Abstract
Objective
To examine pain-related activity interference as a mediator for the relationship between pain intensity and depressive symptoms among older adults with serious mental illness (SMI).
Method
Ordinary least-squares regressions were used to investigate the mediation analysis among older adults with SMI (n = 183) from community mental health centers. Analyses used secondary data from the HOPES intervention study.
Results
Higher pain intensity was associated with greater pain-related activity interference. Higher pain intensity and pain-related activity interference were also associated with elevated depressive symptoms. Finally, greater pain-related activity interference significantly mediated the association between higher pain intensity and elevated depressive symptoms.
Conclusions
These findings demonstrate that pain and depressive symptoms may be linked to functional limitations. Clinicians and researchers in the mental health field should better address pain- related activity interference among older adults with SMI, especially among those with higher pain intensity and elevated depressive symptoms.
Keywords: Depressive symptoms, physical disorders, functional status
Objective
Chronic pain is a disabling symptom reported by approximately 50% of community-dwelling adults aged 65 and older (Patel, Dansie, Guralnik, & Turk, 2013). Complex groupings of biological, psychological, and social factors contribute to and maintain chronic pain (Gatchel, Peng, Peters, Fuchs, & Turk, 2007). These biopsychosocial components interact and negatively influence one another, thereby exacerbating pain intensity and interfering with daily activities inside and outside of the home (Stone & Baker, 2014). Pain intensity and pain-related activity interference often worsen with advancing age (Thomas, Peat, Harris, Wilkie, & Croft, 2004) and are associated with depressive symptoms (Gatchel et al., 2007). Indeed, integrative theories of depression have emphasized the interrelationships among stressful life events (e.g. onset of pain), activity interference, and mood disorders (Lewin-sohn & Graf, 1973). Activity interference, in particular, is negatively associated with positive reinforcement and strongly tied to elevated depressive symptoms (Lee, Chan, & Berven, 2007). Hence, it is critical to consider the role of pain-related activity interference in explaining the pain- depression linkages in later life.
Older adults with serious mental illness (SMI - defined as schizophrenia, schizoaffective disorder, bipolar disorder, or major depression) are a rapidly growing and high-risk, high-cost population (Bartels et al., 2014). Adverse outcomes (e.g. premature death, cancer) are linked to chronic medical conditions among older adults with SMI (Crump, Winkleby, Sundquist, & Sundquist, 2013). Yet, less is known about the physical or psychological dysfunction associated with chronic pain among this group (Stubbs et al., 2014). Older people with SMI may have difficulty recognizing and expressing pain due to illness-related factors, including psychiatric symptoms, antipsychotic medications, and cognitive limitations (Engels et al., 2014). Unhealthy lifestyle (e.g. substance misuse, food insecurity, physical inactivity, poor sleep habits), sociodemographic (e.g. race/ethnicity, poverty), and other contextual factors (e.g. limited social support, inadequate health care) may also conflict with or deprioritize the need for pain treatment. Consequently, clinicians may overlook the potential negative implications of pain-related problems. For instance, depressive symptoms in pain patients can lead to a host of harmful consequences, such as fewer adaptive coping strategies and treatment non-adherence (Harrison et al., 2012). Evaluating pain and related disability as well as associations with depressive symptoms may begin to elucidate understanding of the clinical impact of pain among this highly vulnerable population.
The primary objective of the current study was to examine whether older SMI patients' perceptions of pain-related activity interference mediate the association between their reports of pain intensity and depressive symptoms. Specifically, we hypothesized that the association between higher pain intensity and elevated depressive symptoms would be mediated by greater pain-related activity interference.
Method
Participants
This cross-sectional study included 183 participants from three community mental health centers in the New England region. Eligible participants were: (a) aged 50 years or older; (b) diagnosed with schizophrenia, schizoaffective disorder, bipolar disorder, or chronic major depressive disorder; and (c) functionally impaired. Exclusion criteria included: (a) currently residing in a skilled nursing facility or an inpatient institution; (b) significant cognitive impairment; (c) diagnosis of terminal disease; and (d) substance dependence.
