Abstract
Colorectal cancer (CRC) is a heterogeneous disease, with a diverse and plastic immune cell infiltrate. These immune cells play an important role in regulating tumour growth - progression or elimination. Some populations of cells have a strong correlation with disease-free survival, making them useful prognostic markers. In particular, the infiltrate of CD3+ and CD8+ T cells into CRC tumours has been validated worldwide as a valuable indicator of patient prognosis. However, the heterogeneity of the immune response, both between patients with tumours of different molecular subtypes, and within the tumour itself, necessitates the use of multiparametric analysis in the investigation of tumour-specific immune responses. This review will outline the multiparametric analysis techniques that have been developed and applied to studying the role of immune cells in the tumour, with a focus on colorectal cancer. Because much of the data in this disease relates to T cell subsets and heterogeneity, we have used T cell populations as examples throughout. Flow and mass cytometry give a detailed representation of the cells within the tumour in a single-cell suspension on a per-cell basis. Imaging technologies, such as imaging mass cytometry, are used to investigate increasing numbers of markers whilst retaining the spatial and structural information of the tumour section and the infiltrating immune cells. Together, the analyses of multiple immune parameters can provide valuable information to guide clinical decision-making in CRC.
Keywords: Colorectal cancer, Flow cytometry, Immune cells, Multiparametric analysis, Immunohistochemistry, Mass cytometry, Microscopy
Core tip: Colorectal cancer (CRC) is a heterogeneous disease. The immune response to CRC is highly variable, clinically relevant, and as yet poorly characterised. Clinical management of this disease is still relatively uniform, and does not yet accommodate the influence of immune response on patient outcomes. Multiparametric analysis of CRC is the best approach to describe the immune response. Improved understanding of immune responses to CRC will guide patient management, improved survival, and identify new potential therapeutic biomarkers. This review summarises currently available modes of multiparametric analysis, their advantages and drawbacks, and their clinical relevance.
INTRODUCTION
The immune response has long been established as an important factor in determining the outcome of people with cancer. Studies using immunodeficient animal models of cancer demonstrated that tumour growth was enhanced in the absence of an immune system[1,2]. This phenomenon was later observed in humans undergoing immunosuppressive therapies[3]. Immune based therapies have been developed over the last 30 years, including immune cell transfer, immune priming and inhibition of immune checkpoints (reviewed in[4]), with much success in many types of cancer. These immunotherapies primarily target the adaptive immune system, which coordinates a tumour-specific response. However, despite the fact that a high T cell infiltrate is a better indicator of patient survival than traditional histological based staging methods[5], the immune response in colorectal cancer (CRC) has not yet been reliably and effectively harnessed to treat patients of all stages and types of disease[6,7].
CRC is a highly heterogeneous disease[8] and the immune response in cancer is equally complex. Both factors complicate research into the development of immune therapies for CRC, as well as improving treatment decisions for clinicians. Analyses of tumour-immune cell interactions require the use of a range of parameters to avoid oversimplification, and ensure the disease is captured in its entirety. Technological advances in cytometry and cytometric imaging have exponentially broadened the scope of immunological study. Use of the light and ionic mass spectrums have enabled researchers to label up to 40 parameters, both cell surface and intracellular, to interrogate the immune system on a cell-by-cell basis[9-13]. Analysis of many parameters on each cell is essential to understand the functions of the many cell groups which exist in a heterogeneous population.
In this review, we describe the need for multiparametric analysis to investigate the immune response in CRC, with a focus on how this can improve clinical outcomes. Multiparametric tools such as polychromatic flow cytometry, mass cytometry and imaging technologies, and their use in these studies, will be reviewed. We will argue that use of these techniques is essential to obtain meaningful immune data in the context of fundamental CRC research and that they can be included into ongoing and future clinical trials to improve development of new therapies.
HETEROGENEITY OF CRC AND THE EFFECT ON THE LOCAL IMMUNE RESPONSE
CRC is a heterogeneous disease with different molecular subtypes[14-16]. Patients with microsatellite instability (MSI)high tumours tend to respond better to targeted immunotherapy and have improved disease-free survival than MSInegative patients[17]. A meta-analysis of 18 CRC datasets revealed that tumours could be subtyped into four groups: MSI immune, canonical, metabolic and mesenchymal[18]. These subtypes group tumours by mutational status and chromosomal instability, somatic copy number variation, immune infiltration and metabolic regulation, and could be correlated to staging and survival outcomes[18].
The immune cells infiltrating the tumour in CRC are heterogeneous[19]. Macrophages, dendritic cells, neutrophils and lymphocytes, such as T cells, have all been studied in the context of colorectal tumours, and different populations of these cells control tumour progression or rejection, ultimately affecting overall outcome for the patient[20]. Table 1 summarises the conflicting data between infiltrating immune cell populations and the effect on CRC patient outcome. However, heterogeneity exists within these subsets, which can alter their effect in the tumour. For instance, the study of different neutrophil populations has led to conflicting evidence about their role in colorectal tumours[21-23]. Two studies used immunohistochemistry (IHC) to count myeloperoxidase (MPO)+ cells present in the tumour. Roncucci et al[22] showed that an MPO+ cell infiltrate correlated with colonic inflammation, and that these cells were a CRC risk indicator. Droeser et al[21] later showed that a high MPO+ cell infiltrate was associated with better prognosis, and that MPO was mostly expressed by CD15+ cells. CD15 is a granulocyte marker, which is also expressed at low levels on monocytes[24]. Many studies have highlighted the diversity of T cell infiltrates in CRC, and have linked these subpopulations with patient outcome[25-31].
Table 1.
