incidence rate of invasive cervical cancer in the long run.13,38 Liquid-based cytology has been recommended by the American Cancer Society since 2002 and by the American College of Obstetricians and Gynecologists since 2003, but the U.S. Preventive Services Task Force waited until 2012 to recommend it for cervical screening and concluded that liquid-based cytology had equivalent sensitivity and specificity to conventional cytology.37 Pap testing does not reduce the risk of adenocarcinoma as much as of SCC.39 Furthermore, Pap testing seems to have no beneficial effect on the incidence of adenocarcinoma in the U.S., and may even increase the detection of the incidence of early stage invasive adenocarcinoma.40 In young women aged less than 30 years, the mean time can be as short as 15 months from normal Pap to diagnosis of invasive adenocarcinoma, compared with 38 months from normal Pap to diagnosis of invasive SCC.16 HPV 18 infection is strongly associated with cervical adenocarcinoma rather than SCC.41 A reduction in vaccine-type HPV infections8,24,25 may have an early effect on the incidence of invasive adenocarcinoma, as observed in this study that the incidence of non-SCC was markedly decreased in the vaccine era compared with prevaccine era among young females aged 15–24 years. Therefore, the temporal association of HPV vaccine introduction with reduction in cervical cancer incidence in the vaccine era and reduction in the incidence of cervical adenocarcinoma, especially among young females aged 15–24 years, may at least be partly because of the effect of HPV vaccine introduction. These findings are very promising, considering that the coverage of HPV vaccination among young females in the U.S. is suboptimal.8 These results support the conclusion that HPV vaccination is effective in reducing cervical cancer incidence. With the improving coverage of HPV vaccine in adolescents in recent years,8 the decrease in cervical cancer incidence is expected to be more profound among U.S. women in the near future.
Limitations
One strength of this study is the use of large amount of data from the NPCR and SEER Incidence–USCS database. Data from USCS have high-quality information on sociodemographic characteristics, cervical cancer diagnosis date and histology, and patient age, which allow for examination of the effect of HPV vaccination in various subpopulations of the entire population of young U.S. females. However, this study also had a few limitations. Causal inference cannot be concluded from the findings because this was an ecologic study. Cervical cancer screening may also contribute to this observed decrease in cervical cancer incidence after HPV introduction. Additionally, HPV vaccination status and HPV DNA typing are not available for the participants in USCS.
CONCLUSIONS
This study found a significant decrease in cervical cancer incidence among young females after HPV vaccine introduction. The observed strong decrease in cervical cancer incidence among young females aged 15–24 years is unlikely to entirely result from changes in cervical cancer screening, suggesting HPV vaccination is at least partially responsible for the reduction in cancer incidence. These findings serve as further evidence of the effectiveness of HPV vaccination in preventing cervical cancer. This is important, because despite previous evidence of HPV vaccine effectiveness against HPV infections, genital warts, and cervical lesions, it is essential to know its effect on the target outcome, cervical cancer. Further research is needed to confirm these findings, which may include direct comparison of cervical cancer incidence between vaccinated and unvaccinated women in the U.S. and other countries, trends in cervical cancer incidence among young women in other countries with high HPV vaccine coverage, and HPV types in vaccinated and unvaccinated cervical cancer patients.
Supplementary Material
Acknowledgments
Dr. Guo is currently supported by a research career development award (K12HD052023: Building Interdisciplinary Research Careers in Women’s Health Program; Berenson, PI) from the Office of Research on Women’s Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development at NIH. Dr. Cofie is currently a postdoctoral fellow supported by an institutional training grant (National Research Service Award T32HD055163, Berenson, PI) from the National Institute of Child Health and Human Development at NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript, and decision to submit the manuscript for publication.
A portion of the results was presented in a podium presentation at the 41st Annual American Society of Preventive Oncology Conference. Seattle, WA. March 11–14, 2017.
