Abstract
Currently available pharmacotherapies for treating alcohol use disorder (AUD) suffer from deleterious side effects and are not efficacious in diverse populations. Clinical and preclinical studies provide evidence that the Kcnq family of genes that encode KV7 channels influence alcohol intake and dependence. KV7 channels are a class of slowly activating voltage-dependent K+ channels that regulate neuronal excitability. Studies indicate that the KV7 channel positive modulator retigabine can decrease dopaminergic neuron firing, alter dopamine (DA) release, and reduce alcohol intake in heavy drinking rodents. Given the critical nature of ventral tegmental area (VTA) DA to the addiction process and predominant expression of Kcnq4 in DA neurons, we investigated the role of midbrain Kcnq genes and KV7 channels in the VTA of genetically diverse mice and long-term heavy drinking rats, respectively. Integrative bioinformatics analysis identified negative correlations between midbrain Kcnq4 expression and alcohol intake and seeking behaviors. Kcnq4 expression levels were also correlated with dopaminergic-related phenotypes in BXD strains, and Kcnq4 was present in support intervals for alcohol sensitivity and alcohol withdrawal severity QTLs in rodents. Pharmacological validation studies revealed that VTA KV7 channels regulate excessive alcohol intake in rats with a high-drinking phenotype. Administration of a novel and selective KV7.2/4 channel positive modulator also reduced alcohol drinking in rats. Together, these findings indicate that midbrain Kcnq4 expression regulates alcohol-related behaviors in genetically diverse mice and provide evidence that KV7.4 channels are a critical mediator of excessive alcohol drinking.
Keywords: alcohol drinking, Kcnq4, Kv7 channels, retigabine, ML213
1. Introduction
Current pharmacotherapies for the treatment of alcohol use disorder (AUD) suffer from deleterious side effects and prevent relapse in only a small subset of individuals. Anticonvulsants are a drug class that show promise for treating heavy alcohol (ethanol) drinking and severe alcohol withdrawal associated with AUD (Padula et al., 2013). Emerging evidence indicates that the anticonvulsant retigabine can reduce voluntary alcohol consumption in rodents, especially those with a high-drinking phenotype (McGuier et al., 2016; Rinker et al., 2017). Retigabine is unique among the anticonvulsant class in that it increases the open probability of KV7 channels, which are encoded by the KCNQ family of genes and are responsible for generating the M-current in neurons (Blackburn-Munro et al., 2005). In Drosophila, loss of KV7 channel function increased tolerance and sensitivity to alcohol’s sedative effects (Cavaliere et al., 2012). In addition, genetic variance in KCNQ is associated with alcohol intake in rodents and alcohol dependence in individuals with AUD (Edenberg et al., 2010; Kendler et al., 2011; McGuier et al., 2016; Metten et al., 2014; Rinker et al., 2017). Acute alcohol exposure has been shown to reduce M-current in dopamine (DA) neurons in the ventral tegmental area (VTA) (Koyama et al., 2007), and chronic alcohol exposure alters surface trafficking and reduces function of KV7 channels in the nucleus accumbens (NAc) and lateral habenula, respectively (Kang et al., 2017; McGuier et al., 2016). Thus, this converging evidence supports variations in KV7 channels as a mechanism promoting heavy alcohol drinking and alcohol-induced functional and behavioral adaptations.
Dopamine release from the VTA to the NAc is a central aspect of the addiction process (Koob and Le Moal, 2005). Although activation of KV7 channels can attenuate DA synthesis and efflux in the striatum and reduce VTA dopaminergic neurotransmission (Hansen et al., 2006; Jensen et al., 2011; Martire et al., 2007; Sotty et al., 2009), the role that VTA Kv7 channels play in regulating drinking behaviors is unknown. We previously reported that Kcnq2 and Kcnq3 lie within multiple alcohol-related QTLs in rodents (McGuier et al., 2016), and Kcnq transcript levels in the NAc and prefrontal cortex are negatively correlated with voluntary alcohol drinking in BXD recombinant inbred (RI) strains of mice (Rinker et al., 2017). Whereas Kcnq2/3 expression is relatively low in DA neurons, Kcnq4 expression is predominant in DA neurons in the VTA and substantia nigra pars compacta (SNc) (Hansen et al., 2007; Kharkovets et al., 2000). Thus, it is possible that midbrain Kcnq4 is a critical mediator of alcohol-related behaviors and heavy alcohol drinking.
Here, we performed an integrative functional genomic analysis on midbrain Kcnq4 expression and alcohol- and DA-related phenotypes in BXD RI strains. We also used pharmacological approaches targeting KV7 channels in the VTA using a rat intermittent alcohol access (IAA) model of drinking to validate our genetic findings. Despite its efficacy as adjunct therapy to treat partial onset seizures, production of retigabine was discontinued because of retina and skin pigment changes caused by accumulation of low solubility retigabine dimers after prolonged use (Clark et al., 2015). Retigabine dimers likely form because resonance structures of retigabine are Brandowski’s bases that are self-reactive (Dousa et al., 2014). Because KV7 channels remain a promising target for treating AUD, we explored a novel and selective analog of retigabine (i.e., ML213) with a simplified chemical scaffold that lacks a critical amine group promoting formation of insoluble dimers. Unlike the broad actions and modest potency of retigabine on KV7.2–7.5 channels, ML213 is an opener of KV7 channels with selectivity for KV7.2 (EC50 = 230 nM) and KV7.4 (EC50 = 510 nM) over other KV7 channel subtypes and a panel of 68 other ion channels, transporters, and G-protein coupled receptors (Yu et al., 2010). Results from our studies provide evidence that midbrain Kcnq4 regulates alcohol drinking in genetically diverse mice and pharmacologically targeting these channels reduces excessive alcohol consumption in rats.
2. Materials and methods
2.1. Bioinformatics
To provide evidence for genetic links between Kcnq4 and alcohol-related behaviors, we performed two integrative bioinformatics analyses using open source databases (www.geneweaver.org and www.genenetwork.org) following our previously reported methods (McGuier et al., 2016; Padula et al., 2015; Rinker et al., 2017). First, we identified QTLs related to alcohol that contain Kcnq4. Next, we correlated Kcnq4 midbrain robust multi-array average (RMA) expression levels in alcohol-naïve male BXD RI strains of mice (N = 37 strains and 129 mice) with alcohol-related phenotypes and phenotypes related to monoaminergic signaling in male and female BXD RI strains. When available, PubMed identification numbers for the original manuscripts describing the phenotypes are reported in Tables 1 and 2.
Table 1.