Relationship between different immune cell subsets and colorectal cancer prognosis
| Type of immune cell | Canonical function | Role in colorectal cancer | Ref. |
| CD8+ T cells | Production of cytokines (including IFN-γ) and cytotoxic molecules | High numbers of CD8+ T cells in the invasive margin correlate with favourable prognosis. | [56] |
| RORγT+IL-17+ T cells | Production of IL-17, which recruits inflammatory cells such as neutrophils | Associated with poor prognosis, especially in combination with low levels of Th1 cells (Tbet+IRF1+ IL12Rβ2+STAT4+) | [31] |
| Regulatory T (Treg) cells | Regulation and suppression of effector T cell responses, production of IL-10 and TGF-β. | High density of CD3+FOXP3+ Tregs associated with improved disease-free survival. | [11] |
| Effector Treg cells | Regulation of T cell responses, production of cytokines | CD3+FOXP3+Blimp-1+ associated with increased disease-free survival. | [72] |
| Macrophages | Phagocytic cells with pro- or anti-inflammatory properties, recruit T cells, neutrophils | Associated with favourable prognosis at the invasive margin. | [51] |
| Neutrophils | Phagocytosis of infected, damaged or dying cells, including tumours | Conflicting results, but a high ratio of neutrophils:CD8+T cells associated with poor prognosis. | [21-23,86] |
| Dendritic cells | Antigen presenting cells | Mature tumour-infiltrating (S100+CD83+) dendritic cells associated with improved prognosis. | [87] |
Blimp-1: B lymphocyte-induced maturation protein 1; FOXP3: Forkhead box protein 3; IFN-γ: Interferon-gamma; TGF-β: Transforming growth factor-beta; Treg: Regulatory T cell.
However, the immune response is not independent of the tumour microenvironment. Tumour type and associated molecular profile are associated with different levels of immune infiltrate[18]. The MSI status of a tumour has a strong effect on the number of infiltrating CD3+ lymphocytes: MSIhigh tumours are associated with higher levels of CD8+ T cells within the tumour, and with higher levels of infiltrating forkhead box protein (FOXP3)+ regulatory T (Treg) cells[32,33].
Soluble mediators produced by tumour cells can have inflammatory or immunomodulatory effects, which dictate the function of both tumour and immune cells. Tumours produce angiogenic factors that promote growth, but which can also inhibit activity of the immune infiltrate. Vascular endothelial growth factor (VEGF) is a proangiogenic factor produced by tumour cells[34], and which has immunomodulatory effects on macrophages, T cells and dendritic cells (reviewed in[35]). VEGF led to the maturation of CD34+ haematopoietic stem cells into dendritic cells[36], and also induced conversion of immature dendritic cells into endothelial-like cells, which may contribute to neovascularisation[37].
The cytokine environment has a significant impact on immune cell function and activity. In a Rag-/- mouse model of mucosal inflammatory hypoxia, Treg formation was induced through induction of FoxP3 expression in a transforming growth factor (TGF)-β dependent manner[38]. TGF-β also has a direct pro-oncogenic function in cancer. As well as promoting invasion and metastasis of tumour cells, TGF-β inhibits the anti-tumour immune response by modulating proliferation, function and differentiation of both myeloid and lymphocyte populations (reviewed in[39]). In colorectal cancer patients with MSIhigh tumours, mutations in the TGF-β receptor (TGF-β1 RII) were associated with a higher rate of disease free survival and a lower risk of recurrence than MSIhigh tumours without TGF-β1 RII mutations[40].
IMMUNE CELL PLASTICITY
Immune cells change phenotype and function in response to changes in the cytokine environment or to other stimuli. T cells differentiate into different subtypes when activated by different pathogens, and plasticity between these subtypes has been shown in many models (reviewed in[41]). In a study of T cells from healthy human blood, a high degree of heterogeneity was found within canonical T cell subsets[42]. T cells with an intermediate phenotype between different subsets indicated plasticity even in healthy individuals[42].
The tumour microenvironment induces changes in the phenotype of many types of immune cells. The repolarisation of suppressive Treg cells within the tumour was shown with the discovery of a FOXP3+ population expressing canonical inflammatory markers interleukin (IL)-17 and RORγt, in patients with CRC[43]. Expression of IL-17 by tumour infiltrating lymphocytes was associated with poor survival in CRC patients[31]. These data show that the tumour can manipulate T cells from an immunoregulatory state to an inflammatory pro-tumour state. Both macrophages and neutrophils have been shown to change phenotype and function within the CRC tumour microenvironment[44,45]. For example, soluble factors in the tumour microenvironment, such as TGF-β and IL-13, can drive macrophages into a suppressive phenotype, which promotes tumour progression[46]. In patients with CRC, tumour-associated macrophages (TAMs) had distinct phenotypes to those found in adjacent non-tumour bowel. An increase in populations resembling both pro- and anti-inflammatory macrophages was found within the tumour[19].
TUMOUR-IMMUNE CELL INTERACTIONS-SPATIAL HETEROGENEITY
Tumour-immune cell interactions are spatially heterogeneous, and different infiltrates in different parts of the tumour are associated with different outcomes. As early as 2009, it was shown that the infiltrate of T cells in the invasive margin of CRC tumours was more predictive of patient outcome than T cells in the centre of the tumour[47]. Similarly, a high frequency of CD3+ T cells < 10 μm from metastatic liver CRC tumours correlated with improved overall survival[48]. A comprehensive study of the infiltrating immune cells over the course of tumour progression showed that T cell presence in the tumour decreased over time, whereas the number of B cells and innate immune cells such as macrophages and neutrophils increased with tumour progression[49]. This study also showed that the immune cell populations were distinct between the tumour core and the invasive margin. For instance, in the tumour core, the B cells were closely correlated with the T cell network, despite being present in low numbers[49]. In stage I-II CRC, a low infiltration of neutrophils in the invasive margin was associated with poor patient prognosis[50]. The presence of macrophages at the invasive margin of colorectal tumours was correlated with improved prognosis, but not their presence overall, highlighting the importance of retaining spatial information where possible[51].
Interactions between immune cells also have prognostic power. Co-localisation of CD8+ T cells and CD66b+ neutrophils predicted favourable prognosis in CRC patients[25]. Functional analyses of these cell groups showed that neutrophils from the tumour activated T cells to produce interferon-gamma (IFN-γ). The addition of functional markers into imaging analysis would have increased the power of this study[52].
TOOLS FOR MULTIPARAMETRIC ANALYSIS
Flow cytometry
Flow cytometry is a technique which enables characterisation of cells on a per-cell basis. Cell surface and intracellular proteins are labelled using epitope-specific antibodies conjugated to specific fluorophores[12,53]. Fluorescence-activated cell sorting (FACS) is a related technique, which uses flow to sort viable cells based on the expression of markers which have been tagged. This is a useful tool if further in vitro assays are required to investigate phenotype and function of particular cell types.
