Abstract
Background
Clinical reports link specific medications with the development of antinuclear antibodies (ANA), but population-based evidence is limited.
Objective
The present study investigated associations between prescription medication use and ANA in a representative sample of the adult noninstitutionalized US population, first focusing on medications previously related to ANA and then considering all medications reported in the National Health and Nutrition Examination Survey (NHANES).
Methods
Based on NHANES data (1999–2004) for 3608 adults (ages ≥18 years), we estimated odds ratios (ORs) and 95% confidence intervals (CIs) to assess associations between recent medication use and ANA (overall and in sex and age subgroups), adjusted for potential confounders and the survey sampling design.
Results
We found no evidence that most medications previously associated with ANA in specific individuals were risk factors for ANA in the general population, although statistical power was limited for some medications. Overall, ANA were less prevalent in adults who recently used any prescription medications compared with those who did not (OR=0.73; CI=0.57,0.93), and likewise several classes of medications were inversely associated with ANA, including hormones (OR=0.73; CI=0.55,0.98), thiazide diuretics (OR=0.43; CI=0.24,0.79), sulfonylureas (OR=0.41; CI=0.19,0.89), and selective serotonin reuptake inhibitor antidepressants (OR=0.65; CI=0.42,0.98). Positive associations with ANA were seen for loop diuretics (OR=1.72; CI=1.03,2.88) in all adults, and for benzodiazepines (OR=2.11; CI=1.09,4.10) and bronchodilators (OR=1.83; CI=1.00,3.38) in older (ages ≥60) adults. Estrogens were positively associated with ANA in older women (OR=1.80; CI=1.00,3.23) but inversely associated with ANA in younger (ages 18–59) women (OR=0.43; CI=0.20,0.93). Regarding individual medications, ANA were positively associated with ciprofloxacin (OR=4.23; CI=1.21,14.8), furosemide (OR=1.79; CI=1.09,2.93), and omeprazole (OR=2.05; CI=1.03,4.10) in all adults, and with salmeterol (OR=3.76; CI=1.66,8.52), tolterodine (OR=6.64; CI=1.45,30.5), and triamterene (OR=3.10; CI=1.08,8.88) in older adults. Also, in younger adults, hydrochlorothiazide was inversely associated with ANA (OR=0.44; CI=0.20,0.98).
Conclusions
Our findings in the general population do not confirm most clinically reported positive associations between specific medications and ANA in some individuals. However, novel positive ANA associations with other medications, as well as unexplained inverse associations with certain classes of medications and overall medication use, deserve further research to clarify the possible roles of medications as risk and protective factors in the development of autoantibodies and autoimmune disease.
Keywords: Antinuclear antibodies (ANA), Autoimmune disease, Autoimmunity, Lupus, NHANES, Prescription medication
1. Introduction
Autoimmune diseases are a diverse group of disorders characterized by tissue and organ damage due to an immune response to self-antigens [1] and their causes remain incompletely understood [2]. Antinuclear antibodies (ANA) are observed in patients with many systemic autoimmune diseases. In the general US population, ANA are more common in women, older individuals, African Americans, and persons of normal weight [3]. They are also associated with childbearing [4]; certain genes [5]; and environmental agents, such as chemicals, occupational exposures, infections, and medications [6–10].
The present study focuses on prescription medications, some of which have been reported to induce ANA and symptoms of lupus or other autoimmune diseases in specific individuals. Drug challenge (ANA or disease occurrence after beginning the drug), dechallenge (resolution of ANA or disease after discontinuing the drug), and rechallenge (recurrence of ANA or disease after beginning the drug again) are often considered diagnostic for drug-induced autoimmunity [11]. However, such studies are often small and describe only case reports or case series [8, 12–14]; limited data are available on a population basis to determine the extent of associations from a public health perspective. Also, few if any studies have assessed possible protective effects of medications on autoimmunity, as these cannot be performed in clinical care settings or most drug trials because they require larger sample sizes and population-based approaches.
The purpose of the present study was to investigate associations, positive or negative, between prescription medication use and ANA in the general adult population. We analyzed data from a representative sample of the noninstitutionalized US population, obtained from the National Health and Nutrition Examination Survey (NHANES). First, we examined medications previously reported to induce ANA in specific individuals and sought to determine whether corresponding positive associations could be confirmed in our large, population-based study. Second, we evaluated all prescription medications used by NHANES participants in the month preceding their interview to identify any associations with ANA. The latter analysis was mainly descriptive and exploratory; it assessed individual medications, classes of medications, and overall medication use to generate hypotheses for future studies.
2. Subjects and methods
2.1. Study participants
We analyzed NHANES data from 1999 to 2004, currently the only years with data on ANA. All data are publicly available and no individual follow-up outside NHANES occurred. The NHANES used a multistage strategy to select a nationally representative probability sample of the noninstitutionalized US population. A subsample of 7106 participants at least 12 years old was selected to assess serum levels of organochlorines, and 4754 of these participants gave permission for sera storage and had samples available for ANA analysis. Sampling weights were revised (https://www.cdc.gov/nchs/tutorials/nhanes/SurveyDesign/Weighting/intro.htm) to account for sampling into the organochlorine study and participation in the ANA substudy. The NHANES collected self-reported information on various sociodemographic and health-related factors. Constructed variables, such as body mass index (BMI), defined as weight (kg) divided by height (m) squared, were also included in the published dataset [15]. The NHANES protocol was approved by the human subjects Institutional Review Board of the U.S. Centers for Disease Control and Prevention, and written informed consent was obtained from all participants.
We excluded 236 women who were pregnant, as their physiology and use of medications are not representative of the general population [16, 17]. We also excluded 881 participants under 18 years of age, 20 with missing medication information, and 9 with missing BMI data, which left 3608 adult participants for our analyses. Except for age and pregnancy status, which were used to define our subsample, these exclusions did not lead to any statistically significant differences in other demographic factors compared with the original ANA sample (not shown).
2.2. ANA status
Standard indirect immunofluorescence was used to measure ANA in serum specimens, based on commercial HEp-2 ANA slides (Inova Diagnostics) with 1:80 dilutions of sera and staining with DyLight 488-conjugated donkey anti-human immunoglobulin G (IgG) antibodies (Jackson ImmunoResearch), as previously reported [3]. Staining intensities were graded from 0 to 4 relative to a standard reference gallery, with values of 3 and 4 indicating ANA positivity. Two independent raters, blinded to the sociodemographic data on the subjects, agreed on >95% of the readings for overall intensity ratings, and differences were resolved by consensus or adjudicated by a third blinded rater.
Specific autoantibodies were identified in ANA-positive participants by using immuno-precipitation of 35S-methionine-labeled K562 cell extracts, as previously described [3]. They were classified as autoantibodies against extractable nuclear antigens (ENA) and included Sjögren’s syndrome antigen A (Ro), Sjögren’s syndrome antigen B (La), Argonaute 2 (Su), U1 ribonucleoprotein (U1RNP), Smith antigen (Sm), topoisomerase I, ribosomal proteins or RNA, replication protein A (RPA), isoleucyl-transfer RNA synthetase (OJ), nucleolar organizer region 90kDa antigen or upstream binding protein (NOR90), histidyl-transfer RNA synthetase (Jo-1), threonyl-transfer RNA synthetase (PL-7), alanyl-transfer RNA synthetase (PL-12), glycyl-transfer RNA synthetase (EJ), signal recognition particle (SRP), p70/p80 antigen that is a DNA-binding protein (Ku), polymyositis-scleroderma antigen (PM-Scl), chromodomain helicase DNA binding protein 4 (Mi-2), RNA polymerase, and U3 ribonucleoprotein (U3RNP). The data were too sparse to analyze these autoantibodies individually, but we performed sensitivity analyses that excluded the 483 participants with any of these anti-ENA autoantibodies or certain medical disorders (described later) to focus on those without prior evidence of autoimmune disease.
2.3. Prescription medication information
Medication use was self-reported, but verified with prescription bottles, and referred to use in the month preceding the NHANES interview. Initially, we targeted medications suspected of inducing ANA in specific individuals to assess whether they also were associated with ANA in our population-based sample. After extensive literature reviews of case reports, using PubMed with search terms such as “medications and ANA” and “medications and autoimmunity” and evaluating references in sentinel papers, we (JY and FWM) compiled a list of medications that met predefined criteria for possibly inducing ANA [11, 18]. We refer to this compilation as the medications-associated-with-ANA (MAWA) list. We also investigated associations with ANA for medications previously linked to lupus, an autoimmune disease associated with ANA. We created two lists of such medications: the Rubin list, which refers to medications in Table 2 of Rubin [12] or Table 1 of Rubin [13], and the Chang list, which refers to medications in Table 2 of Chang and Gershwin [8] or Table 1 of Xiao and Chang [14]. Prescription medications used by NHANES participants and included on any of these three lists are shown in our Table 1.