Correlations of midbrain Kcnq4 expression levels from male and female alcohol (EtOH)-naïve BXD recombinant inbred strains of mice with alcohol-related phenotypes. CPP, conditioned-place preference.
| GeneNetwork Record ID | Phenotype | Sex | r | P value | Strain pairs | PubMed ID |
|---|---|---|---|---|---|---|
| 10074 | EtOH (10% v/v) intake | M | −0.5458 | 0.0435 | 14 | 6683363 |
| 12964 | EtOH (15% v/v) intake | M, F | −0.5699 | 0.0420 | 13 | 7978106 |
| 12574 | EtOH (15% v/v) intake | M, F | −0.5148 | 0.0413 | 16 | 27793543 |
| 10090 | CPP - % time in EtOH-paired compartment | M | −0.6766 | 0.0020 | 18 | 7480533 |
| 10097 | CPP - s/min in EtOH-paired compartment | M | −0.621 | 0.0059 | 18 | 7480533 |
| 10081 | EtOH withdrawal severity | M | 0.907 | 0.0048 | 7 | 6683363 |
| 10478 | EtOH withdrawal severity | F | −0.5748 | 0.0198 | 16 | 6683363 |
| 11975 | EtOH-induced ataxia (2.25 g/kg) | M, F | −0.4693 | 0.0059 | 33 | 19958391 |
| 11440 | Locomotor activity after EtOH (2.25 g/kg) | M | −0.4084 | 0.0203 | 32 | 19958391 |
| 11697 | Locomotor activity after EtOH (2.25 g/kg) | F | −0.3994 | 0.0213 | 33 | 19958391 |
| 12391 | Anxiety assay after EtOH (1.8 g/kg) | M, F | −0.3689 | 0.0347 | 33 | |
| 10087 | EtOH-induced hypothermia (2 g/kg) | F | 0.4702 | 0.0364 | 20 | 8627539 |
| 10086 | EtOH-induced hypothermia (3 g/kg) | F | 0.5392 | 0.0142 | 20 | 8627539 |
| 10085 | EtOH-induced hypothermia (4 g/kg) | F | 0.4477 | 0.0478 | 20 | 8627539 |
Table 2.
Correlations of midbrain Kcnq4 expression levels from alcohol-naïve BXD recombinant inbred strains of mice with components of the monoaminergic system. 5-HIAA, 5-Hydroxyindoleacetic acid; DAT1, dopamine transporter; DOPAC, 3,4-Dihydroxyphenylacetic acid; DRD2, dopamine receptor D2; DRD3, dopamine receptor D3; HVA, Homovanillic acid; NE, norepinephrine; TH, tyrosine hydroxylase; PFC, prefrontal cortex, NAc, nucleus accumbens.
| GeneNetwork Record ID | Phenotype | Sex | r | P value | Strain pairs | PubMed ID |
|---|---|---|---|---|---|---|
| 12800 | 5-HIAA level in the medial septal nucleus | M, F | 0.7349 | 0.0378 | 8 | |
| 10234 | DAT1 (SLC6A3) binding maximum in PFC | M | −0.5351 | 0.0182 | 19 | 11454925 |
| 10280 | DAT1 (SLC6A3) binding maximum in PFC | F | 0.5719 | 0.0259 | 15 | 10591541 |
| 10281 | DAT1 (SLC6A3) binding maximum in PFC | M, F | 0.5563 | 0.0252 | 16 | 10591541 |
| 12798 | DOPAC level in the medial septal nucleus in EtOH-dependent mice | M, F | 0.8304 | 0.0107 | 8 | |
| 15146 | DOPAC/DA ratio in striatum | M | −0.8495 | 0.0323 | 6 | 23558233 |
| 10262 | DRD2/DRD3 binding maximum in NAc | F | 0.5362 | 0.0394 | 15 | 10591541 |
| 12801 | HVA level in the medial septal nucleus | M, F | 0.8149 | 0.0137 | 8 | |
| 15161 | TH protein level in striatum | M | 0.9372 | 0.0058 | 6 |
2.2. Animals and housing
Male Wistar rats were purchased from Harlan (Indianapolis, IN) and housed individually in standard home cages in temperature and humidity controlled environments with a 12 h light/dark cycle. Food and water were available ad libitum throughout the study. All procedures were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee and were in accordance with NIH guidelines for the humane care and use of laboratory animals (2011).
2.3. Two-bottle choice drinking
Intermittent access to alcohol (IAA; 20% v/v) or saccharin (0.02% w/v) was performed as previously described (McGuier et al., 2016). After reaching baseline consumption, rats were habituated to systemic injections through administration of saline (3 mL/kg, IP). Rats had access to alcohol and saccharin for 7–8 and 4 weeks, respectively, prior to drug administration.
2.4. Intracranial cannula surgery
After six weeks of alcohol consumption in the IAA paradigm, guide cannula were surgically implanted in the VTA of Wistar rats following our standard procedures (McGuier et al., 2016). Bilateral microinjection guide cannula (23 ga O.D.; PlasticsOne Roanoke, VA) were implanted 2 mm dorsal to the VTA following previously established coordinates (relative to bregma, AP: −5.6 mm, ML: ± 2.1 mm, DV: −7.1, 10°) (Mahler et al., 2013). Rats were allowed 1 week to recover before given alcohol access.
2.5. Drug treatments
In four separate cohorts, rats received vehicle (10% Tween 80 v/v saline) or ML213 (1 or 5 mg/kg in two cohorts and 10 or 20 mg/kg in the other two cohorts; Cayman Chemical, Ann Arbor, MI) 30 min prior to alcohol or saccharin access. Due to its poor solubility (Yu et al., 2010), ML213 was administered systemically rather than into the VTA. Additional cohorts of rats received microinjections beginning 30 min prior to alcohol availability. Injectors were inserted bilaterally to a depth of 2 mm beyond the tip of the guide cannula. Retigabine (5 – 10 ng in 0.01% DMSO v/v aCSF; Axon MedChem, The Netherlands), XE991 (0.225 ng in 0.01% DMSO v/v aCSF; Tocris, Minneapolis, MN) or vehicle (0.01% DMSO v/v aCSF) was delivered in a final volume of 0.5 μl/side at a rate of 0.5 μl/min. Systemic injections of vehicle (10% Tween 80 v/v saline) or 7.5 mg/kg retigabine immediately followed microinfusions. At the completion of the study, brains were Nissl stained to confirm cannula placement.
2.6. Locomotor activity
Alcohol-naïve and long-term drinking rats were habituated to a 57 cm × 58 cm × 63 cm opaque acrylic box for 1 h/day for 2 consecutive days starting two weeks after completion of the drinking paradigms. Over the next three consecutive days, rats were treated with vehicle (10% Tween80 v/v saline) or ML213 (1 – 20 mg/kg, IP) in a Latin square design. Activity was digitally recorded with an overhead video camera under red light illumination. Total movement was automatically scored using EthoVision XT software (Noldus Information Technology, Wageningen, The Netherlands).
2.7. Statistical analysis
Pearson correlations between midbrain Kcnq4 levels and alcohol- and monoamine-related phenotypes were first calculated in GeneNetwork.org, and the data were exported to Excel. Outliers in Kcnq4 RMA levels or phenotype data were determined by the Grubbs’ test with an alpha of 0.05 and were excluded from subsequent correlation analyses in GraphPad Prism software (version 7.04; GraphPad Software, Inc., La Jolla, CA). To increase power and reduce variance associated with individual differences, a within-subjects design was used for all rat behavioral experiments. A mixed data procedure (PROC MIXED) was used in the statistical software language SAS to analyze all drinking and behavioral data using repeated measures factors following our previous methods (McGuier et al., 2016). Rats were separated into low and high alcohol drinkers using a median split. Responsivity to intra-VTA retigabine and systemic ML213 administration was assessed by calculating the difference between alcohol intake under vehicle and drug conditions using hierarchical linear modeling in SAS (Singer, 1998). In this linear mixed-effects analysis, change in drinking after drug was modeled with fixed effects for baseline drinking, time, and dose, including random effects for dose and rat. All data are reported as mean ± SEM and statistical significance was established with p < 0.05.
