Abstract
AIM
To evaluate the correlation between intravoxel incoherent motion (IVIM) diffusion-weighted imaging (DWI) parameters and the degree of hepatic steatosis and fibrosis in children.
METHODS
This retrospective study was approved by the institutional review board. The children (≤ 18 years) who underwent liver IVIM DWI with 8 b-values under the suspicion of hepatic steatosis or fibrosis from February 2013 to November 2016 were included. Subjects were divided into normal, fatty liver (FAT), and fibrotic liver (FIB) groups. The slow diffusion coefficient (D), fast diffusion coefficient (D*), perfusion fraction (f), and apparent diffusion coefficient (ADC) were measured. MR proton density fat fraction (PDFF), MR elastography (MRE), and IVIM values were compared.
RESULTS
A total of 123 children (median age of 12 years old, range: 6-18 years) were included, with 8 in the normal group, 93 in the FAT group, and 22 in the FIB group. The D* values were lower in the FIB group compared with those of the normal (P = 0.015) and FAT (P = 0.003) groups. The f values were lower in the FIB group compared with the FAT group (P = 0.001). In multivariate analyses, PDFF value was positively correlated with f value (β = 3.194, P < 0.001), and MRE value was negatively correlated with D* value (β = -7.031, P = 0.032). The D and ADC values were not influenced by PDFF or MRE value.
CONCLUSION
In liver IVIM DWI with multiple b-values in children, there was a positive correlation between hepatic fat and blood volume, and a negative correlation between hepatic stiffness and endovascular blood flow velocity, while diffusion-related parameters were not affected.
Keywords: Intravoxel incoherent motion, Diffusion-weighted imaging, Fibrosis, Fatty liver, Pediatrics
Core tip: Liver intravoxel incoherent motion (IVIM) diffusion-weighted imaging with multiple b-values was feasible and useful for evaluating hepatic steatosis and fibrosis in children. Hepatic steatosis and fibrosis affected IVIM parameters differently in children. MR proton density fat fraction (PDFF) demonstrated a positive correlation with the perfusion fraction (f) of IVIM. MR elastography (MRE) value was negatively correlated with the fast diffusion coefficient (D*) of IVIM. The other IVIM values were not influenced by PDFF or MRE value.
INTRODUCTION
Liver disease in the pediatric population is not uncommon. Metabolic disease and diverse syndromic conditions commonly affect the liver to varying degrees. Nonalcoholic fatty liver disease (NAFLD) is an important disease entity because it could lead to hepatic steatosis and fibrosis[1]. In addition, the incidence of NAFLD is increasing dramatically in adolescents and in children[1]. Biliary atresia (BA) or neonatal hepatitis also can affect the liver in early childhood, and BA is the most common cause of hepatic fibrosis and reason for liver transplantation in young children[2,3]. Therefore, quantitative and non-invasive characterization of the liver conditions is important in pediatric patients. Biopsy has a limited role in children due to its sampling bias and invasive nature[4]. The intravoxel incoherent motion (IVIM) magnetic resonance imaging (MRI) technique could be one of the alternative methods for assessing hepatic tissue perfusion and diffusion separately in various pediatric liver diseases.
The IVIM technique separates the slow and fast diffusion components of diffusion-weighted imaging (DWI)[5]. The slow component is related to free water diffusion, and the fast component is related to microvascular perfusion[5]. Although the conventional apparent diffusion coefficient (ADC) is influenced by both slow and fast components, IVIM could quantify the two components separately by using multiple b-values[6]. It is because perfusion property has fast diffusion rate while using low b-values, but pure water molecular diffusion has slow diffusion rate while using high number of b-values[6]. Three parameters can be obtained from the IVIM technique: (1) Pure molecular diffusion (D, D slow), which is related to the slow component of diffusion from water; (2) pseudo-diffusion (D*, D fast), which reflects the endovascular blood flow velocity; and (3) perfusion fraction (f), which reflects blood volume[5]. Among them, D* and f are related to blood perfusion.
Many studies have utilized IVIM for the evaluation of liver disease in adults. They demonstrated the better performance of IVIM compared to DWI for staging hepatic steatosis and fibrosis, and fibrosis had different effects on the IVIM parameters[7-9]. However, limited studies included children for assessing liver disease using IVIM. One study included patients 10 years of age or older to evaluate diminished liver microperfusion after Fontan operation[10]. Another study included patients 11 years of age or older to assess the correlations between hepatic steatosis and IVIM perfusion parameters[11]. In the most recent study, Manning et al[12] evaluated D, f, and ADC values derived from three b-values for assessing NAFLD in children. However, there has been no study focused on pediatric livers with enough b-values for IVIM.
Therefore, the purpose of this study was to evaluate the correlation between IVIM DWI parameters and the degree of hepatic steatosis and fibrosis measured with MRI in children.
MATERIALS AND METHODS
Subjects
Our institutional review board approved this retrospective study, and informed consent was waived. Children under the age of 18 years who underwent liver MRI with IVIM DWI from February 2013 to November 2016 were included. There were only two clinical indications: Patients with clinical suspicion of hepatic steatosis or those who were suspected to have hepatic fibrosis after undergoing Kasai operation due to BA. The exclusion criteria were: (1) Subjects who had no laboratory results of aspartate aminotransferase (AST) or alanine aminotransferase (ALT) level within one month of the MRI examination and (2) those who had focal lesions other than fat or fibrosis in the liver, such as hemangioma or cysts on MRI.