Table 1.
Prescription medications used by study participants and included on the MAWA, Rubin [12, 13], or Chang [8, 14] lists (1999–2004 NHANES) a
| Medication | Included on the List of:
|
Medication | Included on the List of:
|
||||
|---|---|---|---|---|---|---|---|
| MAWA | Rubin | Chang | MAWA | Rubin | Chang | ||
| Acebutolol | ---- | Yes | Yes | Mesalamine/Mesalazine | ---- | Yes | Yes |
| Allopurinol | Yes | ---- | Yes | Methimazole | ---- | ---- | Yes |
| Amiodarone | Yes | Yes | ---- | Methyldopa | ---- | Yes | Yes |
| Atenolol b | Yes | Yes | Yes | Metoprolol b | ---- | ---- | Yes |
| Atorvastatin b,c | Yes | Yes | Yes | Minocycline | Yes | Yes | Yes |
| Bupropion | Yes | ---- | ---- | Minoxidil | ---- | Yes | Yes |
| Captopril | Yes | Yes | Yes | Nifedipine b | Yes | ---- | ---- |
| Carbamazepine | Yes | Yes | Yes | Nitrofurantoin | Yes | Yes | Yes |
| Cefuroxime axetil | ---- | ---- | Yes | Penicillin | ---- | ---- | Yes |
| Celecoxib b | Yes | ---- | ---- | Perphenazine | ---- | Yes | Yes |
| Cerivastatin c | ---- | Yes | ---- | Phenytoin b | Yes | Yes | Yes |
| Chlorpromazine | ---- | Yes | Yes | Pindolol | ---- | Yes | Yes |
| Chlorthalidone | ---- | Yes | Yes | Pravastatin b,c | ---- | Yes | Yes |
| Cimetidine | ---- | ---- | Yes | Prazosin | ---- | Yes | Yes |
| Clonidine | ---- | Yes | Yes | Primidone | ---- | Yes | Yes |
| Danazol | ---- | ---- | Yes | Procainamide | ---- | Yes | Yes |
| Diclofenac | ---- | ---- | Yes | Promethazine | ---- | ---- | Yes |
| Diltiazem b | Yes | ---- | ---- | Propranolol b | ---- | ---- | Yes |
| Enalapril b | ---- | Yes | Yes | Propylthiouracil | ---- | Yes | Yes |
| Estrogenic compoundsb | Yes | ---- | ---- | Quinidine | ---- | Yes | Yes |
| Etanercept | ---- | ---- | Yes | Quinine | ---- | Yes | Yes |
| Ethosuximide | ---- | Yes | Yes | Reserpine | ---- | ---- | Yes |
| Fluvastatin c | ---- | Yes | Yes | Simvastatin b,c | Yes | Yes | Yes |
| Griseofulvin | ---- | ---- | Yes | Spironolactone | ---- | ---- | Yes |
| Hydralazine | ---- | Yes | Yes | Sulfasalazine | ---- | Yes | Yes |
| Hydrochlorothiazide b | Yes | Yes | ---- | Sulindac | ---- | ---- | Yes |
| Ibuprofen b | Yes | ---- | Yes | Tetracycline | ---- | ---- | Yes |
| Interferon | ---- | Yes | Yes | Thioridazine | ---- | ---- | Yes |
| Isoniazid | ---- | Yes | Yes | Ticlopidine | ---- | Yes | ---- |
| Labetalol | Yes | Yes | Yes | Timolol | Yes | Yes | Yes |
| Levodopa | ---- | Yes | Yes | Tolazamide | ---- | ---- | Yes |
| Lisinopril b | ---- | Yes | ---- | Tolmetin | ---- | ---- | Yes |
| Lithium | ---- | Yes | Yes | Verapamil b | Yes | ---- | ---- |
| Lovastatin | Yes | Yes | Yes | Zafirlukast | ---- | Yes | ---- |
Abbreviations: ANA = antinuclear antibodies; MAWA = medications associated with ANA.
There are additional medications on the Rubin and Chang lists that were not used by any of the participants and thus are not included in this table.
Included in our covariate-adjusted analysis of individual medications (i.e., used by at least 5 ANA-positive participants).
Included on the Rubin list because Rubin [12] mentioned “lovastatin and other statins.”
In addition to the targeted analysis of medications previously linked to ANA or lupus, we also performed a descriptive analysis to explore whether ANA were positively (or negatively) associated with (1) all prescription medications collectively, (2) prescription medications in established classes, and (3) individual prescription medications, possibly including some not previously reported as being associated with ANA. Overall (collective) use was defined as the use of any medication (yes/no). Medications were then categorized into three sets of classes that coincided with Level 1, Level 2, and Level 3 of the Multum Lexicon Therapeutic Classification Scheme [https://wwwn.cdc.gov/Nchs/Nhanes/1999-2000/RXQ_DRUG.htm]. We also analyzed single medications, used individually or in combinations with other medications.
2.4. Covariates
Previous analyses of these NHANES data documented greater ANA prevalence in women, older participants, non-Hispanic blacks, and participants who were neither overweight nor obese [3, 4]; these participant characteristics were also associated with overall medication use. As a result, we adjusted for them as potential confounders. Age was treated as continuous; race/ethnicity was categorized as non-Hispanic white, non-Hispanic black, or other; and BMI was categorized as obese (≥30 kg/m2), overweight (25 to <30 kg/m2), or neither (<25 kg/m2). The underweight category of BMI (<18.5 kg/m2) was uncommon and did not differ significantly from the healthy category (18.5 to <25 kg/m2) with respect to ANA prevalence; thus, we combined them to form the “neither overweight nor obese” category. We considered additional covariates, such as tobacco smoke exposure, alcohol intake, education, and family poverty income ratio, but none were statistically significantly associated with both ANA and overall medication use, so our analyses did not include them as potential confounders.
2.5. Statistical analyses
Characteristics of the sample are summarized in Table 2, which lists the number and percentage of participants in each category of sex, age, race/ethnicity, and BMI. For each level of these covariates, we also give the number and percentage of participants who were ANA positive or negative and who used or did not use at least one prescription medication. In addition, we give a pair of odds ratios (ORs) and 95% confidence intervals (CIs) for each covariate category to reflect its relationship with ANA status (positive/negative) and with the use of any prescription medications (yes/no). Each OR comes from a logistic regression analysis that adjusted for the complex NHANES sampling design. For these simple covariate summaries, the age analyses did not adjust for any covariates and the other analyses adjusted only for age, via a restricted cubic spline [19] with knots at the 20th, 40th, 60th, and 80th percentiles of the age distribution.
Table 2.
