Summary
Animal-microbe facultative symbioses play a fundamental role in ecosystem and organismal health. Yet, due to the flexible nature of their association, the selection pressures that act on animals and their facultative symbionts remain elusive. Here we apply experimental evolution to Drosophila melanogaster associated with its growth-promoting symbiont Lactobacillus plantarum, representing a well-established model of facultative symbiosis. We find that the diet of the host, rather than the host itself, is a predominant driving force in the evolution of this symbiosis. Furthermore, we identify a mechanism resulting from the bacterium's adaptation to the diet, which confers growth benefits to the colonized host. Our study reveals that bacterial adaptation to the host's diet may be the foremost step in determining the evolutionary course of a facultative animal-microbe symbiosis.
Keywords: symbiosis, lactobacilli, microbiota, Drosophila, experimental evolution
Graphical Abstract
Highlights
-
•
L. plantarum experimental evolution leads to the improvement of its symbiotic benefit
-
•
L. plantarum increases its growth-promotion ability by adapting to Drosophila diet
-
•
Mutation of ackA gene enhances both L. plantarum fitness and benefit to the host
-
•
N-acetyl-glutamine production is sufficient to improve L. plantarum growth promotion
Martino et al. demonstrate that, in the symbiosis between Drosophila and Lactobacillus plantarum, the host diet represents the driving force in the evolution of L. plantarum symbiotic effect. This is a clear example of by-product mutualism, where the host capitalizes on the by-products of the self-serving traits of their symbionts.
Introduction
Animal-microbe symbioses are ubiquitous and their nature can be extremely diverse. Mutualistic relationships are those symbioses whereby both partners benefit from each other (Bronstein, 1994). They can differ in the number of species involved, the duration of the symbiotic relationship, and how dependent the partners are on the interaction for their development, survival, and reproduction (Douglas, 2011). In obligate mutualism, the organisms depend on each other for their survival. In many cases, this co-dependency has occurred over time as each organism adapts to the reciprocal benefits (Holland and Bronstein, 2008). In facultative symbioses, microbes and their hosts are not fully dependent on each other: the host can survive without its bacterial symbionts, which, in turn, can live in different ecosystems regardless of the host presence (Gilbert and Neufeld, 2014). Nevertheless, facultative microbial symbionts confer crucial benefits to their animal partners (Feldhaar, 2011, Ferrari and Vavre, 2011). The flexible nature of facultative mutualism suggests that there are both costs and benefits associated with maintaining such symbiosis (Bronstein, 1994, Douglas, 2011, Engel and Moran, 2013, Fisher et al., 2017). However, the ecological and evolutionary forces that drive the emergence and evolution of the benefits conferred by facultative symbionts to their animal hosts remain largely elusive.
To address this question, we experimentally tested microbial evolution using Drosophila melanogaster associated with one of its most abundant facultative symbionts, Lactobacillus plantarum, with whom it establishes nutritional mutualism (Douglas, 2011, Erkosar et al., 2015, Ma et al., 2015, Matos et al., 2017, Storelli et al., 2011, Storelli et al., 2018). L. plantarum positively affects juvenile growth rate and maturation when Drosophila faces chronic undernutrition (Storelli et al., 2011). Such benefit results, at least in part, from the capacity of L. plantarum to promote the expression of larval intestinal peptidases and the consequent increase of dietary protein digestion and amino acid intake by the host (Erkosar et al., 2015, Matos et al., 2017). Conversely, L. plantarum benefits from its animal partner. Although L. plantarum encounters a strong cost during transit through larval gut, larvae secrete bacterial maintenance factors that counteract this cost and improve microbial fitness, thus perpetuating symbiosis (Storelli et al., 2018). Drosophila/L. plantarum association also represents a prototypical case of facultative symbiosis. Indeed, L. plantarum, as well as most Drosophila facultative symbionts, does not colonize the host intestine, but remains associated with Drosophila during its entire life cycle by constant reassociation through cycles of ingestion and excretion (Blum et al., 2013, Broderick et al., 2014, Storelli et al., 2011, Storelli et al., 2018). In addition, it is vertically transmitted to progenies via the deposition of contaminated mother's feces on the surface of the embryo during egg laying and on the surrounding substratum (Matos and Leulier, 2014).
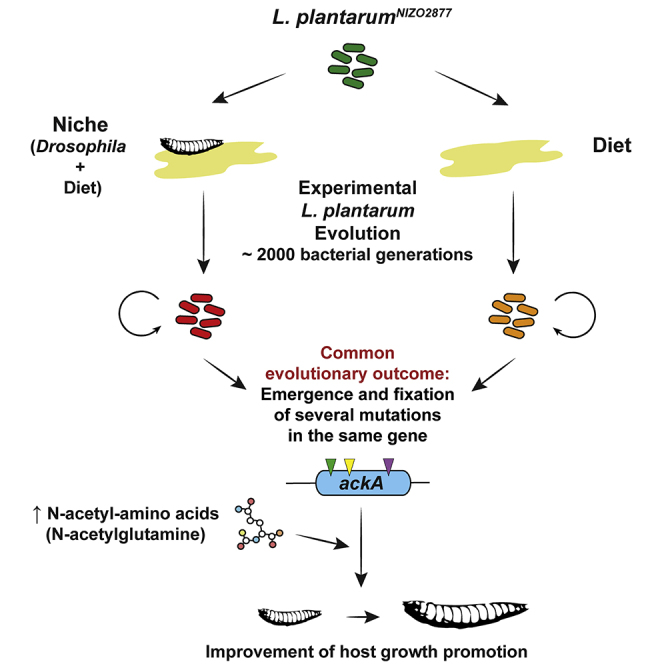
Here we show that the host nutritional environment, instead of the host, is a predominant driving force in the emergence and evolution of symbiotic benefits that L. plantarum confers to its animal partner. By applying experimental evolution to a moderate Drosophila growth-promoting strain L. plantarumNIZO2877 (Schwarzer et al., 2016), we found that the de novo mutations in the same acetate kinase (ackA) locus invariably emerge first and rapidly become fixed, and such evolution occurs with or without the host. Furthermore, we demonstrate that ackA mutations trigger the increased production of N-acetylated amino acids by the evolved strain, including N-acetyl-glutamine, a compound that is sufficient to confer improved Drosophila growth capabilities when provided together with the ancestral bacterial strain. Our study therefore identifies a specific mechanism by which a symbiotic bacterial strain increases its benefit to its animal host, and reveals that adaptation to the host diet is a foremost step in the emergence and perpetuation of facultative animal-microbe symbioses.
Results
Experimental Evolution of L. plantarum with D. melanogaster Improves Its Growth-Promoting Effect
As growth promotion during chronic undernutrition is one of the major advantages conferred by L. plantarum to its animal host (Schwarzer et al., 2016, Storelli et al., 2011), we asked if and how this bacterium can increase its potential to support animal growth while both partners face chronic undernutrition.
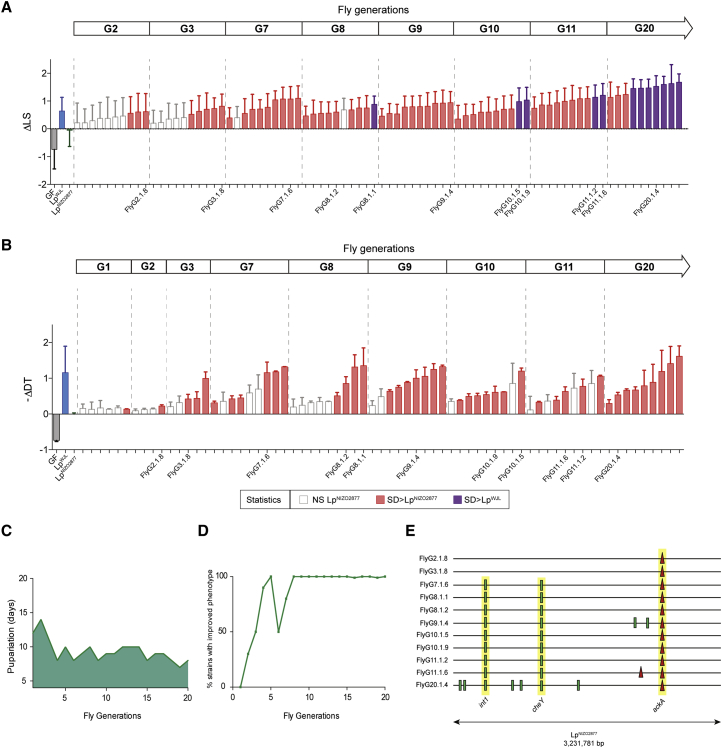
To this end, we performed experimental evolution of NIZO2877 (LpNIZO2877), a strain of L. plantarum isolated from processed human food (Martino et al., 2015a), which was previously shown to moderately promote growth both in Drosophila and mice (Schwarzer et al., 2016). We mono-associated germ-free (GF) Drosophila eggs with a fully sequenced clonal population of LpNIZO2877 on a low-nutritional diet and studied the partners for 20 Drosophila generations (i.e., 313 days, corresponding to about 2,000 bacterial generations; see STAR Methods and Figure S1). At each generation, we selected the first emerging pupae carrying a subpopulation of L. plantarum strains and transferred them to a new sterile diet (STAR Methods and Figure S1A). The adults rapidly emerged from the pupae and deposited the new embryos and their associated L. plantarum strains that subsequently colonized and propagated in the new environment. We then isolated the LpNIZO2877-evolved strains associated with the adult flies that emerged from the transferred pupae, selected a representative set of isolates, and measured individually their growth-promoting capacity on an independent set of naive GF fly larvae. After only two fly generations (i.e., after about 124 bacterial generations, Figures 1A and 1B), we identified a few evolved LpNIZO2877 strains that significantly improved larval growth and accelerated pupariation timing compared with the ancestor strain. Specifically, the evolved strains exhibited the same effect as LpWJL, a potent L. plantarum growth-promoting strain (Martino et al., 2015b, Storelli et al., 2011) (Figures 1A and 1B). These results show that the evolution of LpNIZO2877 in the context of its symbiosis with Drosophila leads to the rapid improvement of L. plantarum animal growth promotion (Figures 1C and 1D).
Figure 1.
