Abstract
Aim: We focused on the ratios of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) to arachidonic acid (AA) and explored the significance of these ratios relative to clinical characteristics by age in ischemic stroke patients.
Methods: We enrolled patients with acute ischemic stroke who underwent radiological investigations and laboratory examinations, including measurement of serum EPA, DHA, and AA levels, and controls. Patients were classified according to age (< 65, 65–74, and ≥ 75 years) and the tertile of EPA/AA and DHA/AA ratios, and clinical aspects were compared with these factors.
Results: We analyzed 373 patients (age 70.2 ± 13.4 years; 245 males) and 105 controls. Among stroke patients, patients aged < 65 years had the lowest EPA/AA (0.35 ± 0.23, p = 0.006) and DHA/AA (0.73 ± 0.27, p < 0.001) ratios. Compared with controls, patients aged < 65 years showed lower EPA/AA (vs. 0.49 ± 0.25, p < 0.001) and DHA/AA (vs. 0.82 ± 0.26, p = 0.009) ratios. From logistic regression analysis, the EPA/AA (odds ratio 0.18, 95% confidence interval 0.04–0.81, p = 0.026) and DHA/AA (odds ratio 0.09, 95% confidence interval 0.02–0.33, p < 0.001) ratios were inversely related to patients aged < 65 years. According to age-stratified analyses, we found an association of aortic arch calcification with a lower EPA/AA ratio for patients aged ≥ 75 years and an association of multiple infarctions and cerebral white matter lesions with a lower EPA/AA ratio for patients aged 65–74 years (p < 0.05).
Conclusions: The ratios of EPA/AA and DHA/AA could be specific markers for younger stroke patients. The EPA/AA ratio may be related to aortic arch calcification for elderly stroke patients and to multiple infarctions and cerebral white matter disease for middle-aged stroke patients.
Keywords: Ischemic stroke, Eicosapentaenoic acid, Docosahexaenoic acid, White matter lesions, Aortic arch calcification
Introduction
Stroke is a leading cause of death and disability worldwide1). Not only elderly patients but also younger adults are affected by ischemic stroke2, 3). Ischemic stroke in elderly patients is related to the progression of atherosclerosis and the high prevalence of atrial fibrillation (AF), whereas non-atherosclerotic mechanisms including patent foramen ovale and mitral valve prolapse could contribute to stroke pathogenesis in young stroke patients4). However, emerging insights have shown that the contributions of atherosclerotic risk factors (e.g., hypertension, diabetes, and dyslipidemia) as well as lifestyle factors (e.g., smoking, alcohol consumption, and obesity) for ischemic stroke are critical for young patients3–6). For middle-aged patients, metabolic syndrome increases the prevalence of ischemic stroke7). Thus, the risk factors for ischemic stroke are diverse for young, middle-aged, and elderly patients. Currently, no evidence is available regarding specific biomarkers for determining ischemic stroke risk for patients according to age.
Aortic arch calcification upon chest radiography and white matter lesions upon magnetic resonance imaging (MRI) are related to the development of ischemic stroke8, 9). We previously showed a close relationship between aortic arch calcification and cerebral white matter lesions for patients with acute ischemic stroke10), and the association of age and atherosclerotic vascular risk factors with those pathological lesions has been reported11–14). However, we could not detect any potential biomarkers for such lesions10).
The n-3 and n-6 polyunsaturated fatty acids (PUFAs), including eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and arachidonic acid (AA), are poorly synthesized in the human body. Large-scale epidemiological and clinical trials demonstrated that foods enriched with EPA, DHA, and fish oil reduce the incidence of cardiovascular diseases and stroke15, 16). In the Japan Lipid Intervention Study, treatment with EPA and low-dose statins significantly reduced coronary artery diseases as well as stroke compared with statin therapy alone17). Some authors have suggested that the ratios of serum n-3 to n-6 PUFAs, such as the EPA/AA and DHA/AA ratios, could be useful markers to determine the incidence of major coronary events, peripheral artery diseases, and early neurological deterioration after acute ischemic stroke18–21).
Aim
We focused on the significance of the ratios of EPA/AA and DHA/AA serum levels by age and the association of these ratios with clinical characteristics including aortic arch calcification and cerebral white matter lesions by age for ischemic stroke patients. In the current study, we aimed to explore the hypothesis that the ratios of EPA/AA and DHA/AA may be linked to younger patients with ischemic stroke, in association with lifestyle risk factors, and may serve as putative biomarkers for aortic arch calcification and cerebral white matter lesions.
Methods
Selection of Subjects
This case series was based on the analysis of data acquired from the prospective registry of 478 patients with acute ischemic stroke who were admitted to the Department of Neurology at Juntendo University Hospital, a secondary referral center, for cerebral ischemic stroke between January 2014 and February 2016. Patients with post-surgical stroke onset including stroke after cardiac surgery, those who were already hospitalized and had received hospital meals for ≥ 7 days, those who were receiving intravenous hyperalimentation, or those taking EPA or DHA agents, which could influence the serum PUFA levels, were excluded. Age, sex, atherosclerotic risk factors, radiological findings, and laboratory findings including the ratios of EPA/AA and DHA/AA serum levels were assessed. To elucidate the contribution of age stratification and the EPA/AA and DHA/AA ratios to clinical characteristics of ischemic stroke patients, patients were classified according to age (< 65, 65–74, and ≥ 75 years) and the tertile of EPA/AA and DHA/AA ratios, and clinical aspects were compared with these factors. We also recruited apparently healthy Japanese subjects who were undergoing a medical check-up at a medical center from December 2004 to January 2005, for whom the data were previously published by Yanagisawa et al.22). Control subjects who were ageand gender-matched to stroke patients aged < 65 years were enrolled in the study. This study was conducted in accordance with the Declaration of Helsinki. The independent ethics committee of Juntendo University Hospital approved this study with an optout consent method. For the control group, the ethics committee of the constitution approved this study, and written informed consent was obtained.
Risk Factors
At baseline, atherosclerotic vascular risk factors were defined according to the description from previous literature8). Vascular risk factors were assessed as follows: 1) hypertension: history of using antihypertensive agents, systolic blood pressure > 140 mmHg, or diastolic blood pressure > 90 mmHg at 14 days after stroke onset, or at rest for more than 5 minutes after arrival for control subjects; 2) diabetes mellitus: use of oral hypoglycemic agents or insulin, or glycosylated hemoglobin (National Glycohemoglobin Standardization Program) ≥ 6.5%; 3) dyslipidemia: use of antihyperlipidemic agents, serum low-density lipoprotein cholesterol (LDL-C) ≥ 140 mg/dL, high-density lipoprotein cholesterol (HDL-C) < 40 mg/dL, or triglyceride ≥ 150 mg/dL; 4) current smoker or history of smoking; 5) AF: a history of AF, or identification of AF upon 12-lead electrocardiography, electrocardiographic monitoring, or Holter electrocardiography; 6) a history of ischemic heart disease; and 7) a history of peripheral artery disease.
Chest Radiograph Study
On chest radiograph, the extent of aortic arch calcification was evaluated and classified into the following four grades according to the method of a previous study: no visible calcification (grade 0); small spots or a single thin area of calcification (grade 1); one or more areas of thick calcification (grade 2); and circumferential calcification (grade 3)23).
MRI Protocol
Diffusion-weighted images, T2-weighted images, fluid-attenuation inversion recovery, and MR angiography (MRA) using a 1.5-Tesla MR scanner equipped with single-shot echo-planar imaging (Visart/EX; Toshiba, Tokyo, Japan) were included in the MRI study. Diagnosis of acute brain infarction was based on the finding of focal hyperintensity that was judged not to be due to normal anisotropic diffusion or magnetic susceptibility artifacts on diffusion-weighted images. The number of infarcts on diffusion-weighted imaging was assessed. Periventricular hyperintensity (PVH) and deep and subcortical white matter hyperintensity (DSWMH) were analyzed to determine the degree of cerebral white matter lesions11). Severe intracranial artery stenosis upon MRA was defined as > 50% or focal signal loss with the presence of signal reduction in the distal artery. Stenoses of bilateral intracranial carotid, anterior cerebral, middle cerebral, and posterior cerebral arteries upon MRA were examined. MRI findings were assessed by two experienced neuroradiologists who were blinded to the patients' status.
Laboratory Findings
Serum fatty acid levels including those of EPA, DHA, and AA were assayed by gas chromatography at an external laboratory (SRL Inc., Tokyo, Japan). We also analyzed serum levels of LDL-C, HDL-C, triglyceride, glucose, and HbA1c. Blood examinations were carried out within 24 hours of admission, or referral to the Department of Neurology for patients who developed ischemic stroke during hospitalization. For control subjects, blood samples were collected after overnight fasting.
