Abstract
Aim: The aims of this study were: 1) to determine whether the accumulation of aortic root calcification (ARC) assessed using coronary computed tomography angiography (CCTA) can predict future cardiovascular events, and 2) to estimate the onset and progression of ARC in patients with familial hypercholesterolemia (FH).
Methods: One hundred thirteen consecutive Japanese patients with heterozygous FH (male = 54, mean age = 52.1 ± 15.6 years, mean LDL-C = 299.0 ± 94.6 mg/dL), without known coronary artery disease, who underwent 64-detector row CCTA were retrospectively evaluated. ARC was defined as the presence of calcium at the aortic root. The extent of ARC was expressed in Agatston units as the ARC-score. Major adverse cardiac events (MACE) were defined as either cardiac death, ST elevated myocardial infarction (STEMI), non-ST elevated myocardial infarction (NSTEMI), unstable angina pectoris (UAP), planned percutaneous coronary intervention (PCI), coronary artery bypass grafting (CABG), or stroke. The periods to MACE were estimated using multivariate logistic regression analysis.
Results: During the follow-up period (median 1635 days), 19 instances of MACE occurred. Multivariate logistic regression analysis revealed that ARC was a significant independent predictor of MACE (OR= 1.48; 95% CI 1.11–1.87, p < 0.001, respectively). The regression equations were Y= 0.09X − 1.59 (R2 = 0.34, p < 0.001) in males and Y = 0.08X − 1.60 (R2 = 0.13, p < 0. 05) in females.
Conclusions: ARC was significantly associated with future MACE in Japanese patients with heterozygous FH. ARC may start to develop, on average, at 17.4 and 19.7 years of age in males and females, respectively, with heterozygous FH.
Keywords: Familial hypercholesterolemia, Coronary computed tomography angiography, Aortic valve calcification
Introduction
Familial hypercholesterolemia (FH; OMIM #143890) is characterized by the triad of (1) primary hyper-LDL-cholesterolemia, (2) tendon xanthomas, and (3) premature coronary artery disease (CAD)1, 2). Coronary computed tomography angiography (CCTA), a noninvasive imaging modality, is quite useful for the accurate detection and exclusion of CAD in the general population3–5). We demonstrated the prognostic utility of coronary plaque burden assessed by CCTA in patients with FH6). On the other hand, it has been shown that the calcium burden of the aortic valve is larger in patients with FH compared to the general population7). CCTA can assess the degree of the calcium burden in the aortic valve, which could be associated with cardiovascular events8, 9). In addition, patients with FH displayed an increased burden of ascending aorta atherosclerosis in comparison with patients with hypercholesterolemia without a gene mutation10). Moreover, CCTA could help us to estimate when and how rapidly aortic root calcification (ARC) develops in patients with FH. Here we aimed to test 2 hypotheses in our FH cohort without known CAD: (1) ARC assessed by CCTA is associated with future coronary events beyond established risk factors in patients with FH; and (2) we can estimate the onset and progression of ARC in patients with FH assuming a linear model of plaque progression.
Methods
The institutional review board approved the study protocol. All patients gave written informed consent.
A total of 134 consecutive patients with FH without known CAD who underwent 64-detector row CCTA between May 2007 and May 2017 due to any clinical indications, including chest symptoms, signs of cardiac diseases, peripheral artery disease, cerebrovascular disease, or multiple coronary risk factors were retrospectively analyzed. We used the diagnostic criteria for FH by the Japan Atherosclerosis Society11). We excluded patients with a known history of coronary disease. We defined major adverse cardiac events (MACE) as either cardiac death, ST elevated myocardial infarction (STEMI), non-ST elevated myocardial infarction (NSTEMI), unstable angina pectoris (UAP), planned percutaneous coronary intervention (PCI), coronary artery bypass grafting (CABG), or stroke.
Hypertension was defined as systolic blood pressure of at least 140 mmHg, diastolic blood pressure of at least 90 mmHg, or use of antihypertensive medication. The presence of diabetes was defined as previously described by the Japan Diabetes Society12) or the use of diabetes medication. Body mass index (BMI) was defined as body weight in kilograms divided by the square of height measured in meters. Serum concentrations of total cholesterol, triglyceride, and HDL-C were determined enzymatically while the patients were not given any lipid lowering drugs as their baseline level. The patients who received some type of a lipid lowering drug had the same examination after administration of statin therapy. LDL-C concentrations were calculated using the Friedewald formula. Most of the patients were assessed simultaneously with CCTA, while some of the subjects were assessed a few months before the assessment with CCTA.