Experimental Evolution of L. plantarum with D. melanogaster Improves Its Growth-Promoting Effect
(A) Longitudinal size of larvae (LS) measured 7 days after egg deposition (AED) on poor-nutrient diet. Larvae were kept germ-free (GF) or associated with LpNIZO2877 (ancestor), LpWJL (growth-promoting L. plantarum strain), or LpNIZO2877-evolved strains. The Delta in larval size (ΔLS) shows the difference between the size of larvae associated with the respective condition and the size of larvae associated with LpNIZO2877 from Drosophila generation 2 (G2) to generation 20 (G20). Each bar refers to an LpNIZO2877-evolved strain isolated from the first replicate of experimental evolution from G2 to G20. LpNIZO2877-evolved strains that exhibited a significant difference (improved) at promoting larval growth compared with the ancestor strain (Student's t test: p < 0.05) are shown in red. LpNIZO2877-evolved strains that exhibited a significant difference (improved) at promoting larval growth compared with the beneficial L. plantarum LpWJL strain are shown in purple.
(B) Developmental timing (DT) of individuals that were kept GF or associated with LpNIZO2877, LpWJL, or LpNIZO2877-evolved strains isolated from Drosophila G1 to G20. The minus Delta in developmental timing (−ΔDT) is calculated from the mean time of emergence of 50% of the pupae associated with the respective condition and the mean time of emergence of 50% of the pupae associated with LpNIZO2877, and shown in the graph. LpNIZO2877-evolved strains that exhibited a significant difference at accelerating DT compared with the ancestor strain (Student's t test: p < 0.05) are shown in red. The evolved strains that have been selected for further analyses are labeled on the x axis.
(C) Difference in maturation time of individuals associated with LpNIZO2877-evolved strains along the first replicate of LpNIZO2877 adaptive evolution. The mean pupariation time of the first 15 individuals at each fly generation is shown on the y axis.
(D) Percentage of LpNIZO2877-evolved strains isolated at each fly generation that were found to be significantly better than the LpNIZO2877 at increasing larval size. Ten bacterial isolates were randomly isolated at the end of each fly generation from newly emerged adult Drosophila (see Figure S1A) and reassociated with new GF Drosophila embryos to quantify their ability to promote larval growth (see A).
(E) Mutations identified in LpNIZO2877-evolved strains from Drosophila generation 2 (G2) to generation 20 (G20) represented along the LpNIZO2877 genome. The genome of each evolved strain is represented as a horizontal line. Red triangles indicate deletions and small green bars show SNPs. Mutations occurring in the same gene of different strains and fixed along the experimental evolution are highlighted in yellow (int1, cheY, ackA).
Genome Sequencing Reveals the Appearance and Fixation of a Single Mutation in L. plantarum ackA Gene
To identify the genetic changes underlying the rapid microbial adaptation responsible for the improved growth of the host, we sequenced the genomes of 11 evolved LpNIZO2877 strains (Table S1, replicate 1) with increased host growth-promoting potential sampled across the 20 Drosophila generations. We identified a total of 11 mutations, including nine SNPs and two small deletions (Figure 1E and Table S2). In particular, in the strain isolated from the second fly generation (FlyG2.1.8), we found a single change in the genome within one of the three acetate kinase genes (ackA). Remarkably, this first mutation was subsequently fixed and strictly correlated with the improved animal growth phenotype (Figure 1E). Following ackA mutation, additional variants appeared along L. plantarum experimental evolution, which seem to correlate with further improvement of symbiotic benefit (Figure 1A).
Independent Replicate of Experimental Evolution Confirms that L. plantarumNIZO2877 Improves Its Growth-Promoting Effect through Mutation of the ackA Gene
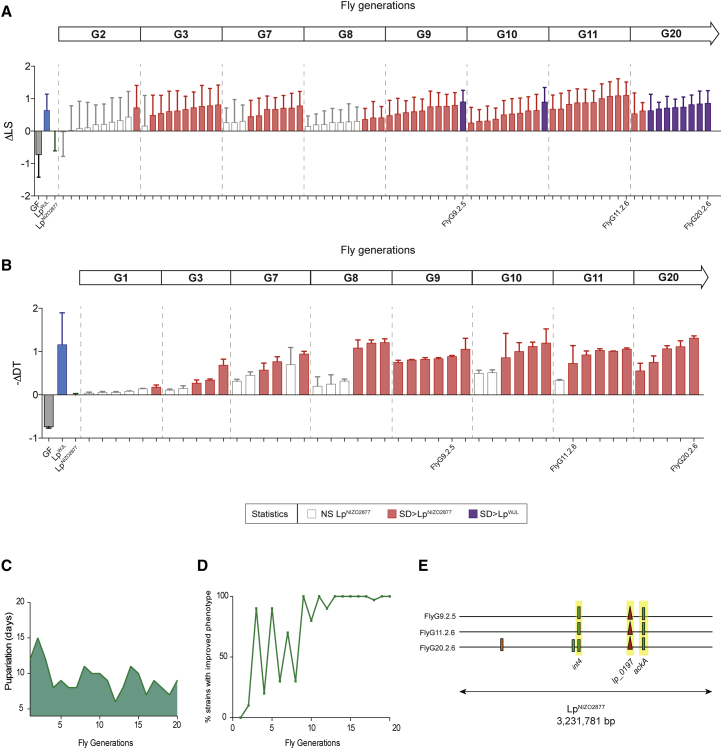
To test the repeatability of our findings, we conducted an independent replicate of L. plantarum experimental evolution while in symbiosis with Drosophila. Both the phenotypic and genomic evolution of L. plantarum were again obtained: LpNIZO2877 improved its animal growth-promoting potential by rapidly acquiring and fixing mutations, including variants in the ackA gene (Figure 2 and Table S2). In the first experiment, the evolved LpNIZO2877 strains with improved animal growth potential all carried a three-nucleotide deletion in the ackA gene that removed one proline residue. From the second replicate, the evolved strains carried a SNP that resulted in a premature stop codon leading to protein truncation (Figure S2). These independently isolated mutations likely generate an inactive ackA protein. Although both replicates of L. plantarum experimental evolution show additional mutations besides the ackA variant (Figures 1E and 2E), the two evolved strains each bearing only one mutation in ackA (FlyG2.1.8 and FlyG3.1.8) already showed a statistically significant Drosophila growth improvement compared with their ancestor (Figures 1A and 1B). This suggests that acquiring an ackA mutation is sufficient to confer an increased Drosophila growth-promotion potential. Therefore, we propose that the de novo appearance of the ackA mutation is the first fundamental step in shaping the evolutionary trajectory in the LpNIZO2877/Drosophila symbiosis model.
Figure 2.
Second Replicate of LpNIZO2877 Adaptive Evolution Confirms the Ability of L. plantarum Evolved Strains to Improve Fly Growth
(A) Difference in longitudinal size of larvae (LS) measured 7 days AED on poor-nutrient diet. Larvae were kept GF or associated with LpNIZO2877, LpWJL, or LpNIZO2877-evolved strains. The Delta in larval size (ΔLS) shows the difference between the size of larvae associated with the respective condition and the size of larvae associated with the ancestor from Drosophila generation 2 (G2) to generation 20 (G20). Each bar refers to an LpNIZO2877-evolved strain isolated from the second replicate of experimental evolution from G2 to G20. LpNIZO2877-evolved strains that exhibited a significant difference (improved) at promoting larval growth compared with the ancestor strain (Student's t test: p < 0.05) are shown in red. LpNIZO2877-evolved strains that exhibited a significant difference (improved) at promoting larval growth compared with the beneficial L. plantarum LpWJL strain are shown in purple.
(B) Developmental timing (DT) of individuals that were kept GF or associated with LpNIZO2877, LpWJL, or LpNIZO2877-evolved strains isolated from Drosophila G1 to G20. The minus Delta in developmental timing (−ΔDT) is calculated from the mean time of emergence of 50% of the whole adult population associated with the respective condition and the mean time of emergence of 50% of the whole adult population associated with LpNIZO2877, and shown in the graph. LpNIZO2877-evolved strains that exhibited a significant difference at accelerating developmental timing compared with the ancestor strain (Student's t test: p < 0.05) are shown in red. The evolved strains that have been selected for further analyses are labeled on the x axis.
(C) Difference in maturation time of individuals associated with LpNIZO2877-evolved strains along the second replicate of LpNIZO2877 adaptive evolution. The pupariation time of the first 15 individuals at each fly generation is shown on the y axis.
(D) Percentage of LpNIZO2877 –evolved strains isolated at each fly generation that were found to be significantly better than the ancestor at increasing larval size during the second replicate of L. plantarum adaptive evolution. Ten bacterial isolates were randomly isolated at the end of each fly generation from newly emerged adult Drosophila (see Figure S1) and reassociated with new GF Drosophila embryos to quantify their ability to promote larval growth.
(E) Mutations identified in LpNIZO2877-evolved strains isolated from the second replicate of experimental evolution from Drosophila generation 9 (G9) to generation 20 (G20) represented along LpNIZO2877 genome. The genome of each evolved strain is represented as a horizontal line. Red triangles indicate deletions and small green bars show SNPs. Mutations occurring in the same gene of different strains and fixed along the experimental evolution are highlighted in yellow (int4, lp_0197, ackA).
ackA Mutation Is Necessary to Improve L. plantarumNIZO2877 Growth-Promoting Effect
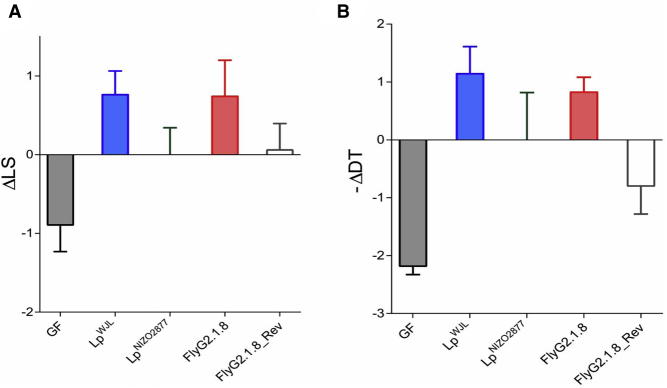
To fully establish that ackA mutation is responsible for the evolution of LpNIZO2877/Drosophila symbiosis, we employed CRISPR/Cas9-based bacterial genetic engineering (Jiang et al., 2013) to reinsert the deleted CCT triplet in the FlyG2.1.8 ackA locus (Figure S3), so that we genetically reverted the ackA allele in the FlyG2.1.8 isolate back to its ancestral form. The reverted strain (FlyG2.1.8Rev) bearing the ancestral ackA allele lost its increased capacity to promote animal growth when compared with the ancestor strain (Figure 3). These results therefore demonstrate that the ackA mutation in LpNIZO2877 is a causative change resulting in faster and increased Drosophila growth.