Subjects were divided into three groups. One was the normal group, for those who had normal MR proton density fat fraction (PDFF) of the liver (< 6% according to a previous study)[13], normal body mass index, and normal transient elastography (TE) results when they had TE results within one year of MRI examinations. The second was the fatty liver (FAT) group, for subjects who had PDFF greater than 6%. The third was the fibrotic liver (FIB) group, for children who underwent MRI after a Kasai operation due to BA. The age, gender, weight (kg), and height (cm) of the children were assessed at the time of MRI examination.
MRI analysis
Each liver MRI was obtained in a 3T system (Discovery 750w, GE Healthcare, Milwaukee, WI, United States). MRI was composed of four sequences including axial single shot fast spine echo (SSFSE) T2 weighted images, 3D volumetric multi-echo gradient sequence (IDEAL-IQ, for proton density fat quantification and T2* decay assessment), MR elastography (MRE), and IVIM DWI. The detailed MR parameters are presented in Table 1. For MRE, a passive pneumatic driver was placed over the liver and was connected to the active driver[14]. The driver power was reduced 20% from the adult level, and a folded towel was placed between the driver and abdominal wall to avoid abdominal discomfort[15,16].
Table 1.
Liver magnetic resonance imaging parameters
| SSFSE T2 weighted image | IDEAL-IQ for PDFF and R2* (1/T2*) map | MRE | IVIM | |
| Acquisition plane | Axial | Axial | Axial | Axial |
| Acquisition scheme | Breath hold | Breath hold | Breath hold | Free breathing |
| TR (millisecond) | 540 | 5.9 | 1000 | 6000 |
| TE (millisecond) | 80 | 2.6 | 62 | 74 |
| Acquisition matrix | 320 × 256 | 128 × 128 | 64 × 64 | 128 × 128 |
| Field of view (cm) | 40 | 42 | 38 | 38 |
| Slice/internal thickness (mm) | 8 | 8 | 8 | 4 |
| Gap (mm) | 10 | 8 | 2 | 5 |
| Flip angle (°) | 90 | 3 | 90 | 90 |
| Number of signal averages | 0.64 | 0.69 | 2 | 2 → 1 |
| Notes | Six gradient echoes (from 0.9 to 4.4 millisecond) | 60 Hz MEG (20% driver power reduction) | Eight b-values (15, 27.3, 49.8, 90.7, 165.3, 301.2, 548.8, 1000) | |
| Scan time (min) | 0:12 | 0:16 | 0:24 (or 0:12 × 2 times) | 4:30 |
SSFSE: Single shot fast spine echo; PDFF: MR proton density fat fraction; MRE: MR elastography; IVIM: Intravoxel incoherent motion; TR: Repetition time; TE: Echo time; MEG: Motion encoding gradient.
In the SSFSE T2 weighted images, liver morphology and focal lesions were assessed. Round regions-of-interest (ROIs) were drawn in the homogenous parenchyma of the right hepatic lobe, avoiding hepatic vessels, by one experienced radiologist in all quantitative imaging sequences. Four ROIs were drawn in the liver parenchyma in each slice, and the mean values were considered as representative values in each sequence, including D (× 10-3 mm2/s), D* (× 10-3 mm2/s), f (%), and ADC (× 10-3 mm2/s) value maps (Figure 1).
Figure 1.
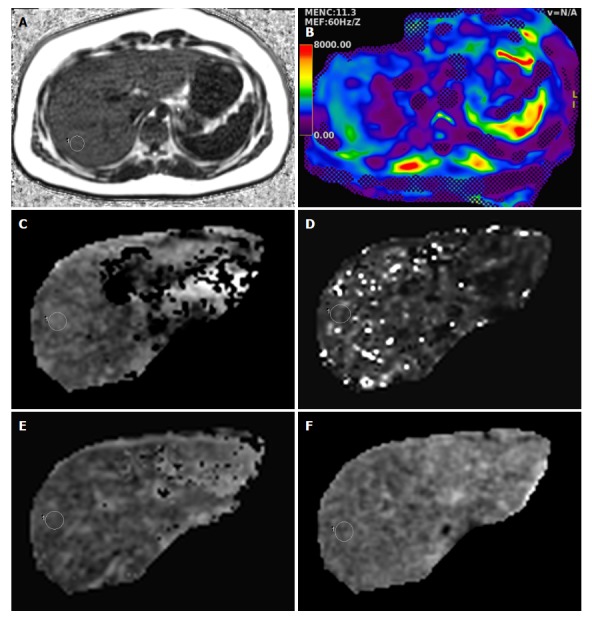
Magnetic resonance imaging of liver from a 16-year-old boy in the fatty liver group. A: MRI-proton density fat fraction = 17.6%; B: MR elasticity = 3.2 kPa; C: D value = 1.45 × 10-3 mm2/s; D: D* value = 90 × 10-3 mm2/s; E: f value = 31.8%; F: ADC value = 1.32 × 10-3 mm2/s.