Often limited by spectral overlap and spread of the fluorophores, and low- or co-expressed antigens, multiparametric flow cytometry using more than 12 markers can require a high level of technical skill and appropriate instrumentation. By rearranging detectors and applying appropriate controls, a 17-colour panel was developed and used to successfully investigate multiple markers on T cell populations[12]. This study was a method development study that highlighted heterogeneity in pre-defined T cell subsets. The development of the bright Brilliant Violet dyes greatly extended the limits of markers which could be analysed in a single panel[54,55]. Further developments in fluorophore chemistry and use of a 50-detector system have resulted in the development of a 27-colour panel for flow cytometry[55].
Flow cytometry has been an essential tool for cancer research. Immunohistological staining showed that more T cells in colorectal tumours correlated with better prognosis, but a causal effect on survival was elucidated using flow cytometry[56,57]. Flow cytometry has been used successfully to identify multiple subsets of immune cells in the context of colorectal cancer[27,30].
Studies using flow cytometry to investigate the role of Tregs in CRC have produced conflicting results. A high infiltrate of Tregs has been associated with negative, positive, or no effect on patient outcome (reviewed in[58]). The heterogeneity and plasticity of Treg populations may explain these conflicting results. A study which defined Tregs as CD4+FOXP3+CD25+CD127lo, using flow cytometry, found that circulating Tregs were reduced in patients with metastatic CRC who had been treated with a VEGF receptor blocker[59]. A study which used flow cytometry to investigate multiple parameters of Tregs in CRC patients revealed a population of highly immunosuppressive CD4+FOXP3- Treg-like cells that were enriched in the tumour, compared to adjacent colon or blood[60]. Cells in this population expressed Helios, CD39, cytotoxic T-lymphocyte antigen (CTLA-4), and latency-associated peptide (LAP). They also produced IL-10 and TGF-β, and were 50 times more suppressive than CD4+FOXP3+ cells[60]. The heterogeneity of the Treg infiltrate necessitates the inclusion of a wide range of markers to understand their role in disease progression.
Flow cytometry has also been used to examine T cell dysfunction in CRC patients. In a study comparing immune cell phenotypes from T cells infiltrating the tumour and non-tumour bowel from matched CRC patients, a higher proportion of cells in the tumour than bowel expressed “exhaustion” markers, including programmed death ligand 1 (PD-1), LAG-3, CTLA-4 and TIM-3[30,61,62]. In a separate study of tumour draining lymph nodes, CD8+ T cells that expressed the activation marker PD-1 were functional in the tumour-free lymph node, but could not produce cytokines in the tumour-draining lymph node[63].
Mass cytometry
The challenges with spectral overlap and compensation experienced by users of flow cytometry have been addressed with the advent of mass cytometry. This technology shares the basic principle of flow cytometry, except monoclonal antibodies are labelled with heavy metal isotopes instead of fluorophores[64]. After cells are labelled, they are nebulised and the isotopes are measured cell-by-cell using mass spectrometry[64]. The use of heavy metal isotopes eliminates spectral overlap, therefore, the number of parameters that can be incorporated is much greater. While the technique is used for 40 parameters currently, the availability of heavy metal ions and lack of spectral overlap mean that this technology can theoretically be used to examine between 70 and 100 markers per cell[65].
The analysis of up to 40 markers gives much better resolution as to the functional and phenotypic properties of the cells of interest. Analysis of heterogeneous cell populations requires the measurement of as many markers as possible, so that the full complexity of the immune cell-tumour interactions can be understood. Mass cytometry was used to develop a detailed model of haematopoietic development in human bone marrow samples and showed functional responses to drug interventions differed across immune cell subtypes[9]. This was one the first papers to use this technique to show the complexity of immune cell development and signalling[9].
Recently, researchers have used mass cytometry to begin studying the complex dyanmics of tumour-immune cell interactions. Spitzer et al[13] demonstrated that the success of immunotherapy in a mouse model required a systemic and diverse immune response. Analysis of the immune infiltrate of bone marrow, blood, tumour, spleen and lymph nodes showed that tumour rejection after immunotherapy was dependent on a system-wide response. The breadth and depth of this study revealed factors essential for immune-mediated tumour rejection in the context of immunotherapy which have until now been overlooked. Mass cytometry has since been used to develop an “immune atlas” of immune cell profiles during the development of lung adenocarcinomas[66]. The use of high dimensional single cell analysis of the early development of lung tumours highlighted modulation of innate cells as an avenue for early therapy.
A caveat of incorporating many parameters is that an analytical framework is needed to visualise the high-dimensional output, and compare datasets within the research community[67]. The tools most useful for multiparametric dataset analysis and visualisation were recently reviewed by Kimball et al[68].
IMAGING TECHNIQUES
The most important advantage of imaging techniques over mass cytometry and flow cytometry is that spatial and geographical information is retained. The imaging techniques described below have been used to determine that the distribution of immune cells, in relation to other immune cells and cancer cells, can not only improve the ability of clinicians to prognosticate patients with CRC but also reveal which patients will benefit from further treatment.
Immunohistochemistry
Lymphocyte infiltration related to CRC patient prognosis was established via histopathology in 1978[69], and IHC has been used in the last 40 years to look at the type, frequency and location of immune cells in colorectal cancer tumours. Galon et al[56] showed in 2006 that higher lymphocytic cell infiltrate in two tumour regions (invasive margin and centre of the tumour) correlated with improved disease-free and overall survival in CRC patients. The T cell infiltrate was a better predictor of patient disease-free survival than current histopathological staging methods used in the clinic. These findings have helped define patient prognosis, better identify high-risk patients, and improve the quality of life by predicting patients who would benefit from further therapy. There have recently been new developments in highly multiplexed tissue imaging. For a comprehensive description of some of the following techniques, see[70].
Immunofluoresence microscopy
Immunofluorescence, using confocal microscopy to look at several markers simultaneously, provides an advantage over standard IHC. Secondary antibodies conjugated to a fluorophore are added to primary antibody-labelled tissue sections. Up to four fluorophores can typically be detected on a confocal microscope. This technique has been used to increase understanding of the immune infiltrate in colorectal tumours and how different cell subsets relate to prognosis, for example, neutrophil and CD8+ T cell interplay has been shown to improve survival in CRC patients[52]. Another example is the validation of the Immunoscore[5] in a New Zealand cohort of stage II CRC patients[71]. Importantly, it was found that analysis of additional markers (to identify Tregs and effector Tregs) provided means to identify patients with the highest risk of recurrent disease, meaning that these patients can be referred for further treatment to improve their prognosis.
