Abstract
Icariin (ICA) is a major bioactive monomer belonging to flavonoid glycosides attracted from Epimedium, being a classic tonic agent in traditional Chinese medicine. ICA commonly presents multiple effects such as regulating sex hormones, relieving atherosclerosis and antioxidant activity, etc. Recently, more and more studies have demonstrated the application of ICA in autoimmune diseases such as rheumatoid arthritis, bronchial asthma, multiple sclerosis and systemic lupus erythematosus due to its anti-inflammatory. Additionally, ICA also has the anti-tumor activities. Multiple targets and mechanisms of ICA are reported which relates to regulate lymphocytes balance, anti-inflammatory/inflammatory cytokines, signal pathways like NF-kappaβ and Erk-p38-JNK, lymphocyte transcription factors and other targets such as TLRs, STAT and PTEN, etc. In this review, we have updated the advance in this field and these studies have suggested that ICA has a potential to treat immunological and inflammatory diseases.
Keywords: Icariin, immune regulation, inflammation, Chinese medicine
Introduction
Icariin (ICA) is one of the major bioactive compounds attracted from Epimedium which is also the most widely studied monomer [1]. Epimedium, belonging to the Berberidaceae familty, in Chinese called Horny Goat Weed or Yin Yang Huo, is a classic tonic agent in traditional Chinese medicine. Epimedium, also known as epimedium, nine-leaf grass, is the aboveground part of herbaceous perennial plants such as Sagittaria Epimedii, Epimediumchinense, Epimediumwushan, or Korean Epimedium. There are more than 40 species in the world, and China is the most important distribution area of this genus. There are 27 species and 4 varieties, accounting for about 70% of the world total species [2].
At early stages, ICA is mainly used for enhancing reproductive function [3] and anti-aging. In addition, now more pharmacological studies suggest that it also possesses various therapeutic capabilities, especially for neuro-protective [4], cardio-protective [5], anti-inflammatory or anti-cancer effects [6], etc. In recent years, there has been an interest in pharmacological investigation of the immune modulator effects of ICA and its derivatives [7]. Evidence from in vitro and in vivo studies has demonstrated that the monomer has effects on regulating immunocyte, relative cytokine and multiple target mechanisms (Figure 2).
Figure 2.
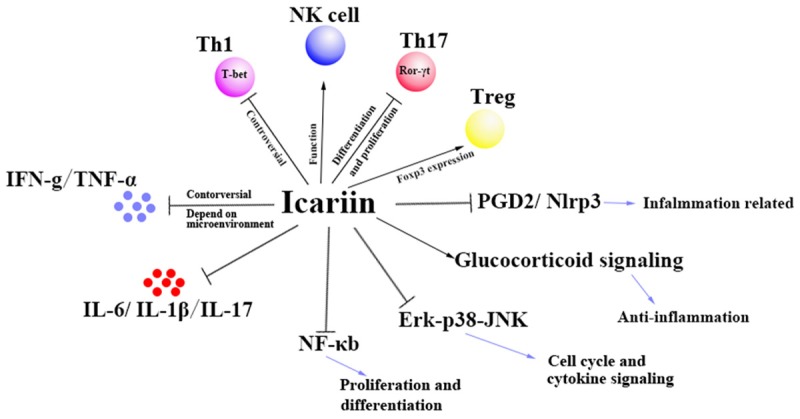
Mechanism of the Effect of ICA on Immunity. Multiple targets and mechanisms of ICA are reported which relates to regulate lymphocytes balance, anti-inflammatory/inflammatory cytokines and signal pathways.
Chemical structure
ICA presents a chemical structure belonging to 8-prenyl flavonoid glycosides, which is pale yellow powder with molecular formula C33H40O15 d (Figure 1). Its molecular weight is 676. 67 with melting point of 231~232°C. ICA can be soluble in ethyl acetate, ethanol while it is insoluble in chloroform, ether, and benzene [2].
Figure 1.