Statistical analysis
Statistical analyses were performed using SPSS version 23 (IBM Corp., Armonk, NY, United States). Clinical findings and MRI values were compared between normal, FAT and FIB groups using Kruskal-Wallis test or Fisher’s exact test, with the use of Dunn’s method for multiple comparisons. We also performed linear regression analysis to determine whether the liver function tests, PDFF, and MRE values influenced the IVIM parameters. All data were presented as median values and interquartile ranges. P-values less than 0.05 were considered statistically significant for all analyses.
RESULTS
During the study period, a total of 130 children (M:F = 86:44) with a median age of 12.2 years (range: 6-18 years) underwent liver MRI including IVIM DWI. From them, seven children were excluded because four had no laboratory results within one month of the MRI, and three had focal hepatic lesions such as hepatic hemangioma or cysts. Therefore, 123 children were ultimately included in this study. There were 83 boys and 40 girls, and the median age was 12 years, with a range of 6-18 years. Among these, 101 children underwent MRI under the suspicion of hepatic steatosis. According to the grouping criteria, eight children were included in the normal group and 93 children were included in the FAT group. During the study period, 22 children underwent MRI after Kasai operation due to BA and were included in the FIB group.
Comparison of clinical findings and MRI parameters between the three groups
The group comparison results are summarized in Table 2. Most clinical parameters did not differ between the normal and FAT groups or between the normal and FIB groups. However, the FIB group had younger children and more girls than the FAT group. The weight was higher in the FAT group than in the normal group. The AST and ALT values were significantly higher in the FAT group compared to the normal group.
Table 2.
Comparison of clinical and magnetic resonance imaging findings between the three groups
| Normal (n = 8)1 | FAT (n = 93)1 | FIB (n = 22)1 | P-value2 | P-value3 | P-value4 | P-value5 | ||
| Clinical findings | Age (yr) | 11 (9.25-13.75) | 12 (10-15.5) | 10 (8.75-11) | < 0.001a | 0.611 | 0.855 | < 0.001a |
| Gender (M:F) | 4:04 | 70:23:00 | 9:13 | 0.005a | 0.615 | 1.000 | 0.012a | |
| Weight (kg) | 36.5 (28.5-49.5) | 65 (51.9-76.5) | 33 (28.3-44) | < 0.001a | 0.001a | 1.000 | < 0.001a | |
| Height (cm) | 145 (137-162) | 158 (145-164) | 136.3 (131.8-148.1) | < 0.001a | 0.543 | 0.563 | < 0.001a | |
| AST (IU/L) | 22.5 (18.3-24.8) | 52.0 (27.0-80.5) | 33.0 (22.8-68.0) | 0.007a | 0.008a | 0.167 | 0.541 | |
| ALT (IU/L) | 11.0 (9.0-12.0) | 87.0 (44.5-138.5) | 28.0 (16.0-55.3) | < 0.001a | < 0.001a | 0.533 | < 0.001a | |
| MRI findings | PDFF (%) | 2.65 (2.4-3.1) | 22 (14.1-34) | 2.6 (2.0-4.1) | < 0.001a | < 0.001a | 1.000 | < 0.001a |
| T2* (millisecond) | 25.8 (22.0-27.3) | 18 (16.1-20.75) | 23.4 (21-26.6) | < 0.001a | 0.007a | 1.000 | < 0.001a | |
| MRE (kPa)6 | 2.0 (1.6-2.5) | 2.6 (2.2-3.1) | 3.2 (2.8-4.6) | < 0.001a | 0.058 | < 0.001a | 0.001a | |
| D (× 10-3 mm2/s) | 1.06 (1.03, 1.1) | 1.07 (0.94, 1.35) | 0.93 (0.81, 1.2) | 0.158 | ||||
| D* (× 10-3 mm2/s) | 70.2 (44.3, 139.7) | 61 (39.5, 81.2) | 36.5 (19.2, 52.5) | 0.001a | 0.937 | 0.015a | 0.003a | |
| f (%) | 28.5 (18.6, 34.8) | 31.5 (27.9, 39.3) | 19.3 (14.0, 33.2) | 0.001a | 0.542 | 1.000 | < 0.001a | |
| ADC (× 10-3 mm2/s) | 1.08 (0.97, 1.2) | 1.05 (0.9, 1.21) | 0.97 (0.89, 1.15) | 0.575 |
P < 0.05;
All values: Median (Q1-Q3);
Overall P-value from Kruskal-Wallis test or Fisher’s exact test, after testing with Dunn’s method for multiple comparisons;
Normal vs FAT groups;
Normal vs FIB groups;
FAT vs FIB groups;
n = 7, 77, 19 for the normal, FAT, and FIB groups, respectively. FAT: Fatty liver; FIB: Fibrotic liver; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; PDFF: MR proton density fat fraction; MRE: MR elastography; ADC: Apparent diffusion coefficient.
The median PDFF values were higher in the FAT group (22.0 %) than in the normal (2.7 %) and FIB (2.6 %) groups (P < 0.001 for both). The median T2* values were lower in the FAT group (18.0 msec) compared with the normal group (25.8 msec, P = 0.007) and the FIB group (23.4 msec, P < 0.001). In addition, the median MRE values were higher in the FIB group (3.2 kPa) compared with the normal group (2 kPa, P < 0.001) and the FAT group (2.6 kPa, P = 0.001).