Measures
Pain intensity was measured through the Dartmouth-Northern New England Primary Care Cooperative Information Project (COOP) Functional Assessment Charts (Nelson et al., 1987). The COOP charts include visual illustrations that depict functioning level for the past two weeks. The COOP has shown reliability and validity for geriatric populations, even when compared to multidimensional measures (Lennon, Carey, Creed, Durcan, & Blake, 2011). We used the pain chart for the current study, which is rated on a 5-point scale ranging from 1 (no pain) to 5 (severe pain). Single-item scales are psychometrically sound and standard assessments for pain intensity (Hawker, Mian, Kendzerska, & French, 2011; Hoffman, Sadosky, Dukes, & Alvir, 2010).
Pain-related activity interference was assessed using the Bodily Pain Scale (BPS) from the 36-item MOS Short form Health Survey (SF-36; Ware et al., 1998). The SF-36 BPS includes two items and has demonstrated reliability and validity among adults ≥ 50 years (Hawker et al., 2011). In line with prior survey research examining pain in older adults (Blyth et al., 2001; Scudds & Østbye, 2001; Thomas et al., 2004), we used the item, 'During the past 4 weeks, how much did pain interfere with your normal work (including both work outside the home and housework)?', which is rated on a 5-point scale ranging from 1 (not at all) to 5 (extremely).
The 20-item Center for Epidemiologic Studies Depression Scale (CES-D) was used to assess depressive symptoms (Radl-off, 1977). Respondents rate their frequency of symptoms over the past week using a 4-point scale ranging from 0 (Rarely or none of the time) to 3 (Most or all of the time). The standard cut-off score for an increased risk of experiencing clinical depression is ≥ 16 (Andresen, Malmgren, Carter, & Patrick, 1994). The CES-D has excellent psychometric properties and has been used in cross-cultural assessments among older populations (Mui, Burnette, & Chen, 2001; Radloff & Teri, 1986), albeit there may be differences in responses to depressive symptom items in racially and ethnically diverse older adult subgroups (Kim, Chiriboga, & Jang, 2009).
Procedures
We conducted a secondary analysis of baseline data collected before the start of the HOPES (Helping Older People Experience Success) intervention study. Study protocol was approved by the Committee for the Protection of Human Subjects at Dartmouth College.
Data analysis
The final dataset included 183 participants. Of these participants, 12 had missing data on key variables. Given that less than 10% of the data was missing (6.6%), we used expectation-maximization imputation to replacing missing data (Scheffer, 2002).
Statistical covariates were selected for model adjustment based on past SMI research (e.g. Sanchez, Rosenthal, Chan, Brooks, & Bezyak, 2016). Covariates included age, gender, race education, residential status, and psychiatric diagnosis.
We used SPSS Version 22 for descriptive statistics. The SPSS INDIRECT macro (Preacher & Hayes, 2008) was used to estimate the ordinary least-squares (OLS) regressions for the path coefficients and to implement a bootstrap testing approach. The following are key terms for the path coefficients:
Direct association of pain intensity with depressive symptoms: c'
Indirect association of pain intensity with depressive symptoms through pain-related activity interference: ab
Total association of pain intensity with depressive symptoms: c = c' + ab
If the bias-corrected bootstrap confidence intervals (CI) for the product of the ab path does not include zero, empirical evidence supports the indirect association for a mediation analysis.
Results
Table 1 presents the descriptive statistics on the sample characteristics.
Table 1.