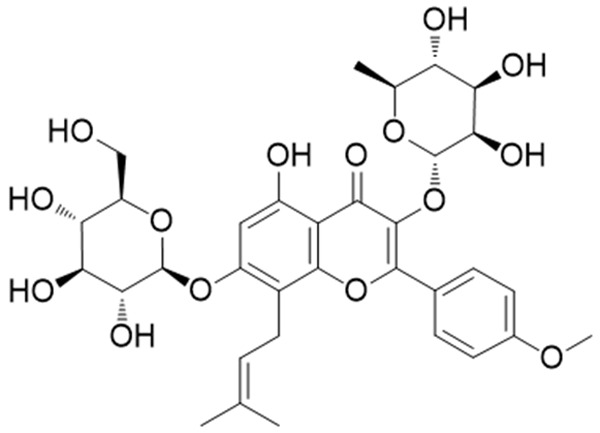
Chemical Structure of ICA. ICA belongs to flavonoid glycosides, of which the molecular formula is C33H40O15. It contains sopentenyl, phenolic hydroxyl and methoxy groups which may related to antioxidant, anti-inflammatory or immune-modulatory activities. The structure suggests its material basis of potential pharmacological activities.
Flavonoid glycosides are widely found in nature and are the active ingredients of various medicinal plants, which are proved to have many pharmacology activities, such as antioxidant, anti-inflammatory and anti-tumor effects, etc. It takes place at different carbon positions by hydroxyl or methoxy substitution, that is, it becomes a variety of flavonoid pigments which has different physicochemical properties. It can also be linked to glycosyl to form glycosides and has other pharmacological activities.
It is proved that in flavonoid glycosides, the number of phenolic hydroxyl groups is related to the function of antioxidant activity. The more hydrogen atoms are bound to the active radicals, the more stable the flavone radicalsare formed after reaction with the active radicals, and the greater the number of hydrogen bonds is formed, the stronger the antioxidant activity will be [8]. The structure of ICA presents hydroxyl groups, suggesting its function in this aspect.
The isopentenyl-substituted compounds on the flavonoid nucleus have the activity of inhibiting tumor cells [9]. Nevertheless, flavonoids may play an anti-inflammatory or immune-modulatory role by affecting cell secretory processes, mitosis, and cell-cell interactions. It is partly related to the methoxy group structure [10]. Both structures exist in ICA, suggesting its material bass is of potential pharmacological activities.
Effects and mechanisms of immune-regulation
Lymphocytes
Th1/Th17 cells
Th17 is closely related to Th1 in development and it has been known that excessive expression of these cells is related to many inflammation diseases [11-15]. ICA can regulate lymphocytes function such as affecting Th1/Th17 or Th2 balance. In Type II Collagen-Induced Arthritis (CIA) model, ICA treatment led to the decreased ratio of CD4+IL-17+ cells and less number of Th17 cells. Serum levels of IgG2a also reduced with alleviated arthritis score following the induced by ICA treatment, while the effects were abolished with additional IL-17 administration [16], suggesting that ICA controls CIA mainly through mediating Th17 cells In experimental autoimmune encephalomyelitis (EAE), mice administrated ICA also displayed the decreased frequencies of Th1 and Th17 cells both in the spleens and lymph node. Lower frequency of Th17 cells also was found in CNS mononuclear cells of ICA-treated mice [17]. Controversially, several studies also demonstrated that ICA may also enhance immune response. Data from these experiments demonstrated that ICA provoked the Th1-lineage development and stimulated IgG production in mice [18].
Treg cells
Regulatory T cells (Treg) are critical mediators of immune homeostasis and hold significant promise in the quest in autoimmunity and infection, while Foxp3 is a representative phenotype of Treg. Treg cells consist of diverse lymphocyte populations that include CD4+ cells, CD8+ cells, and other minor T cell population [19]. Treg population includes thymus-derived CD4+CD25+ natural Treg cells, Tr1 cells producing IL-10, Th3 cells producing TGF-beta [20] and another important cells type like iTreg cells [21], which all have a function of counterbalancing immune hyperactivity. In ovalbumin (OVA)-induced asthma model mice, IICA treatments resulted in a significant suppression on IL-17 as well as a notable increase in Foxp3 mRNA expression in isolated spleen CD4+ T cell, which suggests ICA regulates Th17/Treg balance [7].
NK cells
Natural killer cells are a type of cytotoxic lymphocyte critical to the innate immune system. The role of NK cells played is analogous to that of cytotoxic T cells in the vertebrate adaptive immune response. NK cells provide rapid responses to viral-infected cells, acting at around 3 days after infection, and respond to tumor formation [22]. It is proved that lymphokine-activated killer cell (LAK) and natural killer cell (NK) can be increased by ICA in tumor patients. ICA may also make peripheral blood mononuclear cells to present delayed proliferation [23].
