Abstract
Background
The use of mulitmodal pain regimens have been shown to be an effective technique for the treatment of post-operative pain after total knee arthroplasty. Periarticular injections, of both short and long acting anesthetics, have emerged as an additional method of providing significant improvement in post-operative pain relief. The purpose of this study was to compare the efficacy of periarticular injection using long-acting versus short-acting preparations.
Methods
A randomized prospective study of 80 consecutive patients was performed comparing liposomal bupivacaine versus plain bupivacaine periarticular injection. The primary outcomes included pain relief, total narcotic usage, and completion of physical therapy goals, specifically range-of-motion.
Results
No significant improvements were noted between liposomal bupivacaine and plain bupivacaine injection groups in overall pain reduction, range of motion or total narcotic usage. At 24 hours, small statistically significant differences in physical therapy pain scores were noted with liposomal bupivacaine versus plain bupivacaine and control patients, but these differences did not persist at later time points. Both preparations demonstrated statistically significant improvements in range of motion when compared to historical controls, but no differences were noted between preparations.
Conclusion
Overall, minimal significant differences were noted between liposomal bupivacaine and plain bupivacaine at early and late time points. Both preparations of periarticular injection demonstrated superiority over control pain regimens, but were relatively equivalent to one another in direct comparison
Keywords: total knee arthroplasty, liposomal bupivacaine, bupivacaine, physical therapy, pain
Introduction
Analgesia is a critical post-operative component of total knee arthroplasty (TKA). Multimodal pain regimens have emerged as an effective way to treat the various causes of surgical pain and allow patients early return to activity and satisfactory levels of comfort. Poor control of post-operative pain and high opioid requirements can delay patient mobilization and physical therapy, prolonging hospital stay, and ultimately decrease patient satisfaction and outcomes [1–3]. The numerous side-effects and complications of opioids, a mainstay of conventional TKA pain management, are well documented [4]. Periarticular injections for the treatment of surgical pain have gained widespread traction as an adjunct to oral and intravenous pain regimens, with the goal of providing improved pain control while decreasing the administration of systemic analgesics [5,6]. A variety of periarticular injection ‘cocktails’ have been described, and may include local anesthetics, nonsteroidal antiinflammatory drugs, steroids, or other locally active agents [7].
The local anesthetics bupivacaine or ropivacaine are an important component of TKA periarticular injection. A new slow-release formulation of liposomal bupivacaine has been introduced with the potential to improve clinical efficacy of periarticular injections. However, clinical studies have demonstrated mixed efficacy. There have been a series of studies suggesting this slow-release formulation of bupivacaine may be superior to standard periarticular injections [8,9]. In addition, studies have demonstrated pain control with liposomal bupivacaine to be equivalent to that provided by intrathecal morphine or femoral nerve block (FNB), with less systemic side effects [9–11].These latter FNB comparison studies document improved early ambulation, higher percentage of physical therapy milestones met, decreased opitate consumption and decreased hospital length of stay with liposomal bupivacaine preparation [10–12]. Other studies have demonstrated equivalent or inferior pain control with long-acting liposomal bupivacaine preparations as compared to short actining bupivacaine [13–16].
The conclusions drawn from early comparison studies have been unclear and difficult to apply in clinical practice. Limitations of these studies include retrospective design and comparisons between liposomal bupivacaine periarticular injection to a multiomodal pain regimen cocktail that contains numerous active agents. In addition, they had limited patient reported outcome measures. More recent prospective studies have compared periarticular injection with liposomal bupivacaine to intra-articular bupivacaine infusion (ON-Q*) and liposomal bupivacaine versus normal bupivacaine as part of a larger periarticular injection cocktail. In these studies, no significant differences were noted in narcotic consumption, VAS pain score, or hospital length of stay [17–19]. Though these studies evaluate secondary ‘functional’ outcomes, these studies lack the necessary combination of pain control and objective functional outcome (i.e. knee range of motion, total distance walked, etc.).