Sample characteristics.
| Total (n = 183) | |
|---|---|
| Age (M ± SD) | 60.2 (7.9) |
| Gender | |
| Female (%) | 77 (42.1) |
| Male (%) | 106 (57.9) |
| Race | |
| White (%) | 26 (14.2) |
| Non-White (%) | 157 (85.8) |
| Education | |
| High school graduate (%) | 134 (73.2) |
| Less than high school (%) | 49 (26.8) |
| Residential status | |
| Independent living (%) | 94 (51.4) |
| Supervised/supported housing (%) | 89 (48.6) |
| Psychiatric diagnosis | |
| Schizophrenia or schizoaffective disorder (%) | 103 (56.3) |
| Bipolar or major depressive disorder (%) | 80 (43.7) |
| Pain intensity | |
| COOP pain chart (M ± SD) | 2.8 (1.4) |
| Pain-related activity interference | |
| SF-36 BPS item (M ± SD) | 2.5 (1.3) |
| Depressive symptoms | |
| CES-D (M ± SD) | 22.3 (12.9) |
Note: Non-White = Black/African American, American Indian/Alaska Native, Asian, Native Hawaiian/Pacific Islander, or more than one race; the COOP pain chart = a chart from the Dartmouth-Northern New England Primary Care Cooperative Information Project Functional Assessment Charts; SF-36 BPS item = an item from the Bodily Pain Scale of the 36-item MOS Short form Health Survey; CES-D = Center for Epidemiologic Studies Depression Scale.
Mediation analysis
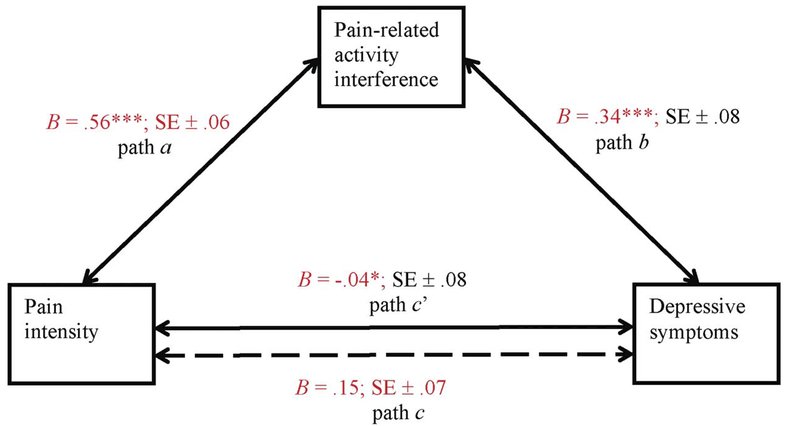
As shown in Figure 1, higher pain intensity was associated with greater pain-related activity interference (B = .56, p < .001; path a). Similarly, higher activity interference was associated with greater depressive symptoms (B = .34, p < .001; path b). Higher pain intensity was also associated with greater depressive symptoms (B = .15, p = .04; path c). After adjusting for activity interference, there was not a significant association between pain and depression (B = −.04, p > .05; path c'). Using 1000 bootstrap samples revealed a significant indirect association of more intense pain with elevated depressive symptoms through greater pain-related activity interference (point estimate = .19, 95% CI [.10, .29]; path ab).
Figure 1.
The solid lines denote path coefficients for the mediation analysis with pain intensity, pain-related activity interference, and depressive symptoms (N = 183). Dotted line denotes the path coefficient between pain intensity and depressive symptoms when pain-related activity interference is not included as a mediator. a, b, c and c’ are standardized ordinary least squares (OLS) regression coefficients.
*p < .05, **p < .01, ***p < .001.
Post hoc test
To examine the stability of findings and determine whether the mediation analysis varied on the basis of psychiatric diagnosis, we estimated models with psychiatric diagnosis as a moderator. Psychiatric diagnosis did not significantly moderate the associations in this study, which suggests that the pattern of findings is stable across mental disorders.
Discussion
As hypothesized, pain-related activity interference significantly mediated the association between higher pain intensity and greater depressive symptoms. Participants reported both more intense pain and elevated depressive symptoms only when their pain interfered with regular work or housework activities. This finding is congruent with biopsychosocial models of functioning, disability, and health that posit stressful life events like pain intensity can interfere with daily activities and trigger consequences that may increase risk for adverse events, including clinical depression (Gatchel et al., 2007; Lewinsohn & Graf, 1973; World Health Organization, 2001). Prior research has found that individuals are more likely to have positive pain adaptation outcomes, such as reduced or subclinical depressive symptoms, when they are able to manage pain and function in spite of it (Harrison et al., 2012; Okifuji, Turk, & Sherman, 2000).
