Abstract
Several recent studies have demonstrated the association of cat-related injuries with major depression and with depressiveness in the general population. It was suggested that cat-scratch disease, the infection with the bacterium Bartonella henselae, can be responsible for the observed association. However, no direct evidence for the role of the Bartonella infection in this association has been published until now. In this preregistered case-controls study performed on 250 healthy subjects tested earlier for the presence of anti-Toxoplasma IgG antibodies, we searched for the positive association between presence of anamnestic anti-Bartonella IgG antibodies and depressiveness measured with Beck II inventory, depression subscale of neuroticism measured with N-70 questionnaire, and self-reported health problems. We found that that Bartonella seropositivity was positively correlated with Beck depression only in Toxoplasma-seronegative men and negatively correlated with health in Toxoplasma-seronegative women. Bartonella seropositivity expressed protective effects against Toxoplasma seropositivity-associated increased neuroticism in men while Toxoplasma-seropositivity expressed protective effects against Bartonella seropositivity-associated health problems in women. A comparison of the patterns of association of mental and physical health problems with Bartonella seropositivity and with reported cat-related injury suggests that different factor, possibly infection with different pathogen transmitted by cat related-injuries than the B. henselae, is responsible for the observed association of cat related-injuries with depressiveness and major depression. The existence of complex interactions between Bartonella seropositivity, Toxoplasma seropositivity, and sex also suggest that the effect of symbionts on the host's phenotype must by always studied in the context of other infections, and separately for men and women.
Keywords: cat-scratch disease, animal-related injuries, depressiveness, major depression, Bartonella, Toxoplasma, bartonellosis, toxoplasmosis
Introduction
Recently, three independent studies have shown that cat-related injuries (1), and namely scratching by a cat (2, 3), is statistically associated with suffering from major depression and also with increased depressiveness as measured by the Beck Depression Inventory in non-clinical populations. Based on these observations we suggested that the cat-scratch disease, the infection with gram-negative bacteria of the genus Bartonella, can be responsible for this association. These bacteria are present in blood and other bodily fluids of about 50% of cats in many parts of world (4). The infection causes relatively common cat-scratch disease as well as several other less frequent but more serious diseases such as infectious endocarditis, bacillary angiomatosis, and Oroya fever (5, 6). Bartonella is transmitted from cats to humans by flea bites and by cat scratches, mostly by contamination of scars by bacteria-containing fleas' feces. It can probably also be transmitted by cat bites, and possibly also by ticks. Cats are most often infected with Bartonella henselae; however, several other species of the genus Bartonella are present also in cats and other hosts (7). The incidence of recognized (or reported) disorder in the USA was only 9.3 per 100,000 inhabitants (8), however, the seroprevalence of bartonellosis in human population is 5–30%. Infected subjects usually develop skin lesions and unilateral lymphadenitis in the lymph-draining region of the site of injury. Patients can suffer from low-grade fever, aching, malaise, headache, or splenomegaly, distortion of vision, anorexia, abdominal pain, severe liver, and spleen tissue abnormalities, sore throat, and conjunctivitis for about 3 to 10 days after the infection. Swollen lymph nodes are typical and take weeks to months to subside. Typically, the disease is self-limiting and patients do not require any treatment. Sometimes, however, the lymphadenopathy persists several months, and more serious sequels, including neuroretinitis, encephalopathy, or osteomyelitis, can occur. Often, neurological symptoms of the infection develop, including severe headache, acute confusion, seizures, ataxia, tremors, and focal neurological deficits (9–11). Some patients also express fatigues, memory loss, and depression (10). No effective method of treatment of cat-scratch disease is currently available (5).
In the present preregistered case-control study, we searched for direct evidences for the association between of Bartonella seropositivity and mental health, namely depressiveness and neuroticism, and physical health in the nonclinical population of 250 subjects. These subjects, mostly former university students of biology, already participated in various studies on the effects of latent infection with protozoan parasite Toxoplasma on human health, behavior, and personality. It was shown recently that the Toxoplasma infection not only influences the physical and mental health of the infected subjects but also modifies the response of the host organism on the effect of other pathogen, namely spirochete Borrelia burgdorferi. The large-scale internet study showed that the increased depressiveness was observed only in Toxoplasma co-infected, Borrelia-infected subjects (12). To enable the detection of a similar interaction between Bartonella and Toxoplasma and to take advantage of the fact that all subjects had been tested for the presence of anti-Toxoplasma antibodies, we included approximately the same number of Toxoplasma seropositive and seronegative subjects into our sample and included the factor Toxoplasma-seropositivity into all statistical models.
Materials and methods
This is a preregistered case-control study Flegr J. (2017, August 30) “Effects of bartonellosis on depression and health,” osf.io/agvbj. The experimental setup, four hypotheses to be tested and statistical tests for testing these particular hypotheses, as well as the most important follow up tests had been registered before the sera were examined for the presence of anti-Bartonella IgG antibodies.
Participants
Data were collected during several projects studying the effects of latent toxoplasmosis on human behavior, personality and health that were performed at the Faculty of Science, Charles University over the past 10 years. During these projects, about 3000 subjects completed various questionnaires in an electronic or paper form and provided a sample of blood for serological testing for Toxoplasma and other pathogens. These samples are kept frozen in −18°C. For the purpose of the current project, we selected a sample of 250 subjects (we tried to find the same number of Toxoplasma seropositive and Toxoplasma-seronegative subjects of similar age) who completed an anamnestic questionnaire containing 20 health-related questions, Beck Depression Inventory (BDI-II—clinical scale measuring depression), and N-70 questionnaire (Czech questionnaire measuring seven subscales of neuroticism, including depression). These sera were examined for the presence of anamnestic titres of anti-Bartonella antibodies. The study was approved by Institutional Review Board of Faculty of Science, Charles University (No: 2016/16).
Questionnaires
In the anamnestic questionnaire (13), the condensed version of the electronic anamnestic questionnaire used in other studies (14, 15), we asked the responders about the existence and intensity of various health problems. They were asked to subjectively rate the intensity of their allergic, dermatologic, cardiovascular, digestive, metabolic including endocrine, orthopedic, neurological, and psychiatric problems using 7-point Likert scales. Using the same scales, they were asked how frequently they were suffering headache, recurrent pain, other recurrent health problems, being tired, being tired after returning from work or after traveling by train, how frequently they have a common viral diseases, how frequently they have to visit medical doctors (except dentist and except for prevention), how many times they used antibiotics during the past 365 days, how many times they used antibiotics during the past three years, how many times they spent more than one week in a hospital within last year, and how many times they spent more than one week in a hospital within last three years. The participants also completed an electronic form of Czech versions of the Beck Depression Inventory (BDI-II) (16) and of N-70 questionnaire (17, 18). BDI-II is a 21-question multiple-choice (scoring from 0 to 3 for each item) self-report inventory. BDI-II is one of the worldwide most used psychometric tests for measuring the severity of depression. Reliability analysis performed on our data showed the Cronbach's alpha (0.920). Higher scores display a higher level of depressive symptomatology. N-70 is a multiple-choice self-report inventory (scoring from 0 to 3 for each item). The instrument assesses several aspects of emotional lability-stability (neuroticism). The N-70 questionnaire is constructed for the assessment of 7 clinical areas—anxiety, depression, phobia, hysteria, hypochondria, vegetative lability (the presence of various psychosomatic symptoms), and psychasthenia. The original purpose of this method was to detect individuals who can be too sensitive for military operations (17), with the expectation that people who are neurotic respond worse to different stressors and are more likely to interpret ordinary situations as threatening. Even though the instrument is old (17), it covers aspects of negative affectivity (i.e., proneness to experiencing negative emotions) and anxiety sensitivity (i.e., the disposition to believe that symptoms of anxiety are harmful) as discussed in DSM-V (19). Vacír (17) expected that soldiers who score low in neuroticism tend to be more emotionally stable, less reactive to stress and better prepared for e.g., psychologically demanding military deployment. The instructions ask how the participants feel during the present time, so the instrument is focused on state rather than traits. The total N-70 score is a sum from all clusters. Reliability analyses performed on our data showed high Cronbach's alpha (0.931) for the total N-70 score, but not for all its subscales (anxiety 0.743, depression 0.826, phobia 0.729, hysteria 0.628, hypochondria 0.651, vegetative lability 0.674, and psychasthenia 0.867). Scores in each subscale can range from 0 to 30. Higher scores signify higher levels of different mental symptoms (often associated with neuroticism) that can be precursors for mental disorders (often labeled as negative affectivity, one of personality traits according to Alternative DSM-V Model for Personality Disorders) (17). During the preparation of the manuscript, in December 2017, we also sent emails to the participants of the study, asking them about sustained animal-related injuries and taking antidepressants and anxiolytics. Two hundred thirteen subjects provided the requested information.