Sample characteristics, their distributions, and odds ratio estimates of their associations with ANA positivity and prescription medication use (1999–2004 NHANES) a
| Characteristic | N (%) | ANA Positivity |
Prescription Medication Use
|
||||
|---|---|---|---|---|---|---|---|
| N+ (%) | N− (%) | OR (95% CI) | D (%) | ND (%) | OR (95% CI) | ||
| Overall | 3608 (100%) | 531 (15%) | 3077 (85%) | ----- | 1963 (54%) | 1645 (46%) | ----- |
| Sex | |||||||
| Male | 1793 (50%) | 196 (11%) | 1597 (89%) | 1.00 (reference) | 863 (48%) | 930 (52%) | 1.00 (reference) |
| Female | 1815 (50%) | 335 (18%) | 1480 (82%) | 2.12 (1.62, 2.80) | 1100 (61%) | 715 (39%) | 1.97 (1.62, 2.38) |
| Age (years) | |||||||
| 18–59 | 2442 (68%) | 345 (14%) | 2097 (86%) | 1.00 (reference) | 1007 (41%) | 1435 (59%) | 1.00 (reference) |
| ≥60 | 1166 (32%) | 186 (16%) | 980 (84%) | 1.27 (0.92, 1.74) | 956 (82%) | 210 (18%) | 5.94 (4.73, 7.47) |
| 18–39 | 1393 (39%) | 194 (14%) | 1199 (86%) | 1.00 (reference) | 423 (30%) | 970 (70%) | 1.00 (reference) |
| 40–59 | 1049 (29%) | 151 (14%) | 898 (86%) | 1.06 (0.79, 1.42) | 584 (56%) | 465 (44%) | 2.50 (2.00, 3.12) |
| ≥60 | 1166 (32%) | 186 (16%) | 980 (84%) | 1.30 (0.93, 1.82) | 956 (82%) | 210 (18%) | 9.22 (7.30, 11.66) |
| 18–29 | 825 (23%) | 114 (14%) | 711 (86%) | 1.00 (reference) | 196 (24%) | 629 (76%) | 1.00 (reference) |
| 30–39 | 568 (16%) | 80 (14%) | 488 (86%) | 0.98 (0.64, 1.50) | 227 (40%) | 341 (60%) | 1.85 (1.38, 2.48) |
| 40–49 | 576 (16%) | 66 (11%) | 510 (89%) | 0.84 (0.60, 1.20) | 279 (48%) | 297 (52%) | 2.59 (1.97, 3.40) |
| 50–59 | 473 (13%) | 85 (18%) | 388 (82%) | 1.34 (0.91, 1.97) | 305 (64%) | 168 (36%) | 5.04 (3.63, 7.00) |
| 60–69 | 523 (14%) | 68 (13%) | 455 (87%) | 1.03 (0.64, 1.66) | 406 (78%) | 117 (22%) | 10.89 (7.75, 15.29) |
| ≥70 | 643 (18%) | 118 (18%) | 525 (82%) | 1.55 (1.02, 2.34) | 550 (86%) | 93 (14%) | 14.58 (10.51, 20.24) |
| Race/Ethnicity | |||||||
| White | 1798 (50%) | 263 (15%) | 1535 (85%) | 1.00 (reference) | 1166 (65%) | 632 (35%) | 1.00 (reference) |
| Black | 658 (18%) | 110 (17%) | 548 (83%) | 1.25 (0.93, 1.68) | 319 (48%) | 339 (52%) | 0.74 (0.59, 0.93) |
| Other | 1152 (32%) | 158 (14%) | 994 (86%) | 0.90 (0.63, 1.27) | 478 (41%) | 674 (59%) | 0.59 (0.46, 0.75) |
| BMI (kg/m2) | |||||||
| Obese | 1105 (31%) | 160 (14%) | 945 (86%) | 0.78 (0.61, 0.99) | 669 (61%) | 436 (39%) | 1.47 (1.21, 1.79) |
| Overweight | 1226 (34%) | 158 (13%) | 1068 (87%) | 0.73 (0.55, 0.98) | 688 (56%) | 538 (44%) | 0.95 (0.73, 1.23) |
| Neither | 1277 (35%) | 213 (17%) | 1064 (83%) | 1.00 (reference) | 606 (47%) | 671 (53%) | 1.00 (reference) |
Abbreviations: ANA = antinuclear antibodies; N (%) = total number (percent) of participants; N+ (%) = number (percent) who were ANA positive; N− (%) = number (percent) who were ANA negative; D (%) = number (percent) who used at least one prescription medication; ND (%) = number (percent) who did not use any prescription medications; OR = odds ratio; CI = confidence interval; White = non-Hispanic white; Black = non-Hispanic black; Other = neither non-Hispanic white nor non-Hispanic black; BMI = body mass index; Obese refers to BMI ≥ 30 kg/m2; Overweight refers to 25 kg/m2 ≤ BMI < 30 kg/m2; Neither refers to BMI < 25 kg/m2.
Each OR was estimated using a logistic regression model for the prevalence of ANA or the prevalence of prescription medication use. All estimates adjusted for the sampling design; the OR estimate for age did not adjust for any covariates; and the other OR estimates adjusted only for continuous age, using a restricted cubic spline with knots at the 20th, 40th, 60th, and 80th percentiles of the age distribution. Participants who were pregnant, under 18 years of age, or had missing data were excluded. Bold typeface indicates a CI that does not include 1 (i.e., is statistically significant at the 0.05 level).
The remaining analyses were based on covariate-adjusted logistic regression models, with a dichotomous indicator of ANA positivity as the response variable and a dichotomous indicator of medication use as the predictor of interest. The one exception was our analysis of the number of medications used, which entered the model as either a linear or categorical predictor. We focused on the association between medication use and ANA positivity, as summarized by the corresponding OR. These analyses adjusted for the complex NHANES sampling design and for sex, age, race/ethnicity, and BMI. A simple linear term was used for age, as the results did not differ materially from those based on a restricted cubic spline. We performed overall analyses of all participants and stratified analyses of sex and age dichotomies.
For each definition of the medication variable, we calculated an OR (and the appropriate 95% CI) to summarize its association with ANA. To avoid problems with interpretation and statistical instability due to limited data, we calculated an OR only if at least 5 medication users were ANA positive. Initial analyses compared ANA prevalence in a test group of participants who used the medication(s) of interest with a reference group who did not, although participants in either group may have also used medications other than the one(s) of interest. A second type of analysis excluded from the reference group all participants who used any medications. Finally, we performed a third type of analysis where, in addition to the reference group excluding those who used any medications, the test group excluded participants who used medications other than the one(s) of interest. These three types of comparisons and analyses are labeled A, B, and C, respectively, and are progressively more homogeneous at the cost of decreasing sample sizes.
Our primary analyses of individual medications aggregated their use alone and in combinations, although secondary analyses assessed single and combined use separately. Most individual medications corresponded to a single drug ID, but we also evaluated a few collections of related medications (e.g., estrogens and statins). We performed sensitivity analyses that excluded 483 participants with a possible autoimmune disease (i.e., those with anti-ENA autoantibodies or self-reported autoimmune disease – thyroid problems, rheumatoid arthritis, or Type 1 diabetes). For medications associated with ANA, we created a categorical variable for duration of use: none, short duration (< median length of use), and long duration (≥ median length of use); the data were too sparse to consider more than three duration-of-use categories.
Our analyses focused on prescription medications as defined by NHANES and did not include dietary supplements or drugs that were exclusively “over the counter.” We could not evaluate medications that were used infrequently in the general population nor those introduced after the 1999–2004 time frame of the ANA study. Extensive medication histories, dosages, and frequencies were not available, and duration of use was only available for medications used in the month preceding the NHANES interview. We could not know if participants stopped using medications prior to the study due to autoimmune complications and there was limited information to exclude autoimmune disease-associated ANA that developed before medication use. Our analysis did not adjust for health behaviors and how they might impact the likelihood of receiving prescription medications. We elaborate on these limitations in our Discussion.
We used the SURVEYLOGISTIC procedure in SAS (version 9.4, SAS Institute, Cary, NC) to perform the logistic regression analyses, which adjusted for the weights, clusters, and strata involved in the complex NHANES sampling design and properly modified the weights in the subgroup analyses. We called a result statistically significant if the 95% CI did not contain 1. We made no corrections for multiple testing because, except for assessing medications on the MAWA list, our analyses were mainly descriptive, exploratory, and hypothesis generating.
3. Results
3.1. Participant characteristics
Of the 3608 participants in our study, 531 (15%) were ANA positive and 1963 (54%) reported using prescription medications in the preceding month (Table 2). Men and women were represented equally; two thirds of the participants were in the younger age group (18–59 years); half were non-Hispanic whites and 18% were non-Hispanic blacks; and one third were overweight and one third were obese. Counts and percentages of participants who were ANA positive or negative and who recently used or did not use prescription medications are listed in Table 2 for each covariate category, along with ORs and 95% CIs for the prevalences of ANA and of overall medication use. Covariate associations with ANA have been reported elsewhere [3]. Prescription medication use was more common in women, non-Hispanic white participants, and obese participants; and, as expected, the prevalence of medication use increased with age.
3.2. Medications previously associated with development of ANA or lupus
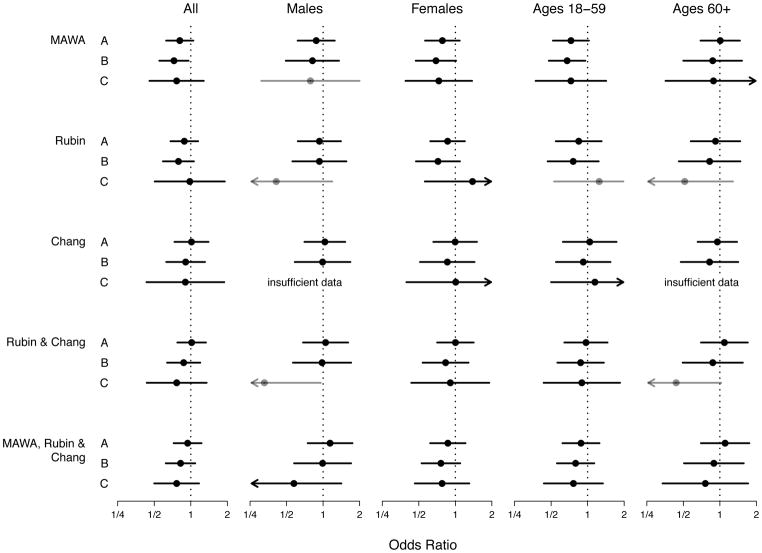
Initially, we focused on the aggregate of all medications on the MAWA list and found no statistically significant association with ANA, either overall or in subgroups of men, women, younger (ages 18–59) participants, and older (ages ≥60) participants (Figure 1, Comparison A). After excluding from the reference group any participants who used medications, the suspected ANA-inducing medications appeared inversely associated with ANA (Figure 1, Comparison B), both overall (OR=0.73; CI=0.55,0.96) and in younger participants (OR=0.68; CI=0.48,0.96). In addition, if the test group excluded participants who used medications not on the MAWA list, none of the associations with ANA were statistically significant (Figure 1, Comparison C), although the CI widths increased because the sample sizes decreased. Across all three types of comparisons and five groups of participants, nearly every OR point estimate was less than 1.