Statistical Analysis
Numerical values are reported as means ± standard deviations. Baseline characteristics, vascular risk factors, chest radiography findings, brain MRI findings, and laboratory data were compared among groups. Data were statistically analyzed using the chisquare test for categorical variables and the Mann-Whitney and Kruskal-Wallis tests for nonparametric analyses. All variables with a value of p < 0.01 on univariate analyses were entered into the multinomial logistic regression analysis. A two-sided p value of < 0.05 was considered significant. All data were analyzed using SPSS version 15.0 for Windows software (SPSS, Chicago, IL, USA).
Results
Study Population
During the study period, 73 patients were excluded due to post-surgical stroke onset after cardiac surgery, hospitalization and receiving hospital meals for ≥ 7 days, administration of intravenous hyperalimentation, or taking EPA and DHA agents, and 405 patients were eligible to participate in the study. Thirty-two patients also were excluded because of missing data including MRI and serum PUFA levels. Thus, 373 patients (age 70.2 ± 13.4 years; 245 males; median National Institute of Health Stroke Scale [NIHSS] score 3 [0–29]) were enrolled. Regarding stroke subtype based on the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification24), 42 patients (11%) had small artery occlusion, 54 (14%) had large artery atherosclerosis, 97 (26%) had cardioembolism, 103 (28%) had stroke with determined etiology, 40 (11%) had stroke with undetermined etiology, and 37 (10%) had a transient ischemic attack. For the controls, 105 subjects whose age and gender were matched to the younger stroke patients aged < 65 years were enrolled in the study.
Clinical Characteristics and Radiological and Laboratory Findings by Age Group for Stroke Patients
Baseline characteristics and radiological and laboratory findings were compared among 113 patients aged < 65 years, 104 patients aged 65 to 74 years, and 156 patients aged ≥ 75 years (Table 1). The frequency of male gender was significantly lower for patients aged ≥ 75, and body mass index (BMI) was significantly higher for younger patients aged < 65 years (p = 0.005 and p < 0.001, respectively). Among atherosclerotic risk factors, the frequencies of hypertension, current cigarette smoking, AF, and coronary artery disease were higher for patients aged 65 to 74, < 65, ≥ 75, and 65 to 74 years, respectively (p = 0.024, p < 0.001, p = 0.011, and p < 0.001, respectively). NIHSS scores on admission were highest for patients aged ≥ 75 years (p = 0.006). On chest X radiograph, older patients aged ≥ 75 years had the highest degree of aortic arch calcification (1.7 ± 0.9, p < 0.001). On MRI, the degree of PVH and DSWMH and the frequency of intracranial large artery stenosis upon MRA were significantly higher for elderly patients aged ≥ 75 years (p < 0.001, p < 0.001, and p = 0.011, respectively). Laboratory data showed that the triglyceride level was highest in younger patients (147.6 ± 100.4 mg/dL, p < 0.001), whereas HbA1c was highest in middle-aged patients (6.2 ± 1.1%, p = 0.003). Regarding the levels of PUFAs, we found no significant differences in EPA level by age group. However, patients aged < 65 years had the highest levels of AA (201.2 ± 61.1 µg/mL, p = 0.005) and the lowest levels of DHA (140.4 ± 55.1 µg/mL, p = 0.007).
Table 1. Baseline characteristics and MRI and laboratory findings of study subjects by age.
| Characteristics | Total n = 373 | Ischemic stroke patients |
Control | p | |||
|---|---|---|---|---|---|---|---|
| Age < 65 years | Age 65–74 years | Age ≥ 75 years | |||||
| n = 113 | n = 104 | n = 156 | n = 105 | Age < 65 vs. 65–74 vs. ≥ 75 years | Age < 65 vs. control | ||
| Sociodemographic | |||||||
| Age, years, mean ± SD | 70.2 ± 13.4 | 54.0 ± 9.3 | 69.9 ± 3.0 | 82.2 ± 5.0 | 54.1 ± 5.5 | < 0.001 | 0.171 |
| Gender, male, no. (%) | 245 (66) | 82 (73) | 77 (74) | 86 (55) | 76 (72) | 0.005 | 0.976 |
| Body mass index | 23.2 ± 3.9 | 24.5 ± 4.3 | 23.3 ± 3.5 | 22.3 ± 3.6 | 23.8 ± 3.2 | < 0.001 | 0.750 |
| Risk factors, no. (%) | |||||||
| Hypertension | 264 (71) | 69 (61) | 79 (76) | 116 (74) | 55 (52) | 0.024 | 0.196 |
| Diabetes mellitus | 111 (30) | 26 (23) | 37 (36) | 48 (31) | 30 (29) | 0.121 | 0.348 |
| Dyslipidemia | 242 (65) | 70 (62) | 76 (73) | 96 (62) | 54 (51) | 0.119 | 0.117 |
| Current cigarette smoking | 74 (20) | 39 (35) | 25 (24) | 10 (6) | 37 (35) | < 0.001 | 0.911 |
| Atrial fibrillation | 79 (21) | 14 (12) | 22 (21) | 43 (28) | 0 (0) | 0.011 | < 0.001 |
| Coronary artery disease | 46 (12) | 2 (2) | 21 (20) | 23 (15) | 3 (3) | < 0.001 | 0.934 |
| Peripheral artery disease | 10 (3) | 0 (0) | 5 (5) | 5 (3) | NA | 0.079 | NA |
| NIHSS score on admission, mean ± SD | 4.4 ± 5.6 | 3.2 ± 4.6 | 4.7 ± 5.9 | 5.2 ± 6.0 | NA | 0.006 | NA |
| Radiological findings, no. (%) | |||||||
| Chest radiograph | |||||||
| Aortic arch calcification, grade 0–3 | 1.2 ± 1.0 | 0.5 ± 0.7 | 1.3 ± 0.9 | 1.7 ± 0.9 | NA | < 0.001 | NA |
| MRI | |||||||
| Multiple lesions, no. (%) | 111 (30) | 29 (26) | 33 (32) | 49 (31) | NA | 0.521 | NA |
| PVH, grade 0–3 | 0.9 ± 0.9 | 0.5 ± 0.7 | 1.0 ± 0.8 | 1.2 ± 1.2 | NA | < 0.001 | NA |
| DSWMH, grade 0–3 | 0.8 ± 0.8 | 0.4 ± 0.7 | 0.8 ± 0.8 | 1.1 ± 1.2 | NA | < 0.001 | NA |
| Intracranial arterial stenosis on MRA | 89 (24) | 17 (15) | 24 (23) | 48 (31) | NA | 0.011 | NA |
| Laboratory findings, mean ± SD | |||||||
| LDL-C | 112.2 ± 36.1 | 117.0 ± 38.3 | 112.5 ± 35.2 | 108.5 ± 34.8 | 122.8 ± 33.2 | 0.157 | 0.158 |
| HDL-C | 50.7 ± 15.7 | 51.8 ± 16.2 | 49.3 ± 14.0 | 50.9 ± 16.5 | 65.8 ± 17.7 | 0.6 | < 0.001 |
| Triglyceride | 123.5 ± 80.9 | 147.6 ± 100.4 | 126.0 ± 83.1 | 104.3 ± 55.0 | 126.6 ± 81.7 | < 0.001 | 0.060 |
| Hemoglobin A1c | 6.1 ± 1.2 | 6.0 ± 1.5 | 6.2 ± 1.1 | 6.1 ± 1.1 | 6.1 ± 1.0, § | 0.003 | NA |
| Glucose | 126.0 ± 52.1 | 124.7 ± 62.2 | 129.9 ± 48.4 | 124.3 ± 46.5 | 106.9 ± 26.7 | 0.09 | 0.036 |
| AA | 186.5 ± 52.8 | 201.2 ± 61.1 | 184.7 ± 42.6 | 177.0 ± 50.3 | 143.2 ± 27.4 | 0.005 | < 0.001 |
| EPA | 70.2 ± 41.2 | 66.6 ± 41.6 | 77.5 ± 45.6 | 68.1 ± 37.4 | 68.6 ± 34.7 | 0.138 | 0.233 |
| DHA | 143.5 ± 48.3 | 140.4 ± 55.1 | 149.6 ± 49.5 | 141.7 ± 41.7 | 114.9 ± 33.9 | 0.007 | < 0.001 |
Chi-square test, the Mann-Whitney U, and Kruskal-Wallis test were used for comparison. MRI = Magnetic resonance imaging; NA = not available; NIHSS = NIH Stroke scale; PVH = Periventricular hyperintensity; DSWMH = deep and subcortical white matter hyperintensity; MRA = Magnetic resonance angiography; LDL-C = Low-density lipoprotein cholesterol; HDL-C = High-density lipoprotein cholesterol; AA = Arachidonic acid; EPA = Eicosapentaenoic acid; DHA = Docosahexaenoic acid.
= Hemoglobin A1c was measured in 86 patients.