Serological tests
The complement-fixation test (CFT), which determines the overall levels of IgM and IgG antibodies of particular specificity, and Enzyme-Linked Immunosorbent Assays (ELISA) (IgG ELISA: SEVAC, Prague) were used to detect the Toxoplasma infection status of the subjects. ELISA assay cut-point values were established using positive and negative standards according to the manufacturer's instructions. In CFT, the titre of antibodies against Toxoplasma in sera was measured in dilutions between 1:4 and 1:1024. The subjects with CFT titres between 1:8 and 1:128 were considered Toxoplasma-seropositive. Only subjects with clearly positive or negative results of CFT and IgG ELISA tests were diagnosed as Toxoplasma-seropositive or Toxoplasma-seronegative, whilst subjects with different results of these tests, or ambiguous results, were retested or excluded from the study. Frozen samples of sera after 1: 64 dilution were also tested for the presence of anamnestic titres of anti-Bartonella IgG antibodies using Indirect Immunofluorescent Assay (FOCUS Diagnostics, U.S.A.). All tests were performed in the reference laboratories of the National Institute of Public Health, Prague.
Statistics
Statistical testing was performed using the programs Statistica v. 10.0 and SPSS v. 21. Before the analyses, the health index was computed for each participant as the average of Z-scores of 20 health-related variables of the anamnestic questionnaire. Participants with intensive or frequent health problems had high (positive) values, while healthy participants had low (negative) values of this index. A logistic regression was used for searching for effects of the predictors age, sex, size of settlement in which a subject spend its childhood, and Toxoplasma (Bartonella) seropositivity on the probability of being Bartonella (Toxoplasma) seropositive. ANCOVA tests with a BDI-II score (or individual N-70 subscales scores, or health score) as an output variable were used to search for the effects of the predictors Bartonella, age, sex, size of place of living in childhood, Toxoplasma, and the sex-Bartonella, Toxoplasma-infection-Bartonella, and sex-Toxoplasma-Bartonella interactions. The effects of these predictors on six (preregistered) or all seven N-70 variables were studied with MANCOVA. In parallel, the effects of Bartonella and Toxoplasma seropositivities were also measured with nonparametric partial Kendall test that allow controlling for one confounding variable, here age (20). This method was also used for searching for the effects of seropositivities on responses to a particular 21 questions of BDI-II and particular health problems in the exploratory part of the study. Ordinal regressions were used with 20 different health-related variables (rated on the 7-points scales) as the dependent variables and sex, age, size of settlements where the responders spent their childhood, Bartonella seropositivity, Toxoplasma seropositivity, and Bartonella-sex, Bartonella-Toxoplasma, and Bartonella-Toxoplasma-sex interactions as the independent variables. The false discovery rate (preset to 0.20) was controlled with the Benjamini-Hochberg procedure (21).
Terminological notes: we abbreviated “Toxoplasma seropositivity” to “Toxoplasma,” and “Bartonella seropositivity” to “Bartonella” in the description of our statistical models. For example, 3-way interaction Toxoplasma seropositivity-Bartonella seropositivity-sex interaction is described in a more condensed way as Toxoplasma-Bartonella-sex interaction. Also, the statistical relations between (formally) dependent and (formally) independent variables are called “effects” in the Result section of the paper, despite the real causality relation between the these variables can be different or even non-existing (as expanded upon in the Discussion).
Differences between preregistered and realized protocol
We had to substitute the preregistered ANCOVA test for depression subscale from N-70 with non-parametric tests as this variable failed in the Levene's test of equity of errors. In addition to the preregistered tests with two 2-way interactions (sex-Bartonella, Toxoplasma-infection-Bartonella) we always additionally analyzed the full model with all three 2-way interactions (sex-Bartonella, Toxoplasma-infection-Bartonella, and sex-Toxoplasma) to show the robustness of our results and to follow recommendation of many statisticians concerning the necessity to analyze full models. However, all inferences have been done exclusively on the basis of results of the preregistered tests. We always used more conservative two-sided, instead of the preregistered one-sided variant of tests because the observed effects of the Bartonella-seropositivity were in the opposite directions in men and women.
Data availability
All data are available at: https://doi.org/10.6084/m9.figshare.5852511.
Results
Descriptive statistics
The final set contained data from 92 men and 158 women, see Table 1. No significant differences were observed in the mean age of men (26.7, S.D. = 7.00) and women (26.4, S.D. = 8.9) in the whole set (p = 0.75, t(248) = −0.316) or between Bartonella-or Toxoplasma-seropositive and seronegative male or female subjects (all p-values > 0.35). Most subjects (31.6% men and 25.0% women) spent their childhood in Prague, 12.0% men and 15.2% women in settlements with < 1000 inhabitants, 17.4% men and 13.9% women in settlements with 1–5 thousands of inhabitants, 26.1% men and 29.8% women in settlements with 5–50 thousands of inhabitants, 10.9% men and 4.4% women in settlements with 50–100 thousands of inhabitants, 8.7% men and 5.1% women in settlements with more than 100 thousands of inhabitants, except Prague. A logistic regression with age, sex, size of settlement in which a subjects spent their childhood as the covariates showed no association between the Bartonella seropositivity (dependent variable) and the Toxoplasma seropositivity (p = 0.468, O.R. = 1.23, C.I.95 = 0.71–2.13) and no association between the Bartonella seropositivity and the covariates. Analogical analysis with the Toxoplasma seropositivity as the dependent variable showed that the probability of being Toxoplasma-seropositive decreased with the increasing size of the settlement in which a subject spent his or her childhood (p = 0.004, O.R.range = 0.35, C.I.95 = 0.17–0.71); other associations of Toxoplasma seropositivity with covariates were nonsignificant.
Table 1.
Seroprevalence of bartonellosis in Toxoplasma-seronegative and Toxoplasma-seropositive subjects.
| Sex | Toxoplasma | Bartonella-negative | Bartonella-positive(%) |
|---|---|---|---|
| Women | Negative | 59 | 22 (27.16) |
| Women | Positive | 51 | 26 (33.77) |
| Men | Negative | 37 | 17 (31.48) |
| Men | Positive | 25 | 13 (34.21) |
Confirmatory section of the study
Four hypotheses have been preregistered before the start of the present study.
Hypothesis 1
Subjects who have been diagnosed as Bartonella-seropositive express higher levels of depression measured with N-70 inventory than the Bartonella-seronegative subjects when sex, age, toxoplasmosis and size of place of living are controlled.
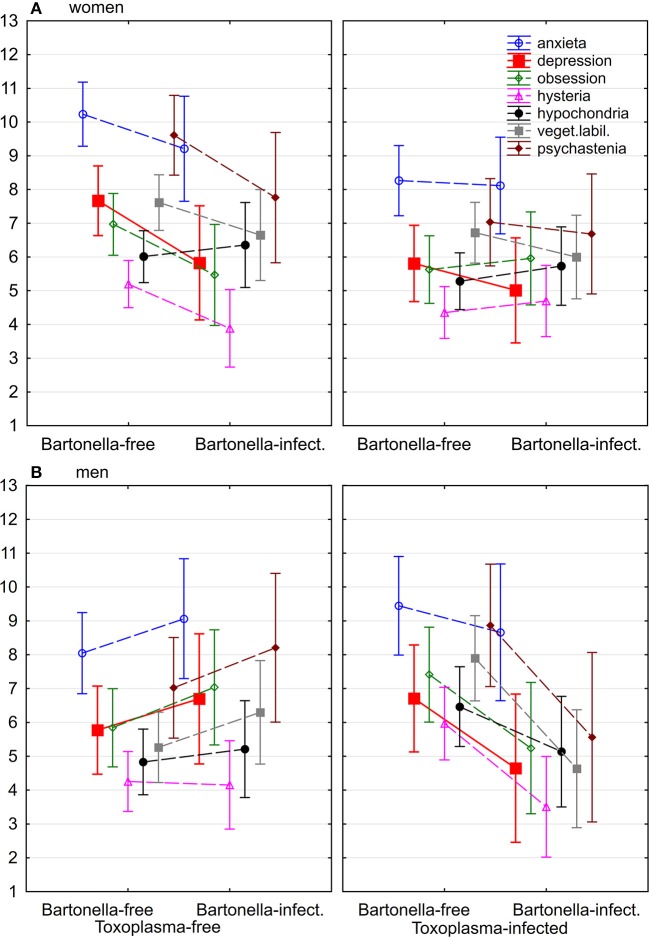
The N-70 inventory was completed by 156 women (30.8% Bartonella-seropositive) and 92 men (32.6% Bartonella-seropositive). The Levene's test of equity of errors showed that a preregistered ANCOVA test cannot be done [F(7,240) = 3.56, p = 0.001]; therefore only the nonparametric partial Kendall correlation tests with age as a covariate were performed. Figure 1 suggests the possible existence of sex-Bartonella-Toxoplasma interaction. The expected higher depression in Bartonella-seropositive subjects was observed only in Toxoplasma-seronegative men. However, even here the association was not significant (p = 0.136, partial Kenadall Tau = 0.139, n = 54). Toxoplasma-seronegative women showed negative nonsignificant association while Toxoplasma-seropositive men showed negative nonsignificant association and Toxoplasma-seropositive women no association between Bartonella and depression. Nonsignificantly lower depression was observed in Toxoplasma-seropositive men (p = 0.063, partial Kenadall Tau = −0.210, n = 38), Toxoplasma-seronegative women (p = 0.072, partial Kenadall Tau = −0.136, n = 81), and Toxoplasma-seropositive women (p = 0.195, partial Kenadall Tau = −0.102, n = 75)–all in comparison with corresponding Bartonella-seronegative subjects.