Fig. 1.
Associations between antinuclear antibodies (ANA) and medications previously linked to ANA or lupus. Each solid circle represents a covariate-adjusted estimate of an odds ratio for medication use in a logistic regression model for ANA prevalence, and each horizontal line represents its 95% confidence interval (CI). An arrowhead signifies that a CI extends beyond the plot limits. Black circles and lines indicate that the number of ANA-positive medication users (D+) was at least 10; gray indicates that D+ was at least 5 but less than 10; and analyses with D+ less than 5 are labeled as having insufficient data. The medication-use variable indicates whether a participant reported using at least one medication on the list of interest (MAWA, Rubin, or Chang) during the month before the NHANES interview. All analyses were adjusted for the sampling design; a linear covariate for age; and categorical covariates for sex (if not stratified on sex), race/ethnicity, and body mass index. Participants who were pregnant, under age 18 years, or had missing data were excluded. Type A analyses compared the use of at least one medication on the list of interest (and possibly other medications) with the use of no medications on that list (but possibly other medications). Type B analyses compared the use of at least one medication on the list of interest (and possibly other medications) with the use of no medications. Type C analyses compared the use of at least one medication on the list of interest (but no medications not on that list) with the use of no medications. Separate analyses were performed for all participants and for sex and age subgroups.
We also investigated associations between individual medications on the MAWA list and ANA. Half of these medications were used by fewer than 5 ANA-positive participants, and of the medications used by a sufficient number of participants, only one was individually associated with ANA. Hydrochlorothiazide was inversely associated with ANA if used alone (OR=0.49; CI=0.27,0.91) or, at least in younger participants, if used in combination with other medications (OR=0.44; CI=0.20,0.98) (Supplemental Table S4).
Next, we examined the Rubin and Chang lists of medications previously linked to lupus. Considered collectively, neither list was associated with ANA, for all types of comparisons and groups of participants (Figure 1). The only statistically significant associations with ANA were negative – one for Comparison C in men, after aggregating the Rubin and Chang lists (OR=0.33; CI=0.11,0.96), and one for hydrochlorothiazide, as mentioned above. For completeness, we also merged the MAWA, Rubin, and Chang lists; this collection of suspected ANA or lupus inducers was not associated with ANA, regardless of comparison type or participant group.
3.3. All medications aggregated
To assess the overall association between recent medication use and ANA, we defined a dichotomous variable that indicated the use of any prescription medication. Of the 1963 participants who used at least one medication, 290 (15%) were ANA positive (Table 3). After adjusting for possible confounders, overall medication use was inversely associated with ANA (OR=0.73; CI=0.57,0.93), implying that participants who used medications during the preceding month had a lower prevalence of ANA than those who reported no medication use. The inverse association between overall medication use and ANA was more pronounced in certain groups, such as participants who were younger, white, or neither overweight nor obese. Men and women did not differ with respect to the inverse association between overall medication use and ANA.
Table 3.
Covariate-adjusted odds ratio estimates of associations between overall prescription medication use and ANA positivity within groups (1999–2004 NHANES) a
| Group Analyzed | N | D | D+ | OR (95% CI) |
|---|---|---|---|---|
| All Participants | 3608 | 1963 | 290 | 0.73 (0.57, 0.93) |
| Males | 1793 | 863 | 93 | 0.72 (0.46, 1.13) |
| Females | 1815 | 1100 | 197 | 0.73 (0.53, 1.00) |
| Ages 18–59 years | 2442 | 1007 | 139 | 0.72 (0.53, 0.97) |
| Ages ≥60 years | 1166 | 956 | 151 | 0.82 (0.48, 1.41) |
| Ages 18–39 years | 1393 | 423 | 52 | 0.57 (0.35, 0.94) |
| Ages 40–59 years | 1049 | 584 | 87 | 0.86 (0.55, 1.35) |
| Ages ≥60 years | 1166 | 956 | 151 | 0.82 (0.48, 1.41) |
| Non-Hispanic Whites | 1798 | 1166 | 165 | 0.67 (0.51, 0.89) |
| Non-Hispanic Blacks | 658 | 319 | 57 | 1.13 (0.59, 2.16) |
| Others | 1152 | 478 | 68 | 0.73 (0.41, 1.28) |
| Obese (BMI ≥30 kg/m2) | 1105 | 669 | 98 | 0.72 (0.50, 1.04) |
| Overweight (BMI 25 to <30 kg/m2) | 1226 | 688 | 100 | 1.15 (0.74, 1.81) |
| Neither (BMI <25 kg/m2) | 1277 | 606 | 92 | 0.52 (0.34, 0.78) |
Abbreviations: ANA = antinuclear antibodies; N = number of participants in the group of interest; D = number of participants in the group of interest who used at least one medication; D+ = number of ANA-positive participants in the group of interest who used at least one medication; OR = odds ratio; CI = confidence interval; BMI = body mass index.
Each OR is for overall prescription medication use and was estimated using a logistic regression model for ANA prevalence. The medication use variable indicates whether a participant used at least one prescription medication during the month before the NHANES interview. All estimates adjusted for the sampling design; a linear covariate for age; and categorical covariates for sex, race/ethnicity, and body mass index. Participants who were pregnant, under 18 years of age, or had missing data were excluded. Bold typeface indicates a CI that does not include 1 (i.e., is statistically significant at the 0.05 level).
We also explored associations between the number of medications used and ANA. One analysis treated the number of medications as a quantitative predictor to assess a trend, while other analyses created categorical predictors to examine nonlinear relationships (Supplemental Table S5). The trend analysis suggested that ANA prevalence decreased as the number of medications increased. Categorical analyses assessed 3, 4, 5, and 6 groups for the number of medications used, with the reference category always being no medications used; no obvious patterns emerged. The main message remained that ANA prevalence was lower in participants who recently used medications (one or more) compared with those who did not, as seen in Table 3.
3.4. Classes of medications
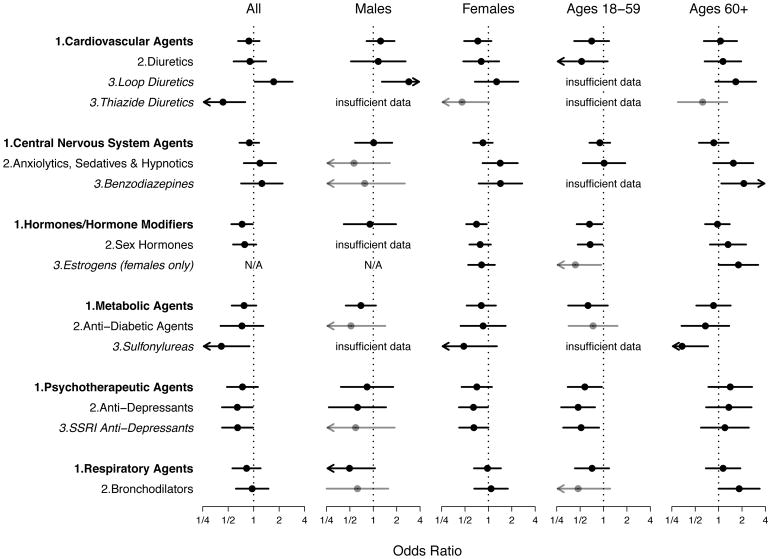
To explore the types of medications driving the inverse association between general medication use and ANA, we examined the Level 1 (broadest), Level 2, and Level 3 (narrowest) medication classes in the Multum Lexicon Therapeutic Classification Scheme. The medication variable referred to the use of any medication in the class of interest. Detailed results for all levels (1, 2, 3) and comparisons types (A, B, C) are given in Supplemental Tables S1A–S3C, while statistically significant results for Type A comparisons are summarized in Figure 2. To provide context, if a result was statistically significant for a given class level, results at broader class levels in the hierarchy are also displayed in Figure 2, regardless of statistical significance.
Fig. 2.
Associations between antinuclear antibodies (ANA) and selected classes of medications. Each solid circle represents a covariate-adjusted estimate of an odds ratio for medication use in a logistic regression model for ANA prevalence, and each horizontal line represents its 95% confidence interval (CI). An arrowhead signifies that a CI extends beyond the plot limits. Black circles and lines indicate that the number of ANA-positive medication users (D+) was at least 10; gray indicates that D+ was at least 5 but less than 10; and analyses with D+ less than 5 are labeled as having insufficient data. The medication-use variable indicates whether a participant reported using at least one medication in the class of interest during the month before the NHANES interview. Level 1, Level 2, and Level 3 classes are identified by bold, plain, and italicized text, respectively, plus the appropriate numeral appended before the class name. All analyses were adjusted for the sampling design; a linear covariate for age; and categorical covariates for sex (if not stratified on sex), race/ethnicity, and body mass index. Participants who were pregnant, under age 18 years, or had missing data were excluded. All results were derived from Type A analyses, which compared the use of at least one medication in the class of interest (and possibly medications in other classes) with the use of no medications in that class (but possibly medications in other classes). Separate analyses were performed for all participants and for sex and age subgroups, except when not applicable (N/A).