The measurement of more than four parameters simultaneously using immunofluorescence has required a change in technology to include iterative staining protocols. An example of this technology is the OpalTM method, developed for fluorescent IHC in formalin-fixed paraffin-embedded (FFPE) tissue of up to seven markers. Using this platform, the tissue sample is stained in cycles with primary antibodies, secondary antibodies conjugated to HRP, and fluorophores which undergo a tyramide signal amplification reaction to covalently bond to the epitope. The antibodies are then removed without disrupting the fluorescence signals, which are measured by multispectral imaging[72]. This multiparameter imaging approach has been used in CRC research to show that TGF-β signalling proteins were increased in cancer tissues compared to normal tissues, and several proteins correlated with improved overall survival[73]. Further, spatial distribution of cytotoxic T cells in proximity to cancer cells correlated with increased overall patient survival in a study of pancreatic cancer patients[74], increased T cells at the tumour border improved overall survival in patients with CRC liver metastases[48], and Treg and CD8+ T cell proximity to cancer cells in non-small cell lung cancer correlate with worse and better lung cancer survival, respectively[75].
Mass cytometry-based imaging methods
Mass cytometry-based imaging methods, such as imaging mass cytometry (IMC[76]) and multiplex ion beam imaging (MIBI[77]) can overcome many difficulties seen with immunofluorescence and allow the detection of up to 40 markers simultaneously using rare earth metal isotopes. The tissue is both ablated by a laser (IMC) or ion beam (MIBI) and carried by a stream of inert gas to a mass cytometer. The metal isotopes detected are mapped to the location of each spot to create an image of the target proteins throughout the tissue of interest[70,77,78]. So far, there are limited studies using these techniques and none in the field of CRC. Giesen et al[76] used IMC and Angelo et al[78] used MIBI on human breast cancer samples. These studies highlight the heterogeneity of tumours and why it is important to look at many parameters together. Because these techniques greatly increase the number of markers that can be looked at simultaneously, it has the potential to significantly expand our understanding of the immune response to CRC. Knowledge of a beneficial or disadvantageous immune infiltrate will enable better prognosis prediction and more personalised treatment.
Digital spatial profiling technology
Digital spatial profiling is a novel platform developed by NanoString Technologies. It combines standard immunofluorescence techniques with digital optical barcoding technology to detect and map up to 800 protein and RNA targets in FFPE samples without destroying the tissue[79]. There are few studies using this technology thus far; however, it has the potential to further increase the volume of data that can be obtained from samples with the inclusion of RNA detection.
MOLECULAR METHODS
Several molecular methods are available to study the immune infiltrate at the genetic, chromatin or transcriptional level, but these methods are outside the scope of this review. Briefly, gene expression analyses using qPCR can show functional differences in the immune infiltrate, but cell-by-cell gene expression techniques are still under development for widespread use. Assay for transposase-accessible chromatin sequencing (ATAC-seq) is used to investigate the chromatin structure of mammalian cells to show the regulation of the chromosomes, which in turn indicates the phenotype and function of a cell, and has been used to distinguish T cell activation from dysfunction[80,81]. ATAC-seq has also been developed to use on a cell-by-cell basis, for heterogeneous populations[82].
CLINICAL PERSPECTIVES
Oncologists and surgeons encounter immunologic, genetic, molecular, and cellular variables involved in CRC patient prognosis. Critical appraisal of the effect of each complex variable is not practical for the clinical specialist also charged with keeping abreast of their own field. This glut of information contributes to slow clinical uptake of promising developments. In particular, advances in the immune response to CRC are complex, often conflicting, and of quality that is difficult for the uninitiated to assess. There is therefore a need not only for detailed analysis of the immune response to CRC, but also a pathway for communicating this information to clinical practice.
The ideal multiparametric analysis of CRC tissue in the clinical setting would more accurately guide postoperative patient management. Chemotherapy is currently excluded from patient treatment when the mortality and morbidity risks inherent with treatment are thought to outweigh the risk of CRC recurrence. But current prognostic techniques are failing up to 25% of patients with apparently “low risk” CRC who experience recurrence despite their good American Joint Committee on Cancer (AJCC) prognosis. These patients often do not receive chemotherapy but with clinical hindsight would benefit from treatment. The current focus of the Immunoscore is improving patient selection for chemotherapy. Gold standard chemotherapy is multiagent[83,84]; however, components are often poorly tolerated and occasionally rationalised to single agent treatment. Future research should include large cohort studies that correlate immune infiltrate with response to chemotherapeutic agents, both single and multiagent. This could further personalise patient management to allow exclusion of risky chemotherapeutic components where appropriate.
CRC tissue banks are ideally suited to prognostic research. Retrospective banks eliminate the usual lag between tissue analysis and patient follow up, enabling immediate correlation of immune infiltrate with long term patient outcome. However, tissue is frequently stored as FFPE, as this is the current histopathological tissue preparation. Not all modes of multiparametric analysis are compatible with FFPE prepared tissue, but most methods of storage have a concordant mode of multiparametric analysis. It can be argued that there is an ethical obligation (with appropriate consent) to donor patients and families in any use of donated tissue; the researcher should collect as much information as possible from as small a sample of tissue as feasible. Multiparametric analysis facilitates this.
Clinical cohort studies have explored crude assessments of inflammation in the context of CRC, such as the neutrophil lymphocyte ratio, with indeterminate results[85-87]. This demonstrates that the importance of immune response to CRC is gradually infiltrating the clinical lexicon. The Immunoscore has far more definitive results[5] but has yet to enter routine clinical practice. However, both these scores are rudimentary quantification of immune cells, and do not reflect the heterogeneity and plasticity of the immune system. Multiparametric analysis addresses this, but adds yet more complexity to a field already overwhelming to the average clinical specialist. Encouraging clinical uptake of new biomarkers will require evolution of existing communication between immunologists and clinicians. Future multidisciplinary cancer management meetings may require incorporation of immunologists, geneticists, and molecular pathologists (Figure 1).
Figure 1.