Figure 1.
Association of Bartonella and Toxoplasma seropositivity with seven neuroticism subscales of N-70 in women (A) and men (B). Markers show the intensity of health problems computed for covariates at their means.
Hypothesis 2
Subjects who have been diagnosed as Bartonella-seropositive express higher levels of depression measured with BDI II questionnaire than Bartonella-seronegative subjects when sex, age, toxoplasmosis, and size of place of living are controlled.
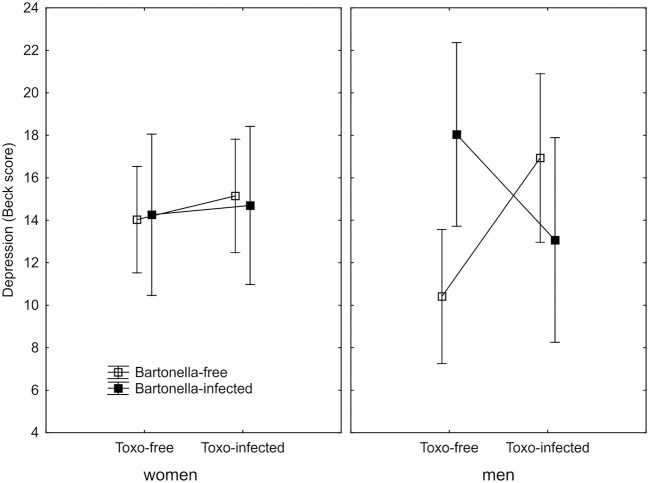
The BDI-II questionnaire was completed by 148 women (30.4% Bartonella-seropositive) and 82 men (34.1% Bartonella-seropositive). An ANCOVA test with a BDI-II score as the output variable and Bartonella, age, sex, size of place of living in childhood, Toxoplasma, and the sex-Bartonella, Toxoplasma-Bartonella and sex-Toxoplasma-Bartonella interactions as the independent variables indicated a significant association of depression with the Toxoplasma-Bartonella interaction (p = 0.034, η2 = 0.020, two sided test and also with sex-Toxoplasma-Bartonella interaction (p = 0.048, η2 = 0.018). Similar results, namely the significant Toxoplasma-Bartonella interaction (p = 0.030, η2 = 0.021) and sex-Toxoplasma-Bartonella interaction (p = 0.023, η2 = 0.023), also provided the full model with three 2-way and one 3-way interactions. Again, the expected positive Bartonella-depression interaction was observed only in Toxoplasma-seronegative men. In all three other subpopulations, the Bartonella-seropositive subjects expressed lower or the same BDI-II depression scores than the Bartonella-seronegative subjects, Figure 2. Nonparametric partial Kendall correlation tests (two-sided, age controlled) showed significantly higher depression in Toxoplasma-seronegative men (p = 0.018, partial Kendall Tau = 0.232, n = 50), non-significantly lower depression in Toxoplasma-seropositive men (p = 0.064, partial Kenadall Tau = −0.230, n = 32), and non-significantly higher depression in Toxoplasma-seronegative (p = 0.986, partial Kenadall Tau = 0.001, n = 77), and Toxoplasma-seropositive women (p = 0.650, partial Kenadall Tau = 0.037, n = 71)–all in comparison with corresponding Bartonella-seronegative subjects.
Figure 2.
Association of Bartonella and Toxoplasma seropositivity with BDI-II depression score. Squares show depression computed for covariates at their means and spreads 95% confidence intervals.
Hypothesis 3
Subjects who have been diagnosed as Bartonella-seropositive report worse health status than Bartonella-seronegative subjects when sex, age, toxoplasmosis, and size of place of living is controlled.
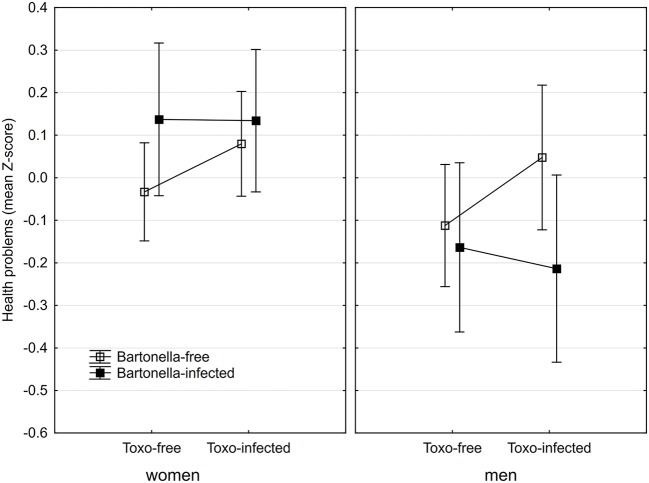
The anamnestic questionnaire was completed by 158 women (30.4% Bartonella-seropositive) and 92 men (32.6% Bartonella-seropositive). An ANCOVA test with the health score as the output variable and Bartonella, age, sex, size of childhood home, Toxoplasma, and the sex-Bartonella, Toxoplasma-Bartonella and sex-Toxoplasma-Bartonella interactions as the independent variables indicated significantly worse health scores in women than in men (p = 0.004, η2 = 0.034) and also a significant association between health score and sex-Bartonella interaction (p = 0.041, η2 = 0.017). Similar results, namely the effects of sex (p = 0.005, η2 = 0.032), and the sex-Bartonella interaction (p = 0.039, η2 = 0.018) also provided the full model with three 2-way and one 3-way interactions. The Figure 3 shows that Bartonella seropositivity had a negative association with health in women and positive association with health in men. Nonparametric partial Kendall correlation tests (age controlled) showed non-significantly better health status of the Bartonella-seropositive subjects in Toxoplasma-seronegative men (p = 0.646, partial Kenadall Tau = −0.043, n = 54), and in Toxoplasma-seropositive positive men (p = 0.080, partial Kenadall Tau = −0.198, n = 38) and also non-significantly worse health status in Toxoplasma-seronegative women (p = 0.084, partial Kenadall Tau = 0.131, n = 81), and in Toxoplasma-seropositive women (p = 0.428, partial Kenadall Tau = 0.062, n = 77)–all in comparison with corresponding Bartonella-seronegative subjects.
Figure 3.
Health status of Bartonella- and Toxoplasma-seropositive subjects. Squares show the intensity of health problems computed for covariates at their means and spreads 95% confidence intervals.
Hypothesis 4
The effects of Bartonella seropositivity on depression and health are stronger in Toxoplasma-seropositive subjects.
The Figures 1–3 show that there is no simple trend in the modulating effects of Toxoplasma seropositivity on the effects of Bartonella seropositivity. Moreover, we did not explicitly state, but we implicitly expected that the associations of Bartonella seropositivity with depression would be positive and with health would be negative. However, the associations of the Bartonella seropositivity with depression in Toxoplasma-seropositive men was negative—Bartonella-seropositive Toxoplasma-seropositive men had better mental health than Bartonella-seronegative Toxoplasma-seropositive men (Figures 1, 2) and a similar paradoxical effect of the Bartonella seropositivity in the Toxoplasma-seropositive men was also observed on physical health (Figure 3).
Preregistered follow-up analyses
A MANCOVA analysis performed with six N-70 facets (except depression) as well as all seven N-70 subscales provided qualitatively the same result; therefore, only the result of the preregistered MANCOVA test with six subscales will be reported. The independent variables were sex, age, size of settlements where the responders spent their childhood, the Bartonella seropositivity, the Toxoplasma seropositivity, and the Bartonella-sex, Bartonella-Toxoplasma, and Bartonella-Toxoplasma-sex interactions. The only significant association observed was that of the Bartonella-Toxoplasma-sex interaction (p = 0.042, η2 = 0.045, Hotelling's Trace method). The results of univariate ANCOVA analyses showed that this interaction was significant for the anxiety (p = 0.018, η2 = 0.033), obsession (p = 0.017, η2 = 0.034), hysteria (p = 0.008, η2 = 0.034), hypochondria (p = 0.048, η2 = 0.025), vegetative lability (p = 0.003, η2 = 0.049), and psychasthenia (p = 0.010, η2 = 0.038); for the direction of these associations see Figure 1. Full models with all three 2-way and one 3-way interactions only showed a trend for the Bartonella-Toxoplasma-sex interaction (MANCOVA: p = 0.070, η2 = 0.048) and the effects of this interactions on obsession (p = 0.011, η2 = 0.027), hysteria (p = 0.011, η2 = 0.027), vegetative lability (p = 0.014, η2 = 0.025), and psychasthenia (p = 0.023, η2 = 0.021). The results of nonparametric tests partial Kendall correlation tests performed separately for Toxoplasma-seropositive and Toxoplasma-seronegative male and female subjects are shown in Table 2.