The only Level 1 class associated with ANA in all participants was hormones or hormone modifiers (OR=0.73; CI=0.55,0.98). In sex and age subgroups, inverse associations with ANA were also noted for hormones or hormone modifiers in women (OR=0.70; CI=0.52,0.95) and in younger participants (OR=0.66; CI=0.45,0.96), as well as for psychotherapeutic agents in younger participants (OR=0.57; CI=0.35,0.95). In addition to the ten Level 1 classes analyzed (see the full list in Supplemental Table S1A), six other Level 1 classes were used by participants but not analyzed and reported due to small counts (alternative medicines, anti-neoplastics, biologicals, immunologic agents, miscellaneous agents, and nutriproducts).
Among the Level 2 classes within the Level 1 classes mentioned above, sex hormones were inversely associated with ANA in younger participants (OR=0.67; CI=0.47,0.97), as were antidepressants in all participants (OR=0.64; CI=0.42,0.97), in women (OR=0.64; CI=0.42,0.98), and especially in younger participants (OR=0.47; CI=0.29,0.78). Among the remaining Level 2 classes, bronchodilators (a subclass of respiratory agents) were positively associated with ANA in older participants (OR=1.83; CI=1.00,3.38).
Among the Level 3 classes within the Level 1 and 2 classes mentioned above, estrogens were inversely associated with ANA in younger women (OR=0.43; CI=0.20,0.93) but positively associated with ANA in older women (OR=1.80; CI=1.00,3.23), and selective serotonin reuptake inhibitor (SSRI) antidepressants were inversely associated with ANA in all participants (OR=0.65; CI=0.42,0.98), in women (OR=0.65; CI=0.42,1.00), and in younger participants (OR=0.52; CI=0.30,0.88). Among the remaining Level 3 classes, as subclasses of diuretics (within the class of cardiovascular agents), loop diuretics were positively associated with ANA in all participants (OR=1.72; CI=1.03,2.88) and especially in men (OR=2.86; CI=1.29,6.33), whereas thiazide diuretics were inversely associated with ANA in all participants (OR=0.43; CI=0.24,0.79). In addition, benzodiazepines (a subclass of anxiolytics, sedatives, and hypnotics within the class of central nervous system agents) were positively associated with ANA in older participants (OR=2.11; CI=1.09,4.10). Finally, sulfonylureas (a subclass of antidiabetic agents within the class of metabolic agents) were inversely associated with ANA in all participants (OR=0.41; CI=0.19,0.89) and especially in older participants (OR=0.34; CI=0.16,0.73).
We also performed Type B analyses, which reduced the reference group by excluding participants who used any medications. Despite fewer participants and fluctuations in statistical significance, most Type B results did not differ materially from the Type A results (see Supplemental Tables S1B, S2B, and S3B for details). With respect to Level 1 classes, previously nonsignificant inverse associations with ANA became significant for psychotherapeutic agents in all participants (OR=0.64; CI=0.43,0.95) and in women (OR=0.58; CI=0.37,0.91), as well as for cardiovascular agents in women (OR=0.64; CI=0.44,0.92). With respect to Level 2 classes, previously nonsignificant inverse associations with ANA became significant for sex hormones in all participants (OR=0.67; CI=0.47,0.94) and in women (OR=0.61; CI=0.43,0.89), whereas the previously significant positive association with ANA became nonsignificant for bronchodilators in older participants. With respect to Level 3 classes, previously nonsignificant inverse associations with ANA in women became significant for estrogens (OR=0.58; CI=0.39,0.87), SSRI antidepressants (OR=0.53; CI=0.33,0.85), and thiazide diuretics (OR=0.39; CI=0.15,0.98), whereas previously significant positive associations with ANA became nonsignificant for loop diuretics in all participants, benzodiazepines in older participants, and estrogens in older females.
Type C analyses, which further excluded those in the test group who used a medication not in the class of interest, had such small numbers of participants that few assessments seemed sensible (Supplemental Tables S1C, S2C, S3C). Only the Level 1 classes of cardiovascular agents, central nervous system agents, and hormones or hormone modifiers had reasonable numbers. Nevertheless, the inverse association between hormones or hormone modifiers and ANA in women remained statistically significant (OR=0.57; CI=0.33,0.96), while cardiovascular agents and central nervous system agents continued to show no association with ANA.
Finally, to assess which medication class(es) best explained the inverse association between overall medication use and ANA, we excluded participants who used medications in a given class and recalculated the OR for the aggregate of all remaining medications; the closer the revised OR was to 1.0, the greater the role played by the excluded class. Relative to the original OR of 0.73, all revised ORs attenuated toward the null, ranging from 0.75 to 0.80 (Supplemental Table S6), but no class appeared to explain a majority of the overall association. However, after excluding users of either hormones or antidepressants, the OR attenuated to 0.89 overall and 0.97 in females, suggesting a sizeable contribution from those two classes, especially in women.
3.5. Individual medications
We also investigated associations with ANA for individual medications, whether used alone or in combination with other medications. Of the 455 medications that participants in our study reported using, we analyzed the 64 that were used by at least 5 ANA-positive participants (Table 4). Of those, 7 were statistically significantly associated with ANA, either overall or in sex or age strata. We did not consider Type B (and Type C) analyses, which shrunk the reference group (and also the test group), because the numbers of participants were already small.
Table 4.
Individual medications (ordered by number of users) and covariate-adjusted odds ratio estimates of their associations with ANA positivity (1999–2004 NHANES) a
| Medication | D | D+ | OR (95% CI) | Medication | D | D+ | OR (95% CI) |
|---|---|---|---|---|---|---|---|
| Statins | 322 | 42 | 0.78 (0.48, 1.27) | Insulin | 56 | 6 | 1.25 (0.40, 3.91) |
| Estrogenic compoundsb | 283 | 49 | 0.81 (0.58, 1.14) | Paroxetine | 54 | 11 | 1.30 (0.64, 2.62) |
| Hydrochlorothiazide | 266 | 44 | 0.80 (0.53, 1.21) | Verapamil | 53 | 11 | 1.38 (0.55, 3.44) |
| Levothyroxine | 182 | 34 | 0.93 (0.53, 1.62) | Amoxicillin | 51 | 10 | 1.55 (0.66, 3.67) |
| Lisinopril | 168 | 21 | 0.79 (0.42, 1.46) | Benazepril | 50 | 9 | 1.52 (0.62, 3.74) |
| Acetaminophen | 167 | 23 | 0.80 (0.46, 1.39) | Ranitidine | 50 | 9 | 1.21 (0.57, 2.57) |
| Estrogensb | 164 | 31 | 0.82 (0.55, 1.21) | Losartan | 48 | 7 | 1.16 (0.42, 3.26) |
| Atorvastatin | 151 | 16 | 0.61 (0.31, 1.19) | Nifedipine | 45 | 8 | 0.96 (0.36, 2.57) |
| Metformin | 131 | 14 | 0.49 (0.21, 1.14) | Valsartan | 44 | 7 | 0.98 (0.42, 2.30) |
| Estradiolsb | 120 | 18 | 0.95 (0.57, 1.57) | Prednisone | 42 | 7 | 0.64 (0.22, 1.89) |
| Amlodipine | 119 | 21 | 0.99 (0.54, 1.80) | Enalapril | 42 | 5 | 0.63 (0.21, 1.93) |
| Atenolol | 118 | 21 | 1.27 (0.64, 2.54) | Naproxen | 41 | 7 | 1.85 (0.64, 5.30) |
| Furosemide | 112 | 22 | 1.79 (1.09, 2.93) | Alendronate | 40 | 8 | 0.56 (0.21, 1.47) |
| Metoprolol | 104 | 22 | 1.27 (0.68, 2.38) | Cetirizine | 40 | 6 | 0.52 (0.18, 1.49) |
| Albuterol | 93 | 13 | 0.75 (0.38, 1.47) | Clopidogrel | 40 | 6 | 1.12 (0.42, 2.98) |
| Hydrocodone | 90 | 8 | 0.85 (0.34, 2.10) | Pravastatin | 39 | 6 | 1.31 (0.47, 3.67) |
| Simvastatin | 85 | 12 | 0.66 (0.31, 1.39) | Salmeterol | 34 | 9 | 1.49 (0.89, 2.47) |
| Ibuprofen | 83 | 7 | 0.71 (0.29, 1.75) | Trazodone | 32 | 5 | 0.41 (0.10, 1.63) |
| Potassium | 80 | 18 | 1.47 (0.82, 2.66) | Esomeprazole | 30 | 7 | 1.38 (0.50, 3.81) |
| Rofecoxib | 79 | 17 | 1.04 (0.59, 1.83) | Propranolol | 30 | 7 | 1.19 (0.42, 3.37) |
| Triamterene | 73 | 17 | 1.43 (0.76, 2.70) | Tramadol | 30 | 5 | 0.58 (0.21, 1.58) |
| Lansoprazole | 73 | 10 | 0.77 (0.31, 1.92) | Doxazosin | 27 | 5 | 0.88 (0.27, 2.90) |
| Fexofenadine | 71 | 12 | 1.11 (0.64, 1.94) | Glimepiride | 26 | 6 | 0.46 (0.13, 1.63) |
| Omeprazole | 69 | 17 | 2.05 (1.03, 4.10) | Norethindroneb | 26 | 6 | 1.34 (0.45, 4.00) |
| Glyburide | 69 | 6 | 0.40 (0.15, 1.07) | Zolpidem | 25 | 7 | 1.87 (0.79, 4.40) |
| Fluticasone | 66 | 9 | 0.68 (0.40, 1.16) | Codeine | 25 | 6 | 1.90 (0.68, 5.33) |
| Diltiazem | 64 | 12 | 1.16 (0.54, 2.48) | Montelukast | 24 | 6 | 1.91 (0.62, 5.89) |
| Warfarin | 64 | 8 | 0.74 (0.27, 2.06) | Lorazepam | 24 | 5 | 1.76 (0.60, 5.12) |
| Celecoxib | 62 | 9 | 0.56 (0.25, 1.27) | Meclizine | 21 | 5 | 1.34 (0.49, 3.63) |
| Digoxin | 60 | 14 | 1.49 (0.67, 3.32) | Phenytoin | 19 | 8 | 3.67 (0.94, 14.3) |
| Medroxyprogesteroneb | 57 | 11 | 1.01 (0.45, 2.27) | Tolterodine | 15 | 6 | 2.10 (0.50, 8.82) |
| Propoxyphene | 56 | 8 | 0.51 (0.18, 1.46) | Ciprofloxacin | 15 | 5 | 4.23 (1.21, 14.8) |
Abbreviations: ANA = antinuclear antibodies; D = number of participants who used the medication of interest; D+ = number of ANA-positive participants who used the medication of interest; OR = odds ratio; CI = confidence interval.