No financial disclosures were reported by the authors of this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 2.Harper DM, Franco EL, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364(9447):1757–1765. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 3.FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 4.Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–723. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 5.Petrosky E, Bocchini JA, Jr, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64(11):300–304. [PMC free article] [PubMed] [Google Scholar]
- 6.Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years – United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(33):850–858. doi: 10.15585/mmwr.mm6533a4. [DOI] [PubMed] [Google Scholar]
- 7.Hariri S, Johnson ML, Bennett NM, et al. Population-based trends in high-grade cervical lesions in the early human papillomavirus vaccine era in the United States. Cancer. 2015;121(16):2775–2781. doi: 10.1002/cncr.29266. [DOI] [PubMed] [Google Scholar]
- 8.Markowitz LE, Hariri S, Lin C, et al. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J Infect Dis. 2013;208(3):385–393. doi: 10.1093/infdis/jit192. [DOI] [PubMed] [Google Scholar]
- 9.Brotherton JM, Fridman M, May CL, Chappell G, Saville AM, Gertig DM. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet. 2011;377(9783):2085–2092. doi: 10.1016/S0140-6736(11)60551-5. [DOI] [PubMed] [Google Scholar]
- 10.Flagg EW, Torrone EA, Weinstock H. Ecological association of human papillomavirus vaccination with cervical dysplasia prevalence in the United States, 2007–2014. Am J Public Health. 2016;106(12):2211–2218. doi: 10.2105/AJPH.2016.303472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berenson AB, Laz TH, Rahman M. Reduction in vaccine-type human papillomavirus prevalence among women in the United States, 2009–2012. J Infect Dis. 2016;214(12):1961–1964. doi: 10.1093/infdis/jiw515. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Preventive Services Task Force. [Accessed March 8, 2018];The Guide to Clinical Preventive Services. 2014 www.ahrq.gov/sites/default/files/wysiwyg/professionals/clinicians-providers/guidelines-recommendations/guide/cpsguide.pdf.
- 13.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin Number 131: Screening for cervical cancer. Obstet Gynecol. 2012;120(5):1222–1238. doi: 10.1097/aog.0b013e318277c92a. [DOI] [PubMed] [Google Scholar]
- 15. [Accessed October 15, 2017];NPCR and SEER Incidence – USCS Public Use Databases. www.cdc.gov/cancer/npcr/public-use/index.htm.
- 16.Nitschmann C, May T, Mirkovic J, Feldman S. Screening history among women with invasive cervical cancer in an academic medical center: will we miss cancers following updated guidelines? J Womens Health (Larchmt) 2016;25(8):826–831. doi: 10.1089/jwh.2015.5394. [DOI] [PubMed] [Google Scholar]
- 17.NAACCR Race and Ethnicity Work Group. NAACCR Guideline for Enhancing Hispanic/Latino Identification: Revised NAACCR Hispanic/Latino Identification Algorithm [NHIA v2.2.1] Springfield, IL: North American Association of Central Cancer Registries; Sep, 2011. [Google Scholar]
- 18.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15(6):547–569. doi: 10.1177/0962280206070621. [DOI] [PubMed] [Google Scholar]
- 19.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. doi: 10.1002/(SICI)1097-0258(20000215)19:3<335::AID-SIM336>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 20.Joinpoint Regression Program, Version 4.5.0.1 - June 2017; Statistical Methodology and Applications Branch, Surveillance Research Program. National Cancer Institute; [Google Scholar]
- 21. [Accessed January 12, 2018];Public-Use Data Files and Documentation. www.cdc.gov/nchs/data_access/ftp_data.htm.