Table 2.
Correlation between Bartonella seropositivity and seven subscales of neuroticism in Toxoplasma-seronegative and Toxoplasma-seropositive subjects.
| N-70 subscale | Toxoplasma-minus women N = 81 | Toxoplasma-plus women N = 75 | Toxoplasma-minus men N = 54 | Toxoplasma-plus men N = 38 | ||||
|---|---|---|---|---|---|---|---|---|
| Tau | p | Tau | p | Tau | p | Tau | p | |
| Anxiety | −0.083 | 0.273 | −0.015 | 0.852 | 0.137 | 0.143 | −0.079 | 0.483 |
| Depression | –0.136 | 0.072 | −0.102 | 0.195 | 0.139 | 0.136 | –0.210 | 0.063 |
| Obsession | –0.114 | 0.131 | 0.076 | 0.332 | 0.139 | 0.137 | –0.210 | 0.064 |
| Hysteria | –0.207 | 0.006 | 0.060 | 0.449 | −0.010 | 0.918 | –0.374 | 0.001 |
| Hypochondria | 0.081 | 0.286 | 0.015 | 0.854 | 0.101 | 0.282 | –0.184 | 0.105 |
| Vegetative lability | –0.129 | 0.089 | −0.107 | 0.175 | 0.191 | 0.042 | –0.420 | < 0.001 |
| Psychasthenia | –0.161 | 0.033 | −0.028 | 0.723 | 0.104 | 0.265 | –0.267 | 0.018 |
Tau and p-values of partial Kendall Tau correlations controlled for age. The results significant after the Benjamini-Hochberg procedure (false discovery rate = 0.2) are printed in bold.
To show which kinds of health problems were responsible for the association of bartonellosis with the health of our responders, we performed 20 ordinal regressions for 20 different health-related variables (rated on the 7-points scales) as the dependent variables and sex, age, size of settlements where the responders spent their childhood, Bartonella seropositivity, Toxoplasma seropositivity, and Bartonella-sex, Bartonella-Toxoplasma, and Bartonella-Toxoplasma-sex interactions as the independent variables. The result showed no effect of the Bartonella-Toxoplasma-sex interaction. At the same time it showed a significant effect of the Bartonella-Toxoplasma interaction on heart disorders (higher problems in the Bartonella-seropositive subjects, especially in those who were also Toxoplasma-seropositive, p = 0.038), recurrent pain (higher in Bartonella-seropositive and Toxoplasma-seronegative, lower in Bartonella-seropositive, Toxoplasma-seropositive, p = 0.026), and frequency of being tired (higher in Bartonella-seropositive and Toxoplasma-seronegative, lower in Bartonella-seropositive, Toxoplasma-seropositive, p = 0.049). The sex-Bartonella- interaction was significant for cardiovascular disorders (higher problems in the Bartonella-seropositive subjects, especially in women, p = 0.009), frequency of being tired (lower in Bartonella-seropositive men, p = 0.022), and frequency of taking antibiotics within the past three years (higher in Bartonella-seropositive women and lower in Bartonella-seropositive men, p = 0.023). The main effect of the Bartonella seropositivity was observed only for variable psychiatric problems, (more serious or frequent problems reported by the seropositive subjects of any sex, p = 0.032). No significant effect of Toxoplasma seropositivity was observed. The results obtained with nonparametric tests (Table 3) were qualitatively similar.
Table 3.
Correlation between Bartonella seropositivity and health status in Toxoplasma-seronegative and Toxoplasma-seropositive subjects.
| Health problems | Toxoplasma-minus women N = 81 | Toxoplasma-plus women N = 77 | Toxoplasma-minus men N = 54 | Toxoplasma-plus men N = 38 | ||||
|---|---|---|---|---|---|---|---|---|
| Tau | p | Tau | p | Tau | p | Tau | p | |
| Allergic problems | 0.163* | 0.031 | 0.003 | 0.973 | 0.056 | 0.549 | −0.044 | 0.696 |
| Dermatologic problems | 0.255* | 0.001 | 0.121 | 0.119 | –0.254* | 0.007 | –0.291* | 0.010 |
| Cardiovascular problems | 0.298* | 0.000 | 0.075 | 0.335 | −0.123 | 0.189 | –0.241* | 0.033 |
| Digestive organs problems | 0.100 | 0.187 | 0.009 | 0.903 | −0.038 | 0.686 | −0.078 | 0.488 |
| Metabolic problems | −0.008 | 0.919 | 0.039 | 0.619 | −0.127 | 0.175 | −0.220 | 0.051 |
| Orthopedic problems | −0.023 | 0.762 | −0.166 | 0.032 | 0.040 | 0.672 | −0.044 | 0.700 |
| Neurological problems | 0.077 | 0.312 | 0.114 | 0.142 | −0.215 | 0.022 | −0.032 | 0.780 |
| Psychiatric problems | 0.189* | 0.013 | 0.125 | 0.113 | 0.156 | 0.096 | 0.075 | 0.506 |
| Headache | −0.066 | 0.380 | 0.095 | 0.219 | −0.028 | 0.768 | −0.053 | 0.639 |
| Other chronical pain | 0.201* | 0.008 | −0.076 | 0.327 | 0.124 | 0.186 | −0.132 | 0.242 |
| Other chronical problems | 0.151* | 0.048 | 0.028 | 0.722 | 0.009 | 0.925 | −0.048 | 0.674 |
| Tired | 0.180* | 0.018 | 0.028 | 0.720 | −0.023 | 0.803 | –0.265* | 0.019 |
| Tired after work | 0.122 | 0.107 | 0.003 | 0.973 | 0.122 | 0.192 | −0.145 | 0.201 |
| Tired after traveling by train | 0.126 | 0.095 | −0.019 | 0.806 | −0.153 | 0.102 | −0.097 | 0.393 |
| Viral infections | 0.114 | 0.133 | 0.020 | 0.797 | 0.118 | 0.208 | 0.131 | 0.246 |
| Doctors' visits per year | 0.156* | 0.039 | −0.036 | 0.640 | 0.065 | 0.484 | 0.087 | 0.443 |
| Antibiotics in last year | −0.014 | 0.853 | 0.144 | 0.063 | −0.058 | 0.537 | 0.034 | 0.761 |
| Antibiotics in last 3 years | −0.063 | 0.404 | 0.294* | 0.000 | −0.094 | 0.316 | −0.214 | 0.058 |
| Hospitals in last year | −0.071 | 0.348 | −0.160 | 0.039 | n.d. | n.d. | ||
| Hospitals in last 3 years | −0.094 | 0.216 | −0.092 | 0.237 | −0.105 | 0.262 | −0.209 | 0.065 |
The table shows partial Kendall Tau (age controlled) and corresponding p-values. Absence/presence of the specific antibodies was coded as 0/1, therefore, the positive Tau means more serious or more frequent problem in the seropositive subjects. Significant results are printed in bold, those significant after the correction for multiple tests are labeled with asterisks. Correlation with number of hospitalizations in the last year (n.d.) could not be computed for men, due to a low number of hospitalized subjects.
To show which problems are responsible for the observed associations of Bartonella seropositivity and depression, we computed partial Kendall correlations of Bartonella seropositivity with responses of subjects to 21 questions of BDI-II, separately for Toxoplasma-seropositive and Toxoplasma-seronegative male and female subjects, Table 4). Only the negative correlation with problems with sleeping was significant for women after the correction for multiple tests. In contrast, the responses to nearly all items of the scale (except pessimism concerning future and problems with sleeping) correlated positively with the Bartonella seropositivity in Toxoplasma-seronegative men. In Toxoplasma-seropositive men, the responses to five questions, namely 7-self-dislike, 11-agitation, 12–loss of interest, 13–indecisiveness, 14–worthlessness, and 15–loss of energy correlated negatively with Bartonella seropositivity, i.e., the men infected with both Bartonella and Toxoplasma reported lower levels of depression than men infected only with Toxoplasma.
Table 4.