The goal of the study was to prospectively compare liposomal bupivacaine to traditional bupivacaine at multiple time points and patient reported outcomes. These endpoints include pain scores, pain medicine usage, and post-operative range of motion. We hypothesized that long-acting, liposomal bupivacaine would provide better pain relief and achieve increased progress with physical therapy when compared to traditional bupivacaine preparations and historical control patients.
Materials and Methods
This prospective, randomized, blinded, institutional review board-approved study enrolled eighty (n = 80) consecutive patients that met inclusion criteria as set forth by the experimental protocol. Inclusion criteria included cemented primary total knee arthroplasty, ability to receive regional anesthetic with intrathecal morphine injection, ability to participate in the institution’s multimodal pain pathway, and no history of prior chronic opioid abuse or withdrawal. Multimodal pain pathway, as defined by our institution, consisted of pre-operative oral dosing of acetaminophen, pregabalin, celecoxib, spinal administration of bupivacaine and morphine and post-operative dosing of oral narcotic, celecoxib, pregabalin. At time of enrollment, the participants were randomized to receive a periarticular injection with either plain bupivacaine (PB) or liposomal bupivacaine (LB). Both patients and staff (nursing and physical therapy) were blinded between the periarticular injection groups. 2 patients were excluded from the LB group secondary to further surgical intervention during the hospitalization (periprosthetic fracture and acute wound dehiscence). In retrospective fashion, 40 consecutive patients that met all the inclusion criteria prior to the intitiation of the study served as a historical control. No pre-study power analysis was performed. This was a trial study where a set number of forty (n = 40) vials of 20 cc liposomal bupivacaine were made available by the health system.
Patients were screened and selected based upon the aforementioned inclusion criteria and were provided written and oral explanations of the goals of study prior to obtaining informed consent. All surgical procedures were performed by two fellowship trained orthopaedic surgeons. Patients received appropriate medication as set forth by the institutional pre-operative multimodal pain pathway prior to initiation of intrathecal medication and surgery. Once in the operating room, 20cc of either 0.5% plain bupivicaine or liposomal bupivacaine was mixed with 70cc normal saline and divided among three 30cc syringes based upon the selected randomized group. This accounted for the manufacturer instructions, which called for 1 vial (20cc) of liposomal bupivacaine to be diluted into 70cc normal saline for a total volume of 90cc. The plain bupivacaine was diluted in the same fashion so that identical volumes were injected in the operating room. After completion of bone preparation, 30cc of the periarticular injection was infiltrated into posterior capsule (avoiding the midline) and the periosteum of the femur and tibia. After cementation of implants, 30cc of the periarticular injection was infiltrated along the arthrotomy including the quadriceps and patellar tendon. The last 20cc was injected throughout the subcutaneous layer. A 21 gauge needle was used for the injection and multiple small injections of the solution was performed at each of the afformentioned sites. We followed the manufacturer’s suggested protocol for injection.
Patients experienced routine care as part of the institutional post-operative multimodal pain and rehabilitation pathways, including the afformentioned post-operative pain regimen and day of surgery mobilization with daily continuous passive motion (CPM).
Data collected in the perioperative period included demographic(s); age, sex, BMI, ASA physical status score. Pain scores, narcotic usage, and completion of physical therapy goals including objective reporting of range of motion were recorded as primary outcomes. All data was reported using summary statistics including means and standard deviations for quantitative data, and frequencies and percentages for qualitative data. P-values for Age and BMI were obtained using F-tests. P-values for ASA and LOS were obtained using Kruskall-Wallis tests. P-values for Surgeon, Gender and D/C disposition were obtained using Fisher’s Exact test. Pain medication usage, PT pain scores, and ROM were analyzed using a linear mixed-effects model controlling for effects of gender and surgeon. Pairwise comparisons between groups were made using t-tests produced by the model fits. P-values were adjusted using a Bonferroni correction to account for multiple comparisons. A simple linear regression model was used to investigate differences in total medication used. Time to first narcotic was analyzed using Wilcoxon Rank Sum tests, and p-values were adjusted for multiple comparisons using a Bonferroni correction.