Each OR is for medication use and was estimated using a logistic regression model for ANA prevalence. The medication use variable indicates whether a participant used the medication of interest, alone or together with other medications, during the month before the NHANES interview. All estimates adjusted for the sampling design; a linear covariate for age; and categorical covariates for sex, race/ethnicity, and body mass index. Participants who were pregnant, under 18 years of age, or had missing data were excluded. Medications used by fewer than 5 ANA-positive participants (i.e., D+ < 5) are not listed. Bold typeface indicates a CI that does not include 1 (i.e., is statistically significant at the 0.05 level).
Results are for females only.
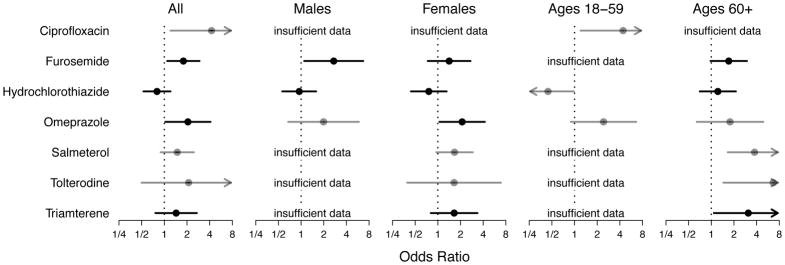
Based on aggregating a given medication’s use alone or in combination, all but one of the statistically significant associations with ANA were positive (Figure 3). Overall, ciprofloxacin (OR=4.23; CI=1.21,14.8), furosemide (OR=1.79; CI=1.09,2.93), and omeprazole (OR=2.05; CI=1.03,4.10) were positively associated with ANA. Stratifying by sex or age, we found positive associations with ANA for furosemide (OR=2.71; CI=1.10,6.69) in men; omeprazole (OR=2.10; CI=1.05,4.21) in women; ciprofloxacin (OR=4.41; CI=1.20,16.2) in younger participants; and salmeterol (OR=3.76; CI=1.66,8.52), tolterodine (OR=6.64; CI=1.45,30.5), and triamterene (OR=3.10; CI=1.08,8.88) in older participants. In contrast, hydrochlorothiazide was inversely associated with ANA in younger participants (OR=0.44; CI=0.20,0.98).
Fig. 3.
Associations between antinuclear antibodies (ANA) and selected individual medications. Each solid circle represents a covariate-adjusted estimate of an odds ratio for medication use in a logistic regression model for ANA prevalence, and each horizontal line represents its 95% confidence interval (CI). An arrowhead signifies that a CI extends beyond the plot limits. Black circles and lines indicate that the number of ANA-positive medication users (D+) was at least 10; gray indicates that D+ was at least 5 but less than 10; and analyses with D+ less than 5 are labeled as having insufficient data. The medication-use variable indicates whether a participant reported using the medication of interest, either alone or in combination with other medications, during the month before the NHANES interview. All analyses were adjusted for the sampling design; a linear covariate for age; and categorical covariates for sex (if not stratified on sex), race/ethnicity, and body mass index. Participants who were pregnant, under age 18 years, or had missing data were excluded. All results were derived from Type A analyses, which compared using the medication of interest (and possibly other medications) with not using that medication (but possibly using other medications). Separate analyses were performed for all participants and for sex and age subgroups.
In addition, we performed separate analyses of single medications used alone and specific combinations of medications used together. Among all participants, hydrochlorothiazide was inversely associated with ANA when used by itself (OR=0.49; CI=0.27,0.91) (see Supplemental Table S4). In contrast, among older participants, we found positive associations with ANA for benazepril alone (OR=5.56; CI=1.31,23.7) and for the combination of hydrochlorothiazide and triamterene (OR=3.93; CI=1.25,12.4).
Finally, we investigated duration of use for the seven medications individually associated with ANA (i.e., those in Figure 3). The data were too sparse to evaluate short-term and long-term use (less than and greater than the median duration, respectively) of ciprofloxacin, salmeterol, and tolterodine. Among all participants, the duration of hydrochlorothiazide and omeprazole use did not significantly alter their associations with ANA, but long-term use of either furosemide or triamterene was associated (positively) with ANA while their short-term use was not associated with ANA (data not shown).
3.6. Other analyses
We examined the nuclear, nucleolar, and cytoplasmic staining patterns for the ANA-positive participants who used any of the seven medications that were individually associated with ANA (Supplemental Table S7). None of those medications was exclusively associated with only one ANA pattern, although the most common pattern was nuclear for all seven medications.
We also performed a sensitivity analysis to assess whether some associations between specific medications and ANA were due to prior autoimmune disease. We excluded participants with anti-ENA autoantibodies or a possible autoimmune disease (of those for which information was available – thyroid problems, rheumatoid arthritis, or Type 1 diabetes). The results of our sensitivity analysis were qualitatively similar to those of our primary analysis; associations were in the same direction and some ORs were even further from the null value of 1 (data not shown).
4. Discussion
Case reports and case series, including some challenge/dechallenge/rechallenge studies, clearly demonstrated that select medications can induce ANA or a lupus-like disease associated with ANA in certain individuals [13]. To shed additional light on those relationships in the general population, and to evaluate a broad range of medications as possible risk or protective factors for autoimmunity, we analyzed data from a large, US population-representative study to assess associations between ANA and various prescription medications, ranging from those previously linked to ANA or lupus to those not having any known relationship with ANA or lupus. To increase participant counts and statistical power, our primary analyses of individual medications aggregated their use alone and in combination with other medications, while secondary analyses also assessed single and combined use separately.
Our targeted analysis of suspected ANA-inducing medications in the general population did not confirm most of the positive ANA associations in clinical reports, whether medications were assessed individually or collectively; one exception was a borderline positive association with ANA for estrogen use in older women. One possible explanation for the dearth of positive associations is that most of the medications studied are not causes of ANA in the general population. Another possibility is that our analysis was not adequately powered to detect many associations. Several medications were not used by enough participants in this general population sample to allow for an adequately powered analysis, especially because some previously reported to induce ANA were no longer prescribed frequently. However, even if statistical significance is ignored, most associations were negative rather than positive. The explanation for this is not clear, and although not well studied, there is little evidence that these medications have general immunosuppressive effects. Thus, our study does not support the hypothesis that medications previously linked to ANA in case reports actually induce ANA in the general population.