The secondary findings in this study were consistent with our hypotheses. Higher pain intensity was associated with greater pain-related activity interference. Activity interference was associated with elevated depressive symptoms. In addition, there was a significant pain-depression link, which is aligned with past documentation on the relationships between severe pain and depression in later life (Stone & Baker, 2014). As expected, there was no longer a significant association between pain and depression after adjusting for activity interference. This result might be explained by the finding of pain-related activity interference as a mediator between pain intensity and depressive symptoms.
These results suggest the need to target health services for older adults with SMI toward providing assistance with daily activities affected by pain problems through home and community-based mental health programs. Interventions that address adaptive coping strategies for chronic pain and depression, such as physical activity, mindfulness, self-efficacy, or social support, may be particularly beneficial for improving opportunities for positive reinforcement and behavioral activation among this population. More research is also needed to examine the role of contextual factors (e.g. race/ethnicity, medical comorbidities, social support) as key variables modifying the associations in this study and as key factors identifying those who may be most at risk for adverse events. While fatal outcomes from pain medication misuse have decreased in recent years for younger adults, adults aged 60+ years have shown a rising trend in prescription opioid misuse associated with suicidal intent and overdose (West, Severtson, Green, & Dart, 2015). An additional future research direction, therefore, might be to determine whether mild, moderate, or severe mental disorders are risk factors for hazardous opioid misuse among older adults with chronic pain (Davis, Lin, Liu, & Sites, 2017).
Several limitations should be taken into account. Because this was a secondary data analysis, we were constrained by the original self-report measures. For example, although 1-item pain rating scales are commonly used in survey research (Herr & Garand, 2001), the COOP pain chart and SF-36 BPS item have limited response ranges. In addition, the pain- related activity interference measure was specific to work in and outside of the home; therefore, these results may not generalize to other types of activities (e.g. leisure, social, ADLs). We also did not have information on pain duration and could not determine pain chronicity. Furthermore, given that the cut-off score for probable depression is ≥16, elevated depressive symptoms on the CES-D scale does not necessarily relate to screening positive for clinically relevant depression. Last, self-report measures can be influenced by affective bias, poor insight, and recent life events, which may have affected the reporting ability of participants with mild cognitive difficulties (Atkinson, Zibin, & Chuang, 1997).
We also used a cross-sectional design, which precludes us from determining cause-and-effect relationships. There may be bidirectional relationships between pain, activity interference, and depression, which should continue to be investigated. A longitudinal design is needed to estimate the synergistic, adductive, or adjunctive effects among the variables of interest in this study. Because our sample was predominantly composed of non-institutionalized, community-dwelling older adults, our results may not be generalizable to all SMI patients. Institutionalized patients may represent those most affected by SMI, which means our findings may be skewed toward persons with less severe symptoms. Furthermore, there may be issues with cultural equivalence in the depressive symptoms reported with the CES-D, given the differential responses patterns across minority groups. Nevertheless, this study demonstrates the value of a biopsychosocial assessment of functional impairment among older adults with SMI, which lays groundwork for future research to gain a greater understanding of the clinical implications of pain- related problems among this population.
Conclusions
Despite potential difficulties with seeking or receiving pain treatment among older adults with SMI, results of this study suggest that their reports of co-occurring pain and depressive symptoms are associated with functional limitations. Similar to other older adult populations, older adults with SMI suffering from more severe pain and greater depressive symptoms may also experience pain-related interference with normal activities. The current findings indicate that clinicians should consider providing interventions that are useful for managing both pain and depression. Given that there may be a bidirectional relationship between pain and depressive symptoms, clinicians may also consider optimizing existing mental health treatments to reduce pain and related disability. In summary, mental health clinicians and researchers should further evaluate and test intervention options for pain-related activity interference among older adults with SMI, especially among those with higher pain intensity and elevated depressive symptoms.
Acknowledgments
Funding
National Institute of Mental Health [grant numbers T32 MH073553–11 and R01 MH6324].