3. Results
3.1. Kcnq4, alcohol-related behaviors, and dopamine
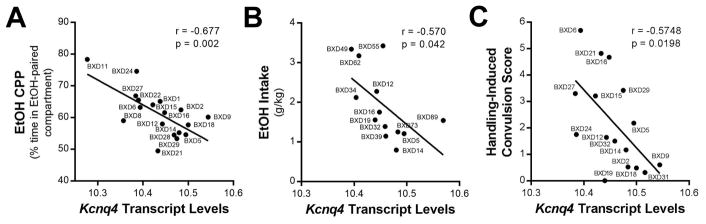
An integrative functional genomic analysis identified Kcnq4 in support intervals for alcohol sensitivity and alcohol withdrawal severity QTLs in rodents (GeneWeaver.org gene set ID GS223356 and GS84162; (Bergeson et al., 2003; Radcliffe et al., 2006)). In male and female alcohol-naïve BXD RI strains, midbrain Kcnq4 transcript levels (but not Kcnq2) significantly correlated with alcohol conditioned place preference and alcohol intake (Table 1; Fig. 1A,B). Moreover, Kcnq4 significantly correlated with the severity of handling-induced convulsions during alcohol withdrawal (Fig. 1C) and other behavioral and physiological measures (e.g., locomotor activity, hypothermia) associated with acute alcohol exposure in BXD RI strains (Table 1). However, not all alcohol-related phenotypes (e.g., alcohol metabolism) correlated with Kcnq4 midbrain expression levels and no other K+ channel gene significantly correlated with the majority of phenotypes related to alcohol-seeking and drinking behaviors. The results from the bioinformatics analysis also indicated that midbrain Kcnq4 levels significantly correlated with NAc expression of D2R/D3R and other components of the monoaminergic system, including positive correlations with 5-HIAA, DOPAC, HVA, and TH protein levels in projection fields of midbrain DA neurons (Table 2).
Fig. 1.
Midbrain Kcnq4 transcript expression levels correlate with alcohol (EtOH) drinking and seeking behaviors in BXD recombinant inbred strains of mice. Negative correlations between Kcnq4 transcript levels in BXD recombinant inbred strains of mice and (A) alcohol conditioned place preference (CPP; N = 18 strain pairs), (B) alcohol drinking (N = 13 strain pairs), and (C) handling-induced convulsions in alcohol-dependent mice (N = 16 strain pairs). GeneNetwork.org data sets: ‘VU BXD Midbrain Agilent SurePrint G3 Mouse GE (May12)’ for Kcnq4 (probe sets A_55_P2086198 and A_55_P2086193) and alcohol-related phenotypic data from male and female BXD RI strains.
3.2. Systemic ML213 administration and alcohol drinking
3.2.1. 1 and 5 mg/kg doses of ML213
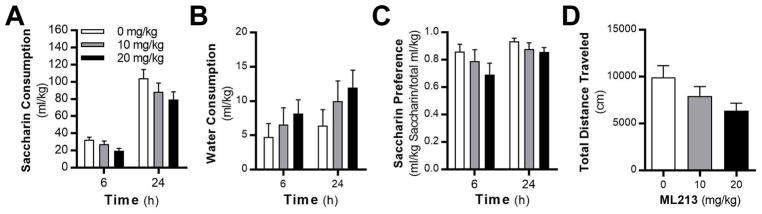
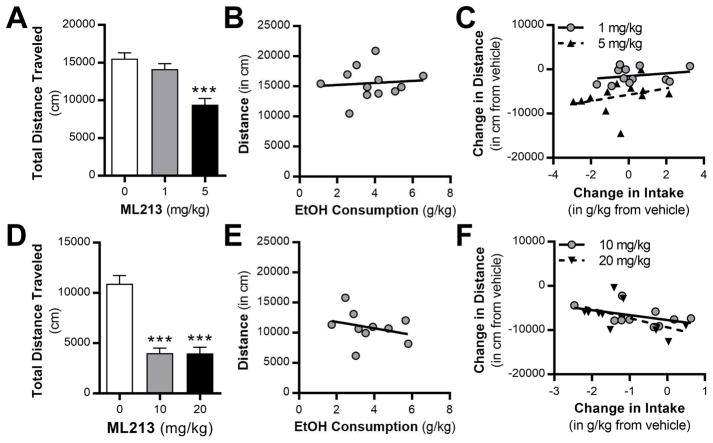
After Wistar rats established baseline drinking the IAA model (Supplemental Fig. 1A), rats were treated with vehicle and ML213 (1 or 5 mg/kg, ip) in weeks 9 through 11. There was a main effect of dose on alcohol intake, and post-hoc analysis revealed that 5 mg/kg ML-213 significantly reduced alcohol drinking compared rats when treated with vehicle (Supplemental Fig. 1B). Administration of ML213 did not affect water (Supplemental Fig. 1C) or total fluid (Supplemental Fig. 1D) intake. ML213 was then tested in alcohol-naïve rats against home cage saccharin drinking and locomotor activity in an open field. Analysis revealed that 1 and 5 mg/kg ML213 did not affect saccharin intake (Supplemental Fig. 2A), water intake (Supplemental Fig. 2B), saccharin preference (Supplemental Fig. 2C), or total locomotor activity (Supplemental Fig. 2D).
3.2.2. 10 and 20 mg/kg doses of ML213
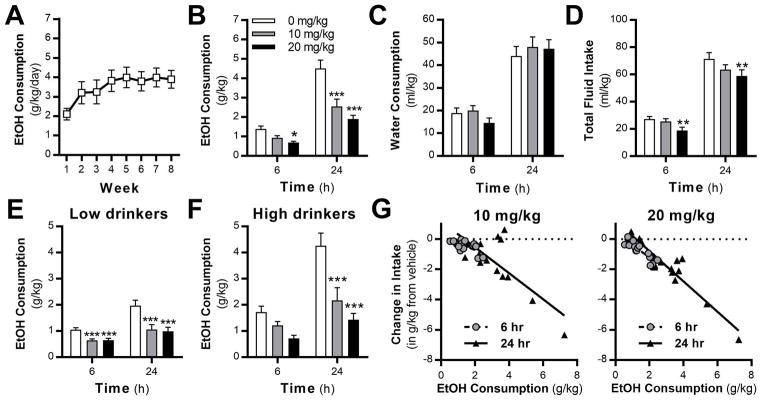
Because of the modest effects of 5 mg/kg ML213 on alcohol intake and the poor brain penetration (Yu et al., 2010), higher doses of ML213 were tested in a separate cohort of alcohol drinking rats (Fig. 2A). A significant treatment by time interaction was observed in alcohol-drinking rats (Fig. 2B). Post-hoc analysis revealed that 20 mg/kg significantly reduced drinking at 6 h, whereas both doses of ML213 reduced drinking at 24 h. While ML213 did not affect water intake at either dose (Fig. 2C), total fluid intake was reduced by the 20 mg/kg dose (Fig. 2D). Our previous results showed that retigabine is more effective at reducing consumption in high- (HD), but not low-drinking (LD) rodents (McGuier et al., 2016; Rinker et al., 2017). When analyzed by drinking phenotype, ML213 attenuated alcohol consumption in low- (Fig. 2E) and high-drinking (Fig. 2F) rats. Post-hoc analyses revealed that both doses of ML213 significantly reduced drinking in the LD rats across time, whereas both doses of ML213 significantly reduced drinking in the HD rats at the 24 h time point. A mixed model analysis of the drinking data showed a strong negative linear relationship between intake after vehicle and the change in drinking produced by ML213 treatment (Fig. 2G). In a separate cohort of alcohol-naïve rats, analysis showed that 10 and 20 mg/kg ML213 did not alter consumption of saccharin (Fig. 3A), water intake (Fig. 3B), or saccharin preference (Fig. 3C). In addition, total locomotor activity was not significantly altered by ML213 treatment in alcohol-naïve rats (Fig. 3D). While there is a trend for a reduction in locomotor activity by ML213, the high dose increased activity in the open field apparatus in some of the alcohol-naive rats.