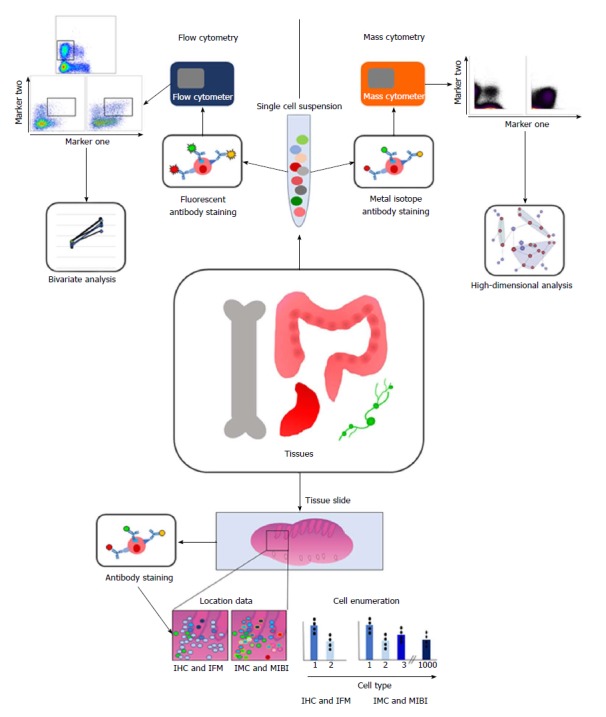
Diagram summarising the methodology and analysis techniques involved in multiparametric analysis of immune cells. Briefly, tissues of interest are taken and either dissociated into a single cell suspension or mounted onto slides. Cells in suspension are stained with fluorescently labelled antibodies for flow cytometry, and then acquired using a flow cytometer. This data is often presented as populations positive or negative for 2 markers. For mass cytometry, cells are stained with antibodies labelled with heavy metal isotopes, and then acquired using a mass cytometer. The high dimensionality of this data means that clustering analyses are preferred to analyse this data. For imaging techniques, the tissue slides are also labelled with antibodies, and can be imaged using IHC, IFM, IMC or MIBI. Imaging techniques enable location of immune cells in situ, as well as enumeration of cell types. IMC and MIBI use more parameters, meaning more cell types can be distinguished, and clustering algorithms can be used on this data too, and related back to the geographical location of each cell. IHC: Immunohistochemistry; IFM: Immunofluorescent microscopy; IMC: Imaging mass cytometry; MIBI: Multiplex ion beam imaging.
CONCLUSION
The complexity and heterogeneity of tumour-immune cell interactions necessitate the use of multiparametric analyses. CRC tumours are highly heterogeneous, and have correspondingly heterogeneous immune infiltrates. The immune cells in a single tumour are also dynamic and varied, and interact with each other and the tumour cells to affect patient prognosis, both positively and negatively. The spatial distribution of these cells can also play a role in the clinical outcome of a CRC patient.
The integration of single cell analysis, functional assays and imaging techniques can be applied in combination to better understand the role of the immune system in CRC. It should be noted that the techniques presented here are limited in that they are only qualitative, and do not always provide quantitative data. Current techniques are able to quantify and describe in detail different immune populations, but extrapolating the effects of these populations on patient outcome is limited to association studies, which require large cohorts. The next step will be combining phenotypic characterisation with functional and interaction analysis, to better understand the mechanisms through which immune cell subsets interact with the tumour in situ.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: New Zealand
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The authors declare no conflicts of interest.
Peer-review started: March 29, 2018
First decision: May 16, 2018
Article in press: June 16, 2018
P- Reviewer: Roussel M, Sterpetti AV S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y
Contributor Information
Julia KH Leman, Department of Microbiology and Immunology, University of Otago, Dunedin 9010, New Zealand.
Sarah K Sandford, Department of Microbiology and Immunology, University of Otago, Dunedin 9010, New Zealand.
Janet L Rhodes, Department of Microbiology and Immunology, University of Otago, Dunedin 9010, New Zealand.
Roslyn A Kemp, Department of Microbiology and Immunology, University of Otago, Dunedin 9010, New Zealand. roslyn.kemp@otago.ac.nz.
References
- 1.Stutman O. Tumor development after 3-methylcholanthrene in immunologically deficient athymic-nude mice. Science. 1974;183:534–536. doi: 10.1126/science.183.4124.534. [DOI] [PubMed] [Google Scholar]
- 2.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 3.Penn I, Starzl TE. Malignant tumors arising de novo in immunosuppressed organ transplant recipients. Transplantation. 1972;14:407–417. doi: 10.1097/00007890-197210000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. 2016;13:273–290. doi: 10.1038/nrclinonc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galon J, Pagès F, Marincola FM, Angell HK, Thurin M, Lugli A, Zlobec I, Berger A, Bifulco C, Botti G, et al. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10:205. doi: 10.1186/1479-5876-10-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung KY, Gore I, Fong L, Venook A, Beck SB, Dorazio P, Criscitiello PJ, Healey DI, Huang B, Gomez-Navarro J, et al. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J Clin Oncol. 2010;28:3485–3490. doi: 10.1200/JCO.2010.28.3994. [DOI] [PubMed] [Google Scholar]
- 8.Jass JR. Molecular heterogeneity of colorectal cancer: Implications for cancer control. Surg Oncol. 2007;16 Suppl 1:S7–S9. doi: 10.1016/j.suronc.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 9.Bendall SC, Simonds EF, Qiu P, Amir el-AD, Krutzik PO, Finck R, Bruggner RV, Melamed R, Trejo A, Ornatsky OI, Balderas RS, Plevritis SK, Sachs K, Pe’er D, Tanner SD, Nolan GP. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science. 2011;332:687–696. doi: 10.1126/science.1198704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irish JM, Doxie DB. High-dimensional single-cell cancer biology. Curr Top Microbiol Immunol. 2014;377:1–21. doi: 10.1007/82_2014_367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ornatsky O, Bandura D, Baranov V, Nitz M, Winnik MA, Tanner S. Highly multiparametric analysis by mass cytometry. J Immunol Methods. 2010;361:1–20. doi: 10.1016/j.jim.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Perfetto SP, Chattopadhyay PK, Roederer M. Seventeen-colour flow cytometry: unravelling the immune system. Nat Rev Immunol. 2004;4:648–655. doi: 10.1038/nri1416. [DOI] [PubMed] [Google Scholar]