CCTA was performed with a dual source 64-slice system (Somatom Definition Flash; Siemens Medical System, Erlangen, Germany). The detailed scan protocol has been described previously6).
Two experienced radiologists, blinded with regards to the clinical status, evaluated all CCTA scans separately. Lesions were classified as ARC if they were located within the aortic root, inclusive of the aortic annulus or coronary artery ostia, and contained ≥ 3 contiguous pixels with an attenuation value > 130 HU (Fig. 1)7, 13). The ARC score was defined as the quantity of ARC expressed in Agatston units referring to the same definition as for coronary artery calcification, using dedicated software (SYNAPSE VINCENT; Fuji Film Medical, Tokyo, Japan). We referred to contrast-enhanced scans when the exact location of calcified lesions was unclear.
Fig. 1.
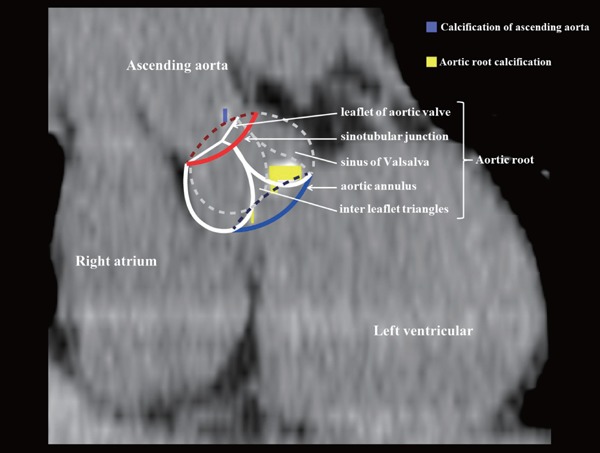
Measurement of aortic root calcification (ARC) score. The purple block indicates calcification of the ascending aorta. The yellow block indicates ARC. The red line indicates the sinotubular junction. The blue line indicates the aortic annulus.
Categorical variables are expressed as percentages. The Fisher exact test or chi-square test was used as appropriate. Continuous variables with a normal distribution are reported as mean ( ± SD), and were compared using unpaired Student's t-tests. Kaplan-Meier curves were compared between the MACE of patients with ARC and those without ARC with the log-rank test. We initially analyzed all available risk factors using a univariate model, then multivariate logistic regression analysis was performed using only the covariates that were significantly associated with MACE in the univariate analysis. Intraobserver/interobserver variability between readers was assessed using the Bland-Altmann method and the coefficient of variation (CV) for 30 randomly selected patients. Most statistical analyses were conducted using JMP® 13 (SAS Institute Inc., Cary, NC, USA) except for receiver-operating characteristic (ROC) curve analysis, which was performed using R version 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org/); p values < 0.05 were considered statistically significant.
Results
Intra- and interobserver reproducibility for measurements of the ARC score are shown in Fig. 2. Bland-Altman analysis demonstrated good agreement, with an intra-observer CV of 13.2% (Fig. 2a) and inter-observer CV of 19.9% (Fig. 2b).
Fig. 2.
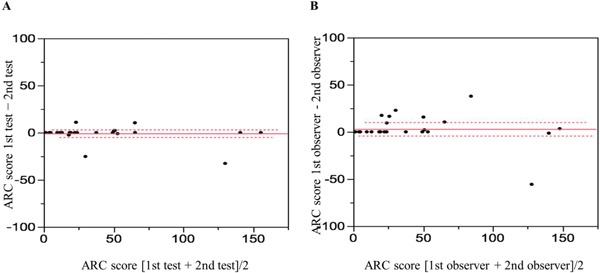
Bland-Altman analysis for the measurement of ARC score
Bland-Altman analysis demonstrated good agreement between intra-observer (a) and inter-observer (b) measurements.