Comparison of clinical characteristics and laboratory data between younger stroke patients and control subjects aged < 65 years
Table 1 also shows the comparison of clinical characteristics of younger stroke patients aged < 65 years and the control subjects that were age- and gender-matched to the younger stroke patients. AF was more common in younger stroke patients (p < 0.001). From laboratory data, HDL-C level was significantly higher in the controls (p < 0.001), whereas glucose level was higher in the younger stroke patients (p = 0.036). Regarding PUFAs, AA and DHA levels were significantly higher in younger stroke patients (both p < 0.001), whereas EPA was not different between these groups.
The Ratios of EPA/AA and DHA/AA Serum Levels among Stroke Patients by Age and for Control Subjects
Fig. 1 shows the EPA/AA and DHA/AA ratios for stroke patients aged < 65, 65–74, and ≥ 75 years and for control subjects aged < 65 years. The ratios of EPA/AA and DHA/AA serum levels were significantly lower for younger patients (0.35 ± 0.23, p = 0.006; 0.73 ± 0.27, p < 0.001) compared with the ratios of patients aged 65–74 and ≥ 75 years, as well as with controls aged < 65 years (vs. 0.49 ± 0.25, p < 0.001; vs. 0.82 ± 0.26, p = 0.009). On the other hand, no significant differences in the EPA/AA and DHA/AA ratios were found between brain infarction and transient ischemic attack, or among stroke subtypes.
Fig. 1.
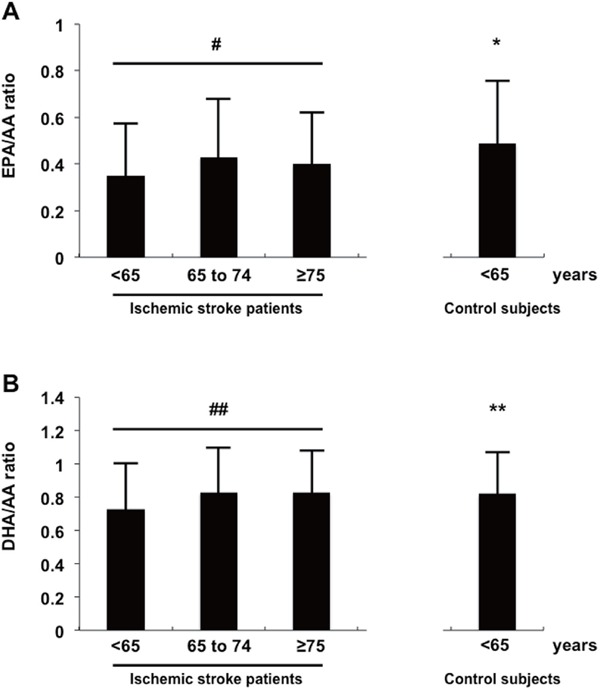
Comparison of the ratios of EPA/AA (A) and DHA/AA (B) serum levels among ischemic stroke patients and control subjects. #p = 0.006; *p < 0.001 vs. ischemic stroke patients aged < 65 years; ##p < 0.001; **p = 0.009 vs. ischemic stroke patients aged < 65 years.
Independent Factors among Age Groups in Multinomial Logistic Regression Analysis
Age, male gender, BMI, current smoking, coronary artery disease, NIHSS score, aortic arch calcification, PVH, DSWMH, triglyceride level, HbA1c level, EPA/AA ratio, and DHA/AA ratio were selected for multinomial logistic regression analyses. We excluded DSWMH from the covariates, because PVH and DSWMH could cause coincidental or interfering effects. Age was considered a confounding factor and was also excluded from the covariates. In Models 1 and 2, lower ratios of EPA/AA (odds ratio [OR] 0.18, 95% confidence interval [CI] 0.04–0.81, p = 0.026) and DHA/AA (OR 0.09, 95% CI 0.02–0.33, p < 0.001) were significantly associated with patients aged < 65 years compared with those of elderly patients (Table 2). In Models 1 and 2, BMI, current smoking, and triglyceride levels were significantly related to younger patients (p < 0.05), whereas aortic arch calcification and PVH were inversely related to younger patients (p < 0.001) (Table 2).
Table 2. Associations of underlying characteristics and radiological and laboratory findings with younger and middle-aged patients on multinomial regression analysis.
| Variables | Age < 65 years vs. ≥ 75 years |
Age 65–74 years vs. ≥ 75 years |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95%CI | p | |
| Model 1 | ||||||
| Male gender | 1.08 | 0.53–2.23 | 0.829 | 1.54 | 0.85–2.82 | 0.157 |
| BMI | 1.14 | 1.04–1.24 | 0.005 | 1.06 | 0.98–1.15 | 0.13 |
| Current smoker | 12.79 | 4.87–33.60 | < 0.001 | 5.45 | 2.32–12.77 | 0 |
| Coronary artery disease | 0.29 | 0.06–1.50 | 0.139 | 2.00 | 0.95–4.19 | 0.068 |
| NIHSS score, § | 0.96 | 0.90–1.02 | 0.157 | 1.00 | 0.96–1.05 | 0.961 |
| Aortic arch calcification, §§ | 0.24 | 0.16–0.36 | < 0.001 | 0.61 | 0.45–0.83 | 0.001 |
| PVH, §§ | 0.37 | 0.23–0.60 | < 0.001 | 0.72 | 0.51–1.03 | 0.071 |
| Triglyceride | 1.01 | 1.00–1.01 | 0.017 | 1.01 | 1.00–1.01 | 0.048 |
| Hemoglobin A1c | 0.79 | 0.59–1.06 | 0.123 | 0.96 | 0.77–1.2 | 0.722 |
| EPA/AA ratio | 0.18 | 0.04–0.81 | 0.026 | 1.08 | 0.35–3.35 | 0.891 |
| Model 2 | ||||||
| Male gender | 1.10 | 0.53–2.28 | 0.801 | 1.59 | 0.88–2.89 | 0.127 |
| BMI | 1.13 | 1.03–1.24 | 0.011 | 1.06 | 0.98–1.15 | 0.14 |
| Current smoker | 11.79 | 4.43–31.43 | < 0.001 | 5.43 | 2.31–12.76 | < 0.001 |
| Coronary artery disease | 0.22 | 0.04–1.22 | 0.083 | 1.92 | 0.91–4.06 | 0.087 |
| NIHSS score, § | 0.96 | 0.9–1.03 | 0.224 | 1.00 | 0.96–1.05 | 0.972 |
| Aortic arch calcification, §§ | 0.23 | 0.15–0.35 | < 0.001 | 0.6 | 0.44–0.81 | 0.001 |
| PVH, §§ | 0.38 | 0.23–0.6 | < 0.001 | 0.71 | 0.50–1.01 | 0.056 |
| Triglyceride | 1.01 | 1.00–1.01 | 0.003 | 1.01 | 1.00–1.01 | 0.032 |
| Hemoglobin A1c | 0.77 | 0.57–1.05 | 0.093 | 0.96 | 0.77–1.19 | 0.696 |
| DHA/AA ratio | 0.09 | 0.02–0.33 | < 0.001 | 0.57 | 0.19–1.65 | 0.297 |
All variables with a p value < 0.05 on univariate analysis were entered into the multinomial logistic regression analysis. OR = Odds ratio; CI = Confidence interval; BMI = Body mass index; NIHSS = NIH Stroke scale; PVH = Periventricular hyperintensity; DSWMH = deep and subcortical white matter hyperintensity; AA = Arachidonic acid; EPA = Eicosapentaenoic acid; DHA = Docosahexaenoic acid.
= per 1-point of increase,
= per 1-grade of increase.
Relationship between age Stratification and the Tertiles of EPA/AA and DHA/AA Ratios
Patients were classified into three tertiles of the EPA/AA ratio (Tertile I, < 0.2444; Tertile II, 0.2444 to 0.444; Tertile III, > 0.444). The comparisons of baseline characteristics and radiological and laboratory data according to the EPA/AA (Table 3) and DHA/AA (Table 4) tertiles of different ages are shown. Among patients aged < 65 years, patients in EPA/AA Tertile I were the youngest (51.5 ± 9.5 years, p = 0.010) and had the highest frequency of dyslipidemia (71%, p = 0.030) (Table 3). Among patients aged 65 to 74 years, patients in EPA/AA Tertile II were the youngest (68.9 ± 2.9 years, p = 0.040). We found significant differences in the degree of PVH and DSWMH among tertiles in the middle-aged group (1.3 ± 0.8, p = 0.042; 1.1 ± 0.8, p = 0.025, respectively). Multiple infarctions were most common in Tertile I in the middle-aged group (54%, p = 0.008). In the elderly age group, patients in EPA/AA Tertile I had the highest grade of aortic arch calcification upon chest radiograph (2.0 ± 0.9, p = 0.012).