Correlation between Bartonella seropositivity and responses to BDI II questions in Toxoplasma-seronegative and Toxoplasma-seropositive subjects.
| BDI II-R item | Toxoplasma-minus women N = 77 | Toxoplasma-plus women N = 71 | Toxoplasma-minus men N = 50 | Toxoplasma-plus men N = 32 | ||||
|---|---|---|---|---|---|---|---|---|
| Tau | p | Tau | p | Tau | p | Tau | p | |
| Sadness | −0.102 | 0.189 | 0.006 | 0.946 | 0.204* | 0.036 | −0.229 | 0.065 |
| Pessimism | 0.055 | 0.481 | 0.042 | 0.602 | −0.017 | 0.863 | 0.040 | 0.746 |
| Past failure | −0.024 | 0.758 | 0.064 | 0.428 | 0.154* | 0.115 | −0.172 | 0.167 |
| Loss of pleasure | −0.021 | 0.784 | −0.007 | 0.932 | 0.212* | 0.030 | −0.136 | 0.275 |
| Guilty feelings | −0.011 | 0.890 | −0.101 | 0.215 | 0.292* | 0.003 | −0.145 | 0.243 |
| Punishment feelings | 0.007 | 0.924 | −0.060 | 0.456 | 0.280* | 0.004 | −0.158 | 0.203 |
| Self–dislike | −0.029 | 0.707 | 0.048 | 0.554 | 0.181* | 0.064 | −0.249* | 0.045 |
| Self–criticalness | −0.120 | 0.121 | 0.067 | 0.406 | 0.305* | 0.002 | 0.193 | 0.120 |
| Suicidal thoughts or wishes | 0.059 | 0.447 | −0.038 | 0.640 | 0.200* | 0.040 | −0.045 | 0.719 |
| Crying | −0.015 | 0.848 | 0.051 | 0.532 | 0.205* | 0.036 | 0.057 | 0.647 |
| Agitation | 0.039 | 0.613 | −0.155 | 0.055 | 0.284* | 0.004 | −0.291* | 0.019 |
| Loss of interest | −0.158 | 0.042 | 0.086 | 0.287 | 0.252* | 0.010 | −0.264* | 0.034 |
| Indecisiveness | −0.062 | 0.425 | 0.064 | 0.429 | 0.176* | 0.071 | −0.322* | 0.010 |
| Worthlessness | 0.044 | 0.568 | 0.106 | 0.191 | 0.177* | 0.070 | −0.338* | 0.007 |
| Loss of energy | 0.093 | 0.229 | 0.119 | 0.145 | 0.362* | 0.000 | −0.174 | 0.161 |
| Changes in sleeping pattern | 0.030 | 0.697 | −0.221* | 0.006 | 0.000 | 0.997 | −0.114 | 0.357 |
| Irritability | 0.027 | 0.726 | −0.023 | 0.775 | 0.164* | 0.092 | −0.149 | 0.232 |
| Changes in appetite | −0.073 | 0.348 | −0.039 | 0.633 | 0.248* | 0.011 | −0.040 | 0.747 |
| Concentration difficulty | −0.028 | 0.714 | 0.047 | 0.566 | 0.183* | 0.061 | 0.025 | 0.839 |
| Tiredness or fatigue | 0.069 | 0.378 | −0.075 | 0.356 | 0.357* | 0.000 | −0.082 | 0.512 |
| Loss of interest in sex | −0.080 | 0.301 | −0.050 | 0.540 | 0.172* | 0.077 | 0.001 | 0.996 |
Tau and p-values of partial Kendall Tau correlations controlled for age. The results that are significant after a correction for multiple tests are labeled with asterisks.
Nonregistered exploratory analyses
To see whether the deteriorated health status played a role in the effect of Bartonella-Toxoplasma-sex association on depression, we also included health status as another covariate into the ANCOVA test (dependent variable: Beck depression score; independent variables: health score, Bartonella, Toxoplasma, age, sex, size of place of living in childhood, and the sex-Bartonella, Toxoplasma-Bartonella and sex-Toxoplasma-Bartonella interactions). The health score had a strong effect on the BDI-II depression score (p < 0.00001, η2 = 0.108). However, the strength of the association of depression with Bartonella-Toxoplasma-sex remained approximately the same (p = 0.043, η2 = 0.028) as was detected in the model without the health score (p = 0.066, η2 = 0.024). The same analysis for depression measured with N-70 could not be done due to the highly significant result of Levene's test of equality of errors [F(7,240) = 3.00, p = 0.005].
To see whether deteriorated health status played a role in the effect of Bartonella-Toxoplasma-sex association on the neuroticism estimated using N-70 inventory, we also included health status as another covariate into the MANCOVA test and following six ANCOVA tests (independent variables: health score, Bartonella, age, sex, size of place of living in childhood, Toxoplasma, and the sex-Bartonella, Toxoplasma-Bartonella and sex-Toxoplasma-Bartonella interactions). The results showed that health status had a strong effect on total neuroticism (p < 0.002, η2 = 0.086) and on five of six of it's subscales, the anxiety (p = 0.004, η2 = 0.034), obsession (p < 0.0001, η2 = 0.077), hysteria (p = 0.104, η2 = 0.011), hypochondria (p = 0.012, η2 = 0.026), vegetative lability (p = 0.004, η2 = 0.034), and psychasthenia (p = 0.034, η2 = 0.018). Again, the strength of association between neuroticism and Bartonella-Toxoplasma-sex was approximately the same as it was detected in the model without the covariate health—(MANCOVA: p = 0.043, η2 = 0.045, ANCOVAS: anxiety p = 0.023, η2 = 0.031, obsession p = 0.015, η2 = 0.035, hysteria p = 0.009, η2 = 0.039, hypochondria p = 0.060, η2 = 0.023, vegetative lability p = 0.003, η2 = 0.048, and psychasthenia p = 0.011, η2 = 0.037).
Toxoplasmosis is known to have negative effects on the health of infected subjects. To check whether these effects can also be detected in our dataset, we performed nonparametric analyses searching for the association between toxoplasmosis and health-related variables on the whole population (not on Bartonella-seronegative and Bartonella seropositive subpopulations). Analogically, we searched for the associations between bartonellosis and health-related variables on the whole population (not on Toxoplasma-seronegative and Toxoplasma-seropositive subpopulations). Table 5 shows that some effects of the infections can be also recognized by this simplified approach; however, some associations were obscured by the opposite-direction shifts in particular subpopulations.
Table 5.
Effects of Toxoplasma and Bartonella seropositivity on health—one-way analyses.
| Health problems | Toxoplasmosis | Bartonellosis | ||||
|---|---|---|---|---|---|---|
| All | Women | Men | All | Women | Men | |
| N = 250 | N = 158 | N = 92 | N = 250 | N = 158 | N = 92 | |
| Allergic problems | 0.002 | 0.014 | −0.024 | 0.054 | 0.079 | 0.015 |
| Dermatologic problems | 0.035 | −0.027 | 0.150* | 0.026 | 0.185* | –0.250 |
| Cardiovascular problems | 0.034 | –0.109 | 0.266* | 0.052 | 0.193* | –0.165* |
| Digestive organs problems | 0.056 | 0.040 | 0.063 | 0.018 | 0.058 | −0.050 |
| Metabolic problems | 0.068 | −0.008 | 0.202* | −0.059 | 0.015 | –0.167* |
| Orthopedic problems | 0.071 | 0.067 | 0.058 | −0.061 | −0.089 | −0.002 |
| Neurological problems | 0.062 | 0.048 | 0.086 | 0.020 | 0.100* | −0.122* |
| Psychiatric problems | 0.088 | 0.074 | 0.114 | 0.147* | 0.162* | 0.121* |
| Headache | 0.057 | −0.002 | 0.143* | −0.011 | 0.020 | −0.038 |
| Other chronical pain | 0.073 | 0.028 | 0.101 | 0.029 | 0.067 | 0.005 |
| Other chronical problems | 0.024 | −0.061 | 0.165* | 0.041 | 0.084 | −0.016 |
| Tired | 0.025 | −0.004 | 0.051 | 0.005 | 0.098* | −0.132* |
| Tired after work | 0.017 | −0.032 | 0.070 | 0.036 | 0.065 | −0.003 |
| Tired after traveling by train | 0.040 | 0.051 | −0.010 | −0.011 | 0.058 | −0.123* |
| Virosis | 0.011 | −0.071 | 0.146* | 0.079 | 0.060 | 0.130* |
| Doctors' visits per year | 0.119* | 0.057 | 0.199* | 0.067 | 0.065 | 0.078 |
| Antibiotics in last year | 0.023 | 0.076 | −0.083 | 0.040 | 0.075 | −0.021 |
| Antibiotics in last 3 years | 0.023 | 0.026 | 0.014 | 0.034 | 0.132* | −0.137* |
| Hospitals in last year | 0.098* | 0.114 | n.d. | –0.096* | –0.119* | n.d. |
| Hospitals in last 3 years | 0.068 | 0.127 | −0.052 | –0.108* | −0.086 | –0.150* |
The table shows partial Kendall Tau (age controlled). Absence/presence of the infection was coded as 0/1, therefore, the positive Tau means more serious or frequent problems in the seropositive subjects. Significant associations are printed in bold; those significant after the correction for multiple tests are labeled with asterisks. Correlation with number of the hospitalization in the last year (n.d.) could not be computed for men, due to low number of hospitalized subjects.
Information about cat-related injuries was available for 213 subjects who responded to our email message in autumn of 2017. A Spearman correlation test showed no significant association between reported scratching by a cat and presence of anti-Bartonella antibodies. The results of a Spearman correlation of cat scratching with Beck depression score, N-70 depression, health, the presence of anti-Bartonella antibodies and presence of anti-Toxoplasma antibodies is shown in Table 6. The relation between cat scratching, Toxoplasma seropositivity and Beck depression score is shown in Figure 4.
Table 6.