Fig. 2.
Systemic administration of ML213 reduced alcohol drinking in rats. (A) Weekly average alcohol intake levels across 8 weeks of intermittent access to alcohol (N = 16). Systemic administration of ML213 significantly reduces (B) alcohol drinking (interaction: F (2,30) = 5.12; P = 0.0112; post hoc, 6 h: *P = 0.021, 10 mg/kg vs vehicle; 24 h: ***P < 0.0001, 10 and 20 mk/kg vs vehicle), but not (C) water consumption (F (2,30) = .34; P = 0.7147). (D) 20 mg/kg ML213 significantly reduces total fluid intake (main effect of dose: F (2,30) = 5.46, P = 0.0095; post-hoc, **P = 0.0025, 20 mg/kg vs vehicle). (E,F) Systemic ML213 treatment reduces alcohol intake in rats with low- (main effect of drug: F (2,14) = 15.8; P = 0.0003; post hoc, ***P < 0.0004, 10 and 20 mg/kg vs vehicle) and high- (interaction: F (2,14) = 4.24; P = 0.0362; post hoc, ***P < 0.0004, 10 and 20 mg/kg vs vehicle at 24 h) alcohol drinking phenotypes. (G) HLM analysis showing a negative linear relationship between vehicle drinking values and ML213-induced change in alcohol intake across the 6 and 24 h time points (β = −0.95; main effect of drug, F (1,30) = 176.67, P < 0.0001).
Fig. 3.
Systemic administration of 10 and 20 mg/kg doses of ML213 does not affect (A) saccharin drinking (F (2,18) = 3.21; P = 0.0641), (B) water consumption (F( 2,18) = 1.32; P = 0.2915), or (C) saccharin preference (F (2,18) = 1.81; P = 0.1929) in alcohol-naïve rats (N = 10). (D) Total locomotor activity in alcohol-naïve rats is not affected by ML213 administration (F (2,18) = 2.54; P = 0.107).
3.3. ML213 and locomotor activity in alcohol-drinking rats
To determine if ML213 treatment affects locomotor behavior in rats with a history of alcohol drinking, both cohorts of rats were tested in the open field apparatus two weeks after the last alcohol drinking session. Unlike the alcohol-naïve rats, administration of ML213 significantly reduced total locomotor activity in the open field test (Fig. 4A,D). Post hoc analysis revealed a significant reduction in total distance traveled at the 5, 10, and 20 mg/kg doses of ML213 in the alcohol-drinking rats. The correlations between alcohol drinking and locomotor activity after vehicle injection (Fig. 4B,E) and the correlations between the change in alcohol intake and locomotor activity after ML213 treatment (Fig. 4C,F) were not significant.
Fig. 4.
Systemic administration of ML213 reduces locomotor activity during protracted abstinence in rats with a history of long-term alcohol drinking in the intermittent access model. (A,D) ML213 administration reduces total distance traveled in an open field apparatus (0, 1, and 5 mg/kg: (F (2,22) = 22.42, P < 0.0001; post-hoc test, *** P < 0.0001, 5 mg/kg vs vehicle and 1 mg/kg; 0, 10, and 20 mg/kg: F (2,18) = 40.61, P < 0.0001; post-hoc test, * P < 0.0001, 10 and 20 mg/kg vs vehicle). (B,E) Total distance traveled does not significantly correlate with alcohol intake in vehicle treated rats (0, 1, and 5 mg/kg: r = 0.092, P = 0.776; 0, 10, and 20 mg/kg: r = −0.2971, P = 0.435). (C,F) Lack of correlation between the change in distance and alcohol intake in rats treated with systemic ML213 (0, 1, and 5 mg/kg: 1 mg/kg: r = 0.248, P = 0.436; 5 mg/kg, r = 0.306, P = 0.334; 0, 10, and 20 mg/kg: 10 mg/kg: r = −0.460, P = 0.181; 20 mg/kg: r = −0.539, P = 0.108).
3.4. Microinfusions of retigabine into the VTA and alcohol drinking
3.4.1. Retigabine microinfusions
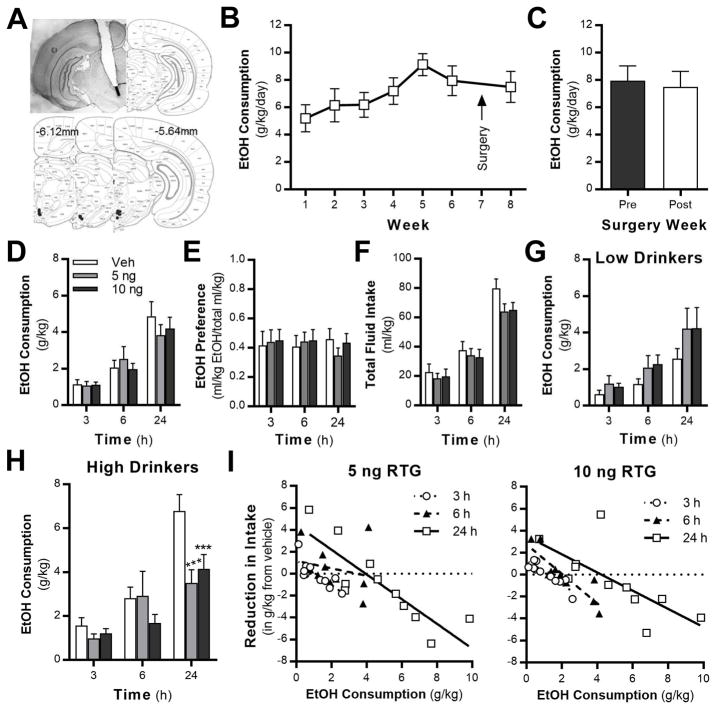
To validate these genetic findings between midbrain Kcnq4 levels and alcohol-seeking behaviors, we performed a number of experiments to investigate the role of VTA KV7 channels in regulating alcohol intake. First, the pan-KV7 channel positive modulator retigabine was microinfused into the VTA (Fig. 5A) after Wistar rats established baseline drinking the IAA model (Fig. 5B). Baseline drinking was not significantly different before and after the surgery (Fig. 5C). Similar to our study that targeted the NAc core (McGuier et al., 2016), retigabine had no effect on alcohol consumption (Fig. 5D), preference (Fig. 5E), or total fluid consumption (Fig. 5F) in the overall population of Wistar rats. Neither dose of retigabine affected alcohol intake in LD rats (Fig. 5G). In the HD group, there was a significant interaction of time and alcohol consumption (Fig. 5H). Post-hoc analysis indicates that both doses of retigabine reduced drinking at the 24 h time point. A mixed model analysis identified a strong negative linear relationship between vehicle treatment and the reduction in intake after intra-VTA retigabine administration across all time points (Fig. 5I).
Fig. 5.