Table 3. Baseline characteristics and radiological and laboratory findings of study subjects according to the tertile of EPA/AA ratio in patients aged > 65, 65–74, and ≥ 75 years.
| Characteristics | Age < 65 years |
Age 65–74 years |
Age ≥ 75 years |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EPA/AA ratio |
EPA/AA ratio |
EPA/AA ratio |
|||||||||||||
| All | Tertile I | Tertile II | Tertile III | All | Tertile I | Tertile II | Tertile III | All | Tertile I | Tertile II | Tertile III | ||||
| n = 113 | n = 51, 45% | n = 35, 31% | n = 27, 24% | p | n = 104 | n = 28, 27% | n = 35, 34% | n = 41, 39% | p | n = 156 | n = 46, 29% | n = 54, 35% | n = 56, 36% | p | |
| Sociodemographic | |||||||||||||||
| Age, years, mean ± SD | 54.0 ± 9.3 | 51.5 ± 9.5 | 55.8 ± 8.1 | 56.4 ± 9.4 | 0.01 | 69.9 ± 3.0 | 70.8 ± 2.8 | 68.9 ± 2.9 | 70.2 ± 3.1 | 0.04 | 82.2 ± 5.0 | 83.2 ± 4.9 | 81.4 ± 4.9 | 82.1 ± 5.0 | 0.181 |
| Gender, male, no. (%) | 82 (73) | 35 (68) | 26 (74) | 21 (78) | 0.664 | 77 (74) | 21 (75) | 26 (74) | 30 (73) | 0.985 | 86 (55) | 22 (48) | 25 (46) | 39 (70) | 0.024 |
| Body mass index | 24.5 ± 4.3 | 25.0 ± 4.9 | 24.4 ± 3.8 | 23.7 ± 3.7 | 0.582 | 23.3 ± 3.5 | 22.3 ± 3.1 | 23.4 ± 2.5 | 23.9 ± 4.4 | 0.242 | 22.3 ± 3.6 | 22.3 ± 4.5 | 22.1 ± 3.2 | 22.6 ± 3.0 | 0.65 |
| Risk factors, no. (%) | |||||||||||||||
| Hypertension | 69 (61) | 31 (61) | 24 (69) | 14 (52) | 0.408 | 79 (76) | 23 (82) | 22 (63) | 34 (83) | 0.083 | 116 (74) | 35 (76) | 41 (76) | 40 (71) | 0.821 |
| Diabetes mellitus | 26 (23) | 9 (18) | 10 (29) | 7 (26) | 0.456 | 37 (36) | 13 (46) | 11 (31) | 13 (32) | 0.374 | 48 (31) | 11 (24) | 18 (33) | 19 (34) | 0.486 |
| Dyslipidemia | 70 (62) | 36 (71) | 23 (66) | 11 (41) | 0.03 | 76 (73) | 22 (79) | 22 (63) | 32 (78) | 0.246 | 96 (62) | 32 (70) | 30 (56) | 34 (61) | 0.352 |
| Cigarette smoking | 39 (35) | 20 (39) | 12 (34) | 7 (26) | 0.501 | 25 (24) | 7 (25) | 10 (29) | 8 (20) | 0.648 | 10 (6) | 4 (9) | 3 (6) | 3 (5) | 0.776 |
| Atrial fibrillation | 14 (12) | 3 (6) | 5 (14) | 6 (22) | 0.105 | 22 (21) | 5 (18) | 6 (17) | 11 (27) | 0.519 | 43 (28) | 8 (17) | 14 (26) | 21 (38) | 0.073 |
| Coronary artery disease | 2 (2) | 1 (2) | 0 (0) | 1 (4) | 0.535 | 21 (20) | 9 (32) | 7 (20) | 5 (12) | 0.128 | 23 (15) | 6 (13) | 9 (17) | 8 (14) | 0.872 |
| Peripheral artery disease | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA | 5 (5) | 2 (7) | 1 (3) | 2 (5) | 0.758 | 5 (3) | 1 (2) | 2 (4) | 2 (4) | 0.899 |
| NIHSS score on admission, mean ± SD | 3.2 ± 4.6 | 3.7 ± 5.1 | 2.1 ± 2.1 | 3.7 ± 5.8 | 0.279 | 4.7 ± 5.9 | 4.7 ± 4.7 | 6.0 ± 7.2 | 3.7 ± 5.2 | 0.168 | 5.2 ± 6.0 | 6.0 ± 6.0 | 5.1 ± 5.9 | 4.5 ± 6.1 | 0.06 |
| Radiological findings, no. (%) | |||||||||||||||
| Chest X-ray | |||||||||||||||
| Aortic arch calcification | 0.5 ± 0.7 | 0.5 ± 0.8 | 0.4 ± 0.7 | 0.4 ± 0.6 | 0.641 | 1.3 ± 0.9 | 1.5 ± 0.8 | 1.1 ± 0.9 | 1.2 ± 1.0 | 0.398 | 1.7 ± 0.9 | 2.0 ± 0.9 | 1.7 ± 0.9 | 1.5 ± 1.0 | 0.012 |
| MRI | |||||||||||||||
| Multiple lesions, no. (%) | 29 (26) | 11 (22) | 11 (31) | 7 (26) | 0.589 | 33 (32) | 15 (54) | 6 (17) | 12 (29) | 0.008 | 49 (31) | 13 (28) | 20 (37) | 16 (29) | 0.545 |
| PVH, grade 0–3 | 0.5 ± 0.7 | 0.5 ± 0.8 | 0.6 ± 0.6 | 0.2 ± 0.5 | 0.028 | 1.0 ± 0.8 | 1.3 ± 0.8 | 0.8 ± 0.8 | 0.9 ± 0.8 | 0.042 | 1.2 ± 1.2 | 1.3 ± 0.8 | 1.2 ± 0.8 | 1.2 ± 0.7 | 0.851 |
| DSWMH, grade 0–3 | 0.4 ± 0.7 | 0.3 ± 0.6 | 0.6 ± 0.8 | 0.3 ± 0.5 | 0.133 | 0.8 ± 0.8 | 1.1 ± 0.8 | 0.6 ± 0.7 | 0.7 ± 0.8 | 0.025 | 1.1 ± 1.2 | 1.3 ± 0.8 | 1.1 ± 0.8 | 1.0 ± 0.7 | 0.245 |
| Intracranial arterial stenosis on MRA | 17 (15) | 4 (8) | 8 (23) | 5 (19) | 0.136 | 24 (23) | 6 (21) | 10 (29) | 8 (20) | 0.628 | 48 (31) | 17 (37) | 13 (24) | 18 (32) | 0.366 |
| Laboratory findings, mean ± SD | |||||||||||||||
| LDL-C | 117.0 ± 38.3 | 121.7 ± 42.1 | 115.7 ± 36.2 | 109.9 ± 33.1 | 0.383 | 112.5 ± 35.2 | 100.2 ± 30.3 | 112.6 ± 29.2 | 121.0 ± 40.7 | 0.079 | 108.5 ± 34.8 | 113.8 ± 44.8 | 108.1 ± 29.7 | 104.6 ± 29.6 | 0.625 |
| HDL-C | 51.8 ± 16.2 | 48.4 ± 14.3 | 53.7 ± 18.4 | 55.8 ± 15.6 | 0.083 | 49.3 ± 14.0 | 46.2 ± 12.2 | 48.6 ± 13.5 | 51.9 ± 15.3 | 0.389 | 50.9 ± 16.5 | 49.2 ± 18.2 | 51.6 ± 15.5 | 51.7 ± 16.1 | 0.501 |
| Triglycerides | 147.6 ± 100.4 | 149.4 ± 82.1 | 171.5 ± 139.9 | 113.1 ± 53.3 | 0.157 | 126.0 ± 83.1 | 125.3 ± 89.0 | 118.8 ± 40.8 | 132.7 ± 104.5 | 0.575 | 104.3 ± 55.0 | 105.3 ± 53.1 | 105.4 ± 58.5 | 102.4 ± 53.9 | 0.959 |
| Hemoglobin A1c | 6.0 ± 1.5 | 6.0 ± 1.5 | 6.2 ± 1.8 | 5.9 ± 1.3 | 0.38 | 6.2 ± 1.1 | 6.2 ± 0.8 | 6.1 ± 0.9 | 6.3 ± 1.3 | 0.451 | 6.1 ± 1.1 | 6.0 ± 1.1 | 6.2 ± 1.4 | 6.1 ± 0.6 | 0.11 |
| Glucose | 124.7 ± 62.2 | 127.5 ± 57.2 | 125.7 ± 75.8 | 117.8 ± 52.5 | 0.608 | 129.9 ± 48.4 | 125.3 ± 45.6 | 132.2 ± 47.6 | 131.0 ± 51.9 | 0.769 | 124.3 ± 46.5 | 119.8 ± 41.7 | 129.2 ± 61.3 | 123.4 ± 31.5 | 0.279 |
The Chi-square test and the Kruskal-Wallis test were used for comparison. † mean ± SD. MRI = Magnetic resonance imaging; NIHSS = NIH Stroke scale; PVH = Periventricular hyperintensity; DSWMH = deep and subcortical white matter hyperintensity; MRA = Magnetic resonance angiography; NA = Not available; LDL-C = low-density lipoprotein cholesterol; HDL-C = high-density lipoprotein cholesterol; AA = Arachidonic acid; EPA = Eicosapentaenoic acid. Tertile I, < 0.2444; Tertile II, 0.2444 to 0.444; Tertile III, > 0.444.