- 22.Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination - updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65(49):1405–1408. doi: 10.15585/mmwr.mm6549a5. [DOI] [PubMed] [Google Scholar]
- 23.Drolet M, Bénard É, Boily MC, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2015;15(5):565–580. doi: 10.1016/S1473-3099(14)71073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markowitz LE, Liu G, Hariri S, Steinau M, Dunne EF, Unger ER. Prevalence of HPV after introduction of the vaccination program in the United States. Pediatrics. 2016;137(3):1–9. doi: 10.1542/peds.2015-1968. [DOI] [PubMed] [Google Scholar]
- 25.Guo F, Hirth JM, Berenson AB. Comparison of HPV prevalence between HPV-vaccinated and non-vaccinated young adult women (20–26 years) Hum Vaccin Immunother. 2015;11(10):2337–2344. doi: 10.1080/21645515.2015.1066948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliver SE, Unger ER, Lewis R, et al. Prevalence of human papillomavirus among females after vaccine introduction-National Health and Nutrition Examination Survey, United States, 2003–2014. J Infect Dis. 2017;216(5):594–603. doi: 10.1093/infdis/jix244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirth JM, Chang M, Resto VA, Group HS. Prevalence of oral human papillomavirus by vaccination status among young adults (18–30 years old) Vaccine. 2017;35(27):3446–3451. doi: 10.1016/j.vaccine.2017.05.025. [DOI] [PubMed] [Google Scholar]
- 28.Berenson AB, Hirth JM, Chang M. Change in human papillomavirus prevalence among U.S. women aged 18–59 years, 2009–2014. Obstet Gynecol. 2017;130(4):693–701. doi: 10.1097/AOG.0000000000002193. [DOI] [PubMed] [Google Scholar]
- 29.Hariri S, Bennett NM, Niccolai LM, et al. Reduction in HPV 16/18-associated high grade cervical lesions following HPV vaccine introduction in the United States – 2008–2012. Vaccine. 2015;33(13):1608–1613. doi: 10.1016/j.vaccine.2015.01.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahmud SM, Kliewer EV, Lambert P, Bozat-Emre S, Demers AA. Effectiveness of the quadrivalent human papillomavirus vaccine against cervical dysplasia in Manitoba, Canada. J Clin Oncol. 2014;32(5):438–443. doi: 10.1200/JCO.2013.52.4645. [DOI] [PubMed] [Google Scholar]
- 31.Pollock KG, Kavanagh K, Potts A, et al. Reduction of low- and high-grade cervical abnormalities associated with high uptake of the HPV bivalent vaccine in Scotland. Br J Cancer. 2014;111(9):1824–1830. doi: 10.1038/bjc.2014.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baldur-Felskov B, Dehlendorff C, Junge J, Munk C, Kjaer SK. Incidence of cervical lesions in Danish women before and after implementation of a national HPV vaccination program. Cancer Causes Control. 2014;25(7):915–922. doi: 10.1007/s10552-014-0392-4. [DOI] [PubMed] [Google Scholar]
- 33.Benard VB, Thomas CC, King J, Massetti GM, Doria-Rose VP, Saraiya M. Vital signs: cervical cancer incidence, mortality, and screening – United States, 2007–2012. MMWR Morb Mortal Wkly Rep. 2014;63(44):1004–1009. [PMC free article] [PubMed] [Google Scholar]
- 34.Beachler DC, Tota JE, Silver MI, et al. Trends in cervical cancer incidence in younger U.S. women from 2000 to 2013. Gynecol Oncol. 2017;144(2):391–395. doi: 10.1016/j.ygyno.2016.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.CDC. Sexually Transmitted Disease Surveillance 2016. Atlanta, GA: U.S. DHHS; 2017. [Google Scholar]
- 36.ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin no. 109: Cervical cytology screening. Obstet Gynecol. 2009;114(6):1409–1420. doi: 10.1097/AOG.0b013e3181c6f8a4. [DOI] [PubMed] [Google Scholar]
- 37.Whitlock EP, Vesco KK, Eder M, Lin JS, Senger CA, Burda BU. Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155(10):687–697. doi: 10.7326/0003-4819-155-10-201111150-00376. [DOI] [PubMed] [Google Scholar]
- 38.Saslow D, Runowicz CD, Solomon D, et al. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002;52(6):342–362. doi: 10.3322/canjclin.52.6.342. [DOI] [PubMed] [Google Scholar]
- 39.Castanon A, Landy R, Sasieni PD. Is cervical screening preventing adenocarcinoma and adenosquamous carcinoma of the cervix? Int J Cancer. 2016;139(5):1040–1045. doi: 10.1002/ijc.30152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang SS, Sherman ME, Hildesheim A, Lacey JV, Devesa S. Cervical adenocarcinoma and squamous cell carcinoma incidence trends among white women and black women in the United States for 1976–2000. Cancer. 2004;100(5):1035–1044. doi: 10.1002/cncr.20064. [DOI] [PubMed] [Google Scholar]
- 41.Castellsagué X, Díaz M, de Sanjosé S, et al. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: implications for screening and prevention. J Natl Cancer Inst. 2006;98(5):303–315. doi: 10.1093/jnci/djj067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.