Association between animal-related injuries pathogens' seropositivity and mental and physical health.
| Bartonella IgG | Toxoplasma IgG | Health problems | Back depression | N-70 depression | ||
|---|---|---|---|---|---|---|
| dog biting | All | 0.002 | −0.01 | 0.001 | −0.068 | −0.030 |
| Women | −0.046 | −0.036 | 0.007 | −0.061 | 0.12 | |
| Men | 0.084 | 0.043 | 0.021 | −0.068 | −0.075 | |
| cat biting | All | −0.066 | 0.110 | 0.079 | 0.149* | 0.068 |
| Women | −0.032 | 0.185* | 0.053 | 0.186* | 0.134 | |
| Men | 0.033 | −0.054 | 0.091 | 0.078 | 0.079 | |
| cat scratching | All | 0.006 | 0.127* | 0.043 | 0.159* | 0.058 |
| Women | −0.082 | 0.179* | 0.088 | 0.116 | 0.035 | |
| Men | 0.156 | 0.036 | −0.086 | 0.246* | −0.107 |
The positive Spearman R always means positive association between the presence of anamnestic titres of anti-Bartonella or anti-Toxoplasma antibodies or with health, Beck depression, or N-70 depression (neuroticism) scores. Significant associations are printed in bold; those significant after the correction for multiple tests are labeled with asterisks.
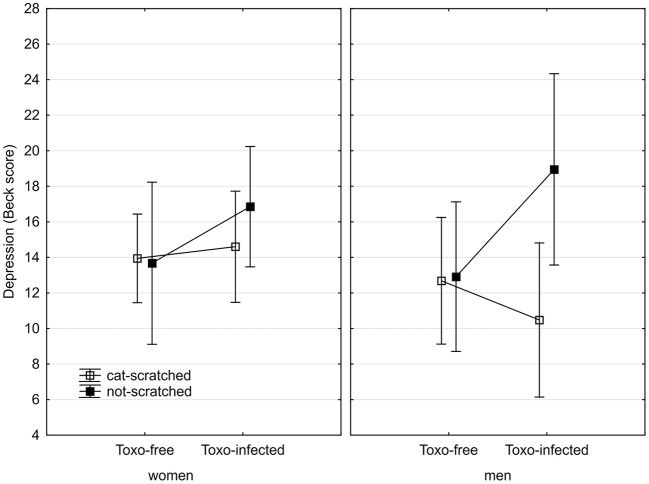
Figure 4.
Association of cat scratching and Toxoplasma seropositivity with BDI-II depression score. Squares show depression computed for covariates at their means and spreads 95% confidence intervals.
Twenty-eight (20.7%) of 130 women and eight of (13.9%) of 67 men reported using antidepressants and twenty-seven (20.6%) of 131 women and five (7.6%) of 66 men reported using anxiolytics. A strong correlation between taking antidepressants and anxiolytics was observed in both women (Spearman R = 0.681, p < 0.0001) and men (Spearman R = 0.452, p < 0.0001). The Beck depression score of the antidepressants-taking women was higher (Spearman R = 0.190, p = 0.030) while that of the antidepressants-taking men was lower (Spearman R = −0.223, p = 0.069) than the score of their non-drug-taking peers. No such correlations or trends were observed between taking antidepressants and the subscale of depression measured with N-70 (women: Spearman R = 0.053, p = 0.538, men: Spearman R = 0.019, p = 0.871) and no association was observed between scores of depression and taking anxiolytic drugs (Beck depression score, women: Spearman R = 0.142, p = 0.105, Beck depression score, men: Spearman R = −0.002, p = 0.986, N-70 depression score, women: Spearman R = 0.017, p = 0.849, N-70 depression score, men: Spearman R = 0.058, p = 0.624). The correlation between the Beck depression score and the N-70 depression score was insignificant both for women (Spearman R = 0.120, p = 0.147), and for men (Spearman R = 0.202, p = 0.069).
Discussion
Our results suggest that the Bartonella-seropositive and Bartonella-seronegative subjects differ in mental and physical health. However, the observed pattern was different and more complex than we expected a priori on the basis of published data. The higher depression in Bartonella-seropositive subjects (hypotheses 1-2) was observed only in Toxoplasma-seronegative men and only when measured with the 21 questions of BDI-II test of depression (hypothesis 2), not with the 10 items of the depression subscale from N-70 (hypothesis 1). The men seropositive for both Bartonella and Toxoplasma expressed lower level of depression than the subjects seropositive only for the Bartonella. This contrasted with the expectations of hypothesis 4, but agreed with recently published data, see below. No difference in the depression scores between Bartonella-seropositive and Bartonella-seronegative subjects was observed in women. Worse physical health, especially more frequent cardiovascular and dermatological problems, of Bartonella-seropositive subjects was observed only in Toxoplasma-seronegative women. Similarly, the Bartonella- and Toxoplasma-seropositive men reported better health, especially less frequent dermatological and cardiovascular problems, than the men seropositive only with Bartonella (which again contrasted with the expectations of hypothesis 4).
Our results seem to contradict the outcomes of two previous studies. The first study revealed a very strong association between having suffered a cat-related injury and being diagnosed with unipolar depression (1). The association was especially strong in women. The 9% prevalence of subjects diagnosed with major depression was observed among all 1.3 million patients of the University Hospital in Michigan. However, among those patients who were treated for a cat-related injury, 24% of men and 48% of women were also diagnosed with major depression within the period of 10 years (1). Based on these data we expected the positive association of depression and Bartonella seropositivity in both sexes, the stronger one in women. In reality, while our data show a relatively strong negative association between Bartonella seropositivity and physical health in women, the strong positive association with depression was observed only in (Toxoplasma-seronegative) men.
The second study (2) showed a positive association between suffering a cat-related injury, especially between being scratched by a cat, and reporting a major depression, and also between the cat-related injury and higher depression score measured with the BDI-II. Again, these associations were observed both in men and in women. However, after a careful inspection of the published data we realized that the odd phenomenon of the negative association between depression and suffering a cat scratching, the proxy for the Bartonella seropositivity, in Toxoplasma-seropositive men was also observed (but not commented) in the previous study (2). Figure 4 of that study shows that the Beck depression score for 15 non-scratched Toxoplasma-seropositive men was 18 (C.I.95 = ±5), while this score for 31 scratched Toxoplasma-seropositive men was 14 (C.I.95 = ±3.5). Corresponding scores for Toxoplasma-seronegative men were 10.2 (C.I.95 = ±2.1) for 88 non-scratched men and 12.8 (C.I.95 = ±2.4) for 90 scratched men. These scores are rather similar to those shown in Figure 2 of the present study (means 18, 13.2, 10.5, and 16.8), despite the fact that scratching by a cat and the presence of anti-Bartonella antibodies was used as a proxy of the Bartonella seropositivity in the past and the present study, respectively.
The third study (3) showed a significant correlation between reporting to be scratched by cat and thirteen of twenty-four studied mental health disorders on a cohort of nearly nine thousand of internet users, see the Table 7. The results of that study seem to agree with our results. Unfortunately, the study did not analyze men and women separately.
Table 7.
Association between mental health disorders and cat scratching.
| OR | CI95 | |
|---|---|---|
| Unipolar depression | 2.97 | 1.86–4.77 |
| Anxiety disorder | 1.4 | 0.98–2.00 |
| Alcohol use disorder | 1.61 | 0.93–2.81 |
| Drug use disorder | 3.30 | 1.45–7.53 |
| Obsessive compulsive disorder | 1.63 | 1.02–2.61 |
| Panic disorder | 2.18 | 1.31–3.63 |
| Borderline personality disorder | 2.16 | 1.00–4.68 |
| Antisocial personality disorder | 2.02 | 1.10–3.70 |
| Phobia | 1.28 | 1.00–1.65 |
| Bulimia, anorexia | 1.94 | 0.95–3.96 |
| Burn–out syndrome | 1.88 | 1.33–2.66 |
| Sexual disorder | 1.77 | 1.06–2.97 |
| Other disorder | 1.67 | 0.89–3.14 |
Thirteen mental health disorders (from the list of 24) showing associations with the reported intensity of cat scratching. The false discovery rate was set to 0.25, therefore about three disorders on the list (probably some with low odds ratio) represent the false discoveries. The table was drown based on data presented in (3).
It must be reminded that the present study, in order to test the prediction of the hypothesis 4 (existence of Toxoplasma-Bartonella interaction), used a case-control study design with a similar number of Toxoplasma-seropositive and Toxoplasma-seronegative subjects while the earlier cohort studies analyzed data drawn from general populations of internet users. Typically, the Toxoplasma-seronegative subjects are much more common than those Toxoplasma-seropositive subjects (who seem to be protected against negative effects of Bartonella) in a general population, especially among young people. Lower representation of Toxoplasma-infected individuals in the participants of the cohort studies explains why being injured by a cat, the proxy of Bartonella seropositivity, has been shown to be positively associated with depression and with the probability of reporting having been diagnosed with major depression in all three cohort studies.