Results
There were a total of 40 patients in the plain bupivicaine group that received periarticular injection with plain bupivacaine, 38 patients in the liposomal bupivacaine group that received periarticular injection with liposomal bupivacaine, and 40 historical controls that received no periarticular injection but were enrolled in the institutional pre- and post-operative pain pathway. There were no statistical differences between age, BMI, ASA status, but a statistically significant imbalance in gender was noted (Table 1). Further multivariable analysis demonstrated this gender imbalance did not alter results.
Table 1.
Patient Demographics Characteristics
| All (N=118) |
Liposomal Bupivacaine (N=38) |
Plain Bupivicaine (N=40) |
Control (N=40) |
P-value | |
|---|---|---|---|---|---|
|
| |||||
| Age, mean (SD) | 62.7 (8.5) | 63.2 (7.2) | 64.3 (8.8) | 60.7 (9.0) | 0.1454 |
|
| |||||
| BMI, mean (SD) | 35.4 (6.7) | 35.5 (7.4) | 35.4 (6.6) | 35.2 (6.3) | 0.9773 |
|
| |||||
| ASA, median [IQR] | 3 [2 – 3] | 3 [2 – 3] | 3 [2 – 3] | 2 [2 – 3] | 0.0595 |
|
| |||||
| Gender, N (%) | |||||
| Male | 42 (64.4) | 19 (50.0) | 14 (35.0) | 9 (22.5) | 0.0439 |
| Female | 76 (35.6) | 19 (50.0) | 26 (65.0) | 31 (77.5) | |
No significant statistical differences were noted in patiet age, BMI, ASA, but a statistically significant imbalance in gender was noted (p = .0439).
Post-operative physical therapy pain scores and objective measure pain scores were obtained at 24 and 48 hr timepoints, and objective measurements of flexion/extension were recorded on postoperative day 1 and at time of discharge. A statistically significant decrease in pain score during PT was noted during the first 24 hours in comparison of liposomal bupivacaine group to controls and liposomal bupivacaine to plain bupivacaine, but this was not observed at later time points (Table 2). In terms of motion, a statistically significant improvement in early motion was noted for both periarticular injection groups as compared to control. Periarticular injection demonstrated an improvement in post-operative flexion, 82.7 deg (LB) and 80.0 deg (PB) compared to 66.4 deg (controls) on post-operative day 1. These differences were not observed at later time points, and there was no statistically significant difference in direct comparison between liposomal and plain bupivacaine groups (Table 3).
Table 2.
Physical Therapy Pain Scores
| Time | PT Pain Score | P-Value(s) | ||||
|---|---|---|---|---|---|---|
| LB | PB | Control | LB v C | PB v C | LB v PB | |
| 24 hrs | 5.4 | 6.9 | 7.3 | 0.005 | 1.00 | 0.03 |
| 48 hrs | 3.9 | 5 | 5.2 | 0.10 | 1.00 | 0.17 |
Physical therapy pain scores at both 24 hrs and 48 hrs post-op were recorded for both treatment groups and historical controls. A significant decrease in pain score during physical therapy was noted at early time point between the LB group and historical controls, but this was not observed at later time points.
Table 3.
Post-operative Range-of-Motion (ROM)
| Time | Motion | Measurement (degrees) | P-value(s) | ||||
|---|---|---|---|---|---|---|---|
| LB | PB | Control | LB v C | PB v C | LB v PB | ||
| POD#1 | Extension | −7.6 | −7.7 | −8.5 | 0.45 | 0.79 | 1.00 |
| Flexion | 82.7 | 80.0 | 66.4 | <0.0001 | <0.0001 | 1.00 | |
| Discharge | Extension | −5.8 | −6.2 | −4.9 | 0.01 | 0.38 | 1.00 |
| Flexion | 87.2 | 89.1 | 85.3 | 1.00 | 0.56 | 1.00 | |
Post-operative range-of-motion (ROM) data was recorded for both treatment groups and historical controls. A significant improvement in early motion was noted for both periarticular injection groups, but these differences were not observed at later time points.