One hundred and thirteen patients with heterozygous FH, whose ages ranged from 13 to 84 years, were included in this analysis (male = 54, mean age = 52.1 ± 15.6 years, mean LDL-C = 299.0 ± 94.6 mg/dL). The median follow-up period was 1635 days. The clinical characteristics of patients with or without subsequent MACE are shown in Table 1. The frequencies of the traditional coronary risk factors, such as age, hypertension, and diabetes mellitus were significantly higher, while HDL-C and post-treatment LDL-C were significantly lower, in patients with FH that developed MACE compared to those without MACE. Interestingly, the extent of ARC was significantly greater in patients with FH with MACE than those without MACE. Table 2 shows the MACE that occurred during the follow-up period. Supplementary Table 1 shows the clinical characteristics of patients with or without ARC. We found that the patients who were ARC positive were older, had a higher BMI, and more likely to have hypertension and diabetes than expected. In addition, when we divided the patients based on the ARC score higher or lower than the threshold value for MACE, the patients with high ARC scores were older and much more likely to have hypertension (Supplementary Table 2).
Table 1. Baseline characteristics.
| All Subjects | MACE (+) | MACE (−) | p value | |
|---|---|---|---|---|
| (n = 113) | (n = 19) | (n = 94) | ||
| Male | 54 (47.8%) | 7 (36.8%) | 47 (50.0%) | n.s. |
| Age, yrs | 52.1 ± 15.6 | 61.2 ± 14.2 | 50.3 ± 15.2 | < 0.05 |
| Body mass index, kg/m2 | 23.6 ± 3.2 | 24.1 ± 3.8 | 23.5 ± 3.1 | n.s. |
| Smoking (Current/former) | 32 (30.5%) | 8 (42.1%) | 24 (27.9%) | n.s. |
| Hypertension | 49 (43.4%) | 13 (68.4%) | 36 (38.3%) | < 0.05 |
| Diabetes mellitus | 26 (23.2%) | 9 (47.4%) | 17 (18.3%) | < 0.05 |
| HbA1c, % | 5.9 ± 1.0 | 6.7 ± 1.8 | 5.8 ± 0.7 | < 0.001 |
| Defined mutation | 78 (69.0%) | 15 (78.9%) | 63 (67.0%) | n.s. |
| Lipids | ||||
| Total cholesterol, mg/dl | 385.2 ± 99.1 | 389.9 ± 104.2 | 384.2 ± 98.7 | n.s. |
| LDL-C, mg/dl | 299.0 ± 94.6 | 309.7 ± 113.4 | 296.7 ± 90.9 | n.s. |
| HDL-C, mg/dl | 54.2 ± 13.6 | 49.0 ± 12.8 | 55.3 ± 13.6 | n.s. |
| Triglyceride, mg/dl | 147.5 ± 93.0 | 169.3 ± 85.9 | 142.9 ± 94.4 | n.s. |
| Lp(a), mg/dl | 34.9 ± 36.0 | 34.0 ± 20.2 | 35.1 ± 38.5 | n.s. |
| Post-treatment LDL-C, mg/dl | 140.7 ± 46.4 | 109.2 ± 28.5 | 148.2 ± 46.8 | < 0.05 |
| Percent reduction of LDL-C, % | 52.3 ± 14.6 | 61.5 ± 16.3 | 50.1 ± 13.3 | < 0.05 |
| Statin use | 107 (94.7%) | 18 (94.7%) | 89 (94.7%) | n.s. |
| Ezetimibe use | 51 (45.1%) | 13 (68.4%) | 38 (40.4%) | n.s. |
| Cholestimide use | 19 (16.8%) | 5 (26.3%) | 14 (14.9%) | n.s. |
| ARC positive | 67 (59.3%) | 16 (84.2%) | 51 (54.3%) | < 0.05 |
| ARC score log | 2.9 ± 2.7 | 5.0 ± 2.4 | 2.4 ± 2.6 | < 0.001 |
MACE: major adverse cardiac events, ARC: aortic root calcification
Table 2. Factors associated with major adverse cardiac events.
| patient (s) | |
|---|---|
| Cardiac death | 0 |
| ST elevated myocardial infarction | 0 |
| Non-ST elevated myocardial infarction/unstable angina pectoris | 4 |
| Planned percutaneous coronary intervention/coronary artery bypass grafting | 11 |
| Congestive heart failure | 3 |
| Stroke | 1 |
Supplementary Table 1. Characteristics divided by ARCS positive or negative.