From the IVIM parameters, the D and ADC values were not significantly different in the three groups. In contrast, the median D* values were significantly lower in the FIB group (36.5 × 10-3 mm2/s) compared with those of the normal group (70.2 × 10-3 mm2/s, P = 0.015) and the FAT group (61.0 × 10-3 mm2/s, P = 0.003). The median f values were significantly lower only in the FIB group compared with the FAT group (19.3% vs 31.5%, P = 0.001).
Assessment of effects of laboratory and MRI results on IVIM parameters in all groups
In univariate linear regression analysis, the D values demonstrated negative correlation with AST (P = 0.020), T2* (P = 0.040), and MRE (P = 0.010) values (Table 3). The D* values were also negatively correlated with AST (P = 0.047) and MRE (P = 0.002) values. The f values were affected positively by PDFF values (P < 0.001) and negatively by T2* and MRE values (P = 0.005 and P < 0.001, respectively). In addition, the AST results were associated with decreased ADC values (P = 0.004), while other MR parameters did not influence ADC values.
Table 3.
Univariate linear regression analysis for intravoxel incoherent motion parameters in all groups
|
D (× 10-3 mm2/s) |
D* (× 10-3 mm2/s) |
f (%) |
ADC (× 10-3 mm2/s) |
|||||||||
| β | SE | P value | β | SE | P value | β | SE | P value | β | SE | P value | |
| AST (IU/L) | -1.389 | 0.588 | 0.020a | -0.145 | 0. 072 | 0.047a | -0.238 | 0.266 | 0.372 | -1.146 | 0.551 | 0.040a |
| ALT (IU/L) | -0.304 | 0.316 | 0.339 | -0.030 | 0.039 | 0.445 | 0.096 | 0.141 | 0.496 | -0.446 | 0.294 | 0.132 |
| PDFF (%) | 1.785 | 1.949 | 0.362 | 0.255 | 0.237 | 0.284 | 4.289 | 0.775 | < 0.001a | -0.263 | 1.826 | 0.886 |
| T2* (millisecond) | -8.400 | 4.042 | 0.040a | 0.818 | 0.495 | 0.101 | -5.002 | 1.767 | 0.005a | -4.978 | 3.814 | 0.194 |
| MRE (kPa) (n = 103) | -58.819 | 22.493 | 0.010a | -8.582 | 2.672 | 0.002a | -27.982 | 7.408 | < 0.001a | -33.675 | 20.407 | 0.102 |
P < 0.05; IVIM: Intravoxel incoherent motion; ADC: Apparent diffusion coefficient; β: Coefficient; SE: Standard error of the estimated coefficient; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; PDFF: MR proton density fat fraction; MRE: MR elastography.
In multivariate linear regression analysis, neither AST nor ALT affected IVIM parameters (Table 4). Increased PDFF value was a significant independent factor for increasing f value (β = 3.194, P < 0.001, Figure 2), which meant a positive correlation between hepatic fat and blood volume. The T2* values affected D values negatively (P = 0.019) and D* values positively (P = 0.022). In addition, increased MRE values were a significant independent factor for decreasing D* values (β = -7.031, P = 0.032, Figure 3), which indicated a negative correlation between hepatic elasticity and endovascular blood flow velocity. However, the values of the diffusion-related parameters D and ADC were not significantly influenced by PDFF or MRE values.
Table 4.
Multivariate linear regression analysis for intravoxel incoherent motion parameters in all groups
| D (× 10-3 mm2/s) | D* (× 10-3 mm2/s) | f (%) | ADC (× 10-3 mm2/s) | |||||||||
| β | SE | P value | β | SE | P value | β | SE | P value | β | SE | P value | |
| AST (IU/L) | -2.222 | 1.337 | 0.100 | -0.063 | 0.161 | 0.697 | -0.485 | 0.408 | 0.238 | -1.343 | 1.227 | 0.277 |
| ALT (IU/L) | 0.718 | 0.673 | 0.289 | 0.013 | 0.081 | 0.877 | 0.116 | 0.206 | 0.574 | 0.419 | 0.618 | 0.499 |
| PDFF (%) | -1.228 | 2.622 | 0.640 | 0.381 | 0.315 | 0.230 | 3.194 | 0.801 | < 0.001a | -3.663 | 2.407 | 0.131 |
| T2* (millisecond) | -11.189 | 4.697 | 0.019a | 1.316 | 0.565 | 0.022a | -0.847 | 1.435 | 0.557 | -8.473 | 4.313 | 0.052 |
| MRE (kPa) (n = 103) | -37.042 | 26.853 | 0.171 | -7.031 | 3.231 | 0.032a | -11.667 | 8.206 | 0.158 | -29.679 | 24.658 | 0.232 |
P < 0.05; IVIM: Intravoxel incoherent motion; ADC: Apparent diffusion coefficient; β: Coefficient; SE: Standard error of the estimated coefficient; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; PDFF: MR proton density fat fraction; MRE: MR elastography.
Figure 2.