Retigabine microinfusions into the VTA reduces alcohol intake in high-drinking rats. (A) Schematic of localization of guide cannula placement. The black line represents the placement of the injection. The black dots mark the sites of microinfusions. (B,C) Weekly average alcohol intake values before and after guide cannula surgery (F (1,11) = 0.06, P = 0.8046; N = 12). (D,E) Alcohol consumption (F (2,20) = 0.23, P = 0.8001) and preference (F (2,20) = 0.29, P = 0.7546) in rats that received microinfusions of retigabine into the VTA. (F) Total intake of alcohol and water after vehicle and retigabine VTA microinfusions (F (2,20) = 3.15, P = 0.0649). (G,H) Retigabine microinfusion reduces alcohol intake in high-, but not low-drinking rats (low-drinkers: F(2,8) = 2.72, P = 0.1255; high-drinkers: interaction, F (4,20) = 3.04; P = 0.0412; post hoc, 5 ng: ***P = 0.0004 vs vehicle, 10 ng: *** P = 0.003 vs vehicle). (I) HLM analysis revealed a negative linear relationship between the reduction in alcohol intake after 5 and 10 ng retigabine microinfusion and the amount of alcohol consumed after infusion of vehicle across time (3, 6, and 24 h time points; β = −0.91; main effect of drug, F (1,41) = 51.84, P < 0.0001).
3.4.2. XE-991 microinfusions
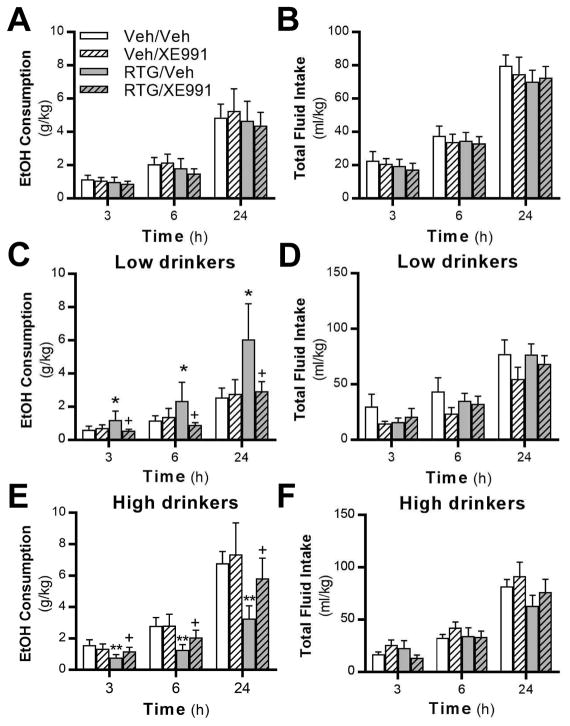
To test if KV7 channels in the VTA are necessary for the effect of retigabine on alcohol consumption, we microinfused the selective KV7 channel antagonist XE-991 (0.225 ng) immediately followed by systemic retigabine (7.5 mg/kg). When considering the total cohort of rats, we observed no main effect of drug on alcohol consumption (Fig. 6A), preference (F (3,31) = 0.80; P = 0.5030), or total fluid intake (Fig. 6B). When separated based on drinking phenotype, there was a main effect of treatment on alcohol consumption in LD rats (Fig. 6C). Consistent with our previous data showing that systemic retigabine treatment increases alcohol consumption in rats with a low drinking phenotype (McGuier et al., 2016), post-hoc analysis indicated that retigabine treatment increased intake compared to rats treated with vehicle. Importantly, microinfusion of XE991 into the VTA completely blocked the increase in drinking induced by systemic retigabine in LD rats. There was no difference between XE991 and vehicle microinfusions on any measure, and there was no effect of treatment on total fluid intake in LD rats (Fig. 6D). In HD rats, there was a main effect of treatment on alcohol consumption (Fig. 6E). Post-hoc analysis showed that retigabine reduced drinking compared to vehicle-treated rats, an effect that was blocked when retigabine treated rats received microinfusions of XE991. There was no effect of treatment on total fluid intake (Fig. 6F). In sum, systemic retigabine increased alcohol consumption in LD rats and decreased consumption in HD rats, and XE-991 microinfusion into the VTA blocked the actions of retigabine.
Fig. 6.
Blockade of KV7 channels in the VTA prevents the bidirectional effects of systemic retigabine treatment in low- and high-drinking rats. (A,B) Neither systemic retigabine administration nor XE991 microinfusion into the VTA affected alcohol (F (3,31) = 0.74; P = 0.5538; N = 12) or total fluid intake (F (3,31) = 0.59; P = 0.6234) in the total populations of rats. XE991 microinfusion blocked the ability of systemic retigabine treatment to (C) increase consumption in low-drinking rats (main effect of drug, F (3,13) = 3.77; P = 0.0388; post-hoc: * P = 0.0147, RTG/Veh vs Veh/Veh; + P = 0.0116, RTG/Veh vs RTG/XE991) and (E) reduce alcohol intake in rats with a high-drinking phenotype (main effect of drug, F (3,15) = 5.29; P = 0.0109; post-hoc: ** P = 0.0044, RTG/Vehicle (Veh) vs Veh/Veh; + P = 0.0029, RTG/Veh vs RTG/XE991). (D,F) Total fluid intake in low- (F (3,13) = 2.27; P = 0.1284) and high-drinking (F (3,15) = 2.32; P = 0.1168) rats was not affected by systemic retigabine administration or XE991 microinfusion.
4. Discussion
The experiments described here provide genetic and pharmacological evidence that midbrain KV7 channels are critically involved in the regulation of heavy alcohol drinking in mice and rats. Midbrain Kcnq4 expression levels negatively correlated with alcohol intake and the rewarding effects of alcohol in BXD RI mouse strains. Consistent with functional links between KV7 channels and monoaminergic signaling, Kcnq4 expression correlated with multiple components of the mesocorticolimbic DA circuitry. Systemic administration of the novel KV7.2/4-selective positive modulator ML213 decreased alcohol intake regardless of phenotype without affecting consumption of a sweetened solution. During prolonged abstinence from alcohol drinking, ML213 administration revealed a greater response to the locomotor impairing effects of KV7 channel activation. We also found that retigabine decreased voluntary alcohol consumption in high-drinking rats, while producing the opposite effect in rats with a low-drinking phenotype. Importantly, blocking KV7 channels in the VTA prevented the changes in drinking induced by systemic retigabine administration. Together, these experiments provide the first evidence that midbrain Kcnq4 and VTA KV7 channels regulate alcohol reward and heavy alcohol drinking.
4.1. Bioinformatics
Our bioinformatics query of midbrain Kcnq genes identified Kcnq4 as a candidate gene that regulates alcohol seeking and reward-related behaviors in BXD RI strains of mice. Kcnq4 was also correlated with alcohol withdrawal convulsion severity and other behaviors and physiological responses to acute alcohol exposure in genetically diverse strains of mice. Moreover, we identified multiple links between Kcnq4 and the monoaminergic system within the mesocorticolimbic DA circuitry. These genetic findings are consistent with a published study showing that KV7.4 channels functionally couple with DA D2-type receptors (Ljungstrom et al., 2003) and co-localize with a subpopulation of serotonin-positive neurons in the dorsal raphe nucleus (Hansen et al., 2008). We found that midbrain Kcnq4 expression positively correlated with the main metabolites of serotonin and DA in the medial septal nucleus and D2R/D3R receptor and TH expression in the striatum of BXD RI strains. These latter observations are intriguing given that TH activity positively regulates dopaminergic activity (Jones et al., 1998; Salvatore, 2014) and low DA activity correlates with high alcohol drinking in BXD RI strains (Siciliano et al., 2017). Moreover, adaptations in D2-type autoreceptors are an important mechanism underpinning the development of heavy drinking (Karkhanis et al., 2015; Siciliano et al., 2016). These findings stemming from mouse genetic studies suggest that midbrain Kcnq4 may regulate alcohol intake, possibly through control of monoaminergic signaling in the mesocorticolimbic circuitry.