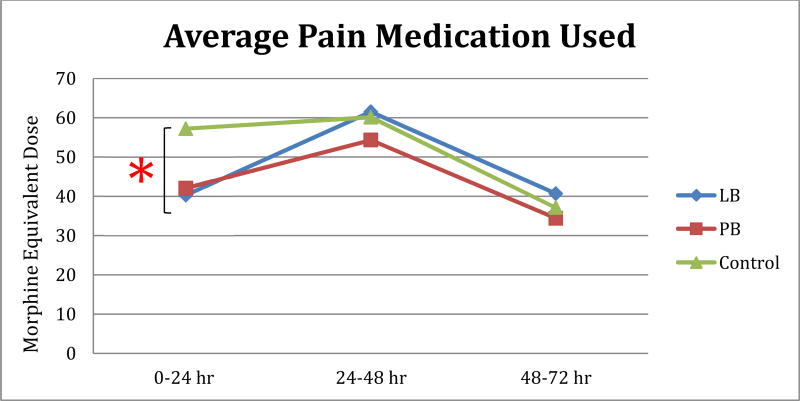
Pain control was assessed by observing total morphine equivalent dosage (MED) between groups (Figure 1). Average morphine equivalent dose required by patients at time points 0–24 hr, 24–48, hr and 48–72 hr and average total morphine equivalent dose were recorded and comparison tests were performed. The only statistically significant difference noted was in the comparison between liposomal bupivacaine and historical control patients at the 0–24 hr (early) time point. This significant difference was not observed at later time points. There were no differences observed between the liposomal bupivacaine and plain bupivicaine group in any of the measures of total pain medication administered. In terms of total morphine equivalent dose used within the hospitalization, no significant differences were noted.
Figure 1. Average Pain Medication Used.
Average Morphine Equivalent Dose (MED). A significant difference in average morphine equivalent dose (MED) required at early time point (0–24 hr) was noted in comparison of control subjects and the LB group, but this difference did not persist at later time points
Discussion
Periarticular injections for the treatment of surgical pain have gained widespread traction as an adjunct to oral and intravenous pain regimens, with the goal of providing improved pain control while decreasing the administration of systemic analgesics [5,6]. In our prospective, randomized study, periarticular injection, whether liposomal bupivacaine or plain bupivicaine, resulted in decreased pain and improved functional performance in early physical therapy as compared to the control group. We could not clinically differentiate any meaningful difference in pain or functional outcomes between the liposomal bupivacaine or plain bupivicaine group. Despite this lack of significance, interesting differences were seen in both pain medication use and physical therapy achievements across all groups (no periarticular injection, traditional periarticular injection, etc.)
Post-operative pain is a critical component of a successful arthroplasty. Poor control of post-operative pain and high opioid requirements can delay patient mobilization and physical therapy, prolonging hospital stay, and ultimately decrease patient satisfaction and outcomes [1–3]. In this study, our data demonstrated decreases in pain medication used at early post-operative time points with the use of periarticular injection. This effect was statistically significant for liposomal bupivacaine and as compared to the control group at up to 24 hrs postoperatively but the effects after that point were equivocal. Though plain bupivicaine did not demonstrate statistical significance (p = 0.06), large, clinically significant decreases in pain medication consumption were observed within this group compared to control group, These findings are comparable to other findings in the current literature. Surdam et al. and Yu et al. demonstrated decreased opiate consumption with liposomal bupivacaine compared to femoral nerve block [10,11]. In our study, average pain medicine administed was decreased in the early time point for liposomal bupivacaine and plain bupivicaine groups, showing an early decrease in post-operative pain and requirement for opiate. Though these results were noted in multiple time points by Barrington et al., the effect of liposomal bupivacaine and plain bupivicaine was only realized in the early time point when compared to control groups. Additionally, there was no statistically significant difference between the liposomal bupivacaine and plain bupivicaine groups.