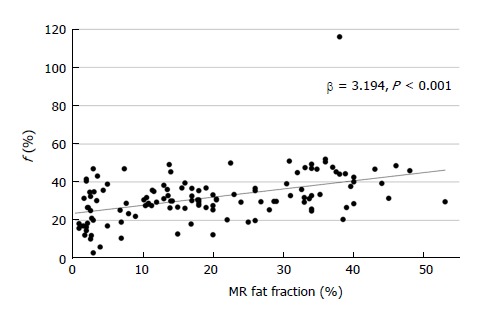
Scatter plot of MR fat fraction and f value, showing a positive correlation.
Figure 3.
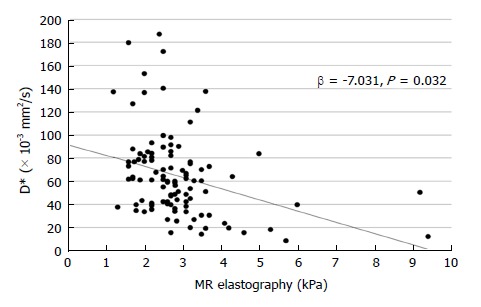
Scatter plot of MR elasticity and D* value, showing a negative correlation.
DISCUSSION
The IVIM technique has been used for kidney and liver diseases in a few previous studies of children[10-12,17,18]. Among the studies that included children, only one included only a pediatric population for assessing the usefulness of liver IVIM technique using three b-values in pathologically proven NAFLD[12]. In addition, there was no study that evaluated the meanings of IVIM parameters only in children, including normal, fatty, and fibrotic livers with sufficient b-values for IVIM, as in our study.
Our study is meaningful because we demonstrated the feasibility of IVIM with multiple b-values in pediatric liver MRI. The ages of the included children ranged from 6 to 18 years, younger than those in previous studies, and the included number of children was large. In addition, multiple b-values are needed to differentiate slow and fast diffusion with higher robustness and accuracy[19,20], because fast diffusion (D*) can be differentiated from low b-values, and slow diffusion (D) can be differentiated from high b-values. Therefore, our methods using multiple b-values could lead to more accurate quantification and separation of IVIM parameters than previous studies.
The results revealed that hepatic steatosis and fibrosis influenced perfusion and diffusion parameters differently, while ADC did not show any significant correlation for these conditions; these results were concordant with previous studies[7,8]. In our study, increased PDFF value was a significant independent factor for increasing f value, which meant a positive correlation between hepatic steatosis and blood volume in pediatric NAFLD. In addition, increased MRE value was a significant independent factor for decreasing D* value, which signified a negative correlation between hepatic fibrosis and endovascular blood flow velocity in children with BA. We performed multivariate logistic regression tests including liver function tests and PDFF and MRE values because liver inflammation, steatosis, and fibrosis could together influence histopathologic changes of the liver and slow and fast diffusions, and we could not obtain pathologic results from the included children.
In the previous studies, the patterns of increasing or decreasing IVIM parameters with hepatic steatosis or fibrosis varied. A previous study by Manning et al[12] revealed that hepatic steatosis and fibrosis had different effects on IVIM parameters, even though the relationships between parameters and hepatic conditions were different from those in our study. They showed decreased ADC and D values with increasing hepatic steatosis, while they observed decreased f values with increasing hepatic fibrosis in pediatric NAFLD. In hepatic steatosis, there are also studies that demonstrated a positive correlation between hepatic steatosis and f value in adults with NAFLD[21] and diffuse liver disease[6], similar to our results. Yu et al[22] also demonstrated the relationships between histopathologic change and IVIM parameters resulting in a positive correlation between hepatic steatosis and not only f, but also D value in a rat model of NAFLD. On the other hand, there is a study that demonstrated no correlation between IVIM parameters and the degree of hepatic steatosis in adults with various chronic liver diseases[23]. Franca et al[6] mentioned that f value was increased more prominently in a mild hepatic steatosis group than in a severe steatosis group compared with a normal group. This could explain why the results of the IVIM parameters on hepatic steatosis were different according to study. We could suggest that the degree of hepatic steatosis in each study group influences the results of the affected IVIM parameters. In addition, another possible explanation of the increasing f value in pediatric hepatic steatosis is based on the histologic characteristics of hepatic steatosis of children. Pediatric hepatic steatosis has mainly portal inflammation with or without fibrosis, rather than ballooning degeneration or perisinusoidal fibrosis, which are more predominantly found in adult hepatic steatosis[12,24-26]. Perisinusoidal fibrosis and ballooning could lead to decreased microperfusion. Instead, increased portal inflammation could lead to increased perfusion fraction due to increased blood volume within the liver. Further studies with pathologic correlation of various degrees and etiologies of hepatic steatosis are needed.