Figure 3.
ackA Mutation Is Sufficient to Improve L. plantarumNIZO2877 Growth-Promoting Effect
(A) Longitudinal size of larvae measured 7 days AED on poor-nutrient diet. Larvae were kept germ-free (GF) or associated with LpNIZO2877, LpWJL, FlyG2.1.8, or with FlyG2.1.8-reverted strain (FlyG2.1.8Rev). The Delta in larval size (ΔLS), the difference between the size of larvae associated with the respective L. plantarum strain and the size of larvae associated with LpNIZO2877, is shown. FlyG8.2.1 strain, exhibiting a significant difference (improved) at promoting larval growth compared with LpNIZO2877 (Student's t test: p < 0.05), is shown in red. Strains that did not exhibit a significant difference (improved) at promoting larval growth compared with LpNIZO2877 are shown in white.
(B) Developmental timing (DT) of individuals that were kept GF or associated with LpNIZO2877, LpWJL, FlyG2.1.8 or with FlyG2.1.8Rev strain. The minus Delta in developmental timing (−ΔDT) between the mean time of emergence of 50% of the pupae associated with the respective condition and the mean time of emergence of 50% of the pupae associated with LpNIZO2877 is shown in the graph. FlyG8.2.1 strain, exhibiting a significant difference (improved) at promoting larval growth compared with LpNIZO2877 (Student's t test: p < 0.05), is shown in red. Strains that did not exhibit a significant difference (improved) at promoting larval growth compared with LpNIZO2877 are shown in white.
ackA Confers Competitive Advantage to L. plantarum Evolved Strains in Both Presence and Absence of the Host
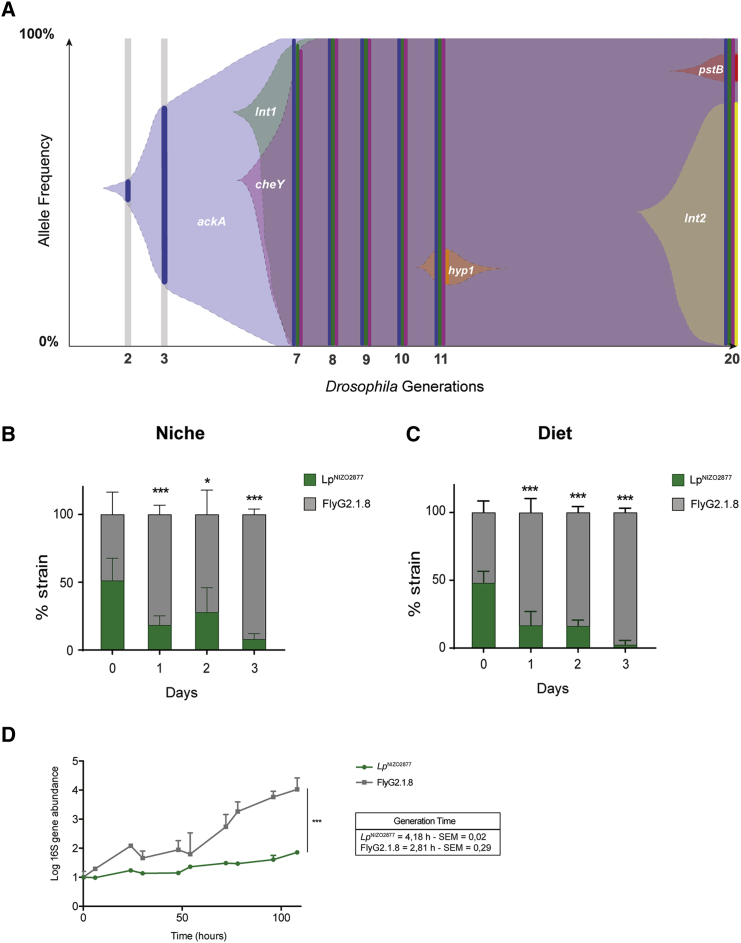
To investigate the complete L. plantarum population dynamics while in symbiosis with Drosophila, we sequenced the metagenome of whole bacterial population samples across the 20 Drosophila generations of the first replicate experiment. We identified both segregating and fixed mutations and tracked their frequencies through time (STAR Methods). We found that the ackA mutation was the first variant to appear in the population. Remarkably, the ackA variant showed a rapid selective sweep and became fixed as early as after three Drosophila generations (Figure 4A). This observation suggests a competitive advantage of the evolved LpNIZO2877 strains bearing this variant. To test this hypothesis, we performed a competition assay between the ancestral LpNIZO2877 strain and the derived FlyG2.1.8 isolate in symbiosis with Drosophila (STAR Methods; Figures 4B and S4). We found that the evolved strain bearing only the ackA mutation started outcompeting the ancestor strain as early as after 1 day, demonstrating that the ackA mutation confers a strong competitive advantage in symbiosis with Drosophila. To test whether such advantage requires the host's presence, we performed the same competition assay by inoculating only the bacterial strains on the Drosophila nutritional environment (i.e., the diet). Surprisingly, we observed that FlyG2.1.8 outcompeted the ancestral strain even when the Drosophila host was absent (Figure 4C). To characterize the nature of such competitive advantage, we measured the growth rate of both strains on the Drosophila nutritional environment. We find that the evolved strain FlyG2.1.8 was able to replicate much faster than its ancestral strain LpNIZO2877 in the Drosophila diet (Figure 4D), which contributes to establishing its competitive advantage. Taken together, these results show that L. plantarum evolved strains bearing the ackA variant shows higher fitness compared with their ancestor in our experimental settings, and that their competitive advantage is likely independent of the animal host.
Figure 4.
LpNIZO2877-Evolved Strain Shows Higher Fitness Compared with the Ancestor
(A) Muller diagram showing the genome evolutionary dynamics of LpNIZO2877 population (replicate I) along 20 Drosophila generations. The y axis shows the percentage of the detected frequencies of each mutation (plain colors). Shaded areas represent the inferred allele frequencies. Lower axis shows the fly generation where the sampling took place.
(B and C) 1:1 competitive assay between LpNIZO2877 and LpNIZO2877-evolved strain (FlyG2.1.8) in poor-nutrient diet with Drosophila larvae (B) and without Drosophila larvae (C). Error bars represent the percentage of each strain detected in each sample (Niche or Diet) by qPCR. ∗p < 0.05, ∗∗∗p < 0.01 obtained by Student's t-test.
(D) 16S rRNA kinetics of LpNIZO2877 and FlyG2.1.8 in the Drosophila nutritional environment. Absolute quantification of the 16rRNA gene (ng/μL) has been conducted for each time point. 16S rRNA gene quantification is shown in logarithmic scale. The values have been normalized by the mean of t0 (time 0). The mean generation time (h) of each strain ± SEM is reported on the graph (see STAR Methods). The result of the non-parametric analysis of covariance (sm.ancova function in R) between the curves is reported (∗∗∗p < 0.005).
L. plantarum Evolution and Improvement in Symbiotic Benefit Is Driven by the Adaptation to the Host Nutritional Environment Rather Than to Its Host
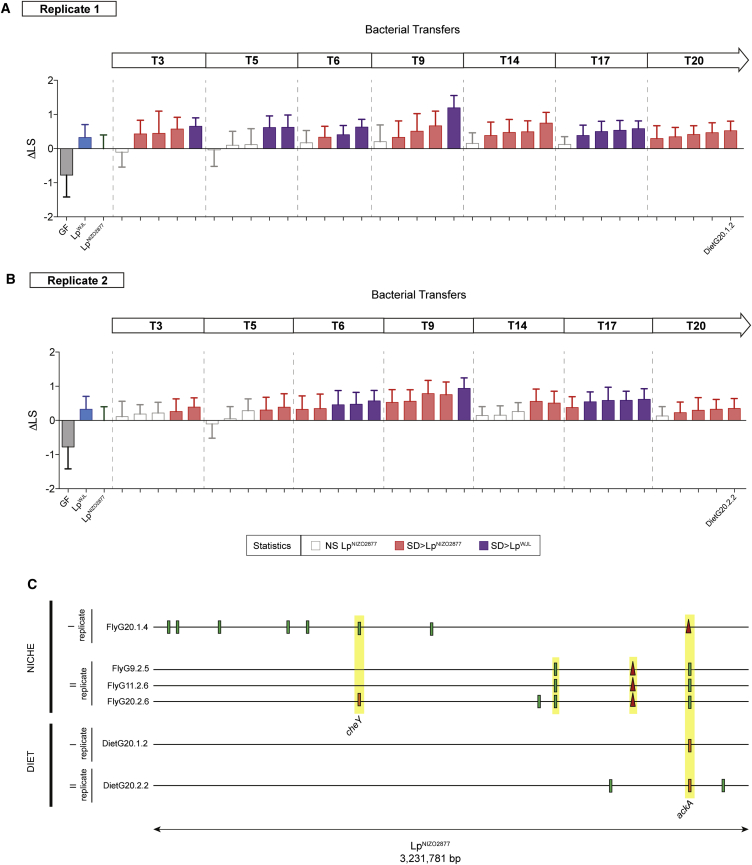
To determine whether the animal host has an influence on the evolution of its symbiotic bacteria, we experimentally evolved LpNIZO2877 in the same low-yeast fly diet, but without Drosophila (STAR Methods and Figure S5) and tested the capacity of isolates sampled throughout the course of the experimental evolution to promote fly growth on a set of naive GF fly larvae. Strikingly, in two parallel experiments, the LpNIZO2877 strains evolved in the absence of the host also show an increased ability to promote Drosophila growth when mono-associated with naive GF fly larvae (Figures 5A and 5B). Furthermore, genome sequencing of single evolved isolates from both experiments again revealed the acquisition of novel mutations in the ackA gene (Figures 5C and S5). Taken together, these findings show that the genomic evolution of L. plantarum is driven by the adaptation to the host nutritional environment, rather than to its host per se; the acquisition of the ackA variant is sufficient to drive the adaptive process to the fly diet, which ultimately results in the improvement of the L. plantarum symbiotic effect on Drosophila.
Figure 5.