- 13.Spitzer MH, Carmi Y, Reticker-Flynn NE, Kwek SS, Madhireddy D, Martins MM, Gherardini PF, Prestwood TR, Chabon J, Bendall SC, et al. Systemic Immunity Is Required for Effective Cancer Immunotherapy. Cell. 2017;168:487–502.e15. doi: 10.1016/j.cell.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Compton CC. Colorectal carcinoma: diagnostic, prognostic, and molecular features. Mod Pathol. 2003;16:376–388. doi: 10.1097/01.MP.0000062859.46942.93. [DOI] [PubMed] [Google Scholar]
- 15.De Sousa E Melo F, Wang X, Jansen M, Fessler E, Trinh A, de Rooij LP, de Jong JH, de Boer OJ, van Leersum R, Bijlsma MF, et al. Poor-prognosis colon cancer is defined by a molecularly distinct subtype and develops from serrated precursor lesions. Nat Med. 2013;19:614–618. doi: 10.1038/nm.3174. [DOI] [PubMed] [Google Scholar]
- 16.Lengauer C, Kinzler KW, Vogelstein B. Genetic instability in colorectal cancers. Nature. 1997;386:623–627. doi: 10.1038/386623a0. [DOI] [PubMed] [Google Scholar]
- 17.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norton SE, Ward-Hartstonge KA, Taylor ES, Kemp RA. Immune cell interplay in colorectal cancer prognosis. World J Gastrointest Oncol. 2015;7:221–232. doi: 10.4251/wjgo.v7.i10.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 21.Droeser RA, Hirt C, Eppenberger-Castori S, Zlobec I, Viehl CT, Frey DM, Nebiker CA, Rosso R, Zuber M, Amicarella F, et al. High myeloperoxidase positive cell infiltration in colorectal cancer is an independent favorable prognostic factor. PLoS One. 2013;8:e64814. doi: 10.1371/journal.pone.0064814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roncucci L, Mora E, Mariani F, Bursi S, Pezzi A, Rossi G, Pedroni M, Luppi D, Santoro L, Monni S, et al. Myeloperoxidase-positive cell infiltration in colorectal carcinogenesis as indicator of colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2008;17:2291–2297. doi: 10.1158/1055-9965.EPI-08-0224. [DOI] [PubMed] [Google Scholar]
- 23.Rao HL, Chen JW, Li M, Xiao YB, Fu J, Zeng YX, Cai MY, Xie D. Increased intratumoral neutrophil in colorectal carcinomas correlates closely with malignant phenotype and predicts patients’ adverse prognosis. PLoS One. 2012;7:e30806. doi: 10.1371/journal.pone.0030806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gustafson MP, Lin Y, Maas ML, Van Keulen VP, Johnston PB, Peikert T, Gastineau DA, Dietz AB. A method for identification and analysis of non-overlapping myeloid immunophenotypes in humans. PLoS One. 2015;10:e0121546. doi: 10.1371/journal.pone.0121546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amicarella F, Muraro MG, Hirt C, Cremonesi E, Padovan E, Mele V, Governa V, Han J, Huber X, Droeser RA, et al. Dual role of tumour-infiltrating T helper 17 cells in human colorectal cancer. Gut. 2017;66:692–704. doi: 10.1136/gutjnl-2015-310016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frey O, Reichel A, Bonhagen K, Morawietz L, Rauchhaus U, Kamradt T. Regulatory T cells control the transition from acute into chronic inflammation in glucose-6-phosphate isomerase-induced arthritis. Ann Rheum Dis. 2010;69:1511–1518. doi: 10.1136/ard.2009.123422. [DOI] [PubMed] [Google Scholar]
- 27.Girardin A, McCall J, Black MA, Edwards F, Phillips V, Taylor ES, Reeve AE, Kemp RA. Inflammatory and regulatory T cells contribute to a unique immune microenvironment in tumor tissue of colorectal cancer patients. Int J Cancer. 2013;132:1842–1850. doi: 10.1002/ijc.27855. [DOI] [PubMed] [Google Scholar]
- 28.Saito T, Nishikawa H, Wada H, Nagano Y, Sugiyama D, Atarashi K, Maeda Y, Hamaguchi M, Ohkura N, Sato E, et al. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med. 2016;22:679–684. doi: 10.1038/nm.4086. [DOI] [PubMed] [Google Scholar]
- 29.Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 30.Taylor ES, McCall JL, Girardin A, Munro FM, Black MA, Kemp RA. Functional impairment of infiltrating T cells in human colorectal cancer. Oncoimmunology. 2016;5:e1234573. doi: 10.1080/2162402X.2016.1234573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pagès F, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263–1271. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 32.Michel S, Benner A, Tariverdian M, Wentzensen N, Hoefler P, Pommerencke T, Grabe N, von Knebel Doeberitz M, Kloor M. High density of FOXP3-positive T cells infiltrating colorectal cancers with microsatellite instability. Br J Cancer. 2008;99:1867–1873. doi: 10.1038/sj.bjc.6604756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mlecnik B, Bindea G, Angell HK, Maby P, Angelova M, Tougeron D, Church SE, Lafontaine L, Fischer M, Fredriksen T, et al. Integrative Analyses of Colorectal Cancer Show Immunoscore Is a Stronger Predictor of Patient Survival Than Microsatellite Instability. Immunity. 2016;44:698–711. doi: 10.1016/j.immuni.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 34.Khorana AA, Ryan CK, Cox C, Eberly S, Sahasrabudhe DM. Vascular endothelial growth factor, CD68, and epidermal growth factor receptor expression and survival in patients with Stage II and Stage III colon carcinoma: a role for the host response in prognosis. Cancer. 2003;97:960–968. doi: 10.1002/cncr.11152. [DOI] [PubMed] [Google Scholar]
- 35.Li YL, Zhao H, Ren XB. Relationship of VEGF/VEGFR with immune and cancer cells: staggering or forward? Cancer Biol Med. 2016;13:206–214. doi: 10.20892/j.issn.2095-3941.2015.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 37.Fernandez Pujol B, Lucibello FC, Zuzarte M, Lütjens P, Müller R, Havemann K. Dendritic cells derived from peripheral monocytes express endothelial markers and in the presence of angiogenic growth factors differentiate into endothelial-like cells. Eur J Cell Biol. 2001;80:99–110. doi: 10.1078/0171-9335-00136. [DOI] [PubMed] [Google Scholar]