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Andresen EM, Malmgren JA, Carter WB, & Patrick DL (1994). Screening for depression in well older adults: Evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). American Journal of Preventive Medicine , 10, 77–84. [PubMed] [Google Scholar]
- Atkinson M, Zibin S, & Chuang H (1997). Characterizing quality of life among patients with chronic mental illness: A critical examination of the self-report methodology. American Journal of Psychiatry , 154 99–105. [DOI] [PubMed] [Google Scholar]
- Bartels SJ, Pratt SI, Mueser KT, Forester BP, Wolfe R, Cather C, … Naslund JA (2014). Long-term outcomes of a randomized trial of integrated skills training and preventive healthcare for older adults with serious mental illness. The American Journal of Geriatric Psychiatry , 22(11), 1251–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyth FM, March LM, Brnabic AJ, Jorm LR, Williamson M, & Cousins MJ (2001). Chronic pain in Australia: A prevalence study. Pain , 89 (2), 127–134. [DOI] [PubMed] [Google Scholar]
- Crump C, Winkleby MA, Sundquist K, & Sundquist J (2013). Comorbidities and mortality in persons with schizophrenia: A Swedish national cohort study. American Journal of Psychiatry , 170(3), 324–333. doi: 10.1001/jamapsychiatry.2013.1394 [DOI] [PubMed] [Google Scholar]
- Davis MA, Lin LA, Liu H, & Sites BD (2017). Prescription opioid use among adults with mental health disorders in the United States. The Journal of the American Board of Family Medicine , 30(4), 407–417. [DOI] [PubMed] [Google Scholar]
- Engels G, Francke AL, van Meijel B, Douma JG, de Kam H, Wesse- link W, Scherder EJ (2014). Clinical pain in schizophrenia: A systematic review. The Journal of Pain, 15(5), 457–467. doi: 10.1016/j.jpain.2013.11.005 [DOI] [PubMed] [Google Scholar]
- Gatchel RJ, Peng YB, Peters ML, Fuchs PN, & Turk DC (2007). The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychological Bulletin , 133(4), 581–624. doi: 10.1037/0033-2909.133.4.581 [DOI] [PubMed] [Google Scholar]
- Harrison M, Reeves D, Harkness E, Valderas J, Kennedy A, Rogers A, Bower P (2012). A secondary analysis of the moderating effects of depression and multimorbidity on the effectiveness of a chronic disease self-management programme. Patient Education and Counseling , 87(1), 67–73. doi: 10.1016/j.pec.2011.06.007 [DOI] [PubMed] [Google Scholar]
- Hawker GA, Mian S, Kendzerska T, & French M (2011). Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), Mcgill Pain Questionnaire (MPQ), Short Form Mcgill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form 36 Bodily Pain Scale (SF 36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care & Research , 63(S11), 240–252. [DOI] [PubMed] [Google Scholar]
- Herr KA, & Garand L (2001). Assessment and measurement of pain in older adults. Clinics in Geriatric Medicine , 17(3), 457–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DL, Sadosky A, Dukes EM, & Alvir J (2010). How do changes in pain severity levels correspond to changes in health status and function in patients with painful diabetic peripheral neuropathy? Pain , 149(2), 194–201. [DOI] [PubMed] [Google Scholar]
- Kim G, Chiriboga DA, & Jang Y (2009). Cultural equivalence in depressive symptoms in older White, Black, and Mexican American Adults. Journal of the American Geriatrics Society , 57(5), 790–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GK, Chan F, & Berven NL (2007). Factors affecting depression among people with chronic musculoskeletal pain: A structural equation model. Rehabilitation Psychology , 52(1), 33–43. doi: 10.1037/0090-5550.52.1.33 [DOI] [Google Scholar]
- Lennon OC, Carey A, Creed A, Durcan S, & Blake C (2011). Relaibility and validity of COOP/WONCA functional health status charts for stroke patients in primary care. Journal of Stroke and Cerebrovascular Diseases , 20(5), 465–473. doi: 10.1016/j.jstrokecerebrovasdis.2010.02.020 [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, & Graf M (1973). Pleasant activities and depression. Journal of Consulting and Clinical Psychology , 41(2), 261–268. doi: 10.1037/h0035142 [DOI] [PubMed] [Google Scholar]