| All Subjects | ARCS positive | ARCS negative | p value | |
|---|---|---|---|---|
| (n = 113) | (n = 67) | (n = 46) | ||
| Male | 54 (47.8%) | 33 (49.3%) | 21 (45.7%) | n.s. |
| Age, yrs | 52.1 ± 15.6 | 57.3 ± 13.1 | 44.5 ± 15.8 | < 0.001 |
| Body mass index, kg/m2 | 23.6 ± 3.2 | 24.2 ± 3.5 | 22.7 ± 2.5 | < 0.05 |
| Smoking (Current/former) | 32 (30.5%) | 23 (34.3%) | 9 (19.6%) | n.s. |
| Hypertension | 49 (43.4%) | 35 (52.2%) | 14 (30.4%) | < 0.05 |
| Diabetes mellitus | 26 (23.2%) | 20 (29.9%) | 6 (13.0%) | < 0.05 |
| HbA1c, % | 5.9 ± 1.0 | 6.1 ± 1.2 | 5.7 ± 0.7 | < 0.05 |
| Defined mutation | 78 (69.0%) | 51 (76.1%) | 27 (58.7%) | < 0.05 |
| Lipids | ||||
| Total cholesterol, mg/dl | 385.2 ± 99.1 | 405.2 ± 112.9 | 358.4 ± 66.3 | < 0.05 |
| LDL-C, mg/dl | 299.0 ± 94.6 | 320.6 ± 105.2 | 270.9 ± 68.8 | < 0.05 |
| HDL-C, mg/dl | 54.2 ± 13.6 | 51.6 ± 14.8 | 57.4 ± 11.4 | < 0.05 |
| TG, mg/dl | 147.5 ± 93.0 | 144.4 ± 95.8 | 150.4 ± 90.1 | n.s. |
| Lp(a), mg/dl | 34.9 ± 36.0 | 36.9 ± 30.0 | 32.5 ± 42.6 | n.s. |
| Post-treatment levels of LDL-C, mg/dl | 140.7 ± 46.4 | 136.0 ± 42.9 | 149.1 ± 51.9 | n.s. |
| Mean percent reduction of LDL-C, % | 52.3 ± 14.6 | 55.6 ± 13.8 | 46.4 ± 14.1 | < 0.05 |
| Statin use | 107 (94.7%) | 66 (98.5%) | 41 (89.1%) | n.s. |
| Ezetimibe use | 51 (45.1%) | 39 (58.2%) | 12 (26.1%) | n.s. |
| Cholestimide use | 19 (16.8%) | 14 (20.1%) | 5 (10.9%) | n.s. |
ARCS: aortic root calcification score
Supplementary Table 2. Characteristics divided by ARCS high or low.
| All Subjects | ARCS ≧ 4.74 | ARCS < 4.74 | p value | |
|---|---|---|---|---|
| (n = 113) | (n = 41) | (n = 72) | ||
| Male | 54 (47.8%) | 18 (43.93%) | 36 (50.0%) | n.s. |
| Age, yrs | 52.1 ± 15.6 | 60.5 ± 11.8 | 47.3 ± 15.5 | < 0.001 |
| Body mass index, kg/m2 | 23.6 ± 3.2 | 24.1 ± 3.3 | 23.3 ± 3.2 | n.s. |
| Smoking (Current/former) | 32 (30.5%) | 13 (31.7%) | 19 (26.4%) | n.s. |
| Hypertension | 49 (43.4%) | 23 (56.1%) | 26 (36.1%) | < 0.05 |
| Diabetes mellitus | 26 (23.2%) | 13 (31.7%) | 13 (18.3%) | n.s. |
| HbA1c, % | 5.9 ± 1.0 | 6.1 ± 1.4 | 5.8 ± 0.8 | n.s. |
| Defined mutation | 78 (69.0%) | 32 (78.0%) | 46 (63.9%) | n.s. |
| Lipids | ||||
| Total cholesterol, mg/dl | 385.2 ± 99.1 | 403.4 ± 113.3 | 374.5 ± 87.4 | n.s. |
| LDL-C, mg/dl | 299.0 ± 94.6 | 323.9 ± 118.1 | 284.3 ± 74.0 | n.s. |
| HDL-C, mg/dl | 54.2 ± 13.6 | 50.7 ± 16.6 | 56.2 ± 11.4 | n.s. |
| TG, mg/dl | 147.5 ± 93.0 | 143.3 ± 80.4 | 149.3 ± 100.1 | n.s. |
| Lp(a), mg/dl | 34.9 ± 36.0 | 41.4 ± 33.2 | 31.2 ± 37.3 | n.s. |
| Post-treatment levels of LDL-C, mg/dl | 140.7 ± 46.4 | 135.7 ± 45.7 | 143.9 ± 47.0 | n.s. |
| Mean percent reduction of LDL-C, % | 52.3 ± 14.6 | 55.3 ± 15.3 | 50.2 ± 13.8 | n.s. |
| Statin use | 107 (94.7%) | 40 (97.6%) | 67 (93.1%) | n.s. |
| Ezetimibe use | 51 (45.1%) | 23 (56.1%) | 28 (38.9%) | < 0.05 |
| Cholebine use | 19 (16.8%) | 10 (24.4%) | 9 (12.5%) | n.s. |
ARCS: aortic root calcification score
ARCS = 4.74 was determined by ROC analysis predicting MACE
Univariable logistic regression analysis showed that age, hypertension, diabetes mellitus, post-treatment LDL-C, and ARC score were significantly associated with MACE (Table 3). In the multivariable logistic regression analysis, the ARC score remained significantly associated with MACE.