- 38.Clambey ET, McNamee EN, Westrich JA, Glover LE, Campbell EL, Jedlicka P, de Zoeten EF, Cambier JC, Stenmark KR, Colgan SP, et al. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci U S A. 2012;109:E2784–E2793. doi: 10.1073/pnas.1202366109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kubiczkova L, Sedlarikova L, Hajek R, Sevcikova S. TGF-β - an excellent servant but a bad master. J Transl Med. 2012;10:183. doi: 10.1186/1479-5876-10-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watanabe T, Wu TT, Catalano PJ, Ueki T, Satriano R, Haller DG, Benson AB 3rd, Hamilton SR. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N Engl J Med. 2001;344:1196–1206. doi: 10.1056/NEJM200104193441603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy KM, Stockinger B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nat Immunol. 2010;11:674–680. doi: 10.1038/ni.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kunicki MA, Amaya Hernandez LC, Davis KL, Bacchetta R, Roncarolo MG. Identity and Diversity of Human Peripheral Th and T Regulatory Cells Defined by Single-Cell Mass Cytometry. J Immunol. 2018;200:336–346. doi: 10.4049/jimmunol.1701025. [DOI] [PubMed] [Google Scholar]
- 43.Blatner NR, Mulcahy MF, Dennis KL, Scholtens D, Bentrem DJ, Phillips JD, Ham S, Sandall BP, Khan MW, Mahvi DM, et al. Expression of RORγt marks a pathogenic regulatory T cell subset in human colon cancer. Sci Transl Med. 2012;4:164ra159. doi: 10.1126/scitranslmed.3004566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Norton SE, Dunn ET, McCall JL, Munro F, Kemp RA. Gut macrophage phenotype is dependent on the tumor microenvironment in colorectal cancer. Clin Transl Immunology. 2016;5:e76. doi: 10.1038/cti.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang F, Wang H, Wang X, Jiang G, Liu H, Zhang G, Wang H, Fang R, Bu X, Cai S, et al. TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget. 2016;7:52294–52306. doi: 10.18632/oncotarget.10561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laghi L, Bianchi P, Miranda E, Balladore E, Pacetti V, Grizzi F, Allavena P, Torri V, Repici A, Santoro A, et al. CD3+ cells at the invasive margin of deeply invading (pT3-T4) colorectal cancer and risk of post-surgical metastasis: a longitudinal study. Lancet Oncol. 2009;10:877–884. doi: 10.1016/S1470-2045(09)70186-X. [DOI] [PubMed] [Google Scholar]
- 48.Berthel A, Zoernig I, Valous NA, Kahlert C, Klupp F, Ulrich A, Weitz J, Jaeger D, Halama N. Detailed resolution analysis reveals spatial T cell heterogeneity in the invasive margin of colorectal cancer liver metastases associated with improved survival. Oncoimmunology. 2017;6:e1286436. doi: 10.1080/2162402X.2017.1286436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 50.Wikberg ML, Ling A, Li X, Öberg Å, Edin S, Palmqvist R. Neutrophil infiltration is a favorable prognostic factor in early stages of colon cancer. Hum Pathol. 2017;68:193–202. doi: 10.1016/j.humpath.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 51.Nagorsen D, Voigt S, Berg E, Stein H, Thiel E, Loddenkemper C. Tumor-infiltrating macrophages and dendritic cells in human colorectal cancer: relation to local regulatory T cells, systemic T-cell response against tumor-associated antigens and survival. J Transl Med. 2007;5:62. doi: 10.1186/1479-5876-5-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Governa V, Trella E, Mele V, Tornillo L, Amicarella F, Cremonesi E, Muraro MG, Xu H, Droeser R, Däster SR, et al. The Interplay Between Neutrophils and CD8+ T Cells Improves Survival in Human Colorectal Cancer. Clin Cancer Res. 2017;23:3847–3858. doi: 10.1158/1078-0432.CCR-16-2047. [DOI] [PubMed] [Google Scholar]
- 53.Hulett HR, Bonner WA, Barrett J, Herzenberg LA. Cell sorting: automated separation of mammalian cells as a function of intracellular fluorescence. Science. 1969;166:747–749. doi: 10.1126/science.166.3906.747. [DOI] [PubMed] [Google Scholar]
- 54.Chattopadhyay PK, Gaylord B, Palmer A, Jiang N, Raven MA, Lewis G, Reuter MA, Nur-ur Rahman AK, Price DA, Betts MR, et al. Brilliant violet fluorophores: a new class of ultrabright fluorescent compounds for immunofluorescence experiments. Cytometry A. 2012;81:456–466. doi: 10.1002/cyto.a.22043. [DOI] [PubMed] [Google Scholar]
- 55.Ashhurst TM, Smith AL, King NJC. High-Dimensional Fluorescence Cytometry. Curr Protoc Immunol. 2017;119:5.8.1–5.8.38. doi: 10.1002/cpim.37. [DOI] [PubMed] [Google Scholar]
- 56.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 57.Reissfelder C, Stamova S, Gossmann C, Braun M, Bonertz A, Walliczek U, Grimm M, Rahbari NN, Koch M, Saadati M, et al. Tumor-specific cytotoxic T lymphocyte activity determines colorectal cancer patient prognosis. J Clin Invest. 2015;125:739–751. doi: 10.1172/JCI74894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ward-Hartstonge KA, Kemp RA. Regulatory T-cell heterogeneity and the cancer immune response. Clin Transl Immunology. 2017;6:e154. doi: 10.1038/cti.2017.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Terme M, Pernot S, Marcheteau E, Sandoval F, Benhamouda N, Colussi O, Dubreuil O, Carpentier AF, Tartour E, Taieb J. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 2013;73:539–549. doi: 10.1158/0008-5472.CAN-12-2325. [DOI] [PubMed] [Google Scholar]