In hepatic fibrosis, our study demonstrated a negative correlation between fibrosis and D* in children after a Kasai operation for BA. In pediatric NAFLD, hepatic fibrosis showed a negative correlation with f[12]. Another study of adolescents and adults found that decreased microperfusion (D* and f) resulted in decreased ADC value in hepatic fibrosis, rather than alterations in molecular diffusion, after a Fontan operation[10]. However, there was no study that included pediatric patients for assessing hepatic fibrosis from BA. Previous studies of hepatic fibrosis from chronic liver disease in adults also demonstrated a negative correlation between hepatic fibrosis and D*[5,7,23], which are consistent with our study even though they focused on a different etiology of fibrosis. The reason for this inverse correlation could be because the increased protein in the extracellular matrix can lead to sinusoidal space obliteration and result in decreased hepatic microperfusion[5,7]. Yoon et al[7] mentioned that a decreased D* value could detect early changes in hepatic fibrosis better than ADC value. Even though there are similar characteristics of microperfusion change in pediatric BA (D* change), chronic liver disease in adults (D* change), and pediatric NAFLD (f change), additional studies on the effects of hepatic fibrosis from different etiologies on IVIM parameters are needed.
There were several limitations in this study. First, there were disproportional subject numbers in the groups, because it was a retrospective study. Second, we did not obtain the pathologic results of the liver in the children. However, we used the radiological and clinical data of the children to define three groups as reasonably as possible. Moreover, the obtained IVIM values of our normal group were similar to the normal values of a recently published meta-analysis study[27]. Third, several factors might influence liver perfusion, including inflammation and edema, were not analyzed. However, most of the children in our study were from an outpatient clinic and were undergoing routine follow-up, and we performed regression analysis including liver function tests to evaluate the effects of liver inflammation on IVIM parameters.
In conclusion, the liver IVIM DWI technique with multiple b-values was feasible and useful for evaluating hepatic steatosis and fibrosis in children using perfusion-related parameters of D* and f. There was a positive correlation between hepatic fat and blood volume, and a negative correlation between hepatic fibrosis and endovascular blood flow velocity, while diffusion-related parameters were not affected. Further study is needed to determine whether the IVIM parameters are affected by different histopathologies and etiologies of pediatric hepatic steatosis and fibrosis.
ARTICLE HIGHLIGHTS
Research background
Liver disease including steatosis and fibrosis is not uncommon in pediatric population. Quantitative and non-invasive examination of liver status is important in children because biopsy has a limited role from its sampling error and invasiveness.
Research motivation
Intravoxel incoherent motion (IVIM) magnetic resonance imaging (MRI) is an advanced diffusion-weighted imaging (DWI) technique. It can separate the perfusion and diffusion components from the conventional apparent diffusion coefficient (ADC) in the liver. Previous studies demonstrated IVIM values were more useful than ADC value for the evaluation of liver disease in adults, using the pure molecular diffusion (D), pseudo-diffusion (D*), and perfusion fracture (f) parameters. However, limited studies have assessed hepatic steatosis and fibrosis in children using IVIM. In addition, no study has focused on pediatric livers with enough b-values for IVIM.
Research objectives
The purpose of the study was to evaluate the usefulness of IVIM DWI technique with multiple b-values for evaluating hepatic steatosis and fibrosis in children and to know the correlation between IVIM parameters and the degree of hepatic steatosis and fibrosis measured with MRI in children.
Research methods
We retrospectively reviewed liver IVIM MRI of children (≤ 18 years) who performed MRI with 8 b-values under the suspicion of liver steatosis or fibrosis from 2013 to 2016. In addition to the conventional ADC value, perfusion related parameters (D*, f) and diffusion related parameter (D) were measured in the liver. Degree of hepatic steatosis was measured using MR proton density fat fracture (PDFF) and degree of hepatic fibrosis was measured using MR elastography (MRE). Perfusion and diffusion parameters were compared between normal, fatty liver, and fibrotic liver groups. Effects of hepatic steatosis and fibrosis on IVIM parameters were assessed.
Research results
Hepatic steatosis and fibrosis affected IVIM parameters differently in children. Perfusion related parameters (D*, f) were significantly lower in the fibrosis group compared with other groups. MR PDFF demonstrated a positive correlation with the f, representing blood volume. MRE value was negatively correlated with the D*, representing endovascular blood flow velocity. However, D and ADC value were not affected.
Research conclusions
Liver IVIM MRI with multiple b-values was useful for evaluating hepatic steatosis and fibrosis in children. Hepatic steatosis and fibrosis influenced perfusion and diffusion parameters differently. As increasing hepatic steatosis, blood volume was significantly increased. As increasing hepatic fibrosis, endovascular blood flow velocity was significantly decreased. However, true molecular diffusion and conventional ADC were not affected. Therefore, IVIM MRI can be a useful tool for evaluating hepatic steatosis and fibrosis in children.
Research perspectives
Perfusion related parameters from IVIM MRI can be used for the evaluation of hepatic steatosis and fibrosis in children. Further study is needed to determine whether the IVIM parameters are affected by different histopathologies and etiologies of pediatric hepatic steatosis and fibrosis.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This study was reviewed and approved by the local ethics committee of the Severance Hospital, Yonsei University (register no. 1-2017-0014).
Informed consent statement: Because of the retrospective and anonymous character of this study, the need for informed consent was waived by the institutional review board.
Conflict-of-interest statement: All authors declare no conflicts-of-interest related to this article.
Data sharing statement: No additional data are available.
Peer-review started: March 17, 2018
First decision: April 24, 2018
Article in press: June 9, 2018
P- Reviewer: Ulaşoğlu C S- Editor: Wang XJ L- Editor: Logan S E- Editor: Huang Y
Contributor Information
Hyun Joo Shin, Department of Radiology, Severance Hospital, Severance Pediatric Liver Disease Research Group, Research Institute of Radiological Science, Yonsei University College of Medicine, Seoul 03722, South Korea.