Table 3. Major adverse cardiac events during follow-up period.
| univariable |
multivariable |
|||||
|---|---|---|---|---|---|---|
| odds ratio | 95% CI | p value | odds ratio | 95% CI | p value | |
| Male | 1.714 | 0.633–4.964 | 0.292 | |||
| Age | 1.053 | 1.016–1.096 | 0.004 | 1.018 | 0.958–1.082 | 0.557 |
| BMI | 1.054 | 0.902–1.227 | 0.502 | |||
| Smoking | 1.879 | 0.656–5.225 | 0.234 | |||
| Hypertension | 3.491 | 1.261–10.702 | 0.016 | 1.325 | 0.288–6.433 | 0.717 |
| Diabetes mellitus | 4.024 | 1.405–11.559 | 0.010 | 1.727 | 0.450–6.369 | 0.418 |
| Total cholesterol | 1.001 | 0.995–1.006 | 0.836 | |||
| LDL-C | 1.001 | 0.997–1.008 | 0.389 | |||
| Post-treatment LDL-C | 0.965 | 0.942–0.984 | < 0.001 | 0.969 | 0.943–0.990 | 0.003 |
| ARC score | 1.500 | 1.210–1.944 | < 0.001 | 1.408 | 1.110–1.869 | 0.029 |
ARC: aortic root calcification
We investigated whether the addition of the ARC score increased the accuracy of risk discrimination beyond established traditional risk factors, including age, sex, BMI, hypertension, diabetes, smoking, and post-treatment LDL-C. The C-statistic increased by adding the ARC score to the traditional risk factors from 0.811 to 0.852, although it did not reach statistical significance (Fig. 3, p = n.s.). Kaplan-Meier curves revealed that patients with any ARC had a significantly higher event rate than those without ARC (Fig. 4).
Fig. 3.
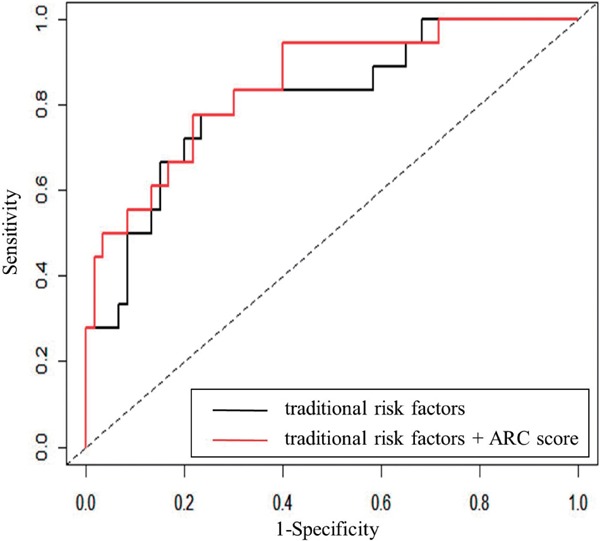
Receiver-operating characteristic (ROC) curves of established risk factors and ARC score in predicting MACE.
The black line indicates traditional risk factors, including age, sex, BMI, hypertension, diabetes, post-treatment levels of LDL-C, and smoking.
The red line indicates traditional risk factors and ARC score.
Fig. 4.
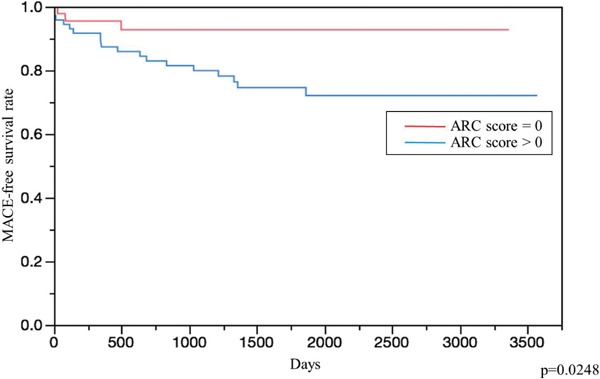
Kaplan-Meier event curves for MACE.