Inflammatory cytokines
ICA exerts diversified effect on inflammatory cytokines in treatment of different diseases. In cultivation of bronchoalveolar lavage fluid (BALF) cell, ICA caused a significant suppression in interleukin-6 (IL-6), IL-17 but not in IL-10 level [7]. In rats with brain dysfunction induced by LPS, the study demonstrated that ICA significantly improved spatial learning and memory abilities with decreased TNF-alpha, IL-1beta and COX-2 expression in the hippocampus [24]. As to titanium-stimulated mice, it is observed that ICA significantly reduced tumor necrosis factor-alpha (TNF-α) secretion, IL-6 and IL-1βin the calvariae [25]. The ICA administration also inhibited the gene expression of IL-1β, IL-8, TACR1 and ICAM-1 in HaCaT cells in a doseand time-dependent manner. The differential production of IFN-gamma-R1 and TNF-alpha-R1also presented after the stimulation of IFN-gamma/TNF-alpha, which was notably normalized after the ICA treatment [26]. However, in another in vitro pharmacological study of T lymphocytes stimulated by concanvalin A (ConA), ICA decreased the production of IL-2, IL-4 and IL-10 but up-regulated TNF-alpha and IFN-gamma [27]. It is possible that ICA affects immune responses depending upon the microenvironments.
Lymphocyte transcription factors
RORgamma
RORγ is a key transcription factor which controls the function and development of CD4+ Th17 and CD8+ Tc17 cells. RORγ enhances the function of Th17 cells by increasing the secretion of cytokines or chemokines such as GM-CSF and IL-17 [28]. In ovalbumin (OVA)-induced asthma model, ICA decreased the number of CD4+ RORgammat+ T cells and presented a significant decrease in RORγ [7].
T-bet
T-box factor expressed in T cells (T-bet), is conceptualized not only a ‘master regulator’ of Th1 cells but also a broad, conserved regulator in immune responses [29]. Asthmatic rats administrated by ICA showed decreased GATA-3 mRNA expression and T-bet in pulmonary tissue and relieved by regulating the imbalance of Th1/Th2.
TLRs
Toll-like receptors (TLRs), as the important pattern recognition receptors in innate immunity, are also proved to be targets of ICA [30]. Ana-1 murine macrophages stimulated with ICA induced a notable expression of TLR9 which was dose-dependent. Several molecules, such as, IL-6 and TNF-alpha, which are playing an important role in downstream signaling pathway of TLR9, were obviously up-regulated by ICA [31]. On the other hand, ICA treatment also decreased the expression of toll-like receptor 4 (TLR4) of human PBMCs [32].
STAT
The signal transducer and activator of transcription (STAT) family are involved in regulating cellular proliferation, apoptosis, angiogenesis and the immune system response [33]. In CIA mice, ICA inhibited STAT3 activation in T cells and STAT3 inhibitor resulted in decreased IL-17 production and alleviated rheumatoid arthritis [16]. As to Inflammatory bowel disease (IBD), ICA treatment inhibited the phosphorylations of STAT1 and STAT3 in CD4(+) T cells, both are the crucial transcription factors for Th1 and Th17 respectively [34].
Signal pathways
NF-kapaβ
The NF-kappaβ is a family of transcription factors which are involved in adaptive immune functions and associated with resistance to infection [35]. The phosphorylation of c-Jun N-terminal kinases (JNK), which is the degradation of inhibitor of kappaβ, is the nuclear translocation of nuclear factor kappaβ (NFkappaβ) p65 in LPS treated H9c2 cells. The pathway was blocked by ICA treatment. These results of studies suggested that ICA may prevent cardiomyocytes from apoptosis and inflammatory response, which may be mediated by inhibition of JNK/NFkappaβ pathway [36]. In GBC-SD cells, ICA notably inhibited both constitutive and gemcitabine-induced NF-kappaβ activity. Nevertheless, it also enhanced induced G(0)-G(1) phase arrest, caspase-3 activity, and decreased the expression of Bcl-2, Bcl-xL [37]. ICA also potentially presented the anti-tumor effect of arsenic trioxide in hepatocellular carcinoma by decreasing NF-kappaβ activity [38]. In colorectal cancer, ICA also enhanced the activity of 5-FU and suppressed tumor growth through inhibiting NF-kappaβ activity [39].
Erk-p38-JNK
Erk-p38-JNK plays important role in immunology functions such as affecting signals stimulating interleukin-2 (IL-2) in T lymphocytes [40-42]. It was found in B16 cell differentiation that ICA could cause cell cycle arrest at G0/G1 phase and the cell differentiation with the mechanism of inhibiting Erk1/2-p38-JNK signaling molecules [43]. In isolated human nucleus pulposus cells, ICA demonstrated significant anti-inflammatory effect, such as suppressing Erk-p38-JNK induced by IL-1bet and the activation of NF-kappaβ signaling pathways [44].