Haesung Yoon, Department of Radiology, Severance Hospital, Severance Pediatric Liver Disease Research Group, Research Institute of Radiological Science, Yonsei University College of Medicine, Seoul 03722, South Korea.
Myung-Joon Kim, Department of Radiology, Severance Hospital, Severance Pediatric Liver Disease Research Group, Research Institute of Radiological Science, Yonsei University College of Medicine, Seoul 03722, South Korea.
Seok Joo Han, Department of Surgery, Severance Hospital, Severance Pediatric Liver Disease Research Group, Yonsei University College of Medicine, Seoul 03722, South Korea.
Hong Koh, Division of Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, Severance Children’s Hospital, Severance Pediatric Liver Disease Research Group, Yonsei University College of Medicine, Seoul 03722, South Korea.
Seung Kim, Division of Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, Severance Children’s Hospital, Severance Pediatric Liver Disease Research Group, Yonsei University College of Medicine, Seoul 03722, South Korea.
Mi-Jung Lee, Department of Radiology, Severance Hospital, Severance Pediatric Liver Disease Research Group, Research Institute of Radiological Science, Yonsei University College of Medicine, Seoul 03722, South Korea. mjl1213@yuhs.ac.
References
- 1.Clemente MG, Mandato C, Poeta M, Vajro P. Pediatric non-alcoholic fatty liver disease: Recent solutions, unresolved issues, and future research directions. World J Gastroenterol. 2016;22:8078–8093. doi: 10.3748/wjg.v22.i36.8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kasahara M, Umeshita K, Sakamoto S, Fukuda A, Furukawa H, Uemoto S. Liver transplantation for biliary atresia: a systematic review. Pediatr Surg Int. 2017;33:1289–1295. doi: 10.1007/s00383-017-4173-5. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez-Valle A, Kassira N, Varela VC, Radu SC, Paidas C, Kirby RS. Biliary Atresia: Epidemiology, Genetics, Clinical Update, and Public Health Perspective. Adv Pediatr. 2017;64:285–305. doi: 10.1016/j.yapd.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi Y, Fukusato T. Histopathology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20:15539–15548. doi: 10.3748/wjg.v20.i42.15539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J, Guo Y, Tan X, Zheng Z, He M, Xu J, Mei Y, Zhang J, Zhao X, Wang C, et al. MRI-based estimation of liver function by intravoxel incoherent motion diffusion-weighted imaging. Magn Reson Imaging. 2016;34:1220–1225. doi: 10.1016/j.mri.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 6.França M, Martí-Bonmatí L, Alberich-Bayarri Á, Oliveira P, Guimaraes S, Oliveira J, Amorim J, Gonzalez JS, Vizcaíno JR, Miranda HP. Evaluation of fibrosis and inflammation in diffuse liver diseases using intravoxel incoherent motion diffusion-weighted MR imaging. Abdom Radiol (NY) 2017;42:468–477. doi: 10.1007/s00261-016-0899-0. [DOI] [PubMed] [Google Scholar]
- 7.Yoon JH, Lee JM, Baek JH, Shin CI, Kiefer B, Han JK, Choi BI. Evaluation of hepatic fibrosis using intravoxel incoherent motion in diffusion-weighted liver MRI. J Comput Assist Tomogr. 2014;38:110–116. doi: 10.1097/RCT.0b013e3182a589be. [DOI] [PubMed] [Google Scholar]
- 8.Murphy P, Hooker J, Ang B, Wolfson T, Gamst A, Bydder M, Middleton M, Peterson M, Behling C, Loomba R, et al. Associations between histologic features of nonalcoholic fatty liver disease (NAFLD) and quantitative diffusion-weighted MRI measurements in adults. J Magn Reson Imaging. 2015;41:1629–1638. doi: 10.1002/jmri.24755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang H, Chen J, Gao R, Huang Z, Wu M, Song B. Liver fibrosis staging with diffusion-weighted imaging: a systematic review and meta-analysis. Abdom Radiol (NY) 2017;42:490–501. doi: 10.1007/s00261-016-0913-6. [DOI] [PubMed] [Google Scholar]
- 10.Dijkstra H, Wolff D, van Melle JP, Bartelds B, Willems TP, Oudkerk M, Hillege H, van den Berg AP, Ebels T, Berger RM, et al. Diminished liver microperfusion in Fontan patients: A biexponential DWI study. PLoS One. 2017;12:e0173149. doi: 10.1371/journal.pone.0173149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JT, Liau J, Murphy P, Schroeder ME, Sirlin CB, Bydder M. Cross-sectional investigation of correlation between hepatic steatosis and IVIM perfusion on MR imaging. Magn Reson Imaging. 2012;30:572–578. doi: 10.1016/j.mri.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manning P, Murphy P, Wang K, Hooker J, Wolfson T, Middleton MS, Newton KP, Behling C, Awai HI, Durelle J, et al. Liver histology and diffusion-weighted MRI in children with nonalcoholic fatty liver disease: A MAGNET study. J Magn Reson Imaging. 