The red line indicates patients whose ARC score = 0.
The blue line indicates patients whose ARC score > 0.
Finally, we evaluated the correlation coefficient between age and ARC score for each sex (Fig. 5), because a previous study demonstrated that the onset of coronary disease was significantly earlier in males with FH than in females6). The regression equations were Y = 0.09X − 1.59 (R2 = 0.34, p < 0.001) in males and Y = 0.08X − 1.60 (R2 = 0.13, p < 0.05) in females with heterozygous FH. These results suggest that ARC may start to develop, on average, at 17.4 and 19.7 years of age in male and female patients with heterozygous FH.
Fig. 5.
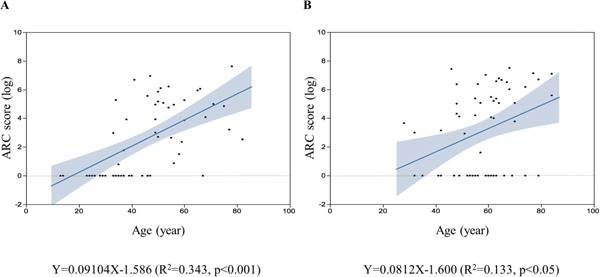
Plots of the correlation between age (X) and aortic root calcification score (Y) in male (a) and female (b) patients with FH. The regression equations are Y = 0.09X − 1.59 (R2 = 0.34, p < 0.05) in males and Y = 0.08X − 1.60 (R2 = 0.13, p < 0.05) in females with heterozygote FH. The solid lines indicate the regression lines. The dotted lines indicate the 95% confidence interval.
To quantify the ARC, as well as the coronary calcium score, CCTA without contrast was performed. Calcification was attributed to the aortic root if it was clearly part of the sinuses of the valsalva, valve cusps, aortic annulus, and the sinotubular junction. Calcifications above the sinotubular junction and calcifications of coronary arteries other than the ostia were removed by manual segmentation.
Discussion
In this study, we evaluated ARC assessed with CCTA among patients with FH and found that the extent of ARC was associated with future coronary or cardiovascular events beyond established risk factors, and that we can estimate the onset and progression of ARC in patients with FH, assuming a linear model of progression.
The patients with FH developed premature coronary atherosclerosis due to extremely high LDL-C levels, thus their risk of future coronary events needs to be assessed, since their lifetime risk is still diverse14). In the current study, ARC assessed with CCTA successfully estimated future MACE in this high-risk population. There are several reports investigating the significance of aortic calcification in patients with FH15–17); however, few studies exist concerning the association between aortic calcification and cardiovascular events in patients with FH. Our study adds evidence that the extent of ARC is significantly associated with cardiovascular events in patients with FH. We speculate the cause of the strong association between ARC and MACE is the fact that ARC can reflect different risk factors, including unknown ones.
In our previous report, we found that the coronary plaque burden might start to develop at 23 and 34 years of age in male and female patients with heterozygous FH, respectively6). In this study, we could estimate the onset and progression of ARC in patients with FH assuming a linear model of plaque progression. The regression lines from age and ARC suggested that ARC might start to develop in the teenage years in both genders, even under statin therapy. In addition, we showed that ARC might start to develop much earlier than coronary plaques in this high-risk population. ARC can be assessed less invasively without using any contrast agents, compared to coronary plaque burden. Accordingly, we suggest the assessment of ARC prior to coronary plaque burden at this younger age.
The pathologic mechanism of aortic calcification is not understood completely in heterozygous FH. In addition, mechanistic insight into the development of aortic calcification, which is earlier than that of coronary plaque formation is still unclear in this study. Aortic calcifications are thought to be due to a complex interplay between inflammation, vascular injury, and osteogenesis18). On the other hand, there are some reports that statins might lead to an increase in coronary calcium score and contribute to vascular calcifications, although this remains controversial19–21). In this study, multivariate analysis suggested that the post-treatment levels of LDL-C were inversely correlated with MACE. Accordingly, lowering LDL-C aggressively could be an effective way to manage such highrisk patients. Further studies, investigating the effects of lipid-lowering agents on the development of calcification as well as plaque formation in patients with/without FH will give us insights into those points.