Glucocorticoid receptor
Glucocorticoid receptor (GR) has anti-inflammatory effect interacting with several signaling pathways such as components of the T cell receptor (TCR) signaling, PI3K, JNK and pro-inflammatory gene expression [45]. In rat model of depression induced by unpredictable chronic mild stress (CMS), oral administration of ICA for 35 days presented effect of relieving the development of depression behaviors caused by exposure to CMS with increasing mRNA expression of GR [46]. In experimental autoimmune encephalomyelitis (EAE) mice, ICA induced estrogen-like activity which modulated HPA function and increased the expression of GR of cerebral white matter [47].
PTEN
Phosphatase and tensin homolog (PTEN) has effect on the development and regulation of adaptive immune cells [48,49]. These functions of PTEN are mainly related to regulating PI3K signaling pathway [50]. Cultured with ICA, ovarian cancer A2780 cells expressed increased PTEN and RECK protein expression levels [51,52].
PGD2
ProstaglandinD-2 (PGD2) generated from immunologically stimulated mast cells, is the major cyclooxygenase metabolite which is thought to contribute to the pathogenesis of allergic diseases. It demonstrates various inflammatory effects [53]. In RSV-infected and OVA-induced asthma mode, BALF and PGD2 in serum were suppressed in ICA treated group [54], which shows the potential of this target in asthma by the monomer.
Nlrp3
The Nlrp3 plays an notable role in inflammatory responses which can adapt immunity [55]. It can support of both Th17 and Th1 responses, partially dependent on IL-18 level [56,57]. In the hippocampus of chronic mild stress (CMS) rats, the axis of NLRP3/caspase-1/IL-1β was negatively regulated by ICA [58]. ICA also relieved renal damage of IgAN rats by inhibiting NF-kappaβ-mediated Nlrp3 activation [59].
Conclusion
Although at early stages in traditional Chinese medicine, ICA is mainly used for enhancing reproductive function, more and more application and emerging evidence indicated that ICA is a promising compound attracted from nature herb that presents multiple immunity functions. The molecular mechanisms may include multi aspects such as the involvement of Th1/Th17 or Th2 balance, Th17/Treg regulation, NK proliferation, anti-inflammatory/inflammatory cytokines, signal pathways like NF-kappaβ and Erk-p38-JNK, lymphocyte transcription factors and other targets such as TLRs, STAT and PTEN, etc. ICA has also demonstrated common immune-suppression effects on many autoimmune diseases like rheumatoid arthritis and autoimmune encephalomyelitis while it also presents anti-tumor results. Further understanding of underlying mechanism(s) of action of ICA will help us gain a comprehensive understanding of its immunity regulatory effects.
Acknowledgements
We acknowledge the work supported by National Natural Science Foundation of China: 81303012.
Disclosure of conflict of interest
None.
References
- 1.Tan HL, Chan KG, Pusparajah P, Saokaew S, Duangjai A, Lee LH, Goh BH. Anti-cancer properties of the naturally occurring aphrodisiacs: ICA and its derivatives. Front Pharmacol. 2016;7:191. doi: 10.3389/fphar.2016.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makarova MN, Pozharitskaya ON, Shikov AN, Tesakova SV, Makarov VG, Tikhonov VP. Effect of lipid-based suspension of epimedium koreanum Nakai extract on sexual behavior in rats. J Ethnopharmacol. 2007;114:412–416. doi: 10.1016/j.jep.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 3.Pan J, Guo B. Effects of light intensity on the growth, photosynthetic characteristics, and flavonoid content of epimedium pseudowushanense B.L.Guo. Molecules. 2016;21 doi: 10.3390/molecules21111475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang GQ, Li DD, Huang C, Lu DS, Zhang C, Zhou SY, Liu J, Zhang F. ICA reduces dopaminergic neuronal loss and microglia-mediated inflammation in vivo and in vitro. Front Mol Neurosci. 2017;10:441. doi: 10.3389/fnmol.2017.00441. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Ke Z, Liu J, Xu P, Gao A, Wang L, Ji L. The cardioprotective effect of ICA on ischemia-reperfusion injury in isolated rat heart: potential involvement of the PI3K-Akt signaling pathway. Cardiovasc Ther. 2015;33:134–140. doi: 10.1111/1755-5922.12121. [DOI] [PubMed] [Google Scholar]