- Mui AC, Burnette D, & Chen LM (2001). Cross-cultural assessment of geriatric depression: A review of the CES-D and the GDS. Journal of Mental Health and Aging , 7(1), 137–164. [Google Scholar]
- Nelson E, Wasson J, Kirk J, Keller A, Clark D, Dietrich A, Zubkoff M (1987). Assessment of function in routine clinical practice: Description of the COOP Chart method and preliminary findings. Journal of Chronic Diseases , 40, 55–63. doi: 10.1016/S0021-9681(87)80033-4 [DOI] [PubMed] [Google Scholar]
- Okifuji A, Turk DC, & Sherman JJ (2000). Evaluation of the relationship between depression and fibromyalgia syndrome: Why aren't all patients depressed? The Journal of Rheumatology , 27(1), 212–219. [PubMed] [Google Scholar]
- Patel K, Dansie E, Guralnik J, & Turk D (2013). Prevalence and impact of pain among older adults in the United States: Findings from the National Health and Aging Trends Study. The Journal of Pain , 14(4), 2649–2657. doi: 10.1016/j.pain.2013.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, & Hayes AF (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods , 40(3), 879–891. doi: 10.3758/BRM.40.3.879 [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement , 1, 385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- Radloff LS, & Teri L (1986). Use of the center for epidemiological studies-depression scale with older adults. Clinical Gerontologist , 5(1–2), 119–136. doi: 10.1300/J018v05n01_06 [DOI] [Google Scholar]
- Sánchez J, Rosenthal DA, Chan F, Brooks J, & Bezyak JL (2016). Relationships between World Health Organization International Classification of Functioning, Disability and Health constructs and participation in adults with severe mental illness. Rehabilitation Research, Policy, and Education , 30(3), 286–304. [Google Scholar]
- Scheffer J (2002). Dealing with missing data. Research Letters in the Information and Mathematical Sciences , 3,153–160. [Google Scholar]
- Scudds RJ, & Østbye T (2001). Pain and pain-related interference with function in older Canadians: The Canadian study of health and aging. Disability and Rehabilitation , 23(15), 654–664. [DOI] [PubMed] [Google Scholar]
- Stone RC, & Baker J (2014). Physical activity, age, and arthritis: Exploring the relationships of major risk factors on biopsychosocial symptomology and disease status. Journal of Aging & Physical Activity , 22(3), 314–323. doi: 10.1123/japa.2012-0293 [DOI] [PubMed] [Google Scholar]
- Stubbs B, Mitchell AJ, De Hert M, Correll CU, Soundy A, Stroobants M, & Vancampfort D (2014). The prevalence and moderators of clinical pain in people with schizophrenia: A systematic review and large scale meta-analysis. Schizophrenia Research , 160(1), 1–8. doi: 10.1016/j.schres.2014.10.017 [DOI] [PubMed] [Google Scholar]
- Thomas E, Peat G, Harris L, Wilkie R, & Croft PR (2004). The prevalence of pain and pain interference in a general population of older adults: Cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP). Pain , 110(1), 361–368. doi: 10.1016/j.pain.2004.04.017 [DOI] [PubMed] [Google Scholar]
- Ware JE Jr., Kosinski M, Gandek B, Aaronson NK, Apolone G, Bech P, Prieto L (1998). The factor structure of the SF-36 Health Survey in 10 countries: Results from the IQOLA Project. Journal of Clinical Epidemiology , 51(11), 1159–1165. doi: 10.1016/S0895-4356(98)00107-3 [DOI] [PubMed] [Google Scholar]
- West NA, Severtson SG, Green JL, & Dart RC (2015). Trends in abuse and misuse of prescription opioids among older adults. Drug and Alcohol Dependence , 149,117–121. [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2001). International classification of functioning, disability, and health (ICF) . Geneva, Switzerland: WHO Document Production Services. [Google Scholar]