Table 4. Baseline characteristics and radiological and laboratory findings of study subjects according to the tertile of DHA/AA ratio in patients aged > 65, 65–74, and ≥ 75 years.
| Characteristics | Age < 65 years |
Age 65–74 years |
Age ≥ 75 years |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DHA/AA ratio |
DHA/AA ratio |
DHA/AA ratio |
|||||||||||||
| All | Tertile I | Tertile II | Tertile III | All | Tertile I | Tertile II | Tertile III | All | Tertile I | Tertile II | Tertile III | ||||
| n = 113 | n = 49, 43% | n = 39, 35% | n = 25, 22% | p | n = 104 | n = 34, 33% | n = 31, 30% | n = 39, 38% | p | n = 156 | n = 42, 27% | n = 54, 35% | n = 60, 38% | p | |
| Sociodemographic | |||||||||||||||
| Age, years, mean ± SD | 54.0 ± 9.3 | 52.1 ± 9.0 | 54.1 ± 10.1 | 57.6 ± 7.5 | 0.024 | 69.9 ± 3.0 | 70.0 ± 3.0 | 69.1 ± 3.0 | 70.5 ± 3.0 | 0.148 | 82.2 ± 5.0 | 82.6 ± 5.6 | 82.4 ± 4.4 | 81.7 ± 5.0 | 0.534 |
| Gender, male, no. (%) | 82 (73) | 35 (71) | 29 (74) | 18 (72) | 0.952 | 77 (74) | 26 (76) | 24 (77) | 27 (69) | 0.685 | 86 (55) | 18 (43) | 32 (59) | 36 (60) | 0.173 |
| Body height | 165.2 ± 8.4 | 165.1 ± 7.8 | 165.9 ± 8.4 | 164.2 ± 9.8 | 0.731 | 162.5 ± 8.9 | 162.8 ± 8.3 | 161.9 ± 9.5 | 162.6 ± 9.1 | 0.98 | 156.6 ± 10.1 | 153.8 ± 10.4 | 158.4 ± 10.3 | 157.1 ± 9.3 | 0.119 |
| Body weight | 67.2 ± 14.6 | 67.2 ± 15.0 | 70.0 ± 16.2 | 62.9 ± 10.2 | 0.302 | 61.5 ± 10.2 | 60.3 ± 9.3 | 61.2 ± 10.4 | 62.8 ± 10.9 | 0.409 | 55.1 ± 11.3 | 53.7 ± 10.4 | 55.8 ± 11.3 | 55.3 ± 10.9 | 0.647 |
| Body mass index | 24.5 ± 4.3 | 24.6 ± 5.0 | 25.1 ± 4.1 | 23.3 ± 3.0 | 0.236 | 23.3 ± 3.5 | 22.8 ± 3.0 | 23.4 ± 4.5 | 23.7 ± 3.1 | 0.468 | 22.3 ± 3.6 | 22.7 ± 4.7 | 22.0 ± 2.7 | 22.3 ± 3.3 | 0.719 |
| Risk factors, no. (%) | |||||||||||||||
| Hypertension | 69 (61) | 32 (65) | 22 (56) | 15 (60) | 0.691 | 79 (76) | 26 (76) | 24 (77) | 29 (74) | 0.953 | 116 (74) | 33 (79) | 43 (80) | 40 (67) | 0.219 |
| Diabetes mellitus | 26 (23) | 10 (20) | 8 (21) | 8 (32) | 0.481 | 37 (36) | 15 (44) | 13 (42) | 9 (23) | 0.117 | 48 (31) | 13 (31) | 16 (30) | 19 (32) | 0.972 |
| Dyslipidemia | 70 (62) | 32 (65) | 23 (59) | 15 (60) | 0.81 | 76 (73) | 26 (76) | 21 (68) | 29 (74) | 0.712 | 96 (62) | 27 (64) | 34 (63) | 35 (58) | 0.802 |
| Cigarette smoking | 39 (35) | 22 (45) | 9 (23) | 8 (32) | 0.097 | 25 (24) | 11 (32) | 6 (19) | 8 (21) | 0.382 | 10 (6) | 4 (10) | 3 (6) | 3 (5) | 0.663 |
| Atrial fibrillation | 14 (12) | 3 (6) | 6 (15) | 5 (20) | 0.180 | 22 (21) | 5 (15) | 6 (19) | 11 (28) | 0.355 | 43 (28) | 8 (19) | 18 (33) | 17 (28) | 0.295 |
| Coronary artery disease | 2 (2) | 1 (2) | 0 (0) | 1 (4) | 0.491 | 21 (20) | 9 (26) | 8 (26) | 4 (10) | 0.148 | 23 (15) | 5 (12) | 11 (20) | 7 (12) | 0.353 |
| Peripheral artery disease | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA | 5 (5) | 2 (6) | 0 (0) | 3 (8) | 0.193 | 5 (3) | 0 (0) | 3 (6) | 2 (3) | 0.211 |
| NIHSS score on admission, mean ± SD | 3.2 ± 4.6 | 2.4 ± 2.9 | 4.0 ± 5.9 | 3.5 ± 5.0 | 0.713 | 4.7 ± 5.9 | 4.0 ± 4.2 | 5.5 ± 5.9 | 4.8 ± 7.0 | 0.481 | 5.2 ± 6.0 | 5.8 ± 5.9 | 4.8 ± 5.9 | 5.0 ± 6.2 | 0.379 |
| Radiological findings, no. (%) | |||||||||||||||
| Chest X-ray | |||||||||||||||
| Aortic arch calcification | 0.5 ± 0.7 | 0.5 ± 0.8 | 0.4 ± 0.7 | 0.4 ± 0.7 | 0.765 | 1.3 ± 0.9 | 1.4 ± 0.9 | 1.1 ± 0.9 | 1.3 ± 1.0 | 0.46 | 1.7 ± 0.9 | 1.9 ± 0.9 | 1.8 ± 0.8 | 1.6 ± 1.0 | 0.211 |
| MRI | |||||||||||||||
| Multiple lesions, no. (%) | 29 (26) | 7 (14) | 19 (49) | 3 (12) | < 0.001 | 33 (32) | 15 (44) | 6 (19) | 12 (31) | 0.099 | 49 (31) | 12 (29) | 17 (32) | 20 (33) | 0.878 |
| PVH | 0.5 ± 0.7 | 0.5 ± 0.8 | 0.4 ± 0.6 | 0.4 ± 0.6 | 0.956 | 1.0 ± 0.8 | 1.1 ± 0.7 | 0.9 ± 0.9 | 0.9 ± 0.8 | 0.444 | 1.2 ± 01.2 | 1.3 ± 0.9 | 1.2 ± 0.7 | 1.3 ± 0.8 | 0.935 |
| DSWMH | 0.4 ± 0.7 | 0.3 ± 0.6 | 0.4 ± 0.6 | 0.5 ± 0.8 | 0.488 | 0.8 ± 0.8 | 1.0 ± 0.8 | 0.7 ± 0.9 | 0.7 ± 0.7 | 0.205 | 1.1 ± 1.2 | 1.1 ± 0.9 | 1.1 ± 0.8 | 1.0 ± 0.7 | 0.665 |
| Intracranial arterial stenosis on MRA | 17 (15) | 7 (14) | 7 (18) | 3 (12) | 0.794 | 24 (23) | 10 (29) | 5 (16) | 9 (23) | 0.447 | 48 (31) | 17 (40) | 13 (24) | 18 (30) | 0.222 |
| Laboratory findings, mean ± SD | |||||||||||||||
| LDL-C | 117.0 ± 38.3 | 121.0 ± 41.2 | 121.5 ± 35.3 | 102.4 ± 34.4 | 0.078 | 112.5 ± 35.2 | 102.6 ± 32.1 | 109.1 ± 28.5 | 124.0 ± 39.8 | 0.034 | 108.5 ± 34.8 | 111.2 ± 42.0 | 109.8 ± 35.6 | 105.4 ± 28.3 | 0.774 |
| HDL-C | 51.8 ± 16.2 | 52.6 ± 18.4 | 49.8 ± 13.1 | 53.4 ± 16.2 | 0.567 | 49.3 ± 14.0 | 44.6 ± 11.2 | 53.6 ± 14.9 | 49.8 ± 14.6 | 0.036 | 50.9 ± 16.5 | 53.2 ± 17.0 | 52.5 ± 17.6 | 47.9 ± 14.8 | 0.228 |
| TG | 147.6 ± 100.4 | 142.9 ± 100.6 | 142.9 ± 63.3 | 164.1 ± 141.7 | 0.552 | 126.0 ± 83.1 | 106.5 ± 42.4 | 130.5 ± 83.6 | 139.5 ± 105.3 | 0.389 | 104.3 ± 55.0 | 102.1 ± 60.1 | 105.2 ± 49.4 | 105.0 ± 56.9 | 0.733 |
| HbA1c | 6.0 ± 1.5 | 6.2 ± 1.9 | 5.9 ± 1.1 | 5.8 ± 1.0 | 0.68 | 6.2 ± 1.1 | 6.3 ± 0.9 | 6.2 ± 0.9 | 6.2 ± 1.3 | 0.751 | 6.1 ± 1.1 | 5.9 ± 0.8 | 6.2 ± 1.2 | 6.1 ± 1.2 | 0.5 |
| Glucose | 124.7 ± 62.2 | 134.3 ± 77.9 | 117.2 ± 52.5 | 116.5 ± 32.6 | 0.489 | 129.9 ± 48.4 | 129.2 ± 46.7 | 133.5 ± 47.6 | 127.6 ± 51.6 | 0.969 | 124.3 ± 46.5 | 119.3 ± 44.0 | 127.7 ± 57.7 | 124.9 ± 36.2 | 0.249 |
The Chi-square test and the Kruskal-Wallis test were used for comparison. MRI = Magnetic resonance imaging; NIHSS = NIH Stroke scale; PVH = Periventricular hyperintensity; DSWMH = deep and subcortical white matter hyperintensity; MRA = Magnetic resonance angiography; LDL-C = low-density lipoprotein cholesterol; HDL-C = high-density lipoprotein cholesterol; TG = triglyceride; AA = Arachidonic acid; EPA = Eicosapentaenoic acid. Tertile I, < 0.666; Tertile II, 0.666 to 0.87; Tertile III, > 0.87.