LpNIZO2877 Adaptation to the Diet Increases Its Host's Growth
(A and B) Longitudinal size of larvae (LS) measured 7 days AED on poor-nutrient diet. Larvae were kept germ-free (GF) or associated with LpNIZO2877, LpWJL and with LpNIZO2877-evolved strains evolved in poor-nutrient diet in the absence of Drosophila. The Delta in larval size (ΔLS) shows the difference between the size of larvae associated with LpNIZO2877-evolved strains and the size of larvae associated with LpNIZO2877 from transfer 3 (T3) to transfer 20 (T20) for the first replicate (A) and the second replicate (B) of evolution. LpNIZO2877-evolved strains that exhibited a significant difference (improved) at promoting larval growth compared with the ancestor strain (Student's t test: p < 0.05) are shown in red. LpNIZO2877-evolved strains that exhibited a significant difference (improved) at promoting larval growth compared with the beneficial L. plantarum LpWJL strain are shown in purple. The evolved strains that have been selected for further analyses are labeled on the x axis.
(C) Mutations identified in LpNIZO2877-derived strains of all replicates evolved in poor-nutrient diet with Drosophila larvae (Niche) and in poor-nutrient diet without Drosophila larvae (Diet). Each evolved strain genome is represented as a horizontal line. Red triangles indicate deletions and small bars show SNPs. Different colors indicate different variants. Mutations occurring in the same gene and fixed along the experimental evolution are highlighted in yellow. The genes mutated in independent replicates of experimental evolution are labeled (cheY, ackA).
L. plantarum Improves Drosophila Growth through Secretion of N-Acetyl-Glutamine
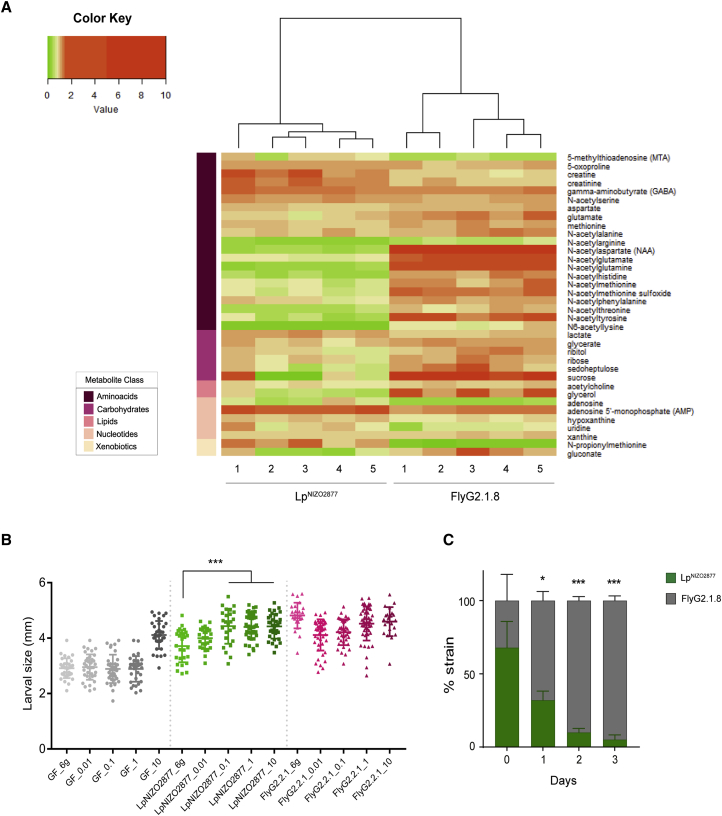
We next investigated how L. plantarum adaptation to the nutritional environment enhances Drosophila growth. We postulated that L. plantarum adaptation to the specific nutritional environment of Drosophila would lead to the production of metabolites that are beneficial for Drosophila growth. To test this hypothesis, we analyzed the metabolome of Drosophila diets colonized with either LpNIZO2877 or the evolved FlyG2.1.8 strain that bears only the ackA variant. Among all of the metabolites differentially detected in the substrate (Table S6), we observed a significant and robust increase in the levels of N-acetyl-amino acids in the diet processed by the evolved strain compared with the diet processed by the ancestral strain (Figure 6A). Specifically, N-acetyl-glutamine is one of the most differentially represented compounds between the two conditions. We therefore tested whether N-acetyl-glutamine is sufficient to improve the animal growth-promoting capacity of LpNIZO2877. Remarkably we find that, when N-acetyl-glutamine is added in a dose-dependent manner in the diet, the ancestor strain LpNIZO2877 is now able to recapitulate the beneficial effect conferred by FlyG2.1.8 on Drosophila growth (Figure 6B). However, the molecule alone is not sufficient to improve the growth of GF flies at low concentrations, but benefits the larvae when added at the highest concentration (10g/L) (Figure 6B). We then asked whether N-acetyl-glutamine enhances fly growth by improving LpNIZO2877 fitness. To test this, we performed a competition assay between LpNIZO2877 and FlyG2.1.8 strains in the host diet supplemented with 0.1 g/L of N-acetyl-glutamine (a concentration sufficient to confer improved symbiotic benefit to the ancestral strain). We find that FlyG2.1.8 still outcompetes the ancestor strain even in presence of N-acetyl-glutamine (Figure 6C). This result indicates that N-acetyl-glutamine does not confer a competitive advantage to LpNIZO2877 over FlyG2.1.8 while growing on the diet; nevertheless, it benefits the host physiology. Taken together, these findings establish N-acetyl-amino acids, and in particular N-acetyl-glutamine, as molecules produced by the evolved L. plantarum strains during growth on the Drosophila diet, which enhance Drosophila growth but not LpNIZO2877 fitness.
Figure 6.
N-Acetyl-Glutamine Recapitulates the Beneficial Effect of FlyG2.1.8 on LpNIZO2877-Associated Larvae
(A) Heatmap showing the metabolites that differ significantly between experimental groups (LpNIZO2877 and FlyG2.1.8) (two-sided t tests, p < 0.05). The heatmap was generated with heatmap.2 function in R. The compounds are ordered by the metabolite class given by the left scale.
(B) Longitudinal size of larvae (n > 60 larvae/group) measured 7 days AED on poor-nutrient diet supplemented with different concentrations (g/L) of N-acetyl-glutamine (x axis). Larvae were kept germ-free (GF) or associated with LpNIZO2877 (ancestor) and with Fly.G2.1.8 (evolved strain). Larval size is shown as mean ± SEM. ∗∗∗p < 0.01.
(C) 1:1 competitive assay between LpNIZO2877 and LpNIZO2877-evolved strain (FlyG2.1.8) in poor-nutrient diet supplemented with 0.1 g/L of N-acetyl-glutamine. Error bars represent the percentage of each strain detected by qPCR. ∗p < 0.05, ∗∗∗p < 0.01, obtained by Student's t test.
Discussion
Our results uncover the nature of the adaptive process of L. plantarum while in symbiosis with its fly host. We report direct experimental evidence showing that the host nutritional environment, and not the host per se, drives microbial adaptation and metabolic changes that alter the functional outputs of a facultative nutritional symbiosis. In our experimental context, the dietary substrate asserts the predominant selective pressure dictating the evolutionary change of facultative symbiotic bacteria and their consequent benefits to host physiology. Rapid adaptation of the L. plantarumNIZO2877 strain to the host nutritional environment occurred in multiple independent experimental lineages through the parallel fixations of different variants of a single gene, the acetate kinase ackA. This is a spectacular case of parallel evolution, indicating that the ackA mutation is the preferred or possibly the unique means for L. plantarumNIZO2877 to adapt to its host nutritional environment. Our experimental settings represent a harsh nutritional condition, which only allows L. plantarum slow growth (calculated generation time: 3.2 hr; Figure S1B). It was shown that the expression of L. plantarum ackA (ack2 in the L. plantarum reference strain WCFS1) is downregulated at low growth rates, suggesting that silencing ackA would be required to cope with poor growth condition (Goffin et al., 2010). This observation may explain the observed strong selection pressure on ackA in our experimental settings, which led to the rapid de novo emergence of variants in the population (Figures 1 and 2). ackA mutation significantly improved L. plantarum fitness on the fly diet (Figure 4D); this conferred a strong competitive advantage to the evolved strains bearing these mutations and led to their fixation (Figure 4). The appearance of the ackA variants provoked a significant modification of L. plantarum metabolite production, leading to the accumulation of N-acetyl-glutamine. This molecule does not per se improve bacterial fitness, so it remains elusive how ackA variants confers competitive advantage to L. plantarum cells on the fly diet. Nevertheless, N-acetyl-glutamine is directly involved in L. plantarum/Drosophila symbiosis, as it is sufficient to improve L. plantarum benefit on fly growth (Figure 6B). Our results indicate that ackA mutations possibly cause a shift in the metabolism of L. plantarum by modifying the usage of cellular acetyl groups, which would confer benefits to L. plantarum growth on the fly diet, thus improving its symbiotic effect. ackA participates in the reversible conversion of acetate to acetyl-phosphate; ackA variants might impede this reaction, and therefore shunt the pools of cellular acetyl groups into different metabolic routes leading to the accumulation of other acetylated compounds, such as N-acetyl-amino acids, which, once secreted, are consumed and beneficial to the host. These interpretations stem from the hypothesis that all the ackA variants obtained along L. plantarum experimental evolution likely generate inactive proteins (Figures S2 and S5B). Nevertheless, further work is needed to establish that all the variants lead to strict loss of function of ackA. Generating an ackA knockout in the ancestral LpNIZO2877 strain, measuring the activity of ackA protein variants, and probing the metabolic consequences of ackA variants in all the ancestral and evolved strains will likely provide insights into the specific molecular mechanisms underlying our findings.
Our results identify ackA as the first target of selection exerted by the nutritional environment on LpNIZO2877. Of note, LpWJL, a potent growth-promoting strain isolated from D. melanogaster gut (Martino et al., 2015b, Ryu et al., 2008, Storelli et al., 2011), shows two nucleotide substitutions in the ackA gene, compared with LpNIZO2877 (Figure S6), which might concur with its high beneficial effect in our experimental settings. Yet, due to the high genetic diversity of L. plantarum strains (Martino et al., 2016), we posit that such a genetic target hinges upon the genomic background of LpNIZO2877. According to their specific network of genetic polymorphisms, other non-beneficial isolates might fix mutations affecting other genes in order to adapt to the host environment and improve their fitness.