This study has several limitations. First, this study was conducted retrospectively from a single center using a relatively small sample size. Second, selection bias exists regarding the indication of CCTA, leading to an attenuation of the estimated age of the development of atherosclerosis. Third, our assumption concerning the development of ARC in FH is based on a linear model that may not be applicable to younger patients with FH. The significance of ARC assessed with CCTA may be high, although future prospective, multi-center studies are necessary to confirm the present results.
Acknowledgements
We express our special thanks to Kazuko Honda and Sachio Yamamoto (staff of Kanazawa University) for their outstanding technical assistance.
Funding Sources
This work has been partially supported by a scientific research grant from the Ministry of Education, Science, and Culture of Japan (No. 16K19394).
Conflicts of Interest
Hayato Tada has received research grants from Takeda Science Foundation, Mochida Memorial Foundation, Japan Research Promotion Society for Cardiovascular Diseases, Sanofi K.K, and Astellas Foundation for Research on Metabolic Disorders. Kenshi Hayashi has received research grants from Takeda Science Foundation and Mitsubishi Tanabe Pharma. Atsushi Nohara and Hiroshi Mabuchi have received research grants from MSD K.K., Sanofi K.K., Shionogi & Co., Ltd., Kowa Co., Ltd., Astellas Pharma Inc., AstraZeneca K.K., Keiai-Kai Medical Corp., and Biopharm of Japan Co. Masakazu Yamagishi has received lecture fees from Astellas Pharma Inc., Daiichi-Sankyo Co., Ltd., Shionogi & Co., Ltd., and Kowa Co., Ltd. Masaaki Kawashiri has received lecture fees from Amgen Astellas Biopharma K.K., Astellas Pharma Inc., and Sanofi K.K.
References
- 1). Goldstein JL, Hobbs HH, Brown MS: Familial hypercholesterolemia. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The metabolic and molecular bases of inherited disease, ed 8, vol 2 New York: McGraw-Hill; 2001: 2863-2913 [Google Scholar]
- 2). Mabuchi H: Half a century tales of familial hypercholesterolemia (FH) in Japan. J Atheroscler Thromb 2017; 24: 189-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, Paul N, Clouse ME, Shapiro EP, Hoe J, Lardo AC, Bush DE, de Roos A, Cox C, Brinker J, Lima JA: Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med 2008; 359: 2324-2336 [DOI] [PubMed] [Google Scholar]
- 4). Meijboom WB1, Meijs MF, Schuijf JD, Cramer MJ, Mollet NR, van Mieghem CA, Nieman K, van Werkhoven JM, Pundziute G, Weustink AC, de Vos AM, Pugliese F, Rensing B, Jukema JW, Bax JJ, Prokop M, Doevendans PA, Hunink MG, Krestin GP, de Feyter PJ: Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol 2008; 52: 2135-2144 [DOI] [PubMed] [Google Scholar]
- 5). Zeb I, Abbas N, Nasir K, Budoff MJ: Coronary computed tomography as a cost-effective test strategy for coronary artery disease assessment - A systematic review. Atherosclerosis 2014; 234: 426-435 [DOI] [PubMed] [Google Scholar]
- 6). Tada H, Kawashiri MA, Okada H, Teramoto R, Konno T, Yoshimuta T, Sakata K, Nohara A, Inazu A, Kobayashi J, Mabuchi H, Yamagishi M, Hayashi K: Assessment of coronary atherosclerosis in patients with familial hypercholesterolemia by coronary computed tomography angiography. Am J Cardiol 2015; 115: 724-729 [DOI] [PubMed] [Google Scholar]
- 7). ten Kate GJ, Bos S, Dedic A, Neefjes LA, Kurata A, Langendonk JG, Liem A, Moelker A, Krestin GP, de Feyter PJ, Roeters van Lennep JE, Nieman K, Sijbrands EJ: Increased aortic valve calcification in familial hypercholesterolemia: Prevalence, extent, and associated risk factors. J Am Coll Cardiol 2015; 66: 2687-2695 [DOI] [PubMed] [Google Scholar]
- 8). Alqahtani AM, Boczar KE, Kansal V, Chan K, Dwivedi G, Chow BJ: Quantifying aortic valve calcification using coronary computed tomography angiography. J Cardiovasc Comput Tomogr 2017; 11: 99-104 [DOI] [PubMed] [Google Scholar]