- 6.Fan C, Yang Y, Liu Y, Jiang S, Di S, Hu W, Ma Z, Li T, Zhu Y, Xin Z, Wu G, Han J, Li X, Yan X. ICA displays anticancer activity against human esophageal cancer cells via regulating endoplasmic reticulum stress-mediated apoptotic signaling. Sci Rep. 2016;6:21145. doi: 10.1038/srep21145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei Y, Liu B, Sun J, Lv Y, Luo Q, Liu F, Dong J. Regulation of Th17/Treg function contributes to the attenuation of chronic airway inflammation by ICA in ovalbumin-induced murine asthma model. Immunobiology. 2015;220:789–797. doi: 10.1016/j.imbio.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Pradhan AB, Bhuiya S, Haque L, Das S. Role of hydroxyl groups in the B-ring of flavonoids in stabilization of the Hoogsteen paired third strand of Poly(U). Poly(A)*Poly(U) triplex. Arch Biochem Biophys. 2018;637:9–20. doi: 10.1016/j.abb.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Choi SU, Ryu SY, Yoon SK, Jung NP, Park SH, Kim KH, Choi EJ, Lee CO. Effects of flavonoids on the growth and cell cycle of cancer cells. Anticancer Res. 1999;19:5229–5233. [PubMed] [Google Scholar]
- 10.Sartor L, Pezzato E, Dell’Aica I, Caniato R, Biggin S, Garbisa S. Inhibition of matrix-proteases by polyphenols: chemical insights for anti-inflammatory and anti-invasion drug design. Biochem Pharmacol. 2002;64:229–237. doi: 10.1016/s0006-2952(02)01069-9. [DOI] [PubMed] [Google Scholar]
- 11.Kong N, Lan Q, Chen M, Zheng T, Su W, Wang J, Yang Z, Park R, Dagliyan G, Conti PS, Brand D, Liu Z, Zou H, Stohl W, Zheng SG. Induced T regulatory cells suppress osteoclastogenesis and bone erosion in collagen-induced arthritis better than natural T regulatory cells. Ann Rheum Dis. 2012;71:1567–1572. doi: 10.1136/annrheumdis-2011-201052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen M, Su W, Lin X, Guo Z, Wang J, Zhang Q, Brand D, Ryffel B, Huang J, Liu Z, He X, Le AD, Zheng SG. Adoptive transfer of human gingiva-derived mesenchymal stem cells ameliorates collagen-induced arthritis via suppression of Th1 and Th17 cells and enhancement of regulatory T cell differentiation. Arthritis Rheum. 2013;65:1181–1193. doi: 10.1002/art.37894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su W, Fan H, Chen M, Wang J, Brand D, He X, Quesniaux V, Ryffel B, Zhu L, Liang D, Zheng SG. Induced CD4+ forkhead box protein-positive T cells inhibit mast cell function and established contact hypersensitivity through TGF-β1. J Allergy Clin Immunol. 2012;130:444–452. e447. doi: 10.1016/j.jaci.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Gao Y, Tang J, Chen W, Li Q, Nie J, Lin F, Wu Q, Chen Z, Gao Z, Fan H, Tsun A, Shen J, Chen G, Liu Z, Lou Z, Olsen NJ, Zheng SG, Li B. Inflammation negatively regulates FOXP3 and regulatory T-cell function via DBC1. Proc Natl Acad Sci U S A. 2015;112:E3246–3254. doi: 10.1073/pnas.1421463112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan Z, Jiang R, Wang X, Wang Y, Lu L, Liu Q, Zheng SG, Sun B, Ryffel B. RORgammat+ IL-17+ neutrophils play a critical role in hepatic ischemia-reperfusion injury. J Mol Cell Biol. 2013;5:143–146. doi: 10.1093/jmcb/mjs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chi L, Gao W, Shu X, Lu X. A natural flavonoid glucoside, ICA, regulates Th17 and alleviates rheumatoid arthritis in a murine model. Mediators Inflamm. 