Patients were also divided into three DHA/AA tertiles (Tertile I, < 0.666; Tertile II, 0.666 to 0.87; Tertile III, > 0.87). Table 4 shows that patients in Tertile I were youngest (52.1 ± 9.0, p = 0.024) among those aged < 65 years. Multiple infarctions were most frequently found in Tertile II of younger stroke patients (49%, p < 0.001). For patients aged 65 to 74 years, LDL-C and HDL-C levels were lowest in Tertile I (102.6 ± 32.1, p = 0.034; 44.6 ± 11.2, p = 0.036, respectively). However, we did not observe significant increases in advanced aortic arch calcification or white matter lesions for patients of any age, which was not consistent with the classification according to EPA/AA tertiles.
Discussion
In the present study, patients' characteristics and radiological and laboratory findings including the ratios of EPA/AA and DHA/AA serum levels were analyzed by age, and age-stratified clinical aspects related to the EPA/AA and DHA/AA ratios were explored. Current data showed that the ratios of EPA/AA and DHA/AA were substantially lower for stroke patients aged < 65 years than those for patients aged 65–74 and ≥ 75 years, as well as those for controls. Further, a lower ratio of EPA/AA but not DHA/AA was related to aortic arch calcification for patients aged ≥ 75 years. Cerebral white matter lesions and multiple infarctions were associated with a lower EPA/AA ratio for patients aged 65–74 years.
From the current investigations, our data showed that the ratios of EPA/AA and DHA/AA serum levels for ischemic stroke patients aged < 65, 65–74, and ≥ 75 years were 0.35 ± 0.23, 0.43 ± 0.25, and 0.40 ± 0.22, and 0.73 ± 0.27, 0.83 ± 0.27, and 0.83 ± 0.25, respectively, and, for control subjects aged < 65 years, the ratios were 0.49 ± 0.25 and 0.82 ± 0.26, respectively, indicating that younger stroke patients aged < 65 years displayed a substantial reduction in EPA/AA and DHA/AA ratios compared with the ratios of older stroke patients as well as healthy subjects of the same age. A previous study showed that the EPA/AA ratio in healthy Japanese subjects aged < 35 years is 0.26 but significantly increases with age and reaches 0.68 by ages of ≥ 65 years22). Another study explored the PUFA levels of White, Japanese, and Japanese American men aged 40–49 years, and the estimation of EPA/AA and DHA/AA ratios were about 0.09, 0.39, and 0.12, and 0.27, 0.91, and 0.37, respectively25). Because PUFAs are poorly synthesized in the human body and must be obtained through dietary sources, research has suggested that Westernized dietary habits of young Japanese, Whites, and Japanese Americans, as well as of young stroke patients in the current study, may be related to a reduction in the EPA/AA and DHA/AA ratios22, 25). Moreover, EPA levels were higher in older subjects than in young subjects in the setting of n-3 PUFA supplementation, indicating that the ability to incorporate dietary EPA into plasma phospholipids is greater in older than in younger subjects26, 27). On the other hand, BMI, current smoking, and triglyceride levels were associated with young stroke patients, while those lifestyle risk factors were not different from those of the controls. Although BMI and serum levels of triglycerides may also be affected by diet, our data did not suggest a link between PUFAs and lifestyle risk factors28, 29). As stated above, the ratios of EPA/AA and DHA/AA could be related to younger patients, for the first time indicating that the EPA/AA and DHA/AA ratios might be possible additional risk factors for ischemic stroke for younger patients. However, precise dietary data from enrolled subjects, including other lifestyle-related factors (e.g., physical activity and abdominal circumference), were not investigated in the current study, and, thus, further studies are warranted.
According to TOAST criteria, ischemic stroke subtypes include small artery occlusion, large artery atherosclerosis, cardioembolism, stroke with determined etiology, and stroke with undetermined etiology. Pathologically, a variety of mechanisms for ischemic stroke such as thrombosis related to the burden of atherosclerotic plaques, blood stagnation, small artery disorders, and coagulation abnormalities exist. n-3 PUFAs have potent anti-inflammatory effects and inhibitory effects on platelet aggregation, and they enhance nitric oxide-mediated vasodilation30–32). In particular, n-3 PUFAs stabilize atherosclerotic plaques, which may be a fundamental preventive factor for ischemic stroke33). Our data did not indicate any significant differences among stroke subtypes. Although n-3 PUFAs have been reported to reduce the incidence of ischemic stroke from large-scale studies17), the contribution of PUFAs to stroke mechanisms and subtypes is yet to be elucidated34, 35).
In the current study, a low EPA/AA ratio was significantly associated with aortic arch calcifications upon chest radiograph for elderly stroke patients aged ≥ 75 years and with the degree of cerebral white matter lesions and multiple infarctions for middle-aged patients aged 65–74 years. Aortic arch calcification and cerebral white matter lesions are commonly correlated with age and atherosclerotic risk factors10–14). More importantly, aortic arch calcification and cerebral white matter lesions may share common pathophysiological mechanisms including inflammation, oxidative stress, apoptosis of vascular smooth muscle cells, and endothelial injury36–40). In experimental studies, EPA reduces aneurysm formation as well as vascular calcification in the mouse abdominal aorta via inhibition of matrix metalloproteinase 2 and 9 expression36, 41). Moreover, n-3 PUFAs have potent anti-inflammatory effects, and proatherogenic and proinflammatory effects on endothelial cells30–32). Thus, n-3 PUFAs may suppress those pathologic processes and thereby inhibit aortic arch calcification and cerebral white matter lesions. On the other hand, multiple infarctions could indicate the presence of atherosclerotic embolic sources in the carotid artery or aortic arch42, 43). n-3 PUFAs also decrease the accumulation of inflammatory cytokines such as tumor necrosis factor-α, interleukin-6, and matrix metalloproteinase 1/2 in carotid atherosclerotic plaques33), and, thus, low EPA levels may lead to the development of embolic stroke owing to atherosclerotic plaques. To date, several studies have documented that cerebral white matter lesions are related to PUFAs for patients with atherosclerotic risk factors and for stroke patients, whereas no clinical studies have explored the association of aortic arch calcification and multiple infarctions with PUFAs44, 45). Our data showed that these findings did not correspond to the DHA/AA ratio, which was consistent with previous studies19, 20). Thus, a low EPA/AA ratio might be linked to aortic arch calcification for elderly stroke patients as well as to cerebral white matter disease and multiple infarctions for middle-aged stroke patients.
Some potential limitations must be considered when interpreting the results of this study. First, the data from the current study were derived from a single center, and the number of patients in each tertile in the different age groups was quite small. Additionally, we excluded 73 patients owing to post-surgical stroke onset after cardiac surgery, hospitalization and receiving hospital meals for ≥ 7 days, administration of intravenous hyperalimentation, or taking EPA and DHA agents, as well as 32 patients because of missing data including MRI findings and serum PUFA levels, thus raising the issue of the generalizability of the results. Second, the blood examinations were done within 24 hours of admission, or referral to the Department of Neurology for patients who developed ischemic stroke during hospitalization. Therefore, an additional issue is that the EPA/AA and DHA/AA ratios could have been affected by the stroke itself, diet, or infusion therapy after admission. These ratios were analyzed only once after admission. Third, the cross-sectional nature of the present study limits the interpretation of the potential importance of the duration of hypertension, diabetes mellitus, and dyslipidemia, as well as the history of receiving treatments such as statins, anti-thrombotic agents, and angiotensin-converting enzyme inhibitors, before the onset of ischemic stroke. These factors may have affected the patients' characteristics. Accordingly, the current data need to be interpreted with caution.