Understanding how evolutionary forces shape host-microbe symbiosis is essential to comprehend the mechanisms of their functional influence. Using the facultative nutritional mutualism between Drosophila and L. plantarum as a model, our results reveal that the primary selection pressure acting on L. plantarum originates from the nutritional substrate alone, which is strong enough to drive the rapid fixation of a de novo mutation. The resulting genetic changes confer a fitness advantage to the evolved bacteria and trigger a metabolic adaptation in bacterial cells, which is quickly capitalized by Drosophila as a physiological growth advantage, hence symbiosis can be perpetuated. This is a clear example of by-product mutualism, whereby animal hosts enjoy benefits from the by-products of the self-serving traits of their microbial symbionts (Bronstein, 1994, Connor, 1995, Holland and Bronstein, 2008). By showing that bacterial adaptation to the host nutritional medium results in a higher microbial competitive advantage and improvement of symbiotic benefit, we posit that such a process represents the first step in the emergence and evolution of facultative mutualism. Our results do not rule out the possibility that the animal host might exert additional selection pressure on its bacterial partners. Indeed, Drosophila is also known to directly affect the fitness of its own microbiota through the activity of innate immune effectors (Guo et al., 2014, Ryu et al., 2008) or the secretion of bacterial maintenance factors (Storelli et al., 2018). Nevertheless, our findings demonstrate the utmost importance of the shared nutritional substrate in the evolution of Drosophila-L. plantarum symbiosis.
Symbiosis is an evolutionary imperative and facultative symbioses are widespread in nature. Despite their unequivocal diversity, animal-microbe symbioses share striking similarities (Foster et al., 2017) and nutrition often plays a major role in shaping the composition of symbiotic microbial communities (Conlon and Bird, 2015, David et al., 2015, Groussin et al., 2017, Hacquard et al., 2015, Lozupone et al., 2012, Muegge et al., 2011). Our results provide direct experimental evidence that nutrition drives the evolution of a bacterial symbiont and, given that other animal and microbe partners have likely faced nutritional challenges over time, common evolutionary trajectories might have occurred. We therefore posit that bacterial adaptation to the diet can be the first step in the emergence and perpetuation of facultative animal-microbe symbioses. Our work provides another angle from which to help unravel the complex adaptive processes in the context of evolving symbiosis.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and Virus Strains | ||
| L. plantarum: LpNIZO2877 | Martino et al., 2015a | LKHZ01000000 |
| L. plantarum: LpWJL | Martino et al., 2015a, Martino et al., 2015b | LKLZ00000000 |
| L. plantarum: FlyG2.1.8 | This paper | PEBE00000000 |
| L. plantarum: FlyG3.1.8 | This paper | PEGI00000000 |
| L. plantarum: FlyG7.1.6 | This paper | PEGJ00000000 |
| L. plantarum: FlyG8.1.1 | This paper | PEGK00000000 |
| L. plantarum: FlyG8.1.2 | This paper | PEGL00000000 |
| L. plantarum: FlyG9.1.4 | This paper | PEGM00000000 |
| L. plantarum: FlyG10.1.5 | This paper | PEGN00000000 |
| L. plantarum: FlyG10.1.9 | This paper | PEGO00000000 |
| L. plantarum: FlyG11.1.2 | This paper | PEGP00000000 |
| L. plantarum: FlyG11.1.6 | This paper | PEGQ00000000 |
| L. plantarum: FlyG20.1.4 | This paper | PEGR00000000 |
| L. plantarum: FlyG2.1.8Rev | This paper | N/A |
| L. plantarum: FlyG9.2.5 | This paper | PEGS00000000 |
| L. plantarum: FlyG11.2.6 | This paper | PEGT00000000 |
| L. plantarum: FlyG20.2.6 | This paper | PEGU00000000 |
| L. plantarum: DietG20.1.2 | This paper | PEGV00000000 |
| L. plantarum: DietG20.2.2 | This paper | PEGW00000000 |
| E. coli E135 | Zhang et al., 2012 | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Inactivated Dried Yeast | Bio Springer | Springaline BA95/0-PW |
| Cornmeal | Westhove | Farigel maize H1 |
| Agar | VWR | #20768.361 |
| Methylparaben Sodium Salt | MERCK | #106756 |
| Propionic Acid | Sigma-Aldrich | P1386 |
| Man, Rogosa and Sharpe (MRS) Broth Medium | Difco | #288130 |
| Man, Rogosa and Sharpe (MRS) Agar Medium | Difco | #288210 |
| N-Acetyl Glutamine | Sigma-Aldrich | A9125-25G |
| PBS | Dutscher | NA.25 |
| Glycerol | Sigma-Aldrich | G5516 |
| SuperScript™ II Reverse Transcriptase | Invitrogen, Thermofisher Scientific | 18064014 |
| SYBR GreenER™ qPCR SuperMix Universal | Invitrogen, Thermofisher Scientific | 1176202K |
| Rifampicin | Sigma-Aldrich | R3501 |
| Chloramphenicol | Sigma-Aldrich | C0378 |
| Erythromycin | Sigma-Aldrich | E1300000 |
| Ampicillin | Sigma-Aldrich | A9393 |
| Glycine | Sigma-Aldrich | 67419 |
| Sucrose | Sigma-Aldrich | S7903 |
| Magnesium chloride | Sigma-Aldrich | M8266 |
| Critical Commercial Assays | ||
| NucleoSpin RNA Isolation Kit | Macherey-Nagel | 740955.240C |
| UltraClean Microbial DNA Isolation Kit | MOBIO Laboratories, Inc. | 12224-250 |
| Ion Xpress™ Plus Fragment Library Kit | Ion Torrent | 4471269 |
| Gibson Assembly Master Mix | NEB | E2611S |
| T4 Polynucleotide Kinase | NEB | M0201S |
| T4 DNA Ligase | NEB | M0202S |
| Deposited Data | ||
| Metabolomic Dataset of Diet | This paper | Table S3 |
| L. plantarum FlyG2.1.8 genome | This paper | PEBE00000000 |
| L. plantarum FlyG3.1.8 genome | This paper | PEGI00000000 |
| L. plantarum FlyG7.1.6 genome | This paper | PEGJ00000000 |
| L. plantarum FlyG8.1.1 genome | This paper | PEGK00000000 |
| L. plantarum FlyG8.1.2 genome | This paper | PEGL00000000 |
| L. plantarum FlyG9.1.4 genome | This paper | PEGM00000000 |
| L. plantarum FlyG10.1.5 genome | This paper | PEGN00000000 |
| L. plantarum FlyG10.1.9 genome | This paper | PEGO00000000 |
| L. plantarum FlyG11.1.2 genome | This paper | PEGP00000000 |
| L. plantarum FlyG11.1.6 genome | This paper | PEGQ00000000 |
| L. plantarum FlyG20.1.4 genome | This paper | PEGR00000000 |
| L. plantarum FlyG9.2.5 genome | This paper | PEGS00000000 |
| L. plantarum FlyG11.2.6 genome | This paper | PEGT00000000 |
| L. plantarum FlyG20.2.6 genome | This paper | PEGU00000000 |
| L. plantarum DietG20.1.2 genome | This paper | PEGV00000000 |
| L. plantarum DietG20.2.2 genome | This paper | PEGW00000000 |
| Experimental Models: Organisms/Strains | ||
| D.melanogaster: y,w (reference strain for this work) | N/A | |
| Oligonucleotides | ||
| Primer: 16S_UniF: GTGSTGCAYGGYTGTCGTCA | Packey et al., 2013 | N/A |
| Primer: 16S_UniR: ACGTCRTCCMCACCTTCCTC | Packey et al., 2013 | N/A |
| Primers for SNP verification, see Table S4 | This paper | N/A |
| Primers for competition tests, see Table S4 | This paper | N/A |
| Primers for engineering L. plantarum with CRISPR-Cas9, see Table S6 | This paper | N/A |
| Recombinant DNA | ||
| Plasmids used to engineer L. plantarum with CRISPR-Cas9, see Table S5 | This paper | N/A |
| pJP005 | CB651 | |
| pMSP3545 | Addgene 46888 | |
| pCas9 | Jiang et al., 2013 | Addgene 42876 |
| Software and Algorithms | ||
| ImageJ | NIH Image | https://imagej.net/ImageJ |
| Leica application suite (LAS) | Leica | N/A |
| Scan 1200 Automatic HD colony counter and Software | Intersciences | Ref. 437 000 |
| Breseq | Deatherage and Barrick, 2014 | http://barricklab.org/twiki/bin/view/Lab/ToolsBacterialGenomeResequencing |
| R Studio | RStudio Team, 2015 | https://www.rstudio.com/ |
| PROVEAN | Choi et al., 2012 | http://provean.jcvi.org |
| Geneious | Kearse et al., 2012 | https://www.geneious.com |
| Gibson assembly | Choi et al., 2012 | http://provean.jcvi.org |
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, François Leulier (francois.leulier@ens-lyon.fr).
Experimental Model and Subject Details
Bacterial Strains and Culture Conditions
The strains used in the present study are listed in Table S1. All experimentally evolved strains were derived from L. plantarumNIZO2877 (kind gift from Prof. Michiel Kleerebezem, NIZO Food Research BV, Netherlands) that was originally isolated from a sausage in Vietnam (Martino et al., 2015a). All strains were routinely grown overnight at 37°C in Man, Rogosa and Sharpe (MRS) medium (BD Bioscience) without agitation. Strains were stored at -80°C in MRS broth containing 20% glycerol.
Drosophila Stocks and Breeding
Drosophila yw flies were used as the reference strain in this work. Drosophila stocks were cultured at 25°C with 12/12 hour dark/light cycles on a yeast/cornmeal medium containing 50g/l inactivated yeast (rich diet) as described by Storelli et al. (2011). Poor-nutrient diet was obtained by reducing the amount of yeast extract to 8 g/l. Germ-free (GF) stocks were established and maintained as described in Storelli et al. (Storelli et al., 2011).
Fly Diets Used in This Study
Poor Yeast Diet: 8g inactivated dried yeast, 80g cornmeal, 7.2g Agar, 5.2g methylparaben sodium salt, 4 mL 99% propionic acid for 1 litre.
PYD + N-acetyl-Glutamine. Fly food is prepared by mixing 8g of inactive dried yeast, 80g of cornmeal, 7,2g of agar, 5,2g of methylparaben sodium salt, 4 mL of 99% propionic acid in 800 mL water. After cooking and before solidification, fly food is mixed with serial dilutions of N-acetyl-Glutamine solution (prepared from a stock solution at 1g N-acetyl-Glutamine/L sterile water). Fly food is then mixed vigorously by vortexing, and then poured in microtubes.
Fly food was poured in petri dishes (diameter=55mm; fly food volume ≈ 7ml) to grow larvae used for larval longitudinal length analysis. Fly food was poured in 50 ml tubes for Experimental Evolution setup. Fly food was poured in 1.5 ml microtubes (fly food volume=100μl) for metabolites profiling.