Currently, we have no explanation for the negative association between the depression score and Bartonella seropositivity in Toxoplasma-seropositive men. It can be speculated that Toxoplasma seropositivity has in some respect a positive influence on certain facets of the mental health of Bartonella-seropositive subjects. Toxoplasma seropositivity does not increase the risk of major depression (22), or even decreases such risk in men (12, 23). The Toxoplasma genome is known to contain two genes for tyrosine hydroxylases, the enzymes that catalyze the rate-limiting step in the synthesis of dopamine (24). Dopamine is synthesized in and nearby the cysts of the parasite in the brain tissue of the infected host (25, 26), but see also (27). This could positively influence the risk of schizophrenia and obsessive compulsive disorder. However, it could also decrease the risk of major depression and also depressiveness measured with BDI-II in members of a nonclinical population. Also, many studies showed a strong positive association between molecular markers of inflammation and depression (28–31). It is possible that the increased concentration of IL-10, which is characteristic for Toxoplasma-infected hosts (32, 33) [but see also (34)], can decrease depression by its immunosuppressive and anti-inflammation activities (35, 36). It is important in the context of the observed gender-toxoplasmosis-bartonellosis interaction that the negative correlation between the inflammation marker CRP (C-reactive protein) and depression was also reported to exist in men, but not in women; for a survey and discussion see (37). It seems urgent to compare the level of CRP in Toxoplasma-seropositive and Toxoplasma-seronegative, Bartonella-seropositive subjects.
The alternative explanation of the observed pattern is that the men infected with both Toxoplasma and Bartonella could have such severe depressions that they must regularly use antidepressants and therefore report lower suffering from depressions. This explanation was supported by the fact that taking antidepressants correlated negatively with depressiveness measured by BDI-II in 67 male participants of the present study. In the Toxoplasma-seropositive subjects, both in men and women, cat scratching (in contrast to Bartonella seropositivity) had a positive, not negative, effect on the Beck depression score, compare the Figures 2, 4. This suggest that another factor, possibly another cat scratching-transmitted pathogen beyond B. henselae, could be responsible for the observed positive association between the cat-related injuries and depression.
The strength of the statistical effect (η2) of Bartonella seropositivity increased rather than decreased when the effect of health was controlled. This suggests that the association between Bartonella seropositivity and depression is the result of some relatively specific effect, rather than being a non-specific side effect of the impaired health of Bartonella seropositive subjects.
The positive association between Bartonella seropositivity and depression (observed only in Toxoplasma-seronegative men) was much stronger when measured with the Beck inventory, which was used in the previous study (2), than with the N-70 questionnaire. We found no significant association between depressiveness measured with these two psychodiagnostic instruments. Moreover, our preliminary factor analysis of all 31 questions from these two questionnaires showed the existence of two distinct factors, the first one loaded exclusively with 21 questions from the Beck questionnaire and the second exclusively with 10 questions focusing on depression from the N-70 questionnaire (results not shown). Another factor analysis for just 70 questions of the N-70 questionnaire showed that this psychodiagnostic instrument measured only one factor, which is loaded with all ten questions focusing on psychasthenia and seven of ten questions focusing on depression. These alarming results should be confirmed on a larger set of data; however, for the present, we can conclude that the published results concerning particular subscales of neuroticism measured with the 50 years old N-70 questionnaire should be interpreted, at least, with caution.
Limits and strength of present study
The number of participants in the present study (250) was high enough for the reliable detection of main effects of infections and their 2-way interaction. However, for a reliable analysis of 3-way interaction, collecting a larger dataset would be highly desirable. It is also known that toxoplasmosis affects Rh-positive and Rh-negative subjects differently (38–41). For example, the positive association between toxoplasmosis and six of seven N-70 subscales of neuroticism has been recently observed only in Rh-negative women (13). Again, to detect such a 3-way (and possibly even 4-way, bartonellosis-toxoplasmosis-Rh-Sex) interaction, a much larger set of participants should be obtained and analyzed.
It is not possible to decide based on associations detected with observational studies what is the cause and what is the effect. Toxoplasma has been shown to affect hosts' behavior by the experimental infection of laboratory animals (42–45); no such data, however, are available for Bartonella. The probability that depression or impaired health status would cause the presence of the anamnestic anti-Bartonella antibodies is rather low. However, it is possible that some unknown third factor, for example immunodeficiency, could cause both impaired mental health (or depression) and the infection by Bartonella.
Conclusions
At the present time, the effects of various symbionts on a phenotype of humans is the subject of a growing number of studies. Our results, however, suggest that rather than main effects of particular symbionts, the interaction of different species symbionts, parasites and mutualists, and their interactions with genotype and environment should be always studied. As a minimum, the effect of symbionts should be always studied separately on men and women as they usually react differently, often in an opposite way, to the infection. The opposite behavioral reaction to the Toxoplasma infection has been explained by the opposite reactions of men and women on the chronic stress that could be associated with life-long infection (46, 47). However, the present results, namely the negative effect of Bartonella seropositivity on physical health and the positive effect of Bartonella seropositivity on the mental health of Toxoplasma-seronegative women, and the positive effect of Bartonella seropositivity on both physical and mental health in Toxoplasma-seropositive men, suggest that the stress-coping hypothesis alone cannot explain all observed phenomena and that the physiological state of men and women could really react differently to some specific environmental factors, including pathogen infections.
Ethics statement
This study was carried out in accordance with the recommendations of European Communities guidelines. The protocol was approved by the by Institutional Review Board of Faculty of Science, Charles University. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
Author contributions
JF designed the study and performed the statistical analyses. PB analyzed the sera. All authors participated on interpretation of results and writing the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Charlie Lotterman for his help with the final version of the paper.
Footnotes
Funding. The work was supported by project UNCE 204004 (Charles University in Prague) and the Czech Science Foundation (Grant No. P303/16/20958).
References
- 1.Hanauer DA, Ramakrishnan N, Seyfried LS. Describing the relationship between cat bites and human depression using data from an electronic health record. Plos ONE (2013) 8:e70585. 10.1371/journal.pone.0070585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flegr J, Hodny Z. Cat scratches, not bites, are associated with unipolar depression - cross-sectional study. Parasites Vectors (2016) 9:8 10.1186/s13071-015-1290-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flegr J, Vedralova M. Specificity and nature of the associations of twenty-four neuropsychiatric disorders with contacts with cats and dogs. Schizophr Res. (2017) 189:219–20. 10.1016/j.schres.2017.02.007 [DOI] [PubMed] [Google Scholar]
- 4.Massei F, Gori L, Macchia P, Maggiore G. The expanded spectrum of bartonellosis in children. Infect Dis Clin North Am. (2005) 19:691–711. 10.1016/j.idc.2005.06.001 [DOI] [PubMed] [Google Scholar]
- 5.Prutsky G, Domecq JP, Mori L, Bebko S, Matzumura M, Sabouni A, et al. Treatment outcomes of human bartonellosis: a systematic review and meta-analysis. Int J Infect Dis. (2013) 17:E811–9. 10.1016/j.ijid.2013.02.016 [DOI] [PubMed] [Google Scholar]
- 6.Breitschwerdt EB. Bartonellosis: one health perspectives for an emerging infectious disease. Ilar J. (2014) 55:46–58. 10.1093/Ilar/Ilu015 [DOI] [PubMed] [Google Scholar]
- 7.Angelakis E, Raoult D. Pathogenicity and treatment of Bartonella infections. Int J Antimicrob Agents (2014) 44:16–25. 10.1016/j.ijantimicag.2014.04.006 [DOI] [PubMed] [Google Scholar]