4.2. VTA microinfusions of retigabine reduce excessive drinking
In a series of microinjection studies to validate the bioinformatics data, we found that KV7 channels in the VTA regulate excessive voluntary drinking in Wistar rats. VTA microinjection of the pan KV7 channel positive modulator retigabine reduced alcohol intake in rats with a high-drinking phenotype. Similar to previous evidence (McGuier et al., 2016), systemic retigabine administration produced bidirectional effects on drinking in the low- and high-drinking rats. Importantly, intra-VTA microinfusion of XE-991 blocked retigabine’s ability to affect drinking in these two populations. While the functional mechanisms underpinning these bidirectional effects on drinking are unknown and beyond the scope of this study, a recent study described divergent VTA DA neuron firing patterns and functional adaptations in low- and high-drinking mice (Juarez et al., 2017). While VTA DA neuron firing in mice with a high alcohol drinking phenotype was similar to controls, low alcohol drinking mice displayed increased phasic/bursting activity, and phasic, but not tonic, stimulation of VTA DA neurons reduced alcohol intake in high-drinking mice (Juarez et al., 2017). In contrast to these findings, a separate study showed that tonic, but not phasic stimulation of the VTA reduced alcohol intake in high-drinking rats (Bass et al., 2013). Given that both KV7 channel function and retigabine can regulate dopaminergic neurotransmission, increasing KV7 channel function with retigabine may alter baseline and anticipatory phasic dopaminergic responses to alcohol in the different drinking populations. It is also possible that retigabine differentially affects firing in low vs high alcohol drinking rats. However, we consider this latter possibility unlikely because retigabine potently reduces tonic and phasic firing of VTA DA neurons (Hansen et al., 2006; Sotty et al., 2009). Despite the unknown neural mechanism driving the bidirectional effects of retigabine on alcohol consumption in rats with different drinking phenotypes, it remains that VTA KV7 channels contribute to alcohol drinking and alcohol-seeking behaviors and are targets for reducing heavy alcohol intake.
4.3. KV7 channels and low-drinking rodents
Although retigabine reduces alcohol consumption in rats and mice with a high-drinking phenotype, it elevates intake in low drinkers when administered systemically and has side effects that limit its clinical use. Notably, administration of the novel and selective KV7.2/4 channel positive modulator ML213 reduced drinking in the total population of rats without increasing alcohol drinking in rats with a low-drinking phenotype. ML213 administration did not reduce saccharin intake demonstrating that the effects on intake of rewarding solutions are selective for alcohol. Because ML213 enhances activity of channels containing the KV7.2/4 subunits (Yu et al., 2010), this suggests that the ability of retigabine to increase drinking in low-drinking rats is through modulation of a different KV7 channel subunit. In addition to the unknown subunit, the locus of the mechanism underpinning the elevation in drinking is unidentified. Whereas systemic administration of ML213 reduced alcohol intake in low-drinking rats, microinjection of retigabine into the NAc (McGuier et al., 2016) or VTA (this study) did not affect consumption in low alcohol drinkers. This suggests that Kcnq4 channels expressed outside of the VTA may also contribute to the effect of ML213 on alcohol intake. XE991 is known to increase spontaneous firing of VTA DA neurons (Koyama et al., 2007), but microinfusion of XE991 into the VTA did not increase voluntary alcohol drinking at the concentration used in this study, as might be expected. While these mixed results demonstrate a complex role for KV7 channel regulation of alcohol drinking, our results show that targeting KV7.2/4 channel subtypes with ML213 is a promising strategy for reducing heavy alcohol drinking.
4.4. Locomotor behavior and neuroadaptations
Hypoactivity is commonly observed during acute and protracted withdrawal from chronic alcohol exposure (Broadwater et al., 2011; Zhao et al., 2007), and high doses of KV7 channel positive modulators can reduce locomotor activity in alcohol-naïve rats (Hansen et al., 2007; Roeloffs et al., 2008). Although we did not directly compare alcohol drinking and non-drinking rats, doses of ML213 that were innocuous in alcohol-naïve rats produced hypoactivity during abstinence in the alcohol-drinking rats. Only the highest dose of ML213 reduced total fluid intake when alcohol was available, and the locomotor response did not correlate with alcohol consumption after vehicle or ML213 treatment. Thus, the locomotor impairing effects of ML213 appear specific to the open field apparatus, suggesting that long-term drinking increases responses to the locomotor-impairing effects of KV7 channel activation. We demonstrated previously that rats with a history of alcohol drinking showed an enhanced sensitivity to hyperactivity when KV7 channels were blocked with XE991 (McGuier et al., 2016). One possible explanation is that long-term drinking and protracted withdrawal produce adaptations in the balance of excitatory and inhibitory processes that are unmasked after manipulation of KV7 channel activity. Alternatively, the augmented behavioral responses to KV7 channel activation and blockade may reflect neuroadaptations in KV7 channels in a yet to be identified region or circuitry.
In support of the latter hypothesis, studies have shown that chronic alcohol exposure altered KV7.2 channel surface trafficking in the NAc (McGuier et al., 2016) and reduced KV7 channel function and expression in the lateral habenula (Kang et al., 2017). Regulation of Kcnq transcript levels by alcohol is complex and varies by direction of change, Kcnq variant, species, and brain region (Rinker and Mulholland, 2017). For example, KCNQ2 expression is increased in the amygdala and frontal cortex in postmortem tissue samples from alcoholics, whereas VTA Kcnq4 expression is not altered in rodent alcohol drinking models (Marballi et al., 2016; McBride et al., 2013; McBride et al., 2012). Importantly, there are SNPs in KCNQ1/5 that associate with alcohol dependence in humans (Edenberg et al., 2010; Kendler et al., 2011), and chronic retigabine treatment prevented the development of alcohol-induced disruptions in brain EEG rhythms in a rabbit model of dependence (Zwierzynska et al., 2016). Given the emerging evidence for alcohol-induced adaptations in KV7 channels, it will be important to continue to investigate the role of KV7 channels in the development of alcohol-seeking behaviors and alcohol dependence and determine the effects of excessive drinking on protein expression levels of KV7 channels in other brain regions, like the VTA and prefrontal cortex.
5. Conclusions
In summary, these data provide several lines of genetic and pharmacological evidence implicating midbrain Kcnq4 and VTA KV7 channels as modulators of heavy alcohol drinking. Our findings showing that treatment with a subtype selective and novel KV7 channel positive modulator and intra-VTA infusions of retigabine reduced heavy alcohol drinking add to a growing literature that KV7 channels are a target for treating excessive alcohol drinking associated with AUD. These data provide the first evidence that genetic variation in midbrain Kcnq4 associates with alcohol intake and alcohol-seeking behaviors, as well as multiple components of the monoaminergic system. This class of drugs was well tolerated in non-treatment seeking moderate drinkers (Crean and Tompson, 2013) and should be further investigated as a pharmacotherapeutic approach for treating individuals with AUD.