2014;2014:392062. doi: 10.1155/2014/392062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen R, Deng W, Li C, Zeng G. A natural flavonoid glucoside ICA inhibits Th1 and Th17 cell differentiation and ameliorates experimental autoimmune encephalomyelitis. Int Immunopharmacol. 2015;24:224–231. doi: 10.1016/j.intimp.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Rhew KY, Han Y. Immunoadjuvant activity of ICA that induces Th1-type antibody in mice. Arch Pharm Res. 2012;35:1685–1691. doi: 10.1007/s12272-012-0920-2. [DOI] [PubMed] [Google Scholar]
- 19.Horwitz DA, Zheng SG, Wang J, Gray JD. Critical role of IL-2 and TGF-beta in generation, function and stabilization of Foxp3+CD4+ Treg. Eur J Immunol. 2008;38:912–915. doi: 10.1002/eji.200738109. [DOI] [PubMed] [Google Scholar]
- 20.Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+ CD25- precursors. J Immunol. 2002;169:4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- 21.Zheng SG. The critical role of TGF-beta1 in the development of induced Foxp3+ regulatory T cells. Int J Clin Exp Med. 2008;1:192–202. [PMC free article] [PubMed] [Google Scholar]
- 22.Kristensen AB, Kent SJ, Parsons MS. Contribution of NK cell education to both direct and anti-HIV-1 antibody-dependent NK cell functions. J Virol. 2018;92 doi: 10.1128/JVI.02146-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He W, Sun H, Yang B, Zhang D, Kabelitz D. Immunoregulatory effects of the herba epimediia glycoside ICA. Arzneimittelforschung. 1995;45:910–913. [PubMed] [Google Scholar]
- 24.Guo J, Li F, Wu Q, Gong Q, Lu Y, Shi J. Protective effects of ICA on brain dysfunction induced by lipopolysaccharide in rats. Phytomedicine. 2010;17:950–955. doi: 10.1016/j.phymed.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Shao H, Shen J, Wang M, Cui J, Wang Y, Zhu S, Zhang W, Yang H, Xu Y, Geng D. ICA protects against titanium particle-induced osteolysis and inflammatory response in a mouse calvarial model. Biomaterials. 2015;60:92–99. doi: 10.1016/j.biomaterials.2015.04.048. [DOI] [PubMed] [Google Scholar]
- 26.Kong L, Liu J, Wang J, Luo Q, Zhang H, Liu B, Xu F, Pang Q, Liu Y, Dong J. ICA inhibits TNF-alpha/IFN-gamma induced inflammatory response via inhibition of the substance P and p38-MAPK signaling pathway in human keratinocytes. Int Immunopharmacol. 2015;29:401–407. doi: 10.1016/j.intimp.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 27.Teng F, Zeng YY, Huang XY, Yang Z, Yao ML, Song B, Li L. [Effect of ICA on intermediate and advanced activation of murine T lymphocytes in vitro] . Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2008;24:1059–1061. [PubMed] [Google Scholar]
- 28.Hu X, Liu X, Moisan J, Wang Y, Lesch CA, Spooner C, Morgan RW, Zawidzka EM, Mertz D, Bousley D, Majchrzak K, Kryczek I, Taylor C, Van Huis C, Skalitzky D, Hurd A, Aicher TD, Toogood PL, Glick GD, Paulos CM, Zou W, Carter LL. Synthetic RORgamma agonists regulate multiple pathways to enhance antitumor immunity. Oncoimmunology. 2016;5:e1254854. doi: 10.1080/2162402X.2016.1254854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohamed R, Lord GM. T-bet as a key regulator of mucosal immunity. Immunology. 2016;147:367–376. doi: 10.1111/imm.12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi M, Chen X, Ye K, Yao Y, Li Y. Application potential of toll-like receptors in cancer immunotherapy: Systematic review. Medicine (Baltimore) 2016;95:e3951. doi: 10.1097/MD.0000000000003951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li L, Peng L, Miao J, Qiu Y, Zhou Y, Gao X, Xu Y, Shi Z, Shao D, Ma Z. ICA induces the expression of toll-like receptor 9 in ana-1 murine macrophages. Phytother Res. 2011;25:1732–1735. doi: 10.1002/ptr.3514. [DOI] [PubMed] [Google Scholar]