Colonization and Infection of Larvae
40 embryos collected from GF females were transferred to a fresh poor nutrient GF medium in a 55 mm petri dish. Bacterial strains were cultured to stationary phase (18h) in MRS broth at 37°C. The embryos and the fly medium were mono-associated with 300 μl (7 x107 CFU) of the respective bacteria. Emerging larvae were allowed to develop on the contaminated medium at 25°C.
Method Details
Experimental Evolution Design
The experimental “Adaptive Evolution” (AE) model of L. plantarum in Drosophila Niche (Drosophila + Diet) was designed as follows: for the first generation of the AE model, GF female flies laid GF embryos on poor nutrient GF medium. Forty GF eggs were transferred on 10 ml of new poor nutrient GF medium. L. plantarumNIZO2877 (ancestor) was cultured in MRS broth to stationary phase (18h). The culture was washed in sterile PBS and 300 μl of PBS-washed culture containing 108 CFU of L. plantarumNIZO2877 were added directly on the embryos and the fly food (bacterial load = 107 CFU/ml) (Figure S1A). No further inoculation of the ancestor strain L. plantarumNIZO2877 has been performed after the beginning of the first generation until the end of the experimental evolution. Emerging larvae were allowed to develop on the medium inoculated with the bacterial culture at 25°C. The first 15 pupae were transferred to a new poor nutrient GF medium, and allowed to complete metamorphosis. Once the pupae were transferred, the bacterial community associated with them (on average 106 CFU/ml) was also indirectly transferred to the new medium by inoculation of the substratum from the surface of the transferred pupae and predominantly by defecation of the adults emerging from the transferred pupae. This allowed the propagation of an evolving bacterial subpopulation derived from the ancestor on the new medium. Once the emerging adults had laid eggs (a minimum of 40 and no more than 80 per tube), which were the founders for the next fly generation, they were collected and homogenized using the Precellys 24 tissue homogenizer (Bertin Technologies, France) and 0.75/1 mm glass beads in 800 μl of MRS broth and stored at -80°C by adding 20% glycerol. Single bacterial isolates were isolated at the end of each fly generation by plating the crushed adult flies on MRS agar plates at 37°C for 48h. Ten colonies were randomly selected from the plates and tested individually for Drosophila growth promotion (larval size and developmental timing assays) on new ancestral GF yw Drosophila embryos (see below). The AE model has been propagated for 20 fly generations.
The experimental evolution of L. plantarum in Drosophila’s nutritional medium (Diet) was designed as follows: L. plantarumNIZO2877 (ancestor) was cultured to stationary phase (18h). The culture was washed in sterile PBS and 3 μl (106 CFU) were added directly on 100 μl of poor nutrient GF diet (bacterial load = 107 CFU/ml) (Figure S5A) and kept at 25°C. After four days (time necessary for the microbial load to reach the same value found on the 15 pupae used for propagating the bacterial population in the Niche adaptive evolution setup), the medium was crushed using the Precellys 24 tissue homogenizer (Bertin Technologies, France) and 0.75/1 mm glass beads in 500 μl of PBS using glass beads and 10 μl of the crushed medium (105 CFU) were used to inoculate 100 μl of new poor nutrient GF medium (106 CFU/ml). This protocol has been repeated 20 times.
Strain-specific PCR tests were performed to confirm the unique presence of L. plantarumNIZO2877 throughout both experimental evolution models as reported in Schwarzer et al. (Schwarzer et al., 2016).
Generation Time of L. plantarum
To determine the generation time of L. plantarum strains in the Drosophila Niche (Drosophila + Diet) and Diet, we used a modified version of a method that reported the correlation between bacterial growth rate and 16SrRNA content (Poulsen et al., 1995). L. plantarum strains were cultured to stationary phase (18h) and washed in sterile PBS. Serial dilutions have been prepared and 5 μl containing a total of 103 colony-forming units (CFUs) were added to 100 μl of GF poor nutrient diet with and without Drosophila larvae (Diet and Niche setup respectively) and kept at 25°C. Samples were snap-frozen in liquid nitrogen at different time points across five days of growth. Bacterial RNA was extracted using NucleoSpin RNA Isolation kit (Macherey-Nagel, Germany) following manufacturer's instructions. Reverse transcription of total extracted RNA into cDNA has been performed using Superscript II (Invitrogen, USA) according to manufacturer’s instructions. Quantitative PCR was performed in a total of 20 μl on a Biorad CFX96 apparatus (Biorad) using SYBR GreenER qPCR Supermix (Invitrogen, USA). The reaction mixture consisted of 0.5 μl of each primer (10 μM each), 12,5 μl of SYBR GreenER mix, 10 μl of water and 1,5 μl of template cDNA. The PCR conditions included 1 cycle of initial denaturation at 95°C for 2 min, followed by 45 cycles of 95°C for 10 sec and 60°C for 40 sec. Absolute quantification of 16rRNA was conducted as follows: five 1:10 serial dilutions of the standard sample (100 ng/μl of cDNA extracted from L. plantarumNIZO2877 culture) were quantified by Real-time PCR using universal 16S primers (forward primer, UniF 5'-GTGSTGCAYGGYTGTCGTCA-3' and reverse primer, UniR 5'-ACGTCRTCCMCACCTTCCTC-3', Table S4) (Packey et al., 2013). Each dilution has been tested in triplicate. Melting curves of the detected amplicons were analysed to ensure specific and unique amplification. Standard curves were generated plotting threshold cycle (Ct) values against the log of the standard sample amount. Based on the data obtained from the standard curve, the Ct values of the Niche and Diet samples have been used to obtain the log of their 16SrRNA concentration at each time point. The 16S rRNA values during exponential phase have been used to infer the bacterial generation time following the equation reported by Widdel et al. (Widdel, 2007).
Larval Size Measurements
Larvae (n ≥ 120) were collected 7 days after inoculation, washed in distilled water, transferred on a microscopy slide, killed with a short heat treatment (5s at 90°C) and mounted in 80% glycerol/PBS. The larvae were imaged under a Leica steromicroscope M205FA and larval longitudinal size (length) was measured using ImageJ software (Schneider et al., 2012).
Developmental Timing
Developmental timing of Drosophila associated with different bacteria was quantified by counting the number of pupae emerging over time. These results are represented as the day at which 50% of the whole population pupariated (D50). Each graph represents the mean of 3 biological replicates, including at least 30 individuals each.
Genome Sequencing
Genomic DNA of single bacterial strains was extracted from cultures grown to stationary phase in MRS broth using the UltraClean Microbial DNA isolation kit (Mo Bio, Qiagen, USA). For all single strain sequencing genomic libraries were prepared following Ion Xpress Plus gDNA Fragment Library construction protocol for 400bp reads. The strains were sequenced using the Ion Torrent PGM platform. The DNA library construction and sequencing was performed on the IGFL sequencing platform (Lyon, France). For community sequencing, the lysate obtained from the crushed adult flies was plated out on MRS agar and cultured at 37°C for 48h. A mixture of >1000 clones was used to extract the genomic DNA using the UltraClean Microbial DNA isolation kit (Mo Bio, Qiagen, USA). The DNA library construction and sequencing was carried out by the EMBL Genomics Core Facilities (Heidelberg, Germany). Each sample was pair-end sequenced on an Illumina MiSeq Benchtop Sequencer. Standard procedures produced data sets of Illumina paired-end 250 bp read pairs. The mean coverage per sample was 99x. Processed reads were aligned and analysed against their respective reference strain (ancestor) genome to identify mutations, using default settings in breseq (Deatherage and Barrick, 2014) for single isolate genomes and using the ‘-polymorphism’ setting for libraries constructed for bacterial communities. In order to discard false positive mutations, we generated an R script (RStudio Team, 2015) which used the breseq file as input to derive the real percentage of reads affected by mutation. Candidate mutations were verified by targeted PCR amplification and Sanger sequencing. These data were used to build a decision tree in order to correlate the frequency of reads affected by a given mutation (%) and its real presence in the genome (obtained by Sanger sequencing). This allowed us to establish that the real mutation calls were those predicted by frequency values higher than 83,5%. All candidate mutations were subsequently confirmed by targeted PCR amplification and Sanger sequencing by using specific primers (Table S2). Non-synonymous mutations in genes belonging to pathway of interest were analysed with PROVEAN (Protein Variation Effect Analyzer) (Choi et al., 2012) to predict the functional impact of the genetic variant. The score threshold used was set to -2.5.
L. plantarum Genomic Editing with CRISPR-Cas9
Plasmid Generation
The Cas9 targeting plasmid was assembled by first amplifying the pMSP3545 plasmid backbone (Table S5) and the Cas9+tracrRNA from pCas9 with oligos oRL1-oRL4 (Table S6). These PCR fragments were stitched together using Gibson assembly (Gibson et al., 2009) (NEB CN#E2611S) and transformed into E. coli NovaBlue cells. This synthesized plasmid was named p3545Cas9 and served as the non-targeting control vector used for subsequent transformation assays (Figure S3A). The targeting sgRNA was synthesized as a repeat-spacer-repeat array under a constitutive Lactobacillus promoter (Ppgm)(Duong et al., 2011). This array (gBlock 1) was amplified with oligos oRL5-oRL6. The p3545Cas9 plasmid was digested with XbaI and PstI and the two fragments were inserted by Gibson assembly. This step created the pCas9+RSR plasmid. To insert a spacer to specifically targeting the mutated ackA gene, oligos oRL13-14 were phosphorylated with T4 PNK (NEB CN#M0201S), annealed, and ligated into the pCas9+RSR plasmid after digestion with PvuI and NotI. The spacer was designed so the CCT triplet (absent in Fly.G2.1.8) was inserted within the seed portion of the target sequence (Figure S3B).
The repair template plasmid was assembled by amplifying the LpNIZO2877 ackA gene with oRL7-oRL8, digesting this PCR product and the pJP005 plasmid with SpeI and SacI, and ligating them together with T4 DNA ligase (NEB CN#M0202S). 300ng of backbone was ligated with 422 ng of insert, ethanol precipitated, and entirely transformed into L. plantarum WCFS1. The center of the amplified region contained the CCT triplet absent in Fly.G2.1.8 ackA gene (Table S5). Colony PCR was used to screen for successful clones using oligos oRL09-10. Colony PCR was performed by picking a single colony into 20 μL of 20 mM NaOH and incubating at 98°C for 20 minutes. These tubes were then microwaved for 1 minute with the cap open, and 5 μL of this mixture was added to the PCR mix and amplified with PFU polymerase isolated from Pyrococcus furiosus (gift from R.M. Kelly).