The lack of positive associations between previously reported medications and ANA in our study parallels the finding from a population-based, matched, nested case-control study in the UK of medications and lupus [20]. That study found that lupus was positively associated with carbamazepine, minocycline, and possibly hydralazine (none of which we analyzed because fewer than 5 ANA-positive participants in our study sample used them). However, the authors concluded overall that not all medications suggested by case reports and series as causing lupus show a positive association with lupus in a larger, population-based sample.
Given the female predominance for many autoimmune diseases and autoimmunity, our findings for estrogens and ANA are notable. We observed a positive association with ANA for estrogen use in older women (OR=1.80; CI=1.00,3.23), which is consistent with previous findings by Parks et al [4], who investigated reproductive factors in the same NHANES data and reported a positive association with ANA for current use (versus never) of estrogen pills or patches (OR=1.34; CI=0.75,2.37) in postmenopausal women. Also, their estimate of ANA prevalence was lower for premenopausal women who were currently using estrogens or birth control pills (versus never), which agrees with our observation that estrogen use was inversely associated with ANA in younger women (OR=0.43; CI=0.20,0.93). Oral contraceptives and postmenopausal estrogens have both been positively associated with lupus [21, 22], so our findings of an inverse association with ANA in younger/premenopausal women warrants further consideration. Estrogen’s effects on immunity and autoimmunity are complex and vary by age and other hormonal exposures [23, 24]. Moreover, associations of exogenous hormones with ANA may depend on the timing of exposure relative to the onset of autoimmunity, which cannot be determined in a cross-sectional study.
Our exploratory analysis revealed positive associations with ANA for some medications not commonly reported to induce autoimmunity. There are published case reports, however, associating several of these medications with autoimmunity or other autoimmune diseases, even though they are not considered high risk for development of ANA or lupus. For example, ciprofloxacin has been associated with bullous pemphigoid [25] and hemolytic anemia [26]; furosemide with bullous pemphigoid [27] and linear IgA bullous dermatosis [28]; omeprazole with hemolytic anemia [29], interstitial nephritis [30], lymphocytic colitis [31], and ANA with an autoimmune-like syndrome [32]; and triamterene with immune thrombocytopenia [33]. As is the case with medications associated with ANA or lupus, there is no clear explanation for these associations, as these medications do not share any common structures or mechanisms of action.
Our analyses suggested that some of the individual medications associated with ANA (i.e., those in Figure 3) may account for the associations between certain medication classes and ANA (i.e., those in Figure 2). For example, salmeterol is a respiratory agent (Level 1) sometimes used with other agents as a bronchodilator (Level 2), so the positive association with ANA in older participants for this individual medication may partially explain that for bronchodilators as a class. Likewise, furosemide is a cardiovascular agent (Level 1) classified as a loop diuretic (Level 3), so the positive associations with ANA in all participants and in men for this medication may partially explain those for loop diuretics as a class. On the other hand, hydrochlorothiazide used by itself was inversely associated with ANA; this medication is a cardiovascular agent (Level 1) sometimes prescribed as a thiazide diuretic (Level 3), and thus it may partially explain the inverse association observed between thiazide diuretics and ANA.
Interestingly, our analyses revealed that ANA prevalence was lower in participants who recently used medications in general than in those not using any medications. This pattern was similar for men and women, but was more apparent in younger adults, non-Hispanic whites, and persons of normal body weight. We also saw inverse ANA associations with certain common classes of medications. For example, psychotherapeutic medications were inversely associated with ANA, especially in younger adults, and specifically for SSRIs. The observed association of ANA with serotonin reuptake inhibitors was unexpected. However, a growing literature supports a plausible mechanistic role for the neurotransmitter, serotonin, in regulation and modification of immune response [34] and clearance of apoptotic cells [35]. In addition to anti-inflammatory effects, SSRIs may suppress antigen presentation to effector T-cells [36]. Because of their common use in the general population, these novel findings warrant further examination.
The juxtaposition of positive associations for some individual medications with inverse associations for medications in general and in many classes suggests that these inconsistencies deserve additional attention. A few positive ANA associations for certain individual medications may be overwhelmed by many inverse associations for other medications, such that an inverse association is observed when considering all medications together or focusing on a sizable class of medications. Even if the few positive associations are statistically significant and the many inverse associations are not, aggregating all medications (or a large class of medications) could still yield an inverse and statistically significant association with ANA. Our ability to interpret the findings in this cross-sectional analysis is limited by temporal ambiguity, as we do not know if ANA developed before or after medication use. In addition, a number of human leukocyte antigen and other genetic polymorphisms have been reported as either risk or protective factors for drug-induced autoimmunity [13, 37]. Therefore, it is possible that individual genetic risk factors account for medication-associated case reports and small series of ANA and lupus in the literature, but that these genes are not common enough in the general population to allow for significant positive associations in larger cohorts. In contrast, possible medication-associated protective genetic factors may be more common, which may explain the inverse associations seen. The observed inconsistencies warrant additional inquiry with appropriately powered prospective studies to establish the temporal relationship between medication use and ANA, as well as possible genetic risk and protective factors for medication-associated autoimmunity.
Our study had the following strengths: it analyzed a large and population-representative sample; it adjusted for multiple covariates to minimize confounding; and medication use was confirmed by participants, who brought their prescription bottles to the evaluation sites. Further, our study examined potential protective relationships between medications and ANA, which is not possible in individual case reports, series, or pharmaceutical studies.
Nonetheless, our study also had limitations, such as exclusion of institutionalized adults by NHANES; examination of only one titer to define ANA positivity; low power to assess medications infrequently used in the general population; assessment of medication usage only in the month preceding the NHANES interview; lack of information on medication dosage or frequency; inability to investigate recently approved medications and biologic agents given the time frame of the NHANES data; incomplete knowledge of other factors possibly associated with ANA, such as dietary supplements and strictly over-the-counter agents; inability to assess certain health behaviors and how they might impact the likelihood of receiving prescription medications; no knowledge of whether participants stopped using medications prior to the study due to autoimmune complications; and limited clinical information to exclude autoimmune disease-associated ANA that developed before medication use.
Regarding the last limitation, it can be difficult to assess whether ANA were associated with a particular medication, the disease the medication was treating, or a risk factor for the disease the medication was treating (i.e., confounding by indication). The two medications for which we saw stronger positive associations with longer duration of use, furosemide and triamterene, are diuretics that increase urine production through different mechanisms. Another diuretic, hydrochlorothiazide, was inversely associated with ANA. All are used to treat a diversity of conditions, and the small numbers of individuals with ANA in this population sample and limited clinical data preclude further analyses of the medications stratified by specific underlying conditions such as heart failure or kidney disease. However, the fact that some individual medications in our study were associated with ANA while the broader classes containing them were not suggests that the diseases for which a class of medications may be prescribed are themselves not necessarily related to ANA, which increases the likelihood that the specific medications in the class are truly associated with ANA. In other cases, a class of medications was associated with ANA, but not all members of that class were associated with ANA. Our sensitivity analysis excluded participants with a possible autoimmune disease (i.e., those with anti-ENA autoantibodies or self-reported autoimmune disease – thyroid problems, rheumatoid arthritis, or Type 1 diabetes) and obtained results similar to those of our primary analysis. These observations suggest that sometimes ANA were indeed associated with a specific medication rather than the disease being treated by the corresponding class of medications.
5. Conclusions
For the first time, associations between prescription medications and ANA were assessed in a large, US population-representative sample. Most medications reportedly associated with autoimmunity in individuals do not appear to be major causes of ANA in the general US population. However, we discovered that several other medications were positively associated with ANA, whereas medication use in general and many classes of medications were inversely associated with ANA. Further investigation is needed to fully understand the magnitude and significance of these interesting relationships and their impact as risk and protective factors for the development of autoimmunity.
Supplementary Material
Highlights.
Most case reports of medications inducing ANA were not confirmed in the population.
Several novel positive associations between medications and ANA were identified.
Some classes of medications and overall use were inversely associated with ANA.
Clarifying which medications are risk and protective factors requires more research.
Acknowledgments
We are grateful to Drs. Aubrey Miller and Srishti Shrestha for helpful comments, to Lisa Maroski for technical editing, and to members of the NHANES Autoimmunity Study Group (including Drs. M. Satoh, R. Cohn, N. Walker, D. Germolec, I. Whitt, L. Birnbaum, and D. Zeldin) for initiating the studies that motivated much of this research.
Funding support statement
This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences under project ES101074 and contract HHSN273201600011C to Social & Scientific Systems, Inc.
Footnotes
Declarations of interest
The authors have no known conflicts of interest.