Adequate post-operative pain control and avoidance of opiate-driven systemic side effects allows for earlier mobilization and more effective physical therapy. Improved early ambulation and a higher percentage of physical therapy milestones met has been demonstrated in the literature with liposomal bupivacaine periarticular injection [10–12]. In our study, we hypothesized that liposomal bupivacaine would demonstrate significant improvements in objective knee range of motion values when compared to plain bupivacaine and control groups. Likewise, these earlier improvements in therapy milestones would allow for persistent, improved motion at discharge. The data revealed that statistically significant improvements were noted in both pain scores during physical therapy and objective ROM at early time point (0–24 hr) in liposomal bupivacaine versus controls (p < 0.05). Statistically significant improvements in pain control with plain bupivicaine versus controls at early time point were also observed. In addition, the improvements seen at early time points did not persist in comparison to non-periarticular injection control subjects at later time points and discharge. This decrease in early PT pain and improved flexion noted with liposomal bupivacaine and plain bupivicaine compared to control supports the notion of improved motion in the early post-operative period with periarticular injection, but the effect does not appear to be resilient in comparison to non-periarticular injection groups.
A cost comparison between the two different periarticular injections demonstrated a cost of $2.28 for 20 cc vial of 0.5% plain bupivacaine versus $285.00 for a 20 cc vial of liposomal bupivacaine, minus the cost of 70 cc of normal saline that was added to both substances for dilution. This amounts to an added cost of $282.72 for liposomal bupivacaine in comparison to plain bupivacaine. This is an important consideration in an era of value-based orthopaedic care that accounts for both outcome and cost. From the results of our study, the added cost of liposomal bupivacaine does not improve the quality of care provided in post-operative pain management.
The collection of objective physical therapy measurements are a strength of our study. A significant improvement is noted in the early post-operative motion for both liposomal bupivacaine and plain bupivacaine periarticular injection. This highlights an inherent strength of periarticular injection in the post-operative pain regimen; local anesthesia aimed directly at the soft tissue locations about the knee can allow patients to achieve greater motion at earlier time points. This suggests that periarticular injections significantly increase objective physical therapy flexion in early time points. Liposomal bupivacaine was observed to be more effective in reducing pain during the first 24 hours. However, this effect was not seen at later time points. This is interesting, as the theorized advantage of liposomal bupivacaine is a prolonged release and analgesic effect due to a slower, more controlled release of active medication.
There were limitations in our study deisgn. First, the use of a historical control group did not allow all groups to be prospective. This was necessary to allow the liposomal bupivacaine and plain bupivicaine groups to be completely blinded. Adding a group that receieved no injection into the prospective arm would have introduced bias. The decision to use of 40 consecutive patients that all underwent surgical intervention prior to the initiation of the study was aimed at eliminating or limiting any aspect of bias. Second, there were no pre-study power analyses or sample size calculations performed prior to execution of the study. However, it is important to note that a total of forty (n = 40) vials of 20 cc liposomal bupivacaine were made available to our study group by health system, and subsequent study size was determined based on this limit. In addition, no post-hoc power analyses were performed based on results of the study to avoid the introduction of biased results. Finally, an unexpected but statistically significant heterogeneity was identified in patient demographics related to male versus female gender (p < 0.05). Multivariate analysis demonstrated the gender was not a statistical significant variable that predicted differences in pain scores or functional outcomes.
In TKA, multimodal pain management that includes either liposomal bupivacaine or plain bupivicaine periarticular injection as compared to no periarticular injection results in better pain control as measured by patient reported outcome scores and functional assements. In our study, patients who received liposomal bupivacaine had better pain experience at 24 hrs postoperatively in terms of total morphine equivalent dosage and pain score during physical therapy when compared to non-PA control subjects. This benefit was not observed at later time points nor was it statistically different than the plain bupivicaine preparation group. Statistically significant improvements in early post-operative flexion were observed with the use of periarticular injection, whether liposomal bupivacaine or plain bupivacaine, compared to historical controls, but no statistically significant differences were observed between periarticular injection group in terms of objective physical therapy outcome. These findings continue to document the success of periarticular injection in the treatment of post-operative pain in TKA, and does not support any clinical difference between long-acting liposomal and standard bupivacaine in periarticular injection.