Conclusions
The ratios of EPA/AA and DHA/AA serum levels could be specifically associated with younger stroke patients, and it is suggested that the EPA/AA and DHA/AA ratios might be possible additional risk factors for ischemic stroke for younger patients. Additionally, the EPA/AA ratio may be related to aortic arch calcification for elderly stroke patients and to cerebral white matter disease and multiple infarctions for middle-aged stroke patients. The current results could be promising but have some limitations and, therefore, should be validated in large-scale clinical trials.
Acknowledgements
None.
Funding
None.
Conflicts of Interest/Disclosures
R.T. received research funds from Bayer Pharmaceutical Co., Ltd., Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd.
T.U. received lecture fees from Boehringer Ingelheim, Bristol-Myers Squibb, AstraZeneca K.K., Bayer Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Mitsubishi-Tanabe Pharma Co., Ltd., Sanofi K.K., Shionogi & Co., Ltd., Novartis Pharmaceuticals, UCB Japan Co., Ltd., Kowa Shinyaku Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., ONO Pharmaceutical Co., Ltd., Pfizer Japan Inc., Merck Sharp and Dohme (MSD) K.K., Astellas Pharma Inc., GlaxoSmithkline K.K., and research funds from Pfizer Japan Inc., Boehringer Ingelheim, AstraZeneca K.K., Otsuka Pharmaceutical Co., Ltd., Astellas Pharma Inc., Eisai Co., Ltd.
K.S. received lecture fees from Mochida Pharmaceutical Company Ltd. and Takeda Pharmaceutical Company Ltd.
H.D. received scholarship funds and lecture fees from Mochida Pharmaceutical Company Ltd. and Takeda Pharmaceutical Company Ltd.
N.H. was an advisory member of Hisamitsu Pharmaceutical, Dai-Nippon Sumitomo Pharma, Otsuka Pharmaceutical, Novartis Pharma, Takeda Pharmaceutical, Abbie, received lecture fees from GSK, Nippon Boehringer Ingelheim, FP Pharmaceutical, Dai-Nippon Sumitomo Pharma, Eisai, Kissei Pharmaceutical, Nihon Medi-physics, Kyowa Hakko-Kirin, Novartis Pharma, Biogen, Otsuka Pharmaceutical, Medtronic, Abbie, research funds from Kyowa Hakko-Kirin, Nihon Medi-physics, FP Pharmaceutical, Takeda Pharmaceutical, and scholarship funds from Astellas Pharma, Daiichi-Sankyo, Pfizer Japan Inc.
The remaining authors report no conflicts of interest.
References
- 1). Donnan GA, Fisher M, Macleod M, Davis SM: Stroke. Lancet, 2008; 371: 1612-1623 [DOI] [PubMed] [Google Scholar]
- 2). Bevan H, Sharma K, Bradley W: Stroke in young adults. Stroke, 1990; 21: 382-386 [DOI] [PubMed] [Google Scholar]
- 3). Ji R, Schwamm LH, Pervez MA, Singhal AB: Ischemic stroke and transient ischemic attack in young adults: risk factors, diagnostic yield, neuroimaging, and thrombolysis. JAMA Neurol, 2013; 70: 51-57 [DOI] [PubMed] [Google Scholar]
- 4). Park TH, Ko Y, Lee SJ, Lee KB, Lee J, Han MK, Park JM, Kim DE, Cho YJ, Hong KS, Kim JT, Cho KH, Kim DH, Cha JK, Yu KH, Lee BC, Yoon BW, Lee JS, Lee J, Gorelick PB, Bae HJ: Gender differences in the age-stratified prevalence of risk factors in Korean ischemic stroke patients: a nationwide stroke registry-based cross-sectional study. Int J Stroke, 2014; 9: 759-765 [DOI] [PubMed] [Google Scholar]
- 5). Zeiler K, Siostrzonek P, Lang W, Gossinger H, Oder W, Ciciyasvilli H, Kollegger H, Mosslacher H, Deecke L: Different risk factor profiles in young and elderly stroke patients with special reference to cardiac disorders. J Clin Epidemiol, 1992; 45: 1383-1389 [DOI] [PubMed] [Google Scholar]
- 6). Andersen KK, Andersen ZJ, Olsen TS: Age- and genderspecific prevalence of cardiovascular risk factors in 40,102 patients with first-ever ischemic stroke: a Nationwide Danish Study. Stroke, 2010; 41: 2768-2774 [DOI] [PubMed] [Google Scholar]
- 7). Kurl S, Laukkanen JA, Niskanen L, Laaksonen D, Sivenius J, Nyyssonen K, Salonen JT: Metabolic syndrome and the risk of stroke in middle-aged men. Stroke, 2006; 37: 806-811 [DOI] [PubMed] [Google Scholar]
- 8). Iribarren C, Sidney S, Sternfeld B, Browner WS: Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA, 2000; 283: 2810-2815 [DOI] [PubMed] [Google Scholar]
- 9). Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM, Rotterdam Scan Study : Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke, 2003; 34: 1126-1129 [DOI] [PubMed] [Google Scholar]
- 10). Ueno Y, Okuzumi A, Watanabe M, Tanaka Y, Shimada Y, Yamashiro K, Tanaka R, Hattori N, Urabe T: Cerebral small artery diseases may be associated with aortic arch calcification in stroke patients. J Atheroscler Thromb, 2014; 21: 1011-1021 [DOI] [PubMed] [Google Scholar]
- 11). Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA: MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol, 1987; 149: 351-356 [DOI] [PubMed] [Google Scholar]
- 12). Dao HH, Essalihi R, Bouvet C, Moreau P: Evolution and modulation of age-related medial elastocalcinosis: impact on large artery stiffness and isolated systolic hypertension. Cardiovasc Res, 2005; 66: 307-317 [DOI] [PubMed] [Google Scholar]
- 13). Chen NX, Moe SM: Arterial calcification in diabetes. Curr Diab Rep, 2003; 3: 28-32 [DOI] [PubMed] [Google Scholar]
- 14). de Leeuw FE, de Groot JC, Oudkerk M, Witteman JC, Hofman A, van Gijn J, Breteler MM: Hypertension and cerebral white matter lesions in a prospective cohort study. Brain, 2002; 125: 765-772 [DOI] [PubMed] [Google Scholar]
- 15). He K, Song Y, Daviglus ML, Liu K, Van Horn L, Dyer AR, Goldbourt U, Greenland P: Fish consumption and incidence of stroke: a meta-analysis of cohort studies. Stroke, 2004; 35: 1538-1542 [DOI] [PubMed] [Google Scholar]
- 16). Kromhout D, Bosschieter EB, de Lezenne Coulander C: The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N Engl J Med, 1985; 312: 1205-1209 [DOI] [PubMed] [Google Scholar]
- 17). Tanaka K, Ishikawa Y, Yokoyama M, Origasa H, Matsuzaki M, Saito Y, Matsuzawa Y, Sasaki J, Oikawa S, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K, JELIS Investigators J : Reduction in the recurrence of stroke by eicosapentaenoic acid for hypercholesterolemic patients: subanalysis of the JELIS trial. Stroke, 2008; 39: 2052-2058 [DOI] [PubMed] [Google Scholar]
- 18). Nishizaki Y, Shimada K, Tani S, Ogawa T, Ando J, Takahashi M, Yamamoto M, Shinozaki T, Miyauchi K, Nagao K, Hirayama A, Yoshimura M, Komuro I, Nagai R, Daida H: Significance of imbalance in the ratio of serum n-3 to n-6 polyunsaturated fatty acids in patients with acute coronary syndrome. Am J Cardiol, 2014; 113: 441-445 [DOI] [PubMed] [Google Scholar]
- 19). Domei T, Yokoi H, Kuramitsu S, Soga Y, Arita T, Ando K, Shirai S, Kondo K, Sakai K, Goya M, Iwabuchi M, Ueeda M, Nobuyoshi M: Ratio of serum n-3 to n-6 polyunsaturated fatty acids and the incidence of major adverse cardiac events in patients undergoing percutaneous coronary intervention. Circ J, 2012; 76: 423-429 [DOI] [PubMed] [Google Scholar]
- 20). Hishikari K, Kimura S, Yamakami Y, Kojima K, Sagawa Y, Otani H, Sugiyama T, Kuwahara T, Hikita H, Takahashi A, Isobe M: The prognostic value of the serum eicosapentaenoic acid to arachidonic acid ratio in relation to clinical outcomes after endovascular therapy in patients with peripheral artery disease caused by femoropopliteal artery lesions. Atherosclerosis, 2015; 239: 583-588 [DOI] [PubMed] [Google Scholar]