Growth Conditions and Electroporation
All L. plantarum strains were grown on MRS liquid broth and MRS agar and incubated at 37°C. Antibiotic concentrations for L. plantarum were as follows: rifampicin (25μg/mL), chloramphenicol (10μg/mL), and erythromycin (10μg/mL). All E. coli strains were cultured in LB medium (10 g/L NaCl, 5 g/L yeast extract, 10 g/L tryptone) while being shaken at 250 RPM at 37°C. Plasmids were maintained at the following antibiotic concentrations: erythromycin (50μg/mL), chloramphenicol (34μg/mL), and ampicillin (50μg/mL). Electroporation of L. plantarum was adapted from numerous protocols (Spath et al., 2012, Teresa Alegre et al., 2004, Thompson and Collins, 1996). To prepare cells for electroporation, 1 mL of overnight culture of L. plantarum was back-diluted into 25 mL of MRS liquid broth containing 0.41 M glycine and any necessary antibiotics. This was performed in sealed 50-mL falcon tubes to prevent aeration of the bacteria. These tubes were cultured at 37°C and 250 RPM for ∼3 hours, or until the OD600 was approximately 0.85. Cells were centrifuged at 5,000 RPM for 10 minutes at 4°C. The pelleted cells were kept on ice, then washed twice with ice-cold 10 mM MgCl2 followed by a wash in SacGly (10% glycerol with 0.5 M sucrose). The washed cell pellet was then re-suspended in 1mL of SacGly and centrifuged at 20,000 RPM for 1 minute and the final pellet was re-suspended in 500 μL of SacGly. For all transformations, 60μL of this suspension was added to a 1-mm gap cuvette and transformed at 1.8kV, 200Ω resistance, and 25 μF capacitance. Following electroporation, cells were resuspended in 1 mL of MRS broth and transferred to a sterile tube and incubated at 37°C without shaking for 3 hours. 250 μL of the recovered cells was then plated on MRS agar with appropriate antibiotics. Any dilutions prior to plating was done in MRS media.
CRISPR-Cas9 Repair-Template Editing
To perform the genomic edits, the ackA_pJP005 plasmid was transferred from WCFS1 into Fly.G2.1.8 and prepared for electroporation using the previously described conditions, selecting for the ackA_pJP005 plasmid with chloramphenicol. Electrocompetent cells were then transformed with 5μg of p3545_Cas9 or pCas+RSR isolated from a methylation-free E. coli strain (EC135) (Zhang et al., 2012). Transformation efficiencies of the Cas9 plasmid into Fly.G2.1.8 were vastly improved in the presence of the homologous-recombination template (Figure S3C). After transformation, cells were plated on solid MRS media supplemented with erythromycin to only select for the CRISPR-Cas9 plasmid. The ackA gene from the resultant colonies was amplified with oRL11-12 and subjected to Sanger sequencing (Figure S3D). Seven colonies harbored the inserted CCT triplet (FlyG2.1.8Rev, Figure S3D), while two colonies were unedited and one colony did not yield an amplification product. The lack of editing in the two colonies may be due to mutation of Cas9 or the spacer as reported previously (Gomaa et al., 2014). To clear the plasmids from the edited strains, colonies were subjected to multiple rounds of non-selective outgrowths in liquid medium (Figure S3E). After each round of non-selective outgrowths, edited Lactobacillus cultures were struck out on non-selective plates and colonies were then struck out on selective chloramphenicol or erythromycin plates to determine if plasmids were successfully cleared. Once colonies were no longer able to grow on selective medium, antibiotic susceptibility was tested in liquid culture and strains were analyzed in vivo. Two isolates from separate colonies were subjected to whole-genome sequencing (using an Ion torrent PGM platform) to confirm the insertion of the CCT triplet in ackA and the absence of any mutations elsewhere in the genome (Table S1).
Bacterial Competitions Tests
Competition assays between LpNIZO2877 (ancestor) and FlyG2.1.8 (evolved beneficial strain) have been tested in Drosophila Niche and Diet. The competitions were performed at a ratio of 1:1, over 3 days of co-colonization, following the same inoculation procedure described for the Diet evolution experiment. A total of 104 CFUs has been used as inoculum. During the three days of co-colonization, the samples have been crushed using the Precellys 24 tissue homogenizer (Bertin Technologies, France) and 0.75/1 mm glass beads in 500 μl of PBS. The lysate has been plated out on MRS agar and cultured at 37°C for 48h. 10000 colonies have been collected and bacterial DNA was extracted using the UltraClean Microbial DNA isolation kit (Mo Bio, Qiagen, USA). A specific Real-time PCR assay has been developed to distinguish and quantify the presence of LpNIZO2877 (ancestor) and FlyG2.1.8 (evolved beneficial strain). Specific primer pairs have been designed on the ackA gene sequence using Geneious 9 (Kearse et al., 2012). Quantitative PCR was performed in a total of 20 μl on a Biorad CFX96 apparatus (Biorad) using SYBR GreenER qPCR Supermix (Invitrogen, USA), bacterial DNA and the gene specific primer sets (forward primer LpNIZO2877-specific: ackA_NIZO2877_F_RT; forward primer FlyG2.1.8-specific: ackA_FlyG2_F_RT; and common reverse primer: ackA_R_RT; Table S4). The reaction mixture consisted of 0.5 μl of each primer (10 μM each), 12,5 μl of SYBR GreenER mix, 10 μl of water and 1,5 μl of template cDNA. Each sample has been tested in triplicate. The PCR conditions included 1 cycle of initial denaturation at 95°C for 2 min, followed by 45 cycles of 95°C for 10 sec and 60°C for 40 sec. Melting curves of the detected amplicons were analysed to ensure specific and unique amplification. PCR efficiency was calculated for each primer set using six serial dilutions of DNA starting from 2 ng/μl. Relative quantification of each bacterial strain has been performed using 16SrRNA as reference gene (UniF-UniR primers, Table S4).
Metabolite Profiling
Microtubes containing axenic poor nutrient diet were inoculated with bacterial suspension (103 CFU/ml) or with PBS and incubated for 3 days at 25°C. Microtubes were then snap-frozen in liquid nitrogen and stored at -80°C before sending to Metabolon Inc. (www.metabolon.com). Five biological replicates per condition were generated. Samples were then extracted and prepared for analysis using Metabolon’s standard solvent extraction method. Each resulting extract was divided into five fractions: two for analysis by two separate reverse phase/Ultrahigh Performance Liquid Chromatography-Tandem Mass Spectroscopy (RP/UPLC-MS/MS) methods with positive ion mode electrospray ionization (ESI), one for analysis by RP/UPLC-MS/MS with negative ion mode ESI, one for analysis by HILIC/UPLC-MS/MS with negative ion mode ESI, and one sample was reserved for backup. Compounds were identified by comparison to library entries of purified standards or recurrent unknown entities.
Information Related to Experimental Design
Blinding was not used in the course of our study. No data or subjects were excluded from our analyses.
Quantification and Statistical Analysis
Data representation and statistical analysis were performed using Graphpad PRISM 6 software (www.graphpad.com). For metabolite profiling, we performed Student’s t test with Welch correction to determine if differences in metabolites levels between two conditions are statistically significant. For all the other pairwise comparisons throughout our study, we performed Mann Whitney’s test. We applied Kruskal Wallis test to perform statistical analyses of multiple (n>2) conditions. No particular method was used to determine whether the data met assumptions of the statistical approach.
Data and Software Availability
The accession number for FlyG2.1.8 genome reported in this paper is NCBI: PEBE00000000. The accession number for FlyG3.1.8 genome reported in this paper is NCBI: PEGI00000000. The accession number for FlyG7.1.6 genome reported in this paper is NCBI: PEGJ00000000. The accession number for FlyG8.1.1 genome reported in this paper is NCBI: PEGK00000000. The accession number for FlyG8.1.2 genome reported in this paper is NCBI: PEGL00000000. The accession number for FlyG9.1.4 genome reported in this paper is NCBI: PEGM00000000. The accession number for FlyG10.1.5 genome reported in this paper is NCBI: PEGN00000000. The accession number for FlyG10.1.9 genome reported in this paper is NCBI: PEGO00000000. The accession number for FlyG11.1.2 genome reported in this paper is NCBI: PEGP00000000. The accession number for FlyG11.1.6 genome reported in this paper is NCBI: PEGQ00000000. The accession number for FlyG20.1.4 genome reported in this paper is NCBI: PEGR00000000. The accession number for FlyG9.2.5 genome reported in this paper is NCBI: PEGS00000000. The accession number for FlyG11.2.6 genome reported in this paper is NCBI: PEGT00000000. The accession number for FlyG20.2.6 genome reported in this paper is NCBI: PEGU00000000. The accession number for DietG20.1.2 genome reported in this paper is NCBI: PEGV00000000. The accession number for DietG20.2.2 genome reported in this paper is NCBI: PEGW00000000.
Acknowledgments
We thank B. Prud'homme and colleagues at the Institut de Génomique Fonctionnelle de Lyon for critical reading of the manuscript. We gratefully acknowledge support from the PSMN (Pôle Scientifique de Modélisation Numérique) of the ENS de Lyon for the computing resources. This work was funded by an ERC starting grant (FP7/2007-2013-N° 309704). M.E.M. was funded by the European Union's Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement N° 659510. The lab of F.L. is supported by the FINOVI foundation and the EMBO Young Investigator Program. The CRISPR/Cas9 work was supported through funding from the National Science Foundation (MCB-1452902 to C.B.). WCFS1 was a kind gift from Dr. Nikhil U. Nair. EC135 was provided to us by Tingyi Wen. We thank the University of Padua and Dr. Barbara Cardazzo for hosting M.E.M. during the last stages of this work.
Author Contributions
M.E.M. and F.L. designed the project; M.E.M. and H.G. conducted the experiments; M.E.M. and P.J. conducted the bioinformatics analyses; R.L., M.S., and C.B. designed and performed the CRISPR/Cas9 engineering experiments; S.H. and B.G. generated the sequencing data; M.E.M. and F.L. analyzed the data and wrote the paper.
Declaration of Interests
M.E.M. and F.L. are inventors of a pending patent application (INPI-n° FR1757717) which applies to bacterial strains presented in this article.
Published: June 28, 2018
Footnotes
Supplemental Information includes six figures and six tables and can be found with this article online at https://doi.org/10.1016/j.chom.2018.06.001.
Contributor Information
Maria Elena Martino, Email: maria-elena.martino@ens-lyon.fr.
François Leulier, Email: francois.leulier@ens-lyon.fr.