References
- 1.Davidson A, Diamond B. Autoimmune diseases. N Engl J Med. 2001;345:340–50. doi: 10.1056/NEJM200108023450506. [DOI] [PubMed] [Google Scholar]
- 2.Giacomelli R, Afeltra A, Alunno A, Baldini C, Bartoloni-Bocci E, Berardicurti O, et al. International consensus: What else can we do to improve diagnosis and therapeutic strategies in patients affected by autoimmune rheumatic diseases (rheumatoid arthritis, spondyloarthritides, systemic sclerosis, systemic lupus erythematosus, antiphospholipid syndrome and Sjogren’s syndrome)?: The unmet needs and the clinical grey zone in autoimmune disease management. Autoimmun Rev. 2017;16:911–24. doi: 10.1016/j.autrev.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Satoh M, Chan EK, Ho LA, Rose KM, Parks CG, Cohn RD, et al. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum. 2012;64:2319–27. doi: 10.1002/art.34380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parks CG, Miller FW, Satoh M, Chan EK, Andrushchenko Z, Birnbaum LS, et al. Reproductive and hormonal risk factors for antinuclear antibodies (ANA) in a representative sample of U.S. women. Cancer Epidemiol Biomarkers Prev. 2014;23:2492–502. doi: 10.1158/1055-9965.EPI-14-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liao KP, Kurreeman F, Li G, Duclos G, Murphy S, Guzman R, et al. Associations of autoantibodies, autoimmune risk alleles, and clinical diagnoses from the electronic medical records in rheumatoid arthritis cases and non-rheumatoid arthritis controls. Arthritis Rheum. 2013;65:571–81. doi: 10.1002/art.37801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansen KE, Arnason J, Bridges AJ. Autoantibodies and common viral illnesses. Semin Arthritis Rheum. 1998;27:263–71. doi: 10.1016/s0049-0172(98)80047-4. [DOI] [PubMed] [Google Scholar]
- 7.Mongey AB, Hess EV. Drug insight: autoimmune effects of medications-what’s new? Nat Clin Pract Rheumatol. 2008;4:136–44. doi: 10.1038/ncprheum0708. [DOI] [PubMed] [Google Scholar]
- 8.Chang C, Gershwin ME. Drugs and autoimmunity--a contemporary review and mechanistic approach. J Autoimmun. 2010;34:J266–J75. doi: 10.1016/j.jaut.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Miller FW, Alfredsson L, Costenbader KH, Kamen DL, Nelson LM, Norris JM, et al. Epidemiology of environmental exposures and human autoimmune diseases: findings from a National Institute of Environmental Health Sciences Expert Panel Workshop. J Autoimmun. 2012;39:259–71. doi: 10.1016/j.jaut.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinse GE, Jusko TA, Whitt IZ, Co CA, Parks CG, Satoh M, et al. Associations Between Selected Xenobiotics and Antinuclear Antibodies in the National Health and Nutrition Examination Survey, 1999–2004. Environ Health Perspect. 2016;124:426–36. doi: 10.1289/ehp.1409345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller FW, Pollard KM, Parks CG, Germolec DR, Leung PS, Selmi C, et al. Criteria for environmentally associated autoimmune diseases. J Autoimmun. 2012;39:253–8. doi: 10.1016/j.jaut.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubin RL. Drug-induced lupus. Toxicology. 2005;209:135–47. doi: 10.1016/j.tox.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 13.Rubin RL. Drug-induced lupus. Expert Opin Drug Saf. 2015;14:361–78. doi: 10.1517/14740338.2015.995089. [DOI] [PubMed] [Google Scholar]
- 14.Xiao X, Chang C. Diagnosis and classification of drug-induced autoimmunity (DIA) J Autoimmun. 2014;48–49:66–72. doi: 10.1016/j.jaut.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual. Human Kinetics Books; Champaign, IL: 1988. [Google Scholar]
- 16.Cardonick E. Pregnancy-associated breast cancer: optimal treatment options. Int J Womens Health. 2014;6:935–43. doi: 10.2147/IJWH.S52381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rich-Edwards JW, Fraser A, Lawlor DA, Catov JM. Pregnancy characteristics and women’s future cardiovascular health: an underused opportunity to improve women’s health? Epidemiol Rev. 2014;36:57–70. doi: 10.1093/epirev/mxt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller FW, Hess EV, Clauw DJ, Hertzman PA, Pincus T, Silver RM, et al. Approaches for identifying and defining environmentally associated rheumatic disorders. Arthritis Rheum. 2000;43:243–9. doi: 10.1002/1529-0131(200002)43:2<243::AID-ANR2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 19.Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. Springer International Publishing; 2015. [Google Scholar]
- 20.Schoonen WM, Thomas SL, Somers EC, Smeeth L, Kim J, Evans S, et al. Do selected drugs increase the risk of lupus? A matched case-control study. Br J Clin Pharmacol. 2010;70:588–96. doi: 10.1111/j.1365-2125.2010.03733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costenbader KH, Feskanich D, Stampfer MJ, Karlson EW. Reproductive and menopausal factors and risk of systemic lupus erythematosus in women. Arthritis Rheum. 2007;56:1251–62. doi: 10.1002/art.22510. [DOI] [PubMed] [Google Scholar]
- 22.Bernier Mo, Mikaeloff Y, Hudson M, SuissA S. Combined oral contraceptive use and the risk of systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2009;61:476–81. doi: 10.1002/art.24398. [DOI] [PubMed] [Google Scholar]
- 23.Hughes GC, Choubey D. Modulation of autoimmune rheumatic diseases by oestrogen and progesterone. Nat Rev Rheumatol. 2014;10:740–51. doi: 10.1038/nrrheum.2014.144. [DOI] [PubMed] [Google Scholar]
- 24.Giefing-Kroll C, Berger P, Lepperdinger G, Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015;14:309–21. doi: 10.1111/acel.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cozzani E, Chinazzo C, Burlando M, Romagnoli M, Parodi A. Ciprofloxacin as a Trigger for Bullous Pemphigoid: The Second Case in the Literature. Am J Ther. 2016;23:e1202–4. doi: 10.1097/MJT.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 26.Garbe E, Andersohn F, Bronder E, Klimpel A, Thomae M, Schrezenmeier H, et al. Drug induced immune haemolytic anaemia in the Berlin Case-Control Surveillance Study. Br J Haematol. 2011;154:644–53. doi: 10.1111/j.1365-2141.2011.08784.x. [DOI] [PubMed] [Google Scholar]
- 27.Lee JJ, Downham TF., 2nd Furosemide-induced bullous pemphigoid: case report and review of literature. J Drugs Dermatol. 2006;5:562–4. [PubMed] [Google Scholar]
- 28.Cerottini JP, Ricci C, Guggisberg D, Panizzon RG. Drug-induced linear IgA bullous dermatosis probably induced by furosemide. J Am Acad Dermatol. 1999;41:103–5. doi: 10.1016/s0190-9622(99)70414-7. [DOI] [PubMed] [Google Scholar]
- 29.Butt MI, Sajid S, Sobolewski S. Autoimmune haemolytic anaemia due to Omeprazole. Ir Med J. 2007;100:372. [PubMed] [Google Scholar]
- 30.Yip D, Kovac S, Jardine M, Horvath J, Findlay M. Omeprazole-induced interstitial nephritis. J Clin Gastroenterol. 1997;25:450–2. doi: 10.1097/00004836-199709000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez-Banares F, de Sousa MR, Salas A, Beltran B, Piqueras M, Iglesias E, et al. Epidemiological risk factors in microscopic colitis: a prospective case-control study. Inflamm Bowel Dis. 2013;19:411–7. doi: 10.1002/ibd.23009. [DOI] [PubMed] [Google Scholar]
- 32.Sivakumar K, Dalakas MC. Autoimmune syndrome induced by omeprazole [letter] Lancet. 1994;344:619–20. doi: 10.1016/s0140-6736(94)92008-7. [DOI] [PubMed] [Google Scholar]
- 33.Garbe E, Andersohn F, Bronder E, Salama A, Klimpel A, Thomae M, et al. Drug-induced immune thrombocytopaenia: results from the Berlin Case-Control Surveillance Study. Eur J Clin Pharmacol. 2012;68:821–32. doi: 10.1007/s00228-011-1184-3. [DOI] [PubMed] [Google Scholar]
- 34.Ahern GP. 5-HT and the Immune System. Curr Opin Pharmacol. 2011;11:29–33. doi: 10.1016/j.coph.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka T, Doe JM, Horstmann SA, Ahmad S, Ahmad A, Min SJ, et al. Neuroendocrine signaling via the serotonin transporter regulates clearance of apoptotic cells. J Biol Chem. 2014;289:10466–75. doi: 10.1074/jbc.M113.482299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Branco-de-Almeida LS, Kajiya M, Cardoso CR, Silva MJB, Ohta K, Rosalen PL, et al. Selective serotonin reuptake inhibitors attenuate the antigen presentation from dendritic cells to effector T lymphocytes. FEMS Immunol Med Microbiol. 2011;62:283–94. doi: 10.1111/j.1574-695X.2011.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller FW. Genetics of environmentally-associated rheumatic disease. In: Kaufman LD, Varga J, editors. Rheumatic Diseases and the Environment. London: Arnold Publishers; 1999. pp. 33–45. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.