2017;46:1149–1158. doi: 10.1002/jmri.25663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin HJ, Kim HG, Kim MJ, Koh H, Kim HY, Roh YH, Lee MJ. Normal range of hepatic fat fraction on dual- and triple-echo fat quantification MR in children. PLoS One. 2015;10:e0117480. doi: 10.1371/journal.pone.0117480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venkatesh SK, Ehman RL. Magnetic resonance elastography of liver. Magn Reson Imaging Clin N Am. 2014;22:433–446. doi: 10.1016/j.mric.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serai SD, Towbin AJ, Podberesky DJ. Pediatric liver MR elastography. Dig Dis Sci. 2012;57:2713–2719. doi: 10.1007/s10620-012-2196-2. [DOI] [PubMed] [Google Scholar]
- 16.Towbin AJ, Serai SD, Podberesky DJ. Magnetic resonance imaging of the pediatric liver: imaging of steatosis, iron deposition, and fibrosis. Magn Reson Imaging Clin N Am. 2013;21:669–680. doi: 10.1016/j.mric.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Poynton CB, Lee MM, Li Y, Laszik Z, Worters PW, Mackenzie JD, Courtier J. Intravoxel incoherent motion analysis of renal allograft diffusion with clinical and histopathological correlation in pediatric kidney transplant patients: A preliminary cross-sectional observational study. Pediatr Transplant. 2017:21. doi: 10.1111/petr.12996. [DOI] [PubMed] [Google Scholar]
- 18.Kim JW, Lee CH, Yoo KH, Je BK, Kiefer B, Park YS, Kim KA, Park CM. Intravoxel incoherent motion magnetic resonance imaging to predict vesicoureteral reflux in children with urinary tract infection. Eur Radiol. 2016;26:1670–1677. doi: 10.1007/s00330-015-3986-7. [DOI] [PubMed] [Google Scholar]
- 19.ter Voert EE, Delso G, Porto M, Huellner M, Veit-Haibach P. Intravoxel Incoherent Motion Protocol Evaluation and Data Quality in Normal and Malignant Liver Tissue and Comparison to the Literature. Invest Radiol. 2016;51:90–99. doi: 10.1097/RLI.0000000000000207. [DOI] [PubMed] [Google Scholar]
- 20.Koh DM, Collins DJ, Orton MR. Intravoxel incoherent motion in body diffusion-weighted MRI: reality and challenges. AJR Am J Roentgenol. 2011;196:1351–1361. doi: 10.2214/AJR.10.5515. [DOI] [PubMed] [Google Scholar]
- 21.Guiu B, Petit JM, Capitan V, Aho S, Masson D, Lefevre PH, Favelier S, Loffroy R, Vergès B, Hillon P, et al. Intravoxel incoherent motion diffusion-weighted imaging in nonalcoholic fatty liver disease: a 3.0-T MR study. Radiology. 2012;265:96–103. doi: 10.1148/radiol.12112478. [DOI] [PubMed] [Google Scholar]
- 22.Yu SM, Ki SH, Baek HM. Nonalcoholic Fatty Liver Disease: Correlation of the Liver Parenchyma Fatty Acid with Intravoxel Incoherent Motion MR Imaging-An Experimental Study in a Rat Model. PLoS One. 2015;10:e0139874. doi: 10.1371/journal.pone.0139874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandrasegaran K, Territo P, Elkady RM, Lin Y, Gasparis P, Borthakur G, Lin C. Does intravoxel incoherent motion reliably stage hepatic fibrosis, steatosis, and inflammation? Abdom Radiol (NY) 2018;43:600–606. doi: 10.1007/s00261-017-1263-8. [DOI] [PubMed] [Google Scholar]
- 24.Schwimmer JB, Behling C, Newbury R, Deutsch R, Nievergelt C, Schork NJ, Lavine JE. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42:641–649. doi: 10.1002/hep.20842. [DOI] [PubMed] [Google Scholar]
- 25.Brunt EM, Kleiner DE, Wilson LA, Unalp A, Behling CE, Lavine JE, Neuschwander-Tetri BA; NASH Clinical Research NetworkA list of members of the Nonalcoholic Steatohepatitis Clinical Research Network can be found in the Appendix. Portal chronic inflammation in nonalcoholic fatty liver disease (NAFLD): a histologic marker of advanced NAFLD-Clinicopathologic correlations from the nonalcoholic steatohepatitis clinical research network. Hepatology. 2009;49:809–820. doi: 10.1002/hep.22724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dillman JR, Trout AT, Costello EN, Serai SD, Bramlage KS, Kohli R, Xanthakos SA. Quantitative Liver MRI-Biopsy Correlation in Pediatric and Young Adult Patients With Nonalcoholic Fatty Liver Disease: Can One Be Used to Predict the Other? AJR Am J Roentgenol. 2018;210:166–174. doi: 10.2214/AJR.17.18446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li YT, Cercueil JP, Yuan J, Chen W, Loffroy R, Wáng YX. Liver intravoxel incoherent motion (IVIM) magnetic resonance imaging: a comprehensive review of published data on normal values and applications for fibrosis and tumor evaluation. Quant Imaging Med Surg. 2017;7:59–78. doi: 10.21037/qims.2017.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]