- 32.Zhou J, Wu J, Chen X, Fortenbery N, Eksioglu E, Kodumudi KN, Pk EB, Dong J, Djeu JY, Wei S. ICA and its derivative, ICT, exert anti-inflammatory, anti-tumor effects, and modulate myeloid derived suppressive cells (MDSCs) functions. Int Immunopharmacol. 2011;11:890–898. doi: 10.1016/j.intimp.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shahmarvand N, Nagy A, Shahryari J, Ohgami RS. Mutations in the STAT family of genes in cancer. Cancer Sci. 2018;109:926–933. doi: 10.1111/cas.13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tao F, Qian C, Guo W, Luo Q, Xu Q, Sun Y. Inhibition of Th1/Th17 responses via suppression of STAT1 and STAT3 activation contributes to the amelioration of murine experimental colitis by a natural flavonoid glucoside ICA. Biochem Pharmacol. 2013;85:798–807. doi: 10.1016/j.bcp.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Caamano J, Tato C, Cai G, Villegas EN, Speirs K, Craig L, Alexander J, Hunter CA. Identification of a role for NF-kappa B2 in the regulation of apoptosis and in maintenance of T cell-mediated immunity to toxoplasma gondii. J Immunol. 2000;165:5720–5728. doi: 10.4049/jimmunol.165.10.5720. [DOI] [PubMed] [Google Scholar]
- 36.Zhou H, Yuan Y, Liu Y, Ni J, Deng W, Bian ZY, Dai J, Tang QZ. ICA protects H9c2 cardiomyocytes from lipopolysaccharideinduced injury via inhibition of the reactive oxygen speciesdependent cJun Nterminal kinases/nuclear factor-kappaB pathway. Mol Med Rep. 2015;11:4327–4332. doi: 10.3892/mmr.2015.3289. [DOI] [PubMed] [Google Scholar]
- 37.Zhang DC, Liu JL, Ding YB, Xia JG, Chen GY. ICA potentiates the antitumor activity of gemcitabine in gallbladder cancer by suppressing NF-kappaB. Acta Pharmacol Sin. 2013;34:301–308. doi: 10.1038/aps.2012.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li W, Wang M, Wang L, Ji S, Zhang J, Zhang C. ICA synergizes with arsenic trioxide to suppress human hepatocellular carcinoma. Cell Biochem Biophys. 2014;68:427–436. doi: 10.1007/s12013-013-9724-3. [DOI] [PubMed] [Google Scholar]
- 39.Shi DB, Li XX, Zheng HT, Li DW, Cai GX, Peng JJ, Gu WL, Guan ZQ, Xu Y, Cai SJ. ICA-mediated inhibition of NF-kappaB activity enhances the in vitro and in vivo antitumour effect of 5-fluorouracil in colorectal cancer. Cell Biochem Biophys. 2014;69:523–530. doi: 10.1007/s12013-014-9827-5. [DOI] [PubMed] [Google Scholar]
- 40.Matsuda S, Moriguchi T, Koyasu S, Nishida E. T lymphocyte activation signals for interleukin-2 production involve activation of MKK6-p38 and MKK7-SAPK/JNK signaling pathways sensitive to cyclosporin A. J Biol Chem. 1998;273:12378–12382. doi: 10.1074/jbc.273.20.12378. [DOI] [PubMed] [Google Scholar]
- 41.Li W, Whaley CD, Mondino A, Mueller DL. Blocked signal transduction to the ERK and JNK protein kinases in anergic CD4+ T cells. Science. 1996;271:1272–1276. doi: 10.1126/science.271.5253.1272. [DOI] [PubMed] [Google Scholar]
- 42.DeSilva DR, Feeser WS, Tancula EJ, Scherle PA. Anergic T cells are defective in both jun NH2-terminal kinase and mitogen-activated protein kinase signaling pathways. J Exp Med. 1996;183:2017–2023. doi: 10.1084/jem.183.5.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang D, Xu W, Chen X, Han J, Yu L, Gao C, Hao W, Liu X, Zheng Q, Li D. ICA induces cell differentiation and cell cycle arrest in mouse melanoma B16 cells via Erk1/2-p38-JNK-dependent pathway. Oncotarget. 2017;8:99504–99513. doi: 10.18632/oncotarget.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hua WB, Zhang YK, Wu XH, Kang L, Tu J, Zhao KC, Li S, Wang K, Song Y, Luo RJ, Shao ZW, Yang C. ICA attenuates interleukin-1beta-induced inflammatory response in human nucleus pulposus cells. Curr Pharm Des. 2017;23 doi: 10.2174/1381612823666170615112158. [DOI] [PubMed] [Google Scholar]