Supplemental Information
References
- Blum J.E., Fischer C.N., Miles J., Handelsman J. Frequent replenishment sustains the beneficial microbiome of Drosophila melanogaster. MBio. 2013;4 doi: 10.1128/mBio.00860-13. e00860–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick N.A., Buchon N., Lemaitre B. Microbiota-induced changes in Drosophila melanogaster host gene expression and gut morphology microbiota-induced changes in Drosophila melanogaster host gene. MBio. 2014;5:1–13. doi: 10.1128/mBio.01117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein J.L. Our current understanding of mutualism. Q. Rev. Biol. 1994;69:31–51. [Google Scholar]
- Choi Y., Sims G.E., Murphy S., Miller J.R., Chan A.P. Predicting the functional effect of amino acid substitutions and indels. PLoS One. 2012;7:e46688. doi: 10.1371/journal.pone.0046688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon M.A., Bird A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2015;7:17–44. doi: 10.3390/nu7010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor R.C. The benefits of mutualism: a conceptual framework. Biol. Rev. 1995;70:427–457. [Google Scholar]
- David L.A., Materna A.C., Friedman J., Campos-Baptista M.I., Blackburn M.C., Perrotta A., Erdman S.E., Alm E.J. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 2015;15:R89. doi: 10.1186/gb-2014-15-7-r89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deatherage D.E., Barrick J.E. Identification of mutations in laboratory-evolved microbes from next-generation sequencing data using breseq. Methods Mol. Biol. 2014;1151:165–188. doi: 10.1007/978-1-4939-0554-6_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas A.E. Lessons from studying insect symbioses. Cell Host Microbe. 2011;10:359–367. doi: 10.1016/j.chom.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong T., Miller M.J., Barrangou R., Azcarate-Peril M.A., Klaenhammer T.R. Construction of vectors for inducible and constitutive gene expression in Lactobacillus. Microb. Biotechnol. 2011;4:357–367. doi: 10.1111/j.1751-7915.2010.00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel P., Moran N.A. The gut microbiota of insects—diversity in structure and function. FEMS Microbiol. Rev. 2013;37:699–735. doi: 10.1111/1574-6976.12025. [DOI] [PubMed] [Google Scholar]
- Erkosar B., Storelli G., Mitchell M., Bozonnet L., Bozonnet N., Leulier F. Pathogen virulence impedes mutualist-mediated enhancement of host juvenile growth via inhibition of protein digestion. Cell Host Microbe. 2015;18:445–455. doi: 10.1016/j.chom.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldhaar H. Bacterial symbionts as mediators of ecologically important traits of insect hosts. Ecol. Entomol. 2011;36:533–543. [Google Scholar]
- Ferrari J., Vavre F. Bacterial symbionts in insects or the story of communities affecting communities. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011;366:1389–1400. doi: 10.1098/rstb.2010.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R.M., Henry L.M., Cornwallis C.K., Kiers E.T., West S.A. The evolution of host-symbiont dependence. Nat. Commun. 2017;8:15973. doi: 10.1038/ncomms15973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster K.R., Schluter J., Coyte K.Z., Rakoff-Nahoum S. The evolution of the host microbiome as an ecosystem on a leash. Nature. 2017;548:43–51. doi: 10.1038/nature23292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D.G., Young L., Chuang R.-Y., Venter J.C., Hutchison C.A., Smith H.O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods. 2009;6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- Gilbert J.A., Neufeld J.D. Life in a world without microbes. PLoS Biol. 2014;12:e1002020. doi: 10.1371/journal.pbio.1002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffin P., van de Bunt B., Giovane M., Leveau J.H.J., Höppener-Ogawa S., Teusink B., Hugenholtz J. Understanding the physiology of Lactobacillus plantarum at zero growth. Mol. Syst. Biol. 2010;6:413. doi: 10.1038/msb.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomaa A.A., Klumpe H.E., Luo M.L., Selle K., Barrangou R., Beisel L. Programmable removal of bacterial strains by use of genome-targeting CRISPR/Cas systems. MBio. 2014;5 doi: 10.1128/mBio.00928-13. e00928–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groussin M., Mazel F., Sanders J.G., Smillie C.S., Lavergne S., Thuiller W., Alm E.J. Unraveling the processes shaping mammalian gut microbiomes over evolutionary time. Nat. Commun. 2017;8:14319. doi: 10.1038/ncomms14319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Karpac J., Tran S.L., Jasper H. PGRP-SC2 promotes gut immune homeostasis to limit commensal dysbiosis and extend lifespan. Cell. 2014;156:109–122. doi: 10.1016/j.cell.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacquard S., Garrido-Oter R., González A., Spaepen S., Ackermann G., Lebeis S., McHardy A.C., Dangl J.L., Knight R., Ley R. Microbiota and host nutrition across plant and animal kingdoms. Cell Host Microbe. 2015;17:603–616. doi: 10.1016/j.chom.2015.04.009. [DOI] [PubMed] [Google Scholar]
- Holland J.N., Bronstein J.L. Mutualism. In: Jorgensen S.E., Fath B.D., editors. Encyclopedia of Ecology. Elsevier; 2008. pp. 2485–2491. [Google Scholar]
- Jiang W., Bikard D., Cox D., Zhang F., Marraffini L.A. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat. Biotechnol. 2013;31:233–239. doi: 10.1038/nbt.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., Buxton S., Cooper A., Markowitz S., Duran C. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C.A., Stombaugh J.I., Gordon J.I., Jansson J.K., Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D., Storelli G., Mitchell M.L., Leulier F. Studying host-microbiota mutualism in Drosophila: harnessing the power of gnotobiotic flies. Biomed. J. 2015;38:285–293. doi: 10.4103/2319-4170.158620. [DOI] [PubMed] [Google Scholar]
- Martino M.E., Bayjanov J.R., Joncour P., Hughes S., Gillet B., Kleerebezem M., Siezen R., van Hijum S.A.F.T., Leulier F. Nearly complete genome sequence of Lactobacillus plantarum strain NIZO2877. Genome Announc. 2015;3 doi: 10.1128/genomeA.01370-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino M.E., Bayjanov J.R., Joncour P., Hughes S., Gillet B., Kleerebezem M., Siezen R., van Hijum S.A.F.T., Leulier F. Resequencing of the Lactobacillus plantarum strain WJL genome. Genome Announc. 2015;3 doi: 10.1128/genomeA.01382-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino M.E., Bayjanov J.R., Caffrey B.E., Wels M., Joncour P., Hughes S., Gillet B., Kleerebezem M., van Hijum S.A.F.T., Leulier F. Nomadic lifestyle of Lactobacillus plantarum revealed by comparative genomics of 54 strains isolated from different habitats. Environ. Microbiol. 2016;18:4974–4989. doi: 10.1111/1462-2920.13455. [DOI] [PubMed] [Google Scholar]
- Matos R.C., Leulier F. Lactobacilli-host mutualism: “learning on the fly”. Microb. Cell Fact. 2014;13:S6. doi: 10.1186/1475-2859-13-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos R.C., Schwarzer M., Gervais H., Courtin P., Joncour P., Gillet B., Ma D., Bulteau A.L., Martino M.E., Hughes S. D-Alanylation of teichoic acids contributes to Lactobacillus plantarum-mediated Drosophila growth during chronic undernutrition. Nat. Microbiol. 2017;2:1635–1647. doi: 10.1038/s41564-017-0038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muegge B.D., Kuczynski J., Knights D., Clemente J.C., González A., Fontana L., Henrissat B., Knight R., Gordon J.I. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science. 2011;332:970–974. doi: 10.1126/science.1198719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packey C.D., Shanahan M.T., Manick S., Bower M.A., Ellermann M., Tonkonogy S.L., Carroll I.M., Sartor R.B. Molecular detection of bacterial contamination in gnotobiotic rodent units. Gut Microbes. 2013;4:361–370. doi: 10.4161/gmic.25824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen L.K., Licht T.R., Rang C., Krogfelt K.A., Molin S. Physiological state of Escherichia coli BJ4 growing in the large intestines of streptomycin-treated mice. J. Bacteriol. 1995;177:5840–5845. doi: 10.1128/jb.177.20.5840-5845.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RStudio Team . RStudio; 2015. RStudio: Integrated Development for R. [Google Scholar]
- Ryu J.H., Kim S.H., Lee H.Y., Jin Y.B., Nam Y.D., Bae J.W., Dong G.L., Seung C.S., Ha E.M., Lee W.J. Innate immune homeostasis by the homeobox gene Caudal and commensal-gut mutualism in Drosophila. Science. 2008;319:777–782. doi: 10.1126/science.1149357. [DOI] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer M., Makki K., Storelli G., Machuca-Gayet I., Srutkova D., Hermanova P., Martino M.E., Balmand S., Hudcovic T., Heddi A. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science. 2016;351:854–857. doi: 10.1126/science.aad8588. [DOI] [PubMed] [Google Scholar]
- Spath K., Hein S., Grabherr R. Direct cloning in Lactobacillus plantarum: electroporation with non-methylated plasmid DNA enhances transformation efficiency and makes shuttle vectors obsolete. Microb. Cell Fact. 2012;11:141. doi: 10.1186/1475-2859-11-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storelli G., Defaye A., Erkosar B., Hols P., Royet J., Leulier F. Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metab. 2011;14:403–414. doi: 10.1016/j.cmet.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Storelli G., Strigini M., Grenier T., Bozonnet L., Schwarzer M., Daniel C., Matos R., Leulier F. Drosophila perpetuates nutritional mutualism by promoting the fitness of its intestinal symbiont Lactobacillus plantarum. Cell Metab. 2018;27:362–377. doi: 10.1016/j.cmet.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teresa Alegre M., Carmen Rodríguez M., Mesas J.M. Transformation of Lactobacillus plantarum by electroporation with in vitro modified plasmid DNA. FEMS Microbiol. Lett. 2004;241:73–77. doi: 10.1016/j.femsle.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Thompson K., Collins M.A. Methods Improvement in electroporation efficiency for Lactobacillus plantarum by the inclusion of high concentrations of glycine in the growth medium. J. Microbiol. Methods. 1996;26:73–79. [Google Scholar]
- Widdel F. Universität Bremen; 2007. Theory and Measurement of Bacterial Growth Grundpraktikum Mikrobiologie; p. 11. [Google Scholar]
- Zhang G., Wang W., Deng A., Sun Z., Zhang Y., Liang Y., Che Y., Wen T. A mimicking-of-DNA-methylation-patterns pipeline for overcoming the restriction barrier of bacteria. PLoS Genet. 2012;8:e1002987. doi: 10.1371/journal.pgen.1002987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.