- 21). Suda S, Katsumata T, Okubo S, Kanamaru T, Suzuki K, Watanabe Y, Katsura K, Katayama Y: Low serum n-3 polyunsaturated fatty acid/n-6 polyunsaturated fatty acid ratio predicts neurological deterioration in Japanese patients with acute ischemic stroke. Cerebrovasc Dis, 2013; 36: 388-393 [DOI] [PubMed] [Google Scholar]
- 22). Yanagisawa N, Shimada K, Miyazaki T, Kume A, Kitamura Y, Ichikawa R, Ohmura H, Kiyanagi T, Hiki M, Fukao K, Sumiyoshi K, Hirose K, Matsumori R, Takizawa H, Fujii K, Mokuno H, Inoue N, Daida H: Polyunsaturated fatty acid levels of serum and red blood cells in apparently healthy Japanese subjects living in an urban area. J Atheroscler Thromb, 2010; 17: 285-294 [DOI] [PubMed] [Google Scholar]
- 23). Iijima K, Hashimoto H, Hashimoto M, Son BK, Ota H, Ogawa S, Eto M, Akishita M, Ouchi Y: Aortic arch calcification detectable on chest X-ray is a strong independent predictor of cardiovascular events beyond traditional risk factors. Atherosclerosis, 2010; 210: 137-144 [DOI] [PubMed] [Google Scholar]
- 24). Adams HP, Jr., Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE, 3rd: Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke, 1993; 24: 35-41 [DOI] [PubMed] [Google Scholar]
- 25). Motoyama KR, Curb JD, Kadowaki T, El-Saed A, Abbott RD, Okamura T, Evans RW, Nakamura Y, Sutton-Tyrrell K, Rodriquez BL, Kadota A, Edmundowicz D, Willcox BJ, Choo J, Katsumi N, Otake T, Kadowaki S, Kuller LH, Ueshima H, Sekikawa A: Association of serum n-6 and n-3 polyunsaturated fatty acids with lipids in 3 populations of middle-aged men. Am J Clin Nutr, 2009; 90: 49-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Rees D, Miles EA, Banerjee T, Wells SJ, Roynette CE, Wahle KW, Calder PC: Dose-related effects of eicosapentaenoic acid on innate immune function in healthy humans: a comparison of young and older men. Am J Clin Nutr, 2006; 83: 331-342 [DOI] [PubMed] [Google Scholar]
- 27). Meydani M, Natiello F, Goldin B, Free N, Woods M, Schaefer E, Blumberg JB, Gorbach SL: Effect of longterm fish oil supplementation on vitamin E status and lipid peroxidation in women. J Nutr, 1991; 121: 484-491 [DOI] [PubMed] [Google Scholar]
- 28). Pieke B, von Eckardstein A, Gulbahce E, Chirazi A, Schulte H, Assmann G, Wahrburg U: Treatment of hypertriglyceridemia by two diets rich either in unsaturated fatty acids or in carbohydrates: effects on lipoprotein subclasses, lipolytic enzymes, lipid transfer proteins, insulin and leptin. Int J Obes Relat Metab Disord, 2000; 24: 1286-1296 [DOI] [PubMed] [Google Scholar]
- 29). Jacobs B, De Angelis-Schierbaum G, Egert S, Assmann G, Kratz M: Individual serum triglyceride responses to high-fat and low-fat diets differ in men with modest and severe hypertriglyceridemia. J Nutr, 2004; 134: 1400-1405 [DOI] [PubMed] [Google Scholar]
- 30). Tagawa H, Shimokawa H, Tagawa T, Kuroiwa-Matsumoto M, Hirooka Y, Takeshita A: Long-term treatment with eicosapentaenoic acid augments both nitric oxide-mediated and non-nitric oxide-mediated endothelium-dependent forearm vasodilatation in patients with coronary artery disease. J Cardiovasc Pharmacol, 1999; 33: 633-640 [DOI] [PubMed] [Google Scholar]
- 31). De Caterina R, Cybulsky MI, Clinton SK, Gimbrone MA, Jr., Libby P: The omega-3 fatty acid docosahexaenoate reduces cytokine-induced expression of proatherogenic and proinflammatory proteins in human endothelial cells. Arterioscler Thromb, 1994; 14: 1829-1836 [DOI] [PubMed] [Google Scholar]
- 32). Kramer HJ, Stevens J, Grimminger F, Seeger W: Fish oil fatty acids and human platelets: dose-dependent decrease in dienoic and increase in trienoic thromboxane generation. Biochem Pharmacol, 1996; 52: 1211-1217 [DOI] [PubMed] [Google Scholar]
- 33). Cawood AL, Ding R, Napper FL, Young RH, Williams JA, Ward MJ, Gudmundsen O, Vige R, Payne SP, Ye S, Shearman CP, Gallagher PJ, Grimble RF, Calder PC: Eicosapentaenoic acid (EPA) from highly concentrated n-3 fatty acid ethyl esters is incorporated into advanced atherosclerotic plaques and higher plaque EPA is associated with decreased plaque inflammation and increased stability. Atherosclerosis, 2010; 212: 252-259 [DOI] [PubMed] [Google Scholar]
- 34). Song TJ, Cho HJ, Chang Y, Choi K, Jung AR, Youn M, Shin MJ, Kim YJ: Low Plasma Proportion of Omega 3-Polyunsaturated Fatty Acids Predicts Poor Outcome in Acute Non-Cardiogenic Ischemic Stroke Patients. J Stroke, 2015; 17: 168-176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Ikeya Y, Fukuyama N, Kitajima W, Ogushi Y, Mori H: Comparison of eicosapentaenoic acid concentrations in plasma between patients with ischemic stroke and control subjects. Nutrition, 2013; 29: 127-131 [DOI] [PubMed] [Google Scholar]
- 36). Wang JH, Eguchi K, Matsumoto S, Fujiu K, Komuro I, Nagai R, Manabe I: The omega-3 polyunsaturated fatty acid, eicosapentaenoic acid, attenuates abdominal aortic aneurysm development via suppression of tissue remodeling. PLoS One, 2014; 9: e96286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). Shao JS, Cai J, Towler DA: Molecular mechanisms of vascular calcification: lessons learned from the aorta. Arterioscler Thromb Vasc Biol, 2006; 26: 1423-1430 [DOI] [PubMed] [Google Scholar]
- 38). Demer LL, Tintut Y: Vascular calcification: pathobiology of a multifaceted disease. Circulation, 2008; 117: 2938-2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39). Ueno Y, Zhang N, Miyamoto N, Tanaka R, Hattori N, Urabe T: Edaravone attenuates white matter lesions through endothelial protection in a rat chronic hypoperfusion model. Neuroscience, 2009; 162: 317-327 [DOI] [PubMed] [Google Scholar]
- 40). Ueno Y, Koike M, Shimada Y, Shimura H, Hira K, Tanaka R, Uchiyama Y, Hattori N, Urabe T: L-carnitine enhances axonal plasticity and improves white-matter lesions after chronic hypoperfusion in rat brain. J Cereb Blood Flow Metab, 2015; 35: 382-391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Kanai S, Uto K, Honda K, Hagiwara N, Oda H: Eicosapentaenoic acid reduces warfarin-induced arterial calcification in rats. Atherosclerosis, 2011; 215: 43-51 [DOI] [PubMed] [Google Scholar]
- 42). Szabo K, Kern R, Gass A, Hirsch J, Hennerici M: Acute stroke patterns in patients with internal carotid artery disease: a diffusion-weighted magnetic resonance imaging study. Stroke, 2001; 32: 1323-1329 [DOI] [PubMed] [Google Scholar]
- 43). Ueno Y, Kimura K, Iguchi Y, Shibazaki K, Inoue T, Hattori N, Urabe T: Mobile aortic plaques are a cause of multiple brain infarcts seen on diffusion-weighted imaging. Stroke, 2007; 38: 2470-2476 [DOI] [PubMed] [Google Scholar]
- 44). Nagai K, Koshiba H, Shibata S, Matsui T, Kozaki K: Correlation between the serum eicosapentanoic acid-to-arachidonic acid ratio and the severity of cerebral white matter hyperintensities in older adults with memory disorder. Geriatr Gerontol Int, 2015; 15 Suppl 1: 48-52 [DOI] [PubMed] [Google Scholar]
- 45). Song TJ, Chang Y, Shin MJ, Heo JH, Kim YJ: Low levels of plasma omega 3-polyunsaturated fatty acids are associated with cerebral small vessel diseases in acute ischemic stroke patients. Nutr Res, 2015; 35: 368-374 [DOI] [PubMed] [Google Scholar]