Supplementary Material
Highlights.
Midbrain Kcnq4 expression correlates with alcohol seeking behaviors in mice.
Activating KV7 channels in the VTA reduces excessive alcohol drinking in rats.
A novel and selective KV7 channel activator reduces alcohol intake in rats.
KV7.4 channels are a critical mediator of excessive alcohol drinking.
Acknowledgments
The authors would like to sincerely thank Ms. Julia Moss for her technical assistance in the completion of these studies. The authors also thank Dr. Steve Mahler for his assistance with the VTA cannula placements.
This work was supported by NIH grants AA023288 (PJM), AA020930 (PJM), AA010761 (MH), AA025110 (JAR), AA014091 (SRJ), and AA021618 (NSM). There are no competing financial interests in relation to the work described in this manuscript. The authors declare no conflict of interest.
Footnotes
Author Contributions
PJM, NSM, and JAR planned the experiments. PJM, with assistance from SRJ, performed the bioinformatics analysis. SNM, JAR, RC, and DBF performed the behavioral experiments. NSM, JAR, PJM, and MH analyzed the data. NSM, JAR, and PJM wrote the manuscript, and all authors edited the manuscript and provide intellectual input into data interpretation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bass CE, Grinevich VP, Gioia D, Day-Brown JD, Bonin KD, Stuber GD, Weiner JL, Budygin EA. Optogenetic stimulation of VTA dopamine neurons reveals that tonic but not phasic patterns of dopamine transmission reduce ethanol self-administration. Front Behav Neurosci. 2013;7:173. doi: 10.3389/fnbeh.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeson SE, Kyle Warren R, Crabbe JC, Metten P, Gene Erwin V, Belknap JK. Chromosomal loci influencing chronic alcohol withdrawal severity. Mamm Genome. 2003;14:454–463. doi: 10.1007/s00335-002-2254-4. [DOI] [PubMed] [Google Scholar]
- Blackburn-Munro G, Dalby-Brown W, Mirza NR, Mikkelsen JD, Blackburn-Munro RE. Retigabine: chemical synthesis to clinical application. CNS Drug Rev. 2005;11:1–20. doi: 10.1111/j.1527-3458.2005.tb00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater M, Varlinskaya EI, Spear LP. Chronic intermittent ethanol exposure in early adolescent and adult male rats: effects on tolerance, social behavior, and ethanol intake. Alcohol Clin Exp Res. 2011;35:1392–1403. doi: 10.1111/j.1530-0277.2011.01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaliere S, Gillespie JM, Hodge JJ. KCNQ channels show conserved ethanol block and function in ethanol behaviour. PLoS One. 2012;7:e50279. doi: 10.1371/journal.pone.0050279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S, Antell A, Kaufman K. New antiepileptic medication linked to blue discoloration of the skin and eyes. Ther Adv Drug Saf. 2015;6:15–19. doi: 10.1177/2042098614560736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean CS, Tompson DJ. The effects of ethanol on the pharmacokinetics, pharmacodynamics, safety, and tolerability of ezogabine (retigabine) Clin Ther. 2013;35:87–93. doi: 10.1016/j.clinthera.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Dousa M, Srbek J, Radl S, Cerny J, Klecan O, Havlicek J, Tkadlecova M, Pekarek T, Gibala P, Novakova L. Identification, characterization, synthesis and HPLC quantification of new process-related impurities and degradation products in retigabine. J Pharm Biomed Anal. 2014;94:71–76. doi: 10.1016/j.jpba.2014.01.042. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Koller DL, Xuei X, Wetherill L, McClintick JN, Almasy L, Bierut LJ, Bucholz KK, Goate A, Aliev F, Dick D, Hesselbrock V, Hinrichs A, Kramer J, Kuperman S, Nurnberger JI, Jr, Rice JP, Schuckit MA, Taylor R, Todd Webb B, Tischfield JA, Porjesz B, Foroud T. Genome-wide association study of alcohol dependence implicates a region on chromosome 11. Alcohol Clin Exp Res. 2010;34:840–852. doi: 10.1111/j.1530-0277.2010.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen HH, Andreasen JT, Weikop P, Mirza N, Scheel-Kruger J, Mikkelsen JD. The neuronal KCNQ channel opener retigabine inhibits locomotor activity and reduces forebrain excitatory responses to the psychostimulants cocaine, methylphenidate and phencyclidine. Eur J Pharmacol. 2007;570:77–88. doi: 10.1016/j.ejphar.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Hansen HH, Ebbesen C, Mathiesen C, Weikop P, Ronn LC, Waroux O, Scuvee-Moreau J, Seutin V, Mikkelsen JD. The KCNQ channel opener retigabine inhibits the activity of mesencephalic dopaminergic systems of the rat. J Pharmacol Exp Ther. 2006;318:1006–1019. doi: 10.1124/jpet.106.106757. [DOI] [PubMed] [Google Scholar]
- Hansen HH, Waroux O, Seutin V, Jentsch TJ, Aznar S, Mikkelsen JD. Kv7 channels: interaction with dopaminergic and serotonergic neurotransmission in the CNS. J Physiol. 2008;586:1823–1832. doi: 10.1113/jphysiol.2007.149450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MM, Lange SC, Thomsen MS, Hansen HH, Mikkelsen JD. The pharmacological effect of positive KCNQ (Kv7) modulators on dopamine release from striatal slices. Basic Clin Pharmacol Toxicol. 2011;109:339–342. doi: 10.1111/j.1742-7843.2011.00730.x. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci U S A. 1998;95:4029–4034. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez B, Morel C, Ku SM, Liu Y, Zhang H, Montgomery S, Gregoire H, Ribeiro E, Crumiller M, Roman-Ortiz C, Walsh JJ, Jackson K, Croote DE, Zhu Y, Zhang S, Vendruscolo LF, Edward S, Roberts A, Hodes GE, Lu Y, Calipari ES, Chaudhury D, Friedman AK, Han MH. Midbrain circuit regulation of individual alcohol drinking behaviors in mice. Nat Commun. 2017;8:2220. doi: 10.1038/s41467-017-02365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Li J, Zuo W, Fu R, Gregor D, Krnjevic K, Bekker A, Ye JH. Ethanol Withdrawal Drives Anxiety-Related Behaviors by Reducing M-type Potassium Channel Activity in the Lateral Habenula. Neuropsychopharmacology. 2017;42:1813–1824. doi: 10.1038/npp.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis AN, Rose JH, Huggins KN, Konstantopoulos JK, Jones SR. Chronic intermittent ethanol exposure reduces presynaptic dopamine neurotransmission in the mouse nucleus accumbens. Drug Alcohol Depend. 2015;150:24–30. doi: 10.1016/j.drugalcdep.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Kalsi G, Holmans PA, Sanders AR, Aggen SH, Dick DM, Aliev F, Shi J, Levinson DF, Gejman PV. Genomewide association analysis of symptoms of alcohol dependence in the molecular genetics of schizophrenia (MGS2) control sample. Alcohol Clin Exp Res. 2011;35:963–975. doi: 10.1111/j.1530-0277.2010.01427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharkovets T, Hardelin JP, Safieddine S, Schweizer M, El-Amraoui A, Petit C, Jentsch TJ. KCNQ4, a K+ channel mutated in a form of dominant deafness, is expressed in the inner ear and the central auditory pathway. Proc Natl Acad Sci U S A. 2000;97:4333–4338. doi: 10.1073/pnas.97.8.