- 45.Baschant U, Tuckermann J. The role of the glucocorticoid receptor in inflammation and immunity. J Steroid Biochem Mol Biol. 2010;120:69–75. doi: 10.1016/j.jsbmb.2010.03.058. [DOI] [PubMed] [Google Scholar]
- 46.Wei K, Xu Y, Zhao Z, Wu X, Du Y, Sun J, Yi T, Dong J, Liu B. ICA alters the expression of glucocorticoid receptor, FKBP5 and SGK1 in rat brains following exposure to chronic mild stress. Int J Mol Med. 2016;38:337–344. doi: 10.3892/ijmm.2016.2591. [DOI] [PubMed] [Google Scholar]
- 47.Wei Z, Wang M, Hong M, Diao S, Liu A, Huang Y, Yu Q, Peng Z. ICA exerts estrogen-like activity in ameliorating EAE via mediating estrogen receptor beta, modulating HPA function and glucocorticoid receptor expression. Am J Transl Res. 2016;8:1910–1918. [PMC free article] [PubMed] [Google Scholar]
- 48.Li S, Zhu M, Pan R, Fang T, Cao YY, Chen S, Zhao X, Lei CQ, Guo L, Chen Y, Li CM, Jokitalo E, Yin Y, Shu HB, Guo D. The tumor suppressor PTEN has a critical role in antiviral innate immunity. Nat Immunol. 2016;17:241–249. doi: 10.1038/ni.3311. [DOI] [PubMed] [Google Scholar]
- 49.Toso A, Revandkar A, Di Mitri D, Guccini I, Proietti M, Sarti M, Pinton S, Zhang J, Kalathur M, Civenni G, Jarrossay D, Montani E, Marini C, Garcia-Escudero R, Scanziani E, Grassi F, Pandolfi PP, Catapano CV, Alimonti A. Enhancing chemotherapy efficacy in Pten-deficient prostate tumors by activating the senescence-associated antitumor immunity. Cell Rep. 2014;9:75–89. doi: 10.1016/j.celrep.2014.08.044. [DOI] [PubMed] [Google Scholar]
- 50.Chen L, Guo D. The functions of tumor suppressor PTEN in innate and adaptive immunity. Cell Mol Immunol. 2017;14:581–589. doi: 10.1038/cmi.2017.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arief ZM, Munshi AH, Shawl AS. Evaluation of medicinal value of epimedium elatum on the basis of pharmacologically active constituents, ICA and Icariside-II. Pak J Pharm Sci. 2015;28:1665–1669. [PubMed] [Google Scholar]
- 52.Li J, Jiang K, Zhao F. ICA regulates the proliferation and apoptosis of human ovarian cancer cells through microRNA-21 by targeting PTEN, RECK and Bcl-2. Oncol Rep. 2015;33:2829–2836. doi: 10.3892/or.2015.3891. [DOI] [PubMed] [Google Scholar]
- 53.Mitsumori S. Recent progress in work on PGD2 antagonists for drugs targeting allergic diseases. Curr Pharm Des. 2004;10:3533–3538. doi: 10.2174/1381612043382864. [DOI] [PubMed] [Google Scholar]
- 54.Qiao J, Sun S, Yuan L, Wang J. Effects of ICA on asthma mouse model are associated with regulation of prostaglandin D2 level. Allergol Immunopathol (Madr) 2017;45:567–572. doi: 10.1016/j.aller.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 55.Chen M, Wang H, Chen W, Meng G. Regulation of adaptive immunity by the NLRP3 inflammasome. Int Immunopharmacol. 2011;11:549–554. doi: 10.1016/j.intimp.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 56.Gris D, Ye Z, Iocca HA, Wen H, Craven RR, Gris P, Huang M, Schneider M, Miller SD, Ting JP. NLRP3 plays a critical role in the development of experimental autoimmune encephalomyelitis by mediating Th1 and Th17 responses. J Immunol. 2010;185:974–981. doi: 10.4049/jimmunol.0904145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine Growth Factor Rev. 2001;12:53–72. doi: 10.1016/s1359-6101(00)00015-0. [DOI] [PubMed] [Google Scholar]
- 58.Liu B, Xu C, Wu X, Liu F, Du Y, Sun J, Tao J, Dong J. ICA exerts an antidepressant effect in an unpredictable chronic mild stress model of depression in rats and is associated with the regulation of hippocampal neuroinflammation. Neuroscience. 2015;294:193–205. doi: 10.1016/j.neuroscience.2015.02.053. [DOI] [PubMed] [Google Scholar]
- 59.Zhang L, Wang XZ, Li YS, Zhang L, Hao LR. ICA ameliorates IgA nephropathy by inhibition of nuclear factor kappa b/Nlrp3 pathway. FEBS Open Bio. 2017;7:54–63. doi: 10.1002/2211-5463.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]