Supplementary Material
Acknowledgments
Dr. Kenneth Urish is supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS K08AR071494), the National Center for Advancing Translational Science (NCATS KL2TR0001856), the Orthopaedic Research and Education Foundation, and the Musculoskeletal Tissue Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was performed at the Department of Orthopaedic Surgery and Clinical and Translation Science Institute at the University of Pittsburgh, Pittsburgh, PA, USA
Contributor Information
Jason P. Zlotnicki, Department of Orthopaedic Surgery, University of Pittsburgh School of Medicine, Pittsburgh, PA 15261.
Brian R. Hamlin, The Bone & Joint Center, Magee Womens Hospital of the University of Pittsburgh Medical Center, Pittsburgh, PA 15212.
Anton Y. Plakseychuk, The Bone & Joint Center, Magee Womens Hospital of the University of Pittsburgh Medical Center, Pittsburgh, PA 15212.
Timothy J. Levison, The Bone & Joint Center, Magee Womens Hospital of the University of Pittsburgh Medical Center, Pittsburgh, PA 15212.
Scott Rothenberger, Clinical and Translation Science Institute; Department of Medicine, University of Pittsburgh, Pittsburgh, PA 15213.
Kenneth Urish, Arthritis and Arthroplasty Design Group; The Bone and Joint Center, Magee Womens Hospital of the University of Pittsburgh Medical Center; Department of Orthopaedic Surgery, Department of Bioengineering, and Clinical and Translational Science Institute, University of Pittsburgh; Department of Biomedical Engineering, Carnegie Mellon University, Pittsburgh, PA, 15219; urishk2@upmc.edu.
References
- 1.Röstlund T, Kehlet H. High-dose local infiltration analgesia after hip and knee replacement--what is it, why does it work, and what are the future challenges? Acta Orthop. 2007 Apr;78(2):159–61. doi: 10.1080/17453670710013627. [DOI] [PubMed] [Google Scholar]
- 2.Busch CA, Whitehouse MR, Shore BJ, MacDonald SJ, McCalden RW, Bourne RB. The efficacy of periarticular multimodal drug infiltration in total hip arthroplasty. Clin Orthop Relat Res. 2010 Aug;468(8):2152–9. doi: 10.1007/s11999-009-1198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parvataneni HK, Shah VP, Howard H, Cole N, Ranawat AS, Ranawat CS. Controlling pain after total hip and knee arthroplasty using a multimodal protocol with local periarticular injections: a prospective randomized study. J Arthroplasty. 2007 Sep;22(6 Suppl 2):33–8. doi: 10.1016/j.arth.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 4.Oderda GM, Evans RS, Lloyd J, Lipman A, Chen C, Ashburn M, et al. Cost of opioid-related adverse drug events in surgical patients. J Pain Symptom Manage. 2003;25:276e83. doi: 10.1016/s0885-3924(02)00691-7. [DOI] [PubMed] [Google Scholar]
- 5.Busch CA, Shore BJ, Bhandari R, Ganapathy S, MacDonald SJ, Bourne RB, et al. Efficacy of periarticular multimodal drug injection in total knee arthroplasty. J Bone Joint Surg Am. 2006;88:959e63. doi: 10.2106/JBJS.E.00344. [DOI] [PubMed] [Google Scholar]
- 6.Broome CB, Burnikel B. Novel strategies to improve early outcomes following total knee arthroplasty: a case control study of intra articular injection versus femoral nerve block. Int Orthop. 2014;38 doi: 10.1007/s00264-014-2392-0. 2087e9Barrington et al., 2015. [DOI] [PubMed] [Google Scholar]