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Koyama S, Brodie MS, Appel SB. Ethanol inhibition of m-current and ethanol-induced direct excitation of ventral tegmental area dopamine neurons. J Neurophysiol. 2007;97:1977–1985. doi: 10.1152/jn.00270.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungstrom T, Grunnet M, Jensen BS, Olesen SP. Functional coupling between heterologously expressed dopamine D(2) receptors and KCNQ channels. Pflugers Arch. 2003;446:684–694. doi: 10.1007/s00424-003-1111-2. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Aston-Jones G. Interactions between VTA orexin and glutamate in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2013;226:687–698. doi: 10.1007/s00213-012-2681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marballi K, Genabai NK, Blednov YA, Harris RA, Ponomarev I. Alcohol consumption induces global gene expression changes in VTA dopaminergic neurons. Genes Brain Behav. 2016;15:318–326. doi: 10.1111/gbb.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martire M, D’Amico M, Panza E, Miceli F, Viggiano D, Lavergata F, Iannotti FA, Barrese V, Preziosi P, Annunziato L, Taglialatela M. Involvement of KCNQ2 subunits in [3H]dopamine release triggered by depolarization and pre-synaptic muscarinic receptor activation from rat striatal synaptosomes. J Neurochem. 2007;102:179–193. doi: 10.1111/j.1471-4159.2007.04562.x. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Kimpel MW, McClintick JN, Ding ZM, Hauser SR, Edenberg HJ, Bell RL, Rodd ZA. Changes in gene expression within the ventral tegmental area following repeated excessive binge-like alcohol drinking by alcohol-preferring (P) rats. Alcohol. 2013;47:367–380. doi: 10.1016/j.alcohol.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Kimpel MW, McClintick JN, Ding ZM, Hyytia P, Colombo G, Edenberg HJ, Lumeng L, Bell RL. Gene expression in the ventral tegmental area of 5 pairs of rat lines selectively bred for high or low ethanol consumption. Pharmacol Biochem Behav. 2012;102:275–285. doi: 10.1016/j.pbb.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuier NS, Griffin WC, 3rd, Gass JT, Padula AE, Chesler EJ, Mulholland PJ. Kv7 channels in the nucleus accumbens are altered by chronic drinking and are targets for reducing alcohol consumption. Addict Biol. 2016;21:1097–1112. doi: 10.1111/adb.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metten P, Iancu OD, Spence SE, Walter NA, Oberbeck D, Harrington CA, Colville A, McWeeney S, Phillips TJ, Buck KJ, Crabbe JC, Belknap JK, Hitzemann RJ. Dual-trait selection for ethanol consumption and withdrawal: genetic and transcriptional network effects. Alcohol Clin Exp Res. 2014;38:2915–2924. doi: 10.1111/acer.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula AE, Griffin WC, 3rd, Lopez MF, Nimitvilai S, Cannady R, McGuier NS, Chesler EJ, Miles MF, Williams RW, Randall PK, Woodward JJ, Becker HC, Mulholland PJ. KCNN Genes that Encode Small-Conductance Ca2+-Activated K+ Channels Influence Alcohol and Drug Addiction. Neuropsychopharmacology. 2015;40:1928–1939. doi: 10.1038/npp.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula AE, McGuier NS, Griffin WC, III, Lopez MF, Becker HC, Mulholland PJ. Novel anticonvulsants for reducing alcohol consumption: A review of evidence from preclinical rodent drinking models. OA Alcohol. 2013;1:2. doi: 10.13172/2053-0285-1-1-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radcliffe RA, Bludeau P, Asperi W, Fay T, Deng XS, Erwin VG, Deitrich RA. Confirmation of quantitative trait loci for ethanol sensitivity and neurotensin receptor density in crosses derived from the inbred high and low alcohol sensitive selectively bred rat lines. Psychopharmacology (Berl) 2006;188:343–354. doi: 10.1007/s00213-006-0512-2. [DOI] [PubMed] [Google Scholar]
- Rinker JA, Fulmer DB, Trantham-Davidson H, Smith ML, Williams RW, Lopez MF, Randall PK, Chandler LJ, Miles MF, Becker HC, Mulholland PJ. Differential potassium channel gene regulation in BXD mice reveals novel targets for pharmacogenetic therapies to reduce heavy alcohol drinking. Alcohol. 2017;58:33–45. doi: 10.1016/j.alcohol.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinker JA, Mulholland PJ. Promising pharmacogenetic targets for treating alcohol use disorder: evidence from preclinical models. Pharmacogenomics. 2017;18:555–570. doi: 10.2217/pgs-2016-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeloffs R, Wickenden AD, Crean C, Werness S, McNaughton-Smith G, Stables J, McNamara JO, Ghodadra N, Rigdon GC. In vivo profile of ICA-27243 [N-(6-chloro-pyridin-3-yl)-3,4-difluoro-benzamide], a potent and selective KCNQ2/Q3 (Kv7.2/Kv7.3) activator in rodent anticonvulsant models. J Pharmacol Exp Ther. 2008;326:818–828. doi: 10.1124/jpet.108.137794. [DOI] [PubMed] [Google Scholar]
- Salvatore MF. ser31 Tyrosine hydroxylase phosphorylation parallels differences in dopamine recovery in nigrostriatal pathway following 6-OHDA lesion. J Neurochem. 2014;129:548–558. doi: 10.1111/jnc.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Yorgason JT, Mateo Y, Helms CM, Lovinger DM, Grant KA, Jones SR. Chronic ethanol self-administration in macaques shifts dopamine feedback inhibition to predominantly D2 receptors in nucleus accumbens core. Drug Alcohol Depend. 2016;158:159–163. doi: 10.1016/j.drugalcdep.2015.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Locke JL, Mathews TA, Lopez MF, Becker HC, Jones SR. Dopamine synthesis in alcohol drinking-prone and -resistant mouse strains. Alcohol. 2017;58:25–32. doi: 10.1016/j.alcohol.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD. Using SAS PROC MIXED to Fit Multilevel Models, Hierarchical Models, and Individual Growth Models. Journal of Educational and Behavioral Statistics. 1998;23:323–355. [Google Scholar]
- Sotty F, Damgaard T, Montezinho LP, Mork A, Olsen CK, Bundgaard C, Husum H. Antipsychotic-like effect of retigabine [N-(2-Amino-4-(fluorobenzylamino)-phenyl)carbamic acid ester], a KCNQ potassium channel opener, via modulation of mesolimbic dopaminergic neurotransmission. J Pharmacol Exp Ther. 2009;328:951–962. doi: 10.1124/jpet.108.146944. [DOI] [PubMed] [Google Scholar]
- Yu H, Wu M, Hopkins C, Engers J, Townsend S, Lindsley C, McManus OB, Li M. Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD): 2010. A small molecule activator of KCNQ2 and KCNQ4 channels. [PubMed] [Google Scholar]
- Zhao Y, Weiss F, Zorrilla EP. Remission and resurgence of anxiety-like behavior across protracted withdrawal stages in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31:1505–1515. doi: 10.1111/j.1530-0277.2007.00456.x. [DOI] [PubMed] [Google Scholar]
- Zwierzynska E, Andrzejczak D, Pietrzak B. Does retigabine affect the development of alcohol dependence?--A pharmaco-EEG study. Neurosci Lett. 2016;611:6–13. doi: 10.1016/j.neulet.2015.11.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.