- 60.Scurr M, Ladell K, Besneux M, Christian A, Hockey T, Smart K, Bridgeman H, Hargest R, Phillips S, Davies M, et al. Highly prevalent colorectal cancer-infiltrating LAP+ Foxp3- T cells exhibit more potent immunosuppressive activity than Foxp3+ regulatory T cells. Mucosal Immunol. 2014;7:428–439. doi: 10.1038/mi.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 62.Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci USA. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu X, Zhang H, Xing Q, Cui J, Li J, Li Y, Tan Y, Wang S. PD-1(+) CD8(+) T cells are exhausted in tumours and functional in draining lymph nodes of colorectal cancer patients. Br J Cancer. 2014;111:1391–1399. doi: 10.1038/bjc.2014.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spitzer MH, Nolan GP. Mass Cytometry: Single Cells, Many Features. Cell. 2016;165:780–791. doi: 10.1016/j.cell.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bendall SC, Nolan GP, Roederer M, Chattopadhyay PK. A deep profiler’s guide to cytometry. Trends Immunol. 2012;33:323–332. doi: 10.1016/j.it.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lavin Y, Kobayashi S, Leader A, Amir ED, Elefant N, Bigenwald C, Remark R, Sweeney R, Becker CD, Levine JH, et al. Innate Immune Landscape in Early Lung Adenocarcinoma by Paired Single-Cell Analyses. Cell. 2017;169:750–765.e17. doi: 10.1016/j.cell.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spitzer MH, Gherardini PF, Fragiadakis GK, Bhattacharya N, Yuan RT, Hotson AN, Finck R, Carmi Y, Zunder ER, Fantl WJ, et al. IMMUNOLOGY. An interactive reference framework for modeling a dynamic immune system. Science. 2015;349:1259425. doi: 10.1126/science.1259425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kimball AK, Oko LM, Bullock BL, Nemenoff RA, van Dyk LF, Clambey ET. A Beginner’s Guide to Analyzing and Visualizing Mass Cytometry Data. J Immunol. 2018;200:3–22. doi: 10.4049/jimmunol.1701494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Watt AG, House AK. Colonic carcinoma: a quantitative assessment of lymphocyte infiltration at the periphery of colonic tumors related to prognosis. Cancer. 1978;41:279–282. doi: 10.1002/1097-0142(197801)41:1<279::aid-cncr2820410139>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 70.Bodenmiller B. Multiplexed Epitope-Based Tissue Imaging for Discovery and Healthcare Applications. Cell Syst. 2016;2:225–238. doi: 10.1016/j.cels.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 71.Ward-Hartstonge KA, McCall JL, McCulloch TR, Kamps AK, Girardin A, Cretney E, Munro FM, Kemp RA. Inclusion of BLIMP-1+ effector regulatory T cells improves the Immunoscore in a cohort of New Zealand colorectal cancer patients: a pilot study. Cancer Immunol Immunother. 2017;66:515–522. doi: 10.1007/s00262-016-1951-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stack EC, Wang C, Roman KA, Hoyt CC. Multiplexed immunohistochemistry, imaging, and quantitation: a review, with an assessment of Tyramide signal amplification, multispectral imaging and multiplex analysis. Methods. 2014;70:46–58. doi: 10.1016/j.ymeth.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 73.Yang L, Liu Z, Tan J, Dong H, Zhang X. Multispectral imaging reveals hyper active TGF-β signaling in colorectal cancer. Cancer Biol Ther. 2018;19:105–112. doi: 10.1080/15384047.2017.1395116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carstens JL, Correa de Sampaio P, Yang D, Barua S, Wang H, Rao A, Allison JP, LeBleu VS, Kalluri R. Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer. Nat Commun. 2017;8:15095. doi: 10.1038/ncomms15095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barua S, Fang P, Sharma A, Fujimoto J, Wistuba I, Rao AUK, Lin SH. Spatial interaction of tumor cells and regulatory T cells correlates with survival in non-small cell lung cancer. Lung Cancer. 2018;117:73–79. doi: 10.1016/j.lungcan.2018.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Giesen C, Wang HA, Schapiro D, Zivanovic N, Jacobs A, Hattendorf B, Schüffler PJ, Grolimund D, Buhmann JM, Brandt S, et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat Methods. 2014;11:417–422. doi: 10.1038/nmeth.2869. [DOI] [PubMed] [Google Scholar]
- 77.Angelo M, Bendall SC, Finck R, Hale MB, Hitzman C, Borowsky AD, Levenson RM, Lowe JB, Liu SD, Zhao S, et al. Multiplexed ion beam imaging of human breast tumors. Nat Med. 2014;20:436–442. doi: 10.1038/nm.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chang Q, Ornatsky OI, Siddiqui I, Loboda A, Baranov VI, Hedley DW. Imaging Mass Cytometry. Cytometry A. 2017;91:160–169. doi: 10.1002/cyto.a.23053. [DOI] [PubMed] [Google Scholar]
- 79.Kulkarni MM. Digital multiplexed gene expression analysis using the NanoString nCounter system. Curr Protoc Mol Biol. 2011;25:Unit25B.10. doi: 10.1002/0471142727.mb25b10s94. [DOI] [PubMed] [Google Scholar]
- 80.Philip M, Fairchild L, Sun L, Horste EL, Camara S, Shakiba M, Scott AC, Viale A, Lauer P, Merghoub T, et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature. 2017;545:452–456. doi: 10.1038/nature22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Buenrostro JD, Wu B, Chang HY, Greenleaf WJ. ATAC-seq: A Method for Assaying Chromatin Accessibility Genome-Wide. Curr Protoc Mol Biol. 2015;109:21.29.1–21.29.9. doi: 10.1002/0471142727.mb2129s109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Buenrostro JD, Wu B, Litzenburger UM, Ruff D, Gonzales ML, Snyder MP, Chang HY, Greenleaf WJ. Single-cell chromatin accessibility reveals principles of regulatory variation. Nature. 2015;523:486–490. doi: 10.1038/nature14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 84.Tournigand C, André T, Bonnetain F, Chibaudel B, Lledo G, Hickish T, Tabernero J, Boni C, Bachet JB, Teixeira L, et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol. 2012;30:3353–3360. doi: 10.1200/JCO.2012.42.5645. [DOI] [PubMed] [Google Scholar]
- 85.Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218–230. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 86.Haram A, Boland MR, Kelly ME, Bolger JC, Waldron RM, Kerin MJ. The prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer: A systematic review. J Surg Oncol. 2017;115:470–479. doi: 10.1002/jso.24523. [DOI] [PubMed] [Google Scholar]
- 87.Gulubova MV, Ananiev JR, Vlaykova TI, Yovchev Y, Tsoneva V, Manolova IM. Role of dendritic cells in progression and clinical outcome of colon cancer. Int J Colorectal Dis. 2012;27:159–169. doi: 10.1007/s00384-011-1334-1. [DOI] [PubMed] [Google Scholar]