- 7.Barrington JW, Olugbode O, Lovald S, Ong K, Watson H, Emerson RH Jr. Liposomal Bupivacaine: A Comparative Study of More Than 1000 Total Joint Arthroplasty Cases. Orthop Clin North Am. 2015 Oct;46(4):469–77. doi: 10.1016/j.ocl.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Bramlett K, Onel E, Viscusi ER, et al. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee. 2012;19(5):530. doi: 10.1016/j.knee.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Barrington JW, Emerson RH, Lovald ST, Lombardi AV, Berend KR. No Difference in Early Analgesia Between Liposomal Bupivacaine Injection and Intrathecal Morphine After TKA. Clin Orthop Relat Res. 2017 Jan;475(1):94–105. doi: 10.1007/s11999-016-4931-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Surdam JW, Licini DJ, Baynes NT, Arce BR. The use of exparel (liposomal bupivacaine) to manage postoperative pain in unilateral total knee arthroplasty patients. J Arthroplasty. 2015 Feb;30(2):325–9. doi: 10.1016/j.arth.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Yu S, Szulc A, Walton S, Bosco J, Iorio R. Pain Control and Functional Milestones in Total Knee Arthroplasty: Liposomal Bupivacaine versus Femoral Nerve Block. Clin Orthop Relat Res. 2017 Jan;475(1):110–117. doi: 10.1007/s11999-016-4740-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chughtai M, Cherian JJ, Mistry JB, Elmallah RD, Bennett A, Mont MA. Liposomal Bupivacaine Suspension Can Reduce Lengths of Stay and Improve Discharge Status of Patients Undergoing Total Knee Arthroplasty. J Knee Surg. 2016 Jul;29(5):e3. doi: 10.1055/s-0036-1584272. [DOI] [PubMed] [Google Scholar]
- 13.Bagsby DT, Ireland PH, Meneghini RM. Liposomal bupivacaine versus traditional periarticular injection for pain control after total knee arthroplasty. J Arthroplasty. 2014 Aug;29(8):1687–90. doi: 10.1016/j.arth.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 14.Klug MJ, Rivey MP, Carter JT. Comparison of Intraoperative Periarticular Injections Versus Liposomal Bupivacaine as Part of a Multimodal Approach to Pain Management in Total Knee Arthroplasty. Hosp Pharm. 2016 Apr;51(4):305–11. doi: 10.1310/hpj5104-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuang MJ, Du Y, Ma JX, He W, Fu L, Ma XL. The Efficacy of Liposomal Bupivacaine Using Periarticular Injection in Total Knee Arthroplasty: A Systematic Review and Meta-Analysis. J Arthroplasty. 2017 Apr;32(4):1395–1402. doi: 10.1016/j.arth.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 16.White S, Vaughan C, Raiff D, Eward W, Bolognesi M. Impact of liposomal bupivacaine administration on postoperative pain in patients undergoing total knee replacement. Pharmacotherapy. 2015 May;35(5):477–81. doi: 10.1002/phar.1587. [DOI] [PubMed] [Google Scholar]
- 17.DeClaire JH, Aiello PM, Warritay OK, Freeman DC. Effectiveness of Bupivacaine Liposome Injectable Suspension for Postoperative Pain Control in Total Knee Arthroplasty: A Prospective, Randomized, Double Blind, Controlled Study. J Arthroplasty. 2017 Sep;32(9S):S268–S271. doi: 10.1016/j.arth.2017.03.062. [DOI] [PubMed] [Google Scholar]
- 18.Smith EB, Kazarian GS, Maltenfort MG, Lonner JH, Sharkey PF, Good RP. Periarticular Liposomal Bupivacaine Injection Versus Intra-Articular Bupivacaine Infusion Catheter for Analgesia After Total Knee Arthroplasty: A Double-Blinded, Randomized Controlled Trial. J Bone Joint Surg Am. 2017 Aug 16;99(16):1337–1344. doi: 10.2106/JBJS.16.00571. [DOI] [PubMed] [Google Scholar]
- 19.Schroer WC, Diesfeld PG, LeMarr AR, Morton DJ, Reedy ME. Does Extended-Release Liposomal Bupivacaine Better Control Pain Than Bupivacaine After Total Knee Arthroplasty (TKA)? A Prospective, Randomized Clinical Trial. J Arthroplasty. 2015 Sep;30(9 Suppl):64–7. doi: 10.1016/j.arth.2015.01.059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.