- 8.Jackson LA, Perkins BA, Wenger JD. Cat-scratch disease in the United-States - an analysis of 3 national databases. Am J Public Health (1993) 83:1707–11. 10.2105/Ajph.83.12.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perkins BA, Swaminathan B, Jackson LA, Brenner DJ, Wenger JD, Regnery RL, et al. Case-22-1992 - pathogenesis of cat scratch disease. N Eng J Med. (1992) 327:1599–600. [DOI] [PubMed] [Google Scholar]
- 10.Breitschwerdt EB, Maggi RG, Nicholson WL, Cherry NA, Woods CW. Bartonella sp bacteremia in patients with neurological and neurocognitive dysfunction. J Clin Microbiol. (2008) 46:2856–61. 10.1128/Jcm.00832-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rondet B, Sarret C, Lacombe P, Rouveyrol F, Chenel C, Romaszko JP, et al. Neurological symptoms with Bartonella henselae infection: Report on 2 pediatric cases. Arch De Pediatrie (2012) 19:823–6. 10.1016/j.arcped.2012.05.011 [DOI] [PubMed] [Google Scholar]
- 12.Flegr J, Horacek J. (2017). Toxoplasmosis, but not borreliosis, is associated with psychiatric disorders: a cross-sectional survey on 46 thousand of subjects. BioRxiv [preprint]. 10.1101/231803 [DOI] [Google Scholar]
- 13.Šebánková B, Flegr J. Physical and mental health status in Toxoplasma-infected women before and three years after they learn about their infection: Manipulation or side-effects of impaired health? Front Ecol Evol. (2017) 5:144 10.3389/fevo.2017.00144 [DOI] [Google Scholar]
- 14.Flegr J, Escudero DQ. Impaired health status and increased incidence of diseases in Toxoplasma-seropositive subjects - an explorative cross-sectional study. Parasitology (2016) 143:1974–89. 10.1017/s0031182016001785 [DOI] [PubMed] [Google Scholar]
- 15.Frost P, Kleisner K, Flegr J. Health status by gender, hair color, and eye color: Red-haired women are the most divergent. PLoS ONE (2017) 12:e0190238. 10.1371/journal.pone.0190238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck AT, Steer RA, Brown GK. (1996). Manual for the Beck Depression Inventory-II. Sant Antonio: Psychological Corporation. [Google Scholar]
- 17.Vacír K. (1973). Monitoring of Decision Making Processes Under Time Pressure (Sledování Rozhodovacích Procesu v Casové Tísni). Ph.D. thesis, Charles University, Prague. [Google Scholar]
- 18.Flegr J, Hampl R, Cernochová D, Preiss M, Bičíkova M, Sieger L, et al. The relation of cortisol and sex hormone levels to results of psychological, performance, IQ and memory tests in military men and women. Neuroendocrinol Lett. (2012) 33:224–35. [PubMed] [Google Scholar]
- 19.APA (2013) Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington: American Psychiatric Publishing. [Google Scholar]
- 20.Siegel S, Castellan NJ. (1988). Nonparametric Statistics for the Behavioral Sciences. New York, NY: McGraw-Hill. [Google Scholar]
- 21.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc Series B-Methodol. (1995) 57:289–300. [Google Scholar]
- 22.Sutterland AL, Fond G, Kuin A, Koeter MW, Lutter R, van Gool T, et al. Beyond the association. Toxoplasma gondii in schizophrenia, bipolar disorder, and addiction: systematic review and meta-analysis. Acta Psychiatr Scandinav. (2015) 132:161–79. 10.1111/acps.12423 [DOI] [PubMed] [Google Scholar]
- 23.Flegr J. Neurological and neuropsychiatric consequences of chronic Toxoplasma infection. Clin Microbiol Rep. (2015) 2:163–72. 10.1007/s40588-40015-40024-40580 [DOI] [Google Scholar]
- 24.Gaskell EA, Smith JE, Pinney JW, Westhead DR, McConkey GA. A unique dual activity amino acid hydroxylase in Toxoplasma gondii. PloS ONE (2009) 4:e4801. 10.1371/journal.pone.0004801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prandovszky E, Gaskell E, Martin H, Dubey JP, Webster JP, McConkey GA. The neurotropic parasite Toxoplasma gondii increases dopamine metabolism. PLoS ONE (2011) 6:e23866. 10.1371/journal.pone.0023866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin HL, Alsaady I, Howell G, Prandovszky E, Peers C, Robinson P, et al. Effect of parasitic infection on dopamine biosynthesis in dopaminergic cells. Neuroscience (2015) 306:50–62. 10.1016/j.neuroscience.2015.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang ZT, Harmon S, O'Malley KL, Sibley LD. Reassessment of the role of aromatic amino acid hydroxylases and the effect of infection by Toxoplasma gondii on host dopamine. Infect Immun. (2015) 83:1039–47. 10.1128/iai.02465-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danner M, Kasl SV, Abramson JL, Vaccarino V. Association between depression and elevated C-reactive protein. Psychosom Med. (2003) 65:347–56. 10.1097/01.Psy.0000041542.29808.01 [DOI] [PubMed] [Google Scholar]
- 29.Penninx BWJH, Kritchevsky SB, Yaffe K, Newman AB, Simonsick EM, Rubin S, et al. Inflammatory markers and depressed mood in older persons: Results from the Health, Aging and Body Composition study. Biol Psychiatry (2003) 54:566–72. 10.1016/S0006-3223(03)01811-5 [DOI] [PubMed] [Google Scholar]
- 30.Ford DE, Erlinger TP. Depression and C-reactive protein in US adults - data from the third national health and nutrition examination survey. Arch Intern Med. (2004) 164:1010–4. 10.1001/archinte.164.9.1010 [DOI] [PubMed] [Google Scholar]
- 31.Elovainio M, Aalto AM, Kivimaki M, Pirkola S, Sundvall J, Lonnqvist J, et al. Depression and C-reactive protein: population-based Health 2000 study. Psychosom Med. (2009) 71:423–30. 10.1097/PSY.0b013e31819e333a [DOI] [PubMed] [Google Scholar]
- 32.Gaddi PJ, Yap GS. Cytokine regulation of immunopathology in toxoplasmosis. Immunol Cell Biol. (2007) 85:155–9. 10.1038/sj.icb.7100038 [DOI] [PubMed] [Google Scholar]
- 33.Matowicka-Karna J, Dymicka-Piekarska V, Kemona H. Does Toxoplasma gondii infection affect the levels of IgE and cytokines (IL-5, IL-6, IL-10, IL-12, and TNF-alpha)? Clin Dev Immunol. (2009) 2009:374696. 10.1155/2009/374696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanková Š, Holán V, Zajícová A, Kodym P, Flegr J. Modulation of immunity in mice with latent toxoplasmosis - the experimental support for the immunosupression hypothesis of Toxoplasma-induced changes in reproduction of mice and humans. Parasitol Res. (2010) 107:1421–7. 10.1007/s00436-010-2013-9 [DOI] [PubMed] [Google Scholar]
- 35.DeckertSchluter M, Buck C, Weiner D, Kaefer N, Rang A, Hof H, et al. Interleukin-10 downregulates the intracerebral immune response in chronic Toxoplasma encephalitis. J Neuroimmunol. (1997) 76:167–76. 10.1016/s0165-5728(97)00047-7 [DOI] [PubMed] [Google Scholar]
- 36.Wilson EH, Wille-Reece U, Dzersznski F, Hunter CA. A critical role for IL-10 in limiting inflammation during toxoplasmic encephalitis. J Neuroimmunol. (2005) 165:63–74. 10.1016/j.jneuroim.2005.04.018 [DOI] [PubMed] [Google Scholar]
- 37.Vetter ML, Wadden TA, Vinnard C, Moore RH, Khan Z, Volger S, et al. Gender differences in the relationship between symptoms of depression and high-sensitivity CRP. Int J Obes. (2013) 37:S38–43. 10.1038/ijo.2013.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flegr J, Novotná M, Lindová J, Havlíček J. Neurophysiological effect of the Rh factor. Protective role of the RhD molecule against Toxoplasma-induced impairment of reaction times in women. Neuroendocrinol Lett. (2008) 29:475–81. [PubMed] [Google Scholar]
- 39.Novotná M, Havlíček J, Smith AP, Kolbeková P, Skallová A, Klose J, et al. Toxoplasma and reaction time: Role of toxoplasmosis in the origin, preservation and geographical distribution of Rh blood group polymorphism. Parasitology (2008) 135:1253–61. [DOI] [PubMed] [Google Scholar]
- 40.Flegr J, Novotná M, Fialová A, Kolbeková P, Gašová Z. The influence of RhD phenotype on toxoplasmosis- and age-associated changes in personality profile of blood donors. Folia Parasitol. (2010) 57:143–50. 10.14411/fp.2010.018 [DOI] [PubMed] [Google Scholar]
- 41.Kanková Š, Šulc J, Flegr J. Increased pregnancy weight gain in women with latent toxoplasmosis and RhD-positivity protection against this effect. Parasitology (2010) 137:1773–9. 10.1017/S0031182010000661 [DOI] [PubMed] [Google Scholar]
- 42.Berdoy M, Webster JP, Macdonald DW. Fatal attraction in rats infected with Toxoplasma gondii. Proc R Soc B Biol Sci. (2000) 267:1591–4. 10.1098/rspb.2000.1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hodková H, Kodym P, Flegr J. Poorer results of mice with latent toxoplasmosis in learning tests: impaired learning processes or the novelty discrimination mechanism? Parasitology (2007) 134:1329–37. 10.1017/S0031182007002673 [DOI] [PubMed] [Google Scholar]
- 44.Vyas A, Kim SK, Giacomini N, Boothroyd JC, Sapolsky RM. Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc Natl Acad Sci USA. (2007) 104:6442–7. 10.1073/pnas.0608310104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Webster JP. The effect of Toxoplasma gondii on animal behavior: playing cat and mouse. Schizophr Bull. (2007) 33:752–6. 10.1093/schbul/sbl073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindová J, Kuběna AA, Šturcová A, Krivohlavá R, Novotná M, Rubešová A, et al. Pattern of money allocation in experimental games supports the stress hypothesis of gender differences in Toxoplasma gondii-induced behavioural changes. Folia Parasitol. (2010) 57:136–42. 10.14411/fp.2010.017 [DOI] [PubMed] [Google Scholar]
- 47.Lindová J, Novotná M, Havlíček J, Jozífková E, Skallov,á A, Kolbeková P, et al. Gender differences in behavioural changes induced by latent toxoplasmosis. Int J Parasitol. (2006) 36:1485–92. 10.1016/j.ijpara.2006.07.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available at: https://doi.org/10.6084/m9.figshare.5852511.