- 9). Pradelli D, Faden G, Mureddu G, Rossi A, Cioffi G, Gaibazzi N, Soranna D, Corrao G, Faggiano P: Impact of aortic or mitral valve sclerosis and calcification on cardiovascular events and mortality: A meta-analysis. Int J Cardiol 2013; 170: e51-e55 [DOI] [PubMed] [Google Scholar]
- 10). Galaska R, Kulawiak-Galaska D, Wegrzyn A, Wasag B, Chmara M, Borowiec J, Studniarek M, Fijalkowski M, Rynkiewicz A, Gruchala M: Assessment of subclinical atherosclerosis using computed tomography calcium scores in patients with familial and nonfamilial hypercholesterolemia. J Atheroscler Thromb 2016; 23: 588-595 [DOI] [PubMed] [Google Scholar]
- 11). Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, Egusa G, Hiro T, Hirobe K, Iida M, Kihara S, Kinoshita M, Maruyama C, Ohta T, Okamura T, Yamashita S, Yokode M, Yokote K, Harada-Shiba M, Arai H, Bujo H, Nohara A, Ohta T, Oikawa S, Okada T, Wakatsuki A: Familial hypercholesterolemia. J Atheroscler Thromb 2014; 21: 6-10 [DOI] [PubMed] [Google Scholar]
- 12). Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus. Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, Araki E, Ito C, Inagaki N, Iwamoto Y, Kasuga M, Hanafusa T, Haneda M, Ueki K: Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig 2010; 1: 212-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Charitos EI, Sievers HH. Anatomy of the aortic root: implications for valve-sparing surgery. Ann Cardiothorac Surg. 2013; 2: 53-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Tada H, Kawashiri MA, Nohara A, Inazu A, Mabuchi H, Yamagishi M: Impact of clinical signs and genetic diagnosis of familial hypercholesterolaemia on the prevalence of coronary artery disease in patients with severe hypercholesterolaemia. Eur Heart J 2017; 38: 1573-1579 [DOI] [PubMed] [Google Scholar]
- 15). Summers RM, Andrasko-Bourgeois J, Feuerstein IM, Hill SC, Jones EC, Busse MK, Wise B, Bove KE, Rishforth BA, Tucker E, Spray TL, Hoeg JM: Evaluation of the aortic root by MRI: insights from patients with homozygous familial hypercholesterolemia. Circulation 1998; 98: 509-518 [DOI] [PubMed] [Google Scholar]
- 16). Alrasadi K, Alwaili K, Awan Z, Valenti D, Couture P, Genest J: Aortic calcifications in familial hypercholesterolemia: Potential role of the low-density lipoprotein receptor gene. Am Heart J 2009; 157: 170-176 [DOI] [PubMed] [Google Scholar]
- 17). Al Kindi M, Bélanger AM, Sayegh K, Senouci S, Aljenedil S, Sivakumaran L, Ruel I, Al Rasadi K, Al Waili K, Awan Z, Valenti D, Genest J: Aortic calcification progression in heterozygote familial hypercholesterolemia. Can J Cardiol 2017; 33: 658-665 [DOI] [PubMed] [Google Scholar]
- 18). Shao JS, Cheng SL, Sadhu J, Towler DA. Inflammation and the osteogenic regulation of vascular calcification: a review and perspective. Hypertension. 2010; 55: 579-592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Puri R, Nicholls SJ, Shao M, Kataoka Y, Uno K, Kapadia SR, Tuzcu EM, Nissen SE. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015; 65: 1273-1282 [DOI] [PubMed] [Google Scholar]
- 20). Shin S, Park HB, Chang HJ, Arsanjani R, Min JK, Kim YJ, Lee BK, Choi JH, Hong GR, Chung N. Impact of Intensive LDL Cholesterol Lowering on Coronary Artery Atherosclerosis Progression: A Serial CT Angiography Study. JACC Cardiovasc Imaging. 2017; 10: 437-446 [DOI] [PubMed] [Google Scholar]
- 21). Achenbach S, Ropers D, Pohle K, Leber A, Thilo C, Knez A, Menendez T, Maeffert R, Kusus M, Regenfus M, Bickel A, Haberl R, Steinbeck G, Moshage W, Daniel WG. Influence of lipid-lowering therapy on the progression of coronary artery calcification: a prospective evaluation. Circulation. 2002; 106: 1077-1082 [DOI] [PubMed] [Google Scholar]


